Influence of Er- and Al-Doped ZnO on Mixed-Matrix Membranes of Chitosan Derivatives in Bioelectrochemical Systems
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization of the Catalyst
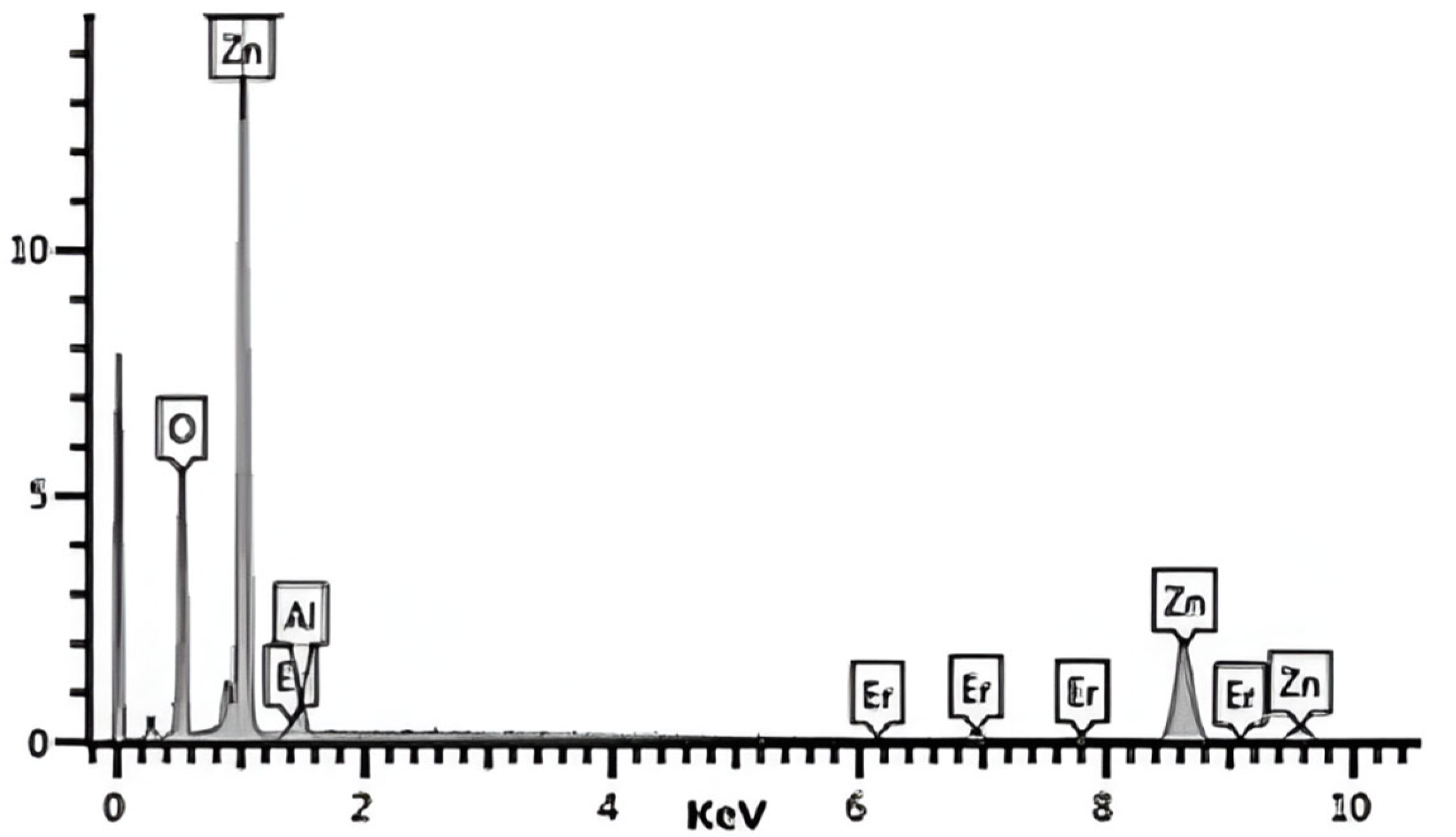
2.1.1. Composition of Catalyst
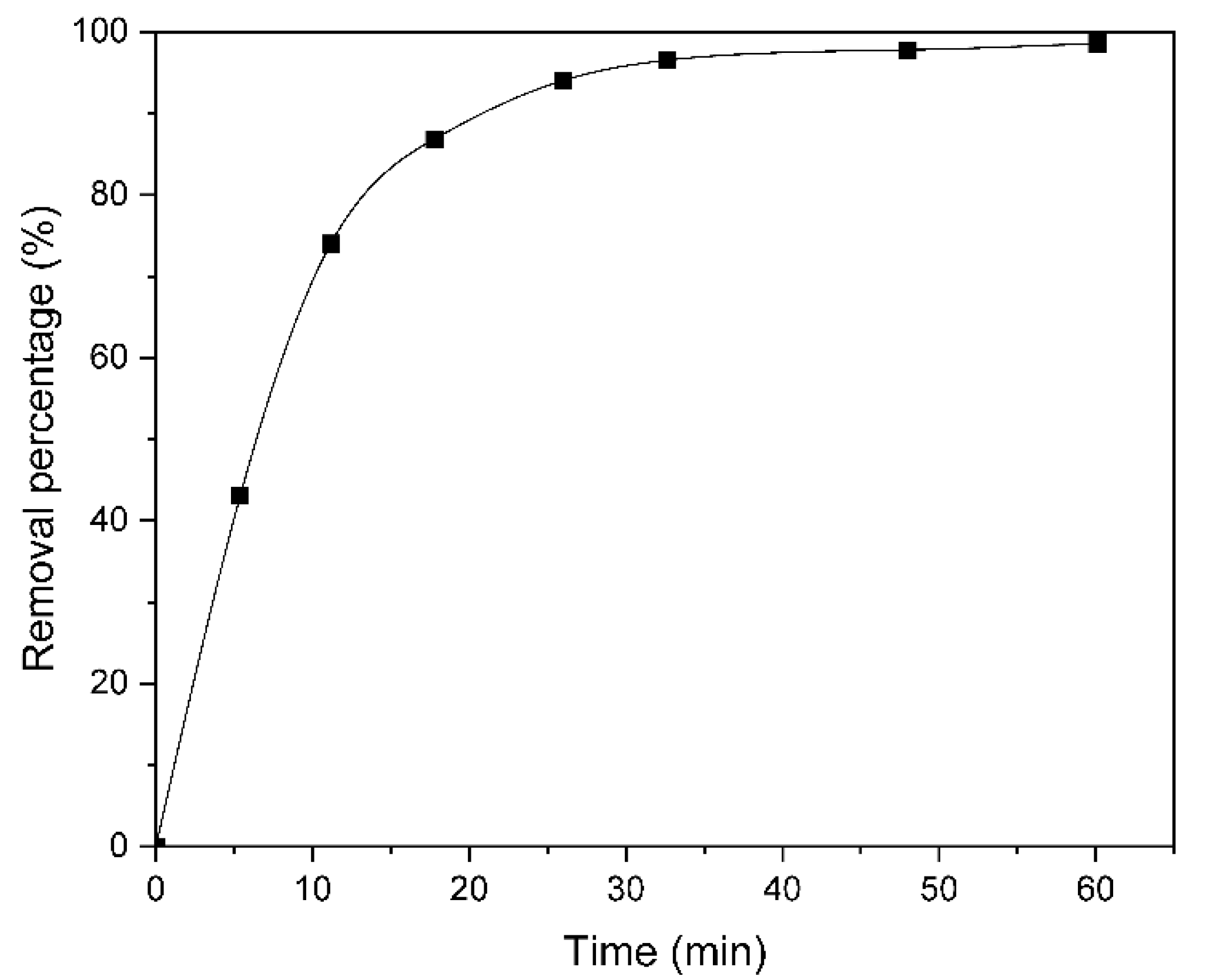
2.1.2. Evaluation of Catalyst
2.2. Evaluation of Membranes
2.2.1. Tensile Test of Membranes
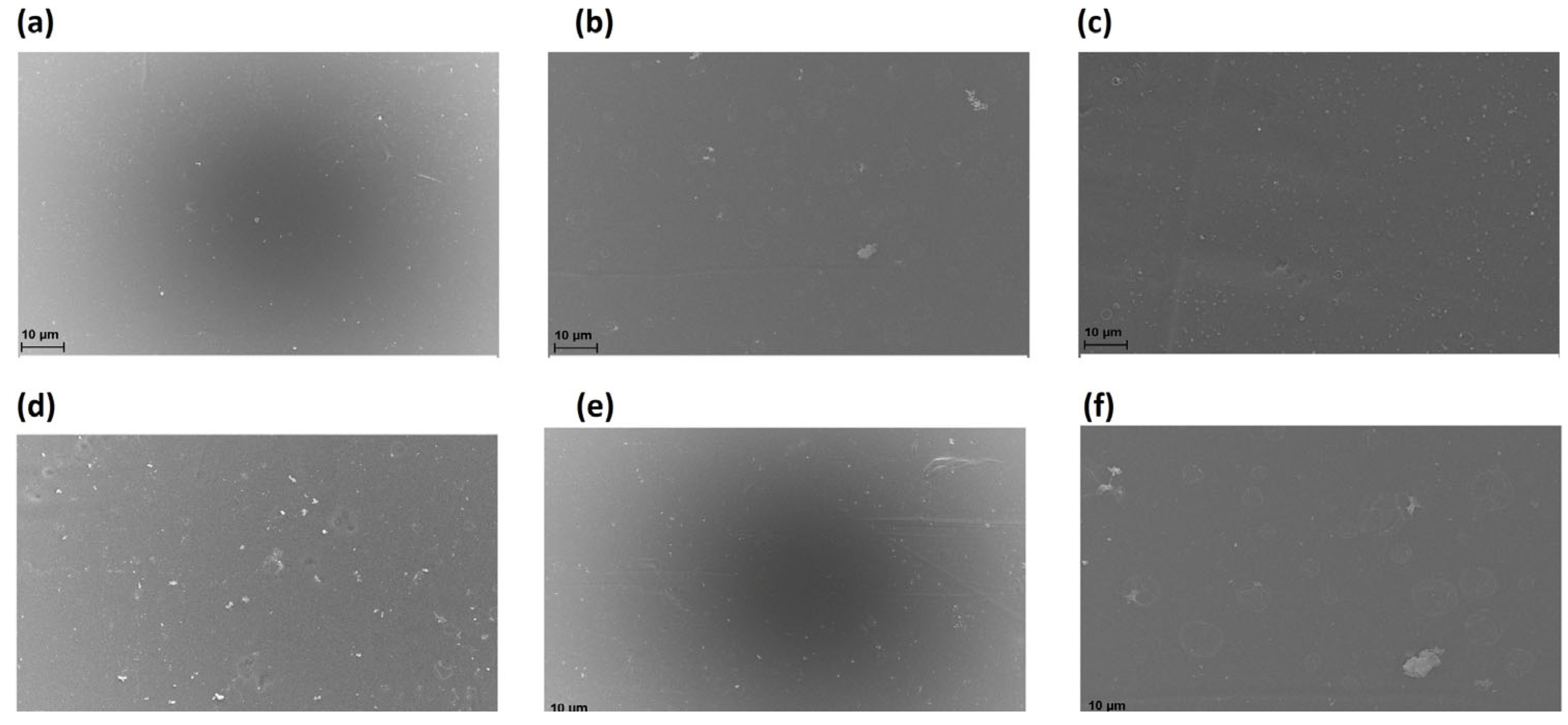
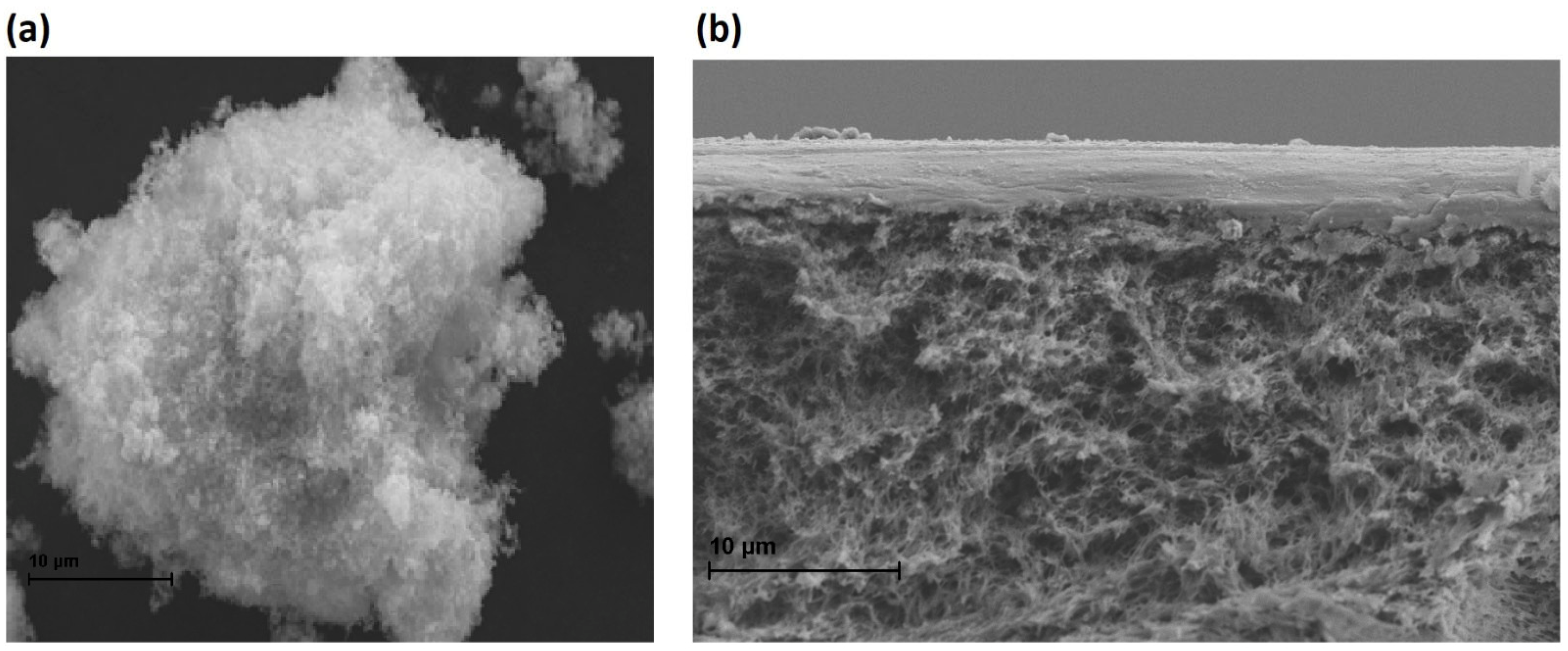
2.2.2. Membrane Morphology
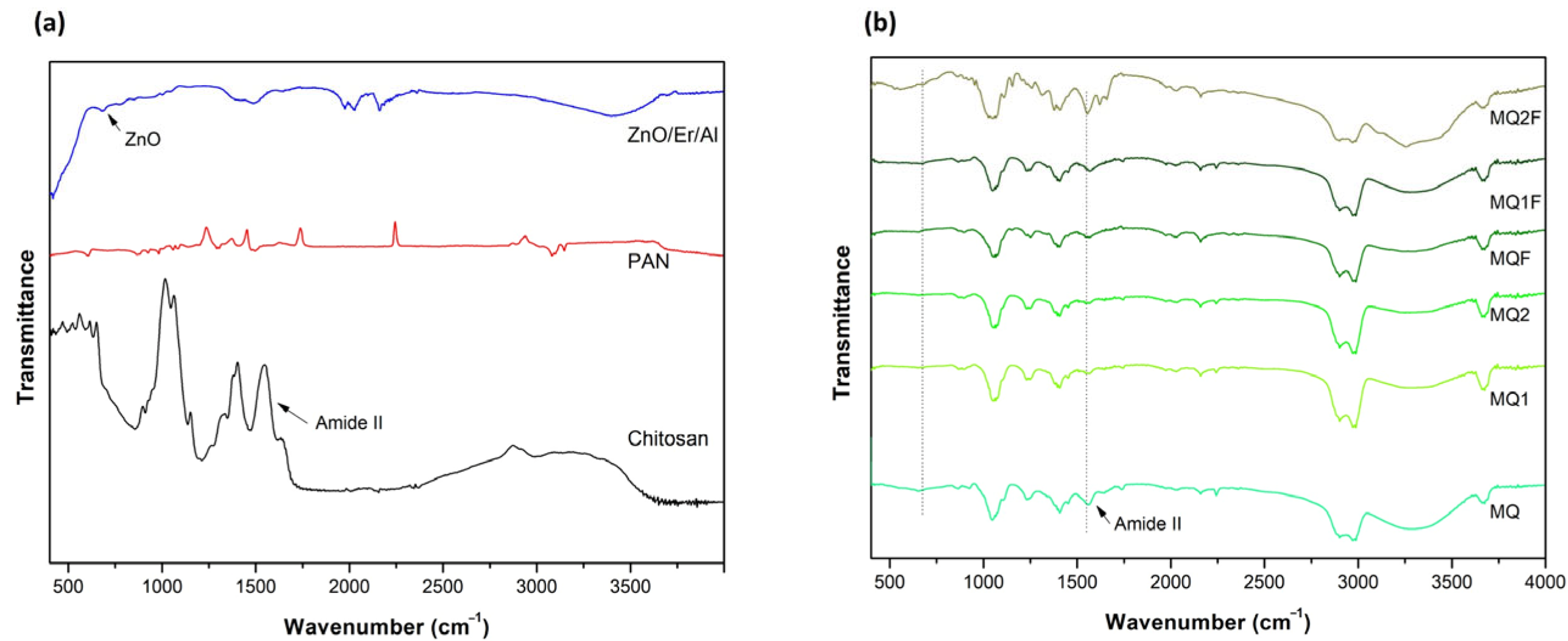
2.2.3. FTIR Spectroscopic Analysis of the Membranes
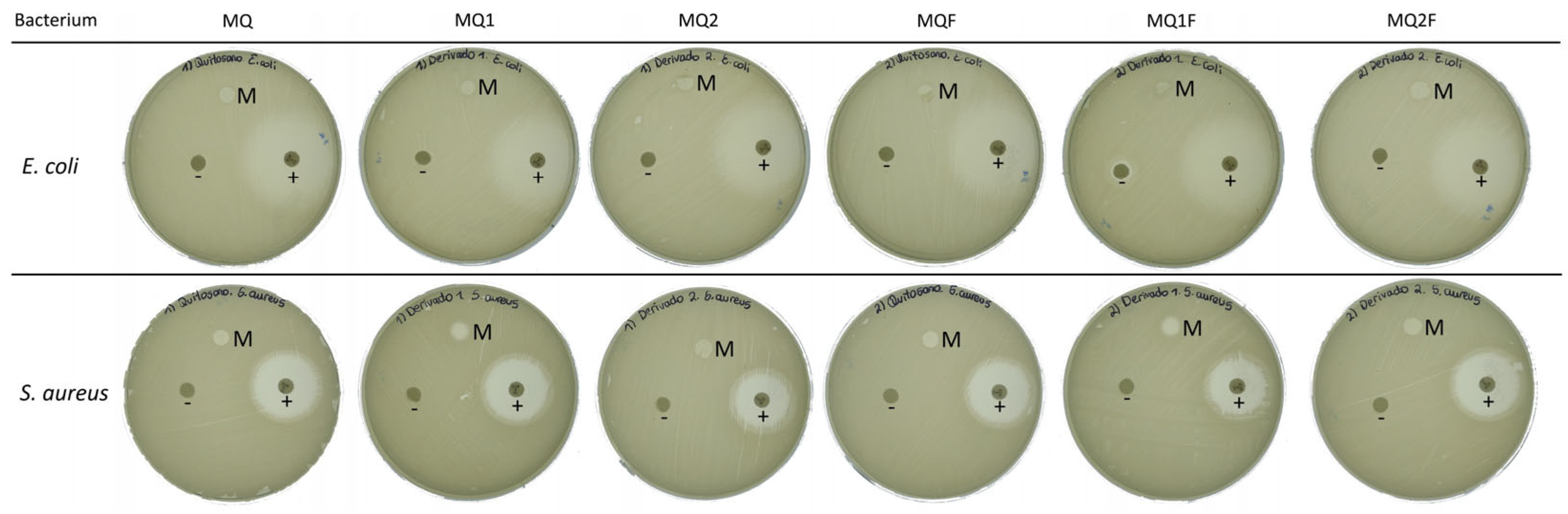
2.2.4. Antimicrobial Capacity
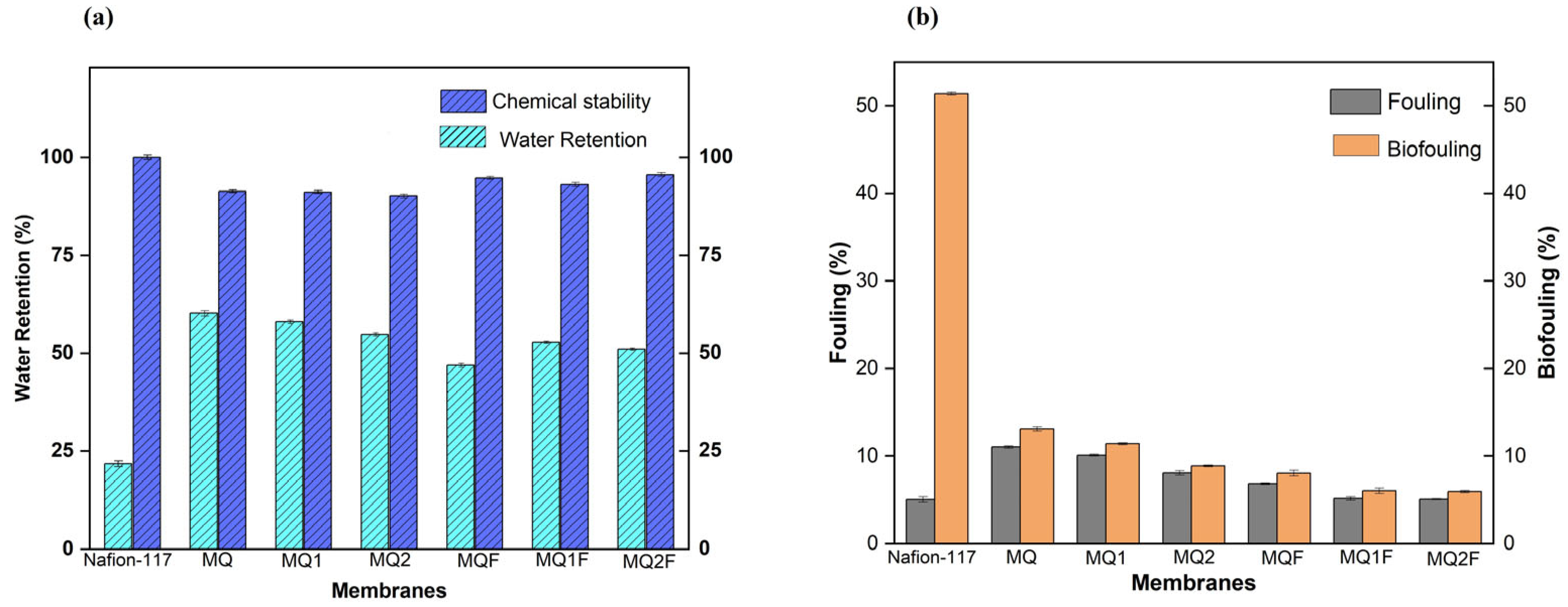
2.2.5. Water-Holding Capacity
2.2.6. Chemical Stability
2.2.7. Fouling
2.2.8. Biofouling
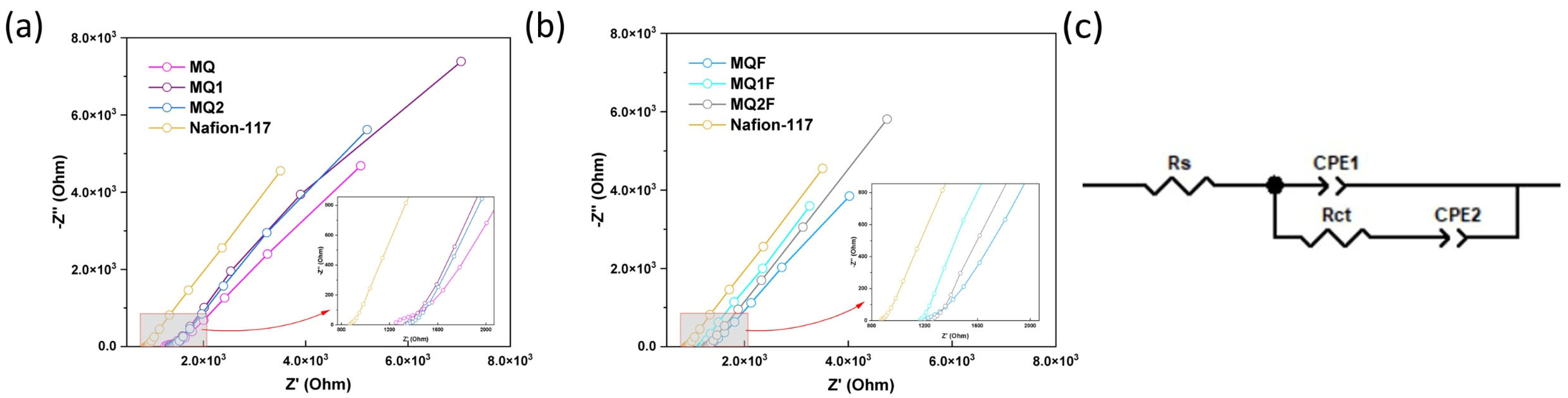
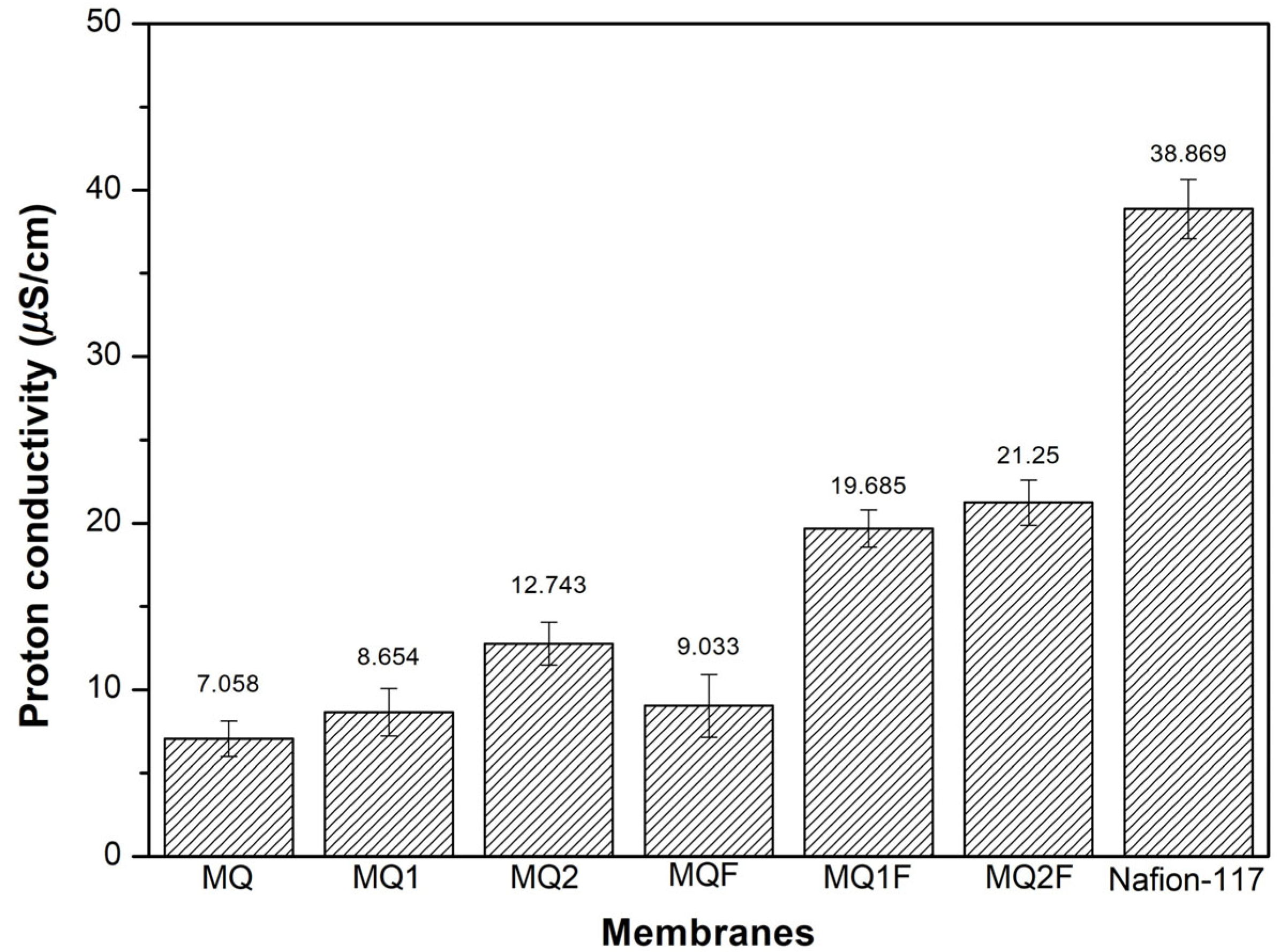
2.2.9. Proton Exchange Capacity
3. Material and Methods
3.1. Materials and Reactives
3.2. Chitosan Synthesis
3.3. Synthesis of Chitosan Derivatives
3.4. Synthesis of Er/Al/ZnO
3.5. Evaluation of Photocatalytic Capacity
3.6. Preparation of Mixed-Matrix Membranes
3.7. Membrane Morphology
3.8. Antimicrobial Capacity
3.9. Water-Holding Capacity
3.10. Chemical Stability
3.11. Fouling and Biofouling
