Conformational Landscape, Polymorphism, and Solid Forms Design of Bicalutamide: A Review
Abstract
1. Introduction
2. Structural and Conformational Characteristics of Bicalutamide
2.1. Structural Features of Bicalutamide
2.2. Polymorphism, Conformational Classification, and Energetics
2.3. Solvent Effects, Non-Covalent Interactions, and Stabilisation Mechanisms
3. Experimental Methods in the Structural and Physicochemical Characterisation of BCL
3.1. Analytical Techniques Applied to BCL
3.2. Polymorphism and Crystalline Structures
3.3. Amorphous State and Phase Transformation Behaviour
3.4. Solvates and Co-Crystals
3.5. Solid Dispersions and Carrier-Based Systems
3.6. Studies on BCL Conformation in Solution
3.7. Quantitative Analysis of Conformer Populations: Concentration Dependence and Influence of Solvent Acceptor Number
3.8. Summary and Implications for Further Research
4. Bicalutamide in Supercritical Fluid Media
4.1. Initial Solubility Studies
4.2. scCO2 Processing of BCL Solid Dispersions
4.3. Alternative Polymer Matrices
4.4. Conformational Analysis in scCO2
4.5. Integration of SCF Processing Insights into Advanced Formulation Strategies
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BCL | Bicalutamide |
| SCF | Supercritical fluid |
| DHT | Dihydrotestosterone |
| AR | Androgen receptor |
| SAR | Structure–activity relationship |
| BCS | Biopharmaceutics Classification System |
| SDDS | Supersaturating drug delivery systems |
| LBD | Ligand-binding domain |
| LogP | Logarithm of the octanol–water partition coefficient |
| PCM | Polarisable continuum model |
| IEFPCM | Integral equation formalism polarisable continuum model |
| DMSO | Dimethyl sulfoxide |
| QTAIM | Quantum theory of atoms in molecules |
| NMR | Nuclear magnetic resonance |
| IR | Infrared |
| FT-IR | Fourier transform infrared spectroscopy |
| VCD | Vibrational circular dichroism |
| SEM | Scanning electron microscopy |
| XRPD | X-ray powder diffraction |
| DSC | Differential scanning calorimetry |
| BDS | Broadband dielectric spectroscopy |
| SPR | Surface plasmon resonance |
| UV–vis | Ultraviolet–visible |
| m.f. | Mole fraction |
| m.p. | Melting point |
| SD | Solid dispersion |
| PVP K30 | Polyvinylpyrrolidone (Povidone K30) |
| BSA | Bovine serum albumin |
| DFT | Density functional theory |
| ISPA | Isolated spin-pair approximation |
| CD3CN | Trideuteroacetonitrile |
| C6D6 | Deuterated benzene |
| DMSO-d6 | Deuterated dimethyl sulfoxide |
| CDCl3 | Deuterated chloroform |
| AN | Gutmann’s acceptor number |
| API | Active pharmaceutical ingredient |
| RESS | Rapid expansion of supercritical solutions |
| SAS | Supercritical anti-solvent |
| SBCW | Subcritical water |
| scCO2 | Supercritical CO2 |
| PEG | Polyethylene glycol |
| PLX | Poloxamer |
| NOE | Nuclear Overhauser effect |
| BZA | Benzamide |
| 2OHBZA | Salicylamide |
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Deng, X.; Shao, Z.; Zhao, Y. Solutions to the Drawbacks of Photothermal and Photodynamic Cancer Therapy. Adv. Sci. 2021, 8, 2002504. [Google Scholar] [CrossRef]
- Gavas, S.; Quazi, S.; Karpiński, T.M. Nanoparticles for Cancer Therapy: Current Progress and Challenges. Nanoscale Res. Lett. 2021, 16, 173. [Google Scholar] [CrossRef]
- Rawla, P. Epidemiology of Prostate Cancer. World J. Oncol. 2019, 10, 63–89. [Google Scholar] [CrossRef]
- Feldman, B.J.; Feldman, D. The Development of Androgen-Independent Prostate Cancer. Nat. Rev. Cancer 2001, 1, 34–45. [Google Scholar] [CrossRef]
- Garje, R.; Chennamadhavuni, A.; Mott, S.L.; Chambers, I.M.; Gellhaus, P.; Zakharia, Y.; Brown, J.A. Utilization and Outcomes of Surgical Castration in Comparison to Medical Castration in Metastatic Prostate Cancer. Clin. Genitourin. Cancer 2020, 18, e157–e166. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.R.; Hussain, M.; Saad, F.; Fizazi, K.; Sternberg, C.N.; Crawford, E.D.; Kopyltsov, E.; Park, C.H.; Alekseev, B.; Montesa-Pino, Á.; et al. Darolutamide and Survival in Metastatic, Hormone-Sensitive Prostate Cancer. N. Engl. J. Med. 2022, 386, 1132–1142. [Google Scholar] [CrossRef] [PubMed]
- Fizazi, K.; Foulon, S.; Carles, J.; Roubaud, G.; McDermott, R.; Fléchon, A.; Tombal, B.; Supiot, S.; Berthold, D.; Ronchin, P.; et al. Abiraterone plus Prednisone Added to Androgen Deprivation Therapy and Docetaxel in de Novo Metastatic Castration-Sensitive Prostate Cancer (PEACE-1): A Multicentre, Open-Label, Randomised, Phase 3 Study with a 2 × 2 Factorial Design. Lancet 2022, 399, 1695–1707. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, A.J.; Azad, A.A.; Iguchi, T.; Szmulewitz, R.Z.; Petrylak, D.P.; Holzbeierlein, J.; Villers, A.; Alcaraz, A.; Alekseev, B.; Shore, N.D.; et al. Improved Survival with Enzalutamide in Patients with Metastatic Hormone-Sensitive Prostate Cancer. J. Clin. Oncol. 2022, 40, 1616–1622. [Google Scholar] [CrossRef]
- James, N.D.; de Bono, J.S.; Spears, M.R.; Clarke, N.W.; Mason, M.D.; Dearnaley, D.P.; Ritchie, A.W.S.; Amos, C.L.; Gilson, C.; Jones, R.J.; et al. Abiraterone for Prostate Cancer Not Previously Treated with Hormone Therapy. N. Engl. J. Med. 2017, 377, 338–351. [Google Scholar] [CrossRef]
- Šlechta, P.; Viták, R.; Bárta, P.; Koucká, K.; Berková, M.; Žďárová, D.; Petríková, A.; Kuneš, J.; Kubíček, V.; Doležal, M.; et al. Replacement of Nitro Function by Free Boronic Acid in Non-Steroidal Anti-Androgens. RSC Med. Chem. 2024, 15, 4018–4038. [Google Scholar] [CrossRef] [PubMed]
