Lewis Acid–Base Adducts of α-Amino Isobutyric Acid-Derived Silaheterocycles and Amines
Abstract
1. Introduction
2. Results and Discussion
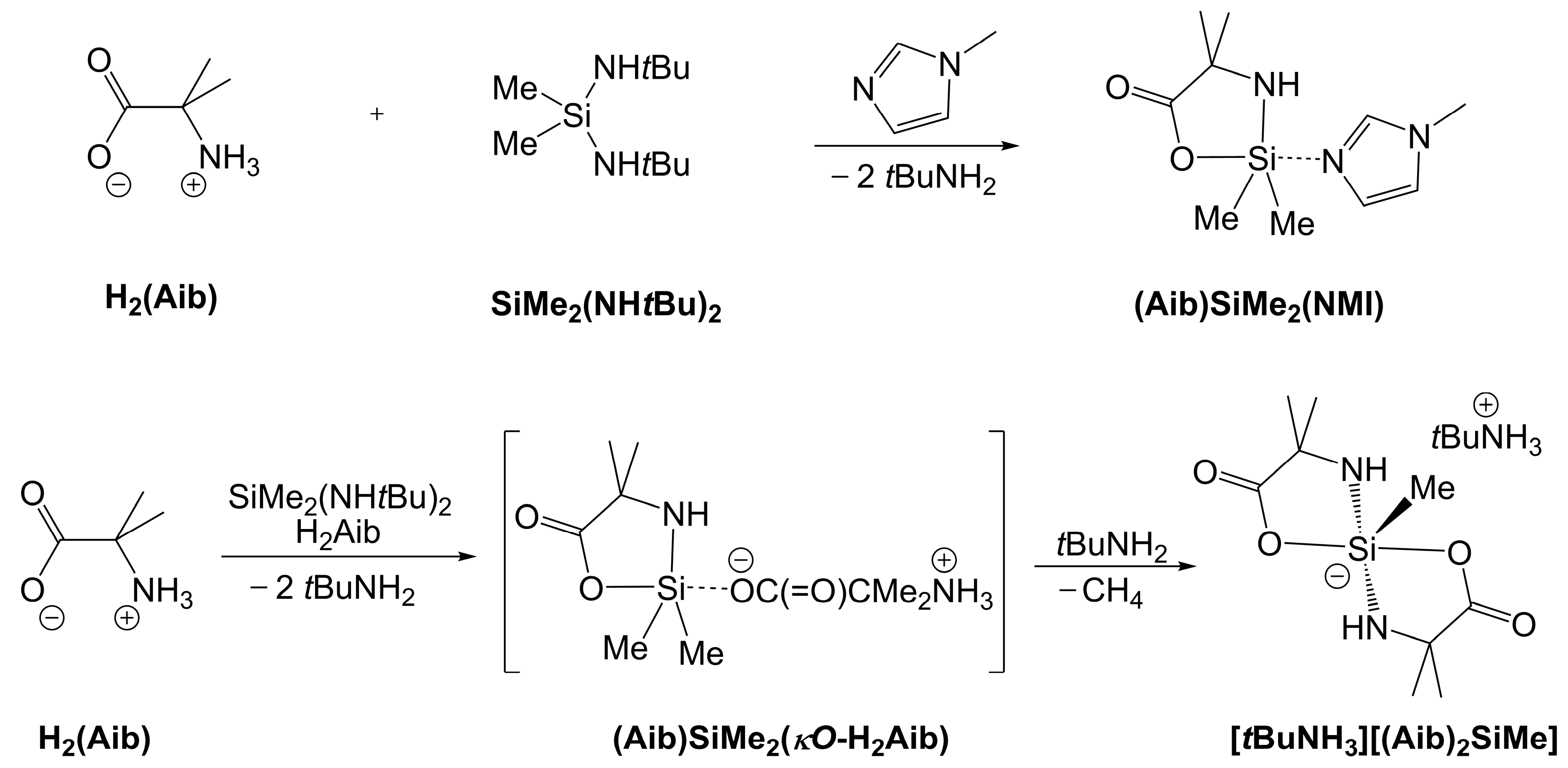
2.1. Syntheses and Molecular Structures of Lewis–Base Adducts of the Type (Aib)SiRR′(LB)
2.2. Spectroscopic Characterization (29Si and 13C Solid-State NMR Spectroscopy) of Lewis-Base Adducts of the Type (Aib)SiRR′(LB)
2.3. Reaction of H2Aib and SiMe2(NHtBu)2 Without an Additional Lewis Base
3. Materials and Methods
3.1. General Considerations
3.2. Synthesis and Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Parameter | SiMe3(Im) 1 | SiMe2(Im)2 |
|---|---|---|
| Formula | C6H12N2Si | C8H12N4Si |
| Mr | 140.27 | 192.31 |
| T(K) | 200(2) | 180(2) |
| λ(Å) | 0.71073 | 0.71073 |
| Crystal system | monoclinic | orthorhombic |
| Space group | P21/c | Pccn |
| a(Å) | 12.7551(4) | 21.6042(8) |
| b(Å) | 10.6258(2) | 7.8214(2) |
| c(Å) | 13.0791(3) | 12.0118(3) |
| β(°) | 106.961(2) | 90 |
| V(Å3) | 1695.55(8) | 2029.70(10) |
| Z | 8 | 8 |
| ρcalc(g·cm−1) | 1.10 | 1.26 |
| μMoKα (mm−1) | 0.2 | 0.2 |
| F(000) | 608 | 816 |
| θmax(°), Rint | 26.0, 0.1085 1 | 26.0, 0.0854 |
| Completeness | 99.9% | 99.9% |
| Reflns collected | 22,510 | 21,792 |
| Reflns unique | 3323 | 1999 |
| Restraints | 0 | 0 |
| Parameters | 170 | 120 |
| GoF | 1.040 | 0.995 |
| R1, wR2 [I > 2σ(I)] | 0.0401, 0.1098 | 0.0424, 0.1142 |
| R1, wR2 (all data) | 0.0426, 0.1117 | 0.0562, 0.1215 |
| Largest peak/hole (e·Å−3) | 0.22, −0.32 | 0.40, −0.30 |
| Parameter | (Aib)SiMe2(HIm)·(CHCl3) | 2[(Aib)SiMe2(HPyr)]·3(CHCl3) 1 |
|---|---|---|
| Formula | C10H18Cl3N3O2Si | C23H47Cl9N4O4Si2 |
| Mr | 346.71 | 818.87 |
| T(K) | 180(2) | 180(2) |
| λ(Å) | 0.71073 | 0.71073 |
| Crystal system | orthorhombic | orthorhombic |
| Space group | Pbca | Pccn |
| a(Å) | 10.9384(4) | 15.6692(14) |
| b(Å) | 16.7355(6) | 21.4914(18) |
| c(Å) | 17.7731(9) | 11.6660(9) |
| V(Å3) | 3253.5(2) | 3928.6(6) |
| Z | 8 | 4 |
| ρcalc(g·cm−1) | 1.42 | 1.38 |
| μMoKα (mm−1) | 0.6 | 0.7 |
| F(000) | 1440 | 1704 |
| θmax(°), Rint | 27.0, 0.0533 | 23.5, 0.1998 1 |
| Completeness | 100% | 99.9% |
| Reflns collected | 33,809 | 25,213 |
| Reflns unique | 3541 | 2905 |
| Restraints | 0 | 94 2 |
| Parameters | 184 | 276 |
| GoF | 1.049 | 1.019 |
| R1, wR2 [I > 2σ(I)] | 0.0293, 0.0677 | 0.0493, 0.0804 |
| R1, wR2 (all data) | 0.0459, 0.0729 | 0.1359, 0.1077 1 |
| Largest peak/hole (e·Å−3) | 0.27, −0.23 | 0.22, −0.26 |
| Parameter | (Aib)SiMe2(HPyr) | (Aib)SiMe2(H2NnPr) |
|---|---|---|
| Formula | C10H22N2O2Si | C9H22N2O2Si |
| Mr | 230.38 | 218.37 |
| T(K) | 140(2) | 140(2) |
| λ(Å) | 0.71073 | 0.71073 |
| Crystal system | monoclinic | monoclinic |
| Space group | P21/n | Pn |
| a(Å) | 10.1535(3) | 9.9795(4) |
| b(Å) | 13.9171(3) | 13.4515(8) |
| c(Å) | 10.3250(3) | 10.2116(4) |
| β(°) | 110.254(2) | 111.317(3) |
| V(Å3) | 1368.78(7) | 1277.01(11) |
| Z | 4 | 4 |
| ρcalc(g·cm−1) | 1.12 | 1.14 |
| μMoKα (mm−1) | 0.2 | 0.2 |
| F(000) | 504 | 480 |
| θmax(°), Rint | 28.0, 0.0515 | 26.0, 0.1060 |
| Completeness | 100% | 100% |
| Reflns collected | 24,955 | 22,161 |
| Reflns unique | 3304 | 4861 |
| Restraints | 7 1 | 4 2 |
| Parameters | 156 | 289 |
| GoF | 1.049 | 0.993 |
| R1, wR2 [I > 2σ(I)] | 0.0344, 0.0877 | 0.0474, 0.0920 |
| R1, wR2 (all data) | 0.0467, 0.0924 | 0.0855, 0.1030 |
| χFlack | n/a | 0.33(15) |
| Largest peak/hole (e·Å−3) | 0.21, −0.21 | 0.18, −0.24 |
| Parameter | (Aib)SiEt2(HPyr) | (Aib)SiMeVi(HPyr) | (Aib)SiMeH(HPyr) |
|---|---|---|---|
| Formula | C12H26N2O2Si | C11H22N2O2Si | C9H20N2O2Si |
| Mr | 258.44 | 242.39 | 216.36 |
| T(K) | 180(2) | 180(2) | 160(2) |
| λ(Å) | 0.71073 | 0.71073 | 0.71073 |
| Crystal system | monoclinic | monoclinic | monoclinic |
| Space group | P21/n | P21/c | C2/c |
| a(Å) | 10.1751(4) | 12.9780(4) | 12.9491(8) |
| b(Å) | 14.8904(6) | 12.1486(3) | 17.6134(8) |
| c(Å) | 10.5661(3) | 17.9866(6) | 11.0312(5) |
| β(°) | 109.834(2) | 102.055(3) | 97.538(4) |
| V(Å3) | 1505.92(10) | 2773.31(15) | 2494.2(2) |
| Z | 4 | 8 | 8 |
| ρcalc(g·cm−1) | 1.14 | 1.16 | 1.15 |
| μMoKα (mm−1) | 0.2 | 0.2 | 0.2 |
| F(000) | 568 | 1056 | 944 |
| θmax(°), Rint | 28.0, 0.0524 | 26.0, 0.0443 | 25.0, 0.1084 |
| Completeness | 100% | 100% | 99.9% |
| Reflns collected | 13,638 | 26,472 | 12,357 |
| Reflns unique | 3627 | 5428 | 2186 |
| Restraints | 21 1 | 31 2 | 0 |
| Parameters | 182 | 393 | 139 |
| GoF | 1.021 | 1.041 | 0.971 |
