Fluorescence of 4-Cyanophenylhydrazones: From Molecular Design to Electrospun Polymer Fibers
Abstract
1. Introduction
2. Results and Discussion
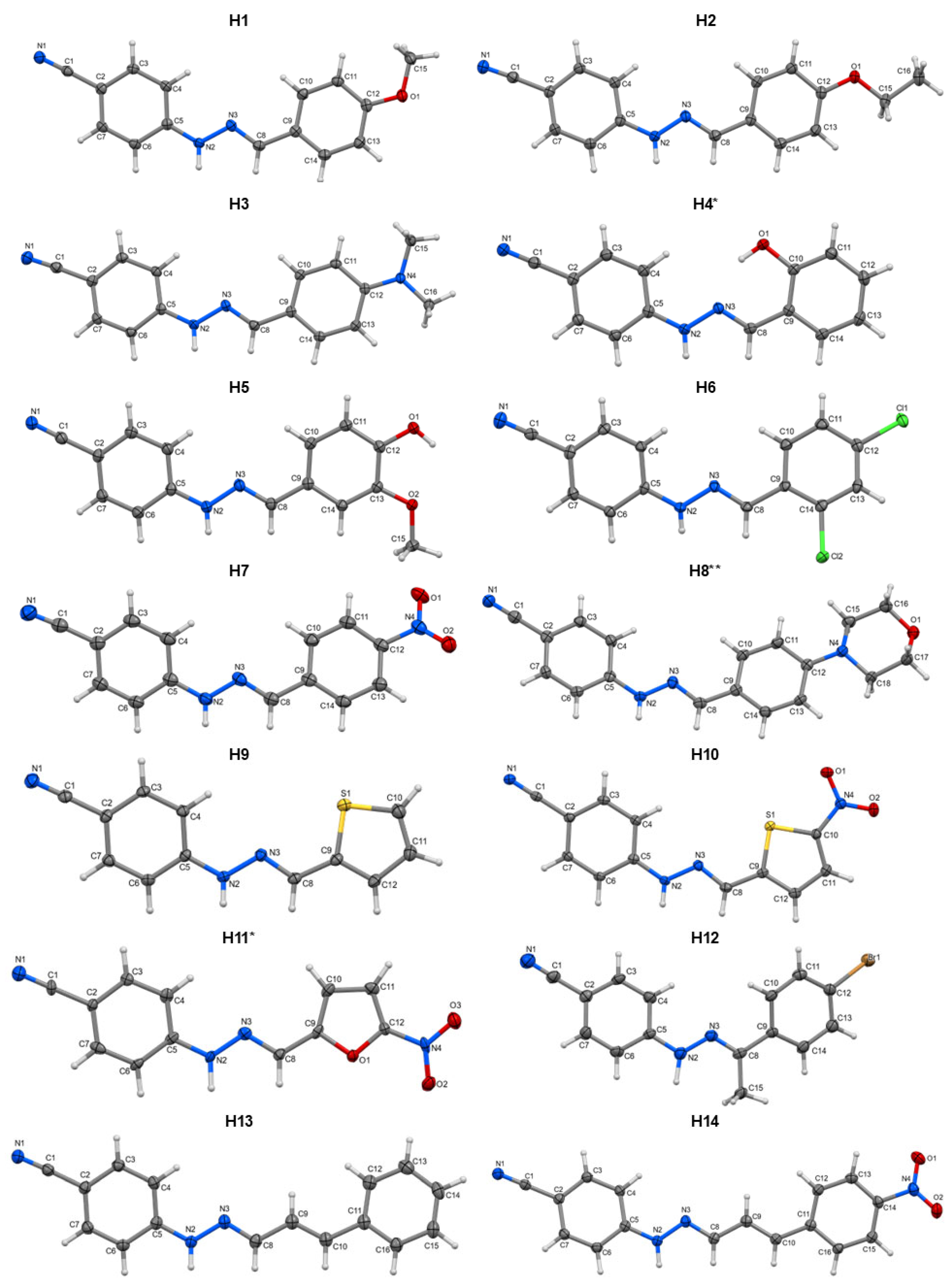
2.1. Crystal Structure Analysis
2.2. Analysis of Absorption Properties of Phenylhydrazones
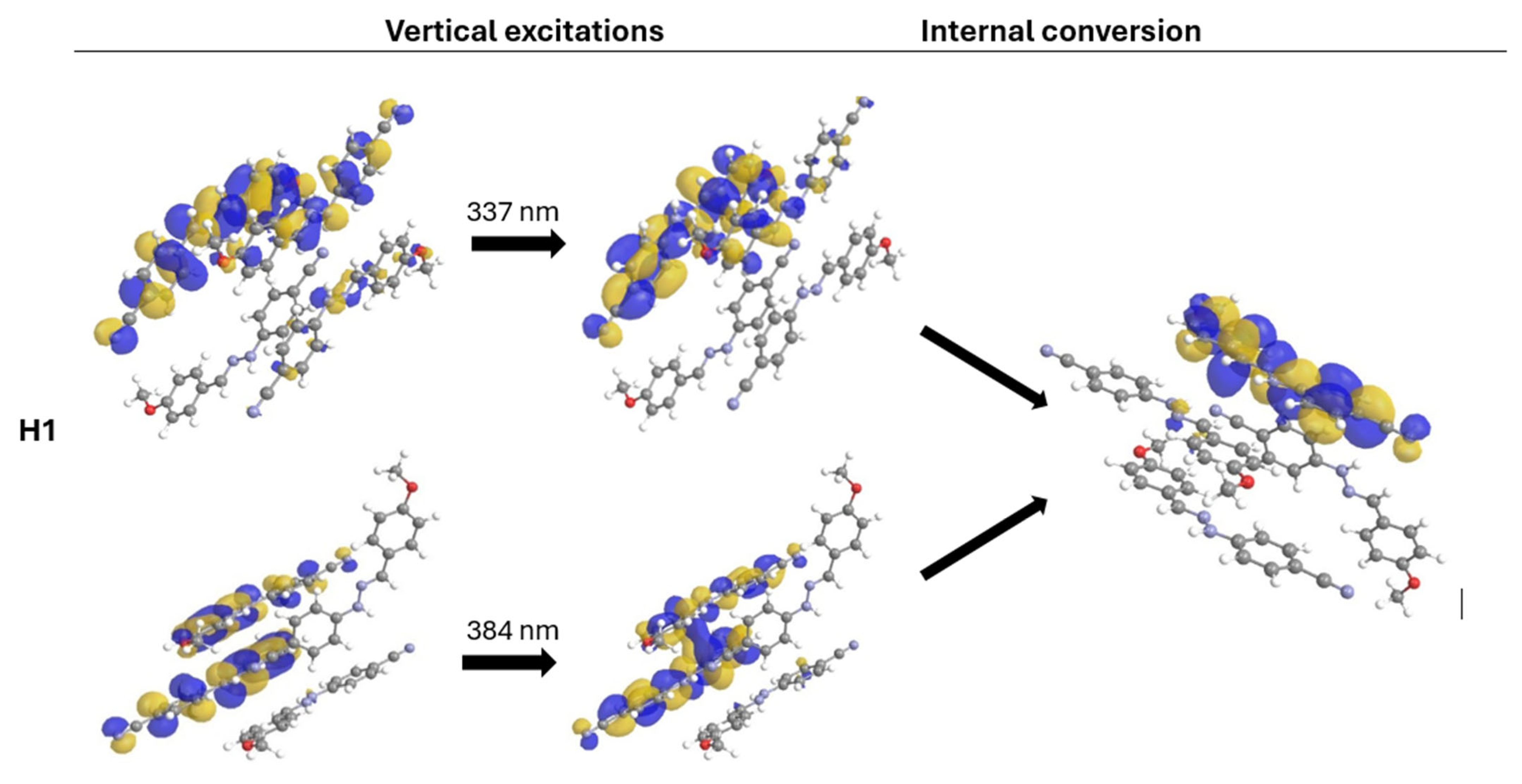
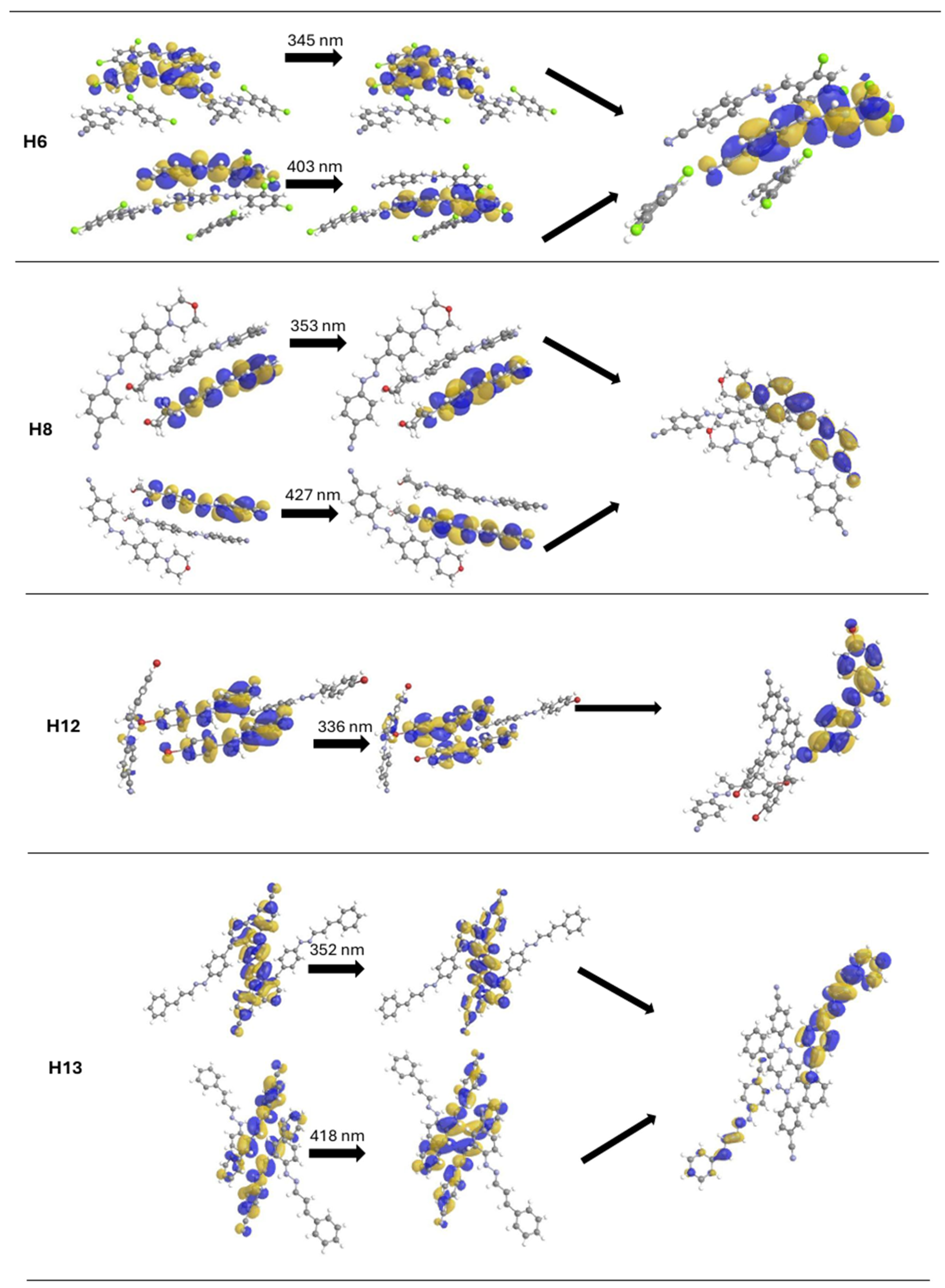
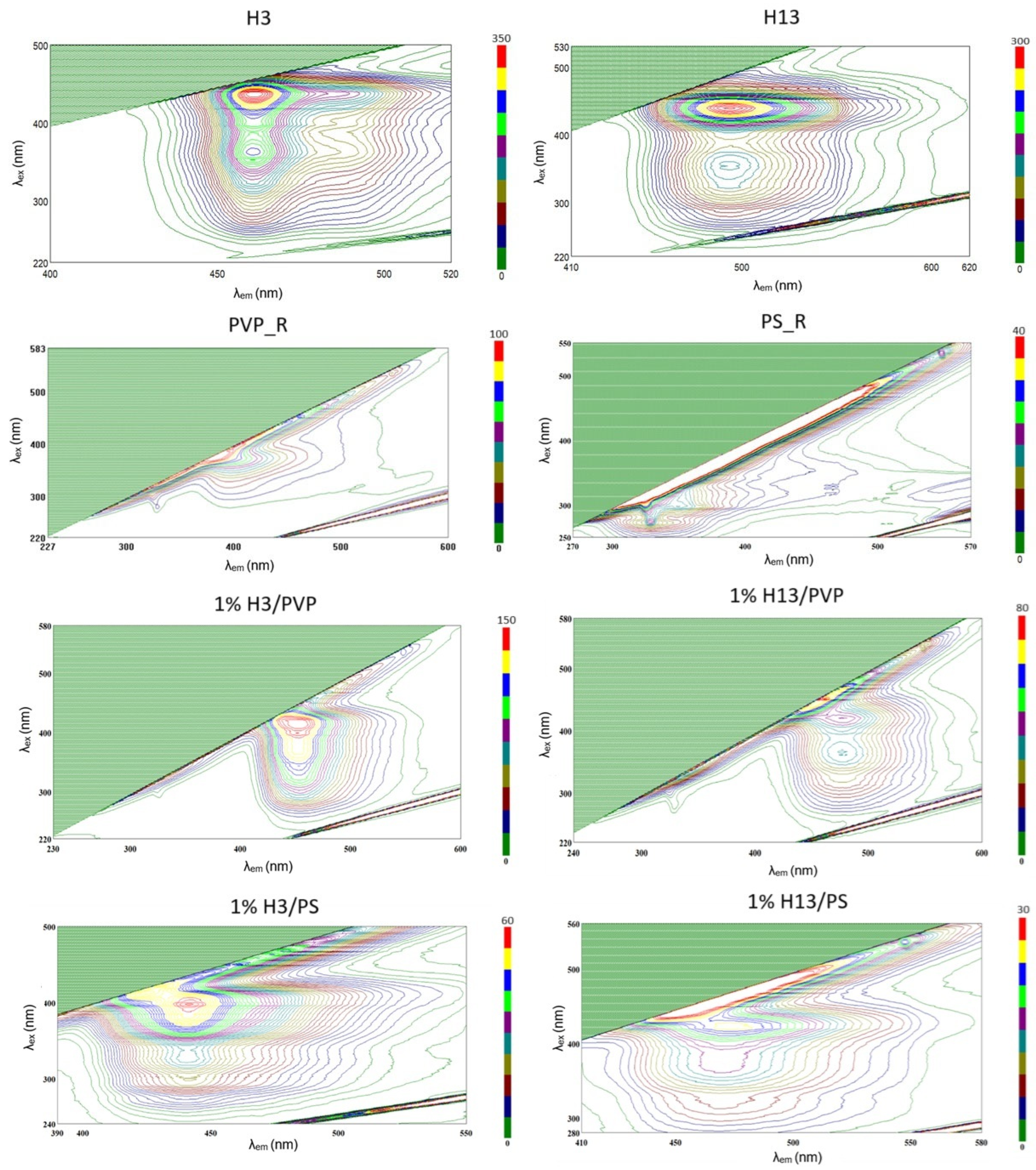
2.3. Analysis of Fluorescent Properties of Phenylhydrazones
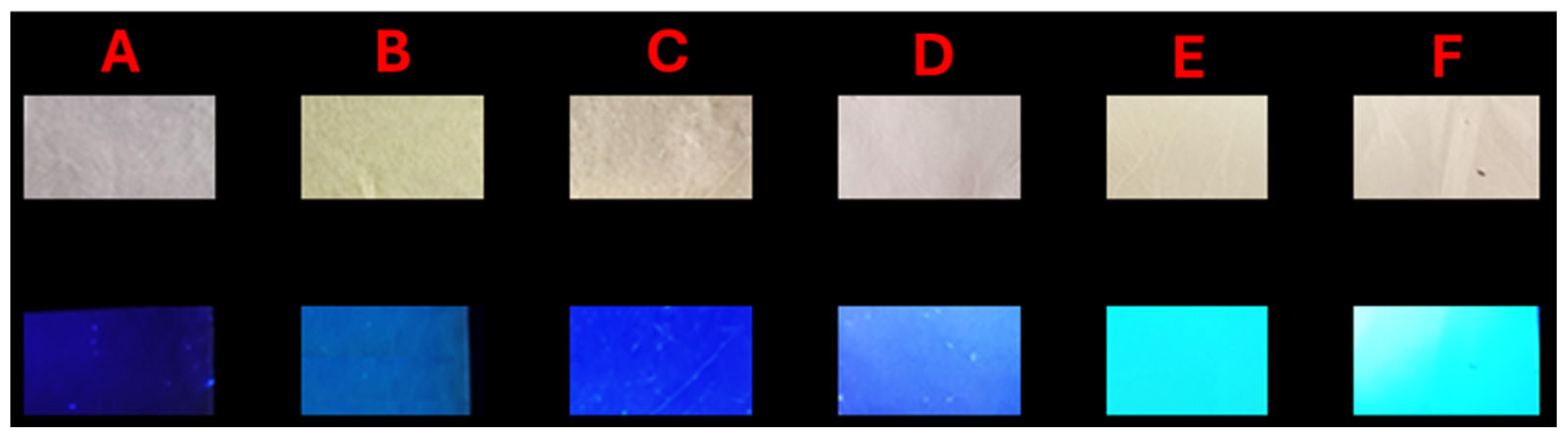
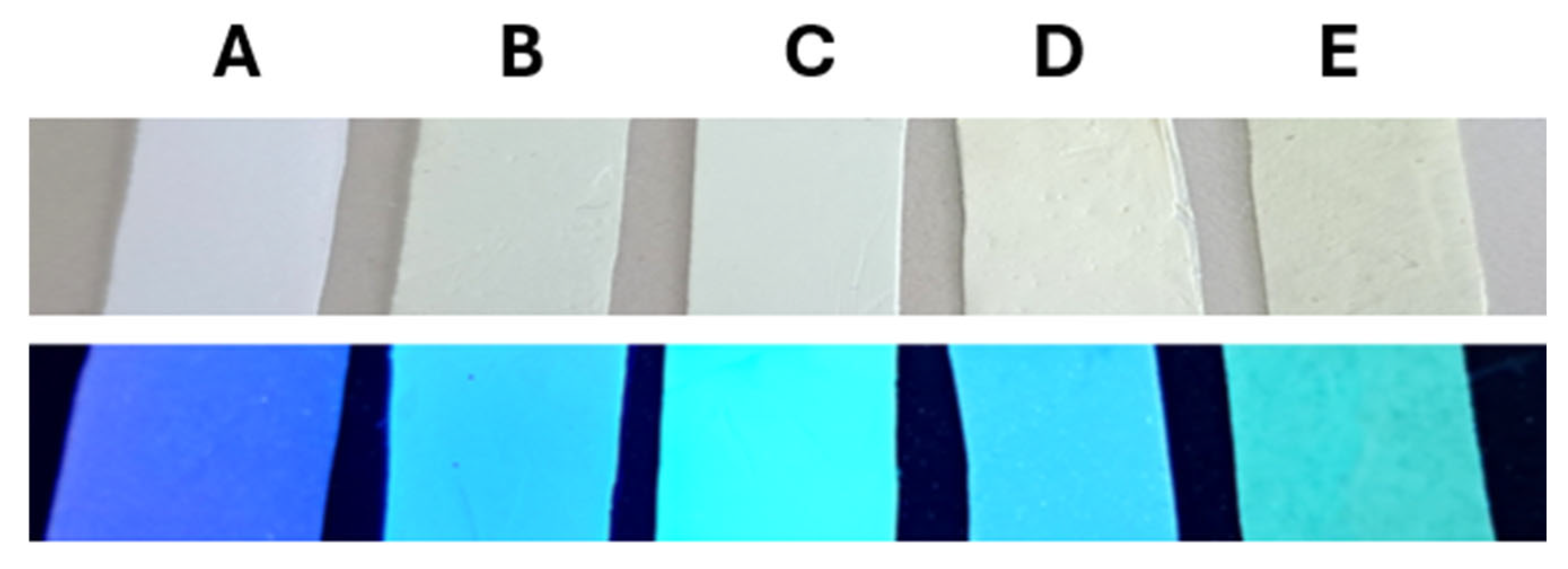
