Portable Bacterial Cellulose-Based Fluorescent Sensor for Rapid and Sensitive Detection of Copper in Food and Environmental Samples
Abstract
1. Introduction
2. Results
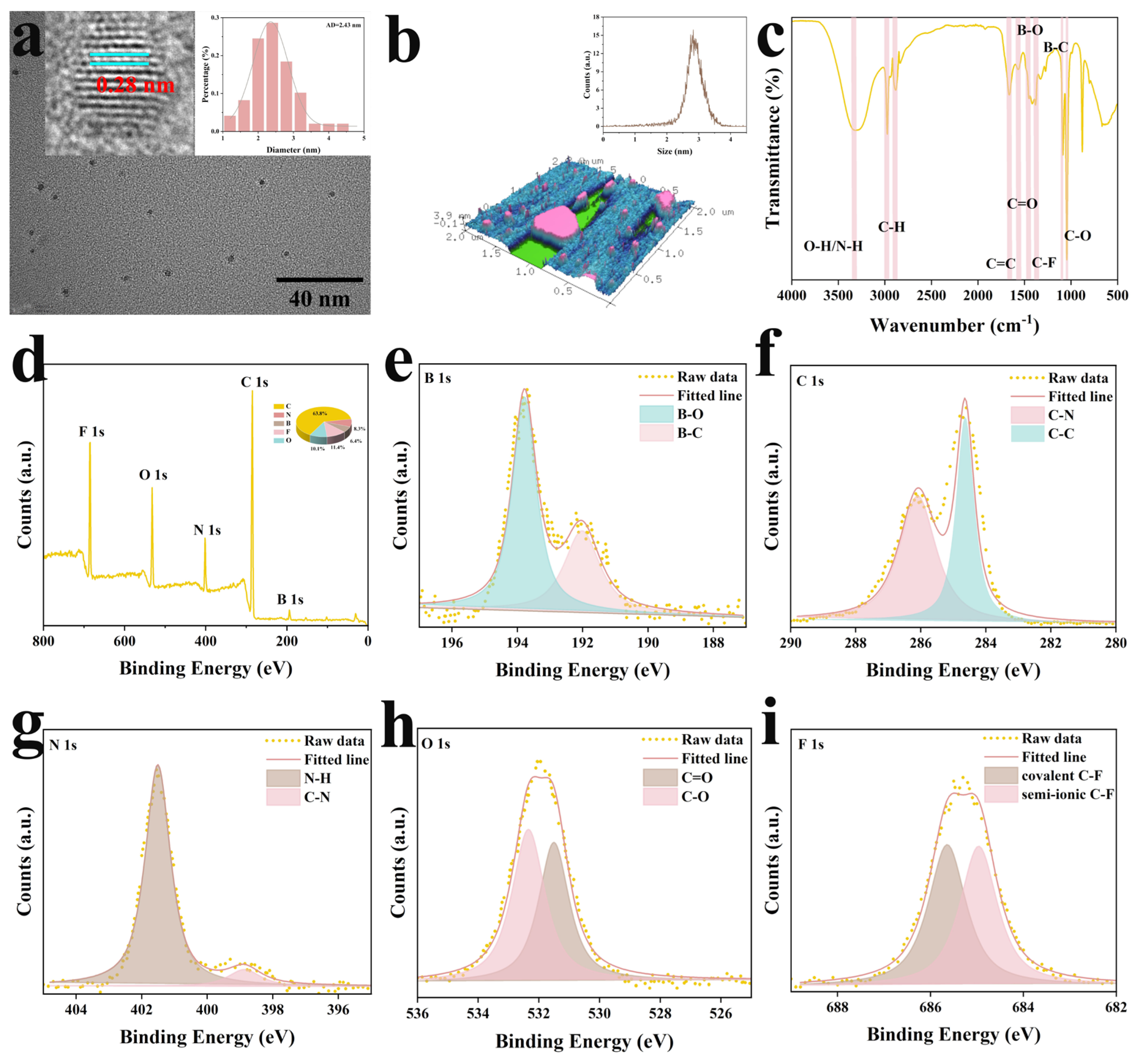
2.1. Synthesis and Characterization of Y-CDs
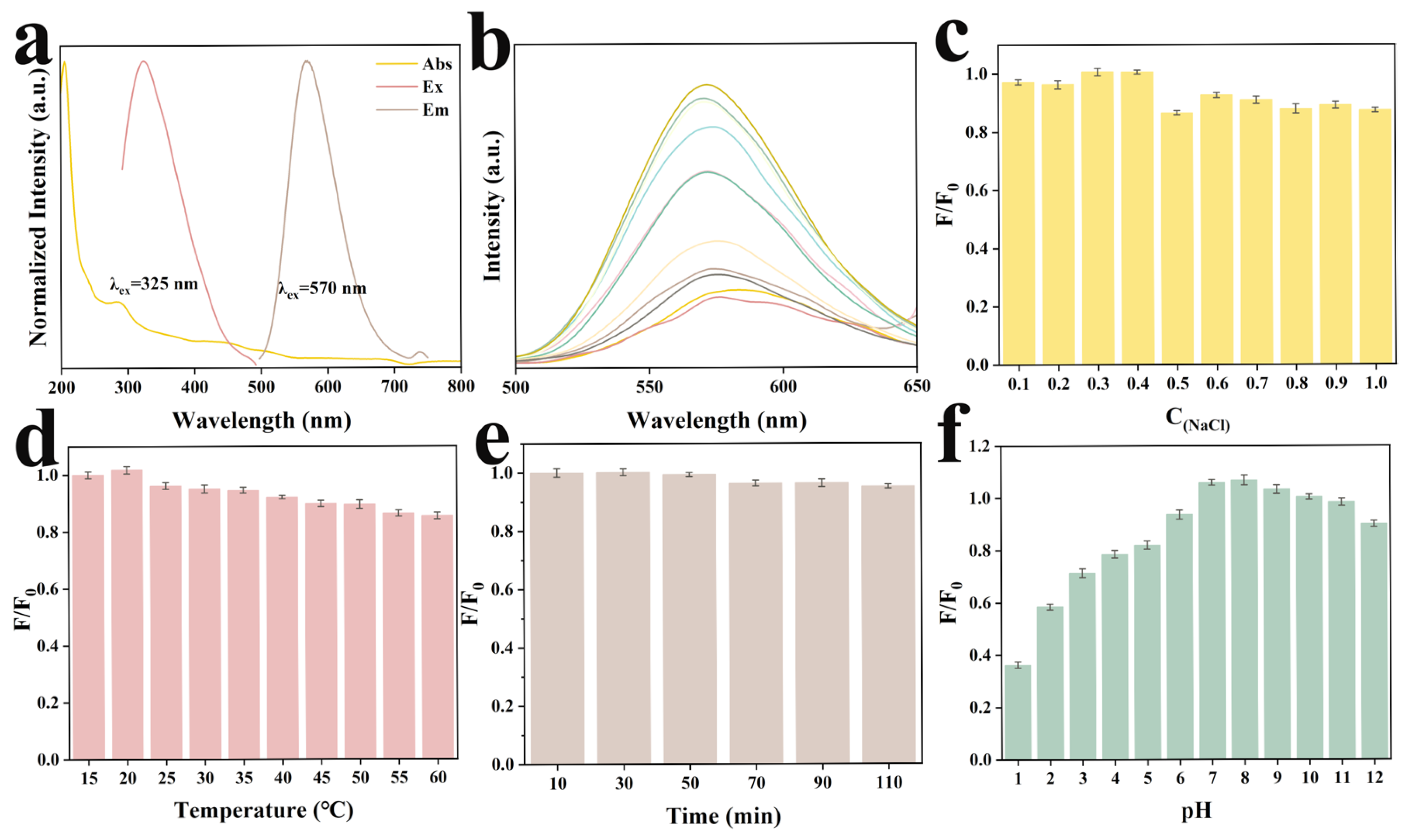
2.2. Optical Properties of Y-CDs
2.3. Quenching Mechanism
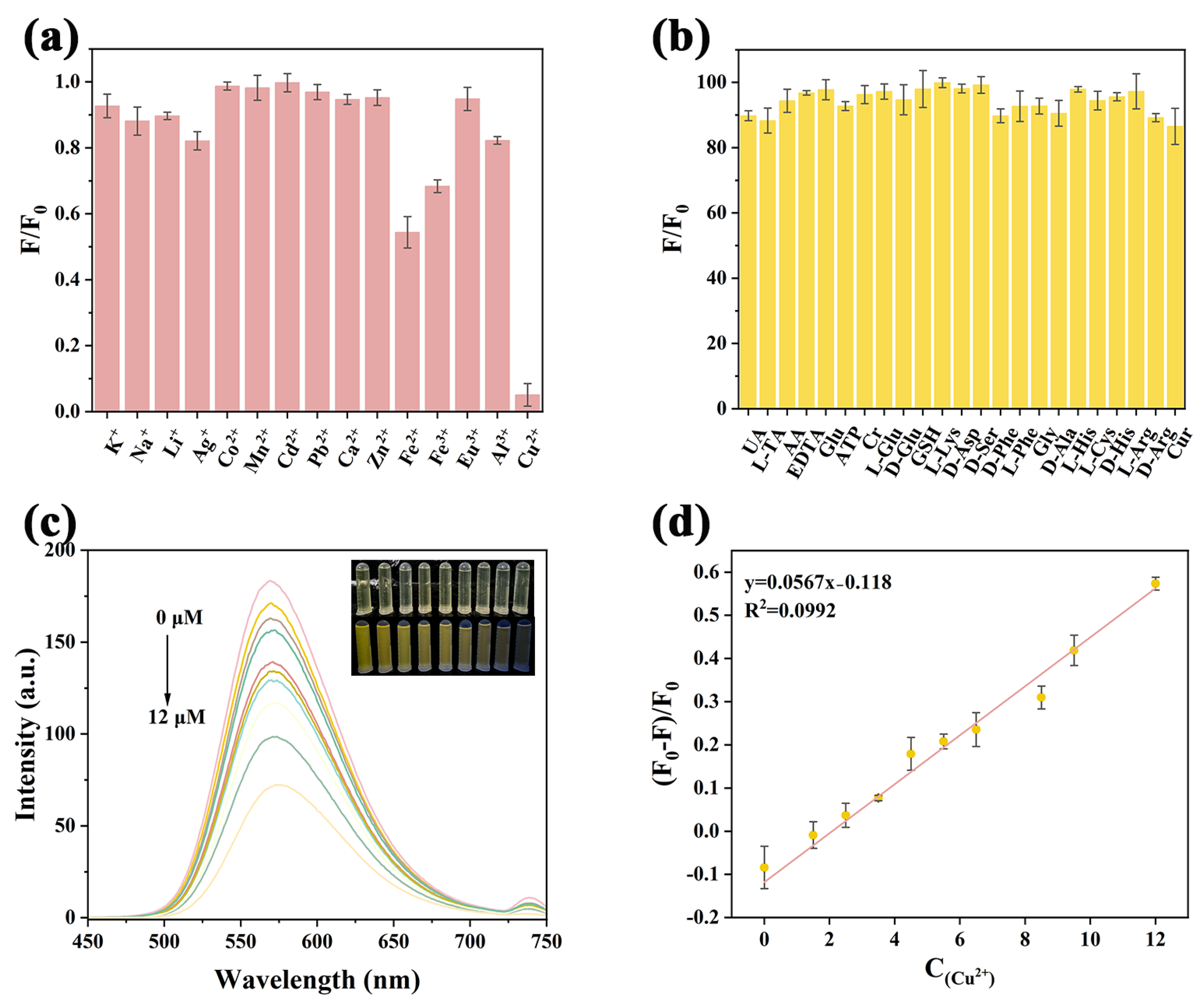
2.4. Selectivity and Sensitivity
2.5. Actual Sample Analysis
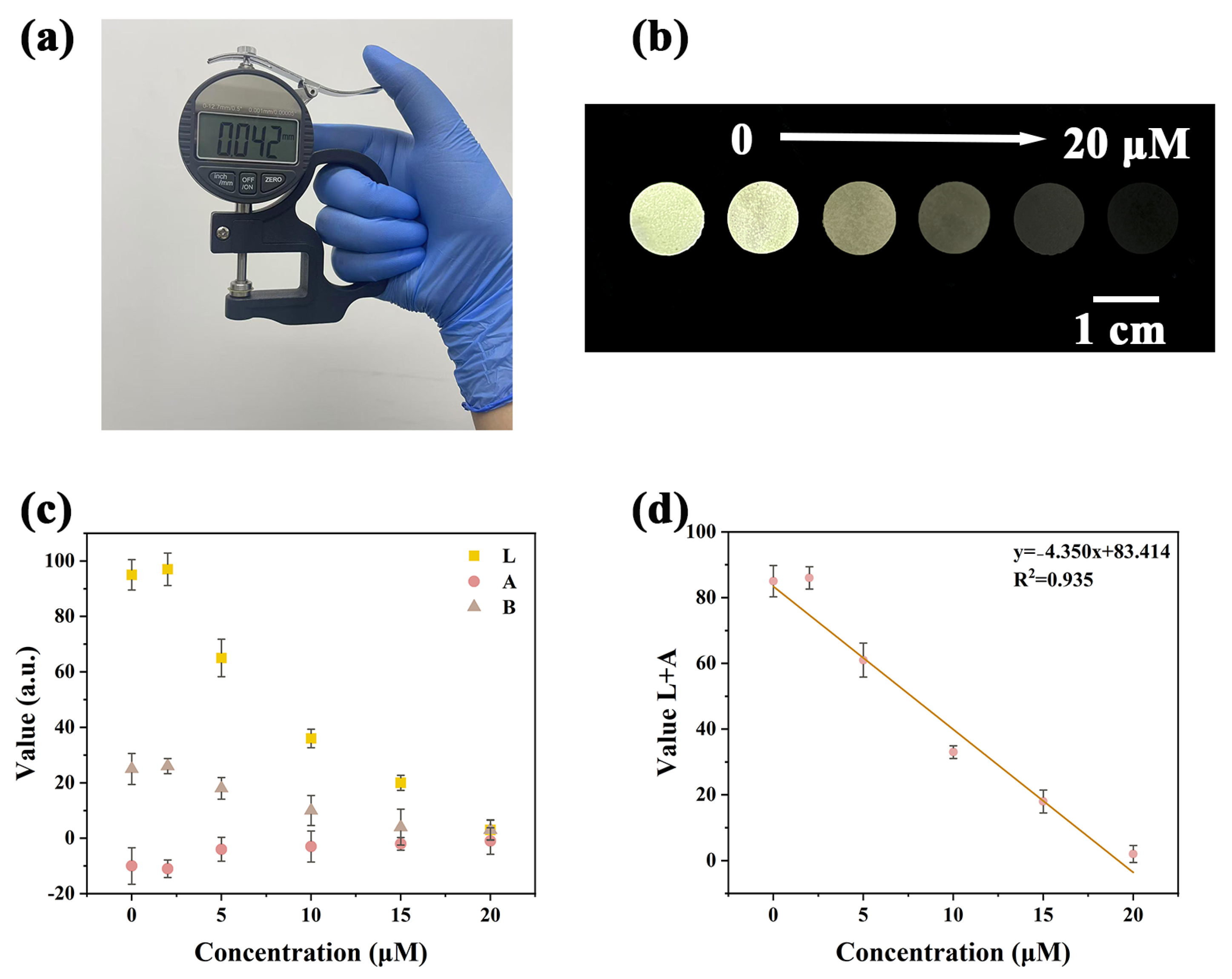
2.6. Y-CDs@BCM Portable Fluorescence Sensor
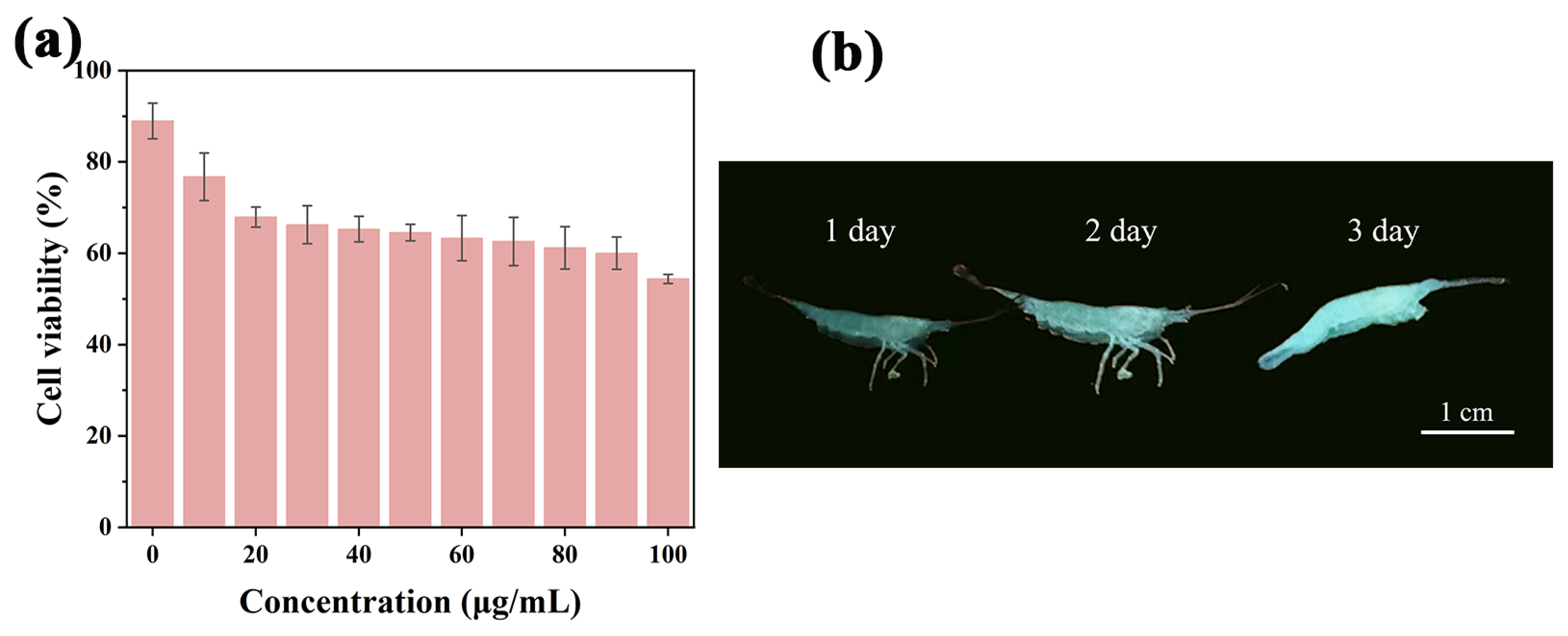
2.7. Cytotoxicity Test and Imaging
3. Materials and Methods
3.1. Reagents and Materials
3.2. Instrument
3.3. Preparation of Y-CDs
3.4. Stability of Y-CDs
3.5. Preparation of Y-CDs@BCM Sensors
3.6. Detection of Cu2+
3.7. Pretreatment of Actual Samples
3.8. Cytotoxicity Assessment of Live Cells and In Vivo Imaging
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, M.; Xu, C.; Wang, C.; Huang, N.; Bian, Z.; Xiao, Y.; Ruan, J.; Sun, F.; Shi, S. Three Birds with One Stone: Copper Ions Assisted Synergistic Cuproptosis/Chemodynamic/Photothermal Therapy by a Three-Pronged Approach. Adv. Healthc. Mater. 2024, 13, 2401567. [Google Scholar] [CrossRef]
- Tapiero, H.; Townsend, D.M.; Tew, K.D. Trace elements in human physiology and pathology. Copper. Biomed. Pharmacother. 2003, 57, 386–398. [Google Scholar] [CrossRef]
