Tunable Crosslinked Polyvinyl Alcohol/Polyethylene Glycol (cPVA/PEG) Nanofiber Membranes with Enhanced Mechanical and Hydrophilic Balance
Abstract
1. Introduction
2. Results and Discussion
2.1. Morphology of Nanofiber Membranes
2.2. Infrared Analysis
2.3. XRD Analysis
2.4. Thermal Analysis
2.5. Porosity
2.6. Contraction upon Contact with Water
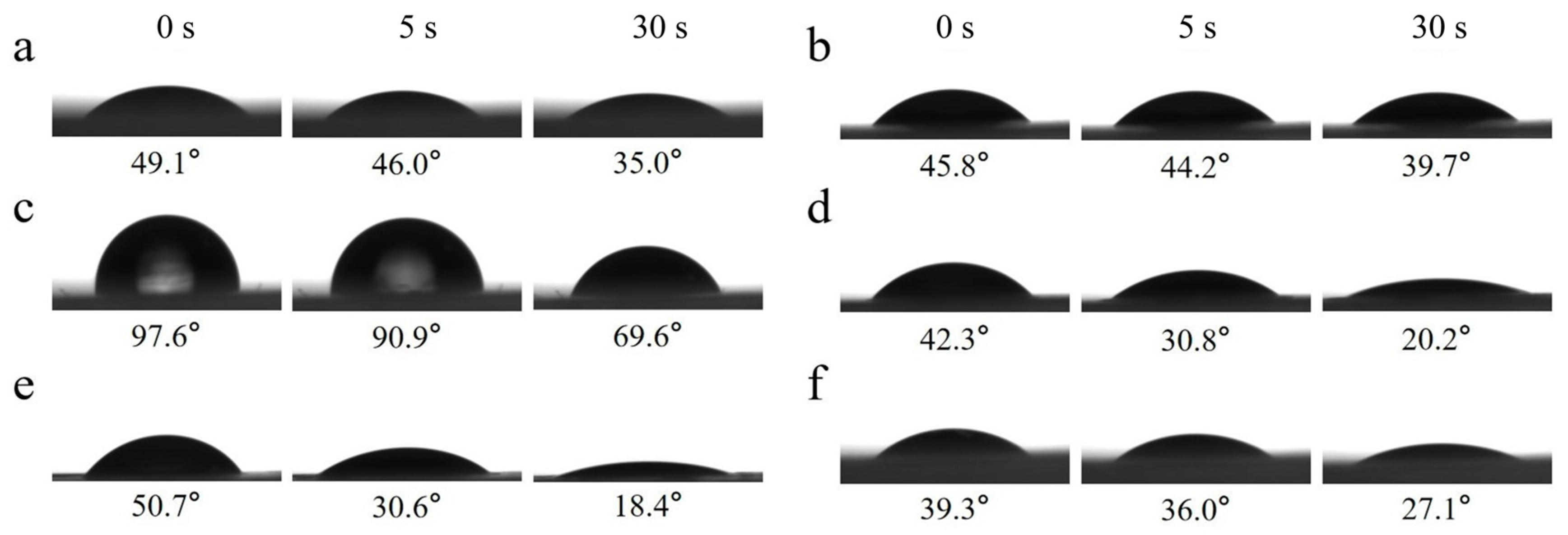
2.7. Surface Water Wettability
2.8. Surface Oil Wettability
2.9. Tensile Properties
3. Materials and Methods
3.1. Materials
3.2. Preparation of Crosslinked PVA/PEG Hydrophilic Nanofiber Membranes
3.3. Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Greenlee, L.F.; Lawler, D.F.; Freeman, B.D.; Marrot, B.; Moulin, P. Reverse Osmosis Desalination: Water Sources, Technology, and Today’s Challenges. Water Res. 2009, 43, 2317–2348. [Google Scholar] [CrossRef]
- Juda, W.; Mcrae, W.A. Coherent Ion-Exchange Gels and Membranes. J. Am. Chem. Soc. 1950, 72, 1043–1044. [Google Scholar] [CrossRef]
- Loeb, S.; Manjikian, S. 6-Month Field Test of a Reverse Osmosis Desalination Membrane. Ind. Eng. Chem. Process Des. Dev. 1965, 4, 207–212. [Google Scholar] [CrossRef]
- Wehle, B.; Asaba, H.; Castenfors, J.; Furst, P.; Gunnarsson, B.; Shaldon, S.; Bergstrom, J. Hemodynamic-Changes during Sequential Ultrafiltration and Dialysis. Kidney Int. 1979, 15, 411–418. [Google Scholar] [CrossRef]
- Schulz, G.; Michele, H.; Werner, U. Gas Separation with Membranes. Chem. Ing. Tech. 1982, 54, 351–362. [Google Scholar] [CrossRef]
- Hu, H.L.; Lee, K.R.; Lai, J.Y. Chemical-Modified Nylon-4 Membrane for Pervaporation. J. Macromol. Sci. Pure 1993, A30, 815–827. [Google Scholar] [CrossRef]
- Zhao, G.J.; Han, W.J.; Dong, L.L.; Fan, H.W.; Qu, Z.; Gu, J.H.; Meng, H. Sprayed Separation Membranes: A Systematic Review and Prospective Opportunities. Green Energy Environ. 2022, 7, 1143–1160. [Google Scholar] [CrossRef]
- Ma, R.; Li, J.; Zeng, P.; Duan, L.; Dong, J.; Ma, Y.; Yang, L. The Application of Membrane Separation Technology in the Pharmaceutical Industry. Membranes 2024, 14, 24. [Google Scholar] [CrossRef]
- Yadav, D.; Karki, S.; Ingole, P.G. Nanofiltration (NF) Membrane Processing in the Food Industry. Food Eng. Rev. 2022, 14, 579–595. [Google Scholar] [CrossRef]
- Al-Amshawee, S.; Yunus, M.Y.B.; Azoddein, A.A.M.; Hassell, D.G.; Dakhil, I.H.; Abu Hasan, H. Electrodialysis Desalination for Water and Wastewater: A Review. Chem. Eng. J. 2020, 380, 122231. [Google Scholar] [CrossRef]
- Lee, A.; Elam, J.W.; Darling, S.B. Membrane Materials for Water Purification: Design, Development, and Application. Environ. Sci.-Water Res. Technol. 2016, 2, 17–42. [Google Scholar] [CrossRef]
