Recycling Spent LFP Batteries: From Resource Recovery to High-Value Functional Materials
Abstract
1. Introduction
2. Principles of Recycling Current Collectors
2.1. Failure Mechanisms of LFP Batteries
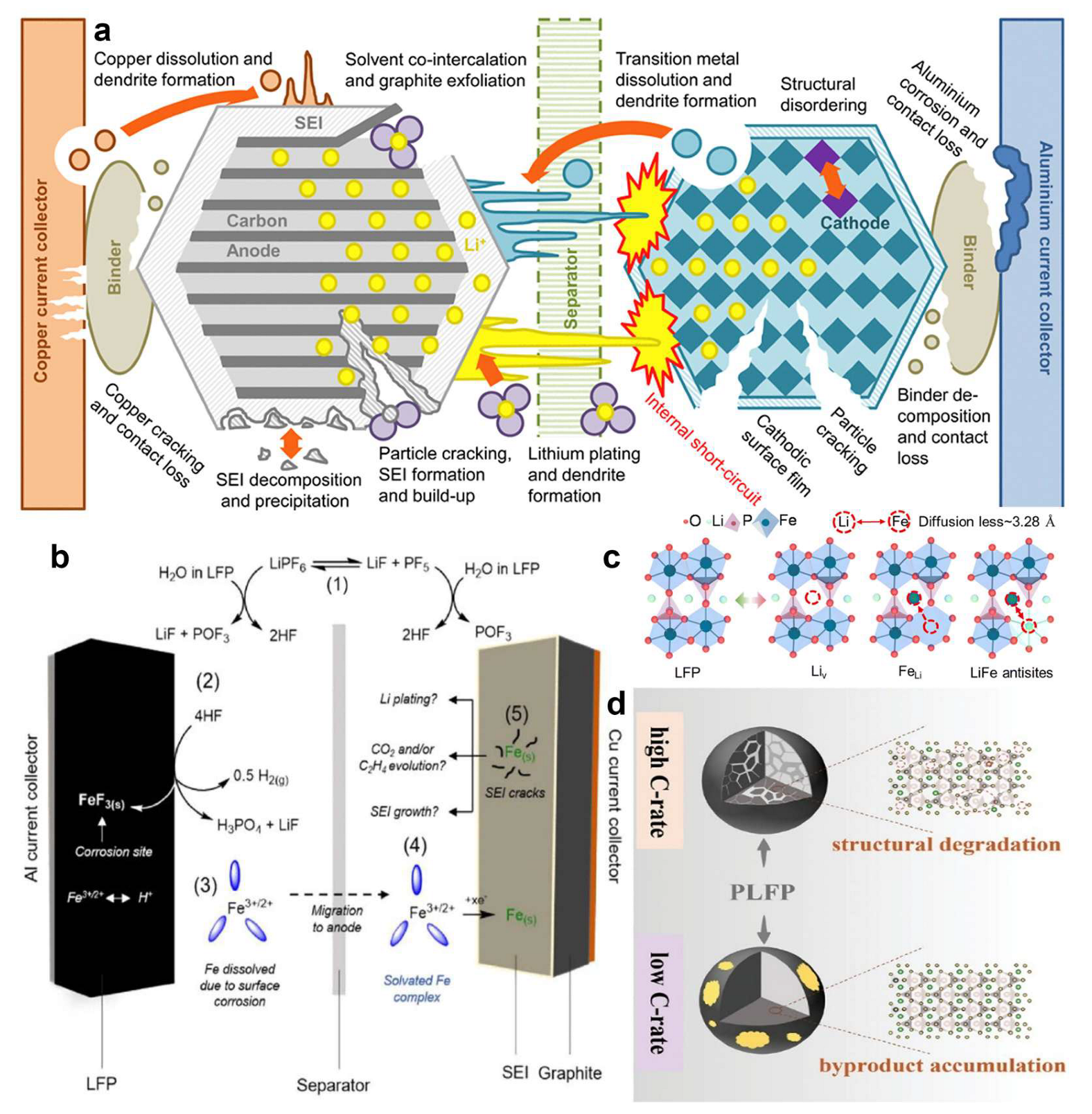
2.2. Current Collector Separation Strategies
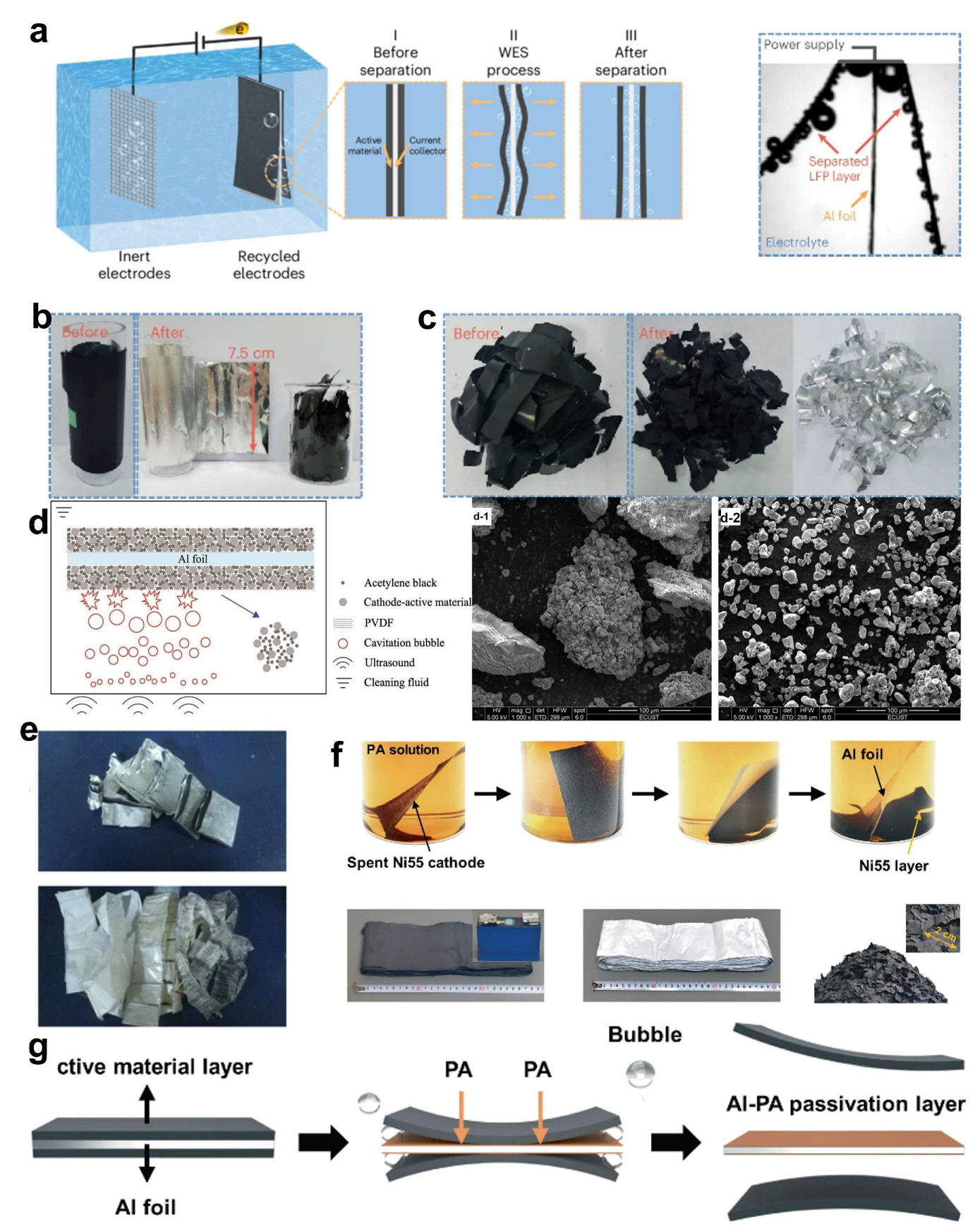
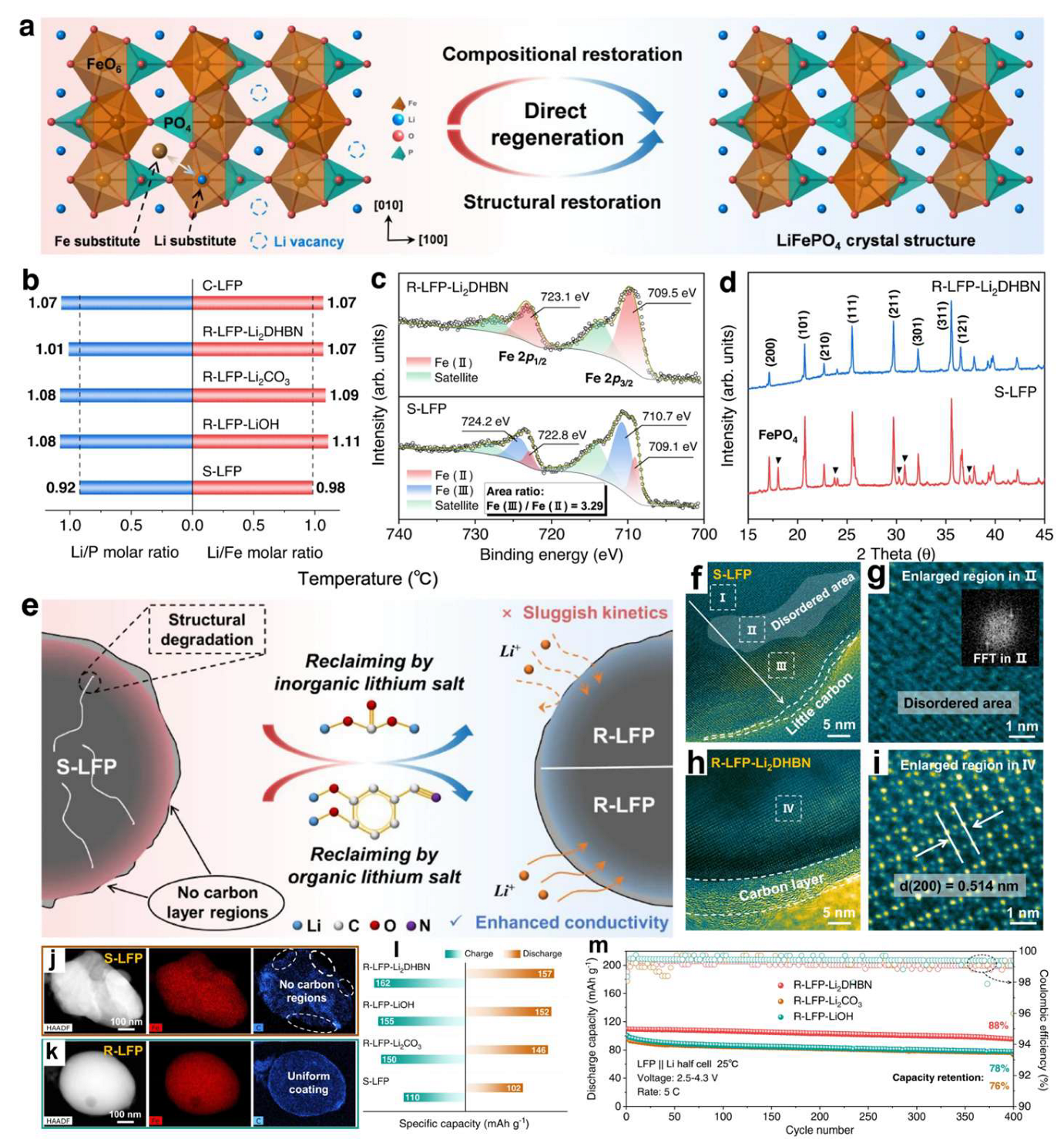
3. Regeneration/Refurbishment of LFP Electrodes
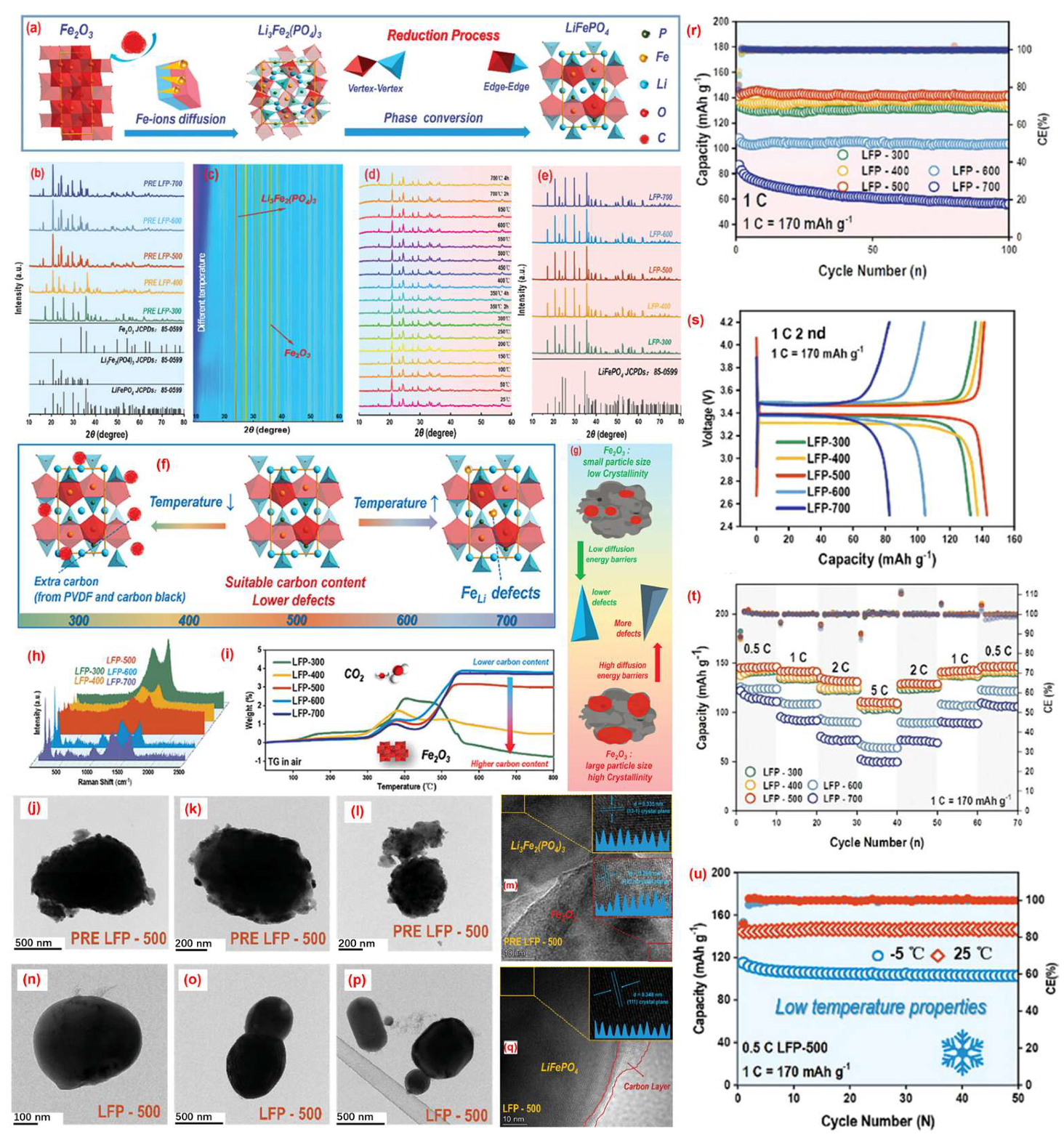
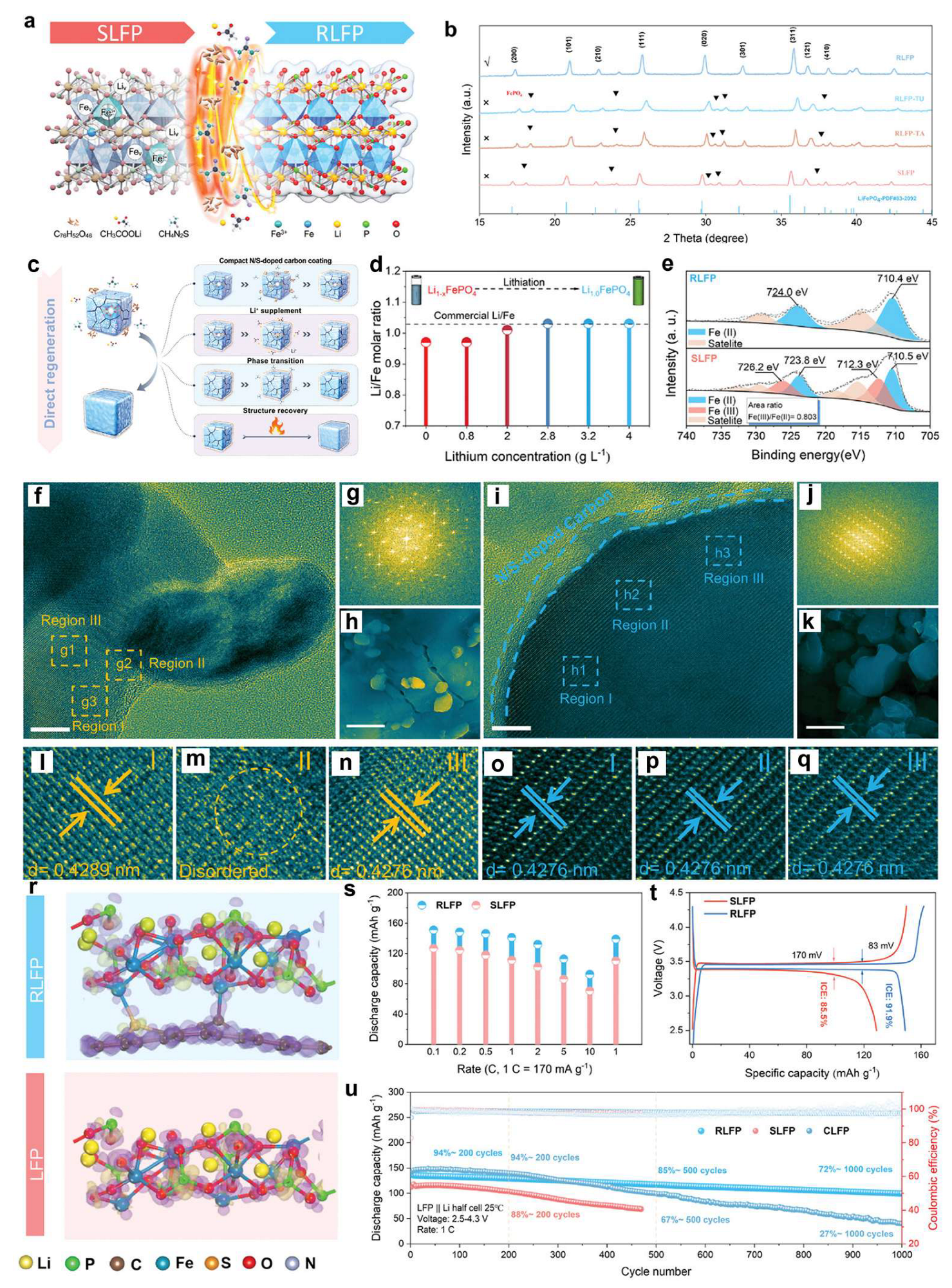
3.1. Pyro-Regeneration of Spent LFP Cathodes
3.2. Hydro-Regeneration of Spent LFP Cathodes
4. Upcycling Spent LFP Batteries as Functional Materials
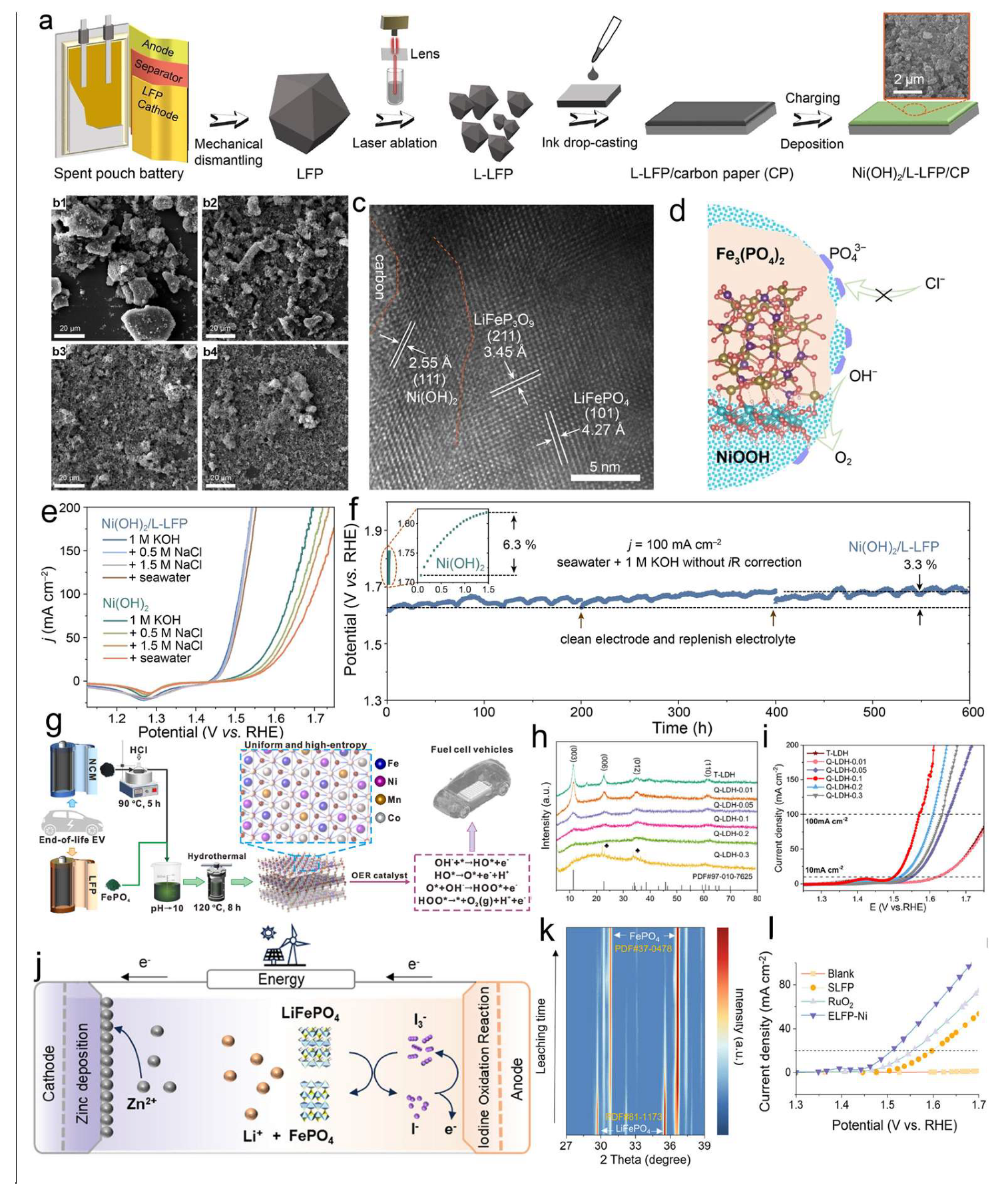
4.1. Upcycling for Electrochemical Water Splitting
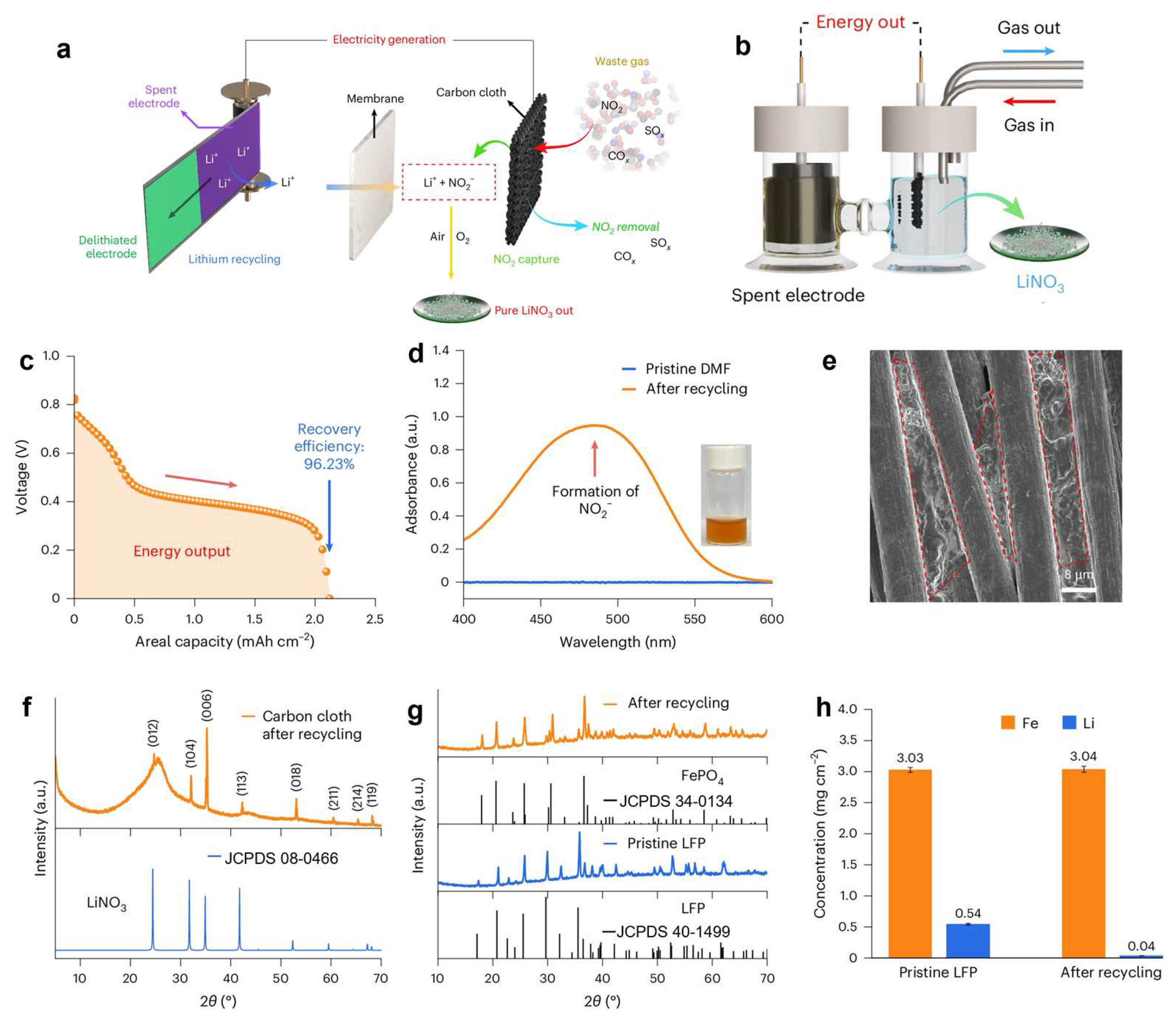
4.2. Upcycling for NO Reduction
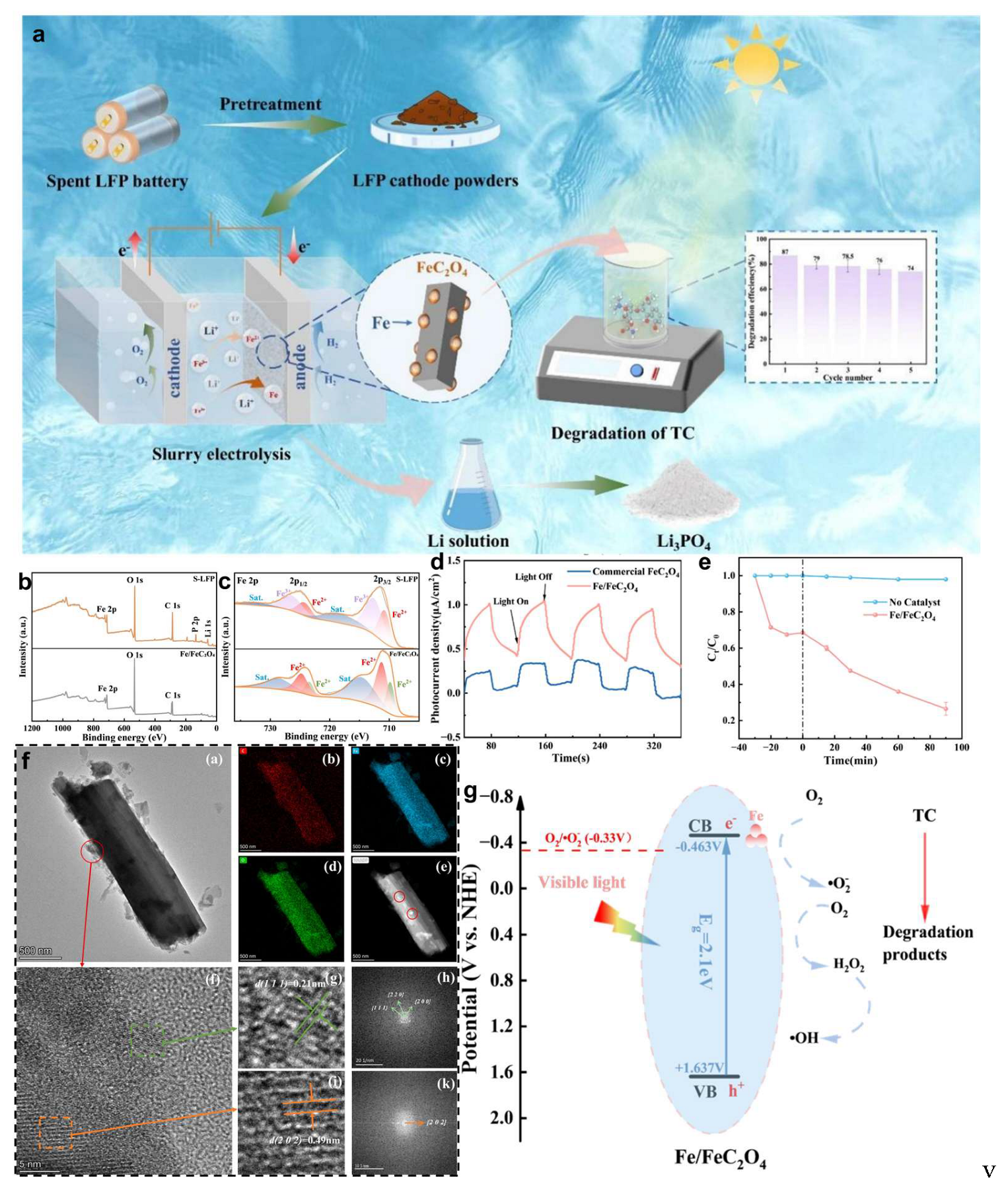
4.3. Upcycling for Photocatalytic Degradation of Tetracycline
4.4. Upcycling for Ferricyanide-Assisted Hydrogen Production
5. Elemental Recycling Toward Value-Added Products
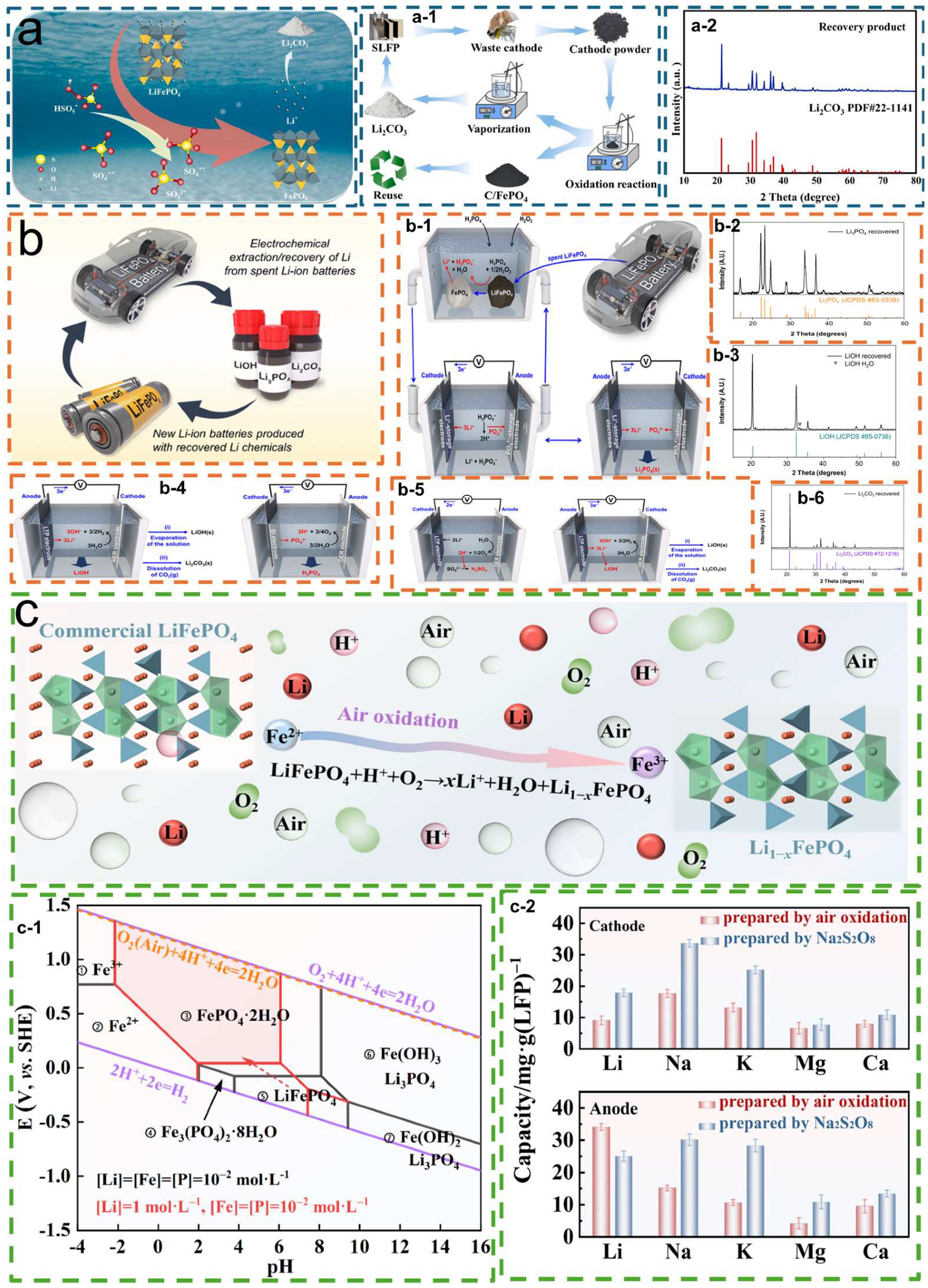
5.1. Selective Lithium Recovery and Conversion
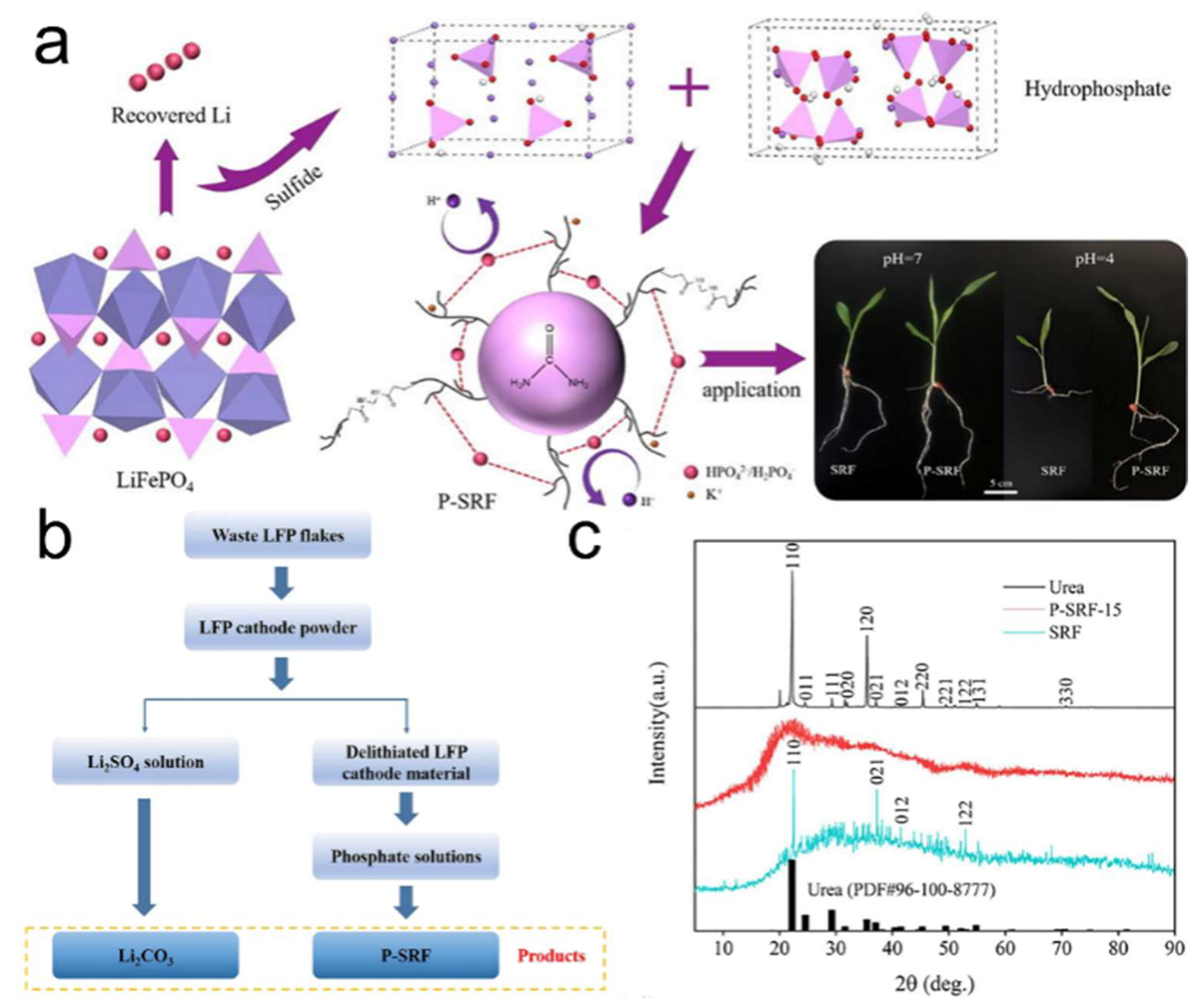
5.2. Phosphorus Recovery and Functional Upcycling
6. Summary and Outlook
| Technology | Methods | Advantages | Reference |
|---|---|---|---|
| Separation of the current collector | Water-dissociation-induced separation (WES) technology | Fast stripping speed with minimal pollution | [52] |
| Ultrasound-assisted cavitation exfoliation | Rapid delamination with excellent structural integrity of the recovered materials | [53] | |
| Chemical passivation method | Simple operation, scalable for large-scale use | [55] | |
| LFP regeneration and repair | Chelating organolithium salt-assisted repair | The surface carbon layer significantly enhances both conductivity and stability. | [56] |
| Redox-mediated regeneration | Enables more complete regeneration of LFP | [57] | |
| Thiourea-assisted solid-state sintering process | Effectively suppresses particle agglomeration | [58] | |
| Synergistic repair using tannic acid (TA) and thiourea (TU) | C–Fe and S–Fe coordination bonds promote surface charge delocalization, markedly enhancing ionic conductivity | [60] | |
| Direct regeneration of spent LiFePO4 with glycerol | Effectively promotes interfacial reconnection and restores the layered micro-crystalline structure | [63] | |
| Multifunctional lithium acetylacetonate (Li(acac))-assisted repair | During repair, it efficiently reconfigures the amorphous by-products. | [69] | |
| Upcycling of LFP | Upcycling via electrochemical water splitting | Upgraded into a green, clean-energy catalyst for hydrogen production | [81,82] |
| Upcycling for nitrogen-oxide reduction recycling | When LFP is upcycled into LiNO3, it simultaneously captures nitrogen oxides from flue gas. | [83] | |
| Upcycling for tetracycline photocatalytic degradation | Upcycled into an economical and environmentally friendly photocatalyst | [86] | |
| Upcycling for ferricyanide-assisted hydrogen production | Reduces the cost of hydrogen production while simultaneously recovering LFP into LiOH. | [90] | |
| Element recovery | Simple and efficient selective Li leaching with peroxymonosulfate (PMS) | The residual FePO4 retains the original olivine structural framework. | [91] |
| Dual-chamber system for Li+ extraction and Li+ recovery units | One chamber consumes the leachate while the other electrochemically reduces it, drastically reducing chemical reagent use and pollution. | [92] | |
| Direct air-oxidative leaching | High selectivity—only Li is recovered | [93] | |
