Effect of Phenological Stage and Leaf Age on Changes of Chlorophyll and Carotenoid Contents in Some Weeds and Invasive Species
Abstract
1. Introduction
2. Results
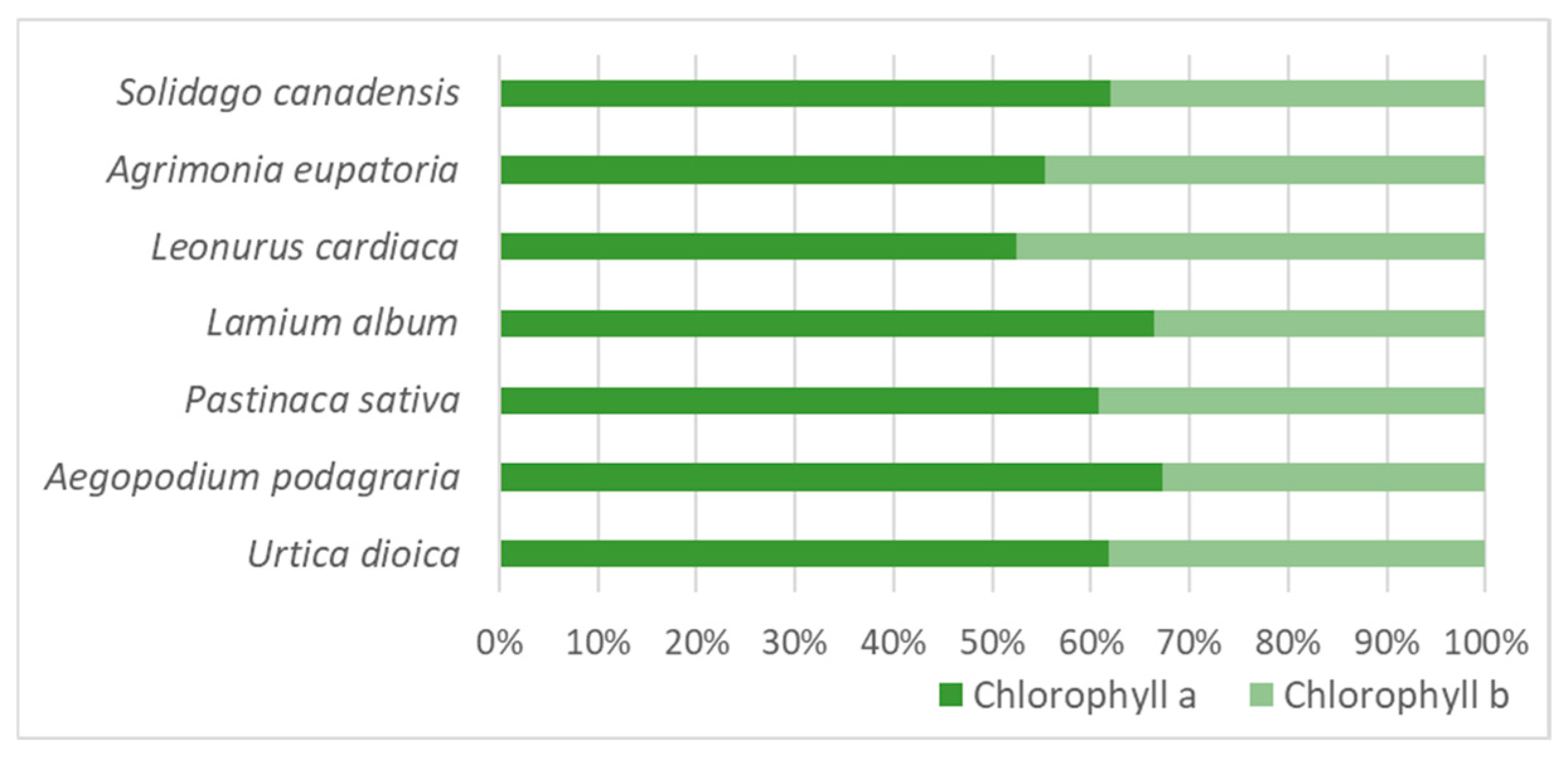
2.1. Changes in Total Chlorophyll (a + b) Content and Chlorophyll a/b Ratio During Plant Vegetation
2.2. Changes in Total Carotenoid Content During Plant Vegetation
2.3. Comparison of the Average Content of Total Chlorophyll (a + b) and Total Carotenoid Between Species, Leaves of Different Ages, Regardless of the Phenological Stage
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Sample Preparation and Determination of Pigment Content
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Calinoiu, L.F.; Mitrea, L.; Teleky, B.E.; Szabo, K.; Martau, A.G.; Nemes, S.A.; Plamada, D.; Pascuta, M.S.; Barta, G.; Varvara, R.A.; et al. Fruit and vegetable waste and by-products for pigments and colour. In Fruit and Vegetable Waste Utilization and Sustainability; Mandavgane, S.A., Chakravarty, I., Jaiswal, A.K., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2023. [Google Scholar]
- Solovchenko, A.; Yahia, E.M.; Chen, C. Pigments. In Postharvest Physiology and Biochemistry of Fruits and Vegetables; Yahia, E.M., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2019; pp. 225–252. [Google Scholar]
- Młodzińska, E. Survey of plant pigments: Molecular and environmental determinants of plant colours. Acta Biol. Cracoviensia Ser. Bot. 2009, 51, 7–16. [Google Scholar]
- Pareek, S.; Sagar, N.A.; Sharma, S.; Kumar, V.; Agarwal, T.; González-Aguilar, G.A.; Yahia, E.M. Chlorophylls: Chemistry and biological functions. Fruit Veg. Phytochem. 2017, 29, 269–284. [Google Scholar]
- Saini, R.K.; Nile, S.H.; Park, S.W. Carotenoids from fruits and vegetables: Chemistry, analysis, occurrence, bioavailability and biological activities. Food Res. Int. 2015, 76, 735–750. [Google Scholar] [CrossRef]
- Holt, N.E.; Zigmantas, D.; Valkunas, L.; Li, X.P.; Niyogi, K.K.; Fleming, G.R. Carotenoid cation formation and the regulation of photosynthetic light harvesting. Science 2005, 307, 433–436. [Google Scholar] [CrossRef]
- Ribeiro, B.D.; Grando de Oliveira, R. Carotenoids as colourants. In Natural Products. Phytochemistry, Botany and Metabolism of Alkaloids, Phenolics and Terpenes; Ramawat, K., Mérillon, J.M., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 4017–4036. [Google Scholar]
