A Para-Substituted 2-Phenoxy-1,10-Phenanthroline Ligand for Lanthanide Sensitization: Asymmetric Coordination and Enhanced Emission from Eu3+, Tb3+, Sm3+ and Dy3+ Complexes
Abstract
1. Introduction
2. Results and Discussion
2.1. Structural Characterization by FTIR
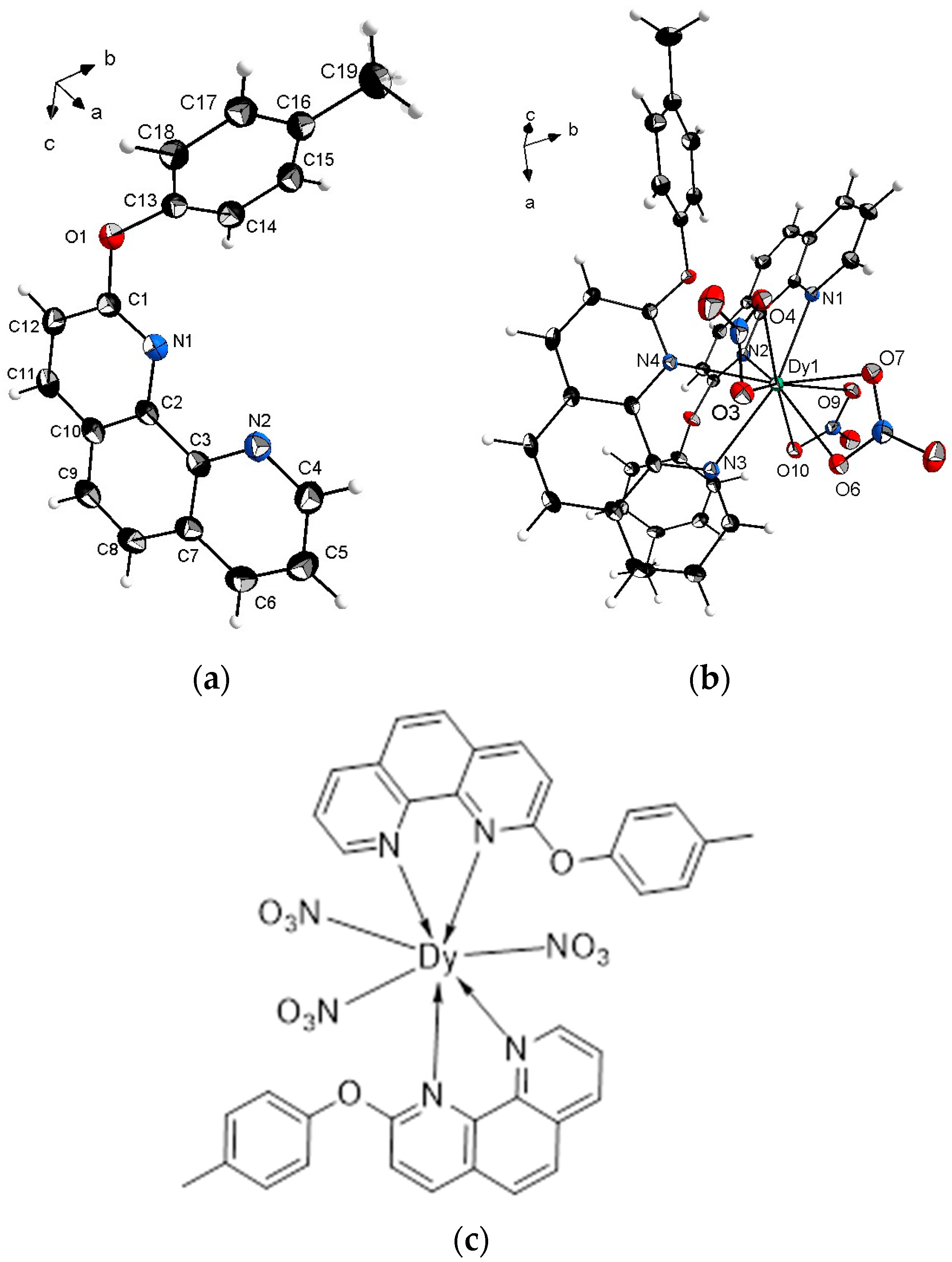
2.2. Crystal Structure
2.3. Photophysical Properties
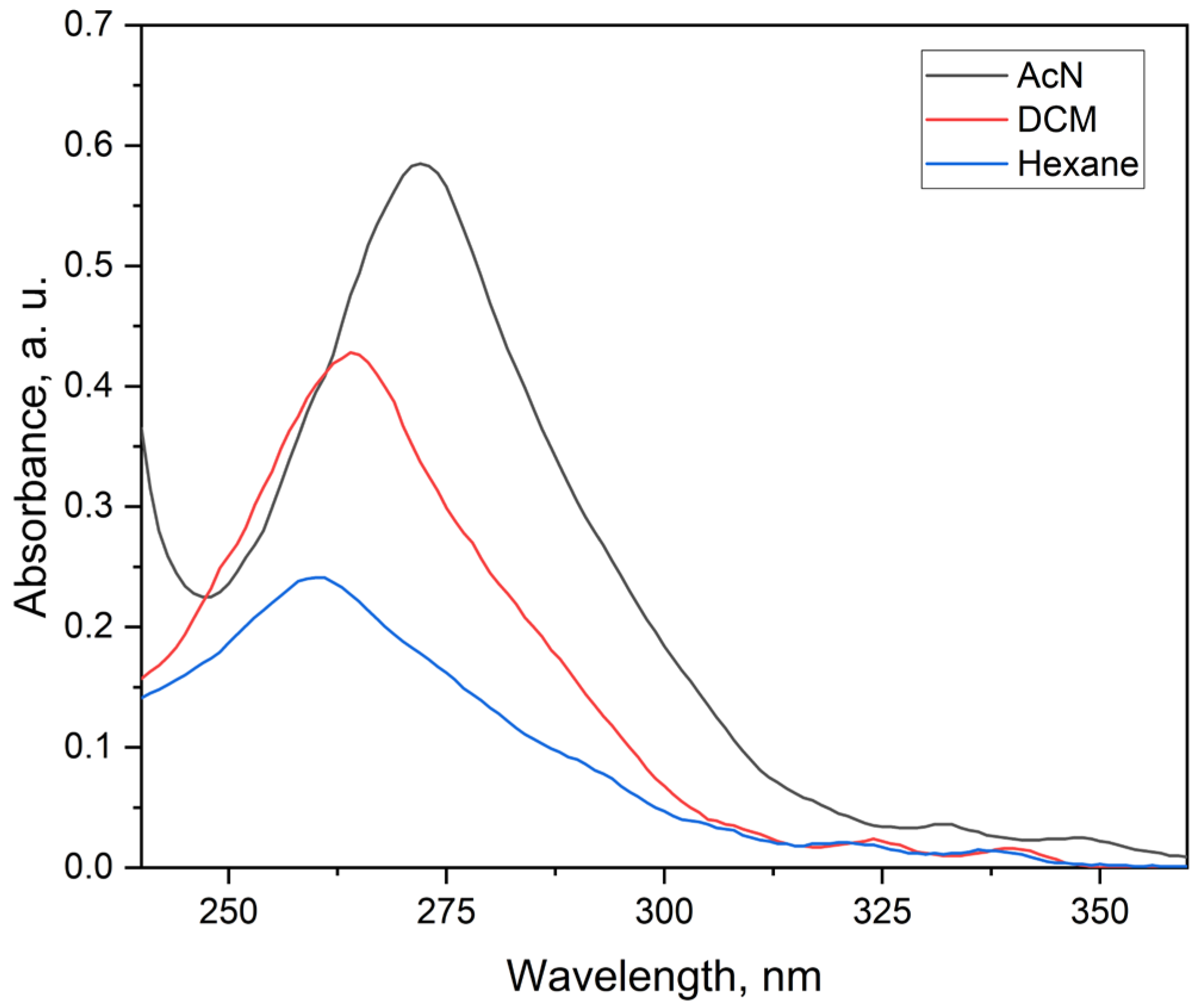
2.3.1. Electronic Spectra
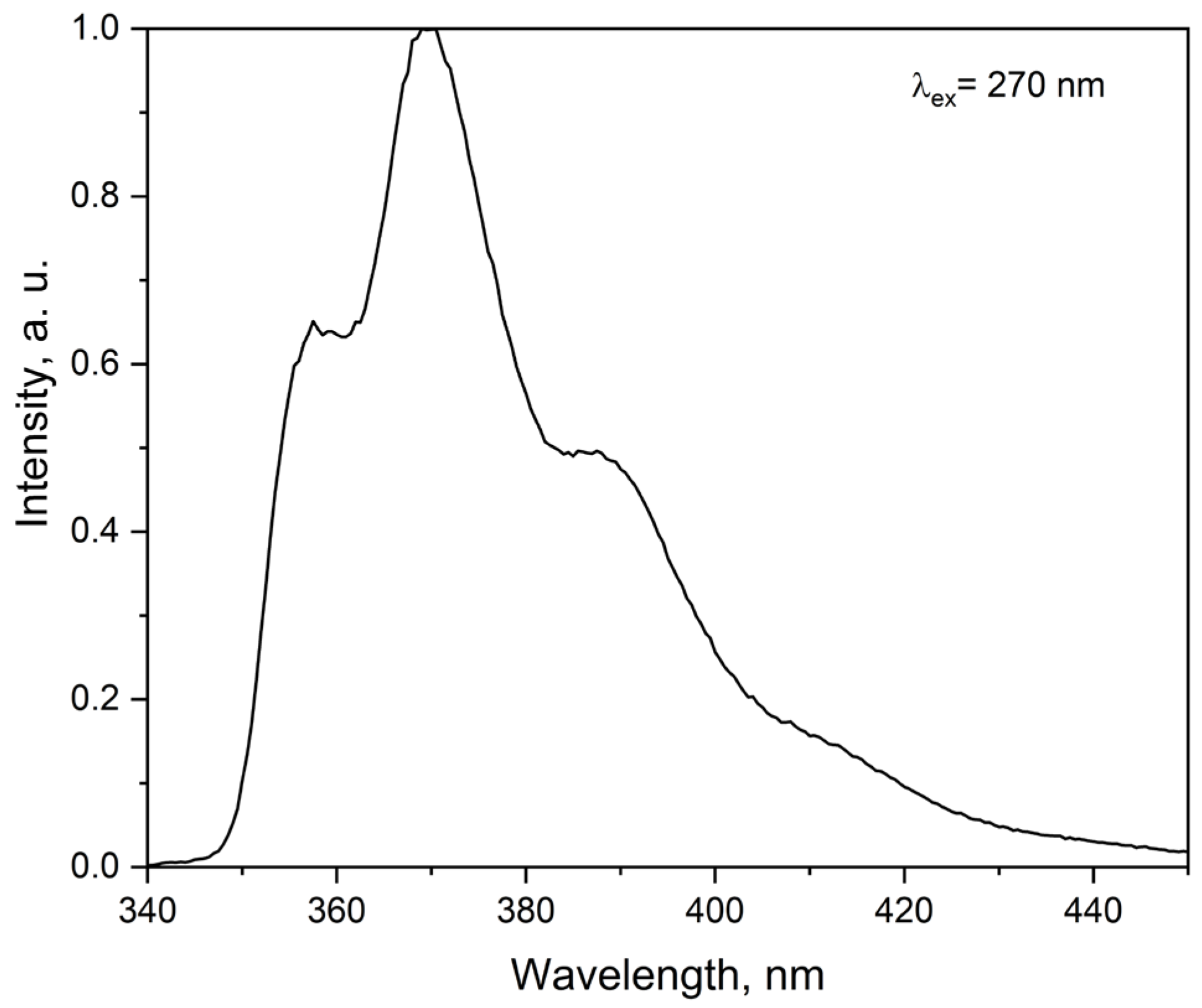
2.3.2. Emission Spectra at Room Temperature
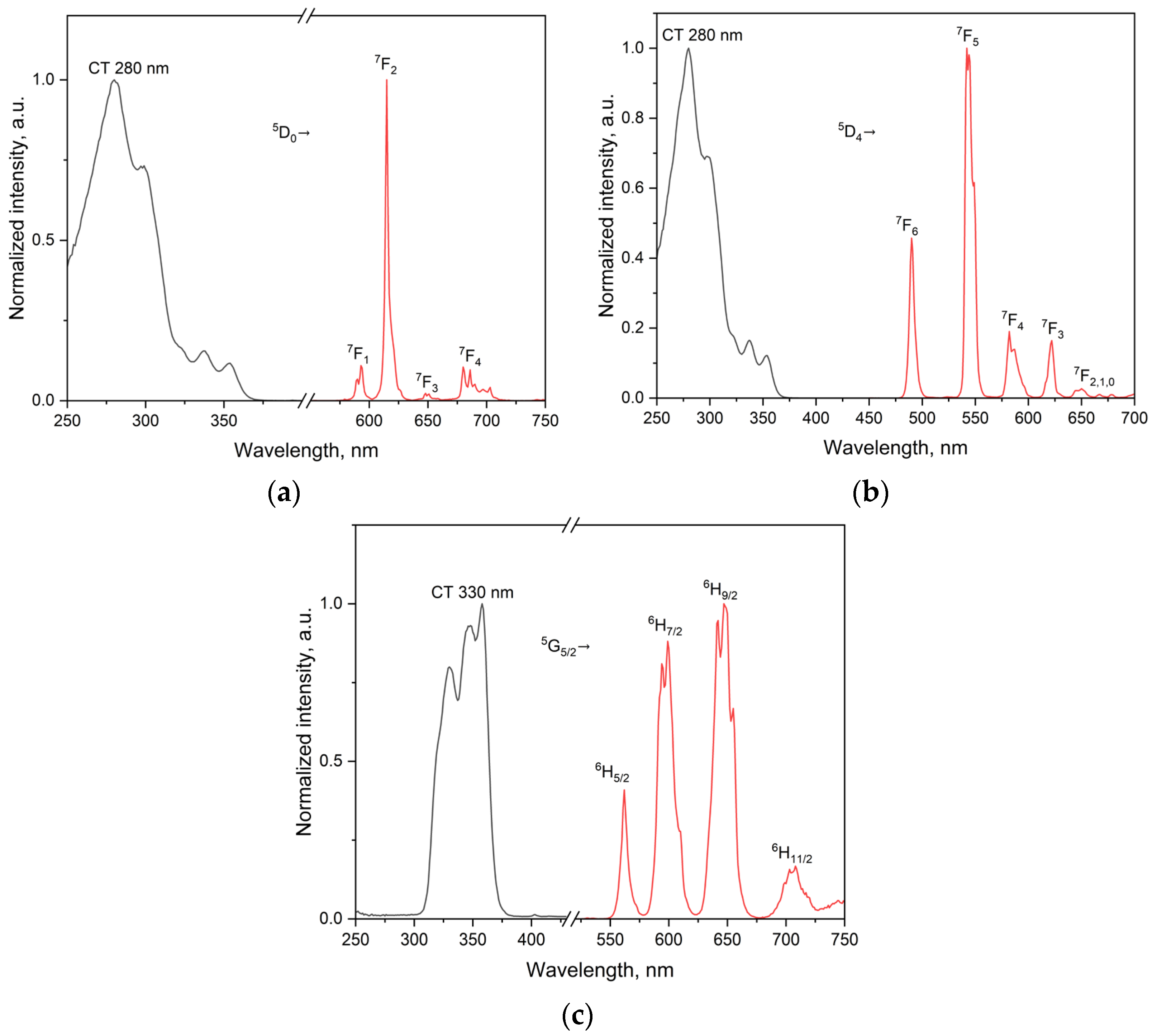
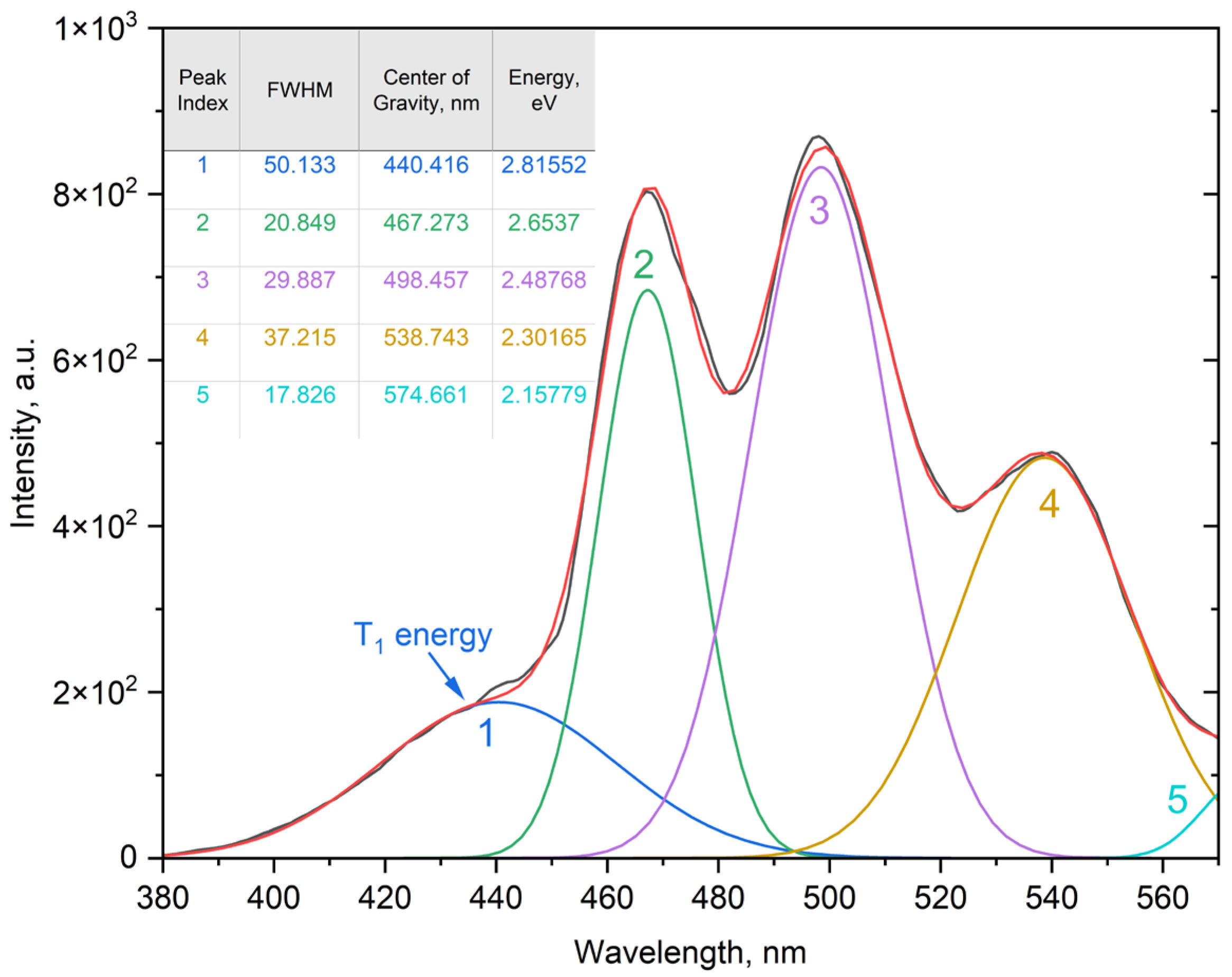
2.3.3. Spectral Deconvolution and Band Assignment
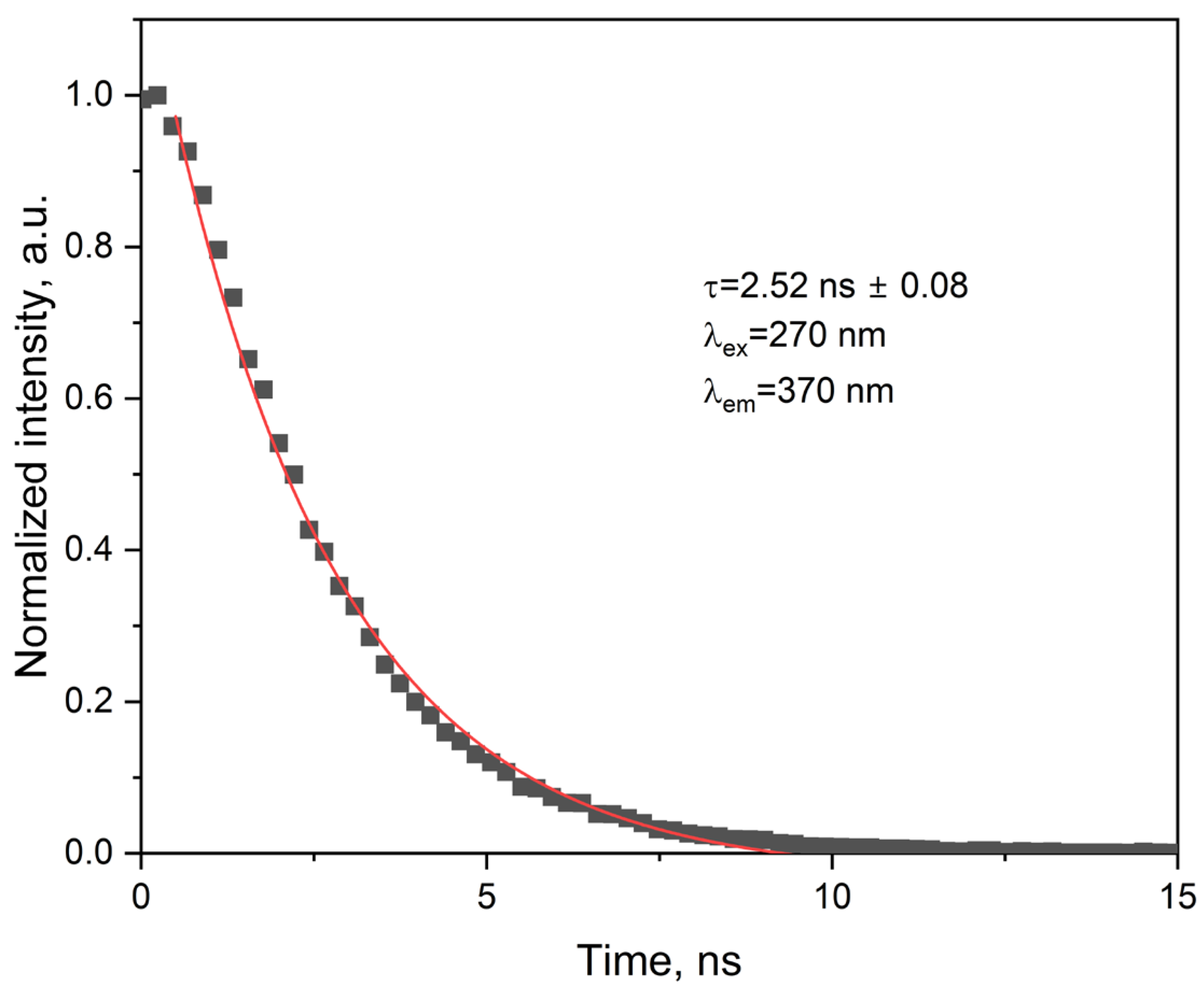
2.3.4. Photophysical Parameters Derived from Emission Studies
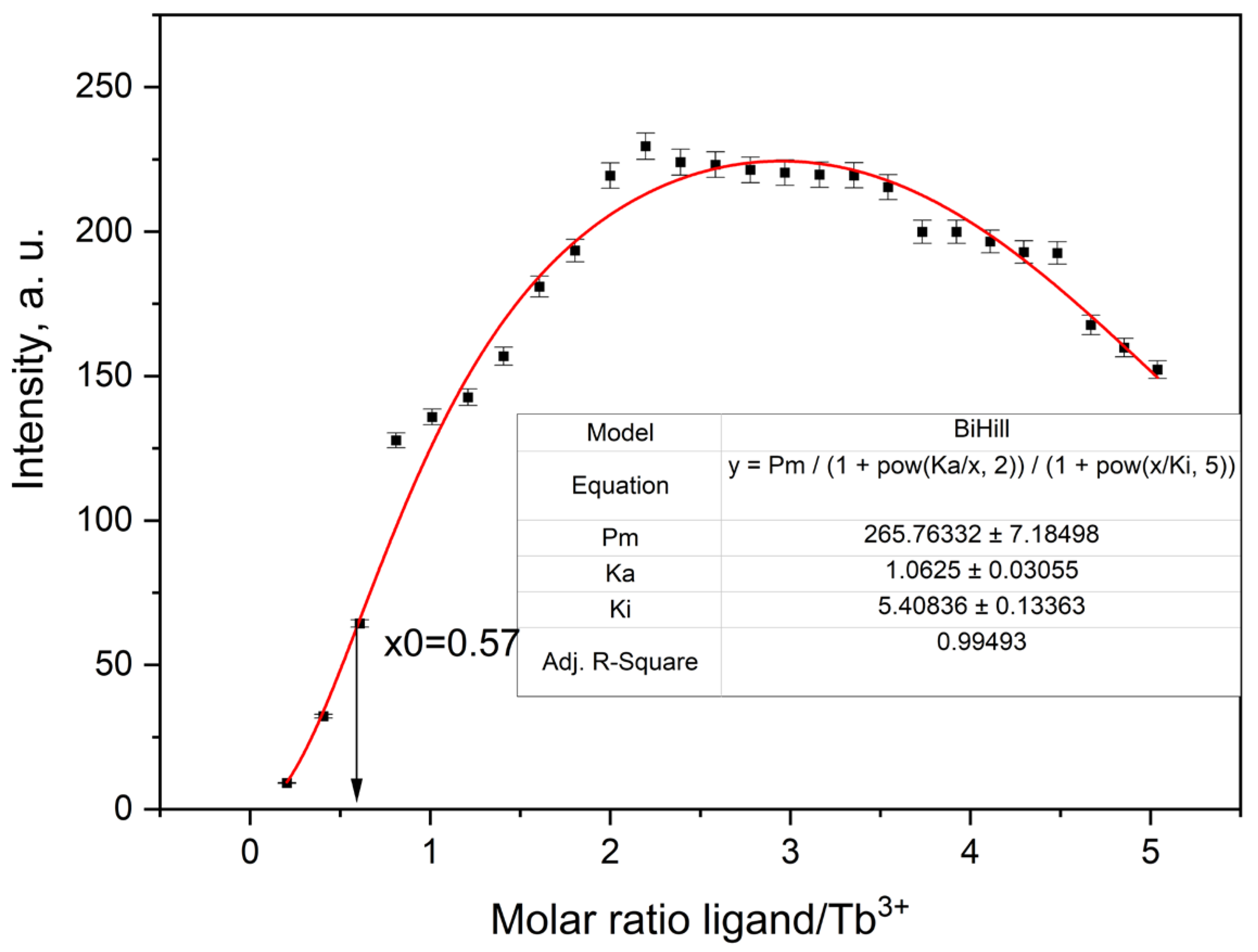
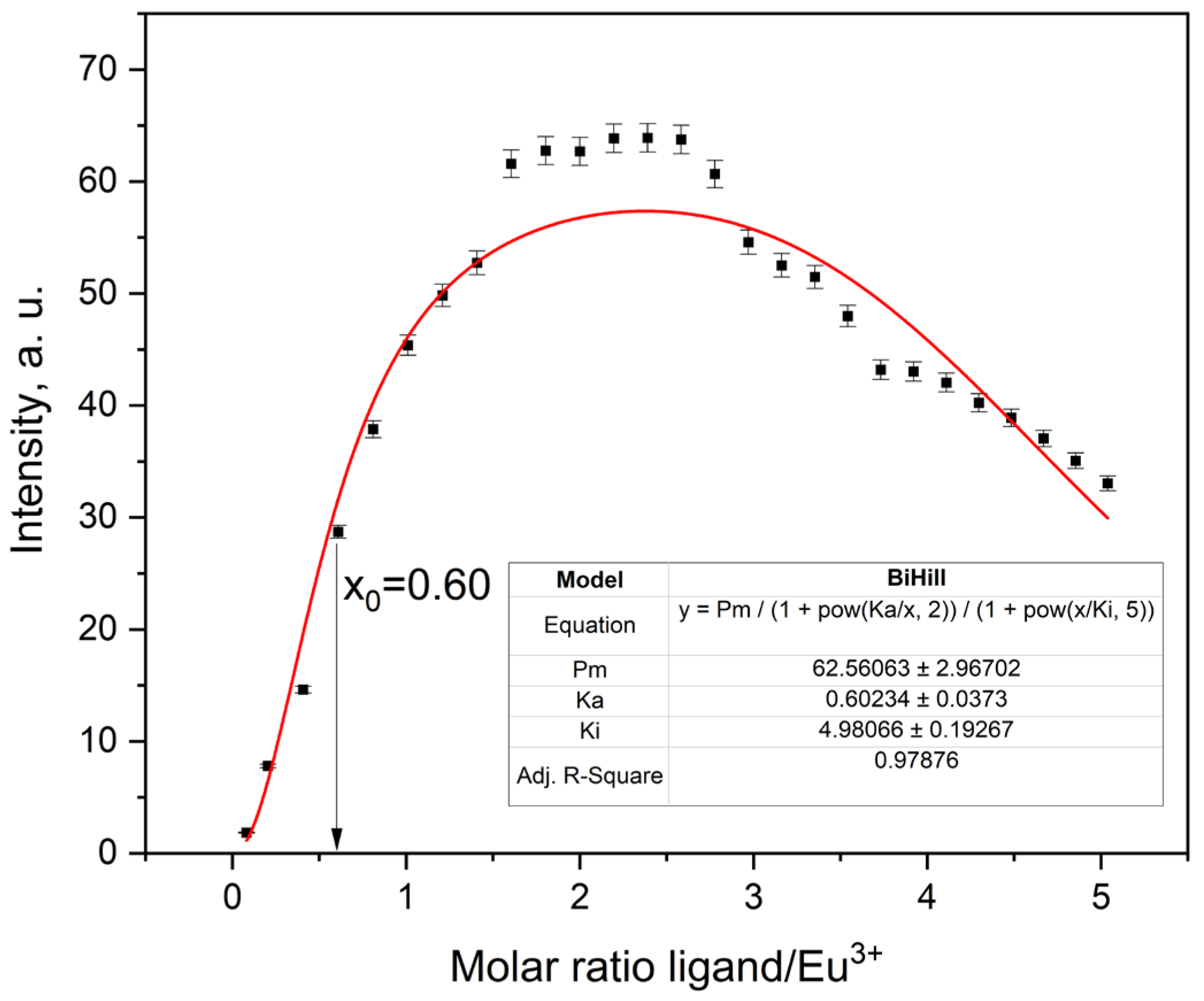
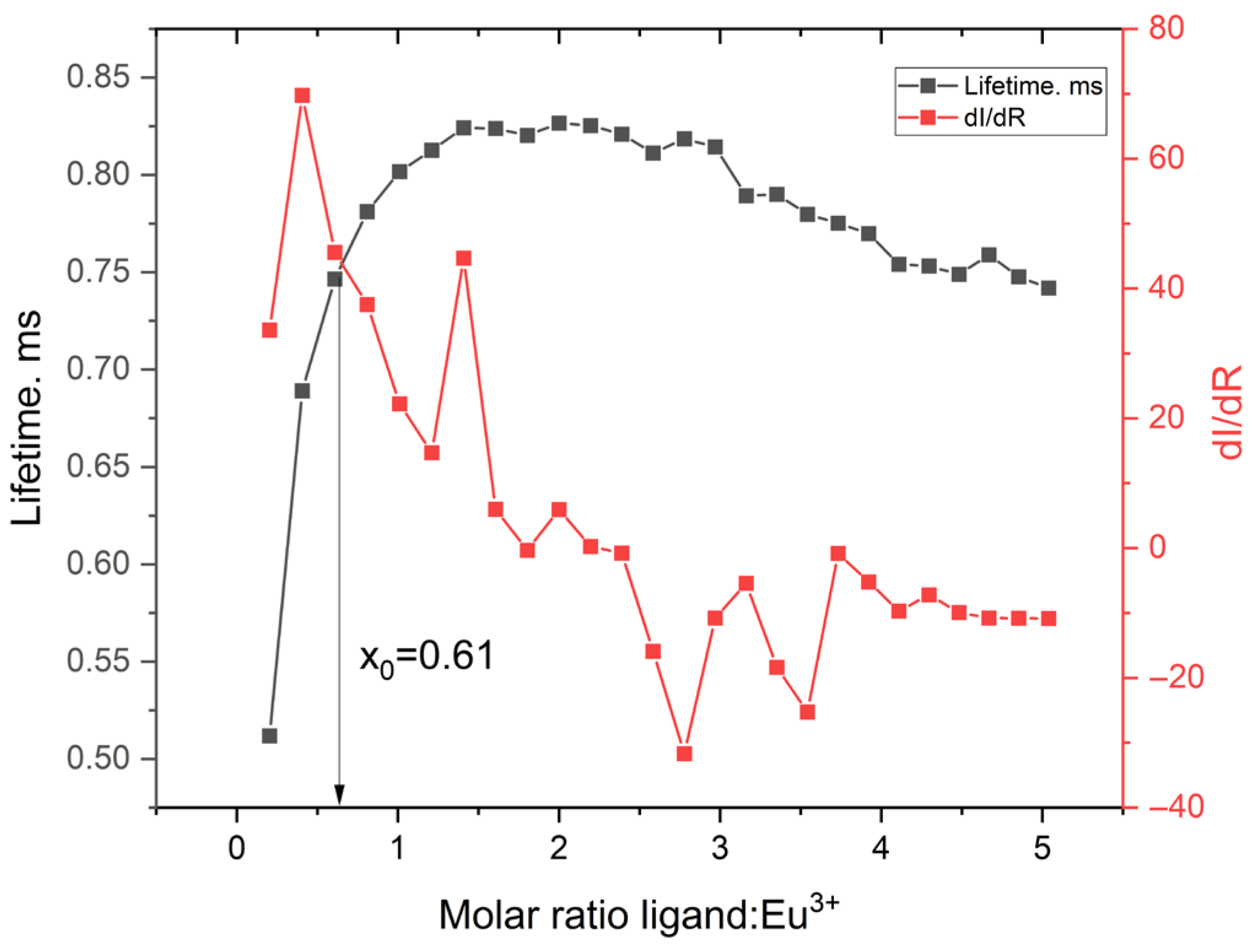
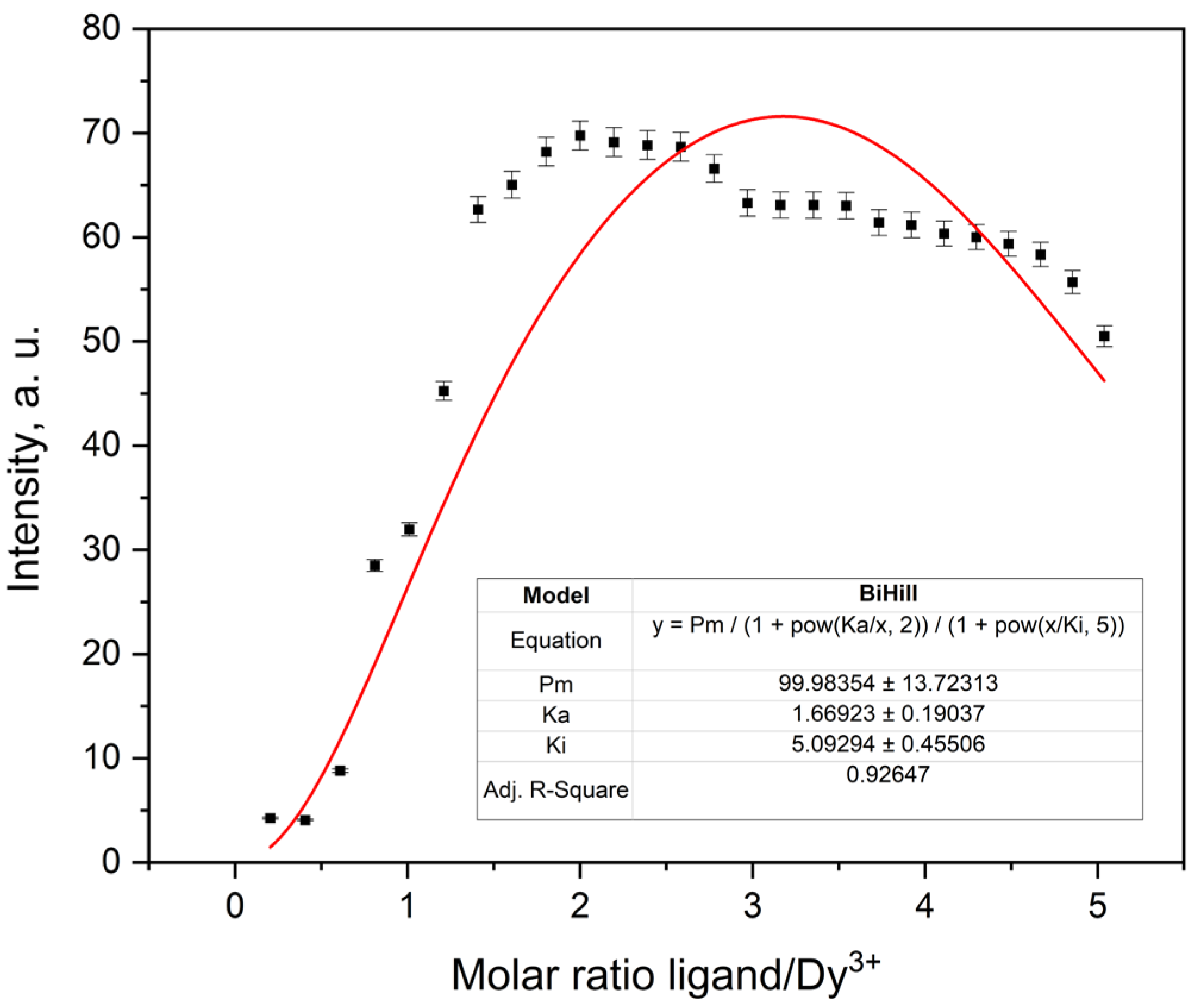
2.3.5. Fluorescence Titration and Binding Analysis
3. Materials and Methods
3.1. Synthesis of 2-(4-Methylphenoxy)-1,10-Phenanthroline
3.2. Synthesis of Lanthanide(III) Complexes
3.3. Photophysical Measurements
3.4. Fluorescence Titration Procedure
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bünzli, J.-C.G. Lanthanide Luminescence for Biomedical Analyses and Imaging. Chem. Rev. 2010, 110, 2729–2755. [Google Scholar] [CrossRef]
- Bünzli, J.-C.G.; Piguet, C. Taking Advantage of Luminescent Lanthanide Ions. Chem. Soc. Rev. 2005, 34, 1048. [Google Scholar] [CrossRef] [PubMed]
- Latva, M.; Takalo, H.; Mukkala, V.-M.; Matachescu, C.; Rodríguez-Ubis, J.C.; Kankare, J. Correlation between the Lowest Triplet State Energy Level of the Ligand and Lanthanide(III) Luminescence Quantum Yield. J. Lumin. 1997, 75, 149–169. [Google Scholar] [CrossRef]
