Myricetin Attenuates Hyperexcitability of Trigeminal Nociceptive Second-Order Neurons in Inflammatory Hyperalgesia: Celecoxib-like Effects
Abstract
1. Introduction
2. Results
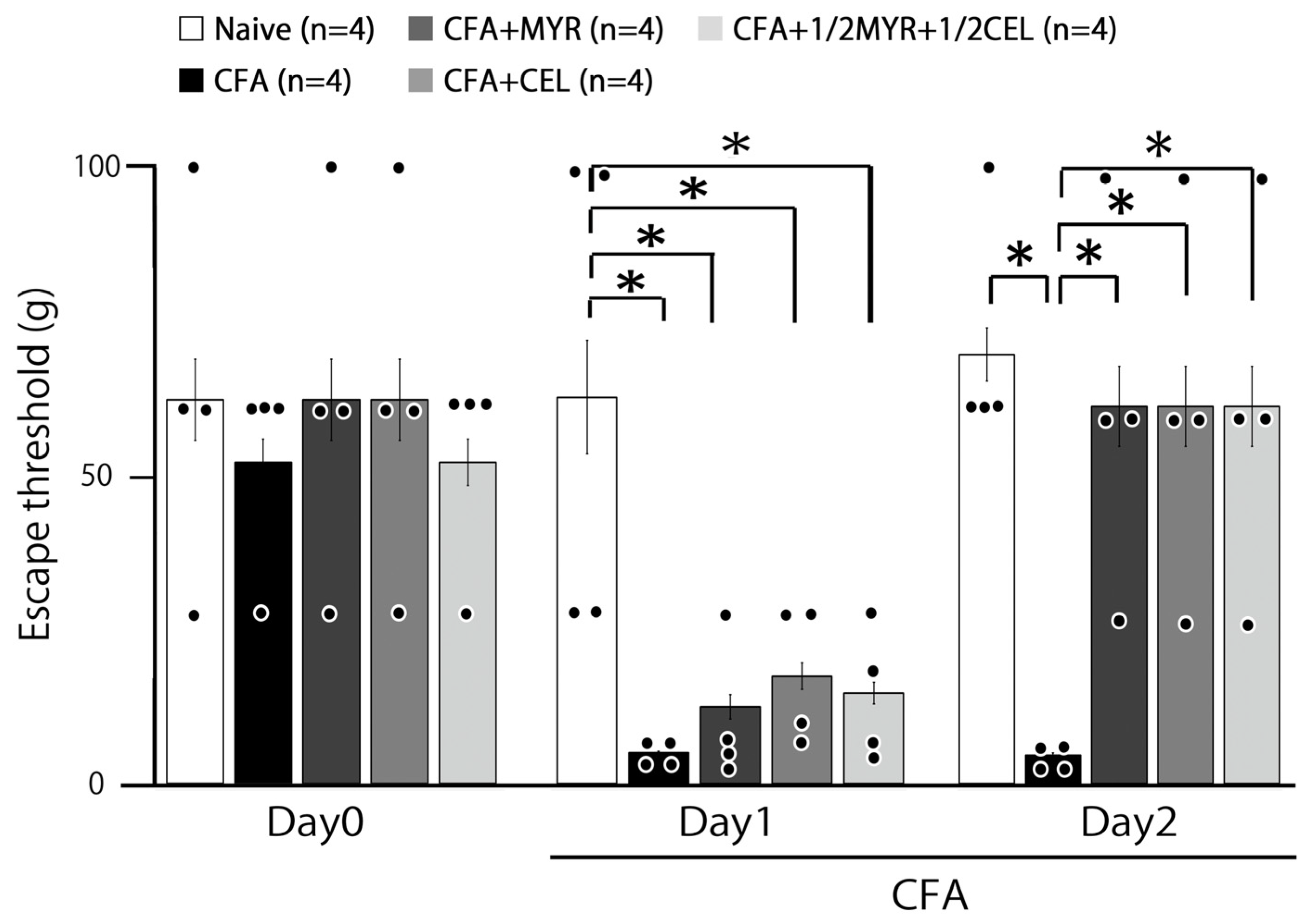
2.1. Inflammation-Induced Hyperalgesia
2.2. Chronic Treatment with Myricetin, Celecoxib, and Their Combination for Hyperalgesia
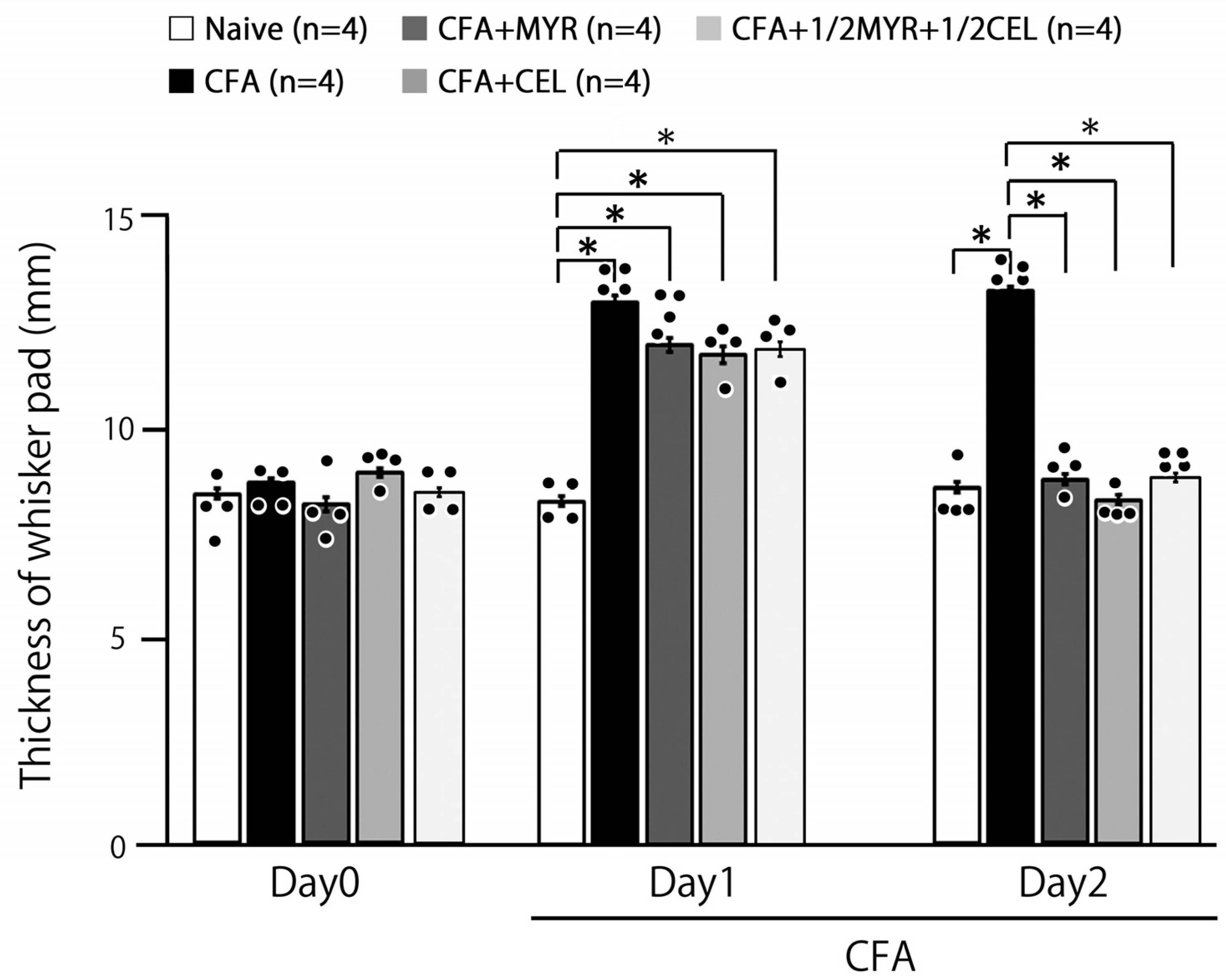
2.3. Myricetin (MYR) Reduces Inflammatory Edema: Whisker Pad Thickness
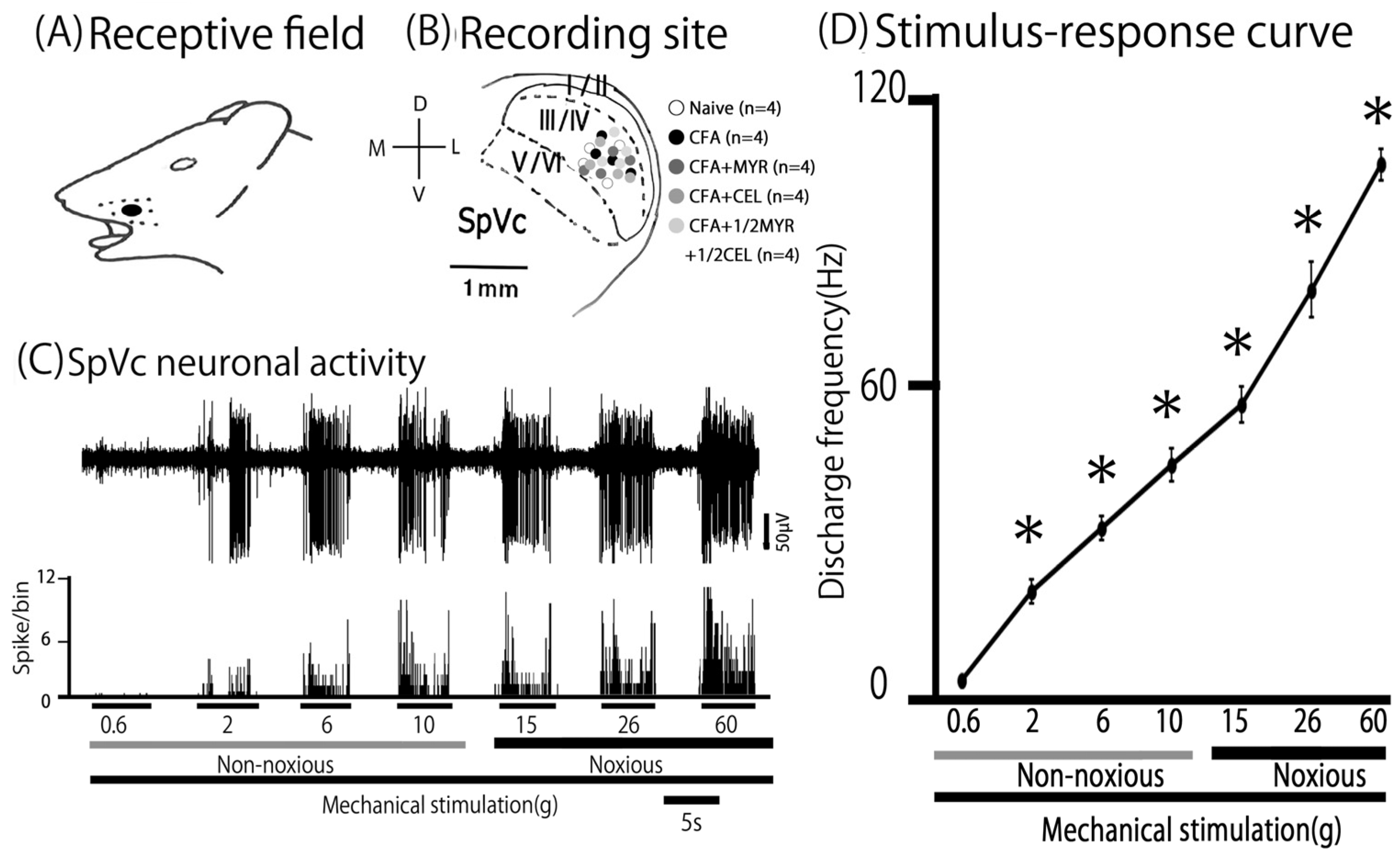
2.4. Altered Excitability of SpVc WDR Neurons Following Inflammation
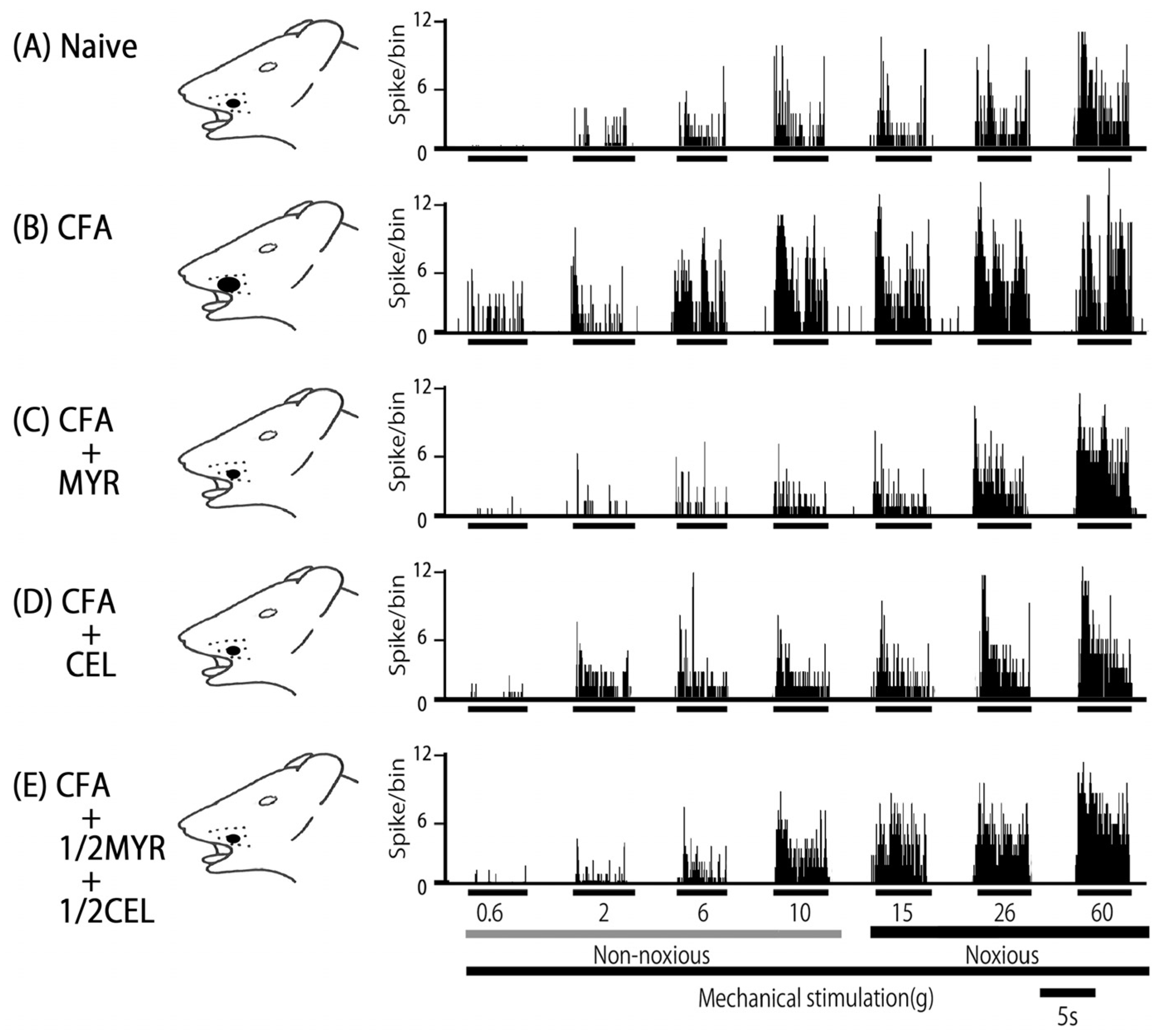
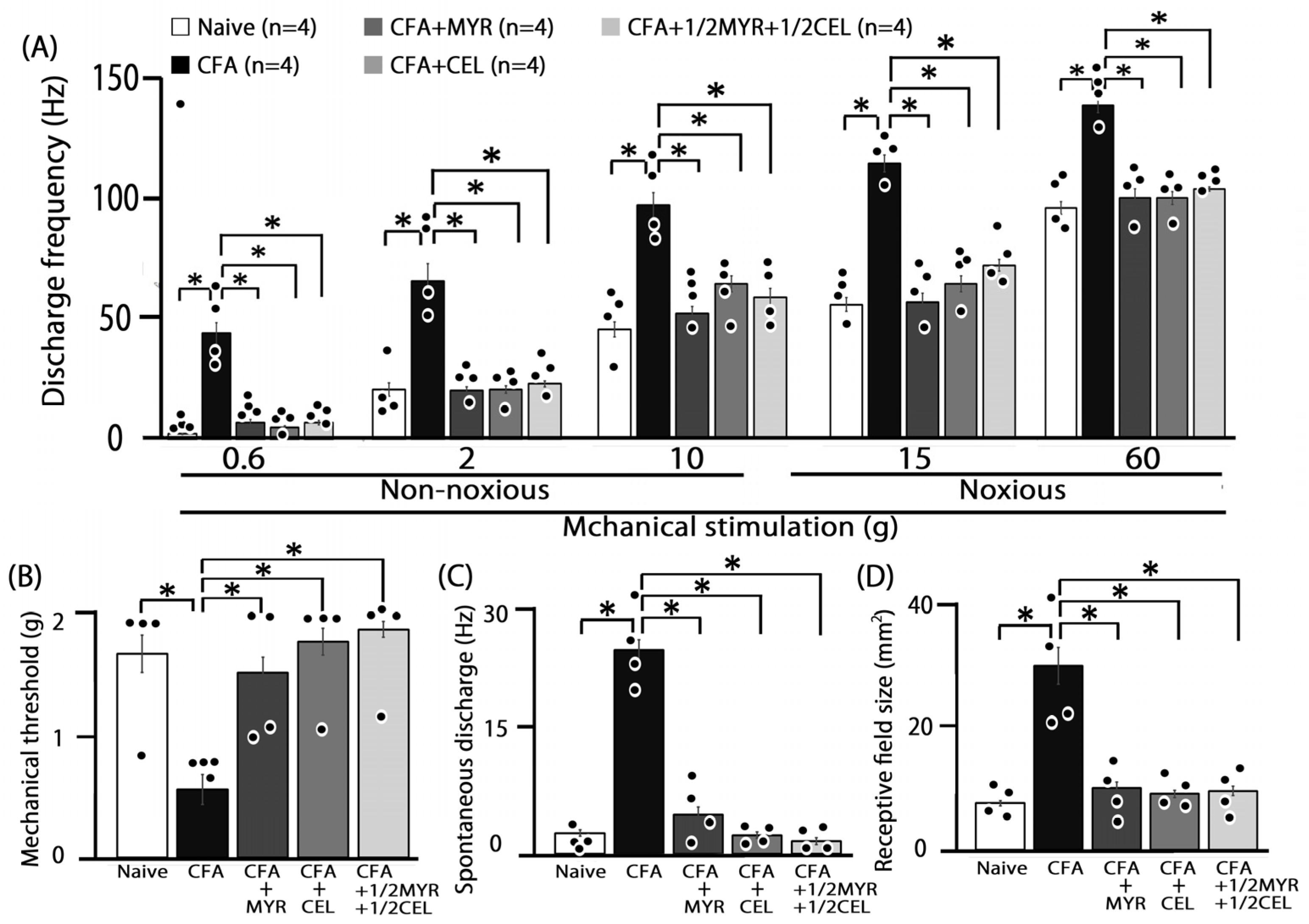
2.5. Chronic Myricetin (MYR) Inhibits SpVc WDR Neuronal Hyperexcitability in Inflamed Rats
2.6. Chronic Celecoxib (CEL) Inhibits SpVc WDR Neuronal Hyperexcitability in Inflamed Rats
2.7. Chronic Half-Dose Myricetin (MYR) + Celecoxib (CEL) Inhibits SpVc WDR Neuronal Hyperexcitability in Inflamed Rats
3. Discussion
3.1. Myricetin (MYR) Attenuates Trigeminal Inflammatory Hyperalgesia
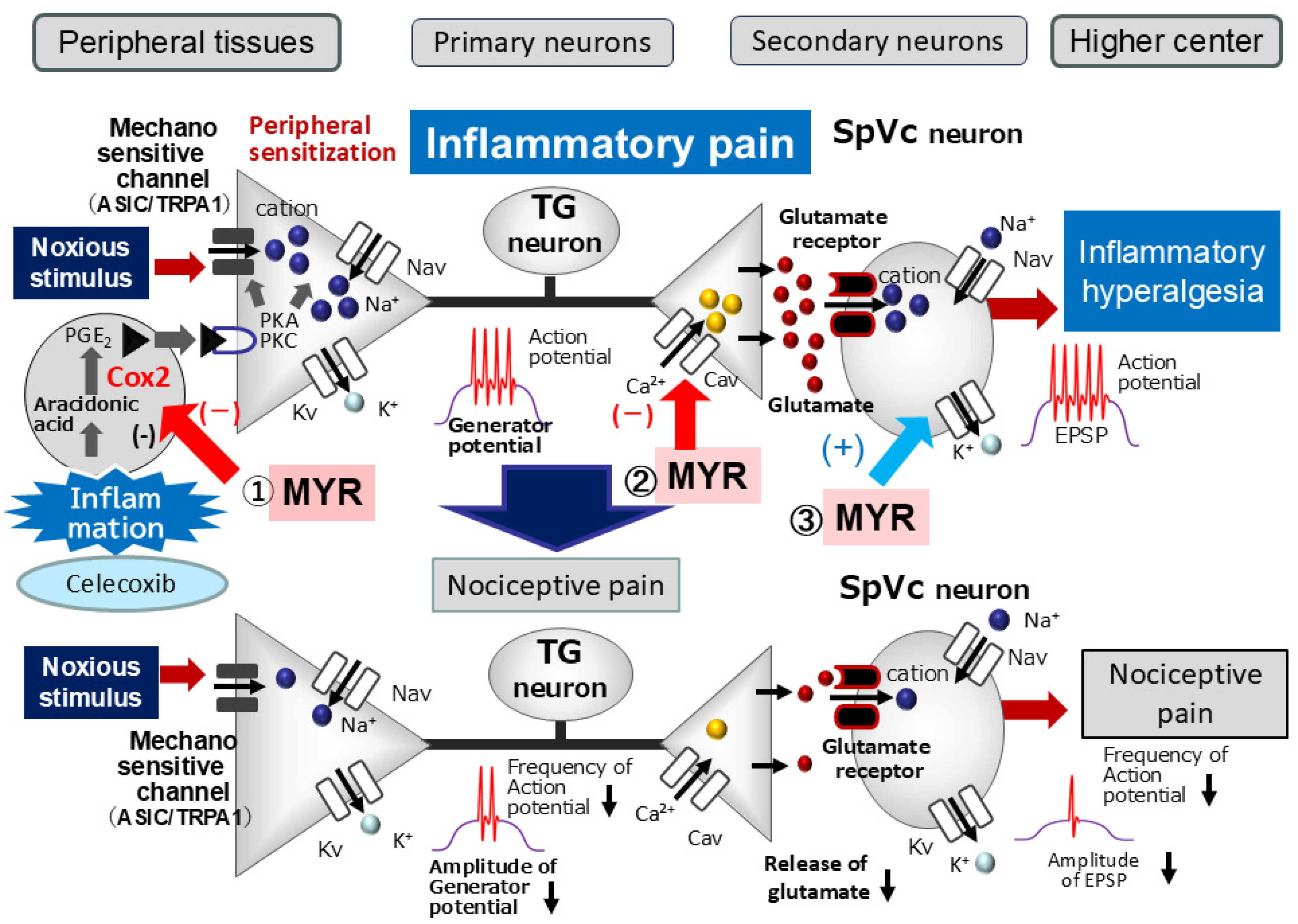
3.2. Myricetin (MYR) Suppresses SpVc WDR Neuronal Hyperexcitability in Inflammation-Induced Hyperalgesia
3.3. Functional Significance of Myricetin (MYR)’s Suppressive Effect on SpVc Neuronal Hyperexcitability in Hyperalgesia
4. Materials and Methods
4.1. Inducing Inflammation and Administering Myricetin (MYR) and NSAIDs
4.2. Mechanical Escape Threshold
4.3. Extracellular Single-Unit Recording of SpVc WDR Neuronal Activity
4.4. Experimental Protocols
4.5. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MYR | Myricetin |
| NSAID | Non-steroidal anti-inflammatory drug |
| CEL | Celecoxib |
| SpVc | Spinal trigeminal nucleus caudalis |