- Lee, I.-C.; Ko, J.-W.; Park, S.-H.; Shin, N.-R.; Shin, I.-S.; Moon, C.; Kim, J.-H.; Kim, H.-C.; Kim, J.-C. Comparative toxicity and biodistribution assessments in rats following subchronic oral exposure to copper nanoparticles and microparticles. Part. Fibre Toxicol. 2016, 13, 56. [Google Scholar] [CrossRef]
- Ghaedi, M.; Ahmadi, F.; Shokrollahi, A. Simultaneous preconcentration and determination of copper, nickel, cobalt and lead ions content by flame atomic absorption spectrometry. J. Hazard. Mater. 2007, 142, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Feng, J.; Tang, L.; Yu, C.; Mo, G.; Deng, B. A highly efficient introduction system for single cell-ICP-MS and its application to detection of copper in single human red blood cells. Talanta 2020, 206, 120174. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Cho, M.; Choe, W.-S.; Son, Y.; Lee, Y. Electrochemical detection of copper ion using a modified copolythiophene electrode. Electrochim. Acta 2009, 54, 7012–7017. [Google Scholar] [CrossRef]
- Liu, J.; Li, R.; Yang, B. Carbon Dots: A New Type of Carbon-Based Nanomaterial with Wide Applications. ACS Cent. Sci. 2020, 6, 2179–2195. [Google Scholar] [CrossRef]
- Liu, M.; Wei, S.; Xie, Y.; Su, K.; Yin, X.; Song, X.; Hu, K.; Sun, G.; Liu, Y. Ratiometric fluorescence sensor based on chiral europium-doped carbon dots for specific and portable detection of tetracycline. Sens. Actuators B Chem. 2025, 423, 136753. [Google Scholar] [CrossRef]
