High Selectivity and Yield in Catalytic Transfer Hydrogenation of Furfural to Furfuryl Alcohol by Zirconium Propoxide Modified Mesoporous Silica
Abstract
1. Introduction
2. Results
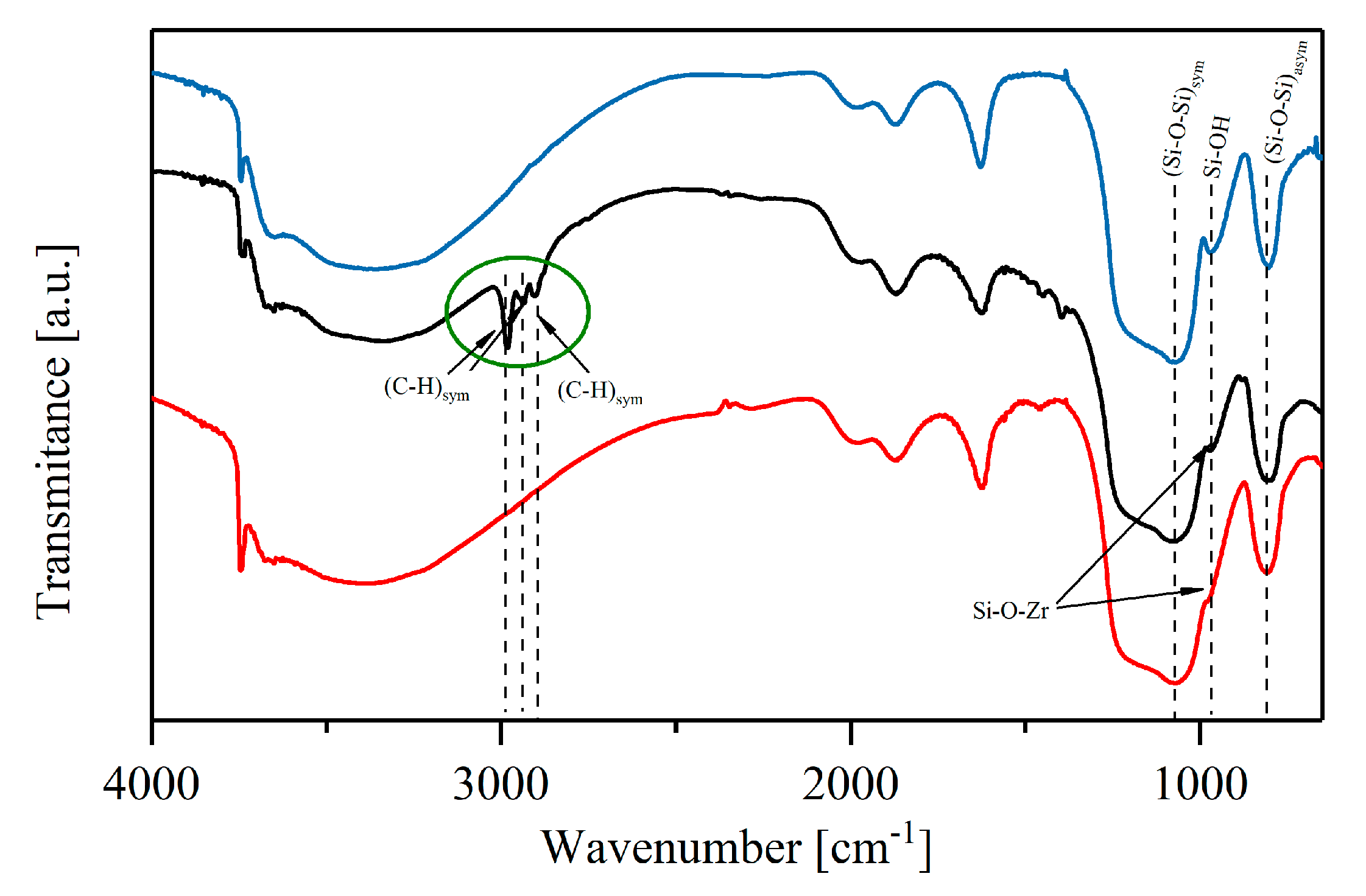
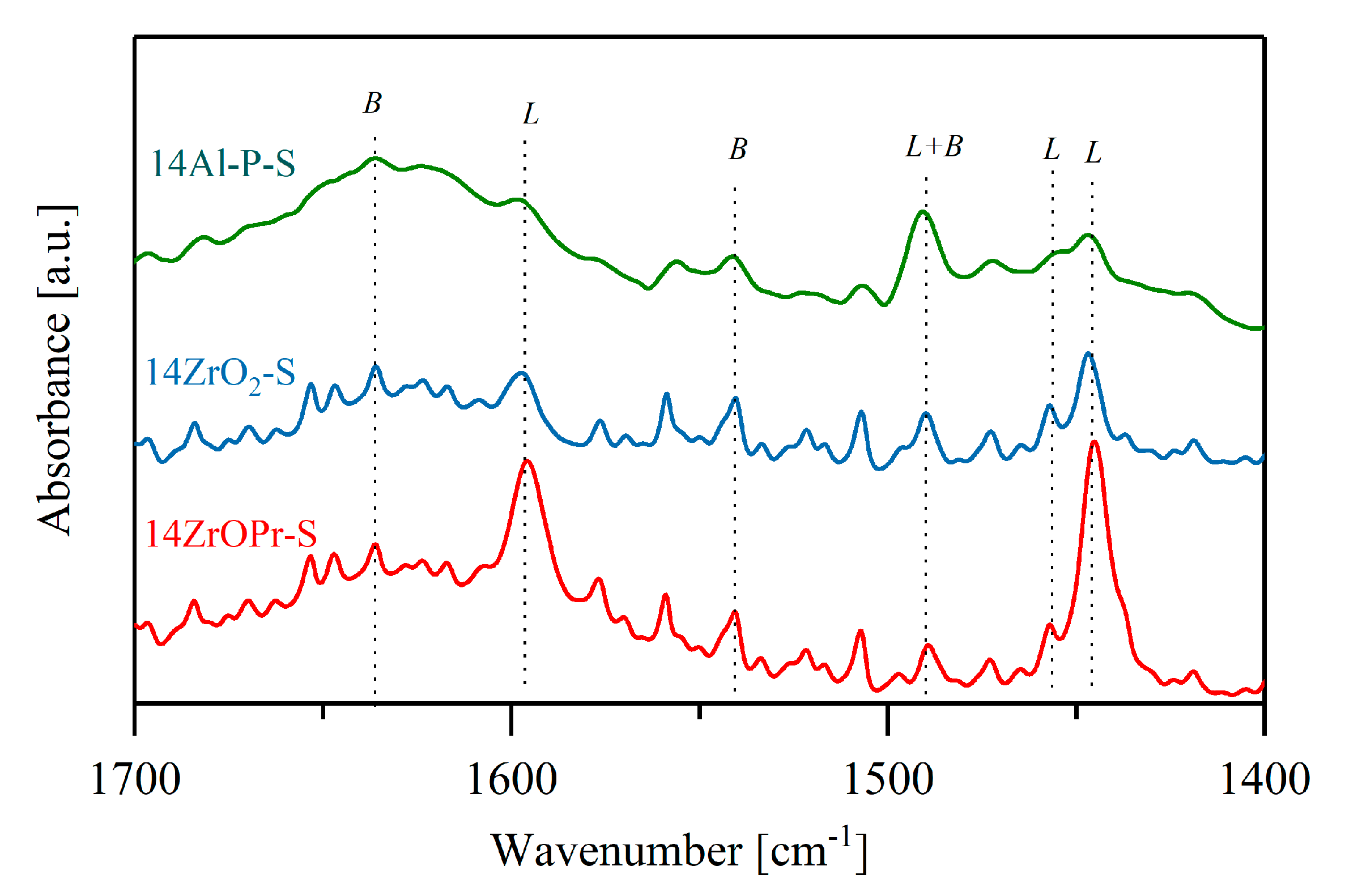
2.1. Properties of the Materials
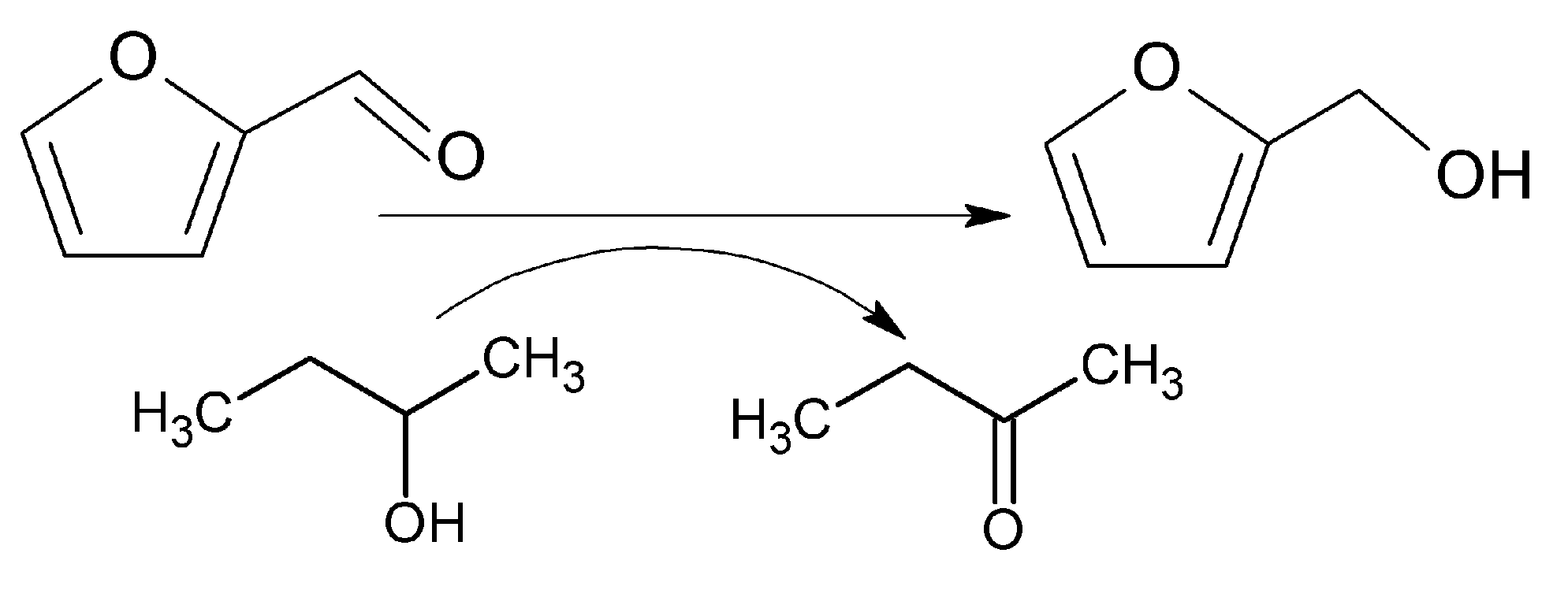
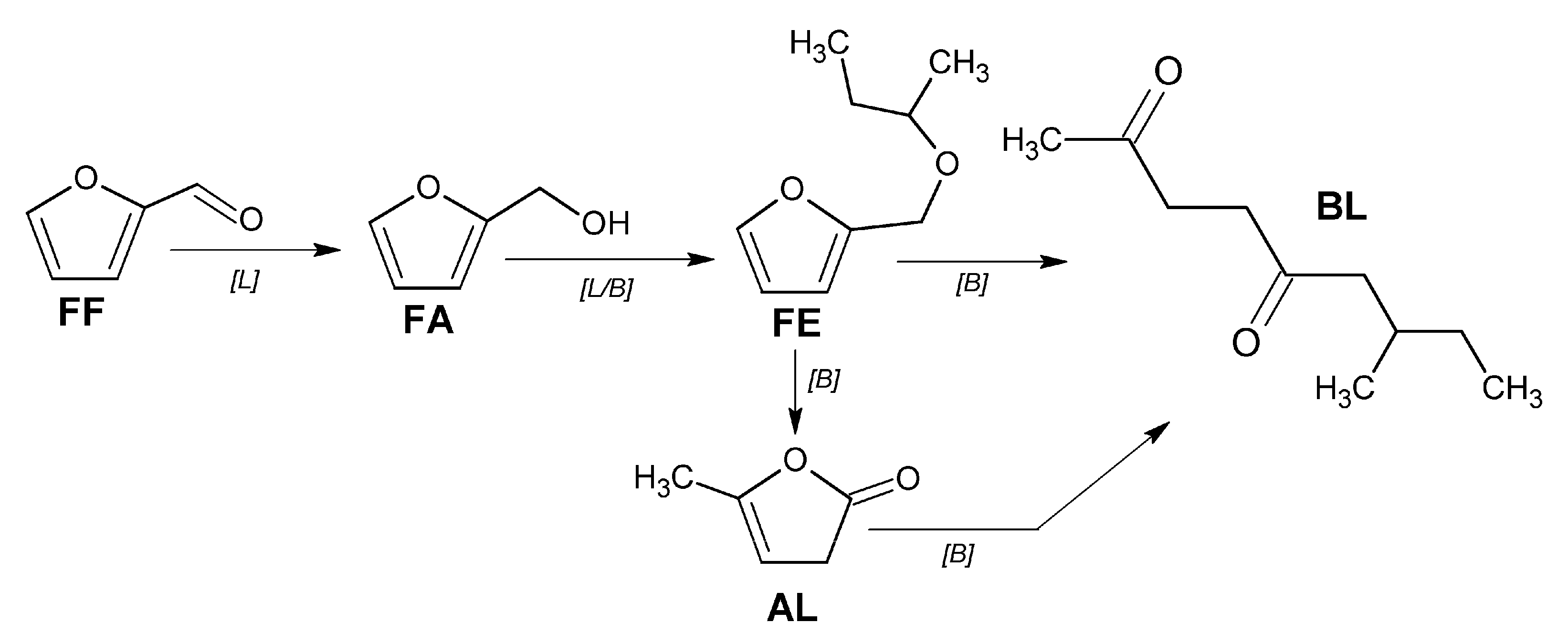
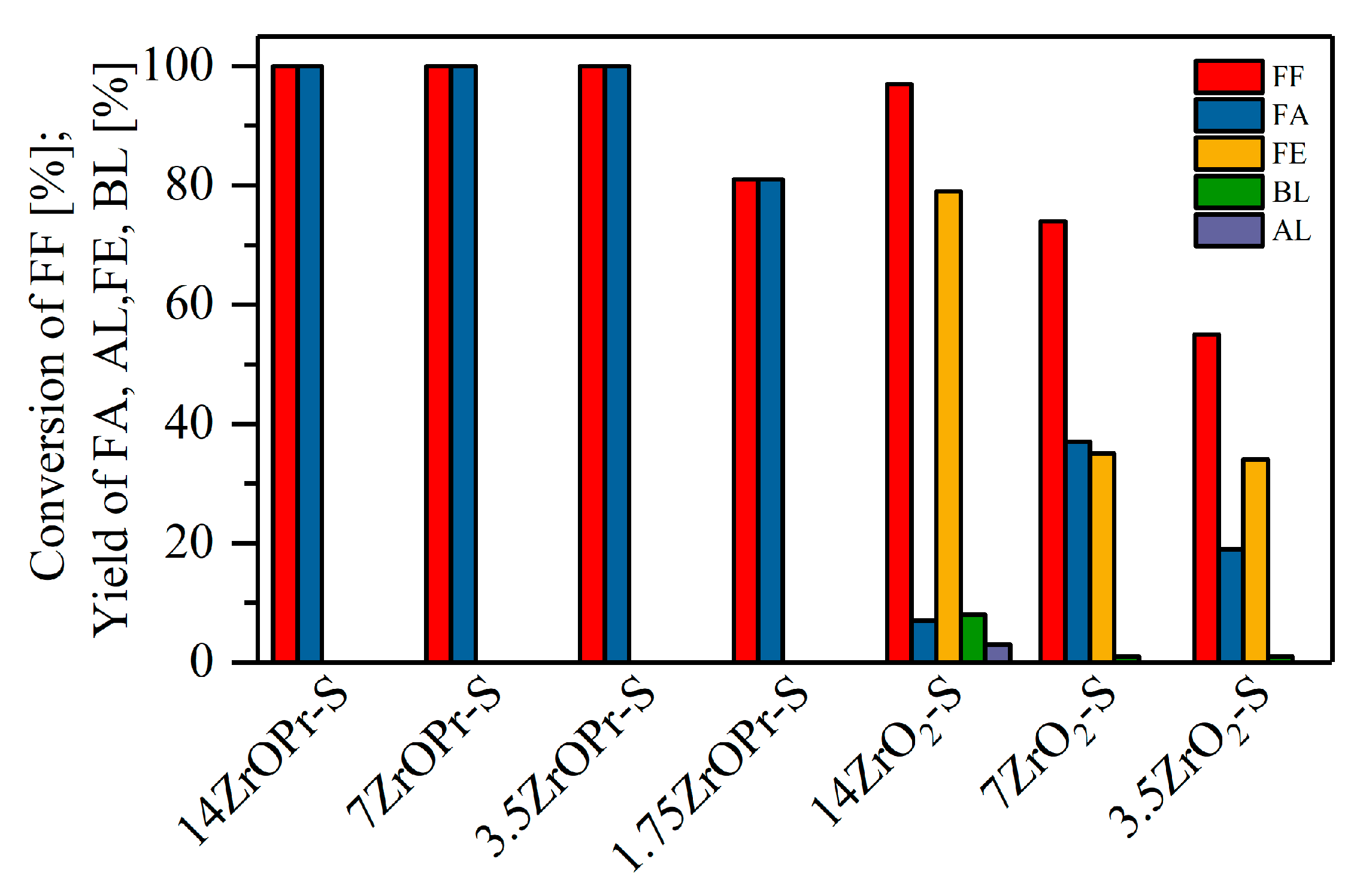
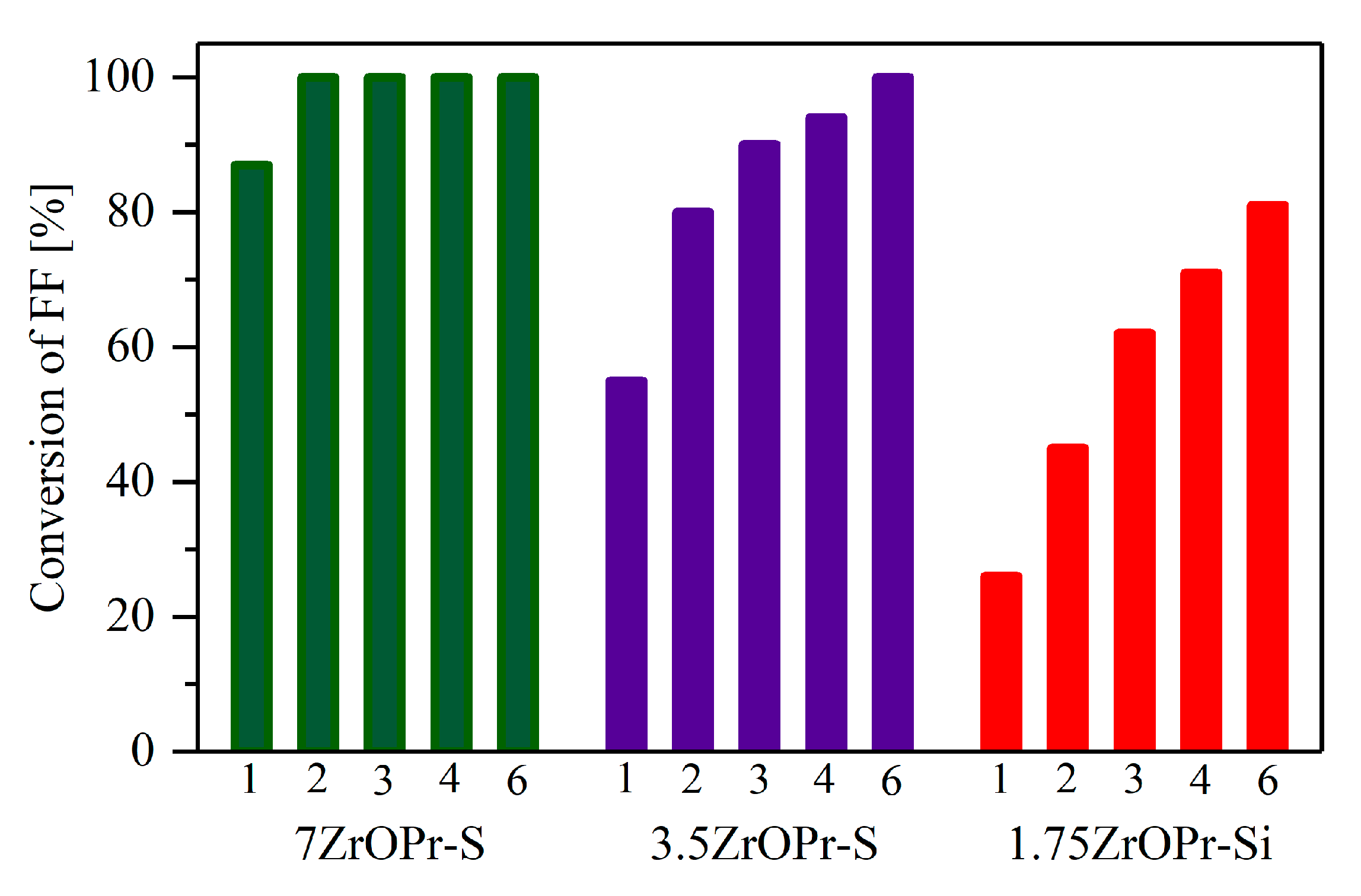
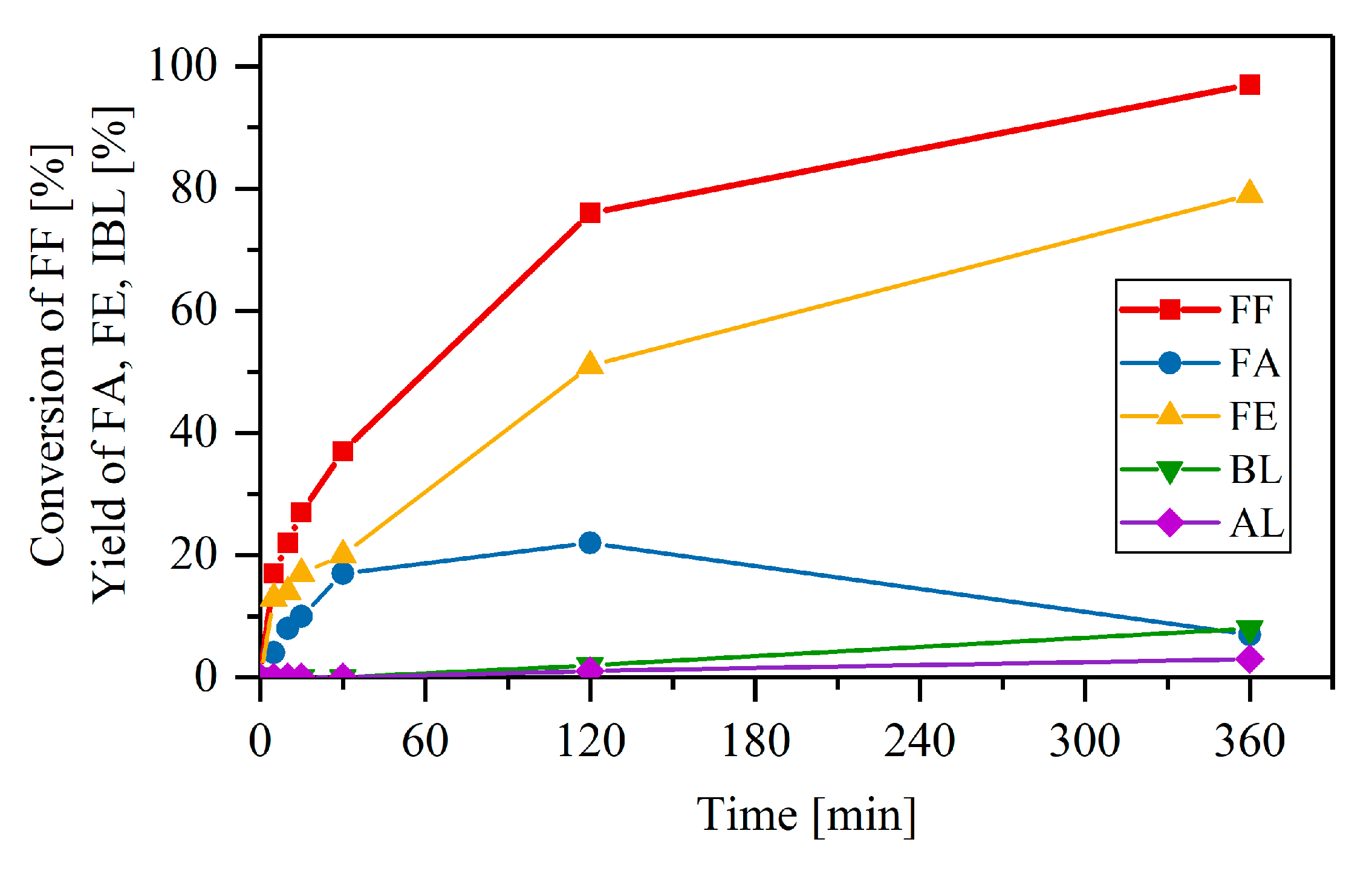
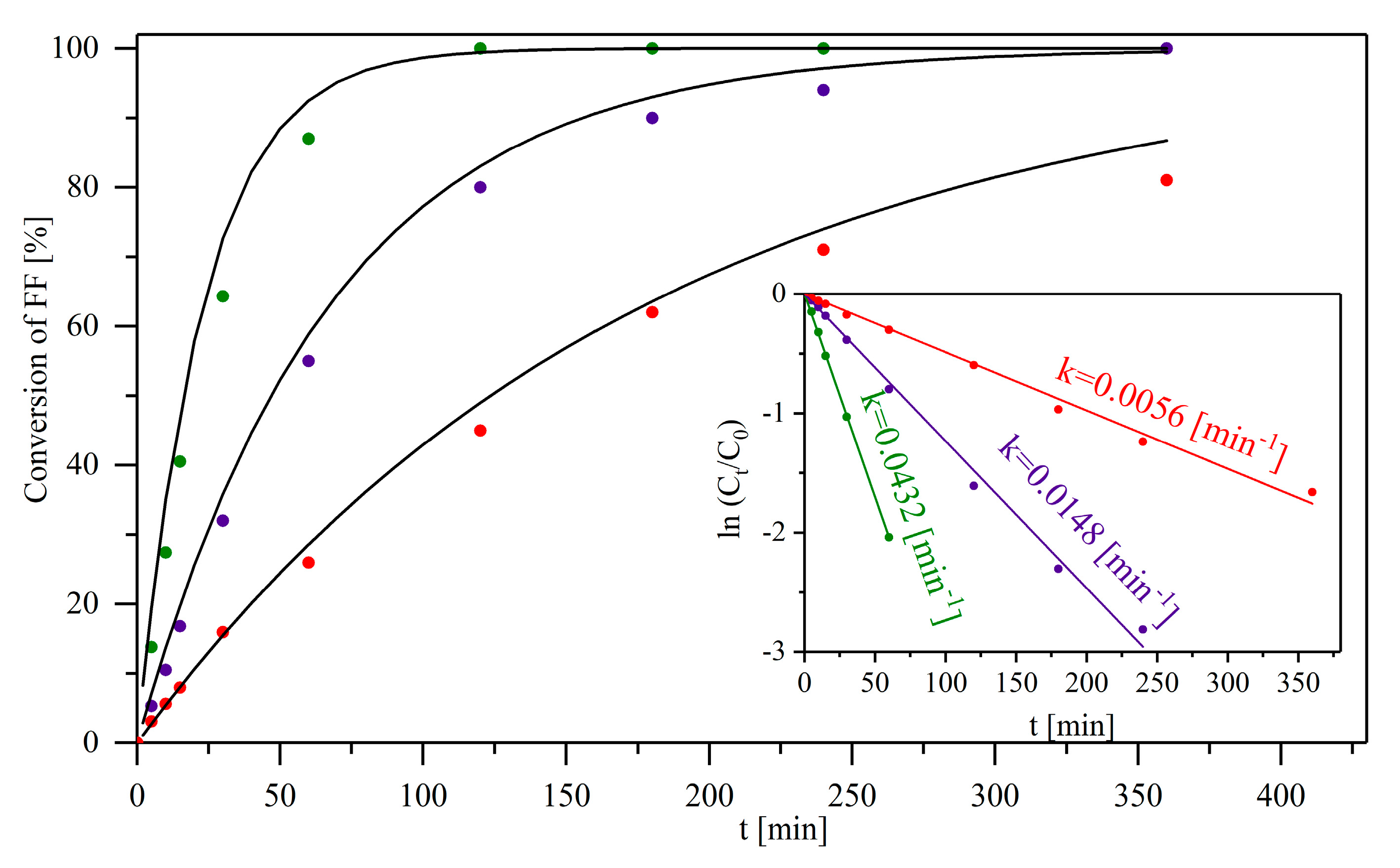
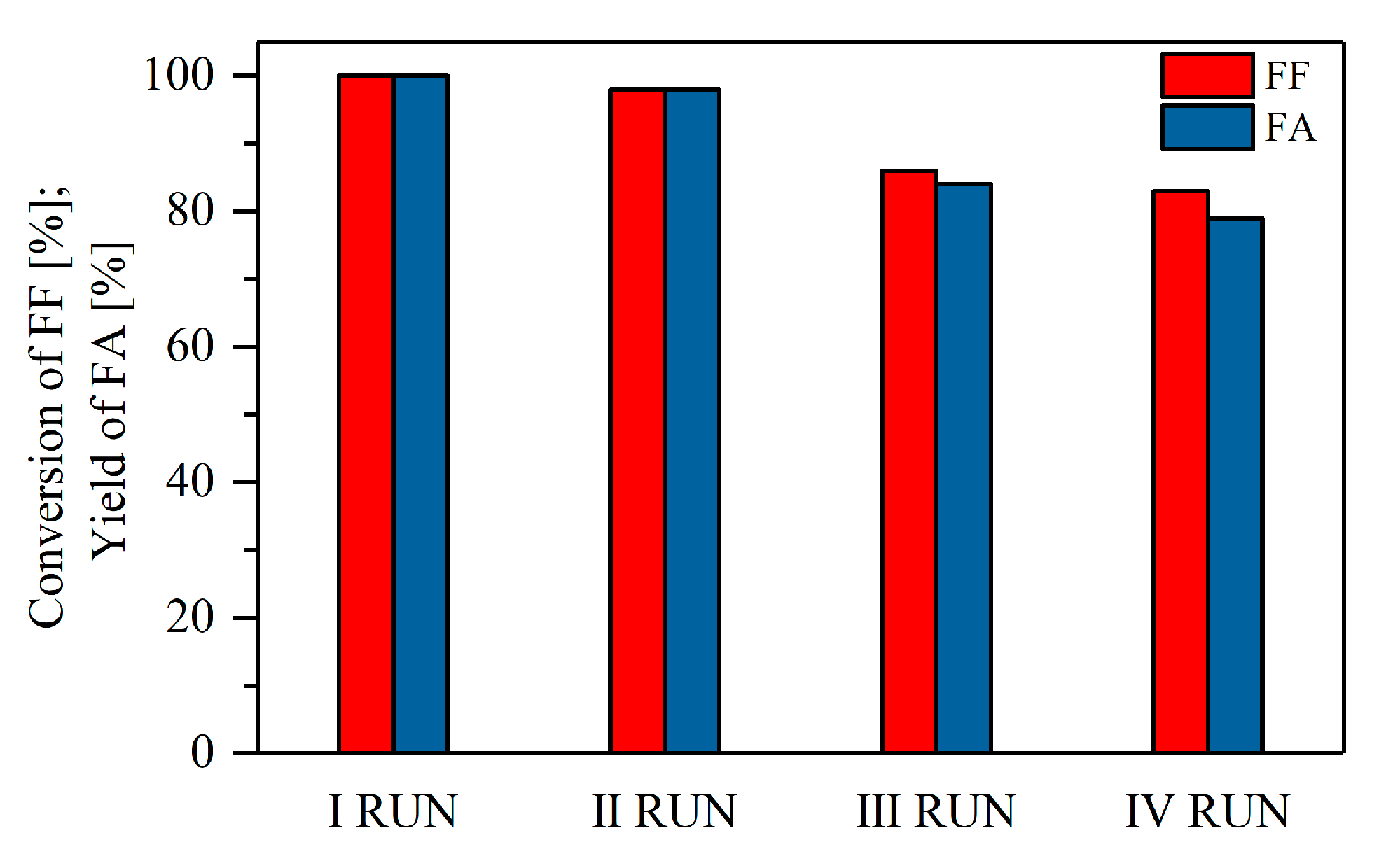
2.2. Catalytic Performance of the Materials in the MPV Reduction
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Catalysts
3.3. Characterisation Techniques
3.4. Catalytic Activity Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baldenhofer, R.; Lange, J.P.; Kersten, S.R.A.; Ruiz, M.P. Furfural to Cyclopentanone—A Search for Putative Oligomeric By-products. Chemsuschem 2024, 17, e202400108. [Google Scholar] [CrossRef]
- Bui, L.; Luo, H.; Gunther, W.R.; Román-Leshkov, Y. Domino Reaction Catalyzed by Zeolites with Brönsted and Lewis Acid Sites for the Production of γ-Valerolactone from Furfural. Angew. Chem. Int. Edit. 2013, 52, 8022–8025. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wang, K.; Zhao, X.P.; Chen, Z.J.; Yin, X.Y.; Cai, T.T.; Zhang, X.L.; Xu, J.M.; Hu, J.; Meng, X.Z.; et al. Integrated lignocellulosic biorefinery for efficient production of furans and photothermal materials. Chem. Eng. J. 2023, 453, 139688. [Google Scholar] [CrossRef]
- Yan, K.; Wu, G.S.; Lafleur, T.; Jarvis, C. Production, properties and catalytic hydrogenation of furfural to fuel additives and value-added chemicals. Renew. Sust. Energ. Rev. 2014, 38, 663–676. [Google Scholar] [CrossRef]
- Yong, K.J.; Wu, T.Y.; Lee, C.B.T.L.; Lee, Z.J.; Liu, Q.; Jahim, J.M.; Zhou, Q.Q.; Zhang, L. Furfural production from biomass residues: Current technologies, challenges and future prospects. Biomass Bioenerg. 2022, 161, 106458. [Google Scholar] [CrossRef]
- Millán, G.G.; Sixta, H. Towards the Green Synthesis of Furfuryl Alcohol in A One-Pot System from Xylose: A Review. Catalysts 2020, 10, 1101. [Google Scholar] [CrossRef]
- Pophali, A.; Lee, K.M.; Zhang, L.X.; Chuang, Y.C.; Ehm, L.; Cuiffo, M.A.; Halada, G.P.; Rafailovich, M.; Verma, N.; Kim, T. First synthesis of poly(furfuryl) alcohol precursor-based porous carbon beads as an efficient adsorbent for volatile organic compounds. Chem. Eng. J. 2019, 373, 365–374. [Google Scholar] [CrossRef]
