The Use of Crude Glycerol as a Co-Substrate for Anaerobic Digestion
Abstract
1. Introduction
- (i)
- Dilution of toxic substances;
- (ii)
- Increased organic loading rate;
- (iii)
- Nutrient balance;
- (iv)
- Synergistic effects on microorganisms;
- (v)
- Adjustment of the pH and moisture content.
- (i)
- Crude glycerol;
- (ii)
- Substrates; used for anaerobic co-digestion, as well as:
- (iii)
- The impact of selected factors (CG content, C/N ratio, temperature and pH) on the AcoD performance with the use of crude glycerol.
2. Characteristics of Feedstocks
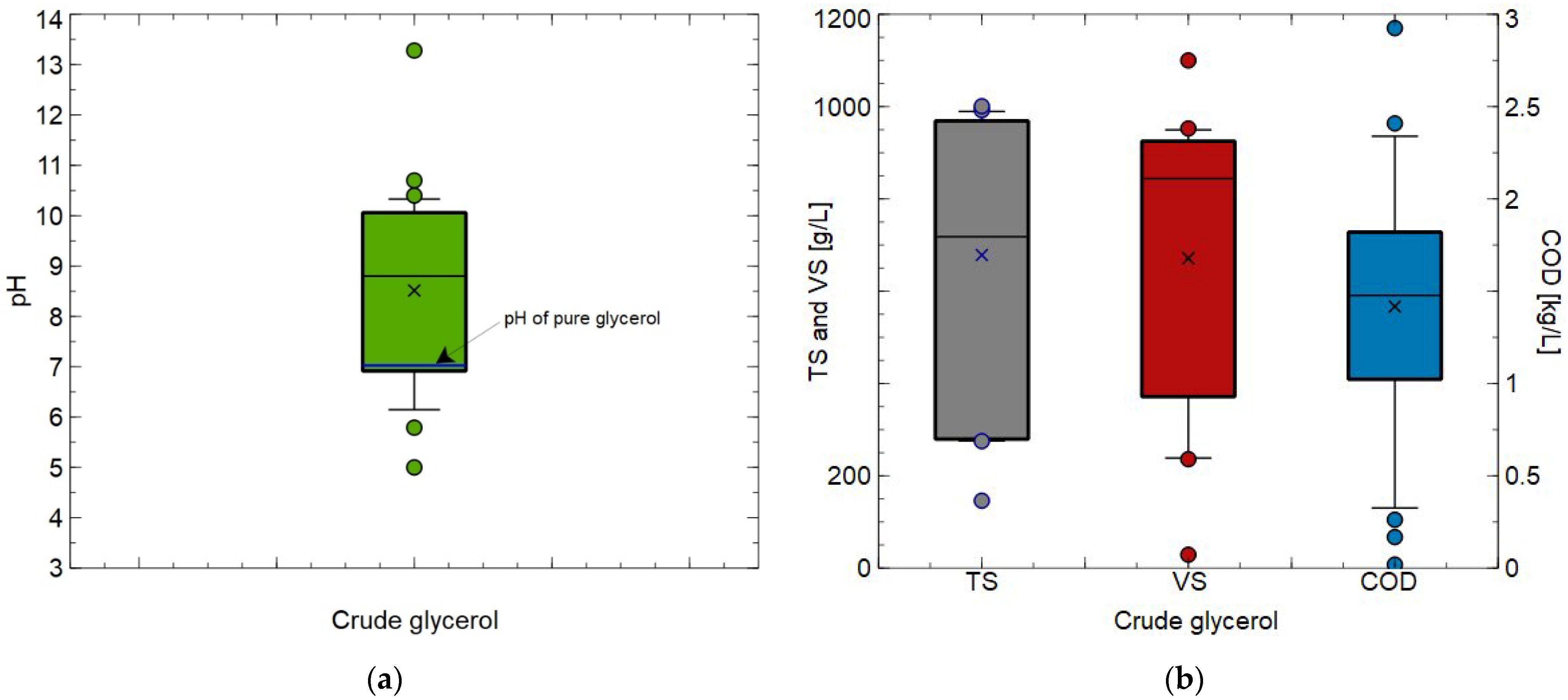
2.1. Crude Glycerol
| Glycerol [g/L] or [%] | pH | TS [g/L] or [%] or [(g/kg)] | VS [g/L] or [%] or [(g/kg)] | Density [kg/L] | Ash [g/kg] or [%] or [(g/L]) | COD [kg/L] | TN [mg/L] or [%] | TP [mg/L] or [g/kg] | C [%] | Na [mg/L] or [mg/kg] | Methanol [g/L] or [%] | C/N [–] | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| - | 8.39 ± 0.02 | 488 ± 3.64 | 488 ± 3.64 | - | - | 1.83000 ± 0.02121 | - | - | - | - | - | - | [3] |
| 46.5 | 10.4 | 78.24 | 95.03 | - | - | - | - | - | - | - | 5.05 | - | [17] |
| 70 | 6.35 ± 0.09 | 1000.8 | 947.8; 94.8 | - | 4.8 | 1.023 | - | - | - | - | - | - | [40] |
| - | 5.0 ± 0.1 | - | - | 1.25 ± 0.1 | 2.8 ± 0.1 | - | 372 ± 21 | 9.6 ± 1.3 | - | - | - | - | [53] |
| 660 ± 19 | - | 933 ± 27 | 844 ± 24 | - | - | 0.660 ± 0.019 | - | - | - | - | 1.2 ± 0.1 | - | [54] |
| 80 | 8–9 | - | - | - | - | 1.140 | - | - | - | 16,939 | - | - | [58] |
| - | 5.0 ± 0.1 | - | - | 1.25 ± 0.1 | 2.8 ± 0.1 | - | 372 ± 21 | 9.6 ± 1.3 | - | - | - | - | [59] |
| 41.67 | 13.28 | 80.98 | 75.23 | - | - | - | - | - | - | - | - | - | [61] |
| - | 6.4 | 146 | 29 | - | - | 1.0286 | - | - | - | - | - | - | [62] |
| - | - | - | 1100 | - | - | 1.43 | - | - | - | - | - | - | [65] |
| - | 9.7 | 87.47 | - | 1.29 | - | 0.01936 | 1.47 | - | 18.88 | - | - | 12.84 | [66] |
| - | 8.86 ± 0.01 | 279.53 ± 3.34 | 254.96 ± 2.94 | - | - | 2.925 ± 0.007 | 1.76 ± 0.01 | - | 720.03 ± 0.27 | - | - | 409 ± 0.03 | [67] |
| - | 9.5 ± 0.1 | 662 ± 28 | 623 ± 27 | - | - | 2.409 ± 0.454 | 100 | - | - | - | - | - | [68] |
| - | 8.8 | 969 | 910 | - | - | 1.76 | 1700 | - | - | - | - | 949 | [72] |
| - | 10.3 ± 0.3 | 717.99 ± 4.1 | 649.12 ± 2.1 | - | - | 1.82312 ± 0.0063 | - | - | - | - | - | - | [73] |
| - | 8.8 | 969 | 910 | - | - | 1.76 | 1700 | - | - | - | - | 949 | [74] |
| 394 | 8.5 | 986 | 940 | - | - | 1.85 | - | - | - | - | - | - | [75] |
| - | - | 80.98 | 75.23 | - | - | - | - | - | - | - | - | - | [76] |
| - | - | 95 | - | - | - | 1.532 | - | - | - | - | - | - | [77] |
| - | - | 81.77 | 72.93 | - | - | - | - | - | - | - | - | - | [78] |
| - | 6.3 | - | - | - | - | - | - | - | - | - | - | - | [79] |
| 70 | 6.35 | 1000.8 | 947.8; 94.8 | - | - | 1.023 | - | - | - | - | - | - | [80] |
| - | 9.0 ± 0.1 | 277 ± 12 | 240.2 ± 9.5 | 1.10 ± 0.1 | - | 1.631 ± 0.025 | - | - | - | - | - | - | [80] |
| - | 10.2 ± 0.1 | 275 ± 12 | 236.0 ± 9.0 | 1.10 ± 0.1 | - | 1.486 ± 0.021 | - | - | - | - | - | - | [81] |
| - | 6.83 | 24.44 | 54.42 | - | - | - | - | - | - | - | - | - | [82] |
| 49.4 ± 0.3 | 7.6 ± 0.2 | - | - | - | 4.8 ± 0.5 | - | - | - | - | 16760 ± 0053 | 5.6 ± 0.8 | - | [83] |
| - | - | (787.6 ± 8.2) | (761.4 ± 9.2) | - | - | - | <0.06 ± 0.1 | - | 35.6 ± 0.2 | 49.3 ± 7.5 | - | - | [84] |
| 47 ± 8.6 | - | 79.5 | 72.1 | 1.02 | - | 1.477 ± 0.235 | - | - | - | - | - | - | [57] |
| 74 | 8.0 | - | - | 1.26 | - | 1.119 | - | - | - | - | 0.019 | - | [85] |
| 50.6 | 10.7 | - | - | - | - | 1.000 | - | - | - | - | 7.1 | - | [86] |
| - | 10.02 ± 0.01 | (62.69 ± 9.00) | (56.61 ± 9.29) | - | 6.08 ± 0.33 | 0.16850 ± 0.00495 | <0.01 | - | 55.90 ± 0.10 | - | - | - | [87] |
| - | 8.8 | 969 | 910 | - | - | 1.760 | 1670 | 71500 | - | - | - | 949 | [88] |
| - | 7.0 | (850.5) | (850.3) | 1.3 | - | - | - | - | - | - | - | - | [89] |
| - | 10.1 ± 0.1 | - | - | 1.052 ± 0.1 | 7.2 ± 0.4 | 0.262 ± 0.009 | - | - | 59.4 ± 0.1 | - | - | - | [90] |
| - | 10.30 ± 0.10 | 870.34 ± 0.10 | 870.10 ± 0.10 | - | - | 1.9740 ± 0.0031 | <0.05 | - | 88.04 ± 0.05 | - | - | - | [91] |
| - | - | 992.2 ± 4.2 | 952.6 ± 4.5 | - | - | - | - | - | - | - | - | - | [92] |
| 75 | - | - | - | - | 5 | - | - | - | - | - | <1 | - | [93] |
2.2. Substrates
| Substrate | pH | TS [g/L] or [%] or [(g/kg)] | VS [g/L] or [%] or [(g/kg)] | VFA [g/L] | Total COD [g/L] or [g/kg] | TN [mg/L] or [%] | TP [mg/L] or [%] or [(g/kg)] | C [%] | Alkalinity [mg/L] | NH4+-N [g/L] or [g/kg] | C/N [–] | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| animal manure | - | 64.6 | 47.5 | 3.3 | 43.8 | - | - | - | 13600 | - | - | [62] |
| animal manure | 7.1 | 25.07 | 4.76 | - | - | 0.84 | - | 15.39 | - | - | 18.32 | [65] |
| animal manure | 6.92 ± 0.2 | 255.97 ± 1.2 | 211.43 ± 2.7 | - | 171.26 ± 3.4 | - | - | - | - | - | - | [72] |
| animal manure | 7.7 ± 0.1 | 30.7 ± 1.5 | 21.2 ± 1.0 | 2.0 ± 0.5 | 33.0 ± 3.2 | - | - | - | 7400 ± 400 | 0.46 ± 0.01 | - | [78] |
| animal manure | - | - | - | - | - | 2.3 ± 0.1 | - | 44.9 ± 0.2 | - | - | - | [83] |
| animal manure | 7.5 | - | - | - | - | - | - | - | - | 4.4 | 16.4 | [89] |
| animal manure | - | 20.7 ± 0.1 | 13.6 ± 0.2 | - | 27.5 ± 0.4 | - | - | - | - | 4.4 | - | [90] |
| animal manure | - | 46.2 ± 0.2 | 32.1 ± 0.2 | - | 58.7 ± 0.4 | - | - | - | - | 4.4 | - | [90] |
| animal manure | 6.51 ± 0.54 | 199.86 ± 0.10 | 167.55 ± 0.10 | - | 137.83 ± 1.34 | 4.08 ± 0.05 | - | 49.62 ± 0.05 | - | - | - | [91] |
| animal manure | - | 71.0 ± 0.6 | 48.0 ± 0.8 | - | - | - | - | - | - | - | - | [92] |
| animal manure | 7.5 ± 0.1 | 23.34 ± 0.24 | 15.49 ± 0.43 | - | - | - | - | - | - | - | - | [93] |
| sewage sludge | 6.52 ± 0.09 | 41.1 ± 1.0 | 22.3 ± 0.6; 54.2 | - | 38.7 ± 0.1 | 15.2 ± 0.1 | - | 29.8 | 960 | - | 17 | [40] |
| sewage sludge | 6.8 ± 0.2 | 35.4 ± 3.1 | 26.1 ± 2.8 | - | 35.2 ± 2.4 | 1042 ± 157 | 845 ± 58 | - | - | - | - | [53] |
| sewage sludge | 6.8 ± 0.5 | 41.1 ± 3.6 | 34.3 ± 3.1 | 0.2 ± 0.1 | 51.9 ± 6.4 | - | - | - | - | 0.8 ± 0.3 | - | [54] |
| sewage sludge | 7.3 | - | - | - | 40 | 1200 | 300 | - | 1600 | - | - | [67] |
| sewage sludge | 7.1 ± 0.4 | 26.3 ± 4.5 | 19.7 ± 5.6 | - | 38.8 ± 14.2 | 1200 ± 600 | - | - | - | - | - | [77] |
| sewage sludge | - | 2.03 | 0.83 | 3.48996 | 21.98333 | - | - | - | - | - | - | [79] |
| sewage sludge | 6.52 | 41.1 ± 1.0 | 22.3 ± 0.6; 54.2 | - | 38.7 ± 0.1 | 392.3 | 15.2 ± 0.1 | 29.8 | 960 | 0.0837 ± 0.0004 | 17 | [84] |
| sewage sludge | - | - | - | - | 17.4 ±3.6 | 540 | - | - | - | 0.0998 | - | [57] |
| sewage sludge | 7.1 | 4.85 | 61.69 | - | - | - | - | - | - | - | - | [57] |
| sewage sludge | 7.0 | 25.6 | 15.8 | - | - | 5.4 | 3.4 | 34.7 | - | - | - | [85] |
| sewage sludge | 7.12 | - | 19.6 | - | - | - | - | - | - | - | - | [86] |
| sewage sludge | 5.65 ± 0.11 | - | - | - | 49.41 ± 5.53 | - | - | - | - | - | - | [107] |
| cattle manure | - | 19 | 83 | - | 293 | - | - | - | - | - | - | [76] |
| cattle manure | 7.5 ± 0.5 | - | - | - | - | - | - | - | - | - | - | [82] |
| cattle manure | 7.16 ± 0.06 | - | - | 1.691 ± 0.012 | 8.000 ± 0.354 | 2.75 ± 0.39 | - | 36.05 ± 1.33 | - | 0.1661 ± 0.0004 | - | [87] |
| cattle manure | 7.4 ± 0.02 | (84.10 ± 0.26) | (40.31 ± 0.55) | - | (48.11 ± 0.27) | - | - | - | - | - | - | [109] |