| Dual-element recovery of Li and P for the production of phosphorus fertilizer | Converting spent LFP into cost-effective phosphorus fertilizer | [95] |
Author Contributions
Funding
Conflicts of Interest
References
- Pan, H.; Wang, L.; Shi, Y.; Sheng, C.C.; Yang, S.X.; He, P.; Zhou, H.S. A solid-state lithium-ion battery with micron-sized silicon anode operating free from external pressure. Nat. Commun. 2024, 15, 2263. [Google Scholar] [CrossRef]
- Lei, S.Y.; Sun, W.; Yang, Y. Comprehensive Technology for Recycling and Regenerating Materials from Spent Lithium Iron Phosphate Battery. Environ. Sci. Technol. 2024, 58, 3609–3628. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Yang, S.C.; Li, J.X.; Zhao, C.; Chen, M.M.; Li, P.Z.; Chen, C.; Gao, Y.; Yan, L.Y.; Mao, J.; et al. Sustainable recovery of LiCoO2 from spent lithium-ion batteries: Simplicity, scalability, and superior electrochemical performance. Chem. Eng. J. 2024, 479, 147710. [Google Scholar] [CrossRef]
- Wang, J.; Sun, X. Olivine LiFePO4: The remaining challenges for future energy storage. Energy Environ. Sci. 2015, 8, 1110–1138. [Google Scholar] [CrossRef]
- Liu, L.; Xue, J.; Gao, Y.; Zhang, S.; Zhang, H.; Peng, K.; Zhang, X.; Lu, S.; Weng, S.; Tu, H.; et al. Polymerized-ionic-liquid-based solid polymer electrolyte for ultra-stable lithium metal batteries enabled by structural design of monomer and crosslinked 3D network. Mater. Rep. Energy 2025, 5, 100311. [Google Scholar] [CrossRef]
- Zheng, F.; Wu, P.; Wang, L.; Shi, Y.; Jiang, J.; Chen, Y.; Zhuang, Q.; Liu, Q.; Ju, Z.; Kong, X. Activated Graphite with Richly Oxygenated Surface from Spent Lithium-ion Batteries for Microwave Absorption. Small 2025, 21, 2409454. [Google Scholar] [CrossRef]
- Zhan, R.T.; Zhang, H.B.; Pei, J.N.; Yang, H.; Lu, W.; Ye, P.Y.; He, B.; Luo, S.L.; Lu, Z.G.; Jiang, F.; et al. Recent Advances in Recycling of Copper-Based Waste Materials: A Critical Review. cMat 2025, 2, e70005. [Google Scholar] [CrossRef]
- Li, H.; Yan, C.; Wang, S. Solvation chemistry in liquid electrolytes for rechargeable lithium batteries at low temperatures. EcoEnergy 2025, 3, 387–421. [Google Scholar] [CrossRef]
- Shi, P.; Jie, Y.; Hu, F.; Yang, S.; Chang, D.; Fang, G.; Ding, J.; Qu, X.; Wu, G.; Zhu, H.; et al. From waste to material strategy: A closed-loop regeneration process of spent LiFePO4 batteries. Sep. Purif. Technol. 2025, 359, 130502. [Google Scholar] [CrossRef]
- Wolf, A.; Flegler, A.; Prieschl, J.; Stuebinger, T.; Witt, W.; Seiser, F.; Vinnay, T.; Sinn, T.; Gleiß, M.; Nirschl, H.; et al. Centrifugation based separation of lithium iron phosphate (LFP) and carbon black for lithium-ion battery recycling. Chem. Eng. Process.-Process Intensif. 2021, 160, 108310. [Google Scholar] [CrossRef]
- Geng, J.; Zhang, S.; Hu, X.; Ling, W.; Peng, X.; Zhong, S.; Liang, F.; Zou, Z. A review of graphene-decorated LiFePO4 cathode materials for lithium-ion batteries. Ionics 2022, 28, 4899–4922. [Google Scholar] [CrossRef]
- Perdana, I.; Aprilianto, D.R.; Fadillah, F.A.; Fadli, R.; Petrus, H.T.B.M.; Astuti, W.; Muflikhun, M.A.; Nilasary, H.; Oktaviano, H.S.; Fathoni, F.; et al. Lithium recovery from mixed spent LFP-NMC batteries through atmospheric water leaching. Sci. Rep. 2025, 15, 2591. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Kong, X.; Wang, L.; Yin, F.; Zheng, F.; Liu, S.; Liu, Q.; Zhuang, Q.; Ju, Z.; Zhang, J. Mechanistically Engineered Heterojunction from Spent LFP for Efficient Oxygen Evolution Electrocatalysis. Angew. Chem. Int. Ed. 2025, 64, e202516122. [Google Scholar] [CrossRef]