- Iroegbu, A.O.C.; Ray, S.S. On the chemistry of furfuryl alcohol polymerization: A review. J. Polym. Sci. 2024, 62, 1044–1060. [Google Scholar] [CrossRef]
- Luckeneder, P.; Gavino, J.; Kuchernig, R.; Petutschnigg, A.; Tondi, G. Sustainable Phenolic Fractions as Basis for Furfuryl Alcohol-Based Co-Polymers and Their Use as Wood Adhesives. Polymers 2016, 8, 396. [Google Scholar] [CrossRef]
- Hao, Y.C.; Zong, M.H.; Wang, Z.L.; Li, N. Chemoenzymatic access to enantiopure N-containing furfuryl alcohol from chitin-derived N-acetyl-D-glucosamine. Bioresour. Bioprocess. 2021, 8, 80. [Google Scholar] [CrossRef]
- Saluta, E.D.; Gebresillase, M.N.; Seo, J.G. A Recent Progress on Upgrading of Furfural and Derivatives into Green Transport Fuels and Precursors via C-C Coupling Reactions. Adv. Energ. Sust. Res. 2025, 6, 202400369. [Google Scholar] [CrossRef]
- Mascal, M.; Dutta, S. Synthesis of ranitidine (Zantac) from cellulose-derived 5-(chloromethyl)furfural. Green Chem. 2011, 13, 3101–3102. [Google Scholar] [CrossRef]
- Tukacs, J.M.; Bohus, M.; Dibó, G.; Mika, L.T. Ruthenium-catalyzed solvent-free conversion of furfural to furfuryl alcohol. Rsc. Adv. 2017, 7, 3331–3335. [Google Scholar] [CrossRef]
- Jiménez-Gómez, C.P.; Cecilia, J.A.; García-Sancho, C.; Moreno-Tost, R.; Maireles-Torres, P. Gas phase hydrogenation of furfural to obtain valuable products using commercial Cr-free catalysts as an environmentally sustainable alternative to copper chromite. J. Environ. Chem. Eng. 2021, 9, 105468. [Google Scholar] [CrossRef]
- Huang, R.J.; Cui, Q.Q.; Yuan, Q.Q.; Wu, H.H.; Guan, Y.J.; Wu, P. Total Hydrogenation of Furfural over Pd/Al2O3 and Ru/ZrO Mixture under Mild Conditions: Essential Role of Tetrahydrofurfural as an Intermediate and Support Effect. ACS Sust. Chem. Eng. 2018, 6, 6957–6964. [Google Scholar] [CrossRef]
- Nehra, P.; Betsy, K.J.; Vinod, C.P. Selective Hydrogenation of Furfural to Furfuryl Alcohol over Pd Supported on Ternary Oxide in Aqueous Medium Under Mild Conditions. Chemcatchem 2025, 15, e02092. [Google Scholar] [CrossRef]
- Sivec, R.; Hus, M.; Likozar, B.; Grilc, M. Furfural hydrogenation over Cu, Ni, Pd, Pt, Re, Rh and Ru catalysts: Modelling of adsorption, desorption and reaction micro-kinetics. Chem. Eng. J. 2022, 436, 135070. [Google Scholar] [CrossRef]