- Morbiato, L.; Cardo, L.; Sturabotti, E.; Gobbo, P.; Filippini, G.; Prato, M. Structure Matters: Tailored Graphitization of Carbon Dots Enhances Photocatalytic Performance. ACS Nano 2025, 19, 4887–4900. [Google Scholar] [CrossRef]
- Li, M.; Gong, W.; Wang, F.; Li, L.; Nie, Y.; Chen, Q. A novel carbon dot-based degradable pre-formed particle gel for sensitive detection of iron ions. Colloids Surf. A Physicochem. Eng. Asp. 2025, 711, 136414. [Google Scholar] [CrossRef]
- Girard, V.-D.; Chaussé, J.; Vermette, P. Bacterial cellulose: A comprehensive review. J. Appl. Polym. Sci. 2024, 141, e55163. [Google Scholar] [CrossRef]
- Zheng, Z.-J.; Ye, H.; Guo, Z.-P. Bacterial Cellulose Applications in Electrochemical Energy Storage Devices. Adv. Mater. 2025, 37, 2412908. [Google Scholar] [CrossRef] [PubMed]
- Lv, P.; Yao, Y.; Li, D.; Zhou, H.; Naeem, M.A.; Feng, Q.; Huang, J.; Cai, Y.; Wei, Q. Self-assembly of nitrogen-doped carbon dots anchored on bacterial cellulose and their application in iron ion detection. Carbohydr. Polym. 2017, 172, 93–101. [Google Scholar] [CrossRef]
- Zhai, X.; Wu, Z.; Sun, Q.; Sun, J.; Chen, F.; Zhang, M.; Duan, H. Bioinspired Bacterial Cellulose Carbon Nanofibers/AgO Composite for Sensitive and Selective Detection of H2O2 Vapor at Room Temperature. J. Electron. Mater. 2023, 52, 5377–5387. [Google Scholar] [CrossRef]
