Effective Degradation of Venlafaxine via Biochar Activated Persulfate: Kinetics, Transformation Products, and Toxicity Assessment
Abstract
1. Introduction
2. Results
2.1. Physicochemical Characterization
2.2. Catalytic Results
2.2.1. Effect of Pyrolysis Temperature on the Catalytic Activity of PPBC
2.2.2. Factors Affecting the Performance of the PPBC/Persulfate System
2.2.3. Impact of Natural Water Constituents on the PPBC800/Persulfate System
2.3. Identification of Transformation Products
2.4. Toxicity Assessment of Transformation Products
2.5. Life Cycle Assessment
3. Materials and Methods
3.1. Chemical Reagents
3.2. Catalyst Preparation
3.3. Characterization Methods
3.4. Experimental Procedure
3.5. Analytical Methods
3.6. Toxicity Evaluation of VEN and Its TPs-ECOSAR
3.7. Life Cycle Analysis
3.7.1. Goal and Scope Definition
3.7.2. Life Cycle Inventory (LCI)
3.7.3. Life Cycle Impact Assessment (LCIA)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Andrews, J.M.; Ninan, P.T.; Nemeroff, C.B. Venlafaxine: A Novel Antidepressant That Has a Dual Mechanism of Action. Depression 1996, 4, 48–56. [Google Scholar] [CrossRef]
- Aboelwafa, A.A.; Basalious, E.B. Optimization and In Vivo Pharmacokinetic Study of a Novel Controlled Release Venlafaxine Hydrochloride Three-Layer Tablet. AAPS PharmSciTech 2010, 11, 1026–1037. [Google Scholar] [CrossRef][Green Version]
- Gill, D.; Hatcher, S. A Systematic Review of the Treatment of Depression with Antidepressant Drugs in Patients Who Also Have a Physical Illness. J. Psychosom. Res. 1999, 47, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Peano, A.; Calabrese, F.; Pechlivanidis, K.; Mimmo, R.; Politano, G.; Martella, M.; Gianino, M.M. International Trends in Antidepressant Consumption: A 10-Year Comparative Analysis (2010–2020). Psychiatr. Q. 2025, 96, 241–255. [Google Scholar] [CrossRef] [PubMed]
- Damiens, J.; Junna, L.; Tarkiainen, L.; Martikainen, P. Individual and Parental Housing Tenure and Mental Healthcare Use among Finnish Men and Women in Early Mid-Adulthood. SSM Ment. Health 2025, 7, 100444. [Google Scholar] [CrossRef]
- Metcalfe, C.D.; Chu, S.; Judt, C.; Li, H.; Oakes, K.D.; Servos, M.R.; Andrews, D.M. Antidepressants and Their Metabolites in Municipal Wastewater, and Downstream Exposure in an Urban Watershed. Environ. Toxicol. Chem. 2010, 29, 79–89. [Google Scholar] [CrossRef]
- Petromelidou, S.; Evgenidou, E.; Tziouvalekas, M.; Lambropoulou, D.A. Unravelling Psychoactive Substances and Their Metabolites and Transformation Products: High-Resolution Mass Spectrometry Approaches for Comprehensive Target and Suspect Screening in Wastewater. Sci. Total Environ. 2024, 931, 172867. [Google Scholar] [CrossRef]
- Golbaz, S.; Zamanzadeh, M.; Yaghmaeian, K.; Nabizadeh, R.; Rastkari, N.; Esfahani, H. Occurrence and Removal of Psychiatric Pharmaceuticals in the Tehran South Municipal Wastewater Treatment Plant. Environ. Sci. Pollut. Res. 2023, 30, 27041–27055. [Google Scholar] [CrossRef]
- Alygizakis, N.A.; Gago-Ferrero, P.; Borova, V.L.; Pavlidou, A.; Hatzianestis, I.; Thomaidis, N.S. Occurrence and Spatial Distribution of 158 Pharmaceuticals, Drugs of Abuse and Related Metabolites in Offshore Seawater. Sci. Total Environ. 2016, 541, 1097–1105. [Google Scholar] [CrossRef]
