Advances in Polyimide Membranes for Gas Separation: Synthesis, Modification, and Application
Abstract
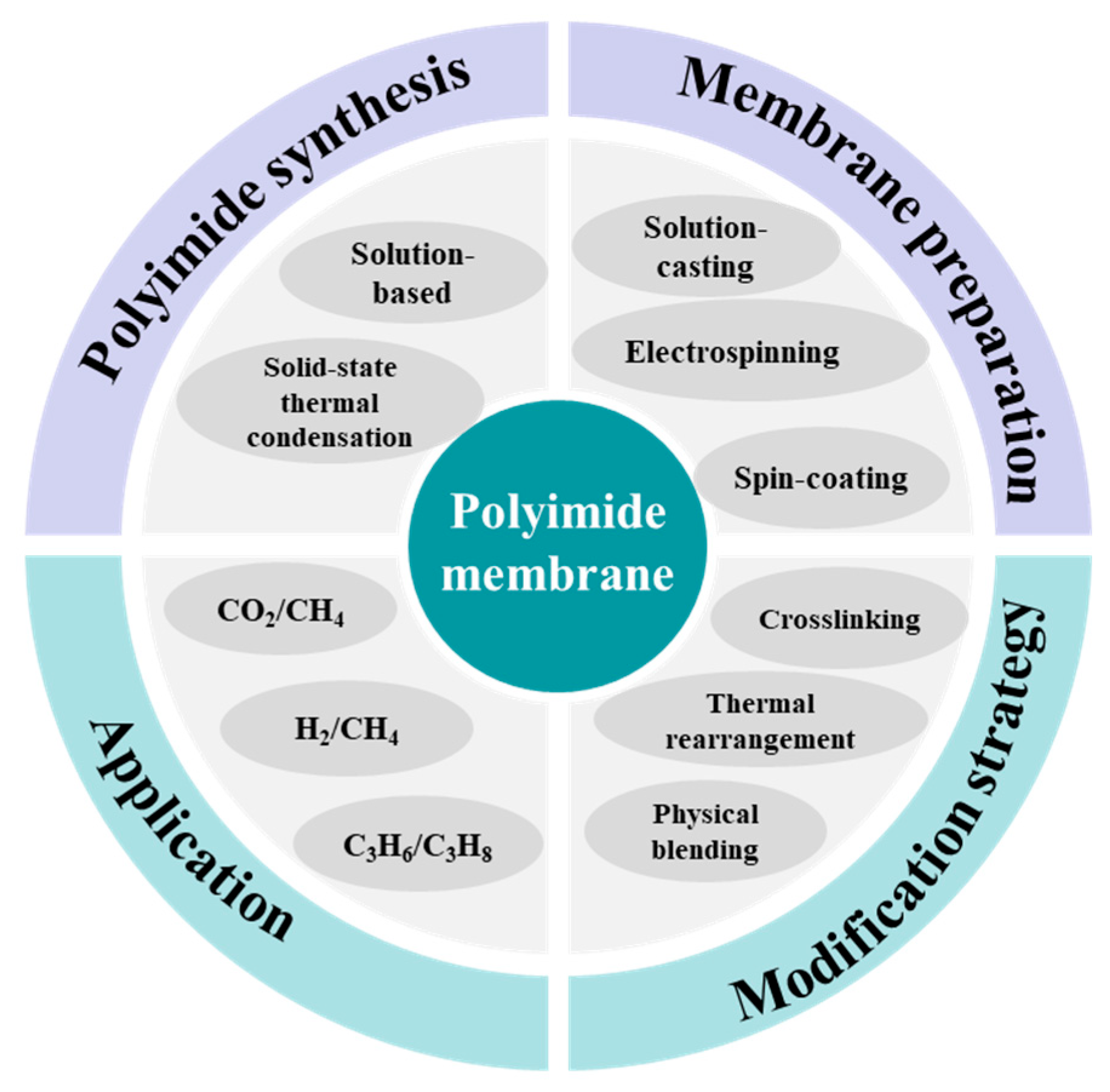
1. Introduction
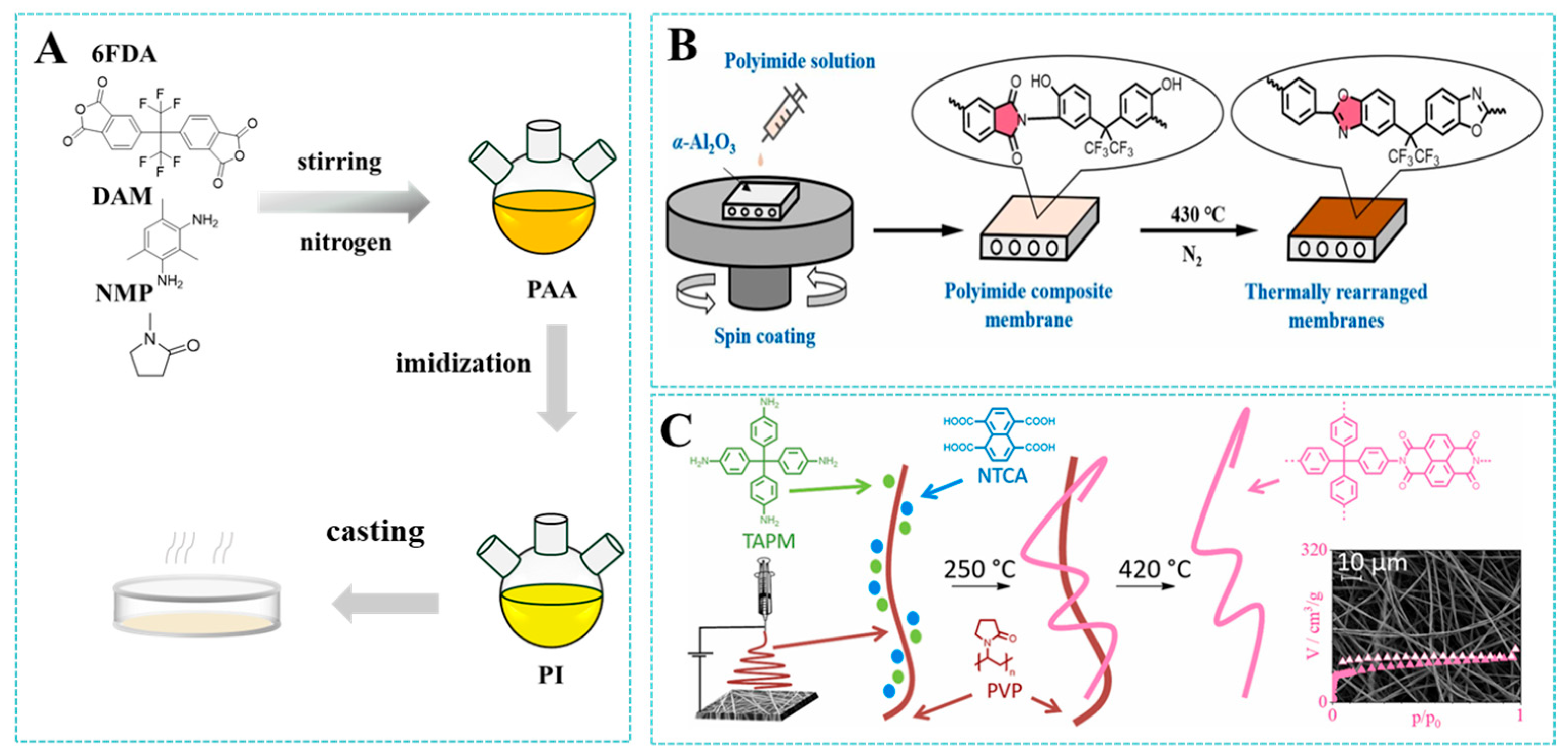
2. Synthesis of Polyimide
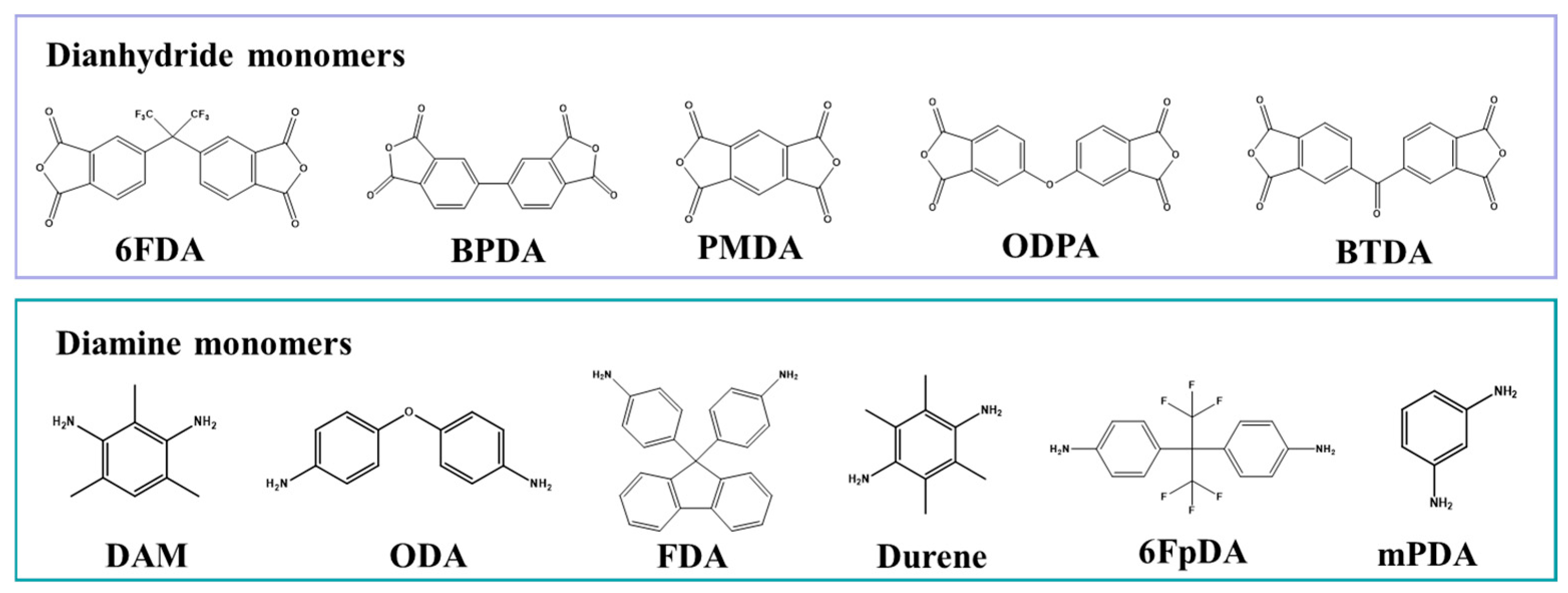
2.1. Monomer Structures
2.2. Polyimide Synthesis
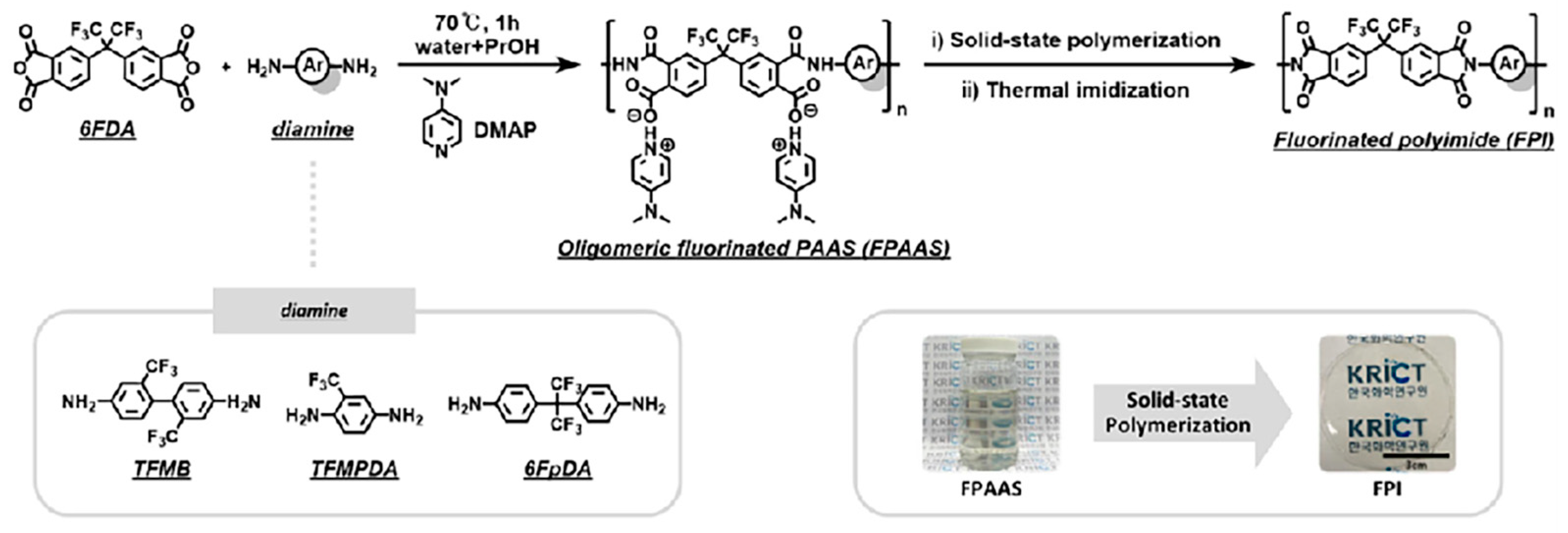
2.2.1. Solution-Based Method
2.2.2. Solid-State Thermal Condensation Method
3. Fabrication and Modification of Polyimide Membranes
3.1. Fabrication Strategies
3.2. Modification Strategies
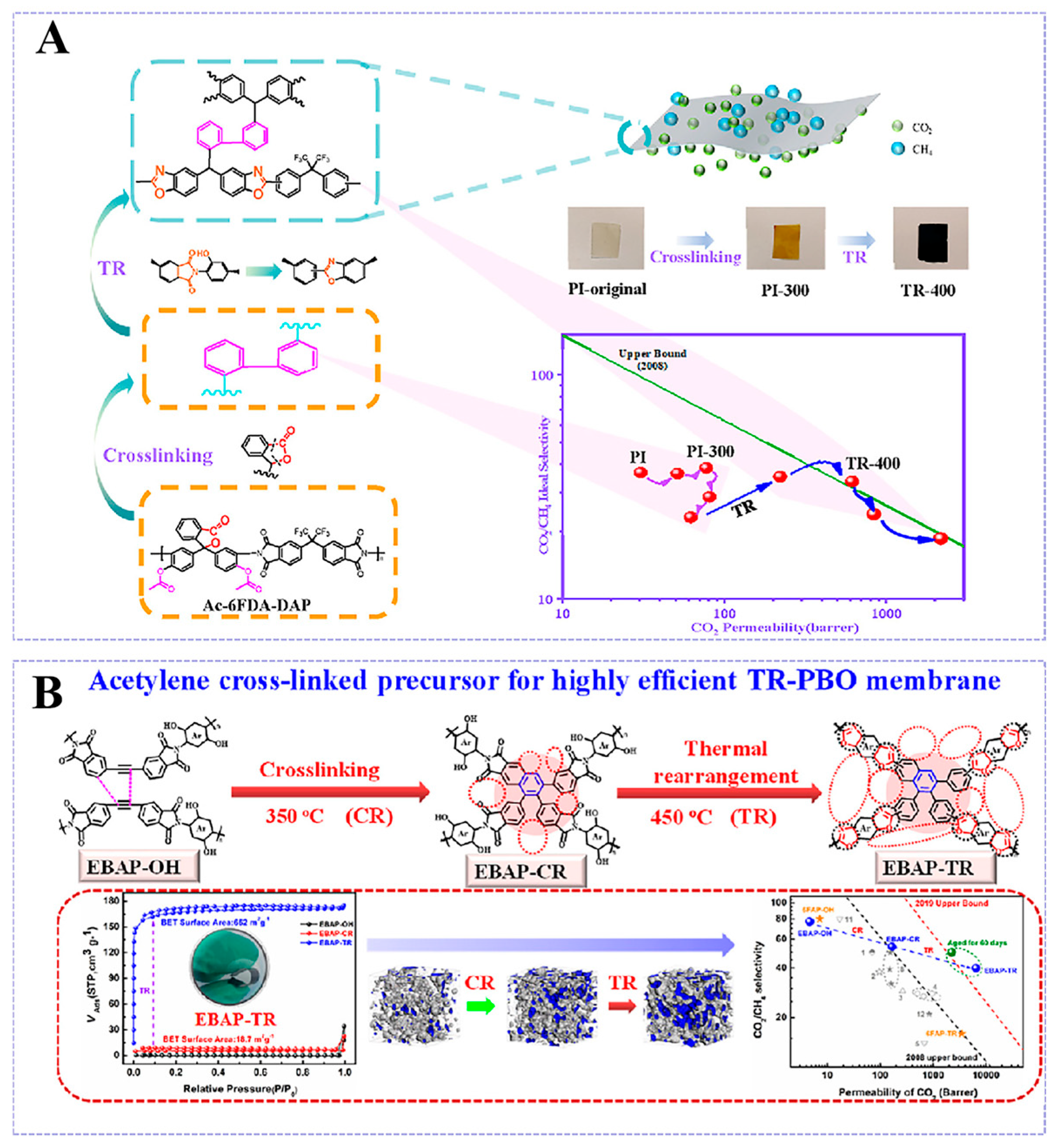
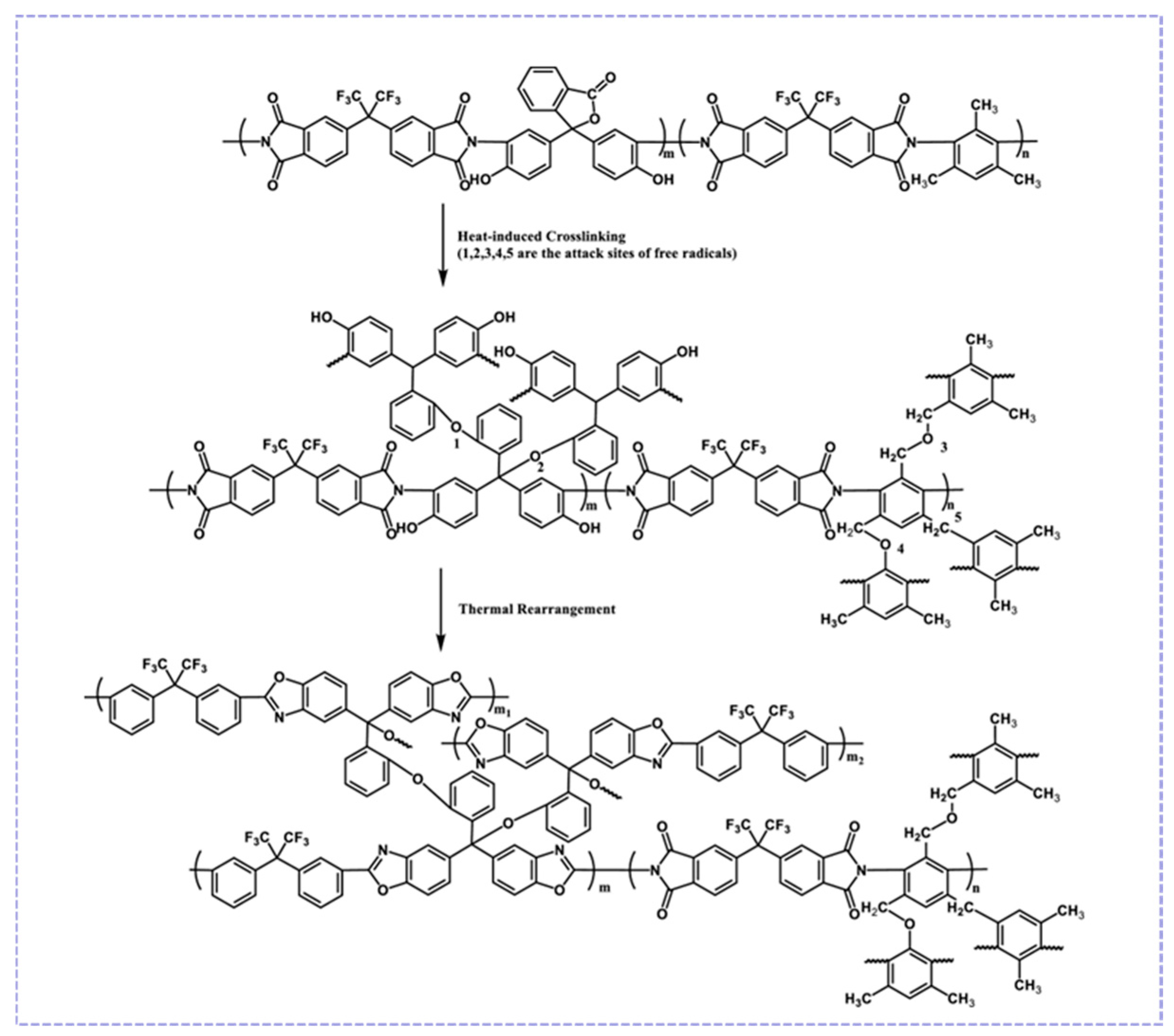
3.2.1. Thermal Rearrangement
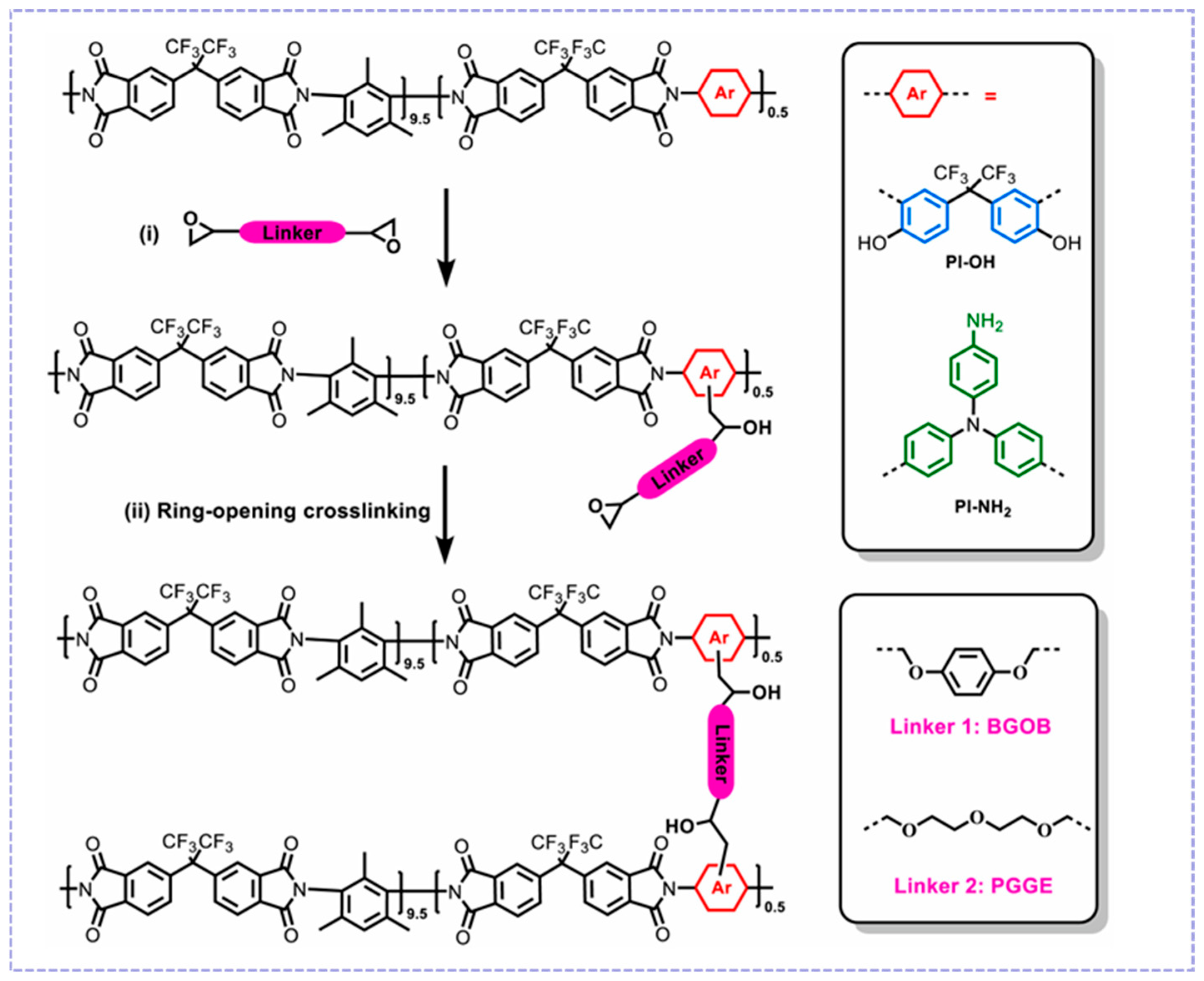
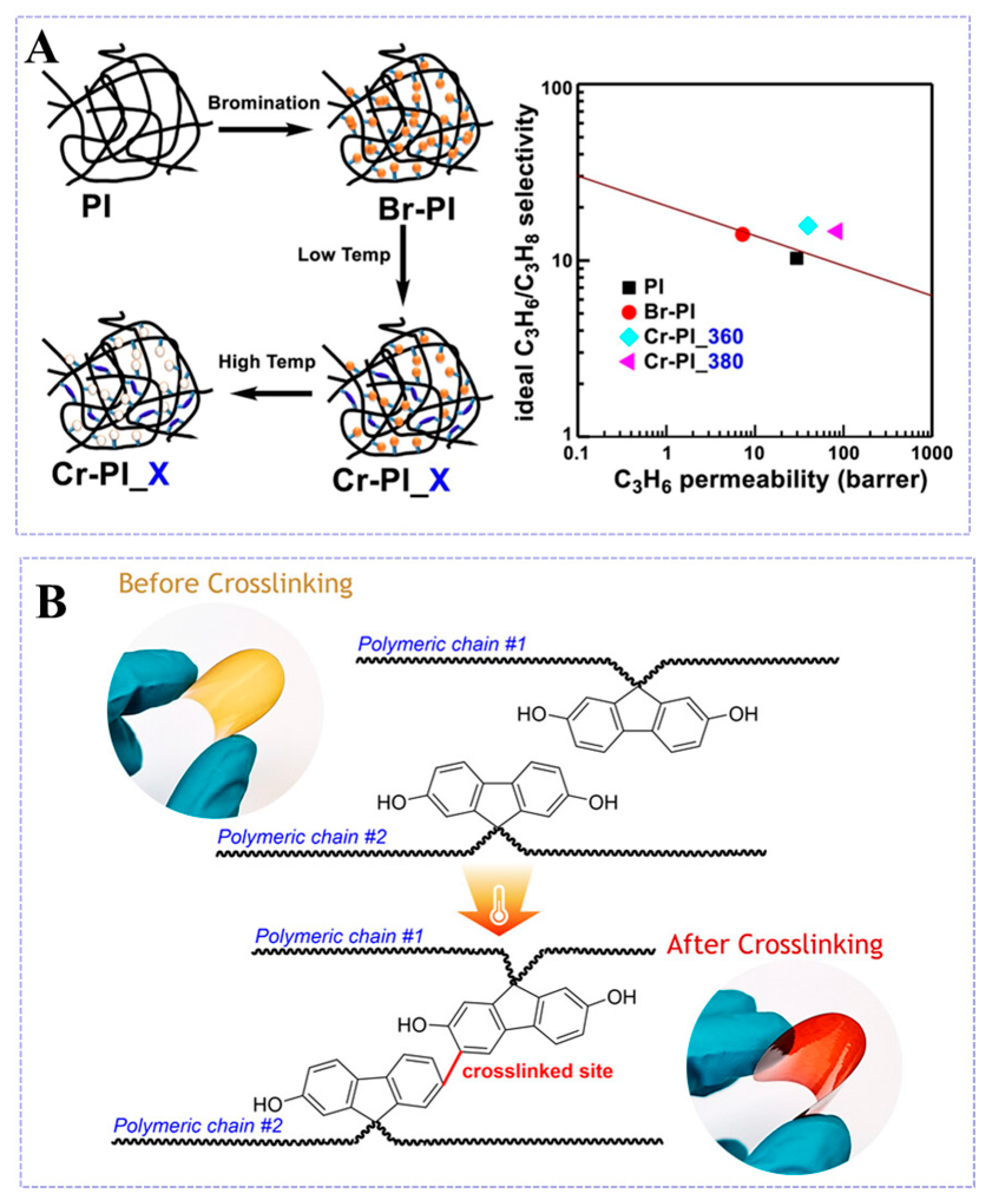
3.2.2. Cross-Linking Modification
3.2.3. Physical Blending
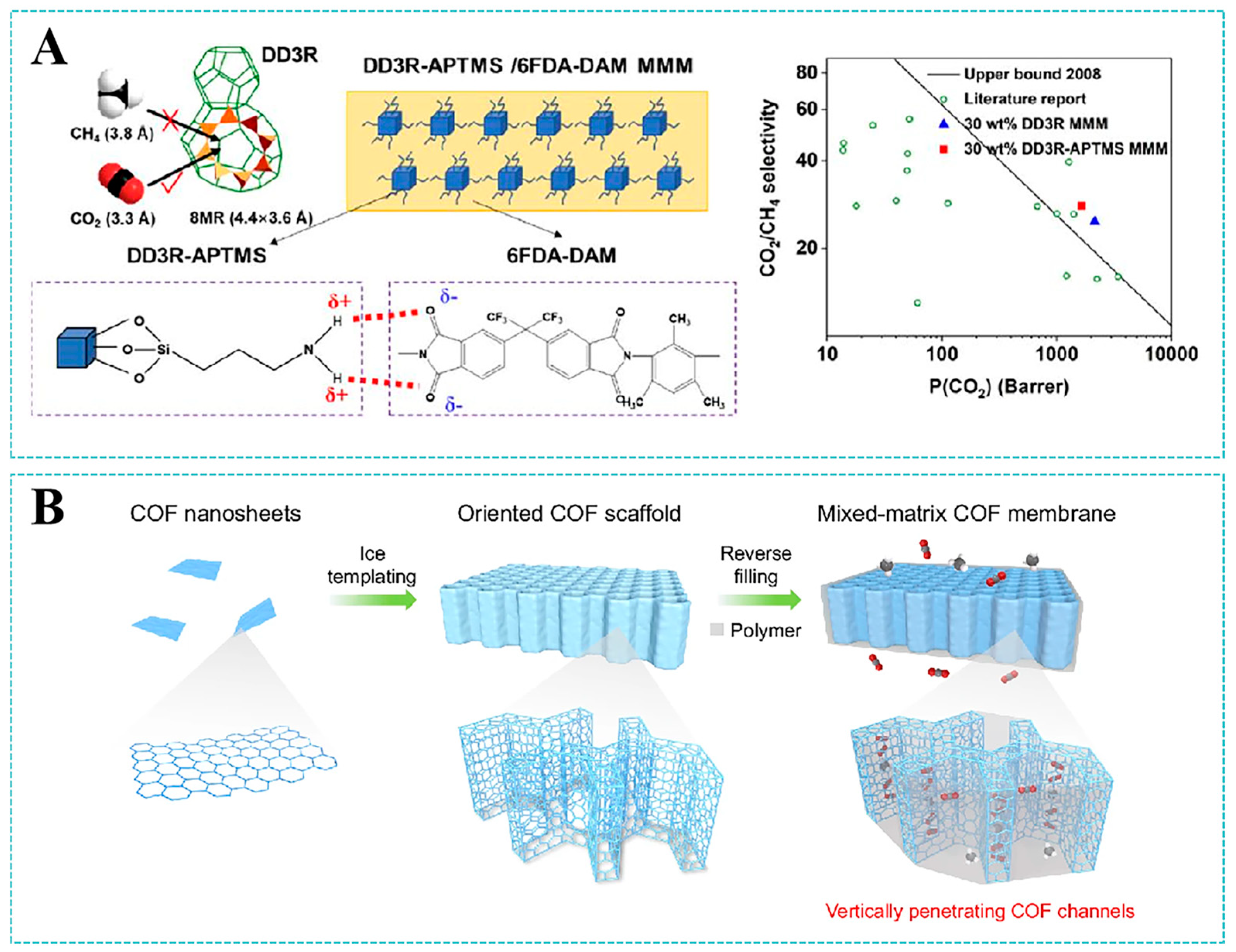
4. Applications in Gas Separation
4.1. CO2/CH4 Separation
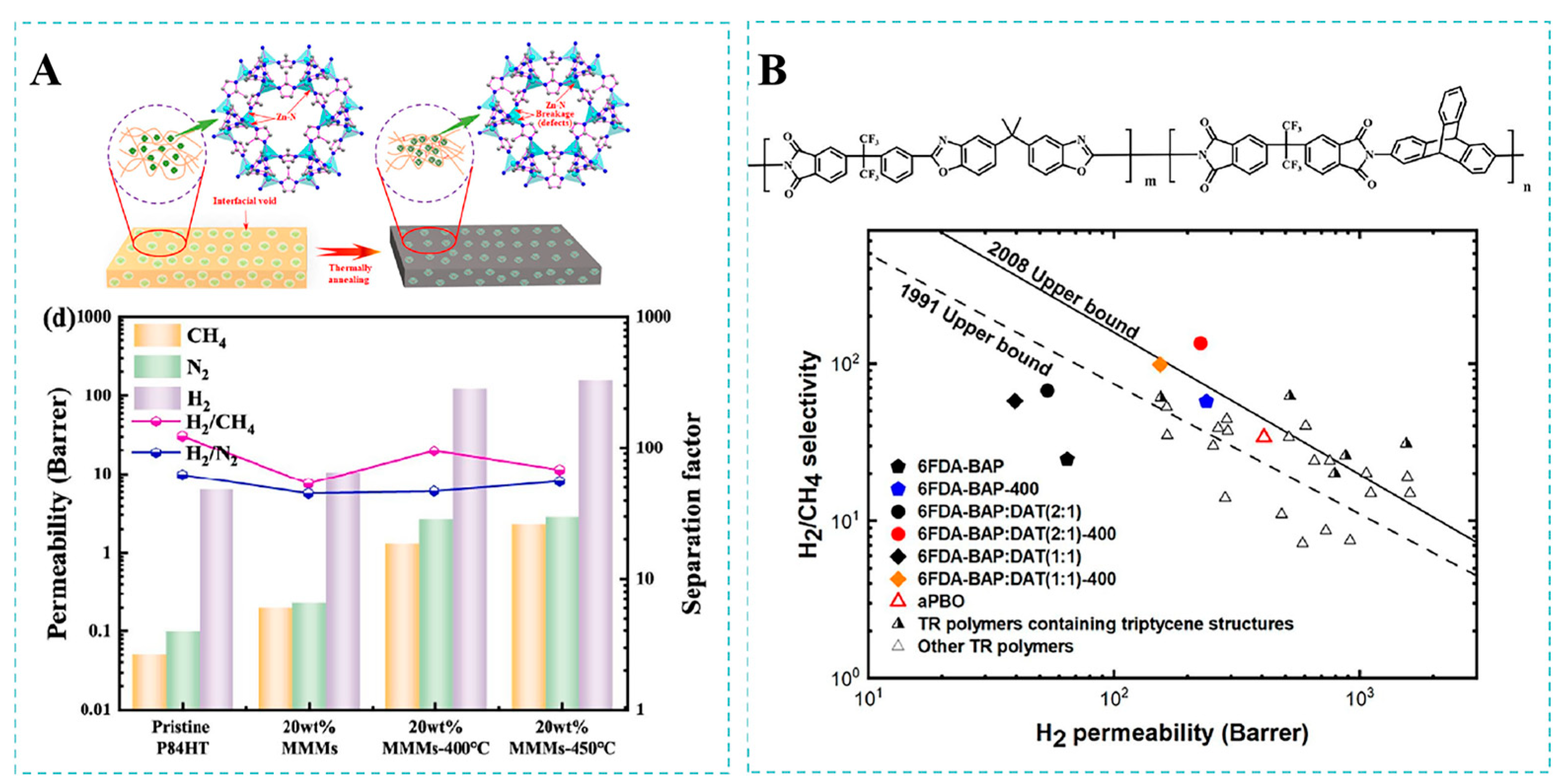
4.2. H2/CH4 Separation
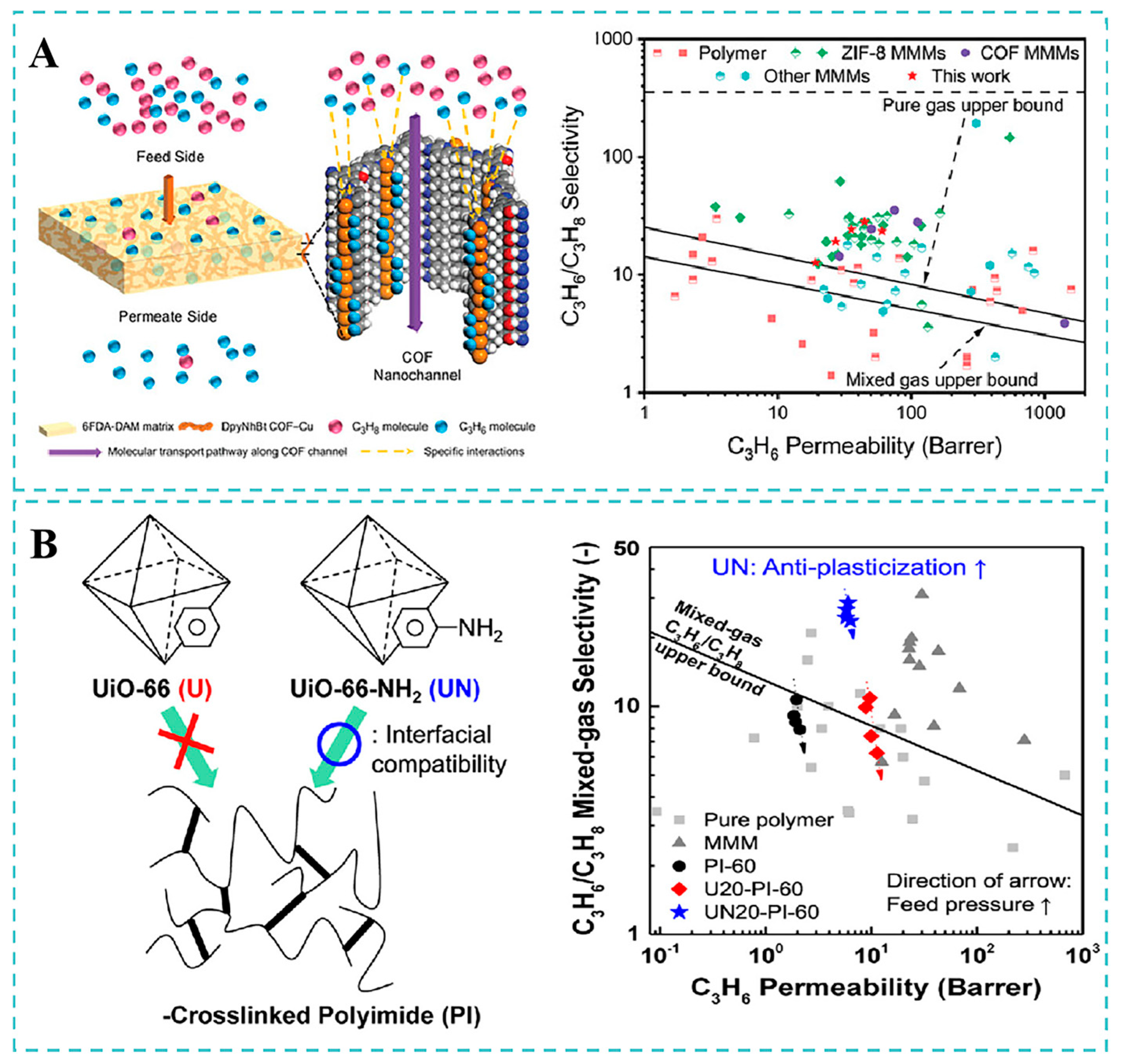
4.3. C3H6/C3H8 Separation
5. Conclusions and Outlook
- (1)
- PI membranes are prone to plasticization when exposed to high-pressure gases such as CO2, leading to diminished selectivity. A series of novel polyimides with good anti-plasticization properties have been synthesized by monomer structure design, thermal rearrangement, and cross-linking modification of polyimides.
- (2)
- As with most membrane materials, the persistent challenge for polyimide membranes is to balance selectivity and permeability. Achieving simultaneous enhancement in both properties remains a core goal in the development of high-performance gas separation membranes. Preparation of MMMs by incorporating nanofillers into the PI matrix can improve the separation performance of membranes. However, the compatibility between polymer matrices and inorganic fillers in MMMs remains a critical challenge. This issue can be addressed through the chemical structure modification of fillers or polymers to reduce or eliminate interfacial defects.
- (3)
- Designing high-performance PI membranes is still difficult, often relying on time-consuming empirical trial-and-error experiments. Machine learning accelerates the discovery of high-performance PI materials through the effective screening of extensive databases using active learning and multi-objective methods. This approach enhances the precision of property predictions, reduces reliance on experimental data, and provides guidance for the design of molecular structures.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PSf | Polysulfone |
| CA | Cellulose acetate |
| PI | Polyimide |
| Tg | Glass transition temperature |
| MMMs | Mixed matrix membranes |
| PMDA | Pyromellitic dianhydride |
| 6FDA | 4,4′-(hexafluoroisopropylidene)-diphthalic anhydride |
| ODPA | 3,3′,4,4′-oxydiphthalic anhydride |
| BPDA | 3,3′,4,4′-biphenyltetracarboxylic dianhydride |
| BTDA | 3,3′,4,4′-benzophenonetetracarboxylic dianhydride |
| DAM | 2,4,6-trimethyl-m-phenylenediamine |
| ODA | 4,4′-oxydianiline |
| FDA | 9,9-bis(4-aminophenyl) fluorene |
| Durene | 2,3,5,6-tetramethyl-1,4-phenylenediamine |
| PAA | Polyamide acid |
| DMF | N, N-dimethylformamide |
| NMP | 1-methyl-2-pyrrolidone |
| GVL | Gamma-Valerolactone |
| TR | Thermally rearranged |
| PBO | Polybenzoxazole |
| TB | Tröger’s Base |
| UV | Ultraviolet |
| FFV | Fractional free volume |
| FFDA | Fluorinated-cardo-based diamine |
| CTCs | Charge-transfer complexes |
| MOF | Metal–Organic Framework |
| COF | Covalent Organic Framework |
References
- Zhang, Y.; Sunarso, J.; Liu, S.M.; Wang, R. Current status and development of membranes for CO2/CH4 separation: A review. Int. J. Greenh. Gas Control 2013, 12, 84–107. [Google Scholar] [CrossRef]
- Ma, C.; Wang, M.; Wang, Z.; Gao, M.; Wang, J. Recent progress on thin film composite membranes for CO2 separation. J. CO2 Util. 2020, 42, 101296. [Google Scholar] [CrossRef]
- Dubey, A.; Arora, A. Advancements in carbon capture technologies: A review. J. Clean. Prod. 2022, 373, 133932. [Google Scholar] [CrossRef]
- Baker, R.W. Future Directions of Membrane Gas Separation Technology. Ind. Eng. Chem. Res. 2002, 41, 1393–1411. [Google Scholar] [CrossRef]
- Sanders, D.E.; Smith, Z.P.; Guo, R.L.; Robeson, L.M.; McGrath, J.E.; Paul, D.R.; Freeman, B.D. Energy-efficient polymeric gas separation membranes for a sustainable future: A review. Polymer 2013, 54, 4729–4761. [Google Scholar] [CrossRef]
- Dutta, R.C.; Bhatia, S.K. Interfacial engineering of MOF-based mixed matrix membrane through atomistic simulations. J. Phys. Chem. C 2020, 124, 594–604. [Google Scholar] [CrossRef]