- Zhang, X.; Yao, J.; Yan, Y.; Zhang, Y.; Tang, Y.; Yang, Y. Bacterial Cellulose Incorporating Multicolor Fluorescent Probes for Visual Acidity Detection in Paper-Based Cultural Relics. ACS Appl. Mater. Interfaces 2024, 16, 60902–60911. [Google Scholar] [CrossRef]
- Liu, K.; Wang, Y.; Liu, W.; Zheng, C.; Xu, T.; Du, H.; Yuan, Z.; Si, C. Bacterial cellulose/chitosan composite materials for biomedical applications. Chem. Eng. J. 2024, 494, 153014. [Google Scholar] [CrossRef]
- Mir, I.S.; Riaz, A.; Roy, J.S.; Fréchette, J.; Morency, S.; Ponce Gomes, O.; Dumée, L.F.; Greener, J.; Messaddeq, Y. Removal of cadmium and chromium heavy metals from aqueous medium using composite bacterial cellulose membrane. Chem. Eng. J. 2024, 490, 151665. [Google Scholar] [CrossRef]
- Filippini, G.; Amato, F.; Rosso, C.; Ragazzon, G.; Vega-Peñaloza, A.; Companyó, X.; Dell’Amico, L.; Bonchio, M.; Prato, M. Mapping the Surface Groups of Amine-Rich Carbon Dots Enables Covalent Catalysis in Aqueous Media. Chem 2020, 6, 3022–3037. [Google Scholar] [CrossRef]
- Zaca-Moran, O.; Díaz-Monge, F.; Rodríguez-Juárez, A.; Gómez-Muñoz, C.L.; Zaca-Moran, P.; Secundino-Sánchez, O.; Díaz-Reyes, J. Synthesis and characterization of fluorescent carbon dots obtained from citrus x sinensis by an eco-friendly method. Results Chem. 2024, 11, 101788. [Google Scholar] [CrossRef]
- Dyrda-Terniuk, T.; Sugajski, M.; Pryshchepa, O.; Śliwiak, J.; Buszewska-Forajta, M.; Pomastowski, P.; Buszewski, B. The Study of Protein-Cyclitol Interactions. Int. J. Mol. Sci. 2022, 23, 2940. [Google Scholar] [CrossRef]
