Multimodal Detection of Magnetically and Fluorescently Dual-Labeled Murine Macrophages After Intravenous Administration
Abstract
1. Introduction
2. Results and Discussion
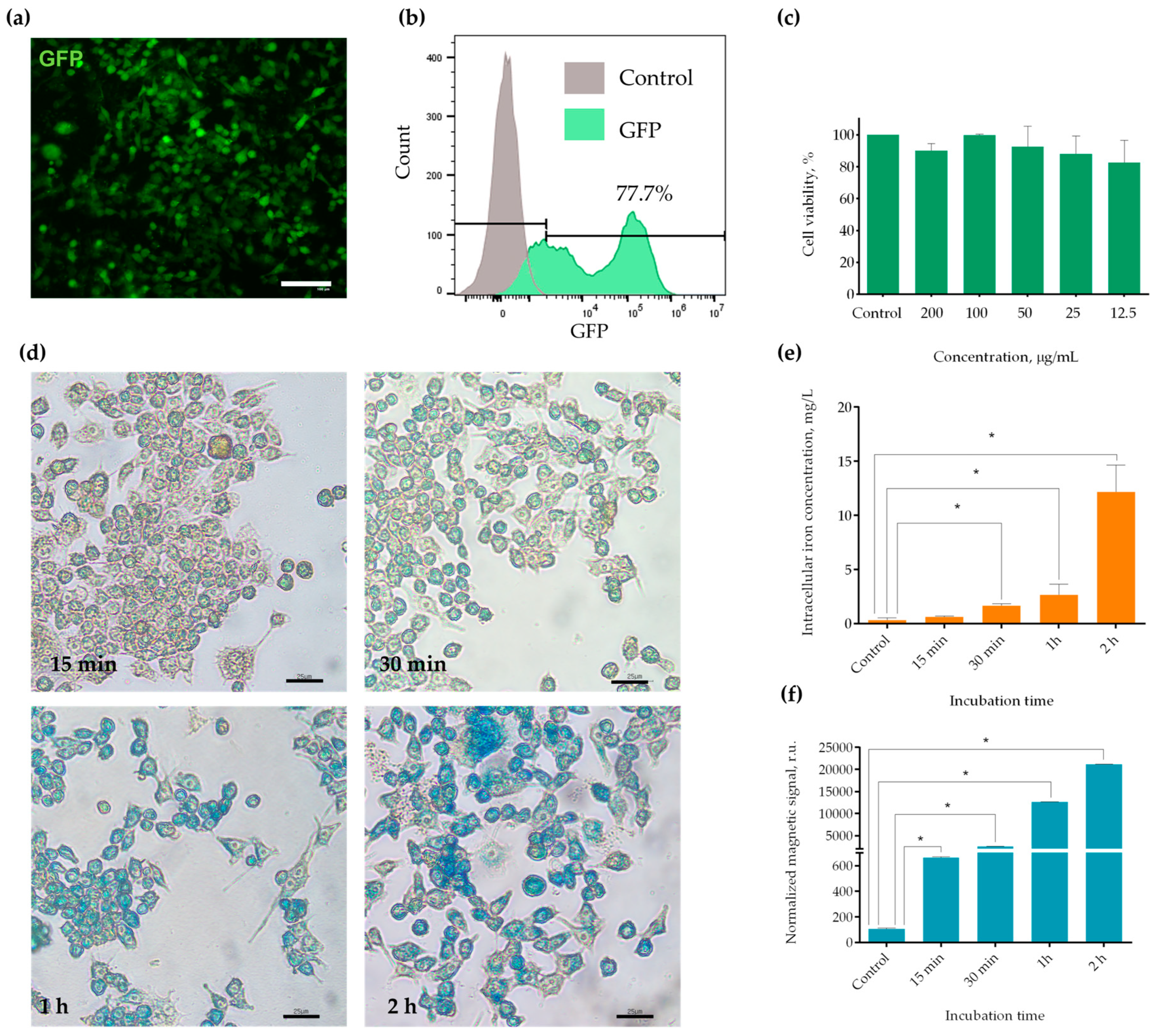
2.1. Establishment of the RAW-GFP Cell Line and MNPs Uptake by the Obtained Cells
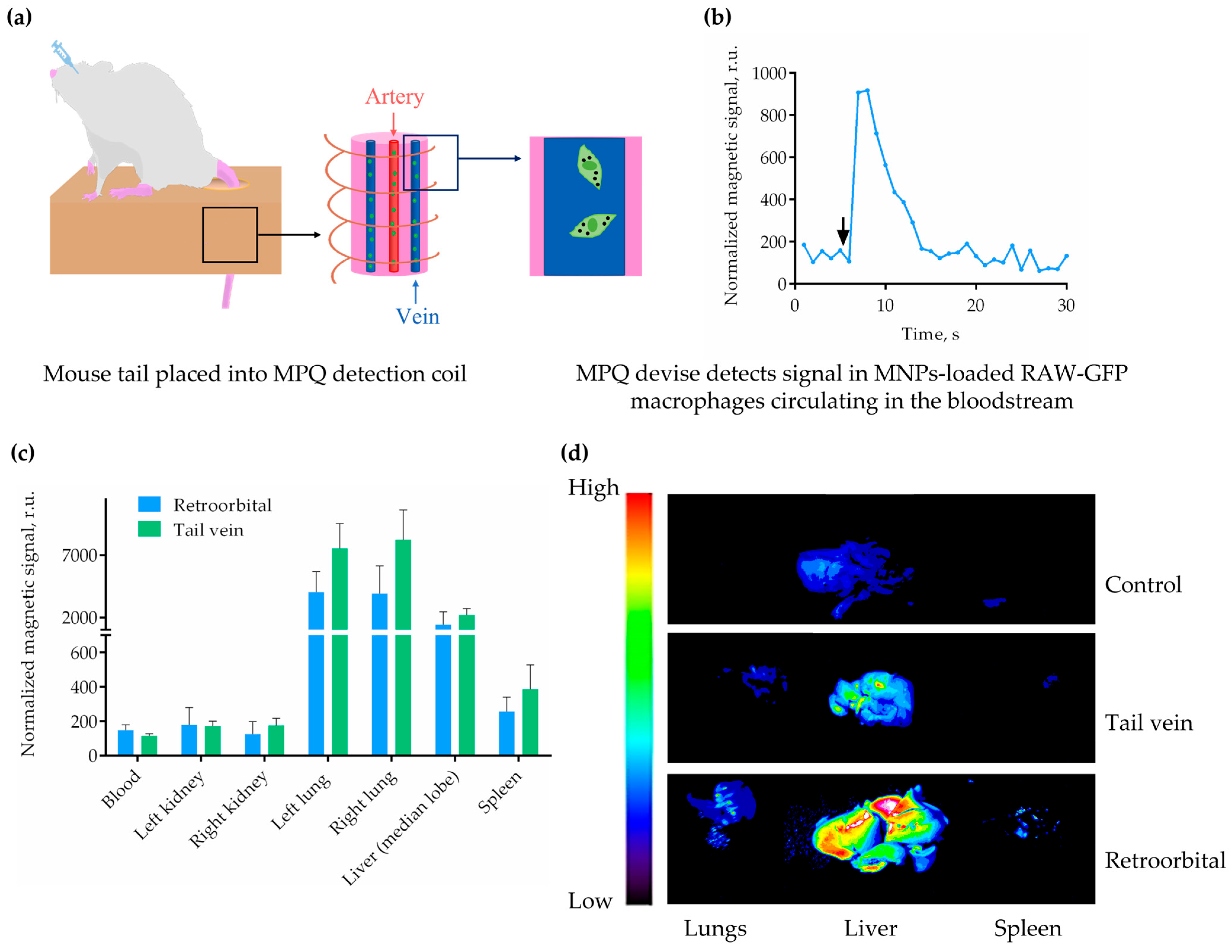
2.2. Multimodal Detection of MNP-Labeled Macrophages In Vivo and Ex Vivo
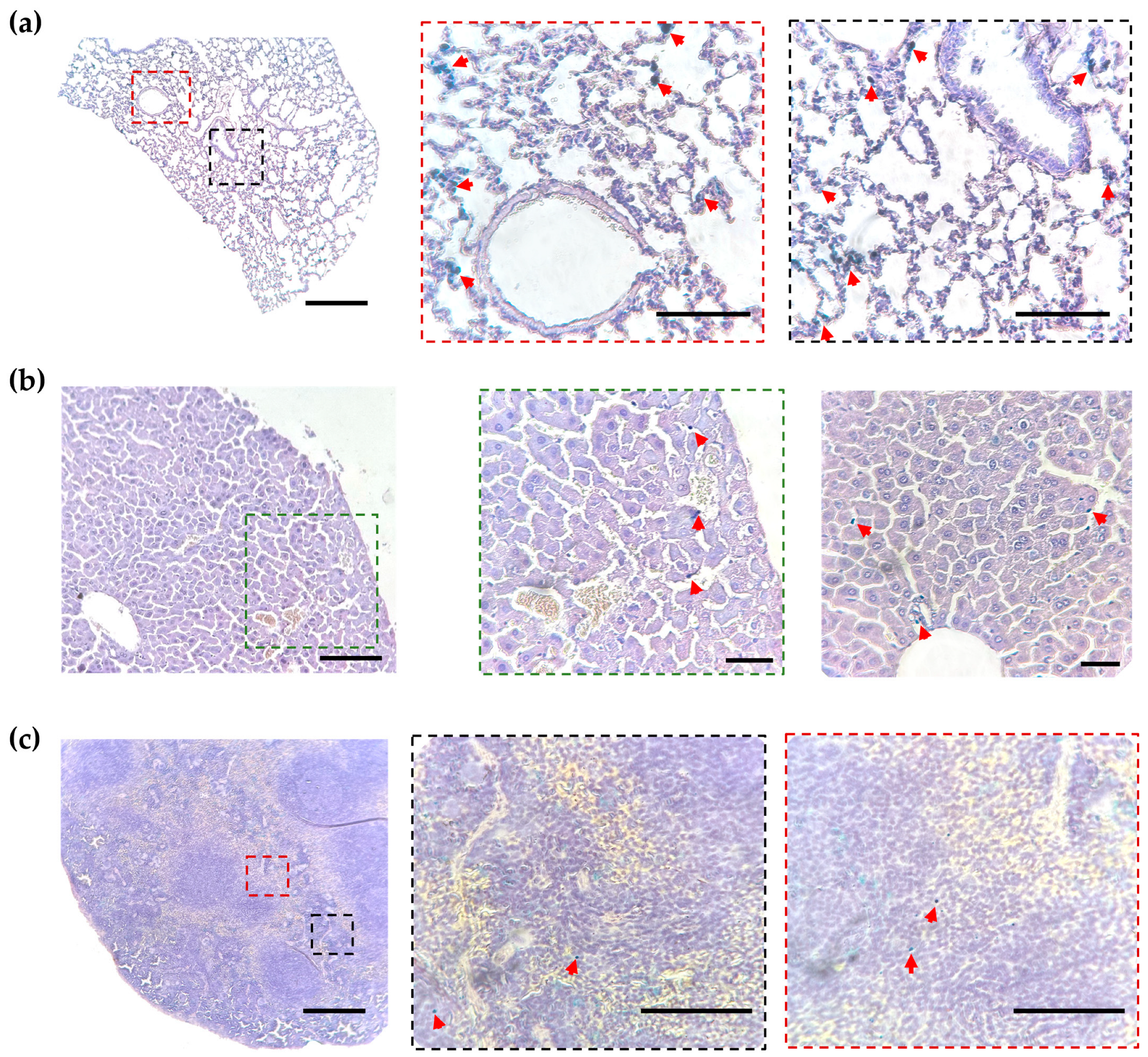
2.3. Histological Analysis of MNP-Loaded Cell Distribution
3. Materials and Methods
3.1. Materials
3.2. Cell Lines and Culture
3.3. Lentiviral Vector Construction and the Generation of GFP-Expressing RAW 264.7 Cells
3.4. Fluorescent Microscopy
3.5. Flow Cytometry
3.6. Cytotoxicity Assay
3.7. Prussian Blue Staining of Cells and Tissue Sections
3.8. Inductively Coupled Plasma Mass Spectrometry (ICP-MS)
3.9. Magnetic Particle Quantification (MPQ)
3.10. Animals
3.11. Histological Study
3.12. Optical Tomography
3.13. Laser Scanning Confocal Microscopy
3.14. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shaim, H.; Shanley, M.; Basar, R.; Daher, M.; Gumin, J.; Zamler, D.B.; Uprety, N.; Wang, F.; Huang, Y.; Gabrusiewicz, K.; et al. Targeting the Alphav Integrin/TGF-Beta Axis Improves Natural Killer Cell Function Against Glioblastoma Stem Cells. J. Clin. Investig. 2021, 131, 142116. [Google Scholar] [CrossRef] [PubMed]
- Albelda, S.M. CAR T Cell Therapy for Patients with Solid Tumours: Key Lessons to Learn and Unlearn. Nat. Rev. Clin. Oncol. 2024, 21, 47–66. [Google Scholar] [CrossRef]
- Zhang, J.; Hu, Y.; Yang, J.; Li, W.; Zhang, M.; Wang, Q.; Zhang, L.; Wei, G.; Tian, Y.; Zhao, K.; et al. Non-Viral, Specifically Targeted CAR-T Cells Achieve High Safety and Efficacy in B-NHL. Nature 2022, 609, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Gabashvili, A.N.; Vasiukova, A.A.; Rakitina, A.S.; Garanina, A.S. The Issue on Dualistic Role of Neutrophils in Carcinogenesis and Their Possible Use for Treatment of Malignant Neoplasms. Biochemistry 2025, 90, 303–320. [Google Scholar] [CrossRef]