| WDR | Wide-dynamic range |
| TG | Trigeminal ganglion |
| CFA | Complete Freund’s adjuvant |
| CAM | Complementary alternative medicine |
| COX-2 | Cyclooxygenase-2 |
| PGE2 | Prostaglandine E2 |
| MAPK | Mitogen activated protein kinase |
| PKA | Protein kinase A |
| PKC | Protein kinase C |
| NMDA | N-methyl-D-aspartate |
| NR2B | NMDA receptor subtype 2B |
| Nav | Voltage-gated Na channels |
| Kv | Voltage-gated K channels |
| Cav | Voltage-gated Ca channels |
| TRPA1 | Transient receptor protein ankyrin 1 |
| ASIC3 | Acid sensing ion channels 3 |
| EPSP | Excitatory postsynaptic potential |
| EP | Prostanoid E |
References
- Sessle, B.J. Chronic orofacial pain: Models, mechanisms, and Genetic and related environmental influences. Int. J. Mol. Sci. 2021, 22, 7112. [Google Scholar] [CrossRef]
- Sessle, B.J. Peripheral and central mechanisms of orofacial pain and their clinical correlates. Minerva Anestesiol. 2005, 71, 117–136. [Google Scholar] [PubMed]
- Shinoda, M.; Suzuro, H.; Iwata, K.; Hayashi, Y. Plastic changes in nociceptive pathways contributing to persistent orofacial pain. J. Oral Biosci. 2022, 64, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Iwata, K.; Takeda, M.; Oh, S.; Shinoda, M. Neurophysiology of Orofacial Pain. In Contemporary Oral Medicine; Farah, C.S., Balasubramaniam, R., McCullough, M.J., Eds.; Springer International Publishing: New York, NY, USA, 2017. [Google Scholar]
- Iwata, K.; Tashiro, A.; Tsuboi, Y.; Imai, T.; Sumino, R.; Morimoto, T.; Dubber, R.; Ren, K. Medullary dorsal horn neuronal activity in rats with persistent temporomandibular joint and perioral inflammation. J. Neurophysiol. 1999, 82, 1244–1253. [Google Scholar] [CrossRef] [PubMed]
