Conformational and Intermolecular Interaction Analysis of Tiaprofenic Acid: A X-Ray Powder Diffraction and First Principle Modeling Analysis
Abstract
1. Introduction
2. Results and Discussion
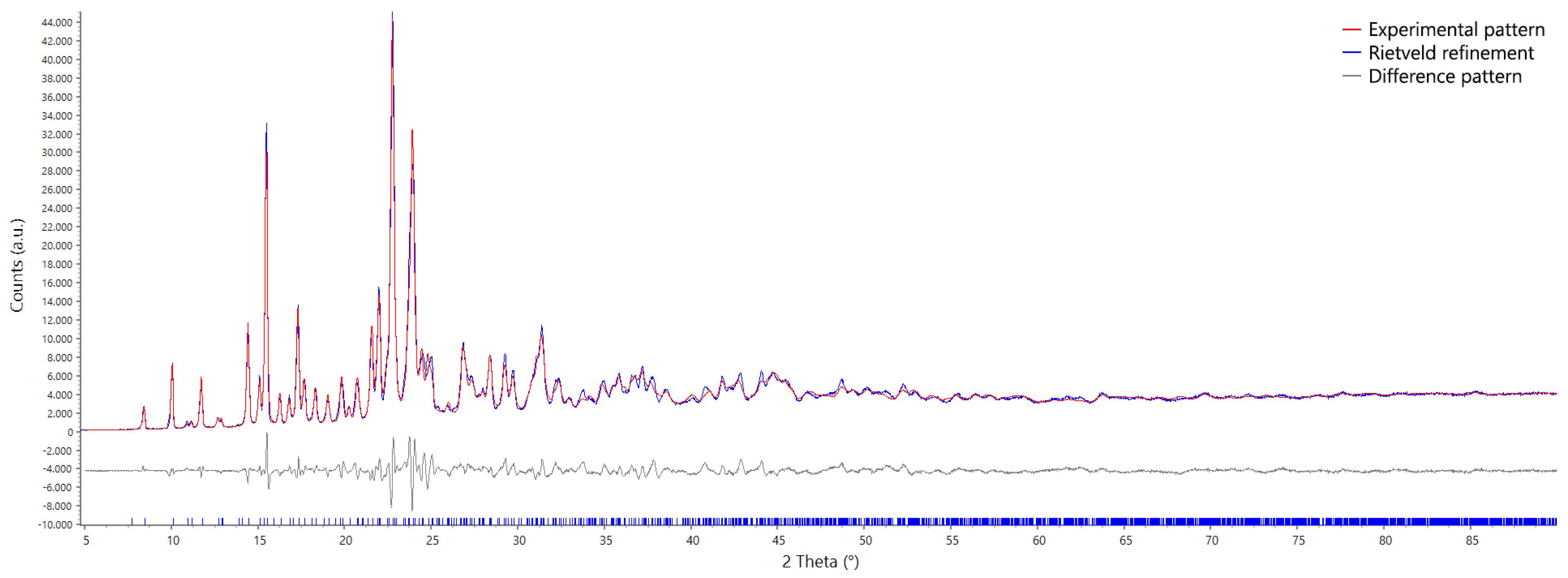
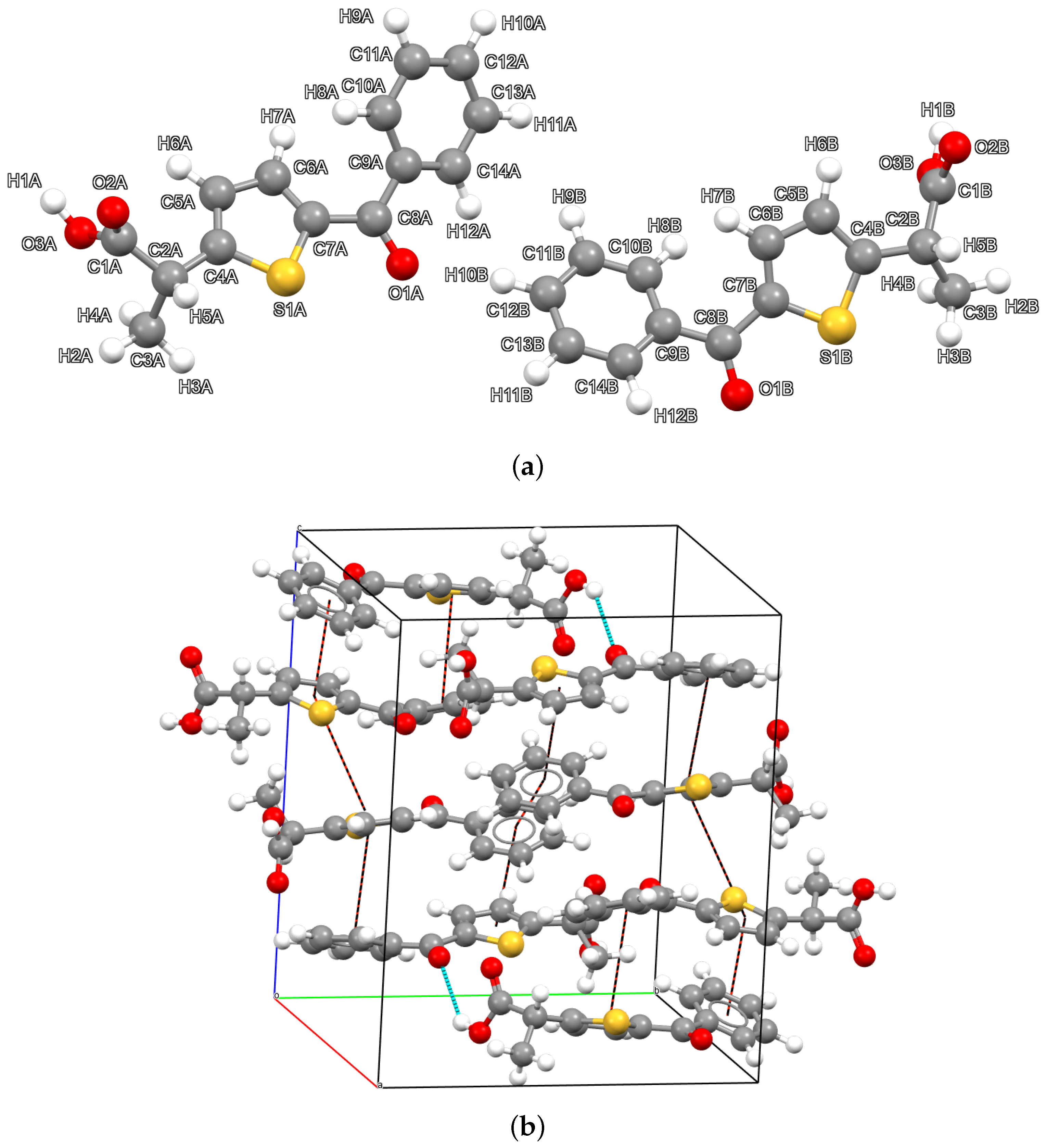
2.1. Description of the Crystal Packing and Refinement
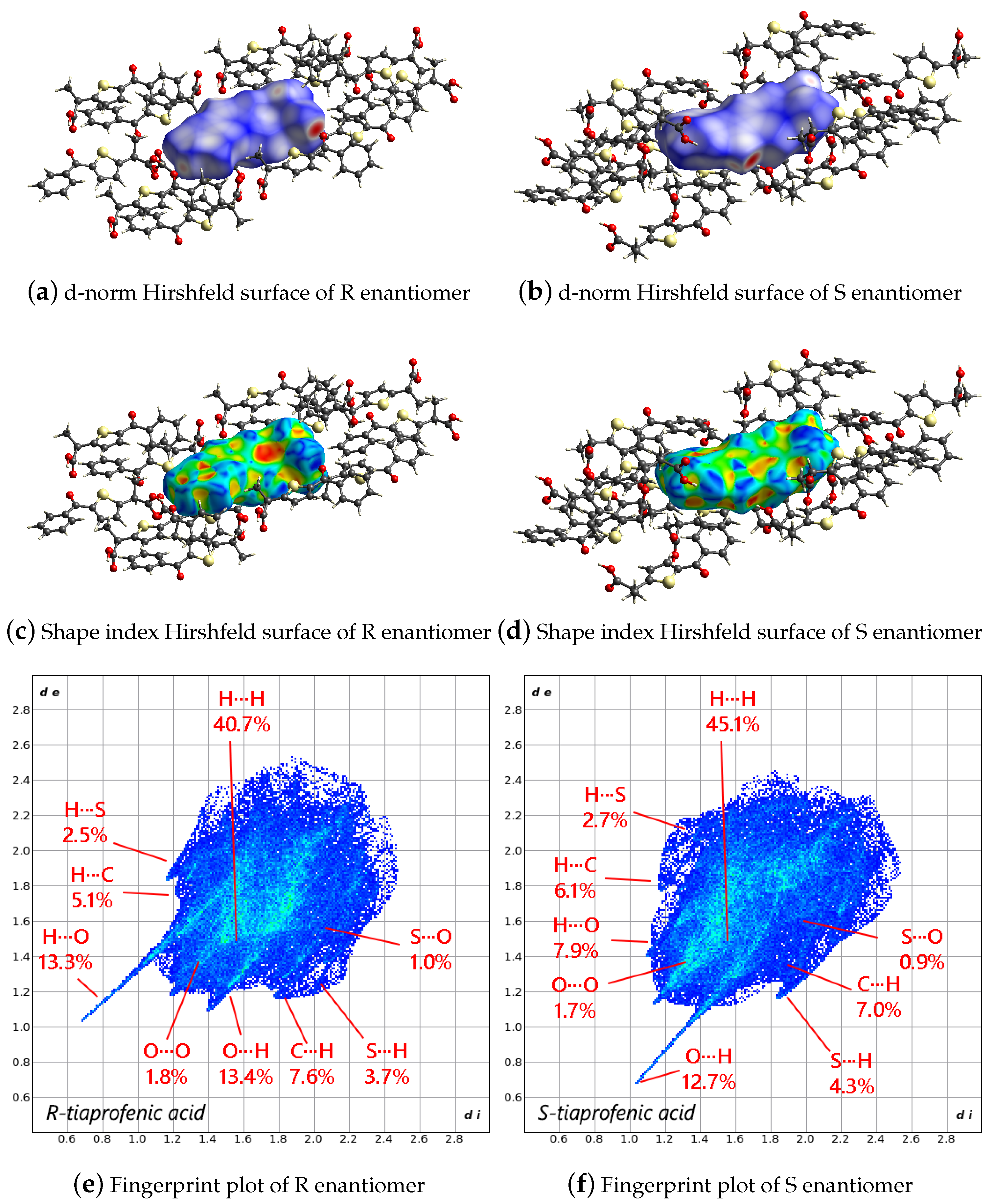
2.2. Hirshfeld Surface Analysis
2.3. Energetic Considerations
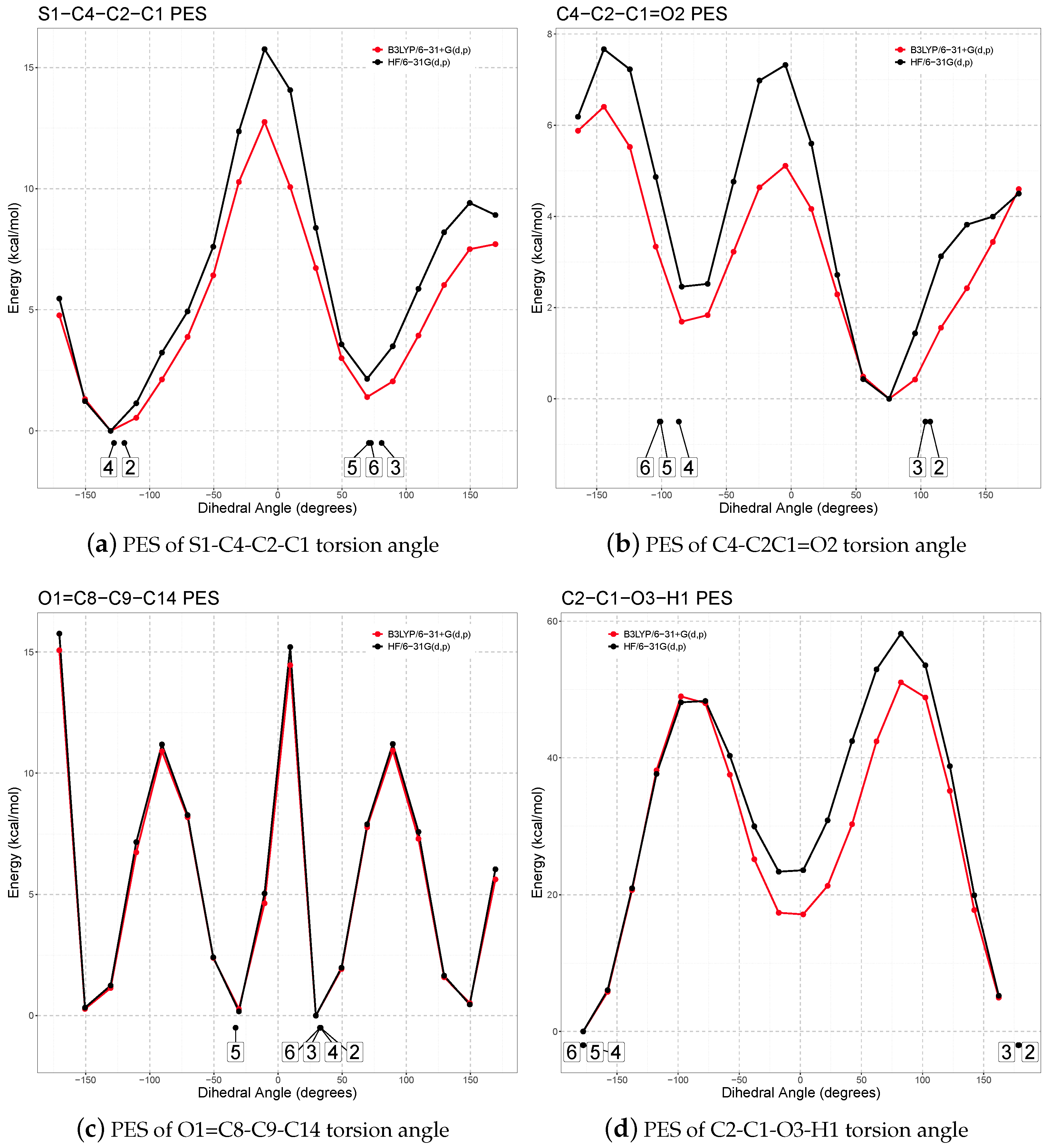
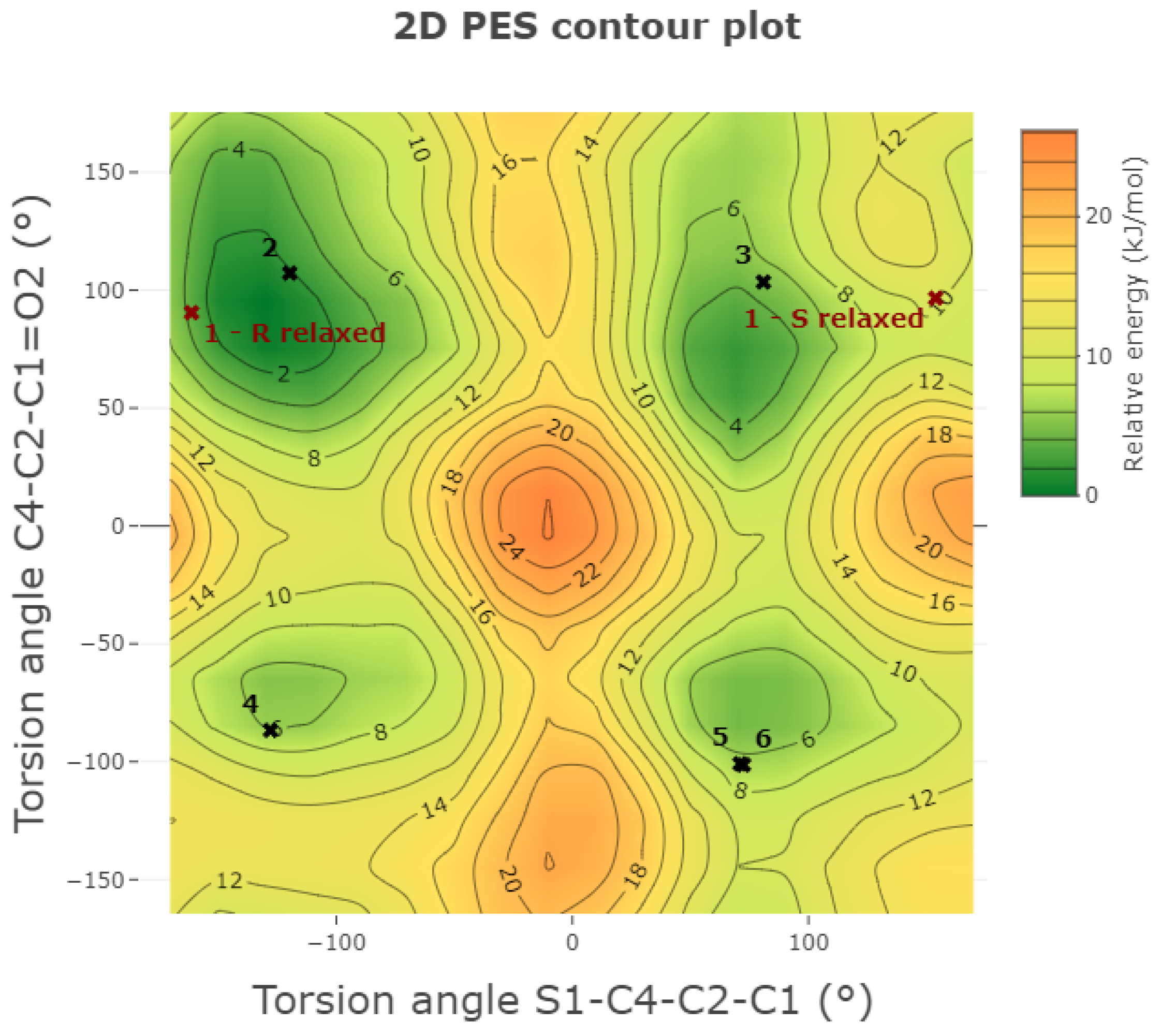
2.3.1. Conformational Analysis on the Isolated Molecule
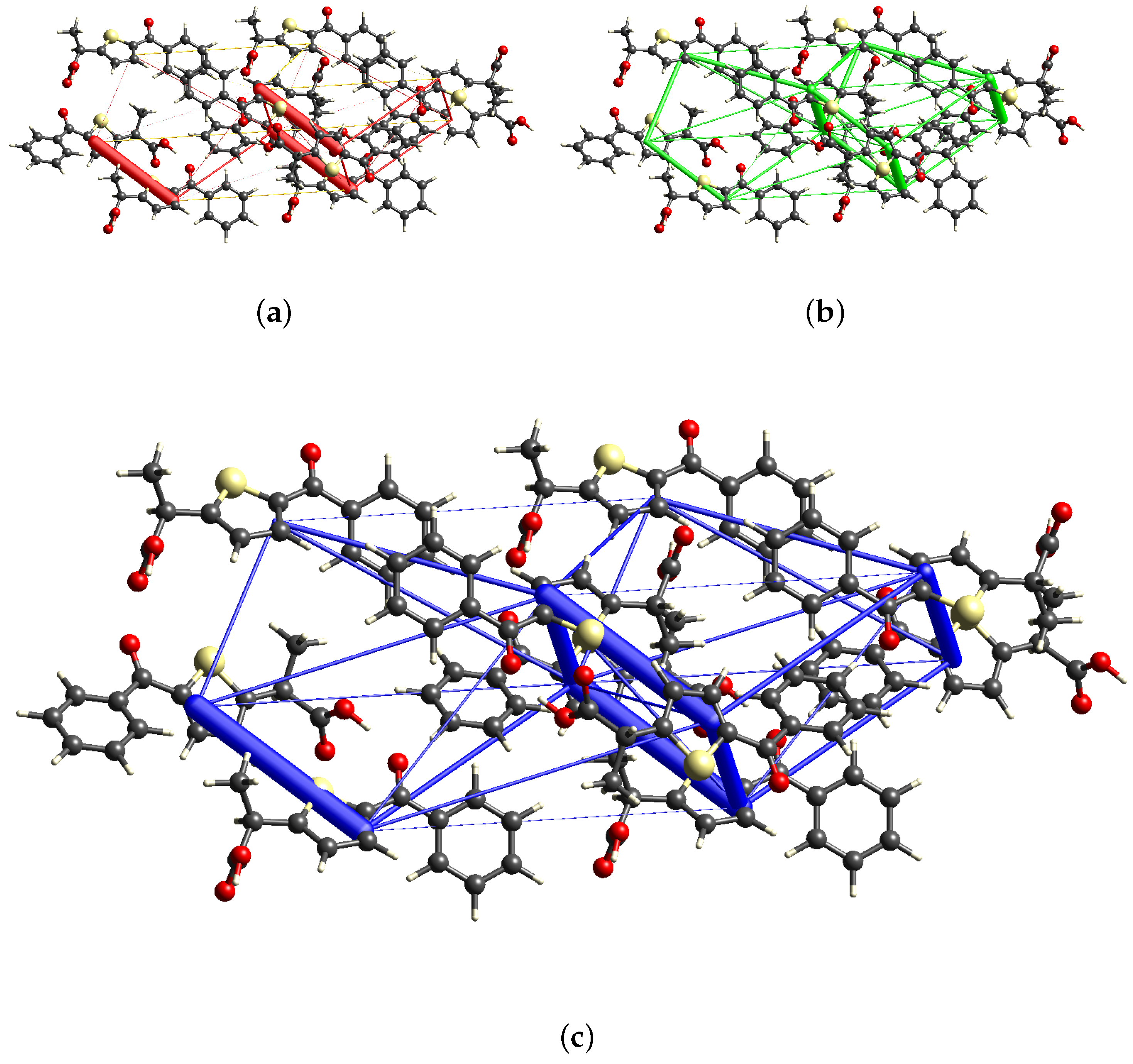
2.3.2. Energy Frameworks Analysis and Lattice Energy Calculation
3. Materials and Methods
3.1. XRPD Characterization and Refinement
3.2. In Silico Analyses
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lapi, F.; Marconi, E.; Magni, A.; Aprile, P.L.; Lagolio, E.; Grattagliano, I.; Fornasari, D.; Rossi, A.; Cricelli, C. Relative effectiveness and gastrointestinal safety of NSAIDs being prescribed for upper respiratory tract infections: An explorative cohort study in primary care. Int. J. Clin. Pharm. 2025, 47, 873–877. [Google Scholar] [CrossRef]
- Nowicki, K.D.; Rogers, N.D.; Keeter, C.L.; Donaldson, N.J.; Soep, J.B.; Zhao, Y. Factors associated with treatment response in chronic nonbacterial osteomyelitis at a single center: A retrospective cohort study. Pediatr. Rheumatol. 2025, 23, 2. [Google Scholar] [CrossRef]