- Armelao, L.; Quici, S.; Barigelletti, F.; Accorsi, G.; Bottaro, G.; Cavazzini, M.; Tondello, E. Design of Luminescent Lanthanide Complexes: From Molecules to Highly Efficient Photo-Emitting Materials. Coord. Chem. Rev. 2010, 254, 487–505. [Google Scholar] [CrossRef]
- Binnemans, K. Interpretation of Europium(III) Spectra. Coord. Chem. Rev. 2015, 295, 1–45. [Google Scholar] [CrossRef]
- Nicolás, Á.; Quero, J.G.; Barroso, M.; Gándara, Z.; Gude, L. DNA Interactions and Biological Activity of 2,9-Disubstituted 1,10-Phenanthroline Thiosemicarbazone-Based Ligands and a 4-Phenylthiazole Derivative. Biology 2024, 13, 60. [Google Scholar] [CrossRef]
- Thor, W.; Carneiro Neto, A.N.; Moura, R.T.; Wong, K.-L.; Tanner, P.A. Europium(III) Coordination Chemistry: Structure, Spectra and Hypersensitivity. Coord. Chem. Rev. 2024, 517, 215927. [Google Scholar] [CrossRef]
- Bryleva, Y.A.; Mikheylis, A.V.; Agafontsev, A.M.; Glinskaya, L.A.; Tkachev, A.V. Antenna Effect of 1,10-Phenanthroline Derivative Bearing (−)-Borneol Moieties in Luminescent Lanthanide(III) Complexes. J. Lumin. 2025, 281, 121144. [Google Scholar] [CrossRef]
- Clarke, J.K.A.; Pullin, A.D.E. An Apparatus for the Spectroscopic Investigation of Adsorbed Molecules and the Infra-Red Spectrum of Formic Acid on Silica-Supported Nickel. Trans. Faraday Soc. 1960, 56, 534. [Google Scholar] [CrossRef]
- Tamiru, G.; Abebe, A.; Abebe, M.; Liyew, M. Synthesis, Structural Investigation and Biological Application of New Mono- and Binuclear Cobalt (II) Mixed-Ligand Complexes Containing 1,10-Phenanthroline, Acetamide and Ethylenediamine. Ethiop. J. Sci. Technol. 2019, 12, 69. [Google Scholar] [CrossRef]