| R1, wR2 [I > 2σ(I)] | 0.0405, 0.0972 | 0.0412, 0.0945 | 0.0446, 0.1023 |
| R1, wR2 (all data) | 0.0617, 0.1061 | 0.0620, 0.1031 | 0.0893, 0.1163 |
| Largest peak/hole (e·Å−3) | 0.32, −0.19 | 0.25, −0.21 | 0.19, −0.23 |
| Parameter | 2[(Aib)SiMeH(HPyr)] ·3(CHCl3) 1 | [tBuNH3][(Aib)2SiMe] ·(CHCl3)·(tBuNH2) |
|---|---|---|
| Formula | C21H43Cl9N4O4Si2 | C18H41Cl3N4O4Si |
| Mr | 790.82 | 511.99 |
| T(K) | 230(2) 2 | 100(2) |
| λ(Å) | 0.71073 | 1.54184 |
| Crystal system | triclinic | triclinic |
| Space group | ||
| a(Å) | 10.3734(3) | 10.4171(1) |
| b(Å) | 11.2006(4) | 11.9432(1) |
| c(Å) | 17.7795(6) | 12.8295(1) |
| α(°) | 105.412(3) | 96.129(1) |
| β(°) | 93.183(3) | 106.033(1) |
| γ(°) | 98.289(3) | 105.231(1) |
| V(Å3) | 1961.12(12) | 1452.25(2) |
| Z | 2 | 2 |
| ρcalc(g·cm−1) | 1.34 | 1.17 |
| μMoKα (mm−1) | 0.7 | 3.5 |
| F(000) | 820 | 548 |
| θmax(°), Rint | 26.0, 0.0648 | 74.5, 0.0480 |
| Completeness | 100% | 99.7% |
| Reflns collected | 33,664 | 113,590 |
| Reflns unique | 7701 | 5891 |
| Restraints | 173 3 | 0 |
| Parameters | 482 | 310 |
| GoF | 1.016 | 1.040 |
| R1, wR2 [I > 2σ(I)] | 0.0446, 0.1360 | 0.0379, 0.0993 |
| R1, wR2 (all data) | 0.0656, 0.1443 | 0.0390, 0.1002 |
| Largest peak/hole (e·Å−3) | 0.19, −0.25 | 0.70, −0.49 |
Appendix B
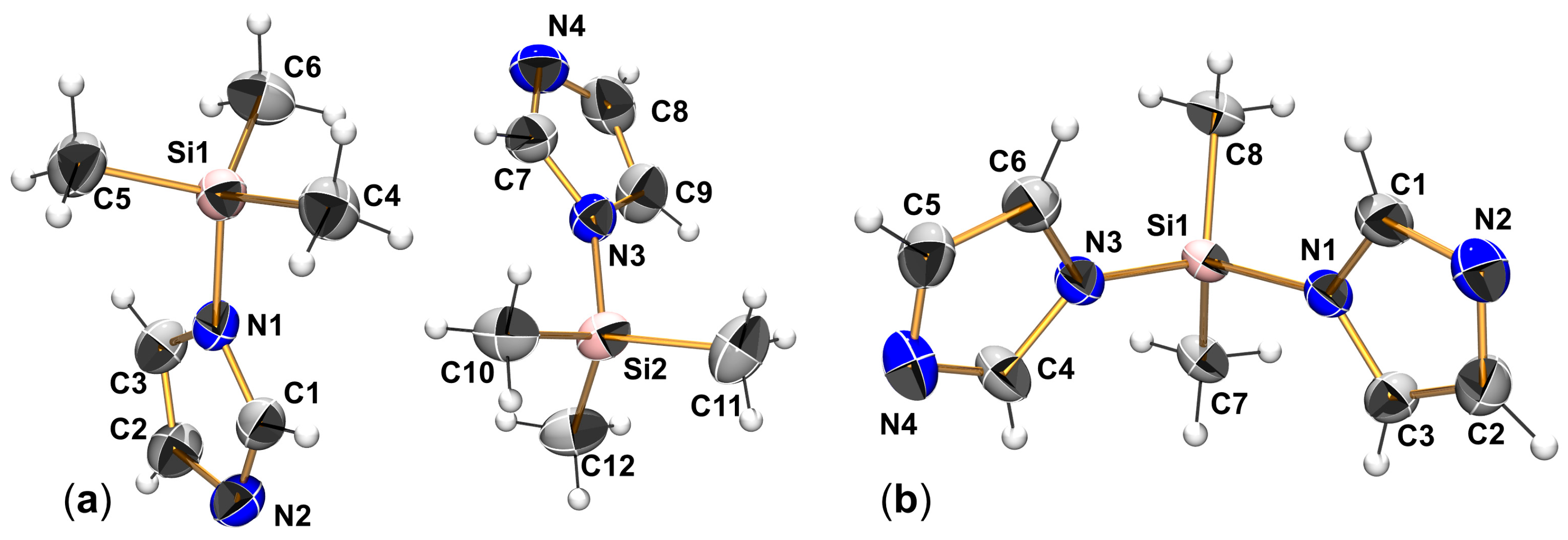
| SiMe3(Im) | SiMe2(Im)2 | ||
|---|---|---|---|
| Si1–N1, Si2–N3 | 1.778(2), 1.780(2) | Si1–N1 | 1.770(2) |
| Si1–C4, Si2–C10 | 1.846(2), 1.846(2) | Si1–N3 | 1.753(2) |
| Si1–C5, Si2–C11 | 1.849(2), 1.845(2) | Si1–C7 | 1.833(2) |
| Si1–C6, Si2–C12 | 1.843(2), 1.854(2) | Si1–C8 | 1.826(2) |
| N1–C1, N3–C7 | 1.359(2), 1.361(2) | N1–C1, N3–C4 | 1.372(2), 1.368(2) |
| N2–C1, N4–C7 | 1.310(2), 1.305(2) | N2–C1, N4–C4 | 1.308(3), 1.307(2) |
| N1–C3, N3–C9 | 1.382(2), 1.383(2) | N1–C3, N3–C6 | 1.380(2), 1.388(2) |
| N2–C2, N4–C8 | 1.378(2), 1.370(2) | N2–C2, N4–C5 | 1.375(3), 1.376(3) |
| C2–C3, C8–C9 | 1.352(2), 1.346(3) | C2–C3, C5–C6 | 1.351(3), 1.338(3) |
| N1–Si1–C4, N3–Si2–C10 | 106.1(1), 106.2(1) | N1–Si1–N3 | 106.9(1) |
| N1–Si1–C5, N3–Si2–C11 | 107.1(1), 107.7(1) | N1–Si1–C7 | 108.4(1) |
| N1–Si1–C6, N3–Si2–C12 | 106.0(1), 106.3(1) | N1–Si1–C8 | 107.6(1) |
| C4–Si1–C5, C10–Si2–C11 | 111.5(1), 112.5(1) | N3–Si1–C7 | 109.1(1) |
| C4–Si1–C6, C10–Si2–C12 | 113.5(1), 112.2(1) | N3–Si1–C8 | 109.3(1) |
| C5–Si1–C6, C11–Si2–C12 | 112.1(1), 111.6(1) | C7–Si1–C8 | 115.3(1) |
References
- Sivaramakrishna, A.; Pete, S.; Mhaskar, C.M.; Ramann, H.; Ramanaiah, D.V.; Arbaaz, M.; Niyaz, M.; Janardan, S.; Suman, P. Role of hypercoordinated silicon(IV) complexes in activation of carbon–silicon bonds: An overview on utility in synthetic chemistry. Coord. Chem. Rev. 2023, 485, 215140. [Google Scholar] [CrossRef]
- Singh, G.; Kaur, G.; Singh, J. Progressions in hyper–coordinate silicon complexes. Inorg. Chem. Commun. 2018, 88, 11–20. [Google Scholar] [CrossRef]
- Lemière, G.; Millanvois, A.; Ollivier, C.; Fensterbank, L. A Parisian Vision of the Chemistry of Hypercoordinated Silicon Derivatives. Chem. Rec. 2021, 21, 1119–1129. [Google Scholar] [CrossRef]
- Wagler, J.; Böhme, U.; Kroke, E. Higher-Coordinated Molecular Silicon Compounds. In Functional Molecular Silicon Compounds I—Regular Oxidation States; Scheschkewitz, D., Ed.; Springer: Berlin/Heidelberg, Germany, 2013; Volume 115, pp. 29–105. [Google Scholar] [CrossRef]
- Chuit, C.; Corriu, R.J.P.; Reye, C.; Young, J.C. Reactivity of Penta- and Hexacoordinate Silicon Compounds and Their Role as Reaction Intermediates. Chem. Rev. 1993, 93, 1371–1448. [Google Scholar] [CrossRef]
- Peloquin, D.M.; Schmedake, T.A. Recent advances in hexacoordinate silicon with pyridine-containing ligands: Chemistry and emerging applications. Coord. Chem. Rev. 2016, 323, 107–119. [Google Scholar] [CrossRef]
- Davy, J., XVIII. An Account of some Experiments on different Combinations of Fluoric Acid. Philos. Trans. R. Soc. Lond. 1812, 102, 352–369. [Google Scholar] [CrossRef][Green Version]
- Kowalke, J.; Brendler, E.; Wagler, J. Valinate and SiMe2—An interesting couple in pentacoordinate Si-complexes: Templated generation of the dipeptide val-val and formation of an organosilicon-ammonia-adduct. J. Organomet. Chem. 2021, 956, 122126. [Google Scholar] [CrossRef]
- Rutt, O.J.; Cowley, A.R.; Clarke, S.J. Bis(ethylamino)tetrafluorosilicon. Acta Crystallogr. E 2007, 63, o3406. [Google Scholar] [CrossRef]