| leachate | 7.3 | 10.79 | 5.53 | - | 4.04 | - | 18.51 | - | 4160 | 0.67328 | - | [61] |
| distillery wastewater | 3.52 ± 0.02 | 44.08 ± 0.60 | 40.67 ± 0.58 | 2.21426 ± 0.00001 | 57.00 ± 0.71 | 1.88 ± 0.02 | - | 47.06 ± 0.54 | 6.67 ± 0.02 | - | 25.03 ± 0.07 | [66] |
| food waste | - | 41.90 | 40.43 | 41.26332 | - | - | - | - | - | - | - | [77] |
| dairy wastewater | - | 12.50 | 11 | - | - | - | - | - | - | - | - | [75] |
| dairy wastewater | 7.86 | 0.55 | 77.84 | - | - | - | - | - | - | - | - | [81] |
| milk wastewater | - | 4.42 | 3.59 | 21.56523 | 54.650 | - | - | - | - | - | - | [77] |
| seafood wastewater | 6.3 | 9.37 | 7.76 | 2.230 | 10.4 | 870 | 53.6 | - | 2560 | - | 11 | [88] |
| agro-industrial waste | 4.80 ± 0.20 | 15.00 ± 2.23 | 13.00 ± 1.98 | - | 99.00 ± 8.77 | 2200 ± 1150 | 2500 ± 3760 | - | - | - | - | [138] |
| sardine wastewater | 6.8 | 8.5 | 6.4 | - | 12.0 | 1500 | - | - | - | - | 11 | [68] |
| sardine wastewater | 6.8 | 8.5 | 6.4 | - | 14.4 | 870 | - | - | - | - | 11 | [73] |
| meat and bone meal | - | 98.46 | 68.47 | - | - | 10.52 | 4.07 | 44.09 | - | - | 4.19 | [59] |
| meat and bone meal | - | 98.46 | 68.47 | - | - | - | - | - | - | - | - | [75] |
| domestic sewage | 7.0 ± 0.3 | 0.183 ± 0.073 | - | - | - | - | - | - | - | - | - | [56] |
| municipal solid waste | 4.3 ± 0.5 | 40.8 ± 14.4 | 30.5 ± 10.9 | - | 27.6 ± 2.9 | 480 ± 29 | 64 ± 12 | - | - | 0.014. ± 0.0025 | - | [58] |
| palm oil mill final | 4.7 | 66.20 | 44.12 | 13.70 | 88.80 | - | - | - | 940 | - | - | [74] |
3. Performance of Anaerobic Co-Digestion Process
| Feedstock | Scale | S/I Ratio | Glycerol Content [%v/v] or [g/L] | C/N | AcoD Conditions | AcoD Performance with the CG Addition | AD Performance Without CG | Ref. | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T [°C] | pH | HRT [d] or [h] | OLR [kgCODred/m3/d] or [kgVSred/m3/d] or [(gVSred/L)] | Biogas [mL/gVSred] or [mL/gCODred] or (L/L/d) | Methane [mLCH4/gVS] or [mLCH4/gCODred] or [(mL/d)] | Biogas [mL/gVSred/d] or [mL/gCODred/d] or [(L/d)] | Methane [mLCH4/gVSred/d] or [molCH4/m3/d] or [(L/L/d)] | CH4 [%] | |||||||
| CG + animal manure | laboratory | - | 13.8 | - | 35 | - | - | - | - | - | - | 79 | - | Methane: 36 mL/gVS/d | [62] |
| CG + animal manure | laboratory | - | 27.5 | - | 35 | - | - | - | - | - | - | 57 | - | [62] | |
| CG + animal manure | laboratory | - | 55.0 | - | 35 | - | - | - | - | - | - | 3 | - | [62] | |
| CG + animal manure | laboratory | - | 110.0 | - | 35 | - | - | - | - | - | - | 0 | - | [62] | |
| CG + animal manure | laboratory | - | 5 | - | 25.4–28.8 | initial: 8.2, final: 5.4 | - | - | 235 | - | - | - | 14.9 | Biogas: 230 mL/gVS | [65] |
| CG + animal manure | laboratory | - | 10 | - | 25.4–28.8 | initial: 8.8, final: 5.4 | - | - | 380 | - | - | - | 3.5 | [65] | |
| CG + animal manure | laboratory | - | 15 | - | 25.4–28.8 | initial: 9.2, final: 6.4 | - | - | 0 | 0 | 0 | 0 | 0 | [65] | |
| CG + animal manure | laboratory | 1.1 | 6 | 29.08 | - | initial: 8.18 ± 0.18, final: 7.38 ± 0.12 | - | - | - | 340.01 ± 5.52 | - | - | 52.04–72.16 | - | [72] |
| CG + animal manure | laboratory | 1.7 | 6 | 17.82 | - | initial: 8.13 ± 0.19, final: 7.29 ± 0.19 | - | - | - | 330.33 ± 8.05 | - | - | 61.90 | [72] | |
| CG + animal manure | laboratory | 2.3 | 15 | 53.89 | - | initial: 8.56 ± 0.15, final: 6.33 ± 0.11 | - | - | - | 172.75 ± 4.82 | - | - | 59.31 | [72] | |
| CG + animal manure | laboratory | 2.9 | 15 | 26.28 | - | initial: 8.52 ± 0.11, final: 7.50 | - | - | - | 344.13 ± 12.31 | - | - | 55.43 | [72] | |
| CG + animal manure | laboratory | 1.1 | 3.75 | 17.47 | - | initial: 7.95 ± 0.23, final: 7.26 ± 0.03 | - | - | - | 328.62 ± 8.56 | - | - | 52.04 | [72] | |
| CG + animal manure | laboratory | 2.8 | 16.86 | 35.85 | - | initial: 8.60 ± 0.04, final: 7.00 ± 0.19 | - | - | - | 199.48 ± 8.69 | - | - | 61.33 | [72] | |
| CG + animal manure | laboratory | 1.5 | 10.5 | 61.82 | - | initial: 8.43 ± 0.06, final: 7.52 ± 0.14 | - | - | - | 287.13 ± 6.45 | - | - | 60.68 | [72] | |
| CG + animal manure | laboratory | 2.4 | 10.5 | 20.86 | - | initial: 8.31 ± 0.03, final: 7.45 ± 0.05 | - | - | - | 264.38 ± 12.45 | - | - | 71.94 | [72] | |
| CG + animal manure | laboratory | 2.0 | 10.5 | 26.98 | - | initial: 8.27 ± 0.03, final: 7.56 | - | - | - | 226.75 ± 16.38 | - | - | 69.95 | [72] | |
| CG + animal manure | laboratory | - | 3 | 27 | 55 | 7.7 ± 0.1 | - | 2.6 ± 0.1 | 470 | - | - | - | - | Biogas: 170 ± 0.01 mL/gVS | [78] |
| CG + animal manure | laboratory | - | 2 | - | 34 ± 1.0 | 7.61 ± 0.15 | 30 | 2.2 | - | 336 ± 53 | (28.6 ± 1.5) | - | 71.3 ± 1.5 | - | [83] |
| CG + animal manure | laboratory | - | 4 | - | 34 ± 1.0 | 7.72 ± 0.08 | 30 | 2.5 | - | 340 ± 62 | (30.8 ± 1.4) | - | 68.2 ± 3.0 | [83] | |
| CG + animal manure | laboratory | - | 6 | - | 34 ± 1.0 | 7.85 ± 0.09 | 30 | 2.9 | - | 423 ± 41 | (43.6 ± 2.7) | - | 69.8 ± 1.9 | [83] | |
| CG + animal manure | laboratory | - | 8 | - | 34 ± 1.0 | 7.85 ± 0.10 | 30 | 3.7 | - | 380 ± 59 | (51.1 ± 7.1) | - | 68.7 ± 0.8 | [83] | |
| CG + animal manure | laboratory | 3:4 | 20 | - | 35 | - | - | - | - | 249.6 | - | - | - | Methane: 187.9 mLCH4/gVS | [89] |
| CG + animal manure | laboratory | 3:4 | 40 | - | 35 | - | - | - | - | 134.1 | - | - | - | [89] | |
| CG + animal manure | laboratory | 3:4 | 60 | - | 35 | - | - | - | - | 92.9 | - | - | - | [89] | |
| CG + animal manure | laboratory | 3:4 | 80 | - | 35 | - | - | - | - | 73.3 | - | - | - | [89] | |
| CG + animal manure | laboratory | - | 2 | - | 34 ± 1 | 8.5 ± 0.1 | 30 | - a | - | 100 ± 20 | (2.13 ± 0.2) | - | 62.6 ± 2.4 | - | [90] |
| CG + animal manure | laboratory | - | 5 | - | 34 ± 1 | 8.4 ± 0.1 | 30 | - b | - | 140 ± 30 | (3.84 ± 0.3) | - | 62.4 ± 2.1 | [90] | |
| CG + animal manure | laboratory | - | 8 | - | 34 ± 1 | 8.3 ± 0.2 | 30 | - c | - | 170 ± 30 | (5.37 ± 0.3) | - | 62.4 ± 2.1 | [90] | |
| CG + animal manure | laboratory | 0.86–2.27 | - | 17.88–63.63 | 30 ± 1.0 | 7.2–7.6 | - | - | 458.38–834.57; 278.19–521.46 | - | 8.64–14.75 | - | - | - | [91] |
| CG + animal manure | laboratory | 1:2 | 4 | - | 34 ± 1 | - | 30 | - | - | 349.0 ± 27.0 | - | - | - | Methane: 202.0 ± 14.2 mLCH4/gVS | [92] |
| CG + animal manure | laboratory | 1:2 | 8 | - | 34 ± 1 | - | 30 | - | - | 413.2 ± 28.8 | - | - | - | [92] | |
| CG + animal manure | laboratory | 1:2 | 12 | - | 34 ± 1 | - | 30 | - | - | 408.6 ± 25.3 | - | - | - | [92] | |
| CG + animal manure | laboratory | 1:2 | 16 | - | 34 ± 1 | - | 30 | - | - | 467.5 ± 26.0 | - | - | - | [92] | |
| CG + animal manure | laboratory | - | 1 | - | 37 | 8.3 ± 0.1 | 17 | 1.17 ± 0.07 | (0.81 ± 0.06) | 480 ± 40 | - | - | - | Biogas: 0.46 ± 0.02 L/L/d; 0.41 ± 0.02 L/L/d Methane: 330 ± 80 mL/gVS; 350 ± 70 mL/gVS | [93] |
| CG + animal manure | laboratory | - | 1 | - | 37 | 8.3 ± 0.1 | 22 | 0.91 ± 0.09 | (0.60 ± 0.04) | 470 ± 40 | - | - | - | [93] | |
| CG + animal manure | laboratory | - | 3 | - | 37 | 8.2 | 17 | 1.91 ± 0.13 | (1.48 ± 0.13) | 480 ± 40 | - | - | - | [93] | |
| CG + animal manure | laboratory | - | 3 | - | 37 | 8.2 ± 0.1 | 22 | 1.42 ± 0.08 | (1.01 ± 0.07) | 470 ± 20 | - | - | - | [93] | |
| CG + sewage sludge | laboratory | - | - | - | 37 | 7.29 ± 0.25 | 32 | - | - | - | 920 | - | - | Biogas: 350 mL/gVS/d | [17] |