2.4. Modulation of Hydrazone Properties in Electrospun Polymer Matrices
2.4.1. Morphological Analysis and Structural Stability
2.4.2. Photophysical Properties and Dye–Polymer Interactions
2.4.3. Influence of Dye Concentration on Quantum Yield in the PVP Matrix
2.4.4. Thermal Stability of Fluorescence Properties
3. Materials and Methods
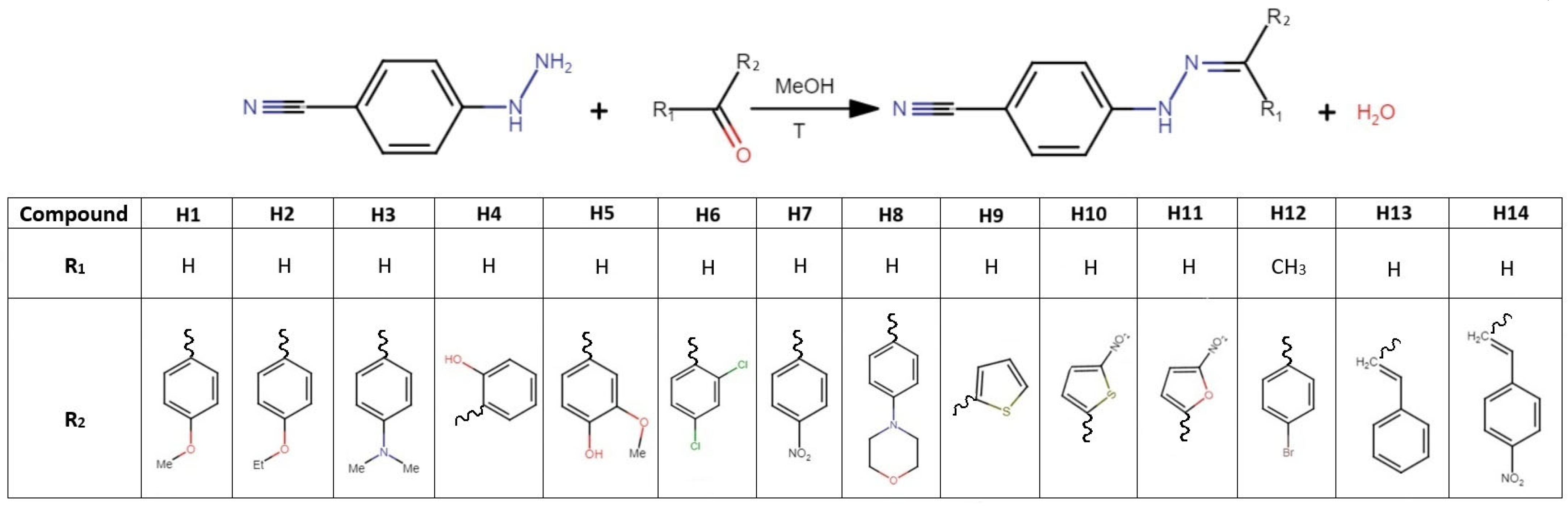
3.1. Synthesis of Phenylhydrazones
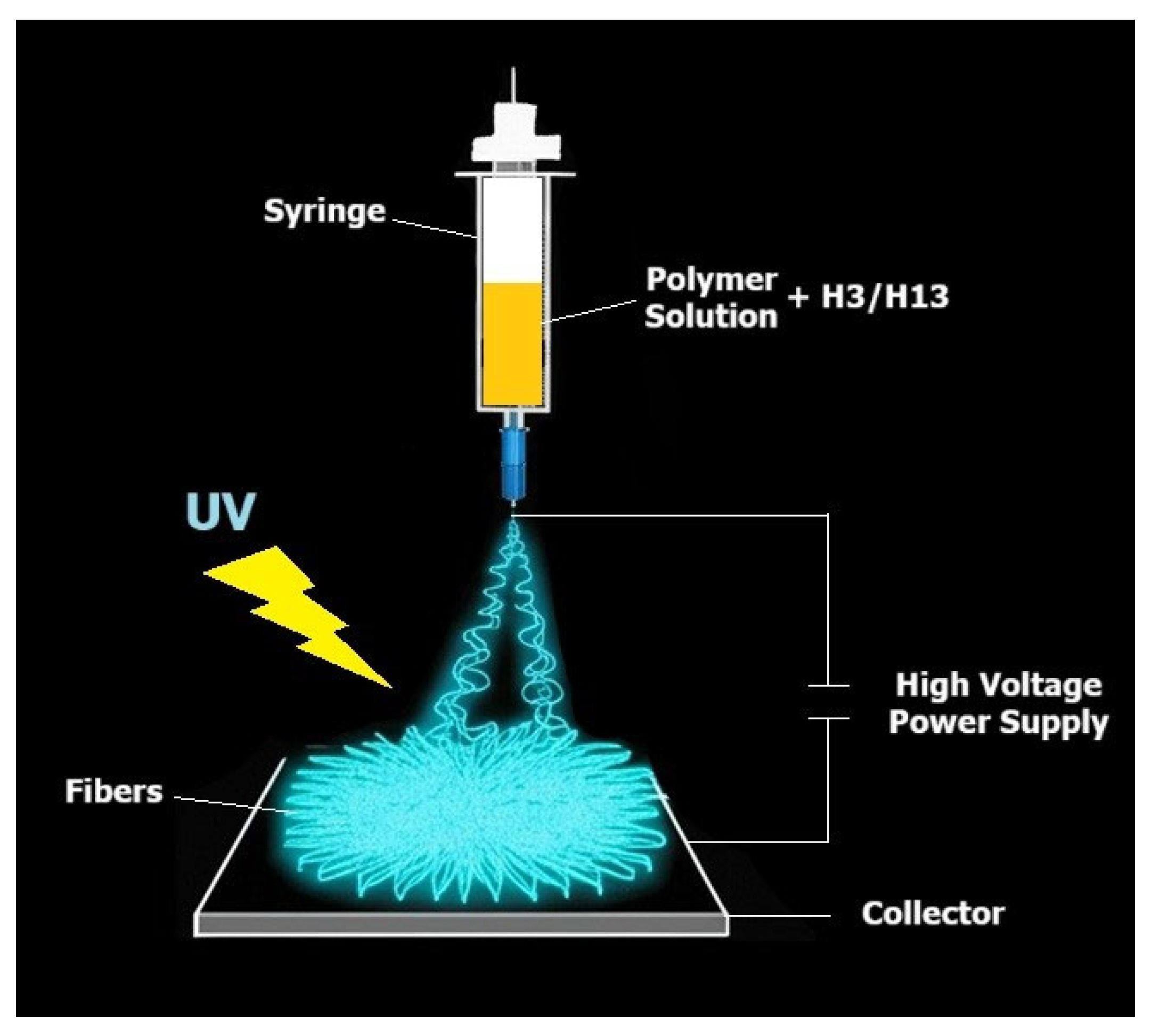
3.2. Preparation of Electrospinning Solutions
3.3. Electrospinning Process
3.4. Instrumental Studies
3.4.1. Single-Crystal X-Ray Diffraction (SC-XRD) Studies
3.4.2. FTIR and ATR Spectroscopy
3.4.3. NMR
3.4.4. DSC
- Results of FTIR, NMR and DSC measurements:
3.4.5. UV-VIS Spectroscopy
3.4.6. Fluorescence Spectroscopy
- Excitation and Emission Spectra: High-resolution excitation spectra were recorded over a focused range (250–450 nm) while monitoring the emission at the respective maxima identified in the initial screening (464 nm for H3 and 485 nm for H13 systems). Correspondingly, detailed emission spectra were recorded upon excitation at several distinct wavelengths (280 nm, 320 nm, 380 nm) to verify the principle of excitation-emission independence within the polymer host.
- Quantum Yield of Doped Matrices: The absolute quantum yields of the dye-doped mats were also determined using the FS5 instrument’s integrating sphere. The excitation wavelengths were specifically selected to match the absorption maxima of the hydrazones once embedded within the polymer matrix (415 nm for H3-doped matrices and 405 nm for H13-doped matrices), which can differ slightly from the pure powder form.