- Thorwart, T.; Hartmann, D.; Greb, L. Dihydrogen Activation with a Neutral, Intermolecular Silicon(IV)-Amine Frustrated Lewis Pair. Chem. Eur. J. 2022, 28, e202202273. [Google Scholar] [CrossRef]
- Mitzel, N.W.; Vojinović, K.; Foerster, T.; Robertson, H.E.; Borisenko, K.B.; Rankin, D.W.H. (Dimethylaminomethyl)trifluorosilane, Me2NCH2SiF3—A Model for the α-Effect in Aminomethylsilanes. Chem. Eur. J. 2005, 11, 5114–5125. [Google Scholar] [CrossRef]
- Seidel, A.; Wagler, J. Anions of α-Amino Acids as (O,N)-Donor Ligands in Si-, Ge- and Sn-Coordination Chemistry. Molecules 2025, 30, 834. [Google Scholar] [CrossRef] [PubMed]
- Seidel, A.; Gericke, R.; Kutzner, B.; Wagler, J. Lewis Acid-Base Adducts of α-Amino Acid-Derived Silaheterocycles and N-Methylimidazole. Molecules 2023, 28, 7816. [Google Scholar] [CrossRef] [PubMed]
- Cota, S.; Beyer, M.; Bertermann, R.; Burschka, C.; Götz, K.; Kaupp, M.; Tacke, R. Neutral Penta- and Hexacoordinate Silicon(IV) Complexes Containing Two Bidentate Ligands Derived from the α-Amino Acids (S)-Alanine, (S)-Phenylalanine, and (S)-tert-Leucine. Chem. Eur. J. 2010, 16, 6582–6589. [Google Scholar] [CrossRef]
- Addison, A.W.; Rao, T.N.; Reedijk, J.; van Rijn, J.; Verschoor, G.C. Synthesis, Structure, and Spectroscopic Properties of Copper(II) Compounds containing Nitrogen-Sulphur Donor Ligands; the Crystal and Molecular Structure of Aqua [1,7-bis(N-methylbenzimidazol-2′-yl)-2,6-dithiaheptane]copper(II) Perchlorate. J. Chem. Soc. Dalton. Trans. 1984, 13, 1349–1356. [Google Scholar] [CrossRef]
- Wagler, J.; Böhme, U.; Brendler, E.; Roewer, G. First X-Ray Structure of a Cationic Silicon Complex with Salen-Type Ligand: An Unusual Compound with Two Different Si-N Dative Bonds. Z. Naturforsch. B 2004, 59, 1348–1352. [Google Scholar] [CrossRef]
- Puri, J.K.; Singh, R.; Kaur Chahal, V. Silatranes: A review on their synthesis, structure, reactivity and applications. Chem. Soc. Rev. 2011, 40, 1791–1840. [Google Scholar] [CrossRef]
- Chen, L.; Xie, Q.; Sun, L.; Wang, H. Synthesis and characterization of 1-ferrocenecarboxysilatranes and crystal structures of FcC(CH3)=CHCOOSi(OCH2CH2)3N and p-FcC6H4COOSi(OCH2CH2)3N. J. Organomet. Chem. 2003, 678, 90–94. [Google Scholar] [CrossRef]
- Garant, R.J.; Daniels, L.M.; Das, S.K.; Janakiraman, M.N.; Jacobson, R.A.; Verkade, J.G. Lewis Basicity of Silatranes and the Molecular Structures of EtOSi(OCH2CH2)3N, Me2O+Si(OCH2CH2)3N, and CF3CO2HEtOSi(OCH2CH2)3N. J. Am. Chem. Soc. 1991, 113, 5728–5735. [Google Scholar] [CrossRef]
- Seiler, O.; Burschka, C.; Fenske, T.; Troegel, D.; Tacke, R. Neutral Hexa- and Pentacoordinate Silicon(IV) Complexes with SiO6 and SiO4N Skeletons. Inorg. Chem. 2007, 46, 5419–5424. [Google Scholar] [CrossRef]
- Bernstein, J.; Davis, R.E.; Shimoni, L.; Chang, N.-L. Patterns in Hydrogen Bonding: Functionality and Graph Set Analysis in Crystals. Angew. Chem. Int. Ed. 1995, 34, 1555–1573. [Google Scholar] [CrossRef]
- Iwamiya, J.H.; Maciel, G.E. Chemical Shifts in Silatrane and Its Derivatives: A Study of the Transannular Interaction. J. Am. Chem. Soc. 1993, 115, 6835–6842. [Google Scholar] [CrossRef]
- Bitto, F.; Kraushaar, K.; Böhme, U.; Brendler, E.; Wagler, J.; Kroke, E. Chlorosilanes and 3,5-Dimethylpyrazole: Multinuclear Complexes, Acetonitrile Insertion and 29Si NMR Chemical-Shift Anisotropy Studies. Eur. J. Inorg. Chem. 2013, 2013, 2954–2962. [Google Scholar] [CrossRef]
- Gerlach, D.; Brendler, E.; Heine, T.; Wagler, J. Dianion of Pyrrole-2-N-(o-hydroxyphenyl)carbaldimine as an Interesting Tridentate (ONN) Ligand System in Hypercoordinate Silicon Complexes. Organometallics 2007, 26, 234–240. [Google Scholar] [CrossRef]
- Bertermann, R.; Biller, A.; Kaupp, M.; Penka, M.; Seiler, O.; Tacke, R. New Zwitterionic Spirocyclic λ5 Si-Silicates with SiX4C Skeleton (X = S, O) Containing Two Ligands of the Dithiolato(2-) or Diolato(2-) Type: Synthesis, Structure and Bonding Situation. Organometallics 2003, 22, 4104–4110. [Google Scholar] [CrossRef]
- Brendler, E.; Heine, T.; Hill, A.F.; Wagler, J. A Pentacoordinate Chlorotrimethylsilane Derivate: A very Polar Snapshot of a Nucleophilic Substitution and its Influence on 29Si Solid State NMR Properties. Z. Anorg. Allg. Chem. 2009, 635, 1300–1305. [Google Scholar] [CrossRef]