3.12. Proton Exchange Capacity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Al-Sahari, M.; Al-Gheethi, A.; Mohamed, R.M.S.R.; Noman, E.; Naushad, M.; Rizuan, M.B.; Vo, D.-V.N.; Ismail, N. Green approach and strategies for wastewater treatment using bioelectrochemical systems: A critical review of fundamental concepts, applications, mechanism, and future trends. Chemosphere 2021, 285, 131373. [Google Scholar] [CrossRef]
- Mahendiravarman, E.; Rajamohan, N.; Rajasimman, M.; Rameshwar, S.S.; Abrar, I. Advancements in tailored polymeric membranes for microbial fuel cells: A comprehensive review of recent developments and challenges. RSC Adv. 2025, 15, 15842–15869. [Google Scholar] [CrossRef] [PubMed]
- Jumaah, A.F.; Abedini, R. Fabrication and performance evaluation of mixed matrix membrane comprising Pebax and graphene hydroxyl in olefin/paraffin separation. Polyolefins J. 2025, 12, 31–44. [Google Scholar]
- Xu, X.; Hartanto, Y.; Nikolaeva, D.; He, Z.; Chergaoui, S.; Luis, P. High-performance ZIF-8/biopolymer chitosan mixed-matrix pervaporation membrane for methanol/dimethyl carbonate separation. Sep. Purif. Technol. 2022, 293, 121085. [Google Scholar] [CrossRef]
- Chen, Q.; Qi, Y.; Jiang, Y.; Quan, W.; Luo, H.; Wu, K.; Li, S.; Ouyang, Q. Progress in Research of Chitosan Chemical Modifi-cation Technologies and Their Applications. Mar. Drugs 2022, 20, 536. [Google Scholar] [CrossRef]