- Zhuravlev, K.P.; Kudryashova, V.A.; Tsaryuk, V.I. Luminescence and Energy Transfer Processes in Europium and Terbium Complexes with 2-Substituted Cycloalkanones and 1,10-Phenanthroline Derivatives. J. Photochem. Photobiol. A Chem. 2016, 314, 14–21. [Google Scholar] [CrossRef]
- Avagyan, N.A.; Lemport, P.S.; Evsiunina, M.V.; Matveev, P.I.; Aksenova, S.A.; Nelyubina, Y.V.; Yatsenko, A.V.; Tafeenko, V.A.; Petrov, V.G.; Ustynyuk, Y.A.; et al. Pyrrolidine-Derived Phenanthroline Diamides: An Influence of Fluorine Atoms on the Coordination of Lu(III) and Some Other f-Elements and Their Solvent Extraction. Int. J. Mol. Sci. 2023, 24, 5569. [Google Scholar] [CrossRef] [PubMed]
- Casanova, D.; Llunell, M.; Alemany, P.; Alvarez, S. The Rich Stereochemistry of Eight-Vertex Polyhedra: A Continuous Shape Measures Study. Chem. A Eur. J. 2005, 11, 1479–1494. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Shang, Y.; Zhang, H.; Li, R.; Wang, W.; Zhang, D.; Wang, L.; Li, Z. Enhanced Luminescence and Tunable Magnetic Properties of Lanthanide Coordination Polymers Based on Fluorine Substitution and Phenanthroline Ligand. RSC Adv. 2019, 9, 16328–16338. [Google Scholar] [CrossRef]
- Zhang, Y.-L.; Liu, W.-S.; Dou, W.; Qin, W.-W. Synthesis and Infrared and Fluorescence Spectra of New Europium and Terbium Polynuclear Complexes with an Amide-Based 1,10-Phenanthroline Derivative. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2004, 60, 1707–1711. [Google Scholar] [CrossRef]
- Eliseeva, S.V.; Bünzli, J.-C.G. Lanthanide Luminescence for Functional Materials and Bio-Sciences. Chem. Soc. Rev. 2010, 39, 189–227. [Google Scholar] [CrossRef]