- Kosma, C.I.; Nannou, C.I.; Boti, V.I.; Albanis, T.A. Psychiatrics and Selected Metabolites in Hospital and Urban Wastewaters: Occurrence, Removal, Mass Loading, Seasonal Influence and Risk Assessment. Sci. Total Environ. 2019, 659, 1473–1483. [Google Scholar] [CrossRef]
- Puga, A.; Moreira, M.M.; Sanromán, M.A.; Pazos, M.M.; Delerue-Matos, C. Antidepressants and COVID-19: Increased Use, Occurrence in Water and Effects and Consequences on Aquatic Environment. A Review. Sci. Total Environ. 2024, 953, 175993. [Google Scholar] [CrossRef]
- Valcárcel, Y.; González Alonso, S.; Rodríguez-Gil, J.L.; Gil, A.; Catalá, M. Detection of Pharmaceutically Active Compounds in the Rivers and Tap Water of the Madrid Region (Spain) and Potential Ecotoxicological Risk. Chemosphere 2011, 84, 1336–1348. [Google Scholar] [CrossRef] [PubMed]
- Gros, M.; Rodríguez-Mozaz, S.; Barceló, D. Fast and Comprehensive Multi-Residue Analysis of a Broad Range of Human and Veterinary Pharmaceuticals and Some of Their Metabolites in Surface and Treated Waters by Ultra-High-Performance Liquid Chromatography Coupled to Quadrupole-Linear Ion Trap Tandem Mass Spectrometry. J. Chromatogr. A 2012, 1248, 104–121. [Google Scholar] [CrossRef]
- Sehonova, P.; Svobodova, Z.; Dolezelova, P.; Vosmerova, P.; Faggio, C. Effects of Waterborne Antidepressants on Non-Target Animals Living in the Aquatic Environment: A Review. Sci. Total Environ. 2018, 631–632, 789–794. [Google Scholar] [CrossRef]
- Chen, Q.; Gu, S.; Lan, Y.; Xu, J.; Lin, W.; Qin, Y.; Ren, Y. Study on the Developmental, Behavioral Toxicity, and Toxicological Mechanism of the Antidepressant Drug Venlafaxine and Its Active Metabolites in Zebrafish. Environ. Toxicol. Chem. 2025, 44, 552–562. [Google Scholar] [CrossRef]
- Paulis, M.G.; Hafez, E.M.; El-Tahawy, N.F.; Aly, M.K.M. Toxicological Assessment of Venlafaxine: Acute and Subchronic Toxicity Study in Rats. Int. J. Toxicol. 2018, 37, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhang, M.; Wang, Y. The Roles of Histone Modifications in Tumorigenesis and Associated Inhibitors in Cancer Therapy. J. Natl. Cancer Cent. 2022, 2, 277–290. [Google Scholar] [CrossRef]
- Rúa-Gómez, P.C.; Püttmann, W. Occurrence and Removal of Lidocaine, Tramadol, Venlafaxine, and Their Metabolites in German Wastewater Treatment Plants. Environ. Sci. Pollut. Res. 2012, 19, 689–699. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhang, J.; Hu, M.; Liu, X.; Sun, T.; Zhang, H. Antidepressants in Wastewater Treatment Plants: Occurrence, Transformation and Acute Toxicity Evaluation. Sci. Total Environ. 2023, 903, 166120. [Google Scholar] [CrossRef]
- Arvaniti, O.S.; Arvaniti, E.S.; Gyparakis, S.; Sabathianakis, I.; Karagiannis, E.; Pettas, E.; Gkotsis, G.; Nika, M.C.; Thomaidis, N.S.; Manios, T.; et al. Occurrence of Pharmaceuticals in the Wastewater of a Greek Hospital: Combining Consumption Data Collection and LC-QTOF-MS Analysis. Sci. Total Environ. 2023, 858, 160153. [Google Scholar] [CrossRef]