- Thomas, B.; Paasch, S.; Steuernagel, S.; Eichele, K. Residual 31P, 35,37Cl Dipolar Coupling in 31P MAS Spectra of Chlorocyclophosphazenes. Solid State Nucl. Magn. Res. 2001, 20, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Tacke, R.; Bertermann, R.; Biller, A.; Dannappel, O.; Penka, M.; Pülm, M.; Willeke, R. Zwitterionic Spirocyclic λ5Si-Silicates with Two Bidentate Acetohydroximato(2-) or Benzohydroximato(2-) Ligands: Synthesis, Structure, and Dynamic Stereochemistry. Z. Anorg. Allg. Chem. 2000, 626, 1159–1173. [Google Scholar] [CrossRef]
- Richter, I.; Penka, M.; Tacke, R. [Benzene-1,2-diolato(2-)][benzene-1,2-diolato(1-)]methyl-[(2,2,6,6-tetramethylpiperidinio)methyl]silicate: Isolation, Structural Characterization, and Thermally Induced Methane Elimination. Organometallics 2002, 21, 3050–3053. [Google Scholar] [CrossRef]
- Tacke, R.; Bertermann, R.; Burschka, C.; Dragota, S.; Penka, M.; Richter, I. Spirocyclic Zwitterionic λ5Si-Silicates with Two Bidentate Ligands Derived from α-Amino Acids or α-Hydroxycarboxylic Acids: Synthesis, Structure, and Stereodynamics. J. Am. Chem. Soc. 2004, 126, 14493–14505. [Google Scholar] [CrossRef]
- Dragota, S.; Bertermann, R.; Burschka, C.; Penka, M.; Tacke, R. Diastereo- and Enantiomerically Pure Zwitterionic Spirocyclic λ5Si-[(Ammonio)methyl]silicates with an SiO2N2C Skeleton Containing Two Bidentate Chelate Ligands Derived from α-Amino Acids. Organometallics 2005, 24, 5560–5568. [Google Scholar] [CrossRef]
- Hensen, K.; Zengerly, T.; Müller, T.; Pickel, P. Ionic Structures of 4- and 5-coordinated Silicon. Novel Ionic Crystal Structures of 4- and 5-coordinated Silicon: [Me3Si(NMI)]+ Cl−, [Me2HSi(NMI)2]+ Cl−, [Me2Si(NMI)3]2+ 2 Cl−·NMI. Z. Anorg. Allg. Chem. 1988, 558, 21–27. [Google Scholar] [CrossRef]
- Kost, D.; Kingston, J.; Gostevskii, B.; Ellern, A.; Stalke, D.; Walfort, B.; Kalikhman, I. Donor-Stabilized Silyl Cations. 3. Ionic Dissociation of Hexacoordinate Silicon Complexes to Pentacoordinate Siliconium Salts Driven by Ion Solvation. Organometallics 2002, 21, 2293–2305. [Google Scholar] [CrossRef]
- Kraushaar, K.; Wiltzsch, C.; Wagler, J.; Böhme, U.; Schwarzer, A.; Roewer, G.; Kroke, E. From CO2 to Polysiloxanes: Di(carbamoyloxy)silanes Me2Si[(OCO)NRR′]2 as Precursors for PDMS. Organometallics 2012, 31, 4779–4785. [Google Scholar] [CrossRef]
- Passarelli, V.; Benetollo, F.; Zanella, P.; Carta, G.; Rossetto, G. Synthesis and characterisation of novel zirconium(IV) derivatives containing the bis-amido ligand SiMe2(NRR′)2. Dalton Trans. 2003, 32, 1411–1418. [Google Scholar] [CrossRef]
- Ryll, C. Carbamoyloxymono-und-Disilane—Synthese, Substituentenaustauschverhalten und Zersetzung. Ph.D. Thesis, Technische Universität Bergakademie Freiberg, Freiberg, Germany, 1 December 2022. [Google Scholar]
- Eichele, K. Herzfeld-Berger Analysis Program; HBA (Version 1.7.3 23.8.2012); University of Tübingen: Tübingen, Germany, 2012. [Google Scholar]
- Massiot, D.; Fayon, F.; Capron, M.; King, I.; Le Calvlé, S.; Alonso, B.; Durand, J.-O.; Bujoli, B.; Gan, Z.; Hoatson, G. Modeling one and two-dimensional Solid State NMR spectra. Magn. Reson. Chem. 2002, 40, 70–76. [Google Scholar] [CrossRef]
- Herzfeld, J.; Berger, A.E. Sideband intensities in NMR spectra of samples spinning at the magic angle. J. Chem. Phys. 1980, 73, 6021–6030. [Google Scholar] [CrossRef]
- Mason, J. Conventions for the reporting of nuclear magnetic shielding (or shift) tensors suggested by participants in the NATO ARW on NMR shielding constants at the University of Maryland, College Park, July 1992. Solid State Nucl. Magn. Reson. 1993, 2, 285–288. [Google Scholar] [CrossRef] [PubMed]
- Wagler, J.; Gericke, R. Molecular structures of various alkyldichlorosilanes in the solid state. Dalton Trans. 2017, 46, 8875–8882. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. A 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Program for the Refinement of Crystal Structures; SHELXL-2018/3; University of Göttingen: Göttingen, Germany, 2018. [Google Scholar]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. C 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Farrugia, L.J. ORTEP-3 for windows—A version of ORTEP-III with a graphical user interface (GUI). J. Appl. Crystallogr. 1997, 30, 565. [Google Scholar] [CrossRef]
- Farrugia, L.J. WinGX and ORTEP for Windows: An update. J. Appl. Crystallogr. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- POV-RAY (Version 3.7), Trademark of Persistence of Vision Raytracer Pty. Ltd., Williamstown, Victoria (Australia). Copyright Hallam Oaks Pty. Ltd., 1994–2004. Available online: http://www.povray.org/download/ (accessed on 28 June 2021).