- Accorsi, G.; Listorti, A.; Yoosaf, K.; Armaroli, N. 1,10-Phenanthrolines: Versatile Building Blocks for Luminescent Molecules, Materials and Metal Complexes. Chem. Soc. Rev. 2009, 38, 1690–1700. [Google Scholar] [CrossRef]
- Romanova, J.; Lyapchev, R.; Kolarski, M.; Tsvetkov, M.; Elenkova, D.; Morgenstern, B.; Zaharieva, J. Molecular Design of Luminescent Complexes of Eu(III): What Can We Learn from the Ligands. Molecules 2023, 28, 4113. [Google Scholar] [CrossRef]
- Al Riyami, N.A.; Al-Rasbi, N.K. Factors That Influence the Energy Transfer Pathways in Luminescent Lanthanide Schiff Base Complexes. Inorg. Chim. Acta 2024, 572, 122324. [Google Scholar] [CrossRef]
- Váradi, B.; Lihi, N.; Bunda, S.; Nagy, A.; Simon, G.; Kéri, M.; Papp, G.; Tircsó, G.; Esteban-Gómez, D.; Platas-Iglesias, C.; et al. Physico-Chemical Characterization of a Highly Rigid Gd(III) Complex Formed with a Phenanthroline Derivative Ligand. Inorg. Chem. 2022, 61, 13497–13509. [Google Scholar] [CrossRef]
- Valeur, B.; Berberan-Santos, M.N. Molecular Fluorescence; John Wiley and Son: Hoboken, NJ, USA, 2012; ISBN 9783527328376. [Google Scholar]
- Abraham, M.K.; Chinnu, S.S.; Madanan, A.S.; Varghese, S.; Shkhair, A.I.; Indongo, G.; Rajeevan, G.; Arathy, B.K.; George, S. Terbium Phenanthroline Complex as a Luminescent Probe for the Detection of Anthrax Biomarker: Dipicolinic Acid. J. Fluoresc. 2024, 35, 3321–3330. [Google Scholar] [CrossRef] [PubMed]
- Omagari, S.; Nakanishi, T.; Kitagawa, Y.; Seki, T.; Fushimi, K.; Ito, H.; Meijerink, A.; Hasegawa, Y. Critical Role of Energy Transfer Between Terbium Ions for Suppression of Back Energy Transfer in Nonanuclear Terbium Clusters. Sci. Rep. 2016, 6, 37008. [Google Scholar] [CrossRef] [PubMed]
