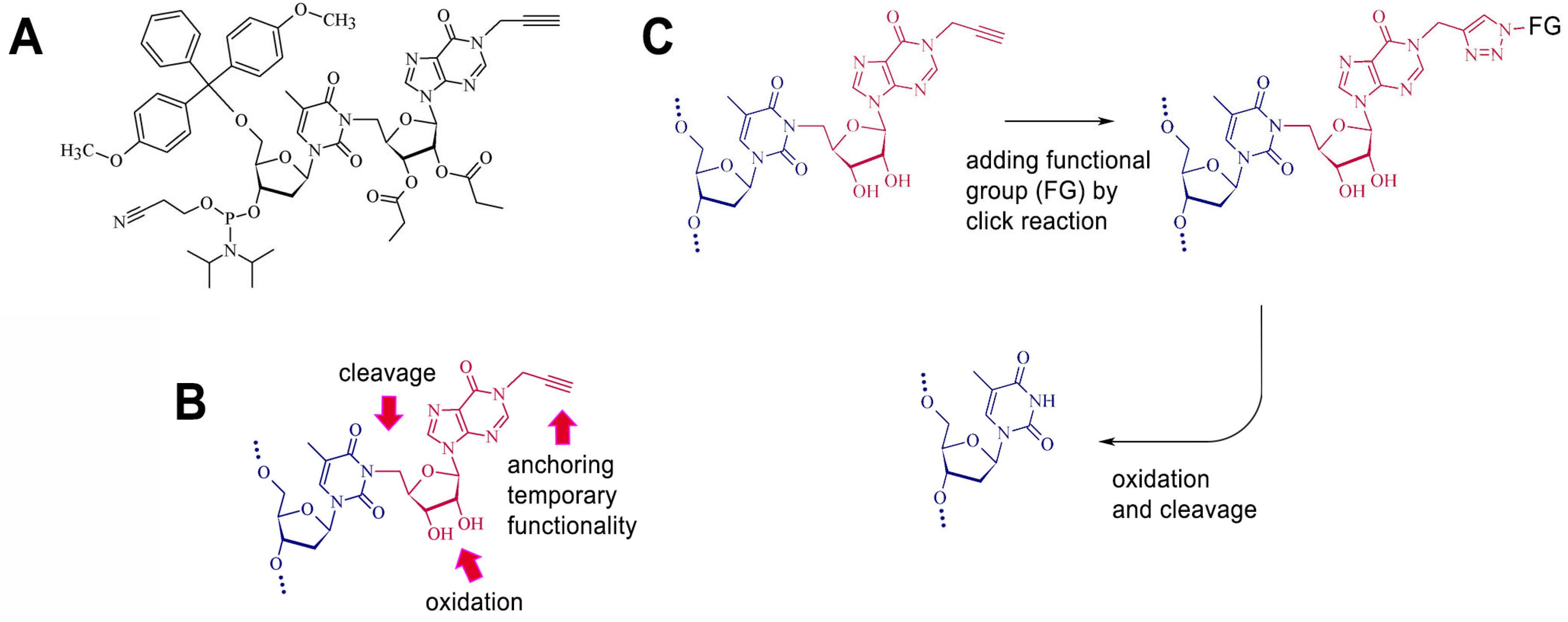
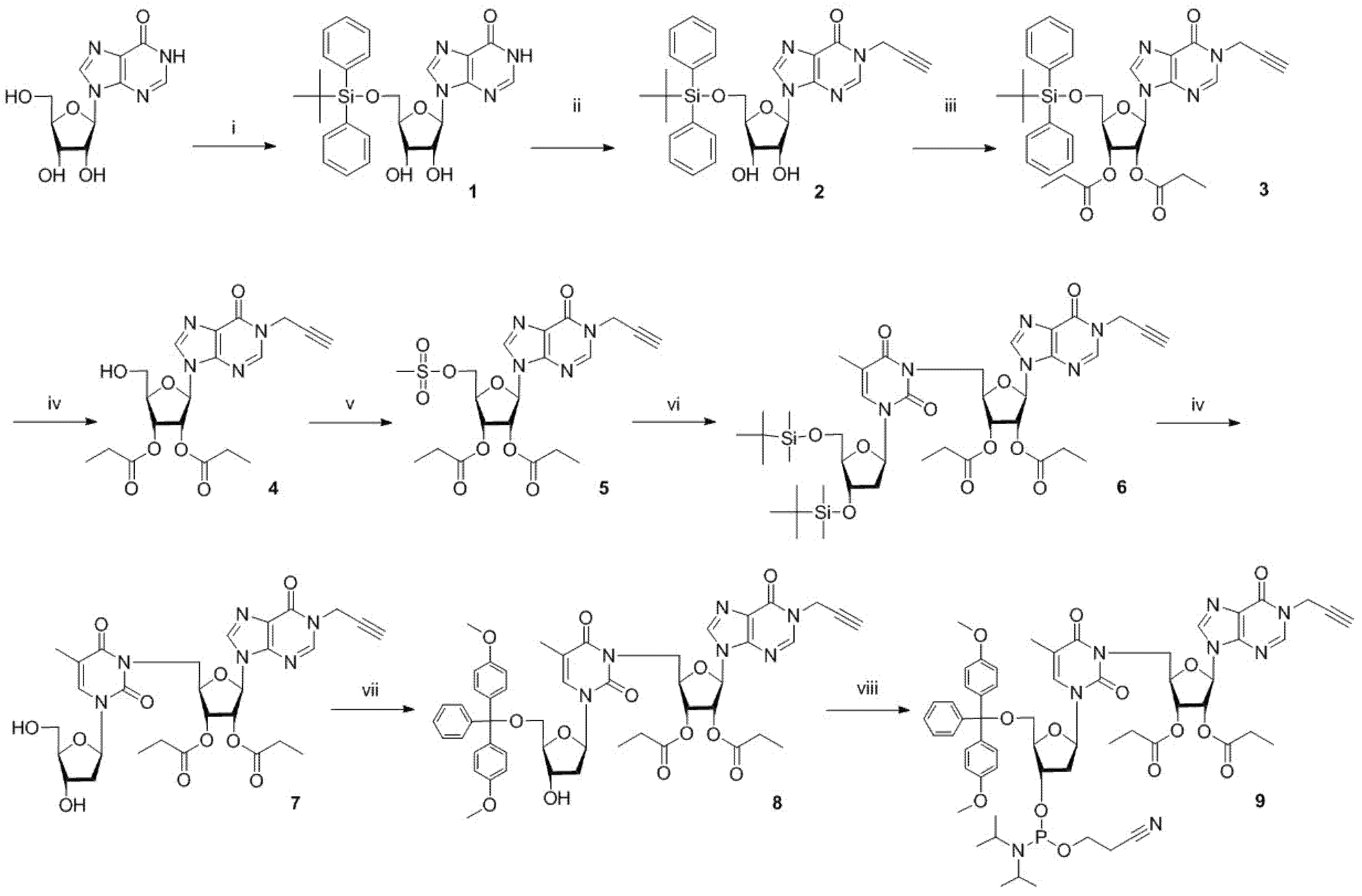
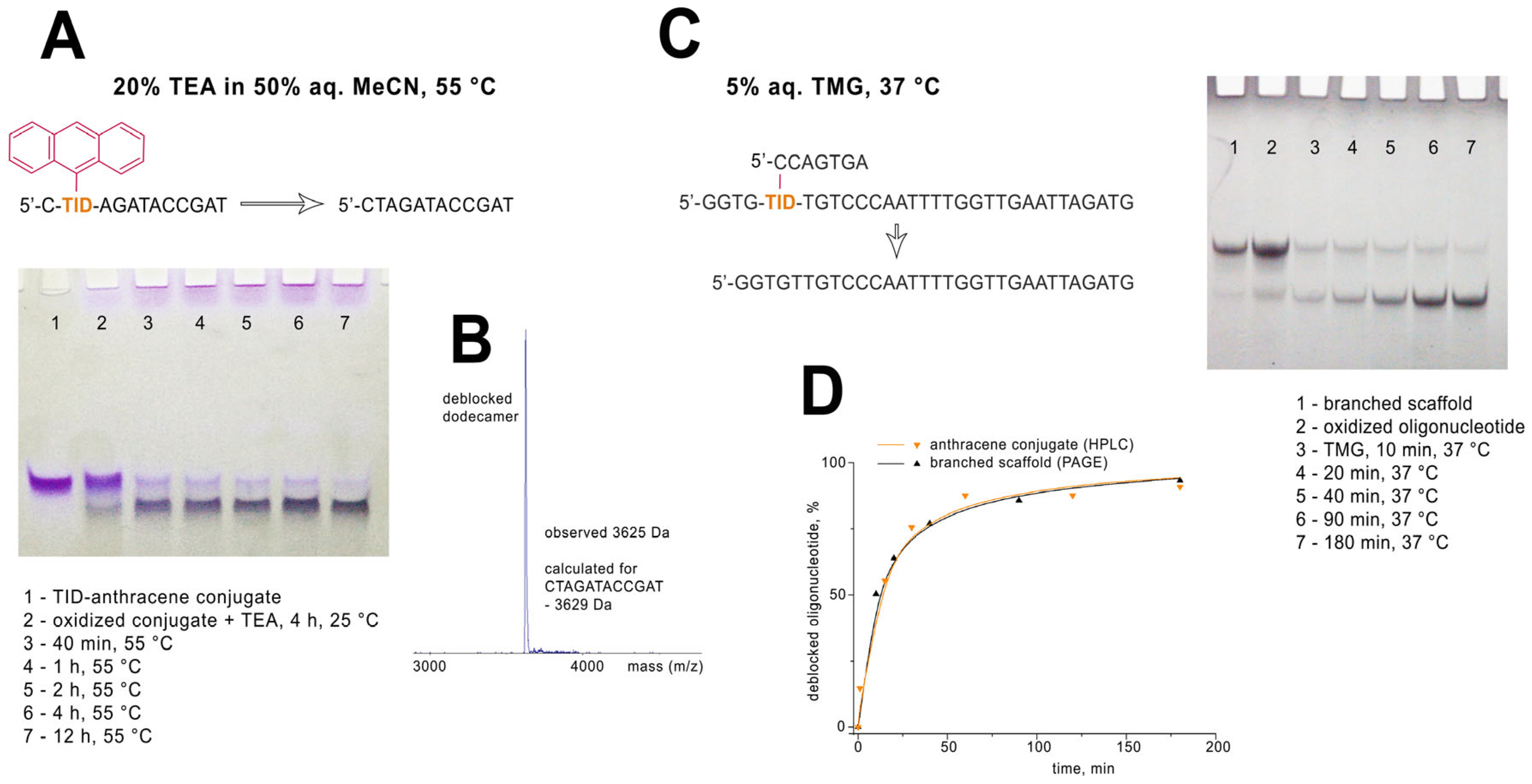
4.2. Synthesis
4.2.1. 5′-O-Tert-Butyldiphenylsilyl Inosine (1)
Imidazole (0.5 g, 7.35 mmol) and t-butyldiphenylsilyl chloride (2 g, 7.35 mmol) were added to a stirred suspension of inosine (1.92 g, 7.16 mmol) in a mixture of pyridine (10 mL) and DMF (2 mL). The mixture was heated to 40 °C and stirred at 25 °C for 24 h. Pyridine was evaporated under reduced pressure. The residue was diluted with DCM (150 mL) and washed successively with saturated aqueous NaHCO3 (100 mL) and water (100 mL). The organic layer was dried over anhydrous Na2SO4 and concentrated in vacuum. The residue (5 mL) was precipitated with diethyl ether (50 mL) yielding 2.76 g (76%) of the title compound. Rf (A): 0.4. 1H-NMR (300.1 MHz, DMSO-d6): δ = 12.3 (brs, 1H, NH), 8.21 (s, 1H, H-2), 7.99 (s, 1H, H-8), 7.67–7.56 and 7.51–7.32 (m, 10H, aromatic), 5.92 (d, 1H, J1′,2′ = 4.8 Hz, H1′), 5.58 (d, 1H, J2′-OH = 5.6 Hz, 2′-OH), 5.25 (d, 1H, J3′-OH = 5.6 Hz, 3′-OH), 4.55 (dd, 1H, J2′,3′ = 5.0 Hz, H2′), 4.30 (dd, 1H, J3′,4′ = 4.6 Hz, H3′), 4.04 (dd, 1H, J4′,5′a = 3.6 Hz, J4′,5′b = 4.9 Hz, H4′), 3.93 (dd, 1H, J5′a′,5′b = −11.4 Hz, H5′a), 3.79 (dd, 1H, H5′b), 0.99 (s, 9H, tBu). 