| CG + sewage sludge | laboratory | 1:2 | 1 | 14.1 | - | initial: 7.55, final: 7.16 | - | - | 269.2 | 223.8 | 30.6 | 21.4 | - | Biogas: 161 mL/gVS, Methane: 138.2 mL/gVS | [40] |
| CG + sewage sludge | laboratory | 1:2 | 3 | 17.3 | - | initial: 7.48, final: 7.02 | - | - | 484.7 | 368.8 | 56.8 | - | - | [40] | |
| CG + sewage sludge | laboratory | - | 1 | - | 35 | 6.8-7.4 | - | - | - | (2353 ± 94) | (1.253 ± 0.163) | - | - | - | [53] |
| CG + sewage sludge | laboratory | - | 0.5 | - | 37 | - | 17 | 0.4 | - | - | - | (0.799 ± 0.069) | - | Methane: 0.522 ± 0.048 L/L/d | [54] |
| CG + sewage sludge | laboratory | - | 0.5 | - | 37 | - | 17 | 0.4 | - | - | - | (0.941 ± 0.112) | - | [54] | |
| CG + sewage sludge | laboratory | - | 2 | - | 37 | - | 17 | 1.5 | - | - | - | (1.248 ± 0.058) | - | [54] | |
| CG + sewage sludge | laboratory | - | 0.5 | - | 37 | - | 17 | 0.4 | - | - | - | (0.780 ± 0.053) | - | [54] | |
| CG + sewage sludge | pilot | - | - | - | 35 | - | - | 1.2 | - | 358 | - | - | - | - | [57] |
| CG + sewage sludge | pilot | - | 3 | - | 35.0 ± 0.1 | - | 20 | - | - e | - | - | - | 59.4; 59.7 | [57] | |
| CG + sewage sludge | laboratory | - | 10 | - | 38 | initial: 7.90, final: 7.93 | 56 | - | - | 428 ± 1 | - | - | 57 g | Methane: 85 ± 1 mL/gVS | [77] |
| CG + sewage sludge | laboratory | - | 5 | - | - | initial: 7.3, final: 4.9 | - | - | - | 0 | - | - | 0.4 | Methane: 79.0 ± 0.5% | [85] |
| CG + sewage sludge | laboratory | - | 10 | - | - | initial: 7.2, final: 6.7 | - | - | - | 0 | - | - | 0.2 | [85] | |
| CG + sewage sludge | laboratory | - | 15 | - | - | initial: 7.2, final: 5.5 | - | - | - | 0 | - | - | 0.2 | [85] | |
| CG + sewage sludge | laboratory | - | 20 | - | - | initial: 7.3, final: 5.2 | - | - | - | 0 | - | - | 0.1 | [85] | |
| CG + sewage sludge | pilot | - | 2 | - | 37 | 6.8–7.2 | 12.3 | 1.0–1.7 | - | - | (114 ± 1.7) | - | - | Biogas: 30 ± 2.1 L/d | [86] |
| CG + sewage sludge | pilot | - | 2 | - | 37 | 6.8–7.2 | 14.0 | 1.0–1.7 | - | - | (100 ± 8.0) | - | - | [86] | |
| CG + sewage sludge | pilot | - | 2 | - | 37 | 6.8–7.2 | 16.4 | 1.0–1.7 | - | - | (90 ± 2.8) | - | - | [86] | |
| CG + sewage sludge | pilot | - | 2 | - | 37 | 6.8–7.2 | 19.7 | 1.0–1.7 | - | - | (80 ± 2.6) | - | - | [86] | |
| CG + sewage sludge | pilot | - | 2 | - | 37 | 6.8–7.2 | 12.3 | 1.0–1.7 | - | - | (139 ± 7.4) | - | - | [86] | |
| CG + sewage sludge | pilot | - | 2 | - | 37 | 6.8–7.2 | 14.0 | 1.0–1.7 | - | - | (130 ± 5.0) | - | - | [86] | |
| CG + sewage sludge | pilot | - | 2 | - | 37 | 6.8–7.2 | 16.4 | 1.0–1.7 | - | - | (105 ± 5.5) | - | - | [86] | |
| CG + sewage sludge | pilot | - | 3 | - | 37 | 6.8–7.2 | 19.7 | 1.0–1.7 | - | - | (86.5 ± 3.8) | - | - | [86] | |
| CG + sewage sludge | pilot | - | 4 | - | 37 | 6.8–7.2 | 19.7 | 1.0–1.7 | - | - | 0 | - | - | [86] | |
| CG + sewage sludge | laboratory | - | 1 | - | 35 | - | 5–20 | 1.03–4.05 | - | - | - | (0.6–0.9) | - | - | [107] |
| CG + cattle manure | laboratory | - | 5 | - | - | initial: 8.0, final: 7.8 | - | - | 360 mLCH4/gVSad | 270 mLCH4/gVSad | - | - | 74 | Biogas: 250 mL/gVSad, Methane: 240 mL/gVSad, Methane: 68.1% | [76] |
| CG + cattle manure | laboratory | - | 10 | - | - | initial: 8.0, final: 7.7 | - | - | 350 mLCH4/gVSad | 260 mLCH4/gVSad | - | - | 73 | [76] | |
| CG + cattle manure | laboratory | - | 15 | - | - | initial: 8.0, final: 7.6 | - | - | 330 mLCH4/gVSad | 210 mLCH4/gVSad | - | - | 71 | [76] | |
| CG + cattle manure | laboratory | - | 20 | - | - | initial: 8.0, final: 7.3 | - | - | 230 mLCH4/gVSad | 160 mLCH4/gVSad | - | - | 67 | [76] | |
| CG + cattle manure | laboratory | - | 6 | - | 55 ± 0.1 | initial: 7.2 ± 0.1, final: 6.7 ± 0.3 | 18 | 6.01; 3.24 | - | 600 | - | - | 67 | - | [82] |
| CG + cattle manure | laboratory | - | 6 | - | 55 ± 0.1 | initial: 7.2 ± 0.1, final: 6.7 ± 0.5 | 20 | 5.41; 2.91 | - | 0 | - | - | 0 | [82] | |
| CG + cattle manure | laboratory | - | 6 | - | 55 ± 0.1 | initial: 7.1 ± 0.2, final: 6.8 ± 0.1 | 22 | 4.65; 2.35 | - | 0 | - | - | 0 | [82] | |
| CG + cattle manure | laboratory | - | - | - | 39 | 7.53 | 30 | 2.3 | - | 0.4 g | - | (0.8) g | 69 | - | [87] |
| CG + cattle manure | pilot | - | 6 | 28.0 ± 0.50 | 55 ± 1 | initial: 7.82 ± 0.01, final: 7.35 ± 0.07 | 20 | 5.8 | - | - | - | - | 64.48 ± 0.17 | - | [109] |
| CG + cattle manure | laboratory | - | 5 | - | 35–37 | - | - | - | 825.3 | - | - | - | 9.5 | Biogas: 268.6 mL/gVS | [139] |
| CG + cattle manure | laboratory | - | 10 | - | 35–37 | - | - | - | 825.7 | - | - | - | 14.3 | [139] | |
| CG + cattle manure | laboratory | - | 15 | - | 35–37 | - | - | - | 387.9 | - | - | - | 14.6 | [139] | |
| CG + leachate | laboratory | - | 5 | - | 30 ± 1 | final: 8.02 ± 0.28 | - | 2 | - | 110 ± 60; (360 ± 170) | - | (0.12 ± 0.35) | - | - | [61] |
| CG + leachate | laboratory | - | 5 | - | 30 ± 1 | final: 8.67 ± 0.20 | - | 3.5 | - | 180 ± 90; (3290 ± 1500) | - | (0.86 ± 0.43) | - | [61] | |
| CG + leachate | laboratory | - | 5 | - | 30 ± 1 | final: 8.01 ± 0.55 | 35.2 | 7.1 | - | 180 ± 76; (4970 ± 1840) | - | (1.51 ± 0.66) | - | [61] | |
| CG + leachate | laboratory | - | 5 | - | 30 ± 1 | final: 7.97 ± 0.41 | 32.3 | 11.6 | - | 80 ± 30; (3770 ± 1440) | - | (1.27 ± 0.45) | - | [61] | |
| CG + distillery wastewater | laboratory | - | 5 | 27.02 | - f | 7.85 | 20–35 | - | - | 339 | - | - | 27.02 | Methane: 265 mL/gCOD | [66] |
| CG + distillery wastewater | laboratory | - | 1 | 25.40 | - f | 7.73 | 20–35 | - | - | 289 | - | - | 25.40 | [66] | |
| CG + distillery wastewater | laboratory | - | 2 | 25.78 | - f | 7.75 | 20–35 | - | - | 277 | - | - | 25.78 | [66] | |
| CG + distillery wastewater | laboratory | - | 3 | 26.18 | - f | 7.80 | 20–35 | - | - | 271 | - | - | 26.18 | [66] | |
| CG + distillery wastewater | laboratory | - | 4 | 25.60 | - f | 7.81 | 20–35 | - | - | 270 | - | - | 25.60 | [66] | |
| CG + food waste | laboratory | - | 10 | - | 38 | initial: 8.16, final: 7.94 | 56 | - | 882 | 442 ± 15 | - | - | 60.5 g | Methane: 316 ± 7 mL/gVS | [77] |
| CG + milk sludge | laboratory | - | 10 | - | 38 | initial: 7.88, final: 8.04 | 56 | - | 858 | 496 ± 12 | - | - | 61.58 | Methane: 263 ± 1 mL/gVS | [77] |
| CG + milk sludge + food waste | laboratory | - | 10 | - | 38 | initial: 7.89, final: 7.40 | 56 | - | - | 409 ± 10 | - | - | 58 g | - | [77] |
| CG + sewage sludge + food waste | laboratory | - | 10 | - | 38 | initial: 8.37, final: 7.32 | 56 | - | - | 338 ± 3 | - | - | 56 g | - | [77] |
| CG + sewage sludge + food waste | laboratory | 1:2 | 1 | 18.3 | - | initial: 7.48, final: 7.07 | - | - | 432.4 | 343.3 | 55.1 | 36.2 | - | - | [79] |
| CG + sewage sludge + food waste | laboratory | 1:2 | 3 | 38.4 | - | initial: 7.13, final: 7.05 | - | - | 692.6 | 525.7 | 79.9 | - | - | [79] | |
| CG + seafood wastewater | laboratory | - | 1–10 | - | - | 6.9–8.3 | 13–51 | 2 | 2–577 | - | - | - | - | CH4: 278 mL/gVS | [88] |
| CG + agro-industrial waste | pilot | - | 2.5 | - | 38 | 7.1–7.5 | 28.9 ± 0.5 | - | - | - | (480 ± 230) | - | - | - | [138] |
| CG + agro-industrial waste | pilot | - | 2.5 | - | 38 | 7.1–7.5 | 86.8 ± 0.2 | - | - | - | (144 ± 35) | - | - | [138] | |
| CG + agro-industrial waste | pilot | - | 2.5 | - | 38 | 7.1-7.5 | 43.4 ± 0.3 | - | - | - | (360 ± 103) | - | - | [138] | |
