Review of Modern Eschweiler–Clarke Methylation Reaction
Abstract
1. Introduction
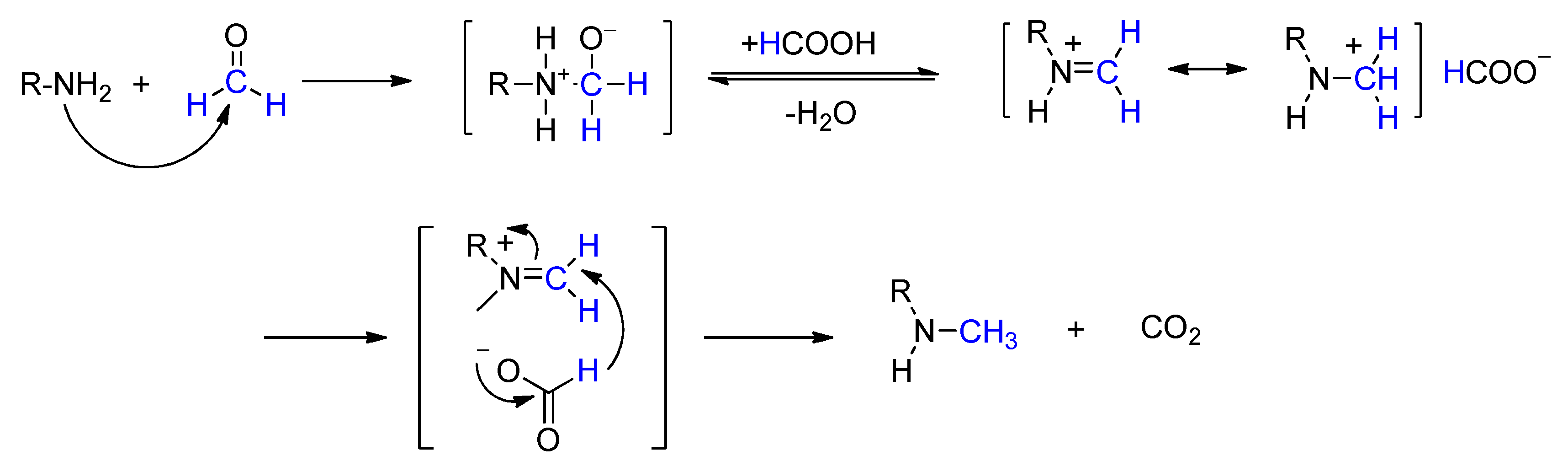
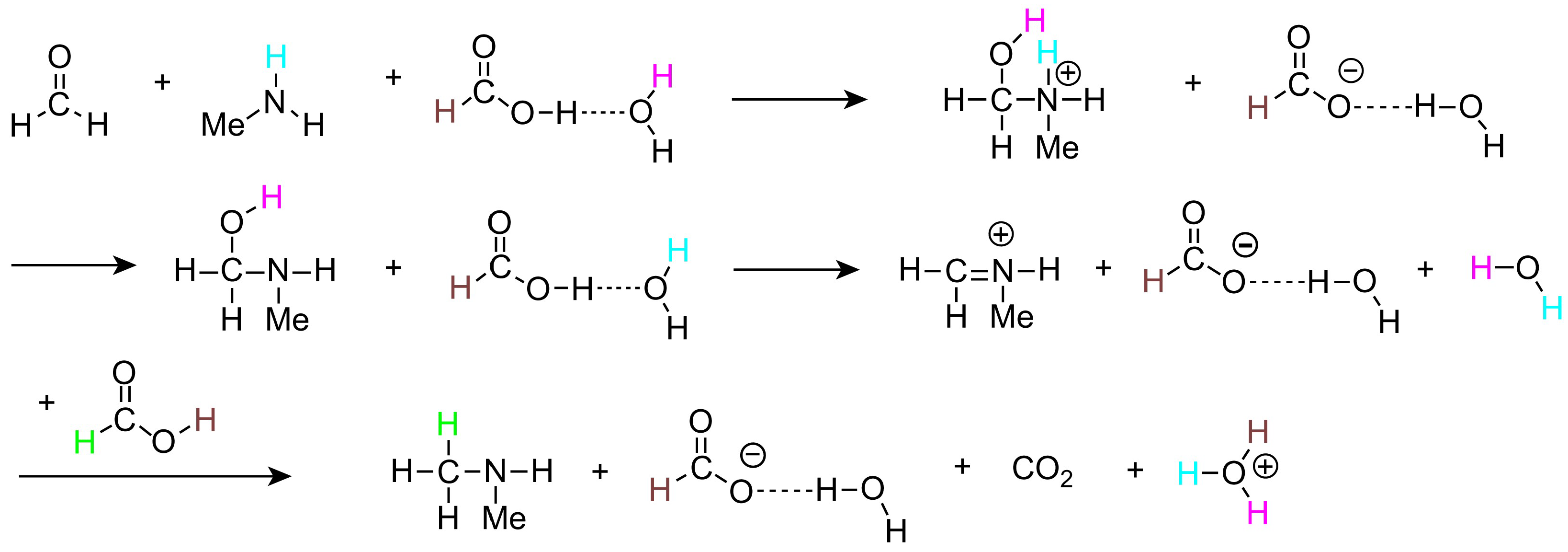
2. Mechanism of the Eschweiler–Clarke Methylation Reaction
3. Applications of the Typical Eschweiler–Clarke Methylation Reaction
4. Development of the Eschweiler–Clarke Methylation Reaction
4.1. Eschweiler–Clarke Methylation Reaction with Formic Acid Substitutes
4.2. Eschweiler–Clarke Methylation Reaction with Formaldehyde Substitutes
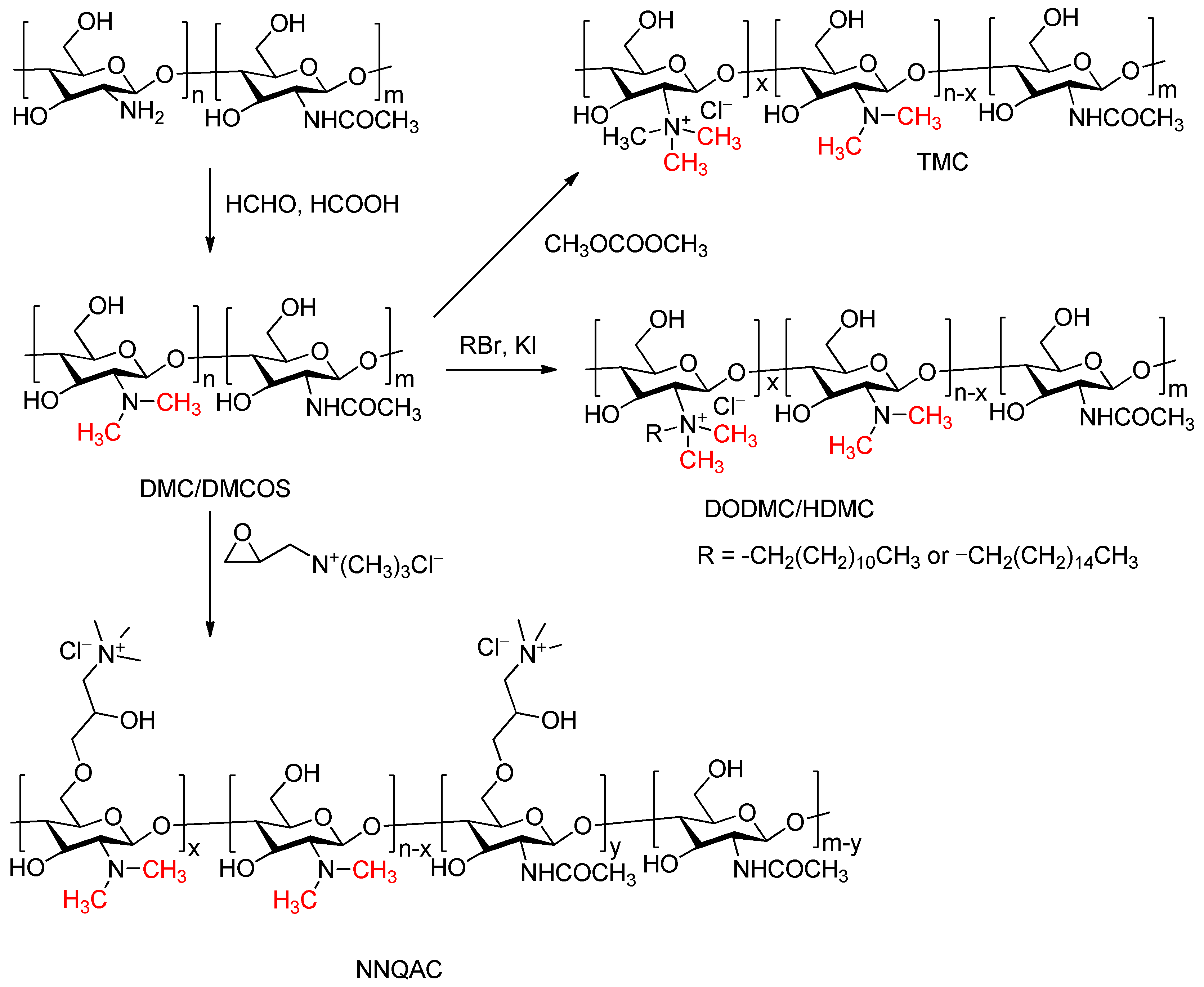
5. Special Cases of the Eschweiler–Clarke Methylation Reaction
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| DFT | Density functional theory |
| DHA | Dehydroabietylamine |
| DMC | N,N-dimethyl chitosan |
| DMCOS | N,N-dimethyl chitosan oligosaccharide |
| DMDHA | N,N-dimethyl dehydroabietyl |
| NMDA | N-methyl-D-aspartic acid |
References
- Barreiro, E.J.; Kümmerle, A.E.; Fraga, C.A.M. The methylation effect in medicinal chemistry. Chem. Rev. 2011, 111, 5215–5246. [Google Scholar] [CrossRef] [PubMed]
- Schönherr, H.; Cernak, T. Profound methyl effects in drug discovery and a call for new C-H methylation reactions. Angew. Chem. Int. Ed. Engl. 2013, 52, 12256–12267. [Google Scholar] [CrossRef]
- Moulay, S. Methyl, the smallest alkyl groups with stunning effects. Chemistry 2018, 27, 759–770. [Google Scholar]
- Moulay, S. N-Methylation of Nitrogen-Containing Organic Substrates: A comprehensive overview. Curr. Org. Chem. 2019, 23, 1695–1737. [Google Scholar] [CrossRef]
- Aurelio, L.; Brownlee, R.T.; Hughes, A.B. Synthetic preparation of N methyl-alpha-amino acids. Chem. Rev. 2004, 104, 5823–5846. [Google Scholar] [CrossRef]
- Chatterjee, J.; Gilon, C.; Hoffman, A.; Kessler, H. N-methylation of peptides: A new perspective in medicinal chemistry. Acc. Chem. Res. 2008, 41, 1331–1342. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, J.; Rechenmacher, F.; Kessler, H. N-methylation of peptides and proteins: An important element for modulating biological functions. Angew. Chem. Int. Ed. Engl. 2013, 52, 254–269. [Google Scholar] [CrossRef]
- Koay, Y.C.; Richardson, N.L.; Zaiter, S.S.; Kho, J.; Nguyen, S.Y.; Tran, D.H.; Lee, K.W.; Buckton, L.K.; McAlpine, S.R. Hitting a moving target: How does an N!methyl group impact biological activity? Chem. Med. Chem. 2016, 11, 881–892. [Google Scholar] [CrossRef]
- Di Gioia, M.L.; Leggio, A.; Malagrinò, F.; Romio, E.; Siciliano, C.; Liguori, A. N-Methylated α-amino acids and peptides: Synthesis and biological activity. Mini Rev. Med. Chem. 2016, 16, 683–690. [Google Scholar] [CrossRef]
- Räder, A.F.B.; Reichart, F.; Weinmüller, M.; Kessler, H. Improving oral bioavailability of cyclic peptides by N-methylation. Bioorg. Med. Chem. 2018, 26, 2766–2773. [Google Scholar] [CrossRef]