- Laursen, C.C.W.; Lunn, T.H.; Hägi-Pedersen, D.; Brorson, S.; Lindberg-Larsen, M.; Overgaard, S.; Jakobsen, J.C.; Mathiesen, O. Recommended Use of Non-Steroidal Anti-Inflammatory Drugs for Pain Treatment Following Primary Total Hip and Knee Arthroplasties in Denmark. A National Survey. Acta Anaesthesiol. Scand. 2025, 69, e70055. [Google Scholar] [CrossRef]
- Clephas, P.R.; Orbach-Zinger, S.; Gosteli-Peter, M.A.; Hoshen, M.; Halpern, S.; Hilber, N.D.; Leo, C.; Heesen, M. Regional analgesia techniques for postoperative pain after breast cancer surgery: A network meta-analysis. Cochrane Database Syst. Rev. 2025, 2025, CD014818. [Google Scholar] [CrossRef]
- Varrassi, G.; Pergolizzi, J.V.; Dowling, P.; Paladini, A. Ibuprofen Safety at the Golden Anniversary: Are all NSAIDs the Same? A Narrative Review. Adv. Ther. 2019, 37, 61–82. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Ro, D.H.; Han, H.S.; Won, S. Overall compilation of adverse effects of non-steroidal anti-inflammatory drugs: A hypothesis-free systematic investigation using a nationwide cohort study. Front. Pharmacol. 2025, 16, 1539328. [Google Scholar] [CrossRef] [PubMed]
- Angel Nieto, I.; Bernès, S.; Pérez-Benítez, A. Crystal structure of a new hydrate form of the NSAID sodium diclofenac. Acta Crystallogr. Sect. E Crystallogr. Commun. 2020, 76, 1846–1850. [Google Scholar] [CrossRef] [PubMed]