- Imbe, H.; Iwata, K.; Zhou, Q.-Q.; Zou, S.; Dubner, R.; Ren, K. Orofacial deep and cutaneous tissue inflammation and trigeminal neuronal activation. Cells Tissues Organs 2001, 169, 238–247. [Google Scholar] [CrossRef]
- Konvicka, J.J.; Meyer, T.A.; McDavid, A.J.; Roberson, C.R. Complementary/alternative medicine use among chronic pain clinic patients. J. PeriAnesth. Nurs. 2008, 23, 17–23. [Google Scholar] [CrossRef]
- Syoji, Y.; Kobayashi, R.; Miyamura, N.; Hirohara, T.; Kubota, Y.; Uotsu, N.; Yui, K.; Shimazu, Y.; Takeda, M. Suppression of hyperexcitability of trigeminal nociceptive neurons associated with inflammatory hyperalgesia following systemic administration of lutein via inhibition of cyclooxygenase-2 cascade signaling. J. Inflamm. 2018, 15, 24. [Google Scholar] [CrossRef]
- Yajima, S.; Sakata, R.; Watanuki, Y.; Sashide, Y.; Takeda, M. Naringenin suppresses the hyperexcitability of trigeminal nociceptive neurons associated with inflammatory hyperalgesia: Replacement of NSAIDs with phytochemicals. Nutrients 2024, 16, 389. [Google Scholar] [CrossRef]
- Hakkinen, S.H.; Karenlampi, S.O.; Heinonen, I.M.; Mykkanen, H.M.; Torronen, A.R. Content of the flavonols quercetin, myricetin, and kaempferol in 25 edible berries. J. Agric. Food Chem. 1999, 47, 2274–2279. [Google Scholar] [CrossRef]
- Miean, K.H.; Mohamed, S. Flavonoid (myricetin, quercetin, kaempferol, luteolin, and apigenin) content of edible tropical plants. J. Agric. Food Chem. 2001, 49, 3106–3112. [Google Scholar] [CrossRef]
- Valdez, L.B.; Alvarez, S.; Zaobornyj, T.; Boveris, A. Polyphenols and red wine as antioxidants against peroxynitrite and other oxidants. Biol. Res. 2004, 37, 279–286. [Google Scholar] [CrossRef]