- Temperature-Dependent Studies: The thermal stability of the polymer mats was assessed using a temperature-controlled reflectance setup integrated with the FS5 spectrometer. For the analysis, the sample was positioned on a heating plate equipped with an adjacent thermocouple to monitor the surface temperature. A 460 nm LED was employed as a stable excitation source, and emission was recorded at 25 °C, 50 °C, and 100 °C. Each measurement was taken at the moment the thermocouple confirmed that the setpoint temperature had been reached.
3.4.7. Scanning Electron Microscopy (SEM)
3.5. Computational Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khattab, T.A. From chromic switchable hydrazones to smart materials. Mater. Chem. Phys. 2020, 254, 123456. [Google Scholar] [CrossRef]
- Gao, L.-L.; Wang, B.-B.; Chen, X.; Wang, Y.; Wu, W.-N.; Zhao, X.-L.; Yan, L.-L.; Fan, Y.-C.; Xu, Z.-H. Hydrazone derivative bearing coumarin for the relay detection of Cu2+ and H2S in an almost neat aqueous solution and bioimaging in lysosomes. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 255, 119693. [Google Scholar] [CrossRef] [PubMed]
- Alsharif, M.A.; Naeem, N.; Mughal, E.U.; Sadiq, A.; Jassas, R.S.; Kausar, S.; Altaf, A.A.; Zafar, M.N.; Mumtaz, A.; Obaid, R.J.; et al. Experimental and theoretical insights into the photophysical and electrochemical properties of flavone-based hydrazones. J. Mol. Struct. 2021, 1244, 130965. [Google Scholar] [CrossRef]
- Wu, B.-X.; Chang, H.-Y.; Liao, Y.-S.; Yeh, M.-Y. Synthesis, photochemical isomerization and photophysical properties of hydrazide–hydrazone derivatives. New J. Chem. 2021, 45, 1651–1657. [Google Scholar] [CrossRef]
- Mellado, M.; Sariego-Kluge, R.; González, C.; Díaz, K.; Aguilar, L.F.; Bravo, M.A. Systematic study of the fluorescent properties of cinnamaldehyde phenylhydrazone and its interactions with metals: Synthesis and photophysical evaluation. J. Mol. Struct. 2020, 1217, 128430. [Google Scholar] [CrossRef]
- Niu, R.; Chen, S.; Zhou, W.; Wu, X.; Yang, J.; Wang, Y.; Zhang, X.; Song, Y. Investigation of ultrafast broadband optical nonlinearity and intensity-dependent nonlinear absorption in novel hydrazone derivatives. Opt. Laser Technol. 2022, 149, 107798. [Google Scholar] [CrossRef]
- Sivadas, D.K.; Ravi, S.; Nagarasu, P.; Johnson, D.; Thiruvenkatam, V.; Anthony, S.P.; Madhu, V. Fluorophore unit controlled photoswitching of hydrazone derivatives: Reversible and irreversible off–on/dual-color fluorescence photoswitches. Mol. Syst. Des. Eng. 2023, 8, 1462–1469. [Google Scholar] [CrossRef]
- Idemudia, O.; Sadimenko, A.; Hosten, E. Metal Complexes of New Bioactive Pyrazolone Phenylhydrazones; Crystal Structure of 4-Acetyl-3-methyl-1-phenyl-2-pyrazoline-5-one phenylhydrazone Ampp-Ph. J. Mol. Sci. 2016, 17, 687. [Google Scholar] [CrossRef]
- Tcelykh, L.O.; Vashchenko, A.A.; Medved’ko, A.V.; Marciniak, Ł.; Aleksandrov, A.E.; Goloveshkin, A.S.; Lepnev, L.S.; Latipov, E.V.; Burlov, A.S.; Utochnikova, V.V. Ytterbium complexes with 2-(tosylamino)-benzylidene-N-(2-halobenzoyl)-hydrazones for solution-processable NIR OLEDs. J. Mater. Chem. C 2022, 10, 1371–1380. [Google Scholar] [CrossRef]
- Makhlouf, M.M.; Shehata, M.M. Improvement of photoresponse in an isatin hydrazone/CdTe hybrid heterostructure device via ZnO chelation for photovoltaic applications. Sol. Energy 2020, 211, 1128–1136. [Google Scholar] [CrossRef]
- Decavoli, C.; Boldrini, C.L.; Abbotto, A.; Manfredi, N. Economical and Environmentally Friendly Organic hydrazone Derivatives Characterized by a Heteroaromatic Core as Potential Hole Transporting Materials in Perovskite Solar Cells. Eur. J. Org. Chem. 2023, 26, e202201511. [Google Scholar] [CrossRef]
- Sakr, M.A.S.; Sherbiny, F.F.; El-Etrawy, A.-A.S. Hydrazone-based Materials; DFT, TD-DFT, NBO Analysis, Fukui Function, MESP Analysis, and Solar Cell Applications. J. Fluoresc. 2022, 32, 1857–1871. [Google Scholar] [CrossRef]
- Emami, M.; Shahroosvand, H.; Bikas, R.; Lis, T.; Daneluik, C.; Pilkington, M. Synthesis, Study, and Application of Pd(II) Hydrazone Complexes as the Emissive Components of Single-Layer Light-Emitting Electrochemical Cells. Inorg. Chem. 2021, 60, 982–994. [Google Scholar] [CrossRef]
- Doğan, K.; Gülkaya, A.; Forough, M.; Persil Çetinkol, Ö. Novel Fluorescent Azacyanine Compounds: Improved Synthesis and Optical Properties. ACS Omega 2020, 5, 22874–22882. [Google Scholar] [CrossRef]
- Porobić, S.J.; Božić, B.Đ.; Dramićanin, M.D.; Vitnik, V.; Vitnik, Ž.; Marinović-Cincović, M.; Mijin, D.Ž. Absorption and fluorescence spectral properties of azo dyes based on 3-amido-6-hydroxy-4-methyl-2-pyridone: Solvent and substituent effects. Dye. Pigment. 2020, 175, 108139. [Google Scholar] [CrossRef]
- Sun, X.; Yan, F.; Jiang, Y.; Zhang, H.; Sun, Z.; Wang, R.; Cui, Y. Designing enhanced and ratiometric probes for detecting OCl − based on substituents influencing the fluorescence of HBT and their application in strips and bioimaging. Analyst 2020, 145, 5933–5939. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.N.; Lyngkhoi, D.L.; Gaikwad, S.; Samanta, D.; Khatua, S.; Pramanik, S. The effect of substituents on the aggregation-induced emission of 9,10-phenanthraquinone-hydrazones. New J. Chem. 2023, 47, 15066–15075. [Google Scholar] [CrossRef]
- Fei-Hua, L. Theoretical Study of the Substituent Effect on the Electronic Structure and Spectral Properties of Six Salicylaldehyde Schiff Bases. Russ. J. Phys. Chem. 2020, 94, 352–359. [Google Scholar] [CrossRef]
- Dilek, O.; Bane, S.L. Synthesis and Spectroscopic Characterization of Fluorescent Boron Dipyrromethene-Derived Hydrazones. J. Fluoresc. 2011, 21, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Jois, H.S.V.; Kalluraya, B.; Vishwanath, T. Synthesis, Spectroscopic Properties and Antioxidant Activity of Bis-Hydrazones and Schiff’s bases Derived from Terephthalic Dihydrazide. J. Fluoresc. 2015, 25, 481–488. [Google Scholar] [CrossRef]