- Kiadehi, A.D.; Rahimpour, A.; Jahanshahi, M.; Ghoreyshi, A.A. Novel carbon nano-fibers (CNF)/polysulfone (PSf) mixed matrix membranes for gas separation. J. Ind. Eng. Chem. 2015, 22, 199–207. [Google Scholar] [CrossRef]
- Waheed, N.; Mushtaq, A.; Tabassum, S.; Gilani, M.A.; Ilyas, A.; Ashraf, F.; Jamal, Y.; Bilad, M.R.; Khan, A.U.; Khan, A.L. Mixed matrix membranes based on polysulfone and rice husk extracted silica for CO2 separation. Sep. Purif. Technol. 2016, 170, 122–129. [Google Scholar] [CrossRef]
- Vatanpour, V.; Pasaoglu, M.E.; Barzegar, H.; Teber, O.O.; Kaya, R.; Bastug, M.; Khataee, A.; Koyuncu, I. Cellulose acetate in fabrication of polymeric membranes: A review. Chemosphere 2022, 295, 133914. [Google Scholar] [CrossRef]
- Sanaeepur, H.; Amooghin, A.E.; Bandehali, S.; Moghadassi, A.; Matsuura, T.; Van der Bruggen, B. Polyimides in membrane gas separation: Monomer’s molecular design and structural engineering. Prog. Polym. Sci. 2019, 91, 80–125. [Google Scholar] [CrossRef]
- Hauenstein, O.; Rahman, M.M.; Elsayed, M.; Krause-Rehberg, R.; Agarwal, S.; Abetz, V.; Greiner, A. Biobased polycarbonate as a gas separation membrane and “breathing glass” for energy saving applications. Adv. Mater. Technol. 2017, 2, 1700026. [Google Scholar] [CrossRef]
- Xing, L.X.; Qin, J.L.; Wang, J.M.; Wang, K.D.; Wang, H.L.; Yang, Z.D.; He, G.H.; Ruan, X.H. Polyimide membrane materials with multiple trifluoromethyl groups for helium enrichment from natural gases. Ind. Eng. Chem. Res. 2023, 62, 16081–16092. [Google Scholar] [CrossRef]
- Suleman, M.S.; Lau, K.K.; Yeong, Y.F. Plasticization and swelling in polymeric membranes in CO2 removal from natural gas. Chem. Eng. Technol. 2016, 39, 1604–1616. [Google Scholar] [CrossRef]
- Lu, Y.; Hu, X.; Lee, W.H.; Bae, J.Y.; Zhao, J.; Nie, W.; Wang, Z.; Yan, J.; Lee, Y.M. Effects of bulky 2,2′-substituents in dianhydrides on the microstructures and gas transport properties of thermally rearranged polybenzoxazoles. J. Membr. Sci. 2021, 639, 119777. [Google Scholar] [CrossRef]
- Wang, T.J.; Yao, H.Y.; Song, N.N.; Yang, Y.C.; Shi, K.X.; Guan, S.W. Microporous polymer networks constructed from cross-linkable linear polyimides for CO2 adsorption. Microporous Mesoporous Mater. 2021, 311, 110708. [Google Scholar] [CrossRef]
- Song, S.Q.; Jiang, H.F.; Wu, H.; Zhao, M.G.; Guo, Z.Y.; Li, B.Y.; Ren, Y.X.; Wang, Y.H.; Ye, C.M.; Guiver, M.D.; et al. Weakly pressure-dependent molecular sieving of propylene/propane mixtures through mixed matrix membrane with ZIF-8 direct-through channels. J. Membr. Sci. 2022, 648, 120366. [Google Scholar] [CrossRef]
- Bronnikov, S.V.; Sukhanova, T.E.; Goikhman, M.Y. Evolution of statistical ensembles of microdomains on the surface of films of rigid-chain polyimide during thermal imidization. Russ. J. Appl. Chem. 2003, 76, 967–971. [Google Scholar] [CrossRef]
- Zhu, Y.Q.; Zhao, P.Q.; Cai, X.D.; Meng, W.D.; Qing, F.L. Synthesis and characterization of novel fluorinated polyimides derived from bis 4-(4′-aminophenoxy)phenyl -3,5-bis(trifluoromethyl)phenyl phosphine oxide. Polymer 2007, 48, 3116–3124. [Google Scholar] [CrossRef]
- Matsumoto, T. Alicyclic polyimides: An approach from monomer synthesis. J. Synth. Org. Chem. Jpn. 2000, 58, 776–786. [Google Scholar] [CrossRef]
- Zhang, L.M.; Mu, J.X.; Jiang, Z.H.; Zhang, H.B.; Yue, X.G. Fully aromatic macrocycle-terminated polyimide: Synthesis and cross-linking. Polym. Adv. Technol. 2013, 24, 415–420. [Google Scholar] [CrossRef]
- Imai, Y. Recent advances in synthesis of high-temperature aromatic polymers. React. Funct. Polym. 1996, 30, 3–15. [Google Scholar] [CrossRef]
- Cosutchi, A.I.; Nica, S.L.; Hulubei, C.; Homocianu, M.; Ioan, S. Effects of the aliphatic/aromatic structure on the miscibility, thermal, optical, and rheological properties of some polyimide blends. Polym. Eng. Sci. 2012, 52, 1429–1439. [Google Scholar] [CrossRef]
- Dujardin, W.; Van Goethem, C.; Zhang, Z.; Verbeke, R.; Dickmann, M.; Egger, W.; Nies, E.; Vankelecom, I.; Koeckelberghs, G. Fine-tuning the molecular structure of binaphthalene polyimides for gas separations. Eur. Polym. J. 2019, 114, 134–143. [Google Scholar] [CrossRef]
- Saeed, M.B.; Zhan, M.-S. Effects of monomer structure and imidization degree on mechanical properties and viscoelastic behavior of thermoplastic polyimide films. Eur. Polym. J. 2006, 42, 1844–1854. [Google Scholar] [CrossRef]
- Chen, G.N.; Li, D.Q.; Chen, L.Y.; Lin, Z.Y.; Li, W.Y.; Zhao, B.Q.; Zhao, Z.W.; Liu, J.D.; Sun, Y.R.; Pang, J.H.; et al. Innovative thermal crosslinked polyimide gas separation membrane with highly selective and resistance to physical aging base on phenyl ethynyl. Chem. Eng. J. 2024, 500, 156642. [Google Scholar] [CrossRef]