- Chang, Y.; Chang, C.-Y.; Wang, S.-J.; Huang, S.-K. Myricetin inhibits the release of glutamate in rat cerebrocortical nerve terminals. J. Med. Food 2015, 18, 516–523. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Liu, T. Myricetin facilitates potassium currents and inhibits neuronal activity of PVN neurons. Neurochem. Res. 2012, 37, 1450–1456. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.H.; Ma, Z.G.; Rowlands, D.K.; Gou, Y.L.; Fok, K.L.; Wong, H.Y.; Yu, M.K.; Tsang, L.L.; Mu, L.; Chen, L.; et al. Flavonoid myricetin modulates GABAA receptor activity through activation of Ca2+ channels and CaMK-II pathway. Evid. Based Complement. Altern. Med. 2012, 2012, 758097. [Google Scholar] [CrossRef] [PubMed]
- Youdim, K.A.; Qaiser, M.Z.; Begley, D.J.; Rice-Evans, C.A.; Abbott, N.J. Flavonoid permeability across an in situ model of the blood-brain barrier. Free Radic. Biol. Med. 2004, 36, 592–604. [Google Scholar] [CrossRef]
- Yamaguchi, S.; Chida, R.; Utugi, S.; Sashide, Y.; Takeda, M. Systemic administration of the phytochemical, myricetin, attenuates the excitability of rat nociceptive secondary trigeminal neuron. Molecules 2025, 30, 1019. [Google Scholar] [CrossRef]
- Jang, J.-H.; Lee, S.-H.; Jung, K.; Yoo, H.; Park, G. Inhibitory effects of myricetin on lipopolysaccharide -induced neuroinflammation. Brain Sci. 2020, 10, 32. [Google Scholar] [CrossRef]
- Rosas-Martinez, M.; Gutierrez-Vegas, G. Myricetin inhibition of peptidoglycan-induced Cox-2 expression in H9c2 cardiomyocytes. Prev. Nutr. Food Sci. 2019, 24, 202–209. [Google Scholar] [CrossRef]
- Gutierrez-Venegas, G.; Luna, O.A.; Arreguin-Cano, J.A.; Hernandez-Bermudez, C. Myricetin blocks lipoteichoic acid-inducedCox-2 expression in human gingival fibroblasts. Cell. Mol. Biol. Lett. 2014, 19, 126–139. [Google Scholar] [CrossRef]
