Chitosan as an Elicitor in Plant Tissue Cultures: Methodological Challenges
Abstract
1. Introduction
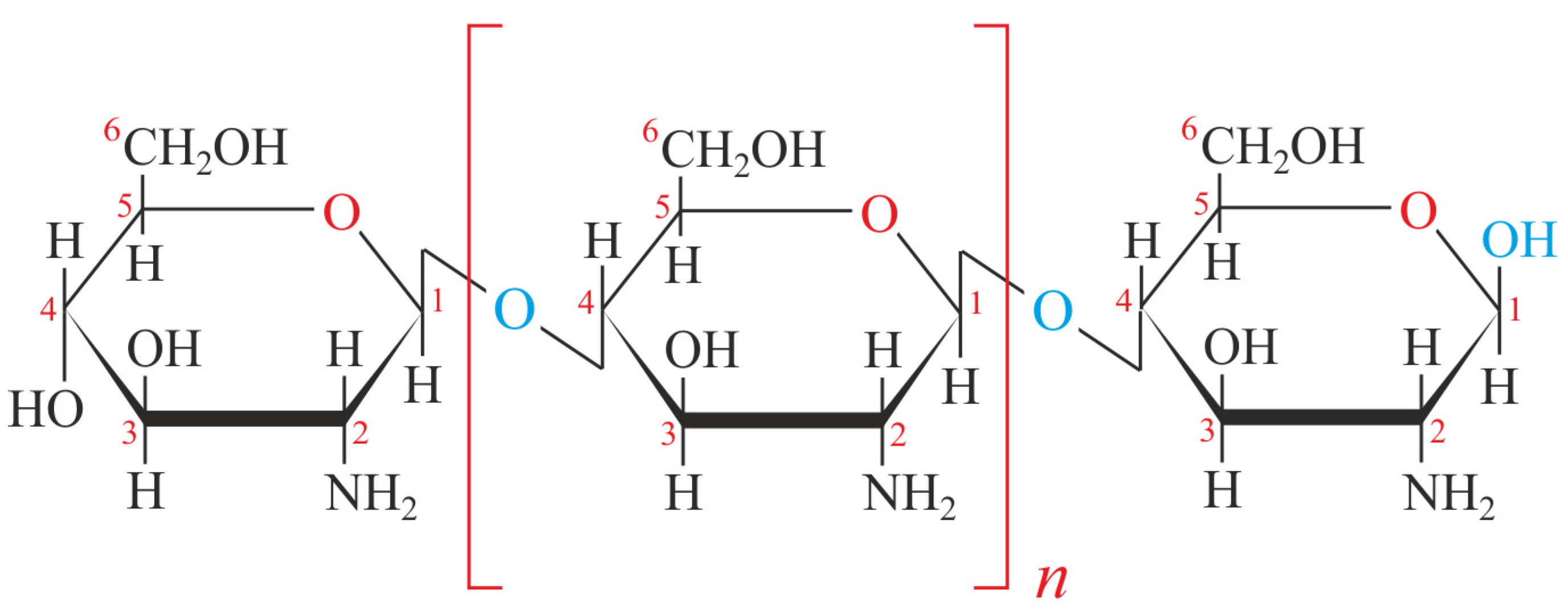
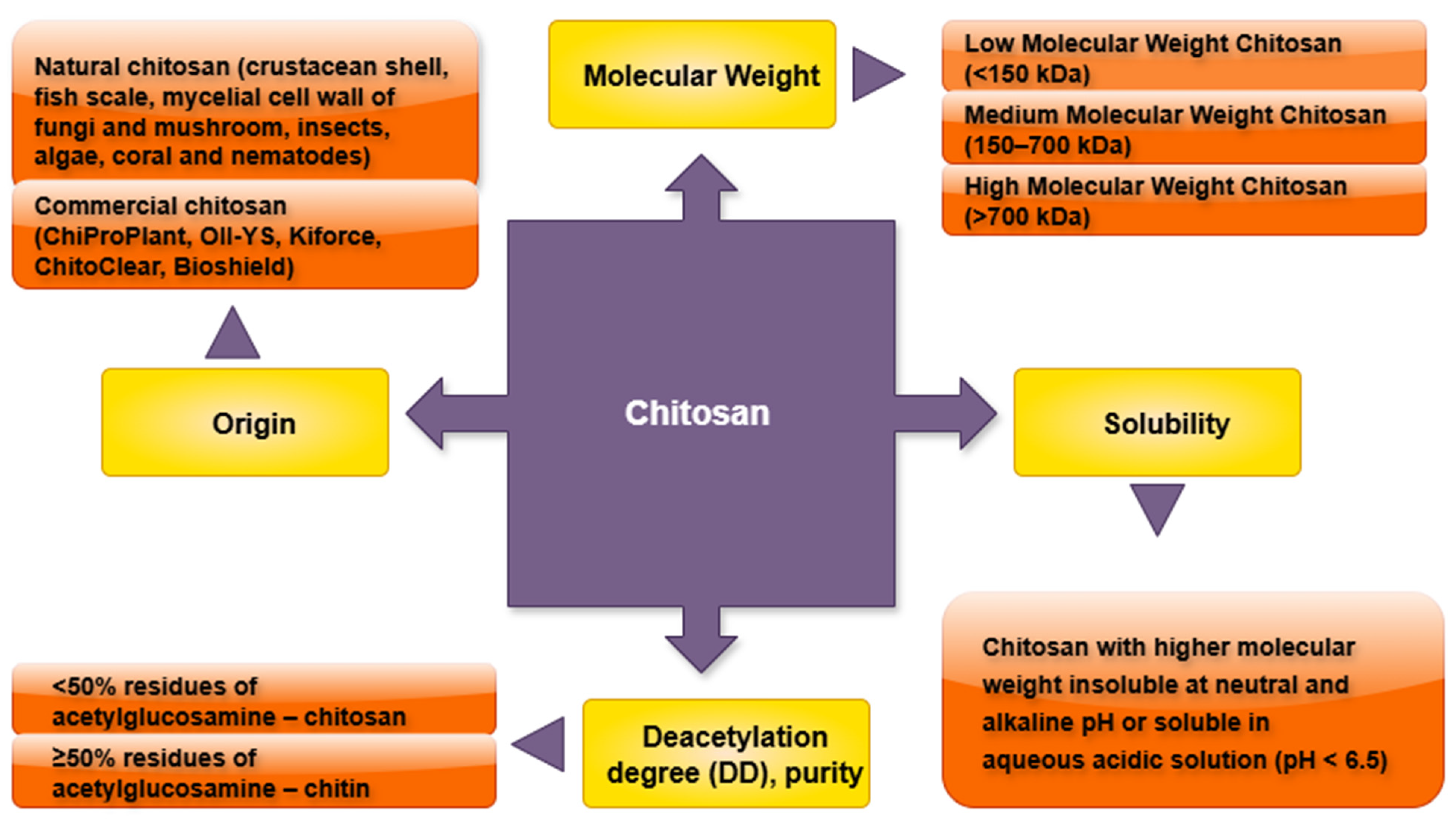
2. Chitosan (CTS): Structure and Biochemical Properties
3. Molecular Mechanisms of CTS Action
3.1. CTS Effects on the Induction of Defence Genes
3.2. Physiological and Biochemical Effects of CTS on Plant Cellular Functions
4. CTS’s Potential Under In Vitro Conditions
4.1. Methods of Plant Tissue Culture: Techniques, Applications, and Advantages
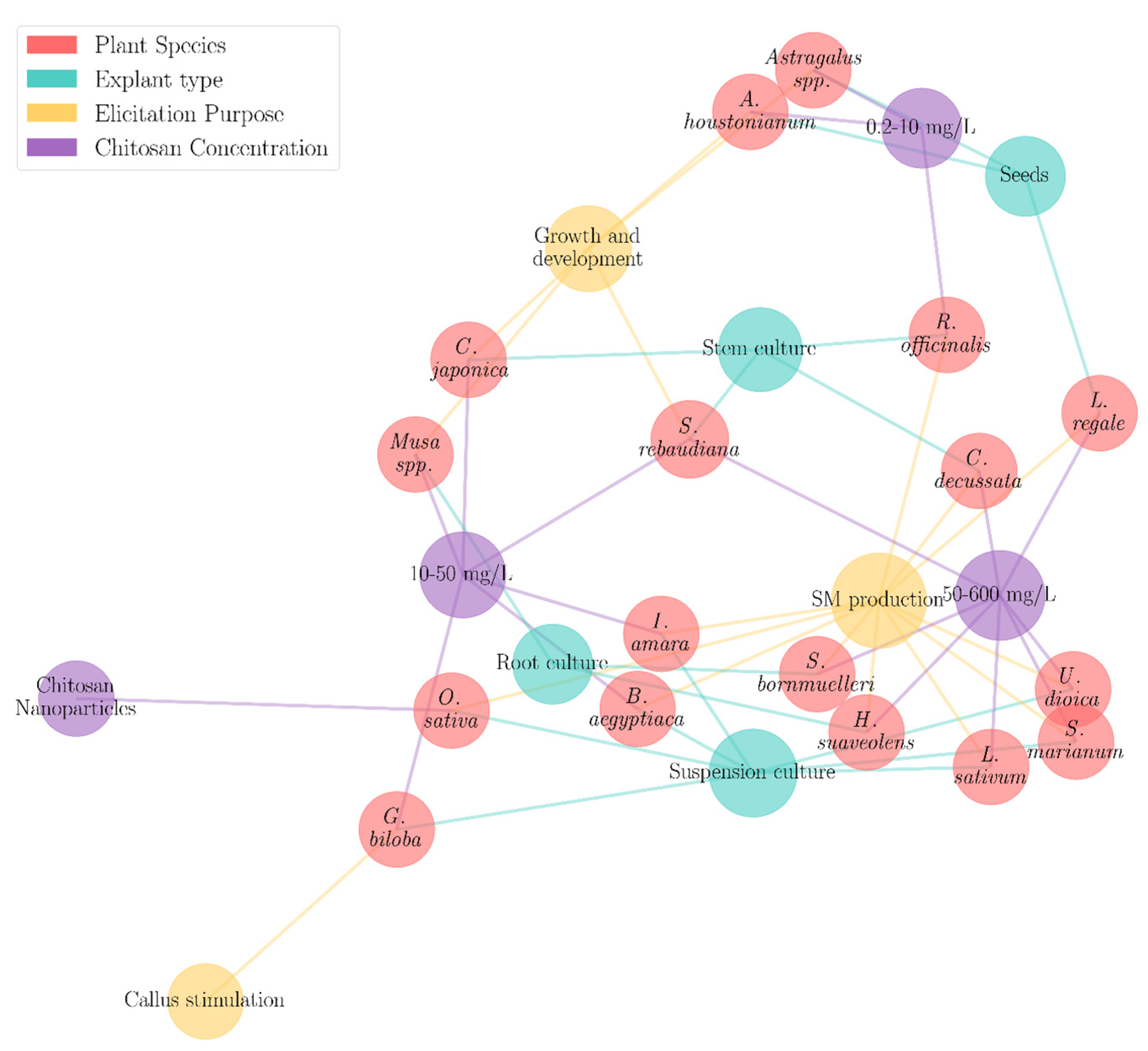
4.2. CTS Application in Plant Tissue Cultures
5. Inadequacies in Current Insights into CTS Elicitation Methods
- CTS type based on the molecular weight, degree of deacetylation, and purity: Variations in the molecular weight, degree of deacetylation, and purity of CTS may lead to differential responses in the plant tissue cultures. These physicochemical properties are likely to influence its role as a growth enhancer or elicitor of secondary metabolites.
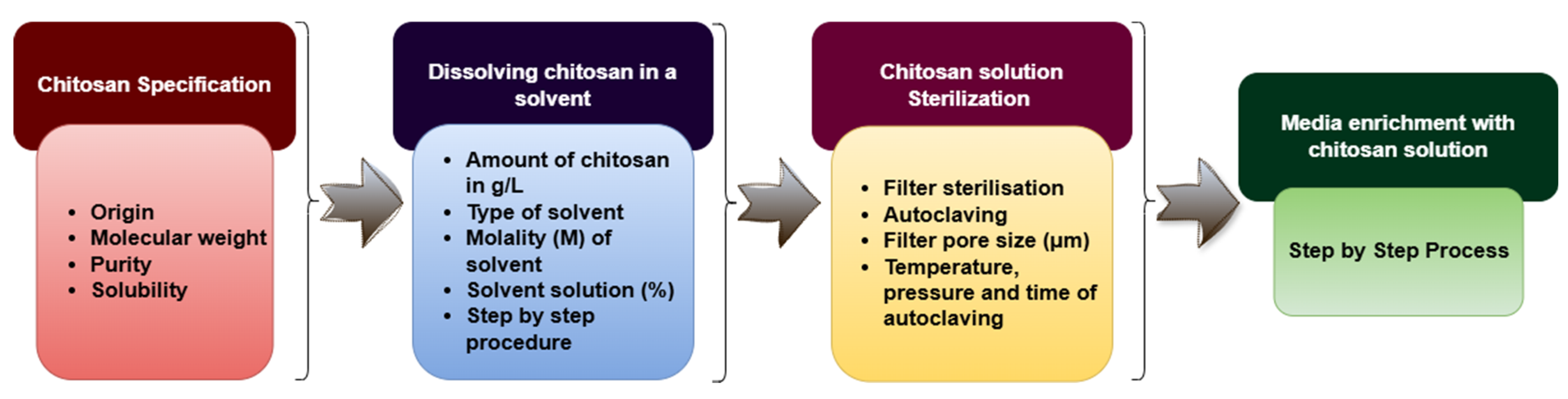
- Method of preparing the CTS stock solutions and the subsequent sterilization approach: The procedure used to dissolve and sterilize CTS may alter its structural integrity and effectiveness. Inappropriate preparation methods could reduce its bioavailability or biological activity in cultured tissues.
- Method of adding the required CTS concentration to the medium: The timing and method of incorporating CTS into the culture medium may impact its uniformity and interaction with plant cells. This could influence both the growth outcomes and secondary metabolite production.
6. Future Perspectives and Concluding Remarks
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, H.; Zhao, Y.; Zhu, J.-K. Thriving under Stress: How Plants Balance Growth and the Stress Response. Dev. Cell 2020, 55, 529–543. [Google Scholar] [CrossRef] [PubMed]
- Mishra, D.; Kumar, A.; Tripathi, S.; Chitara, M.K.; Chaturvedi, P. Chapter 16-Endophytic Fungi as Biostimulants: An Efficient Tool for Plant Growth Promotion under Biotic and Abiotic Stress Conditions. In Biostimulants for Crops from Seed Germination to Plant Development; Gupta, S., Van Staden, J., Eds.; Academic Press: Amsterdam, The Netherlands, 2021; pp. 365–391. [Google Scholar]
- Roy Chowdhury, M.; Basak, J. Tiny Yet Indispensable Plant MicroRNAs Are Worth to Explore as Key Components for Combating Genotoxic Stresses. Front. Plant Sci. 2019, 10. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, J.; Zhao, L.; Wu, H.; Zhu, Y.; Ahmad, I.; Zhou, G. Abiotic Stress Responses in Crop Plants: A Multi-Scale Approach. J. Integr. Agric. 2024. [Google Scholar] [CrossRef]
- Kalariya, K.A.; Mevada, R.R.; Meena, R.P.; Das, M. Biotic Stress Nexus: Integrating Various Physiological Processes in Medicinal and Aromatic Plants. J. Appl. Res. Med. Aromat. Plants 2024, 43, 100574. [Google Scholar] [CrossRef]
- Jan, R.; Asaf, S.; Numan, M.; Lubna; Kim, K.-M. Plant Secondary Metabolite Biosynthesis and Transcriptional Regulation in Response to Biotic and Abiotic Stress Conditions. Agronomy 2021, 11, 968. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, J.; Li, R.; Ge, Y.; Li, Y.; Li, R. Plants’ Response to Abiotic Stress: Mechanisms and Strategies. Int. J. Mol. Sci. 2023, 24, 10915. [Google Scholar] [CrossRef]
- Du, B.; Haensch, R.; Alfarraj, S.; Rennenberg, H. Strategies of Plants to Overcome Abiotic and Biotic Stresses. Biol. Rev. 2024, 99, 1524–1536. [Google Scholar] [CrossRef]
- Oyebamiji, Y.O.; Adigun, B.A.; Shamsudin, N.A.A.; Ikmal, A.M.; Salisu, M.A.; Malike, F.A.; Lateef, A.A. Recent Advancements in Mitigating Abiotic Stresses in Crops. Horticulturae 2024, 10, 156. [Google Scholar] [CrossRef]
- Thakur, M.; Bhattacharya, S.; Khosla, P.K.; Puri, S. Improving Production of Plant Secondary Metabolites through Biotic and Abiotic Elicitation. J. Appl. Res. Med. Aromat. Plants 2019, 12, 1–12. [Google Scholar] [CrossRef]
- Salam, U.; Ullah, S.; Tang, Z.-H.; Elateeq, A.A.; Khan, Y.; Khan, J.; Khan, A.; Ali, S. Plant Metabolomics: An Overview of the Role of Primary and Secondary Metabolites against Different Environmental Stress Factors. Life 2023, 13, 706. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Kanwal, F.; Ullah, S.; Fahad, M.; Tariq, L.; Altaf, M.T.; Riaz, A.; Zhang, G. Plant Secondary Metabolites—Central Regulators Against Abiotic and Biotic Stresses. Metabolites 2025, 15, 276. [Google Scholar] [CrossRef]
- Ramirez-Estrada, K.; Vidal-Limon, H.; Hidalgo, D.; Moyano, E.; Golenioswki, M.; Cusidó, R.M.; Palazon, J. Elicitation, an Effective Strategy for the Biotechnological Production of Bioactive High-Added Value Compounds in Plant Cell Factories. Molecules 2016, 21, 182. [Google Scholar] [CrossRef]
- Gowthami, L. Role of Elicitors in Plant Defense Mechanism. J. Pharmacogn. Phytochem. 2018, 7, 2806–2812. [Google Scholar]
- Khan, E.H.; Liaqat, W.; Jan, M.F.; Altaf, M.T.; Ali, A.; Ahmad, H. Elicitors in Plant Biology: Types and Defense Mechanisms. In Elicitors for Sustainable Crop Production: Overcoming Biotic Stress Challenges in Plants; Abd-Elsalam, K.A., Mohamed, H.I., Eds.; Springer Nature Singapore: Singapore, 2025; pp. 41–61. [Google Scholar]
- Angelova, Z.; Georgiev, S.; Roos, W. Elicitation of Plants. Biotechnol. Biotechnol. Equip. 2006, 20, 72–83. [Google Scholar] [CrossRef]
- Bruce, R.J.; West, C.A. Elicitation of Lignin Biosynthesis and Isoperoxidase Activity by Pectic Fragments in Suspension Cultures of Castor Bean. Plant Physiol. 1989, 91, 889–897. [Google Scholar] [CrossRef]