- Mercury (Version 2021.3.0, build 333817). Available online: http://www.ccdc.cam.ac.uk/ (accessed on 30 November 2021).
- FIA Calculator. Available online: https://www.grebgroup.de/fia-gnn (accessed on 19 July 2025).
- Sigmund, L.M.; Shree Sowndarya, S.V.; Albers, A.; Erdmann, P.; Paton, R.S.; Greb, L. Predicting Lewis Acidity: Machine Learning the Fluoride Ion Affinity of p-Block-Atom-Based Molecules. Angew. Chem. Int. Ed. 2024, 63, e202401084. [Google Scholar] [CrossRef]
- Spek, A.L. Single-crystal structure validation with the program PLATON. J. Appl. Cryst. 2003, 36, 7–13. [Google Scholar] [CrossRef]
- Spek, A.L. Structure validation in chemical crystallography. Acta Crystallogr. D 2009, 65, 148–155. [Google Scholar] [CrossRef]
- Spek, A.L. PLATON SQUEEZE: A tool for the calculation of the disordered solvent contribution to the calculated structure factors. Acta Crystallogr. C 2015, 71, 9–18. [Google Scholar] [CrossRef]
- Reveley, M.J.; Feld, J.; Temerova, D.; Yang, E.S.; Goicoechea, J.M. Hydroelementation and Phosphinidene Transfer: Reactivity of Phosphagermenes and Phosphastannenes Towards Small Molecule Substrates. Chem. Eur. J. 2023, 29, e202301542. [Google Scholar] [CrossRef]
- Weiss, A.; Pritzkow, H.; Siebert, W. Synthesis, Structures and Reactivity of N-Borane-Protected 1,1′-Bisimidazoles with Different Bridging Functions. Eur. J. Inorg. Chem. 2002, 2002, 1607–1614. [Google Scholar] [CrossRef]
- Hensen, K.; Lemke, A.; Näther, C. Synthesis and Crystal Structure Determination of Hexachloro-μ-dichloro-bis[N-(trimethylsilyl)imidazol]dititanium Chloroform (1/2). Z. Anorg. Allg. Chem. 1997, 623, 1973–1977. [Google Scholar] [CrossRef]
