Perspectives on the Catalytic Processes for the Deep Valorization of Carbohydrates into Fuels and Chemicals
Abstract
1. Introduction
2. Conversion of Carbohydrates into Platform Molecules
2.1. Furfural Production
2.1.1. Carbon-Based Catalysts
2.1.2. Zeolite-Based Catalysts
2.1.3. Other Solid Acid Catalysts
2.2. Production of 5-HMF and Levulinic Acid
3. Hydrogenation of Platform Molecules Using Ru-Catalysts
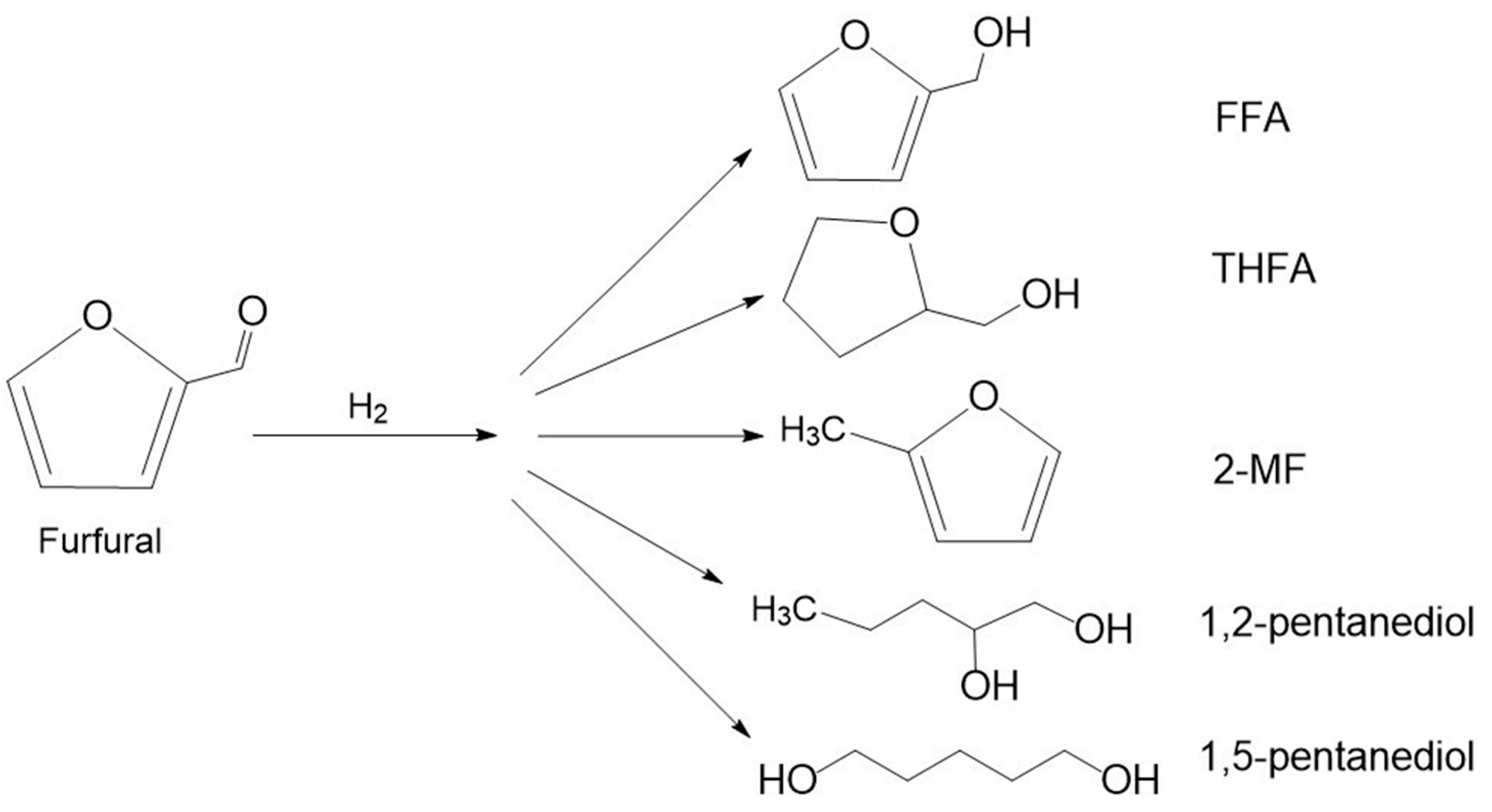
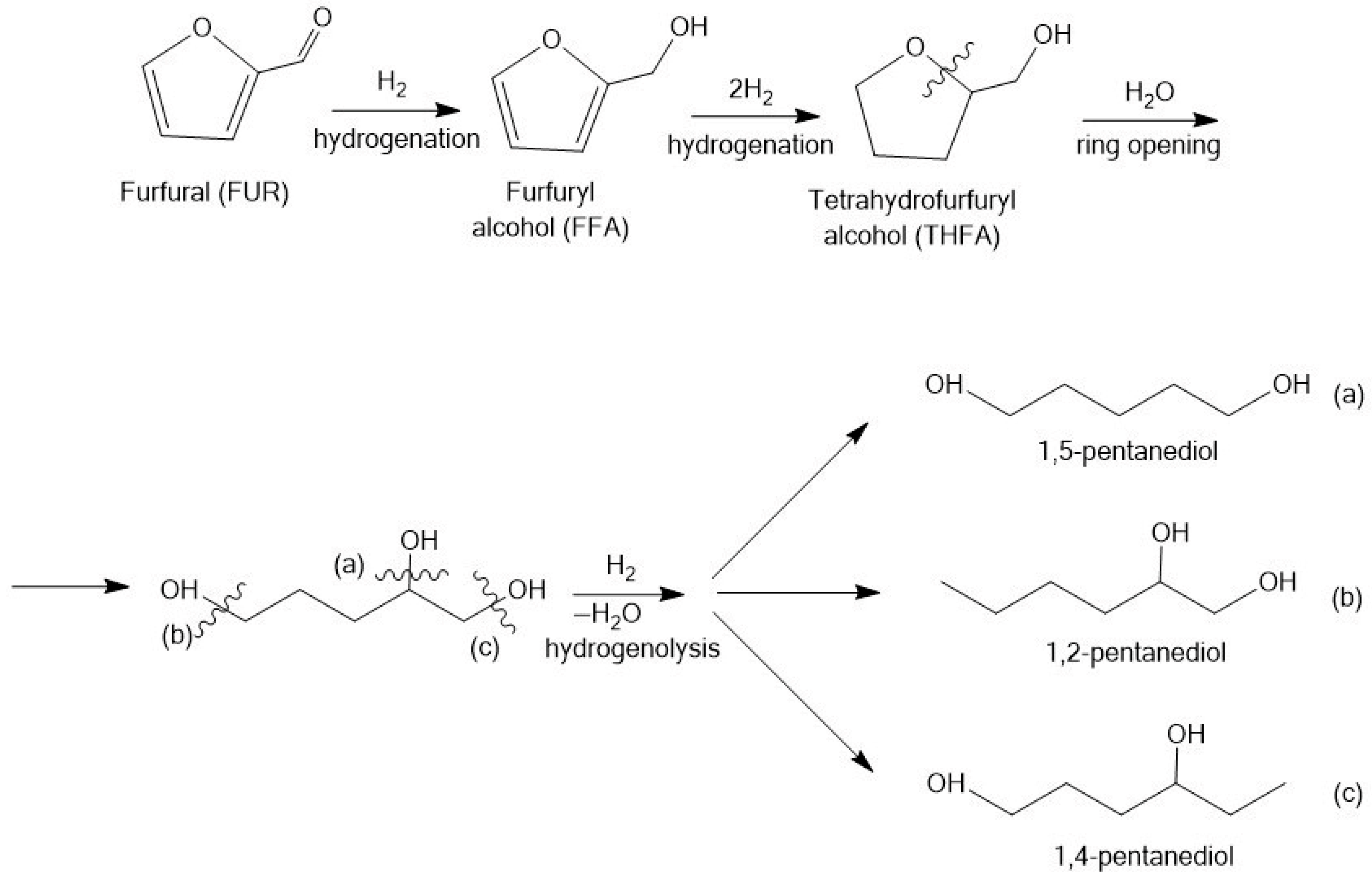
3.1. Hydrogenation of Furfural
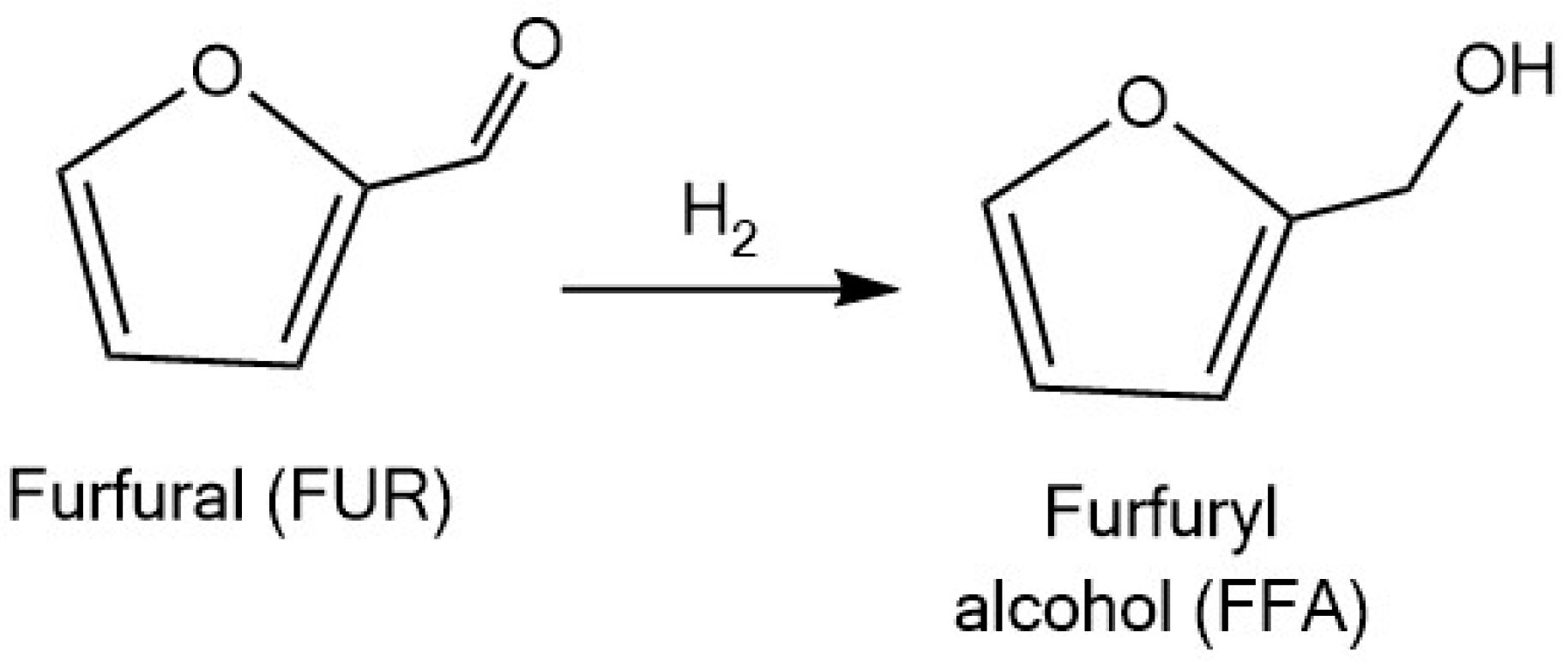
3.1.1. Furfuryl Alcohol
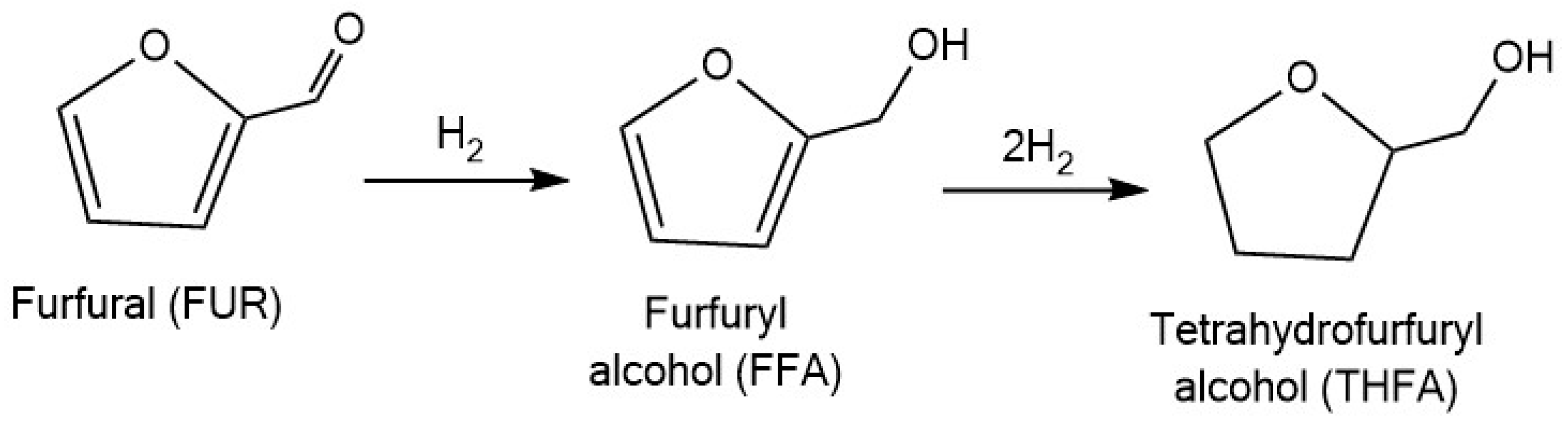
3.1.2. Tetrahydrofurfuryl Alcohol
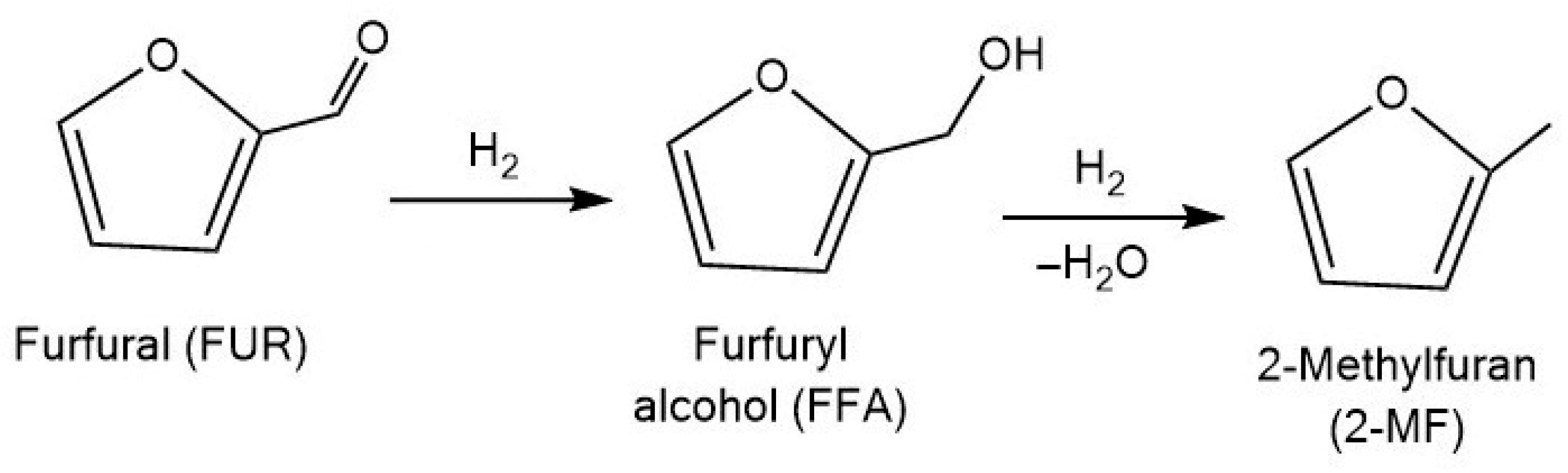
3.1.3. 2-Methylfuran
3.1.4. Pentanediols
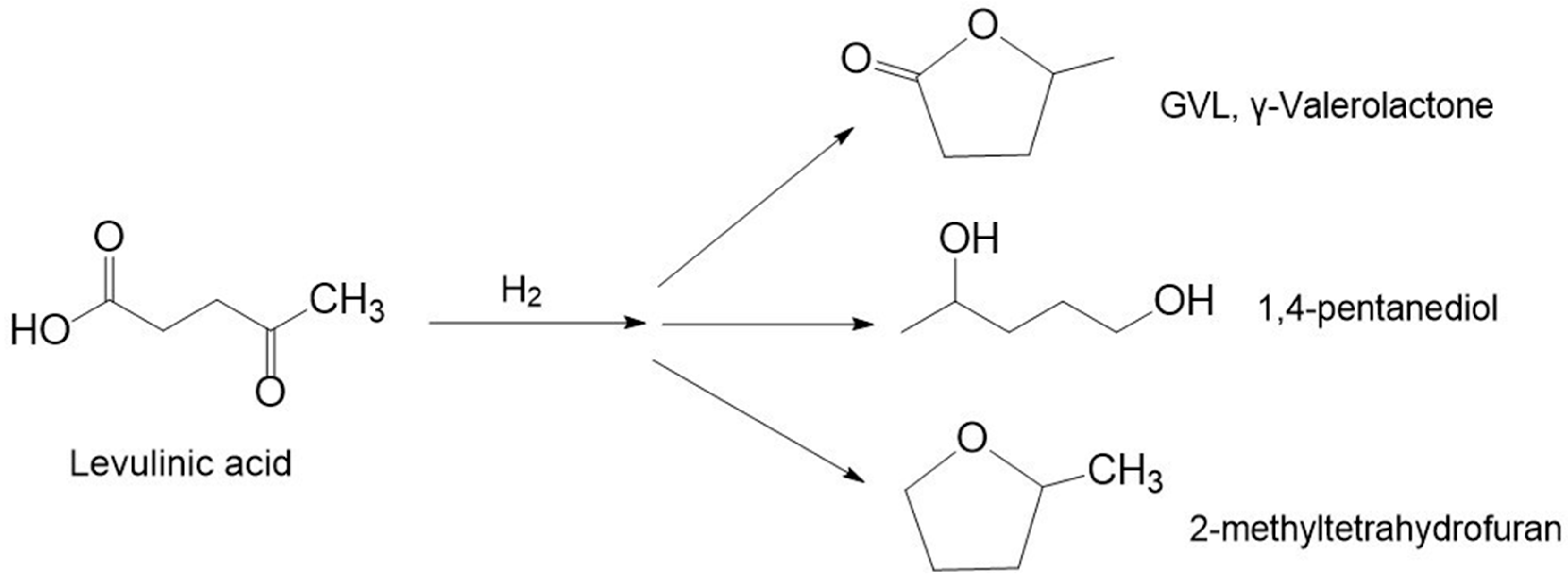
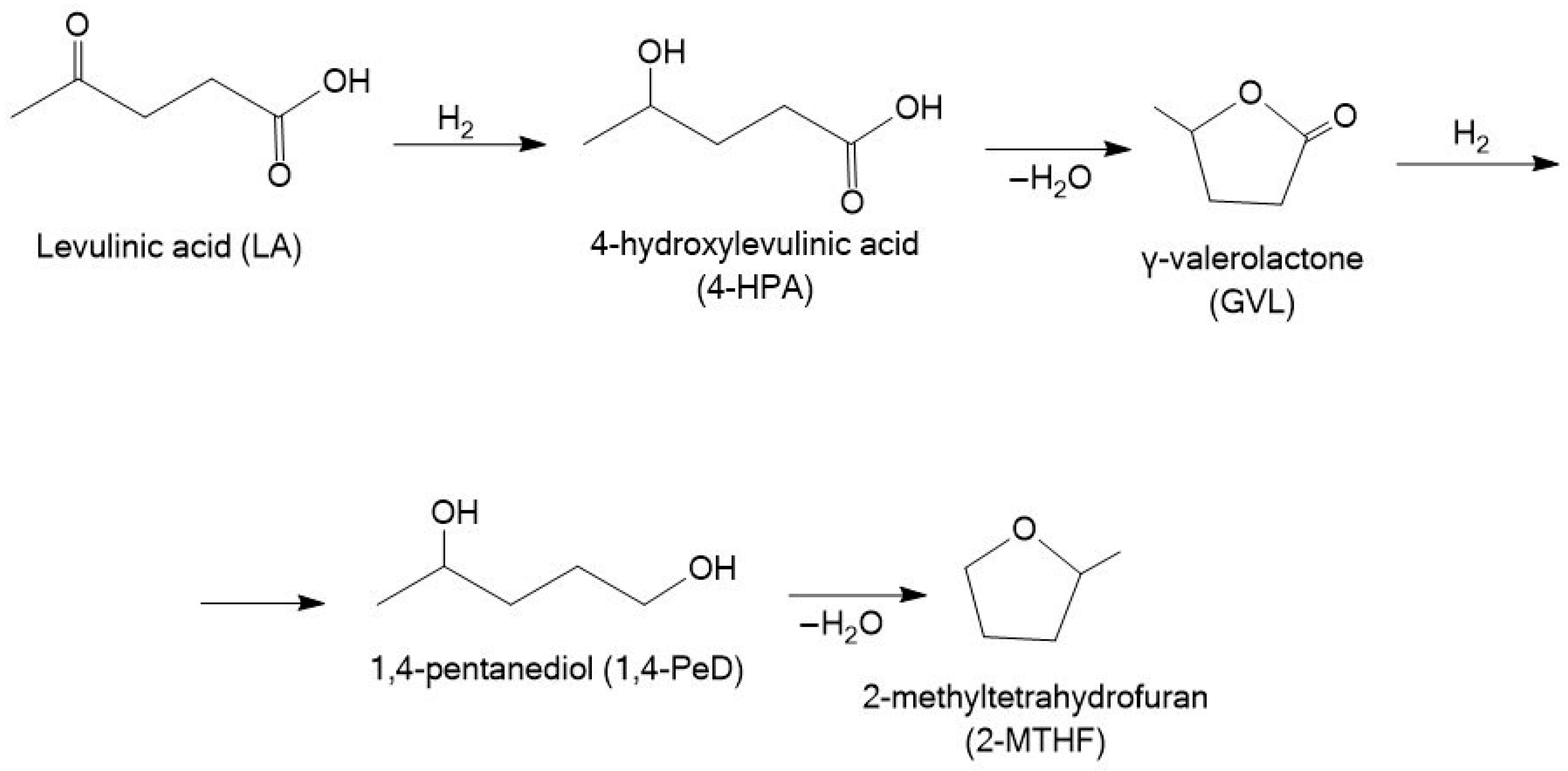
3.2. Hydrogenation of Levulinic Acid
3.2.1. γ-Valerolactone
3.2.2. 1,4-Pentanediol (1,4-PeD)
3.2.3. 2-Methyltetrahydrofuran
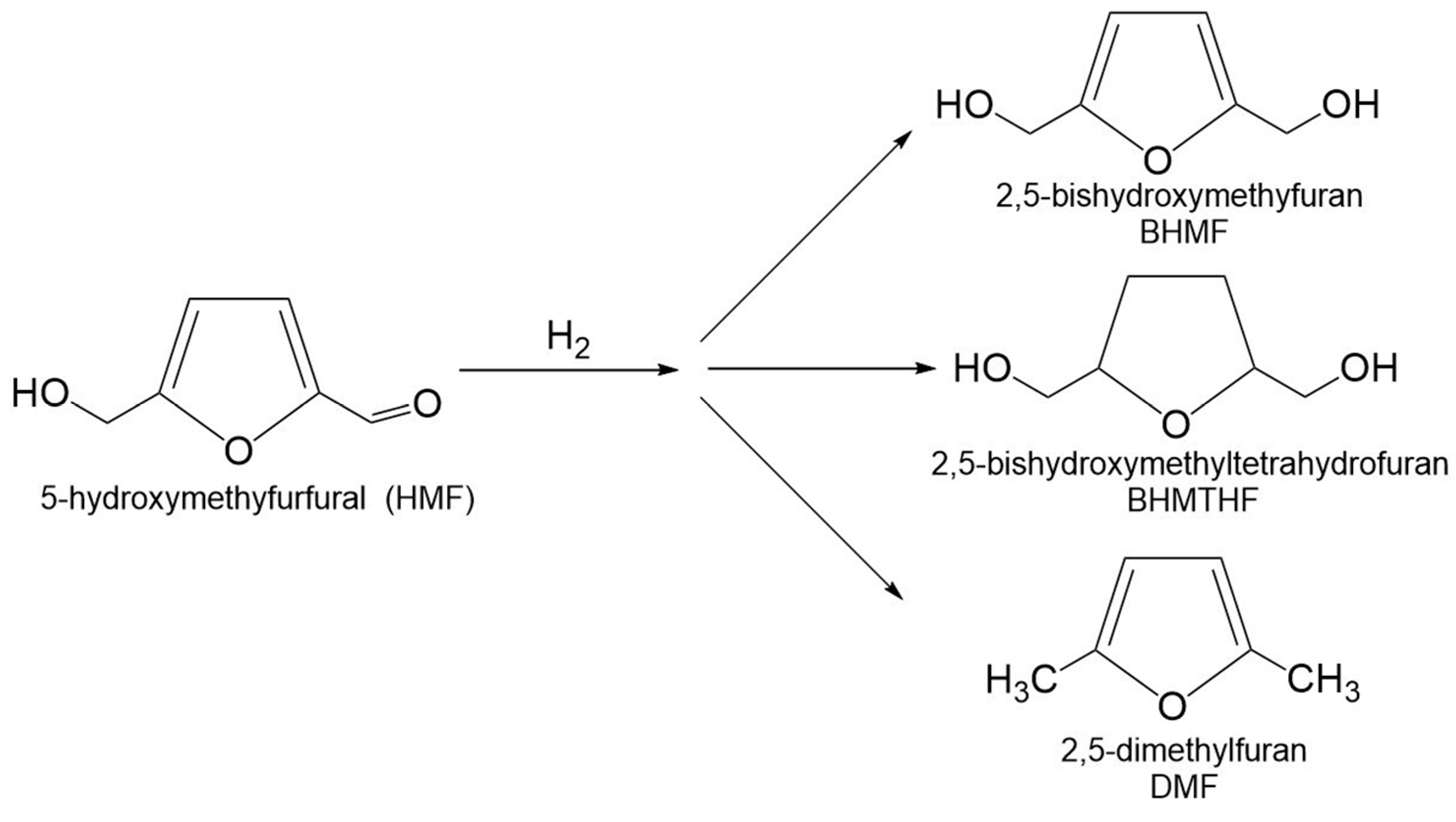
3.3. Hydrogenation of 5-Hydroxymethylfurfural
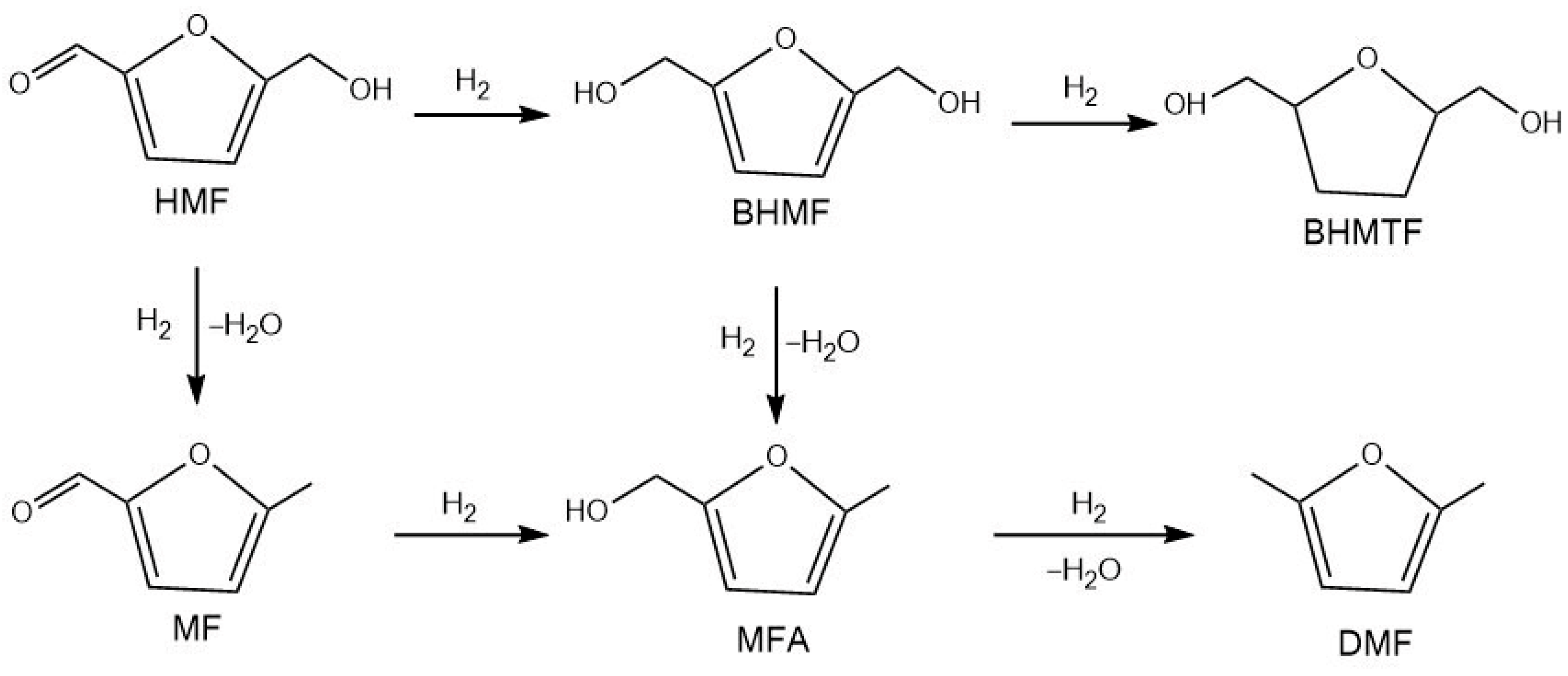
3.3.1. 2,5-Bis(hydroxymethyl)furan and 2,5-Bis(hydroxymethyl)tetrahydrofuran
3.3.2. 2,5-Dimethylfuran
4. Ru-Catalyzed One-Pot Conversion of Carbohydrates into Value Added Products
5. Conclusions
- -
- Development of advanced supports with tailored acid–base properties and high surface area, enhancing Ru dispersion, catalytic activity, and resistance to deactivation;
- -
- Design and synthesis of bifunctional Ru-based catalysts that integrate hydrogenation and acid-catalyzed functionalities within a single material for efficient one-pot carbohydrate conversion;
- -
- Optimization of catalyst preparation conditions to control the size, morphology, and dispersion of Ru nanoparticles;
- -
- Development of bi- and multi-metallic systems, including doping of Ru nanoparticles with oxophilic promoters (e.g., Re, Mo, W) or additional metals such as Pd, to improve hydrogenation activity, guide selectivity, and suppress side reactions;
- -
- Surface modification by acidic groups (Bronsted centers), organic ligands, or transition-metal compounds (to introduce or strengthen Lewis acidity);
- -
- Adjustment of catalyst pretreatment protocols to achieve the desired Ru0/RuOx ratio, which directly influences activity and selectivity.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 1,2-PeD | 1,2-pentanediol |
| 1,4-PeD | 1,4-pentanediol |
| 1,5-PeD | 1,5-pentanediol |
| 2-MF | 2-methylfuran |
| 2-MTHF | 2-methyltetrahydrofuran |
| 4-HPA | 4-hydroxylevulinic acid |
| 5-HMF | 5-hydroxymethylfurfural |
| AC | Activated carbon |
| AI | Aluminum isopropoxide |
| ARD-O | Post-oxidative activated reductive deposition |
| AS | Aluminum sulfate |
| BEA | Beta zeolites |
| BEATUD | Beta zeolite nanocrystals embedded in silicon mesoporous matrix |
| BHMF | 2,5-Bis(hydroxymethyl)furan |
| BHMTHF | 2,5-Bis(hydroxymethyl)tetrahydrofuran |
| CB | Carbon black |
| CMK-3 | Mesoporous carbon |
| CNF | Carbon nanofiber |
| CNT | Carbon nanotubes |
| CTAB | Cetyltrimethylammonium bromide |
| DMF | 2,5-dimethylfuran |
| DMSO | Dimethyl sulfoxide |
| EAC | Eucalyptus-derived activated carbon |
| EMIMBr | ionic liquid 1-ethyl-3-methylimidazolium bromide |
| FA | Formic acid |
| FFA | Furfuryl alcohol |
| FUR | Furfural |
| GVL | γ-valerolactone |
| HDA | Hexadecylamine |
| HHD | 1-hydroxyhexane-2,5-dione |
| LA | Levulinic acid |
| LC | Crude lignin |
| Lys-PTA | Lysine-functionalized phosphotungstic acid |
| MCSA | Magnetic carbon-based solid acid |
| MFA | 5-methylfurfuryl alcohol |
| [MIMPS]4SiW | 1-methyl-3-(3-sulfopropylimidazolium)silicotungstate |
| MOF | Metal-organic framework |
| NBP | Niobium phosphate |
| SA | Sodium aluminate |
| THF | Tetrahydrofuran |
| THFA | Tetrahydrofurfuryl alcohol |
| THFDM | Tetrahydro-2,5-furandimethanol |
| TsOH | p-toluenesulfonic acid |
| WHSV | Weight hourly space velocity |
References
- Du, X.; Zhang, J.; Wang, Y.; Qu, Y. Conversion of Carbohydrates into Platform Chemicals Catalyzed by Alkaline Ionic Liquids. Catalysts 2017, 7, 245. [Google Scholar] [CrossRef]
- Vaithyanathan, V.K.; Goyette, B.; Rajagopal, R. A critical review of the transformation of biomass into commodity chemicals: Prominence of pretreatments. Environ. Chall. 2023, 11, 100700. [Google Scholar] [CrossRef]
- Hommes, A.; Heeres, H.J.; Yue, J. Catalytic Transformation of Biomass Derivatives to Value-Added Chemicals and Fuels in Continuous Flow Microreactors. ChemCatChem 2019, 11, 4671–4708. [Google Scholar] [CrossRef]
- Dutta, S. Catalytic Transformation of Carbohydrates into Renewable Organic Chemicals by Revering the Principles of Green Chemistry. ACS Omega 2024, 9, 26805–26825. [Google Scholar] [CrossRef]
- da Silva, M.J.; Rodrigues, A.A.; Batalha, D.C. Furfural and Levulinic Acid: Synthesis of Platform Molecules from Keggin Heteropolyacid-Catalyzed Biomass Conversion Reactions. Reactions 2024, 5, 361–378. [Google Scholar] [CrossRef]
- Esteban, J.; Yustos, P.; Ladero, M. Catalytic Processes from Biomass-Derived Hexoses and Pentoses: A Recent Literature Overview. Catalysts 2018, 8, 637. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, S.; Yang, Y.; Wang, L.; Xu, E.; Hou, Q.; Zhao, S.; Liu, T.; Hong, S.; Zheng, L.; et al. A switchable hydrogenation chemoselectivity of biomass platform compounds based on solvent regulation. Appl. Catal. B: Environ. Energy 2024, 346, 123719. [Google Scholar] [CrossRef]
- Xia, H.; Xu, S.; Hu, H.; An, J.; Li, C. Efficient conversion of 5-hydroxymethylfurfural to high-value chemicals by chemo- and bio-catalysis. RSC Adv. 2018, 8, 30875–30886. [Google Scholar] [CrossRef]
- Li, X.; Zhang, L.; Wang, S.; Wu, Y. Recent Advances in Aqueous-Phase Catalytic Conversions of Biomass Platform Chemicals Over Heterogeneous Catalysts. Front. Chem. 2020, 7, 948–968. [Google Scholar] [CrossRef]
- Zhang, K.; Wang, J.; Tian, Y.; Zhang, S.; Chen, S.S.; Cao, L.; Zhang, J.; Clark, J.H.; Zhang, S. Ruthenium catalysts for hydrogenation of biomass-based levulinic acid for efficient γ-valerolactone synthesis. iScience 2025, 28, 112734. [Google Scholar] [CrossRef]
- Seretis, A.; Diamantopoulou, P.; Thanou, I.; Tzevelekidis, P.; Fakas, C.; Lilas, P.; Papadogianakis, G. Recent Advances in Ruthenium-Catalyzed Hydrogenation Reactions of Renewable Biomass-Derived Levulinic Acid in Aqueous Media. Front. Chem. 2020, 8, 221–243. [Google Scholar] [CrossRef]