- Jiménez-Gómez, C.P.; Cecilia, J.A.; Moreno-Tost, R.; Maireles-Torres, P. Nickel Phosphide/Silica Catalysts for the Gas-Phase Hydrogenation of Furfural to High-Added-Value Chemicals. Chemcatchem 2017, 9, 2881–2889. [Google Scholar] [CrossRef]
- Kalumpha, M.; Charlie, L.; Makhubela, B.C.E. Synthesis and Characterization of Nickel(II) Homogeneous and Supported Complexes for the Hydrogenation of Furfural to Furfuryl Alcohol. Catalysts 2021, 11, 684. [Google Scholar] [CrossRef]
- Assary, R.S.; Curtiss, L.A.; Dumesic, J.A. Exploring Meerwein-Ponndorf-Verley Reduction Chemistry for Biomass Catalysis Using a First-Principles Approach. ACS Catal. 2013, 3, 2694–2704. [Google Scholar] [CrossRef]
- Antunes, M.M.; Lima, S.; Neves, P.; Magalhaes, A.L.; Fazio, E.; Neri, F.; Pereira, M.T.; Silva, A.F.; Silva, C.M.; Rocha, S.M.; et al. Integrated reduction and acid-catalysed conversion of furfural in alcohol medium using Zr, Al-containing ordered micro/mesoporous silicates. Appl. Catal. B-Environ. 2016, 182, 485–503. [Google Scholar] [CrossRef]
- Lu, Y.J.; Li, W.Z.; Zhu, Y.S.; Zhang, T.W.; Zhang, Q.; Liu, Q.Y. One-pot Synthesis of High Value-added Chemicals from Furfural over Bimetal-doped Beta Zeolite and Carbon Solid Acid Catalysts. Bioresources 2018, 13, 5925–5941. [Google Scholar] [CrossRef]
- López-Asensio, R.; Gómez, C.P.J.; Sancho, C.G.; Moreno-Tost, R.; Cecilia, J.A.; Maireles-Torres, P. Influence of Structure-modifying Agents in the Synthesis of Zr-doped SBA-15 Silica and Their Use as Catalysts in the Furfural Hydrogenation to Obtain High Value-added Products through the Meerwein-Ponndorf-Verley Reduction. Int. J. Mol. Sci. 2019, 20, 828. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.H.; Liu, Y.; Yang, S.B.; Wei, J.N.; He, L.; Peng, L.C.; Tang, X.; Ni, Y.H. Highly Selective Conversion of Furfural to Furfural Alcohol or Levulinate Ester in One Pot over ZrO2@SBA-15 and Its Kinetic Behavior. ACS Sust. Chem. Eng. 2020, 8, 5584–5594. [Google Scholar] [CrossRef]
- Vasconcelos, S.C.; Pinhel, L.F.C.; Madriaga, V.G.C.; Rossa, V.; Batinga, L.G.S.; Silva, D.S.A.; dos Santos, R.D.; Soares, A.V.H.; Urquieta-González, E.A.; Passos, F.B.; et al. Selective Synthesis of Levulinic Ester from Furfural Catalyzed by Hierarchical Zeolites. Catalysts 2022, 12, 783. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Lu, T.L.; Yang, X.M.; Zhou, L.P. Regulation of acid/basic properties of Zr-Beta zeolite for efficient conversion of furfural to furfuryl alcohol. Mol. Catal. 2024, 559, 114093. [Google Scholar] [CrossRef]