- Lee, J.; Park, H.G.; Hyeon, M.H.; Kim, B.G.; Kim, S.K.; Moon, S.Y. Low-temperature CO2 hydrogenation overcoming equilibrium limitations with polyimide hollow fiber membrane reactor. Chem. Eng. J. 2021, 403, 126457. [Google Scholar] [CrossRef]
- Hu, C.P.; Polintan, C.K.; Tayo, L.L.; Chou, S.C.; Tsai, H.A.; Hung, W.S.; Hu, C.C.; Lee, K.R.; Lai, J.Y. The gas separation performance adjustment of carbon molecular sieve membrane depending on the chain rigidity and free volume characteristic of the polymeric precursor. Carbon 2019, 143, 343–351. [Google Scholar] [CrossRef]
- Lee, C.; Shul, Y.; Han, H. Dielectric properties of oxydianiline-based polyimide thin films according to the water uptake. J. Polym. Sci. Part B Polym. Phys. 2002, 40, 2190–2198. [Google Scholar] [CrossRef]
- Dingemans, T.J.; Mendes, E.; Hinkley, J.J.; Weiser, E.S.; StClair, T.L. Poly(ether imide)s from diamines with para-, meta-, and ortho-arylene substitutions: Synthesis, characterization, and liquid crystalline properties. Macromolecules 2008, 41, 2474–2483. [Google Scholar] [CrossRef]
- Seo, J.; Jeon, J.; Shul, Y.G.; Han, H. Water sorption and activation energy in polyimide thin films. J. Polym. Sci. Part B-Polym. Phys. 2000, 38, 2714–2720. [Google Scholar] [CrossRef]
- Qiu, W.L.; Xu, L.R.; Chen, C.C.; Paul, D.R.; Koros, W.J. Gas separation performance of 6FDA-based polyimides with different chemical structures. Polymer 2013, 54, 6226–6235. [Google Scholar] [CrossRef]
- Wang, M.; Jiang, J.W. Accelerating discovery of polyimides with intrinsic microporosity for membrane-based gas separation: Synergizing physics-informed performance metrics and active learning. Adv. Funct. Mater. 2024, 34, 2314683. [Google Scholar] [CrossRef]
- Staudt-Bickel, C.; Koros, W.J. Olefin/paraffin gas separations with 6FDA-based polyimide membranes. J. Membr. Sci. 2000, 170, 205–214. [Google Scholar] [CrossRef]
- Liu, Z.Y.; Liu, Y.; Qiu, W.L.; Koros, W.J. Molecularly engineered 6FDA-based polyimide membranes for sour natural gas separation. Angew. Chem.-Int. Ed. 2020, 59, 14877–14883. [Google Scholar] [CrossRef]
- Jia, Y.; Yang, J.H.; Liu, P.X.; Chen, K.Y.; Li, J.C.; Pi, X.J.; Han, C.G.; Zhang, Y. Mixed matrix membranes containing oriented two-dimensional ZIF-L nanosheets for efficient H2/CO2 separation. Sep. Purif. Technol. 2024, 338, 126589. [Google Scholar] [CrossRef]
- Zhang, C.L. Synthesis and characterization of bis(phenyl)fluorene-based cardo polyimide membranes for H2/CH4 separation. J. Mater. Sci. 2019, 54, 10560–10569. [Google Scholar] [CrossRef]
- Soh, L.S.; Hong, S.U.; Liang, C.Z.; Yong, W.F. Green solvent-synthesized polyimide membranes for gas separation: Coupling Hansen solubility parameters and synthesis optimization. Chem. Eng. J. 2023, 478, 147451. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, Y.C.; Hao, X.Q.; Ma, C.H.; Wang, Y.; Zou, Z.H. Controllable conformation transfer of conjugated polymer toward high photoelectrical performance: The role of solvent in induced-crystallization route. J. Phys. Chem. C 2018, 122, 1037–1043. [Google Scholar] [CrossRef]
- Baumgartner, B.; Svirkova, A.; Bintinger, J.; Hametner, C.; Marchetti-Deschmann, M.; Unterlass, M.M. Green and highly efficient synthesis of perylene and naphthalene bisimides in nothing but water. Chem. Commun. 2017, 53, 1229–1232. [Google Scholar] [CrossRef]
- Baumgartner, B.; Bojdys, M.J.; Unterlass, M.M. Geomimetics for green polymer synthesis: Highly ordered polyimides via hydrothermal techniques. Polym. Chem. 2014, 5, 3771–3776. [Google Scholar] [CrossRef]
- Park, S.; So, Y.J.; Kim, K.W.; Park, J.; Kim, H.; Kim, L.K.; Kim, J.; Jung, H.T.; Kim, D.W.; Won, J.C.; et al. A novel approach for environmentally benign synthesis and solid-state polymerization of fluorinated polyimides in aqueous co-solvent. Chem. Eng. J. 2024, 495, 153288. [Google Scholar] [CrossRef]
- Zhou, L.R.; Li, Y.Z.; Wang, Z.Q.; Zhang, M.Y.; Wang, X.D.; Niu, H.Q.; Wu, D.Z. Preparation of polyimide films via microwave-assisted thermal imidization. RSC Adv. 2019, 9, 7314–7320. [Google Scholar] [CrossRef] [PubMed]
- Bäte, M.; Neuber, C.; Giesa, R.; Schmidt, H.W. Combinatorial investigation of the vapor deposition polymerization of aromatic polyimides. Macromol. Rapid Commun. 2004, 25, 371–376. [Google Scholar] [CrossRef]
- Liu, T.X.; Zhang, R.L.; Huang, G.C.; Xie, Y.B.; Xie, L.H.; Li, J.R. Mixed matrix membranes based on soluble perfluorinated metal-organic cage and polyimide for CO2/CH4 separation. Sep. Purif. Technol. 2023, 318, 124006. [Google Scholar] [CrossRef]
- Cheng, Y.D.; Wang, X.R.; Jia, C.K.; Wang, Y.X.; Zhai, L.Z.; Wang, Q.; Zhao, D. Ultrathin mixed matrix membranes containing two-dimensional metal-organic framework nanosheets for efficient CO2/CH4 separation. J. Membr. Sci. 2017, 539, 213–223. [Google Scholar] [CrossRef]