- Akram, M.; Bhutto, S.U.A.; Aftab, S.; Wang, F.; Xu, X.; Xia, M. Ruthenium based with carbon supported catalysts for the catalytic transfer hydrogenation of furfural: A review. Nano Energy 2023, 117, 108808. [Google Scholar] [CrossRef]
- Gebresillase, M.N.; Errol, D.S.; Jeong, G.S. Interplay Between Metallicity and Acidity in the Hydrogenation of Levulinic Acid. ACS Sustain. Chem. Eng. 2024, 12, 7026–7039. [Google Scholar] [CrossRef]
- Liu, M.; Liu, Y. Highly efficient hydrogenation of levulinic acid into γ-valerolactone over modified bifunctional yolkshell catalyst. J. Phys. Conf. SeriesJ. Phys. Conf. Ser. 2024, 2873, 012016. [Google Scholar] [CrossRef]
- Yu, I.K.M.; Tsang, D.C.W. Insights into the kinetics of furfural production from different monomers and polymers derived from biomass in a subcritial water reaction medium intensified by CO2 as pressurization agent. Biomass Bioenergy 2025, 193, 107550. [Google Scholar] [CrossRef]
- Sener, C.; Motagamwala, A.H.; Alonso, D.M.; Dumesic, J.A. Enhanced Furfural Yields from Xylose Dehydration in the γ-Valerolactone/Water Solvent System at Elevated Temperatures. ChemSusChem 2018, 11, 2262–2463. [Google Scholar] [CrossRef]
- Kabbour, M.; Luque, R. Furfural as a platform chemical: From production to applications. In Biomass, Biofuels, Biochemicals Recent Advances in Development of Platform Chemicals; Saravanamurugan, S., Pandey, A., Li, H., Riisager, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 283–297. [Google Scholar] [CrossRef]
- Mariscal, R.; Maireles-Torres, P.; Ojeda, M.; Sádaba, I.; López Granados, M. Furfural: A renewable and versatile platform molecule for the synthesis of chemicals and fuels. Energy Environ. Sci. 2016, 9, 1144–1189. [Google Scholar] [CrossRef]
- Edumujeze, D.; Fournier-Salaün, M.-C.; Leveneur, S. Production of furfural: From kinetics to process assessment. Fuel 2025, 381, 133423. [Google Scholar] [CrossRef]
- Gan, P.; Zhang, K.; Yang, G.; Li, J.; Zhao, Y.; Chen, J. Catalytic Production and Upgrading of Furfural: A Platform Compound. Int. J. Mol. Sci. 2024, 25, 11992. [Google Scholar] [CrossRef]
- Wang, Y.; Li, M.; Wang, Z.; Liu, S.; O’Young, L. Furfural production: A review on reaction mechanism and conventional production process. Ind. Crops Prod. 2025, 230, 121103. [Google Scholar] [CrossRef]
- Illera, A.E.; Candela, H.; Bermejo-López, A.; Barea, P.; Alonso-Riaño, P.; Benito-Román, Ó.; Beltrán, S.; Sanz, M.T. Evaluation of homogeneous and heterogeneous catalytic strategies for furfural production from sugar-derived biomass in a solvent-free green pressurized reaction media (subcritical water-CO2). Biomass Bioenergy 2024, 187, 107304. [Google Scholar] [CrossRef]
- Choudhary, V.; Sandler, S.I.; Vlachos, D.G. Conversion of xylose to furfural using Lewis and Brønsted acid catalysts in aqueous media. ACS Catal. 2012, 2, 2022–2028. [Google Scholar] [CrossRef]
- Tongtummachat, T.; Jaree, A.; Akkarawatkhoosith, N. Continuous hydrothermal furfural production from xylose in a microreactor with dual-acid catalysts. RSC Adv. 2022, 12, 23366–23378. [Google Scholar] [CrossRef]
- Lamminpää, K.; Ahola, J.; Tanskanen, J. Acid-catalysed xylose dehydration into furfural in the presence of Kraft lignin. Bioresour. Technol. 2015, 177, 94–101. [Google Scholar] [CrossRef]
- Delbecq, F.; Wang, Y.; Muralidhara, A.; El Ouardi, K.E.; Marlair, G.; Len, C. Hydrolysis of hemicellulose and derivatives-a review of recent advances in the production of furfural. Front. Chem. 2018, 6, 2018. [Google Scholar] [CrossRef]
- Zhang, L.; Yu, H.; Wang, P.; Dong, H.; Peng, X. Conversion of xylan, D-xylose and lignocellulosic biomass into furfural using AlCl3 as catalyst in ionic liquid. Bioresour. Technol. 2013, 130, 110–116. [Google Scholar] [CrossRef]
- Yang, T.; Zhou, Y.H.; Zhu, S.Z.; Pan, H.; Huang, Y.B. Insight into Aluminum Sulfate-Catalyzed Xylan Conversion into Furfural in a γ-Valerolactone/Water Biphasic Solvent under Microwave Conditions. ChemSusChem 2017, 10, 4066–4079. [Google Scholar] [CrossRef] [PubMed]
- Lima, S.; Antunes, M.M.; Fernandes, A.; Pillinger, M.; Ribeiro, M.F.; Valente, A.A. Catalytic cyclodehydration of xylose to furfural in the presence of zeolite H-Beta and a micro/mesoporous Beta/TUD-1 composite material. Appl. Catal. A Gen. 2010, 388, 141–148. [Google Scholar] [CrossRef]
- Gao, H.; Liu, H.; Pang, B.; Yu, G.; Du, J.; Zhang, Y.; Wang, H.; Mu, X. Production of furfural from waste aqueous hemicellulose solution of hardwood over ZSM-5 zeolite. Bioresour. Technol. 2014, 172, 453–456. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Pan, D.; Wu, Y.; Song, X.; Gao, L.; Li, W.; Das, L.; Xiao, G. Efficient production of furfural from xylose and wheat straw by bifunctional chromium phosphate catalyst in biphasic systems. Fuel Process. Technol. 2018, 175, 90–96. [Google Scholar] [CrossRef]
- Cheng, L.; Guo, X.; Song, C.; Yu, G.; Cui, Y.; Xue, N.; Peng, L.; Guo, X.; Ding, W. High performance mesoporous zirconium phosphate for dehydration of xylose to furfural in aqueous-phase. RSC Adv. 2013, 3, 23228–23235. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, G.; Wang, K. Efficient conversion of biomass derivatives to furfural with a novel carbon-based solid acid catalyst. Catal. Commun. 2023, 175, 106608. [Google Scholar] [CrossRef]
- Yang, T.; Li, W.; Su, M.; Liu, Y.; Liu, M. Production of furfural from xylose catalyzed by a novel calcium gluconate derived carbon solid acid in 1,4-dioxane. New J. Chem. 2020, 44, 7968–7975. [Google Scholar] [CrossRef]
- Zhou, S.; Zhang, X.; Zhan, L.; Song, X.; Li, R.; Wu, Y. Future development for furfural production: Comparison of one-step and two-step strategies and life cycle assessment. Ind. Crops Prod. 2025, 227, 120807. [Google Scholar] [CrossRef]
- Li, W.; Zhu, Y.; Lu, Y.; Liu, Q.; Guan, S.; Chang, H.; Jameel, H.; Ma, L. Enhanced furfural production from raw corn stover employing a novel heterogeneous acid catalyst. Bioresour. Technol. 2017, 245, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Qiu, B.; Shi, J.; Hu, W.; Wang, Y.; Zhang, D.; Chu, H. Efficient and selective conversion of xylose to furfural over carbon-based solid acid catalyst in water-γ-valerolactone. Energy 2024, 294, 130774. [Google Scholar] [CrossRef]
- Antonyraj, C.A.; Haridas, A. A lignin-derived sulphated carbon for acid catalyzed transformations of bio-derived sugars. Catal. Commun. 2018, 104, 101–105. [Google Scholar] [CrossRef]
- Zhang, Z.; Lu, L.; Xu, H.; Lu, X.; Li, X. A magnetic carbon-based solid acid catalyst derived from tobacco stalk for efficient valorization of tobacco stalk to furfural. Ind. Crops Prod. 2025, 227, 120816. [Google Scholar] [CrossRef]
