Can Sirtuin 1 Serve as a Therapeutic Target in Pulmonary Arterial Hypertension? A Comprehensive Review
Abstract
1. Introduction
2. Molecular and Cellular Pathophysiology of Pulmonary (Arterial) Hypertension
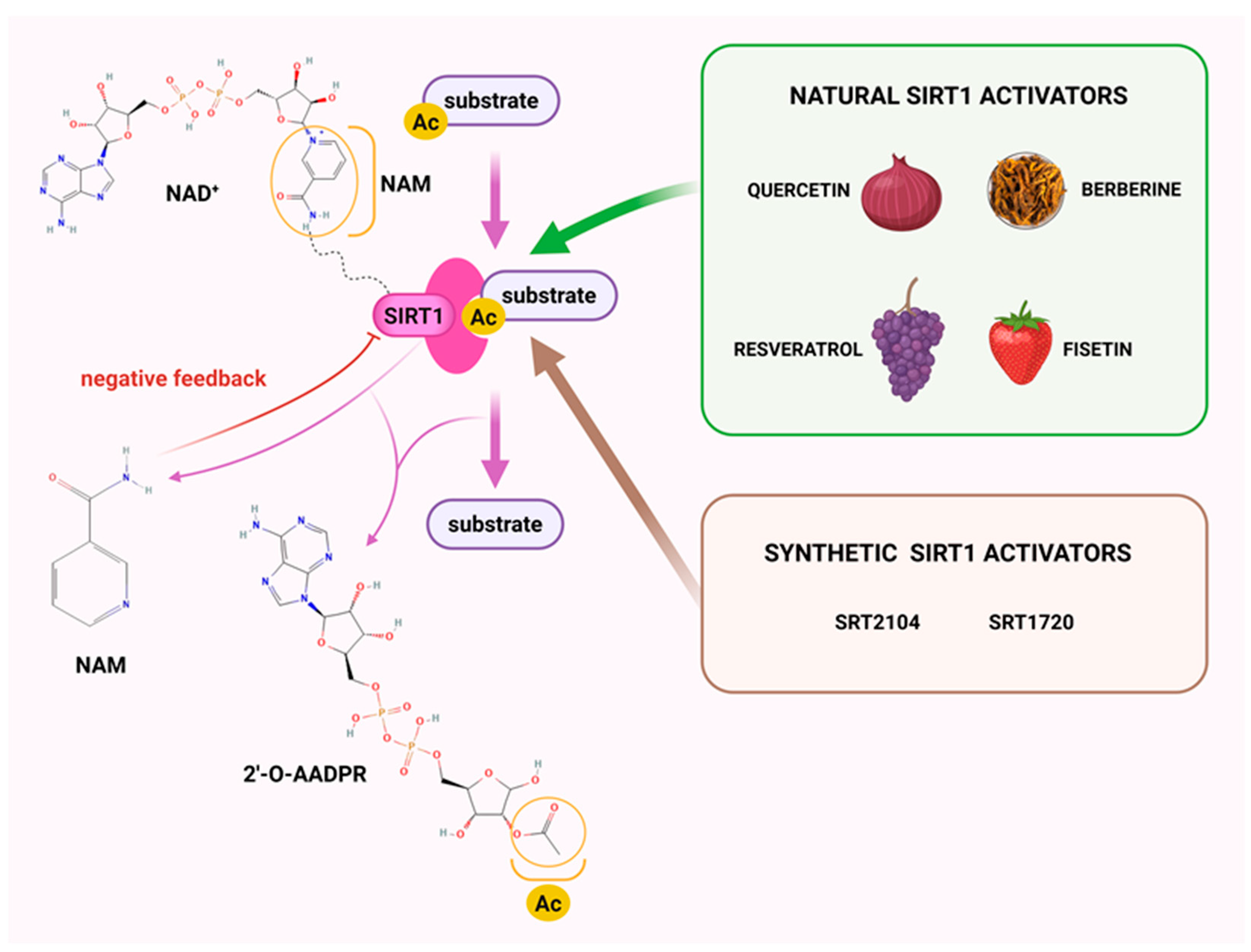
3. Sirtuin 1
| Modulators | Potency | References | |
|---|---|---|---|
| resveratrol– activator |  | SIRT1: EC50 = 100 μM *EC1.5 = 31.6 μM SIRT2: EC50 = 1.92 μM *EC1.5 > 300 μM SIRT3: *EC1.5 > 300 μM | [100,102] |
| SRT1720– activator | 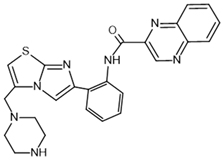 | SIRT1: EC50 = 0.10 μM *EC1.5 = 0.16 μM SIRT2: *EC1.5 = 37 μM SIRT3: *EC1.5 > 300 μM | [100,102] |
| SRT2104– activator |  | SIRT1: *EC1.5 = 450 nM | [113] |
| SRT1460– activator |  | SIRT1: *EC1.5 = 2.9 μM SIRT2: *EC1.5 > 300 μM SIRT3: *EC1.5 > 300 μM | [102] |
| SRT2183– activator | 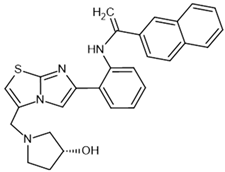 | SIRT1: *EC1.5 = 0.36 μM | [102] |
| SRT3025– activator | 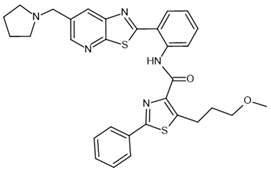 | SIRT1: *EC1.5 < 1 µm | [114] |
| EX-527– inhibitor | 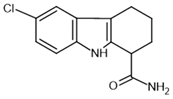 | SIRT1: IC50 = 38 nM SIRT2: IC50 = 19.6 μM SIRT3: IC50 = 48.7 μM | [115] |
| nicotinamide– inhibitor | 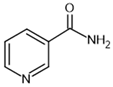 | SIRT1: IC50 = 62 μM SIRT2: IC50 = 10 μM SIRT3: IC50 = 31 μM | [116] |
| sirtinol– inhibitor | 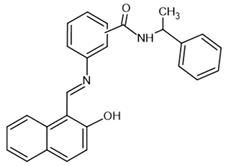 | SIRT1: IC50 = 123.2 μM SIRT2: IC50 = 38 μM SIRT3: IC50: 189.0 μM | [117,118] |
| tenovin-6– inhibitor | 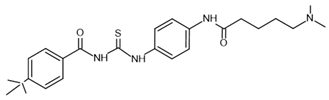 | SIRT1: IC50 = 21 μM SIRT2: IC50 = 10 μM SIRT3: IC50 = 67 μM | [119] |
| suramin– inhibitor |  | SIRT1: IC50 = 297 nM SIRT2: IC50 = 799 nM | [120] |
Sirtuin 1 in the Cardiovascular System
4. Sirtuin 1 in Pulmonary (Arterial) Hypertension
4.1. Monocrotaline-Induced Pulmonary Hypertension
4.2. Hypoxia- and Sugen/Hypoxia-Induced Pulmonary Hypertension
4.3. Sirtuin 1 Expression in Pulmonary (Arterial) Hypertension
4.4. Implications of Sirtuin 1 in the Pathogenesis of Pulmonary (Arterial) Hypertension
5. Limitations
6. Conclusions
7. Methods
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ac-eNOS | acetylated endothelial nitric synthase |
| Ach | acetylcholine |
| Ac-Lys | acetyl-lysine |
| ADP | adenosine diphosphate |
| Akt | protein kinase B |
| AMPK | adenosine monophosphate-activated kinase |
| ANP | atrial natriuretic peptide |
| APC | adenomatous polyposis coli |
| ASC | apoptosis-associated speck-like protein containing a CARD |
| ATP | adenosine triphosphate |
| Bax | Bcl-2-associated x-protein |
| Bcl-2 | B-cell lymphoma-2 |
| Bcl-xl | B-cell lymphoma-extra-large |
| BMP | bone morphogenetic protein |
| BNP | brain natriuretic peptide |
| cAMP | cyclic adenosine monophosphate |
| CCL5 | CC Motif Chemokine Ligand 5 |
| CD68 | cluster of differentiation 68 |
| CDK2 | cyclin-dependent kinase 2 |
| circ-SIRT1 | circular RNA derived from the SIRT1 gene |
| CKIα | casein kinase I alpha |
| c-Myc | cellular myelocytomatosis oncogene |
| COL1A1 | collagen type I alpha I chain |
| COX2 | cyclooxygenase 2 |
| CR | calorie restriction |
| CS | citrate synthase |
| CTGF | connective tissue growth factor |
| CVD | cardiovascular diseases |
| Drp1 | dynamin-related protein 1 |
| EC | endothelial cells |
| EC1.5 | 1.5-fold effective concentration |
| EC50 | half-maximal effective concentration |
| EGFR | epidermal growth factor receptor |
| EMA | European Medicines Agency |
| EndMT | endothelial-to-mesenchymal transition |
| eNOS | endothelial nitric oxide synthase |
| ERRα | estrogen-related receptor alpha |
| ET | ejection time |
| ET-1 | endothelin-1 |
| FDA | Food and Drug Administration |
| FOXO1 | Forkhead box protein O1 |
| FOXO3a | Forkhead box protein O3a |
| G0 | gap 0 |
| G1 | gap 1 |
| Gclc | glutamate-cysteine ligase catalytic subunit |
| Gclm | glutamate-cysteine ligase modifier subunit |
| GLUT1 | glucose transporter 1 |
| gp91phox | glycoprotein 91-phagocyte oxidase |
| GSK3β | glycogen synthase kinase 3β |
| H1 | histone H1 |
| HFpEF | heart failure with preserved ejection fraction |
| HIFs | hypoxia-inducible factors |
| HIF-α | hypoxia-inducible factor-alpha |
| HMGB1 | high-mobility group box 1 |
| HO-1 | heme oxygenase-1 |
| HUVEC | human umbilical vein endothelial cells |
| I/R | ischemia/reperfusion |
| IC50 | half-maximal inhibitory concentration |
| ICAM-1 | intercellular adhesion molecule 1 |
| IFN-γ | interferon-gamma |
| IIA-Fc | activin receptor type IIA fusion protein |
| IL-10 | interleukin-10 |
| IL-18 | interleukin-18 |
| IL-1β | interleukin-1beta |
| IL-2 | interleukin-2 |
| IL-4 | interleukin-4 |
| IL-6 | interleukin-6 |
| iNOS | inducible nitric oxide synthase |
| iPAH | idiopathic pulmonary arterial hypertension |
| IκBα | NF-κB inhibitor alpha |
| Keap1 | Kelch-like ECH-associated protein 1 |
| Kv1.5 | voltage-gated potassium channel |
| LDH | lactate dehydrogenase |
| LEF | lymphoid enhancer-binding factor |
| L-NAME | N-nitro-L-arginine methyl ester |
| LRP | lipoprotein receptor-related protein |
| LV | left ventricle |
| MCP-1 | monocyte chemotactic protein-1 |
| MCT | monocrotaline |
| MDA | malondialdehyde |
| MI | myocardial infarction |
| miR | microRNA |
| MMP2 | matrix metalloproteinase 2 |
| MMP9 | matrix metalloproteinase 9 |
| MnSOD | manganese-dependent superoxide dismutase |
| mPAP | mean pulmonary arterial pressure |
| MPO | myeloperoxidase |
| mPT | mitochondrial permeability transition |
| MSC | mesenchymal stem cells |
| mTOR | mechanistic target of rapamycin |
| mTORC1 | mechanistic target of rapamycin complex 1 |
| mTORC2 | mechanistic target of rapamycin complex 2 |
| MuRF-1 | muscle RING-finger protein-1 |
| NAD+ | nicotinamide adenine nucleotide |
| NADPH | nicotinamide adenine dinucleotide phosphate |
| NAM | nicotinamide |
| NAMPT | nicotinamide phosphoribosyltransferase |
| NF-κB | nuclear factor-kappa B |
| NLRP3 | nucleotide-binding oligomerization domain-like receptor protein 3 |
| NNT | nicotinamide nucleotide transhydrogenase |
| NO | nitric oxide |
| Notch1 | neurogenic locus notch homolog protein 1 |
| NOX 4 | NADPH oxidase 4 |
| NOX-1 | NADPH oxidase-1 |
| Nqo1 | NAD(P)H: quinone oxidoreductase-1 |
| Nrf2 | nuclear factor erythroid 2-related factor 2 |
| p21 | cyclin-dependent kinase inhibitor 1A |
| p47phox | neutrophil cytosolic factor 1 |
| p53 | tumor protein |
| PA | pulmonary arteries |
| PAAT | pulmonary artery acceleration time |
| PAEC | pulmonary artery endothelial cells |
| PAH | pulmonary arterial hypertension |
| PAH-CTD | PAH associated with connective tissue disease |
| p-Akt | phosphorylated-protein kinase B |
| PASMC | pulmonary arterial smooth muscle cells |
| PCNA | proliferating cell nuclear antigen |
| PDGF | platelet-derived growth factor |
| p-eNOS | phosphorylated endothelial nitric oxide synthase |
| PGC-1α | peroxisome proliferator-activated receptor-gamma coactivator-1 alpha |
| PGI2 | prostacyclin I2 |
| PH | pulmonary hypertension |
| PI3K | phosphoinositide 3-kinase |
| PINK1 | PTEN-induced putative kinase 1 |
| p-JNK | phosphorylated Jun N-terminal kinase |
| PKA | cAMP-dependent protein kinase |
| PKIA | cAMP-dependent protein kinase inhibitor |
| PMEC | pulmonary microvascular endothelial cells |
| PPARα | peroxisome proliferator-activated receptor alpha |
| PTEN | phosphatase and tensin homologue deleted on chromosome 10 |
| PTGS2 | prostaglandin-endoperoxide synthase 2 |
| ROS | reactive oxygen species |
| RV | right ventricle |
| RVH | right ventricle hypertrophy |
| RVSP | right ventricle systolic pressure |
| S | septum |
| SD | Sprague-Dawley |
| si-Jag2 | short interfering RNA targeting Jagged2 |
| Sir2 | silent information regulator 2 |
| SIRT1 | sirtuin 1 |
| SIRT2 | sirtuin 2 |
| SIRT3 | sirtuin 3 |
| SIRT7 | sirtuin 7 |
| SIRTs | sirtuins |
| SKL | secreted Klotho |
| Smad1/5/8 | mothers against decapentaplegic homolog 1/5/8 |
| Smad2/3 | mothers against decapentaplegic homolog 2/3 |
| Smad4 | mothers against decapentaplegic homolog 4 |
| Smad7 | mothers against decapentaplegic homolog 7 |
| SOD2 | superoxide dismutase 2 |
| SRC | non-receptor tyrosine kinase Src |
| STAT3 | signal transducer and activator of transcription 3 |
| SU5416 | Sugen 5416 |
| TAPSE | tricuspid annular plane systolic excursion |
| TCF | T-cell factor |
| TFAM | mitochondrial transcription factor |
| TGF-β | transforming growth factor-β |
| TnC | troponin C |
| TNF-α | tumor necrosis factor-alpha |