- Miklos, D.B.; Remy, C.; Jekel, M.; Linden, K.G.; Drewes, J.E.; Hübner, U. Evaluation of Advanced Oxidation Processes for Water and Wastewater Treatment—A Critical Review. Water Res. 2018, 139, 118–131. [Google Scholar] [CrossRef]
- Priyadarshini, M.; Das, I.; Ghangrekar, M.M.; Blaney, L. Advanced Oxidation Processes: Performance, Advantages, and Scale-up of Emerging Technologies. J. Environ. Manag. 2022, 316, 115295. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.L.; Xu, L.J. Advanced Oxidation Processes for Wastewater Treatment: Formation of Hydroxyl Radical and Application. Crit. Rev. Environ. Sci. Technol. 2012, 42, 251–325. [Google Scholar] [CrossRef]
- Dong, C.; Fang, W.; Yi, Q.; Zhang, J. A Comprehensive Review on Reactive Oxygen Species (ROS) in Advanced Oxidation Processes (AOPs). Chemosphere 2022, 308, 136205. [Google Scholar] [CrossRef]
- Arvaniti, O.S.; Ioannidi, A.A.; Mantzavinos, D.; Frontistis, Z. Heat-Activated Persulfate for the Degradation of Micropollutants in Water: A Comprehensive Review and Future Perspectives. J. Environ. Manag. 2022, 318, 115568. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Wang, B.; Gao, B.; Cheng, N.; Feng, Q.; Chen, M.; Wang, S. Degradation of Organic Pollutants from Water by Biochar-Assisted Advanced Oxidation Processes: Mechanisms and Applications. J. Hazard. Mater. 2023, 442, 130075. [Google Scholar] [CrossRef]
- Zhao, C.; Shao, B.; Yan, M.; Liu, Z.; Liang, Q.; He, Q.; Wu, T.; Liu, Y.; Pan, Y.; Huang, J.; et al. Activation of Peroxymonosulfate by Biochar-Based Catalysts and Applications in the Degradation of Organic Contaminants: A Review. Chem. Eng. J. 2021, 416, 128829. [Google Scholar] [CrossRef]
- Ioannidi, A.A.; Logginou, O.; Kouvelis, K.; Petala, A.; Antonopoulou, M.; Mantzavinos, D.; Frontistis, Z. Peroxydisulfate Activation by Biochar from Banana Peel Promoted with Copper Phosphide for Bisphenol S Degradation in Aqueous Media. Catalysts 2024, 14, 789. [Google Scholar] [CrossRef]
- Nidheesh, P.V.; Gopinath, A.; Ranjith, N.; Praveen Akre, A.; Sreedharan, V.; Suresh Kumar, M. Potential Role of Biochar in Advanced Oxidation Processes: A Sustainable Approach. Chem. Eng. J. 2021, 405, 126582. [Google Scholar] [CrossRef]
- Daimary, N.; Eldiehy, K.S.H.; Boruah, P.; Deka, D.; Bora, U.; Kakati, B.K. Potato Peels as a Sustainable Source for Biochar, Bio-Oil and a Green Heterogeneous Catalyst for Biodiesel Production. J. Environ. Chem. Eng. 2022, 10, 107108. [Google Scholar] [CrossRef]
- García-Galán, M.J.; Anfruns, A.; Gonzalez-Olmos, R.; Rodríguez-Mozaz, S.; Comas, J. UV/H2O2degradation of the Antidepressants Venlafaxine and O-Desmethylvenlafaxine: Elucidation of Their Transformation Pathway and Environmental Fate. J. Hazard. Mater. 2016, 311, 70–80. [Google Scholar] [CrossRef]
- Zhu, T.; Deng, J.; Zhu, S.; Cai, A.; Ye, C.; Ling, X.; Guo, H.; Wang, Q.; Li, X. Kinetic and Mechanism Insights into the Degradation of Venlafaxine by UV/Chlorine Process: A Modelling Study. Chem. Eng. J. 2022, 431, 133473. [Google Scholar] [CrossRef]
- Lambropoulou, D.; Evgenidou, E.; Saliverou, V.; Kosma, C.; Konstantinou, I. Degradation of Venlafaxine Using TiO2/UV Process: Kinetic Studies, RSM Optimization, Identification of Transformation Products and Toxicity Evaluation. J. Hazard. Mater. 2017, 323, 513–526. [Google Scholar] [CrossRef] [PubMed]