- Rossi, P.; Paoli, P.; Ienco, A.; Biagi, D.; Valleri, M.; Conti, L. A new crystal form of the NSAID dexketoprofen. Acta Crystallogr. Sect. C Struct. Chem. 2019, 75, 783–792. [Google Scholar] [CrossRef]
- Konovalova, I.S.; Kovalenko, S.M.; Kravchenko, D.V.; Chuev, V.P. Crystal structure of the non-steroidal anti-inflammatory drug (NSAID) tolmetin sodium. Acta Crystallogr. Sect. E Crystallogr. Commun. 2021, 77, 134–137. [Google Scholar] [CrossRef]
- Harris, K.D.M.; Tremayne, M.; Kariuki, B.M. Contemporary Advances in the Use of Powder X-Ray Diffraction for Structure Determination. Angew. Chem. Int. Ed. 2001, 40, 1626–1651. [Google Scholar] [CrossRef]
- Dey, T.; Chatterjee, P.; Bhattacharya, A.; Pal, S.; Mukherjee, A.K. Three Nimesulide Derivatives: Synthesis, Ab Initio Structure Determination from Powder X-Ray Diffraction, and Quantitative Analysis of Molecular Surface Electrostatic Potential. Cryst. Growth Des. 2016, 16, 1442–1452. [Google Scholar] [CrossRef]
- Flanagan, J.U.; Yosaatmadja, Y.; Teague, R.M.; Chai, M.Z.L.; Turnbull, A.P.; Squire, C.J. Crystal Structures of Three Classes of Non-Steroidal Anti-Inflammatory Drugs in Complex with Aldo-Keto Reductase 1C3. PLoS ONE 2012, 7, e43965. [Google Scholar] [CrossRef]
- Barcellini, W.; Borghi, M.O.; Fain, C.; Del Papa, N.; Favini, P.; Meroni, P.L. In vitro and ex vivo effect of tiaprofenic acid on human peripheral blood mononuclear cells. Int. J. Immunopharmacol. 1992, 14, 1279–1284. [Google Scholar] [CrossRef]