- Shin, H.S.; Chimborazo, M.E.M.; Rivas, J.M.E.; Lorenzo-Felipe, Á.; Soler, M.M.; Serrano, M.J.Z.; Martín, J.F.; Artiles, J.S.R.; Sánchez, A.P.; Navarro, J.L.; et al. Genetic parameters for growth and morphological traits of the Pacific white shrimp Penaeus vannamei from a selective breeding programme in the industrial sector of Ecuador. Aquac. Rep. 2023, 31, 101649. [Google Scholar] [CrossRef]
- Pupiales, H.; Soria, R.B.; Arboleda, D.; Cevallos, C.; Alcivar, C.; Francis, L.; Xu, X.; Luis, P. ZIF-8/Chitosan Composite Hydrogel as a High-Performance Separator for Bioelectrochemical Systems. 2025; preprint repository Preprints.org. [Google Scholar] [CrossRef]
- Soria, R.B.; Chinchin, B.D.; Arboleda, D.; Zhao, Y.; Bonilla, P.; Van der Bruggen, B.; Luis, P. Effect of the bio-inspired modifi-cation of low-cost membranes with TiO2:ZnO as microbial fuel cell membranes. Chemosphere 2022, 291, 132840. [Google Scholar] [CrossRef] [PubMed]
- Vevers, R.; Kulkarni, A.; Seifert, A.; Pöschel, K.; Schlenstedt, K.; Meier-Haack, J.; Mezule, L. Photocatalytic Zinc Oxide Na-noparticles in Antibacterial Ultrafiltration Membranes for Biofouling Control. Molecules 2024, 29, 1274. [Google Scholar] [CrossRef]
- Kumar, S.; Ye, F.; Mazinani, B.; Dobretsov, S.; Dutta, J. Chitosan Nanocomposite Coatings Containing Chemically Resistant ZnO–SnOx Core–shell Nanoparticles for Photocatalytic Antifouling. Int. J. Mol. Sci. 2021, 22, 4513. [Google Scholar] [CrossRef]
- Siddique, T.; Gangadoo, S.; Pham, D.Q.; Dutta, N.K.; Choudhury, N.R. Antifouling and Antimicrobial Study of Nanostruc-tured Mixed-Matrix Membranes for Arsenic Filtration. Nanomaterials 2023, 13, 738. [Google Scholar] [CrossRef]
