Abstract
Capsaicin, the major pungent alkaloid in Capsicum species, has been reported to exhibit cytotoxic activity through various mechanisms. In this study, capsaicin and 37 structurally related vanillylamide and ester analogs were synthesized and evaluated for cytotoxic activity and tumor cell/non-tumor cell selectivity in vitro and compared with a Capsicum baccatum (Aji mochero) extract. Seven analogs with superior potency and selectivity compared to capsaicin were identified. Notably, vanillylamides with a C16–C18 chain exhibited IC50 values five-fold lower than capsaicin (15–84 µM), with selectivity indices up to 35. The extract obtained from the dried chili fruit, known to hold capsaicin as its primary component, however, exhibited significantly lower cytotoxic activity against tumor cells than pure capsaicin. These data demonstrate that even minor modifications to the acyl chain (as exemplified for the nonivamide analogs) can enhance the cytotoxicity and selectivity of these derivatives and that isolated compounds are able to offer even greater efficacy than whole-fruit extracts.
1. Introduction
Peppers are native to Central and South America and Mexico, with their use as spices dating back more than 6000 years [1]. It was C. Columbus who introduced chilis to Spain in 1493 [2], and in the following years, these spices spread all over Europe, and later on to India, Asia, and Africa.
Plants of the genus Capsicum are rich in a class of compounds known as capsaicinoids, with capsaicin (A, 8-methyl-N-vanillyl-6-nonenamide, Figure 1) being the most important. The “chemistry” of A dates back to 1816: C.F. Buchholz [3] extracted A in impure form for the first time, and A was obtained in almost pure form by J.C. Tresh in 1876 [4]. Its burning and even painful sensations were first reported by R. Buchheim [5,6] and E. Högyes [7] in 1872 and 1877, respectively. Finally, the structure of A was elucidated by D.J. Bennett and G.W. Kirby almost a century later, in 1968 [8].
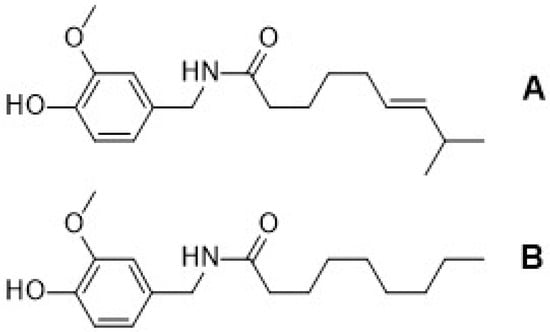
Figure 1.
Structure of capsaicin (A) and nonivamide (B).
These sometimes extremely painful sensations are due to the binding of A and analogs thereof to the vanilloid receptor subtype 1 (TRPV1) [9], resulting in a hypersensitivity reaction in the skin.
For many years, A has been reported as a cytotoxic agent for more than 70 different tumor cell lines (e.g., carcinomas, sarcomas, leukemias, and lymphomas) [10,11] and several different modes of action have been reported, more or less irrespective of the presence of the TRPV1 receptor in these malignant cells [12,13,14,15].
However, prolonged exposure to low concentrations of A (0.1–10 μM) has been reported to lead to the development of aggressive phenotypes in tumor cells [10,16]. There is a suggestion that low concentrations might act as co-carcinogens [17], which has been supported by epidemiological studies revealing a positive correlation between chili consumption and the development of certain malignancies [18,19,20], but contradicted by recent results that did not find an increase in tumor incidence [21].
There have been several excellent reviews on this topic (also referred to in the literature as “the double-edged sword“ postulate) [11,22,23,24]. In addition, the role of the amide bond in capsaicin and analogs has been studied quite recently [25].
Cancer remains one of the leading causes of morbidity and mortality worldwide [26]. While chemotherapy is a cornerstone of cancer treatment, many conventional chemotherapeutic agents suffer from significant limitations, including severe side effects, limited tumor selectivity, and the emergence of drug resistance [27]. There is therefore an urgent need to discover and develop new anticancer compounds that are more effective, less toxic to normal cells, and capable of overcoming resistance mechanisms. Natural products and their derivatives continue to be a valuable source of novel chemical structures with potential anticancer activity, offering promising avenues for the development of improved therapeutics.
Recently, we reported on the strong inhibitory activity of capsaicin (A) and nonivamide (B, Figure 1) against acetylcholinesterase and butyrylcholinesterase [28]. Furthermore, B (also known as pseudocapsaicin) was shown to protect model cells from Alzheimer’s disease-related damage [23,29]. In this study, we characterized novel capsaicin-derivative compounds in comparison to capsaicin and whole-chili extract, focusing on their anticancer activity and selectivity. A mandatory prerequisite for the application of such compounds as potential anticancer agents is proof of low (cyto)toxicity to normal, non-malignant cells.
To accomplish this, we performed cytotoxicity assays to analyze the impact of new compounds on tumor cells and normal fibroblasts and to assess general cytotoxic activity and tumor cell selectivity. For this purpose, we used our standard cell line panel comprising tumor cell lines from various sources—including breast, lung, and colorectal cancers—representing the most common cancer types. As a result, we identified three compounds that showed cytotoxic activity in various tumor models while being non-toxic to normal fibroblasts. Their selectivity indices were up to 35, thereby outperforming capsaicin and whole-chili extract.
2. Results
2.1. Synthesis
To investigate the activity/selectivity pattern in more detail, compounds 1–37 were prepared as previously reported [28] and—together with capsaicin—subjected to cytotoxicity assays. The data were compared with the characteristics of a prepared whole-chili extract, known to have capsaicin as its primary component. Capsaicin (A) was obtained from a local supplier and purified by chromatography [30] followed by repeated re-crystallization. For the synthesis of 1–34, briefly, a panel of alkanoic acids were converted into their respective vanillyl amides (Scheme 1) and di-acylation products; products 35–37 were obtained from divanillylamine.