- Meyer-Carrive, I.; Ghosh, P. Effects of tiaprofenic acid (Surgam) on cartilage proteoglycans in the rabbit joint immobilisation model. Ann. Rheum. Dis. 1992, 51, 448–455. [Google Scholar] [CrossRef]
- Varley, G.; Fagg, P.; Ghosh, M.; Hobbs, N. NSAIDs and the rate of recovery from arthroscopy: A double-blind randomized study of tiaprofenic acid versus placebo. Knee 1994, 1, 54–56. [Google Scholar] [CrossRef]
- van der Westhuijzen, A.; Roelofse, J.; Grotepass, F.; Becker, P. Randomized double-blind comparison of tiaprofenic acid and diclophenac sodium after third molar surgery. Oral Surg. Oral Med. Oral Pathol. 1994, 78, 557–566. [Google Scholar] [CrossRef]
- Hayles, C.D.; Andrews, F.J.; O’brien, P.E. Tiaprofenic acid inhibits mucosal prostaglandin E2 synthesis without delaying experimental gastric ulcer healing. J. Gastroenterol. Hepatol. 1998, 13, 572–578. [Google Scholar] [CrossRef] [PubMed]
- Boscá, F.; Miranda, M.A.; Morera, I.M.; Samadi, A. Involvement of type I and type II mechanisms in the linoleic acid peroxidation photosensitized by tiaprofenic acid. J. Photochem. Photobiol. B Biol. 2000, 58, 1–5. [Google Scholar] [CrossRef]
- Greene, G.F.; Millard, O.H.; Norman, R.W.; Boudreau, S.F.; Auld, R.B.; Awad, S.A. Cystitis Associated with Tiaprofenic Acid. J. Urol. 1994, 152, 1101–1102. [Google Scholar] [CrossRef] [PubMed]