- López-Asensio, R.; Cecilia, J.A.; Jiménez-Gómez, C.P.; García-Sancho, C.; Moreno-Tost, R.; Maireles-Torres, P. Selective production of furfuryl alcohol from furfural by catalytic transfer hydrogenation over commercial aluminas. Appl. Catal. A-Gen. 2018, 556, 1–9. [Google Scholar] [CrossRef]
- Hidalgo-Carrillo, J.; Parejas, A.; Cuesta-Rioboo, M.J.; Marinas, A.; Urbano, F.J. MPV Reduction of Furfural to Furfuryl Alcohol on Mg, Zr, Ti, Zr-Ti, and Mg-Ti Solids: Influence of Acid-Base Properties. Catalysts 2018, 8, 539. [Google Scholar] [CrossRef]
- Peng, L.C.; Gao, X.Y.; Yu, X.; Li, H.; Zhang, J.H.; He, L. Facile and High-Yield Synthesis of Alkyl Levulinate Directly from Furfural by Combining Zr-MCM-41 and Amberlyst-15 without External H. Energ. Fuel 2019, 33, 330–339. [Google Scholar] [CrossRef]
- Bi, S.F.; Lu, J.Y.; Zheng, S.R.; Xu, Z.Y. Zirconium-grafted mesoporous SiO2 (Zr-SBA-15) as Brönsted acid sites-free catalyst enables selective catalytic transfer hydrogenation of furfural to furfuryl alcohol. Sustain. Chem. Pharm. 2025, 46, 102084. [Google Scholar] [CrossRef]
- Valekar, A.H.; Lee, M.; Yoon, J.W.; Kwak, J.; Hong, D.Y.; Oh, K.R.; Cha, G.Y.; Kwon, Y.U.; Jung, J.; Chang, J.S.; et al. Catalytic Transfer Hydrogenation of Furfural to Furfuryl Alcohol under Mild Conditions over Zr-MOFs: Exploring the Role of Metal Node Coordination and Modification. ACS Catal. 2020, 10, 3720–3732. [Google Scholar] [CrossRef]
- Zhang, W.L.; Zhou, Y.J.; Wu, Z.Z.; Pan, C.; Cheng, Q.P.; Fan, G.Z. One-pot cascade conversion of furfural to isopropyl levulinate catalyzed by sulfonic acid-functionalized UiO-66. Fuel 2024, 378, 132912. [Google Scholar] [CrossRef]
- Iglesias, J.; Melero, J.A.; Morales, G.; Moreno, J.; Segura, Y.; Paniagua, M.; Cambra, A.; Hernández, B. Zr-SBA-15 Lewis Acid Catalyst: Activity in Meerwein Ponndorf Verley Reduction. Catalysts 2015, 5, 1911–1927. [Google Scholar] [CrossRef]
- Li, W.L.; Li, M.Z.; Liu, H.; Jia, W.L.; Yu, X.; Wang, S.; Zeng, X.H.; Sun, Y.; Wei, J.N.; Tang, X.; et al. Domino transformation of furfural to γ-valerolactone over SAPO-34 zeolite supported zirconium phosphate catalysts with tunable Lewis and Brönsted acid sites. Mol. Catal. 2021, 506, 111538. [Google Scholar] [CrossRef]
- Maderuelo-Solera, R.; Richter, S.; Jiménez-Gómez, C.P.; García-Sancho, C.; García-Mateos, F.J.; Rosas, J.M.; Moreno-Tost, R.; Cecilia, J.A.; Maireles-Torres, P. Porous SiO2 Nanospheres Modified with ZrO2 and Their Use in One-Pot Catalytic Processes to Obtain Value-Added Chemicals from Furfural. Ind. Eng. Chem. Res. 2021, 60, 18791–18805. [Google Scholar] [CrossRef]