- Pine, S.H. Eschweiler-Clarke methylation of amines. An organic chemistry experiment. J. Chem. Educ. 1968, 45, 118. [Google Scholar] [CrossRef]
- Cope, A.C.; Ciganek, E.; Fleckenstein, L.J.; Meisinger, M.A.P. Tertiary amines from methiodides and lithium aluminum hydride. J. Am. Chem. Soc. 1960, 82, 4651–4655. [Google Scholar] [CrossRef]
- Eschweiler, W. Ersatz von an Stickstoff gebundenen Wasserstoffatomen durch die Methylgruppe mit Hülfe von Formaldehyd. Ber. Dtsch. Chem. Ges. 1905, 38, 880–882. [Google Scholar] [CrossRef]
- Clarke, H.T.; Gillespie, H.B.; Weisshaus, S.Z. The action of formaldehyde on amines and amino acids. J. Am. Chem. Soc. 1933, 55, 4571–4587. [Google Scholar] [CrossRef]
- Xu, G.-M.; Chen, B.; Guo, B.; He, D.; Yao, S. Detection of intermediates for the Eschweiler-Clarke reaction by liquid-phase reactive desorption electrospray ionization mass spectrometry. Analyst 2011, 136, 2385–2390. [Google Scholar] [CrossRef]
- Yamabe, S.; Tsuchida, N.; Yamazaki, S. A density functional theory study of the hydride shift in the Eschweiler-Clarke reaction. J. Phys. Org. Chem. 2021, 34, e4253. [Google Scholar] [CrossRef]
- Zhou, L.; Zhou, M.; Wu, B.; Deng, Y. Synthesis of Rivastigmine Hydrogen Tartrate. Zhongguo Yiyao Gongye Zazhi 2007, 38, 327–329. [Google Scholar]
- Wang, F.; Zhu, J.; Chen, J. Preparation of 3-[1-(Dimethylamino)Ethyl]Phenol as Intermediate of Rivastigmine for Treating Central Nervous System Diseases. CN Patent CN104987294 A, 21 October 2015. [Google Scholar]
- Giridhar, T.; Gudipati, S.; Rao, K.S. An Improved Process for the Preparation of Rivastigmine and Its Salts and Intermediates. WO Patent WO2011070585 A1, 16 June 2011. [Google Scholar]
- Zeng, P.; He, X.; Wu, J.; He, L.; Liu, J. Preparation of Dapoxetine Hydrochloride. CN Patent CN105061230 A, 18 November 2015. [Google Scholar]
- Liao, X.; Wu, X.; Ge, D.; Cheng, Y.; Wu, S. Preparation of 3-dimethylamino-3-phenylpropanol. Zhongguo Yiyao Gongye Zazhi 2006, 37, 152–153. [Google Scholar]
- Xu, D.; Xia, A.; Bai, L.; Pan, G. Synthesis method of dapoxetine and intermediate. CN Patent CN110845369 A, 28 February 2020. [Google Scholar]
- Gu, S.; Li, T.; Zhu, Y. Graphical Synthetic Routes of Venlafaxine Hyderochoride. Chin. J. Pharm. 2014, 45, 187–189. [Google Scholar]
- Saigal, J.C.; Gupta, R.P.; Pandit, V.V.; Desai, A.J.; Mehta, N.V.; Rane, S.H. Manufacture of phenyl ethylamine compounds, in particular venlafaxine, as antidepressants. WO Patent WO2003080560 A1, 2 October 2003. [Google Scholar]
- Gokhale, U.B.; Parenky, C. A process for the manufacture of venlafaxine and intermediates thereof. WO Patent WO2006035457, 6 April 2006. [Google Scholar]
- Zhang, T.; Yu, Y.; Yin, S.; Chen, W.; Liang, C.; Shao, T.; Chang, S. Improved synthesis of desvenlafaxine succinate monohydrate. Huaxue Shiji 2020, 42, 1268–1272. [Google Scholar]