- Chen, C.; Xiong, L.L.; Cui, Y.H.; Wang, C.S. Preparation, air filtration performance of a fluorinated polyimide/polyacrylonitrile nanofibrous membrane by electrospinning. Polymers 2024, 16, 1240. [Google Scholar] [CrossRef]
- Kanehashi, S.; Nakagawa, T.; Nagai, K.; Duthie, X.; Kentish, S.; Stevens, G. Effects of carbon dioxide-induced plasticization on the gas transport properties of glassy polyimide membranes. J. Membr. Sci. 2007, 298, 147–155. [Google Scholar] [CrossRef]
- Xie, C.; Yang, Z.; Ma, W.J.; Chen, X.L.; Zhao, L.; Liu, G.P.; Li, N.W. Facile fabrication of 6FDA-DAM/PGMA blend membranes for advanced gas separation. Sep. Purif. Technol. 2025, 360, 130895. [Google Scholar] [CrossRef]
- Rasool, M.A.; Vankelecom, I.F.J. γ-Valerolactone as Bio-Based Solvent for Nanofiltration Membrane Preparation. Membranes 2021, 11, 418. [Google Scholar] [CrossRef]
- Burmann, P.; Zornoza, B.; Téllez, C.; Coronas, J. Mixed matrix membranes comprising MOFs and porous silicate fillers prepared via spin coating for gas separation. Chem. Eng. Sci. 2014, 107, 66–75. [Google Scholar] [CrossRef]
- Zhou, X.T.; Tian, H.J.; Ling, H.L.; Yang, Y.L.; Luo, J.Z.; Zong, X.P.; Xue, S. Thermally rearranged OH-containing polyimide composite membranes with enhanced gas separation performance and physical aging resistance. J. Environ. Chem. Eng. 2024, 12, 112275. [Google Scholar] [CrossRef]
- Pei, F.Y.; Yin, B.H.; Jia, H.G.; Liu, L.J.; Shan, Y.T.; Wang, H.J. Multifunctional carboxylated polyimide fiber membrane for gravity-driven efficient simultaneous separation of oil-in-water emulsions and methylene blue dye. Sep. Purif. Technol. 2025, 359, 130760. [Google Scholar] [CrossRef]
- Breunig, M.; Zhu, J.; Ding, C.; Siegel, R.; Agarwal, S.; Senker, J. Electrospun, non-woven fiber membranes of porous polyimides with high carbon dioxide uptakes and selectivities. Microporous Mesoporous Mater. 2022, 329, 111519. [Google Scholar] [CrossRef]
- Huo, G.L.; Guo, Z.Y.; Zhang, Z.G.; Zhou, X.W.; Xin, J.H.; Zhang, Y.C.; Kang, S.Y.; Yang, Y.Q.; Li, N.W. Effect of uniaxial stretching on gas separation performance of polyimide membranes. J. Membr. Sci. 2023, 687, 122046. [Google Scholar] [CrossRef]
- Darban, M.A.; Lock, S.S.M.; Ilyas, S.U.; Waqas, S.; Lim, L.G.; Lock, I.S.M.; Kang, D.Y.; Othman, M.H.D.; Yiin, C.L.; Hira, N.E.; et al. A critical review on advancements and challenges in CO2 gas separation via 6FDA-based membranes. Carbon Capture Sci. Technol. 2025, 15, 35. [Google Scholar] [CrossRef]
- Shi, Y.S.; Liang, J.C.; Shrestha, B.B.; Wang, Z.G.; Zhang, Y.T.; Jin, J. Enhancing the CO2 plasticization resistance of thin polymeric membranes by designing Metal-polymer complexes. Sep. Purif. Technol. 2022, 289, 120699. [Google Scholar] [CrossRef]
- Bernardo, G.; Araújo, T.; da Silva Lopes, T.; Sousa, J.; Mendes, A. Recent advances in membrane technologies for hydrogen purification. Int. J. Hydrogen Energy 2020, 45, 7313–7338. [Google Scholar] [CrossRef]
- Ogieglo, W.; Puspasari, T.; Alabdulaaly, A.; Nguyen, T.P.N.; Lai, Z.P.; Pinnau, I. Gas separation performance and physical aging of tubular thin-film composite carbon molecular sieve membranes based on a polyimide of intrinsic microporosity precursor. J. Membr. Sci. 2022, 652, 120497. [Google Scholar] [CrossRef]
- Scholes, C.A.; Ribeiro, C.P.; Kentish, S.E.; Freeman, B.D. Thermal rearranged poly(benzoxazole-co-imide) membranes for CO2 separation. J. Membr. Sci. 2014, 450, 72–80. [Google Scholar] [CrossRef]
- Park, H.B.; Jung, C.H.; Lee, Y.M.; Hill, A.J.; Pas, S.J.; Mudie, S.T.; Van Wagner, E.; Freeman, B.D.; Cookson, D.J. Polymers with cavities tuned for fast selective transport of small molecules and ions. Science 2007, 318, 254–258. [Google Scholar] [CrossRef]
- Moon, J.D.; Bridge, A.T.; D’Ambra, C.; Freeman, B.D.; Paul, D.R. Gas separation properties of polybenzimidazole/thermally-rearranged polymer blends. J. Membr. Sci. 2019, 582, 182–193. [Google Scholar] [CrossRef]
- Cai, M.W.; Chen, J.C.; Wang, H.X.; Wu, J.H.; Zhang, S.Y.; Min, Y.G. Polyimide-based thermal rearranged (TR) membrane for highly efficient natural gas separation: A review. Sep. Purif. Technol. 2025, 355, 129624. [Google Scholar] [CrossRef]
- Park, H.B.; Han, S.H.; Jung, C.H.; Lee, Y.M.; Hill, A.J. Thermally rearranged (TR) polymer membranes for CO2 separation. J. Membr. Sci. 2010, 359, 11–24. [Google Scholar] [CrossRef]
- Du, P.Y.; Wang, Z.Y.; Zhang, T.; Lau, C.H.; Liu, S.M.; Li, P. Crosslinked thermally rearranged polybenzoxazole derived from phenolphthalein-based polyimide for gas separation. J. Membr. Sci. 2022, 662, 120934. [Google Scholar] [CrossRef]
- Zhang, S.Y.; Xu, Z.L.; Weng, Y.T.; Cai, M.W.; Wang, Y.L.; Zhu, W.J.; Min, Y.G.; Ma, X.H. Remarkable gas separation performance of a thermally rearranged membrane derived from an alkynyl self-crosslinkable precursor. J. Membr. Sci. 2023, 672, 121464. [Google Scholar] [CrossRef]