- Takeda, M.; Takehana, S.; Sekiguchi, K.; Kubota, Y.; Shimazu, Y. Modulatory Mechanism of Nociceptive Neuronal Activity by Dietary Constituents Resveratrol. Int. J. Mol. Sci. 2016, 17, 1702. [Google Scholar] [CrossRef]
- Ahmadi, S.; Lippross, S.; Neuhuber, W.L.; Zeilhofer, H.U. PGE2 Selectively Blocks Inhibitory Glycinergic Neurotransmission onto Rat Superficial Dorsal Horn Neurons. Nat. Neurosci. 2002, 5, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Gan, T.J. Diclofenac: An Update on Its Mechanism of Action and Safety Profile. Curr. Med. Res. Opin. 2010, 26, 1715–1731. [Google Scholar] [CrossRef] [PubMed]
- Craft, R.M.; Hewitt, K.A.; Britch, S.C. Antinociception Produced by Non-Steroidal Anti-Inflammatory Drugs in Female and Male Rats. Behav. Pharmacol. 2021, 32, 153–169. [Google Scholar] [CrossRef] [PubMed]
- Harriott, A.M.; Gold, M.S. Contribution of primary afferent channels to neuropathic pain. Curr. Pain Headache Rep. 2009, 13, 197–207. [Google Scholar] [CrossRef]
- Zamponi, G.W.; Lewis, R.J.; Todorovic, S.M.; Arneric, S.P.; Snutch, T.P. Role of Voltage-Gated Calcium Channels in Ascending Pain Pathways. Brain Res. Rev. 2009, 60, 84–89. [Google Scholar] [CrossRef]
- Schaible, H.G.; Richter, F. Pathophysiology of Pain. Langenbecks Arch. Surg. 2004, 389, 237–243. [Google Scholar] [CrossRef]
- Hildebrand, M.E.; Snutch, T.P. Contributions of T-Type Ca Channels to the Pathophysiology of Pain Signaling. Drug Discov. Today Dis. Mech. 2006, 3, 335–341. [Google Scholar] [CrossRef]
- Ficke, E.; Heinemann, U. Slow and fast transient potassium current in cultured rat hippocampus cells. J. Physiol. 2001, 445, 431–455. [Google Scholar] [CrossRef]