- Ghomri, R.; Shaikh, M.N.; Ahmed, M.I.; Song, W.; Cai, W.; Bououdina, M.; Ghers, M. Pure and (Er, Al) co-doped ZnO na-noparticles: Synthesis, characterization, magnetic and photocatalytic properties. J. Mater. Sci. Mater. Electron. 2018, 29, 10677–10685. [Google Scholar] [CrossRef]
- Azizah, N.; Muhammady, S.; Purbayanto, M.A.K.; Nurfani, E.; Winata, T.; Sustini, E.; Widita, R.; Darma, Y. Influence of Al doping on the crystal structure, optical properties, and photodetecting performance of ZnO film. Prog. Nat. Sci. Mater. Int. 2020, 30, 28–34. [Google Scholar] [CrossRef]
- Pratomo, U.; Fransisca, N.; Adzani, M.D.; Irkham, I.; Sulaeman, A.P.; Eddy, D.R.; Mulyana, J.Y.; Primadona, I. Doping of rare earth element: The effects in elevated physical and optical properties of ZnO. Talanta Open 2025, 11, 100411. [Google Scholar] [CrossRef]
- Shukla, S.; Sharma, D.K. A review on rare earth (Ce and Er)-doped zinc oxide nanostructures. Mater. Today: Proc. 2021, 34, 793–801. [Google Scholar] [CrossRef]
- Soria, R.B.; Estupiñan, J.; Gonza, I.; Naranjo, M.; Chinchin, B.D.; Manangón, L.E.; Vaca, K.; Romero-Bastidas, M.; Pupiales, H.; Taco, V.; et al. Enhancing Photocatalytic Performance of ZnO Nanoparticles through Er/Al Co-doping for Solar-driven Environmental Remediation. 2025. [Google Scholar] [CrossRef]