| Trx-1 | thioredoxin-1 |
| TSC2 | tuberous sclerosis complex subunit 2 |
| VCAM-1 | vascular cell adhesion molecule 1 |
| VDAC | voltage dependent anion channel |
| VEGFA | vascular endothelial growth factor A |
| VEGFR-2 | vascular endothelial growth factor receptor 2 |
| VSMC | vascular smooth muscle cells |
| Wnt | wingless-type mouse mammary tumor virus integration site family |
| YAP | yes-associated protein |
| α-SMA | alpha smooth muscle actin |
References
- Kovacs, G.; Bartolome, S.; Denton, C.P.; Gatzoulis, M.A.; Gu, S.; Khanna, D.; Badesch, D.; Montani, D. Definition, classification and diagnosis of pulmonary hypertension. Eur. Respir. J. 2024, 64, 2401324. [Google Scholar] [CrossRef]
- Guignabert, C.; Aman, J.; Bonnet, S.; Dorfmüller, P.; Olschewski, A.J.; Pullamsetti, S.; Rabinovitch, M.; Schermuly, R.T.; Humbert, M.; Stenmark, K.R. Pathology and pathobiology of pulmonary hypertension: Current insights and future directions. Eur. Respir. J. 2024, 64, 2401095. [Google Scholar] [CrossRef]
- Humbert, M.; McLaughlin, V.; Gibbs, J.S.R.; Gomberg-Maitland, M.; Hoeper, M.M.; Preston, I.R.; Souza, R.; Waxman, A.; Escribano Subias, P.; Feldman, J.; et al. Sotatercept for the treatment of pulmonary arterial hypertension. N. Engl. J. Med. 2021, 384, 1204–1215. [Google Scholar] [CrossRef]
- Hoeper, M.M.; Badesch, D.B.; Ghofrani, H.A.; Gibbs, J.S.R.; Gomberg-Maitland, M.; McLaughlin, V.V.; Preston, I.R.; Souza, R.; Waxman, A.B.; Grünig, E.; et al. STELLAR Trial Investigators. Phase 3 trial of sotatercept for treatment of pulmonary arterial hypertension. N. Engl. J. Med. 2023, 388, 1478–1490. [Google Scholar] [CrossRef] [PubMed]
- Campagna, R.; Vignini, A. NAD+ homeostasis and NAD+-consuming enzymes: Implications for vascular health. Antioxidant 2023, 12, 376. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Barnes, K.; Gibson, S.; Fillmore, N. Dual-edged role of SIRT1 in energy metabolism and cardiovascular disease. Am. J. Physiol. Heart Circ. Physiol. 2024, 327, 1162–1173. [Google Scholar] [CrossRef] [PubMed]
- Zurlo, G.; Piquereau, J.; Moulin, M.; Pires Da Silva, J.; Gressette, M.; Ranchoux, B.; Garnier, A.; Ventura-Clapier, R.; Fadel, E.; Humbert, M.; et al. Sirtuin 1 regulates pulmonary artery smooth muscle cell proliferation: Role in pulmonary arterial hypertension. J. Hypertens. 2018, 36, 1164–1177. [Google Scholar] [CrossRef]
- Cheng, X.W.; Narisawa, M.; Jin, X.; Murohara, T.; Kuzuya, M. Sirtuin 1 as a potential therapeutic target in pulmonary artery hypertension. J. Hypertens. 2018, 36, 1032–1035. [Google Scholar] [CrossRef]
- Kurakula, K.; Smolders, V.; Tura-Ceide, O.; Jukema, J.W.; Quax, P.H.A.; Goumans, M.J. Endothelial dysfunction in pulmonary hypertension: Cause or consequence? Biomedicines 2021, 9, 57. [Google Scholar] [CrossRef]
- Zhu, J.; Yang, L.; Jia, Y.; Balistrieri, A.; Fraidenburg, D.R.; Wang, J.; Tang, H.; Yuan, J.X. Pathogenic mechanisms of pulmonary arterial hypertension: Homeostasis imbalance of endothelium-derived relaxing and contracting factors. JACC Asia 2022, 2, 787–802. [Google Scholar] [CrossRef]
- Humbert, M.; Morrell, N.W.; Archer, S.L.; Stenmark, K.R.; MacLean, M.R.; Lang, I.M.; Christman, B.W.; Weir, E.K.; Eickelberg, O.; Voelkel, N.F.; et al. Cellular and molecular pathobiology of pulmonary arterial hypertension. J. Am. Coll. Cardiol. 2004, 43 (Suppl. S12), 13S–24S. [Google Scholar] [CrossRef] [PubMed]
- Bousseau, S.; Sobrano Fais, R.; Gu, S.; Frump, A.; Lahm, T. Pathophysiology and new advances in pulmonary hypertension. BMJ Med. 2023, 2, e000137. [Google Scholar] [CrossRef] [PubMed]
- Andersen, S.; Nielsen-Kudsk, J.E.; Vonk Noordegraaf, A.; de Man, F.S. Right ventricular fibrosis. Circulation 2019, 139, 269–285. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Chi, L.; Kuebler, W.M.; Goldenberg, N.M. Perivascular inflammation in pulmonary arterial hypertension. Cells 2020, 9, 2338. [Google Scholar] [CrossRef]
- Xu, D.; Hu, Y.H.; Gou, X.; Li, F.Y.; Yang, X.Y.; Li, Y.M.; Chen, F. Oxidative stress and antioxidative therapy in pulmonary arterial hypertension. Molecules 2022, 27, 3724. [Google Scholar] [CrossRef]
- Shen, Y.; Goncharov, D.A.; Pena, A.; Baust, J.; Chavez Barragan, A.; Ray, A.; Rode, A.; Bachman, T.N.; Chang, B.; Jiang, L.; et al. Cross-talk between TSC2 and the extracellular matrix controls pulmonary vascular proliferation and pulmonary hypertension. Sci. Signal. 2022, 15, eabn2743. [Google Scholar] [CrossRef]
- Liu, S.F.; Nambiar Veetil, N.; Li, Q.; Kucherenko, M.M.; Knosalla, C.; Kuebler, W.M. Pulmonary hypertension: Linking inflammation and pulmonary arterial stiffening. Front. Immunol. 2022, 13, 959209. [Google Scholar] [CrossRef]
- Wang, R.R.; Yuan, T.Y.; Wang, J.M.; Chen, Y.C.; Zhao, J.L.; Li, M.T.; Fang, L.H.; Du, G.H. Immunity and inflammation in pulmonary arterial hypertension: From pathophysiology mechanisms to treatment perspective. Pharmacol. Res. 2022, 180, 106238. [Google Scholar] [CrossRef]
- Li, C.; Liu, P.; Song, R.; Zhang, Y.; Lei, S.; Wu, S. Immune cells and autoantibodies in pulmonary arterial hypertension. Acta Biochim. Biophys. Sin. 2017, 49, 1047–1057. [Google Scholar] [CrossRef]
- Stacher, E.; Graham, B.B.; Hunt, J.M.; Gandjeva, A.; Groshong, S.D.; McLaughlin, V.V.; Jessup, M.; Grizzle, W.E.; Aldred, M.A.; Cool, C.D.; et al. Modern age pathology of pulmonary arterial hypertension. Am. J. Respir. Crit. Care Med. 2012, 186, 261–272. [Google Scholar] [CrossRef]
- Savai, R.; Pullamsetti, S.S.; Kolbe, J.; Bieniek, E.; Voswinckel, R.; Fink, L.; Scheed, A.; Ritter, C.; Dahal, B.K.; Vater, A.; et al. Immune and inflammatory cell involvement in the pathology of idiopathic pulmonary arterial hypertension. Am. J. Respir. Crit. Care Med. 2012, 186, 897–908. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Song, J.; Li, X.; Xia, Z.; Wang, Q.; Fu, J.; Miao, Y.; Wang, D.; Wang, X. The role of immune cells and inflammation in pulmonary hypertension: Mechanisms and implications. Front. Immunol. 2024, 15, 1374506. [Google Scholar] [CrossRef]
- Song, J.L.; Zheng, S.Y.; He, R.L.; Gui, L.X.; Lin, M.J.; Sham, J.S.K. Serotonin and chronic hypoxic pulmonary hypertension activate a NADPH oxidase 4 and TRPM2 dependent pathway for pulmonary arterial smooth muscle cell proliferation and migration. Vascul. Pharmacol. 2021, 138, 106860. [Google Scholar] [CrossRef] [PubMed]
- Archer, S.L. Pyruvate Kinase and Warburg Metabolism in Pulmonary Arterial Hypertension: Uncoupled Glycolysis and the Cancer-Like Phenotype of Pulmonary Arterial Hypertension. Circulation 2017, 136, 2486–2490. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Liu, B.; Wang, Y.; Zhang, H.; He, L.; Wang, P.; Dong, M. Mitochondrial dysfunction in pulmonary arterial hypertension. Front. Physiol. 2022, 13, 1079989. [Google Scholar] [CrossRef]
- Liu, R.; Xu, C.; Zhang, W.; Cao, Y.; Ye, J.; Li, B.; Jia, S.; Weng, L.; Liu, Y.; Liu, L.; et al. FUNDC1-mediated mitophagy and HIF1α activation drives pulmonary hypertension during hypoxia. Cell Death Dis. 2022, 13, 634. [Google Scholar] [CrossRef]
- Liang, S.; Yegambaram, M.; Wang, T.; Wang, J.; Black, S.M.; Tang, H. Mitochondrial Metabolism, Redox, and Calcium Homeostasis in Pulmonary Arterial Hypertension. Biomedicines 2022, 10, 341. [Google Scholar] [CrossRef]
- Pokharel, M.D.; Marciano, D.P.; Fu, P.; Franco, M.C.; Unwalla, H.; Tieu, K.; Fineman, J.R.; Wang, T.; Black, S.M. Metabolic reprogramming, oxidative stress, and pulmonary hypertension. Redox Biol. 2023, 64, 102797. [Google Scholar] [CrossRef]
- Ryanto, G.R.T.; Suraya, R.; Nagano, T. Mitochondrial Dysfunction in Pulmonary Hypertension. Antioxidants 2023, 12, 372. [Google Scholar] [CrossRef]
- Ling, H.; Peng, L.; Wang, J.; Rahhal, R.; Seto, E. Histone Deacetylase SIRT1 Targets Plk2 to Regulate Centriole Duplication. Cell Rep. 2018, 25, 2851–2865.e3. [Google Scholar] [CrossRef]
- Teixeira, C.S.S.; Cerqueira, N.M.F.S.A.; Gomes, P.; Sousa, S.F. A Molecular Perspective on Sirtuin Activity. Int. J. Mol. Sci. 2020, 21, 8609. [Google Scholar] [CrossRef]
- Majeed, Y.; Halabi, N.; Madani, A.Y.; Engelke, R.; Bhagwat, A.M.; Abdesselem, H.; Agha, M.V.; Vakayil, M.; Courjaret, R.; Goswami, N.; et al. SIRT1 promotes lipid metabolism and mitochondrial biogenesis in adipocytes and coordinates adipogenesis by targeting key enzymatic pathways. Sci. Rep. 2021, 11, 8177. [Google Scholar] [CrossRef]