- Solymosi, K.; Mysliwa-Kurdziel, B. Chlorophylls and their derivatives used in food industry and medicine. Mini Rev. Med. Chem. 2017, 17, 1194–1222. [Google Scholar] [CrossRef]
- Junker, L.V.; Ensminger, I. Relationship between leaf optical properties, chlorophyll fluorescence and pigment changes in senescing Acer saccharum leaves. Tree Physiol. 2016, 36, 694–711. [Google Scholar] [CrossRef]
- Jiménez-Lao, R.; Garcia-Caparros, P.; Pérez-Saiz, M.; Llanderal, A.; Lao, M.T. Monitoring optical tool to determine the chlorophyll concentration in ornamental plants. Agronomy 2021, 11, 2197. [Google Scholar] [CrossRef]
- Mishra, V.K.; Bacheti, R.K.; Husen, A. Medicinal uses of chlorophyll: A critical overview. In Chlorophyll: Structure, Function and Medicinal Uses; Le, H., Salcedo, E., Eds.; Nova Science Publishers Inc.: Hauppauge, NY, USA, 2011; pp. 177–196. [Google Scholar]
- Hayes, M.; Ferruzzi, M.G. Update on the bioavailability and chemopreventative mechanisms of dietary chlorophyll derivatives. Nutr. Res. 2020, 81, 19–37. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Sarkar, T.; Das, A.; Chakraborty, R. Natural colourants from plant pigments and their encapsulation: An emerging window for the food industry. Food Sci. Technol. 2022, 153, 112527. [Google Scholar]
- Buchovec, I.; Lukševičiūtė, V.; Kokstaitė, R.; Labeikytė, D.; Kaziukonytė, L.; Lukšienė, Z. Inactivation of gram (–) bacteria Salmonella enterica by chlorophyllin-based photosensitization: Mechanism of action and new strategies to enhance the inactivation efficiency. J. Photochem. Photobiol. B Biol. 2017, 172, 1–10. [Google Scholar] [CrossRef]
- Kendrick, A. Natural food and beverage colourings. In Natural Food Additives, Ingredients and Flavourings; Baines, D., Seal, R., Eds.; Woodhead Publishing Limited: Cambridge, UK, 2012; pp. 25–40. [Google Scholar]
- Martins, T.; Barros, A.N.; Rosa, E.; Antunes, L. Enhancing health benefits through chlorophylls and chlorophyll-rich agro-food: A comprehensive review. Molecules 2023, 28, 5344. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, C.; Gökmen, V. Chlorophyll. In Encyclopedia of Food and Health; Finglas, P.M., Toldra, F., Caballero, B., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 37–41. [Google Scholar]
- Sharma, M.; Usmani, Z.; Gupta, V.K.; Bhat, R. Valorization of fruits and vegetable wastes and by-products to produce natural pigments. Rit. Rev. Biotechnol. 2021, 41, 535563. [Google Scholar] [CrossRef] [PubMed]