- Henley, M.; Harriss, D.; Bishop, M. Cystitis associated with tiaprofenic acid: A survey of British and Irish urologists. Br. J. Urol. 1997, 79, 585–587. [Google Scholar] [CrossRef]
- Geisslinger, G.; Menzel, S.; Brune, K. Stereospecific determination of tiaprofenic acid in plasma: Problems with drug degradation. J. Chromatogr. Biomed. Sci. Appl. 1996, 675, 77–81. [Google Scholar] [CrossRef]
- Ferretti, R.; Gallinella, B.; La Torre, F.; Villani, C. Direct high-performance liquid chromatography resolution on chiral columns of tiaprofenic acid and related compounds in bulk powder and pharmaceutical formulations. J. Chromatogr. A 1995, 704, 217–223. [Google Scholar] [CrossRef]
- Nakagita, T.; Taketani, C.; Narukawa, M.; Hirokawa, T.; Kobayashi, T.; Misaka, T. Ibuprofen, a Nonsteroidal Anti-Inflammatory Drug, is a Potent Inhibitor of the Human Sweet Taste Receptor. Chem. Senses 2020, 45, 667–673. [Google Scholar] [CrossRef] [PubMed]
- Mirocki, A.; Lopresti, M.; Palin, L.; Conterosito, E.; Sikorski, A.; Milanesio, M. Exploring the molecular landscape of multicomponent crystals formed by naproxen drug and acridines. CrystEngComm 2022, 24, 6839–6853. [Google Scholar] [CrossRef]
- Mirocki, A.; Conterosito, E.; Palin, L.; Sikorski, A.; Milanesio, M.; Lopresti, M. Crystal Structure of a New 1:1 Acridine-Diclofenac Salt, Obtained with High Yield by a Mechanochemical Approach. Crystals 2022, 12, 1573. [Google Scholar] [CrossRef]