- Peng, X.L.; Chen, X.J.; Tang, C.X.; Weng, S.J.; Hu, X.R.; Xiang, Y. Self-Healing Binder for High-Voltage Batteries. ACS Appl. Mater. Interfaces 2023, 15, 21517–21525. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Fan, S.; Sun, Z.; Peng, Y.; Wang, Q.; Ye, F. Highly stable Li+ deposition guided by a lithiophilic microchannel. Mater. Rep. Energy 2025, 5, 100316. [Google Scholar] [CrossRef]
- Wu, W.; Liang, J.; Ye, S.; Chen, Z.; Chen, W.; Zhao, X.; Zheng, L.; Zhang, Q.; Liu, J. Cu/Co binary-transition metal glycerolates as anode materials for lithium-ion batteries. EcoEnergy 2024, 2, 169–180. [Google Scholar] [CrossRef]
- Wang, Z.R.; Zhang, Y.-L.; Zhang, R.-Z.; Li, W.-W.; Song, J.; Liu, S.-L. The Road Ahead for Aqueous Lithium-ion Batteries. cMat 2025, 2, e70010. [Google Scholar] [CrossRef]
- Birkl, C.R.; Roberts, M.R.; McTurk, E.; Bruce, P.G.; Howey, D.A. Degradation diagnostics for lithium ion cells. J. Power Sources 2016, 341, 373–386. [Google Scholar] [CrossRef]
- Fan, M.; Meng, Q.H.; Chang, X.; Gu, C.F.; Meng, X.H.; Yin, Y.X.; Li, H.L.; Wan, L.J.; Guo, Y.G. In Situ Electrochemical Regeneration of Degraded LiFePO4 Electrode with Functionalized Prelithiation Separator. Adv. Energy Mater. 2022, 12, 2103630. [Google Scholar] [CrossRef]
- Bai, Y.C.; Muralidharan, N.; Sun, Y.K.; Passerini, S.; Whittingham, M.S.; Belharouak, I. Energy and environmental aspects in recycling lithium-ion batteries: Concept of Battery Identity Global Passport. Mater. Today 2020, 41, 304–315. [Google Scholar] [CrossRef]
- Liu, W.; Li, K.; Wang, W.; Hu, Y.L.; Ren, Z.Q.; Zhou, Z.Y. Selective leaching of lithium ions from LiFePO4 powders using hydrochloric acid and sodium hypochlorite system. Can. J. Chem. Eng. 2023, 101, 1831–1841. [Google Scholar] [CrossRef]
- Geng, Z.W.; Liu, J.J.; Geng, Y.N.; Peng, M.M.; Xiong, M.P.; Shi, H.; Luo, X.B. Separation and recovery of graphite from spent lithium-ion batteries for synthesizing micro-expanded sorbents. New J. Chem. 2022, 46, 20250–20259. [Google Scholar] [CrossRef]
- Jin, Y.C.; Zhang, T.; Zhang, M.D. Advances in Intelligent Regeneration of Cathode Materials for Sustainable Lithium-Ion Batteries. Adv. Energy Mater. 2022, 12, 2001526. [Google Scholar] [CrossRef]
- Qi, C.; Zu, S.; Zhu, X.K.; Zhang, T.; Li, L.Y.; Song, L.; Jin, Y.C.; Zhang, M.D. Bamboo-shaped Co@NCNTs as superior sulfur host for Li-S batteries. Appl. Surf. Sci. 2022, 601, 154245. [Google Scholar] [CrossRef]
- Chen, B.B.; Liu, M.; Cao, S.; Hu, H.; Chen, G.R.; Guo, X.W.; Wang, X.Y. Direct regeneration and performance of spent LiFePO4 via a green efficient hydrothermal technique. J. Alloys Compd. 2022, 924, 166487. [Google Scholar] [CrossRef]
- Wang, M.M.; Liu, K.; Dutta, S.; Alessi, D.S.; Rinklebe, J.; Ok, Y.S.; Tsang, D.C.W. Recycling of lithium iron phosphate batteries: Status, technologies, challenges, and prospects. Renew. Sustain. Energy Rev. 2022, 163, 112515. [Google Scholar] [CrossRef]
- Song, L.; Qi, C.; Wang, S.H.; Zhu, X.K.; Zhang, T.; Jin, Y.C.; Zhang, M.D. Direct regeneration of waste LiFePO4 cathode materials with a solid-phase method promoted by activated CNTs. Waste Manag. 2023, 157, 141–148. [Google Scholar] [CrossRef]
- Li, W.; Li, H.; He, Z.; Ji, W.J.; Zeng, J.; Li, X.; Zhang, Y.Y.; Zhang, P.; Zhao, J.B. Electrochemical Failure Results Inevitable Capacity Degradation in Li-Ion Batteries-A Review. Energies 2022, 15, 9165. [Google Scholar] [CrossRef]
- Li, P.W.; Luo, S.H.; Zhang, L.; Wang, Y.K.; Zhang, H.R.; Wang, J.H.; Yan, S.X.; Hou, P.Q.; Wang, Q.; Zhang, Y.H.; et al. Study on efficient and synergistic leaching of valuable metals from spent lithium iron phosphate using the phosphoric acid-oxalic acid system. Sep. Purif. Technol. 2022, 303, 122247. [Google Scholar] [CrossRef]