- Xue, W.; Ma, M.W.; Hou, P.; Zhang, P.; Liu, W.Z.; Liu, Y.C.; Yue, H.J.; Wang, X.F.; Tian, G.; Feng, S.H. One-pot tandem conversion of furfural to γ-valerolactone over a series of modified zirconium-based metal-organic frameworks with variational Lewis and Brønsted acid sites. Fuel 2024, 371, 132115. [Google Scholar] [CrossRef]
- Podobinski, J.; Sliwa, M.; Datka, J. Ethoxy Groups on ZrO2, CuO, and CuO/ZrO2 Studied by IR Spectroscopy. Molecules 2022, 27, 4790. [Google Scholar] [CrossRef]
- Ivanchikova, I.D.; Zalomaeva, O.; Maksimchuk, N.; Stonkus, O.A.; Glazneva, T.S.; Chesalov, Y.A.; Shmakov, A.N.; Guidotti, M.; Kholdeeva, O.A. Alkene Epoxidation and Thioether Oxidation with Hydrogen Peroxide Catalyzed by Mesoporous Zirconium-Silicates. Catalysts 2022, 12, 742. [Google Scholar] [CrossRef]
- Koreniuk, A.; Maresz, K.; Mrowiec-Białoń, J. Supported zirconium-based continuous-flow microreactor for effective Meerwein-Ponndorf-Verley reduction of cyclohexanone. Catal. Commun. 2015, 64, 48–51. [Google Scholar] [CrossRef]
- Ciemięga, A.; Maresz, K.; Mrowiec-Białoń, J. Continuous-flow chemoselective reduction of cyclohexanone in a monolithic silica-supported Zr(OPri)4 multichannel microreactor. Microporous Mesoporous Mater. 2017, 252, 140–145. [Google Scholar] [CrossRef]
- Marques, J.P.; Gener, I.; Ayrault, P.; Bordado, J.C.; Lopes, J.M.; Ribeiro, F.R.; Guisnet, M. Infrared spectroscopic study of the acid properties of dealuminated BEA zeolites. Microporous Mesoporous Mater. 2003, 60, 251–262. [Google Scholar] [CrossRef]
- Travert, A.; Vimont, A.; Sahibed-Dine, A.; Daturi, M.; Lavalley, J.C. Use of pyridine CH(D) vibrations for the study of Lewis acidity of metal oxides. Appl. Catal. A-Gen 2006, 307, 98–107. [Google Scholar] [CrossRef]
- Emeis, C.A. Determination of Integrated Molar Extinction Coefficients for Infrared-Absorption Bands of Pyridine Adsorbed on Solid Acid Catalysts. J. Catal. 1993, 141, 347–354. [Google Scholar] [CrossRef]
- Wang, J.H.; Wang, S.H.; Peng, H.Y.; Xiao, Z.S.; Xu, Q.; Liu, X.X.; Yin, D.L.; Zhong, W.Z. Two salicylic acid derivatives welded Zr-based hybrids relay catalysis: One-pot conversion of bio-based furfural to additive γ-valerolactone. Ind. Crop. Prod. 2025, 228, 120878. [Google Scholar] [CrossRef]