- Wu, Q.J.; Zhang, T.N.; Chen, H.H.; Yu, X.F.; Lv, J.L.; Liu, Y.Y.; Liu, Y.S.; Zheng, G.; Zhao, J.Q.; Wei, Y.F.; et al. The sirtuin family in health and disease. Signal Transduct. Target. Ther. 2022, 7, 402. [Google Scholar] [CrossRef] [PubMed]
- Kassan, M.; Vikram, A.; Li, Q.; Kim, Y.R.; Kumar, S.; Gabani, M.; Liu, J.; Jacobs, J.S.; Irani, K. MicroRNA-204 promotes vascular endoplasmic reticulum stress and endothelial dysfunction by targeting Sirtuin1. Sci. Rep. 2017, 7, 9308. [Google Scholar] [CrossRef]
- Lu, C.L.; Liao, M.T.; Hou, Y.C.; Fang, Y.W.; Zheng, C.M.; Liu, W.C.; Chao, C.T.; Lu, K.C.; Ng, Y.Y. Sirtuin-1 and Its Relevance in Vascular Calcification. Int. J. Mol. Sci. 2020, 21, 1593. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Gan, D.; Luo, Z.; Yang, Q.; An, D.; Zhang, H.; Hu, Y.; Ma, Z.; Zeng, Q.; Xu, D.; et al. α-Ketoglutarate improves cardiac insufficiency through NAD+-SIRT1 signaling-mediated mitophagy and ferroptosis in pressure overload-induced mice. Mol. Med. 2024, 30, 15. [Google Scholar] [CrossRef] [PubMed]
- Poljšak, B.; Kovač, V.; Špalj, S.; Milisav, I. The Central Role of the NAD+ Molecule in the Development of Aging and the Prevention of Chronic Age-Related Diseases: Strategies for NAD+ Modulation. Int. J. Mol. Sci. 2023, 24, 2959. [Google Scholar] [CrossRef]
- Hwang, E.S.; Song, S.B. Nicotinamide is an inhibitor of SIRT1 in vitro, but can be a stimulator in cells. Cell Mol. Life Sci. 2017, 74, 3347–3362. [Google Scholar] [CrossRef]
- Zhang, Q.J.; Wang, Z.; Chen, H.Z.; Zhou, S.; Zheng, W.; Liu, G.; Wei, Y.S.; Cai, H.; Liu, D.P.; Liang, C.C. Endothelium-specific overexpression of class III deacetylase SIRT1 decreases atherosclerosis in apolipoprotein E-deficient mice. Cardiovasc. Res. 2008, 80, 191–199. [Google Scholar] [CrossRef]
- Zhou, S.; Chen, H.Z.; Wan, Y.Z.; Zhang, Q.J.; Wei, Y.S.; Huang, S.; Liu, J.J.; Lu, Y.B.; Zhang, Z.Q.; Yang, R.F.; et al. Repression of P66Shc expression by SIRT1 contributes to the prevention of hyperglycemia-induced endothelial dysfunction. Circ. Res. 2011, 109, 639–648. [Google Scholar] [CrossRef]
- Man, A.W.C.; Li, H.; Xia, N. The Role of Sirtuin1 in Regulating Endothelial Function, Arterial Remodeling and Vascular Aging. Front. Physiol. 2019, 10, 1173. [Google Scholar] [CrossRef] [PubMed]
- Potente, M.; Ghaeni, L.; Baldessari, D.; Mostoslavsky, R.; Rossig, L.; Dequiedt, F.; Haendeler, J.; Mione, M.; Dejana, E.; Alt, F.W.; et al. SIRT1 controls endothelial angiogenic functions during vascular growth. Genes Dev. 2007, 21, 2644–2658. [Google Scholar] [CrossRef] [PubMed]
- Budbazar, E.; Rodriguez, F.; Sanchez, J.M.; Seta, F. The Role of Sirtuin-1 in the Vasculature: Focus on Aortic Aneurysm. Front. Physiol. 2020, 11, 1047. [Google Scholar] [CrossRef]
- Mengozzi, A.; Costantino, S.; Paneni, F.; Duranti, E.; Nannipieri, M.; Mancini, R.; Lai, M.; La Rocca, V.; Puxeddu, I.; Antonioli, L.; et al. Targeting SIRT1 Rescues Age- and Obesity-Induced Microvascular Dysfunction in Ex Vivo Human Vessels. Circ. Res. 2022, 131, 476–491. [Google Scholar] [CrossRef]
- Bartoli-Leonard, F.; Wilkinson, F.L.; Schiro, A.; Serracino Inglott, F.; Alexander, M.Y.; Weston, R. Loss of SIRT1 in diabetes accelerates DNA damage-induced vascular calcification. Cardiovasc. Res. 2021, 117, 836–849. [Google Scholar] [CrossRef]
- Gorenne, I.; Kumar, S.; Gray, K.; Figg, N.; Yu, H.; Mercer, J.; Bennett, M. Vascular smooth muscle cell sirtuin 1 protects against DNA damage and inhibits atherosclerosis. Circulation 2013, 127, 386–396. [Google Scholar] [CrossRef]
- Gao, P.; Xu, T.T.; Lu, J.; Li, L.; Xu, J.; Hao, D.L.; Chen, H.Z.; Liu, D.P. Overexpression of SIRT1 in vascular smooth muscle cells attenuates angiotensin II-induced vascular remodeling and hypertension in mice. J Mol. Med. 2014, 92, 347–357. [Google Scholar] [CrossRef]
- Chen, H.Z.; Wang, F.; Gao, P.; Pei, J.F.; Liu, Y.; Xu, T.T.; Tang, X.; Fu, W.Y.; Lu, J.; Yan, Y.F. Age-Associated Sirtuin 1 Reduction in Vascular Smooth Muscle Links Vascular Senescence and Inflammation to Abdominal Aortic Aneurysm. Circ. Res. 2016, 119, 1076–1088. [Google Scholar] [CrossRef]
- Kloza, M.; Krzyżewska, A.; Kozłowska, H.; Budziak, S.; Baranowska-Kuczko, M. Empagliflozin Plays Vasoprotective Role in Spontaneously Hypertensive Rats via Activation of the SIRT1/AMPK Pathway. Cells 2025, 14, 507. [Google Scholar] [CrossRef]
- Wang, A.J.; Zhang, J.; Xiao, M.; Wang, S.; Wang, B.J.; Guo, Y.; Tang, Y.; Gu, J. Molecular mechanisms of doxorubicin-induced cardiotoxicity: Novel roles of sirtuin 1-mediated signaling pathways. Cell Mol. Life Sci. 2021, 78, 3105–3125. [Google Scholar] [CrossRef]
- Hsu, C.P.; Zhai, P.; Yamamoto, T.; Maejima, Y.; Matsushima, S.; Hariharan, N.; Shao, D.; Takagi, H.; Oka, S.; Sadoshima, J. Silent information regulator 1 protects the heart from ischemia/reperfusion. Circulation 2010, 122, 2170–2182. [Google Scholar] [CrossRef]
- Kuno, A.; Hosoda, R.; Tsukamoto, M.; Sato, T.; Sakuragi, H.; Ajima, N.; Saga, Y.; Tada, K.; Taniguchi, Y.; Iwahara, N.; et al. SIRT1 in the cardiomyocyte counteracts doxorubicin-induced cardiotoxicity via regulating histone H2AX. Cardiovasc. Res. 2023, 118, 3360–3373. [Google Scholar] [CrossRef]
- Costantino, S.; Mengozzi, A.; Velagapudi, S.; Mohammed, S.A.; Gorica, E.; Akhmedov, A.; Mongelli, A.; Pugliese, N.R.; Masi, S.; Virdis, A.; et al. Treatment with recombinant Sirt1 rewires the cardiac lipidome and rescues diabetes-related metabolic cardiomyopathy. Cardiovasc. Diabetol. 2023, 22, 312. [Google Scholar] [CrossRef]
- Tang, Y.J.; Zhang, Z.; Yan, T.; Chen, K.; Xu, G.F.; Xiong, S.Q.; Wu, D.Q.; Chen, J.; Jose, P.A.; Zeng, C.Y.; et al. Irisin attenuates type 1 diabetic cardiomyopathy by anti-ferroptosis via SIRT1-mediated deacetylation of p53. Cardiovasc. Diabetol. 2024, 23, 116. [Google Scholar] [CrossRef] [PubMed]
- Qiu, H.; Sun, Y.; Wang, X.; Gong, T.; Su, J.; Shen, J.; Zhou, J.; Xia, J.; Wang, H.; Meng, X.; et al. Lamin A/C deficiency-mediated ROS elevation contributes to pathogenic phenotypes of dilated cardiomyopathy in iPSC model. Nat. Commun. 2024, 15, 7000. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liu, Y.; Wang, Y.; Chao, Y.; Zhang, J.; Jia, Y.; Tie, J.; Hu, D. Regulation of SIRT1 and Its Roles in Inflammation. Front. Immunol. 2022, 13, 831168. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Chen, H.Z. Histone Deacetylase SIRT1, Smooth Muscle Cell Function, and Vascular Diseases. Front. Pharmacol. 2020, 11, 537519. [Google Scholar] [CrossRef]
- Ding, Y.N.; Wang, H.Y.; Chen, X.F.; Tang, X.; Chen, H.Z. Roles of Sirtuins in Cardiovascular Diseases: Mechanisms and Therapeutics. Circ. Res. 2025, 136, 524–550. [Google Scholar] [CrossRef]
- Singh, C.K.; Chhabra, G.; Ndiaye, M.A.; Garcia-Peterson, L.M.; Mack, N.J.; Ahmad, N. The Role of Sirtuins in Antioxidant and Redox Signaling. Antioxid. Redox Signal. 2018, 28, 643–661. [Google Scholar] [CrossRef]
- Liu, Y.P.; Wen, R.; Liu, C.F.; Zhang, T.N.; Yang, N. Cellular and molecular biology of sirtuins in cardiovascular disease. Biomed. Pharmacother. 2023, 164, 114931. [Google Scholar] [CrossRef]
- Li, Y.; Kang, K.; Bao, H.; Liu, S.; Zhao, B.; Hu, G.; Wu, J. Research Progress on the Interaction Between SIRT1 and Mitochondrial Biochemistry in the Aging of the Reproductive System. Biology 2025, 14, 643. [Google Scholar] [CrossRef]
- Mortuza, R.; Feng, B.; Chakrabarti, S. SIRT1 reduction causes renal and retinal injury in diabetes through endothelin 1 and transforming growth factor β1. J. Cell Mol. Med. 2015, 19, 1857–1867. [Google Scholar] [CrossRef]
- Diao, W.; Liu, G.; Shi, C.; Jiang, Y.; Li, H.; Meng, J.; Shi, Y.; Chang, M.; Liu, X. Evaluating the Effect of Circ-Sirt1 on the Expression of SIRT1 and Its Role in Pathology of Pulmonary Hypertension. Cell Transplant. 2022, 31, 9636897221081479. [Google Scholar] [CrossRef] [PubMed]
- Zhan, H.; Huang, F.; Niu, Q.; Jiao, M.; Han, X.; Zhang, K.; Ma, W.; Mi, S.; Guo, S.; Zhao, Z. Downregulation of miR-128 Ameliorates Ang II-Induced Cardiac Remodeling via SIRT1/PIK3R1 Multiple Targets. Oxidative Med. Cell. Longev. 2021, 2021, 8889195. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.W.; Wu, M.S.; Liu, Y.; Lu, M.; Guo, J.D.; Meng, Y.H.; Zhou, Y.H. SIRT1-mediated deacetylation of NF-κB inhibits the MLCK/MLC2 pathway and the expression of ET-1, thus alleviating the development of coronary artery spasm. Am. J. Physiol. Heart Circ. Physiol. 2021, 320, H458–H468. [Google Scholar] [CrossRef]