| CG + agro-industrial waste | pilot | - | 2.5 | - | 38 | 7.1-7.5 | 33.3 ± 0.4 | - | - | 681 ± 98 | (576 ± 84) | - | 60–70 | [138] | |
| CG + sardine wastewater | laboratory | 1:1 | - | 27 | 37 | final: 7.28 | - | - | - | 244.85 | - | - | 73.15 | Methane: 62.15% | [68] |
| CG + sardine wastewater | laboratory | 2:1 | - | 43 | 50 | final: 7.52 | - | - | - | 255.21 | - | - | 68.48 | Methane: 37.28% | [68] |
| CG + sardine wastewater | laboratory | 1:1 | 1 | 27 | 37 | 7 | - | - | - | 244.85 | - | - | 73.15 | Methane: 68.21 mL/gCOD | [73] |
| CG + sardine wastewater | laboratory | 1:1 | 2 | 43 | 37 | 7 | - | - | - | 78.42 | - | - | 60.81 | [73] | |
| CG + sardine wastewater | laboratory | 1:1 | 3 | 51 | 37 | 7 | - | - | - | 67.45 | - | - | 64.12 | [73] | |
| CG + sardine wastewater | laboratory | 1:1 | 4 | 63 | 37 | 7 | - | - | - | 27.38 | - | - | 63.71 | [73] | |
| CG + sardine wastewater | laboratory | 1:1 | 5 | 73 | 37 | 7 | - | - | - | 21.92 | - | - | 60.25 | [73] | |
| CG + sardine wastewater | laboratory | 1:1 | 1 | 27 | 50 | 7 | - | - | - | 210.5 | - | - | 70.57 | Methane: 16.29 mL/gCOD | [73] |
| CG + sardine wastewater | laboratory | 1:1 | 2 | 43 | 50 | 7 | - | - | - | 255.21 | - | - | 68.48 | [73] | |
| CG + sardine wastewater | laboratory | 1:1 | 3 | 51 | 50 | 7 | - | - | - | 36.79 | - | - | 51.40 | [73] | |
| CG + sardine wastewater | laboratory | 1:1 | 4 | 63 | 50 | 7 | - | - | - | 18.03 | - | - | 33.78 | [73] | |
| CG + sardine wastewater | laboratory | 1:1 | 5 | 73 | 50 | 7 | - | - | - | 10.28 | - | - | 26.39 | [73] | |
| CG + meat and bone meal | laboratory | 1:1 | - | - | 38 | initial: 7.57, final: 7.80 | - | (27) | - | 320 | - | - | 66.39 | - | [59] |
| CG + meat and bone meal | laboratory | 1:1 | - | - | 38 | initial: 7.60, final: 7.79 | - | (27) | - | 350 | - | - | 66.33 | [59] | |
| CG + meat and bone meal | laboratory | 1:1 | - | - | 38 | initial: 7.64, final: 7.77 | - | (27) | - | 400 | - | - | 58.67 | [59] | |
| CG + dairy wastewater | laboratory | - | 2 | - | - f | 7.21 ± 0.05 | - | - | 722.0 ± 20.6 | - | - | - | 74.99 ± 13.52 | Biogas: 414.9 ± 57.0 mL/gVS, Methane: 70.40 ± 17.06% | [81] |
| CG + dairy wastewater | laboratory | - | 4 | - | - f | 7.13 ± 0.04 | - | - | 1310.0 ± 144.4 | - | - | - | 73.97 ± 18.44 | [81] | |
| CG + dairy wastewater | laboratory | - | 8 | - | - f | 7.07 ± 0.03 | - | - | 2307.2 ± 312.8 | - | - | - | 73.10 ± 24.03 | [81] | |
| CG + dairy wastewater + meat and bone meal | laboratory | - | 13 | 13 | 38 | initial: 6.77, final: 7.67 | 30 | 2.65 | 1390 ± 10 | 900 ± 10 | - | - | - | - | [75] |
| CG + municipal wastewater sludge | pilot | - | - | - | 36 ± 1 | final: 7.25 ± 0.07 | - | 2.34 ± 0.08; 1.03 ± 0.01 | (1.45) | - | - | (0.93) | 64.00 ± 1.17 | Methane: 60.00 ± 0.84%; 60.00 ± 0.74%; 60.00 ± 0.63% | [3] |
| CG + municipal wastewater sludge | pilot | - | 1.1 | - | 36 ± 1 | final: 7.18 ± 0.05 | - | 2.38 ± 0.06; 1.04 ± 0.04 | - | - | - | - | 66.50 ± 2.02 | [3] | |
| CG + municipal wastewater sludge | pilot | - | 1.8 | - | 36 ± 1 | final: 7.09 ± 0.05 | - | 2.88 ± 0.11; 1.18 ± 0.04 | - | - | - | - | 56 ± 1.68 | [3] | |
| CG + municipal wastewater sludge | laboratory | - | 1.25 | - | 37 | 7.45 ± 0.03 | - | 4.82 | - | - | (12.2 ± 0.2) | - | - | Biogas: 8.2 ± 0.1 L/d; 4.5 ± 0.1 L/d | [80] |
| CG + municipal wastewater sludge | laboratory | - | 1.35 | - | 37 | 7.30 ± 0.03 | - | 3.02 | - | - | (9.0 ± 0.1) | - | - | [80] | |
| CG + municipal wastewater sludge | laboratory | - | 2.72 | - | 37 | 6.20 ± 0.10 | - | 4.01 | - | - | (4.8 ± 0.1) | - | - | [80] | |
| CG + municipal solid waste | laboratory | 1:1 | - | - | 35 | 6.8-7.0 | - | - | - | (2094 ± 92) | - | - | - | Metane: 1400 ± 305 mL/d | [58] |
| CG + palm oil mill final | laboratory | - | 1 | - | 37 | initial: 6.89, final: 7.35 | - | - | - | 553.46, 276.73, (45.66) | - | - | - | Methane: 278.64 mL/gVS; 73.34 mL/d | [74] |
| CG + palm oil mill final | laboratory | - | 2 | - | 37 | initial: 6.92, final: 5.34 | - | - | - | 98.24, 49.12, (26.51) | - | - | - | [74] | |
| CG + palm oil mill final | laboratory | - | 3 | - | 37 | initial: 6.99, final: 5.35 | - | - | - | 77.48, 38.74, (11.97) | - | - | - | [74] | |
| CG + palm oil mill final | laboratory | - | 4 | - | 37 | initial: 7.08, final: 5.30 | - | - | - | 62.36, 31.18, (10.09) | - | - | - | [74] | |
| CG + palm oil mill final | laboratory | - | 5 | - | 37 | initial: 7.15, final: 5.30 | - | - | - | 55.47, 27.87, (8.95) | - | - | - | [74] | |
| CG + sugarcane stillage | laboratory | - | 1.53 | - | 30 | initial: 7.9 ± 0.2, final: 7.7 ± 0.2 | - | 5 | - | - | - | 60.23 | 81.9 ± 0.2 | Methane: 83.2 ± 0.5% | [140] |
| CG + sugarcane stillage | laboratory | - | 1.53 | - | 30 | initial: 7.55 ± 0.04, final: 7.5 ± 0.1 | - | 5 | - | - | - | 59.61 | 83 ± 1 | [140] | |
| CG + sugarcane stillage | laboratory | - | 1.53 | - | 30 | initial: 7.4 ± 0.3, final: 7.7 ± 0.2 | - | 5 | - | - | - | 58.57 | 83.5 ± 0.3 | [140] | |
| CG + sugarcane stillage | laboratory | - | 1.53 | - | 30 | initial: 7.6 ± 0.1, final: 8.2 ± 0.3 | - | 5 | - | - | - | 65.39 | 80.5 ± 0.1 | [140] | |
| CG + sugarcane stillage | laboratory | - | 1.53 | - | 30 | initial: 7.40 ± 0.09, final: 7.3 ± 0.2 | - | 5 | - | - | - | 68.57 | 84.0 ± 0.6 | [140] | |
| CG + sugarcane stillage | laboratory | - | 1.53 | - | 30 | initial: 7.5 ± 0.1, final: 7.4 ± 0.3 | - | 5 | - | - | - | 52.10 | 86.0 ± 0.4 | [140] | |
| CG + sugarcane stillage | laboratory | - | 1.53 | - | 30 | initial: 7.5 ± 0.1, final: 7.6 ± 0.1 | - | 7.5 | - | - | - | 95.07 | 82.6 ± 0.2 | [140] | |
| CG + sugarcane stillage | laboratory | - | 1.53 | - | 30 | initial: 7.4 ± 0.1, final: 7.7 ± 0.3 | - | 10 | - | - | - | 139.32 | 83.1 ± 0.1 | [140] | |
| CG + sugarcane stillage | laboratory | - | 1.53 | - | 35 | initial: 7.5 ± 0.2, final: 8.2 ± 0.2 | - | 10 | - | - | - | 122.99 | 83.2 ± 0.7 | [140] | |
| CG + olive mill wastewater + slaughterhouse wastewater | laboratory | 1:1 | - | - | 35 | 6.9–7.6 | - | - | - | (1210 ± 205) | - | - | - | Methane: 479 mL/d | [58] |
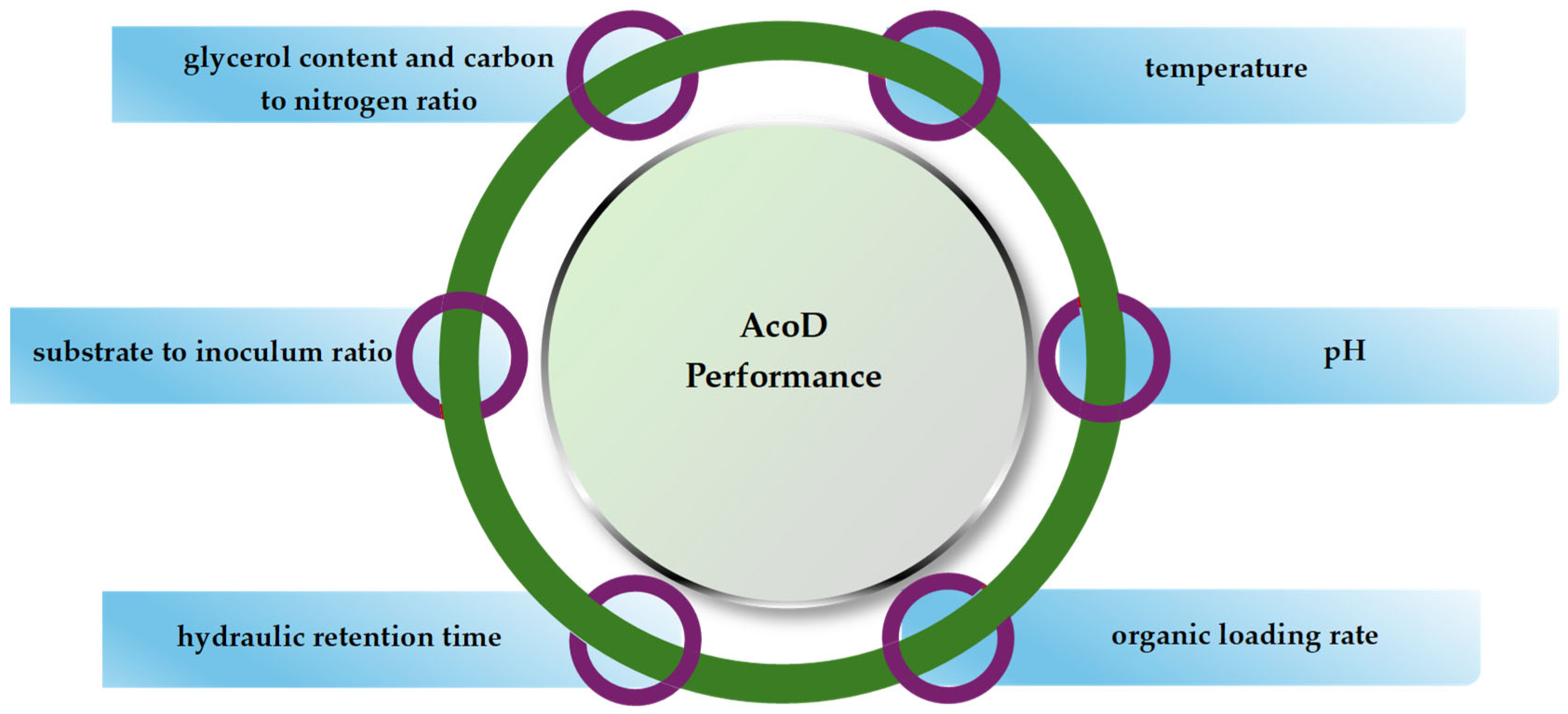
3.1. Crude Glycerol Content and C/N Ratio
- (i)
- Low methane production of methane;
- (ii)
- Long retention time of digestion process;
- (iii)
- Low efficiency of volatile solids reduction.
- (i)
- Type of substrate;
- (ii)
- Content of trace elements;
- (iii)
- Chemical components present in the feedstock;
- (iv)
- Biodegradability.
3.2. Temperature and pH
4. Conclusions and Perspectives
- (i)
- Values of key parameters characterizing the CG (glycerol concentration, pH, TS and VS content as well as COD) used for the AcoD were within wide ranges. It can be attributed to the fact that the CG composition depends mainly on the source of oil used for the biodiesel production, processing technology, ratio of reactants, type of catalyst and procedure applied.
- (ii)
- Adding CG to the feedstock caused a significant enhancement of CH4 and biogas yield compared to results obtained for the mono-digestion process of various substrates. Indeed, raw glycerol is a source of additional carbon, which can be consumed by specific microorganisms and converted to CH4.
- (iii)
- The increase in the CG concentration in the feedstock leads to an enhancement of the AcoD performance; nevertheless, the substrate inhibition effect should be considered.