- Shylesh, S.; Kapoor, M.P.; Juneja, L.R.; Samuel, P.R.; Srilakshmi, C.; Singh, A.R. Catalytic Meerwein-Ponndorf-Verley reductions over mesoporous silica supports: Rational design of hydrophobic mesoporous silica for enhanced stability of aluminum doped mesoporous catalysts. J. Mol. Catal. A-Chem 2009, 301, 118–126. [Google Scholar] [CrossRef]
- Caillot, M.; Chaumonnot, A.; Digne, M.; Poleunis, C.; Debecker, D.P.; van Bokhoven, J.A. Synthesis of amorphous aluminosilicates by grafting: Tuning the building and final structure of the deposit by selecting the appropriate synthesis conditions. Microporous Mesoporous Mater. 2014, 185, 179–189. [Google Scholar] [CrossRef]
- Wu, D.; Hernández, W.Y.; Zhang, S.W.; Vovk, E.I.; Zhou, X.H.; Yang, Y.; Khodakov, A.Y.; Ordomsky, V.V. In Situ Generation of Bronsted Acidity in the Pd-I Bifunctional Catalysts for Selective Reductive Etherification of Carbonyl Compounds under Mild Conditions. ACS Catal. 2019, 9, 2940–2948. [Google Scholar] [CrossRef]
- Panagiotopoulou, P.; Martin, N.; Vlachos, D.G. Effect of hydrogen donor on liquid phase catalytic transfer hydrogenation of furfural over a Ru/RuO/C catalyst. J. Mol. Catal. A-Chem. 2014, 392, 223–228. [Google Scholar] [CrossRef]
- Luo, H.Y.; Consoli, D.F.; Gunther, W.R.; Román-Leshkov, Y. Investigation of the reaction kinetics of isolated Lewis acid sites in Beta zeolites for the Meerwein-Ponndorf-Verley reduction of methyl levulinate to γ-valerolactone. J. Catal. 2014, 320, 198–207. [Google Scholar] [CrossRef]
- Nielsen, M. Catalyst Kinetics and Stability in Homogeneous Alcohol Acceptorless Dehydrogenation. In Advanced Chemical Kinetics; Farrukh, M.A., Ed.; IntechOpen: London, UK, 2017. [Google Scholar] [CrossRef]
- Corma, A.; Domine, M.E.; Valencia, S. Water-resistant solid Lewis acid catalysts: Meerwein-Ponndorf-Verley and Oppenauer reactions catalyzed by tin-beta zeolite. J. Catal. 2003, 215, 294–304. [Google Scholar] [CrossRef]
- Ye, L.; Han, Y.W.; Zhang, M.; Yu, Z.H.; Lu, X.B. Enhanced sorption of carbonyl groups by zirconium hydroxide modified with polydopamine for highly selective production of alcohols via MPV reduction under mild conditions. Fuel 2023, 331, 125786. [Google Scholar] [CrossRef]
- Iglesias, J.; Melero, J.A.; Morales, G.; Paniagua, M.; Hernández, B.; Osatiashtiani, A.; Lee, A.F.; Wilson, K. ZrO2-SBA-15 catalysts for the one-pot cascade synthesis of GVL from furfural. Catal. Sci. Technol. 2018, 8, 4485–4493. [Google Scholar] [CrossRef]