- Elmorsy, E.A.; Elashry, H.A.; Alkhamiss, A.S.; Alsaykhan, H.; Hamad, R.S.; Abdel-Reheim, M.A.; Alsoghair, M.; Alharbi, M.S.; Gabr, A.M.; Ellethy, A.T.; et al. E1231/NMN protects against experimental metabolic syndrome: The central role of SIRT1 in modulating AKT/Nrf2/NFκB signaling. Front. Pharmacol. 2025, 16, 1558709. [Google Scholar] [CrossRef]
- Breitenstein, A.; Stein, S.; Holy, E.W.; Camici, G.G.; Lohmann, C.; Akhmedov, A.; Spescha, R.; Elliott, P.J.; Westphal, C.H.; Matter, C.M.; et al. Sirt1 inhibition promotes in vivo arterial thrombosis and tissue factor expression in stimulated cells. Cardiovasc. Res. 2011, 89, 464–472. [Google Scholar] [CrossRef]
- López-Fernández-Sobrino, R.; Soliz-Rueda, J.R.; Ávila-Román, J.; Arola-Arnal, A.; Suárez, M.; Muguerza, B.; Bravo, F.I. Blood Pressure-Lowering Effect of Wine Lees Phenolic Compounds Is Mediated by Endothelial-Derived Factors: Role of Sirtuin 1. Antioxidants 2021, 10, 1073. [Google Scholar] [CrossRef]
- Kim, K.T.; Heo, J.B.; Roh, T.; Jeon, S.M.; Heo, H.J.; Choi, Y.J.; Jo, E.K.; Song, G.Y.; Paik, S. Resveratrol derivative SH-707 inhibits NLRP3 inflammasome activation via a sirtuin 1-dependent pathway. Int. Immunopharmacol. 2025, 161, 115049. [Google Scholar] [CrossRef]
- Chen, X.; Yang, Q.; Shen, Y.; Hou, J.; Yuan, Q.; Zhong, Z.; Liu, Y. Research progress on the role of exosomes in the pathogenesis, diagnosis, and treatment of pulmonary hypertension. Respir. Res. 2023, 24, 144. [Google Scholar]
- Yang, H.; Zhang, W.; Guo, S.; Zhang, M.; Hu, L.; Li, X.; Liu, J.; Wang, J.; Yin, Y. Progress of pyroptosis in pulmonary hypertension. Heart Fail. Rev. 2023, 28, 835–847. [Google Scholar]
- Wang, H.; Zhao, Q.; Zhang, Y.; Li, T.; Zhao, M.; Yang, W.; Wang, G. The role of ferroptosis in pulmonary hypertension. Front. Pharmacol. 2022, 13, 1032481. [Google Scholar]
- Zhao, L.; Li, M.; Hu, H.; Liu, Z.; Zhang, Y. Mitochondria and mitochondrial regulators in the development of pulmonary hypertension. Front. Med. 2022, 9, 994474. [Google Scholar]
- Li, Y.; Yang, Y.; Luo, J.; Włodarski, P.K.; Wang, G. Molecular mechanisms involved in the development of pulmonary arterial hypertension (PAH). J. Physiol. Pharmacol. 2022, 73, 163–177. [Google Scholar]
- Włodarski, A.; Strycharz, J.; Wróblewski, A.; Kasznicki, J.; Drzewoski, J.; Śliwińska, A. The Role of microRNAs in Metabolic Syndrome-Related Oxidative Stress. Int. J. Mol. Sci. 2020, 21, 6902. [Google Scholar] [CrossRef]
- Jin, Q.; Xie, X.; Wang, C.; Zhang, W.; Wang, T.; Li, S.; Yang, Y.; Li, J.; Zhang, H. Endothelial cell metabolism in pulmonary arterial hypertension. Front. Pharmacol. 2021, 12, 767480. [Google Scholar]
- Zhang, Y.; He, J.; Wang, Y.; Chen, H.; Lin, J.; Zhong, C.; Li, L.; Huang, J.; Wang, H.; Liang, G.; et al. Mitochondrial metabolic reprogramming-mediated immunogenic cell death reveals immune and prognostic features of pulmonary arterial hypertension. Front. Immunol. 2023, 14, 1221181. [Google Scholar]
- Ho, M.F.; Walseth, T.F.; Anderson, S.M.; Gerrity, R.; Hohmeier, K.C.; Johnson, L.W.; Croatt, A.J.; Nath, K.A.; Limper, A.H.; Leof, E.B.; et al. Increased CD38 in the lungs of patients with pulmonary arterial hypertension. Am. J. Physiol. Lung Cell Mol. Physiol. 2021, 320, L872–L885. [Google Scholar]
- Waldman, M.; Nudelman, V.; Shainberg, A.; Kornwoski, R.; Aravot, D.; Abraham, N.G.; Arad, M.; Hochhauser, E. Regulation of oxidative stress and apoptosis in pulmonary hypertension: The role of heme oxygenase-1. J. Cardiovasc. Pharmacol. 2008, 51, 367–375. [Google Scholar]
- Hou, L.; Guo, D.; Wang, D.; He, W.; Wu, C.; Yang, J. Research progress on the mechanism of vascular remodeling in pulmonary hypertension: The role of endothelial cells and endothelial microenvironment. Biomed. Pharmacother. 2023, 159, 114224. [Google Scholar]
- Guo, J.; Wang, Y.; Guo, H.; Zhou, S.; Wu, B.; Zhu, L.; Wang, Y. Molecular mechanisms of vascular remodeling in idiopathic pulmonary arterial hypertension. Front. Pharmacol. 2022, 13, 1047672. [Google Scholar]
- Fan, C.; Luo, Y.; Ma, Y.; Chen, Y.; Li, X.; Yang, W.; Yang, X.; Li, W.; Sun, L. Epigenetics in pulmonary hypertension: Mechanisms and therapeutic targets. Front. Genet. 2022, 13, 963333. [Google Scholar]
- Scisciola, L.; Fusi, F.; Iside, C.; Fiore, D.; Liccardo, D.; Iannone, M.; Marfella, R.; Barbieri, M.; Paolisso, G.; D’Onofrio, N. Epigenetic mechanisms in pulmonary arterial hypertension: The key to precision medicine. Front. Cardiovasc. Med. 2022, 9, 965954. [Google Scholar]
- Fusi, J.; Bianchi, S.; Daniele, S.; Pellegrini, S.; Martini, C.; Galetta, F.; Giovannini, L.; Franzoni, F. An in vitro comparative study of the antioxidant activity and SIRT1 modulation of natural compounds. Biomed. Pharmacother. 2018, 101, 805–819. [Google Scholar] [CrossRef]
- Iside, C.; Scafuro, M.; Nebbioso, A.; Altucci, L. SIRT1 Activation by Natural Phytochemicals: An Overview. Front. Pharmacol. 2020, 11, 1225. [Google Scholar] [CrossRef]
- Kimira, Y.; Kasahara, Y.; Matsubara, H. Current understanding and future therapeutic prospects for pulmonary arterial hypertension with BMPR2 mutations. Int. J. Mol. Sci. 2023, 24, 1520. [Google Scholar]
- Baskaran, D.; Suresh, K. Pulmonary hypertension: Biomarkers and role of microRNAs. Am. J. Physiol. Lung Cell Mol. Physiol. 2023, 324, L293–L308. [Google Scholar]
- Ho, J.H.; Baskaran, R.; Wang, M.F.; Mohammedsaleh, Z.M.; Yang, H.S.; Balasubramanian, B.; Lin, W.T. Dipeptide IF and exercise training attenuate hypertension in SHR rats by inhibiting fibrosis and hypertrophy and activating AMPKα1, SIRT1, and PGC1α. Int. J. Mol. Sci. 2022, 23, 8167. [Google Scholar] [CrossRef]
- Galiniak, S.; Walczak, M.; Wilińska, M.; Kukla, M.; Michalski, Ł.; Biesiada, G. Role of microRNAs in pulmonary arterial hypertension: Review and exploratory analysis. Biomedicines 2021, 9, 438. [Google Scholar]
- Kumar, A.; Sharma, R.; Rehman, M.U.; Shah, B.A.; Goyal, S.N. Pharmacological overview of microRNA-based drugs for pulmonary arterial hypertension. Naunyn-Schmiedebergs Arch. Pharmacol. 2022, 395, 1159–1175. [Google Scholar]
- Sharifi-Rad, J.; Rayess, Y.E.; Rizk, A.A.; Sadaka, C.; Zgheib, R.; Zam, W.; Sestito, S.; Rapposelli, S.; Neffe-Skocińska, K.; Zielińska, D.; et al. Turmeric and its major compound curcumin on health: Bioactive effects and safety profiles for food, pharmaceutical, biotechnological and medicinal applications. Front. Pharmacol. 2020, 11, 1021. [Google Scholar] [CrossRef]
- Neag, M.A.; Mocan, A.; Echeverría, J.; Pop, R.M.; Bocsan, C.I.; Crişan, G.; Buzoianu, A.D. Berberine: Botanical occurrence, traditional uses, extraction methods, and relevance in cardiovascular, metabolic, hepatic, and renal disorders. Front. Pharmacol. 2018, 9, 557. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Ahmad, F.; Philp, A.; Baar, K.; Williams, T.; Luo, H.; Ke, H.; Rehmann, H.; Taussig, R.; Brown, A.L.; et al. Resveratrol ameliorates aging-related metabolic phenotypes by inhibiting cAMP phosphodiesterases. Cell 2012, 148, 421–433. [Google Scholar] [CrossRef] [PubMed]
- Houghton, M.J.; Kerimi, A.; Tumova, S.; Boyle, J.P.; Williamson, G. Quercetin preserves redox status and stimulates mitochondrial function in metabolically-stressed HepG2 cells. Free Radic. Biol. Med. 2018, 129, 296–309. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Kou, J.; Wang, P.; Ye, T.; Wang, Z.; Gao, Z.; Cong, L.; Li, M.; Dong, B.; Yang, W.; et al. Berberine-induced TFEB deacetylation by SIRT1 promotes autophagy in peritoneal macrophages. Aging 2021, 13, 7096–7119. [Google Scholar] [CrossRef]
- Guo, F.; Xu, F.; Li, S.; Zhang, Y.; Lv, D.; Zheng, L.; Gan, Y.; Zhou, M.; Zhao, K.; Xu, S.; et al. Amifostine ameliorates bleomycin-induced murine pulmonary fibrosis via NAD+/SIRT1/AMPK pathway-mediated effects on mitochondrial function and cellular metabolism. Eur. J. Med. Res. 2024, 29, 68. [Google Scholar] [CrossRef]
- Jadeja, R.N.; Powell, F.L.; Jones, M.A.; Fuller, J.; Joseph, E.; Thounaojam, M.C.; Bartoli, M.; Martin, P.M. Loss of NAMPT in aging retinal pigment epithelium reduces NAD+ availability and promotes cellular senescence. Aging 2018, 10, 1306–1323. [Google Scholar] [CrossRef]
- Cantó, C.; Gerhart-Hines, Z.; Feige, J.N.; Lagouge, M.; Noriega, L.; Milne, J.C.; Elliott, P.J.; Puigserver, P.; Auwerx, J. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature 2009, 458, 1056–1060. [Google Scholar] [CrossRef]