- Vyas, S.K. Process for the Preparation of Sibutramine. WO Patent WO2002036540 A2, 10 May 2002. [Google Scholar]
- Zhang, L.; Xie, L.; Xiong, F.; Yang, P. Synthesis of sibutramine hydrochloride. Chin. J. Pharm. 2001, 32, 1–2. [Google Scholar]
- Chen, Z.; Yu, S. Synthesis Method of Antitussive Dextromethorphan. CN Patent CN116283775 A, 23 June 2023. [Google Scholar]
- Ma, M.; Yao, G.; Shi, Y.; Deng, Y. Synthesis of azithromycin. Jingxi Huagong 2006, 23, 781–783. [Google Scholar]
- Jin, Y.; Qiao, W.; Wang, Z.; Hou, Z. Improvement on synthesis of azithromycin. Fine Chem. Intermed. 2014, 44, 49–51. [Google Scholar]
- Yao, D.; Jiang, Y.; Sun, Y.; Hou, Q.; He, Q. Method for Preparation of Baquiloprim. CN Patent CN103755684 A, 30 April 2014. [Google Scholar]
- Pu, L.; Li, Z.; Li, L.; Ma, Y.; Hu, S.; Wu, Z. Concise enantioselective total synthesis of isopavine alkaloids. J. Org. Chem. 2023, 88, 4317–4324. [Google Scholar] [CrossRef]
- Ruchirawat, S.; Bhavakul, V.; Chaisupakitsin, M. A One-Pot Synthesis of (±) Cryptostylines I, II, III. Synth. Commun. 2003, 33, 621–625. [Google Scholar] [CrossRef]
- Shibata, K.; Tarui, A.; Todoroki, N.; Kawamoto, S.; Takahashi, S.; Kera, Y.; Yamada, R. Occurrence of N-methyl-L-aspartate in bivalves and its distribution compared with that of N-methyl-D-aspartate and D,L-aspartate. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2001, 130, 493–500. [Google Scholar] [CrossRef]
- D’Aniello, A.; De Simone, A.; Spinelli, P.; D’Aniello, S.; Branno, M.; Aniello, F.; Rios, J.; Tsesarskaja, M.; Fisher, G. A specific enzymatic high-performance liquid chromatography method to determine N-methyl-Daspartic acid in biological tissues. Anal. Biochem. 2002, 308, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, A.; Oka, T. Free D-aspartate and D-serine in the mammalian brain and periphery. Prog. Neurobiol. 1997, 52, 325–353. [Google Scholar] [CrossRef]
- Roher, A.E.; Lowenson, J.D.; Clarke, S.; Wolkow, C.; Wang, R.; Cotter, R.J.; Reardon, I.M.; Zürcher-Neely, H.A.; Heinrikson, R.L.; Ball, M.J. Structural alterations in the peptide backbone of β-amyloid core protein may account for its deposition and stability in Alzheimer’s disease. J. Biol. Chem. 1993, 268, 3072–3083. [Google Scholar] [CrossRef]
- Orpiszewski, J.; Schormann, N.; Kluve-Beckerman, B.; Liepnieks, J.J.; Benson, M.D. Protein aging hypothesis of Alzheimer disease. FASEB J. 2000, 14, 1255–1263. [Google Scholar] [CrossRef]
- Xi, L.; Wu, D.; Zhu, H.-Y.; Zhang, C.-H.; Jin, Y.; Lin, J. Improved and large-scale synthesis of N-methyl-D-aspartic acid. Curr. Org. Synth. 2015, 12, 197–201. [Google Scholar] [CrossRef]
- Long, Z.; Wu, M.; Zhang, D.; Wang, C. Preparation Method of Chitosan Quaternary Ammonium Salt. CN Patent CN106167532 A, 30 November 2016. [Google Scholar]