- Xu, Y.Y.; Liu, X.N.; Guo, H.X.; Qiu, M.; Qi, X.H. Efficient catalytic transfer hydrogenation of furfural to furfuryl alcohol over Zr-doped ordered mesoporous carbon synthesized by Zr-arbutin coordinated self-assembly. Fuel 2023, 331, 125834. [Google Scholar] [CrossRef]
- Zhang, J.; Dong, K.J.; Luo, W.M.; Guan, H.F. Selective Transfer Hydrogenation of Furfural into Furfuryl Alcohol on Zr-Containing Catalysts Using Lower Alcohols as Hydrogen Donors. ACS Omega 2018, 3, 6206–6216. [Google Scholar] [CrossRef]
- Yang, J.R.; Zhang, Y.W.; Shen, F.; Qi, X.H. Glucose-derived zirconium-containing mesoporous composite for efficient catalytic transfer hydrogenation of furfural to furfuryl alcohol. Biomass Bioenerg. 2023, 170, 106723. [Google Scholar] [CrossRef]
- Zhou, S.H.; Dai, F.L.; Xiang, Z.Y.; Song, T.; Liu, D.T.; Lu, F.C.; Qi, H.S. Zirconium-lignosulfonate polyphenolic polymer for highly efficient hydrogen transfer of biomass-derived oxygenates under mild conditions. Appl. Catal. B-Environ. 2019, 248, 31–43. [Google Scholar] [CrossRef]
- Ye, L.; Han, Y.W.; Yu, Z.H.; Zhang, M.; Xiong, J.; Zhang, R.; Li, X.Y.; Qiao, Y.N.; Lu, X.B. Efficient MPV reductions of biomass-derived aldehydes and ketones over an assembling zirconium-gallic acid hybrid under mild condition. Fuel 2022, 328, 125233. [Google Scholar] [CrossRef]
- Liu, Z.H.; Zhang, Z.Z.; Fu, R.; Xu, J.; Lu, J.Y.; Wen, Z.; Xue, B. One-Pot Conversion of Furfural to Gamma-Valerolactone over Zr-SBA-15: Cooperation of Lewis and Bronsted Acidic Sites. ACS Appl. Nano. Mater. 2023, 6, 13196–13207. [Google Scholar] [CrossRef]
- Nakanishi, K.; Takahashi, R.; Nagakane, T.; Kitayama, K.; Koheiya, N.; Shikata, H.; Soga, N. Formation of hierarchical pore structure in silica gel. J. Sol-Gel Sci. Technol. 2000, 17, 191–210. [Google Scholar] [CrossRef]
- Smatt, J.H.; Schunk, S.; Linden, M. Versatile double-templating synthesis route to silica monoliths exhibiting a multimodal hierarchical porosity. Chem. Mater. 2003, 15, 2354–2361. [Google Scholar] [CrossRef]
- Koreniuk, A.; Maresz, K.; Odrozek, K.; Jarzębski, A.B.; Mrowiec-Białoń, J. Highly effective continuous-flow monolithic silica microreactors for acid catalyzed processes. Appl. Catal. A-Gen. 2015, 489, 203–208. [Google Scholar] [CrossRef]



| Support/Catalyst | SBET [m2/g] | Vp [cm3/g] |
|---|---|---|
| S | 358 | 1.23 |
| 14ZrOPr-S | 337 | 0.95 |
| 14ZrO2-S | 340 | 1.06 |
| 14Al-P-S | 330 | 1.04 |
| Catalyst | Relative L/B Ratio |
|---|---|
| 14ZrOPr-S | 8.5 |
| 14ZrO2-S | 2.5 |