- Liu, Y.; Lin, Q.; Zhao, L.; Zhu, R.; Wang, X.; Ren, J.; Qi, W.; Li, L. Mechanism insights into the upgrading of xylose to furfural over the carbon-based catalysts in aqueous media. Fuel 2024, 378, 132844. [Google Scholar] [CrossRef]
- Yusuff, A.S.; Gu, Y. Studies on effective catalytic conversion of xylose to furfural using green sulfonated carbon catalysts: Process optimization by Taguchi approach. Arab. J. Chem. 2024, 17, 105892. [Google Scholar] [CrossRef]
- Ma, J.; Li, W.; Guan, S.; Liu, Q.; Li, Q.; Zhu, C.; Yang, T.; Ogunbiyi, A.T.; Ma, L. Efficient catalytic conversion of corn stalk and xylose into furfural over sulfonated graphene in γ-valerolactone. RSC Adv. 2019, 9, 10569–10577. [Google Scholar] [CrossRef] [PubMed]
- Bruce, S.M.; Zong, Z.; Chatzidimitriou, A.; Avci, L.E.; Bond, J.Q.; Carreon, M.A.; Wettstein, S.G. Small pore zeolite catalysts for furfural synthesis from xylose and switchgrass in a γ-valerolactone/water solvent. J. Mol. Catal. A Chem. 2016, 422, 18–22. [Google Scholar] [CrossRef]
- Gallo, J.M.R.; Alonso, D.M.; Mellmer, M.A.; Yeap, J.H.; Wong, H.C.; Dumesic, J.A. Production of Furfural from Lignocellulosic Biomass Using Beta Zeolite and Biomass-Derived Solvent. Top. Catal. 2013, 56, 1775–1781. [Google Scholar] [CrossRef]
- Wang, Y.; Dai, Y.; Wang, T.; Li, M.; Zhu, Y.; Zhang, L. Efficient conversion of biomass xylose to furfural over modified zeolite in the recyclable water/n-butanol system. Fuel Process. Technol. 2022, 237, 107472. [Google Scholar] [CrossRef]
- Wang, L.; Guo, H.; Wang, Q.; Hou, B.; Jia, L.; Cui, J.; Li, D. The study of active sites for producing furfural and soluble oligomers in fructose conversion over HZSM-5 zeolites. Mol. Catal. 2019, 474, 110411. [Google Scholar] [CrossRef]
- Hoang, P.H.; Cuong, T.D. Simultaneous Direct Production of 5-Hydroxymethylfurfural (HMF) and Furfural from Corncob Biomass Using Porous HSO3-ZSM-5 Zeolite Catalyst. Energy Fuels 2021, 35, 546–551. [Google Scholar] [CrossRef]
- Shringi, N.; Sidana, C.; Rani, A. SO42−/SnO2-fly ash as bifunctional catalyst for microwave-assisted single-step condensation of 2-naphthol and aromatic aldehydes. Arab. J. Sci. Eng. 2023, 48, 483–495. [Google Scholar] [CrossRef]
- Zhang, L.; Tian, L.; Sun, R.; Liu, C.; Kou, Q.; Zuo, H. Transformation of corncob into furfural by a bifunctional solid acid catalyst. Bioresour. Technol. 2019, 276, 60–64. [Google Scholar] [CrossRef] [PubMed]
- Bakili, S.; Kivevele, T.; King’ondu, C.K. Optimization of furfural production from xylose over sulfated titanium-niobium mixed oxides catalyst in biphasic system. Clean. Chem. Eng. 2024, 10, 100125. [Google Scholar] [CrossRef]
- Zhong, Y.; Liu, Y.; Wang, S.; Hou, S.; Fan, Y. Incorporation of tin oxide nanoparticles on sulfonated carbon microspheres as a bifunctional catalyst for efficient conversion of biomass-derived monosaccharides to 5-Hydroxymethylfurfural and furfural. Ind. Crops Prod. 2024, 208, 117913. [Google Scholar] [CrossRef]
- Fúnez-Núñez, I.; García-Sancho, C.; Cecilia, J.A.; Moreno-Tost, R.; Pérez-Inestrosa, E.; Serrano-Cantador, L.; Maireles-Torres, P. Synergistic effect between CaCl2 and γ-Al2O3 for furfural production by dehydration of hemicellulosic carbohydrates. Appl. Catal. A Gen. 2019, 585, 117188. [Google Scholar] [CrossRef]
- García-Sancho, C.; Agirrezabal-Telleria, I.; Güemez, M.B.; Maireles-Torres, P. Dehydration of D-xylose to furfural using different supported niobia catalysts. Appl. Catal. B Environ. 2014, 152–153, 1–10. [Google Scholar] [CrossRef]
- Chareonlimkun, A.; Champreda, V.; Shotipruk, A.; Laosiripojana, N. Catalytic conversion of sugarcane bagasse, rice husk and corncob in the presence of TiO2, ZrO2 and mixed-oxide TiO2–ZrO2 under hot compressed water (HCW) condition. Bioresour. Technol. 2010, 101, 4179–4186. [Google Scholar] [CrossRef]
- García-Sancho, C.; Rubio-Caballero, J.M.; Mérida-Robles, J.M.; Moreno-Tost, R.; Santamaría-González, J.; Maireles-Torres, P. Mesoporous Nb2O5 as solid acid catalyst for dehydration of D-xylose into furfural. Catal. Today 2014, 234, 119–124. [Google Scholar] [CrossRef]
- Kumar Mishra, R.; Bhooshan Kumar, V.; Victor, A.; Neel Pulidindi, I.; Gedanken, A. Selective Production of Furfural from the Dehydration of Xylose using Zn doped CuO Catalyst. Ultrason. Sonochem. 2019, 56, 55–62. [Google Scholar] [CrossRef]
- Moreno-Marrodan, C.; Barbaro, P.; Caporali, S.; Bossola, F. Low temperature continuous flow dehydration of xylose over water-tolerant niobia-titania heterogeneous catalysts. ChemSusChem 2018, 24, 3649–3660. [Google Scholar] [CrossRef]
- Rakngam, I.; Osakoo, N.; Wittayakun, J.; Chanlek, N.; Pengsawang, A.; Sosa, N.; Butburee, T.; Faungnawakij, K.; Khemthong, P. Properties of mesoporous Al-SBA-15 from one-pot hydrothermal synthesis with. Microporous Mesoporous Mater. 2021, 317, 110999. [Google Scholar] [CrossRef]
- Xu, S.; Pan, D.; Wu, Y.; Fan, J.; Wu, N.; Gao, L.; Li, W.; Xiao, G. Catalytic conversion of xylose and xylan into furfural over Cr3+/P-SBA-15 catalyst derived from spent adsorbent. Ind. Eng. Chem. Res. 2019, 58, 13013–13020. [Google Scholar] [CrossRef]
- Yu, I.K.M.; Tsang, D.C.W. Conversion of biomass to hydroxymethylfurfural: A review of catalytic systems and underlying mechanisms. Bioresour Technol. 2017, 238, 716–732. [Google Scholar] [CrossRef]
- Jeanmard, L.; Rongwong, W.; Chisti, Y. Biomass-derived levulinic acid as a platform chemical for making diverse products. Biomass Bioenergy 2025, 195, 107683. [Google Scholar] [CrossRef]
- Yang, F.; Fu, J.; Mo, J.; Lu, X. Synergy of Lewis and Brønsted Acids on Catalytic Hydrothermal Decomposition of Hexose to Levulinic Acid. Energy Fuels 2013, 27, 6973–6978. [Google Scholar] [CrossRef]
- Hurst, G.; Teklemariam, A.; Brierley, S.; Diaz De Rienzo, M.A.; Tedesco, S. Lignocellulosic biomass conversion to levulinic acid via acid catalysis: Current methods, opportunities and challenges for self-sustaining biorefineries. Int. J. Thermofluids 2025, 27, 101175. [Google Scholar] [CrossRef]
- Di Menno Di Bucchianico, D.; Wang, Y.; Buvat, J.-C.; Pan, Y.; Casson Moreno, V.; Leveneur, S. Production of levulinic acid and alkyl levulinates: A process insight. Green Chem. 2022, 24, 614–646. [Google Scholar] [CrossRef]
- Conti Silva, J.A.; Grilo, L.M.; Vasconcelos, M.H.; Lacerda, T.M. 13—Levulinic acid: Perspectives of its biobased production and most promising derivatives. In Production of Top 12 Biochemicals Selected by USDOE from Renewable Resources. Status and Innovation; Elsevier: Amsterdam, The Netherlands, 2022; pp. 387–414. [Google Scholar] [CrossRef]