- Li, M.; Zhou, S.; Xin, M.; Song, M.; Wang, C. Synthesis and antibacterial activity of N-alkyl quaternary ammonium chitosan derivatives. Gongneng Cailiao 2012, 43, 2338–2342. [Google Scholar]
- Cao, Z.L.; Liu, W.W.; Xiong, J.J.; Qu, N.; Li, H.X.; Yao, G.W. Synthesis and properties of N,N-dimethyl-O-quaternary ammonium chitosan. Adv. Mater. Res. 2011, 152, 1337–1341. [Google Scholar] [CrossRef]
- Dong, P.; Shi, Q.; Peng, R.; Yuan, Y.; Xie, X. N,N-dimethyl chitosan oligosaccharide (DMCOS) promotes antifungal activity by causing mitochondrial damage. Carbohydr. Polym. 2023, 303, 120459. [Google Scholar] [CrossRef] [PubMed]
- Sudarma, I.M.; Satriani, A.R.; Darmayanti, M.G. N,N-dimethylation of nitro-eugenol to its new 4-allyl-2-(dimethylamino)-6-methoxyphenol via Eschweiler-Clarke methylation reaction. Asian J. Chem. 2017, 29, 867–869. [Google Scholar] [CrossRef]
- Higgins, T.F.; Winkler, J.D. Synthesis and applications of the C2-symmetrical diamine 2,7-diazabicyclo[4.4.1]undecane. J. Org. Chem. 2020, 85, 7424–7432. [Google Scholar] [CrossRef]
- Ren, B.; Chen, T. Halogen-Free Environment-Protective Cationic Antistatic Agent and Preparation Method Thereof. CN Patent CN102775697 A, 14 November 2012. [Google Scholar]
- Zhu, X.-M.; Jiang, S.-M.; Cai, Z.-S.; Chen, Z.-D. Preparation of 3-chloro-2-hydroxypropyl dimethyl dehydroabietyl ammonium chloride and its surface activities. Linchan Huaxue Yu Gongye 2014, 34, 116–120. [Google Scholar]
- Pei, L.-J.; Cai, Z.-S.; Song, Z.-Q.; Zhu, X.-M.; Shang, S.-B. 3-chloro-2-hydroxypropyl dimethyl dehydroabietyl ammonium chloride: Synthesis, characterization, and physicochemical properties. J. Surfactants Deterg. 2014, 17, 493–499. [Google Scholar] [CrossRef]
- Xu, D.; Cai, Z.; Chen, Z.; Xu, Q. Synthesis of allyl dimethyl dehydroabietyl ammonium chloride and optimization of its synthetic conditions. Huaxue Shijie 2014, 55, 619–623. [Google Scholar]
- Liu, H.; Hu, J.; Xu, B.; Zhao, T.; Shi, G.; Zhang, G. Synthesis, surface activities and toluene solubilization by amine-oxide gemini surfactants. J. Surfactants Deterg. 2016, 19, 673–680. [Google Scholar] [CrossRef]
- Döbbelin, M.; Azcune, I.; Bedu, M.; Ruiz de Luzuriaga, A.R.; Genua, A.; Jovanovski, V.; Cabañero, G.; Odriozola, I. Synthesis of Pyrrolidinium-Based Poly(ionic liquid) Electrolytes with Poly(ethylene glycol) Side Chains. Chem. Mater. 2012, 24, 1583–1590. [Google Scholar] [CrossRef]
- Wang, C.; Mu, C.; Li, L.; Lin, W. Zwitterionic Waterborne Polyurethane for Resisting Protein and Microbial Adsorption and Its Preparation Method. CN Patent CN105732953 A, 6 July 2016. [Google Scholar]
- Dong, P.; Xie, X.; Gan, S.; Shi, Q. Preparing Method and Application of 5-Dimethylamino-1,3-dioxane-2-one. CN Patent CN109467547 A, 15 March 2019. [Google Scholar]