- Lonkar, S.P.; Raquez, J.M.; Dubois, P. One-Pot Microwave-Assisted Synthesis of Graphene/Layered Double Hydroxide (LDH) Nanohybrids. Nano-Micro Lett. 2015, 7, 332–340. [Google Scholar] [CrossRef]
- Zeeshan, Q.M.; Qiu, S.; Gu, J.; Abbew, A.W.; Wu, Z.; Chen, Z.; Xu, S.; Ge, S. Unravelling multiple removal pathways of oseltamivir in wastewater by microalgae through experimentation and computation. J. Hazard Mater. 2022, 427, 128139. [Google Scholar] [CrossRef]
- Chen, M.; Zhang, Q.; Zhou, M.; Andrada, D.M.; Frenking, G. Carbon Monoxide Bonding With BeO and BeCO3: Surprisingly High CO Stretching Frequency of OCBeCO3. Angew. Chem. Int. Ed. 2015, 54, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, W.; Liu, Y.; Ren, M.; Xiao, H.; Liu, X. Characterization of Conformation and Locations of C–F Bonds in Graphene Derivative by Polarized ATR-FTIR. Anal. Chem. 2016, 88, 3926–3934. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, H.; Zhao, Y.; Han, X.; Wang, H.; Gao, Y. B/N co-doped carbon derived from the sustainable chitin for CH bond oxidation. Appl. Surf. Sci. 2018, 457, 439–448. [Google Scholar] [CrossRef]
- Daniyal, W.M.; Fen, Y.W.; Saleviter, S.; Chanlek, N.; Nakajima, H.; Abdullah, J.; Yusof, N.A. X-ray Photoelectron Spectroscopy Analysis of Chitosan–Graphene Oxide-Based Composite Thin Films for Potential Optical Sensing Applications. Polymers 2021, 13, 478. [Google Scholar] [CrossRef]