- Qi, C.; Yao, T.; Zhai, W.; Zhang, M.; Song, L.; He, J. Advances in degradation mechanism and sustainable recycling of LiFePO4-type lithium-ion batteries. Energy Storage Mater. 2024, 71, 103623. [Google Scholar] [CrossRef]
- Kumar, J.; Neiber, R.R.; Park, J.; Ali Soomro, R.; Greene, G.W.; Ali Mazari, S.; Young Seo, H.; Hong Lee, J.; Shon, M.; Wook Chang, D.; et al. Recent Progress in Sustainable Recycling of LiFePO4-type Lithium-ion Batteries: Strategies for Highly Selective Lithium Recovery. Chem. Eng. J. 2021, 431, 133993. [Google Scholar] [CrossRef]
- Huang, B.; Pan, Z.; Su, X.; An, L. Recycling of lithium-ion batteries: Recent advances and perspectives. J. Power Sources 2018, 399, 274–286. [Google Scholar] [CrossRef]
- Rufino Júnior, C.A.; Sanseverino, E.R.; Gallo, P.; Amaral, M.M.; Koch, D.; Kotak, Y.; Diel, S.; Walter, G.; Schweiger, H.-G.; Zanin, H. Unraveling the Degradation Mechanisms of Lithium-Ion Batteries. Energies 2024, 17, 3372. [Google Scholar] [CrossRef]
- Hu, G.R.; Gong, Y.F.; Peng, Z.D.; Du, K.; Huang, M.; Wu, J.H.; Guan, D.C.; Zeng, J.Y.; Zhang, B.C.; Cao, Y.B. Direct Recycling Strategy for Spent Lithium Iron Phosphate Powder: An Efficient and Wastewater-Free Process. Acs Sustain. Chem. Eng. 2022, 10, 11606–11616. [Google Scholar] [CrossRef]
- Qi, C.; Wang, S.H.; Zhu, X.K.; Zhang, T.; Gou, Y.J.; Xie, Z.X.; Jin, Y.C.; Wang, Y.; Song, L.; Zhang, M.D. Environmental-friendly low-cost direct regeneration of cathode material from spent LiFePO4. J. Alloys Compd. 2022, 924, 166612. [Google Scholar] [CrossRef]
- Hu, A.J.; Li, F.; Chen, W.; Lei, T.Y.; Li, Y.Y.; Fan, Y.X.; He, M.; Wang, F.; Zhou, M.J.; Hu, Y.; et al. Ion Transport Kinetics in Low-Temperature Lithium Metal Batteries. Adv. Energy Mater. 2022, 12, 2202432. [Google Scholar] [CrossRef]
- Pidaparthy, S.; Luo, M.; Rodrigues, M.T.F.; Zuo, J.M.; Abraham, D.P. Physicochemical Heterogeneity in Silicon Anodes from Cycled Lithium-Ion Cells. ACS Appl. Mater. Interfaces 2022, 14, 38660–38668. [Google Scholar] [CrossRef]
- Eldesoky, A.; Logan, E.R.; Johnson, M.B.; McFarlane, C.R.M.; Dahn, J.R. Scanning Micro X-ray Fluorescence (μXRF) as an Effective Tool in Quantifying Fe Dissolution in LiFePO4 Cells: Towards a Mechanistic Understanding of Fe Dissolution. J. Electrochem. Soc. 2020, 167, 130539. [Google Scholar] [CrossRef]
- Jie, Y.; Yang, S.; Hu, F.; Li, Y.; Ye, L.; Zhao, D.; Jin, W.; Chang, C.; Lai, Y.; Chen, Y. Gas evolution characterization and phase transformation during thermal treatment of cathode plates from spent LiFePO4 batteries. Thermochim. Acta 2020, 684, 178483. [Google Scholar] [CrossRef]
- Liu, W.; Zhong, X.; Han, J.; Qin, W.; Liu, T.; Zhao, C.; Chang, Z. Kinetic Study and Pyrolysis Behaviors of Spent LiFePO4 Batteries. ACS Sustain. Chem. Eng. 2019, 7, 1289–1299. [Google Scholar] [CrossRef]
- Fan, Y.; Song, J.; Su, Y.; Luo, Y.; Tian, L.; Liu, Q.; Fei, Z.; Li, C.; Meng, Q.; Dong, P.; et al. Structural reinforcement and nucleation regulation via Fe vacancy engineering for high-performance regenerated LiFePO4 cathodes. Sep. Purif. Technol. 2025, 376, 134183. [Google Scholar] [CrossRef]
- Rikka, V.R.; Sahu, S.R.; Gurumurthy, M.; Chatterjee, A.; Chandran, S.; Sundararajan, G.; Gopalan, R.; Prakash, R. Temperature-Derived Fe Dissolution of a LiFePO4/Graphite Cell at Fast Charging and High State-of-Charge Condition. Energy Technol. 2023, 11, 2201388. [Google Scholar] [CrossRef]