13C-NMR (75.5 MHz, DMSO-d6): 157.00 (C6), 148.66 (C4), 146.26 (C2), 138.97 (C8), 135.57, 135.48, 133.25, 133.09, 130.34, 128.34, and 128.30 (CH Ph), 124.98 (C5), 88.13 (C1′), 84.87 (C4′), 74.07 (C2′), 70.23 (C3′), 64.32 (C5′), 27.10 (CH3 tBu), 19.25 (C tBu). HRMS (ESI) calculated [M+H]+ for C26H30N4O5Si: 507.2058, found: 507.2061.
4.2.2. N1-Propargyl-5′-O-Tert-Butyldiphenylsilyl Inosine (2)
DBU (2.4 mL, 16 mmol) and propargyl bromide (80% soln. in toluene, 2 mL, 16 mmol) were added to a stirred solution of compound 1 (2.7 g, 5.3 mmol) in 10 mL of a mixture of MeCN-EtOH (3:1 v/v). The reaction mixture was heated to 40 °C for 5 min and stirred at room temperature for an additional 30 min. Then, the solvents were evaporated under reduced pressure. The residue was dissolved in 150 mL of DCM and washed with water (100 mL). The organic layer was dried over anhydrous Na2SO4 and concentrated in vacuum. The residue was dissolved in 5 mL of