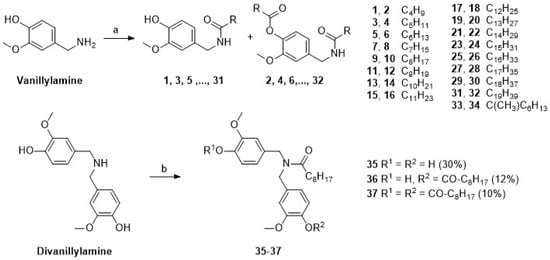
Scheme 1.
Synthesis of compounds 1–37; reactions and conditions: (a) DCM, 5 h, 20 °C; (b) nonanoic acid, (COCl)2, DMF (cat.), and then divanillylamine, 5 h, 20 °C.
2.2. Biological Evaluation
All compounds (1–37) and capsaicin were screened in SRB assays for their cytotoxic activity. For an initial screening, A2780 (human ovarian carcinoma) cells were used, with CCD18Co (non-malignant human fibroblast) cells to obtain some indication about the general cytotoxic activity and the tumor/non-tumor-cell selectivity. The results from these assays are depicted in Figure 2.
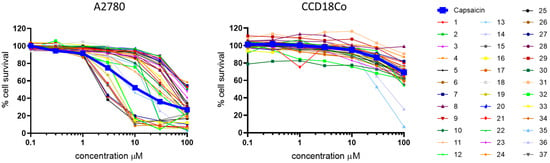
Figure 2.
Dose–response pattern of new compounds (colored lines) compared to capsaicin (bold blue line) in A2780 cells and non-malignant fibroblasts.
A potential tumor cell selectivity of compounds can be assumed if the dose–response pattern clearly differs between tumor and normal cells. Most compounds exhibited no toxicity and caused only minimal growth inhibition in normal fibroblasts, even at 100 µM, thus not reaching an IC50 value (Figure 2, right). As a result, 7 out of 37 compounds (15, 17, 19, 21, 25, 27, 29), with IC50 values well below 10 µM, demonstrated tumor cell selectivity over non-tumor cells and were more active than capsaicin. An analysis (Figure 3) of cytotoxic activity at 3 μM (from dose–response curves) revealed the three most active and selective compounds (red) compared to capsaicin (blue).
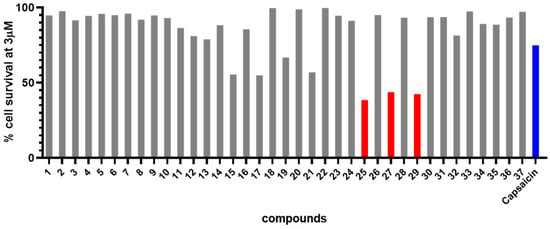
Figure 3.
Analysis of cytotoxic activity of capsaicin (blue) and compounds 1–37 employing a concentration of 3 μM and A2780 tumor cells. Most active and selective compounds (25, 27, 29) are depicted in red color.
The three most active compounds (25, 27, 29) were further investigated concerning their cytotoxic activity using a panel of five different human tumor cell lines compared to non-malignant fibroblasts. The results from these investigations are summarized in Table 1.

Table 1.
Cytotoxic activity (IC50, means with SD ≤ 15%, in μM) from SRB assays; three independent experiments of the three most active compounds—25, 27, and 29—and capsaicin (A) in a panel of human cancer cell lines: A2780, A2780cis (ovarian carcinoma), A549 (lung carcinoma), MCF7 (breast carcinoma), HT29 (colorectal carcinoma), and CCD18Co (non-malignant human fibroblasts). A selectivity index (SI, as a ration of the respective IC50 values (IC50 CCD18Co/IC50 A2780) is provided too.
These results confirm the superior activity/selectivity pattern of the new compounds compared to capsaicin. Overall, A2780 ovarian carcinoma and MCF7 breast carcinoma cells proved to be the most susceptible tumor cell types to capsaicin and new derivatives, with A2780 being the most sensitive.
To complement our investigations, we were also interested in the cytotoxic effect of whole-chili extract given that capsaicin is its main component. Ultrasonic extraction of capsaicinoids from hot chili peppers was thereby shown to significantly improve the extraction yield since power ultrasound promotes cell wall disruption and hydration by forcing the solvent into the plant cells. This results in an increased mass transfer, where intracellular substances are washed out of the plant cell into the solvent. As a purely mechanical, non-thermal processing method, ultrasonic extraction prevents thermal damage to thermally labile compounds. Recently, Olguín-Rojas et al. [31] reported the extraction and isolation of capsaicinoids with a yield of more than 96% from three Capsicum chinense varieties. As a consequence, we decided to use a modified version of this method for the extract preparation, and Aji mochero (Capsicum baccatum) chilis were therefore used. Chili extracts are used as an ingredient in what is considered one of the most delicious extract-based sauces (“vicious but delicious”) in food production. However, the extract is also promoted as an appetite suppressant and as a weight loss agent through increased fat burning. We were therefore interested in determining the cytotoxic activity and selectivity of the extract. The results from these investigations are summarized in Table 2 and graphically illustrated in Figure 4.

Table 2.
Cytotoxicity of a chili extract and of capsaicin (A) in a panel of different cancer cell lines and non-malignant CCD18Co cells. IC50 values of the extract (μg/mL) were calculated from dose–response curves shown in Figure 4 (three independent experiments); IC50 values for capsaicin are taken from Table 1 and were converted into μg/mL.
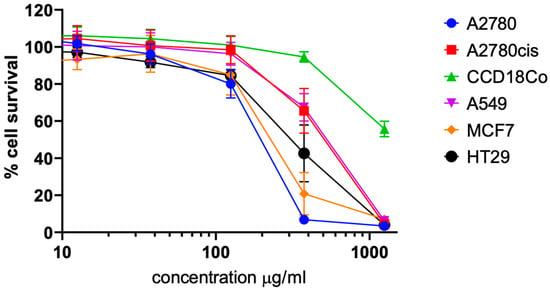
Figure 4.