- Aysha, T.; Luňák, S.; Lyčka, A.; Hrdina, R. Synthesis, absorption and fluorescence of hydrazone colorants based on pyrrolinone esters. Dye. Pigment. 2011, 91, 170–176. [Google Scholar] [CrossRef]
- Coto, P.B.; Serrano-Andrés, L.; Gustavsson, T.; Fujiwara, T.; Lim, E.C. Intramolecular charge transfer and dual fluorescence of 4-(dimethylamino)benzonitrile: Ultrafast branching followed by a two-fold decay mechanism. Phys. Chem. Chem. Phys. 2011, 13, 15182–15188. [Google Scholar] [CrossRef]
- Grabowski, Z.R.; Rotkiewicz, K.; Rettig, W. Structural Changes Accompanying Intramolecular Electron Transfer: Focus on Twisted Intramolecular Charge-Transfer States and Structures. Chem. Rev. 2003, 103, 3899–4032. [Google Scholar] [CrossRef]
- Pijeau, S.; Foster, D.; Hohenstein, E.G. Effect of Nonplanarity on Excited-State Proton Transfer and Internal Conversion in Salicylideneaniline. J. Phys. Chem. A 2018, 122, 5555–5562. [Google Scholar] [CrossRef] [PubMed]
- Ziółek, M.; Kubicki, J.; Maciejewski, A.; Naskręcki, R.; Grabowska, A. An ultrafast excited state intramolecular proton transfer (ESPIT) and photochromism of salicylideneaniline (SA) and its “double” analogue salicylaldehyde azine (SAA). A controversial case. Phys. Chem. Chem. Phys. 2004, 6, 4682–4689. [Google Scholar] [CrossRef]
- Zachariasse, K.A. Comment on “Pseudo-Jahn–Teller and TICT-models: A photophysical comparison of meta-and para-DMABN derivatives” [Chem. Phys. Lett. 305 (1999) 8]. Chem. Phys. Lett. 2000, 320, 8–13. [Google Scholar] [CrossRef]
- Zhang, X.-F. The effect of phenyl substitution on the fluorescence characteristics of fluorescein derivatives via intramolecular photoinduced electron transfer. Photochem. Photobiol. Sci. 2010, 9, 1261–1268. [Google Scholar] [CrossRef]
- Bismillah, A.N.; Aprahamian, I. Boron difluoride hydrazone (BODIHY) complexes: A new class of fluorescent molecular rotors. J. Phys. Org. Chem. 2023, 36, e4485. [Google Scholar] [CrossRef]
- Hoelm, M.; Adamczyk, J.; Wzgarda-Raj, K.; Palusiak, M. Effect of a Substituent on the Properties of Salicylaldehyde Hydrazone Derivatives. J. Org. Chem. 2023, 88, 2132–2139. [Google Scholar] [CrossRef] [PubMed]
- Villa-Martínez, C.A.; Magaña-Vergara, N.E.; Rodríguez, M.; Mojica-Sánchez, J.P.; Ramos-Organillo, Á.A.; Barroso-Flores, J.; Padilla-Martínez, I.I.; Martínez-Martínez, F.J. Synthesis, Optical Characterization in Solution and Solid-State, and DFT Calculations of 3-Acetyl and 3-(1′-(2′-Phenylhydrazono)ethyl)-coumarin-(7)-substituted Derivatives. Molecules 2022, 27, 3677. [Google Scholar] [CrossRef]
- Dey, D.; Chopra, D. Quantitative analysis of solid-state diversity in trifluoromethylated phenylhydrazones. Acta Crystallogr. B Struct. Sci. Cryst. Eng. Mater. 2017, 73, 781–793. [Google Scholar] [CrossRef]
- Tatum, L.A.; Su, X.; Aprahamian, I. Simple Hydrazone Building Blocks for Complicated Functional Materials. Acc. Chem. Res. 2014, 47, 2141–2149. [Google Scholar] [CrossRef]
- Zhao, J.; Ji, S.; Chen, Y.; Guo, H.; Yang, P. Excited state intramolecular proton transfer (ESIPT): From principal photophysics to the development of new chromophores and applications in fluorescent molecular probes and luminescent materials. Phys. Chem. Chem. Phys. 2012, 14, 8803–8817. [Google Scholar] [CrossRef]
- Sedgwick, A.C.; Wu, L.; Han, H.-H.; Bull, S.D.; He, X.-P.; James, T.D.; Sessler, J.L.; Tang, B.Z.; Tian, H.; Yoon, J. Excited-state intramolecular proton-transfer (ESIPT) based fluorescence sensors and imaging agents. Chem. Soc. Rev. 2018, 47, 8842–8880. [Google Scholar] [CrossRef]
- Traven, V.F.; Cheptsov, D.A.; Lodeiro, C. Control of Fluorescence of Organic Dyes in the Solid-State by Supramolecular Interactions. J. Fluoresc. 2023, 33, 799–847. [Google Scholar] [CrossRef]
- Li, J.; Yang, W.; Zhou, W.; Li, C.; Cheng, Z.; Li, M.; Xie, L.; Li, Y. Aggregation-induced emission in fluorophores containing a hydrazone structure and a central sulfone: Restricted molecular rotation. RSC Adv. 2016, 6, 35833–35841. [Google Scholar] [CrossRef]
- Sobczak, P.; Sierański, T.; Świątkowski, M.; Trzęsowska-Kruszyńska, A. New Schiff base salts as sources of blue and green light in the solid state: The role of the anion and protonation. CrystEngComm 2023, 25, 6185–6193. [Google Scholar] [CrossRef]
- Hong, Y.; Lam, J.W.Y.; Tang, B.Z. Aggregation-induced emission: Phenomenon, mechanism and applications. Chem. Commun. 2009, 29, 4332–4353. [Google Scholar] [CrossRef] [PubMed]
- Ostrowska, K.; Stadnicka, K.M.; Gryl, M.; Klimas, O.; Brela, M.Z.; Goszczycki, P.; Liberka, M.; Grolik, J.; Węgrzyn, A. Influence of Hydrogen Bonds and π–π Interactions on the Fluorescence of Crystalline (N-Alkylpyridyl)enamino-pyrrolo[2,3-b]quinoxalin-2-one Derivatives. Cryst. Growth Des. 2022, 22, 1571–1582. [Google Scholar] [CrossRef]
- Traven, V.F.; Cheptsov, D.A.; Svetlova, J.I.; Ivanov, I.V.; Cuerva, C.; Lodeiro, C.; Duarte, F.; Dunaev, S.F.; Chernyshev, V.V. The role of the intermolecular π···π interactions in the luminescence behavior of novel coumarin-based pyrazoline materials. Dye. Pigment. 2021, 186, 108942. [Google Scholar] [CrossRef]
- Lübtow, M.; Helmers, I.; Stepanenko, V.; Albuquerque, R.Q.; Marder, T.B.; Fernández, G. Self-Assembly of 9,10-Bis(phenylethynyl) Anthracene (BPEA) Derivatives: Influence of π–π and Hydrogen-Bonding Interactions on Aggregate Morphology and Self-Assembly Mechanism. Chem. A Eur. J. 2017, 23, 6198–6205. [Google Scholar] [CrossRef]
- Afanasenko, A.M.; Novikov, A.S.; Chulkova, T.G.; Grigoriev, Y.M.; Kolesnikov, I.E.; Selivanov, S.I.; Starova, G.L.; Zolotarev, A.A.; Vereshchagin, A.N.; Elinson, M.N. Intermolecular interactions-photophysical properties relationships in phenanthrene-9,10-dicarbonitrile assemblies. J. Mol. Struct. 2020, 1199, 126789. [Google Scholar] [CrossRef]