- Hu, X.F.; Lee, W.H.; Bae, J.Y.; Kim, J.S.; Jung, J.T.; Wang, H.H.; Park, H.J.; Lee, Y.M. Thermally rearranged polybenzoxazole copolymers incorporating Troger’s base for high flux gas separation membranes. J. Membr. Sci. 2020, 612, 118437. [Google Scholar] [CrossRef]
- Wang, F.W.; Zhao, G.K.; Liu, Y.Q.; Tang, G.Q.; Qin, P.Y.; Li, P. Development of phenolphthalein-based copolyimides and their derivative cross-linked and thermally rearranged polymers for gas separation. Macromolecules 2024, 57, 1370–1382. [Google Scholar] [CrossRef]
- Iwasa, R.; Suizu, T.; Yamaji, H.; Yoshioka, T.; Nagai, K. Gas separation in polyimide membranes with molecular sieve-like chemical/physical dual crosslink elements onto the top of surface. J. Membr. Sci. 2018, 550, 80–90. [Google Scholar] [CrossRef]
- Yerzhankyzy, A.; Wang, Y.; Ghanem, B.S.; Puspasari, T.; Pinnau, I. Gas separation performance of solid-state in-situ thermally crosslinked 6FDA-based polyimides. J. Membr. Sci. 2022, 641, 119885. [Google Scholar] [CrossRef]
- Staudt-Bickel, C.; Koros, W.J. Improvement of CO2/CH4 separation characteristics of polyimides by chemical crosslinking. J. Membr. Sci. 1999, 155, 145–154. [Google Scholar] [CrossRef]
- Kita, H.; Inada, T.; Tanaka, K.; Okamoto, K.-I. Effect of photocrosslinking on permeability and permselectivity of gases through benzophenone- containing polyimide. J. Membr. Sci. 1994, 87, 139–147. [Google Scholar] [CrossRef]
- Wu, S.S.; Liang, J.C.; Shi, Y.P.; Huang, M.H.; Bi, X.Y.; Wang, Z.G.; Jin, J. Design of interchain hydrogen bond in polyimide membrane for improved gas selectivity and membrane stability. J. Membr. Sci. 2021, 618, 118659. [Google Scholar] [CrossRef]
- Wind, J.D.; Staudt-Bickel, C.; Paul, D.R.; Koros, W.J. The effects of crosslinking chemistry on CO2 plasticization of polyimide gas separation membranes. Ind. Eng. Chem. Res. 2002, 41, 6139–6148. [Google Scholar] [CrossRef]
- Vanherck, K.; Koeckelberghs, G.; Vankelecom, I.F.J. Crosslinking polyimides for membrane applications: A review. Prog. Polym. Sci. 2013, 38, 874–896. [Google Scholar] [CrossRef]
- Esteban, N.; Juan-y-Seva, M.; Aguilar-Lugo, C.; Miguel, J.A.; Staudt, C.; de la Campa, J.G.; Alvarez, C.; Lozano, A.E. Aromatic polyimide membranes with tert-butyl and carboxylic side groups for gas separation applications-covalent crosslinking study. Polymers 2022, 14, 5517. [Google Scholar] [CrossRef]
- Yang, K.; Ling, H.L.; Jiang, H.; Luo, J.Z.; Zong, X.P.; Xue, S. Enhanced gas separation performance of polyimide membranes through nucleophilic ring-opening crosslinking with diepoxides. J. Membr. Sci. 2025, 716, 123534. [Google Scholar] [CrossRef]
- Wang, C.; Cai, Z.L.; Xie, W.; Jiao, Y.; Liu, L.; Gong, L.L.; Zhang, Q.W.; Ma, X.H.; Zhang, H.J.; Luo, S.J. Finely tuning the microporosity in dual thermally crosslinked polyimide membranes for plasticization resistance gas separations. J. Membr. Sci. 2022, 659, 120769. [Google Scholar] [CrossRef]
- Do, X.H.; Nguyen, Q.T.; Kim, S.; Lee, A.S.; Baek, K.Y. Effect of thermal processing on brominated 6FDA-DAM for effective propylene/propane separation. Sep. Purif. Technol. 2021, 262, 118331. [Google Scholar] [CrossRef]
- Hayek, A.; Alsamah, A.; Saleem, Q.; Alhajry, R.H.; Alsuwailem, A.A. Enhanced High-Pressure Mixed-Gas Sieving Properties of Thermally Cross-Linked Polyimide Membranes. ACS Appl. Polym. Mater. 2024, 6, 6108–6120. [Google Scholar] [CrossRef]
- Chen, G.N.; Wang, T.L.; Zhang, G.R.; Liu, G.P.; Jin, W.Q. Membrane materials targeting carbon capture and utilization. Adv. Membr. 2022, 2, 100025. [Google Scholar] [CrossRef]
- Sunder, N.; Fong, Y.Y.; Bustam, M.A.; Suhaimi, N.H. Development of Amine-Functionalized Metal-Organic Frameworks Hollow Fiber Mixed Matrix Membranes for CO2 and CH4 Separation: A Review. Polymers 2022, 14, 1408. [Google Scholar] [CrossRef]
- Datta, S.J.; Mayoral, A.; Bettahalli, N.M.S.; Bhatt, P.M.; Karunakaran, M.; Carja, I.D.; Fan, D.; Mileo, P.G.M.; Semino, R.; Maurin, G.; et al. Rational design of mixed-matrix metal-organic framework membranes for molecular separations. Science 2022, 376, 1080–1087. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, Z.G.; Yang, Z.; Liu, G.Z.; Chen, G.N.; Liu, G.P.; Meng, X.X.; Yang, N.T.; Jin, W.Q. Simultaneously enhanced permeability, selectivity and anti-plasticization of polyimide membrane for natural gas purification by incorporating surface-engineered DD3R zeolite. J. Membr. Sci. 2024, 707, 122993. [Google Scholar] [CrossRef]