- Hille, B. Potassium channels and chloride channels. In Ion Channels of Excitable Membranes, 3rd ed.; Hille, B., Ed.; Sinauer Associates: Sunderland, MA, USA, 2001; pp. 134–167. [Google Scholar]
- Pearce, R.J.; Duchen, M.R. Differential expression of membrane currents in dissociated mouse primary sensory neurons. Neuroscience 1994, 63, 1041–1056. [Google Scholar] [CrossRef]
- Lawson, K. Potassium channels as targets for the management of pain. Cent. Nerv. Syst. Agents Med. Chem. 2006, 6, 119–128. [Google Scholar] [CrossRef]
- Takeda, M.; Tanimoto, T.; Ikeda, M.; Nasu, M.; Kadoi, J.; Yoshida, S.; Matsumoto, S. Enhanced excitability of rat trigeminal root ganglion neurons via decrease in A-type potassium currents following temporomandibular joint inflammation. Neuroscience 2006, 138, 621–630. [Google Scholar] [CrossRef] [PubMed]
- Kakita, K.; Tsubouchi, H.; Adachi, M.; Takehana, S.; Shimazu, Y.; Takeda, M. Local subcutaneous injection of chlorogenic acid inhibits the nociceptive trigeminal spinal nucleus caudalis neurons in rats. Neurosci. Res. 2018, 134, 49–55. [Google Scholar] [CrossRef]
- Hara, N.; Takeda, M.; Takahashi, M.; Matsumoto, S. Iontophoretic application of an A-type potassium channel blocker to the trigeminal ganglion neurons enhances the excitability of Aδ- and C-neurons innervating the temporomandibular joint. Neurosci. Res. 2012, 74, 216–222. [Google Scholar] [CrossRef]
- Burstein, R.; Cutrer, M.F.; Yarnitsky, D. The Development of Cutaneous Allodynia during a Migraine Attack: Clinical Evidence for the Sequential Recruitment of Spinal and Supraspinal Nociceptive Neurons in Migraine. Brain 2009, 123, 1703–1709. [Google Scholar]