Analysis of the cytotoxic activity (from SRM, n = 3; means shown and standard deviation) of a chili extract in a panel of cancer cell lines from different entities and non-malignant fibroblasts. Chart shows mean curves from 3 independent experiments ± standard deviation. Cytotoxicity testing was performed in the concentration range of 0.125–1250 μg/mL; the area of cytotoxic activity is shown.
As a result, the extract exhibited a cytotoxicity and selectivity pattern similar to that of capsaicin and the new derivatives, with the most pronounced effect observed in A2780 ovarian carcinoma and MCF7 breast carcinoma cells. Overall, there was a tumor cell selectivity over non-tumor cells, with a selectivity index (IC50 CCD18Co/IC50 A2780) of more than six-fold, which was very similar to that of capsaicin.
Interestingly, these results also revealed that the extract (with a capsaicin content of approximately 80%) [32,33,34] exhibited significantly lower cytotoxic activity against tumor cells than pure capsaicin (Table 2). In A2780 cells, for example, there was an approximately 30-fold difference between the IC50 values of capsaicin (15.9 µM ≈ 4.856 µg/mL) and the extract (80% of 183.4 µg/mL ≈ 146.72 µg/mL).
Finally, these results also suggest that even minor structural modifications (cf. compounds 25, 27, 29 compared to all other structures in this study) can lead to significant changes in their biological activity—for example, increased cytotoxic activity against tumor cells was observed accompanied by improved tumor cell/non-tumor cell selectivity.
Different modes of action for capsaicin have been reported for various cells. Our current investigations do not allow us to draw conclusions about how the derivatives exert their cytotoxic effect. Since the target(s) of our compounds are unknown and will be the subject of future investigations, we cannot perform structure–activity analyses or corresponding molecular modeling calculations.
3. Discussion
Capsaicin, the major pungent alkaloid in Capsicum species, exhibits well-documented anticancer activity across various tumor models, mediated by multiple mechanisms. This comprehensive study investigated capsaicin alongside 37 structurally related vanillylamide and ester analogs synthesized to evaluate cytotoxicity and tumor selectivity in vitro. A Capsicum baccatum (Aji mochero) extract was assessed comparatively. Overall, 37 structurally related vanillylamide and ester derivatives (compounds 1–37) were synthesized via the conversion of various alkanoic acids into their respective vanillyl amides, including di-acylation and divanillylamine derivatives. As a result, seven analogs demonstrated superior potency and selectivity relative to capsaicin, with vanillylamides featuring C16-C18 chains showing five-fold-lower IC50 values (15–84 µM) and selectivity indices (SI) up to 35. Whole-fruit extract, despite capsaicin being its major component, exhibited significantly lower cytotoxic activity than pure capsaicin. These findings highlight the impact of minor acyl chain modifications on cytotoxicity and selectivity, underlining the potential of purified compounds over extracts for therapeutic applications in oncology.
Compounds 25, 27, and 29 emerged as the most active and selective derivatives, exhibiting IC50 values in A2780 cells of 2.8 µM, 3.4 µM, and 3.0 µM, respectively, with selectivity indices exceeding 29, compared to capsaicin’s IC50 of 15.9 µM and SI of 6.3.
Despite the high activity of compounds 25, 27, and 29, it would be very interesting to synthesize and study the cytotoxic activity of analogs of compounds 35–37, i.e., divanillylamides holding C16–C18 carboxylic acid residues. However, it has to be noted that solubility issues were encountered even with monomeric vanillylamides carrying longer alkyl chains. Hence, it can assumed that these issues will increase with divanillylamines holding three long alkyl chains, thus making them unsuitable for in vitro testing.
This systematic evaluation underscores the following: Capsaicin and structurally related vanillylamide analogs exhibit significant cytotoxicity toward various cancer cell lines, with a high degree of selectivity. Structural modifications, particularly in acyl chain length, can dramatically enhance anticancer efficacy and selectivity. Whole-capsicum extracts, despite high capsaicin content, display lower cytotoxic activity, indicating the benefit of purified compound use in therapeutic approaches. Several factors might explain this discrepancy, including matrix effects due to the presence of other compounds (that might interfere), reduced purity, degradation, and binding to other extract constituents.
4. Materials and Methods
4.1. General
Reagents were purchased from commercial suppliers and used without further purification. The solvents were dried according to usual procedures. TLC was performed on silica gel (Macherey-Nagel, detection with UV absorption; Macherey-Nagel, Düren Germany). Melting points have been measured with a Büchi M-565 instrument (Büchi Labortechnik, Flawill, Switzerland). NMR spectra were recorded using VARIAN spectrometers (Varian Germany, Darmstadt, Germany) at 27 °C (δ given in ppm; J in Hz, typical experiments for assignments: 13C APT, HMBC, HSQC). ASAP-MS spectra were taken on an Advion (Advion, Ithaca, NY, USA) expression CMS-L with an ASAP/APCI Ion source (capillary voltage 150 V, capillary temperature 220 °C, and voltage of the ion source: 15 V; APCI source temperature 300 °C with 5 μA). TLC plates (SiO2, F254 from Macherey-Nagel) were impregnated with AgNO3 (15% followed by drying at 110 °C). AgNO3-impregnated silica gel was freshly prepared from silica gel (180 g, 0.040–0.063 mm, Merck, Darmstadt, Germany) and AgNO3 (22.5 g in 45 mL water) followed by drying at 110 °C for 1 h. Aji mochero chilis were obtained from a local supplier, and used as received.