- Vega, D.R.; Polla, G.; Martinez, A.; Mendioroz, E.; Reinoso, M. Conformational Polymorphism in Bicalutamide. Int. J. Pharm. 2007, 328, 112–118. [Google Scholar] [CrossRef]
- Korlyukov, A.A.; Malinska, M.; Vologzhanina, A.V.; Goizman, M.S.; Trzybinski, D.; Wozniak, K. Charge Density View on Bicalutamide Molecular Interactions in the Monoclinic Polymorph and Androgen Receptor Binding Pocket. IUCrJ 2020, 7, 71–82. [Google Scholar] [CrossRef]
- Bruchovsky, N.; Wilson, J.D. The Conversion of Testosterone to 5α-Androstan-17β-Ol-3-One by Rat Prostate in Vivo and in Vitro. Elsevier 1968, 243, 2012–2021. [Google Scholar] [CrossRef]
- Petrow, V. The Dihydrotestosterone (DHT) Hypothesis of Prostate Cancer and Its Therapeutic Implications. Prostate 1986, 9, 343–361. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.D. Role of Dihydrotestosterone in Androgen Action. Prostate 1996, 29, 88–92. [Google Scholar] [CrossRef]
- Huggins, C. Endocrine-Induced Regression of Cancers. Science 1967, 156, 1050–1054. [Google Scholar] [CrossRef]
- Greenlee, R.T.; Murray, T.; Bolden, S.; Wingo, P.A. Cancer Statistics, 2000. CA Cancer J. Clin. 2000, 50, 7–33. [Google Scholar] [CrossRef]
- Payen, O.; Top, S.; Vessières, A.; Brulé, E.; Plamont, M.A.; McGlinchey, M.J.; Müller-Bunz, H.; Jaouen, G. Synthesis and Structure-Activity Relationships of the First Ferrocenyl-Aryl-Hydantoin Derivatives of the Nonsteroidal Antiandrogen Nilutamide. J. Med. Chem. 2008, 51, 1791–1799. [Google Scholar] [CrossRef]
- Slater, S.; Oliver, R.T.D. Testosterone: Its Role in Development of Prostate Cancer and Potential Risk from Use as Hormone Replacement Therapy. Drugs Aging 2000, 17, 431–439. [Google Scholar] [CrossRef]
- Eaton, N.E.; Reeves, G.K.; Appleby, P.N.; Key, T.J. Endogenous Sex Hormones and Prostate Cancer: A Quantitative Review of Prospective Studies. Br. J. Cancer 1999, 80, 930–934. [Google Scholar] [CrossRef]
- Andersson, S.O.; Adami, H.O.; Bergstrom, R.; Wide, L. Serum Pituitary and Sex Steroid Hormone Levels in the Etiology of Prostatic Cancer—A Population-Based Case-Control Study. Br. J. Cancer 1993, 68, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Culig, Z.; Hoffmann, J.; Erdel, M.; Eder, I.E.; Hobisch, A.; Hittmair, A.; Bartsch, G.; Utermann, G.; Schneider, M.R.; Parczyk, K.; et al. Switch from Antagonist to Agonist of the Androgen Receptor Blocker Bicalutamide Is Associated with Prostate Tumour Progression in a New Model System. Br. J. Cancer 1999, 81, 242–251. [Google Scholar] [CrossRef]
- Gur, M.; Dil, E.; Akdeniz, E.; Cobanoglu, U.; Kalyoncu, N.I.; Topbas, M.; Ozyavuz, R.; Author, C.; Kalyoncu, N.İ. The Toxic Effects on the Testis of Flutamide vs. Bicalutamide vs. Cyproterone Acetate: An Experimental Rat Study. New Trends Med. Sci. 2024, 5, 84–90. [Google Scholar] [CrossRef]
- Chen, C.S.; Gao, G.L.; Ho, D.R.; Lin, C.Y.; Chou, Y.T.; Chen, S.C.; Huang, M.C.; Kao, W.Y.; Su, J.G.J. Cyproterone Acetate Acts as a Disruptor of the Aryl Hydrocarbon Receptor. Sci. Rep. 2021, 11, 5457. [Google Scholar] [CrossRef]
- Russell, N.; Hoermann, R.; Cheung, A.S.; Zajac, J.D.; Grossmann, M. Effects of Oestradiol Treatment on Hot Flushes in Men Undergoing Androgen Deprivation Therapy for Prostate Cancer: A Randomised Placebo-Controlled Trial. Eur. J. Endocrinol. 2022, 187, 617–627. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Namadchian, M.; Baghayeri, M. Follow up of the Prostate Cancer Treatment Based on a Novel Sensing Method for Anti-Prostate Cancer Drug (Flutamide). Environ. Res. 2023, 238, 117261. [Google Scholar] [CrossRef]
- Pavlik, T.; Gudkova, V.; Razvolyaeva, D.; Pavlova, M.; Kostukova, N.; Miloykovich, L.; Kolik, L.; Konchekov, E.; Shimanovskii, N. The Role of Autophagy and Apoptosis in the Combined Action of Plasma-Treated Saline, Doxorubicin, and Medroxyprogesterone Acetate on K562 Myeloid Leukaemia Cells. Int. J. Mol. Sci. 2023, 24, 5100. [Google Scholar] [CrossRef]
- La Vecchia, M.; Galanti, D.; Fazio, I.; Paratore, R.; Borsellino, N. Megestrol Acetate for Heavily Pretreated Metastatic Castration-Resistant Prostate Cancer: An Old Answer for a New Problem. Case Rep. Oncol. 2022, 15, 312–317. [Google Scholar] [CrossRef] [PubMed]
- Sanad, M.H.; Challan, S.B.; Essam, H.M.; Abdou, F.Y.; Farag, A.B. Design of a Novel Complex 99mTc-Nilutamide as a Tracer for Prostate Cancer Disorder Detection in Mice. Radiochim. Acta 2025, 113, 213–228. [Google Scholar] [CrossRef]
- Keerthika Devi, R.; Ganesan, M.; Chen, T.W.; Chen, S.M.; Lou, B.S.; Ajmal Ali, M.; Al-Hemaid, F.M.; Li, R.H. Gadolinium Vanadate Nanosheets Entrapped with 1D-Halloysite Nanotubes-Based Nanocomposite for the Determination of Prostate Anticancer Drug Nilutamide. J. Electroanal. Chem. 2022, 923, 116817. [Google Scholar] [CrossRef]
- Ueda, T.; Shiraishi, T.; Miyashita, M.; Kayukawa, N.; Gabata, Y.; Sako, S.; Ogura, R.; Fujihara, A.; Okihara, K.; Ukimura, O. Apalutamide versus Bicalutamide in Combination with Androgen Deprivation Therapy for Metastatic Hormone Sensitive Prostate Cancer. Sci. Rep. 2024, 14, 705. [Google Scholar] [CrossRef]
- Schellhammer, P.F. An Evaluation of Bicalutamide in the Treatment of Prostate Cancer. Expert Opin. Pharmacother. 2002, 3, 1313–1328. [Google Scholar] [CrossRef]
- Beebe-Dimmer, J.L.; Ruterbusch, J.J.; Bylsma, L.C.; Gillezeau, C.; Fryzek, J.; Schultz, N.M.; Flanders, S.C.; Barlev, A.; Heath, E.; Quek, R.G.W. Patterns of Bicalutamide Use in Prostate Cancer Treatment: A U.S. Real-World Analysis Using the SEER-Medicare Database. Adv. Ther. 2018, 35, 1438–1451. [Google Scholar] [CrossRef] [PubMed]