- Mirocki, A.; Lopresti, M.; Palin, L.; Conterosito, E.; Sikorska, E.; Sikorski, A.; Milanesio, M. Crystallization from solution versus mechanochemistry to obtain double-drug multicomponent crystals of ethacridine with salicylic/acetylsalicylic acids. Sci. Rep. 2024, 14, 1834. [Google Scholar] [CrossRef]
- Mirocki, A.; Lopresti, M. The role of the solvent molecule in the crystal packing arrangements of hydrated salts formed by ethacridine and fluorobenzoic acids. Acta Crystallogr. Sect. B Struct. Sci. Cryst. Eng. Mater. 2025, B81, 395–406. [Google Scholar] [CrossRef]
- Scherlis, D.A.; Marzari, N. π-Stacking in Thiophene Oligomers as the Driving Force for Electroactive Materials and Devices. J. Am. Chem. Soc. 2005, 127, 3207–3212. [Google Scholar] [CrossRef]
- Jing, L.; Li, P.; Li, Z.; Ma, D.; Hu, J. Influence of π–π interactions on organic photocatalytic materials and their performance. Chem. Soc. Rev. 2025, 54, 2054–2090. [Google Scholar] [CrossRef]
- Janiak, C. A critical account on π–π stacking in metal complexes with aromatic nitrogen-containing ligands. J. Chem. Soc. Dalton Trans. 2000, 21, 3885–3896. [Google Scholar] [CrossRef]
- Chen, Z.; Yu, J.; Wang, H.; Xu, P.; Fan, L.; Sun, F.; Huang, S.; Zhang, P.; Huang, H.; Gu, S.; et al. Flexible scaffold-based cheminformatics approach for polypharmacological drug design. Cell 2024, 187, 2194–2208.e22. [Google Scholar] [CrossRef]