- Moradi, Z.; Khorasani-Motlagh, M.; Noroozifar, M. Synthesis and Biological Evaluation of a New Dysprosium(III) Complex Containing 2,9-Dimethyl 1,10-Phenanthroline. J. Biomol. Struct. Dyn. 2017, 35, 300–311. [Google Scholar] [CrossRef] [PubMed]
- Bünzli, J.-C.G. On the Design of Highly Luminescent Lanthanide Complexes. Coord. Chem. Rev. 2015, 293–294, 19–47. [Google Scholar] [CrossRef]
- Vázquez López, M.; Eliseeva, S.V.; Blanco, J.M.; Rama, G.; Bermejo, M.R.; Vázquez, M.E.; Bünzli, J.G. Synthesis and Photophysical Properties of Ln III –DOTA–Bipy Complexes and Ln III –DOTA–Bipy–Ru II Coordination Conjugates. Eur. J. Inorg. Chem. 2010, 2010, 4532–4545. [Google Scholar] [CrossRef]
- Bünzli, J.G.; Milicic-Tang, A.; Mabillard, C. Lanthanide-nitrate Interaction in Anhydrous Acetonitrile and Coordination Numbers of the Lanthanide Ions: FT-IR Study. Helv. Chim. Acta 1993, 76, 1292–1304. [Google Scholar] [CrossRef]
- Summers, T.J.; Sobrinho, J.A.; de Bettencourt-Dias, A.; Kelly, S.D.; Fulton, J.L.; Cantu, D.C. Solution Structures of Europium Terpyridyl Complexes with Nitrate and Triflate Counterions in Acetonitrile. Inorg. Chem. 2023, 62, 5207–5218. [Google Scholar] [CrossRef]
- Shavaleev, N.M.; Eliseeva, S.V.; Scopelliti, R.; Bünzli, J.-C.G. Influence of Symmetry on the Luminescence and Radiative Lifetime of Nine-Coordinate Europium Complexes. Inorg. Chem. 2015, 54, 9166–9173. [Google Scholar] [CrossRef]
- Dexter, D.L. A Theory of Sensitized Luminescence in Solids. J. Chem. Phys. 1953, 21, 836–850. [Google Scholar] [CrossRef]
- Larionov, S.V.; Bryleva, Y.A.; Glinskaya, L.A.; Plyusnin, V.F.; Kupryakov, A.S.; Agafontsev, A.M.; Tkachev, A.V.; Bogomyakov, A.S.; Piryazev, D.A.; Korolkov, I.V. LnIII Complexes (Ln = Eu, Gd, Tb, Dy) with a Chiral Ligand Containing 1,10-Phenanthroline and (–)-Menthol Fragments: Synthesis, Structure, Magnetic Properties and Photoluminescence. Dalton. Trans. 2017, 46, 11440–11450. [Google Scholar] [CrossRef]