- Stanisławska, I.J.; Piwowarski, J.P.; Granica, S.; Kiss, A.K. The Effects of Urolithins on the Response of Prostate Cancer Cells to Non-Steroidal Antiandrogen Bicalutamide. Phytomedicine 2018, 46, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Charalabidis, A.; Sfouni, M.; Bergström, C.; Macheras, P. The Biopharmaceutics Classification System (BCS) and the Biopharmaceutics Drug Disposition Classification System (BDDCS): Beyond Guidelines. Int. J. Pharm. 2019, 566, 264–281. [Google Scholar] [CrossRef]
- Zhang, X.; Lionberger, R.A.; Davit, B.M.; Yu, L.X. Utility of Physiologically Based Absorption Modeling in Implementing Quality by Design in Drug Development. AAPS J. 2011, 13, 59–71. [Google Scholar] [CrossRef] [PubMed]
- Johnson, T.N.; Bonner, J.J.; Tucker, G.T.; Turner, D.B.; Jamei, M. Development and Applications of a Physiologically-Based Model of Paediatric Oral Drug Absorption. Eur. J. Pharm. Sci. 2018, 115, 57–67. [Google Scholar] [CrossRef]
- Kesisoglou, F.; Wu, Y. Understanding the Effect of API Properties on Bioavailability through Absorption Modeling. AAPS J. 2008, 10, 516–525. [Google Scholar] [CrossRef]
- Lloyd, A.; Penson, D.; Dewilde, S.; Kleinman, L. Eliciting Patient Preferences for Hormonal Therapy Options in the Treatment of Metastatic Prostate Cancer. Prostate Cancer Prostatic Dis. 2007, 11, 153–159. [Google Scholar] [CrossRef]
- Fradet, Y. Bicalutamide (Casodex®) in the Treatment of Prostate Cancer. Expert Rev. Anticancer. Ther. 2004, 4, 37–48. [Google Scholar] [CrossRef]
- Manso, G.; Thole, Z.; Salgueiro, E.; Revuelta, P.; Hidalgo, A. Spontaneous Reporting of Hepatotoxicity Associated with Antiandrogens: Data from the Spanish Pharmacovigilance System. Pharmacoepidemiol. Drug Saf. 2006, 15, 253–259. [Google Scholar] [CrossRef]
- Savjani, K.T.; Gajjar, A.K.; Savjani, J.K. Drug Solubility: Importance and Enhancement Techniques. ISRN Pharm. 2012, 2012, 195727. [Google Scholar] [CrossRef]
- Khadka, P.; Ro, J.; Kim, H.; Kim, I.; Kim, J.T.; Kim, H.; Cho, J.M.; Yun, G.; Lee, J. Pharmaceutical Particle Technologies: An Approach to Improve Drug Solubility, Dissolution and Bioavailability. Asian J. Pharm. Sci. 2014, 9, 304–316. [Google Scholar] [CrossRef]
- Khan, A.D.; Singh, L. Various Techniques of Bioavailability Enhancement: A Review. J. Drug Deliv. Ther. 2016, 6, 34–41. [Google Scholar] [CrossRef]
- Seedher, N.; Kanojia, M. Micellar Solubilization of Some Poorly Soluble Antidiabetic Drugs: A Technical Note. AAPS PharmSciTech 2008, 9, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Vandana, K.R.; Prasanna Raju, Y.; Harini Chowdary, V.; Sushma, M.; Vijay Kumar, N. An Overview on in Situ Micronization Technique—An Emerging Novel Concept in Advanced Drug Delivery. Saudi Pharm. J. 2014, 22, 283–289. [Google Scholar] [CrossRef]
- Han, X.; Ghoroi, C.; To, D.; Chen, Y.; Davé, R. Simultaneous Micronization and Surface Modification for Improvement of Flow and Dissolution of Drug Particles. Int. J. Pharm. 2011, 415, 185–195. [Google Scholar] [CrossRef]
- Loh, Z.H.; Samanta, A.K.; Sia Heng, P.W. Overview of Milling Techniques for Improving the Solubility of Poorly Water-Soluble Drugs. Asian J. Pharm. Sci. 2014, 10, 255–274. [Google Scholar] [CrossRef]
- Choudhury, H.; Gorain, B.; Madheswaran, T.; Pandey, M.; Kesharwani, P.; Tekade, R.K. Drug Complexation: Implications in Drug Solubilization and Oral Bioavailability Enhancement. Dos. Form Des. Consid. 2018, 1, 473–512. [Google Scholar] [CrossRef]
- Takenaka, H.; Kawashima, Y.; Lin, S.Y.; Ando, Y. Preparations of Solid Particulates of Theophylline-ethylenediamine Complex by a Spray-drying Technique. J. Pharm. Sci. 1982, 71, 914–919. [Google Scholar] [CrossRef]
- Brouwers, J.; Brewster, M.E.; Augustijns, P. Supersaturating Drug Delivery Systems: The Answer to Solubility-Limited Oral Bioavailability? J. Pharm. Sci. 2009, 98, 2549–2572. [Google Scholar] [CrossRef]
- Hens, B.; Bermejo, M.; Tsume, Y.; Gonzalez-Alvarez, I.; Ruan, H.; Matsui, K.; Amidon, G.E.; Cavanagh, K.L.; Kuminek, G.; Benninghoff, G.; et al. Evaluation and Optimized Selection of Supersaturating Drug Delivery Systems of Posaconazole (BCS Class 2b) in the Gastrointestinal Simulator (GIS): An in Vitro-in Silico-in Vivo Approach. Eur. J. Pharm. Sci. 2018, 115, 258–269. [Google Scholar] [CrossRef]
- Vaksler, Y.A.; Benedis, D.; Dyshin, A.A.; Oparin, R.D.; Correia, N.T.; Capet, F.; Shishkina, S.V.; Kiselev, M.G.; Idrissi, A. Spectroscopic Characterization of Single Co-Crystal of Mefenamic Acid and Nicotinamide Using Supercritical CO2. J. Mol. Liq. 2021, 334, 116117. [Google Scholar] [CrossRef]
- Singhal, D.; Curatolo, W. Drug Polymorphism and Dosage Form Design: A Practical Perspective. Adv. Drug Deliv. Rev. 2004, 56, 335–347. [Google Scholar] [CrossRef]
- Stanton, M.K.; Kelly, R.C.; Colletti, A.; Kiang, Y.H.; Langley, M.; Munson, E.J.; Peterson, M.L.; Roberts, J.; Wells, M. Improved Pharmacokinetics of AMG 517 through Co-Crystallization Part 1: Comparison of Two Acids with Corresponding Amide Co-Crystals. J. Pharm. Sci. 2010, 99, 3769–3778. [Google Scholar] [CrossRef]
- Nascimento, A.L.C.S.; Fernandes, R.P.; Charpentier, M.D.; ter Horst, J.H.; Caires, F.J.; Chorilli, M. Co-Crystals of Non-Steroidal Anti-Inflammatory Drugs (NSAIDs): Insight toward Formation, Methods, and Drug Enhancement. Particuology 2021, 58, 227–241. [Google Scholar] [CrossRef]