- Soria, R.B. Removal of Azo Dyes in Textile Wastewater Using Microbial Fuel Cells and Membrane Technology. 2022. Available online: http://hdl.handle.net/2078.1/264175 (accessed on 26 September 2024).
- Wu, M.; Zhang, X.; Zhao, Y.; Yang, C.; Jing, S.; Wu, Q.; Brozena, A.; Miller, J.T.; Libretto, N.J.; Wu, T.; et al. A high-performance hydroxide exchange membrane enabled by Cu2+-crosslinked chitosan. Nat. Nanotechnol. 2022, 17, 629–636. [Google Scholar] [CrossRef]
- Tazeen, S.K.; Niazi, M.B.K.; Binobead, M.A.; Ahmed, T.; Shahid, M. Characterization and toxicity evaluation of chitosan/ZnO nanocompoite as promising nano-biopolymer for treatment of synthetic wastewater. J. King Saud Univ. Sci. 2024, 36, 103432. [Google Scholar] [CrossRef]
- Madih, K.; El-Shazly, A.; Elkady, M.; Aziz, A.N.; Youssef, M.E.; Khalifa, R.E. A facile synthesis of cellulose acetate reinforced graphene oxide nanosheets as proton exchange membranes for fuel cell applications. J. Saudi Chem. Soc. 2022, 26, 101435. [Google Scholar] [CrossRef]
- Bahamonde Soria, R.; Zhu, J.; Gonza, I.; Van der Bruggen, B.; Luis, P. Effect of (TiO2:ZnO) Ratio on the Anti-Fouling Properties of Bio-Inspired Nanofiltration Membranes. Sep. Purif. Technol. 2020, 251, 117280. [Google Scholar] [CrossRef]
- Chakraborty, S.; Bashir, Y.; Sirotiya, V.; Ahirwar, A.; Das, S.; Vinayak, V. Role of bacterial quorum sensing and quenching mechanism in the efficient operation of microbial electrochemical technologies: A state-of-the-art review. Heliyon 2023, 9, e16205. [Google Scholar] [CrossRef]