| 14Al-P-S | 1.3 |
| Solvent | b. p. [°C] | ε | Conversion of FF [%] |
|---|---|---|---|
| 2-Propanol | 82 | 18.3 | 36 |
| 2-Butanol | 99 | 15.8 | 55 |
| 2-Pentanol | 119 | 13.6 | 38 |
| 2-Heptanol | 159 | 9.21 | 3 |
| 1-Propanol | 97 | 20.1 | 6 |
| 1-Butanol | 118 | 17.8 | 10 |
| Catalyst | Temp. [°C] | Time [h] | Solvent | FF Conv. [%] | P * | FA Selec. [%] | Ref. |
|---|---|---|---|---|---|---|---|
| Zr@OMCs | 180 | 4 | 2-PrOH | 100 | 3.75 | 93.2 | [54] |
| Zr(OH)4 | 170 | 2.5 | 2-PrOH | 100 | 0.01 | 99 | [55] |
| Zr-LS | 100 | 1 | 2-PrOH | 97.5 | 4.37 | 98.5 | [57] |
| Zr5-GAF | 100 | 1 | 2-PrOH | 96.5 | 13.2 | 98.1 | [58] |
| meso-Zr-glu | 180 | 3 | 2-PrOH | 100 | 2.52 | 92 | [56] |
| M-MOF-808 | 100 | 2 | 2-PrOH | 99.2 | 17.6 | 93.8 | [31] |
| UiO-66 | 150 | 6 | 2-PrOH | 82.1 | N/A | ~92 | [32] |
| ZrO2-SBA-15(1) | 130 | 1.5 | 2-PrOH | 75 | 9.28 | 45 | [53] |
| Zr1-SBA-15 | 120 | 3 | 2-PrOH | 99 | 10.38 ** | 98.5 | [30] |
| Zr-SBA-15_25 | 190 | 1 | 2-PrOH | >99 | N/A | N/A | [59] |
| RT (Zr-SBA-15) | 110 | 4 | 2-BuOH | 95 | 3.85 | 74 | [23] |
| ZrO2(20)@SBA-15 | 140 | 3 | 2-PrOH | 93.2 | 10 | 96.9 | [24] |
| Zr-MCM-41 | 130 | 24 | 2-PrOH | 96.9 | N/A | N/A | [29] |
| 7ZrOPr-Si | 120 | 1.5 | 2-BuOH | 99 | 15.2 | >99 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciemięga, A.; Maresz, K.; Janoszka, K.; Mrowiec-Białoń, J. High Selectivity and Yield in Catalytic Transfer Hydrogenation of Furfural to Furfuryl Alcohol by Zirconium Propoxide Modified Mesoporous Silica. Molecules 2025, 30, 3600. https://doi.org/10.3390/molecules30173600
Ciemięga A, Maresz K, Janoszka K, Mrowiec-Białoń J. High Selectivity and Yield in Catalytic Transfer Hydrogenation of Furfural to Furfuryl Alcohol by Zirconium Propoxide Modified Mesoporous Silica. Molecules. 2025; 30(17):3600. https://doi.org/10.3390/molecules30173600
Chicago/Turabian StyleCiemięga, Agnieszka, Katarzyna Maresz, Katarzyna Janoszka, and Julita Mrowiec-Białoń. 2025. "High Selectivity and Yield in Catalytic Transfer Hydrogenation of Furfural to Furfuryl Alcohol by Zirconium Propoxide Modified Mesoporous Silica" Molecules 30, no. 17: 3600. https://doi.org/10.3390/molecules30173600
APA StyleCiemięga, A., Maresz, K., Janoszka, K., & Mrowiec-Białoń, J. (2025). High Selectivity and Yield in Catalytic Transfer Hydrogenation of Furfural to Furfuryl Alcohol by Zirconium Propoxide Modified Mesoporous Silica. Molecules, 30(17), 3600. https://doi.org/10.3390/molecules30173600