- Carollo, C.; Sorce, A.; Cirafici, E.; Mulè, G.; Caimi, G. Sirtuins and resveratrol in cardiorenal diseases: A narrative review of mechanisms and therapeutic potential. Nutrients 2025, 17, 1212. [Google Scholar] [CrossRef]
- Pacholec, M.; Bleasdale, J.E.; Chrunyk, B.; Cunningham, D.; Flynn, D.; Garofalo, R.S.; Griffith, D.; Griffor, M.; Loulakis, P.; Pabst, B.; et al. SRT1720, SRT2183, SRT1460, and resveratrol are not direct activators of SIRT1. J. Biol. Chem. 2010, 285, 8340–8351. [Google Scholar] [CrossRef]
- Villalba, J.M.; Alcaín, F.J. Sirtuin activators and inhibitors. Biofactors 2012, 38, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Milne, J.C.; Lambert, P.D.; Schenk, S.; Carney, D.P.; Smith, J.J.; Gagne, D.J.; Jin, L.; Boss, O.; Perni, R.B.; Vu, C.B.; et al. Small molecule activators of SIRT1 as therapeutics for the treatment of type 2 diabetes. Nature 2007, 450, 712–716. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Dong, X.; Wang, X.; Zheng, Y.; Qiu, J.; Peng, Y.; Xu, J.; Chai, Z.; Liu, C. Multiple roles of SIRT2 in regulating physiological and pathological signal transduction. Genet. Res. (Camb.) 2022, 2022, 9282484. [Google Scholar] [CrossRef] [PubMed]
- Murugasamy, K.; Munjal, A.; Sundaresan, N.R. Emerging roles of SIRT3 in cardiac metabolism. Front. Cardiovasc. Med. 2022, 9, 850340. [Google Scholar] [CrossRef]
- Ma, H.; Yu, Y.; Mo, L.; Chen, Q.; Dong, H.; Xu, Y.; Zhuan, B. Exosomal miR-663b from “M1” macrophages promotes pulmonary artery vascular smooth muscle cell dysfunction through inhibiting the AMPK/Sirt1 axis. Aging 2023, 15, 3549–3571. [Google Scholar] [CrossRef]
- Varshney, R.; Ali, Q.; Wu, C.; Sun, Z. Monocrotaline-Induced Pulmonary Hypertension Involves Downregulation of Antiaging Protein Klotho and eNOS Activity. Hypertension 2016, 68, 1255–1263. [Google Scholar] [CrossRef]
- Xi, L.; Ruan, L.; Yao, X.; Zhang, D.; Yuan, H.; Li, Q.; Yan, C. SIRT1 promotes pulmonary artery endothelial cell proliferation by targeting the Akt signaling pathway. Exp. Ther. Med 2020, 20, 179. [Google Scholar] [CrossRef]
- Yu, L.; Tu, Y.; Jia, X.; Fang, K.; Liu, L.; Wan, L.; Xiang, C.; Wang, Y.; Sun, X.; Liu, T.; et al. Resveratrol Protects Against Pulmonary Arterial Hypertension in Rats via Activation of Silent Information Regulator 1. Cell Physiol. Biochem. 2017, 42, 55–67. [Google Scholar] [CrossRef]
- Liu, H.; Pan, Z.; Wu, X.; Gong, C.; Hu, J. Jagged 2 inhibition attenuates hypoxia-induced mitochondrial damage and pulmonary hypertension through Sirtuin 1 signaling. PLoS ONE 2024, 19, e0297525. [Google Scholar] [CrossRef]
- Ding, M.; Lei, J.; Qu, Y.; Zhang, H.; Xin, W.; Ma, F.; Liu, S.; Li, Z.; Jin, F.; Fu, E. Calorie Restriction Attenuates Monocrotaline-induced Pulmonary Arterial Hypertension in Rats. J. Cardiovasc. Pharmacol. 2015, 65, 562–570, Erratum in: J. Cardiovasc. Pharmacol. 2015, 66, 514; Erratum in J. Cardiovasc. Pharmacol. 2015, 66, 322. [Google Scholar] [CrossRef]
- Zhou, S.; Li, M.T.; Jia, Y.Y.; Liu, J.J.; Wang, Q.; Tian, Z.; Liu, Y.T.; Chen, H.Z.; Liu, D.P.; Zeng, X.F. Regulation of Cell Cycle Regulators by SIRT1 Contributes to Resveratrol-Mediated Prevention of Pulmonary Arterial Hypertension. Biomed. Res. Int. 2015, 2015, 762349. [Google Scholar] [CrossRef]
- Vázquez-Garza, E.; Bernal-Ramírez, L.; Jerjes-Sánchez, C.; Lozano, O.; Acuña-Morín, E.; Vanoye-Tamez, M.; Ramos-González, M.R.; Chapoy-Villanueva, H.; Pérez-Plata, L.; Sánchez-Trujillo, L.; et al. Resveratrol Prevents Right Ventricle Remodeling and Dysfunction in Monocrotaline-Induced Pulmonary Arterial Hypertension with a Limited Improvement in the Lung Vasculature. Oxidative Med. Cell. Longev. 2020, 2020, 1841527. [Google Scholar] [CrossRef]
- Hoffmann, E.; Wald, J.; Lavu, S.; Roberts, J.; Beaumont, C.; Haddad, J.; Elliott, P.; Westphal, C.; Jacobson, E. Pharmacokinetics and tolerability of SRT2104, a first-in-class small molecule activator of SIRT1, after single and repeated oral administration in man. Br. J. Clin. Pharmacol. 2013, 75, 186–196. [Google Scholar] [CrossRef]
- Miranda, M.X.; van Tits, L.J.; Lohmann, C.; Arsiwala, T.; Winnik, S.; Tailleux, A.; Stein, S.; Gomes, A.P.; Suri, V.; Ellis, J.L.; et al. The Sirt1 activator SRT3025 provides atheroprotection in Apoe-/- mice by reducing hepatic Pcsk9 secretion and enhancing Ldlr expression. Eur. Heart J. 2015, 36, 51–59. [Google Scholar] [CrossRef]
- Solomon, J.M.; Pasupuleti, R.; Xu, L.; McDonagh, T.; Curtis, R.; DiStefano, P.S.; Huber, L.J. Inhibition of SIRT1 catalytic activity increases p53 acetylation but does not alter cell survival following DNA damage. Mol. Cell. Biol. 2006, 26, 28–38. [Google Scholar] [CrossRef]
- Rye, P.T.; Frick, L.E.; Ozbal, C.C.; Lamarr, W.A. Advances in label-free screening approaches for studying sirtuin-mediated deacetylation. J. Biomol. Screen. 2011, 16, 1217–1226. [Google Scholar] [CrossRef]
- Kahyo, T.; Ichikawa, S.; Hatanaka, T.; Yamada, M.K.; Setou, M. A novel chalcone polyphenol inhibits the deacetylase activity of SIRT1 and cell growth in HEK293T cells. J. Pharmacol. Sci. 2008, 108, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Grozinger, C.M.; Chao, E.D.; Blackwell, H.E.; Moazed, D.; Schreiber, S.L. Identification of a class of small molecule inhibitors of the sirtuin family of NAD-dependent deacetylases by phenotypic screening. J. Biol. Chem. 2001, 276, 38837–38843. [Google Scholar] [CrossRef] [PubMed]
- Lain, S.; Hollick, J.J.; Campbell, J.; Staples, O.D.; Higgins, M.; Aoubala, M.; McCarthy, A.; Appleyard, V.; Murray, K.E.; Baker, L.; et al. Discovery, in vivo activity, and mechanism of action of a small-molecule p53 activator. Cancer Cell 2008, 13, 454–463. [Google Scholar] [CrossRef] [PubMed]
- Trapp, J.; Meier, R.; Hongwiset, D.; Kassack, M.U.; Sippl, W.; Jung, M. Structure-activity studies on suramin analogues as inhibitors of NAD+-dependent histone deacetylases (sirtuins). ChemMedChem 2007, 2, 1419–1431. [Google Scholar] [CrossRef]
- Bononi, G.; Citi, V.; Martelli, A.; Poli, G.; Tuccinardi, T.; Granchi, C.; Testai, L.; Calderone, V.; Minutolo, F. Sirtuin 1-activating derivatives belonging to the anilinopyridine class displaying in vivo cardioprotective activities. RSC Med. Chem. 2023, 15, 267–282. [Google Scholar] [CrossRef] [PubMed]
- Campagna, R.; Mazzanti, L.; Pompei, V.; Alia, S.; Vignini, A.; Emanuelli, M. The Multifaceted Role of Endothelial Sirt1 in Vascular Aging: An Update. Cells 2024, 13, 1469. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Xu, C.; Man, A.W.C.; Bai, B.; Luo, C.; Huang, Y.; Xu, A.; Vanhoutte, P.M.; Wang, Y. Endothelial SIRT1 prevents age-induced impairment of vasodilator responses by enhancing the expression and activity of soluble guanylyl cyclase in smooth muscle cells. Cardiovasc. Res. 2019, 115, 678–690. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Zhao, H.; Liu, Y.; Yang, Z.; Yao, H.; Liu, T.; Gou, T.; Wang, L.; Zhang, J.; Tian, Y.; et al. Novel Role of the SIRT1 in Endocrine and Metabolic Diseases. Int. J. Biol. Sci. 2023, 19, 484–501. [Google Scholar] [CrossRef]
- Toulassi, I.A.; Al Saedi, U.A.; Gutlapalli, S.D.; Poudel, S.; Kondapaneni, V.; Zeb, M.; Cancarevic, I. A Paradigm Shift in the Management of Atherosclerosis: Protective Role of Sirtuins in Atherosclerosis. Cureus 2021, 13, e12735. [Google Scholar] [CrossRef]
- Zhang, T.; Xu, L.; Guo, X.; Tao, H.; Liu, Y.; Liu, X.; Zhang, Y.; Meng, X. The potential of herbal drugs to treat heart failure: The roles of Sirt1/AMPK. J. Pharm. Anal. 2024, 14, 157–176. [Google Scholar] [CrossRef]
- Chen, L.; Li, S.; Zhu, J.; You, A.; Huang, X.; Yi, X.; Xue, M. Mangiferin prevents myocardial infarction-induced apoptosis and heart failure in mice by activating the Sirt1/FoxO3a pathway. J. Cell. Mol. Med. 2021, 25, 2944–2955. [Google Scholar] [CrossRef]
- Ding, X.; Zhu, C.; Wang, W.; Li, M.; Ma, C.; Gao, B. SIRT1 is a regulator of autophagy: Implications for the progression and treatment of myocardial ischemia-reperfusion. Pharmacol. Res. 2024, 199, 106957. [Google Scholar] [CrossRef]
- Podyacheva, E.; Toropova, Y. SIRT1 activation and its effect on intercalated disc proteins as a way to reduce doxorubicin cardiotoxicity. Front Pharmacol 2022, 13, 1035387, Erratum in Front. Pharmacol. 2023, 14, 1154384. [Google Scholar] [CrossRef]