- Hensen, K.; Gebhardt, F.; Bolte, M. Synthese und Kristallstrukturbestimmung von Additionsverbindungen aus Alkyldimethylbromsilanen und N-Trimethylsilylimidazol. Z. Naturforsch. B 1997, 52, 1491–1496. [Google Scholar] [CrossRef]
- Purdy, A.P.; Gilardi, R.; Luther, J.; Butcher, R.J. Synthesis, crystal structure, and reactivity of alkali and silver salts of sulfonated imidazoles. Polyhedron 2007, 26, 3930–3938. [Google Scholar] [CrossRef]
- Junold, K.; Burschka, C.; Tacke, R. Activation of Nitriles by Trichloro [2-(dialkylphosphanyl)imidazol-1-yl]silanes—Synthesis and Characterization of New Dinuclear Pentacoordinate Silicon(IV) Complexes with Bridging Imido-Nitrogen Ligand Atoms. Eur. J. Inorg. Chem. 2012, 2012, 189–193. [Google Scholar] [CrossRef]
- Kocherga, M.; Boyle, K.M.; Merkert, J.; Schmedake, T.A.; Walter, M.G. Exploring the molecular electronic device applications of synthetically versatile silicon pincer complexes as charge transport and electroluminescent layers. Mater. Adv. 2022, 3, 2373–2379. [Google Scholar] [CrossRef]



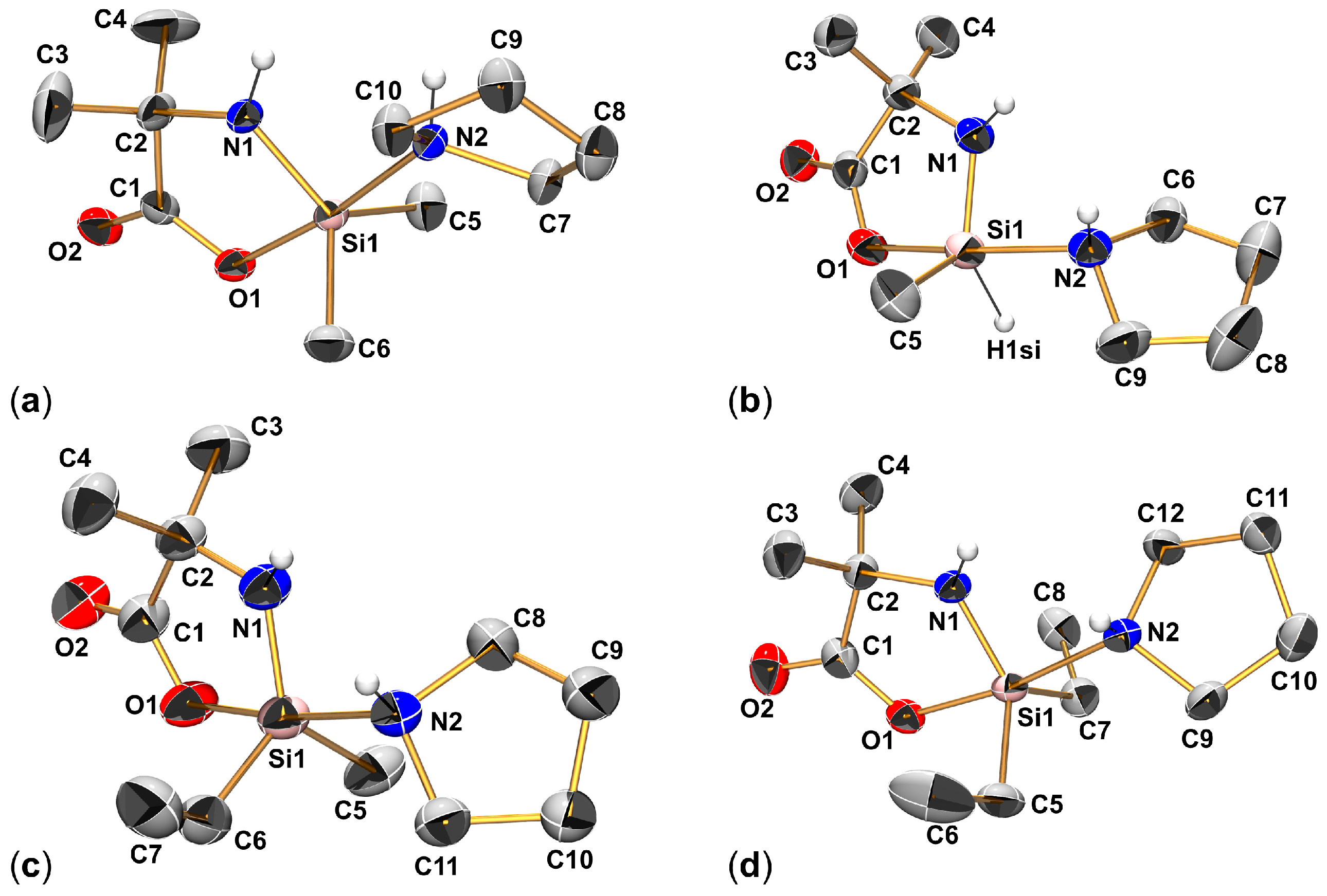




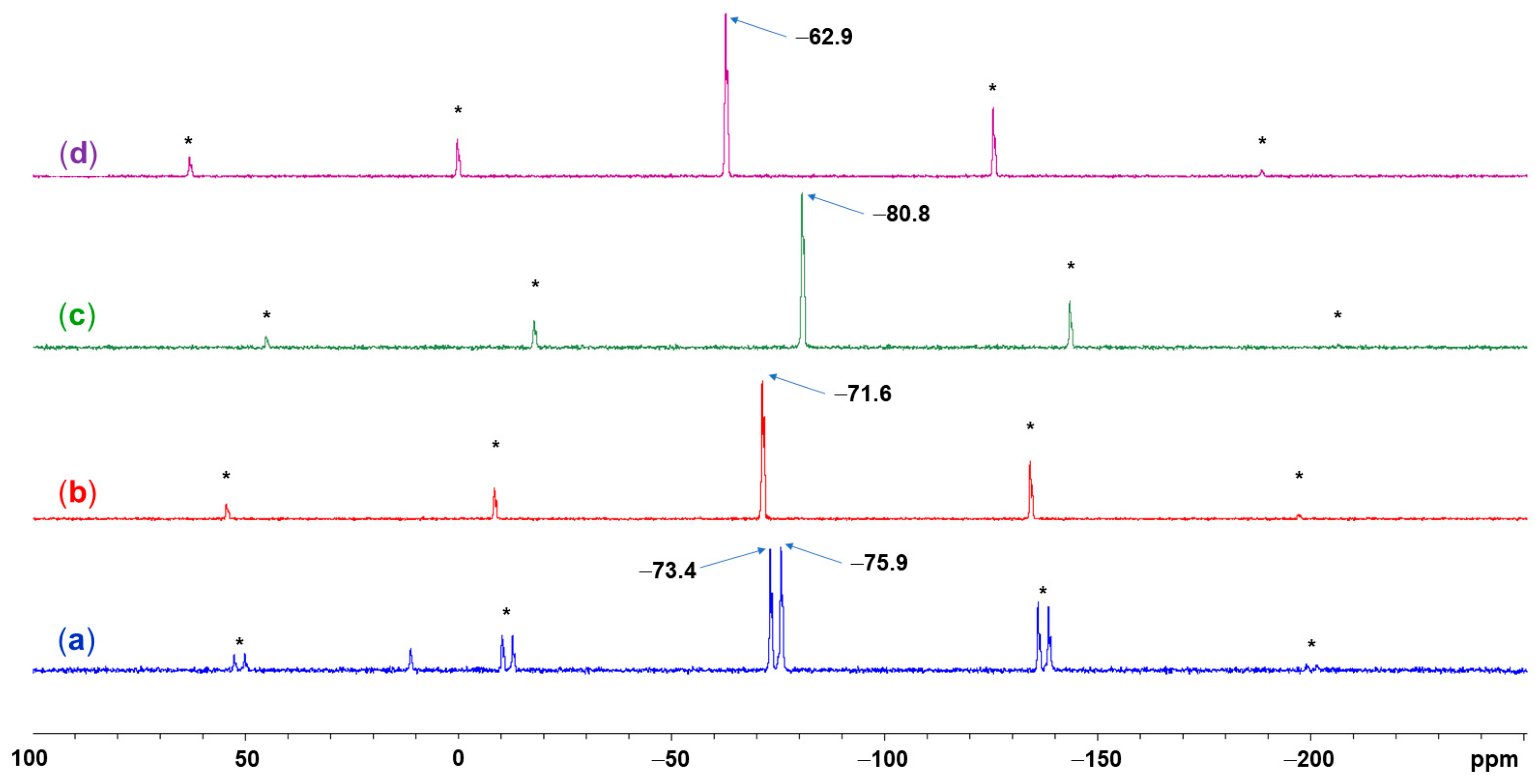

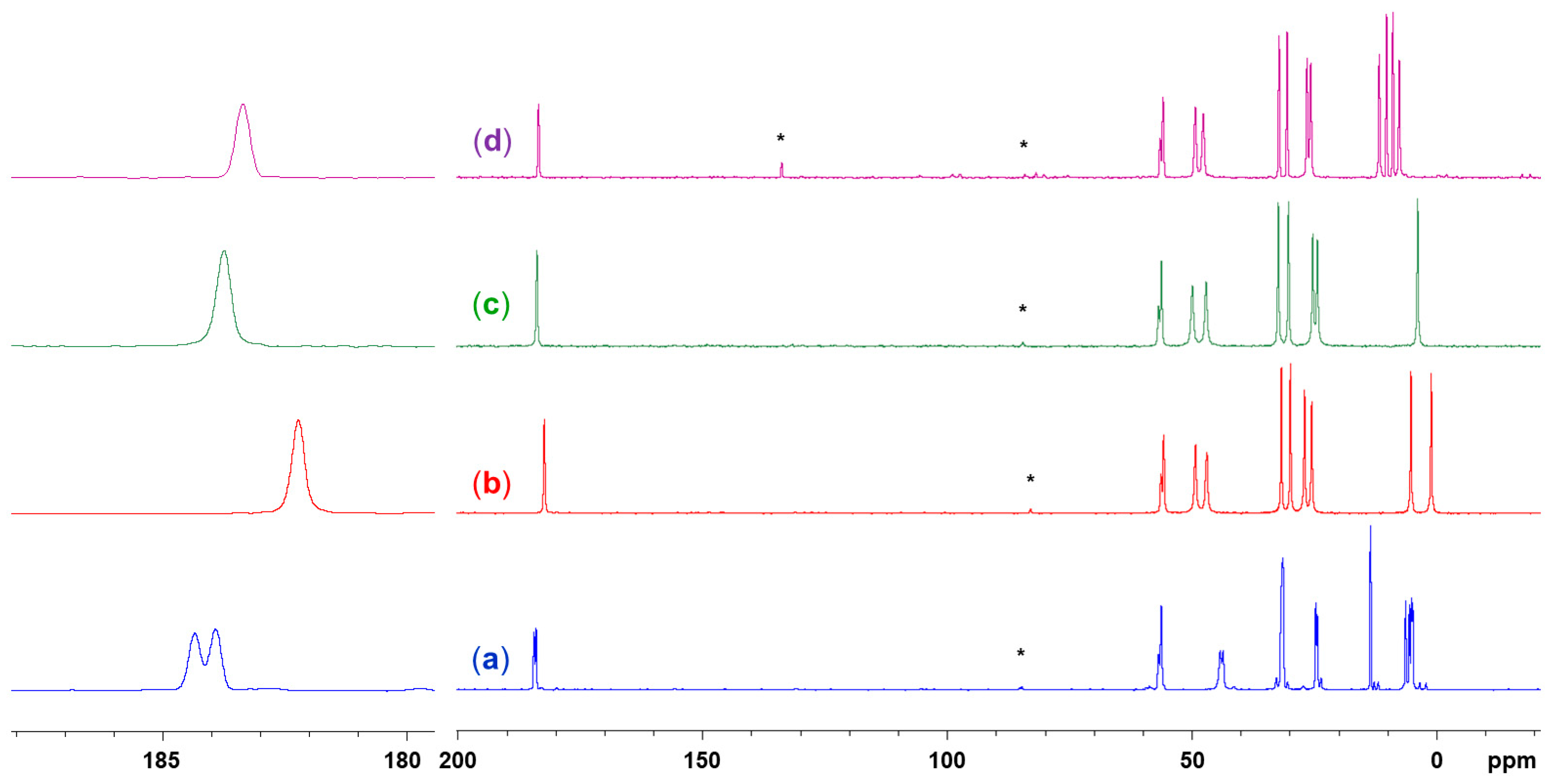
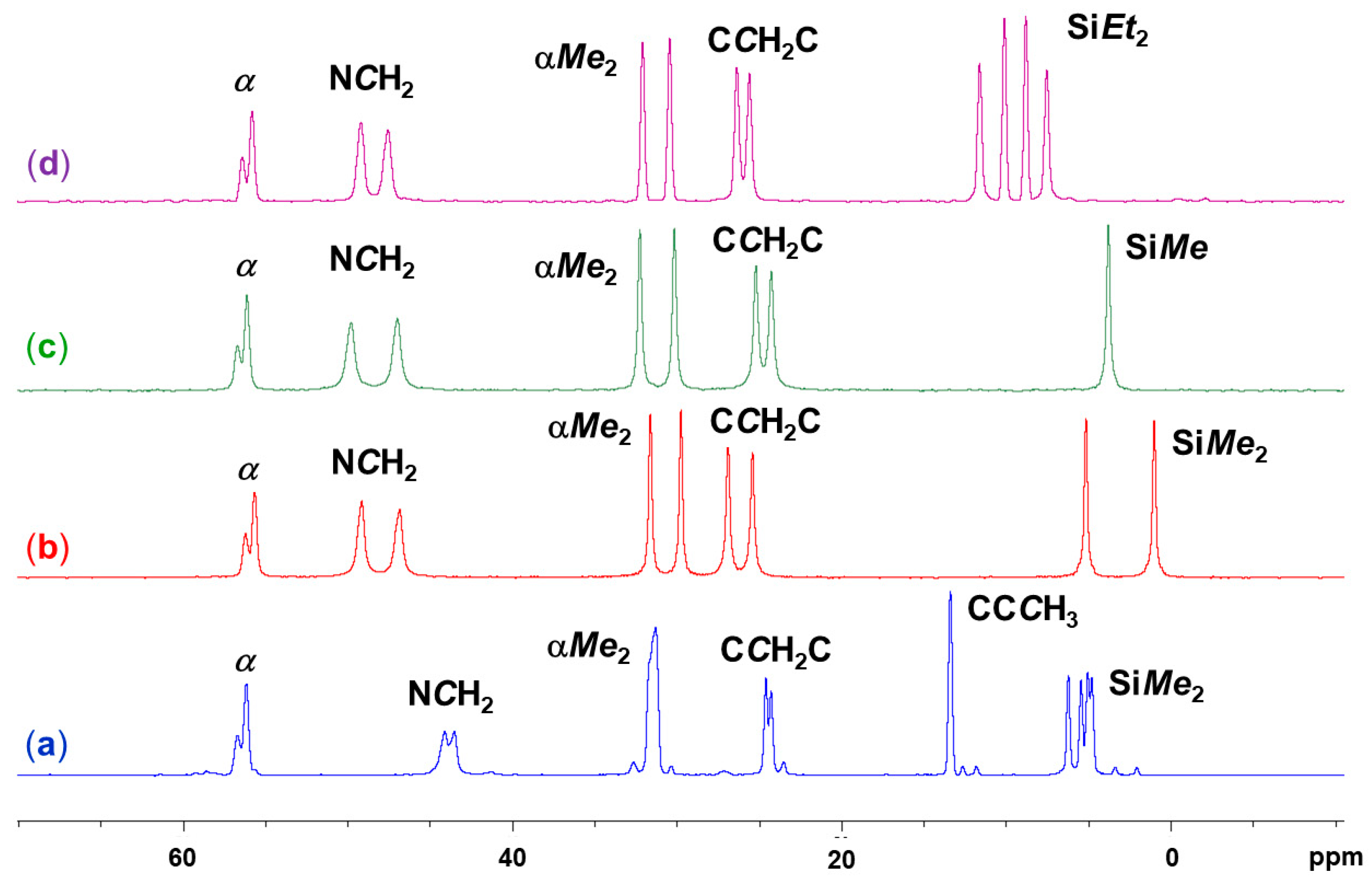
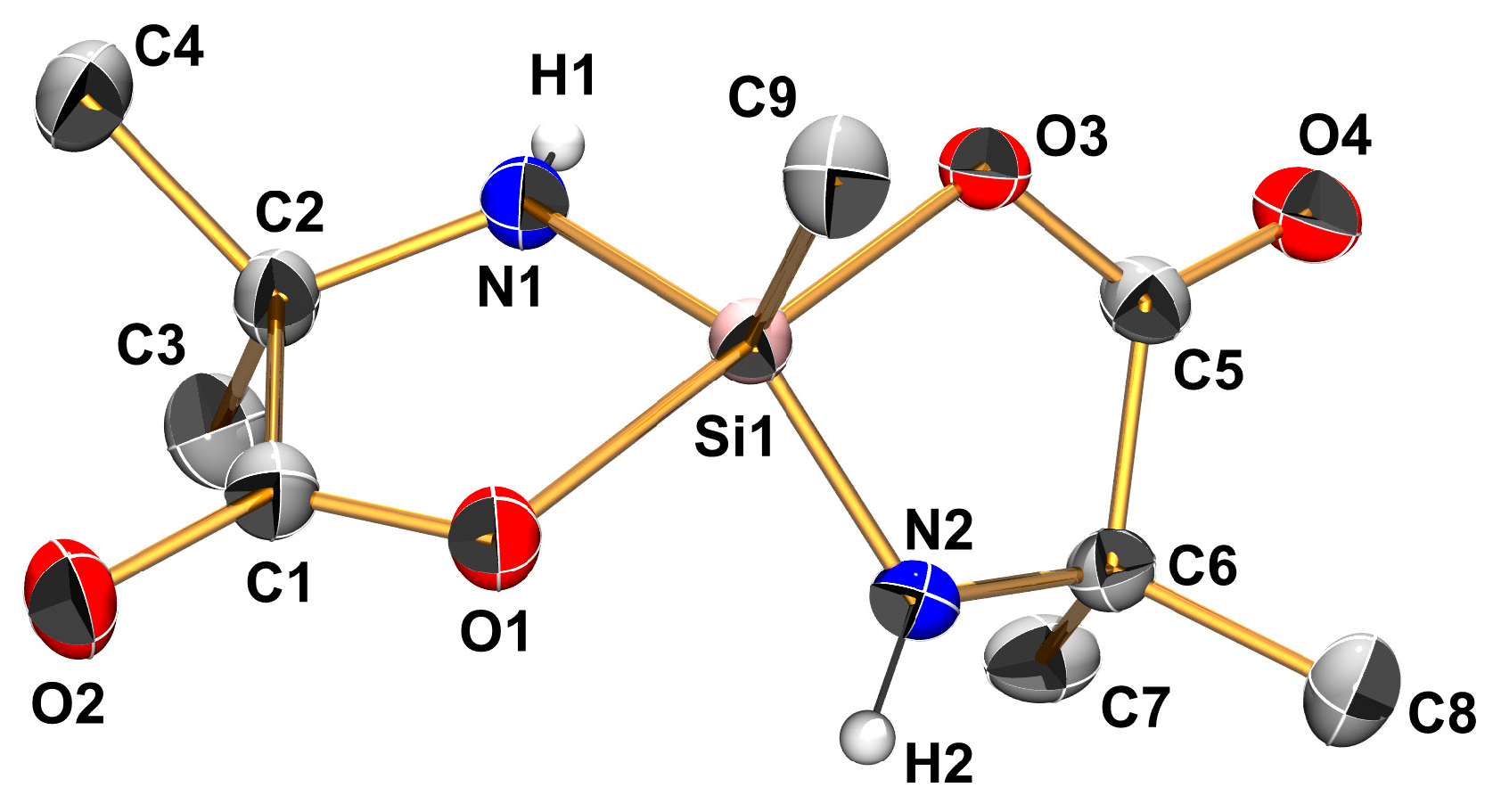
| Compound | Si1–O1 | Si1–N1 | Si1–N2 | O1–Si1–N2 | N1–Si1–C5 | N1–Si1–A3 | C5–Si1–A3 | τ5 |
|---|---|---|---|---|---|---|---|---|
| (Aib)SiMe2(NMI) 1 | 1.852(2) | 1.713(2) | 2.036(2) | 172.3(2) | 123.4(2) | 122.7(2) | 113.9(2) | 0.82 |
| (Aib)SiMe2(HIm) 2 | 1.867(2) | 1.720(2) | 2.034(2) | 172.2(1) | 121.9(1) | 124.1(1) | 114.0(1) | 0.80 |
| (Aib)SiMe2(H2NnPr) | 1.877(3) | 1.717(4) | 2.031(4) | 172.5(2) | 120.6(2) | 123.9(2) | 115.4(2) | 0.81 |