- Kang, S.; Fu, J.; Zhang, G. From lignocellulosic biomass to levulinic acid: A review on acid-catalyzed hydrolysis. Renew. Sustain. Energy Rev. 2018, 94, 340–362. [Google Scholar] [CrossRef]
- Nakason, K.; Kuboon, S.; Phanthuwongpakdee, J.; Kraithong, W.; Jiratanachotikul, A.; Panyapinyopol, B.; Kanokkantapong, V. Economic viability and life cycle assessment of levulinic acid and hydrochar production via catalytic hydrothermal process of waste lignocellulosic biomass: A comparison of feedstock types. Case Stud. Chem. Environ. Eng. 2025, 11, 101217. [Google Scholar] [CrossRef]
- Li, X.; Xu, R.; Yang, J.; Nie, S.; Liu, D.; Liu, Y.; Si, C. Production of 5-hydroxymethylfurfural and levulinic acid from lignocellulosic biomass and catalytic upgradation. Ind. Crops Prod. 2019, 130, 184–197. [Google Scholar] [CrossRef]
- Šivec, R.; Grilc, M.; Huš, M.; Likozar, B. Multiscale modeling of (hemi)cellulose hydrolysis and cascade hydrotreatment of 5-hydroxymethylfurfural, furfural, and levulinic acid. Ind. Eng. Chem. Res. 2019, 58, 16018–16032. [Google Scholar] [CrossRef]
- Ukawa-Sato, R.; Hirano, N.; Fushimi, C. Design and techno–economic analysis of levulinic acid production process from biomass by using co-product formic acid as a catalyst with minimal waste generation. Chem. Eng. Res. Des. 2023, 192, 389–401. [Google Scholar] [CrossRef]
- Toftgaard Pedersen, A.; Ringborg, R.; Grotkjær, T.; Pedersen, S.; Woodley, J.M. Synthesis of 5-hydroxymethylfurfural (HMF) by acid catalyzed dehydration of glucose–fructose mixtures. Chem. Eng. J. 2015, 273, 455–464. [Google Scholar] [CrossRef]
- Huang, H.; Denard, C.A.; Alamillo, R.; Crisci, A.J.; Miao, Y.; Dumesic, J.A.; Scott, S.L.; Zhao, H. Tandem catalytic conversion of glucose to 5-hydroxymethylfurfural with an immobilized enzyme and a solid acid. ACS Catal. 2014, 4, 2165–2168. [Google Scholar] [CrossRef]
- Guo, W.; Zhang, Z.; Hacking, J.; Heeres, H.J.; Yue, J. Selective fructose dehydration to 5-hydroxymethylfurfural from a fructose-glucose mixture over a sulfuric acid catalyst in a biphasic system: Experimental study and kinetic modeling. Chem. Eng. J. 2021, 409, 128182. [Google Scholar] [CrossRef]
- Zhang, X.; Murria, P.; Jiang, Y.; Xiao, W. Maleic Acid and Aluminum Chloride Catalyzed Conversion of Glucose to 5-(Hydroxymethyl) furfural and Levulinic Acid in Aqueous Media. Green Chem. 2016, 18, 5219–5229. [Google Scholar] [CrossRef]
- Yu, I.K.M.; Tsang, D.C.W.; Chen, S.S.; Ok, Y.S.; Poon, C.S. Valorization of food waste into hydroxymethylfurfural: Dual role of metal ions in successive conversion steps. Bioresour. Technol. 2016, 219, 338–347. [Google Scholar] [CrossRef]
- Sun, A.; Ying, Y.; Wang, M.; Zhu, L.; Wang, Y.; Zhang, Q.; Li, L.; Cao, C.; Xu, H.; Cheng, D. Efficient conversion of fructose to 5-hydroxymethylfurfural by hydrophobic modified SAPO-34 molecular sieve. J. Catal. 2025, 446, 116059. [Google Scholar] [CrossRef]
- Jeong, H.; Park, S.-Y.; Ryu, G.-H.; Choi, J.-H.; Kim, J.-H.; Choi, W.-S.; Lee, S.M.; Choi, J.W.; Choi, I.-G. Catalytic conversion of hemicellulosic sugars derived from biomass to levulinic acid. Catal. Commun. 2018, 117, 19–25. [Google Scholar] [CrossRef]
- Hu, Y.; Li, H.; Wu, D.; Li, L.; Hu, C.; Zhu, L. Boosting catalytic performance of Amberlyst-15 by modulating surface properties for synthesis of 5-hydroxymethylfurfural from high-concentration fructose. Catal. Today 2024, 442, 114939. [Google Scholar] [CrossRef]
- Wrigstedt, P.; Keskivali, J.; Repo, T. Microwave-enhanced aqueous biphasic dehydration of carbohydrates to 5 hydroxymethylfurfural. RSC Adv. 2016, 6, 18973. [Google Scholar] [CrossRef]
- Hou, Q.; Zhen, M.; Liu, L.; Chen, Y.; Huang, F.; Zhang, S.; Li, W.; Ju, M. Tin phosphate as a heterogeneous catalyst for efficient dehydration of glucose into 5-hydroxymethylfurfural in ionic liquid. Appl. Catal. B Environ. 2018, 224, 183–193. [Google Scholar] [CrossRef]
- Weingarten, R.; Kim, Y.T.; Tompsett, G.A.; Fernández, A.; Han, K.S.; Hagaman, E.W.; Conner, W.C., Jr.; Dumesic, J.A.; Huber, G.W. Conversion of glucose into levulinic acid with solid metal (IV) phosphate catalysts. J. Catal. 2013, 304, 123–134. [Google Scholar] [CrossRef]
- Cheng, X.; Feng, Q.; Ma, D.; Chen, H.; Zeng, X.; Xing, F.; Teng, J. Efficient catalytic production of levulinic acid over hydrothermally stable propyl sulfonic acid functionalized SBA-15 in γ-valerolactone-water system. J. Environ. Chem. Eng. 2021, 9, 105747. [Google Scholar] [CrossRef]
- Lee, B.W.; Seo, J.Y.; Jeong, K.; Choi, J.; Cho, K.Y.; Cho, S.; Baek, K.-Y. Efficient production of levulinic acid using metal–organic framework catalyst: Role of Brønsted acid and flexibility. Chem. Eng. J. 2022, 444, 136566. [Google Scholar] [CrossRef]
- Souzanchi, S.; Nazari, L.; Rao, K.T.V.; Yuan, Z.; Tan, Z.; Xu, C. 5-HMF production from industrial grade sugar syrups derived from corn and wood using niobium phosphate catalyst in a biphasic continuous-flow tubular reactor. Catal. Today 2021, 407, 274–280. [Google Scholar] [CrossRef]
- Liu, B.; Ba, C.; Jin, M.; Zhang, Z. Effective conversion of carbohydrates into biofuel precursor 5-hydroxymethylfurfural (HMF) over Cr-incorporated mesoporous zirconium phosphate. Ind. Crops Prod. 2015, 76, 781–786. [Google Scholar] [CrossRef]
- Van der Graaff, W.N.P.; Olvera, K.G.; Pidko, E.A.; Hensen, E.J.M. Stability and catalytic properties of porous acidic (organo)silica materials for conversion of carbohydrates. J. Mol. Catal. A Chem. 2014, 388, 81–89. [Google Scholar] [CrossRef]
- Agirrezabal-Telleria, I.; Requies, J.; Gueemez, M.B.; Arias, P.L. Dehydration of D-xylose to furfural using selective and hydrothermally stable arenesulfonic SBA-15 catalysts. Appl. Catal. B 2014, 145, 34–42. [Google Scholar] [CrossRef]
- Wei, W.; Wu, S. Experimental and kinetic study of glucose conversion to levulinic acid in aqueous medium over Cr/HZSM-5 catalyst. Fuel 2018, 225, 311–321. [Google Scholar] [CrossRef]
- Ramli, N.A.S.; Amin, N.A.S. Kinetic study of glucose conversion to levulinic acid over Fe/HY zeolite catalyst. Chem. Eng. J. 2016, 283, 150–159. [Google Scholar] [CrossRef]
- Qu, H.; Liu, B.; Gao, G.; Ma, Y.; Zhou, Y.; Zhou, H.; Li, L.; Li, Y.; Liu, S. Metal-organic framework containing Brønsted acidity and Lewis acidity for efficient conversion glucose to levulinic acid. Fuel Process. Technol. 2019, 193, 1–6. [Google Scholar] [CrossRef]