- Hu, C.; Zhang, X.; Teng, T.; Ma, Z.G.; Tang, Q.Z. Cellular Senescence in Cardiovascular Diseases: A Systematic Review. Aging Dis. 2022, 13, 103–128. [Google Scholar] [CrossRef]
- Zheng, S.; Yang, L.; Dai, Q.; Li, X.; Masuoka, T.; Lv, J. Role of sirtuin 1 in depression-induced coronary heart disease: Molecular pathways and therapeutic potential (Review). Biomed. Rep. 2025, 22, 46. [Google Scholar] [CrossRef] [PubMed]
- Fang, W.J.; Wang, C.J.; He, Y.; Zhou, Y.L.; Peng, X.D.; Liu, S.K. Resveratrol alleviates diabetic cardiomyopathy in rats by improving mitochondrial function through PGC-1α deacetylation. Acta Pharmacol. Sin. 2018, 39, 59–73. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.L.; Mostoslavsky, R.; Saito, S.; Manis, J.P.; Gu, Y.; Patel, P.; Bronson, R.; Appella, E.; Alt, F.W.; Chua, K.F. Developmental defects and p53 hyperacetylation in Sir2 homolog (SIRT1)-deficient mice. Proc. Natl. Acad. Sci. USA 2003, 100, 10794–10799. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Li, Y.; Zhang, Y.; Ye, T.; Wang, K.; Li, S.; Zhang, Y. SIRT1 mediates the inhibitory effect of Dapagliflozin on EndMT by inhibiting the acetylation of endothelium Notch1. Cardiovasc. Diabetol. 2023, 22, 331. [Google Scholar] [CrossRef]
- Mattagajasingh, I.; Kim, C.S.; Naqvi, A.; Yamamori, T.; Hoffman, T.A.; Jung, S.B.; DeRicco, J.; Kasuno, K.; Irani, K. SIRT1 promotes endothelium-dependent vascular relaxation by activating endothelial nitric oxide synthase. Proc. Natl. Acad. Sci. USA 2007, 104, 14855–14860. [Google Scholar] [CrossRef]
- Pan, Q.; Gao, Z.; Zhu, C.; Peng, Z.; Song, M.; Li, L. Overexpression of histone deacetylase SIRT1 exerts an antiangiogenic role in diabetic retinopathy via miR-20a elevation and YAP/HIF1α/VEGFA depletion. Am. J. Physiol. Endocrinol. Metab. 2020, 319, E932–E943. [Google Scholar] [CrossRef]
- Chen, C.; Hu, S.; Hu, H.J.; Liu, Z.X.; Wu, X.T.; Zou, T.; Su, H. Dronedarone Attenuates Ang II-Induced Myocardial Hypertrophy Through Regulating SIRT1/FOXO3/PKIA Axis. Korean Circ. J. 2024, 54, 172–186. [Google Scholar] [CrossRef]
- Yang, J.; Li, L.; Zheng, X.; Lu, Z.; Zhou, H. Dapagliflozin attenuates myocardial hypertrophy via activating the SIRT1/HIF-1α signaling pathway. Biomed. Pharmacother. 2023, 165, 115125. [Google Scholar] [CrossRef]
- Jiang, Q.; Lu, M.; Li, J.; Zhu, Z. Ginkgolide B Protects Cardiomyocytes from Angiotensin II-Induced Hypertrophy via Regulation of Autophagy through SIRT1-FoxO1. Cardiovasc. Ther. 2021, 2021, 5554569. [Google Scholar] [CrossRef]
- Zhang, Y.; Connelly, K.A.; Thai, K.; Wu, X.; Kapus, A.; Kepecs, D.; Gilbert, R.E. Sirtuin 1 Activation Reduces Transforming Growth Factor-β1-Induced Fibrogenesis and Affords Organ Protection in a Model of Progressive, Experimental Kidney and Associated Cardiac Disease. Am. J. Pathol. 2017, 187, 80–90. [Google Scholar]
- Yin, B.; Wang, Y.B.; Li, X.; Hou, X.W. β-aminoisobutyric acid ameliorates hypertensive vascular remodeling via activating the AMPK/SIRT1 pathway in VSMCs. Bioengineered 2022, 13, 14382–14401. [Google Scholar] [CrossRef] [PubMed]
- Bugyei-Twum, A.; Ford, C.; Civitarese, R.; Seegobin, J.; Advani, S.L.; Desjardins, J.F.; Kabir, G.; Zhang, Y.; Mitchell, M.; Switzer, J.; et al. Sirtuin 1 activation attenuates cardiac fibrosis in a rodent pressure overload model by modifying Smad2/3 transactivation. Cardiovasc. Res. 2018, 114, 1629–1641. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.; Zuo, Z.; Tian, J.; Ali, Q.; Lin, Y.; Lei, H.; Sun, Z. Activation of SIRT1 Attenuates Klotho Deficiency-Induced Arterial Stiffness and Hypertension by Enhancing AMP-Activated Protein Kinase Activity. Hypertension 2016, 68, 1191–1199. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yan, J.; Ni, X.; Hu, S.; Zhang, M.; Ying, Y. Phloretin targets SIRT1 to alleviate oxidative stress, apoptosis, and inflammation in deep venous thrombosis. Toxicol. Res. 2023, 40, 83–96. [Google Scholar] [CrossRef]
- Pai, P.Y.; Wong, J.K.S.; Cui, Z.Y.; Lin, Y.Y.; Lee, S.D. Angiotensin II Receptor Blocker Irbesartan Enhanced SIRT1 longevity Signaling Replaces the Mitochondrial Biogenetic Survival Pathway to Attenuate Hypertension-Induced Heart Apoptosis. J. Cardiovasc. Dev. Dis. 2022, 9, 266. [Google Scholar] [CrossRef]
- Han, Y.; Sun, W.; Ren, D.; Zhang, J.; He, Z.; Fedorova, J.; Sun, X.; Han, F.; Li, J. SIRT1 agonism modulates cardiac NLRP3 inflammasome through pyruvate dehydrogenase during ischemia and reperfusion. Redox Biol. 2020, 34, 101538. [Google Scholar] [CrossRef]
- Ni, Y.; Deng, J.; Liu, X.; Li, Q.; Zhang, J.; Bai, H.; Zhang, J. Echinacoside reverses myocardial remodeling and improves heart function via regulating SIRT1/FOXO3a/MnSOD axis in HF rats induced by isoproterenol. J. Cell. Mol. Med. 2021, 25, 203–216. [Google Scholar] [CrossRef]
- Zhu, H.Z.; Zhang, L.Y.; Zhai, M.E.; Xia, L.; Cao, Y.; Xu, L.; Li, K.F.; Jiang, L.Q.; Shi, H.; Li, X.; et al. GDF11 Alleviates Pathological Myocardial Remodeling in Diabetic Cardiomyopathy Through SIRT1-Dependent Regulation of Oxidative Stress and Apoptosis. Front. Cell Dev. Biol. 2021, 9, 686848. [Google Scholar] [CrossRef]
- Hao, Z.; Xu, G.; Yuan, M.; Tan, R.; Xia, Y.; Liu, Y.; Yin, X. Leucine Supplementation in Middle-Aged Male Mice Improved Aging-Induced Vascular Remodeling and Dysfunction via Activating the Sirt1-Foxo1 Axis. Nutrients 2022, 14, 3856. [Google Scholar] [CrossRef]
- Yang, K.; Velagapudi, S.; Akhmedov, A.; Kraler, S.; Lapikova-Bryhinska, T.; Schmiady, M.O.; Wu, X.; Geng, L.; Camici, G.G.; Xu, A.; et al. Chronic SIRT1 supplementation in diabetic mice improves endothelial function by suppressing oxidative stress. Cardiovasc Res. 2023, 119, 2190–2201. [Google Scholar] [CrossRef]
- Corbi, G.; Conti, V.; Troisi, J.; Colucci, A.; Manzo, V.; Di Pietro, P.; Calabrese, M.C.; Carrizzo, A.; Vecchione, C.; Ferrara, N.; et al. Cardiac rehabilitation increases SIRT1 activity and β-hydroxybutyrate levels and decreases oxidative stress in patients with HF with preserved ejection fraction. Oxidative Med. Cell. Longev. 2019, 2019, 7049237. [Google Scholar] [CrossRef]
- Pei, J.; Liu, Z.; Wang, C.; Chu, N.; Liu, L.; Tang, Y.; Liu, H.; Xiang, Q.; Cheng, H.; Li, M.; et al. Progesterone Attenuates SIRT1-Deficiency-Mediated Pre-Eclampsia. Biomolecules 2022, 12, 422. [Google Scholar] [CrossRef]
- Sasaki, Y.; Ikeda, Y.; Miyauchi, T.; Uchikado, Y.; Akasaki, Y.; Ohishi, M. Estrogen-SIRT1 Axis Plays a Pivotal Role in Protecting Arteries Against Menopause-Induced Senescence and Atherosclerosis. J. Atheroscler. Thromb. 2020, 27, 47–59. [Google Scholar] [CrossRef]
- Karolczak, K.; Watala, C. Estradiol as the Trigger of Sirtuin-1-Dependent Cell Signaling with a Potential Utility in Anti-Aging Therapies. Int. J. Mol. Sci. 2023, 24, 13753. [Google Scholar] [CrossRef]
- Bendale, D.S.; Karpe, P.A.; Chhabra, R.; Shete, S.P.; Shah, H.; Tikoo, K. 17-β Oestradiol prevents cardiovascular dysfunction in post-menopausal metabolic syndrome by affecting SIRT1/AMPK/H3 acetylation. Br. J. Pharmacol. 2013, 170, 779–795. [Google Scholar] [CrossRef]
- Csiszar, A.; Labinskyy, N.; Olson, S.; Pinto, J.T.; Gupte, S.; Wu, J.M.; Hu, F.; Ballabh, P.; Podlutsky, A.; Losonczy, G.; et al. Resveratrol prevents monocrotaline-induced pulmonary hypertension in rats. Hypertension 2009, 54, 668–675. [Google Scholar] [CrossRef]
- Tang, H.; Ning, K.; Wu, B.; Wang, X.; He, J.; Li, P.; Pan, L.; Zhang, J.; He, Y.; Bian, S.; et al. Scutellarein ameliorates pulmonary arterial hypertension via sirtuin 1 mediated deacetylation of nicotinamide nucleotide transhydrogenase. Biochem. Pharmacol. 2025, 237, 116932. [Google Scholar] [CrossRef] [PubMed]
- Chai, Y.; Gu, X.; Zhang, H.; Xu, X.; Chen, L. Phoenixin 20 ameliorates pulmonary arterial hypertension via inhibiting inflammation and oxidative stress. Aging 2024, 16, 5027–5037. [Google Scholar] [CrossRef] [PubMed]
- Paffett, M.L.; Lucas, S.N.; Campen, M.J. Resveratrol reverses monocrotaline-induced pulmonary vascular and cardiac dysfunction: A potential role for atrogin-1 in smooth muscle. Vascul Pharmacol. 2012, 56, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; You, Y.; Zhu, H. 15-HETE protects pulmonary artery smooth muscle cells against apoptosis via SIRT1 regulation during hypoxia. Biomed. Pharmacother. 2018, 108, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Hei, B.; Hao, W.; Lin, S.; Liu, X.; Meng, X.; Wang, Y.; Zhao, M.; Yu, H.; Yang, L.; et al. Shionone Exhibits Anti-inflammatory and Antiproliferative Effects in Pulmonary Arterial Endothelial Cells and Smooth Muscle Cells via SIRT1 in Pulmonary Arterial Hypertension. Rev. Bras. Farmacogn. 2024, 34, 1287–1297. [Google Scholar] [CrossRef]