- Cha, Y.; Park, T.Y.; Leblanc, P.; Kim, K.S. Current Status and Future Perspectives on Stem Cell-Based Therapies for Parkinson’s Disease. J. Mov. Disord. 2023, 16, 22–41. [Google Scholar] [CrossRef]
- Baloh, R.H.; Johnson, J.P.; Avalos, P.; Allred, P.; Svendsen, S.; Gowing, G.; Roxas, K.; Wu, A.; Donahue, B.; Osborne, S.; et al. Transplantation of Human Neural Progenitor Cells Secreting GDNF Into the Spinal Cord of Patients with ALS: A Phase 1/2a Trial. Nat. Med. 2022, 28, 1813–1822. [Google Scholar] [CrossRef]
- Revah, O.; Gore, F.; Kelley, K.W.; Andersen, J.; Sakai, N.; Chen, X.; Li, M.Y.; Birey, F.; Yang, X.; Saw, N.L.; et al. Maturation and Circuit Integration of Transplanted Human Cortical Organoids. Nature 2022, 610, 319–326. [Google Scholar] [CrossRef]
- Sung, P.H.; Chiang, H.J.; Li, Y.C.; Chiang, J.Y.; Chu, C.H.; Shao, P.L.; Lee, F.Y.; Lee, M.S.; Yip, H.K. Baseline Factors Identified for the Prediction of Good Responders in Patients with end-Stage Diffuse Coronary Artery Disease Undergoing Intracoronary CD34+ Cell Therapy. Stem Cell Res. Ther. 2020, 11, 324. [Google Scholar] [CrossRef] [PubMed]
- Makkar, R.R.; Kereiakes, D.J.; Aguirre, F.; Kowalchuk, G.; Chakravarty, T.; Malliaras, K.; Francis, G.S.; Povsic, T.J.; Schatz, R.; Traverse, J.H.; et al. Intracoronary ALLogeneic Heart Stem Cells to Achieve Myocardial Regeneration (ALLSTAR): A Randomized, Placebo-Controlled, Double-Blinded Trial. Eur. Heart J. 2020, 41, 3451–3458. [Google Scholar] [CrossRef] [PubMed]
- Bolli, R.; Mitrani, R.D.; Hare, J.M.; Pepine, C.J.; Perin, E.C.; Willerson, J.T.; Traverse, J.H.; Henry, T.D.; Yang, P.C.; Murphy, M.P.; et al. A Phase II Study of Autologous Mesenchymal Stromal Cells and C-Kit Positive Cardiac Cells, Alone or in Combination, in Patients with Ischaemic Heart Failure: The CCTRN CONCERT-HF Trial. Eur. J. Heart Fail. 2021, 23, 661–674. [Google Scholar] [CrossRef]