- Kumar Bandaru, R.; Rout, S.R.; Kenguva, G.; Gorain, B.; Alhakamy, N.A.; Kesharwani, P.; Dandela, R. Recent Advances in Pharmaceutical Cocrystals: From Bench to Market. Front. Pharmacol. 2021, 12, 780582. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Johnston, K.P.; Williams, R.O. Nanoparticle Engineering Processes for Enhancing the Dissolution Rates of Poorly Water Soluble Drugs. Drug Dev. Ind. Pharm. 2004, 30, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Chandel, S.; Bioavailability Enhancement, A.; Kumar Bolla, P.; Bhalani, D.V.; Nutan, B.; Kumar, A.; Singh Chandel, A.K. Bioavailability Enhancement Techniques for Poorly Aqueous Soluble Drugs and Therapeutics. Biomedicines 2022, 10, 2055. [Google Scholar] [CrossRef]
- Costa, P.; Sousa Lobo, J.M. Modeling and Comparison of Dissolution Profiles. Eur. J. Pharm. Sci. 2001, 13, 123–133. [Google Scholar] [CrossRef]
- Wei, W.; Evseenko, V.I.; Khvostov, M.V.; Borisov, S.A.; Tolstikova, T.G.; Polyakov, N.E.; Dushkin, A.V.; Xu, W.; Min, L.; Su, W. Solubility, Permeability, Anti-Inflammatory Action and In Vivo Pharmacokinetic Properties of Several Mechanochemically Obtained Pharmaceutical Solid Dispersions of Nimesulide. Molecules 2021, 26, 1513. [Google Scholar] [CrossRef]
- Aaltonen, J.; Allesø, M.; Mirza, S.; Koradia, V.; Gordon, K.C.; Rantanen, J. Solid Form Screening—A Review. Eur. J. Pharm. Biopharm. 2008, 71, 23–37. [Google Scholar] [CrossRef] [PubMed]
- Baghel, S.; Cathcart, H.; O’Reilly, N.J. Polymeric Amorphous Solid Dispersions: A Review of Amorphization, Crystallization, Stabilization, Solid-State Characterization, and Aqueous Solubilization of Biopharmaceutical Classification System Class II Drugs. J. Pharm. Sci. 2016, 105, 2527–2544. [Google Scholar] [CrossRef] [PubMed]
- Laitinen, R.; Löbmann, K.; Strachan, C.J.; Grohganz, H.; Rades, T. Emerging Trends in the Stabilization of Amorphous Drugs. Int. J. Pharm. 2013, 453, 65–79. [Google Scholar] [CrossRef]
- Liu, L.; Wang, J.R.; Mei, X. Enhancing the Stability of Active Pharmaceutical Ingredients by the Cocrystal Strategy. CrystEngComm. 2022, 24, 2002–2022. [Google Scholar] [CrossRef]
- Mannava, M.K.C.; Gunnam, A.; Lodagekar, A.; Shastri, N.R.; Nangia, A.K.; Solomon, K.A. Enhanced Solubility, Permeability, and Tabletability of Nicorandil by Salt and Cocrystal Formation. CrystEngComm 2021, 23, 227–237. [Google Scholar] [CrossRef]
- Zhou, Z.; Li, W.; Sun, W.J.; Lu, T.; Tong, H.H.Y.; Sun, C.C.; Zheng, Y. Resveratrol Cocrystals with Enhanced Solubility and Tabletability. Int. J. Pharm. 2016, 509, 391–399. [Google Scholar] [CrossRef]
- Bolla, G.; Sarma, B.; Nangia, A.K. Crystal Engineering of Pharmaceutical Cocrystals in the Discovery and Development of Improved Drugs. Chem. Rev. 2022, 122, 11514–11603. [Google Scholar] [CrossRef]
- Wang, J.; Dai, X.L.; Lu, T.B.; Chen, J.M. Temozolomide-Hesperetin Drug-Drug Cocrystal with Optimized Performance in Stability, Dissolution, and Tabletability. Cryst. Growth Des. 2021, 21, 838–846. [Google Scholar] [CrossRef]
- Wong, H.-H.; Jessup, A.; Sertkaya, A.; Birkenbach, A.; Berlind, A.; Eyraud, J. Examination of Clinical Trial Costs and Barriers for Drug Development Final; Office of Assistant Secretary for Planning and Evaluation: Washington, DC, USA, 2014. [Google Scholar]
- Sinha, S.; Vohora, D. Drug Discovery and Development: An Overview. Pharm. Med. Transl. Clin. Res. 2018, 19–32. [Google Scholar] [CrossRef]
- Liberti, L.; Stolk, P.; McAuslane, N.; Somauroo, A.; Breckenridge, A.M.; Leufkens, H.G.M. Adaptive Licensing and Facilitated Regulatory Pathways: A Survey of Stakeholder Perceptions. Clin. Pharmacol. Ther. 2015, 98, 477–479. [Google Scholar] [CrossRef] [PubMed]
- Surov, A.O.; Ramazanova, A.G.; Voronin, A.P.; Drozd, K.V.; Churakov, A.V.; Perlovich, G.L. Virtual Screening, Structural Analysis, and Formation Thermodynamics of Carbamazepine Cocrystals. Pharmaceutics 2023, 15, 836. [Google Scholar] [CrossRef] [PubMed]
- Karimi-Jafari, M.; Padrela, L.; Walker, G.M.; Croker, D.M. Creating Cocrystals: A Review of Pharmaceutical Cocrystal Preparation Routes and Applications. Cryst. Growth Des. 2018, 18, 6370–6387. [Google Scholar] [CrossRef]
- Joo, H.; Kraka, E.; Cremer, D. Environmental Effects on Molecular Conformation: Bicalutamide Analogs. J. Mol. Struct. Theochem. 2008, 862, 66–73. [Google Scholar] [CrossRef]
- Sobornova, V.V.; Belov, K.V.; Krestyaninov, M.A.; Khodov, I.A. Influence of Solvent Polarity on the Conformer Ratio of Bicalutamide in Saturated Solutions: Insights from NOESY NMR Analysis and Quantum-Chemical Calculations. Int. J. Mol. Sci. 2024, 25, 8254. [Google Scholar] [CrossRef]
- Marhefka, C.A.; Gao, W.; Chung, K.; Kim, J.; He, Y.; Yin, D.; Bohl, C.; Dalton, J.T.; Miller, D.D. Design, Synthesis, and Biological Characterization of Metabolically Stable Selective Androgen Receptor Modulators. J. Med. Chem. 2004, 47, 993–998. [Google Scholar] [CrossRef]
- Hwang, D.J.; Yang, J.; Xu, H.; Rakov, I.M.; Mohler, M.L.; Dalton, J.T.; Miller, D.D. Arylisothiocyanato Selective Androgen Receptor Modulators (SARMs) for Prostate Cancer. Bioorg. Med. Chem. 2006, 14, 6525–6538. [Google Scholar] [CrossRef]
- Dhaked, D.K.; Jain, V.; Kasetti, Y.; Bharatam, P.V. Conformational Polymorphism in Bicalutamide: A Quantum Chemical Study. Struct. Chem. 2012, 23, 1857–1866. [Google Scholar] [CrossRef]
- Mukherjee, A.; Kirkovsky, L.; Yao, X.T.; Yates, R.C.; Miller, D.D.; Dalton, J.T. Enantioselective Binding of Casodex to the Androgen Receptor. Xenobiotica 1996, 26, 117–122. [Google Scholar] [CrossRef]