- Sampath, G.; Kannan, S. Fructose dehydration to 5-hydroxymethylfurfural: Remarkable solvent influence on recyclability of Amberlyst-15 catalyst and regeneration studies. Catal. Commun. 2013, 37, 41–44. [Google Scholar] [CrossRef]
- Dou, Y.; Zhou, S.; Oldani, C.; Fang, W.; Cao, Q. 5-Hydroxymethylfurfural production from dehydration of fructose catalyzed by Aquivion@silica solid acid. Fuel 2018, 214, 45–54. [Google Scholar] [CrossRef]
- Perveen, F.; Farooq, M.; Ramli, A.; Naeem, A.; Khan, I.W.; Saeed, T.; Khan, J. Levulinic Acid Production from Waste Corncob Biomass Using an Environmentally Benign WO3-Grafted ZnCo2O4@CeO2 Bifunctional Heterogeneous Catalyst. ACS Omega 2023, 8, 333–345. [Google Scholar] [CrossRef]
- Michel, C.; Gallezot, P. Why Is Ruthenium an Efficient Catalyst for the Aqueous-Phase Hydrogenation of Biosourced Carbonyl Compounds? ACS Catal. 2015, 5, 4130–4132. [Google Scholar] [CrossRef]
- Patel, A.; Shah, A.R. Integrated lignocellulosic biorefinery: Gateway for production of second generation ethanol and value added products. J. Bioresour. Bioprod. 2021, 6, 108–128. [Google Scholar] [CrossRef]
- Roldugina, E.A.; Kardashev, S.V.; Maximov, A.L. Hydrogenation of Furfural on Pt- and Pd-Containing Catalysts in an Aqueous Medium. Russ. J. Appl. Chem. 2024, 96, 953–961. [Google Scholar] [CrossRef]
- Kumaravel, S.; Durai, M.; Kaliyamoorthy, S.; Kumaravel, S.; Chandramoorthy, C.; Avula, B.; Hasan, I.; Kim, M.-J.; Balu, K.; Ahn, Y.-H. Ru Nanoparticles Supported on Mesoporous Al-SBA-15Catalysts for Highly Selective Hydrogenation of Furfural to Furfuryl Alcohol. ChemistrySelect 2023, 8, e202301787. [Google Scholar] [CrossRef]
- Yuan, Q.; Zhang, D.; van Haandel, L.; Ye, F.; Xue, T.; Hensen, E.J.M.; Guan, Y. Selective liquid phase hydrogenation of furfural to furfuryl alcohol by Ru/Zr-MOFs. J. Mol. Catal. A Chem. 2015, 406, 58–64. [Google Scholar] [CrossRef]
- Bardestani, R.; Biriaei, R.; Kaliaguine, S. Hydrogenation of Furfural to Furfuryl Alcohol over Ru Particles Supported on Mildly Oxidized Biochar. Catalysts 2020, 10, 934. [Google Scholar] [CrossRef]
- Tolek, W.; Nanthasanti, N.; Pongthawornsakun, B.; Praserthdam, P.; Panpranot, J. Effects of TiO2 structure and Co addition as a second metal on Ru-based catalysts supported on TiO2 for selective hydrogenation of furfural to FA. Sci. Rep. 2021, 11, 9786. [Google Scholar] [CrossRef]
- Tang, Q.; Sun, X.; Duan, Z.; Xiao, Z.; Liu, X. Efficient hydrogenation of biomass-derived furfural to tetrahydrofurfuryl alcohol over a non-noble Ni/CeO2 catalyst under mild conditions. Mol. Catal. 2025, 584, 115261. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, R.; Huang, L.; Liu, P. Highly efficient selective hydrogenation of furfural to tetrahydrofurfuryl alcohol over MOF-derived Co-Ni bimetallic catalysts: The effects of Co-Ni alloy and adsorption configuration. J. Catal. 2024, 440, 115824. [Google Scholar] [CrossRef]
- Huang, R.; Cui, Q.; Yuan, Q.; Wu, H.; Guan, Y.; Wu, P. Total Hydrogenation of Furfural over Pd/Al2O3 and Ru/ZrO2 Mixture under Mild Conditions: Essential Role of Tetrahydrofurfural as an Intermediate and Support Effect. ACS Sustain. Chem. Eng. 2018, 6, 6957–6964. [Google Scholar] [CrossRef]
- Bruna, L.; Miquel, C.-F.; Colliere, V.; Philippot, K.; Rosa Axet, M. In Situ Ruthenium Catalyst Modification for the Conversion of Furfural to 1,2-Pentanediol. Nanomaterials 2022, 12, 328. [Google Scholar] [CrossRef] [PubMed]
- Kasad, M.R.; Jackson, J.E.; Saffron, C.M. Electrocatalytic hydrogenation of the formyl group and heteroaromatic ring in furfural on activated carbon cloth-supported ruthenium. RSC Sustain. 2024, 2, 3001–3013. [Google Scholar] [CrossRef]
- Yan, K.; Wu, G.; Lafleur, T.; Jarvis, C. Production, properties and catalytic hydrogenation of furfural to fuel additives and value-added chemicals. Renew. Sustain. Energy Rev. 2014, 38, 663–676. [Google Scholar] [CrossRef]
- Li, H.; Fang, Z.; Smith, R.L., Jr.; Yang, S. Efficient valorization of biomass to biofuels with bifunctional solid catalytic materials. Prog. Energy Combust. Sci. 2016, 55, 98–194. [Google Scholar] [CrossRef]
- Aldosari, O.F.; Iqbal, S.; Miedziak, P.J.; Brett, G.L.; Jones, D.R.; Liu, X.; Edwards, J.K.; Morgan, D.J.; Knight, D.K.; Hutchings, G.J. Pd–Ru/TiO2 catalyst—An active and selective catalyst for furfural hydrogenation. Catal. Sci. Technol. 2016, 6, 234–242. [Google Scholar] [CrossRef]
- Kumaravel, S.; Avula, B.; Alagarasan, J.K.; Lee, M.; Ali, W.; Khan, M.E.; Ali, S.K.; Bashiri, A.H.; Khan, A.U.; Balu, K. Development of bimetallic Ru/Ni/SBA-16 catalysts for catalytic hydrogenation of bio-based furfural to 2-methylfuran and furfuryl alcohol. Mater. Chem. Phys. 2024, 316, 129083. [Google Scholar] [CrossRef]
- Sun, X.; Wen, B.; Wang, F.; Zhang, W.; Zhao, K.; Liu, X. Research advances on the catalytic conversion of biomass-derived furfural into pentanediols. Catal. Commun. 2024, 187, 106864. [Google Scholar] [CrossRef]
- Upare, P.P.; Kim, Y.; Oh, K.-R.; Han, S.J.; Kim, S.K.; Hong, D.Y.; Lee, M.; Manjunathan, P.; Hwang, D.W.; Hwang, Y.K. Bimetallic Ru3Sn7 Nanoalloy on ZnO Catalyst for Selective Conversion of Biomass-derived Furfural into 1, 2-Pentanediol. ACS Sustain. Chem. Eng. 2021, 9, 17242–17253. [Google Scholar] [CrossRef]
- Rodiansono; Azzahra, A.S.; Santoso, U.T.; Mikrianto, E.; Suarso, E.; Sembiring, K.C.; Adilina, I.B.; Sunnardiantoe, G.K.; Afandi, A. Highly efficient and selective aqueous phase hydrogenolysis of furfural to 1,5-pentanediol using bimetallic Ru–SnOx/γ-Al2O3 catalysts. Catal. Sci. Technol. 2025, 15, 808–821. [Google Scholar] [CrossRef]
- Kamble, P.A.; Vinod, C.P.; Rathod, V.K.; Kantam, M.L. Hydrogenation of levulinic acid to gamma-valerolactone over nickel supported organoclay catalyst. Catal. Today 2023, 408, 36–49. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, L.; Guo, P.; Yao, G. Recent advances in the production of γ-valerolactone with liquid hydrogen source. IOP Conf. Ser. Earth Environ. Sci. 2020, 571, 012116. [Google Scholar] [CrossRef]
- Córdova-Pérez, G.E.; Cortez-Elizalde, J.; Silahua-Pavón, A.A.; Cervantes-Uribe, A.; Arévalo-Pérez, J.C.; Cordero-Garcia, A.; Espinosa de los Monteros, A.E.; Espinosa-González, C.G.; Godavarthi, S.; Ortiz-Chi, F.; et al. γ-Valerolactone Production from Levulinic Acid Hydrogenation Using Ni Supported Nanoparticles: Influence of Tungsten Loading and pH of Synthesis. Nanomaterials 2022, 12, 2017. [Google Scholar] [CrossRef] [PubMed]
- Piskun, A.; Winkelman, J.G.M.; Tang, Z.; Heeres, H.J. Support Screening Studies on the Hydrogenation of Levulinic Acid to γ-Valerolactone in Water Using Ru Catalysts. Catalysts 2016, 6, 131. [Google Scholar] [CrossRef]