- Dogan, F.; Vaughey, J.T.; Iddir, H.; Key, B. Direct Observation of Lattice Aluminum Environments in Li-ion Cathodes NCA and Al-doped NMC via Al MAS NMR Spectroscopy. ACS Appl. Mater. Interfaces 2016, 8, 16708–16717. [Google Scholar] [CrossRef]
- Banerjee, H.; Grey, C.P.; Morris, A.J. Stability and Redox Mechanisms of Ni-Rich NMC Cathodes: Insights from First-Principles Many-Body Calculations. Chem. Mater. 2024, 36, 6575–6587. [Google Scholar] [CrossRef]
- Wang, F.-M.; Kuo, Y.-L.; Huang, L.-S.; Ramar, A.; Su, C.-H. Fabrication of in operando, self-growing, core-shell solid electrolyte interphase on LiFePO4 electrodes for preventing undesirable high-temperature effects in Li-ion batteries. Electrochim. Acta 2018, 268, 260–267. [Google Scholar] [CrossRef]
- Markey, B.; Zhang, M.H.; Robb, I.; Xu, P.P.; Gao, H.P.; Zhang, D.W.; Holoubek, J.; Xia, D.; Zhao, Y.F.; Guo, J.C.; et al. Effective Upcycling of Graphite Anode: Healing and Doping Enabled Direct Regeneration. J. Electrochem. Soc. 2020, 167, 160511. [Google Scholar] [CrossRef]
- Tang, R.; Dong, J.; Wang, C.; Guan, Y.; Yin, A.; Yan, K.; Lu, Y.; Li, N.; Zhao, G.; Li, B.; et al. Rate-Dependent Failure Behavior Regulation of LiFePO4 Cathode via Functional Interface Engineering. Adv. Funct. Mater. 2025, 35, 2421284. [Google Scholar] [CrossRef]
- Yadav, P.; Jie, C.J.; Tan, S.; Srinivasan, M. Recycling of cathode from spent lithium iron phosphate batteries. J. Hazard. Mater. 2020, 399, 123068. [Google Scholar] [CrossRef]
- Van Beek, C.B.; Yilmaz, E.; Boom, D.H.A. Sustainable Hydrometallurgical LFP Battery Recycling: Electrochemical Approaches. ChemSusChem 2025, 18, e202402699. [Google Scholar] [CrossRef]
- Wu, Y.H.Z.; Zhou, K.G.; Zhang, X.K.; Peng, C.H.; Jiang, Y.; Chen, W. Aluminum separation by sulfuric acid leaching-solvent extraction from Al-bearing LiFePO4/C powder for recycling of Fe/P. Waste Manag. 2022, 144, 303–312. [Google Scholar] [CrossRef]
- Guo, Y.; Yao, Y.; Guo, C.; Song, Y.; Huang, P.; Liao, X.; He, K.; Zhang, H.; Liu, H.; Hu, R.; et al. Atomistic observation and transient reordering of antisite Li/Fe defects toward sustainable LiFePO4. Energy Environ. Sci. 2024, 17, 7749–7761. [Google Scholar] [CrossRef]
- Yang, F.; Chen, X.; Qu, G.; Nie, Q.; Liu, G.; Wan, W.; Wang, T.; Li, S.; Huang, Y.; Li, J.; et al. Electrode separation via water electrolysis for sustainable battery recycling. Nat. Sustain. 2025, 8, 520–529. [Google Scholar] [CrossRef]
- Chen, X.; Li, S.; Wang, Y.; Jiang, Y.; Tan, X.; Han, W.; Wang, S. Recycling of LiFePO4 cathode materials from spent lithium-ion batteries through ultrasound-assisted Fenton reaction and lithium compensation. Waste Manag. 2021, 136, 67–75. [Google Scholar] [CrossRef]
- Sun, Q.; Li, X.; Zhang, H.; Song, D.; Shi, X.; Song, J.; Li, C.; Zhang, L. Resynthesizing LiFePO4/C materials from the recycled cathode via a green full-solid route. J. Alloys Compd. 2020, 818, 153292. [Google Scholar] [CrossRef]
- Chen, Z.; Feng, R.; Wang, W.; Tu, S.; Hu, Y.; Wang, X.; Zhan, R.; Wang, J.; Zhao, J.; Liu, S.; et al. Reaction-passivation mechanism driven materials separation for recycling of spent lithium-ion batteries. Nat. Commun. 2023, 14, 4648. [Google Scholar] [CrossRef] [PubMed]
- Ji, G.; Wang, J.; Liang, Z.; Jia, K.; Ma, J.; Zhuang, Z.; Zhou, G.; Cheng, H.-M. Direct regeneration of degraded lithium-ion battery cathodes with a multifunctional organic lithium salt. Nat. Commun. 2023, 14, 584. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Xu, P.; Li, J.; Yi, C.; Zhao, W.; Sun, W.; Ji, X.; Yang, Y.; Ge, P. Large-Scale and Homogenized Strategies of Spent LiFePO4 Recycling: Reconstruction of Targeted Lattice. Adv. Funct. Mater. 2023, 34, 2308671. [Google Scholar] [CrossRef]