- Morin-Crini, N.; Lichtfouse, E.; Torri, G.; Crini, G. Applications of Chitosan in Food, Pharmaceuticals, Medicine, Cosmetics, Agriculture, Textiles, Pulp and Paper, Biotechnology, and Environmental Chemistry. Environ. Chem. Lett. 2019, 17, 1667–1692. [Google Scholar] [CrossRef]
- Bertrand, M.; Simonin, S.; Bach, B. Applications of Chitosan in the Agri-Food Sector: A Review. Carbohydr. Res. 2024, 543, 109219. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Pirela, M.; Carillo, P.; Lárez-Velásquez, C.; Romanazzi, G. Effects of Chitosan on Plant Growth under Stress Conditions: Similarities with Plant Growth Promoting Bacteria. Front. Plant Sci. 2024, 15, 1423949. [Google Scholar] [CrossRef]
- Almeida, L.G.; Silva, E.M.D.; Magalhães, P.C.; Karam, D.; Reis, C.O.D.; Júnior, C.C.G.; Marques, D.M. Root System in Maize Plants Cultivated Under Water Deficit and Application of Chitosan. Rev. Bras. Milho Sorgo 2020, 19, 11. [Google Scholar]
- Suwanchaikasem, P.; Idnurm, A.; Selby-Pham, J.; Walker, R.; Boughton, B.A. The Impacts of Chitosan on Plant Root Systems and Its Potential to Be Used for Controlling Fungal Diseases in Agriculture. J. Plant Growth Regul. 2024, 43, 3424–3445. [Google Scholar] [CrossRef]
- Goudarzian, A.; Pirbalouti, A.G.; Hossaynzadeh, M. Menthol, Balance of Menthol/Menthone, and Essential Oil Contents of Mentha × Piperita L. under Foliar-Applied Chitosan and Inoculation of Arbuscular Mycorrhizal Fungi. J. Essent. Oil Bear. Plants 2020, 23, 1012–1021. [Google Scholar] [CrossRef]
- Kousalya, L.; Seethapathy, P.; Pandita, D.; Packiaraj, S.; Venkatesh, S.; Sankaralingam, S.; Arunkumar, M.; Harinathan, B.; Pandita, A.; Casini, R.; et al. Chitosan (CTS) Induced Secondary Metabolite Production in Canscora decussata Schult.-An Endangered Medicinal Plant. Kuwait J. Sci. 2025, 52, 100306. [Google Scholar] [CrossRef]
- Kahromi, S.; Khara, J. Chitosan Stimulates Secondary Metabolite Production and Nutrient Uptake in Medicinal Plant Dracocephalum Kotschyi. J. Sci. Food Agric. 2021, 101, 3898–3907. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Xu, X.; Xue, S.; Gong, D.; Wang, B.; Zheng, X.; Xie, P.; Bi, Y.; Prusky, D. Preharvest Multiple Sprays with Chitosan Promotes the Synthesis and Deposition of Lignin at Wounds of Harvested Muskmelons. Int. J. Biol. Macromol. 2022, 206, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Orlita, A.; Sidwa-Gorycka, M.; Paszkiewicz, M.; Malinski, E.; Kumirska, J.; Siedlecka, E.M.; Łojkowska, E.; Stepnowski, P. Application of Chitin and Chitosan as Elicitors of Coumarins and Furoquinolone Alkaloids in Ruta graveolens L. (Common Rue). Biotechnol. Appl. Biochem. 2008, 51, 91–96. [Google Scholar] [CrossRef]
- Orzali, L.; Corsi, B.; Forni, C.; Riccioni, L.; Orzali, L.; Corsi, B.; Forni, C.; Riccioni, L. Chitosan in Agriculture: A New Challenge for Managing Plant Disease. In Biological Activities and Application of Marine Polysaccharides; IntechOpen: London, UK, 2017; ISBN 978-953-51-2860-1. [Google Scholar]
- Eriksson*, H. Basic Principles of Plant Tissue Culture and Its Applications. Glob. J. Biochem. Biotechnol. 2022, 10, 1–2. [Google Scholar]
- Phillips, G.C.; Garda, M. Plant Tissue Culture Media and Practices: An Overview. In Vitro Cell. Dev. Biol.-Plant 2019, 55, 242–257. [Google Scholar] [CrossRef]
- Bhojwani, S.S.; Dantu, P.K. Plant Tissue Culture: An Introductory Text; Springer: New Delhi, India, 2013; Volume 10, pp. 1–2. [Google Scholar]
- Jalota, K.; Sharma, V.; Agarwal, C.; Jindal, S. Eco-Friendly Approaches to Phytochemical Production: Elicitation and Beyond. Nat. Prod. Bioprospect. 2024, 14, 5. [Google Scholar] [CrossRef]
- Jadoon, L.; Gul, A.; Fatima, H.; Babar, M.M. Nano-Elicitation and Hydroponics: A Synergism to Enhance Plant Productivity and Secondary Metabolism. Planta 2024, 259, 80. [Google Scholar] [CrossRef]
- Jain, D.; Bisht, S.; Parvez, A.; Singh, K.; Bhaskar, P.; Koubouris, G. Effective Biotic Elicitors for Augmentation of Secondary Metabolite Production in Medicinal Plants. Agriculture 2024, 14, 796. [Google Scholar] [CrossRef]
- Selwal, N.; Goutam, U.; Akhtar, N.; Sood, M.; Kukreja, S. Elicitation: “A Trump Card” for Enhancing Secondary Metabolites in Plants. J. Plant Growth Regul. 2024, 43, 3027–3047. [Google Scholar] [CrossRef]
- Kumar, D.A.S.; Parasurama, D.S. Plant Bioactive Compounds: Crucial Pharmacological Properties and Role of Elicitors in Enhancing Production. Indian. J. Pharm. Educ. Res. 2025, 58, 1034–1044. [Google Scholar] [CrossRef]
- Islam, S.; Bhuiyan, M.A.R.; Islam, M.N. Chitin and Chitosan: Structure, Properties and Applications in Biomedical Engineering. J. Polym. Environ. 2017, 25, 854–866. [Google Scholar] [CrossRef]
- Dar, S.A.; Abd Al Galil, F.M. Biodegradation, Biosynthesis, Isolation, and Applications of Chitin and Chitosan. In Handbook of Biodegradable Materials; Ali, G.A.M., Makhlouf, A.S.H., Eds.; Springer: Cham, Switzerland, 2022; pp. 1–42. [Google Scholar]
- Popa, L.; Ghica, M.V.; Dinu-Pîrvu, C.-E. Chitosan Biomaterials: Advances and Challenges—2nd Edition. Int. J. Mol. Sci. 2025, 26, 4836. [Google Scholar] [CrossRef]
- Aranaz, I.; Mengibar, M.; Harris, R.; Panos, I.; Miralles, B.; Acosta, N.; Galed, G.; Heras, A. Functional Characterization of Chitin and Chitosan. Curr. Chem. Biol. 2009, 3, 203–230. [Google Scholar] [CrossRef] [PubMed]
- Kurita, K. Chitin and Chitosan: Functional Biopolymers from Marine Crustaceans. Mar. Biotechnol. 2006, 8, 203–226. [Google Scholar] [CrossRef]
- Hisham, F.; Maziati Akmal, M.H.; Ahmad, F.; Ahmad, K.; Samat, N. Biopolymer Chitosan: Potential Sources, Extraction Methods, and Emerging Applications. Ain Shams Eng. J. 2024, 15, 102424. [Google Scholar] [CrossRef]