- Ohtsubo, N.; Gohda, S.; Gotoh, K.; Sato, S.; Yamada, Y. Bottom-up synthesis of pyridinic nitrogen-containing carbon materials with C–H groups next to pyridinic nitrogen from two-ring aromatics. Carbon 2023, 207, 270–291. [Google Scholar] [CrossRef]
- Ge, L.; Kang, Y.; Li, G.; Wu, J.; Chen, Y.; Liu, C.; Wen, X.; Li, J.; Li, L.; Qu, Q. Regenerating Spent Lithium-Ion Battery Cathodes as Dual-Functional Biocatalysts for Highly-Efficient Oxygen and Hydrogen Evolution Using Aspergillus niger Soluble Extracellular Polymers. ACS Sustain. Chem. Eng. 2024, 12, 7466–7477. [Google Scholar] [CrossRef]
- Liu, C.; Xu, J.A.; Gao, H.; Zhou, M.; Lu, L.H. Nitrogen-skinned carbon nanocone enables non-dynamic electrochemistry of individual metal particles. Sci. China Chem. 2022, 65, 2031–2037. [Google Scholar] [CrossRef]
- Liu, C.; Jiang, Y.T.; Mu, X.D.; Chang, X.L.; Zhang, Y.; He, X.X.; Zhu, X.J.; Sun, W.B.; Lu, L.H. Bionic synapses for real-time epileptic seizure control. Sens. Actuators B Chem. 2025, 429, 137304. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, J.; Wei, S.; Wang, C.; Yin, X.; Song, X.; Jiang, C.; Sun, G. Nitrogen-doped graphene quantum dot-based portable fluorescent sensors for the sensitive detection of Fe3+ and ATP with logic gate operation. J. Mater. Chem. B 2023, 11, 6082–6094. [Google Scholar] [CrossRef]
- Weitner, T.; Friganović, T.; Šakić, D. Inner Filter Effect Correction for Fluorescence Measurements in Microplates Using Variable Vertical Axis Focus. Anal. Chem. 2022, 94, 7107–7114. [Google Scholar] [CrossRef]