- Liu, Y.; Tu, W.; Bai, J.; Wang, P.; Mao, Y.; Xiao, K.; Wang, S.; Qiu, S.; Zhu, X.; Lu, W.; et al. Amino Group-Aided Efficient Regeneration Targeting Structural Defects and Inactive FePO4 Phase for Degraded LiFePO4 Cathodes. Small 2024, 20, 2405362. [Google Scholar] [CrossRef]
- Chen, X.; Yuan, L.; Yan, S.; Ma, X. Self-activation of Ferro-chemistry based advanced oxidation process towards in-situ recycling of spent LiFePO4 batteries. Chem. Eng. J. 2023, 471, 144343. [Google Scholar] [CrossRef]
- Cao, Y.; Li, J.; Tang, D.; Zhou, F.; Yuan, M.; Zhu, Y.; Feng, C.; Shi, R.; Wei, X.; Wang, B.; et al. Targeted Defect Repair and Multi-functional Interface Construction for the Direct Regeneration of Spent LiFePO4 Cathodes. Adv. Mater. 2024, 36, 2414048. [Google Scholar] [CrossRef]
- Zhou, Y.; Fan, Y.; Wang, Z.; Mao, W.; Weng, Y.; Zhang, J.; Pu, Y.; Yang, J.; Li, X.a. Effect of non-metallic X(X=F, N, S) and Cr co-doping on properties of BiFeO3: A first-principles study. Phys. Lett. A 2019, 383, 383–388. [Google Scholar] [CrossRef]
- Jiang, K.; Wang, F.; Liu, M.; Fan, Y.; Chen, Z.; Li, G.; Li, P.; Shi, X.; Hong, W. First principles study of the electronic structure and Li-ion diffusion properties of co-doped LIFex-1MxPyNy-1O4 (M=Co/Mn, N[dbnd]S/Si) Li-ion battery cathode materials. Micro Nanostructures 2024, 196, 207988. [Google Scholar] [CrossRef]
- Feng, C.; Cao, Y.; Song, L.; Zhao, B.; Yang, Q.; Zhang, Y.; Wei, X.; Zhou, G.; Song, Y. Direct Regeneration of Industrial LiFePO4 Black Mass Through A Glycerol-Enabled Granule Reconstruction Strategy. Angew. Chem. Int. Ed. 2024, 64, e202418198. [Google Scholar]
- Xie, J.; Liu, J.; Yang, B. Dehydroxylation of glycerol on Pt surfaces:ab initiomolecular dynamics study. Chin. J. Chem. Phys. 2022, 35, 611–618. [Google Scholar] [CrossRef]
- Li, D.; Zhao, X.; Zhou, Q.; Ding, B.; Zheng, A.; Peng, Q.; Hou, Z. Vicinal hydroxyl group-inspired selective oxidation of glycerol to glyceric acid on hydroxyapatite supported Pd catalyst. Green Energy Environ. 2020, 7, 691–703. [Google Scholar] [CrossRef]
- Hoshina, H.; Chen, J.; Amada, H.; Seko, N. Chelating Fabrics Prepared by an Organic Solvent-Free Process for Boron Removal from Water. Polymers 2021, 13, 1163. [Google Scholar] [CrossRef]
- Yang, M.; Xu, J.; Gan, K.; Qu, Y.; Ma, N.; Wang, X.; Yang, J. Direct coagulation casting of alumina via controlled release of calcium from ammonium polyphosphate chelate complex. J. Mater. Res. 2016, 31, 154–162. [Google Scholar] [CrossRef]
- Supriya, S.; Das, S.K. Reversible Single Crystal to Single Crystal Transformation through Fe−O(H)Me/Fe−OH2Bond Formation/Bond Breaking in a Gas−Solid Reaction at an Ambient Condition. J. Am. Chem. Soc. 2007, 129, 3464–3465. [Google Scholar] [CrossRef]
- Li, J.; Shi, R.; Wang, J.; Cao, Y.; Ji, H.; Tang, J.; Ji, G.; Chen, W.; Zhang, M.; Xiao, X.; et al. Interfacial Metal-Solvent Chelation for Direct Regeneration of LiFePO4 Cathode Black Mass. Adv. Mater. 2024, 37, 2414235. [Google Scholar] [CrossRef]
- Agarwal, G.; Doan, H.A.; Assary, R.S. Molecular Structure and Electron Affinity of Metal-Solvent Complexes: Insights from Density Functional Theory Simulations. J. Electrochem. Soc. 2020, 167, 100545. [Google Scholar] [CrossRef]
- Tanaka, M.; Koyama, K. Theoretical Analysis of the Inflection Point on the Logarithm of the Distribution Ratio vs pH Plot for Metal Solvent Extraction with Chelating Extractants. Ind. Eng. Chem. Res. 1996, 35, 4230–4235. [Google Scholar] [CrossRef]
- Li, Z.; Li, M.; Chen, Y.; Ye, X.; Liu, M.; Lee, L.Y.S. Upcycling of Spent LiFePO4 Cathodes to Heterostructured Electrocatalysts for Stable Direct Seawater Splitting. Angew. Chem. Int. Ed. 2024, 63, 202410396. [Google Scholar] [CrossRef]