DCM, precipitated with diethyl ether (50 mL), and purified by column chromatography on silica gel using gradient elution from DCM/hexane/EtOH 10:10:1 to DCM/hexane/EtOH 10:5:1 yielding 2.37 g (82%) of compound 2. Rf (A): 0.85. 1H-NMR (300.1 MHz, DMSO-d6): δ = 8.42 (s, 1H, H-2), 8.26 (s, 1H, H-8), 7.66–7.58 and 7.50–7.32 (m, 10H, aromatic), 5.91 (d, 1H, J1′,2′ = 4.8 Hz, H1′), 5.58 (d, 1H, J2′-OH = 5.7 Hz, 2′-OH), 5.26 (d, 1H, J3′-OH = 5.6 Hz, 3′-OH), 4.84 (d, 2H, JN-CH2, ≡CH = 2.4 Hz, N-CH2), 4.57 (dd, 1H, J2′,3′ = 5.1 Hz, H2′), 4.31 (dd, 1H, J3′,4′ = 4.8 Hz, H3′), 4.05 (ddd, 1H, J4′,5′a = 3.7 Hz, J4′,5′b = 4.9 Hz, H4′), 3.92 (dd, 1H, J5′a′,5′b = −11.3 Hz, H5′a), 3.79 (dd, 1H, H5′b), 3.43 (t, 1H, ≡CH), 0.98 (s, 9H, tBu). 13C-NMR (75.5 MHz, DMSO-d6): 155.65 (C6), 148.25 (C2), 147.81 (C4), 139.89 (C8), 135.57, 135.48, 133.23, 133.09, 130.35, 128.33, and 128.28 (CH Ph), 124.25 (C5), 88.28 (C1′), 84.97 (C4′), 79.20 (C≡CH), 76.07 (C≡CH), 74.01 (C2′), 70.23 (C3′), 64.28 (C5′), 35.38 (N-CH2 propargyl), 27.09 (CH3 tBu), 19.25 (C tBu). HRMS (ESI) calculated [M+H]+ for C29H32N4O5Si: 545.2215, found: 545.2211.