- Boucherat, O.; Agrawal, V.; Lawrie, A.; Bonnet, S. The Latest in Animal Models of Pulmonary Hypertension and Right Ventricular Failure. Circ. Res. 2022, 130, 1466–1486. [Google Scholar] [CrossRef]
- Bueno-Beti, C.; Sassi, Y.; Hajjar, R.J.; Hadri, L. Pulmonary Artery Hypertension Model in Rats by Monocrotaline Administration. Methods Mol. Biol. 2018, 1816, 233–241. [Google Scholar] [PubMed]
- Corssac, G.B.; Bonetto, J.P.; Campos-Carraro, C.; Cechinel, L.R.; Zimmer, A.; Parmeggiani, B.; Grings, M.; Carregal, V.M.; Massensini, A.R.; Siqueira, I.; et al. Pulmonary arterial hypertension induces the release of circulating extracellular vesicles with oxidative content and alters redox and mitochondrial homeostasis in the brains of rats. Hypertens. Res. 2021, 44, 918–931. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.H.; Ma, J.L.; Ding, D.; Ma, Y.J.; Wei, Y.P.; Jing, Z.C. Experimental animal models of pulmonary hypertension: Development and challenges. Anim. Models Exp. Med. 2022, 5, 207–216. [Google Scholar] [CrossRef]
- Corboz, M.R.; Nguyen, T.L.; Stautberg, A.; Cipolla, D.; Perkins, W.R.; Chapman, R.W. Current Overview of the Biology and Pharmacology in Sugen/Hypoxia-Induced Pulmonary Hypertension in Rats. J. Aerosol Med. Pulm. Drug Deliv. 2024, 37, 241–283. [Google Scholar] [CrossRef]
- Lin, Q.; Fan, C.; Skinner, J.T.; Hunter, E.N.; Macdonald, A.A.; Illei, P.B.; Yamaji-Kegan, K.; Johns, R.A. RELMα Licenses Macrophages for Damage-Associated Molecular Pattern Activation to Instigate Pulmonary Vascular Remodeling. J. Immunol. 2019, 203, 2862–2871. [Google Scholar] [CrossRef]
- Jing, X.; Wu, S.; Liu, Y.; Wang, H.; Huang, Q. Circular RNA Sirtuin1 represses pulmonary artery smooth muscle cell proliferation, migration and autophagy to ameliorate pulmonary hypertension via targeting microRNA-145-5p/protein kinase-B3 axis. Bioengineered 2022, 13, 8759–8771. [Google Scholar] [CrossRef]
- Wilson, D.N.; Schacht, S.E.; Al-Nakkash, L.; Babu, J.R.; Broderick, T.L. Resveratrol prevents pulmonary trunk remodeling but not right ventricular hypertrophy in monocrotaline-induced pulmonary hypertension. Pathophysiology 2016, 23, 243–250. [Google Scholar] [CrossRef]
- Klinke, A.; Berghausen, E.; Friedrichs, K.; Molz, S.; Lau, D.; Remane, L.; Berlin, M.; Kaltwasser, C.; Adam, M.; Mehrkens, D.; et al. Myeloperoxidase aggravates pulmonary arterial hypertension by activation of vascular Rho-kinase. JCI Insight 2018, 3, e97530. [Google Scholar] [CrossRef]
- Yeung, F.; Hoberg, J.E.; Ramsey, C.S.; Keller, M.D.; Jones, D.R.; Frye, R.A.; Mayo, M.W. Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J. 2004, 23, 2369–2380. [Google Scholar] [CrossRef]
- Yu, Q.; Dong, L.; Li, Y.; Liu, G. SIRT1 and HIF1α signaling in metabolism and immune responses. Cancer Lett. 2018, 418, 20–26. [Google Scholar] [CrossRef]
- Planavila, A.; Iglesias, R.; Giralt, M.; Villarroya, F. Sirt1 acts in association with PPARα to protect the heart from hypertrophy, metabolic dysregulation, and inflammation. Cardiovasc. Res. 2011, 90, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, Y.; Li, J.; Li, B.; Chong, Y.; Zheng, G.; Sun, S.; Feng, F. SIRT1 alleviates isoniazid-induced hepatocyte injury by reducing histone acetylation in the IL-6 promoter region. Int. Immunopharmacol. 2019, 67, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Bai, M.; Lu, C.; An, L.; Gao, Q.; Xie, W.; Miao, F.; Chen, X.; Pan, Y.; Wang, Q. SIRT1 relieves Necrotizing Enterocolitis through inactivation of Hypoxia-inducible factor (HIF)-1a. Cell Cycle 2020, 19, 2018–2027. [Google Scholar] [CrossRef] [PubMed]
- Alcendor, R.R.; Gao, S.; Zhai, P.; Zablocki, D.; Holle, E.; Yu, X.; Tian, B.; Wagner, T.; Vatner, S.F.; Sadoshima, J. Sirt1 regulates aging and resistance to oxidative stress in the heart. Circ. Res. 2007, 100, 1512–1521. [Google Scholar] [CrossRef]
- Elibol, B.; Kilic, U. High Levels of SIRT1 Expression as a Protective Mechanism Against Disease-Related Conditions. Front. Endocrinol. 2018, 9, 614. [Google Scholar] [CrossRef]
- Salla, M.; Karaki, N.; El Kaderi, B.; Ayoub, A.J.; Younes, S.; Abou Chahla, M.N.; Baksh, S.; El Khatib, S. Enhancing the Bioavailability of Resveratrol: Combine It, Derivatize It, or Encapsulate It? Pharmaceutics 2024, 16, 569. [Google Scholar] [CrossRef]
- Chimento, A.; De Amicis, F.; Sirianni, R.; Sinicropi, M.S.; Puoci, F.; Casaburi, I.; Saturnino, C.; Pezzi, V. Progress to Improve Oral Bioavailability and Beneficial Effects of Resveratrol. Int. J. Mol. Sci. 2019, 20, 1381. [Google Scholar] [CrossRef]
- Wang, J.; Liu, T.; Chen, P.; Yin, D.; Zhang, H.; Qiu, X.; Zou, S.; Li, W. Pharmacokinetic evaluation of two oral Resveratrol formulations in a randomized, open-label, crossover study in healthy fasting subjects. Sci. Rep. 2025, 15, 24515. [Google Scholar] [CrossRef]
- Patel, K.R.; Scott, E.; Brown, V.A.; Gescher, A.J.; Steward, W.P.; Brown, K. Clinical trials of resveratrol. Ann. N. Y. Acad. Sci. 2011, 1215, 161–169. [Google Scholar] [CrossRef]
- Almeida, L.; Vaz-da-Silva, M.; Falcão, A.; Soares, E.; Costa, R.; Loureiro, A.I.; Fernandes-Lopes, C.; Rocha, J.F.; Nunes, T.; Wright, L.; et al. Pharmacokinetic and safety profile of trans-resveratrol in a rising multiple-dose study in healthy volunteers. Mol. Nutr. Food Res. 2009, 53 (Suppl. 1), S7–S15. [Google Scholar] [CrossRef]
- Tsai, H.Y.; Ho, C.T.; Chen, Y.K. Biological actions and molecular effects of resveratrol, pterostilbene, and 3′-hydroxypterostilbene. J. Food Drug Anal. 2017, 25, 134–147. [Google Scholar] [CrossRef]
- Reddy, R.; Kalra, S.S.; Alzghoul, B.; Khan, A.; Zayed, Y. Effect of Obesity on Mortality in Pulmonary Hypertension-A Systematic Review and Meta-Analysis. J. Cardiovasc. Dev. Dis. 2023, 10, 419. [Google Scholar] [CrossRef]
- Curjuric, I.; Imboden, M.; Bridevaux, P.O.; Gerbase, M.W.; Haun, M.; Keidel, D.; Kumar, A.; Pons, M.; Rochat, T.; Schikowski, T.; et al. Common SIRT1 variants modify the effect of abdominal adipose tissue on aging-related lung function decline. Age 2016, 38, 52. [Google Scholar] [CrossRef]

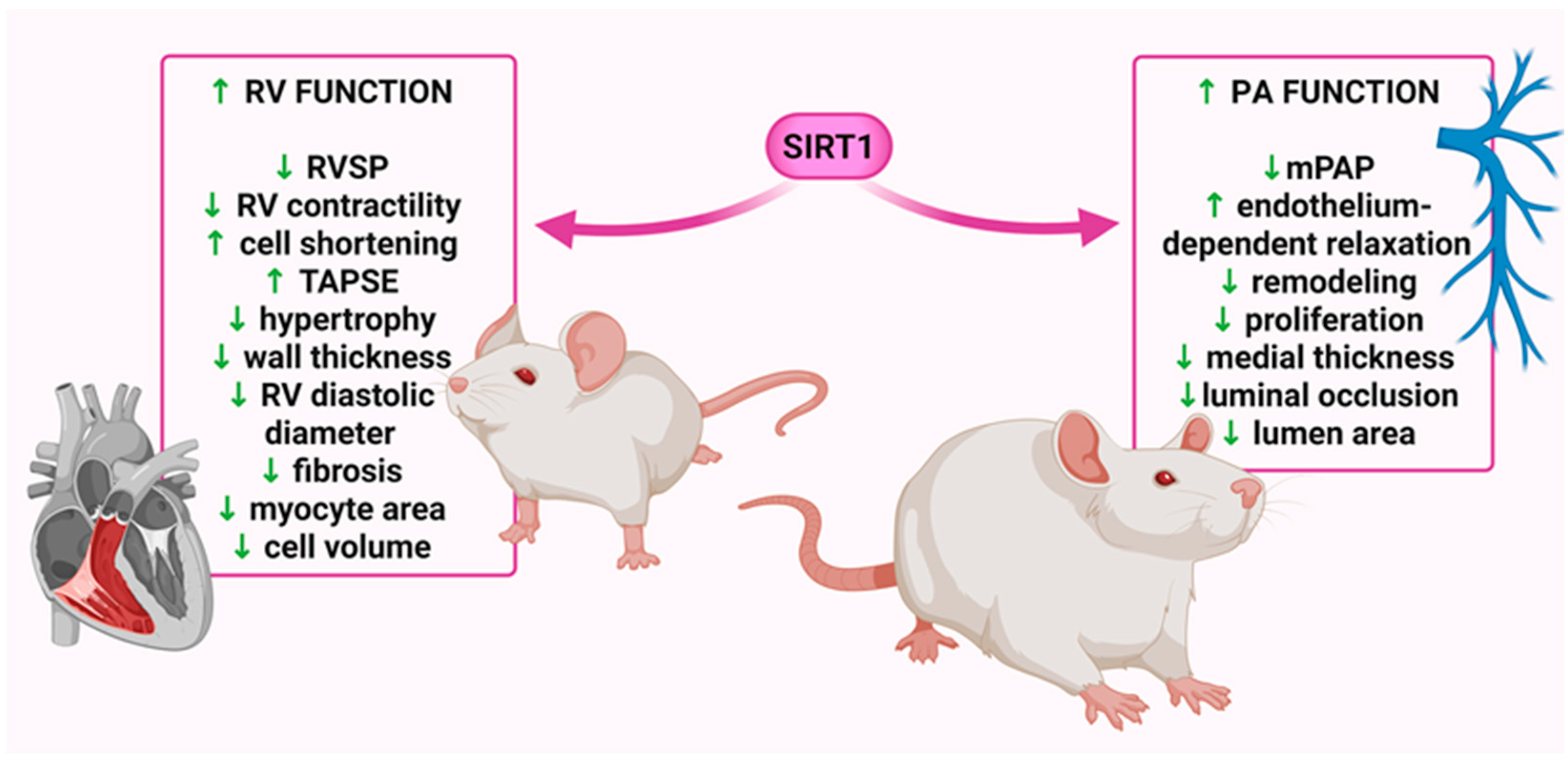
| Rodents Specifications | Conditions/ Comments | Modulator | Administration | SIRT1 Expression in PH | Effects After Modulator | Ref. | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Species/Age/ Weight/Sex | Dose/Concentration/ Route/Duration/Time | RVSP | PAP/ mPAP | RV Hypertrophy | PA Wall Thickness | Result/s | ||||
| Preventive models | ||||||||||
| SIRT1 KO mice (mice C57BL/6J); 5–6 w; n/d; female | 21 d in hypoxia (10% O2) | C57BL/6J carrying both the UBC-Cre-ERT2 and the SIRT1floxDE4/ floxDE4 alleles) | tamoxifene (30 mg/kg/d) for 4 consecutive d to induce SIRT1 deletion, 21 d before exposure to hypoxia | ↓ in lungs | ↑ | n/d | ↑ | ↓↓ non-muscular ↔ part. muscular ↑ tot. muscular | in lungs: ↑ α-SMA, acetylation; ↓ PPARα, PGC-1α, HIF-1α; ↑↑ GLUT1, mitochondrial biogenesis | [7] |
| SD rats; 6 w; 150–200 g; male | 40 d in hypoxia (10% O2) | circ-SIRT1 | 1 × 108 CFU; i.v.; once, at 1 and 20 d of 40 d treatment | ↓ in PASMCs and lungs | ↓ | n/d | ↓ | ↓ pulmonary small blood vessels thickness and lumen stenosis | ↑ SIRT1 expression in PASMCs and lungs; in lungs: ↓ Smad3, Smad7, TGF-β1, VCAM-1, α-SMA, ICAM-1, PCNA, vimentin | [63] |
| Wistar rats; n/d; 200–300 g; male | 21 d, fractional inspired oxygen of 0.21 and 0.12 | resveratrol | 25 mg/kg/d; n/d; 21 d | ↓↓ in PASMCs | ↓↓ | n/d | ↓ | ↓ | ↔ SIRT1 expression in PASMCs | [108] |
| SRT1720 | 25 mg/kg/d; n/d; 21 d | ↓ in PASMCs | ↓ | n/d | n/d | ↓ | ↔ SIRT1 expression in PASMCs | |||
| SD rats; 6–8 w; 180–240 g; male | 28 d in hypoxia (10% O2) | adeno-associated virus serotype 1-Jag2 | n/d; 14 d before exposure to hypoxia | n/d | ↓↓ | n/d | ↓↓ | ↓↓↓ <50 μm, ↓↓ >50 μm | in lungs: ↑↑↑ SOD; ↓↓ MPO, MDA activity; ↑↑ Nrf2, HO-1 | [109] |
| SD rats; n/d; 240–260 g, male | 60 mg/kg of MCT, 21 d (PAP, PA relaxation) or 7 d (eNOS level, SIRT1 expression) before evaluation | adenoviral vectors for the overexpression of SIRT1 | 7.5 × 109 pfu; i.t.; 1 d before MCT injection | n/d | n/d | ↓ | n/d | n/d | ↑↑ SIRT1 expression; in PA: ↑ p-eNOS; ↓↓ ac-eNOS | [110] |