- Salih, A.K.; Irvine, C.P.; Matar, F.; Aditya, L.; Nghiem, L.D.; Ton-That, C. Photocatalytic self-cleansing ZnO-coated ceramic membranes for preconcentrating microalgae. J. Memb. Sci. 2025, 718, 123700. [Google Scholar] [CrossRef]
- Choi, M.-J.; Chae, K.-J.; Ajayi, F.F.; Kim, K.-Y.; Yu, H.-W.; Kim, C.-W.; Kim, I.S. Effects of biofouling on ion transport through cation exchange membranes and microbial fuel cell performance. Bioresour. Technol. 2011, 102, 298–303. [Google Scholar] [CrossRef] [PubMed]
- Lala, S.F.; Shahgaldi, S. Mass and charge transport phenomena in porous transport layer for proton exchange membrane water electrolyzers: A review. Energy Rep. 2025, 13, 162–183. [Google Scholar] [CrossRef]
- Vinothkannan, M.; Kim, A.R.; Yoo, D.J. Potential carbon nanomaterials as additives for state-of-the-art Nafion electrolyte in proton-exchange membrane fuel cells: A concise review. RSC Adv. 2021, 11, 18351–18370. [Google Scholar] [CrossRef]
- Malar, C.G.; Seenuvasan, M.; Kumar, K.S.; Kumar, M.A. Synthesis and applications of Chitosan: A contemporary macro-molecule. In Microbial and Natural Macromolecules: Synthesis and Applications; Academic Press: Cambridge, MA, USA, 2020; pp. 73–86. [Google Scholar] [CrossRef]