4.2.3. N1-Propargyl-2′,3′-di-O-Propionyl, 5′-O-Tert-Butyldiphenylsilyl Inosine (3)
Compound 2 (2.3 g, 4.2 mmol) in pyridine (20 mL) was treated with propionic anhydride (2.7 mL, 21.2 mmol) for 12 h at 25 C. Then, methanol (5 mL) was added to the reaction mixture. After 30 min the mixture was concentrated in vacuum, dissolved in 200 mL of DCM, and washed successively with saturated aqueous NaHCO3 (100 mL) and water (100 mL). The organic layer was dried over anhydrous Na2SO4 and concentrated under reduced pressure. The residue was purified by column chromatography on silica gel using gradient elution from DCM/hexane 1:1 to DCM/hexane/EtOH 7:5:1, yielding 2.24 g (81%) of white sticky foam. Rf (B): 0.65. 1H-NMR (300.1 MHz, DMSO-d6): δ = 8.38 (s, 1H, H-2), 8.27 (s, 1H, H-8), 7.63–7.55 and 7.50–7.32 (m, 10H, aromatic), 6.20 (d, 1H, J1′,2′ = 5.3 Hz, H1′), 5.98 (dd, 1H, J2′,3′ = 5.8 Hz, H2′), 5.75 (dd, 1H, J3′,4′ = 4.8 Hz, H3′), 4.91–4.77 (m, 2H, N-CH2), 4.29 (dd, 1H, J4′,5′a = 3.7 Hz, J4′,5′b = 4.5 Hz, H4′), 3.96 (dd, 1H, J5′a′,5′b = −11.5 Hz, H5′a), 3.85 (dd, 1H, H5′b), 3.42 (t, 1H, JN-CH2, ≡CH = 2.5 Hz, ≡CH), 2.45–2.28 (m, 4H, CH2 propionyl), 1.08 (t, 3H, JC3,CH3 = 7.5 Hz, CH3 propionyl), 1.03–0.95 (m, 12H, CH3 propionyl, tBu). 13C-NMR (75.5 MHz, DMSO-d6): 173.07, 172.94 (O-C=O), 155.53 (C6), 148.46 (C2), 147.54 (C4), 140.36 (C8), 135.53, 135.49, 132.92, 132.77, 130.42, 128.32, and 128.29 (CH Ph), 124.51 (C5), 86.30 (C1′), 82.67 (C4′), 79.01 (C≡CH), 76.22 (C≡CH), 72.87 (C2′), 70.11 (C3′), 63.44 (C5′), 35.46 (N-CH2 propargyl), 27.09 (CH2 propionyl), 26.97 (C tBu), 26.92 (CH2 propionyl), 19.20 (C tBu), 9.37, 9.20 (CH3 propionyl). HRMS (ESI) calculated [M+H]+ for C35H40N4O7Si: 657.2739, found: 657.2740.