- Alimi, B.A.; Pathania, S.; Wilson, J.; Duffy, B.; Frias, J.M.C. Extraction, Quantification, Characterization, and Application in Food Packaging of Chitin and Chitosan from Mushrooms: A Review. Int. J. Biol. Macromol. 2023, 237, 124195. [Google Scholar] [CrossRef]
- Kumari, S.; Kumar Annamareddy, S.H.; Abanti, S.; Kumar Rath, P. Physicochemical Properties and Characterization of Chitosan Synthesized from Fish Scales, Crab and Shrimp Shells. Int. J. Biol. Macromol. 2017, 104, 1697–1705. [Google Scholar] [CrossRef]
- Ali, G.; Sharma, M.; Salama, E.-S.; Ling, Z.; Li, X. Applications of Chitin and Chitosan as Natural Biopolymer: Potential Sources, Pretreatments, and Degradation Pathways. Biomass Conv. Bioref. 2024, 14, 4567–4581. [Google Scholar] [CrossRef]
- Rinaudo, M. Chitin and Chitosan: Properties and Applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Żukiewicz-Sobczak, W.; Sobczak, P.; Zawiślak, K.; Zagórski, J.; Wojtyła-Buciora, P.; Wojtyła, A. Physical and Chemical Properties Comparison of Fungal and Crustaceous Chitosan. J. Health Inequal. 2015, 1, 7–14. [Google Scholar] [CrossRef]
- Huq, T.; Khan, A.; Brown, D.; Dhayagude, N.; He, Z.; Ni, Y. Sources, Production and Commercial Applications of Fungal Chitosan: A Review. J. Bioresour. Bioprod. 2022, 7, 85–98. [Google Scholar] [CrossRef]
- Bilbao-Sainz, C.; Chiou, B.-S.; Williams, T.; Wood, D.; Du, W.-X.; Sedej, I.; Ban, Z.; Rodov, V.; Poverenov, E.; Vinokur, Y.; et al. Vitamin D-Fortified Chitosan Films from Mushroom Waste. Carbohydr. Polym. 2017, 167, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Oberemko, A.; Salaberria, A.M.; Saule, R.; Saulis, G.; Kaya, M.; Labidi, J.; Baublys, V. Physicochemical and in Vitro Cytotoxic Properties of Chitosan from Mushroom Species (Boletus bovinus and Laccaria laccata). Carbohydr. Polym. 2019, 221, 1–9. [Google Scholar] [CrossRef]
- Uragami, T.; Tokura, S. Materials Science of Chitin and Chitosan; Springer: Berlin Heidelberg, Germany, 2006; ISBN 978-364-20-6935-2. Available online: https://books.google.sk/books/about/Material_Science_of_Chitin_and_Chitosan.html?id=PP18cgAACAAJ&redir_esc=y (accessed on 17 August 2025).
- Roy, J.C.; Salaün, F.; Giraud, S.; Ferri, A. Solubility of Chitin: Solvents, Solution Behaviors and Their Related Mechanisms. In Solubility of Polysaccharides; Xu, Z., Ed.; IntechOpen: London, UK, 2017. [Google Scholar]
- Sogias, I.A.; Khutoryanskiy, V.V.; Williams, A.C. Exploring the Factors Affecting the Solubility of Chitosan in Water. Macromol. Chem. Phys. 2010, 211, 426–433. [Google Scholar] [CrossRef]
- Sharif, R.; Mujtaba, M.; Ur Rahman, M.; Shalmani, A.; Ahmad, H.; Anwar, T.; Tianchan, D.; Wang, X. The Multifunctional Role of Chitosan in Horticultural Crops; A Review. Molecules 2018, 23, 872. [Google Scholar] [CrossRef]
- Naveed, M.; Phil, L.; Sohail, M.; Hasnat, M.; Baig, M.M.F.A.; Ihsan, A.U.; Shumzaid, M.; Kakar, M.U.; Mehmood Khan, T.; Akabar, M.D.; et al. Chitosan Oligosaccharide (COS): An Overview. Int. J. Biol. Macromol. 2019, 129, 827–843. [Google Scholar] [CrossRef]
- Sun, W.; Shahrajabian, M.H.; Petropoulos, S.A.; Shahrajabian, N. Developing Sustainable Agriculture Systems in Medicinal and Aromatic Plant Production by Using Chitosan and Chitin-Based Biostimulants. Plants 2023, 12, 2469. [Google Scholar] [CrossRef]
- Hawary, D.L.; Motaleb, M.A.; Farag, H.; Guirguis, O.W.; Elsabee, M.Z. Water-Soluble Derivatives of Chitosan as a Target Delivery System of 99mTc to Some Organs in Vivo for Nuclear Imaging and Biodistribution. J. Radioanal. Nucl. Chem. 2011, 290, 557–567. [Google Scholar] [CrossRef]
- Khanjari, A.; Karabagias, I.K.; Kontominas, M.G. Combined Effect of N,O-Carboxymethyl Chitosan and Oregano Essential Oil to Extend Shelf Life and Control Listeria monocytogenes in Raw Chicken Meat Fillets. LWT-Food Sci. Technol. 2013, 53, 94–99. [Google Scholar] [CrossRef]
- Rathinam, S.; Solodova, S.; Kristjánsdóttir, I.; Hjálmarsdóttir, M.Á.; Másson, M. The Antibacterial Structure-Activity Relationship for Common Chitosan Derivatives. Int. J. Biol. Macromol. 2020, 165, 1686–1693. [Google Scholar] [CrossRef]
- Ingle, P.U.; Shende, S.S.; Shingote, P.R.; Mishra, S.S.; Sarda, V.; Wasule, D.L.; Rajput, V.D.; Minkina, T.; Rai, M.; Sushkova, S.; et al. Chitosan Nanoparticles (ChNPs): A Versatile Growth Promoter in Modern Agricultural Production. Heliyon 2022, 8, e11893. [Google Scholar] [CrossRef] [PubMed]
- Aliasghari, A.; Rabbani Khorasgani, M.; Vaezifar, S.; Rahimi, F.; Younesi, H.; Khoroushi, M. Evaluation of Antibacterial Efficiency of Chitosan and Chitosan Nanoparticles on Cariogenic Streptococci: An in Vitro Study. Iran. J. Microbiol. 2016, 8, 93–100. [Google Scholar]
- Román-Doval, R.; Torres-Arellanes, S.P.; Tenorio-Barajas, A.Y.; Gómez-Sánchez, A.; Valencia-Lazcano, A.A. Chitosan: Properties and Its Application in Agriculture in Context of Molecular Weight. Polymers 2023, 15, 2867. [Google Scholar] [CrossRef]
- Romanazzi, G.; Feliziani, E.; Sivakumar, D. Chitosan, a Biopolymer With Triple Action on Postharvest Decay of Fruit and Vegetables: Eliciting, Antimicrobial and Film-Forming Properties. Front. Microbiol. 2018, 9, 2745. [Google Scholar] [CrossRef]
- Nguyen, V.C.; Nguyen, V.B.; Hsieh, M.-F. Curcumin-Loaded Chitosan/Gelatin Composite Sponge for Wound Healing Application. Int. J. Polym. Sci. 2013, 2013, 106570. [Google Scholar] [CrossRef]
- Geng, W.; Li, Z.; Hassan, M.J.; Peng, Y. Chitosan Regulates Metabolic Balance, Polyamine Accumulation, and Na+ Transport Contributing to Salt Tolerance in Creeping Bentgrass. BMC Plant Biol. 2020, 20, 506. [Google Scholar] [CrossRef] [PubMed]