- (iv)
- For most of the experiments presented in the literature, CH4 content in the biogas higher than 60% has been obtained under CG content up to 8% v/v. A higher concentration may cause a reduction in the biogas yield due to the overload of organic material in digestion.
- (v)
- Most of studies focusing on the AcoD performance with the use of CG have been carried out by applying laboratory-scale installations; hence, further experimental investigations with the use of pilot-scale systems are recommended.
- (vi)
- In order to evaluate the performance of AcoD with the use of CG under thermophilic conditions, more studies are required.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AD | Anaerobic digestion |
| AcoD | Anaerobic co-digestion |
| C | Carbon |
| CG | Crude glycerol |
| COD | Chemical oxygen demand |
| CSTR | Continuously stirred tank reactor |
| C/N | Carbon to nitrogen ratio |
| HRT | Hydraulic retention time |
| IBR | Induced bed reactor |
| MW | Molecular weight |
| N | Nitrogen |
| OLR | Organic loading rate |
| S/I | Substrate to inoculum |
| TN | Total nitrogen |
| TP | Total phosphorus |
| TS | Total solids |
| VS | Volatile solids |
References
- Zhang, J.; Wang, Y.; Muldoon, V.L.; Deng, S. Crude Glycerol and Glycerol as Fuels and Fuel Additives in Combustion Applications. Renew. Sustain. Energy Rev. 2022, 159, 112206. [Google Scholar] [CrossRef]
- Almeida, E.L.; Olivo, J.E.; Andrade, C.M.G. Production of Biofuels from Glycerol from the Biodiesel Production Process—A Brief Review. Fermentation 2023, 9, 869. [Google Scholar] [CrossRef]
- Razaviarani, V.; Buchanan, I.D.; Malik, S.; Katalambula, H. Pilot Scale Anaerobic Co-Digestion of Municipal Wastewater Sludge with Biodiesel Waste Glycerin. Bioresour. Technol. 2013, 133, 206–212. [Google Scholar] [CrossRef]
- Chilakamarry, C.R.; Sakinah, A.M.M.; Zularisam, A.W.; Pandey, A. Glycerol Waste to Value Added Products and Its Potential Applications. Syst. Microbiol. Biomanuf. 2021, 1, 378–396. [Google Scholar] [CrossRef] [PubMed]
- He, Q. (Sophia); McNutt, J.; Yang, J. Utilization of the Residual Glycerol from Biodiesel Production for Renewable Energy Generation. Renew. Sustain. Energy Rev. 2017, 71, 63–76. [Google Scholar] [CrossRef]
- Andriamanohiarisoamanana, F.J.; Yamashiro, T.; Ihara, I.; Iwasaki, M.; Nishida, T.; Umetsu, K. Farm-Scale Thermophilic Co-Digestion of Dairy Manure with a Biodiesel Byproduct in Cold Regions. Energy Convers. Manag. 2016, 128, 273–280. [Google Scholar] [CrossRef]
- Ben, Z.Y.; Samsudin, H.; Yhaya, M.F. Glycerol: Its Properties, Polymer Synthesis, and Applications in Starch Based Films. Eur. Polym. J. 2022, 175, 111377. [Google Scholar] [CrossRef]
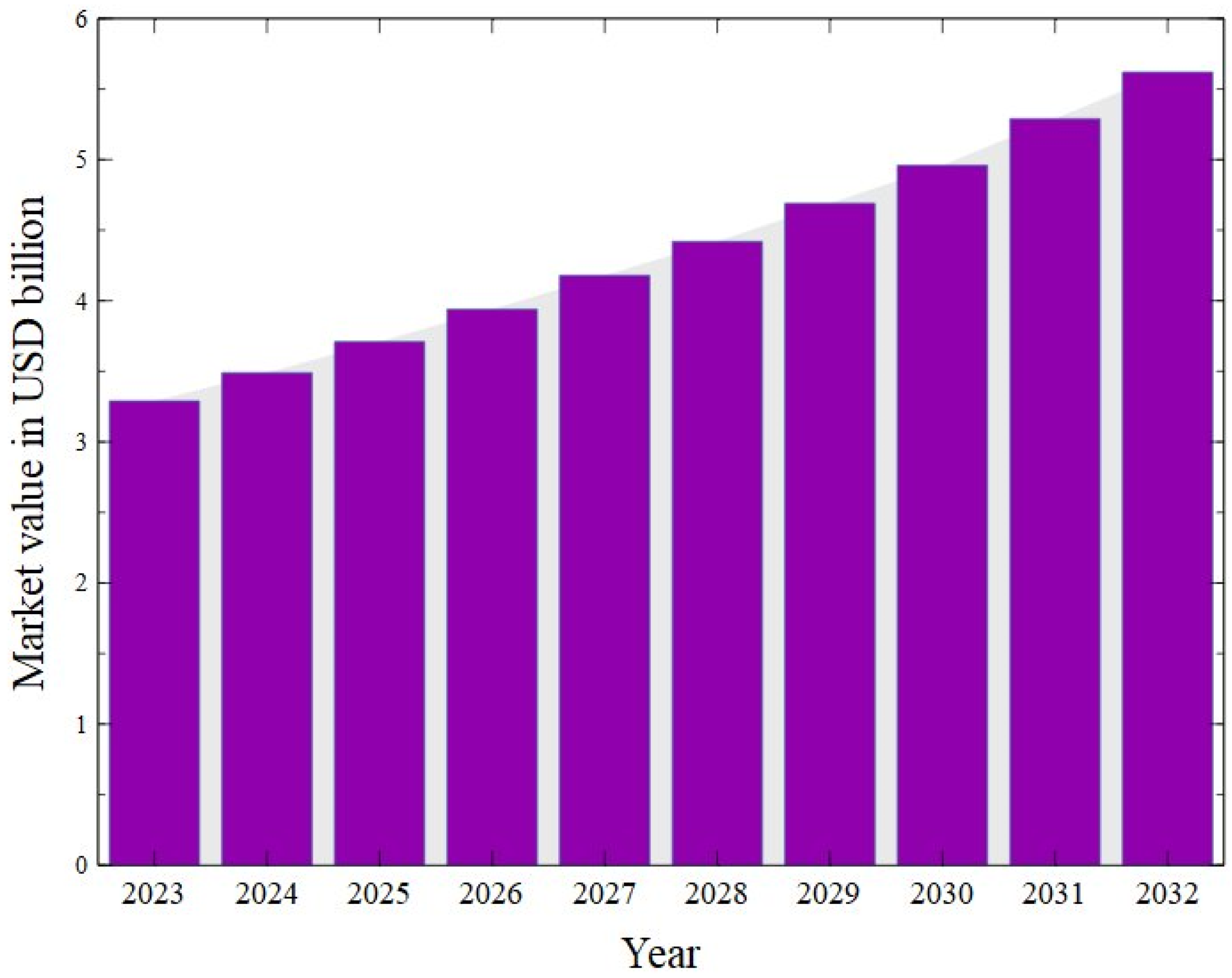
- Available online: https://www.Wiseguyreports.Com/Reports/Crude-Glycerol-Market (accessed on 1 July 2025).
- Lima, P.J.M.; Da Silva, R.M.; Neto, C.A.C.G.; Gomes, E.; Silva, N.C.; Souza, J.E.D.S.; Nunes, Y.L.; Sousa Dos Santos, J.C. An Overview on the Conversion of Glycerol to Value-added Industrial Products via Chemical and Biochemical Routes. Biotech. App. Biochem. 2022, 69, 2794–2818. [Google Scholar] [CrossRef]
- Hambali, E.; Fitria, R.; Sari, V.I. Glycerol and Derivatives. In Biorefinery of Oil Producing Plants for Value-Added Products; Abd-Aziz, S., Gozan, M., Ibrahim, M.F., Phang, L., Eds.; Wiley: Hoboken, NJ, USA, 2022; pp. 469–491. ISBN 978-3-527-34876-3. [Google Scholar]
- Hejna, A.; Kosmela, P.; Formela, K.; Piszczyk, Ł.; Haponiuk, J.T. Potential Applications of Crude Glycerol in Polymer Technology–Current State and Perspectives. Renew. Sustain. Energy Rev. 2016, 66, 449–475. [Google Scholar] [CrossRef]
- Schwingel, A.W.; Orrico, A.C.A.; De Lucas Junior, J.; Orrico Junior, M.A.P.; Aspilcueta Borquis, R.R.; Fava, A.F. Laying Hen Manure in Anaerobic Co-Digestion with Glycerin Containing Different Glycerol and Impurity Levels. J. Clean. Prod. 2019, 215, 1437–1444. [Google Scholar] [CrossRef]
- Kurahashi, K.; Kimura, C.; Fujimoto, Y.; Tokumoto, H. Value-Adding Conversion and Volume Reduction of Sewage Sludge by Anaerobic Co-Digestion with Crude Glycerol. Bioresour. Technol. 2017, 232, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Johnravindar, D.; Wong, J.W.C. Enhanced Volatile Fatty Acid Degradation and Methane Production Efficiency by Biochar Addition in Food Waste-Sludge Co-Digestion: A Step towards Increased Organic Loading Efficiency in Co-Digestion. Bioresour. Technol. 2020, 308, 123250. [Google Scholar] [CrossRef] [PubMed]
- Bhukya, G.; Pilli, S.; Chinthala, S.; Tyagi, R.D. Pre-Treated Crude Glycerol a Valuable Green Energy Source in the Era of Circular Bioeconomy—A Review. Circ. Econ. Sust. 2024, 4, 877–904. [Google Scholar] [CrossRef]
- Anitha, M.; Kamarudin, S.K.; Kofli, N.T. The Potential of Glycerol as a Value-Added Commodity. Chem. Eng. J. 2016, 295, 119–130. [Google Scholar] [CrossRef]
- Nartker, S.; Ammerman, M.; Aurandt, J.; Stogsdil, M.; Hayden, O.; Antle, C. Increasing Biogas Production from Sewage Sludge Anaerobic Co-Digestion Process by Adding Crude Glycerol from Biodiesel Industry. Waste Manag. 2014, 34, 2567–2571. [Google Scholar] [CrossRef]
- Attarbachi, T.; Kingsley, M.D.; Spallina, V. New Trends on Crude Glycerol Purification: A Review. Fuel 2023, 340, 127485. [Google Scholar] [CrossRef]
- Da Silva Ruy, A.D.; Carvalho, A.; Santos, A.; Lima, D.L.S.D.; Pessôa, L.C.; Alves, R.M.B.; Pontes, L.A.M. A Novel Chemical Route for 1,3-Propanediol Production from Crude Glycerol: Process Simulation and Environmental Assessment. J. Environ. Chem. Eng. 2024, 13, 116864. [Google Scholar] [CrossRef]
- Agrawal, D.; Budakoti, M.; Kumar, V. Strategies and Tools for the Biotechnological Valorization of Glycerol to 1, 3-Propanediol: Challenges, Recent Advancements and Future Outlook. Biotechnol. Adv. 2023, 66, 108177. [Google Scholar] [CrossRef]
- Fokum, E.; Zabed, H.M.; Yun, J.; Zhang, G.; Qi, X. Recent Technological and Strategical Developments in the Biomanufacturing of 1,3-Propanediol from Glycerol. Int. J. Environ. Sci. Technol. 2021, 18, 2467–2490. [Google Scholar] [CrossRef]
- Restrepo, J.B.; Paternina-Arboleda, C.D.; Bula, A.J. 1,2—Propanediol Production from Glycerol Derived from Biodiesel’s Production: Technical and Economic Study. Energies 2021, 14, 5081. [Google Scholar] [CrossRef]
- Główka, M.; Krawczyk, T. New Trends and Perspectives in Production of 1,2-Propanediol. ACS Sustain. Chem. Eng. 2023, 11, 7274–7287. [Google Scholar] [CrossRef]
- Sun, S.; Shu, L.; Lu, X.; Wang, Q.; Tišma, M.; Zhu, C.; Shi, J.; Baganz, F.; Lye, G.J.; Hao, J. 1,2-Propanediol Production from Glycerol via an Endogenous Pathway of Klebsiella Pneumoniae. Appl. Microbiol. Biotechnol. 2021, 105, 9003–9016. [Google Scholar] [CrossRef] [PubMed]
- Gujar, J.P.; Verma, A.; Modhera, B. Optimizing Glycerol Conversion to Hydrogen: A Critical Review of Catalytic Reforming Processes and Catalyst Design Strategies. Int. J. Hydrogen Energy 2025, 109, 823–850. [Google Scholar] [CrossRef]
- Qureshi, F.; Yusuf, M.; Pasha, A.A.; Khan, H.W.; Imteyaz, B.; Irshad, K. Sustainable and Energy Efficient Hydrogen Production via Glycerol Reforming Techniques: A Review. Int. J. Hydrogen Energy 2022, 47, 41397–41420. [Google Scholar] [CrossRef]
- Macedo, M.S.; Soria, M.A.; Madeira, L.M. Process Intensification for Hydrogen Production through Glycerol Steam Reforming. Renew. Sustain. Energy Rev. 2021, 146, 111151. [Google Scholar] [CrossRef]
- Abubakar, U.C.; Bansod, Y.; Forster, L.; Spallina, V.; D’Agostino, C. Conversion of Glycerol to Acrylic Acid: A Review of Strategies, Recent Developments and Prospects. React. Chem. Eng. 2023, 8, 1819–1838. [Google Scholar] [CrossRef]
- Ahmad, N.N.R.; Ang, W.L.; Teow, Y.H.; Mohammad, A.W.; Hilal, N. Nanofiltration Membrane Processes for Water Recycling, Reuse and Product Recovery within Various Industries: A Review. J. Water Process Eng. 2022, 45, 102478. [Google Scholar] [CrossRef]
- Jin, C.; Sun, S.; Yang, D.; Sheng, W.; Ma, Y.; He, W.; Li, G. Anaerobic Digestion: An Alternative Resource Treatment Option for Food Waste in China. Sci. Total Environ. 2021, 779, 146397. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, Y.; Wang, Y.; Li, J.; Jia, X.; Wu, Z. Recent Progress in Glycerol Oxidation to Lactic Acid and Pyruvic Acid with Heterogeneous Metal Catalysts. Carbon Resour. Convers. 2025, 8, 100250. [Google Scholar] [CrossRef]
- Akbulut, D.; Özkar, S. A Review of the Catalytic Conversion of Glycerol to Lactic Acid in the Presence of Aqueous Base. RSC Adv. 2022, 12, 18864–18883. [Google Scholar] [CrossRef]
- Wang, K.; Yang, Z.; Ma, Y.; Zhao, W.; Sun, J.; Lu, T.; He, H. Recent Advances in the Utilization of Glycerol for the Production of Lactic Acid by Catalysis. Biofuels Bioprod. Bioref. 2022, 16, 1428–1454. [Google Scholar] [CrossRef]
- Gebreegziabher, B.W.; Dubale, A.A.; Adaramola, M.S.; Morken, J. Advancing Anaerobic Digestion of Biodiesel Byproducts: A Comprehensive Review. Bioenerg. Res. 2025, 18, 15. [Google Scholar] [CrossRef]