- Zuo, K.C.; Wang, K.P.; DuChanois, R.M.; Fang, Q.Y.; Deemer, E.M.; Huang, X.C.; Xin, R.K.; Said, I.A.; He, Z.; Feng, Y.R.; et al. Selective Membranes in Water and Wastewater Treatment: Role of Advanced Materials. Mater. Today 2021, 50, 516–532. [Google Scholar] [CrossRef]
- Gonçalves, I.S.; Costa, J.A.V.; de Morais, M.G. Microfiltration Membranes Developed from Nanofibers via an Electrospinning Process. Mater. Chem. Phys. 2022, 277, 125509. [Google Scholar] [CrossRef]
- Rashad, M.; Logesh, G.; Sabu, U.; Balasubramanian, M. A Novel Monolithic Mullite Microfiltration Membrane for Oil-in-Water Emulsion Separation. J. Membr. Sci. 2021, 620, 118857. [Google Scholar] [CrossRef]
- Sheng, J.L.; Zhang, M.; Xu, Y.; Yu, J.Y.; Ding, B. Tailoring Water-Resistant and Breathable Performance of Polyacrylonitrile Nanofibrous Membranes Modified by Polydimethylsiloxane. ACS Appl. Mater. Interfaces 2016, 8, 27218–27226. [Google Scholar] [CrossRef] [PubMed]
- Xiang, H.S.; Wang, B.C.; Zhong, M.Q.; Liu, W.G.; Yu, D.H.; Wang, Y.L.; Tam, K.C.; Zhou, G.F.; Zhang, Z. Sustainable and Versatile Superhydrophobic Cellulose Nanocrystals. ACS Sustain. Chem. Eng. 2022, 10, 5939–5948. [Google Scholar] [CrossRef]
- Rouhani, S.T.; Fashandi, H. Breathable Dual-layer Textile Composed of Cellulose Dense Membrane and Plasma-treated Fabric with Enhanced Comfort. Cellulose 2018, 25, 5427–5442. [Google Scholar] [CrossRef]
- Yadav, D.; Karunanithi, A.; Saxena, S.; Shukla, S. Modified PVA Membrane for Separation of Micro-emulsion. Sci. Total Environ. 2022, 822, 153610. [Google Scholar] [CrossRef]
- Xu, Y.Z.; He, L.A.; Xie, Z.M.; Wang, Z.L.; Chen, Y.F.; Wu, Q. Influence of PEG on Toughness, Humidity Sensitivity and Structural Color of Cellulose Nanocrystal Films. Cellulose 2024, 31, 6885–6896. [Google Scholar] [CrossRef]
- Manshahia, M.; Das, A. Moisture Management of High Active Sportswear. Fibers Polym. 2014, 15, 1221–1229. [Google Scholar] [CrossRef]
- Akhtar, F.H.; Kumar, M.; Vovusha, H.; Shevate, R.; Villalobos, L.F.; Schwingenschlögl, U.; Peinemann, K.V. Scalable Synthesis of Amphiphilic Copolymers for CO2 and Water-Selective Membranes: Effect of Copolymer Composition and Chain Length. Macromolecules 2019, 52, 6213–6226. [Google Scholar] [CrossRef]
- Pantelic, J.; Teitelbaum, E.; Bozlar, M.; Kim, S.; Meggers, F. Development of Moisture Absorber Based on Hydrophilic Nonporous Membrane Mass exchanger and Alkoxylated Siloxane Liquid Desiccant. Energy Build. 2018, 160, 34–43. [Google Scholar] [CrossRef]
- Kamoun, E.A.; Kenawy, E.R.S.; Chen, X. A Review on Polymeric Hydrogel Membranes for Wound Dressing Applications: PVA-based Hydrogel Dressings. J. Adv. Res. 2017, 8, 217–233. [Google Scholar] [CrossRef]
- Suhail, Z.; Ashfaq, J.; Channa, I.A.; Sadia, M.; Chandio, A.D.; Sharmmari, B.A.; Pervez, T.; Al-Jahwar, F.K. Synthesis and Evaluation of Self-Healing Polyvinyl Alcohol-tannic Acid Membranes for Skin Bio-applications. J. Appl. Polym. Sci. 2025, 142, e56458. [Google Scholar] [CrossRef]