- Sarıoğlu, A.O.; Yalçın, Ş.P.; Ceylan, Ü.; Aygün, M.; Kırpık, H.; Sönmez, M. Photoluminescence Properties of Samarium(III)-Based Complexes: Synthesis, Characterization and Single Crystal X-Ray. J. Lumin. 2020, 227, 117537. [Google Scholar] [CrossRef]
- Conroy, H.; Firestone, R.A. The Intermediate Dienone in the Para-Claisen Rearrangement. J. Am. Chem. Soc. 1956, 78, 2290–2297. [Google Scholar] [CrossRef]
- Krapcho, A.P.; Lanza, J.B. IMPROVED SYNTHESIS OF 2-CHLORO- AND 2, 9-DICHLORO-1, 10-PHENANTHROLINES. Org. Prep. Proced. Int. 2007, 39, 603–608. [Google Scholar] [CrossRef]
- Rodrigues, F.S.; da Silva, V.A.M.; Segovia, G.B.Q.; Bortoluzzi, A.J.; Cuin, A.; de Souza, J.I.R.; da Silva, J.A.B.; Lourenço da Luz, L.; Alves Júnior, S.; Tonon de Almeida, E.; et al. Synthesis, Solid State Characterization, Theoretical and Experimental Spectroscopic Studies of the New Lanthanide Complexes. Spectrochim. Acta. A Mol. Biomol. Spectrosc. 2023, 296, 122677. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. SHELXT—Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. A 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. C. Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Hübschle, C.B.; Sheldrick, G.M.; Dittrich, B. ShelXle: A Qt Graphical User Interface for SHELXL. J. Appl. Crystallogr. 2011, 44, 1281–1284. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09W, Revision A.02; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Elenkova, D.; Lyapchev, R.; Romanova, J.; Morgenstern, B.; Dimitrova, Y.; Dimov, D.; Tsvetkov, M.; Zaharieva, J. Luminescent Complexes of Europium (III) with 2-(Phenylethynyl)-1,10-Phenanthroline: The Role of the Counterions. Molecules 2021, 26, 7272. [Google Scholar] [CrossRef]