| 1.882(3) | 1.711(4) | 2.011(4) | 172.2(2) | 121.5(2) | 125.3(2) | 113.2(2) | 0.78 | |
| (Aib)SiMe2(HPyr) | 1.869(1) | 1.718(2) | 2.031(2) | 172.5(1) | 120.9(1) | 123.3(1) | 115.8(1) | 0.82 |
| (Aib)SiMe2(HPyr) 3 | 1.877(3) | 1.715(4) | 2.034(4) | 172.1(2) | 121.5(2) | 122.5(2) | 116.0(2) | 0.83 |
| (Aib)SiMeH(HPyr) | 1.840(2) | 1.708(2) | 1.986(2) | 170.1(1) | 120.8(2) | 124.6(8) | 114.6(8) | 0.76 |
| (Aib)SiMeH(HPyr) 4 | 1.845(2) | 1.697(2) | 1.996(2) | 170.0(1) | 119.8(1) | 123.2(8) | 116.9(8) | 0.78 |
| 1.861(2) | 1.700(2) | 1.989(2) | 170.9(1) | 120.9(2) | 124.1(8) | 115.0(8) | 0.78 | |
| (Aib)SiMeVi(HPyr) 5 | 1.858(2) | 1.712(2) | 2.014(2) | 173.1(1) | 124.6(2) | 121.3(2) | 114.2(2) | 0.81 |
| 1.852(2) | 1.702(2) | 2.029(2) | 173.8(1) | 125.8(4) | 119.1(4) | 115.1(3) | 0.80 | |
| (Aib)SiEt2(HPyr) | 1.871(2) | 1.713(2) | 2.081(2) | 171.3(1) | 123.2(1) | 122.4(1) | 114.4(1) | 0.80 |
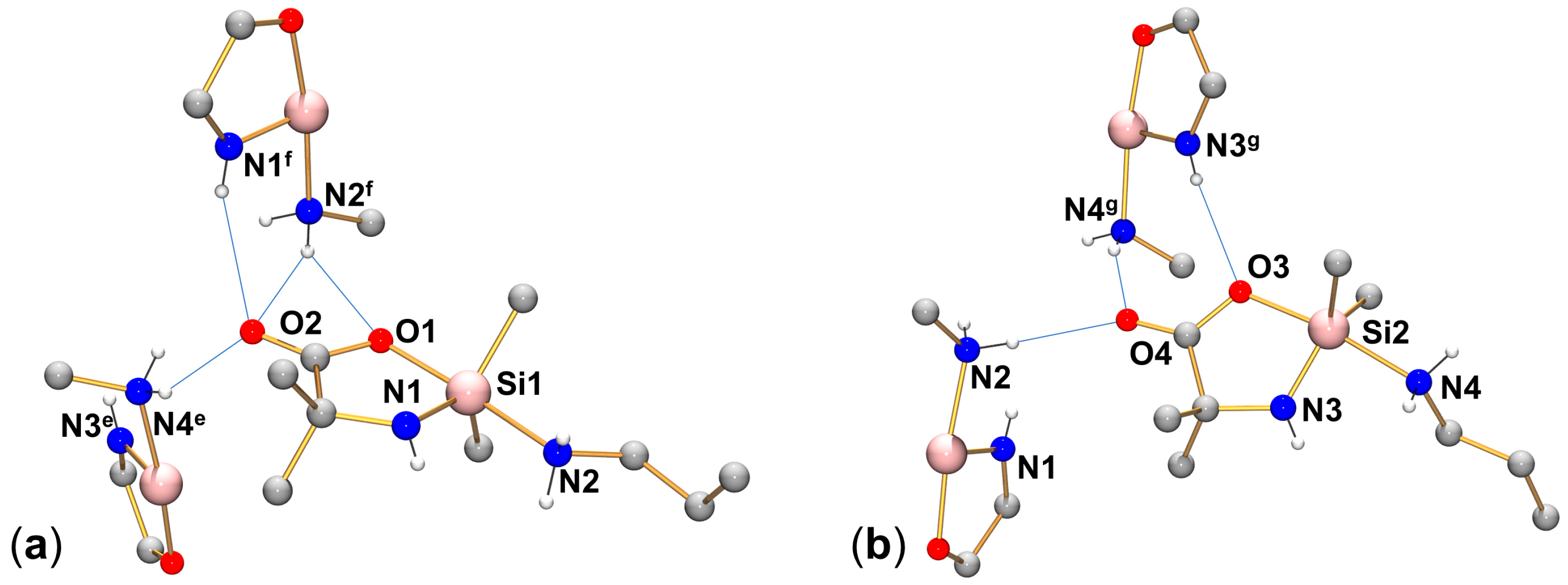
| Compound | C⋯Ocarbonyl | d(D⋯O) D–H⋯O | N⋯Ocarbonyl | d(D⋯O) D–H⋯O | N⋯Ocarboxylato | d(D⋯O) D–H⋯O | Symmetry Operations |
|---|---|---|---|---|---|---|---|
| (Aib)SiMe2(HIm) 1 | C10⋯O2 | 2.940(2) 128 | N3a⋯O2 | 2.755(2) 171 | - | - | a x, 1.5 − y, −0.5 + z |
| (Aib)SiMe2(H2NnPr) molecule 1 | - | - | N4e⋯O2 N1f⋯O2 N2f⋯O2 | 2.980(6) 146 3.247(5) 158 3.177(6) 153 | N2f⋯O1 | 3.279(5) 147 | e x, 1 + y, z; f −0.5 + x, 2 − y, −0.5 + z |
| (Aib)SiMe2(H2NnPr) molecule 2 | - | - | N2⋯O4 N4g⋯O4 | 3.081(6) 177 2.973(5) 157 | N3g⋯O3 | 3.272(5) 174 | g 0.5 + x, 1 − y, 0.5 + z |
| (Aib)SiMe2(HPyr) | - | - | N1c⋯O2 | 3.058(2) 163 | N2c⋯O1 | 3.085(2) 154 | c −0.5 + x, 1.5 − y, −0.5 + z |
| (Aib)SiMe2(HPyr) 2 | C11⋯O2 4 | 3.052(11) 172 | N2b⋯O2 | 2.918(4) 175 | - | - | b 0.5 − x, y, 0.5 + z |