- Alamri, A.A.; Borik, R.M.A.; El-Wahab, A.H.F.A.; Mohamed, H.M.; Ismail, K.S.; El-Aassar, M.R.; Al-Dies, A.-A.M.; El-Agrody, A.M. Synthesis of Schiff bases based on Chitosan, thermal stability and evaluation of antimicrobial and antitumor activities. Sci. Rep. 2025, 15, 892. [Google Scholar] [CrossRef]
- Anzabi, L.C.; Shafiekhani, A.; Darabi, E.; Bramowicz, M.; Kulesza, S.; Sabbaghzadeh, J.; Solaymani, S. Nanoscale 3D spatial analysis of FTO/ZnO/Ag-x films subjected to photocatalytic activity. Sci. Rep. 2025, 15, 7330. [Google Scholar] [CrossRef]
- Xin, Z.; Yanyi, Z.; Xiaobing, W. Research on testing and evaluation technology of proton exchange membrane for fuel cell. Energy Rep. 2023, 10, 1943–1950. [Google Scholar] [CrossRef]
- Xing, Y.; Li, H.; Avgouropoulos, G. Research progress of proton exchange membrane failure and mitigation strategies. Materials 2021, 14, 2591. [Google Scholar] [CrossRef] [PubMed]
- Avilés, D.G.A.; Barrionuevo, O.F.N.; Olmedo, O.F.S.; Piñan, B.D.C.; Briones, D.A.A.; Soria, R.A.B. Application of a direct current circuit to pick up and to store bioelectricity produced by microbial fuel cells|Aplicação de um circuito de corrente contínua para coleta e armazenamento de bioeletricidade produzida por células a combustível microbianas. Rev. Colomb. De Quimica 2019, 48, 26–35. [Google Scholar] [CrossRef]