- Tarpey, W.; Hauptmann, H.; Tolbert, B.M.; Rapoport, H. The preparation of demerol-N-methyl-C14 by reductive methylation. J. Am. Chem. Soc. 1950, 72, 5126–5127. [Google Scholar] [CrossRef]
- Lindeke, B.; Anderson, E.; Jenden, D.J. Specific deuteriomethylation by the Eschweiler-Clarke reaction. Synthesis of differently labelled variants of trimethylamine and their use for the preparation of labelled choline and acetylcholine. Biomed. Mass Spectrom. 1976, 3, 257–259. [Google Scholar] [CrossRef]
- Al-Hadedi, A.A.M.; Sawyer, S.; Elliott, S.J.; Green, R.A.; O’Leary, D.J.; Brown, R.C.D.; Brown, L.J. A flow electrochemistry-enabled synthesis of 2-substituted N-(methyl-d)piperidines. J. Labelled Comp. Radiopharm. 2022, 65, 361–368. [Google Scholar] [CrossRef]
- Harding, J.R.; Jones, J.R.; Lu, S.-Y.; Wood, R. Development of a microwave-enhanced isotopic labeling procedure based on the Eschweiler-Clarke methylation reaction. Tetrahedron Lett. 2002, 43, 9487–9488. [Google Scholar] [CrossRef]
- Tirumalai, P.S.; Shakleya, D.M.; Gannett, P.M.; Callery, P.S.; Bland, T.M.; Tracy, T.S. Conversion of methamphetamine to N-methyl-methamphetamine in formalin solutions. J. Anal. Toxicol. 2005, 29, 48–53. [Google Scholar] [CrossRef]
- Man, N.Y.T.; Li, W.; Stewart, S.G.; Wu, X.-F. Transition metal-free methylation of amines with formaldehyde as the reductant and methyl source. Chimia 2015, 69, 345–347. [Google Scholar] [CrossRef]
- Kumar, R.S.; Almansour, A.I.; Arumugam, N.; Mohammad, F.; Kumar, R.R. In vitro mechanistic exploration of novel spiropyrrolidine heterocyclic hybrids as anticancer agents. Front. Chem. 2020, 8, 465. [Google Scholar] [CrossRef]
- Kumar, R.S.; Almansour, A.I.; Arumugam, N.; Kumar, R.R. Substitution induced switch between Pictet-Spengler and Eschweiler-Clarke reactions: Selective synthesis of spiro acenaphthylene pyrrolo[1,2-b]-isoquinoline/pyrrolidine hybrids. Tetrahedron Lett. 2020, 61, 151606. [Google Scholar] [CrossRef]
- Cheng, J.; Armugam, A.; Yang, Y.; Jin, F.; Zhang, Y.; Yan, N. One-Pot chitin conversion to high-activity antifungal N,N-dimethyl chitosan oligosaccharides. ChemSusChem 2023, 16, e202300591. [Google Scholar] [CrossRef] [PubMed]
- Rosenau, T.; Potthast, A.; Röhrling, J.; Hofinger, A.; Sixta, H.; Kosma, P. Asolvent-free and formalin-free Eschweiler-Clarke methylation for amines. Synth. Commun. Synth. Commun. 2002, 32, 457–466. [Google Scholar] [CrossRef]
- Mulholland, G.K.; Jewett, D.M.; Toorongian, S.A. synthesis of N-[11C-methyl]scopolamine by phosphite mediated reductive methylation with [11C]formaldehyde. Int. J. Rad. Appl. Instrum. A 1988, 39, 373–379. [Google Scholar] [CrossRef]
- Borch, R.F.; Hassid, A.I. A New method for the methylation of amines. J. Org. Chem. 1972, 37, 1673–1674. [Google Scholar] [CrossRef]