- Huang, Y.; Guan, Z.; Dai, X.; Shen, Y.; Wei, Q.; Ren, L.; Jiang, J.; Xiao, Z.; Jiang, Y.; Liu, D.; et al. Engineered Macrophages as Near-Infrared Light Activated Drug Vectors for Chemo-Photodynamic Therapy of Primary and Bone Metastatic Breast Cancer. Nat. Commun. 2021, 12, 4310. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, L.; Liu, Y.; Tang, L.; He, J.; Sun, X.; Younis, M.H.; Cui, D.; Xiao, H.; Gao, D.; et al. Engineering CpG-ASO-Pt-Loaded Macrophages (CAP@M) for Synergistic Chemo-/Gene-/Immuno-Therapy. Adv. Healthc. Mater. 2022, 11, 2201178. [Google Scholar] [CrossRef] [PubMed]
- Taciak, B.; Bialasek, M.; Kubiak, M.; Marszalek, I.; Gorczak, M.; Osadchuk, O.; Kurpiel, D.; Strzemecki, D.; Barwik, K.; Skorzynski, M.; et al. Harnessing Macrophage-Drug Conjugates for Allogeneic Cell-Based Therapy of Solid Tumors via the TRAIN Mechanism. Nat. Commun. 2025, 16, 1327. [Google Scholar] [CrossRef] [PubMed]
- Lendeckel, U.; Venz, S.; Wolke, C. Macrophages: Shapes and Functions. Chem. Texts 2022, 8, 12. [Google Scholar] [CrossRef]
- Christofides, A.; Strauss, L.; Yeo, A.; Cao, C.; Charest, A.; Boussiotis, V.A. The complex role of tumor-infiltrating macrophages. Nat. Immunol. 2022, 23, 1148–1156. [Google Scholar] [CrossRef] [PubMed]
- Guan, F.; Wang, R.; Yi, Z.; Luo, P.; Liu, W.; Xie, Y.; Liu, Z.; Xia, Z.; Zhang, H.; Cheng, Q. Tissue Macrophages: Origin, Heterogenity, Biological Functions, Diseases and Therapeutic Targets. Signal Transduct. Target Ther. 2025, 10, 93. [Google Scholar] [CrossRef]
- Sapach, A.Y.; Sindeeva, O.A.; Nesterchuk, M.V.; Tsitrina, A.A.; Mayorova, O.A.; Prikhozhdenko, E.S.; Verkhovskii, R.A.; Mikaelyan, A.S.; Kotelevtsev, Y.V.; Sukhorukov, G.B. Macrophage In Vitro and In Vivo Tracking via Anchored Microcapsules. ACS Appl. Mater. Interfaces 2022, 14, 51579–51592. [Google Scholar] [CrossRef]
- Gabashvili, A.N.; Chmelyuk, N.S.; Sarkisova, V.A.; Melnikov, P.A.; Semkina, A.S.; Nikitin, A.A.; Abakumov, M.A. Myxococcus xanthus Encapsulin as a Promising Platform for Intracellular Protein Delivery. Int. J. Mol. Sci. 2022, 23, 15591. [Google Scholar] [CrossRef]
- Gabashvili, A.N.; Alexandrushkina, N.A.; Mochalova, E.N.; Goliusova, D.V.; Sapozhnikova, E.N.; Makarevich, P.I.; Nikitin, P.I. Internalization of Transferrin-Tagged Myxococcus xanthus Encapsulins into Mesenchymal Stem Cells. Exp. Biol. Med. 2024, 249, 10055. [Google Scholar] [CrossRef]