4.2.4. N1-Propargyl-2′,3′-di-O-Propionyl Inosine (4)
To a solution of compound 3 (2.2 g, 3.4 mmol) in THF (5 mL) triethylamine (2.5 mL) and triethylamine trihydrofluoride (2.5 mL) were added. After 4 h, the reaction mixture was diluted with DCM (200 mL) and washed with 10% aqueous sodium chloride (100 mL). The organic layer was dried over anhydrous Na2SO4 and concentrated under reduced pressure. The residue was purified by column chromatography on silica gel using gradient elution from DCM/hexane/EtOH 10:10:1 to DCM/hexane/EtOH 5:5:1 yielding 1.0 g (71%) of compound 5. Rf (B): 0.45. 1H-NMR (300.1 MHz, DMSO-d6): δ = 8.53 (s, 1H, H-2), 8.41 (s, 1H, H-8), 6.17 (d, 1H, J1′,2′ = 6.5 Hz, H1′), 5.85 (dd, 1H, J2′,3′ = 5.7 Hz, H2′), 5.52 (dd, 1H, J3′,4′ = 3.1 Hz, H3′), 5.33 (dd, 1H, J5′a,OH = 5.2 Hz, J5′b,OH = 5.8 Hz, 5′-OH), 4.85 (d, 2H, JN-CH2, ≡CH = 2.5 Hz, N-CH2), 4.24 (dd, 1H, J4′,5′a = 3.8 Hz, J4′,5′b = 3.7 Hz, H4′), 3.74 (ddd, 1H, J5′a′,5′b = −12.2 Hz, H5′a), 3.64 (ddd, 1H, H5′b), 3.40 (t, 1H, ≡CH), 2.48–2.37 (m, 2H, CH2 propionyl), 2.35–2.22 (m, 2H, CH2 propionyl), 1.08 (t, 3H, JCH2, -CH3 = 7.5 Hz, CH3 propionyl), 0.96 (t, 3H, JCH2, -CH3 = 7.5 Hz, CH3 propionyl). 13C-NMR (75.5 MHz, DMSO-d6): 173.24, 172.86 (O-C=O x2), 155.54 (C6), 148.79 (C2), 147.72 (C4), 139.98 (C8), 124.23 (C5), 85.67 (C1′), 84.26 (C4′), 79.13 (C≡CH), 76.15 (C≡CH), 73.49 (C2′), 71.46 (C3′), 61.33 (C5′), 35.53 (N-CH2 propargyl), 27.14 (CH2 propionyl), 26.88 (CH2 propionyl), 9.40 (CH3 propionyl), 9.16 (CH3 propionyl). HRMS (ESI) calculated [M+H]+ for C19H22N4O7: 419.1561, found: 419.1561.
4.2.5. N1-Propargyl-5′-O-Methanesulfonyl-2′,3′-di-O-Propionyl Inosine (5)
To a cooled (0 °C) solution of compound 4 (0.95 g, 2.3 mmol) in DCM (10 mL) triethylamine (0.41 mL, 3 mmol) and methanesulfonyl chloride (0.23 mL, 3 mmol) were added while stirring. After 12 h of stirring at 25 °C the reaction mixture was diluted with DCM to 100 mL and washed successively with saturated aqueous NaHCO3 (50 mL) and water (50 mL). The organic layer was dried over anhydrous Na2SO4 and concentrated in vacuum. The residue was dissolved in 3 mL of DCM and precipitated with hexane (60 mL). The title compound was purified by column chromatography on silica gel using gradient elution from DCM/hexane 1:1 to DCM/hexane/EtOH 5:5:1 yielding 0.81 g (72%) of mesylate 6 as sticky white foam. Rf (B): 0.35. 1H-NMR (300.1 MHz, DMSO-d6): δ = 8.54 (s, 1H, H-2), 8.37 (s, 1H, H-8), 6.23 (d, 1H, J1′,2′ = 5.7 Hz, H1′), 5.92 (dd, 1H, J2′,3′ = 5.9 Hz, H2′), 5.61 (dd, 1H, J3′,4′ = 4.4 Hz, H3′), 4.86 (d, 2H, JN-CH2, ≡CH = 2.5 Hz, N-CH2), 4.61–4.44 (m, 3H, H4′, H5′a, H5′b), 3.41 (t, 1H, ≡CH), 3.19 (s, 3H, CH3 mesyl), 2.47–2.38 (m, 2H, CH2 propionyl), 2.37–2.28 (m, 2H, CH2 propionyl), 1.08 (t, 3H, JCH2, -CH3 = 7.5 Hz, CH3 propionyl), 0.99 (t, 3H, JCH2, -CH3 = 7.5 Hz, CH3 propionyl). 13C-NMR (75.5 MHz, DMSO-d6): 173.09 (O-C=O), 172.88 (O-C=O), 155.54 (C6), 148.80 (C2), 147.64 (C4), 140.39 (C8), 124.42 (C5), 86.09 (C1′), 80.02 (C4′), 79.10 (C≡CH), 76.17 (C≡CH), 72.62 (C2′), 70.13 (C3′), 68.98 (C5′), 37.30 (CH3 mesyl), 35.56 (N-CH2 propargyl), 27.06 (CH2 propionyl), 26.87 (CH2 propionyl), 9.31 (CH3 propionyl), 9.17 (CH3 propionyl). HRMS (ESI) calculated [M+H]+ for C20H24N4O9S: 497.1337, found: 497.1336.