- Giannakis, S.; Samoili, S.; Rodríguez-Chueca, J. A Meta-Analysis of the Scientific Literature on (Photo)Fenton and Persulfate Advanced Oxidation Processes: Where Do We Stand and Where Are We Heading To? Curr. Opin. Green Sustain. Chem. 2021, 29, 100456. [Google Scholar] [CrossRef]
- Li, X.; Wang, Y.; Zhao, J.; Wang, H.; Wang, B.; Huang, J.; Deng, S.; Yu, G. Electro-Peroxone Treatment of the Antidepressant Venlafaxine: Operational Parameters and Mechanism. J. Hazard. Mater. 2015, 300, 298–306. [Google Scholar] [CrossRef]
- Huang, W.; Fu, B.; Fang, S.; Wang, F.; Shao, Q.; Du, W.; Fang, F.; Feng, Q.; Cao, J.; Luo, J. Insights into the Accelerated Venlafaxine Degradation by Cysteine-Assisted Fe2+/Persulfate: Key Influencing Factors, Mechanisms and Transformation Pathways with DFT Study. Sci. Total Environ. 2021, 793, 148555. [Google Scholar] [CrossRef]
- Cruz-Alcalde, A.; López-Vinent, N.; Ribeiro, R.S.; Giménez, J.; Sans, C.; Silva, A.M.T. Persulfate Activation by Reduced Graphene Oxide Membranes: Practical and Mechanistic Insights Concerning Organic Pollutants Abatement. Chem. Eng. J. 2022, 427, 130994. [Google Scholar] [CrossRef]
- Ribeiro, R.S.; Vieira, O.; Fernandes, R.; Roman, F.F.; Diaz de Tuesta, J.L.; Silva, A.M.T.; Gomes, H.T. Synthesis of Low-Density Polyethylene Derived Carbon Nanotubes for Activation of Persulfate and Degradation of Water Organic Micropollutants in Continuous Mode. J. Environ. Manag. 2022, 308, 114622. [Google Scholar] [CrossRef] [PubMed]
- Sbardella, L.; Velo Gala, I.; Comas, J.; Morera Carbonell, S.; Rodríguez-Roda, I.; Gernjak, W. Integrated Assessment of Sulfate-Based AOPs for Pharmaceutical Active Compound Removal from Wastewater. J. Clean. Prod. 2020, 260, 121014. [Google Scholar] [CrossRef]
- Fardi, Z.; Shahbeik, H.; Nosrati, M.; Motamedian, E.; Tabatabaei, M.; Aghbashlo, M. Waste-to-Energy: Co-Pyrolysis of Potato Peel and Macroalgae for Biofuels and Biochemicals. Environ. Res. 2024, 242, 117614. [Google Scholar] [CrossRef]
- Sakulkit, P.; Palamanit, A.; Dejchanchaiwong, R.; Reubroycharoen, P. Characteristics of Pyrolysis Products from Pyrolysis and Co-Pyrolysis of Rubber Wood and Oil Palm Trunk Biomass for Biofuel and Value-Added Applications. J. Environ. Chem. Eng. 2020, 8, 104561. [Google Scholar] [CrossRef]
- Oliveira, F.R.; Patel, A.K.; Jaisi, D.P.; Adhikari, S.; Lu, H.; Khanal, S.K. Environmental Application of Biochar: Current Status and Perspectives. Bioresour. Technol. 2017, 246, 110–122. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.-J.; Lu, J.-S.; Chang, J.-S. Pyrolysis Synergy of Municipal Solid Waste (MSW): A Review. Bioresour. Technol. 2020, 318, 123912. [Google Scholar] [CrossRef]
- Luo, Q.; Deng, Y.; Li, Y.; He, Q.; Wu, H.; Fang, X. Effects of Pyrolysis Temperatures on the Structural Properties of Straw Biochar and Its Adsorption of Tris-(1-Chloro-2-Propyl) Phosphate. Sci. Rep. 2024, 14, 25711. [Google Scholar] [CrossRef]
- Taksal, P.A.; Arasavilli, S.; Kanti Das, B.; Chowdhury, S.; Bhattacharya, J. Application of Persulfate-Assisted Graphitic Carbon, Derived through Pyrolysis of Waste Biomass, Driven by Visible Light, for the Removal of Tetracycline from Water: Mechanisms and Performance Influencing Parameters. J. Anal. Appl. Pyrolysis 2024, 177, 106310. [Google Scholar] [CrossRef]