- Wang, R.; Chen, L.; Zhang, X.; Zhang, Q.; Li, Y.; Wang, C.; Ma, L. Conversion of levulinic acid to γ-valerolactone over Ru/Al2O3–TiO2 catalyst under mild conditions. RSC Adv. 2018, 8, 40989–40995. [Google Scholar] [CrossRef]
- Sorokina, S.A.; Mikhailov, S.P.; Kuchkina, N.V.; Bykov, A.V.; Vasiliev, A.L.; Ezernitskaya, M.G.; Golovin, A.L.; Nikoshvili, L.Z.; Sulman, M.G.; Shifrina, Z.B. Ru@hyperbranched Polymer for Hydrogenation of Levulinic Acid to Gamma-Valerolactone: The Role of the Catalyst Support. Int. J. Mol. Sci. 2022, 23, 799. [Google Scholar] [CrossRef]
- Jaya, V.S.; Sudhakar, M.; Kumara, S.N.; Venugopala, A. Selective hydrogenation of levulinic acid to γ-valerolactone over a Ru/Mg–LaO catalyst. RSC Adv. 2015, 5, 9044–9049. [Google Scholar] [CrossRef]
- Rodríguez, V.I.; Mendow, G.; Sánchez, B.S.; García, J.R.; Pujro, R.A.; de Miguel, S.R.; Veizag, N.S. Ruthenium Catalysts Supported on Hydrothermally Treated Carbon from Rice Husk: The Effect of Reduction Temperature on the Hydrogenation Reaction of Levulinic Acid to γ-Valerolactone. Processes 2023, 11, 1421. [Google Scholar] [CrossRef]
- Petcuta, O.A.; Guzo, N.C.; Bordeiasu, M.; Nicolaev, A.; Parvulescu, V.I.; Coman, S.M. Ru/Beta Zeolite Catalysts for Levulinic Acid Hydrogenation: The Importance of Catalyst Synthesis Methodology. Catalysts 2025, 15, 80. [Google Scholar] [CrossRef]
- Rodiansono; Astuti, M.D.; Mustikasari, K.; Husain, S.; Ansyah, F.R.; Harae, T.; Shimazu, S. Unravelling the one-pot conversion of biomassderived furfural and levulinic acid to 1,4-pentanediol catalysed by supported RANEY®® Ni–Sn alloy catalysts. RSC Adv. 2022, 12, 241–250. [Google Scholar] [CrossRef]
- Lv, J.; Rong, Z.; Sun, L.; Liu, C.; Lu, A.-H.; Wanga, Y.; Qu, J. Catalytic conversion of biomass-derived levulinic acid into alcohols over nanoporous Ru catalyst. Catal. Sci. Technol. 2018, 8, 975–979. [Google Scholar] [CrossRef]
- Cui, J.; Tan, J.; Zhu, Y.; Cheng, F. Aqueous Hydrogenation of Levulinic Acid to 1,4-Pentanediol over Mo-Modified Ru/Activated Carbon Catalyst. ChemSusChem 2018, 11, 1316–1320. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Kim, H.U.; Kim, J.R.; Park, Y.-K.; Ha, J.-M.; Jae, J. Insights into the structure–activity relationship in aqueous-phase hydrogenation of levulinic acid to 1,4-pentanediol over bimetallic Ru-Re/C catalysts. J. Ind. Eng. Chem. 2024, 131, 490–502. [Google Scholar] [CrossRef]
- Cañadas, R.; Díaz, I.; Rodríguez, M.; González, E.J.; González-Miquel, M. An integrated approach for sustainable valorization of winery wastewater using bio-based solvents for recovery of natural antioxidants. J. Clean. Prod. 2022, 334, 130181. [Google Scholar] [CrossRef]
- Gundekari, S.; Karmee, S.K. Catalytic Conversion of Levulinic Acid into 2-Methyltetrahydrofuran: A Review. Molecules 2024, 29, 242. [Google Scholar] [CrossRef]
- Phanopoulos, A.; White, A.J.P.; Long, N.J.; Miller, P.W. Catalytic Transformation of Levulinic Acid to 2-Methyltetrahydrofuran Using Ruthenium–N-Triphos Complexes. ACS Catal. 2015, 5, 2500–2512. [Google Scholar] [CrossRef]
- Licursi, D.; Antonetti, C.; Fulignati, S.; Giannoni, M.; Galletti, A.M.R. Cascade Strategy for the Tunable Catalytic Valorization of Levulinic Acid and γ-Valerolactone to 2-Methyltetrahydrofuran and Alcohols. Catalysts 2018, 8, 277. [Google Scholar] [CrossRef]
- Upare, P.P.; Lee, M.; Lee, S.-K.; Yoon, J.W.; Bae, J.; Hwang, D.W.; Lee, U.-H.; Chang, J.-S.; Hwang, Y.K. Ru nanoparticles supported graphene oxide catalyst for hydrogenation of bio-based levulinic acid to cyclic ethers. Catal. Today 2016, 265, 174–183. [Google Scholar] [CrossRef]
- Menegazzo, F.; Ghedini, E.; Signoretto, M. 5-Hydroxymethylfurfural (HMF) Production from Real Biomasses. Molecules 2018, 23, 2201. [Google Scholar] [CrossRef]
- Post, C.; Maniar, D.; Voet, V.S.D.; Folkersma, R.; Loos, K. Biobased 2,5-Bis(hydroxymethyl)furan as a Versatile Building Block for Sustainable Polymeric Materials. ACS Omega 2023, 8, 8991–9003. [Google Scholar] [CrossRef]
- Li, M.; Zheng, T.; Lu, D.; Dai, S.; Chen, X.; Pan, X.; Dong, D.; Weng, R.; Xu, G.; Wang, F. Facet effect on the reconstructed Cu-catalyzed electrochemical hydrogenation of 5-hydroxymethylfurfural (HMF) towards 2,5-bis(hydroxymethy)furan (BHMF). J. Energy Chem. 2023, 84, 101–111. [Google Scholar] [CrossRef]
- Huang, R.; Yuan, S.; Chen, B.; Yang, Z.; Tian, Y.; Li, Z.; Lin, L.; Zeng, X. Selective conversion of 5-hydroxymethylfurfural to 2,5-bis(hydroxymethyl)tetrahydrofuran over Ni-NC/SiO2 at room temperature. Chem. Eng. Sci. 2024, 298, 120243. [Google Scholar] [CrossRef]
- Jain, A.B.; Vaidya, P.D. Kinetics of Catalytic Hydrogenation of 5-Hydroxymethylfurfural to 2,5-bis-Hydroxymethylfuran in Aqueous Solution over Ru/C. Int. J. Chem. Kinet. 2016, 48, 318–328. [Google Scholar] [CrossRef]
- Rodríguez-Padrón, D.; Perosa, A.; Longo, L.; Luque, R.; Selva, M. Tuning the Selectivity of the Hydrogenation/Hydrogenolysis of 5-Hydroxymethylfurfural under Batch Multiphase and Continuous-Flow Conditions. ChemSusChem 2022, 15, e202200503. [Google Scholar] [CrossRef]
- Fulignati, S.; Antonetti, C.; Licursi, D.; Pieraccioni, M.; Wilbers, E.; Heeres, H.J.; Galletti, A.M.R. Insight into the hydrogenation of pure and crude HMF to furan diols using Ru/C as catalyst. Appl. Catal. A Gen. 2019, 578, 122–133. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, J.; Xie, W.; Tang, Y.; Guo, D.; Ni, Y. Catalytic Transfer Hydrogenation of Biobased HMF to 2,5-Bis-(Hydroxymethyl)Furan over Ru/Co3O4. Catalysts 2017, 7, 92. [Google Scholar] [CrossRef]
- Kashyap, P.; Jędrzejczyk, M.; Akhgar, M.; Aubrecht, J.; Kubička, D.; Keller, N.; Ruppert, A. Ru/TiO2 Catalyzed High-Yield Synthesis of Furanic Diols by 5-Hydroxymethylfurfural Hydrogenation with Switchable Selectivity. ChemSusChem 2025, 17, e202401433. [Google Scholar] [CrossRef]
- Mishra, D.K.; Lee, H.J.; Truong, C.C.; Kim, J.; Suh, Y.-W.; Baek, J.; Kim, Y.J. Ru/MnCo2O4 as a catalyst for tunable synthesis of 2,5-bis(hydroxymethyl)furan or 2,5-bis(hydroxymethyl)tetrahydrofuran from hydrogenation of 5-hydroxymethylfurfural. Mol. Catal. 2020, 484, 110722. [Google Scholar] [CrossRef]
- Sindhu, R.; Binod, P.; Pandey, A.; Ankaram, S.; Duan, Y.; Awasthi, M.K. Chapter 5-Biofuel Production From Biomass: Toward Sustainable Development. In Current Developments in Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2019; pp. 79–92. [Google Scholar] [CrossRef]