4.2. Synthesis of the Amides 1–37
Reactions of the carboxylic acid (1.25 eq, all obtained from a local supplier; used as received) in dry DCM (25 mL) with oxalyl chloride (4.0 eq.) in the presence of catalytic amounts of dry DMF at 22 °C for 4 h followed by the evaporation of all volatiles in vacuo gave an oily residue that was re-dissolved in dry DCM (25 mL) and added slowly to a solution of (di)-vanillylamide (1 eq., from a local supplier, used as received) in dry DCM (25 mL). The reactions were completed in about 5 h (as checked by TLC). Standard aqueous work-up followed by chromatography (silica gel, hexanes/ethyl acetate mixtures) gave the products. Identity with previously synthesized compounds [28] was determined by comparing their 1H and 13C NMR spectra, MS and IR spectra, and (where applicable) mixed melting points; the former were identical to authentic material, and the latter showed no melting point depression. Spectra and data are provided in the Supplementary Materials file. CAUTION: skin contact of capsaicin and analogs leads to extremely burning sensations.
4.2.1. N-[(4-Hydroxy-3-methoxy)benzyl]pentanamide (1) and 2-methoxy-4-[(pentanoylamino)methyl]phenyl pentanoate (2)
Following GP from vanillylamine and pentanoic acid, 1 (25%) and 2 (28%) were obtained.
Data for 1: colorless oil; RF = 0.21 (silica gel, n-hexane/ethyl acetate, 6:4); ESI-MS (methanol/chloroform, 4:1): m/z (%) = 102 ([M + Na]+, 100%).
Data for 2: RF = 0.37 (silica gel, n-hexane/ethyl acetate, 6:4); ESI-MS (methanol/chloroform, 4:1): m/z (%) = 344 ([M + Na]+, 100%).
4.2.2. N-[(4-Hydroxy-3-methoxy)benzyl]hexanamide (3) and 2-methoxy-4-[(hexanoylamino)methyl]phenyl hexanoate (4)
Following GP from vanillylamine and hexanoic acid, 3 (27%) and 4 (25%) were obtained.
Data for 3: m.p. 49–49.5 °C; RF = 0.06 (silica gel, n-hexane/ethyl acetate, 7:3); ESI-MS (methanol/chloroform, 4:1): m/z (%) = 274 ([M + Na]+, 100%).
Data for 4: colorless solid; m.p. 104–106 °C; RF = 0.22 (silica gel, n-hexane/ethyl acetate, 7:3); ESI-MS (methanol/chloroform, 4:1): m/z (%) = 372 ([M + Na]+, 100%).
4.2.3. N-[(4-Hydroxy-3-methoxy)benzyl]heptanamide (5) and 2-methoxy-4-[(heptanoylamino)methyl]phenyl heptanoate (6)
Following GP from vanillylamine and heptanoic acid, 5 (31%) and 6 (56%) were obtained.
Data for 5: m.p. 59–62 °C; RF = 0.46 (silica gel, n-hexane/ethyl acetate, 6:4); ESI-MS (methanol/chloroform, 4:1): m/z (%) = 288 ([M + Na]+, 100%).
Data for 6: colorless solid; m.p. 63–65 °C; RF = 0.67 (silica gel, n-hexane/ethyl acetate, 6:4); ESI-MS (methanol/chloroform, 4:1): m/z (%) = 400 ([M + Na]+, 100%).
4.2.4. N-[(4-Hydroxy-3-methoxy)benzyl]octanamide (7) and 2-methoxy-4-[(octanoylamino)methyl]phenyl octanoate (8)
Following GP from vanillylamine and octanoic anhydride, 7 (36%) and 8 (56%) were obtained.
Data for 7: m.p. 41–44 °C; RF = 0.44 (silica gel, n-hexane/ethyl acetate, 6:4); ESI-MS (methanol/chloroform, 4:1): m/z (%) = 304 ([M + Na]+, 100%).
Data for 8: colorless solid; m.p. 71–73 °C; RF = 0.88 (silica gel, n-hexane/ethyl acetate, 6:4); ESI-MS (methanol/chloroform, 4:1): m/z (%) = 428 ([M + Na]+, 100%).
4.2.5. N-[(4-Hydroxy-3-methoxy)benzyl]nonanamide (9) and 2-methoxy-4-[(nonanoylamino)methyl]phenyl nonanoate (10)
Following GP from vanillylamine and nonanoic acid, 9 (67%) and 10 (26%) were obtained.
Data for 9: colorless solid; m.p. 48–50 °C; RF = 0.65 (silica gel, n-hexane/ethyl acetate, 4:6); ESI-MS (methanol/chloroform, 4:1): m/z (%) = 292 ([M − H]−, 100%).
Data for 10: colorless solid; m.p. 74 °C; RF = 0.8 (silica gel, n-hexane/ethyl acetate, 4:6); ESI-MS (methanol/chloroform, 4:1): m/z (%) = 432 ([M − H]−, 100%).
4.2.6. N-[(4-Hydroxy-3-methoxy)benzyl]decanamide (11) and 2-methoxy-4-[(decanoylamino)methyl]phenyl decanoate (12)
Following GP from vanillylamine and decanoic acid, 11 (25%) and 12 (54%) were obtained.
Data for 11: colorless solid; m.p. 55–57 °C; RF = 0.16 (silica gel, n-hexane/ethyl acetate, 6:4); ESI-MS (methanol/chloroform, 4:1): m/z (%) = 331 ([M + Na]+, 100%).
Data for 12: colorless solid; m.p. 99 °C; RF = 0.70 (silica gel, n-hexane/ethyl acetate, 6:4); ESI-MS (methanol/chloroform, 4:1): m/z (%) = 460 ([M − H]−, 100%).
4.2.7. N-[(4-Hydroxy-3-methoxy)benzyl]undecanamide (13) and 22-methoxy-4-[(undecanoylamino)methyl]phenyl undecanoate (14)
Following GP from vanillylamine and undecanoic acid, 13 (23%) and 14 (54%) were obtained.