- De Gaetano, F.; Cristiano, M.C.; Paolino, D.; Celesti, C.; Iannazzo, D.; Pistarà, V.; Iraci, N.; Ventura, C.A. Bicalutamide Anticancer Activity Enhancement by Formulation of Soluble Inclusion Complexes with Cyclodextrins. Biomolecules 2022, 12, 1716. [Google Scholar] [CrossRef]
- Mckillop, D.; Boyle, G.W.; Cockshott, I.D.; Jones, D.C.; Phillips, P.J.; Yates, R.A. Metabolism and Enantioselective Pharmacokinetics of Casodex in Man. Xenobiotica 1993, 23, 1241–1253. [Google Scholar] [CrossRef]
- Kirkovsky, L.; Mukherjee, A.; Yin, D.; Dalton, J.T.; Miller, D.D. Chiral Nonsteroidal Affinity Ligands for the Androgen Receptor. 1. Bicalutamide Analogues Bearing Electrophilic Groups in the B Aromatic Ring. J. Med. Chem. 2000, 43, 581–590. [Google Scholar] [CrossRef]
- Nageswara Rao, R.; Narasa Raju, A.; Nagaraju, D. An Improved and Validated LC Method for Resolution of Bicalutamide Enantiomers Using Amylose Tris-(3,5-Dimethylphenylcarbamate) as a Chiral Stationary Phase. J. Pharm. Biomed. Anal. 2006, 42, 347–353. [Google Scholar] [CrossRef]
- Török, R.; Bor, Á.; Orosz, G.; Lukács, F.; Armstrong, D.W.; Péter, A. High-Performance Liquid Chromatographic Enantioseparation of Bicalutamide and Its Related Compounds. J. Chromatogr. A 2005, 1098, 75–81. [Google Scholar] [CrossRef]
- Sadutto, D.; Ferretti, R.; Zanitti, L.; Casulli, A.; Cirilli, R. Analytical and Semipreparative High Performance Liquid Chromatography Enantioseparation of Bicalutamide and Its Chiral Impurities on an Immobilized Polysaccharide-Based Chiral Stationary Phase. J. Chromatogr. A 2016, 1445, 166–171. [Google Scholar] [CrossRef]
- Osguthorpe, D.J.; Hagler, A.T. Mechanism of Androgen Receptor Antagonism by Bicalutamide in the Treatment of Prostate Cancer. Biochemistry 2011, 50, 4105–4113. [Google Scholar] [CrossRef] [PubMed]
- Bohl, C.E.; Gao, W.; Miller, D.D.; Bell, C.E.; Dalton, J.T. Structural Basis for Antagonism and Resistance of Bicalutamide in Prostate Cancer. Proc. Natl. Acad. Sci. USA 2005, 102, 6201–6206. [Google Scholar] [CrossRef] [PubMed]
- Blaising, J.; Polyak, S.J.; Pécheur, E.I. Arbidol as a Broad-Spectrum Antiviral: An Update. Antiviral Res. 2014, 107, 84–94. [Google Scholar] [CrossRef]
- Perlovich, G.L.; Blokhina, S.V.; Manin, N.G.; Volkova, T.V.; Tkachev, V.V. Polymorphism and Solvatomorphism of Bicalutamide: Thermophysical Study and Solubility. J. Therm. Anal. Calorim. 2013, 111, 655–662. [Google Scholar] [CrossRef]
- Bis, J.A.; Vishweshwar, P.; Weyna, D.; Zaworotko, M.J. Hierarchy of Supramolecular Synthons: Persistent Hydroxyl···Pyridine Hydrogen Bonds in Cocrystals That Contain a Cyano Acceptor. Mol. Pharm. 2007, 4, 401–416. [Google Scholar] [CrossRef]
- Surov, A.O.; Solanko, K.A.; Bond, A.D.; Bauer-Brandl, A.; Perlovich, G.L. Cocrystals of the Antiandrogenic Drug Bicalutamide: Screening, Crystal Structures, Formation Thermodynamics and Lattice Energies. CrystEngComm 2016, 18, 4818–4829. [Google Scholar] [CrossRef]
- Hu, X.R.; Gu, J.M. N-[4-Cyano-3-(Trifluoromethyl)Phenyl]-3-(4-Fluorophenylsulfonyl) -2-Hydroxy-2-Methylpropionamide. Acta Crystallogr. Sect. E Struct. Report Online 2005, 61, o3897–o3898. [Google Scholar] [CrossRef]
- Nair, V.A.; Mustafa, S.M.; Mohler, M.L.; Dalton, J.T.; Miller, D.D. Synthesis of Oxazolidinedione Derived Bicalutamide Analogs. Tetrahedron Lett. 2006, 47, 3953–3955. [Google Scholar] [CrossRef]
- Mololina, A.A.; Sobornova, V.V.; Krestyaninov, M.A.; Belov, K.V.; Khodov, I.A. Conformational Equilibria of Bicalutamide: A Study Based on One-Dimensional Selective Nuclear Overhauser Effect Experiments. Russ. J. Phys. Chem. A 2024, 98, 3530–3537. [Google Scholar] [CrossRef]
- Le, Y.; Ji, H.; Chen, J.F.; Shen, Z.; Yun, J.; Pu, M. Nanosized Bicalutamide and Its Molecular Structure in Solvents. Int. J. Pharm. 2009, 370, 175–180. [Google Scholar] [CrossRef]
- Miertuš, S.; Scrocco, E.; Tomasi, J. Electrostatic Interaction of a Solute with a Continuum. A Direct Utilizaion of AB Initio Molecular Potentials for the Prevision of Solvent Effects. Chem. Phys. 1981, 55, 117–129. [Google Scholar] [CrossRef]
- Pascual-ahuir, J.L.; Silla, E.; Tuñon, I.; Pascual-ahuir, J.L.; Silla, E.; Tuñon, I. GEPOL: An Improved Description of Molecular Surfaces. III. A New Algorithm for the Computation of a Solvent-Excluding Surface. J. Comput. Chem. 1994, 15, 1127–1138. [Google Scholar] [CrossRef]
- Volkova, T.V.; Simonova, O.R.; Perlovich, G.L. Physicochemical Profile of Antiandrogen Drug Bicalutamide: Solubility, Distribution, Permeability. Pharmaceutics 2022, 14, 674. [Google Scholar] [CrossRef]
- Escudero, F.; Yáñez, M. Atoms in Molecules. Mol. Phys. 1982, 45, 617–628. [Google Scholar] [CrossRef]
- Chakalov, E.R.; Tupikina, E.Y.; Ivanov, D.M.; Bartashevich, E.V.; Tolstoy, P.M. The Distance between Minima of Electron Density and Electrostatic Potential as a Measure of Halogen Bond Strength. Molecules 2022, 27, 4848. [Google Scholar] [CrossRef]
- Mololina, A.A.; Sobornova, V.V.; Belov, K.V.; Krestyaninov, M.A.; Khodov, I.A. Role of Non-Covalent Interactions in the Conformational Stability of Bicalutamide in Different Solvent Environments: Insights from Quantum-Chemical Calculations and NMR Spectroscopy. J. Mol. Liq. 2025, 423, 126921. [Google Scholar] [CrossRef]
- Belov, K.V.; Khodov, I.A. NOESY Investigation of Bicalutamide Conformational Changes in DMSO: Role of Solution Concentration in Polymorph Formation. J. Mol. Liq. 2025, 422, 126896. [Google Scholar] [CrossRef]