- Cain, N.; Darbyshire, S.J.; Francis, A.; Nurse, R.E.; Simard, M.-J. The biology of Canadian weeds. 144. Pastinaca sativa L. Can. J. Plant Sci. 2010, 90, 217–240. [Google Scholar] [CrossRef]
- Dębia, K.; Dzięcioł, M.; Wróblewska, A.; Janda-Milczarek, K. Goutweed (Aegopodium podagraria L.)—An edible weed with health-promoting properties. Molecules 2025, 30, 1603. [Google Scholar] [CrossRef]
- Galinis, V. Lūpažiedžiai (Notreliniai)—Labiatae Juss. (Lamiaceae). In Flora of the Lithuanian SSR; Minkevičius, A., Ed.; Lithuanian SSR Academy of Sciences, Institute of Botany: Vilnius, Lithuania, 1976; Volume 5, pp. 275–381. [Google Scholar]
- Poljuha, D.; Sladonja, B.; Uzelac Božac, M.; Šola, I.; Damijanić, D.; Weber, T. The invasive alien plant Solidago canadensis: Phytochemical composition, ecosystem service potential, and application in bioeconomy. Plants 2024, 13, 1745. [Google Scholar] [CrossRef]
- Rendeková, A.; Mičieta, K. Interesting and rare plant taxa and community in the ruderal flora and vegetation of Bratislava and Malacky. Acta Bot. Univ. Comen. 2017, 52, 11–27. [Google Scholar]
- Müllerová, V.; Hejcman, M.; Hejcmanová, P.; Pavlů, V. Effect of fertilizer application on Urtica dioica and its element concentrations in a cut grassland. Acta Oecol. 2014, 59, 1–6. [Google Scholar] [CrossRef]
- Maj, G.; Krzaczek, P.; Stamirowska-Krzaczek, E.; Lipinska, H.; Kornas, R. Assessment of energy and physicochemical biomass properties of selected forecrop plant species. Renew. Energy 2019, 143, 520–529. [Google Scholar] [CrossRef]
- Paulauskienė, A.; Tarasevičienė, Ž.; Laukagalis, V. Influence of harvesting time on the chemical composition of wild stinging nettle (Urtica dioica L.). Plants 2021, 10, 686. [Google Scholar] [CrossRef]
- Doğan, H.; Baş, H.; Fidan, H.; Stankov, S.; Stoyanova, A.; Uskutoğlu, T.; Şenkal, B.C.; Yılmaz, G. Phytochemical profile of Agrimonia eupatoria L. from Bulgaria and effects of its extracts on Galleria mellonella (L.) (Lepidoptera: Pyralidae) larvae. Carpath. J. Food Sci. Technol. 2023, 15, 58–69. [Google Scholar]
- Jankevičienė, R. Skėtiniai—Umbelliferae Juss. In Flora of the Lithuanian SSR; Minkevičius, A., Ed.; Lithuanian SSR Academy of Sciences, Institute of Botany: Vilnius, Lithuania, 1976; Volume 5, pp. 24–97. [Google Scholar]
- European Pharmacopoeia, 6th ed.; European Directorate for the Quality of Medicines & Healthcare (EDQM): Strasbourg, France, 2020.