- Bishwanathan, S.; Gupta, P.K. Breaking the Scaling Relationship of Oxygen Evolution Reaction and Chlorine Oxidation Reaction via MnO2 Polymorphic Engineering for Selective Seawater Electrolysis. ACS Appl. Energy Mater. 2024, 7, 5467–5478. [Google Scholar] [CrossRef]
- Liu, K.; Ye, J.; Lei, L.; Deng, K.; Bo, T.; Han, X.; Guo, X.; Guo, X.; Huang, L.; Wang, D. Chlorine-facilitated amorphous Co-based catalyst with self-termination of surface reconstruction for seawater splitting. Nano Energy 2025, 143, 111288. [Google Scholar] [CrossRef]
- Wang, L.; Huang, K.; Zheng, X.; Liu, Y.; Wu, J.; Dai, Q.; Chen, Z.; Li, Z.; Yang, B.; Lian, C.; et al. A Corrosion-Resistant Amorphous/Crystalline Heterostructured Catalyst for Industrial-Level Seawater Electrolysis in Membrane Electrode Assembly Electrolyzer. Adv. Funct. Mater. 2025, 2417603. [Google Scholar] [CrossRef]
- Jiang, C.; Gan, L.; Liu, J.; Ren, Y.; Li, J.; Tian, J.; Chen, W.; Huang, J.; Zhao, J.; Chen, K.; et al. Dual Vacancy Engineering of CoFePvOv/CF Electrocatalyst for High-Efficiency Seawater Splitting with Enhanced Chloride Corrosion Resistance. J. Alloys Compd. 2025, 1037, 182334. [Google Scholar] [CrossRef]
- Wang, X.; Chen, J.; Xu, L.; Miao, J.; Sunarso, J.; Wang, X.; Cao, W.; Yang, Y.; Zhou, W. Electrocatalytic selective oxygen evolution of FeOOH-modified perovskite for alkaline seawater electrolysis. J. Power Sources 2024, 614, 235017. [Google Scholar] [CrossRef]
- Tong, X.; Xu, H.; Wei, S.; Sun, D.; Lin, S.; Zhou, H.; Ji, X.; Yang, Y.; Zhang, L. Sulfidation adjust the valence states of metal ions enhancing alkaline seawater OER catalytic activity and stability for PBA−derived self–supporting NiFe sulfide. Mater. Sci. Semicond. Process. 2025, 192, 109396. [Google Scholar] [CrossRef]
- Zhou, X.; Dong, H.; Ren, A.-M. The mechanism of hydrogen and oxygen evolution reaction in Ni–NiO/β-Ga2O3 photocatalyst. Int. J. Hydrogen Energy 2016, 71, 674–682. [Google Scholar] [CrossRef]
- Duan, H.; Chen, Z.; Xu, N.; Qiao, S.; Chen, G.; Li, D.; Deng, W.; Jiang, F. Non-stoichiometric NiOx nanocrystals for highly efficient electrocatalytic oxygen evolution reaction. J. Electroanal. Chem. 2021, 68, 19–26. [Google Scholar] [CrossRef]
- Yao, R.; Wu, J.; Kansara, S.; Sun, Z.; Kang, H.; Liu, F.; Wang, K.; Hu, J.; Li, X.; Wu, D.; et al. Recycling Defunct Lithium-Ion Battery Cathodes to Quaternary Layered Double Hydroxides for Efficient Oxygen Evolution Reaction. Adv. Sci. 2025, 12, 2501957. [Google Scholar] [CrossRef]
- Liu, J.; Liu, Z.; Xiao, Z.; Zhu, Y.; Wang, J.; Ji, G.; Liu, Y.; Sun, B.; Zhou, G. Iodine-Mediated Redox Strategy for Sustainable Lithium Extraction From Spent LiFePO4 Cathodes. Adv. Mater. 2025, 37, 2503450. [Google Scholar] [CrossRef]
- Wang, W.; Liu, Z.; Zhu, Z.; Ma, Y.; Zhang, K.; Meng, Y.; Ahmad, T.; Khan, N.A.; Peng, Q.; Xie, Z.; et al. Electrochemical lithium recycling from spent batteries with electricity generation. Nat. Sustain. 2025, 8, 287–296. [Google Scholar] [CrossRef]
- Hashemi, N.; Nandy, S.; Aleshkevych, P.; Chae, K.H.; Najafpour, M.M. Reaction between Nickel Hydroxide and Cerium(IV) Ammonium Nitrate in Aqueous Solution. Inorg. Chem. 2023, 62, 12157–12165. [Google Scholar] [CrossRef]
- Le, H.T.; Chun, H.-J.; Kwon, Y.J.; Ham, M.J.; Kim, S.K.; Jeong, Y.K. Synergistic doping of Iron and selenium on nickel hydroxide nitrate nanoarrays-derived as an efficient electrocatalyst for overall water splitting. Appl. Surf. Sci. 2024, 671, 160657. [Google Scholar] [CrossRef]