- van Essen, M.; Thür, R.; van den Akker, L.; Houben, M.; Vankelecom, I.F.J.; Nijmeijer, K.; Borneman, Z. Tailoring the separation performance of ZIF-based mixed matrix membranes by MOF-matrix interfacial compatibilization. J. Membr. Sci. 2021, 637, 119642. [Google Scholar] [CrossRef]
- Zhao, Q.Z.; Sun, Y.C.; Zhang, J.F.; Fan, F.X.; Li, T.Y.; He, G.H.; Ma, C.H. Mixed matrix membranes incorporating amino-functionalized ZIF-8-NH2 in a carboxylic polyimide for molecularly selective gas separation. J. Membr. Sci. 2024, 693, 122326. [Google Scholar] [CrossRef]
- Guo, Z.Y.; Li, W.P.; Wu, H.; Cao, L.; Song, S.Q.; Ma, X.H.; Shi, J.F.; Ren, Y.X.; Huang, T.; Li, Y.H.; et al. Reverse filling approach to mixed matrix covalent organic framework membranes for gas separation. Nat. Commun. 2025, 16, 3617. [Google Scholar] [CrossRef]
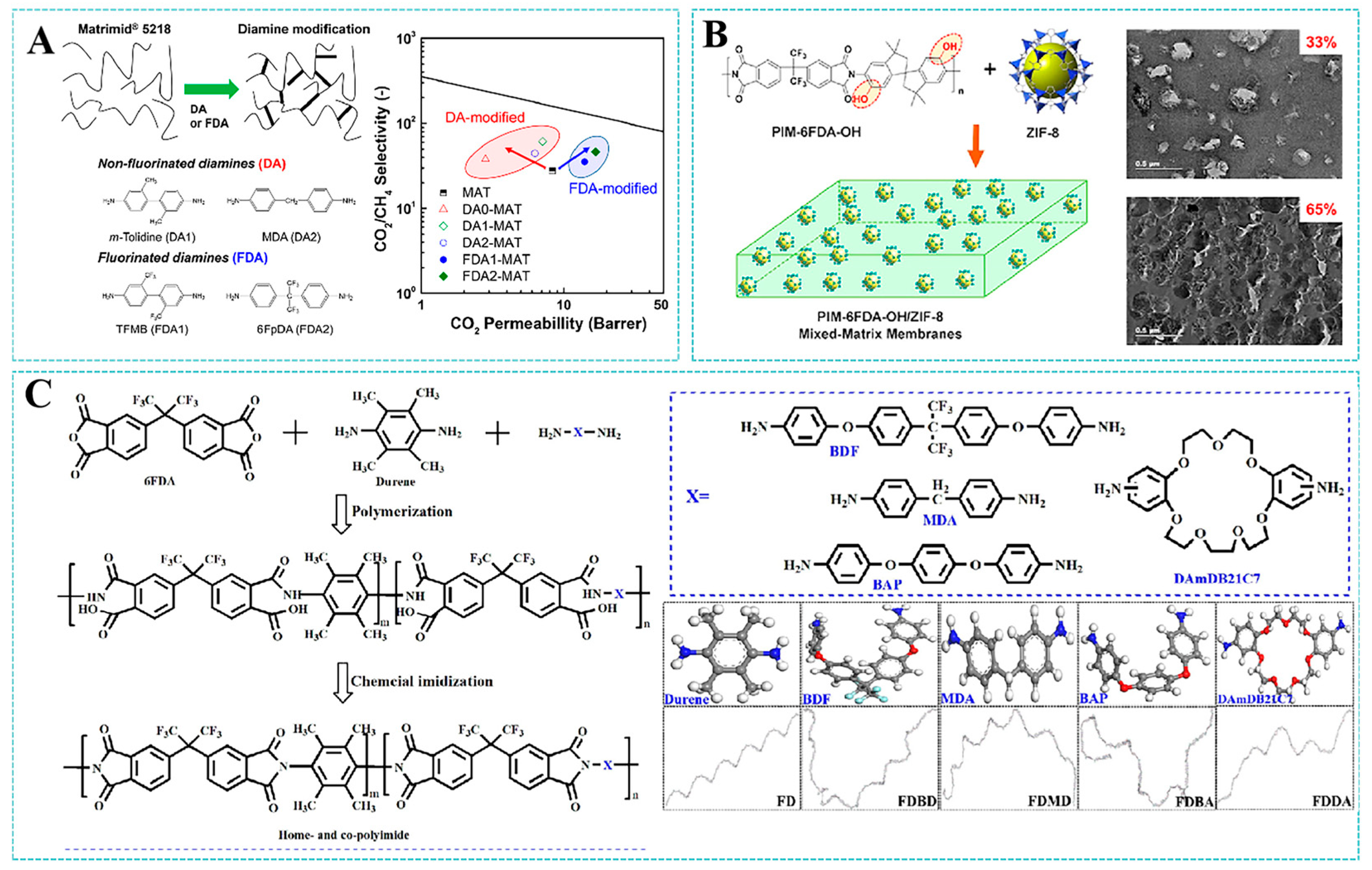
- Lee, T.H.; Lee, B.K.; Park, J.S.; Park, J.; Kang, J.H.; Yoo, S.Y.; Park, I.; Kim, Y.H.; Park, H.B. Surface modification of Matrimid® 5218 polyimide membrane with fluorine-containing diamines for efficient gas separation. Membranes 2022, 12, 256. [Google Scholar] [CrossRef]
- Ma, X.H.; Swaidan, R.J.; Wang, Y.G.; Hsiung, C.E.; Han, Y.; Pinnau, I. Highly compatible hydroxyl-functionalized microporous polyimide-ZIF-8 mixed matrix membranes for energy efficient propylene/propane separation. ACS Appl. Nano Mater. 2018, 1, 3541–3547. [Google Scholar] [CrossRef]
- Wu, D.Y.; Zhang, B.B.; Yuan, J.; Yi, C.H. Structural engineering on 6FDA-Durene based polyimide membranes for highly selective gas separation. Sep. Purif. Technol. 2023, 316, 123786. [Google Scholar] [CrossRef]
- Wang, Y.G.; Alaslai, N.; Ghanem, B.; Ma, X.H.; Hu, X.F.; Balcik, M.; Liu, Q.; Abdulhamid, M.A.; Han, Y.; Eddaoudi, M.; et al. Hydroxyl-Functionalized Polymers of Intrinsic Microporosity and Dual-Functionalized Blends for High-Performance Membrane-Based Gas Separations. Adv. Mater. 2024, 36, 2406076. [Google Scholar] [CrossRef]
- Favvas, E.P.; Katsaros, F.K.; Papageorgiou, S.K.; Sapalidis, A.A.; Mitropoulos, A.C. A review of the latest development of polyimide based membranes for CO2 separations. React. Funct. Polym. 2017, 120, 104–130. [Google Scholar] [CrossRef]
- Tanaka, K.; Kita, H.; Okano, M.; Okamoto, K. Permeability and permselectivity of gases in fluorinated and non-fluorinated polyimides. Polymer 1992, 33, 585–592. [Google Scholar] [CrossRef]
- Lim, W.; He, W.; Ma, J.; Ul Hassan, S.; Du, J.C.; Sun, Q.; Cao, D.; Guan, J.; Zhang, H.J.; Liu, J.T. Membranes with hollow bowl-shaped window for CO2 removal from natural gas. Adv. Membr. 2025, 5, 100129. [Google Scholar] [CrossRef]
- Dong, G.X.; Li, H.Y.; Chen, V. Plasticization mechanisms and effects of thermal annealing of Matrimid hollow fiber membranes for CO2 removal. J. Membr. Sci. 2011, 369, 206–220. [Google Scholar] [CrossRef]
- Chang, Y.S.; Kumari, P.; Munro, C.J.; Szekely, G.; Vega, L.F.; Nunes, S.; Dumée, L.F. Plasticization mitigation strategies for gas and liquid filtration membranes—A review. J. Membr. Sci. 2023, 666, 121125. [Google Scholar] [CrossRef]
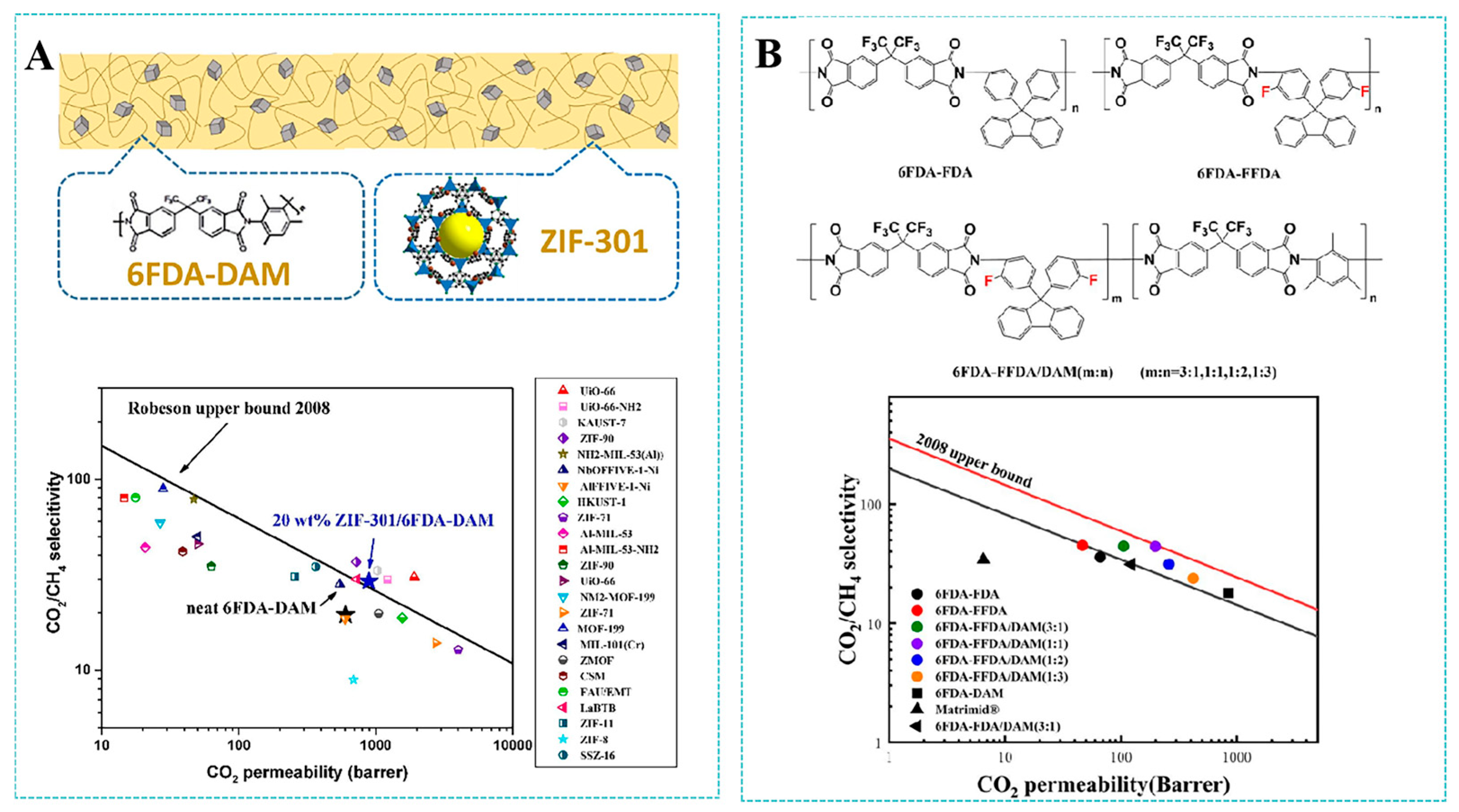
- Wang, Z.G.; Yuan, J.W.; Li, R.H.; Zhu, H.P.; Duan, J.G.; Guo, Y.N.; Liu, G.P.; Jin, W.Q. ZIF-301 MOF/6FDA-DAM polyimide mixed-matrix membranes for CO2/CH4 separation. Sep. Purif. Technol. 2021, 264, 118431. [Google Scholar] [CrossRef]
- Xu, S.; Ren, X.L.; Zhao, N.; Wu, L.; Zhang, Z.G.; Fan, Y.F.; Li, N.W. Self-crosslinking of bromomethylated 6FDA-DAM polyimide for gas separations. J. Membr. Sci. 2021, 636, 119534. [Google Scholar] [CrossRef]
- Fan, F.X.; Sun, Y.C.; Zhao, Q.Z.; Zhang, J.F.; Guan, J.Y.; He, G.H.; Ma, C.H. Fluorinated-cardo-based Co-polyimide membranes with enhanced selectivity for CO2 separation. Sep. Purif. Technol. 2023, 324, 124511. [Google Scholar] [CrossRef]
- Fan, F.X.; Sun, Y.C.; Bai, L.; Li, T.Y.; Gao, Z.Y.; He, G.H.; Ma, C.H. Fluorinated-cardo-based thermally rearranged membranes with enhanced gas separation performance for CO2 capture and hydrogen separation. J. Membr. Sci. 2025, 722, 123843. [Google Scholar] [CrossRef]
- Tin, P.S.; Chung, T.S.; Liu, Y.; Wang, R. Separation of CO2/CH4 through carbon molecular sieve membranes derived from P84 polyimide. Carbon 2004, 42, 3123–3131. [Google Scholar] [CrossRef]
- Ma, J.; He, W.; Lim, W.W.; Du, J.C.; Sun, Q.; Ul Hassan, S.; Cao, D.; Guan, J.; Liu, J.T. Finely tailoring microstructure of hyperbranched polyimide membrane for facile natural gas upgrading. J. Membr. Sci. 2025, 720, 123729. [Google Scholar] [CrossRef]
- Kong, J.J.; Liu, J.J.; Jia, P.Y.; Qi, N.; Chen, Z.Q.; Xu, S.; Li, N.W. Synergistic effect of thermal crosslinking and thermal rearrangement on free volume and gas separation properties of 6FDA based polyimide membranes studied by positron annihilation. J. Membr. Sci. 2022, 645, 120163. [Google Scholar] [CrossRef]