- Kitajima, K.; Hogan, K.P. Increases of chlorophyll a/b ratios during acclimation of tropical woody seedlings to nitrogen limitation and high light. Plant Cell Environ. 2003, 26, 857–865. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, R.; Tanaka, A. Chlorophyll cycle regulates the construction and destruction of the light-harvesting complexes. Biochim. Biophys. Acta (BBA)-Bioenerg. 2011, 1807, 968–976. [Google Scholar] [CrossRef]
- Pizarro, L.; Stange, C. Light-dependent regulation of carotenoid biosynthesis in plants. Cien. Investig. Agr. 2009, 36, 143–162. [Google Scholar] [CrossRef]
- Wellburn, A.R. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]


| Species | Total Chlorophyll (a + b) (mg/g) at Different Times of the Vegetation Period (Month/Day) | CV (%) | H-Value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 12 May | 26 May | 9 June | 23 June | 7 July | 21 July | 4 August | 18 August | 1 September | 15 September | |||
| Aegopodium podagraria | 1.82 ± 0.05 | 1.64 ± 0.06 | 1.74 ± 0.23 | 1.15 ± 0.08 | 1.46 ± 0.11 | 1.04 ± 0.08 | 1.05 ± 0.20 | 0.79 ± 0.16 | 0.32 ± 0.01 | 0.60 ± 0.27 | 43 | 66.1 * |
| Pastinaca sativa | 1.86 ± 0.09 | 1.98 ± 0.04 | 2.14 ± 0.04 | 2.22 ± 0.12 | 1.76 ± 0.09 | 1.54 ± 0.08 | 2.01 ± 0.15 | 1.43 ± 0.13 | 1.70 ± 0.03 | 1.92 ± 0.21 | 13 | 40.7 * |
| Lamium album | 2.02 ± 0.16 | 1.69 ± 0.06 | 1.42 ± 0.06 | 1.40 ± 0.07 | 0.54 ± 0.08 | 0.79 ± 0.06 | 1.35 ± 0.05 | – | – | – | 39 | 49.7 * |
| Leonurus cardiaca | 2.33 ± 0.13 | 2.42 ± 0.05 | 2.21 ± 0.02 | 2.29 ± 0.03 | 2.24 ± 0.11 | 2.28 ± 0.07 | 2.15 ± 0.04 | 2.24 ± 0.11 | 2.32 ± 0.15 | 2.07 ± 0.07 | 4 | 14.8 |
| Agrimonia eupatoria | 1.66 ± 0.17 | 1.67 ± 0.13 | 2.42 ± 0.16 | 2.40 ± 0.18 | 2.61 ± 0.09 | 2.50 ± 0.06 | 2.72 ± 0.27 | 2.44 ± 0.38 | 2.31 ± 0.30 | 1.52 ± 0.05 | 18 | 46.0 * |
| Solidago canadensis | 0.98 ± 0.17 | 1.37 ± 0.07 | 1.50 ± 0.29 | 1.75 ± 0.12 | 1.41 ± 0.06 | 2.38 ± 0.04 | 2.38 ± 0.13 | 2.08 ± 0.08 | 2.07 ± 0.04 | 1.66 ± 0.05 | 26 | 68.9 * |