- Boullais, C.; Oberdorfer, F.; Sastre, J.; Prenant, C.; Crouzel, C. Synthesis of 11C-suriclone. J. Label. Comp. Radiopharm. 1985, 22, 1081–1086. [Google Scholar] [CrossRef]
- Kaljuste, K.; Undén, A. New method for the synthesis of N-methyl amino acids containing peptides by reductive methylation of amino groups on the solid phase. Ini. J. Pept. Protein Res. 1993, 42, 118–124. [Google Scholar] [CrossRef]
- Huang, Z.-P.; Du, J.-T.; Zhao, Y.-F.; Li, Y.-M. Synthesis of site-specifically dimethylated and trimethylated peptides derived from histone H3 N-terminal tail. Int. J. Pept. Res. Ther. 2006, 12, 187–193. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Chatterjee, A.; Duttachowdhury, S.K. Use of zinc borohydride in reductive amination: An efficient and mild method for Nmethylation of amines. J. Chem. Soc. Perkin Trans. 1 1994, 1, 1–2. [Google Scholar] [CrossRef]
- Bhattacharyya, S. Borohydride reductions in dichloromethane: A convenient, environmentally compatible procedure for the methylation of amines. Synth. Commun. 1995, 25, 2061–2069. [Google Scholar] [CrossRef]
- Alinezhad, H.; Tajbakhsh, M.; Zamani, R. Efficient and mild procedure forreductive methylation of amines using N-methylpiperidine zinc borohydride. Synth. Commun. 2006, 36, 3609–3615. [Google Scholar] [CrossRef]
- Alinezhad, H.; Tajbakhsh, M.; Fatemeh, S.; Fazli, K. Safe and efficientreductive methylation of primary and secondary amines using Nmethylpyrrolidine zinc borohydride. Synth. Commun. 2010, 40, 2415–2420. [Google Scholar] [CrossRef]
- da Silva, R.A.; Estevam, I.H.S.; Bieber, L.W. Reductive methylation ofprimary and secondary amines and amino acids by aqueous formaldehydeand zinc. Tetrahedron Lett. 2007, 48, 7680–7682. [Google Scholar] [CrossRef]
- Pearson, D.E.; Bruton, J.D. Reductive methylation of amines. J. Am. Chem. Soc. 1951, 73, 864. [Google Scholar] [CrossRef]
- Guyon, C.; Duclos, M.-C.; Métay, E.; Lemaire, M. Reductive N-methylation of amines with calcium hydride and Pd/C catalyst. Tetrahedron Lett. 2016, 57, 3002–3005. [Google Scholar] [CrossRef]
- Natte, K.; Neumann, H.; Jagadeesh, R.V.; Beller, M. Convenient ironcatalyzed reductive aminations without hydrogen for selective synthesis of N-methylamines. Nat. Commun. 2017, 8, 1344. [Google Scholar] [CrossRef]
- Wang, H.; Yuan, H.; Yang, B.; Dai, X.; Xu, S.; Shi, F. Highly selective Nmonomethylanilines synthesis from nitroarene and formaldehyde via kinetically excluding of the thermodynamically favorable N,N-dimethylation reaction. ACS Catal. 2018, 8, 3943–3949. [Google Scholar] [CrossRef]
- Rong, Z.; Zhang, W.; Zhang, P.; Sun, Z.; Lv, J.; Du, W.; Wang, Y. One-pot synthesis of N, N-dimethylanilines from nitroarenes with skeletal Cu as chemoselective catalyst. Catal. Commun. 2013, 41, 115–118. [Google Scholar] [CrossRef]