- Liang, Z.J.; Tan, H.L.; Li, D.; Liang, Y.; Wang, L.P.; Chen, Y.B.; Niu, H.T. Establishment of a novel pork kidney lavage method and detection of heavy metals and antibiotics. Food Sci. Technol. 2022, 42, e09622. [Google Scholar] [CrossRef]
- Zhang, Y.-N.; Liang, W.; Li, L.; Zhang, H.; Cao, S.; Han, B.; Zhao, Y. Optical fiber fluorescence Cu2+ sensing technology based on CdSe/ZnS quantum dots: Large detection range, low detection limit. Analytica Chimica Acta 2024, 1331, 343300. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Chen, R.; Dai, L.; Ren, B.; Yang, F.; Xu, Y.-J.; Li, Q. Construction of a reaction-based fluorescent sensor for tandem detection of Cu2+ and glutathione in wine. Food Chemistry 2025, 464, 141632. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Hou, X.L.; Lu, D.C.; Chen, Y.Y.; Feng, L.Y. Porphyrin Functionalized Carbon Quantum Dots for Enhanced Electrochemiluminescence and Sensitive Detection of Cu2+. Molecules 2023, 28, 1459. [Google Scholar] [CrossRef] [PubMed]
- Stalin Elanchezhian, V.; Kasirajan, E.; Muthirulan, P.; Muthukrishnan, P.; Kandaswamy, M. Ferrocene-based chemosensor creates molecular logic circuit for selective detection of Hg2+ and Cu2+. J. Mol. Struct. 2024, 1313, 138687. [Google Scholar] [CrossRef]

| Sample | Added (μM) | Found (μM) | Recovery (%) | RSD (%) |
|---|---|---|---|---|