- Krause, L.; Herbst-Irmer, R.; Sheldrick, G.M.; Stalke, D. Comparison of Silver and Molybdenum Microfocus X-Ray Sources for Single-Crystal Structure Determination. J. Appl. Crystallogr. 2015, 48, 3–10. [Google Scholar] [CrossRef]








| Ion | ΔET1 (Ligand) [eV] | ΔET1 (Complex) [eV] | ΔET1 (Complex–Ligand) [eV] |
|---|---|---|---|
| Eu3+ | 2.92 | 2.82 | −0.1 |
| Tb3+ | 2.92 | 2.95 | 0.03 |
| Sm3+ | 2.92 | 2.82 | −0.1 |
| Dy3+ | 2.92 | 3.16 | 0.24 |
| Complex | State | τ (ms) |
|---|---|---|
| Eu | Dichloromethane | 1.799 ± 0.0024 |
| Eu | Acetonitrile | 2.116 ± 0.0815 |
| Eu | Solid | 1.314 ± 0.012 |
| Tb | Dichloromethane | 1.118 ± 0.0124 |
| Tb | Acetonitrile | 0.512 ± 0.0004 |
| Tb | Solid | 0.598 ± 0.0003 |
| Sm | Acetonitrile | 0.105 ± 0.0004 |
| Sm | Dichloromethane | 0.111 ± 0.0001 |
| Sm | Solid | 0.080 ± 0.0003 |
| Dy | Dichloromethane | 0.009 ± 0.0003 |
| Dy | Acetonitrile | 0.010 ± 0.0009 |
| Dy | Solid | 0.011 ± 0.0001 |
| Metal Ion | Pm * (a.u.) | Ka | Ki | R2 | χ2 Reduced |
|---|---|---|---|---|---|
| Tb3+ | 265.76332 ± 7.18498 | 1.0625 ± 0.03055 | 5.40836 ± 0.13363 | 0.99493 | 10.32388 |
| Eu3+ | 62.56063 ± 2.96702 | 0.60234 ± 0.0373 | 4.98066 ± 0.19267 | 0.9804 | 45.25359 |
| Sm3+ | 39.52412 ± 1.52018 | 0.80803 ± 0.03943 | 5.31437 ± 0.18941 | 0.98402 | 25.32979 |
| Dy3+ | 99.98354 ± 13.72313 | 1.66923 ± 0.19037 | 5.09294 ± 0.45506 | 0.93235 | 136.9168 |
| Complex | Φ in Acetonitrile (%) | Φ in DCM (%) | τ in Acetonitrile (ms) | τ in DCM (ms) | τ in Solid (ms) |
|---|---|---|---|---|---|
| Eu | 42.05 ± 0.09 | 37.15 ± 0.09 | 2.12 ± 0.08 | 1.80 ± 0.002 | 1.31 ± 0.012 |