- Lopresti, M.; Milanesio, M.; Palin, L. The Crystal Structure of Calcium Sebacate by X-Ray Powder Diffraction Data. Crystals 2023, 13, 261. [Google Scholar] [CrossRef]
- Lopresti, M.; Palin, L.; Calegari, G.; Milanesio, M. The Peculiar H-Bonding Network of 4-Methylcatechol: A Coupled Diffraction and In Silico Study. Molecules 2024, 29, 2173. [Google Scholar] [CrossRef] [PubMed]
- Mendenhall, M.H.; Henins, A.; Hudson, L.T.; Szabo, C.I.; Windover, D.; Cline, J.P. High-precision measurement of the x-ray Cu spectrum. J. Phys. B At. Mol. Opt. Phys. 2017, 50, 115004. [Google Scholar] [CrossRef]
- Altomare, A.; Giacovazzo, C.; Guagliardi, A.; Moliterni, A.G.G.; Rizzi, R.; Werner, P.E. New techniques for indexing: N-TREORinEXPO. J. Appl. Crystallogr. 2000, 33, 1180–1186. [Google Scholar] [CrossRef]
- Altomare, A.; Cuocci, C.; Giacovazzo, C.; Moliterni, A.; Rizzi, R.; Corriero, N.; Falcicchio, A. EXPO2013: A kit of tools for phasing crystal structures from powder data. J. Appl. Crystallogr. 2013, 46, 1231–1235. [Google Scholar] [CrossRef]
- Coelho, A. TOPAS and TOPAS-Academic: An optimization program integrating computer algebra and crystallographic objects written in C++. J. Appl. Crystallogr. 2018, 51, 210–218. [Google Scholar] [CrossRef]
- Coelho, A. TOPAS-Academic V7. 2020. Available online: https://journals.iucr.org/paper?S1600576718000183 (accessed on 29 August 2025).