- Rashidi, N.; Khavari-Nejad, R.A.; Ramak, P.; Saadatmand, S. The Effect of Chitosan on Gene Expression, Some Morphological and Physiological Traits of Sweet Basil (Ocimum Basilicum L.) Under Salinity Stress. Acta Sci. Pol. Hortorum Cultus 2020, 19, 21–30. [Google Scholar] [CrossRef]
- Hassan, F.A.S.; Ali, E.; Gaber, A.; Fetouh, M.I.; Mazrou, R. Chitosan Nanoparticles Effectively Combat Salinity Stress by Enhancing Antioxidant Activity and Alkaloid Biosynthesis in Catharanthus roseus (L.) G. Don. Plant Physiol. Biochem. 2021, 162, 291–300. [Google Scholar] [CrossRef]
- Hernández-Hernández, H.; Juárez-Maldonado, A.; Benavides-Mendoza, A.; Ortega-Ortiz, H.; Cadenas-Pliego, G.; Sánchez-Aspeytia, D.; González-Morales, S. Chitosan-PVA and Copper Nanoparticles Improve Growth and Overexpress the SOD and JA Genes in Tomato Plants under Salt Stress. Agronomy 2018, 8, 175. [Google Scholar] [CrossRef]
- Mohamed, N.G.; Abdel-Hakeem, M.A. Chitosan Nanoparticles Enhance Drought Tolerance in Tomatoes (Solanum lycopersicum L.) via Gene Expression Modulation. Plant Gene 2023, 34, 100406. [Google Scholar] [CrossRef]
- Ali, E.F.; El-Shehawi, A.M.; Ibrahim, O.H.M.; Abdul-Hafeez, E.Y.; Moussa, M.M.; Hassan, F.A.S. A Vital Role of Chitosan Nanoparticles in Improvisation the Drought Stress Tolerance in Catharanthus roseus (L.) through Biochemical and Gene Expression Modulation. Plant Physiol. Biochem. 2021, 161, 166–175. [Google Scholar] [CrossRef]
- Li, S.; Sun, H.; Zhang, R.; Gao, C.; Yang, P.; He, X.; Hu, T. Chitosan Induced Cold Tolerance in Kobresia Pygmaea by Regulating Photosynthesis, Antioxidant Performance, and Chloroplast Ultrastructure. Front. Plant Sci. 2024, 15, 1441564. [Google Scholar] [CrossRef]
- Hidangmayum, A.; Dwivedi, P.; Katiyar, D.; Hemantaranjan, A. Application of Chitosan on Plant Responses with Special Reference to Abiotic Stress. Physiol. Mol. Biol. Plants 2019, 25, 313–326. [Google Scholar] [CrossRef]
- Sofy, A.R.; Dawoud, R.A.; Sofy, M.R.; Mohamed, H.I.; Hmed, A.A.; El-Dougdoug, N.K. Improving Regulation of Enzymatic and Non-Enzymatic Antioxidants and Stress-Related Gene Stimulation in Cucumber Mosaic Cucumovirus-Infected Cucumber Plants Treated with Glycine Betaine, Chitosan and Combination. Molecules 2020, 25, 2341. [Google Scholar] [CrossRef]
- Nandhini, R.; Rajeswari, E.; Harish, S.; Sivakumar, V.; Gangai Selvi, R.; Jaya Sundrasharmila, D. Role of Chitosan Nanoparticles in Sustainable Plant Disease Management. J. Nanopart. Res. 2025, 27, 13. [Google Scholar] [CrossRef]
- Stasińska-Jakubas, M.; Hawrylak-Nowak, B. Protective, Biostimulating, and Eliciting Effects of Chitosan and Its Derivatives on Crop Plants. Molecules 2022, 27, 2801. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.; Jan, H.; Drouet, S.; Tungmunnithum, D.; Shirazi, J.H.; Hano, C.; Abbasi, B.H. Chitosan Elicitation Impacts Flavonolignan Biosynthesis in Silybum marianum (L.) Gaertn Cell Suspension and Enhances Antioxidant and Anti-Inflammatory Activities of Cell Extracts. Molecules 2021, 26, 791. [Google Scholar] [CrossRef] [PubMed]
- Afsharipour, F.; Mahmoodnia Meimand, M.; Dahajipour Heidarabadi, M.; Dehghani, M.R. Increasing the Content of Flavonoid Compounds and Changing the Antioxidant Enzyme Activities in Chitosan-Elicited Callus Culture of Nettle Plant. Cell Tissue J. 2024, 15, 317–335. [Google Scholar] [CrossRef]
- Ling, Y.; Zhao, Y.; Cheng, B.; Tan, M.; Zhang, Y.; Li, Z. Seed Priming with Chitosan Improves Germination Characteristics Associated with Alterations in Antioxidant Defense and Dehydration-Responsive Pathway in White Clover under Water Stress. Plants 2022, 11, 2015. [Google Scholar] [CrossRef] [PubMed]
- Ruffo Roberto, S.; Youssef, K.; Hashim, A.F.; Ippolito, A. Nanomaterials as Alternative Control Means Against Postharvest Diseases in Fruit Crops. Nanomaterials 2019, 9, 1752. [Google Scholar] [CrossRef]
- Jia, X.; Meng, Q.; Zeng, H.; Wang, W.; Yin, H. Chitosan Oligosaccharide Induces Resistance to Tobacco Mosaic Virus in Arabidopsis via the Salicylic Acid-Mediated Signalling Pathway. Sci. Rep. 2016, 6, 26144. [Google Scholar] [CrossRef]
- Malerba, M.; Cerana, R. Chitosan Effects on Plant Systems. Int. J. Mol. Sci. 2016, 17, 996. [Google Scholar] [CrossRef]
- Poznanski, P.; Hameed, A.; Orczyk, W. Chitosan and Chitosan Nanoparticles: Parameters Enhancing Antifungal Activity. Molecules 2023, 28, 2996. [Google Scholar] [CrossRef]
- Wang, X.; Xie, H.; Wang, P.; Yin, H. Nanoparticles in Plants: Uptake, Transport and Physiological Activity in Leaf and Root. Materials 2023, 16, 3097. [Google Scholar] [CrossRef]
- Pourbeyrami Hir, Y.; Khalafi, M.; Chamani, E.; Maleki Lajayer, H. Effect of Chitosan on Regeneration and Secondary Metabolite Production of Lilium Regale. J. Plant Physiol. Breed. 2021, 11, 147–160. [Google Scholar] [CrossRef]
- Ramadan, K.M.A.; El-Beltagi, H.S.; Makhlouf, B.S.I.; Khalil, S.R.A.E.; Al-Daej, M.I.; Shalaby, T.A.; Bendary, E.S.A.; Saudy, H.S. Carboxymethyl Chitosan Improves Sugar Beet Tolerance to Drought by Controlling Enzyme Activity and Stomatal Conductance. Pol. J. Environ. Stud. 2025, 34, 791–800. [Google Scholar] [CrossRef]
- Almeida, L.G.; Magalhães, P.C.; Karam, D.; da Silva, E.M.; Alvarenga, A.A. Chitosan Application in the Induction of Water Deficit Tolerance in Maize Plants. Acta Sci. Agron. 2020, 42, e42463. [Google Scholar] [CrossRef]