- Li, H.; Gilbert, R.G.; Gidley, M.J. Molecular-Structure Evolution during in Vitro Fermentation of Granular High-Amylose Wheat Starch Is Different to in Vitro Digestion. Food Chem. 2021, 362, 130188. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Wang, Y.; Li, B.; Zhang, J.; Guan, D.; Hu, Y.; Fan, H.; Sun, Y.; Wang, H.; Guo, L. New Insights into Microplastics Inhibiting Kitchen Waste Dry Digestion: Digestion Efficiency, Microbial Communities and Microplastic Aging Mechanisms. Chem. Eng. J. 2025, 508, 160907. [Google Scholar] [CrossRef]
- Kegl, T. Consideration of Biological and Inorganic Additives in Upgraded Anaerobic Digestion BioModel. Bioresour. Technol. 2022, 355, 127252. [Google Scholar] [CrossRef]
- Tomczak, W.; Gryta, M.; Grubecki, I.; Miłek, J. Biogas Production in AnMBRs via Treatment of Municipal and Domestic Wastewater: Opportunities and Fouling Mitigation Strategies. Appl. Sci. 2023, 13, 6466. [Google Scholar] [CrossRef]
- Kegl, T.; Torres Jiménez, E.; Kegl, B.; Kovač Kralj, A.; Kegl, M. Modeling and Optimization of Anaerobic Digestion Technology: Current Status and Future Outlook. Prog. Energy Combust. Sci. 2025, 106, 101199. [Google Scholar] [CrossRef]
- Alves, I.R.F.S.; Mahler, C.F.; Oliveira, L.B.; Reis, M.M.; Bassin, J.P. Assessing the Use of Crude Glycerol from Biodiesel Production as an Alternative to Boost Methane Generation by Anaerobic Co-Digestion of Sewage Sludge. Biomass Bioenergy 2020, 143, 105831. [Google Scholar] [CrossRef]
- Esposito, G.; Frunzo, L.; Giordano, A.; Liotta, F.; Panico, A.; Pirozzi, F. Anaerobic Co-Digestion of Organic Wastes. Rev. Env. Sci. Biotechnol. 2012, 11, 325–341. [Google Scholar] [CrossRef]
- Guo, Q.; Dai, X. Analysis on Carbon Dioxide Emission Reduction during the Anaerobic Synergetic Digestion Technology of Sludge and Kitchen Waste: Taking Kitchen Waste Synergetic Digestion Project in Zhenjiang as an Example. Waste Manag. 2017, 69, 360–364. [Google Scholar] [CrossRef]
- Romero Güiza, M.S.; Mata Alvarez, J.; Chimenos Rivera, J.M.; Astals Garcia, S. Nutrient Recovery Technologies for Anaerobic Digestion Systems: An Overview. Rev. ION 2016, 29, 7–26. [Google Scholar] [CrossRef]
- Vasco-Correa, J.; Khanal, S.; Manandhar, A.; Shah, A. Anaerobic Digestion for Bioenergy Production: Global Status, Environmental and Techno-Economic Implications, and Government Policies. Bioresour. Technol. 2018, 247, 1015–1026. [Google Scholar] [CrossRef] [PubMed]
- Chow, W.; Chong, S.; Lim, J.; Chan, Y.; Chong, M.; Tiong, T.; Chin, J.; Pan, G.-T. Anaerobic Co-Digestion of Wastewater Sludge: A Review of Potential Co-Substrates and Operating Factors for Improved Methane Yield. Processes 2020, 8, 39. [Google Scholar] [CrossRef]
- Praveen, P.; Chatterjee, B.; Mazumder, D. A Review on Biodegradation of Plastics with Organic Fraction of Municipal Solid Wastes by Anaerobic Co-Digestion. Water Air Soil. Pollut. 2025, 236, 177. [Google Scholar] [CrossRef]
- Jasińska, A.; Grosser, A.; Meers, E. Possibilities and Limitations of Anaerobic Co-Digestion of Animal Manure—A Critical Review. Energies 2023, 16, 3885. [Google Scholar] [CrossRef]
- Pramanik, S.K. Anaerobic Co-Digestion of Municipal Organic Solid Waste: Achievements and Perspective. Bioresour. Technol. Rep. 2022, 20, 101284. [Google Scholar] [CrossRef]
- Liu, K.; Lv, L.; Li, W.; Ren, Z.; Wang, P.; Liu, X.; Gao, W.; Sun, L.; Zhang, G. A Comprehensive Review on Food Waste Anaerobic Co-Digestion: Research Progress and Tendencies. Sci. Total Environ. 2023, 878, 163155. [Google Scholar] [CrossRef]
- González, R.; Peña, D.C.; Gómez, X. Anaerobic Co-Digestion of Wastes: Reviewing Current Status and Approaches for Enhancing Biogas Production. Appl. Sci. 2022, 12, 8884. [Google Scholar] [CrossRef]
- Ferdeș, M.; Zăbavă, B.Ș.; Paraschiv, G.; Ionescu, M.; Dincă, M.N.; Moiceanu, G. Food Waste Management for Biogas Production in the Context of Sustainable Development. Energies 2022, 15, 6268. [Google Scholar] [CrossRef]
- Tomczak, W.; Daniluk, M.; Kujawska, A. Food Waste as Feedstock for Anaerobic Mono-Digestion Process. Appl. Sci. 2024, 14, 10593. [Google Scholar] [CrossRef]
- Fountoulakis, M.S.; Petousi, I.; Manios, T. Co-Digestion of Sewage Sludge with Glycerol to Boost Biogas Production. Waste Manag. 2010, 30, 1849–1853. [Google Scholar] [CrossRef]
- Jensen, P.D.; Astals, S.; Lu, Y.; Devadas, M.; Batstone, D.J. Anaerobic Codigestion of Sewage Sludge and Glycerol, Focusing on Process Kinetics, Microbial Dynamics and Sludge Dewaterability. Water Res. 2014, 67, 355–366. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Wu, B.; Yao, F.; He, L.; Chen, F.; Ma, Y.; Shu, X.; Hou, K.; Wang, D.; Li, X. Biogas Production from Anaerobic Co-Digestion of Waste Activated Sludge: Co-Substrates and Influencing Parameters. Rev. Env. Sci. Biotechnol. 2019, 18, 771–793. [Google Scholar] [CrossRef]
- Adames, L.V.; Pires, L.O.; Maintinguer, S.I. Continuous Long-Term Anaerobic Co-Digestion of Crude Glycerol and Domestic Sewage: Plug-Flow In-Series Reactor Performance and Microbiota Acclimatization. Bioenerg. Res. 2023, 16, 1876–1888. [Google Scholar] [CrossRef]
- Nghiem, L.D.; Nguyen, T.T.; Manassa, P.; Fitzgerald, S.K.; Dawson, M.; Vierboom, S. Co-Digestion of Sewage Sludge and Crude Glycerol for on-Demand Biogas Production. Int. Biodeterior. Biodegrad. 2014, 95, 160–166. [Google Scholar] [CrossRef]
- Fountoulakis, M.S.; Manios, T. Enhanced Methane and Hydrogen Production from Municipal Solid Waste and Agro-Industrial by-Products Co-Digested with Crude Glycerol. Bioresour. Technol. 2009, 100, 3043–3047. [Google Scholar] [CrossRef]
- Andriamanohiarisoamanana, F.J.; Saikawa, A.; Tarukawa, K.; Qi, G.; Pan, Z.; Yamashiro, T.; Iwasaki, M.; Ihara, I.; Nishida, T.; Umetsu, K. Anaerobic Co-Digestion of Dairy Manure, Meat and Bone Meal, and Crude Glycerol under Mesophilic Conditions: Synergistic Effect and Kinetic Studies. Energy Sustain. Dev. 2017, 40, 11–18. [Google Scholar] [CrossRef]
- Tan, H.W.; Abdul Aziz, A.R.; Aroua, M.K. Glycerol Production and Its Applications as a Raw Material: A Review. Renew. Sustain. Energy Rev. 2013, 27, 118–127. [Google Scholar] [CrossRef]
- De Castro, T.M.; Arantes, E.J.; De Mendonça Costa, M.S.S.; Gotardo, J.T.; Passig, F.H.; De Carvalho, K.Q.; Gomes, S.D. Anaerobic Co-Digestion of Industrial Waste Landfill Leachate and Glycerin in a Continuous Anaerobic Bioreactor with a Fixed-Structured Bed (ABFSB): Effects of Volumetric Organic Loading Rate and Alkaline Supplementation. Renew. Energy 2021, 164, 1436–1446. [Google Scholar] [CrossRef]
- Kim, S.-H.; Sung, S. Co-Digestion of Waste Glycerol with Swine Manure. J. Korea Org. Resour. Recycl. Assoc. 2010, 18, 71–75. [Google Scholar]
- Bendicho, C.; Lavilla, I. Sewage. In Reference Module in Chemistry, Molecular Sciences and Chemical Engineering; Elsevier: Amsterdam, The Netherlands, 2017; p. B9780124095472115197. ISBN 978-0-12-409547-2. [Google Scholar]
- Meegoda, J.N.; Li, B.; Patel, K.; Wang, L.B. A Review of the Processes, Parameters, and Optimization of Anaerobic Digestion. IJERPH 2018, 15, 2224. [Google Scholar] [CrossRef] [PubMed]
- Goembira, F.; Yuliarningsih, R.; Silvia, S.; Rahmadani, F. The Effects of Crude Glycerol Addition on Biogas Production. IOP Conf. Ser. Earth Environ. Sci. 2024, 1306, 012041. [Google Scholar] [CrossRef]
- Phuket, K.R.N.; Srimachai, T.; Luanunkarb, S.; O-Thong, S. Enhanced Efficiency for Biogas Production from Distillery Wastewater as Mixed with Molasses and Glycerol Waste in the Anaerobic Co-Digestion. Sci. Technol. Indones. 2024, 9, 120–128. [Google Scholar] [CrossRef]
- Maragkaki, A.E.; Fountoulakis, M.; Gypakis, A.; Kyriakou, A.; Lasaridi, K.; Manios, T. Pilot-Scale Anaerobic Co-Digestion of Sewage Sludge with Agro-Industrial by-Products for Increased Biogas Production of Existing Digesters at Wastewater Treatment Plants. Waste Manag. 2017, 59, 362–370. [Google Scholar] [CrossRef]
- Srimachai, T.; Imai, T.; Rattanadilok Na Phuket, K. Biogas and Biohythane Production from Anaerobic Co-Digestion of Canned Sardine Wastewater with Glycerol Waste. ASEAN Sci. Tech. Rept. 2024, 27, 1–13. [Google Scholar] [CrossRef]
- Hutňan, M.; Kolesárová, N.; Bodík, I.; Czölderová, M. Long-Term Monodigestion of Crude Glycerol in a UASB Reactor. Bioresour. Technol. 2013, 130, 88–96. [Google Scholar] [CrossRef]
- Dhabhai, R.; Koranian, P.; Huang, Q.; Scheibelhoffer, D.S.B.; Dalai, A.K. Purification of Glycerol and Its Conversion to Value-Added Chemicals: A Review. Sep. Sci. Technol. 2023, 58, 1383–1402. [Google Scholar] [CrossRef]
- Kumar, L.R.; Yellapu, S.K.; Tyagi, R.D.; Zhang, X. A Review on Variation in Crude Glycerol Composition, Bio-Valorization of Crude and Purified Glycerol as Carbon Source for Lipid Production. Bioresour. Technol. 2019, 293, 122155. [Google Scholar] [CrossRef]
- Aguilar-Aguilar, F.; Adaya, L.; Godoy-Lozano, E.E.; Pantoja, L.A.; Dos Santos, A.S.; Eapen, D.; Sebastian, P.J. Anaerobic Co-Digestion of Raw Glycerol and Swine Manure: Microbial Communities. Biomass Conv. Bioref. 2023, 13, 7127–7138. [Google Scholar] [CrossRef]
- Panpong, K.; Srimachai, T.; Nuithitikul, K.; Kongjan, P.; O-Thong, S.; Imai, T.; Kaewthong, N. Anaerobic Co-Digestion between Canned Sardine Wastewater and Glycerol Waste for Biogas Production: Effect of Different Operating Processes. Energy Procedia 2017, 138, 260–266. [Google Scholar] [CrossRef]