- Dodda, J.M.; Belsky, P.; Chmelar, J.; Remis, T.; Smolná, K.; Tomás, M.; Kullová, L.; Kadlec, J. Comparative Study of PVA/SiO2 and PVA/SiO2/glutaraldehyde (GA) Nanocomposite Membranes Prepared by Single-step Solution Casting Method. J. Mater. Sci. 2015, 50, 6477–6490. [Google Scholar] [CrossRef]
- Alhazime, A.A. Effect of Nano CuO Doping on Structural, Thermal and Optical Properties of PVA/PEG Blend. J. Inorg. Organomet. Polym. Mater. 2020, 30, 4459–4467. [Google Scholar] [CrossRef]
- Jambaladinni, S.; Bhat, J.S. The Role of ZnO Nanofillers in Enhancing the Properties of PVA/PVP Blend Nanocomposites. Iran. J. Sci. Technol. A 2021, 45, 1851–1860. [Google Scholar] [CrossRef]
- Amiri, H.; Mohsennia, M. Impedance Study of PVA/PEG/LiClO4/TiO2 Nanocomposite Solid Polymer Blend Electrolyte. J. Mater. Sci.-Mater. Electron. 2017, 28, 4586–4592. [Google Scholar] [CrossRef]
- do Nascimento, F.C.; de Aguiar, L.C.V.; Costa, L.A.T.; Fernandes, M.T.; Marassi, R.J.; Gomes, A.D.; de Castro, J.A. Formulation and Characterization of Crosslinked Polyvinyl Alcohol (PVA) Membranes: Effects of the Crosslinking Agents. Polym. Bull. 2021, 78, 917–929. [Google Scholar] [CrossRef]
- Zhang, R.; Xu, X.Y.; Cao, B.; Li, P. Fabrication of High-performance PVA/PAN Composite Pervaporation Membranes Crosslinked by PMDA for Wastewater Desalination. Pet. Sci. 2018, 15, 146–156. [Google Scholar] [CrossRef]
- Khan, M.Q.; Kharaghani, D.; Ullah, S.; Waqas, M.; Abbasi, A.M.R.; Saito, Y.; Zhu, C.H.; Kim, I.S. Self-Cleaning Properties of Electrospun PVA/TiO2 and PVA/ZnO Nanofibers Composites. Nanomaterials 2018, 8, 644. [Google Scholar] [CrossRef]
- Hu, W.L.; Han, X.L.; Liu, L.L.; Zhang, X.; Xue, J.Q.; Wang, B.Y.; Zhang, P.; Cao, X.Z. PEG/PVDF Membranes for Separating Organosulphur Compounds from N-Heptane: Effect of Peg Molecular Weight. Can. J. Chem. Eng. 2017, 95, 364–371. [Google Scholar] [CrossRef]
- Bayazidi, S.; Nezhad, S.M.; Pourmousavi, S.A.; Zare, E.N.; Rahmavand, A.; Kousha, A. Sulfonated Crosslinked PVA-CuFe2O4 Nanocomposite as A Sustainable Catalysis for Synthesizing Antioxidant, Antimicrobial, and Anti-cancer Imidazoles and Amino Naphthoquinones Compounds. Inorg. Chem. Commun. 2024, 167, 112743. [Google Scholar] [CrossRef]
- Wu, J.M.; Li, W.Z.; Zhen, X.Q.; Wang, S.L.; Chen, K.M.; Gan, W.J. Conductive Epoxy Nanocomposite with Self-supporting Networks of Silver@carbon Nanocable Sponge and Improved Properties. J. Mater. Sci.-Mater. Electron. 2020, 31, 6488–6496. [Google Scholar] [CrossRef]
- Wang, H.; Peng, L.; Huang, Z.X.; Mei, S. Well-dispersed Hollow Silica Microspheres Synthesis with Silica Sol as Precursor by Template Method. J. Mol. Struct. 2014, 1059, 15–19. [Google Scholar] [CrossRef]