| Tb | 45.24 ± 0.09 | 68.15 ± 0.30 | 0.51 ± 0.0004 | 1.12 ± 0.012 | 0.60 ± 0.0003 |
| Sm | 2.41 ± 0.03 | 2.84 ± 0.05 | 0.105 ± 0.0004 | 0.111 ± 0.0001 | 0.080 ± 0.0003 |
| Dy | 0.32 ± 0.02 | 0.34 ± 0.03 | 0.00976 ± 0.0013 | 0.00943 ± 0.0005 | 0.00997± 0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaharieva, J.; Videva, V.; Kolarski, M.; Lyapchev, R.; Morgenstern, B.; Tsvetkov, M. A Para-Substituted 2-Phenoxy-1,10-Phenanthroline Ligand for Lanthanide Sensitization: Asymmetric Coordination and Enhanced Emission from Eu3+, Tb3+, Sm3+ and Dy3+ Complexes. Molecules 2025, 30, 3548. https://doi.org/10.3390/molecules30173548
Zaharieva J, Videva V, Kolarski M, Lyapchev R, Morgenstern B, Tsvetkov M. A Para-Substituted 2-Phenoxy-1,10-Phenanthroline Ligand for Lanthanide Sensitization: Asymmetric Coordination and Enhanced Emission from Eu3+, Tb3+, Sm3+ and Dy3+ Complexes. Molecules. 2025; 30(17):3548. https://doi.org/10.3390/molecules30173548
Chicago/Turabian StyleZaharieva, Joana, Vladimira Videva, Mihail Kolarski, Rumen Lyapchev, Bernd Morgenstern, and Martin Tsvetkov. 2025. "A Para-Substituted 2-Phenoxy-1,10-Phenanthroline Ligand for Lanthanide Sensitization: Asymmetric Coordination and Enhanced Emission from Eu3+, Tb3+, Sm3+ and Dy3+ Complexes" Molecules 30, no. 17: 3548. https://doi.org/10.3390/molecules30173548
APA StyleZaharieva, J., Videva, V., Kolarski, M., Lyapchev, R., Morgenstern, B., & Tsvetkov, M. (2025). A Para-Substituted 2-Phenoxy-1,10-Phenanthroline Ligand for Lanthanide Sensitization: Asymmetric Coordination and Enhanced Emission from Eu3+, Tb3+, Sm3+ and Dy3+ Complexes. Molecules, 30(17), 3548. https://doi.org/10.3390/molecules30173548