Data for 13: colorless solid; m.p. 60–63 °C; RF = 0.24 (silica gel, n-hexane/ethyl acetate, 6:4); ESI-MS (methanol/chloroform, 4:1): m/z (%) = 344 ([M + Na]+, 100%).
Data for 14: colorless solid; m.p. 81–83 °C; RF = 0.64 (silica gel, n-hexane/ethyl acetate, 6:4); ESI-MS (methanol/chloroform, 4:1): m/z (%) = 513 ([M + Na]+, 100%).
4.2.8. N-[(4-Hydroxy-3-methoxy)benzyl]dodecanamide (15) and 2-methoxy-4-[(dodecanoylamino)methyl]phenyl dodecanoate (16)
Following GP from vanillylamine and dodecanoic acid, 15 (38%) and 16 (59%) were obtained.
Data for 15 colorless solid; m.p. 76–78 °C; RF = 0.25 (silica gel, n-hexane/ethyl acetate, 6:4); ESI-MS (methanol/chloroform, 4:1): m/z (%) = 358 ([M + Na]+, 100%).
Data for 16: colorless solid; m.p. 84 °C; RF = 0.64 (silica gel, n-hexane/ethyl acetate, 6:4); ESI-MS (methanol/chloroform, 4:1): m/z (%) = 540 ([M + Na]+, 100%).
4.2.9. N-[(4-Hydroxy-3-methoxy)benzyl]tridecanamide (17) and 2-methoxy-4-[(tridecanoylamino)methyl]phenyl tridecanoate (18)
Following GP from vanillylamine and tridecanoic acid, 17 (43%) and 18 (33%) were obtained.
Data for 17: colorless solid; m.p. 70–72 °C; RF = 0.11 (silica gel, n-hexane/ethyl acetate, 7:3); ESI-MS (methanol/chloroform, 4:1): m/z (%) = 373 ([M + Na]+, 100%).
Data for 18: m.p. 79 °C; RF = 0.24 (silica gel, n-hexane/ethyl acetate, 7:3); ESI-MS (methanol/chloroform, 4:1): m/z (%) = 569 ([M + Na]+, 100%).
4.2.10. N-[(4-Hydroxy-3-methoxy)benzyl]tetradecanamide (19) and 2-methoxy-4-[(tetradecanoylamino)methyl]phenyl tetradecanoate (20)
Following GP from vanillylamine and tetradecanoic acid, 19 (33%) and 20 (47%) were obtained.
Data for 19: colorless solid; m.p. 76 °C; RF = 0.13 (silica gel, n-hexane/ethyl acetate, 7:3); ESI-MS (methanol/chloroform, 4:1): m/z (%) = 386 ([M + Na]+, 100%).
Data for 20: colorless solid; m.p. 85–87 °C; RF = 0.28 (silica gel, n-hexane/ethyl acetate, 7:3); ESI-MS (methanol/chloroform, 4:1): m/z (%) = 596 ([M + Na]+, 100%).
4.2.11. N-[(4-Hydroxy-3-methoxy)benzyl]pentadecanamide (21) and 2-methoxy-4-[(pentadecanoylamino)methyl]phenyl pentadecanoate (22)
Following GP from vanillylamine and pentadecanoic acid, 21 (48%) and 22 (26%) were obtained.
Data for 21: colorless solid; m.p. 79 °C; RF = 0.11 (silica gel, n-hexane/ethyl acetate, 7:3); ESI-MS (methanol/chloroform, 4:1): m/z (%) = 400 ([M + Na]+, 100%).
Data for 22: colorless solid; m.p. 79–80 °C; RF = 0.75 (silica gel, n-hexane/ethyl acetate, 6:4); ESI-MS (methanol/chloroform, 4:1): m/z (%) = 625 ([M + Na]+, 100%).
4.2.12. N-[(4-Hydroxy-3-methoxy)benzyl]hexadecanamide (23) and 2-methoxy-4-[(hexadecanoylamino)methyl]phenyl hexadecanoate (24)
Following GP from vanillylamine and hexadecanoic acid, 23 (31%) and 24 (30%) were obtained.
Data for 23: colorless solid; m.p. 80 °C; RF = 0.12 (silica gel, n-hexane/ethyl acetate, 7:3); ESI-MS (methanol/chloroform, 4:1): m/z (%) = 390 ([M − H]−, 100%).
Data for 24: m.p. 91–92 °C; RF = 0.45 (silica gel, n-hexane/ethyl acetate, 7:3); ESI-MS (methanol/chloroform, 4:1): m/z (%) = 665 ([M + Na]+, 100%).
4.2.13. N-[(4-Hydroxy-3-methoxy)benzyl]heptadecanamide (25) and 2-methoxy-4-[(heptadecanoylamino)methyl]phenyl heptadecanoate (26)
Following GP from vanillylamine and heptadecanoic acid, 25 (32%) and 26 (30%) were obtained.
Data for 25: colorless solid; m.p. 84 °C; RF = 0.15 (silica gel, n-hexane/ethyl acetate, 7:3); ESI-MS (methanol/chloroform, 4:1): m/z (%) = 404 ([M − H]−, 100%).
Data for 26: m.p. 94 °C; RF = 0.45 (silica gel, n-hexane/ethyl acetate, 7:3); ESI-MS (methanol/chloroform, 4:1): m/z (%) = 680 ([M + Na]+, 100%).
4.2.14. N-[(4-Hydroxy-3-methoxy)benzyl]octadecanamide (27) and 2-methoxy-4-[(octadecanoylamino)methyl]phenyl octadecanoate (28)
Following GP from vanillylamine and octadecanoic acid, 27 (45%) and 28 (34%) were obtained.
Data for 27: colorless solid; m.p. 87–89 °C; RF = 0.10 (silica gel, n-hexane/ethyl acetate, 7:3); ESI-MS (methanol/chloroform, 4:1): m/z (%) = 418 ([M − H]−, 100%).