- Macrae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.A.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M.; et al. Mercury 4.0: From visualization to analysis, design and prediction. J. Appl. Crystallogr. 2020, 53, 226–235. [Google Scholar] [CrossRef]
- Kabekkodu, S.N.; Dosen, A.; Blanton, T.N. PDF-5+: A comprehensive Powder Diffraction File™ for materials characterization. Powder Diffr. 2024, 39, 47–59. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian˜16 Revision B.01; Gaussian Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Spackman, P.R.; Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer: A program for Hirshfeld surface analysis, visualization and quantitative analysis of molecular crystals. J. Appl. Crystallogr. 2021, 54, 1006–1011. [Google Scholar] [CrossRef]
- Mackenzie, C.F.; Spackman, P.R.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer model energies and energy frameworks: Extension to metal coordination compounds, organic salts, solvates and open-shell systems. IUCrJ 2017, 4, 575–587. [Google Scholar] [CrossRef] [PubMed]
- Van Duong, T.; Reekmans, G.; Venkatesham, A.; Van Aerschot, A.; Adriaensens, P.; Van Humbeeck, J.; Van den Mooter, G. Spectroscopic Investigation of the Formation and Disruption of Hydrogen Bonds in Pharmaceutical Semicrystalline Dispersions. Mol. Pharm. 2017, 14, 1726–1741. [Google Scholar] [CrossRef] [PubMed]
| Name | (±)-Tiaprofenic Acid |
|---|---|
| Empirical Formula | C14 H12 O3 S |
| Formula weight/ | |
| Temperature/ | |
| Crystal system | monoclinic |
| Space group | |
| a/ | |
| b/ | |
| c/ | |
| / | 90 |
| / | |
| / | 90 |
| Volume/ | |
| Average atomic volume/ | |
| Z | 8 |
| µ | |
| F(000) | 1088.0 |
| Radiation/ | |
| range for data collection/ | |
| Reflections used for indexing | 36 |
| Rp/Rwp | 5.390/7.461 |
| Chirality | Torsion Angle | S1–C4–C2–C1 | C4–C2–C1=O2 | O1=C8–C9–C14 | C2–C1–O3–H1 | H5–C2–C3–H3 | Relative Energy |
|---|---|---|---|---|---|---|---|
| Conformation | (°) | (°) | (°) | (°) | (°) | ( | |
| S | 1-S relaxed | 153.9 | 96.6 | 27.3 | −180.0 | 58.8 | 8.4 |
| S | 2 | −119.7 | 107.2 | 33.2 | 178.9 | −58.8 | 4.1 |
| S | 3 | 80.9 | 103.5 | 32.8 | 178.3 | −58.5 | 3.0 |
| S | 4 | −127.8 | −86.7 | 33.0 | −177.5 | −56.6 | 0.1 |
| S | 5 | 71.0 | −100.9 | −33.1 | −177.6 | −56.9 | 1.8 |
| S | 6 | 72.5 | −101.4 | 32.7 | −177.9 | −57.0 | 1.5 |
| R | 1-R relaxed | 161.3 | −90.5 | 25.1 | −170.0 | −59.2 | 10.9 |
| R | 2 | 119.6 | −106.1 | −33.2 | −178.9 | −60.6 | 3.8 |
| R | 3 | −77.8 | −104.8 | −32.7 | −178.3 | −60.9 | 3.2 |
| R | 4 | 126.9 | 87.5 | −33.0 | 177.4 | −63.3 | 0.0 |
| R | 5 | −71.0 | 100.9 | 33.1 | 177.6 | −63.1 | 1.8 |
| R | 6 | −71.0 | 100.9 | -33.1 | 177.6 | 57.0 | 1.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lopresti, M.; Palin, L.; Milanesio, M. Conformational and Intermolecular Interaction Analysis of Tiaprofenic Acid: A X-Ray Powder Diffraction and First Principle Modeling Analysis. Molecules 2025, 30, 3593. https://doi.org/10.3390/molecules30173593
Lopresti M, Palin L, Milanesio M. Conformational and Intermolecular Interaction Analysis of Tiaprofenic Acid: A X-Ray Powder Diffraction and First Principle Modeling Analysis. Molecules. 2025; 30(17):3593. https://doi.org/10.3390/molecules30173593
Chicago/Turabian StyleLopresti, Mattia, Luca Palin, and Marco Milanesio. 2025. "Conformational and Intermolecular Interaction Analysis of Tiaprofenic Acid: A X-Ray Powder Diffraction and First Principle Modeling Analysis" Molecules 30, no. 17: 3593. https://doi.org/10.3390/molecules30173593
APA StyleLopresti, M., Palin, L., & Milanesio, M. (2025). Conformational and Intermolecular Interaction Analysis of Tiaprofenic Acid: A X-Ray Powder Diffraction and First Principle Modeling Analysis. Molecules, 30(17), 3593. https://doi.org/10.3390/molecules30173593