| (Aib)SiMeH(HPyr) | - | - | N2d⋯O2 | 2.932(2) 169 | N1d⋯O1 | 3.193(2) 172 | d x, 1 − y, 0.5 + z |
| (Aib)SiMeH(HPyr) 3 molecule 1 | C20⋯O2 5 | 3.069(8) 164 | N4h⋯O2 | 2.887(2) 164 | N3h⋯O1 | 3.214(2) 172 | h x, −1 + y, z |
| (Aib)SiMeH(HPyr) 3 molecule 2 | C19i⋯O4 6 | 3.114(6) 157 | N2⋯O4 | 2.918(2) 167 | N1⋯O3 | 3.223(2) 169 | i −1 + x, y, z |
| (Aib)SiMeVi(HPyr) molecule 1 | - | - | N3j⋯O2 | 3.076(2) 169 | N4j⋯O1 | 3.003(2) 165 | j x, 1 + y, z |
| (Aib)SiMeVi(HPyr) molecule 2 | - | - | N1⋯O4 | 3.173(2) 154 | N2⋯O3 | 3.307(2) 144 | - |
| (Aib)SiEt2(HPyr) | - | - | N1k⋯O2 | 3.013(2) 170 | N2k⋯O1 | 3.178(2) 166 | k 0.5 + x, 0.5 − y, 0.5 + z |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seidel, A.; Brendler, E.; Torvisco, A.; Fischer, R.; Wagler, J. Lewis Acid–Base Adducts of α-Amino Isobutyric Acid-Derived Silaheterocycles and Amines. Molecules 2025, 30, 3501. https://doi.org/10.3390/molecules30173501
Seidel A, Brendler E, Torvisco A, Fischer R, Wagler J. Lewis Acid–Base Adducts of α-Amino Isobutyric Acid-Derived Silaheterocycles and Amines. Molecules. 2025; 30(17):3501. https://doi.org/10.3390/molecules30173501
Chicago/Turabian StyleSeidel, Anne, Erica Brendler, Ana Torvisco, Roland Fischer, and Jörg Wagler. 2025. "Lewis Acid–Base Adducts of α-Amino Isobutyric Acid-Derived Silaheterocycles and Amines" Molecules 30, no. 17: 3501. https://doi.org/10.3390/molecules30173501
APA StyleSeidel, A., Brendler, E., Torvisco, A., Fischer, R., & Wagler, J. (2025). Lewis Acid–Base Adducts of α-Amino Isobutyric Acid-Derived Silaheterocycles and Amines. Molecules, 30(17), 3501. https://doi.org/10.3390/molecules30173501