- Schauer, J.; Brozova, L. Heterogeneous ion-exchange membranes based on sulfonated poly(1,4-phenylene sulfide) and linear polyethylene: Preparation, oxidation stability, methanol permeability and electrochemical properties. J. Membr. Sci. 2005, 250, 151–157. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alvear Méndez, S.; Soria, R.B.; Arboleda, D.; Cevallos, C.; Alcívar, C.; Jimenez, Y.; Teran, R.; Pupiales, H.; Luis, P. Influence of Er- and Al-Doped ZnO on Mixed-Matrix Membranes of Chitosan Derivatives in Bioelectrochemical Systems. Molecules 2025, 30, 3759. https://doi.org/10.3390/molecules30183759
Alvear Méndez S, Soria RB, Arboleda D, Cevallos C, Alcívar C, Jimenez Y, Teran R, Pupiales H, Luis P. Influence of Er- and Al-Doped ZnO on Mixed-Matrix Membranes of Chitosan Derivatives in Bioelectrochemical Systems. Molecules. 2025; 30(18):3759. https://doi.org/10.3390/molecules30183759
Chicago/Turabian StyleAlvear Méndez, Santiago, Raúl Bahamonde Soria, Daniel Arboleda, Carlos Cevallos, Christian Alcívar, Yessenia Jimenez, Rommy Teran, Henry Pupiales, and Patricia Luis. 2025. "Influence of Er- and Al-Doped ZnO on Mixed-Matrix Membranes of Chitosan Derivatives in Bioelectrochemical Systems" Molecules 30, no. 18: 3759. https://doi.org/10.3390/molecules30183759
APA StyleAlvear Méndez, S., Soria, R. B., Arboleda, D., Cevallos, C., Alcívar, C., Jimenez, Y., Teran, R., Pupiales, H., & Luis, P. (2025). Influence of Er- and Al-Doped ZnO on Mixed-Matrix Membranes of Chitosan Derivatives in Bioelectrochemical Systems. Molecules, 30(18), 3759. https://doi.org/10.3390/molecules30183759