- Nichols, J.M.; Crelli, C.V.; Liu, L.; Pham, H.V.; Janjic, J.M.; Shepherd, A.J. Tracking Macrophages in Diabetic Neuropathy with Two-Color Nanoemulsions for Near-Infrared Fluorescent Imaging and Microscopy. J. Neuroinflamm. 2021, 18, 299. [Google Scholar] [CrossRef]
- Tang, S.; Guo, Y.; Yang, Y.; Li, Y.; Gao, Y.; Zhang, C.; Xiong, L. High Resolution Tracking of Macrophage Cells in Deep Organs and Lymphatics Using Fluorescent Polymer Dots. RSC Adv. 2019, 9, 10966–10975. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.P.; Nguyen, L.N.M.; Ouyang, B.; MacMillan, P.; Ngai, J.; Kingston, B.R.; Mladjenovic, S.M.; Chan, W.C.W. Macrophages Actively Transport Nanoparticles in Tumors After Extravasation. ACS Nano 2022, 16, 6080–6092. [Google Scholar] [CrossRef] [PubMed]
- Chu, T.; Zheng, X.; Manshian, B.B.; Soenen, S.J. Optical Imaging in Biomedical Research: Guidelines and Practical Insights. Health Nanotechnol. 2025, 1, 4. [Google Scholar] [CrossRef]
- Miller, D.A.; Xu, Y.; Highland, R.; Nguyen, V.T.; Brown, W.J.; Hong, G.; Yao, J.; Wax, A. Enhanced Penetration Depth in Optical Coherence Tomography and Photoacoustic Microscopy In Vivo Enabled by Absorbing Dye Molecules. Optica 2025, 12, 24–30. [Google Scholar] [CrossRef]
- Zhou, Y.; Que, K.T.; Tang, H.M.; Zhang, P.; Fu, Q.M.; Liu, Z.J. Anti-CD206 Antibody-Conjugated Fe(3)O(4)-Based PLGA Nanoparticles Selectively Promote Tumor-Associated Macrophages to Polarize to the Pro-Inflammatory Subtype. Oncol. Lett. 2020, 20, 298. [Google Scholar] [CrossRef] [PubMed]
- Santana, J.G.; Petukhova-Greenstein, A.; Gross, M.; Hyder, F.; Pekurovsky, V.; Gottwald, L.A.; Boustani, A.; Walsh, J.J.; Kucukkaya, A.S.; Malpani, R.; et al. MR Imaging-Based In Vivo Macrophage Imaging to Monitor Immune Response after Radiofrequency Ablation of the Liver. J. Vasc. Interv. Radiol. 2023, 34, 395–403. [Google Scholar] [CrossRef]