| Urtica dioica | 2.21 ± 0.09 | 2.21 ± 0.21 | 2.18 ± 0.28 | 2.14 ± 0.13 | 1.74 ± 0.16 | 1.00 ± 0.05 | 1.27 ± 0.18 | 1.28 ± 0.16 | 1.92 ± 0.04 | 1.28 ± 0.05 | 27 | 67.0 * |
| Species | Chlorophyll a/b Ratio at Different Times of the Vegetation Period (Month/Day) | CV (%) | H-Value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 12 May | 26 May | 9 June | 23 June | 7 July | 21 July | 4 August | 18 August | 1 September | 15 September | |||
| Aegopodium podagraria | 1.7 ± 0.5 | 2.3 ± 0.2 | 1.8 ± 0.4 | 2.3 ± 0.2 | 1.9 ± 0.3 | 2.7 ± 0.3 | 2.5 ± 0.2 | 2.4 ± 0.1 | 2.7 ± 0.4 | 2.7 ± 0.4 | 22 | 52.0 * |
| Pastinaca sativa | 1.7 ± 0.3 | 1.7 ± 0.2 | 1.3 ± 0.2 | 1.2 ± 0.2 | 1.9 ± 0.5 | 2.1 ± 0.3 | 1.5 ± 0.7 | 2.1 ± 0.4 | 2.2 ± 0.8 | 1.9 ± 0.5 | 33 | 38.4 * |
| Lamium album | 1.5 ± 0.4 | 2.1 ± 0.3 | 2.4 ± 0.2 | 2.3 ± 0.5 | 2.3 ± 0.4 | 2.6 ± 0.2 | 1.9 ± 0.3 | – | – | – | 27 | 30.9 * |
| Leonurus cardiaca | 1.2 ± 0.4 | 0.9 ± 0.2 | 1.3 ± 0.4 | 1.0 ± 0.2 | 1.2 ± 0.3 | 1.1 ± 0.1 | 1.2 ± 0.5 | 1.0 ± 0.2 | 1.3 ± 0.5 | 1.5 ± 0.3 | 33 | 27.4 * |
| Agrimonia eupatoria | 2.5 ± 0.4 | 2.3 ± 0.6 | 1.0 ± 0.2 | 0.9 ± 0.3 | 0.8 ± 0.2 | 0.8 ± 0.3 | 1.3 ± 0.5 | 1.3 ± 0.5 | 2.0 ± 0.4 | 2.1 ± 0.3 | 47 | 56.1 * |
| Solidago canadensis | 4.1 ± 0.7 | 3.0 ± 0.5 | 2.4 ± 0.8 | 2.0 ± 0.2 | 2.6 ± 0.4 | 1.0 ± 0.1 | 1.2 ± 0.5 | 1.3 ± 0.6 | 1.4 ± 0.3 | 2.5 ± 0.7 | 46 | 66.7 * |
| Urtica dioica | 1.3 ± 0.5 | 1.2 ± 0.3 | 1.3 ± 0.4 | 1.3 ± 0.3 | 2.0± 0.7 | 2.8 ± 0.7 | 3.0 ± 0.4 | 2.8 ± 0.5 | 1.7± 0.2 | 3.0 ± 0.5 | 43 | 66.1 * |
| Species | Total Carotenoid (mg/g) at Different Times of the Vegetation Period (Month/Day) | CV (%) | H-Value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 12 May | 26 May | 9 June | 23 June | 7 July | 21 July | 4 August | 18 August | 1 September | 15 September | |||
| Aegopodium podagraria | 0.27 ± 0.00 | 0.31 ± 0.01 | 0.17 ± 0.02 | 0.20 ± 0.01 | 0.21 ± 0.01 | 0.19 ± 0.01 | 0.20 ± 0.03 | 0.15 ± 0.03 | 0.08 ± 0.00 | 0.11 ± 0.03 | 35 | 64.4 * |