Data for 28: colorless solid; 94 °C; RF = 0.49 (silica gel, n-hexane/ethyl acetate, 7:3); ESI-MS (methanol/chloroform, 4:1): m/z (%) = 709 ([M + Na]+, 100%).
4.2.15. N-[(4-Hydroxy-3-methoxy)benzyl]nonadecanamide (29) and 2-methoxy-4-[(nonadecanoylamino)methyl]phenyl nonadecanoate (30)
Following GP from vanillylamine and nonadecanoic acid, 29 (51%) and 30 (34%) were obtained.
Data for 29: colorless solid; m.p. 92 °C; RF = 0.18 (silica gel, n-hexane/ethyl acetate, 7:3); ESI-MS (methanol/chloroform, 4:1): m/z (%) = 432 ([M − H]−, 100%).
Data for 30: colorless solid; m.p. 97 °C; RF = 0.55 (silica gel, n-hexane/ethyl acetate, 7:3); ESI-MS (methanol/chloroform, 4:1): m/z (%) = 737 ([M + Na]+, 100%).
4.2.16. N-[(4-Hydroxy-3-methoxy)benzyl]icosanamide (31) and 2-methoxy-4-[(icosanoylamino)methyl]phenyl icosanoate (32)
Following GP from vanillylamine and icosanoic acid, 31 (20%) and 32 (56%) were obtained.
Data for 31: colorless solid; m.p. 94 °C; RF = 0.38 (silica gel, n-hexane/ethyl acetate, 6:4); ESI-MS (methanol/chloroform, 4:1): m/z (%) = 446 ([M − H]−, 100%).
Data for 32: colorless solid; m.p. 80 °C; RF = 0.75 (silica gel, n-hexane/ethyl acetate, 6:4); ESI-MS (methanol/chloroform, 4:1): m/z (%) = 764 ([M + Na]+, 100%).
4.2.17. N,N-bis(4-hydroxy-3-methoxybenzyl)nonanamide (33), 4-{[4-hydroxy-3-methoxybenzyl)(nonanoyl)amino]methyl}-2-methoxyphenyl nonanoate (34) and (nonanoylimino) bis(methylene-2-methoxy-4,1-phenylene) dinonanoate (35)
Following GP from divanillylamine and nonanoic acid, 33 (30%), 34 (12%), and 35 (10%) were obtained.
Data for 33: colorless oil; RF = 0.55 (n-hexane/ethyl acetate, 5:5); ESI-MS (methanol/chloroform, 4:1): m/z (%) = 430 ([M + H]+, 100%).
Data for 34: colorless oil; RF = 0.65 (n-hexane/ethyl acetate, 5:5); ESI-MS (methanol/chloroform, 4:1): m/z (%) = 592 ([M + Na]+, 100%).
Data for 35: colorless oil; RF = 0.8 (n-hexane/ethyl acetate, 5:5); ESI-MS (methanol/chloroform, 4:1): m/z (%) = 733 ([M + Na]+, 100%).
4.2.18. N-[(4-Hydroxy-3-methoxyphenyl)methyl]-2-methyloctanamide (36) and 4-{[(2-methyloctanoyl)amino]methyl}-2-(methyloxy)phenyl 2-methyloctanoate (37)
Following GP from vanillylamine and 2-methyloctanoic acid, 36 (48%) and 37 (17%) were obtained.
Data for 36: colorless solid; m.p. 78–80 °C; RF = 0.28 (silica gel, n-hexane/ethyl acetate, 4:6); ESI-MS (methanol/chloroform, 4:1): m/z (%) = 292 ([M − H]−, 100%).
Data for 37: colorless solid; m.p. 80–82 °C; RF = 0.55 (silica gel, n-hexane/ethyl acetate, 4:6); ESI-MS (methanol/chloroform, 4:1): m/z (%) = 432 ([M − H]−, 100%).
4.3. Capsaicin
Capsaicin was obtained from a local supplier and purified [30] to remove traces of small amounts of main/secondary capsaicinoids. Chromatography was performed on AgNO3-impregnated silica gel using dry chloroform as the eluent (Rf = 0.03) followed by a first crystallization from 2-propanol/n-hexane (15:85) at −40 °C followed by a second re-crystallization from methyl-tert-butylether/n-pentane (2:1) at −40 °C to yield colorless crystals; m.p. 67–69 °C (lit.: [35] 58–61 °C); NMR and MS as reported by Drosky et al. [30].
4.4. Preparation of the Chili Extract
The chili fruit (Capsicum baccatum, obtained from a local market, 14 g) were cut in small pieces and soaked in methanol for 1 h; all of the material was transferred into an ultrasound device (Hielscher UP200S, Hielscher Ultrasonics GmbH, Teltow, Germany) and treated at 50 °C for 10 min (25 kHz, 50–560 W). After cooling to 22 °C, the reaction mixture was filtered, and the solvent was evaporated under diminished pressure, leading to a brownish amorphous powder (160 mg) that was used in the bio-assays.
4.5. SRB Assay
Cytotoxic activities of compounds were analyzed using the SRB cytotoxicity assay. The human cancer cell lines A2780 (ECACC #93112519), A2780Cis (ECACC #93112517), A549 (ATCC—CCL-185), HT29 (ATCC—HTB-38), and MCF7 (ATCC—HTB-22) were cultivated in RPMI1640 medium, non-malignant human fibroblasts CCD18Co (ATCC—CRL-1459) were grown in MEME (both from Sigma-Aldrich, St. Louis, MO, USA). Both media were supplemented with 10% fetal bovine serum (Biowest, Nuaillé, France) and 1% penicillin-streptomycin (Sigma-Aldrich). Cells were seeded in 96-well plates and, after 24h, were treated with serial dilutions of compounds for 72 h. All subsequent steps were performed according to the previously described SRB assay protocol [26,36,37]. Dose–response curves and calculations of IC50 values, including standard deviations, were performed using GraphPad Prism (version 8) (https://www.graphpad.com, accessed on 17 March 2025).