- Song, J.; Lin, X.; Ee, L.Y.; Li, S.F.Y.; Huang, M. A Review on Electrospinning as Versatile Supports for Diverse Nanofibers and Their Applications in Environmental Sensing. Adv. Fiber Mater. 2023, 5, 429–460. [Google Scholar] [CrossRef] [PubMed]
- Al-Abduljabbar, A.; Farooq, I. Electrospun Polymer Nanofibers: Processing, Properties, and Applications. Polymers 2023, 15, 65. [Google Scholar] [CrossRef]
- Patel, K.D.; Kim, H.-W.; Knowles, J.C.; Poma, A. Molecularly Imprinted Polymers and Electrospinning: Manufacturing Convergence for Next-Level Applications. Adv. Funct. Mater. 2020, 30, 2001955. [Google Scholar] [CrossRef]
- Venmathi Maran, B.A.; Jeyachandran, S.; Kimura, M. A Review on the Electrospinning of Polymer Nanofibers and Its Biomedical Applications. J. Compos. Sci. 2024, 8, 32. [Google Scholar] [CrossRef]
- Li, L.; Wang, Y.; Gao, Z.; Mao, X.; Zhang, X.; Xing, W.; Jia, C.; Huang, L.; Tang, J. Research progress on electrospinning fluorescent nanofibers based on rare earth complex. Dye. Pigment. 2024, 224, 111977. [Google Scholar] [CrossRef]
- Hancharova, M.; Dupla, A.; Cabaj, J.; Zając, D. Electrospinning vs Fluorescent Organic Nano-Dots: A Comparative Review of Nanotechnologies in Organoluminophores Utilization. J. Inorg. Organomet. Polym. 2025, 35, 4242–4262. [Google Scholar] [CrossRef]
- Mohamed, S.K.; Bogdanović, G.A.; Abdelhamid, A.A.; Novaković, S.B.; Potgeiter, H. 4-[(2E)-2-(2-Hy-droxy-benzyl-idene)hydrazin-1-yl]benzonitrile. Acta Cryst. E 2012, 68, o2886–o2887. [Google Scholar] [CrossRef]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Cryst. B 2016, 72, 171–179. [Google Scholar] [CrossRef]
- Allen, F.H.; Kennard, O.; Watson, D.G.; Brammer, L.; Orpen, A.G.; Taylor, R. Tables of Bond Lengths determined by X-Ray and Neutron Diffraction. Part I. Bond Lengths in Organic Compounds. J. Chem. Soc. Perkin Trans. 1987, 12, S1–S19. [Google Scholar] [CrossRef]
- Sobczak, P.; Sierański, T.; Świątkowski, M.; Trzęsowska-Kruszyńska, A.; Kolińska, J. Unraveling the structure–property relationships in fluorescent phenylhydrazones: The role of substituents and molecular interactions. RSC Adv. 2025, 15, 1514–1526. [Google Scholar] [CrossRef]
- Olesiejuk, M.; Kudelko, A.; Swiatkowski, M.; Kruszynski, R. Synthesis of 4-Alkyl-4H-1,2,4-triazole Derivatives by Suzuki Cross-Coupling Reactions and Their Luminescence Properties. Molecules 2019, 24, 652. [Google Scholar] [CrossRef]
- Kędzia, A.; Kudelko, A.; Świątkowski, M.; Kruszyński, R. Microwave-promoted synthesis of highly luminescent s-tetrazine-1,3,4-oxadiazole and s-tetrazine-1,3,4-thiadiazole hybrids. Dye. Pigment. 2020, 172, 107865. [Google Scholar] [CrossRef]
- Kędzia, A.; Kudelko, A.; Świątkowski, M.; Kruszyński, R. Highly fluorescent 1,2,4,5-tetrazine derivatives containing 1,3,4-oxadiazole ring conjugated via a 1,4-phenylene linker. Dye. Pigment. 2020, 183, 108715. [Google Scholar] [CrossRef]
- Maj, A.; Kudelko, A.; Swiatkowski, M. 1,3,4-Thiadiazol-2-ylphenyl-1,2,4,5-tetrazines: Efficient synthesis via Pinner reaction and their luminescent properties. Arkivoc 2021, 2021, 167–178. [Google Scholar] [CrossRef]
- Maj, A.; Kudelko, A.; Świątkowski, M. Novel Conjugated s-Tetrazine Derivatives Bearing a 4H-1,2,4-Triazole Scaffold: Synthesis and Luminescent Properties. Molecules 2022, 27, 459. [Google Scholar] [CrossRef]
- Maj, A.; Kudelko, A.; Świątkowski, M. Synthesis and Luminescent Properties of s-Tetrazine Derivatives Conjugated with the 4H-1,2,4-Triazole Ring. Molecules 2022, 27, 3642. [Google Scholar] [CrossRef]
- Ranjan Gartia, M.; Eichorst, J.P.; Clegg, R.M.; Logan Liu, G. Lifetime imaging of radiative and non-radiative fluorescence decays on nanoplasmonic surface. Appl. Phys. Lett. 2012, 101, 023118. [Google Scholar] [CrossRef]
- Dhar, S.; Rana, D.K.; Singha Roy, S.; Roy, S.; Bhattacharya, S.; Bhattacharya, S.C. Effect of solvent environment on the Photophysics of a newly synthesized bioactive 7-oxy(5-selenocyanato-pentyl)-2H-1-benzopyran-2-one. J. Lumin. 2012, 132, 957–964. [Google Scholar] [CrossRef]
- Kwak, G.; Kim, H.; Kang, I.-K.; Kim, S.-H. Charge Transfer Dye in Various Polymers with Different Polarity: Synthesis, Photophysical Properties, and Unusual Aggregation-Induced Fluorescence Changes. Macromolecules 2009, 42, 1733–1738. [Google Scholar] [CrossRef]
- Shundo, A.; Okada, Y.; Ito, F.; Tanaka, K. Fluorescence Behavior of Dyes in Thin Films of Various Polymers. Macromolecules 2012, 45, 329–335. [Google Scholar] [CrossRef]
- Macrae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.A.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M.; et al. Mercury 4.0: From visualization to analysis, design and prediction. J. Appl. Cryst. 2020, 53, 226–235. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Cheeseman, J.R.; Scalmani, G.; Caricato, M.; Hratchian, H.P.; Li, X.; Barone, V.; Bloino, J.; Zheng, G.; et al. Gaussian 09; Gaussian, Inc.: Wallingford, CT, USA, 2015. [Google Scholar]
- Mardirossian, N.; Head-Gordon, M. Thirty years of density functional theory in computational chemistry: An overview and extensive assessment of 200 density functionals. Mol. Phys. 2017, 115, 2315–2372. [Google Scholar] [CrossRef]
- Ditchfield, R.; Hehre, W.J.; Pople, J.A. Self-Consistent Molecular-Orbital Methods. IX. An Extended Gaussian-Type Basis for Molecular-Orbital Studies of Organic Molecules. J. Chem. Phys. 1971, 54, 724–728. [Google Scholar] [CrossRef]
- Gordon, M.S.; Binkley, J.S.; Pople, J.A.; Pietro, W.J.; Hehre, W.J. Self-consistent molecular-orbital methods. 22. Small split-valence basis sets for second-row elements. J. Am. Chem. Soc. 1982, 104, 2797–2803. [Google Scholar] [CrossRef]
- Chemissian v4.67. Available online: https://www.chemissian.com/ (accessed on 3 September 2025).