- Jheng, L.; Park, J.; Yoon, H.W.; Chang, F.C. Mixed matrix membranes comprising 6FDA-based polyimide blends and UiO-66 with co-continuous structures for gas separations. Sep. Purif. Technol. 2023, 310, 123126. [Google Scholar] [CrossRef]
- Li, C.E.; Qi, A.H.; Ling, Y.; Tao, Y.; Zhang, Y.B.; Li, T. Establishing gas transport highways in MOF-based mixed matrix membranes. Sci. Adv. 2023, 9, eadf5087. [Google Scholar] [CrossRef]
- Krokidas, P.; Spera, M.B.M.; Boutsika, L.G.; Bratsos, I.; Charalambopoulou, G.; Economou, I.G.; Steriotis, T. Nanoengineered ZIF fillers for mixed matrix membranes with enhanced CO2/CH4 selectivity. Sep. Purif. Technol. 2023, 307, 122737. [Google Scholar] [CrossRef]
- Zhou, R.F.; Pan, Y.C.; Xing, W.H.; Xu, N.P. Advanced microporous membranes for H2/CH4 separation: Challenges and perspectives. Adv. Membr. 2021, 1, 100011. [Google Scholar] [CrossRef]
- Shen, Y.B.; Jiang, F.F.; Liu, Q.; Chen, Z.Q.; Ge, K.; Bai, J.F.; Duan, J.G. Size effect of porous filler in mixed matrix membranes for faster hydrogen permeation from methane-containing mixtures. Adv. Membr. 2025, 5, 100136. [Google Scholar] [CrossRef]
- Wang, Z.G.; Wang, D.; Zhang, S.X.; Hu, L.; Jin, J. Interfacial design of mixed matrix membranes for improved gas separation performance. Adv. Mater. 2016, 28, 3399–3405. [Google Scholar] [CrossRef] [PubMed]
- Mu, Q.D.; Sheng, L.J.; Chen, S.H.; Liu, Y.F.; Deng, M.C.; Ren, J.Z. Thermal induced in-situ defect engineering of ZIF-8 in mixed matrix membranes with significantly enhanced gas separation properties. Sep. Purif. Technol. 2025, 360, 131195. [Google Scholar] [CrossRef]
- Huang, Y.J.; Li, K.H.; Zhang, Y.; Wang, G.; Ma, Y.C.; Jiao, L.; Shu, D.K.; Yang, S.; Ma, X.H.; Zhang, Q.; et al. Fine-tuning gas separation performance of copolymer polyimide by the regulation of local microstructure. J. Membr. Sci. 2025, 718, 123689. [Google Scholar] [CrossRef]
- Wang, S.Y.; Zhang, J.F.; Sun, Y.C.; Fan, F.X.; Zhao, Q.Z.; He, G.H.; Ma, C.H. Thermally rearranged polyimide membranes incorporating triptycenes for improved CO2 capture and hydrogen separation. Sep. Purif. Technol. 2025, 364, 132302. [Google Scholar] [CrossRef]
- Peydayesh, M.; Mohammadi, T.; Bakhtiari, O. Effective hydrogen purification from methane via polyimide Matrimid® 5218- Deca-dodecasil 3R type zeolite mixed matrix membrane. Energy 2017, 141, 2100–2107. [Google Scholar] [CrossRef]
- Fan, Y.F.; Yu, H.Y.; Xu, S.; Shen, Q.C.; Ye, H.M.; Li, N.W. Zn(II)-modified imidazole containing polyimide/ZIF-8 mixed matrix membranes for gas separations. J. Membr. Sci. 2020, 597, 117775. [Google Scholar] [CrossRef]
- Liu, Y.; Xie, W.; Liang, S.; Li, X.X.; Fan, Y.F.; Luo, S.J. Polyimide/ZIFs mixed matrix membranes with tunable interfacial interaction for efficient gas separation. J. Membr. Sci. 2022, 646, 120240. [Google Scholar] [CrossRef]
- Wang, Z.G.; Tian, Y.Y.; Fang, W.X.; Shrestha, B.B.; Huang, M.H.; Jin, J. Constructing strong interfacial interactions under mild conditions in MOF-incorporated mixed matrix membranes for gas separation. ACS Appl. Mater. Interfaces 2021, 13, 3166–3174. [Google Scholar] [CrossRef] [PubMed]
- Sholl, D.S.; Lively, R.P. Seven chemical separations to change the world. Nature 2016, 532, 435–437. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, M.; Nakamura, S.; Kita, H.; Okamoto, K.-i.; Tanihara, N.; Kusuki, Y. Olefin/paraffin separation performance of asymmetric hollow fiber membrane of 6FDA/BPDA–DDBT copolyimide. J. Membr. Sci. 2003, 212, 13–27. [Google Scholar] [CrossRef]
- Li, P.; Chung, T.S.; Paul, D.R. Gas sorption and permeation in PIM-1. J. Membr. Sci. 2013, 432, 50–57. [Google Scholar] [CrossRef]
- An, H.; Lee, A.S.; Kammakakam, I.; Hwang, S.S.; Kim, J.H.; Lee, J.H.; Lee, J.S. Bromination/debromination-induced thermal crosslinking of 6FDA-Durene for aggressive gas separations. J. Membr. Sci. 2018, 545, 358–366. [Google Scholar] [CrossRef]
- Das, M.; Koros, W.J. Performance of 6FDA–6FpDA polyimide for propylene/propane separations. J. Membr. Sci. 2010, 365, 399–408. [Google Scholar] [CrossRef]
- Swaidan, R.J.; Ma, X.; Litwiller, E.; Pinnau, I. Enhanced propylene/propane separation by thermal annealing of an intrinsically microporous hydroxyl-functionalized polyimide membrane. J. Membr. Sci. 2015, 495, 235–241. [Google Scholar] [CrossRef]
- Zhang, C.; Dai, Y.; Johnson, J.R.; Karvan, O.; Koros, W.J. High performance ZIF-8/6FDA-DAM mixed matrix membrane for propylene/propane separations. J. Membr. Sci. 2012, 389, 34–42. [Google Scholar] [CrossRef]
- An, H.; Cho, K.Y.; Lyu, Q.; Chiou, D.; Nam, K.J.; Kang, D.Y.; Lin, L.C.; Lee, J.S. Facile Defect Engineering of Zeolitic Imidazolate Frameworks towards Enhanced C3H6/C3H8 Separation Performance. Adv. Funct. Mater. 2021, 31, 2105577. [Google Scholar] [CrossRef]