| Pastinaca sativa | 0.29 ± 0.03 | 0.30 ± 0.00 | 0.23 ± 0.02 | 0.21 ± 0.00 | 0.23 ± 0.01 | 0.26 ± 0.01 | 0.15 ± 0.03 | 0.27 ± 0.01 | 0.26 ± 0.02 | 0.32 ± 0.03 | 24 | 54.3 * |
| Lamium album | 0.31 ± 0.07 | 0.37 ± 0.03 | 0.29 ± 0.03 | 0.31 ± 0.03 | 0.18 ± 0.05 | 0.21 ± 0.03 | 0.21 ± 0.04 | – | – | – | 30 | 46.2 * |
| Leonurus cardiaca | 0.22 ± 0.03 | 0.15 ± 0.03 | 0.25 ± 0.03 | 0.15 ± 0.02 | 0.26 ± 0.01 | 0.17 ± 0.02 | 0.18 ± 0.00 | 0.17 ± 0.02 | 0.20 ± 0.02 | 0.27 ± 0.02 | 35 | 29.8 * |
| Agrimonia eupatoria | 0.33 ± 0.00 | 0.33 ± 0.01 | 0.17 ± 0.05 | 0.13 ± 0.08 | 0.13 ± 0.03 | 0.15 ± 0.03 | 0.12 ± 0.03 | 0.18 ± 0.03 | 0.40 ± 0.04 | 0.37 ± 0.02 | 48 | 60.2 * |
| Solidago canadensis | 0.29 ± 0.04 | 0.32 ± 0.02 | 0.26 ± 0.03 | 0.30 ± 0.01 | 0.28 ± 0.00 | 0.15 ± 0.02 | 0.17 ± 0.07 | 0.19 ± 0.02 | 0.24 ± 0.02 | 0.31 ± 0.01 | 32 | 52.6 * |
| Urtica dioica | 0.22 ± 0.01 | 0.22 ± 0.05 | 0.21 ± 0.03 | 0.21 ± 0.03 | 0.24 ± 0.02 | 0.19 ± 0.00 | 0.28 ± 0.02 | 0.26 ± 0.01 | 0.27 ± 0.00 | 0.29 ± 0.02 | 21 | 43.2 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ložienė, K.; Chochlovaitė, I. Effect of Phenological Stage and Leaf Age on Changes of Chlorophyll and Carotenoid Contents in Some Weeds and Invasive Species. Molecules 2025, 30, 3788. https://doi.org/10.3390/molecules30183788
Ložienė K, Chochlovaitė I. Effect of Phenological Stage and Leaf Age on Changes of Chlorophyll and Carotenoid Contents in Some Weeds and Invasive Species. Molecules. 2025; 30(18):3788. https://doi.org/10.3390/molecules30183788
Chicago/Turabian StyleLožienė, Kristina, and Ineta Chochlovaitė. 2025. "Effect of Phenological Stage and Leaf Age on Changes of Chlorophyll and Carotenoid Contents in Some Weeds and Invasive Species" Molecules 30, no. 18: 3788. https://doi.org/10.3390/molecules30183788
APA StyleLožienė, K., & Chochlovaitė, I. (2025). Effect of Phenological Stage and Leaf Age on Changes of Chlorophyll and Carotenoid Contents in Some Weeds and Invasive Species. Molecules, 30(18), 3788. https://doi.org/10.3390/molecules30183788