- Liu, W.; Wang, S.; Zhao, Y.; Sun, C.; Xu, H.; Zhao, J. PVP-Induced Bi2S3/BiOCl Photocatalyst with Open Hollow Structures for the Removal of Ciprofloxacin under Visible-Light Irradiation. J. Alloys Compd. 2021, 861, 157995. [Google Scholar] [CrossRef]
- Tang, C.; Xu, C.; Zhong, G.; Cen, Z.; Ni, Z.; Yao, Z.; Fang, Y.; Qiu, R.; Zhang, S. Unveiling Activation Mechanism of Persulfate by Homologous Hemp-Derived Biochar Catalysts for Enhanced Tetracycline Wastewater Remediation. Bioresour. Technol. 2024, 400, 130684. [Google Scholar] [CrossRef]
- Yu, M.; Liu, Y.; Yang, X.; Hu, C.; Bao, M.; Yan, N.; Zheng, Y. PH-Dependent Generation of Active Species to Mediate Degradation Pathways of Binary Antibiotics in Persulfate-Based System. Chem. Eng. Sci. 2024, 299, 120514. [Google Scholar] [CrossRef]
- Wei, J.; Xiong, Z.; Ao, M.; Guo, Z.; Zhang, J.; Lai, B.; Song, Y. Selective Degradation of Sulfamethoxazole by N-Doped Iron-Based Carbon Activated Peroxymonosulfate: Collaboration of Singlet Oxygen and High-Valent Iron-Oxo Species. Sep. Purif. Technol. 2022, 297, 121379. [Google Scholar] [CrossRef]
- Liang, J.; Chen, K.; Duan, X.; Zhao, L.; Qiu, H.; Xu, X.; Cao, X. PH-Dependent Generation of Radical and Nonradical Species for Sulfamethoxazole Degradation in Different Carbon/Persulfate Systems. Water Res. 2022, 224, 119113. [Google Scholar] [CrossRef] [PubMed]
- Huo, X.; Zhou, P.; Zhang, J.; Liu, Y.; Cheng, X.; Liu, Y.; Li, W.; Zhang, Y. N, S-Doped Porous Carbons for Persulfate Activation to Remove Tetracycline: Nonradical Mechanism. J. Hazard. Mater. 2020, 391, 122055. [Google Scholar] [CrossRef]
- Zhu, K.; Shen, Y.; Hou, J.; Gao, J.; He, D.; Huang, J.; He, H.; Lei, L.; Chen, W. One-Step Synthesis of Nitrogen and Sulfur Co-Doped Mesoporous Graphite-like Carbon Nanosheets as a Bifunctional Material for Tetracycline Removal via Adsorption and Catalytic Degradation Processes: Performance and Mechanism. Chem. Eng. J. 2021, 412, 128521. [Google Scholar] [CrossRef]
- Li, D.; Feng, Z.; Zhou, B.; Chen, H.; Yuan, R. Impact of Water Matrices on Oxidation Effects and Mechanisms of Pharmaceuticals by Ultraviolet-Based Advanced Oxidation Technologies: A Review. Sci. Total Environ. 2022, 844, 157162. [Google Scholar] [CrossRef]
- Feng, Y.; Song, Q.; Lv, W.; Liu, G. Degradation of Ketoprofen by Sulfate Radical-Based Advanced Oxidation Processes: Kinetics, Mechanisms, and Effects of Natural Water Matrices. Chemosphere 2017, 189, 643–651. [Google Scholar] [CrossRef] [PubMed]
- Lado Ribeiro, A.R.; Moreira, N.F.F.; Li Puma, G.; Silva, A.M.T. Impact of Water Matrix on the Removal of Micropollutants by Advanced Oxidation Technologies. Chem. Eng. J. 2019, 363, 155–173. [Google Scholar] [CrossRef]
- Medina, E.; Paredes, C.; Bustamante, M.A.; Moral, R.; Moreno-Caselles, J. Relationships between Soil Physico-Chemical, Chemical and Biological Properties in a Soil Amended with Spent Mushroom Substrate. Geoderma 2012, 173–174, 152–161. [Google Scholar] [CrossRef]