- Iriti, M.; Picchi, V.; Rossoni, M.; Gomarasca, S.; Ludwig, N.; Gargano, M.; Faoro, F. Chitosan Antitranspirant Activity Is Due to Abscisic Acid-Dependent Stomatal Closure. Environ. Exp. Bot. 2009, 66, 493–500. [Google Scholar] [CrossRef]
- Rout, G.R.; Mohapatra, A.; Jain, S.M. Tissue Culture of Ornamental Pot Plant: A Critical Review on Present Scenario and Future Prospects. Biotechnol. Adv. 2006, 24, 531–560. [Google Scholar] [CrossRef]
- Kulus, D.; Tymoszuk, A. Advancements in In Vitro Technology: A Comprehensive Exploration of Micropropagated Plants. Horticulturae 2024, 10, 88. [Google Scholar] [CrossRef]
- Chawla, S. Introduction to Plant Biotechnology, 3rd ed.; Science Publishers: Enfield, NH, USA, 2009; ISBN 978-1-57808-636-8. [Google Scholar]
- Edwin, F.G.; Sherington, P.D. Plant Propagation by Tissue Culture, 3rd ed.; Springer: Dordrecht, The Netherlands, 2007; ISBN 978-1-4020-5004-6. [Google Scholar]
- Indu, B.K.; Balasubramanya, S.; Anuradha, M.; Shilpa, P. Callus and Cell Suspension Cultures for Secondary Metabolite Production. In In Vitro Production of Plant Secondary Metabolites: Theory and Practice; Anuradha, M., Balasubramanya, S., Eds.; Springer Nature Singapore: Singapore, 2025; pp. 71–88. [Google Scholar]
- Kim, J.-S.; Sato, M.; Kojima, M.; Asrori, M.I.; Uehara-Yamaguchi, Y.; Takebayashi, Y.; Do, T.N.; Do, T.Y.; Thi, K.O.N.; Sakakibara, H.; et al. Multi-Omics Signatures of Diverse Plant Callus Cultures. Plant Biotechnol. 2024, 41, 309–314. [Google Scholar] [CrossRef]
- Häkkinen, S.T.; Legay, S.; Rischer, H.; Renaut, J.; Guerriero, G. Editorial: Plant Cell Factories: Current and Future Uses of Plant Cell Cultures. Front. Plant Sci. 2024, 15. [Google Scholar] [CrossRef]
- Ramachandra Rao, S.; Ravishankar, G.A. Plant Cell Cultures: Chemical Factories of Secondary Metabolites. Biotechnol. Adv. 2002, 20, 101–153. [Google Scholar] [CrossRef] [PubMed]
- Mirmazloum, I.; Slavov, A.K.; Marchev, A.S. The Untapped Potential of Hairy Root Cultures and Their Multiple Applications. Int. J. Mol. Sci. 2024, 25, 12682. [Google Scholar] [CrossRef]
- Singh, R.S.; Singh, P.; Kumari, R.; Kumar, S. Hairy Root Cultures for Secondary Metabolite Production. In Genetic Manipulation of Secondary Metabolites in Medicinal Plant; Singh, R., Kumar, N., Eds.; Springer Nature Singapore: Singapore, 2023; pp. 205–223. [Google Scholar]
- Katiyar, D.; Hemantaranjan, A.; Singh, B. Chitosan as a Promising Natural Compound to Enhance Potential Physiological Responses in Plant: A Review. Ind. J. Plant Physiol. 2015, 20, 1–9. [Google Scholar] [CrossRef]
- Sembada, A.A.; Lenggoro, I.W. Transport of Nanoparticles into Plants and Their Detection Methods. Nanomaterials 2024, 14, 131. [Google Scholar] [CrossRef]
- Arya, S.S.; Rookes, J.E.; Cahill, D.M.; Lenka, S.K. Chitosan Nanoparticles and Their Combination with Methyl Jasmonate for the Elicitation of Phenolics and Flavonoids in Plant Cell Suspension Cultures. Int. J. Biol. Macromol. 2022, 214, 632–641. [Google Scholar] [CrossRef]
- Elateeq, A.A.; Saad, Z.; Eissa, M.; Ullah, S. Effect of Chitosan and Light Conditions on the Production of Callus Biomass, Total Flavonoids and Total Phenolics in Ginkgo biloba L. Al-Azhar J. Agric. Res. 2021, 46, 28–42. [Google Scholar] [CrossRef]
- Taghizadeh, M.; Sabagh Nekonam, M.; Setorki, M. Production of Phenolic Compounds in Iberis Amara L. Cell Suspension Culture under Chitosan Treatment. J. Med. Plants By-Prod. 2024, 13, 1092–1102. [Google Scholar] [CrossRef]
- Barrientos Carvacho, H.; Pérez, C.; Zúñiga, G.; Mahn, A. Effect of Methyl Jasmonate, Sodium Selenate and Chitosan as Exogenous Elicitors on the Phenolic Compounds Profile of Broccoli Sprouts. J. Sci. Food Agric. 2014, 94, 2555–2561. [Google Scholar] [CrossRef] [PubMed]
- Gharari, Z.; Bagheri, K.; Danafar, H.; Sharafi, A. Enhanced Flavonoid Production in Hairy Root Cultures of Scutellaria bornmuelleri by Elicitor Induced over-Expression of MYB7 and FNSП2 Genes. Plant Physiol. Biochem. 2020, 148, 35–44. [Google Scholar] [CrossRef]
- Coelho, A.D.; de Assis, R.M.A.; Leite, J.J.F.; Rocha, J.P.M.; Bertolucci, S.K.V.; Pinto, J.E.B.P. Podophyllotoxin Bioaccumulation with Biotic and Abiotic Elicitors on Root Culture of Hyptis Suaveolens (L.) POIT. In Vitro Cell. Dev. Biol.-Plant 2025, 61, 51–66. [Google Scholar] [CrossRef]
- Faqir, Y.; Ma, J.; Chai, Y. Chitosan in Modern Agriculture Production. Plant Soil. Environ. 2021, 67, 679–699. [Google Scholar] [CrossRef]
- Coskun, Y.; Duran, R.E.; Ozgul, Z.; Yavuz, N.; Turkmen, K. Chitosan Improves Plant Regeneration in Callus Culture of a Medicinal Herb Melissa officinalis L. J. Biotechnol. 2015, 208, S107. [Google Scholar] [CrossRef]
- Fruchterman, T.M.J.; Reingold, E.M. Graph Drawing by Force-Directed Placement. Softw. Pract. Exp. 1991, 21, 1129–1164. [Google Scholar] [CrossRef]
- Gün Polat, E.; Acemi, A.; Özen, F. The Effects of Chitosan and Its Acetylation Degree on in Vitro Seed Germination and Organ Development in Ageratum Houstonianum Mill. Plant Cell Tiss. Organ. Cult. 2022, 149, 809–821. [Google Scholar] [CrossRef]
- Alhaithloul, H.A.S.; Awad, N.S.; Shoala, T.; Alqahtani, M.M.; Alzuaibr, F.M.; Alasmari, A.; Abdein, M.A. Comparison of Various Hormone Combinations and the Potential of Chitosan Nanoparticles for Growth Stimulation in Astragalus Spp. Plant Cell Tiss. Organ. Cult. 2024, 157, 74. [Google Scholar] [CrossRef]
- Abou-Elfadl, R.E.; Ahmad, M.E.; Abdel-Magiud, M.R.; Mahdi, A.A. Comparative Analysis of Bioactive Compounds of Balanites Aegyptiaca L. Callus. Egypt. J. Chem. 2023, 66, 339–349. [Google Scholar] [CrossRef]