4.2.6. 3′,5′-di-O-Tert Butyldimethylsilylthymidine, 2′,3′-di-O-Propionylinosine (N3-C5′) Dimer (6)
To a stirred solution of compound 5 (0.64 g, 1.3 mmol) and 3′,5′-di-O-t-butyldimethylsilyl thymidine (1.8 g, 3.8 mmol) in acetonitrile (5 mL) 1,8-diazabicyclo[5.4.0]undec-7-ene (0.6 mL 4 mmol) was added. The reaction mixture was left for 48 h at room temperature. Then, the solution was diluted with DCM (150 mL) and washed with water (100 mL). The organic layer was dried over anhydrous Na2SO4 and concentrated in vacuum. The residue was purified by column chromatography on silica gel using gradient elution from DCM/hexane 1:1 to DCM/hexane/EtOH 50:50:1 yielding 0.46 g (41%) of compound 6. Rf (B): 0.5. 1H-NMR (300.1 MHz, DMSO-d6): δ = 8.52 (s, 1H, H-2 inosine), 8.38 (s, 1H, H-8 inosine), 7.52 (s, 1H, H-6 thymidine), 6.20–6.12 (m, 2H, H1′ inosine, thymidine), 5.94 (dd, 1H, J1′,2′ = 5.7 Hz, J2′,3′ = 5.4 Hz, H2′ inosine), 5.57 (dd, 1H, J3′,4′ = 4.7 Hz, H3′ inosine), 4.85 (d, 2H, JN-CH2, ≡CH = 2.4 Hz, N-CH2 inosine), 4.43–4.28 (m, 3H, H4′, H5′a, H5′b inosine), 4.21–4.08 (m, 1H, H3′ thymidine), 3.85–3.67 (m, 3H, H4′, H5′a, H5′b thymidine), 3.41 (t, 1H, ≡CH inosine), 2.37–2.26 (m, 4H, CH2 propionyl), 2.23–2.06 (m, 2H, H2′a, H2′b thymidine), 1.84 (s, 3H, CH3 thymidine), 1.04–0.93 (m, 6H, CH3 propionyl), 0.88 (s, 18H, CH3 tBu), 0.09 (s, 12H, Si-CH3). 13C-NMR (75.5 MHz, DMSO-d6): 172.90 (O-C=O), 163.22 (C4 thymidine), 155.54 (C6 inosine), 150.98 (C2 thymidine), 148.60 (C2 inosine), 147.49 (C4 inosine), 140.88 (C8 inosine), 135.01 (C6 thymidine), 124.64 (C5 inosine), 109.13 (C5 thymidine), 87.40 (C1′ thymidine), 86.61 (C1′ inosine), 85.40 (C4′ thymidine), 79.10 (C≡CH), 78.96 (C3′ inosine), 76.18 (C≡CH), 72.77 (C2′ inosine), 72.37 (C3′ thymidine), 72.28 (C4′ inosine), 63.11 (C5′ thymidine), 42.20 (C5′ inosine), 39.91 (C2′, thymidine overlapped with DMSO-d6 signal), 35.51 (N-CH2 propargyl), 26.94 (CH2 propionyl), 26.87 (CH2 propionyl), 26.21 (CH3 tBu), 26.11 (CH3 tBu), 18.46 (C tBu), 18.14 (CH3 tBu), 13.33 (CH3 thymidine), 9.23 (CH3 propionyl), 9.17 (CH3 propionyl), −4.32 (Si-CH3), −4.48 (Si-CH3), −5.01 (Si-CH3). HRMS (ESI) calculated [M+H]+ for C41H62N6O11Si2: 871.4088, found: 871.4090.
4.2.7. Thymidine, 2′,3′-di-O-Propionylinosine (N3-C5′) Dimer (7)
To a solution of compound 6 (0.42 g, 0.48 mmol) in THF (2 mL) triethylamine (0.65 mL) and triethylamine trihydrofluoride (0.65 mL) were added. After 4 h the reaction mixture was diluted with DCM (100 mL) and washed with 10% aqueous sodium chloride (50 mL). The organic layer was dried over anhydrous Na2SO4 and concentrated under reduced pressure. The residue was purified by column chromatography on silica gel using gradient elution from DCM/hexane/EtOH 10:10:1 to DCM/EtOH 9:1 yielding 0.28 g (91%) of compound 7. Rf (A): 0.8. 1H-NMR (300.1 MHz, DMSO-d6): δ = 8.51 (s, 1H, H-2 inosine), 8.37 (s, 1H, H-8 inosine), 7.81 (s, 1H, H-6 Thy), 6.20–6.12 (m, 2H, H1′ inosine, thymidine), 5.94 (dd, 1H, J1′,2′ = 5.6 Hz, J2′,3′ = 5.4 Hz, H2′ inosine), 5.57 (dd, 1H, J3′,4′ = 4.8 Hz, H3′ inosine), 5.23 (d, 1H, J3′-OH = 4.3 Hz, 3′-OH thymidine), 5.04 (t, 1H, J5′a-OH = J5′b-OH = 5.2 Hz, 5′-OH thymidine), 4.85 (d, 2H, JN-CH2, ≡CH = 2.4 Hz, N-CH2), 4.43–4.09 (m, 4H, H4′, H5′a, H5′b inosine, H3′ thymidine), 3.79 (dd, 1H, J4′,3′ = J4′,5′a = J4′,5′b = 3.5 Hz, H4′ thymidine), 3.67–3.52 (m, 2H, H5′a, H5′b thymidine), 3.41 (t, 1H, ≡CH), 2.37–2.27 (m, 4H, CH2 propionyl), 2.16–2.05 (m, 2H, H2′a, H2′b thymidine), 1.83 (s, 3H, CH3 thymidine), 1.06–0.93 (m, 6H, CH3 propionyl). 13C-NMR (75.5 MHz, DMSO-d6): 172.93 (O-C=O), 172.89 (O-C=O), 163.30 (C4 thymidine), 155.56 (C6 inosine), 151.05 (C2 thymidine), 148.59 (C2 inosine), 147.49 (C4 inosine), 140.87 (C8 inosine), 135.56 (C6 thymidine), 124.63 (C5 inosine), 108.89 (C5 thymidine), 87.94 (C1′ thymidine), 86.61 (C1′ inosine), 85.45 (C4′ thymidine), 79.11 (C≡CH), 79.03 (C3′ inosine), 76.18 (C≡CH), 72.78 (C2′ inosine), 72.25 (C4′ inosine), 70.66 (C3′ thymidine), 61.62 (C5′ thymidine), 42.14 (C5′ inosine), 40.16 (C2′ thymidine, overlapped by the DMSO-d6 signal), 35.52 (N-CH2 propargyl), 26.97 (CH2 propionyl), 26.87 (CH2 propionyl), 13.33 (CH3 thymidine), 9.28 (CH3 propionyl), 9.18 (CH3 propionyl). HRMS (ESI) calculated [M+H]+ for C29H34N6O11: 643.2358, found: 643.2358.