- Pornea, A.M.; Puguan, J.M.C.; Deonikar, V.G.; Kim, H. Robust Janus Nanocomposite Membrane with Opposing Surface Wettability for Selective Oil-water Separation. Sep. Purif. Technol. 2020, 236, 116297. [Google Scholar] [CrossRef]
- Todica, M.; Stefan, T.; Trandafir, D.; Simon, S. ESR and XRD Investigation of Effects induced by Gamma Radiation on PVA-TiO2 Membranes. Cent. Eur. J. Phys. 2013, 11, 928–935. [Google Scholar] [CrossRef]
- Araújo, R.C.S.; Pasa, V.M.D. Thermal Study of Polyurethane Elastomers Based on biopitch-PEG-MDI System. J. Therm. Anal. Calorim. 2002, 67, 313–319. [Google Scholar] [CrossRef]
- Baraker, B.M.; Lobo, B. Multistage Thermal Decomposition in Films of Cadmium Chloride-doped PVA-PVP Polymeric Blend. J. Therm. Anal. Calorim. 2018, 134, 865–878. [Google Scholar] [CrossRef]
- Chang, Y.; Liu, F. Review of Waterproof Breathable Membranes: Preparation, Performance and Applications in the Textile Field. Materials 2023, 16, 5339. [Google Scholar] [CrossRef]
- Yekrang, J.; Mohseni, L.; Etemadi, H. Water Treatment Using PVC/TPU/PC Electrospun Nanofiber Membranes. Fibers Polym. 2023, 24, 907–920. [Google Scholar] [CrossRef]
- Wang, Y.; Górecki, R.P.; Stamate, E.; Norrman, K.; Aili, D.; Zuo, M.; Guo, W.; Hélix-Nielsen, C.; Zhang, W. Preparation of Super-Hydrophilic Polyphenylsulfone Nanofiber Membranes for Water Treatment. RSC Adv. 2019, 9, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liu, X.; Chi, E.; Xu, G. Phytic acid modified polyacrylonitrile nanofiber membranes: Fabrication, characterization, and optimization for Pb2+ adsorption. Polym. Eng. Sci. 2024, 64, 3669–3681. [Google Scholar] [CrossRef]
- Li, J.; Li, Y.; Lu, Y.; Shi, W.; Tian, H. PDMS/PVDF Electrospinning Membranes for Water-in-Oil Emulsion Separation and UV Protection. Biomimetics 2022, 7, 217. [Google Scholar] [CrossRef] [PubMed]
- Feng, T.; Fu, L.; Mu, Z.; Wei, W.; Li, W.; Liang, X.; Ma, L.; Wu, Y.; Wang, X.; Wu, T.; et al. Bicomponent Electrospinning of PVDF-Based Nanofiber Membranes for Air Filtration and Oil–Water Separation. Polymers 2025, 17, 703. [Google Scholar] [CrossRef]
- Gao, L.; Gu, H.; Ye, C.; Wang, C.; Jin, Z. Superhydrophilic and Degradable PLA Fiber Membrane with Silica Layer for Oil-in-Water Emulsion Separation. J. Appl. Polym. Sci. 2025, 142, e56947. [Google Scholar] [CrossRef]
- Ma, J.; Gao, L.; Shuai, Y.; Wu, R.; Wu, Y.; Wang, Y.; Wei, H.; Zhou, J.; Zhang, N.; Cui, P.; et al. Superhydrophilic and Underwater Oleophobic SiO2@PEI-PAN/PVB Nanofiber Membrane for Highly Efficient oil-in-water Emulsion Separation. J. Environ. Chem. Eng. 2025, 13, 115953. [Google Scholar] [CrossRef]
- Destaye, A.G.; Lin, C.K.; Lee, C.K. Glutaraldehyde Vapor Crosslinked Nanofibrous PVA Mat with in Situ Formed Silver Nanoparticles. ACS Appl. Mater. Interfaces 2013, 5, 4745–4752. [Google Scholar] [CrossRef]