- Safana, H.S.; Majid Abdulhameed, I.; Abdulkareem Mohammed, A. Impact of Chitosan and Benzyl Adenine on Shoot Multiplication of Kumquat Plant (Citrus Japonica Thumb.) in Vitro. Int. J. Agricult. Stat. Sci. 2022, 18, 359–365. [Google Scholar]
- Bakhtiari, M.A.; Golkar, P. The Effects of Callus Elicitation on Lepidine, Phenolic Content, and Antioxidant Activity of Lepidium Sativum L.: Chitosan and Gibberellic Acid. J. Plant Growth Regul. 2022, 41, 1148–1160. [Google Scholar] [CrossRef]
- Kandha, L.; Kumar, R.; Sethi, S.K.; Bindhani, B.K. Chitosan Enhances Growth and Survival Rate of in Vitro-Cultured Plantlets of Banana Cultivar “Grand Naine”. J. Crop Improv. 2021, 35, 848–865. [Google Scholar] [CrossRef]
- Al-Saeedi, A.A.R.; Al-Rekaby, L.S. Effect of Growth Regulators and Chitosan on the Production of Callus Biomass and Active Compounds of Rosemary in Vitro. Connect. J. 2022, 18, 805–812. [Google Scholar]
- Ikhlef, A.; Ait-Amar, H.; Khelifi, L.; Chemat, S. Optimization of Silybin Production from Silybum Marianum Tissue Cell Cultures Using Different Elicitor Solutions. J. Food Process. Preserv. 2022, 46, e16491. [Google Scholar] [CrossRef]
- Tang, C.-N.; Lamasudin, D.U.; Lamasudin, D.U.; Abdullah, W.M.A.N.W.; Chew, L.M.; Chiew, M.-S.; Chai, Q.-Y.; Ong-Abdullah, J.; Lai, K.-S. Enhanced in Vitro Shoot Regeneration and Biochemical Properties of Stevia Rebaudiana Using Chitosan. Sains Malays. 2021, 50, 667–676. [Google Scholar] [CrossRef]
- Espinosa-Leal, C.A.; Puente-Garza, C.A.; García-Lara, S. In Vitro Plant Tissue Culture: Means for Production of Biological Active Compounds. Planta 2018, 248, 1–18. [Google Scholar] [CrossRef]
- Iqbal, Z.; Javad, S.; Naz, S.; Shah, A.A.; Shah, A.N.; Paray, B.A.; Gulnaz, A.; Abdelsalam, N.R. Elicitation of the in Vitro Cultures of Selected Varieties of Vigna Radiata L. With Zinc Oxide and Copper Oxide Nanoparticles for Enhanced Phytochemicals Production. Front. Plant Sci. 2022, 13, 908532. [Google Scholar] [CrossRef]
- Satdive, R.K.; Fulzele, D.P.; Eapen, S. Studies on Production of Ajmalicine in Shake Flasks by Multiple Shoot Cultures of Catharanthus Roseus. Biotechnol. Prog. 2003, 19, 1071–1075. [Google Scholar] [CrossRef]
- Muxika, A.; Etxabide, A.; Uranga, J.; Guerrero, P.; de la Caba, K. Chitosan as a Bioactive Polymer: Processing, Properties and Applications. Int. J. Biol. Macromol. 2017, 105, 1358–1368. [Google Scholar] [CrossRef] [PubMed]
- Harugade, A.; Sherje, A.P.; Pethe, A. Chitosan: A Review on Properties, Biological Activities and Recent Progress in Biomedical Applications. React. Funct. Polym. 2023, 191, 105634. [Google Scholar] [CrossRef]
- Gabr, A.M.M.; Ghareeb, H.; El Shabrawi, H.M.; Smetanska, I.; Bekheet, S.A. Enhancement of Silymarin and Phenolic Compound Accumulation in Tissue Culture of Milk Thistle Using Elicitor Feeding and Hairy Root Cultures. J. Genet. Eng. Biotechnol. 2016, 14, 327–333. [Google Scholar] [CrossRef]
- Asghari-Zakaria, R.; Maleki-Zanjani, B.; Sedghi, E. Effect of in Vitro Chitosan Application on Growth and Minituber Yield of Solanum tuberosum L. Plant Soil Environ. 2009, 55, 252–256. [Google Scholar] [CrossRef]
- Golkar, P.; Taghizadeh, M.; Yousefian, Z. The Effects of Chitosan and Salicylic Acid on Elicitation of Secondary Metabolites and Antioxidant Activity of Safflower under in Vitro Salinity Stress. Plant Cell Tiss. Organ. Cult. 2019, 137, 575–585. [Google Scholar] [CrossRef]
- Mukarram, M.; Ali, J.; Dadkhah-Aghdash, H.; Kurjak, D.; Kačík, F.; Ďurkovič, J. Chitosan-Induced Biotic Stress Tolerance and Crosstalk with Phytohormones, Antioxidants, and Other Signalling Molecules. Front. Plant Sci. 2023, 14, 1217822. [Google Scholar] [CrossRef] [PubMed]
- Xing, K.; Zhu, X.; Peng, X.; Qin, S. Chitosan Antimicrobial and Eliciting Properties for Pest Control in Agriculture: A Review. Agron. Sustain. Dev. 2015, 35, 569–588. [Google Scholar] [CrossRef]
- Ke, C.-L.; Deng, F.-S.; Chuang, C.-Y.; Lin, C.-H. Antimicrobial Actions and Applications of Chitosan. Polymers 2021, 13, 904. [Google Scholar] [CrossRef] [PubMed]
- Komarova, T.; Shipounova, I.; Kalinina, N.; Taliansky, M. Application of Chitosan and Its Derivatives Against Plant Viruses. Polymers 2024, 16, 3122. [Google Scholar] [CrossRef]
- Pichyangkura, R.; Chadchawan, S. Biostimulant Activity of Chitosan in Horticulture. Sci. Hortic. 2015, 196, 49–65. [Google Scholar] [CrossRef]
- Oshunsanya, S.O.; Nwosu, N.J.; Li, Y. Abiotic Stress in Agricultural Crops Under Climatic Conditions. In Sustainable Agriculture, Forest and Environmental Management; Jhariya, M.K., Banerjee, A., Meena, R.S., Yadav, D.K., Eds.; Springer Singapore: Singapore, 2019; pp. 71–100. [Google Scholar]
- Farouk, S.; EL-Metwally, I.M. Synergistic Responses of Drip-Irrigated Wheat Crop to Chitosan and/or Silicon under Different Irrigation Regimes. Agric. Water Manag. 2019, 226, 105807. [Google Scholar] [CrossRef]
- Turk, H. Chitosan-Induced Enhanced Expression and Activation of Alternative Oxidase Confer Tolerance to Salt Stress in Maize Seedlings. Plant Physiol. Biochem. 2019, 141, 415–422. [Google Scholar] [CrossRef]
- Shater Abdallah, M.; El-Mohsen Ramadan, A.; El-Bassiouny, H.M.; Bakry, B. Regulation of Antioxidant System in Wheat Cultivars by Using Chitosan or Salicylic Acid to Improve Growth and Yield under Salinity Stress. Asian J. Plant Sci. 2020, 19, 114–126. [Google Scholar] [CrossRef]
- Ullah, N.; Basit, A.; Ahmad, I.; Ullah, I.; Shah, S.T.; Mohamed, H.I.; Javed, S. Mitigation the Adverse Effect of Salinity Stress on the Performance of the Tomato Crop by Exogenous Application of Chitosan. Bull. Natl. Res. Cent. 2020, 44, 181. [Google Scholar] [CrossRef]