- Dutta, S.; Mascal, M. Novel Pathways to 2,5-Dimethylfuran via Biomass-Derived 5-(Chloromethyl)furfural. ChemSusChem 2014, 7, 3028–3030. [Google Scholar] [CrossRef]
- Yan, L.; Zhang, Q.; Deng, W.; Zhang, Q.; Wang, Y. Catalytic valorization of biomass and bioplatforms to chemicals through deoxygenation. In Advances in Catalysis; Song, C., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; Volume 66, pp. 1–108. [Google Scholar] [CrossRef]
- Hu, L.; Tang, X.; Xu, J.; Wu, Z.; Lin, L.; Liu, S. Selective Transformation of 5-Hydroxymethylfurfural into the Liquid Fuel 2,5-Dimethylfuran over Carbon-Supported Ruthenium. Ind. Eng. Chem. Res. 2014, 53, 3056–3064. [Google Scholar] [CrossRef]
- Dong, Z.; Zhanga, Y.; Xia, H. Selective hydrogenolysis of 5-hydroxymethylfurfural to 2,5-dimethylfuran with high yield over bimetallic Ru–Co/AC catalysts. RSC Adv. 2024, 14, 14982–14991. [Google Scholar] [CrossRef]
- Zu, Y.; Yang, P.; Wang, J.; Liu, X.; Ren, J.; Lu, G.; Wang, Y. Efficient production of the liquid fuel 2,5-dimethylfuran from 5-hydroxymethylfurfural over Ru/Co3O4 catalyst. Appl. Catal. B Environ. 2014, 146, 244–248. [Google Scholar] [CrossRef]
- Buta, J.G.; Dame, B.; Ayala, T. Nitrogen-doped ordered mesoporous carbon supported ruthenium metallic nanoparticles: Opportunity for efficient hydrogenolysis of biomass-derived 5-hydroxymethylfurfural to 2,5-dimethylfuran by catalytic transfer hydrogenation. Heliyon 2024, 10, e26690. [Google Scholar] [CrossRef] [PubMed]
- Insyani, R.; Barus, A.F.; Gunawan, R.; Park, J.; Jaya, G.T.; Cahyadi, H.S.; Sibi, M.G.; Kwak, S.K.; Verma, D.; Kim, J. RuO2–Ru/Hβ zeolite catalyst for high-yield direct conversion of xylose to tetrahydrofurfuryl alcohol. Appl. Catal. B Environ. 2021, 291, 120120. [Google Scholar] [CrossRef]
- Wang, N.; Chen, Z.; Liu, L. Acid catalysis dominated suppression of xylose hydrogenation with increasing yield of 1,2-pentanediol in the acid-metal dual catalyst system. Appl. Catal. A Gen. 2018, 561, 41–48. [Google Scholar] [CrossRef]
- Ren, H.-F.; Zhu, D.; Li, J.-F.; Liu, C.-L.; Yang, R.-Z.; Dong, W.-S. One-pot conversion of carbohydrates into γ-valerolactone under the coordination of heteropoly acid based ionic liquid and Ru/ZrO2 in water media. Chem. Technol. Biotechnol. 2019, 94, 2355–2363. [Google Scholar] [CrossRef]
- Duan, Y.; Zhang, J.; Li, D.; Deng, D.; Maa, L.-F.; Yang, Y. Direct conversion of carbohydrates to diol by the combination of niobic acid and a hydrophobic ruthenium catalyst. RSC Adv. 2017, 7, 26487–26493. [Google Scholar] [CrossRef]
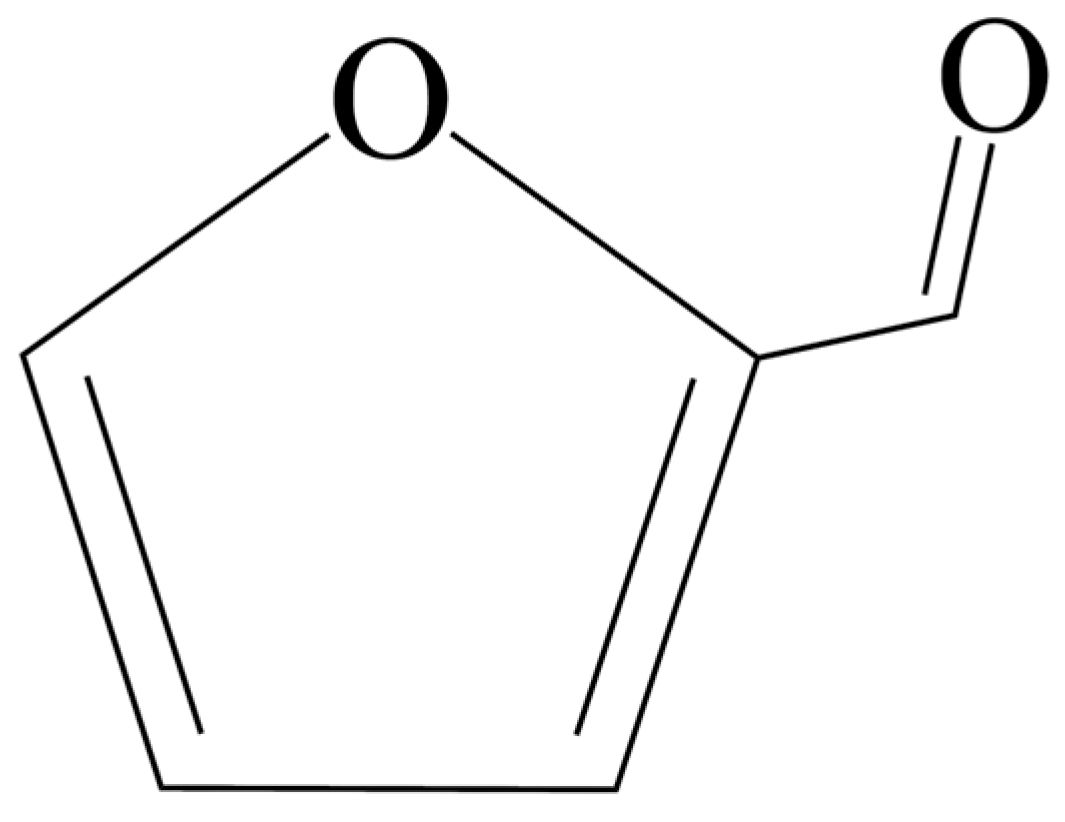
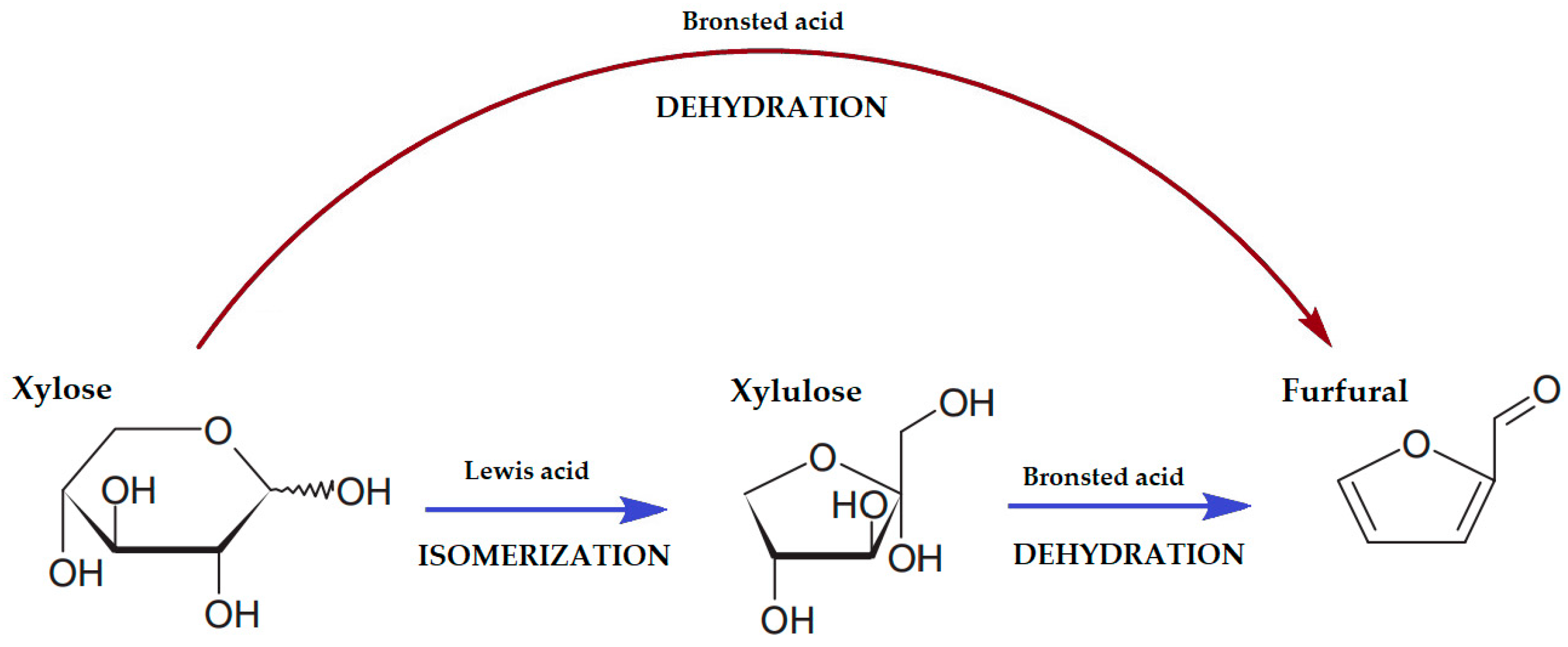
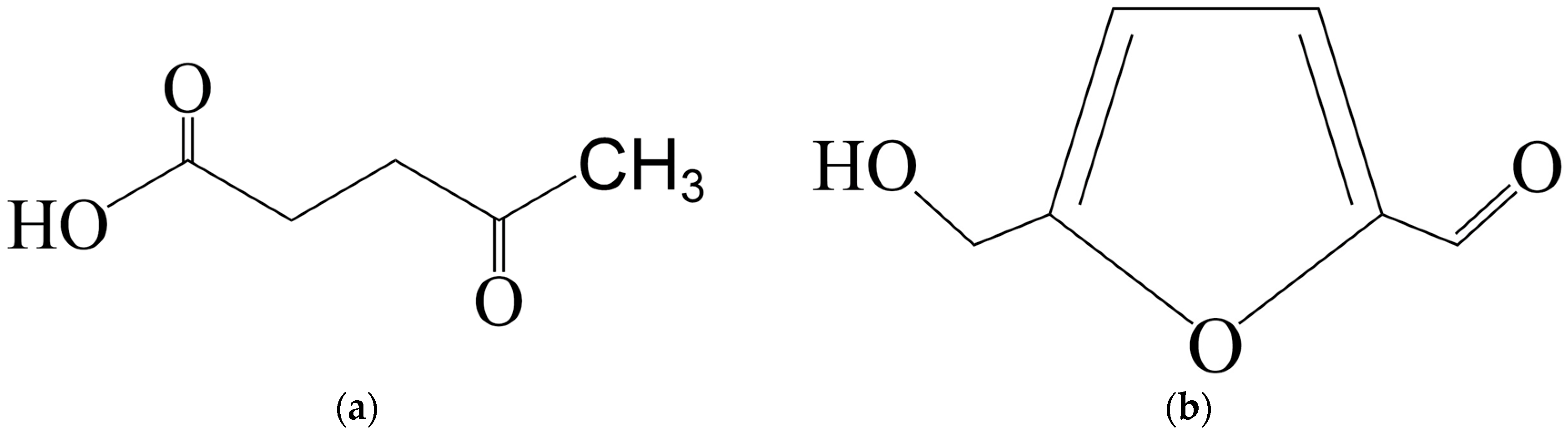
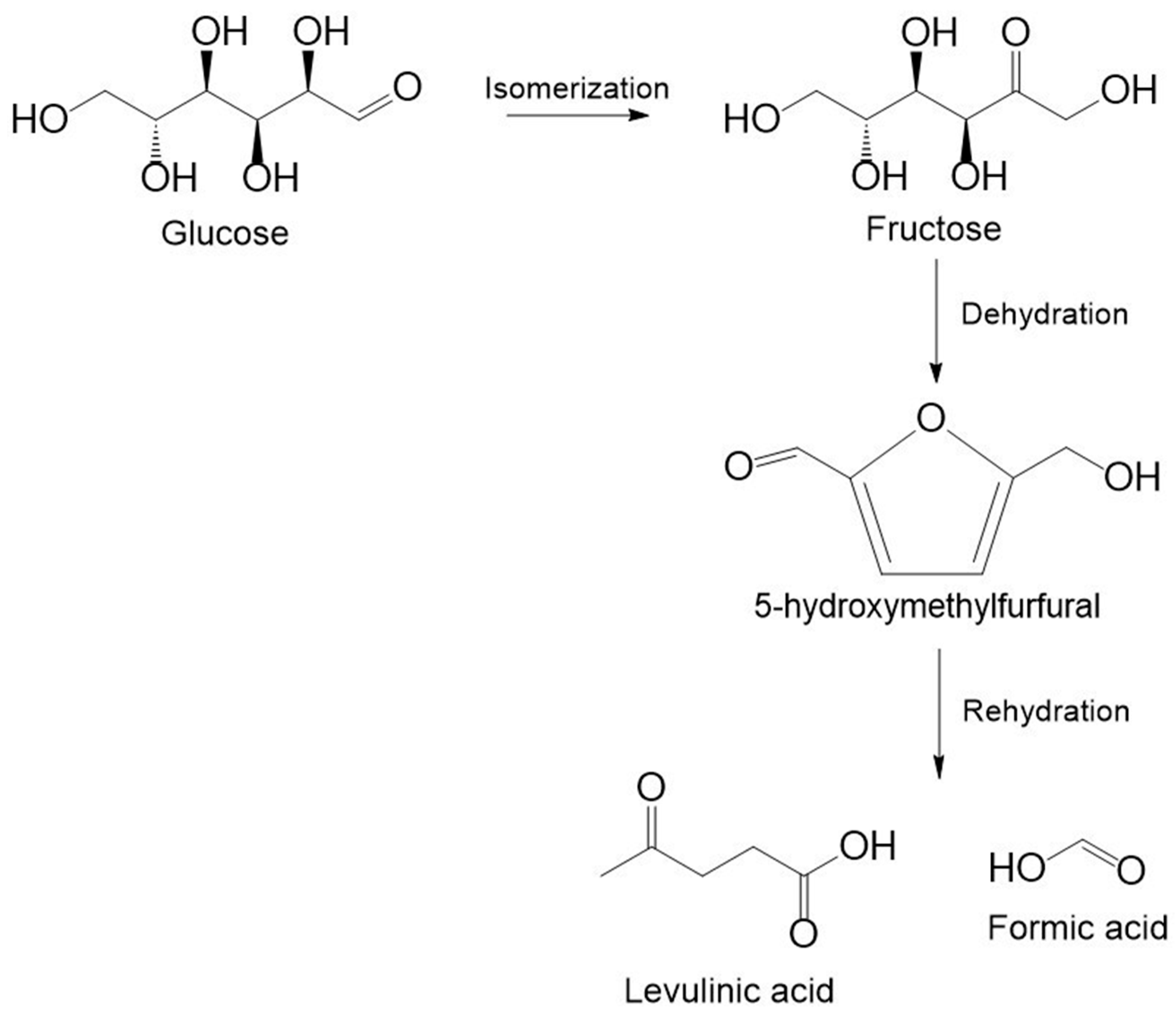
| Catalyst | Reaction Conditions | Furfural Yield, % | Ref. |
|---|---|---|---|
| Carbon based catalysts | |||
| S-800-CG | T = 140 °C t = 40 min mcat = 100 mg solvent—1,4-dioxane xylose—200 mg | 85.9 | [33] |
| SC-GCa-800 | T = 140 °C t = 40 min mcat = 50 mg solvent—1,4-dioxane xylose—100 mg | 76.9 | [34] |
| ZnCS-2 | T = 180 °C t = 60 min mcat = 5 mg solvent—methyl isobutyl ketone /NaCl xylose—100 mg | 72.4 | [35] |
| MCSA | T = 190 °C t = 120 min mcat = 5 mg solvent—H2O/γ-valerolactone tobacco stalks—60 mg | 46.6 | [39] |
| EAC-3H-2T | T = 180 °C t = 3 h mcat = 1.5 wt% solvent—γ-valerolactone xylose—100 mg | 74.6 | [41] |
| SG | T = 150 °C t = 40 min mcat = 28 mg solvent—γ-valerolactone xylose—60 mg | 96.0 | [42] |
| Zeolite based catalysts | |||
| BEATUD | T = 170 °C t = 8 h mcat = 20 mg solvent—H2O D-xylose—30 mg | 74.0 | [29] |
| ZSM-5 | T = 463 K t = 3 h mcat = 1.0 g solvent—organic solvent/aqueous waste aqueous hemicellulose solution—15 mL | 82.4 | [30] |
| SAPO-34 | T = 463 K t = 8 h mcat= 48 mg solvent—γ-valerolactone/H2O xylose or switchgrass—2 wt% | 40.0 | [43] |
| H-Beta | T = 160 °C t = 40 min mcat= 150 mg solvent—H2O monosaccharide—2 wt% | 73.0 | [44] |
| Cr-deAl-Y | T = 180 °C t = 30 min solvent—H2O/n-butanol ratio catalyst/xylose = 2:4 (w/w) | 77.5 | [45] |
| HZ-Na-50 | T = 150 °C t = 60 min mcat = 20 mg solvent—H2O/toluene xylose—625 mg | 63.0 | [46] |
| HSO3-ZSM-5 | T = 160 °C t = 5 h mcat = 20 mg solvent—THF/H2O corncob—20 mg | 89.0 | [47] |
| Metal oxide-based catalysts | |||
| γ-Al2O3 | T = 175 °C t = 30 min mcat = 50 mg solvent—H2O/toluene xylose—150 mg | 83.0 | [52] |
| Mesoporous niobia (Nb450) | T = 170 °C; t = 90 min mcat = 50 mg D-xylose—150 mg solvent—H2O/toluene | 50.0 | [55] |
| Zn doped CuO | T = 150 °C t = 12 h mcat = 100 mg ratio cat/xylose = 1:5 (w/w) solvent—H2O | 86.0 | [56] |
| NbTiO-MNL2 | T = 140 °C t = 141s mcat = 114 mg xylose—0.02 M solvent—H2O/γ –valerolactone | 42.0 | [57] |
| Al-SBA-15(SA) | T = 170 °C t = 7 h mcat = 100 mg solvent—H2O xylose—0.9 g | 63.0 | [58] |
| Cr3+/P-SBA-15 | T = 170 °C t = 90 min mcat = 30 mg xylose—100 mg solvent—H2O/THF | 91.0 | [59] |
| Catalyst | Reaction Conditions | Yield 5-HMF/ LA, % | Ref. |
|---|---|---|---|
| 5-HMF production | |||
| SnPO | T = 120 °C t = 3 h The catalyst/glucose ratio = 1:2 (w/w) solvent—ionic liquid EMIMBr | 58.3 | [80] |
| ZrP-Cr | T = 120 °C t = 2 h mcat = 100 mg fructose—180 mg solvent—1-butyl-3-methylimidazolium chloride | 94.5 | [85] |
| CTAB-modified Amberlyst-15 | T = 140 °C t = 2 h solvent—1,4-dioxane/H2O | 63.1 | [78] |
| NbPO4 | T = 150 °C corn syrup—200 mg/mL aqueous to organic phase ratio of 1:5 (v/v). | 53.1 | [84] |
| Fe/HY zeolite | T = 120 °C t = 60 min solvent—H2O glucose—5000 ppm | 11.4 | [89] |
| Aquivion@silica | T = 90 °C t = 2 h solvent—DMSO fructose—300 mg catalyst—16 μmol H+ | 85.0 | [92] |
| LA production | |||
| AZ25 | T = 190 °C t = 180 min ratio C5 sugar/zeolite = 2:1 (w/w) solvent—H2O | 42.7 | [77] |
| CH3-SBA-15-SO3H | T = 180 °C t = 2.5 h ratio catalyst/glucose = 5:1 (w/w) solvent—GVL/H2O | 61.56 | [82] |
| UiO-66-NH-R-SO3H | T = 170 °C solvent—H2O | 71.6 | [83] |
| 8%Cr/HZSM-5 | T = 180 °C t = 180 min mcat = 0.75 g solvent—H2O | 64.4 | [88] |
| Fe/HY zeolite | T = 180 °C t = 240 min solvent—H2O glucose–5000 ppm | 66.0 | [89] |
| WO3/ZnCo2O4@CeO | T = 180 °C t = 200 min catalyst dosage—4 wt% corncob biomass—5 g solvent—H2O | 78.5 | [93] |
| Product | Catalyst | Reaction Conditions | Results | Reference |
|---|---|---|---|---|
| Furfuryl alcohol (FFA) | 3.5% Ru/Al-SBA-15 | T = 160 °C P(H2) = 1 atm mcat = 150 mg t = 4 h | FUR conversion—100%, selectivity to FFA—99% | [97] |
| Ru/UiO-66 | T = 20°C P(H2) = 5 bar t = 4 h mcat = 100 mg FUR—10 µL solvent—H2O | yield of FFA—94.9% | [98] | |
| Ru/C (mildly oxidized biochar) | T = 105 °C P(H2) = 1035 kPa t = 25 h mcat = 400 mg FUR—1 g | FUR conversion—53%, selectivity to FFA—93% | [99] | |