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules30173488/s1. Spectra and data for compounds, 1H and 13C NMR spectra of compounds 1–37.
Author Contributions
Conceptualization, R.C.; validation, R.C.; investigation: N.V.H., T.M., and R.C.; writing—original draft preparation, R.C., N.V.H., and T.M.; writing—review and editing, N.V.H., T.M., and R.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors upon request.
Acknowledgments
We would like to thank T. Schmidt for the measurement of the MS spectra, and M. Schneider for measuring IR und UV/vis spectra and performing the micro-analyses. NMR spectra were measured by D. Ströhl, Y. Schiller, and S. Ludwig. A special thanks is due to J. Quast for his help with the synthesis of the compounds.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Perry, L.; Dickau, R.; Zarrillo, S.; Holst, I.; Pearsall, D.M.; Piperno, D.R.; Berman, M.J.; Cooke, R.G.; Rademaker, K.; Ranere, A.J.; et al. Starch Fossils and the Domestication and Dispersal of Chili Peppers (Capsicum spp. L.) in the Americas. Science 2007, 315, 986–988. [Google Scholar] [CrossRef]
- Govindarajan, V.S. Capsicum-production, technology, chemistry, and quality. Part 1: History, botany, cultivation, and primary processing. CRC Crit. Rev. Food Sci. Nutr. 1985, 22, 109–176. [Google Scholar] [CrossRef]
- Buchholz, C.F. Chemische Untersuchungen der trockenen, reifen spanischen Pfeffers. Alm. Oder Taschenb. Für Scheidekünste Apoth. 1816, 37, 1–30. [Google Scholar]
- Tresh, J.C. Capsicine: An alkaloid said to be contained in Capsicum fruit: Does it exist? Pharm. J. Transact. 1876, 6, 941–942. [Google Scholar]
- Buchheim, R. Fructus Capsici. Vierteljahresschr. Für Prakt. Pharm. 1872, 4, 507–521. [Google Scholar]
- Buchheim, R. Ueber die “scharfen Stoffe”. Arch. Heilkd. 1873, 13, 1–42. [Google Scholar]
- Högyes, E. Adatok a Capsicum annuum (paprika) alkatrészeinek élettani hatásához. Bull. Med. Sci. Assoc. 1877, 2, 51–56. [Google Scholar]
- Bennett, D.J.; Kirby, G.W. Constitution and biosynthesis of capsaicin. J. Chem. Soc. C 1968, 442–446. [Google Scholar] [CrossRef]
- Caterina, M.J.; Schumacher, M.A.; Tominaga, M.; Rosen, T.A.; Levine, J.D.; Julius, D. The capsaicin receptor: A heat-activated ion channel in the pain pathway. Nature 1997, 389, 816–824. [Google Scholar] [CrossRef]
- Bley, K.; Boorman, G.; Mohammad, B.; McKenzie, D.; Babbar, S. A comprehensive review of the carcinogenic and anticarcinogenic potential of capsaicin. Toxicol. Pathol. 2012, 40, 847–873. [Google Scholar] [CrossRef]
- Lujan-Mendez, F.; Roldan-Padron, O.; Castro-Ruiz, J.E.; Lopez-Martinez, J.; Garcia-Gasca, T. Capsaicinoids and Their Effects on Cancer: The “Double-Edged Sword” Postulate from the Molecular Scale. Cells 2023, 12, 2573. [Google Scholar] [CrossRef]
- Qiao, S.; Li, W.; Tsubouchi, R.; Haneda, M.; Murakami, K.; Yoshino, M. Involvement of peroxynitrite in capsaicin-induced apoptosis of C6 glioma cells. Neurosci. Res. 2005, 51, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, A.M.; Sanchez, M.G.; Malagarie-Cazenave, S.; Olea, N.; Diaz-Laviada, I. Induction of apoptosis in prostate tumor PC-3 cells and inhibition of xenograft prostate tumor growth by the vanilloid capsaicin. Apoptosis 2006, 11, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Athanasiou, A.; Smith, P.A.; Vakilpour, S.; Kumaran, N.M.; Turner, A.E.; Bagiokou, D.; Layfield, R.; Ray, D.E.; Westwell, A.D.; Alexander, S.P.H.; et al. Vanilloid receptor agonists and antagonists are mitochondrial inhibitors: How vanilloids cause non-vanilloid receptor mediated cell death. Biochem. Biophys. Res. Commun. 2007, 354, 50–55. [Google Scholar] [CrossRef]
- Kim, C.S.; Park, W.H.; Park, J.Y.; Kang, J.H.; Kim, M.O.; Kawada, T.; Yoo, H.; Han, I.S.; Yu, R. Capsaicin, a spicy component of hot pepper, induces apoptosis by activation of the peroxisome proliferator-activated receptor γ in HT-29 human colon cancer cells. J. Med. Food 2004, 7, 267–273. [Google Scholar] [CrossRef]
- Surh, Y.-J.; Lee, S.S. Capsaicin, a double-edged sword: Toxicity, metabolism, and chemopreventive potential. Life Sci. 1995, 56, 1845. [Google Scholar] [CrossRef] [PubMed]
- Surh, Y.J.; Lee, S.S. Capsaicin in hot chili pepper: Carcinogen, co-carcinogen or anticarcinogen? Food Chem. Toxicol. 1996, 34, 313–316. [Google Scholar] [CrossRef]
- Archer, V.E.; Jones, D.W. Capsaicin pepper, cancer and ethnicity. Med. Hypotheses 2002, 59, 450–457. [Google Scholar] [CrossRef]