- Jeong, H.J.; Yoo, R.J.; Kim, J.K.; Kim, M.H.; Park, S.H.; Kim, H.; Lim, J.W.; Do, S.H.; Lee, K.C.; Lee, Y.J.; et al. Macrophage Cell Tracking PET Imaging Using Mesoporous Silica Nanoparticles via In Vivo Bioorthogonal F-18 Labeling. Biomaterials 2019, 199, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Toner, Y.C.; Prevot, G.; van Leent, M.M.T.; Munitz, J.; Oosterwijk, R.; Verschuur, A.V.D.; van Elsas, Y.; Peric, V.; Maas, R.J.F.; Ranzenigo, A.; et al. Macrophage PET Imaging in Mouse Models of Cardiovascular Disease and Cancer with an Apolipoprotein-Inspired Radiotracer. Npj Imaging 2024, 2, 12. [Google Scholar] [CrossRef]
- Varasteh, Z.; Braeuer, M.; Mohanta, S.; Steinsiek, A.L.; Habenicht, A.; Omidvari, N.; Topping, G.J.; Rischpler, C.; Weber, W.A.; Sager, H.B.; et al. In vivo Visualization of M2 Macrophages in the Myocardium After Myocardial Infarction (MI) Using (68) Ga-NOTA-Anti-MMR Nb: Targeting Mannose Receptor (MR, CD206) on M2 Macrophages. Front. Cardiovasc. Med. 2022, 9, 889963. [Google Scholar] [CrossRef]
- Chiou, A.E.; Hinckley, J.A.; Khaitan, R.; Varsano, N.; Wang, J.; Malarkey, H.F.t.; Hernandez, C.J.; Williams, R.M.; Estroff, L.A.; Weiner, S.; et al. Fluorescent Silica Nanoparticles to Label Metastatic Tumor Cells in Mineralized Bone Microenvironments. Small 2021, 17, 2001432. [Google Scholar] [CrossRef]
- Harizaj, A.; Descamps, B.; Mangodt, C.; Stremersch, S.; Stoppa, A.; Balcaen, L.; Brans, T.; De Rooster, H.; Devriendt, N.; Fraire, J.C.; et al. Cytosolic Delivery of Gadolinium via Photoporation Enables Improved In Vivo Magnetic Resonance Imaging of Cancer Cells. Biomater. Sci. 2021, 9, 4005–4018. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, J.; Zhou, N.; Fang, Q.; Cai, H.; Du, Z.; An, R.; Liu, D.; Chen, X.; Wang, X.; et al. Protozoan-Derived Cytokine-Transgenic Macrophages Reverse Hepatic Fibrosis. Adv. Sci. 2024, 11, 2308750. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.U.; Kim, Y.; Lee, D.Y.; Park, J.S.; Jeun, M.; Lee, H.K.; Park, C.H. Lentivirus-Based Production of Human Chimeric Antigen Receptor Macrophages from Peripheral Blood. Biomark. Res. 2025, 13, 1. [Google Scholar] [CrossRef]