- Rayaroth, M.P.; Boczkaj, G.; Aubry, O.; Aravind, U.K.; Aravindakumar, C.T. Advanced Oxidation Processes for Degradation of Water Pollutants—Ambivalent Impact of Carbonate Species: A Review. Water 2023, 15, 1615. [Google Scholar] [CrossRef]
- Canonica, S.; Kohn, T.; Mac, M.; Real, F.J.; Wirz, J.; von Gunten, U. Photosensitizer Method to Determine Rate Constants for the Reaction of Carbonate Radical with Organic Compounds. Environ. Sci. Technol. 2005, 39, 9182–9188. [Google Scholar] [CrossRef] [PubMed]
- Oyekunle, D.T.; Cai, J.; Gendy, E.A.; Chen, Z. Impact of Chloride Ions on Activated Persulfates Based Advanced Oxidation Process (AOPs): A Mini Review. Chemosphere 2021, 280, 130949. [Google Scholar] [CrossRef]
- Yuan, R.; Ramjaun, S.N.; Wang, Z.; Liu, J. Effects of Chloride Ion on Degradation of Acid Orange 7 by Sulfate Radical-Based Advanced Oxidation Process: Implications for Formation of Chlorinated Aromatic Compounds. J. Hazard. Mater. 2011, 196, 173–179. [Google Scholar] [CrossRef]
- Anipsitakis, G.P.; Dionysiou, D.D.; Gonzalez, M.A. Cobalt-Mediated Activation of Peroxymonosulfate and Sulfate Radical Attack on Phenolic Compounds. Implications of Chloride Ions. Environ. Sci. Technol. 2006, 40, 1000–1007. [Google Scholar] [CrossRef]
- Irikura, K.; Rojas-Mantilla, H.D.; Ayala-Durán, S.C.; de Souza, J.C.; Rossini, E.L.; Salazar-González, R.; Zanoni, M.V.B. Performance of Ti/TiO2-ZIF-67 Photoanode under LED Irradiation Applied to the Photoelectrodegradation of the Antidepressant Venlafaxine (VEN) in Municipal Wastewater Effluent. J. Environ. Manag. 2025, 373, 123460. [Google Scholar] [CrossRef]
- Osawa, R.A.; Barrocas, B.T.; Monteiro, O.C.; Conceição Oliveira, M.; Florêncio, M.H. Photocatalytic Degradation of Amitriptyline, Trazodone and Venlafaxine Using Modified Cobalt-Titanate Nanowires under UV–Vis Radiation: Transformation Products and in Silico Toxicity. Chem. Eng. J. 2019, 373, 1338–1347. [Google Scholar] [CrossRef]
- Zucker, I.; Mamane, H.; Riani, A.; Gozlan, I.; Avisar, D. Formation and Degradation of N-Oxide Venlafaxine during Ozonation and Biological Post-Treatment. Sci. Total Environ. 2018, 619–620, 578–586. [Google Scholar] [CrossRef] [PubMed]
- Zizzamia, A.R.; Pasquariello, V.; Lelario, F.; Tesoro, C.; Ciriello, R. Electrochemical Degradation of Venlafaxine on Platinum Electrodes: Identification of Transformation Products by LC-MS/MS and In Silico Ecotoxicity Assessment. Molecules 2025, 30, 1881. [Google Scholar] [CrossRef] [PubMed]
- Ayala-Durán, S.C.; Rojas-Mantilla, H.D.; Pérez-Moya, M.; Pupo Nogueira, R.F. Enhanced Degradation of Venlafaxine Induced by Fe2O3/CeO2 Heterostructures through the Thermal Transformation from Type I to Direct Z-Scheme in Heterogeneous Photo-Fenton. J. Environ. Chem. Eng. 2024, 12, 114401. [Google Scholar] [CrossRef]
- Liu, L.; Zhi, S.; Chen, R.; Yang, Y.; Luo, C.; Liang, P.; Liu, Y.; Zhu, G. Highly Selective and Rapid Sequential Degradation of Venlafaxine-Class Antidepressants Using a Novel BiOCl-Based Two-Dimensional Molecularly Imprinted Photocatalyst. Sep. Purif. Technol. 2025, 362, 131777. [Google Scholar] [CrossRef]