- Rams-Baron, M.; Wlodarczyk, P.; Dulski, M.; Wlodarczyk, A.; Kruk, D.; Rachocki, A.; Jachowicz, R.; Paluch, M. The Indications of Tautomeric Conversion in Amorphous Bicalutamide Drug. Eur. J. Pharm. Sci. 2017, 110, 117–123. [Google Scholar] [CrossRef]
- Ren, F.; Jing, Q.; Tang, Y.; Shen, Y.; Chen, J.; Gao, F.; Cui, J. Characteristics of Bicalutamide Solid Dispersions and Improvement of the Dissolution. Drug Dev. Ind. Pharm. 2006, 32, 967–972. [Google Scholar] [CrossRef]
- Cheng, X.; Chen, X.; Liang, C.; Jin, H.; Ren, S.; Xue, R.; Chen, F. Explanation and Prediction for the Crystallized Products from Amorphous Bicalutamide and Bicalutamide Solutions by Using Mid-Frequency Raman Difference Spectra. Vib. Spectrosc. 2023, 127, 103565. [Google Scholar] [CrossRef]
- Tres, F.; Patient, J.D.; Williams, P.M.; Treacher, K.; Booth, J.; Hughes, L.P.; Wren, S.A.C.; Aylott, J.W.; Burley, J.C. Monitoring the Dissolution Mechanisms of Amorphous Bicalutamide Solid Dispersions via Real-Time Raman Mapping. Mol. Pharm. 2015, 12, 1512–1522. [Google Scholar] [CrossRef]
- Ray, S.; Ghosh, S.; Mandal, S. Development of Bicalutamide-Loaded PLGA Nanoparticles: Preparation, Characterization and in-Vitro Evaluation for the Treatment of Prostate Cancer. Artif. Cells Nanomedicine Biotechnol. 2017, 45, 944–954. [Google Scholar] [CrossRef]
- Yang, C.; Di, P.; Fu, J.P.; Xiong, H.; Jing, Q.; Ren, G.; Tang, Y.; Zheng, W.; Liu, G.; Ren, F. Improving the Physicochemical Properties of Bicalutamide by Complex Formation with Bovine Serum Albumin. Eur. J. Pharm. Sci. 2017, 106, 381–392. [Google Scholar] [CrossRef] [PubMed]
- Szafraniec-Szczęsny, J.; Antosik-Rogóż, A.; Knapik-Kowalczuk, J.; Kurek, M.; Szefer, E.; Gawlak, K.; Chmiel, K.; Peralta, S.; Niwiński, K.; Pielichowski, K.; et al. Compression-Induced Phase Transitions of Bicalutamide. Pharmaceutics 2020, 12, 438. [Google Scholar] [CrossRef]
- Szczurek, J.; Rams-Baron, M.; Knapik-Kowalczuk, J.; Antosik, A.; Szafraniec, J.; Jamróz, W.; Dulski, M.; Jachowicz, R.; Paluch, M. Molecular Dynamics, Recrystallization Behavior, and Water Solubility of the Amorphous Anticancer Agent Bicalutamide and Its Polyvinylpyrrolidone Mixtures. Mol. Pharm. 2017, 14, 1071–1081. [Google Scholar] [CrossRef]
- Cruz-Cabeza, A.J.; Bernstein, J. Conformational Polymorphism. Chem. Rev. 2014, 114, 2170–2191. [Google Scholar] [CrossRef] [PubMed]
- Nangia, A. Conformational Polymorphism in Organic Crystals. Acc. Chem. Res. 2008, 41, 595–604. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, J.; Hagler, A.T. Conformational Polymorphism. The Influence of Crystal Structure on Molecular Conformation. J. Am. Chem. Soc. 1978, 100, 673–681. [Google Scholar] [CrossRef]
- Bauer, J.; Spanton, S.; Henry, R.; Quick, J.; Dziki, W.; Porter, W.; Morris, J. Ritonavir: An Extraordinary Example of Conformational Polymorphism. Pharm. Res. 2001, 18, 859–866. [Google Scholar] [CrossRef]
- Shi, P.; Han, Y.; Zhu, Z.; Gong, J. Research Progress on the Molecular Mechanism of Polymorph Nucleation in Solution: A Perspective from Research Mentality and Technique. Crystals 2023, 13, 1206. [Google Scholar] [CrossRef]
- Yu, L.; Reutzel-Edens, S.M.; Mitchell, C.A. Crystallization and Polymorphism of Conformationally Flexible Molecules: Problems, Patterns, and Strategies. Org. Process Res. Dev. 2000, 4, 396–402. [Google Scholar] [CrossRef]
- Shukla, A.K.; Maikap, G.C.; Agarwal, S.K. Process for Preparation of Bicalutamide. US7544828B2, 9 June 2006. [Google Scholar]
- Shintaku, T.; Katsura, T.; Itaya, N. Crystal of Bicalutamide and Production Method Thereof. US20030191337A1, 25 May 2004. [Google Scholar]
- Perlovich, G.L.; Bauer-Brandl, A. Solvation of Drugs as a Key for Understanding Partitioning and Passive Transport Exemplified by NSAIDs. Curr. Drug Deliv. 2008, 1, 213–226. [Google Scholar] [CrossRef]
- Német, Z.; Sztatisz, J.; Demeter, Á. Polymorph Transitions of Bicalutamide: A Remarkable Example of Mechanical Activation. J. Pharm. Sci. 2008, 97, 3222–3232. [Google Scholar] [CrossRef]
- Ito, T.; Byrn, S.; Chen, X.; Carvajal, M.T. Thermal Insight of Mechanically Activated Bile Acid Powders. Int. J. Pharm. 2011, 420, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, N.; Yang, J.; Huang, Y.; Ji, X.; Huang, X.; Wang, T.; Wang, H.; Hao, H. Molecular Conformational Evolution Mechanism during Nucleation of Crystals in Solution. IUCrJ 2020, 7, 542–556. [Google Scholar] [CrossRef]
- Back, K.R.; Davey, R.J.; Grecu, T.; Hunter, C.A.; Taylor, L.S. Molecular Conformation and Crystallization: The Case of Ethenzamide. Cryst. Growth Des. 2012, 12, 6110–6117. [Google Scholar] [CrossRef]
- Shi, P.; Xu, S.; Du, S.; Rohani, S.; Liu, S.; Tang, W.; Jia, L.; Wang, J.; Gong, J. Insight into Solvent-Dependent Conformational Polymorph Selectivity: The Case of Undecanedioic Acid. Cryst. Growth Des. 2018, 18, 5947–5956. [Google Scholar] [CrossRef]
- Oparin, R.D.; Vaksler, Y.A.; Krestyaninov, M.A.; Idrissi, A.; Shishkina, S.V.; Kiselev, M.G. Polymorphism and Conformations of Mefenamic Acid in Supercritical Carbon Dioxide. J. Supercrit. Fluids 2019, 152, 104547. [Google Scholar] [CrossRef]
- Belov, K.V.; Dyshin, A.A.; Krestyaninov, M.A.; Efimov, S.V.; Khodov, I.A.; Kiselev, M.G. Conformational Preferences of Tolfenamic Acid in DMSO-CO2 Solvent System by 2D NOESY. J. Mol. Liq. 2022, 367, 120481. [Google Scholar] [CrossRef]