- Roch, M.; Messlinger, K.; Kulchitsky, V.; Tichonovich, O.; Azev, O.; Koulchitsky, S. Ongoing Activity in Trigeminal Wide-Dynamic Range Neurons Is Driven from the Periphery. Neuroscience 2007, 150, 681–691. [Google Scholar] [CrossRef]
- Takeda, M.; Tanimoto, T.; Matsumoto, S. Change in Mechanical Receptive Field Properties Induced by GABAA Receptor Activation in the Trigeminal Spinal Nucleus Caudalis Neurons in Rats. Exp. Brain Res. 2000, 134, 409–416. [Google Scholar] [CrossRef]
- Ekstrand, B.; Rasmussen, M.K.; Woll, F.; Zlabek, V.; Zamaratskaia, G. In vitro gender-dependent inhibition of porcine cytochrome P450 activity by selected flavonoids and phenolic acids. BioMed Res. Int. 2015, 2015, 387918. [Google Scholar] [CrossRef] [PubMed]
- Riley, J.L.; Robinson, M.E.; Wise, E.A.; Price, D. A Meta-Analytic Review of Pain Perception Across the Menstrual Cycle. Pain 1999, 81, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Bartley, E.J.; Fillingim, R.B. Sex Differences in Pain: A Brief Review of Clinical and Experimental Findings. Br. J. Anaesth. 2013, 111, 52–58. [Google Scholar] [CrossRef]
- Garlet, T.P.; Coelho, U.; Silva, J.S.; Garlet, G.P. Cytokine Expression Pattern in Compression and Tension Sides of Periodontal Ligament During Orthodontic Tooth Movement in Humans. Eur. J. Oral Sci. 2007, 115, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Karthi, M.; Anbuslevan, G.J.; Senthilkumar, K.P.; Tamizharsi, S.; Raja, S.; Prabhakrr, K. NSAIDs in Orthodontic Tooth Movement. J. Pharm. Biol. Sci. 2012, 4 (Suppl. S2), S304–S306. [Google Scholar] [CrossRef] [PubMed]