| 60 mg/kg of MCT, 21 d before evaluation | short-term CR | 10% restriction; 14 d before MCT injection +35% restriction; p.o.; 21 d after MCT injection | ↓ in PA | n/d | ↓ | ↓↓ | ↓ | ↑ SIRT1 expression; in PA: ↔ eNOS; ↑ p-eNOS; ↓↓ ac-eNOS | ||
| SD rats; 6–8 w; 200–220 g; male | 60 mg/kg of MCT, 21 d before evaluation | scutellarein | 50 mg/kg/d; i.p. | ↓↓ in lungs | ↓↓ | n/d | ↓↓ | ↓↓ | ↑↑ SIRT1 expression; in serum: ↓↓ TNF-α, IL-6, IL-1β, α-SMA | [157] |
| C57BL/6 mice; 6–8 w; 20–22 g; male | 28 d in hypoxia (10% O2) + SU5416 injection (20 mg/kg i.p.) once a week | 10 mg/kg/d; i.p. | ↓↓ in lungs | ↓↓ | n/d | ↓↓ | ↓↓ | ↑↑ SIRT1 expression; in serum: ↓↓ TNF-α, IL-6, IL-1β, α-SMA | ||
| n/d rats; 7–9 w; n/d; male | 28 d in hypoxia (10.5% O2) | phoenixin-20 | 100 ng/g/d; 28 d; n/d | n/d | ↓ | ↓ | ↓ | ↓ | in lungs: ↓ TNF-α, IL-6, MCP-1, MDA, NLRP3, ASC; ↑ SOD activity | [158] |
| Therapeutic models | ||||||||||
| SD rats; 6–8 w; n/d; male | 21 d in hypoxia (10% O2) and 35 d in normoxia, SU5416 injection (20 mg/kg) on day 0 of the experiment | SRT2104 | 100 mg/kg/d, by gavage; at the beginning of w 4 of the experiment for 5 w, 5 d/w | n/d | ↓ | ↓ | ↓↓↓ | ↓↓ | n/d | [16] |
| C57BL/6J mice; n/d; n/d; female, male | 35 d in hypoxia (10% O2), SU5416 injections (20 mg/kg) on d 0, 7 and 14 of the experiment | 100 mg/kg/d, by gavage; at w 4, 5 d/w, 7 d from day 15 after PH induction | n/d | ↓ | ↓ | ↓↓↓ | ↓ | n/d | ||
| SD rats; 6–8 w; 200–250 g; male | 28 d in hypobaric conditions (pressure—380 mmHg, PaO2—79.6 mmHg) | exosomes derived from M1 macrophage with miR-663b low expression | 20 μg of M1miR-663b-in-Exo; i.v.; 7 d, from d 30 | ↓↓↓↓ in PASMCs | ↓ | n/d | ↓ | n/d | ↑ SIRT1 expression in PASMCs; in PASMCs: ↓ TNF-α, IL-6, IL-1β, iNOS, COX2; ↑ Nrf2, HO-1, Trx-1, AMPK | [105] |
| SD rats; 6–8 w; n/d; male | 60 mg/kg of MCT, 21 d before evaluation | MSC overexpressing secreted KL | 3.5 × 106 MSC/rat; i.v.; once, 3 d after MCT injection | ↓↓↓ in lungs | ↓ | n/d | ↓ | ↓ PASMC proliferation; ↑ lumen area | ↔ SIRT1 expression; in lungs: ↔ eNOS; ↑↑ p-eNOS; ↓ CD68 | [106] |
| SD rats; adult; 280–300 g; male | 60 mg/kg of MCT, 14 d before evaluation | resveratrol | 2.5 mg/kg/d; p.o.; for 14 d after MCT injection or for 21 d after MCT injection | ↓↓↓ in lungs | ↓ | ↓ | ↔ | ↔ 25–50 μm; ↓↓ 51–100 μm; ↓ 101–500 μm | ↑↑ SIRT1 expression in lungs; ↑↑ p21; ↔ cyclin D | [111] |
| 60 mg/kg of MCT, 21 d before evaluation | ↓↓↓ | ↓↓↓ | ↓↓↓ | ↓↓ 25–50 μm; ↓↓ 51–100 μm; ↓ 101–500 μm | ↑↑ SIRT1 expression in lungs; ↑↑ p21; ↓↓↓ cyclin D | |||||
| 60 mg/kg of MCT, 14 d before evaluation | 20 mg/kg/d; p.o.; for 14 d after MCT injection or for 21 d after MCT injection | ↓ | ↓↓↓ | ↓ | ↔ 25–50 μm; ↓↓ 51–100 μm; ↓↓↓ 101–500 μm | ↑ SIRT1 expression in lungs; ↑↑↑ p21; ↓↓↓ cyclin D | ||||
| 60 mg/kg of MCT, 21 d before evaluation | ↓↓↓ | ↓↓↓ | ↓↓↓ | ↓↓ 25–50 μm; ↓↓ 51–100 μm; ↓↓ 101–500 μm | ↑↑ SIRT1 expression in lungs; ↑ p21; ↓↓↓ cyclin D | |||||
| SD rats; adult; >300 g; male | 60 mg/kg of MCT | resveratrol | 20 mg/kg/d; by gavage for 42 d after MCT injection | ↔ in RV | n/d | ↔ | ↓ | n/d | in RV: ↓ BNP, TnC, Ac-Lys; ↔ Col1, IL-1β, IL-10 | [112] |
| SD rats; adult; 300 g; male | 60 mg/kg of MCT, 14 d before evaluation | resveratrol | 25 mg/kg/d; p.o., in the drinking water; for 14 d after MCT injection or for 21 d after MCT injection | n/d | ↓ | n/d | ↓ | ↓ | n/d | [156] |
| 60 mg/kg of MCT, 21 d before evaluation | n/d | ↓ | n/d | ↓ | ↓ | in lungs: ↓ IL-6, IL-1, TNFα, PDGFα, PDGFβ, MCP-1, iNOS, ICAM-1, in PA: ↓ NOX-1; ↑ eNOS | ||||
| SD rats; 8–10 w; >300 g; male | 50 mg/kg of MCT, 28 d before evaluation | resveratrol | 3 mg/kg/d, p.o. in the drinking water; for 14 d from d 28 after PH induction | n/d | ↓ | n/d | ↓ | ↔ <75 μm, ↓ 75–150 μm, ↔ >150 μm | in PA: ↑ atrogin-1; ↔ MuRF-1, eNOS, Kv1.5 | [159] |
| SD rats; 4–5 w; 180–220 g; male | 60 mg/kg of MCT | sh-circSIRT1 | 2 × 108 TU/mL sh-RNA lentiviral vector of stably targeting circ-SIRT1; i.v. | n/d | n/d | ↓ | ↓ | ↓ | in PA: ↑ miR-145-5p | [168] |
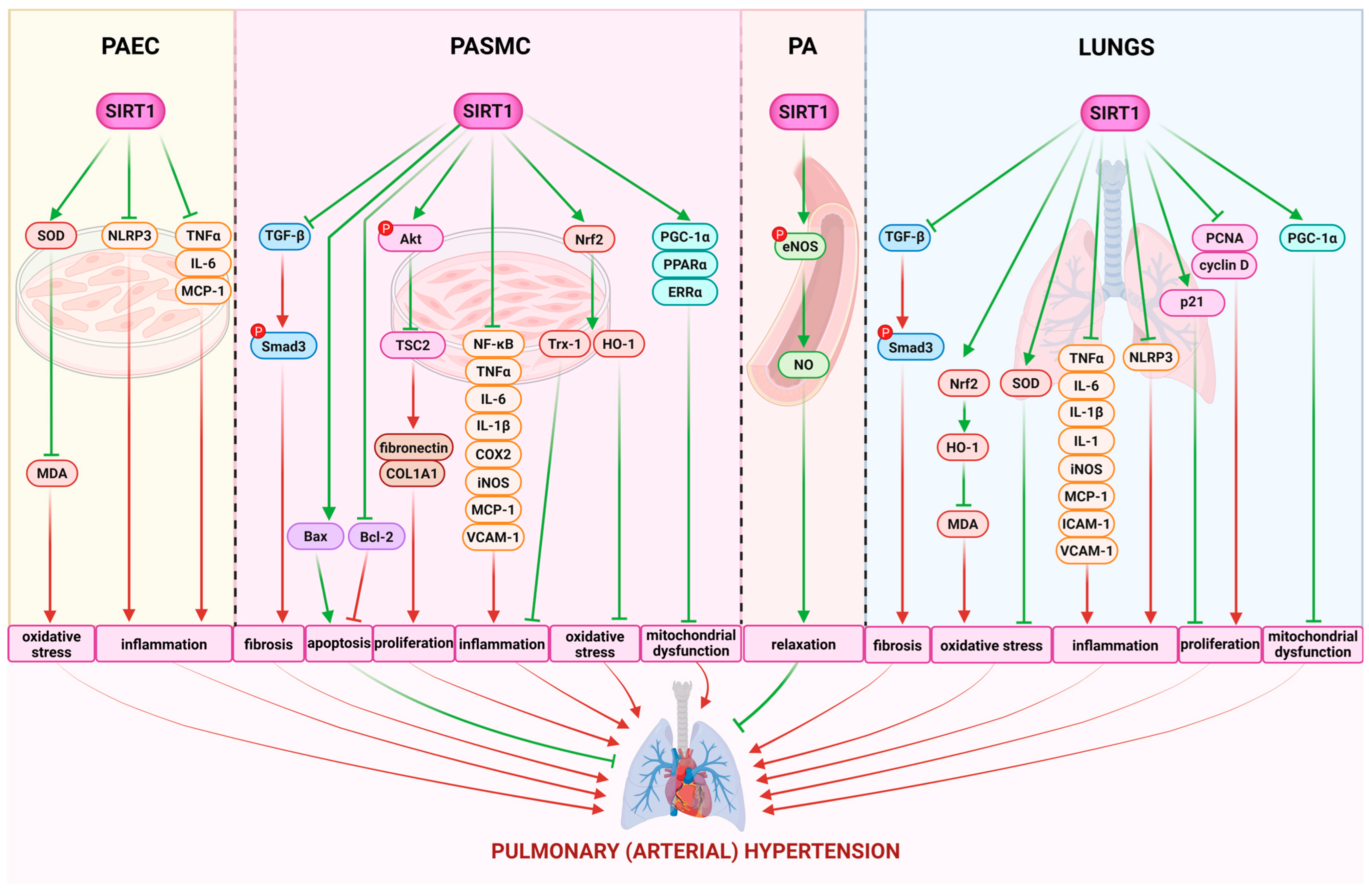
| Cell Culture | Conditions | Modulator | Effects | Ref. | |||||
|---|---|---|---|---|---|---|---|---|---|
| Viability | Regulation of Apoptosis and Proliferation | Anti-Inflammatory, Antioxidant | Mitochondrial Dysfunction | SIRT1 Expression/ Other Mechanisms | |||||
| HUMAN | |||||||||
| iPAH PASMC obtained during lung transplantation | in normoxia | Stac-3; 10 μM | ↔ | ↓ PCNA | ↑ SOD2 | ↑ VDAC, PPARα, CS, ERRα, PGC-1α, GLUT1, LDH | ↔ SIRT1 expression | [7] | |
| iPAH PASMC obtained during lung transplantation | 48 h in normoxia | SRT2104; 10 μM | n/d | ↑ TSC2; ↓ Col1A1, p-Akt, fibronectin | n/d | n/d | - | [16] | |
| PAEC | 72 h in hypoxia (10% O2, 5% CO2) | SRT1720; 4 μM | ↑ | ↑ p-Akt, Bcl2, HIF-1 | ↑ HIF-1 | n/d | ↔ Akt | [107] | |
| NAC; 5000 μM | n/d | ↓ p-Akt, Bcl2, HIF-1 | ↓ HIF-1 | n/d | ↔ Akt | ||||
| PASMC | PDGF (10 ng/mL) for 48 h | resveratrol; 10 µM | n/d | ↓ (proliferation) | ↓ NF-κB | n/d | - | [156] | |
| PASMC | 48 h in hypoxia (1% O2) | scutellarein; 50 and 100 µM | ↓↓ | ↓↓ (proliferation and apoptosis) | ↓↓ IL-6; ↓ TNF-α, IL-1β | n/d | ↑↑ SIRT1 expression | [157] | |
| PASMC | PDGF (10 ng/mL) for 48 h | resveratrol | 10 µM | n/d | ↔ (proliferation and apoptosis) | n/d | n/d | - | [159] |
| 30 µM | ↑ atrogin-1; ↔ (proliferation and apoptosis) | ||||||||
| 100 µM | ↑ atrogin-1; ↓↓ (proliferation and apoptosis) | ||||||||
| PASMC | 48 h in hypoxia | si-circSIRT1 | n/d | ↓ (proliferation) ↑ (apoptosis) | n/d | n/d | ↓ migration, Beclin-1, ATG5, LC3 II, Akt3 ↑ p62, miR-145-5p | [168] | |
| RAT | |||||||||
| PASMC | in normoxia | Stac-3; 10 μM | n/d | ↓↓↓ ac-histone H1, ac-FOXO1; ↓ PCNA | n/d | ↓ ac-PGC-1α | ↔ SIRT1 expression | [7] | |
| PASMC | 24 h in hypoxia (3% O2) | circ-SIRT1 | ↓ | ↓ Smad3, Smad7, TGF-β1, α-SMA | ↓ VCAM-1 | n/d | ↓ migration | [63] | |
| PASMC | 24 h in hypoxia (92% N2, 5% CO2, 3% O2) | resveratrol 30 and 50 μM | n/d | ↓ (proliferation) | n/d | n/d | n/d | [108] | |
| SRT1720 (1, 3, 5, and 10 μM) | ↓ | ↑↑ (apoptosis; 1 μM); ↓ (proliferation; 3, 5, and 10 μM) | n/d | n/d | ↑ SNO; ↓ migration; ↓↓ mPT | ||||
| PASMC | 24 h in hypoxia (5% CO2, 1% O2) | Rat 1-Jag2 | ↓ | ↑ Bax; ↓↓ Bcl2 | n/d | ↑↑ Tom20, Coxiv | n/d | [109] | |
| PMEC | in hypoxia (5% CO2) | phoenixin 20 | 10 nM | n/d | n/d | ↑ SOD; ↓ MDA, TNF-α, IL-6, NLRP3, ASC, MCP-1 | n/d | ↑ SIRT1 expression; ↓ MCP-1 | [158] |
| 20 nM | ↑↑ SOD; ↓↓ MDA, TNF-α, IL-6, NLRP3, ASC, MCP-1 | ||||||||
| PASMC | 48 h in hypoxia (2.5% O2, 5% CO2) | 15-HETE | n/d | n/d | n/d | n/d | ↑ SIRT1 expression | [160] | |
| 15-HETE + serum deprivation | ↑ | ↑ Bcl-xl, Bcl2; ↓ caspase 3 ↓ (apoptosis) | n/d | n/d | n/d | ||||
| PASMC | 24 h in hypoxia (2% O2, 5% CO2) | Shionone | 2 µg/ml | ↔ | ↑ Bax; ↓↓ Bcl2; ↑↑ (apoptosis) | ↓ TNF-α, IL-6; ↔ IL-1β | n/d | ↑↑ SIRT1 expression ↑↑ eNOS; ↓ ET-1 | [161] |
| 4 µg/ml | ↓↓ | ↑↑ Bax; ↓↓ Bcl2; ↑↑ (apoptosis) | ↓↓ TNF-α, IL-1β, IL-6 | n/d | ↑↑ SIRT1 expression ↑↑ eNOS; ↓↓ ET-1 | ||||
| 8 µg/ml | ↓↓ | ↑↑ Bax; ↓↓ Bcl2; ↑↑ (apoptosis) | ↓↓ TNF-α, IL-1β, IL-6 | n/d | ↑↑ SIRT1 expression ↑↑ eNOS; ↓↓ ET-1 | ||||
| PAEC | 24 h in hypoxia (2% O2, 5% CO2) | 2, 4, 8 µg/ml | n/d | n/d | n/d | n/d | ↑↑ SIRT1 expression | ||
| 8 µg/mL + SIRT1-siRNA | ↓↓ | ↑↑ Bax; ↓↓ Bcl2; ↑↑ (apoptosis) | ↑↑ TNF-α, IL-1β, IL-6 | n/d | ↓↓ eNOS; ↑↑ ET-1 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Budziak, S.; Kloza, M.; Krzyżewska, A.; Baranowska-Kuczko, M. Can Sirtuin 1 Serve as a Therapeutic Target in Pulmonary Arterial Hypertension? A Comprehensive Review. Molecules 2025, 30, 3740. https://doi.org/10.3390/molecules30183740
Budziak S, Kloza M, Krzyżewska A, Baranowska-Kuczko M. Can Sirtuin 1 Serve as a Therapeutic Target in Pulmonary Arterial Hypertension? A Comprehensive Review. Molecules. 2025; 30(18):3740. https://doi.org/10.3390/molecules30183740
Chicago/Turabian StyleBudziak, Sandra, Monika Kloza, Anna Krzyżewska, and Marta Baranowska-Kuczko. 2025. "Can Sirtuin 1 Serve as a Therapeutic Target in Pulmonary Arterial Hypertension? A Comprehensive Review" Molecules 30, no. 18: 3740. https://doi.org/10.3390/molecules30183740
APA StyleBudziak, S., Kloza, M., Krzyżewska, A., & Baranowska-Kuczko, M. (2025). Can Sirtuin 1 Serve as a Therapeutic Target in Pulmonary Arterial Hypertension? A Comprehensive Review. Molecules, 30(18), 3740. https://doi.org/10.3390/molecules30183740