- Wang, H.; Huang, Y.; Dai, X.; Shi, F. N-Monomethylation of amines using paraformaldehyde and H2. Chem. Commun. 2017, 53, 5542–5545. [Google Scholar] [CrossRef] [PubMed]
- Sorribes, I.; Junge, K.; Beller, M. General catalytic methylation of amines with formic acid under mild reaction conditions. Chemistry 2014, 20, 7878–7883. [Google Scholar] [CrossRef]
- Pedrajas, E.; Sorribes, I.; Guillamón, E.; Junge, K.; Beller, M.; Llusar, R. Efficient and selective N-methylation of nitroarenes under mild reaction conditions. Chemistry 2017, 23, 13205–13212. [Google Scholar] [CrossRef]
- Zhu, L.; Wang, L.-S.; Li, B.; Li, W.; Fu, B. Methylation of aromatic amines and imines using formic acid over a heterogeneous Pt/C catalyst. Catal. Sci. Technol. 2016, 6, 6172–6176. [Google Scholar] [CrossRef]
- Singh, A.K.; Hwang, Y.-H.; Kim, D.-P. Heterogeneous PdAg alloy catalyst for selective methylation of aromatic amines with formic acid under an additive-free and solvothermal one-pot condition. NPG Asia Mater. 2015, 7, e222. [Google Scholar] [CrossRef]
- Savourey, S.; Lefèvre, G.; Berthet, J.-C.; Cantat, T. Catalytic methylation of aromatic amines with formic acid as the unique carbon and hydrogen source. Chem. Commun. 2014, 50, 14033–14036. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Zhang, Q.; Li, S.-S.; Huang, J.; Liu, Y.-M.; He, H.-Y.; Cao, Y. Gold-catalyzed reductive transformation of nitro compounds using formic acid: Mild, efficient, and versatile. ChemSusChem 2015, 8, 3029–3035. [Google Scholar] [CrossRef] [PubMed]
- Qiao, C.; Liu, X.-F.; Liu, X.; He, L.-N. Copper(II)-catalyzed selective reductive methylation of amines with formic acid: An option for indirect utilization of CO2. Org. Lett. 2017, 19, 1490–1493. [Google Scholar] [CrossRef] [PubMed]
- Fu, M.-C.; Shang, R.; Cheng, W.-M.; Fu, Y. Boron-catalyzed N-alkylation of amines using carboxylic acids. Angew. Chem. Int. Ed. Engl. 2015, 54, 9042–9046. [Google Scholar] [CrossRef]
- Zhang, Q.; Fu, M.-C.; Yu, H.-Z.; Fu, Y. Mechanism of boron-catalyzed N-alkylation of amines with carboxylic acids. J. Org. Chem. 2016, 81, 6235–6243. [Google Scholar] [CrossRef]
- Cope, A.C.; Burrows, W.D. Clarke-Eschweiler cyclization. Scope and mechanism1a. J. Org. Chem. 1966, 31, 3099–3103. [Google Scholar] [CrossRef]
- Castrillón, J.A. Cactus alkaloids. II. Condensation of mescaline with formaldehyde by the Eschweiler-Clarke reaction. J. Am. Chem. Soc. 1952, 74, 558–559. [Google Scholar] [CrossRef]
- Ruchirawat, S.; Chaisupakitsin, M.; Patranuwatana, N.; Cashaw, J.L.; Davis, V.E. A convenient synthesis of simple tetrahydroisoquinolines. Synth. Commun. 1984, 14, 1221–1228. [Google Scholar] [CrossRef]