- Shetty, N.; Patil, A.K.; Ganeshkar, S.V.; Hegde, S. Comparison of the Effects of Ibuprofen and Acetaminophen on PGE2 Levels in the GCF During Orthodontic Tooth Movement: A Human Study. Prog. Orthod. 2013, 14, 6. [Google Scholar] [CrossRef] [PubMed]
- Okubo, N.; Ishikawa, H.; Sano, R.; Shimazu, Y.; Takeda, M. Effect of Resveratrol on the Hyperexcitability of Nociceptive Neurons Associated with Ectopic Hyperalgesia Induced by Experimental Tooth Movement. Eur. J. Oral Biosci. 2020, 128, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, M. Ethical Guidelines for Investigations of Experimental Pain in Conscious Animals. Pain 1983, 16, 109–110. [Google Scholar] [CrossRef]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates, 2nd ed.; Academic Press: New York, NY, USA, 1986. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamaguchi, S.; Takeda, M. Myricetin Attenuates Hyperexcitability of Trigeminal Nociceptive Second-Order Neurons in Inflammatory Hyperalgesia: Celecoxib-like Effects. Molecules 2025, 30, 3789. https://doi.org/10.3390/molecules30183789
Yamaguchi S, Takeda M. Myricetin Attenuates Hyperexcitability of Trigeminal Nociceptive Second-Order Neurons in Inflammatory Hyperalgesia: Celecoxib-like Effects. Molecules. 2025; 30(18):3789. https://doi.org/10.3390/molecules30183789
Chicago/Turabian StyleYamaguchi, Sana, and Mamoru Takeda. 2025. "Myricetin Attenuates Hyperexcitability of Trigeminal Nociceptive Second-Order Neurons in Inflammatory Hyperalgesia: Celecoxib-like Effects" Molecules 30, no. 18: 3789. https://doi.org/10.3390/molecules30183789
APA StyleYamaguchi, S., & Takeda, M. (2025). Myricetin Attenuates Hyperexcitability of Trigeminal Nociceptive Second-Order Neurons in Inflammatory Hyperalgesia: Celecoxib-like Effects. Molecules, 30(18), 3789. https://doi.org/10.3390/molecules30183789