| pig liver | 2.0 | 1.98 | 99.13 | 2.48 |
| 4.0 | 4.57 | 114.34 | 0.77 | |
| 6.0 | 6.97 | 116.13 | 0.35 | |
| serum | 2.0 | 2.01 | 100.73 | 2.74 |
| 4.0 | 4.16 | 103.88 | 0.56 | |
| 6.0 | 5.88 | 98.08 | 1.18 | |
| environmental water | 2.0 | 2.12 | 106.18 | 1.71 |
| 4.0 | 4.05 | 101.33 | 1.34 | |
| 6.0 | 6.58 | 109.59 | 0.62 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Zhang, Q.; Ji, X.; Han, B.; Wang, J.; Han, C. Portable Bacterial Cellulose-Based Fluorescent Sensor for Rapid and Sensitive Detection of Copper in Food and Environmental Samples. Molecules 2025, 30, 3633. https://doi.org/10.3390/molecules30173633
Zhang H, Zhang Q, Ji X, Han B, Wang J, Han C. Portable Bacterial Cellulose-Based Fluorescent Sensor for Rapid and Sensitive Detection of Copper in Food and Environmental Samples. Molecules. 2025; 30(17):3633. https://doi.org/10.3390/molecules30173633
Chicago/Turabian StyleZhang, Hongyuan, Qian Zhang, Xiaona Ji, Bing Han, Jieqiong Wang, and Ce Han. 2025. "Portable Bacterial Cellulose-Based Fluorescent Sensor for Rapid and Sensitive Detection of Copper in Food and Environmental Samples" Molecules 30, no. 17: 3633. https://doi.org/10.3390/molecules30173633
APA StyleZhang, H., Zhang, Q., Ji, X., Han, B., Wang, J., & Han, C. (2025). Portable Bacterial Cellulose-Based Fluorescent Sensor for Rapid and Sensitive Detection of Copper in Food and Environmental Samples. Molecules, 30(17), 3633. https://doi.org/10.3390/molecules30173633