- Klimak, M.; Cimino, A.; Lenz, K.L.; Springer, L.E.; Collins, K.H.; Harasymowicz, N.S.; Xu, N.; Pham, C.T.N.; Guilak, F. Engineered Self-Regulating Macrophages for Targeted Anti-Inflammatory Drug Delivery. Arthritis Res. Ther. 2024, 26, 190. [Google Scholar] [CrossRef]
- Zelepukin, I.V.; Yaremenko, A.V.; Ivanov, I.N.; Yuryev, M.V.; Cherkasov, V.R.; Deyev, S.M.; Nikitin, P.I.; Nikitin, M.P. Long-Term Fate of Magnetic Particles in Mice: A Comprehensive Study. ACS Nano 2021, 15, 11341–11357. [Google Scholar] [CrossRef]
- Yaremenko, A.V.; Zelepukin, I.V.; Ivanov, I.N.; Melikov, R.O.; Pechnikova, N.A.; Dzhalilova, D.S.; Mirkasymov, A.B.; Bragina, V.A.; Nikitin, M.P.; Deyev, S.M.; et al. Influence of Magnetic Nanoparticle Biotransformation on Contrasting Efficiency and Iron Metabolism. J. Nanobiotechnol. 2022, 20, 535. [Google Scholar] [CrossRef]
- Zelepukin, I.V.; Yaremenko, A.V.; Yuryev, M.V.; Mirkasymov, A.B.; Sokolov, I.L.; Deyev, S.M.; Nikitin, P.I.; Nikitin, M.P. Fast Processes of Nanoparticle Blood Clearance: Comprehensive Study. J. Control. Release 2020, 326, 181–191. [Google Scholar] [CrossRef]
- Applegate, C.C.; Nelappana, M.B.; Cui, Y.; Okoro, G.; Nielsen, E.A.; Dovalovsky, N.P.; Smith, A.M.; Dobrucki, I.T.; Dobrucki, L.W. Biodistribution of PET Radiotracers in Tumor-Bearing TRAMP Mice Administered by Retroorbital or Jugular Vein Injections. Sci. Rep. 2024, 14, 20241. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; LoBue, A.; Heuser, S.K.; Li, J.; Engelhardt, E.; Papapetropoulos, A.; Patel, H.H.; Lilley, E.; Ferdinandy, P.; Schulz, R.; et al. Best Practices for Blood Collection and Anaesthesia in Mice: Selection, Application and Reporting. Br. J. Pharmacol. 2025, 182, 2337–2353. [Google Scholar] [CrossRef]
- Welsher, K.; Sherlock, S.P.; Dai, H. Deep-Tissue Anatomical Imaging of Mice Using Carbon Nanotube Fluorophores in the Second Near-Infrared Window. Proc. Natl. Acad. Sci. USA 2011, 108, 8943–8948. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Zhou, Y.Q.; Blaser, M.C.; Steinman, D.A.; Simmons, C.A. Ultrasound Image Velocimetry for High Spatiotemporal Resolution Blood Flow Velocity Field Mapping in Mice. Ultrasound Med. Biol. 2025, 51, 841–851. [Google Scholar] [CrossRef]