4.2.8. 5′-O-Dimethoxytrityl Thymidine, 2′,3′-di-O-Propionylinosine (N3-C5′) Dimer (8)
Compound 7 (0.27 g, 0.42 mmol) was treated with 4,4′-dimethoxytrityl chloride (0.15 g, 0.45 mmol) in pyridine (5 mL) for 24 h at room temperature. Pyridine was removed in vacuum. The residue was dissolved in DCM (100 mL) and washed successively with saturated aqueous NaHCO3 (50 mL) and water (50 mL). The organic layer was dried over anhydrous Na2SO4 and concentrated under reduced pressure. The residue was purified by column chromatography on silica gel using gradient elution from DCM/hexane 1:1 to DCM/hexane/EtOH 10:10:1 yielding 0.31 g (78%) of compound 8. Rf (B): 0.65. 1H-NMR (300.1 MHz, DMSO-d6): δ = 8.52 (s, 1H, H-2 inosine), 8.37 (s, 1H, H-8 inosine), 7.58 (s, 1H, H-6 thymidine), 7.62–7.18 and 6.91–6.88 (m, 13H aromatic), 6.22 (dd, 1H J1′,2a′ = 6.5 Hz, J1′,2b′ = 6.8 Hz, H1′ thymidine), 6.16 (d, 1H, J1′,2′ = 5.1 Hz, H1′ inosine), 5.94 (dd, 1H, J2′,3′ = 5.8 Hz, H2′ inosine), 5.59 (dd, 1H, J3′,4′ = 4.4 Hz, H3′ inosine), 5.34 (d, 1H, J3′-OH = 4.6 Hz, 3′-OH thymidine), 4.84 (d, 2H, JN-CH2, ≡CH = 2.4 Hz, N-CH2), 4.42–4.28 (m, 3H, H4′, H5′a inosine, H3′ thymidine), 4.23–4.12 (m, 1H, H5′b inosine), 3.92 (ddd, 1H, J4′,3′ = 3.5 Hz, J4′,5′a = 4.4 Hz, J4′,5′b = 2.9 Hz, H4′ thymidine), 3.73 (s, 6H, OCH3), 3.40 (t, 1H, ≡CH), 3.28–3.15 (m, 2H, H5′a, H5′b thymidine), 2.38–2.16 (m, 6H, H2′a, H2′b thymidine, CH2 propionyl), 1.50 (s, 3H, CH3 thymidine), 1.07–0.93 (m, 6H, CH3 propionyl). 13C-NMR (75.5 MHz, DMSO-d6): 172.90 (O-C=O), 163.22 (C4 thymidine), 158.64 (C4 PhOMe), 155.55 (C6 inosine), 151.00 (C2 thymidine), 148.60 (C2 inosine), 147.49 (C4 inosine), 145.15 (C1 Ph), 140.83 (C8 inosine), 135.91 (C1 Ph-OMe), 135.73 (C1 Ph-OMe), 135.15 (C6 thymidine), 130.20 (C2,6 Ph-OMe), 127.26 (C4 Ph), 128.36 (C3 Ph), 128,15 (C5 Ph), 124.62 (C5 inosine), 113.73 (C3,5 Ph-OMe), 109.19 (C5 thymidine), 86.65 (C1′ inosine), 86.35 (C1′ thymidine), 86.14 (C4′ thymidine), 85.36 (O-C DMTr), 79.10 (C≡CH), 78.85 (C3′ inosine), 76.16 (C≡CH), 72.87 (C2′ inosine), 72.31 (C4′ inosine), 70.82 (C3′ thymidine), 64.15 (C5′ thymidine), 55.52 (O-CH3 DMTr), 42.31 (C5′ inosine), 40.16 (C2′ thymidine overlapped with the DMSO-d6 signal), 35.52 (N-CH2 propargyl), 26.95 (CH2 propionyl), 26.87 (CH2 propionyl), 12.79 (CH3 thymidine), 9.24 (CH3 propionyl), 9.17 (CH3 propionyl). HRMS (ESI) calculated [M+H]+ for C50H52N6O13: 945.3665, found: 945.3655.