- Alenazi, M.M.; El-Ebidy, A.M.; El-shehaby, O.A.; Seleiman, M.F.; Aldhuwaib, K.J.; Abdel-Aziz, H.M.M. Chitosan and Chitosan Nanoparticles Differentially Alleviate Salinity Stress in Phaseolus vulgaris L. Plants. Plants 2024, 13, 398. [Google Scholar] [CrossRef]
- Dradrach, A.; Iqbal, M.; Lewińska, K.; Jędroszka, N.; Gull-e-Faran; Rana, M.A.K.; Tanzeem-ul-Haq, H.S. Effects of Soil Application of Chitosan and Foliar Melatonin on Growth, Photosynthesis, and Heavy Metals Accumulation in Wheat Growing on Wastewater Polluted Soil. Sustainability 2022, 14, 8293. [Google Scholar] [CrossRef]
- Marzouk, N.M.; Abd-Alrahman, H.A.; El-Sawy, S.M.M. Amino Acids Sources and Chitosan Enhance Cauliflower Yield and Quality under Heat Stress. Asian J. Plant Sci. 2021, 21, 9–23. [Google Scholar] [CrossRef]
- Dawood, M.G.; El-Awadi, M.E.; Sadak, M.S. Chitosan and Its Nanoform Regulates Physiological Processes and Antioxidant Mechanisms to Improve Drought Stress Tolerance of Vicia Faba Plant. J. Soil. Sci. Plant Nutr. 2024, 24, 5696–5709. [Google Scholar] [CrossRef]
- Krupa-Małkiewicz, M.; Fornal, N. Application of Chitosan In Vitro to Minimize the Adverse Effects of Salinity in Petunia × Atkinsiana D. Don. J. Ecol. Eng. 2018, 143–149. [Google Scholar] [CrossRef]
| Factor | Type of Elicitor | Examples |
|---|---|---|
| Site of action | Exogenous elicitors | Molecules produced by pathogens that act externally on the plant. |
| Endogenous elicitors | Compounds synthesized by the plant itself in response to pathogen attack. | |
| Origin | Biotic elicitors | Derived from living organisms or their metabolic products: - polysaccharides (chitin, CTS, glucans, etc.); - oligosaccharides (mannuronate, galacturonides, etc.); - proteins (oligandrin, glycoproteins, etc.). |
| Biotic elicitors with a defined composition: CTS, alginate, pectin, chitin, elicitin, and pectic fragments. | ||
| Biotic elicitors with a complex composition: fungi homogenate, yeast extract, and fungal spores. | ||
| Abiotic elicitors | Originating from non-living sources. | |
| Chemical elicitors: silicon, benzothiadiazole, ethanol, acetic acid, and metal ions (e.g., Cu2+, Zn2+, Ag+). | ||
| Physical elicitors: UV radiation, temperature stress, drought, osmotic and saline stress, and mechanical damage. | ||
| Specificity | General elicitors | Capable of inducing defense responses in a wide range of plant species. |
| Race-specific elicitors | Trigger resistance only in plants carrying specific resistance genes. |
| Properties | Characteristic | |
|---|---|---|
| Molecular weight (MW) | High (HMWC) | >700 kDa |
| Medium (MMWC) | 150–700 kDa | |
| Low (LMWC) | <150 kDa | |
| 100,000–1,200,000 Da, industrial CTS. | >1,000,000 Da, native chitin. | |
| Deacetylation degree (DD) | 100%, the biopolymer contains only monomeric forms of 2-amido-2-deoxy-D-glucopyranose. | 50%, the biopolymer contains 50% of 2-amido-2-deoxy-D-glucopyranose units. |
| 100% deacetylation, CTS. | 0% deacetylation, chitin. | |
| Residues of acetylglucosamine (2-acetamido-2-deoxy-D-glycopyranose) | <50%, the substance is categorized as CTS. | ≥50%, the substance is categorized as chitin. |
| Solubility | 100% DD, soluble in water. | 50% DD, soluble in an aqueous acidic environment. |
| HMWC; lower solubility in water. | LMWC; higher solubility in water. | |
| Crystallinity index (CI) | CTS swelling, its porosity, water absorption, and moisture retention are affected by crystallinity. | Both, chitin (0% deacetylated) and CTS (100% deacetylated) have the highest crystallinity value. The solid form of CTS is semi-crystalline. |
| Particle size | <1 mm, often used in most applications. | |
| Surface area | <10 m2/g, CTS flakes or powder. | |
| High temperature sensitivity | >280 °C; heat degradation of CTS. | |
| Antioxidant activity | LMWC; higher. | HMWC; lower. |
| Bioactivity (in terms of biocompatibility, nontoxicity, biodegradability, antimicrobial activity, etc.) | LMWC; more significant. | HMWC; less significant. |
| Company Name | Country | Focus Areas |
|---|---|---|
| Advanced Biopolymers AS | Norway | Industrial and medical-grade CTS |
| Axio Biosolutions | USA | CTS-based wound care products |
| BIO21 Co. Ltd. | Thailand | CTS for agriculture and water treatment |
| ChytoLytic | Canada | Pure, clean, high-quality, high-grade CTS for advanced R&D and commercial biomedical, pharma, and industrial applications |
| Golden-Shell Pharmaceutical | China | Pharmaceutical-grade CTS |
| Heppe Medical CTS GmbH | Germany | High-purity CTS for biomedical use |
| Kimica Corporation | Japan | Industrial and cosmetic applications |
| KitoZyme S.A. | Belgium | Biopolymers for healthcare and nutrition |
| Meron Biopolymers | Norway | Eco-friendly biopolymers for food and cosmetics |
| Panvo Organics Pvt. Ltd. | India | Sustainable CTS for agriculture, pharma |
| Plant Species | Stress Condition | Genes Affected | Biological Function/Pathway | Reference |
|---|---|---|---|---|
| Various species | General defense response | PAL, PR1, POX | Induces systemic acquired resistance (SAR); enhances phenolic and carbohydrate production. | [54] |
| Agrostis stolonifera L. (creeping bentgrass) | Salinity stress | AsHKT1, AsNHX4, AsNHX5, AsNHX6 | Regulates Na+/H+ exchangers; improves ion homeostasis. | [55] |
| Ocimum basilicum L. (sweet basil) | Salinity stress | PAL, CVOMT | Activates phenylpropanoid pathway; increases phenolic compound synthesis. | [56] |
| Various species | Salinity stress | MAPK3, GS, ORCA3 | Enhances stress signaling and secondary metabolite biosynthesis. | [57] |