| Ru-Co/TiO2 (0.6%Co) | T = 50 °C P(H2) = 2 MPa t = 2 h mcat = 50 mg FUR—50 µL solvent—methanol | FUR conversion—91.7%, selectivity to FFA—97.5% | [100] | |
| Tetrahydro-furfuryl alcohol (THFA) | Pd/Al2O3 (60 mg) + Ru/ZrO2 (100 mg) | T = 30 °C P(H2) = 0.5 MPa t = 3 h FUR—0.1 mL solvent—H2O | yield of THFA—99% | [103] |
| Ru/PVP + HDA | T = 125 °C P(H2) = 20 bar t = 48 h mcat = 10 mg FUR—4 mmol solvent—1-propanol | yield of THFA—64%, yield of 1,2-PeD—36% | [104] | |
| Ru/C (electrocatalysis) | T = 25 °C solvent—mildly acidic catholyte solutions (0.02 M HCl) 100 mA t = 2 h | FUR conversion—97%, yield of THFA—48% | [105] | |
| 2-Methylfuran (2-MF) | 1%Ru:4%Pd/TiO2 | T = 25 °C P(H2) = 3 bar t = 3 h mcat = 100 mg FUR—1 g solvent—octane | FUR conversion—39.3%, selectivity to 2-MF—51.5% | [108] |
| Ru0.8Ni0.2/SBA-16 | T = 180 °C P(H2) = 1 atm t = 4 h mcat = 200 mg FUR—1mL solvent—2-propanol | FUR conversion—100%, selectivity to 2-MF—88% | [109] | |
| Pentanediols | Ru-Sn/ZnO (Ru3Sn7) | T = 140 °C P(H2) = 30 bar t = 6 h mcat = 100 mg | 1,2-PeD—84.3% | [111] |
| Ru-SnOx/γ-Al2O3 | T = 180 °C P(H2) = 30 bar t = 7 h mcat = 50 mg FUR—2.0 mmol solvent—1,4-dioxane | yield of 1,5-PeD—94% | [112] |
| Product | Catalyst | Reaction Conditions | Results | Ref. |
|---|---|---|---|---|
| GVL | Ru/Beta-12.5 | T = 90 °C P(H2) = 45 bar t = 2 h mcat = 60 mg LA—0.6–0.7 mol L−1 | LA conversion—94%, yield of GVL—66% | [116] |
| Ru/Al2O3-TiO2 | T = 80 °C WHSV = 1.8 h−1 P(H2) = 4.0 MPa mcat = 2g LA—5 wt% solvent—H2O | yield of GVL—97% | [117] | |
| Ru@HBPPS/SO3H | T = 100 °C P(H2) = 2.0 MPa t = 4h catalyst loading—0.016 mol.% LA—1g solvent—H2O | yield of GVL—99.9%, selectivity to GVL—100% | [118] | |
| Ru/Mg-LaO | T = 80 °C P(H2) = 0.5 MPa t = 4h mcat = 100 mg LA—2g solvent—toluene | LA conversion—92%, selectivity to GVL > 99% | [119] | |
| Ru/AC (reduced at 200 °C) | T = 70 °C P(H2) = 1.5 MPa t = 2 h mcat = 100 mg LA—2.3g solvent—H2O | yield of GVL—74% | [120] | |
| Ru/BEA (3 mass.%, Si/Al = 150) | T = 130 °C P(H2) = 10 bar mcat = 10 mg t = 24 h LA—1 mmol solvent—1,4-dioxane | LA conversion—96.5%, selectivity to GVL—97.8% | [121] | |
| 1,4-PeD | Nanoporous Ru | T = 120 °C P(H2) = 6 MPa t = 24 h mcat = 10 mg LA—2 mmol solvent—H2O | yield 1,4-PeD—74.6% | [123] |
| Ru–MoOx/AC | T = 70 °C P(H2) = 4 MPa WHSV—0.4h−1 mcat = 4.3 g LA—5 wt% solvent—H2O | yield of 1,4-PeD—96.7% | [124] | |
| RuRe/CB | T = 130 °C P(H2) = 50 bar | LA conversion—99%, selectivity to 1,4-PeD ~ 75% | [125] | |
| 2-MeTHF | [RuH2(PPh3){N(CH2PPh2)3}] + HN(Tf)2 | T = 150 °C P(H2) = 65 bar t = 25 h catalyst—0.5 mol% LA—10 mmol solvent—THF | yield of 2-MeTHF—87% | [128] |
| Ru/C (10 mg) + Re/C (20 mg) + NBP (500 mg) | T = 180 °C P(H2) = 5 MPa t = 3 h LA—1.99 g solvent—H2O | yield of 2-MeTHF ~ 28 mol.% | [129] | |
| Ru/GO | T = 265 °C P(H2) = 25 bar WHSV—0.512 h−1 t = 50 h mcat = 1.0 g solvent—1,4-dioxane | selectivity to ether 92%, to 2-MeTHF—77% | [130] |
| Product | Catalyst | Reaction Conditions | Results | Ref. |
|---|---|---|---|---|
| BHMF | 5% Ru/C | T = 40–70 °C P(H2) = 0.69–2.07 MPa t = 1 h 5-HMF—19.8–39.7 mM catalyst loading—0.3–0.7 kg m–3 aqueous phase | 5-HMF conversion—31–95%, selectivity to BHMF—100% | [135] |
| Ru/C | T = 60 °C P(H2) = 30 bar t = 6 h mcat = 50 mg 5-HMF—0.2 M aqueous phase | 5-HMF conversion—85%, selectivity to BHMF—92% | [136] | |
| Ru/C | T = 50 °C P(H2) = 30 bar t = 240 min Ru/5-HMF ratio = 1 wt% 3 wt% 5-HMF aqueous solution | BHMF yield—93.0 mol% | [137] | |
| Ru/Co3O4 | T = 190 °C t = 6 h 5-HMF—0.5 wt% catalyst loading—0.25 wt% catalytic transfer hydrogenation, isopropanol as the hydrogen donor | 5-HMF conversion—100%, yield of BHMF—82.8% | [138] | |
| Ru/TiO2 reduced at 400 °C | T = 120 °C P(H2) = 70 bar t = 6 h mcat = 150 mg 5-HMF—1 g solvent—1,4-dioxane | BHMF yield—98% | [139] | |
| Ru/MnCo2O4 | T = 100 °C P(H2) = 8.2 MPa 5-HMF/Ru =100 t = 4 h | HMF conversion—100%, BHMF yield —98.5% | [140] | |
| BHMTHF | Ru/C | T = 100 °C P(H2) = 50 bar run time—60 min flow rate = 0.1 mL min−1 mcat = 300 mg 0.05 M 5-HMF in ethyl acetate | 5-HMF conversion—100%, selectivity to BHMTHF—90% | [136] |
| Ru/C | T = 100 °C P(H2) = 50 bar t = 240 min Ru/5-HMF ratio = 1 wt% 3 wt% 5-HMF aqueous solution | BHMTHF yield—95.3% | [137] | |
| Ru/TiO2 reduced at 400 °C | T = 120 °C P(H2) = 70 bar t = 24 h mcat = 150 mg 5-HMF—1 g solvent—1,4-dioxane | BHMTHF yield—100% | [139] | |
| Ru/MnCo2O4 | T = 100 °C P(H2) = 8.2 MPa 5-HMF/Ru = 50 t = 16 h | 5-HMF conversion—98.7%, yield of BHMTHF—97.3% | [140] | |
| DMF | Ru/C | T = 200 °C P(H2) = 2 MPa t = 2 h catalyst—5mol% 5-HMF—2.5 wt% solvent—THF | 5-HMF conversion—100%, yield of DMF—94.7% | [144] |
| Ru–Co/AC (5% Ru, 1% Co) | T = 200 °C P(H2) = 1.0 MPa t = 1.5 h mcat = 25 mg 5-HMF—1.25 wt% solvent—THF | 5-HMF conversion—98.7%, yield of DMF—97.9% | [145] | |
| Ru/Co3O4 | T = 130 °C P(H2) = 0.7 MPa t = 24 h mcat = 100 mg 5-HMF—0.25 g solvent—THF | yield of DMF—93.4% | [146] | |
| 2% Ru/N-CMK-1 | T = 160 °C P(N2) = 20 bar t = 8 h mcat = 10 mg 5-HMF—20mM catalytic transfer hydrogenolys isopropanol as a hydrogen donor | yield of DMF—88% | [147] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zamanbekova, A.T.; Zharmagambetova, A.K.; Auyezkhanova, A.S.; Talgatov, E.T.; Jumekeyeva, A.I.; Akhmetova, S.N.; Kenzheyeva, A.M. Perspectives on the Catalytic Processes for the Deep Valorization of Carbohydrates into Fuels and Chemicals. Molecules 2025, 30, 3498. https://doi.org/10.3390/molecules30173498
Zamanbekova AT, Zharmagambetova AK, Auyezkhanova AS, Talgatov ET, Jumekeyeva AI, Akhmetova SN, Kenzheyeva AM. Perspectives on the Catalytic Processes for the Deep Valorization of Carbohydrates into Fuels and Chemicals. Molecules. 2025; 30(17):3498. https://doi.org/10.3390/molecules30173498
Chicago/Turabian StyleZamanbekova, Aigul T., Alima K. Zharmagambetova, Assemgul S. Auyezkhanova, Eldar T. Talgatov, Aigul I. Jumekeyeva, Sandugash N. Akhmetova, and Alima M. Kenzheyeva. 2025. "Perspectives on the Catalytic Processes for the Deep Valorization of Carbohydrates into Fuels and Chemicals" Molecules 30, no. 17: 3498. https://doi.org/10.3390/molecules30173498
APA StyleZamanbekova, A. T., Zharmagambetova, A. K., Auyezkhanova, A. S., Talgatov, E. T., Jumekeyeva, A. I., Akhmetova, S. N., & Kenzheyeva, A. M. (2025). Perspectives on the Catalytic Processes for the Deep Valorization of Carbohydrates into Fuels and Chemicals. Molecules, 30(17), 3498. https://doi.org/10.3390/molecules30173498