| Samples | Porosity (%) |
|---|---|
| PVA/PEG | 50.69 ± 3.79 |
| cPVA/PEG-3 | 48.23 ± 5.72 |
| cPVA/PEG-9 | 45.12 ± 5.06 |
| cPVA/PEG-15 | 43.89 ± 4.22 |
| cPVA/PEG-30 | 31.95 ± 1.21 |
| cPVA/PEG-50 | 15.02 ± 2.81 |
| Samples | Breaking Stress (MPa) | Breaking Strain (%) |
|---|---|---|
| PVA/PEG | 8.79 ± 0.93 | 115.44 ± 30.58 |
| cPVA/PEG-3 | 21.91 ± 0.61 | 90.57 ± 1.16 |
| cPVA/PEG-9 | 39.21 ± 3.71 | 57.56 ± 6.11 |
| cPVA/PEG-15 | 29.07 ± 2.60 | 77.60 ± 6.02 |
| cPVA/PEG-30 | 22.49 ± 2.67 | 63.61 ± 7.70 |
| cPVA/PEG-50 | 30.58 ± 1.66 | 69.91 ± 5.07 |
| Materials | Preparation Methods | Breaking Stress (MPa) | Breaking Strain (%) | Origin | Manufacturer | References |
|---|---|---|---|---|---|---|
| PVC/TPU/PC electrospun nanofiber membranes | Electrospinning | 3.5~10.3 | 41~56 | Bonab, Iran | Yekrang et al. | [41] |
| Heat-treated PPSU ENM | Electrospinning combined with thermal treatment | 4.1 ± 0.2 | 34.4 ± 4.5 | Kongens Lyngby, Denmark | Wang et al. | [42] |
| PA-modified PAN nanofiber membranes | Electrospinning | 1.65 ± 0.21 | 53.0 ± 10.1 | Jiangmen, China | Li et al. | [43] |
| PDMS/PVDF membranes | Electrospinning | 1.84 | 68.30 | Beijing, China | Li et al. | [44] |
| PVDF-PVDF/PDA NFMs | Double-nozzle electrospinning | <2.052 | <49.382 | Jinan, China | Feng et al. | [45] |
| PLA fiber membrane with silica layer | Electrospinning | 2.395 | 44.1 | Yancheng, China | Gao et al. | [46] |
| SiO2@PEI-PAN/PVB nanofiber membrane | Coaxial electrospinning | 2.4 | 13.3 | Suzhou, China | Ma et al. | [47] |
| cPVA/PEG-15 membrane | Electrospinning | 29.07 ± 2.60 | 77.60 ± 6.02 | Suzhou, China | Chang et al. | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, Y.; Wang, Z.; Liu, F. Tunable Crosslinked Polyvinyl Alcohol/Polyethylene Glycol (cPVA/PEG) Nanofiber Membranes with Enhanced Mechanical and Hydrophilic Balance. Molecules 2025, 30, 3750. https://doi.org/10.3390/molecules30183750
Chang Y, Wang Z, Liu F. Tunable Crosslinked Polyvinyl Alcohol/Polyethylene Glycol (cPVA/PEG) Nanofiber Membranes with Enhanced Mechanical and Hydrophilic Balance. Molecules. 2025; 30(18):3750. https://doi.org/10.3390/molecules30183750
Chicago/Turabian StyleChang, Yawen, Zijia Wang, and Fujuan Liu. 2025. "Tunable Crosslinked Polyvinyl Alcohol/Polyethylene Glycol (cPVA/PEG) Nanofiber Membranes with Enhanced Mechanical and Hydrophilic Balance" Molecules 30, no. 18: 3750. https://doi.org/10.3390/molecules30183750
APA StyleChang, Y., Wang, Z., & Liu, F. (2025). Tunable Crosslinked Polyvinyl Alcohol/Polyethylene Glycol (cPVA/PEG) Nanofiber Membranes with Enhanced Mechanical and Hydrophilic Balance. Molecules, 30(18), 3750. https://doi.org/10.3390/molecules30183750