- Ozyurt, D.; Kobaisi, M.A.; Hocking, R.K.; Fox, B. Properties, synthesis, and applications of carbon dots: A review. Carbon Trends 2023, 12, 100276. [Google Scholar] [CrossRef]
- Alassafi, J.E.; Al-Hadeethi, Y.; Aida, M.S.; Roqan, I.S.; Al-Shehri, S.F.; Ansari, M.S.; Alamodi, S.; Chen, M. Uniform blue emicarbon nanodots synthesized from fig fruit using reverse diffusion purification. Sci. Rep. 2024, 14, 29254. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, S.-W.; Zhang, M.; Wu, C.; Li, W.; Wu, Y.; Yang, C.; Kang, F.; Meng, H.; Wei, G. Highly Efficient Phosphorescent Blue-Emitting [3+2+1] Coordinated Iridium (III) Complex for OLED Application. Front. Chem. 2021, 9, 758357. [Google Scholar] [CrossRef] [PubMed]
- Gusev, A.N.; Kiskin, M.A.; Braga, E.V.; Kryukova, M.A.; Baryshnikov, G.V.; Karaush-Karmazin, N.N.; Minaeva, V.A.; Minaev, B.F.; Ivaniuk, K.; Stakhira, P.; et al. Schiff Base Zinc(II) Complexes as Promising Emitters for Blue Organic Light-Emitting Diodes. ACS Appl. Electron. Mater. 2021, 3, 3436–3444. [Google Scholar] [CrossRef]
- Schinabeck, A.; Rau, N.; Klein, M.; Sundermeyer, J.; Yersin, H. Deep blue emitting Cu(I) tripod complexes. Design of high quantum yield materials showing TADF-assisted phosphorescence. Dalton Trans. 2018, 47, 17067–17076. [Google Scholar] [CrossRef] [PubMed]


| Compound | H-Bond Motifs or NH···π (Ring) Interactions | π···π Ring-Stacking Motifs (System) | π···π Stacking Between Hydrazone Moiety and Ring (System) | π···π Stacking Between Cyano group and Ring |
|---|---|---|---|---|
| H1 | NH···NC, L1C(8) | – | hm···R2 (dimers) | CN···R2 |
| H2 | NH···NC, L1C(8) | – | – | CN···R2 |
| H3 | NH···R2 | – | – | – |
| H4 | NH···NC, L2C22(16)[C(8)] | HT1 and HT2 (columnar) * | – | – |
| H5 | NH···N, L1C(3) OH···NC, L1C(15) | HT2 (dimers) | – | – |
| H6 | NH···NC, L1C(8) | HH2 (columnar) | – | – |
| H7 | NH···NC, L1C(8) NH···ON, L1C(10) | – | hm···PhCN (columnar) | CN···PhCN |
| H8 | NH···NC, L4C44(32)[C(8)] | – | – | CN···PhCN |
| H9 | NH···NC, L1C(8) | HH1 (columnar) | – | – |
| H10 | NH···NC, L1C(8) | HH2 (columnar) | – | – |
| H11 | NH···NC, L1C(8) NH···ON, L1R22(9) | HT2 (columnar) | – | – |
| H12 | NH···NC, L1C(8) | HH2 (columnar) | – | – |
| H13 | NH···NC, L1C(8) | – | hm···R2 (dimers) | – |
| H14 | NH···NC, L1C(8) | HT2 (dimers) | – | – |
| Compound | λex (nm) | λem (nm) | Int | H → L Transition | Orbital Transition | Φf | τ (ns) |
|---|---|---|---|---|---|---|---|
| H1 | 343 | 440 | 252 | H-1 → L + 3 H → L + 4 | π → π* | 0.23 | 0.94 |
| 419 | 440 | 436 | H → L + 2 | π → π* | 0.11 | ||
| H2 | 338 | 425 | 227 | H-1 → L + 2 | π → π* | 0.25 | 0.72 |
| 407 | 425 | 407 | H-1 → L | π → π* (LLCT) | 0.20 | ||
| H3 | 362 | 462 | 249 | H-1 → L + 6 | π → π* (LLCT) | 0.22 | 12.50 |
| 438 | 462 | 359 | H-1 → L | π → π* (LLCT) | 0.10 | ||
| H4 | 372 | 434 | 17 | H-2 → L + 2 H-3 → L | π → π* | 0.02 | 0.45 |
| H5 | 367 | 433 | 16 | H → L + 4 | π → π* | 0.03 | <0.2 # |
| H6 | 357 | 463 | 101 | H-3 → L | π → π* (LLCT) | 0.07 | 1.18 |
| 434 | 463 | 171 | H-2 → L | π → π* (LLCT) | 0.04 | ||
| H8 | 344 | 446 | 121 | H → L + 2 H-2 → L | π → π* | 0.05 | 0.58 |
| 425 | 446 | 244 | H → L | π → π* (LLCT) | 0.03 | ||
| H12 | 366 | 421 | 43 | H-1 → L + 2 | π → π* | 0.02 | 1.33 |
| H13 | 351 | 494 | 135 | H-1 → L + 3 H-3 → L + 1 H-2 → L | π → π* | 0.40 | 5.14 |
| 439 | 494 | 296 | H → L + 1 | π → π* | 0.22 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sobczak-Tyluś, P.; Sierański, T.; Świątkowski, M.; Trzęsowska-Kruszyńska, A.; Bogucki, O. Fluorescence of 4-Cyanophenylhydrazones: From Molecular Design to Electrospun Polymer Fibers. Molecules 2025, 30, 3638. https://doi.org/10.3390/molecules30173638
Sobczak-Tyluś P, Sierański T, Świątkowski M, Trzęsowska-Kruszyńska A, Bogucki O. Fluorescence of 4-Cyanophenylhydrazones: From Molecular Design to Electrospun Polymer Fibers. Molecules. 2025; 30(17):3638. https://doi.org/10.3390/molecules30173638
Chicago/Turabian StyleSobczak-Tyluś, Paulina, Tomasz Sierański, Marcin Świątkowski, Agata Trzęsowska-Kruszyńska, and Oskar Bogucki. 2025. "Fluorescence of 4-Cyanophenylhydrazones: From Molecular Design to Electrospun Polymer Fibers" Molecules 30, no. 17: 3638. https://doi.org/10.3390/molecules30173638
APA StyleSobczak-Tyluś, P., Sierański, T., Świątkowski, M., Trzęsowska-Kruszyńska, A., & Bogucki, O. (2025). Fluorescence of 4-Cyanophenylhydrazones: From Molecular Design to Electrospun Polymer Fibers. Molecules, 30(17), 3638. https://doi.org/10.3390/molecules30173638