- Zuo, A.; Feng, H.; Yuan, T.; Xu, L.; Shu, K.; Tian, J.; Luo, Y.; Xue, K. One-step Fe/Li separation via slurry electrolysis from spent LiFePO4 batteries and iron-based composite formation for photocatalytic tetracycline degradation. Sep. Purif. Technol. 2025, 377, 134207. [Google Scholar] [CrossRef]
- Cao, H.; Wang, Q.; Zhang, J.; Yu, J.; Yu, K.; Zhang, F. Enhanced lithium extraction from spent LiFePO4 via high-throughput electrochemical flow cell technology. J. Clean. Prod. 2025, 503, 145423. [Google Scholar] [CrossRef]
- Kong, X.; Xu, J.; Ju, Z.; Chen, C. Durable Ru Nanocrystal with HfO2 Modification for Acidic Overall Water Splitting. Nano-Micro Lett. 2024, 16, 185. [Google Scholar] [CrossRef]
- Xu, J.; Chen, C.; Kong, X. Ru-O-Cu center constructed by catalytic growth of Ru for efficient hydrogen evolution. Nano Energy 2023, 111, 108403. [Google Scholar] [CrossRef]
- Jia, X.; Kang, H.; Hou, G.; Wu, W.; Lu, S.; Li, Y.; Wang, Q.; Qin, W.; Wu, X. Coupling Ferricyanide/Ferrocyanide Redox Mediated Recycling Spent LiFePO4 with Hydrogen Production. Angew. Chem. Int. Ed. 2024, 63, e202318248. [Google Scholar] [CrossRef]
- Lv, Z.-B.; Xu, L.; Chen, C.; Chen, Z.-H.; Fu, M.-L.; Yuan, B. Selective recovery of lithium from spent LiFePO4 batteries based on PMS advanced oxidation process. J. Environ. Chem. Eng. 2025, 13, 115735. [Google Scholar] [CrossRef]
- Nam, D.-H.; Foster, B.M.; Choi, K.-S. Electrochemical Li Recovery from Spent LiFePO4-Based Li-Ion Batteries. ACS Energy Lett. 2025, 10, 2934–2941. [Google Scholar] [CrossRef]
- Zhu, G.; Yu, D.; Foka Meugang, E.; Li, H.; Huan, H.; Guo, X.; Tian, Q. Powder electrolysis for direct selective lithium recovery from spent LiFePO4 materials. Resour. Conserv. Recycl. 2023, 199, 107282. [Google Scholar] [CrossRef]
- Li, Z.; Liu, D.; Xiong, J.; He, L.; Zhao, Z.; Wang, D. Selective recovery of lithium and iron phosphate/carbon from spent lithium iron phosphate cathode material by anionic membrane slurry electrolysis. Waste Manag. 2020, 107, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Sathya, S.; Soosaimanickam, C.; Bella, F.; Yoo, D.J.; Stephan, A.M. Cycling performance of SiOx-Si-C composite anode with different blend ratios of PAA-CMC as binder for lithium sulfur batteries. J. Polym. Res. 2024, 31, 205. [Google Scholar] [CrossRef]
- Sathya, S.; Kumar, R.S.; Garcia-Ballesteros, S.; Bella, F.; Yoo, D.J.; Stephan, A.M. Interplay Between Composition and Cycling Performance of Pre-Lithiated SiOx-Si-C Composite Anodes for Lithium–Sulfur Full Cells. Materials 2025, 18, 1053. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Wang, L.; Fu, Z.; Yin, F.; Zheng, F.; Wang, J.; Fang, F.; Liu, Q.; Kong, X. Recycling Spent LFP Batteries: From Resource Recovery to High-Value Functional Materials. Molecules 2025, 30, 3557. https://doi.org/10.3390/molecules30173557
Wang C, Wang L, Fu Z, Yin F, Zheng F, Wang J, Fang F, Liu Q, Kong X. Recycling Spent LFP Batteries: From Resource Recovery to High-Value Functional Materials. Molecules. 2025; 30(17):3557. https://doi.org/10.3390/molecules30173557
Chicago/Turabian StyleWang, Chang, Lizhi Wang, Zixuan Fu, Fan Yin, Fangyu Zheng, Jun Wang, Fei Fang, Qiangchun Liu, and Xiangkai Kong. 2025. "Recycling Spent LFP Batteries: From Resource Recovery to High-Value Functional Materials" Molecules 30, no. 17: 3557. https://doi.org/10.3390/molecules30173557
APA StyleWang, C., Wang, L., Fu, Z., Yin, F., Zheng, F., Wang, J., Fang, F., Liu, Q., & Kong, X. (2025). Recycling Spent LFP Batteries: From Resource Recovery to High-Value Functional Materials. Molecules, 30(17), 3557. https://doi.org/10.3390/molecules30173557