- Cheng, Z.L.; Zhang, P.Y.; Wang, Z.Y.; Jiang, H.C.; Wang, W.J.; Liu, D.D.; Wang, L.A.; Zhu, G.S.; Zou, X.Q. A Bipyridyl Covalent Organic Framework with Coordinated Cu(I) for Membrane C3H6/C3H8 Separation. Small 2023, 19, 2300438. [Google Scholar] [CrossRef]
- Lee, T.H.; Lee, B.K.; Youn, C.; Kang, J.H.; Kim, Y.J.; Kim, K.I.; Ha, Y.R.; Han, Y.J.; Park, H.B. Interface engineering in MOF/crosslinked polyimide mixed matrix membranes for enhanced propylene/propane separation performance and plasticization resistance. J. Membr. Sci. 2023, 667, 121182. [Google Scholar] [CrossRef]
- Moghadam, F.; Lee, T.H.; Park, I.; Park, H.B. Thermally annealed polyimide-based mixed matrix membrane containing ZIF-67 decorated porous graphene oxide nanosheets with enhanced propylene/propane selectivity. J. Membr. Sci. 2020, 603, 118019. [Google Scholar] [CrossRef]
- Su, Y.F.; Li, D.Y.; Shan, M.X.; Feng, X.Q.; Gascon, J.; Wang, Y.; Zhang, Y.T. Uniformly distributed mixed matrix membranes via a solution processable strategy for propylene/propane separation. Angew. Chem.-Int. Ed. 2024, 63, e202316093. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.L.; Wang, Z.Y.; Dong, J.C.; Ni, F.; Song, D.D.; Liu, T.X.; Yuan, Z.H.; Wang, K.J.; Zou, X.Q. A zeolitic imidazolate framework with uncoordinated cyano groups in mixed matrix membranes for efficient propylene/propane separation. Chem. Eng. J. 2025, 505, 159352. [Google Scholar] [CrossRef]
| Membrane | Temperature (°C) | Pressure (Bar) | Permeability (Barrer) | Selectivity | Ref. |
|---|---|---|---|---|---|
| P84 | 35 | 10.1 | 1.2 | 50 | [100] |
| Matrimide® 5218 | 35 | 2 | 8.3 | 31 | [87] |
| 6FDA-DABA | 35 | 4.5 | 10.9 | 34.1 | [85] |
| 6FBDA | 35 | 4.6 | 14.0 | 48.3 | [85] |
| 50%ZIF-8-NH2/6FBDA | 35 | 4.7 | 80.8 | 89.4 | [85] |
| 6FDA-FFDA/DAM (1:1) | 35 | 2.0 | 203.4 | 43.4 | [98] |
| 6FDA-6FAP/TAPA (7:3) | 25 | 3.5 | 26.9 | 134.5 | [101] |
| 6FDA-APAF-350 | 35 | 6.9 | 10.5 | 74.0 | [102] |
| 6FDA-APAF/DABA-350 | 35 | 6.9 | 26.6 | 60.0 | [102] |
| 20%UiO-66/6FDA-SDA | 35 | 10.0 | 32.7 | 40.0 | [103] |
| 19%UiO-66-NH2/6FDA-DAM | 35 | 3.0 | 1385.6 | 20.25 | [104] |
| 10%ZIF-8/6FDA-DAM | 25 | 2.0 | 1689.0 | 16.6 | [105] |
| Membrane | Temperature (°C) | Pressure (Bar) | Permeability (Barrer) | Selectivity | Ref. |
|---|---|---|---|---|---|
| P84 HT/20% ZIF-8(400 °C) | 35 | 2.0 | 123.8 | 75 | [109] |
| Matrimide® 5218/15% DDR | 35 | 10.0 | 29.37 | 309.16 | [112] |
| Matrimide® 5218/20% DDR | 35 | 10.0 | 34.9 | 375.27 | [112] |
| 30%ZIF-8/6FDA-BI | 35 | 4.0 | 174 | 140.9 | [113] |
| 6FDA-DAM:DABA (3:2)/10%ZIF-8-90(30) | 35 | 4.0 | 301 | 55.7 | [114] |
| 20%-COOH-PI/NH2-UiO-66 | 25 | 4.0 | 1180 | 27.2 | [115] |
| 50%ZIF-8-NH2/6FBDA | 35 | 4.5 | 127.5 | 141.7 | [85] |
| 6FDA-BAP | 35 | 2.0 | 64.57 | 24.6 | [111] |
| 6FDA-BAP:DAT (2:1) | 35 | 2.0 | 53.61 | 67.6 | [111] |
| 6FDA-BAP:DAT (2:1) 400 °C | 35 | 2.0 | 225.29 | 146.3 | [111] |
| Membrane | Temperature (°C) | Pressure (Bar) | Permeability (Barrer) | Selectivity | Ref. |
|---|---|---|---|---|---|
| 48%ZIF-8/6FDA-DAM | 35 | 2.0 | 56.2 | 31 | [122] |
| 20%ZIF-8/GO-6FDA-DAM | 35 | 2.0 | 43.1 | 13.9 | [126] |
| PIM-6FDA-OH | 35 | 2.0 | 3.5 | 30 | [88] |
| 65%ZIF-8/PIM-6FDA-OH | 35 | 2.0 | 38 | 43 | [88] |
| 40%ZIF-67@Ag4tz4/6FDA-TMPDA | 30 | 2.0 | 127 | 23.3 | [127] |
| 6FDA-TMPDA | 30 | 2.0 | 78 | 9.5 | [127] |
| 30%ZIF-67/6FDA-TMPDA | 30 | 2.0 | 157.6 | 14 | [127] |
| 6FDA-DAM | 35 | 2.0 | 33.29 | 10.28 | [78] |
| Br-6FDA-DAM | 35 | 2.0 | 7.27 | 14.04 | [78] |
| Br-6FDA-DAM@380 °C | 35 | 2.0 | 84.7 | 14.6 | [78] |
| 15%CN-ZIF-8/6FDA-DAM | 35 | 3.0 | 379.8 | 23.6 | [128] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Q.-Y.; Mao, H.; Wen, M.; Chen, B.-H.; Li, Q.-Q.; Zhang, Y.-M.; Zhao, Z.-P. Advances in Polyimide Membranes for Gas Separation: Synthesis, Modification, and Application. Molecules 2025, 30, 3507. https://doi.org/10.3390/molecules30173507
Zhang Q-Y, Mao H, Wen M, Chen B-H, Li Q-Q, Zhang Y-M, Zhao Z-P. Advances in Polyimide Membranes for Gas Separation: Synthesis, Modification, and Application. Molecules. 2025; 30(17):3507. https://doi.org/10.3390/molecules30173507
Chicago/Turabian StyleZhang, Qiu-Ying, Heng Mao, Meng Wen, Bing-Hong Chen, Qian-Qian Li, Yan-Mei Zhang, and Zhi-Ping Zhao. 2025. "Advances in Polyimide Membranes for Gas Separation: Synthesis, Modification, and Application" Molecules 30, no. 17: 3507. https://doi.org/10.3390/molecules30173507
APA StyleZhang, Q.-Y., Mao, H., Wen, M., Chen, B.-H., Li, Q.-Q., Zhang, Y.-M., & Zhao, Z.-P. (2025). Advances in Polyimide Membranes for Gas Separation: Synthesis, Modification, and Application. Molecules, 30(17), 3507. https://doi.org/10.3390/molecules30173507