- Khodov, I.A.; Belov, K.V.; Krestyaninov, M.A.; Dyshin, A.A.; Kiselev, M.G.; Krestov, G.A. Investigation of the Spatial Structure of Flufenamic Acid in Supercritical Carbon Dioxide Media via 2D NOESY. Materials 2023, 16, 1524. [Google Scholar] [CrossRef]
- Yamasaki, R.; Tanatani, A.; Azumaya, I.; Masu, H.; Yamaguchi, K.; Kagechika, H. Solvent-Dependent Conformational Switching of N-Phenylhydroxamic Acid and Its Application in Crystal Engineering. Cryst. Growth Des. 2006, 6, 2007–2010. [Google Scholar] [CrossRef]
- Ischenko, V.; Englert, U.; Jansen, M. Conformational Dimorphism of 1,1,3,3,5,5-Hexachloro-1,3,5-Trigermacyclohexane: Solvent-Induced Crystallization of a Metastable Polymorph Containing Boat-Shaped Molecules. Chem. A Eur. J. 2005, 11, 1375–1383. [Google Scholar] [CrossRef]
- Gutmann, V.; Wychera, E. Coordination Reactions in Non Aqueous Solutions—The Role of the Donor Strength. Inorg. Nucl. Chem. Lett. 1966, 2, 257–260. [Google Scholar] [CrossRef]
- Mayer, U.; Gutmann, V.; Gerger, W. The Acceptor Number—A Quantitative Empirical Parameter for the Electrophilic Properties of Solvents. Monatshefte Chem. 1975, 106, 1235–1257. [Google Scholar] [CrossRef]
- Knez, Ž.; Markočič, E.; Leitgeb, M.; Primožič, M.; Hrnčič, M.K.; Škerget, M. Industrial Applications of Supercritical Fluids: A Review. Energy 2014, 77, 235–243. [Google Scholar] [CrossRef]
- Girotra, P.; Singh, S.K.; Nagpal, K. Supercritical Fluid Technology: A Promising Approach in Pharmaceutical Research. Pharm. Dev. Technol. 2013, 18, 22–38. [Google Scholar] [CrossRef]
- Li, K.; Xu, Z. A Review of Current Progress of Supercritical Fluid Technologies for E-Waste Treatment. J. Clean. Prod. 2019, 227, 794–809. [Google Scholar] [CrossRef]
- Preetam, A.; Jadhao, P.R.; Naik, S.N.; Pant, K.K.; Kumar, V. Supercritical Fluid Technology—An Eco-Friendly Approach for Resource Recovery from e-Waste and Plastic Waste: A Review. Sep. Purif. Technol. 2023, 304, 122314. [Google Scholar] [CrossRef]
- de Oliveira, C.R.S.; de Oliveira, P.V.; Pellenz, L.; de Aguiar, C.R.L.; da Silva Júnior, A.H. Supercritical Fluid Technology as a Sustainable Alternative Method for Textile Dyeing: An Approach on Waste, Energy, and CO2 Emission Reduction. J. Environ. Sci. 2024, 140, 123–145. [Google Scholar] [CrossRef] [PubMed]
- Fraguela-Meissimilly, H.; Bastías-Monte, J.M.; Vergara, C.; Ortiz-Viedma, J.; Lemus-Mondaca, R.; Flores, M.; Toledo-Merma, P.; Alcázar-Alay, S.; Gallón-Bedoya, M. New Trends in Supercritical Fluid Technology and Pressurized Liquids for the Extraction and Recovery of Bioactive Compounds from Agro-Industrial and Marine Food Waste. Molecules 2023, 28, 4421. [Google Scholar] [CrossRef]
- Liu, Z.; Navik, R.; Tan, H.; Xiang, Q.; Wahyudiono; Goto, M.; Ibarra, R.M.; Zhao, Y. Graphene-Based Materials Prepared by Supercritical Fluid Technology and Its Application in Energy Storage. J. Supercrit. Fluids 2022, 188, 105672. [Google Scholar] [CrossRef]
- Zhou, J.; Gullón, B.; Wang, M.; Gullón, P.; Lorenzo, J.M.; Barba, F.J. The Application of Supercritical Fluids Technology to Recover Healthy Valuable Compounds from Marine and Agricultural Food Processing By-Products: A Review. Processes 2021, 9, 357. [Google Scholar] [CrossRef]
- Tran, P.; Park, J.S. Application of Supercritical Fluid Technology for Solid Dispersion to Enhance Solubility and Bioavailability of Poorly Water-Soluble Drugs. Int. J. Pharm. 2021, 610, 121247. [Google Scholar] [CrossRef]
- Ranieri, U.; Formisano, F.; Gorelli, F.A.; Santoro, M.; Koza, M.M.; De Francesco, A.; Bove, L.E. Crossover from Gas-like to Liquid-like Molecular Diffusion in a Simple Supercritical Fluid. Nat. Commun. 2024, 15, 4142. [Google Scholar] [CrossRef]
- Du, Y.; Liao, G.; Zhang, F.; Jiaqiang, E.; Chen, J. Recognition of Supercritical CO2 Liquid-like and Gas-like Molecules Based on Deep Neural Network. J. Supercrit. Fluids 2024, 206, 106164. [Google Scholar] [CrossRef]
- Carlès, P. A Brief Review of the Thermophysical Properties of Supercritical Fluids. J. Supercrit. Fluids 2010, 53, 2–11. [Google Scholar] [CrossRef]
- King, J.W. Modern Supercritical Fluid Technology for Food Applications. Annu. Rev. Food Sci. Technol. 2014, 5, 215–238. [Google Scholar] [CrossRef]
- Braga, M.E.M.; Gaspar, M.C.; de Sousa, H.C. Supercritical Fluid Technology for Agrifood Materials Processing. Curr. Opin. Food Sci. 2023, 50, 100983. [Google Scholar] [CrossRef]
- Abdulagatov, I.M.; Skripov, P.V. Thermodynamic and Transport Properties of Supercritical Fluids. Part 2: Review of Transport Properties. Russ. J. Phys. Chem. B 2021, 15, 1171–1188. [Google Scholar] [CrossRef]
- Martín, A.; Cocero, M.J. Micronization Processes with Supercritical Fluids: Fundamentals and Mechanisms. Adv. Drug Deliv. Rev. 2008, 60, 339–350. [Google Scholar] [CrossRef]
- Kerč, J.; Srčič, S.; Knez, Ž.; Senčar-Božič, P. Micronization of Drugs Using Supercritical Carbon Dioxide. Int. J. Pharm. 1999, 182, 33–39. [Google Scholar] [CrossRef]
- Türk, M. Particle Synthesis by Rapid Expansion of Supercritical Solutions (RESS): Current State, Further Perspectives and Needs. J. Aerosol Sci. 2022, 161, 105950. [Google Scholar] [CrossRef]
- Liu, G.; Li, J.; Deng, S. Applications of Supercritical Anti-Solvent Process in Preparation of Solid Multicomponent Systems. Pharmaceutics 2021, 13, 475. [Google Scholar] [CrossRef] [PubMed]
- Kayrak, D.; Akman, U.; Hortaçsu, Ö. Micronization of Ibuprofen by RESS. J. Supercrit. Fluids 2003, 26, 17–31. [Google Scholar] [CrossRef]