- Bailly, A.L.; Correard, F.; Popov, A.; Tselikov, G.; Chaspoul, F.; Appay, R.; Al-Kattan, A.; Kabashin, A.V.; Braguer, D.; Esteve, M.A. In Vivo Evaluation of Safety, Biodistribution and Pharmacokinetics of Laser-Synthesized Gold Nanoparticles. Sci. Rep. 2019, 9, 12890. [Google Scholar] [CrossRef]
- Huang, Y.; Hao, X.; Lin, Z.; Li, L.; Jiang, H.; Zhang, H.; Geng, X.; Zhu, H.; Wen, H. Bio-Distribution and Toxicity Potential of Human Umbilical Cord Mesenchymal Stem Cells in Cynomolgus Monkeys. Sci. Rep. 2024, 14, 12251. [Google Scholar] [CrossRef]
- Nikitin, M.P.; Orlov, A.V.; Sokolov, I.L.; Minakov, A.A.; Nikitin, P.I.; Ding, J.; Bader, S.D.; Rozhkova, E.A.; Novosad, V. Ultrasensitive Detection Enabled by Nonlinear Magnetization of Nanomagnetic Labels. Nanoscale 2018, 10, 11642–11650. [Google Scholar] [CrossRef]
- da Conceicao Mendonca, J.; Lumsdaine, S.; Burcham, L.R. Comparison of Lateral Tail Vein and Retro-Orbital Venous Sinus as Routes of Inoculation to Study Group B Streptococcal Systemic Infection. Microbiol. Spectr. 2025, 13, 0210424. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Takimoto, H.R.; Hashimoto, K.; Ishii, Y.; Sasaki, N. Effectively Simplified Adriamycin-Induced Chronic Kidney Disease Mouse Model: Retro-Orbital Vein Injection Versus Tail-Vein Injection. Animal Model. Exp. Med. 2025, 8, 568–572. [Google Scholar] [CrossRef] [PubMed]
- Garcia de Jalon, E.; Kleinmanns, K.; Fosse, V.; Davidson, B.; Bjorge, L.; Haug, B.E.; McCormack, E. Comparison of Five Near-Infrared Fluorescent Folate Conjugates in an Ovarian Cancer Model. Mol. Imaging Biol. 2023, 25, 144–155. [Google Scholar] [CrossRef]
- Nikitin, M.P.; Zelepukin, I.V.; Shipunova, V.O.; Sokolov, I.L.; Deyev, S.M.; Nikitin, P.I. Enhancement of the Blood-Circulation Time and Performance of Nanomedicines via the Forced Clearance of Erythrocytes. Nat. Biomed. Eng. 2020, 4, 717–731. [Google Scholar] [CrossRef] [PubMed]
- Weber, K.; Mock, U.; Petrowitz, B.; Bartsch, U.; Fehse, B. Lentiviral Gene Ontology (Lego) Vectors Equipped with Novel Drug-Selectable Fluorescent Proteins: New Building Blocks for Cell Marking and Multi-Gene Analysis. Gene Ther. 2010, 17, 511–520. [Google Scholar] [CrossRef]