- Prasertsan, P.; Leamdum, C.; Chantong, S.; Mamimin, C.; Kongjan, P.; O-Thong, S. Enhanced Biogas Production by Co-Digestion of Crude Glycerol and Ethanol with Palm Oil Mill Effluent and Microbial Community Analysis. Biomass Bioenergy 2021, 148, 106037. [Google Scholar] [CrossRef]
- Andriamanohiarisoamanana, F.J.; Saikawa, A.; Kan, T.; Qi, G.; Pan, Z.; Yamashiro, T.; Iwasaki, M.; Ihara, I.; Nishida, T.; Umetsu, K. Semi-Continuous Anaerobic Co-Digestion of Dairy Manure, Meat and Bone Meal and Crude Glycerol: Process Performance and Digestate Valorization. Renew. Energy 2018, 128, 1–8. [Google Scholar] [CrossRef]
- Simm, S.; Orrico, A.C.A.; Orrico Junior, M.A.P.; Sunada, N.D.S.; Schwingel, A.W.; Mendonça Costa, M.S.S.D. Crude Glycerin in Anaerobic Co-Digestion of Dairy Cattle Manure Increases Methane Production. Sci. Agric. (Piracicaba Braz.) 2017, 74, 175–179. [Google Scholar] [CrossRef]
- Farghali, M.; Mohamed, I.M.A.; Hassan, D.; Iwasaki, M.; Yoshida, G.; Umetsu, K.; Ihara, I. Kinetic Modeling of Anaerobic Co-Digestion with Glycerol: Implications for Process Stability and Organic Overloads. Biochem. Eng. J. 2023, 199, 109061. [Google Scholar] [CrossRef]
- Astals, S.; Nolla-Ardèvol, V.; Mata-Alvarez, J. Thermophilic Co-Digestion of Pig Manure and Crude Glycerol: Process Performance and Digestate Stability. J. Biotechnol. 2013, 166, 97–104. [Google Scholar] [CrossRef]
- Alves, I.R.F.S.; Mahler, C.F.; Oliveira, L.B.; Reis, M.M.; Bassin, J.P. Investigating the Effect of Crude Glycerol from Biodiesel Industry on the Anaerobic Co-Digestion of Sewage Sludge and Food Waste in Ternary Mixtures. Energy 2022, 241, 122818. [Google Scholar] [CrossRef]
- Razaviarani, V.; Buchanan, I.D. Anaerobic Co-Digestion of Biodiesel Waste Glycerin with Municipal Wastewater Sludge: Microbial Community Structure Dynamics and Reactor Performance. Bioresour. Technol. 2015, 182, 8–17. [Google Scholar] [CrossRef]
- Chou, Y.-C.; Su, J.-J. Biogas Production by Anaerobic Co-Digestion of Dairy Wastewater with the Crude Glycerol from Slaughterhouse Sludge Cake Transesterification. Animals 2019, 9, 618. [Google Scholar] [CrossRef]
- Castrillón, L.; Fernández-Nava, Y.; Ormaechea, P.; Marañón, E. Methane Production from Cattle Manure Supplemented with Crude Glycerin from the Biodiesel Industry in CSTR and IBR. Bioresour. Technol. 2013, 127, 312–317. [Google Scholar] [CrossRef]
- Fierro, J.; Martinez, E.J.; Rosas, J.G.; Fernández, R.A.; López, R.; Gomez, X. Co-Digestion of Swine Manure and Crude Glycerine: Increasing Glycerine Ratio Results in Preferential Degradation of Labile Compounds. Water Air Soil. Pollut. 2016, 227, 78. [Google Scholar] [CrossRef]
- Baba, Y.; Tada, C.; Watanabe, R.; Fukuda, Y.; Chida, N.; Nakai, Y. Anaerobic Digestion of Crude Glycerol from Biodiesel Manufacturing Using a Large-Scale Pilot Plant: Methane Production and Application of Digested Sludge as Fertilizer. Bioresour. Technol. 2013, 140, 342–348. [Google Scholar] [CrossRef]
- Dos Santos Ferreira, J.; Volschan, I.; Cammarota, M.C. Co-Digestion of Sewage Sludge with Crude or Pretreated Glycerol to Increase Biogas Production. Env. Sci. Pollut. Res. 2018, 25, 21811–21821. [Google Scholar] [CrossRef] [PubMed]
- Athanasoulia, E.; Melidis, P.; Aivasidis, A. Co-Digestion of Sewage Sludge and Crude Glycerol from Biodiesel Production. Renew. Energy 2014, 62, 73–78. [Google Scholar] [CrossRef]
- Bułkowska, K.; Mikucka, W.; Pokój, T. Enhancement of Biogas Production from Cattle Manure Using Glycerine Phase as a Co-Substrate in Anaerobic Digestion. Fuel 2022, 317, 123456. [Google Scholar] [CrossRef]
- Panpong, K.; Srisuwan, G.; O-Thong, S.; Kongjan, P. Anaerobic Co-Digestion of Canned Seafood Wastewater with Glycerol Waste for Enhanced Biogas Production. Energy Procedia 2014, 52, 328–336. [Google Scholar] [CrossRef]
- Astals, S.; Ariso, M.; Galí, A.; Mata-Alvarez, J. Co-Digestion of Pig Manure and Glycerine: Experimental and Modelling Study. J. Environ. Manag. 2011, 92, 1091–1096. [Google Scholar] [CrossRef]
- Lobato, A.; Cuetos, M.; Gómez, X.; Morán, A. Improvement of Biogas Production by Co-Digestion of Swine Manure and Residual Glycerine. Biofuels 2010, 1, 59–68. [Google Scholar] [CrossRef]
- Aguilar Aguilar, F.A.; Lee Nelson, D.; De Araújo Pantoja, L.; Soares Dos Santos, A. Study of Anaerobic Co-Digestion of Crude Glycerol and Swine Manure for the Production of Biogas. Rev. Virtual Quim. 2017, 9, 2383–2403. [Google Scholar] [CrossRef]
- González, R.; Smith, R.; Blanco, D.; Fierro, J.; Gómez, X. Application of Thermal Analysis for Evaluating the Effect of Glycerine Addition on the Digestion of Swine Manure. J. Therm. Anal. Calorim. 2019, 135, 2277–2286. [Google Scholar] [CrossRef]
- Lymperatou, A.; Skiadas, I.V.; Gavala, H.N. Anaerobic Co-Digestion of Swine Manure and Crude Glycerol Derived from Animal Fat—Effect of Hydraulic Retention Time. AIMS Environ. Sci. 2018, 5, 105–116. [Google Scholar] [CrossRef]
- Shober, A.L.; Maguire, R.O. Manure Management. In Reference Module in Earth Systems and Environmental Sciences; Elsevier: Berlin, Germany, 2018; ISBN 978-0-12-409548-9. [Google Scholar]
- Sommer, S.G.; Hutchings, N.J. Ammonia Emission from Field Applied Manure and Its Reduction—Invited Paper. Eur. J. Agron. 2001, 15, 1–15. [Google Scholar] [CrossRef]
- Mata-Alvarez, J.; Dosta, J.; Romero-Güiza, M.S.; Fonoll, X.; Peces, M.; Astals, S. A Critical Review on Anaerobic Co-Digestion Achievements between 2010 and 2013. Renew. Sustain. Energy Rev. 2014, 36, 412–427. [Google Scholar] [CrossRef]
- Song, Y.; Qiao, W.; Westerholm, M.; Huang, G.; Taherzadeh, M.J.; Dong, R. Microbiological and Technological Insights on Anaerobic Digestion of Animal Manure: A Review. Fermentation 2023, 9, 436. [Google Scholar] [CrossRef]
- Samoraj, M.; Mironiuk, M.; Izydorczyk, G.; Witek-Krowiak, A.; Szopa, D.; Moustakas, K.; Chojnacka, K. The Challenges and Perspectives for Anaerobic Digestion of Animal Waste and Fertilizer Application of the Digestate. Chemosphere 2022, 295, 133799. [Google Scholar] [CrossRef]
- Ankathi, S.K.; Chaudhari, U.S.; Handler, R.M.; Shonnard, D.R. Sustainability of Biogas Production from Anaerobic Digestion of Food Waste and Animal Manure. Appl. Microbiol. 2024, 4, 418–438. [Google Scholar] [CrossRef]
- Gyadi, T.; Bharti, A.; Basack, S.; Kumar, P.; Lucchi, E. Influential Factors in Anaerobic Digestion of Rice-Derived Food Waste and Animal Manure: A Comprehensive Review. Bioresour. Technol. 2024, 413, 131398. [Google Scholar] [CrossRef]
- Nuamah, A.; Malmgren, A.; Riley, G.; Lester, E. Biomass Co-Firing. In Comprehensive Renewable Energy; Elsevier: Berlin, Germany, 2012; pp. 55–73. ISBN 978-0-08-087873-7. [Google Scholar]
- Okopi, S.; Li, Y.; Xu, F. Biomass Digestion. In Encyclopedia of Sustainable Technologies; Elsevier: Berlin, Germany, 2024; pp. 236–251. ISBN 978-0-443-22287-0. [Google Scholar]
- Khawer, M.U.B.; Naqvi, S.R.; Ali, I.; Arshad, M.; Juchelková, D.; Anjum, M.W.; Naqvi, M. Anaerobic Digestion of Sewage Sludge for Biogas & Biohydrogen Production: State-of-the-Art Trends and Prospects. Fuel 2022, 329, 125416. [Google Scholar] [CrossRef]
- Yuan, H.; Zhu, N. Progress in Inhibition Mechanisms and Process Control of Intermediates and By-Products in Sewage Sludge Anaerobic Digestion. Renew. Sustain. Energy Rev. 2016, 58, 429–438. [Google Scholar] [CrossRef]
- Liew, C.S.; Yunus, N.M.; Chidi, B.S.; Lam, M.K.; Goh, P.S.; Mohamad, M.; Sin, J.C.; Lam, S.M.; Lim, J.W.; Lam, S.S. A Review on Recent Disposal of Hazardous Sewage Sludge via Anaerobic Digestion and Novel Composting. J. Hazard. Mater. 2022, 423, 126995. [Google Scholar] [CrossRef]
- Damian, C.S.; Devarajan, Y.; Thandavamoorthy, R. Sewage Sludge as a Sustainable Feedstock for Biodiesel: Advances in Conversion Technologies and Catalytic Applications. Results Eng. 2025, 25, 104000. [Google Scholar] [CrossRef]
- Zahedi, S.; Rivero, M.; Solera, R.; Perez, M. Mesophilic Anaerobic Co-Digestion of Sewage Sludge with Glycerine: Effect of Solids Retention Time. Fuel 2018, 215, 285–289. [Google Scholar] [CrossRef]
- ElMekawy, A.; Srikanth, S.; Bajracharya, S.; Hegab, H.M.; Nigam, P.S.; Singh, A.; Mohan, S.V.; Pant, D. Food and Agricultural Wastes as Substrates for Bioelectrochemical System (BES): The Synchronized Recovery of Sustainable Energy and Waste Treatment. Food Res. Int. 2015, 73, 213–225. [Google Scholar] [CrossRef]
- Ormaechea, P.; Castrillón, L.; Suárez-Peña, B.; Megido, L.; Fernández-Nava, Y.; Negral, L.; Marañón, E.; Rodríguez-Iglesias, J. Enhancement of Biogas Production from Cattle Manure Pretreated and/or Co-Digested at Pilot-Plant Scale. Characterization by SEM. Renew. Energy 2018, 126, 897–904. [Google Scholar] [CrossRef]
- Font-Palma, C. Methods for the Treatment of Cattle Manure—A Review. C 2019, 5, 27. [Google Scholar] [CrossRef]
- Rico, C.; García, H.; Rico, J.L. Physical–Anaerobic–Chemical Process for Treatment of Dairy Cattle Manure. Bioresour. Technol. 2011, 102, 2143–2150. [Google Scholar] [CrossRef] [PubMed]