- Pathak, P.; Meziani, M.J.; Desai, T.; Sun, Y.P. Formation and Stabilization of Ibuprofen Nanoparticles in Supercritical Fluid Processing. J. Supercrit. Fluids 2006, 37, 279–286. [Google Scholar] [CrossRef]
- Jung, J.; Perrut, M. Particle Design Using Supercritical Fluids: Literature and Patent Survey. J. Supercrit. Fluids 2001, 20, 179–219. [Google Scholar] [CrossRef]
- Knez, Ž.; Hrnčič, M.K.; Škerget, M. Particle Formation and Product Formulation Using Supercritical Fluids. Annu. Rev. Chem. Biomol. Eng. 2015, 6, 379–407. [Google Scholar] [CrossRef]
- Jessop, P.G.; Subramaniam, B. Gas-Expanded Liquids. Chem. Rev. 2007, 107, 2666–2694. [Google Scholar] [CrossRef] [PubMed]
- Rossmann, M.; Braeuer, A.; Dowy, S.; Gallinger, T.G.; Leipertz, A.; Schluecker, E. Solute Solubility as Criterion for the Appearance of Amorphous Particle Precipitation or Crystallization in the Supercritical Antisolvent (SAS) Process. J. Supercrit. Fluids 2012, 66, 350–358. [Google Scholar] [CrossRef]
- Chong, G.H.; Yunus, R.; Choong, T.S.Y.; Abdullah, N.; Spotar, S.Y. Simple Guidelines for a Self-Built Laboratory-Scale Supercritical Anti-Solvent System. J. Supercrit. Fluids 2011, 60, 69–74. [Google Scholar] [CrossRef]
- Pu, Y.; Cai, F.; Wang, D.; Li, Y.; Chen, X.; Maimouna, A.G.; Wu, Z.; Wen, X.; Chen, J.F.; Foster, N.R. Solubility of Bicalutamide, Megestrol Acetate, Prednisolone, Beclomethasone Dipropionate, and Clarithromycin in Subcritical Water at Different Temperatures from 383.15 to 443.15 K. J. Chem. Eng. Data 2017, 62, 1139–1145. [Google Scholar] [CrossRef]
- Szafraniec, J.; Antosik, A.; Knapik-Kowalczuk, J.; Kurek, M.; Syrek, K.; Chmiel, K.; Paluch, M.; Jachowicz, R. Planetary Ball Milling and Supercritical Fluid Technology as a Way to Enhance Dissolution of Bicalutamide. Int. J. Pharm. 2017, 533, 470–479. [Google Scholar] [CrossRef]
- Patel, S.; Kou, X.; Hou, H.; Huang, Y.; Strong, J.C.; Zhang, G.G.Z.; Sun, C.C. Mechanical Properties and Tableting Behavior of Amorphous Solid Dispersions. J. Pharm. Sci. 2017, 106, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Antosik-Rogóż, A.; Szafraniec-Szczęsny, J.; Gawlak, K.; Knapik-Kowalczuk, J.; Paluch, M.; Jachowicz, R. Tabletting Solid Dispersions of Bicalutamide Prepared Using Ball-Milling or Supercritical Carbon Dioxide: The Interrelationship between Phase Transition and in-Vitro Dissolution. Pharm. Dev. Technol. 2020, 25, 1109–1117. [Google Scholar] [CrossRef]
- Antosik-Rogóż, A.; Szafraniec-Szczęsny, J.; Chmiel, K.; Knapik-Kowalczuk, J.; Kurek, M.; Gawlak, K.; Danesi, V.P.; Paluch, M.; Jachowicz, R. How Does the CO2 in Supercritical State Affect the Properties of Drug-Polymer Systems, Dissolution Performance and Characteristics of Tablets Containing Bicalutamide? Materials 2020, 13, 2848. [Google Scholar] [CrossRef]
- Belov, K.V.; Khodov, I.A. Open or Closed? Quantifying Bicalutamide Conformers in Supercritical Carbon Dioxide by 2D NOESY. J. Mol. Struct. 2025, 1348, 143449. [Google Scholar] [CrossRef]
- Belov, K.V.; Dyshin, A.A.; Krestyaninov, M.A.; Khodov, I.A. 1D NOESY Study of Bicalutamide Conformations in a Supercritical CO2. J. Mol. Liq. 2025, 436, 128262. [Google Scholar] [CrossRef]
- Pasquali, I.; Bettini, R.; Giordano, F. Supercritical Fluid Technologies: An Innovative Approach for Manipulating the Solid-State of Pharmaceuticals. Adv. Drug Deliv. Rev. 2008, 60, 399–410. [Google Scholar] [CrossRef] [PubMed]
- Moribe, K.; Tozuka, Y.; Yamamoto, K. Supercritical Carbon Dioxide Processing of Active Pharmaceutical Ingredients for Polymorphic Control and for Complex Formation. Adv. Drug Deliv. Rev. 2008, 60, 328–338. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.S.; Borsadia, S. Particle Formation Using Supercritical Fluids: Pharmaceutical Applications. Expert Opin. Ther. Pat. 2001, 11, 861–872. [Google Scholar] [CrossRef]
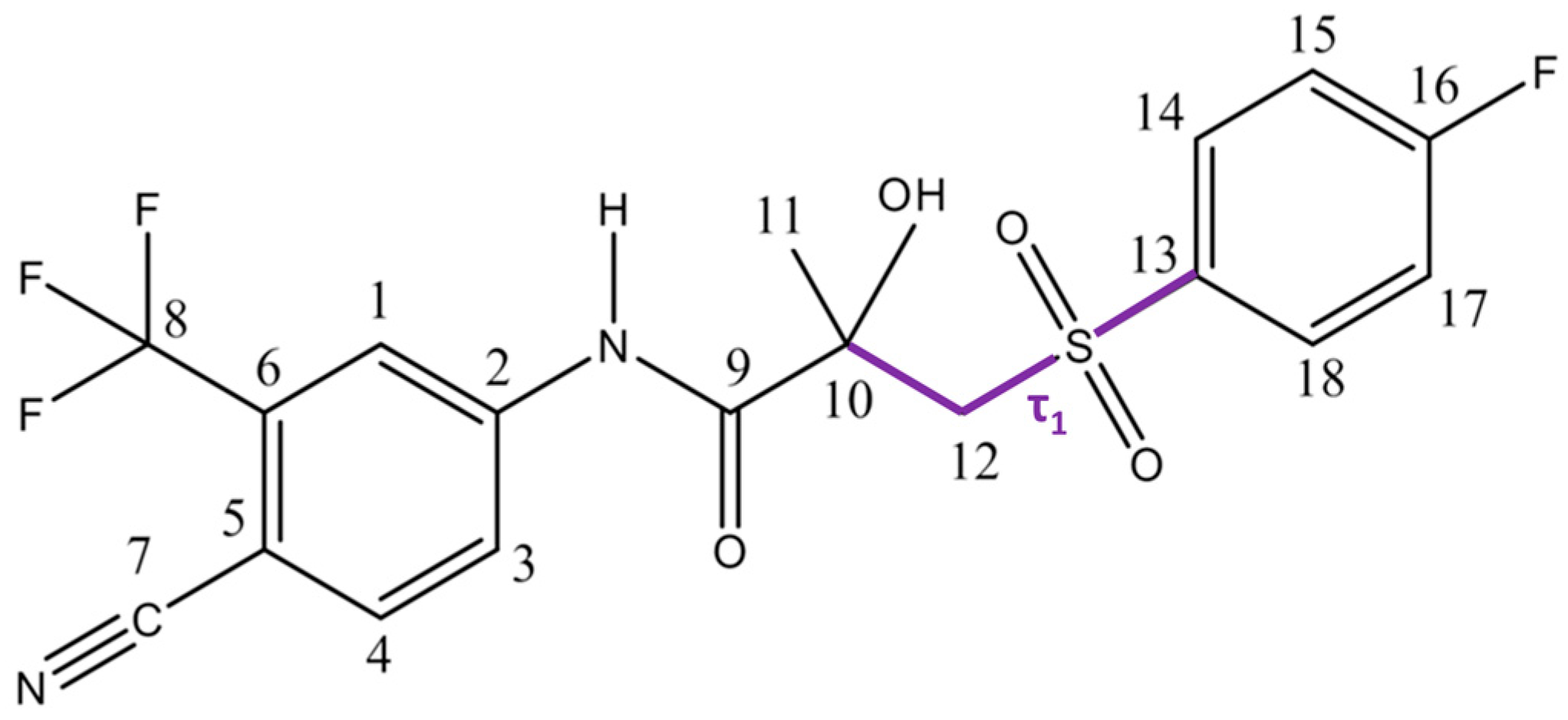

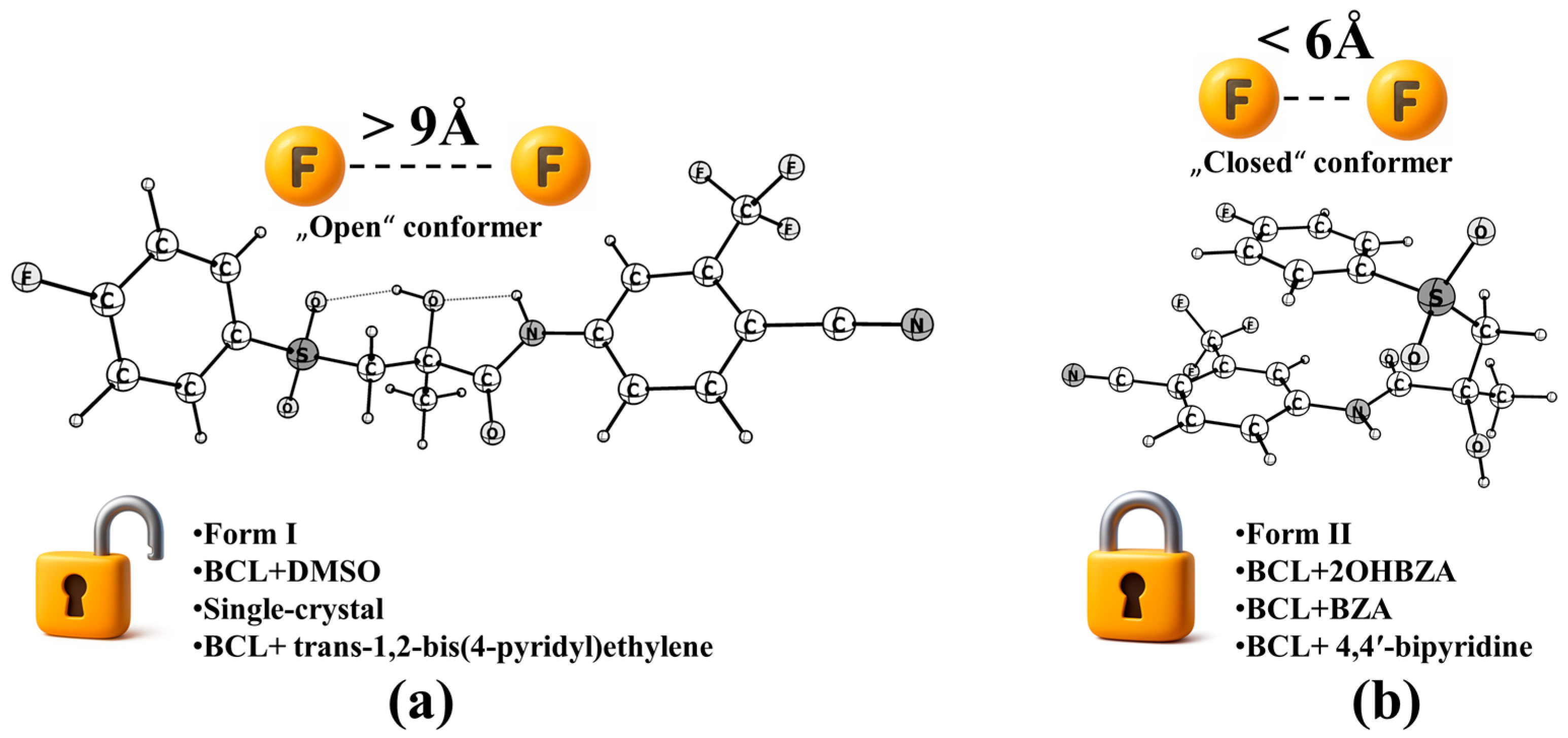

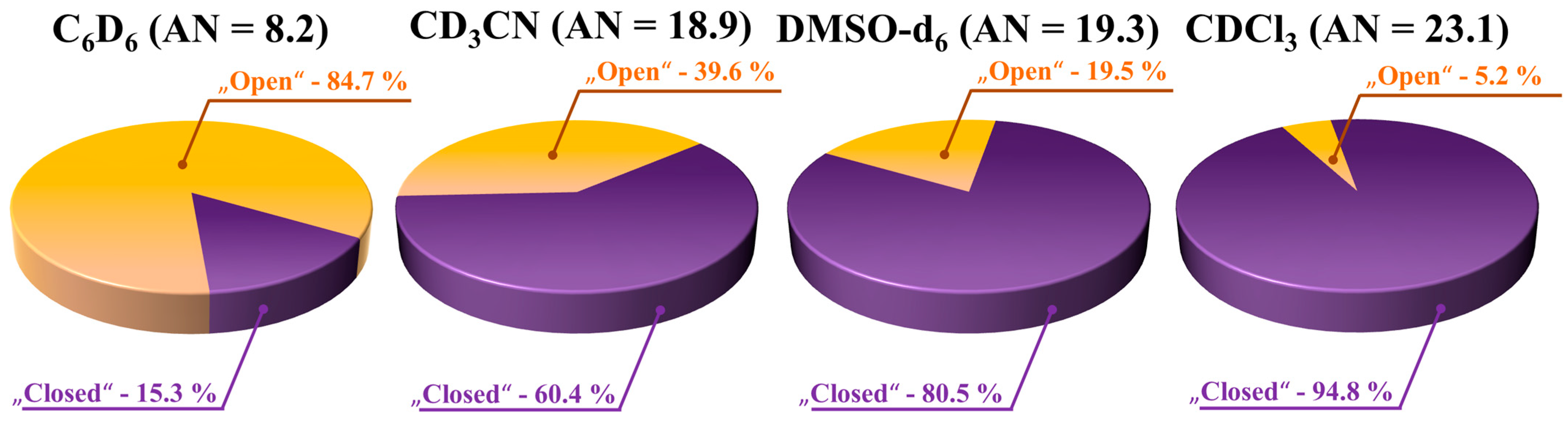

| Conf. | Z’ | τ1 (C10–C12–S–C13) | X–H···Y, | τ1 (C3–C2–N(H)–C9) | χ, M | Structure | |
|---|---|---|---|---|---|---|---|
| Form I (hydrogen-bonded chains) | “Open” | 1 | –88.3° | N–H···O(H) O–H···O(S) C–H3···O=C | −28.5° | 1.38 × 10−7 |  |
| Form II (π–π stacking) | “Closed” | 1 | –72.5° | N–H···O(H) O–H···O(S) C–H1···O=C | −164.4° | 3.38 × 10−7 |  |
| ΔsolH298, kJ/mol | ΔHtr, kJ/mol | m.p., °C | |
|---|---|---|---|
| Form I | 9.6 ± 0.3 | I → II 5.5 ± 0.5 | 193 |
| Form II | 4.1 ± 0.2 | ||
| Amorphous | –14.5 ± 0.3 | I → Am. 24.1 ± 0.6 II → Am. 18.6 ± 0.5 | - |
| BCL+DMSO | 22.0 ± 0.2 | - | 115 |
| BCL+4,4′-bipyridine | - | 163 | |
| BCL+trans-1,2-bis(4-pyridyl)ethylene | 159 | ||
| BCL+benzamide | 132 | ||
| BCL+salicylamide | 157 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belov, K.V.; Khodov, I.A. Conformational Landscape, Polymorphism, and Solid Forms Design of Bicalutamide: A Review. Molecules 2025, 30, 3793. https://doi.org/10.3390/molecules30183793
Belov KV, Khodov IA. Conformational Landscape, Polymorphism, and Solid Forms Design of Bicalutamide: A Review. Molecules. 2025; 30(18):3793. https://doi.org/10.3390/molecules30183793
Chicago/Turabian StyleBelov, Konstantin V., and Ilya A. Khodov. 2025. "Conformational Landscape, Polymorphism, and Solid Forms Design of Bicalutamide: A Review" Molecules 30, no. 18: 3793. https://doi.org/10.3390/molecules30183793
APA StyleBelov, K. V., & Khodov, I. A. (2025). Conformational Landscape, Polymorphism, and Solid Forms Design of Bicalutamide: A Review. Molecules, 30(18), 3793. https://doi.org/10.3390/molecules30183793