4.2.9. 5′-O-Dimethoxytritylthymidine-3′-O-(2-Cyanoethyl-N,N-Diisopropyl)phosphoramidite, 2′,3′-di-O-Propionylinosine (N3-C5′) Dimer (9)
Compound 8 (0.3 g, 0.32 mmol), pyridine (25 μL) and tetrazole (25 mg, 0.35 mmol) were dissolved in 3 mL of anhydrous MeCN, and 3–5 beads of 4 Å molecular sieves (4–8 mesh) were added to the solution. The mixture was flushed with argon and left for 30 min. Then, 2-cyanoethyl N,N,N′,N′-tetraisopropylphosphorodiamidite (105 μL, 0.35 mmol) was added under intensive stirring to the solution. The reaction was allowed to proceed for 30 min at room temperature. Then, the solution was diluted with ethyl acetate (50 mL) and washed twice with cold saturated aqueous NaHCO3 (50 mL) and cold water (50 mL). The organic layer was dried over anhydrous Na2SO4, and the solvent was removed in vacuum. The residue was purified by column chromatography on silica gel using gradient elution with an ethyl acetate/hexane/triethylamine mixture from 50:50:1 to 70:30:1 yielding 0.3 g (83%) of compound 9. Rf (C): 0.55. 1H-NMR (300.1 MHz, DMSO-d6): δ = 8.52 (s, 1H, H-2 inosine), 8.39 (s, 1H, H-8 inosine), 7.62, 7.20 (s, 1H, H-6 thymidine diaster. I+II), 7.45–7.19 and 6.95–6.83 (m, 13H, aromatic), 6.24, 6.22 (t, 1H J1′,2a′ = J1′,2b′ = 6.5 Hz, H1′ thymidine diaster. I+II), 6.16 (d, 1H, J1′,2′ = 5.1 Hz, H1′ inosine), 5.94 (dd, 1H, J2′,3′ = 5.8 Hz, H2′ inosine), 5.59, 5.57 (dd, 1H, J3′,4′ = 8.0 Hz, H3′ inosine), 4.85 (d, 2H, JN-CH2, ≡CH = 2.4 Hz, N-CH2), 4.62–4.49 (m, 1H, H3′ thymidine diaster. I+II), 4.43–4.30 (m, 2H, H4′, H5′a inosine), 4.23–3.98 (m, 2H, H5′b inosine, H4′ thymidine), 3.73 (s, 6H, OCH3), 3.66–3.45 (m, 3H, H5′a, H5′b, CH(CH3)2 thymidine), 3.41 (t, 1H, ≡CH), 3.29–3.22 (m, 1H, CH(CH3)2), 2.77, 2.64 (t 2H, JCH2-C≡N, O-CH2 = 5.9 Hz, CH2CN), 2.44–2.25 (m, 6H, H2′a, H2′b thymidine, CH2 propionyl), 1.54 (s, 3H, CH3 thymidine diaster. I+II), 1.18–0.93 (m, 18H, CH3 propionyl, isopropyl). 13C-NMR (75.5 MHz, DMSO-d6): 172.90 (O-C=O), 163.20 (C4 thymidine), 158.69 (C4 Ph-OMe), 155.55 (C6 inosine), 150.95 (C2 thymidine), 148.61 (C2 inosine), 147.50 (C4 inosine), 145.03 (C1 Ph), 140.84 (C8 inosine), 135.77 (C1 Ph-OMe), 135.63, 135.60 (C6 thymidine diaster. I, II), 135.06 (C1 Ph-OMe), 130.22 (C2,6 Ph-OMe), 128.35 (C4 Ph), 128,17 (C3,5 Ph), 127.32 (C4 Ph), 124.63 (C5 inosine), 119.39, 119.19 (CN diaster. I, II), 113.70 (C3,5 Ph-OMe), 109.39, 109.31 (C5 thymidine diaster. I, II), 86.61 (C1′ inosine), 86.50, 86.44 (C1′ thymidine diaster. I, II), 85.38, 85.31 (C4′ thymidine diaster. I, II, O-C DMTr), 85.08, 85.04 (C3′ thymidine diaster. I, II), 79.11 (C≡CH), 78.92, 78.88 (C3′ diaster. I, II, inosine), 76.17 (C≡CH), 72.83 (C2′ inosine), 72.32 (C4′ inosine), 63.66, 63.50 (C5′ thymidine diaster. I, II), 58.92, 58.67 (-CH2- cyanoethyl diaster. I, II), 55.53 (O-CH3 DMTr), 42.31 (C5′ inosine), 39.07 (C2′ thymidine overlapped by the DMSO-d6 signal), 35.52 (N-CH2), 26.96 (CH2 propionyl), 26.87 (CH2 Propionyl), 24.85, 24.74, 24.70, 24.64 (CH3 isopropyl diaster. I, II), 12.81 (CH3 thymidine), 9.24, 9.17 (CH3 propionyl). 31P-NMR (202 MHz, DMSO-d6): 147.74 (diaster. I) and 147.40 (diaster. II). HRMS (ESI) calculated [M+H]+ for C59H69N8O14P: 1145.4744, found: 1145.4753.