- Lopez-Carrillo, L.; Camargo, M.C.; Schneider, B.G.; Sicinschi, L.A.; Hernandez-Ramirez, R.U.; Correa, P.; Cebrian, M.E. Capsaicin consumption, Helicobacter pylori CagA status and IL1B-31C > T genotypes: A host and environment interaction in gastric cancer. Food Chem. Toxicol. 2012, 50, 2118–2122. [Google Scholar] [CrossRef]
- Pabalan, N.; Jarjanazi, H.; Ozcelik, H. The Impact of Capsaicin Intake on Risk of Developing Gastric Cancers: A Meta-Analysis. J. Gastrointest. Cancer 2014, 45, 334–341. [Google Scholar] [CrossRef]
- Akagi, A.; Sano, N.; Uehara, H.; Minami, T.; Otsuka, H.; Izumi, K. Non-carcinogenicity of capsaicinoids in B6C3F1 mice. Food Chem. Toxicol. 1998, 36, 1065–1071. [Google Scholar] [CrossRef] [PubMed]
- Adetunji, T.L.; Olawale, F.; Olisah, C.; Adetunji, A.E.; Aremu, A.O. Capsaicin: A two decade systematic review of global research output and recent advances against human cancer. Front. Oncol. 1922, 12, 908487. [Google Scholar] [CrossRef]
- Zhang, B.; Zhao, J.; Wang, Z.; Guo, P.; Liu, A.; Du, G. Identification of multi-target anti-AD chemical constituents from traditional Chinese medicine formulae by integrating virtual screening and in vitro validation. Front. Pharmacol. 2021, 12, 709607. [Google Scholar] [CrossRef]
- Radhakrishna, G.K.; Ammunje, D.N.; Kunjiappan, S.; Ravi, K.; Vellingiri, S.; Ramesh, S.H.; Almeida, S.D.; Sireesha, G.; Ramesh, S.; Al-Qahtani, S.; et al. A Comprehensive Review of Capsaicin and Its Role in Cancer Prevention and Treatment. Drug Res. 2024, 74, 195–207. [Google Scholar] [CrossRef]
- Brown, K.C.; Modi, K.J.; Light, R.S.; Cox, A.J.; Long, T.E.; Gadepalli, R.S.; Rimoldi, J.M.; Miles, S.L.; Rankin, G.; Valentovic, M.; et al. Anticancer Activity of Region B Capsaicin Analogs. J. Med. Chem. 2023, 66, 4294–4323. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2001, 71, 209–249. [Google Scholar] [CrossRef]
- Holohan, C.; van Schoeybroek, S.; Longley, D.B.; Johnston, P.G. Cancer drug resistance: An evolving paradigm. Nat. Rev. Cancer 2013, 13, 714–726. [Google Scholar] [CrossRef] [PubMed]
- Heise, N.V.; Quast, J.; Csuk, R. Revisiting capsaicin and nonivamide: Their analogs exert strong inhibitory activity against cholinesterases. Eur. J. Med. Chem. Rep. 2024, 12, 100200. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, H.; Li, R.; Sterling, K.; Song, W. Amyloid β-based therapy for Alzheimer’s disease: Challenges, successes and future. Signal Transduct. Target. Ther. 2023, 8, 248. [Google Scholar] [CrossRef] [PubMed]
- Drosky, P.; Siehl, H.-U.; Zeller, K.-P.; Sieler, J.; Berger, S.; Sicker, D. Sharp, sharper, Capsaicin. Chem. Unserer Zeit 2015, 49, 114–122. [Google Scholar] [CrossRef]
- Olguín-Rojas, J.A.; Fayos, O.; Vázquez-León, L.A.; Ferreiro-González, M.; Rodríguez-Jimenes, G.D.; Palma, M.; Garcés-Claver, A.; Barbero, G.F. Progression of the Total and Individual Capsaicinoids Content in the Fruits of Three Different Cultivars of Capsicum chinense Jacq. Agronomy 2019, 9, 141. [Google Scholar] [CrossRef]
- Attuquayefio, V.K.; Buckle, K.A. Rapid sample preparation method for HPLC analysis of capsaicinoids in capsicum fruits and oleoresins. J. Agric. Food Chem. 1987, 35, 777–779. [Google Scholar] [CrossRef]
- Garces-Claver, A.; Arnedo-Andres, M.S.; Abadia, J.; Gil-Ortega, R.; Alvarez-Fernandez, A. Determination of Capsaicin and Dihydrocapsaicin in Capsicum Fruits by Liquid Chromatography-Electrospray/Time-of-Flight Mass Spectrometry. J. Agric. Food Chem. 2006, 54, 9303–9311. [Google Scholar] [CrossRef]
- Thompson, R.Q.; Phinney, K.W.; Sander, L.C.; Welch, M.J. Reversed-phase liquid chromatography and argentation chromatography of the minor capsaicinoids. Anal. Bioanal. Chem. 2005, 381, 1432–1440. [Google Scholar] [CrossRef]
- Migglautsch, A.K.; Willim, M.; Schweda, B.; Glieder, A.; Breinbauer, R.; Winkler, M. Aliphatic hydroxylation and epoxidation of capsaicin by cytochrome P450 CYP505X. Tetrahedron 2018, 74, 6199–6204. [Google Scholar] [CrossRef]
- Heise, N.; Becker, S.; Mueller, T.; Bache, M.; Csuk, R.; Güttler, A. Mitochondria-Targeting 1,5-Diazacyclooctane-Spacered Triterpene Rhodamine Conjugates Exhibit Cytotoxicity at Sub-Nanomolar Concentration against Breast Cancer Cells. Int. J. Mol. Sci. 2023, 24, 10695. [Google Scholar] [CrossRef] [PubMed]
- Heise, N.; Lehmann, F.; Csuk, R.; Mueller, T. Targeted theranostics: Near-infrared triterpenoic acid-rhodamine conjugates as prerequisites for precise cancer diagnosis and therapy. Eur. J. Med. Chem. 2023, 259, 115663. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).