- Kametani, T.; Terui, T.; Agui, H.; Fukumoto, K. A modified synthesis of codamine under Eschweiler-Clarke conditions. J. Heterocycl. Chem. 1968, 5, 753–755. [Google Scholar] [CrossRef]
- Nelson, W.L. Oxzolidine formation in the attempted Eschweiler-Clarke reductive methylation of cis-3-aminobicyclo[2.2.2]octan-2-ol. J. Heterocycl. Chem. 1968, 5, 231–233. [Google Scholar] [CrossRef]
- Sahakitpichan, P.; Ruchirawat, S. Highly efficient synthesis of buflavine: A unique Amaryllidaceae alkaloid. Tetrahedron Lett. 2003, 44, 5239–5241. [Google Scholar] [CrossRef]
- Eresko, A.B.; Raksha, E.V.; Berestneva, Y.V.; Muratov, A.V.; Viotash, A.A.; Tolkunov, V.S.; Tolkunov, S.V. Synthesis, NMR spectroscopy, and molecular modeling of 2-methyl-2,3,4,5-tetrahydro-1H-[1]benzothieno[2,3-c]azepine. Russ. J. Org. Chem. 2020, 56, 1929–1936. [Google Scholar] [CrossRef]
- Chen, F.-L.; Sung, K. An exception of Eschweiler-Clarke methylation: Cyclocondensation of α-amino amides with formaldehyde and formic acid. J. Heterocycl. Chem. 2004, 41, 697–700. [Google Scholar] [CrossRef]
- Rahal, S.; Badache, L. An anomalous Eschweiler-Clarke reaction. Tetrahedron Lett. 1991, 32, 3847–3848. [Google Scholar] [CrossRef]
- Watanabe, K.; Wakabayashi, T. Synthesis of (1R,9S)-13-methyl-13-azatricyclo-[7.3.1.02,7]trideca-2,4,6-triene. Observation of a novel deaminative fragmentation. J. Org. Chem. 1980, 45, 357–359. [Google Scholar] [CrossRef]
- Alder, R.W.; Colclough, D.; Mowlam, R.W. Fragmentation during the formic acid/formaldehyde (Eschweiler-Clarke) methylation of polyamines. Tetrahedron Lett. 1991, 32, 7755–7758. [Google Scholar] [CrossRef]
- Hou, Z.; Chen, Y.; Li, Y.L. Synthesis of a new dibenziodolium inner salt. Acta Chim. Sin. 1989, 47, 516–517. [Google Scholar]
- Goyal, V.; Naik, G.; Narani, A.; Natte, K.; Jagadeesh, R.V. Recent developments in reductive N-methylation with base-metal catalysts. Tetrahedron 2021, 98, 132414. [Google Scholar] [CrossRef]
- Umar, Q.; Luo, M. A brief review: Advancement in the synthesis of amine through the Leuckart reaction. Reactions 2023, 4, 117–147. [Google Scholar] [CrossRef]

















Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, X.; Ni, X.; Wu, X.; Yin, L. Review of Modern Eschweiler–Clarke Methylation Reaction. Molecules 2025, 30, 3504. https://doi.org/10.3390/molecules30173504
Zhou X, Ni X, Wu X, Yin L. Review of Modern Eschweiler–Clarke Methylation Reaction. Molecules. 2025; 30(17):3504. https://doi.org/10.3390/molecules30173504
Chicago/Turabian StyleZhou, Xiaoli, Xue Ni, Xianfu Wu, and Lihui Yin. 2025. "Review of Modern Eschweiler–Clarke Methylation Reaction" Molecules 30, no. 17: 3504. https://doi.org/10.3390/molecules30173504
APA StyleZhou, X., Ni, X., Wu, X., & Yin, L. (2025). Review of Modern Eschweiler–Clarke Methylation Reaction. Molecules, 30(17), 3504. https://doi.org/10.3390/molecules30173504