- Manyi-Loh, C.; Mamphweli, S.; Meyer, E.; Makaka, G.; Simon, M.; Okoh, A. An Overview of the Control of Bacterial Pathogens in Cattle Manure. IJERPH 2016, 13, 843. [Google Scholar] [CrossRef]
- Tufaner, F.; Avşar, Y. Effects of Co-Substrate on Biogas Production from Cattle Manure: A Review. Int. J. Environ. Sci. Technol. 2016, 13, 2303–2312. [Google Scholar] [CrossRef]
- Youcai, Z. Summary. In Pollution Control Technology for Leachate from Municipal Solid Waste; Elsevier: Berlin, Germany, 2018; ISBN 978-0-12-815813-5. [Google Scholar]
- Ehrig, H.-J.; Stegmann, R. Leachate Quality. In Solid Waste Landfilling; Elsevier: Berlin, Germany, 2018; pp. 511–539. ISBN 978-0-12-818336-6. [Google Scholar]
- Abdel-Shafy, H.I.; Ibrahim, A.M.; Al-Sulaiman, A.M.; Okasha, R.A. Landfill Leachate: Sources, Nature, Organic Composition, and Treatment: An Environmental Overview. Ain Shams Eng. J. 2024, 15, 102293. [Google Scholar] [CrossRef]
- Mojiri, A.; Zhou, J.L.; Ratnaweera, H.; Ohashi, A.; Ozaki, N.; Kindaichi, T.; Asakura, H. Treatment of Landfill Leachate with Different Techniques: An Overview. J. Water Reuse Desalination 2021, 11, 66–96. [Google Scholar] [CrossRef]
- Renou, S.; Givaudan, J.G.; Poulain, S.; Dirassouyan, F.; Moulin, P. Landfill Leachate Treatment: Review and Opportunity. J. Hazard. Mater. 2008, 150, 468–493. [Google Scholar] [CrossRef]
- Babaei, S.; Sabour, M.R.; Moftakhari Anasori Movahed, S. Combined Landfill Leachate Treatment Methods: An Overview. Env. Sci. Pollut. Res. 2021, 28, 59594–59607. [Google Scholar] [CrossRef]
- Mikucka, W.; Zielińska, M. Distillery Stillage: Characteristics, Treatment, and Valorization. Appl. Biochem. Biotechnol. 2020, 192, 770–793. [Google Scholar] [CrossRef]
- Ratna, S.; Rastogi, S.; Kumar, R. Current Trends for Distillery Wastewater Management and Its Emerging Applications for Sustainable Environment. J. Environ. Manag. 2021, 290, 112544. [Google Scholar] [CrossRef] [PubMed]
- Bezuneh, T.T. The Role of Microorganisms in Distillery Wastewater Treatment: A Review. J. Bioremediat. Biodegrad. 2016, 7, 1000375. [Google Scholar] [CrossRef]
- Kumar, V.; Chowdhary, P.; Shah, M.P. Recent Advances in Distillery Waste Management for Environmental Safety; CRC Press: Boca Raton, FL, USA, 2021; ISBN 978-1-00-302988-5. [Google Scholar]
- Tripathi, S.; Sharma, P.; Purchase, D.; Chandra, R. Distillery Wastewater Detoxification and Management through Phytoremediation Employing Ricinus communis L. Bioresour. Technol. 2021, 333, 125192. [Google Scholar] [CrossRef]
- Kohli, K.; Prajapati, R.; Shah, R.; Das, M.; Sharma, B.K. Food Waste: Environmental Impact and Possible Solutions. Sustain. Food Technol. 2024, 2, 70–80. [Google Scholar] [CrossRef]
- Ilakovac, B.; Voca, N.; Pezo, L.; Cerjak, M. Quantification and Determination of Household Food Waste and Its Relation to Sociodemographic Characteristics in Croatia. Waste Manag. 2020, 102, 231–240. [Google Scholar] [CrossRef]
- Li, Y.; Wang, L.; Liu, G.; Cheng, S. Rural Household Food Waste Characteristics and Driving Factors in China. Resour. Conserv. Recycl. 2021, 164, 105209. [Google Scholar] [CrossRef]
- Wang, P.; Wang, H.; Qiu, Y.; Ren, L.; Jiang, B. Microbial Characteristics in Anaerobic Digestion Process of Food Waste for Methane Production–A Review. Bioresour. Technol. 2018, 248, 29–36. [Google Scholar] [CrossRef]
- Paritosh, K.; Kushwaha, S.K.; Yadav, M.; Pareek, N.; Chawade, A.; Vivekanand, V. Food Waste to Energy: An Overview of Sustainable Approaches for Food Waste Management and Nutrient Recycling. BioMed Res. Int. 2017, 2017, 1–19. [Google Scholar] [CrossRef]
- Al-Obadi, M.; Ayad, H.; Pokharel, S.; Ayari, M.A. Perspectives on Food Waste Management: Prevention and Social Innovations. Sustain. Prod. Consum. 2022, 31, 190–208. [Google Scholar] [CrossRef]
- Thi, N.B.D.; Kumar, G.; Lin, C.-Y. An Overview of Food Waste Management in Developing Countries: Current Status and Future Perspective. J. Environ. Manag. 2015, 157, 220–229. [Google Scholar] [CrossRef]
- Ananno, A.A.; Masud, M.H.; Chowdhury, S.A.; Dabnichki, P.; Ahmed, N.; Arefin, A.M.E. Sustainable Food Waste Management Model for Bangladesh. Sustain. Prod. Consum. 2021, 27, 35–51. [Google Scholar] [CrossRef]
- Zhang, C.; Su, H.; Baeyens, J.; Tan, T. Reviewing the Anaerobic Digestion of Food Waste for Biogas Production. Renew. Sustain. Energy Rev. 2014, 38, 383–392. [Google Scholar] [CrossRef]
- Mirmohamadsadeghi, S.; Karimi, K.; Tabatabaei, M.; Aghbashlo, M. Biogas Production from Food Wastes: A Review on Recent Developments and Future Perspectives. Bioresour. Technol. Rep. 2019, 7, 100202. [Google Scholar] [CrossRef]
- Bong, C.P.C.; Lim, L.Y.; Lee, C.T.; Klemeš, J.J.; Ho, C.S.; Ho, W.S. The Characterisation and Treatment of Food Waste for Improvement of Biogas Production during Anaerobic Digestion—A Review. J. Clean. Prod. 2018, 172, 1545–1558. [Google Scholar] [CrossRef]
- Leung, D.Y.C.; Wang, J. An Overview on Biogas Generation from Anaerobic Digestion of Food Waste. Int. J. Green Energy 2016, 13, 119–131. [Google Scholar] [CrossRef]
- Chew, K.R.; Leong, H.Y.; Khoo, K.S.; Vo, D.-V.N.; Anjum, H.; Chang, C.-K.; Show, P.L. Effects of Anaerobic Digestion of Food Waste on Biogas Production and Environmental Impacts: A Review. Environ. Chem. Lett. 2021, 19, 2921–2939. [Google Scholar] [CrossRef]
- Muñoz-Martínez, S.; Salmerón-Alcocer, A.M.; Rodríguez-Casasola, F.N.; Ahuatzi-Chacón, D. Biogas Production by Anaerobic Co-Digestion of Agro-Industrial Waste and Crude Glycerol. Agrociencia 2025, 59, 1–15. [Google Scholar] [CrossRef]
- Robra, S.; Serpa Da Cruz, R.; De Oliveira, A.M.; Neto, J.A.A.; Santos, J.V. Generation of Biogas Using Crude Glycerin from Biodiesel Production as a Supplement to Cattle Slurry. Biomass Bioenergy 2010, 34, 1330–1335. [Google Scholar] [CrossRef]
- Lovato, G.; Batista, L.P.P.; Preite, M.B.; Yamashiro, J.N.; Becker, A.L.S.; Vidal, M.F.G.; Pezini, N.; Albanez, R.; Ratusznei, S.M.; Rodrigues, J.A.D. Viability of Using Glycerin as a Co-Substrate in Anaerobic Digestion of Sugarcane Stillage (Vinasse): Effect of Diversified Operational Strategies. Appl. Biochem. Biotechnol. 2019, 188, 720–740. [Google Scholar] [CrossRef]
- Madondo, N.I.; Chetty, M. Anaerobic Co-Digestion of Sewage Sludge and Bio-Based Glycerol: Optimisation of Process Variables Using One-Factor-at-a-Time (OFAT) and Box-Behnken Design (BBD) Techniques. S. Afr. J. Chem. Eng. 2022, 40, 87–99. [Google Scholar] [CrossRef]
- Li, P.; Zhao, H.; Cheng, C.; Hou, T.; Shen, D.; Jiao, Y. A Review on Anaerobic Co-Digestion of Sewage Sludge with Other Organic Wastes for Methane Production: Mechanism, Process, Improvement and Industrial Application. Biomass Bioenergy 2024, 185, 107241. [Google Scholar] [CrossRef]
- Ibro, M.K.; Ancha, V.R.; Lemma, D.B. Impacts of Anaerobic Co-Digestion on Different Influencing Parameters: A Critical Review. Sustainability 2022, 14, 9387. [Google Scholar] [CrossRef]
- Ryue, J.; Lin, L.; Kakar, F.L.; Elbeshbishy, E.; Al-Mamun, A.; Dhar, B.R. A Critical Review of Conventional and Emerging Methods for Improving Process Stability in Thermophilic Anaerobic Digestion. Energy Sustain. Dev. 2020, 54, 72–84. [Google Scholar] [CrossRef]
- Suryawanshi, P.C.; Chaudhari, A.B.; Kothari, R.M. Thermophilic Anaerobic Digestion: The Best Option for Waste Treatment. Crit. Rev. Biotechnol. 2010, 30, 31–40. [Google Scholar] [CrossRef]
- Rawoof, S.A.A.; Kumar, P.S.; Vo, D.-V.N.; Subramanian, S. Sequential Production of Hydrogen and Methane by Anaerobic Digestion of Organic Wastes: A Review. Environ. Chem. Lett. 2021, 19, 1043–1063. [Google Scholar] [CrossRef]
- Gonde, L.; Wickham, T.; Brink, H.G.; Nicol, W. pH-Based Control of Anaerobic Digestion to Maximise Ammonium Production in Liquid Digestate. Water 2023, 15, 417. [Google Scholar] [CrossRef]
- Hossain, M.S.; Karim, T.U.; Onik, M.H.; Kumar, D.; Rahman, M.A.; Yousuf, A.; Uddin, M.R. Impact of Temperature, Inoculum Flow Pattern, Inoculum Type, and Their Ratio on Dry Anaerobic Digestion for Biogas Production. Sci. Rep. 2022, 12, 6162. [Google Scholar] [CrossRef] [PubMed]
- Cioabla, A.E.; Ionel, I.; Dumitrel, G.-A.; Popescu, F. Comparative Study on Factors Affecting Anaerobic Digestion of Agricultural Vegetal Residues. Biotechnol. Biofuels 2012, 5, 39. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomczak, W.; Żak, S.; Kujawska, A.; Szwast, M. The Use of Crude Glycerol as a Co-Substrate for Anaerobic Digestion. Molecules 2025, 30, 3655. https://doi.org/10.3390/molecules30173655
Tomczak W, Żak S, Kujawska A, Szwast M. The Use of Crude Glycerol as a Co-Substrate for Anaerobic Digestion. Molecules. 2025; 30(17):3655. https://doi.org/10.3390/molecules30173655
Chicago/Turabian StyleTomczak, Wirginia, Sławomir Żak, Anna Kujawska, and Maciej Szwast. 2025. "The Use of Crude Glycerol as a Co-Substrate for Anaerobic Digestion" Molecules 30, no. 17: 3655. https://doi.org/10.3390/molecules30173655
APA StyleTomczak, W., Żak, S., Kujawska, A., & Szwast, M. (2025). The Use of Crude Glycerol as a Co-Substrate for Anaerobic Digestion. Molecules, 30(17), 3655. https://doi.org/10.3390/molecules30173655