- Nikitin, M.P.; Vetoshko, P.M.; Brusentsov, N.A.; Nikitin, P.I. Highly Sensitive Room-Temperature Method of Non-Invasive In Vivo Detection of Magnetic Nanoparticles. J. Magn. Magn Mater. 2009, 321, 1658–1661. [Google Scholar] [CrossRef]
- Nikitin, P.I.; Vetoshko, P.M.; Ksenevich, T.I. New Type of Biosensor Based on Magnetic Nanoparticle Detection. J. Magn. Magn Mater. 2007, 311, 445–449. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gabashvili, A.N.; Znoyko, S.L.; Ryabova, A.V.; Mochalova, E.N.; Griaznova, O.Y.; Tortunova, T.A.; Sheveleva, O.N.; Butorina, N.N.; Kuziaeva, V.I.; Lyadova, I.V.; et al. Multimodal Detection of Magnetically and Fluorescently Dual-Labeled Murine Macrophages After Intravenous Administration. Molecules 2025, 30, 3726. https://doi.org/10.3390/molecules30183726
Gabashvili AN, Znoyko SL, Ryabova AV, Mochalova EN, Griaznova OY, Tortunova TA, Sheveleva ON, Butorina NN, Kuziaeva VI, Lyadova IV, et al. Multimodal Detection of Magnetically and Fluorescently Dual-Labeled Murine Macrophages After Intravenous Administration. Molecules. 2025; 30(18):3726. https://doi.org/10.3390/molecules30183726
Chicago/Turabian StyleGabashvili, Anna N., Sergey L. Znoyko, Anastasia V. Ryabova, Elizaveta N. Mochalova, Olga Yu. Griaznova, Tatiana A. Tortunova, Olga N. Sheveleva, Nina N. Butorina, Valeriia I. Kuziaeva, Irina V. Lyadova, and et al. 2025. "Multimodal Detection of Magnetically and Fluorescently Dual-Labeled Murine Macrophages After Intravenous Administration" Molecules 30, no. 18: 3726. https://doi.org/10.3390/molecules30183726
APA StyleGabashvili, A. N., Znoyko, S. L., Ryabova, A. V., Mochalova, E. N., Griaznova, O. Y., Tortunova, T. A., Sheveleva, O. N., Butorina, N. N., Kuziaeva, V. I., Lyadova, I. V., & Nikitin, P. I. (2025). Multimodal Detection of Magnetically and Fluorescently Dual-Labeled Murine Macrophages After Intravenous Administration. Molecules, 30(18), 3726. https://doi.org/10.3390/molecules30183726