- Schymanski, E.L.; Jeon, J.; Gulde, R.; Fenner, K.; Ruff, M.; Singer, H.P.; Hollender, J. Identifying Small Molecules via High Resolution Mass Spectrometry: Communicating Confidence. Environ. Sci. Technol. 2014, 48, 2097–2098. [Google Scholar] [CrossRef]
- Kouvelis, K.; Ioannidi, A.A.; Petala, A.; Souliotis, M.; Frontistis, Z. Photocatalytic Degradation of Losartan with Bismuth Oxychloride: Batch and Pilot Scale Demonstration. Catalysts 2023, 13, 1175. [Google Scholar] [CrossRef]
- Kokka, A.; Ramantani, T.; Petala, A.; Panagiotopoulou, P. Effect of the Nature of the Support, Operating and Pretreatment Conditions on the Catalytic Performance of Supported Ni Catalysts for the Selective Methanation of CO. Catal. Today 2020, 355, 832–843. [Google Scholar] [CrossRef]
- Kuppireddy, S.; Varghese, A.M.; Al Araj, H.; Hart, P.; Ramantani, T.; Bampos, G.; Karanikolos, G.N. A Combined Experimental and Simulations Assessment of CO2 Capture and CO2/H2 Separation Performance of Aminosilane-Grafted MCM-41 and Pore-Expanded MCM-41. Microporous Mesoporous Mater. 2024, 377, 113220. [Google Scholar] [CrossRef]
- United Nations. Globally Harmonized System of Classification and Labelling of Chemicals (GHS), 9th ed.; UN: New York, NY, USA, 2021. [Google Scholar]
- ISO 14040:2006; Environmental Management—Life Cycle Assessment—Principles and Framework. International Organization for Standardization: Geneva, Switzerland, 2006.
- ISO 14044:2006; Environmental Management—Life Cycle Assessment—Requirements and guidelines. International Organization for Standardization: Geneva, Switzerland, 2006.
- Ioannidou, S.M.; Pateraki, C.; Ladakis, D.; Papapostolou, H.; Tsakona, M.; Vlysidis, A.; Kookos, I.K.; Koutinas, A. Sustainable Production of Bio-Based Chemicals and Polymers via Integrated Biomass Refining and Bioprocessing in a Circular Bioeconomy Context. Bioresour. Technol. 2020, 307, 123093. [Google Scholar] [CrossRef] [PubMed]
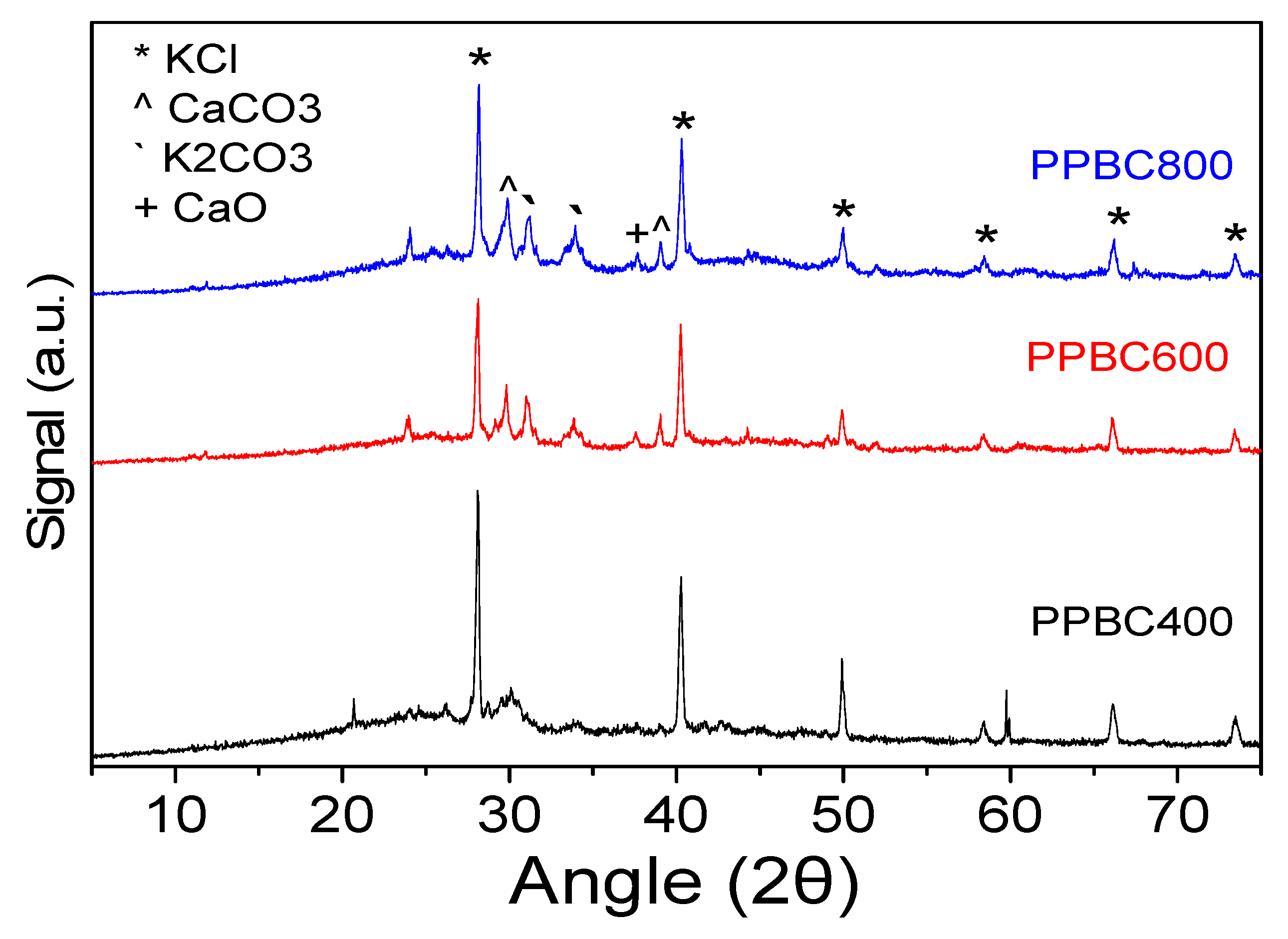

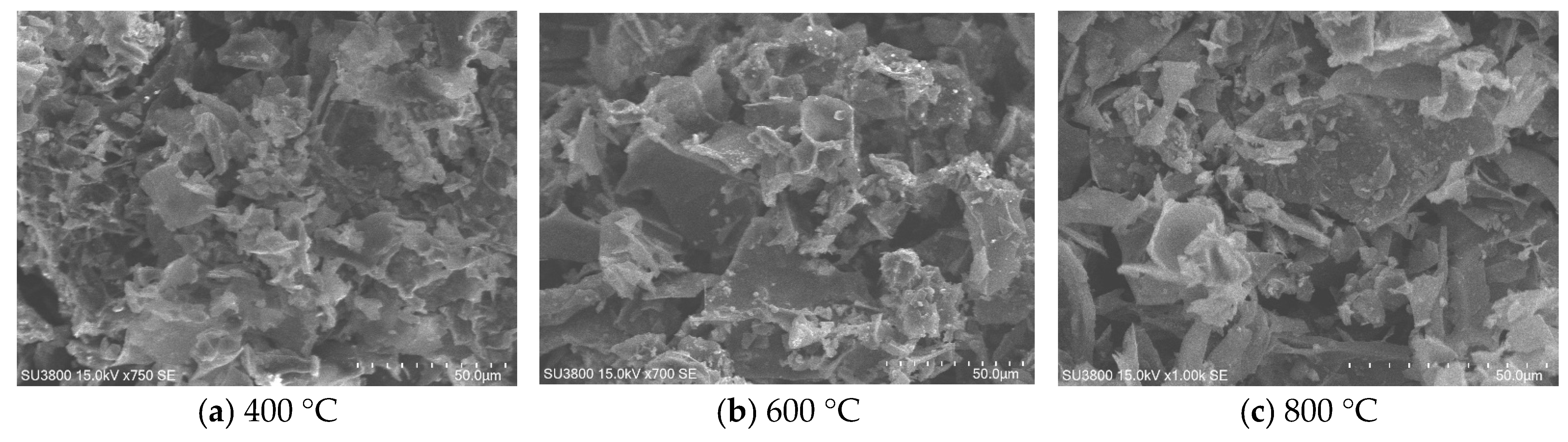
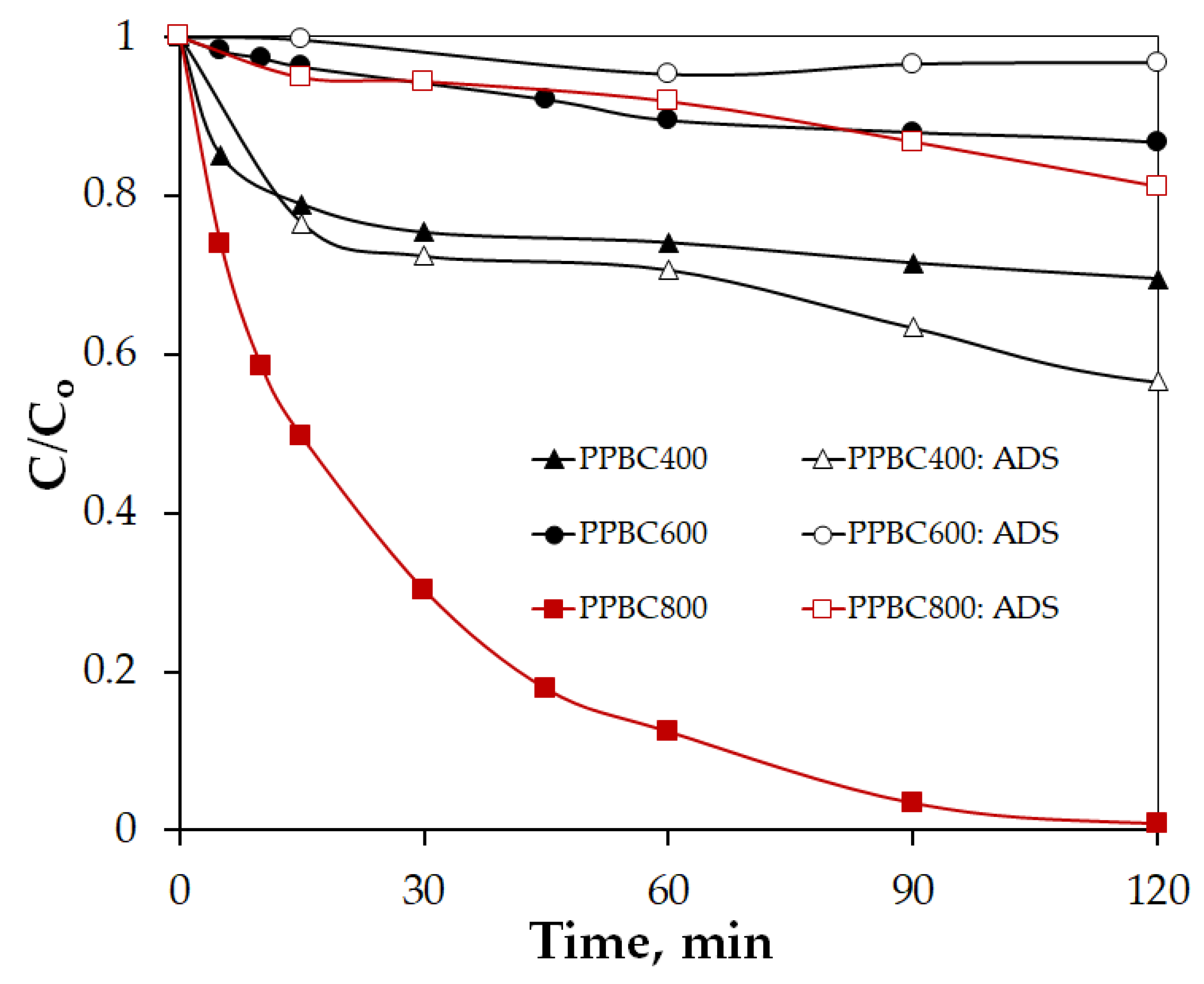

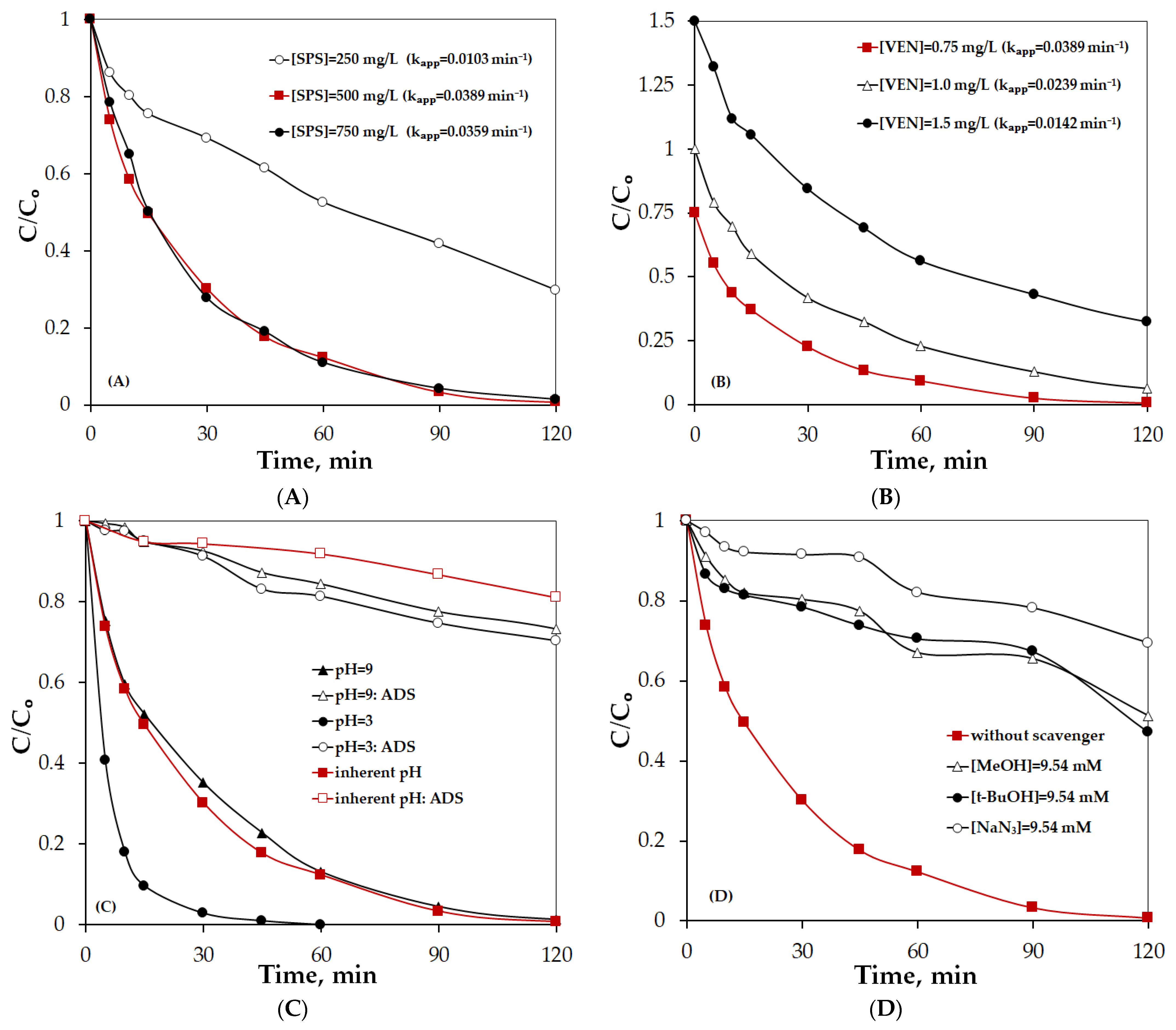
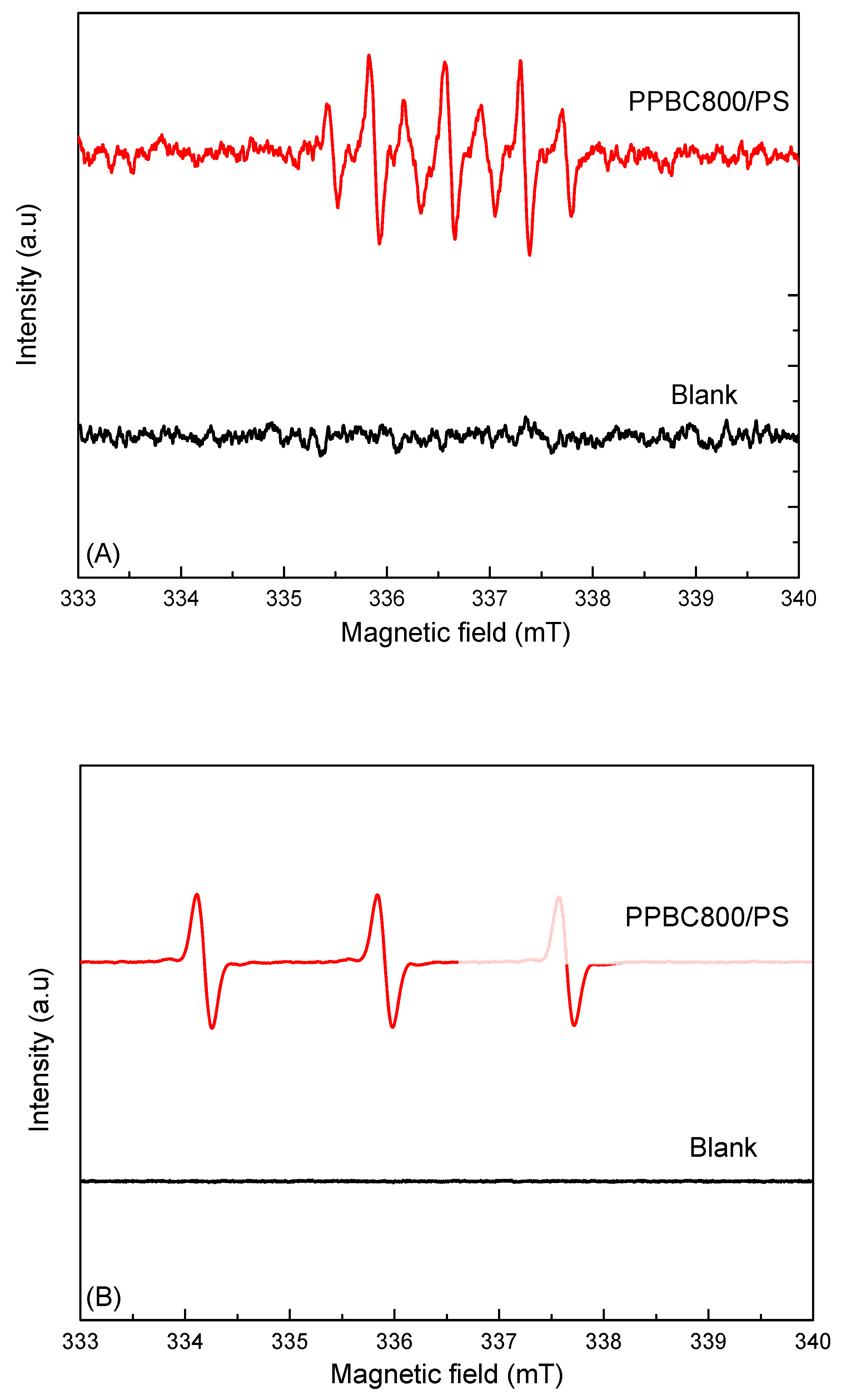
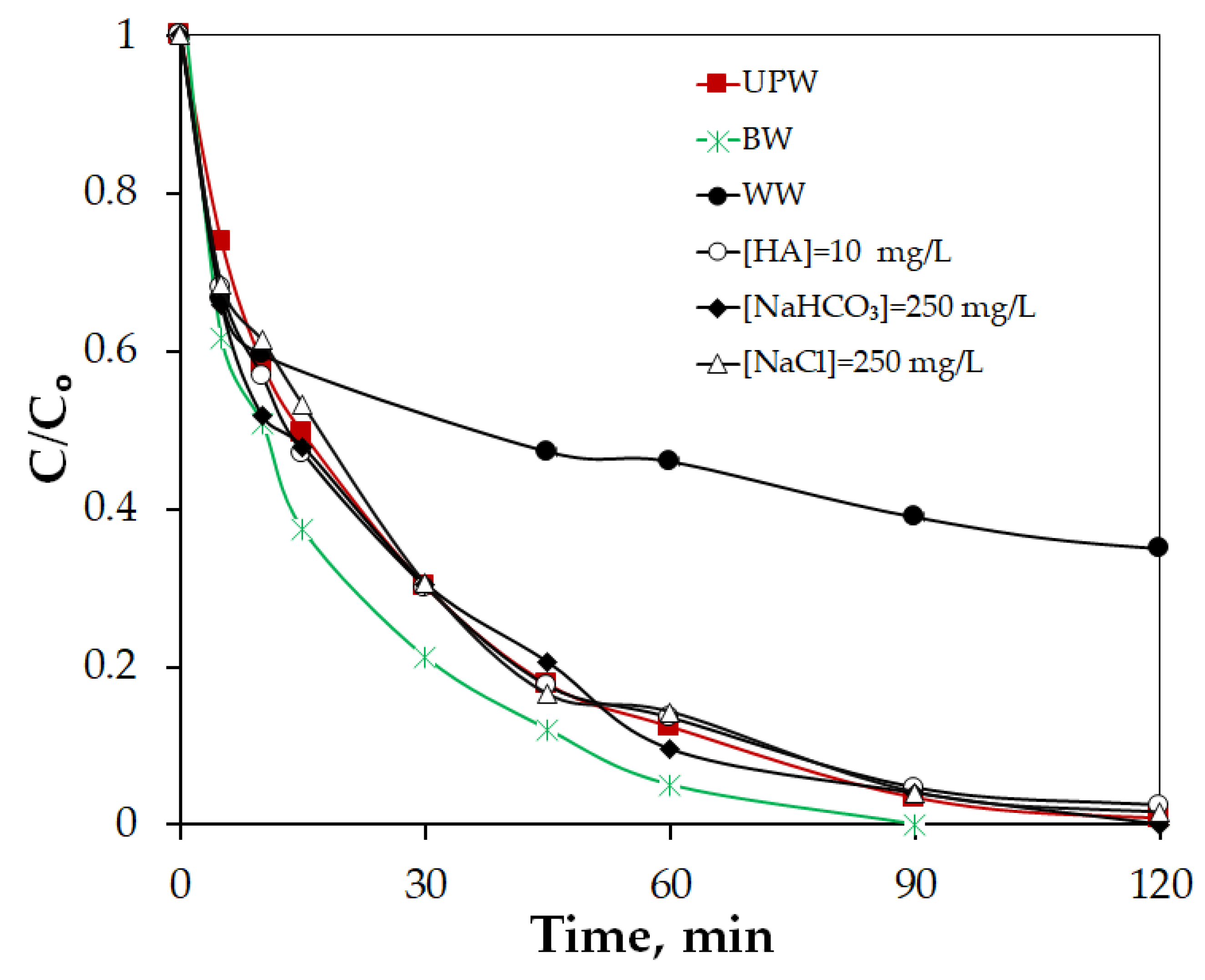
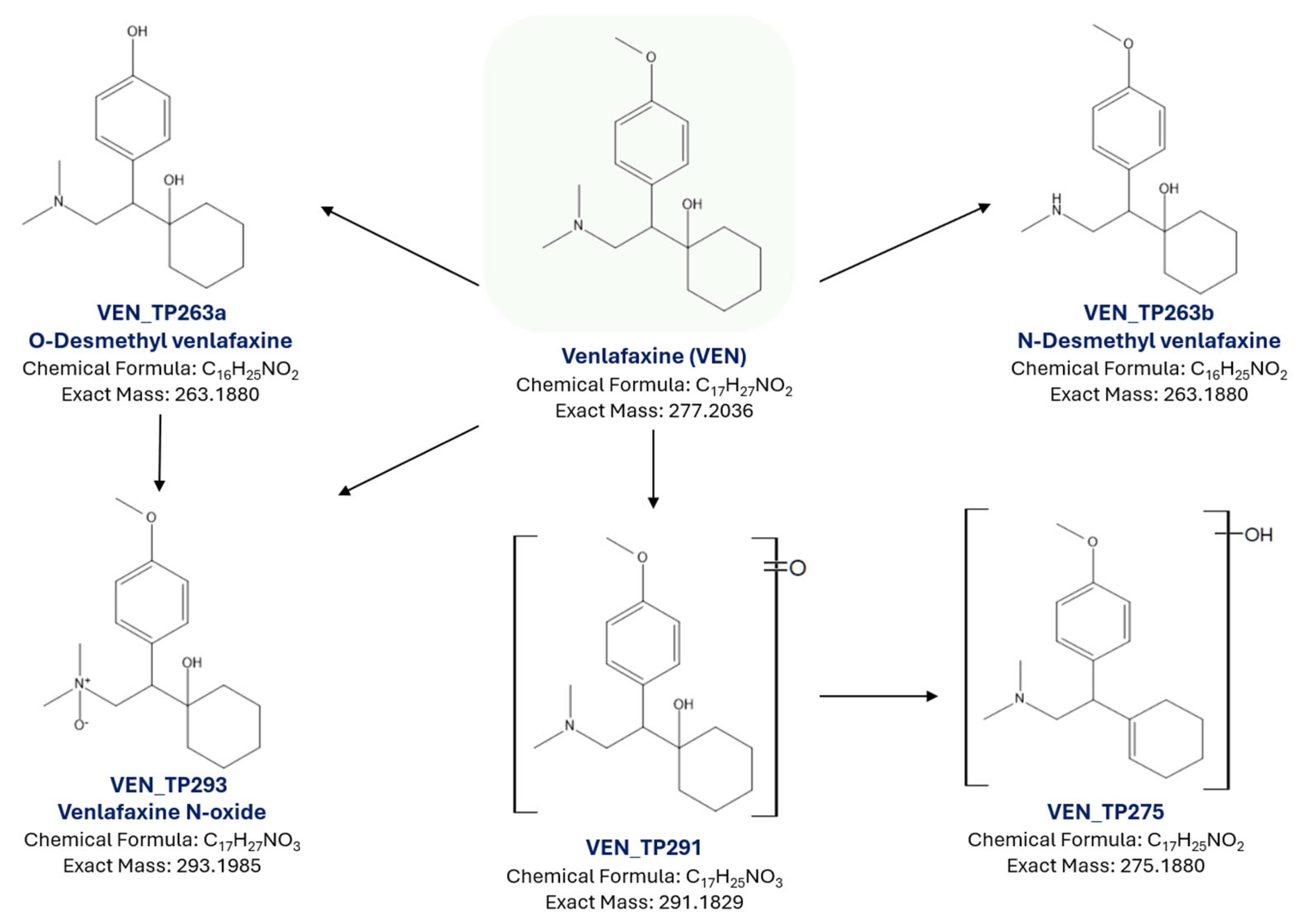
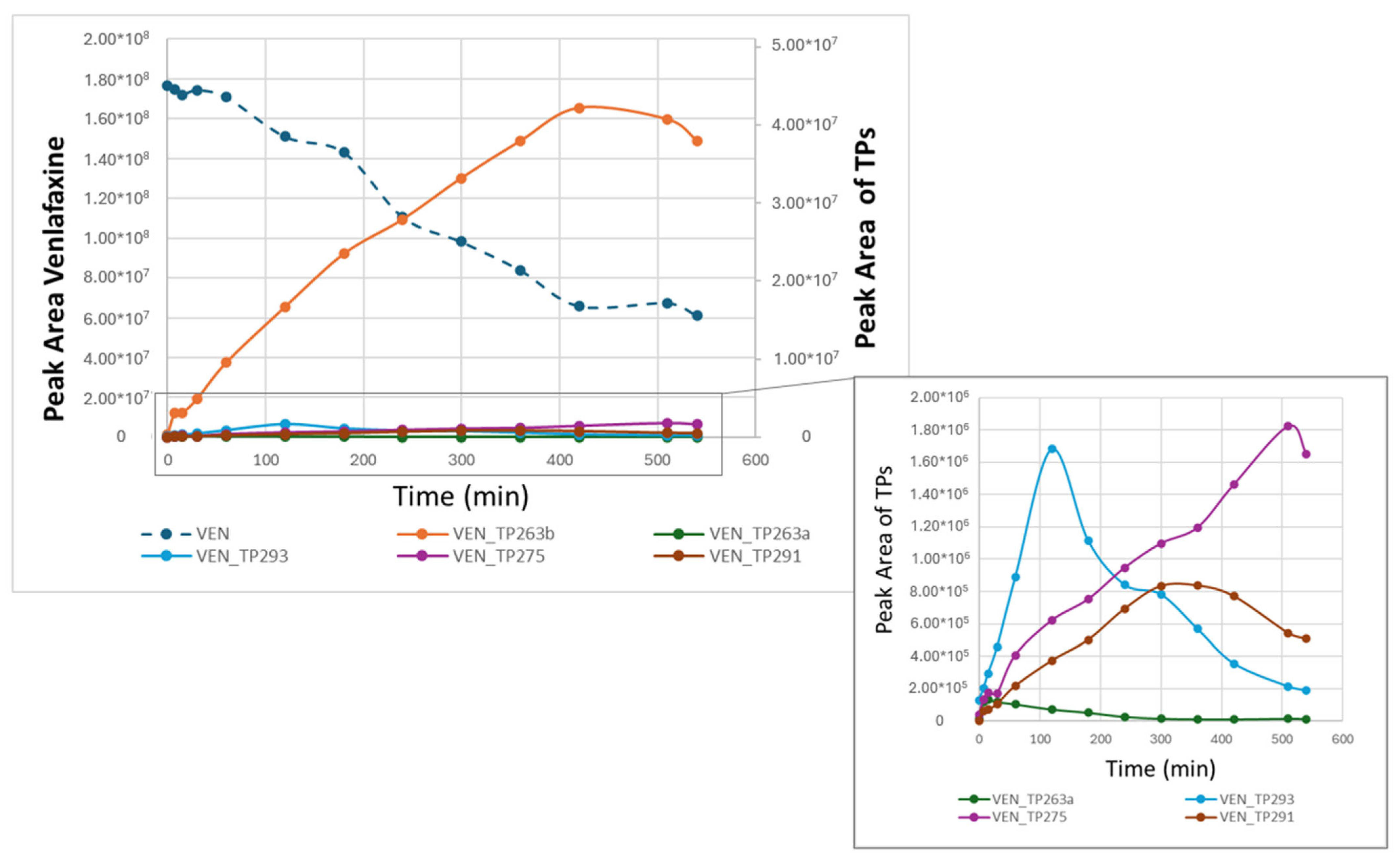

| Sample | Primary Crystallite Size (Å) |
|---|---|
| PPBC 400 | 41.6 |
| PPBC 600 | 41.2 |
| PPBC 800 | 36.3 |
| Sample | , min−1 | , min−1 |
|---|---|---|
| PPBC 400 | 0.0042 | 0.0029 |
| PPBC 600 | 0.0003 | 0.0012 |
| PPBC 800 | 0.0017 | 0.0389 |
| pH | , min−1 | , min−1 |
|---|---|---|
| 3 | 0.0032 | 0.1136 |
| Inherent pH (7) | 0.0017 | 0.0389 |
| 9 | 0.0028 | 0.0352 |
| pH | , min−1 | R2 |
|---|---|---|
| Without scavenger | 0.0389 | 0.99 |
| MeOH | 0.0058 | 0.98 |
| t-BuOH | 0.0063 | 0.98 |
| NaN3 | 0.0030 | 0.99 |
| Compound | Ion Mode | Retention Time (min) | Principal Ion-Molecular Formula | Principal Ion- m/z | Err (ppm) | Fragments Ions- Molecular Formula | Fragments Ions- m/z | Err (ppm) |
|---|---|---|---|---|---|---|---|---|
| Venlafaxine (VEN) | ESI+ | 5.47 | C17H28NO2+ | 278.2124 | −1.2 | C8H9O+ | 121.0648 | −3.8 |
| C10H11O+ | 147.0804 | −2.8 | ||||||
| C11H11O+ | 159.0804 | −2.8 | ||||||
| C12H13O+ | 173.0961 | −1.0 | ||||||
| C15H19O+ | 215.143 | 0.0 | ||||||
| C17H26NO+ | 260.2009 | −0.6 | ||||||
| VEN_TP263a | ESI+ | 4.51 | C16H26NO2+ | 264.196 | −2.1 | C9H11N+ | 133.0886 | 20.1 |
| C8H11+ | 107.0855 | 4.0 | ||||||
| C8H11O+ | 123.0804 | 1.5 | ||||||
| C6H9O+ | 97.0648 | 5.2 | ||||||
| C6H9O+ | 201.1274 | 6.9 | ||||||
| VEN_TP263b | ESI+ | 5.53 | C16H26NO2+ | 264.1958 | −2.3 | C8H9O+ | 121.0648 | 0.3 |
| C10H11O+ | 147.0804 | 1.3 | ||||||
| C11H11O+ | 159.0804 | 1.8 | ||||||
| C15H19O+ | 215.143 | 1 | ||||||
| VEN_TP293 | ESI+ | 6.27 | C17H28NO3+ | 294.2063 | −3.7 | C8H9O+ | 121.0648 | −2.3 |
| C15H19O+ | 215.1430 | −1.8 | ||||||
| C6H9+ | 81.0699 | 0.7 | ||||||
| C10H7NO+ | 157.0522 | 14.8 | ||||||
| C11H16NO+ | 178.1226 | −1.8 | ||||||
| VEN_TP291 | ESI+ | 7.85 | C17H26NO3+ | 292.1912 | 1.0 | C8H9O+ | 121.0648 | 1.2 |
| C15H19O+ | 215.1430 | −0.7 | ||||||
| C14H19O2+ | 219.1380 | 0.4 | ||||||
| C11H11O+ | 159.0804 | −3.1 | ||||||
| VEN_TP275 | ESI+ | 4.73 | C17H26NO2+ | 276.1958 | −2.0 | C10H12NO+ | 162.0913 | −0.8 |
| C9H9NO+ | 147.0679 | 1.6 | ||||||
| C11H14NO+ | 176.107 | −0.9 |
| Acute Toxicity | Chronic Toxicity | |||||
|---|---|---|---|---|---|---|
| Compound | Fish | Daphnia | Green Algae | Fish | Daphnia | Green Algae |
| LC50–96 h | LC50–48 h | EC50–96 h | ChV | ChV | ChV | |
| mg/L | ||||||
| Venlafaxine | 16.1 | 10.3 | 12.5 | 1.81 | 1.40 | 4.27 |
| VEN_TP263a | 48.9 | 29.7 | 29.1 | 5.17 | 3.48 | 8.83 |
| VEN_TP263b | 23.7 | 14.9 | 16.6 | 2.60 | 1.91 | 5.43 |
| VEN_TP293 | 96.8 | 57.3 | 50.5 | 9.93 | 6.26 | 14.5 |
| VEN_TP291 | 1.17 × 103 | 621 | 345 | 10.0 | 49.7 | 77.1 |
| VEN_TP275 | 20.6 | 13.0 | 15.1 | 2.28 | 1.17 | 5.01 |
| Harmful | 10–100 mg L−1 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ioannidi, A.A.; Panagopoulou, E.I.; Kouvelis, K.; Ladakis, D.; Petala, A.; Dasenaki, M.E.; Thomaidis, N.S.; Mantzavinos, D.; Frontistis, Z.; Arvaniti, O.S. Effective Degradation of Venlafaxine via Biochar Activated Persulfate: Kinetics, Transformation Products, and Toxicity Assessment. Molecules 2025, 30, 3720. https://doi.org/10.3390/molecules30183720
Ioannidi AA, Panagopoulou EI, Kouvelis K, Ladakis D, Petala A, Dasenaki ME, Thomaidis NS, Mantzavinos D, Frontistis Z, Arvaniti OS. Effective Degradation of Venlafaxine via Biochar Activated Persulfate: Kinetics, Transformation Products, and Toxicity Assessment. Molecules. 2025; 30(18):3720. https://doi.org/10.3390/molecules30183720
Chicago/Turabian StyleIoannidi, Alexandra A., Eleni I. Panagopoulou, Konstantinos Kouvelis, Dimitrios Ladakis, Athanasia Petala, Marilena E. Dasenaki, Nikolaos S. Thomaidis, Dionissios Mantzavinos, Zacharias Frontistis, and Olga S. Arvaniti. 2025. "Effective Degradation of Venlafaxine via Biochar Activated Persulfate: Kinetics, Transformation Products, and Toxicity Assessment" Molecules 30, no. 18: 3720. https://doi.org/10.3390/molecules30183720
APA StyleIoannidi, A. A., Panagopoulou, E. I., Kouvelis, K., Ladakis, D., Petala, A., Dasenaki, M. E., Thomaidis, N. S., Mantzavinos, D., Frontistis, Z., & Arvaniti, O. S. (2025). Effective Degradation of Venlafaxine via Biochar Activated Persulfate: Kinetics, Transformation Products, and Toxicity Assessment. Molecules, 30(18), 3720. https://doi.org/10.3390/molecules30183720