| Solanum lycopersicum L. (tomato) | Salt stress | SOD, JA | Boosts antioxidant defense and jasmonic acid signaling. | [58] |
| Solanum lycopersicum L. (tomato) | Drought stress | HsfA1a, SlAREB1, LeNCED1, LePIP1 | Improves drought tolerance via ABA signaling and aquaporin regulation | [59] |
| Catharanthus roseus L. | Drought stress | STR, DAT, PRX1, GS | Enhances secondary metabolite production and oxidative stress defense. | [60] |
| Kobresia pygmaea (Willd.) | Cold stress | Chit134, BSK2, ERF, NCED, DRE326 | Activates cold-responsive transcription factors and ABA-related genes. | [61] |
| Various species | Heat stress | ABA-responsive genes | Increases heat tolerance via ABA signaling and defense gene activation. | [62] |
| Species | Types of Tissue/Explants Used in the Culture Media | Purpose | CTS Concentration (mg/L)/Form | Reference |
|---|---|---|---|---|
| Ageratum houstonianum Mill. | Seeds | In vitro seed germination and organ development | 2.5, 5.0, 10.0 mg/L, (10%, 20% DA shrimp CTS) | [109] |
| Astragalus spp. | In vitro seedlings | Growth stimulation | 0.2, 0.5, 1, 2, 3, and 4 mg/L, (CTS nanoparticles) | [110] |
| Balanites aegyptiaca L. | Callus suspension culture | SM production | 40 mg/L | [111] |
| Canscora decussata Schult. | Nodal explants | SM production | 200 mg/L (Sigma-Aldrich) | [24] |
| Citrus japonica THUMB. | Nodule stems | Shoot multiplication | 10, 15, 20, 25 mg/L | [112] |
| Ginkgo biloba L. | Callus suspension culture | Callus stimulation | 50 mg/L | [101] |
| Hyptis suaveolensJACQ. | Root culture | Podophyllotoxin synthesis | 50; 100; 150 mg/L (≥98% purity, Sigma-Aldrich) | [105] |
| Iberis amara L. | Cell suspension culture | SM production | 50 mg/L | [102] |
| Lepidium sativum L. | Callus suspension culture | SM production and antioxidant activity | 100, 250 and 500 mg/L (low molecular weight, 50,000 Da) | [113] |
| Lilium regaleWils. | In vitro seedlings | Flavonoid content, chlorophyll, and regeneration | 50; 100; 150; 200 mg/L | [84] |
| Musa spp. | Rhizome and sucker | Regeneration of shoots and roots | 25 mg/L(shrimp CTS) | [114] |
| Oryza sativa L. japonica | Cell suspension cultures | SM production | CTS nanoparticles (shrimp CTS, ≥75% DA) | [100] |
| Rosmarinus officinalis L. | Apex and lateral buds | Callus biomass, SM production | 5 mg/L | [115] |
| Scutellaria bornmuelleri L. | Hairy root cultures | Flavonoid content | 50,100, 200 mg/L | [104] |
| Silybum marianum L. | Cell suspension cultures | Silybin production | 600 mg/L | [116] |
| Stevia rebaudiana L. | Nodal stems | Shoot regeneration | 20, 40, 60, 80, 100 mg/L (low, medium, high MW CTS) | [117] |
| Urtica dioica L. | Callus suspension culture | SM production | 50 and 100 mg/L | [77] |
| Methodology of CTS Elicitation In Vitro | Missing Information | Reference |
|---|---|---|
| The stock solution of 0.3 g/L CTS (≥ 98% purity; Sigma-Aldrich) was prepared by dissolution in 1000 mL of distilled water, to which 10 mL of acetic acid was added. From this stock solution, solutions with concentrations of 50.0, 100.0, and 150.0 mg/L were prepared. | CTS stock solution sterilization Medium supplementation with CTS | [105] |
| CTS was dissolved in 5% (v/v) 1 N HCl through gentle heating and continuous stirring and added to the callus induction medium at concentrations of 200, 400, and 800 mg/L. | CTS type CTS stock solution sterilization Medium supplementation with CTS | [123] |
| Soluble CTS (ChitoPlant, ChiPro GmbH Bremen, CTS content 99.9%) was added to the rooting medium prior to autoclaving, and the pH was adjusted to 5.7–5.8. The concentrations of soluble CTS of 0, 5, 15, 50, 150, 500, 750, and 1000 mg/L were tested. | CTS solutions preparation | [124] |
| CTS was added in six different combinations into the MS medium prior to the adjustment of pH and medium autoclaving. | CTS type CTS solutions preparation | [115] |
| MS medium containing different molecular weights (low, medium, and high) and concentration (0, 20, 40, 60,80, and 100 mg/L) of CTS. | CTS type CTS solutions preparation and sterilization Medium supplementation with CTS | [117] |
| CTS (Sigma-Aldrich) was added on the 8th day into the medium (200 mg/L). | CTS solutions preparation and sterilization Medium supplementation with CTS | [24] |
| CTS at a concentration of 10, 15, 20, and 25 mg/L. | CTS type CTS solutions preparation and sterilization Medium supplementation with CTS | [112] |
| Commercially purchased Sigma-Aldrich CTS solutions were dissolved in distilled warm water and sterile filtered through a prefilter (0.2 m pore size; Advantec). The sterilized CTS solutions at a concentration of 20, 40, and 80 mg/L were added to the callus culture flasks. | CTS type | [111] |
| Low-molecular-weight CTS (50,000 Da) (Sigma-Aldrich, Taufkirchen, Germany) was used in this study. | Complete information | [113] |
| Low-molecular-weight CTS (50,000 Da) (Sigma-Aldrich, Germany) was dissolved in 3% (v/v) 0.1 M acetic acid using gentle heating and continuous stirring (at 60 °C, 12 h with stirring). The pH was adjusted to 5.8 with 1 N sodium hydroxide (NaOH), and the final concentration was adjusted to 10 mg×mL−1. The solution was stirred to further dissolve the CTS and then autoclaved for 15 min at 121 °C. The solution was kept at 4 °C prior to use. The CTS was added to the sub-cultures at two final concentrations (25 and 50 mg/L). | Complete information | [125] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roy Chowdhury, M.; Mehmet, M.; Mukherjee, J.; Debnath, A.J.; Ražná, K. Chitosan as an Elicitor in Plant Tissue Cultures: Methodological Challenges. Molecules 2025, 30, 3476. https://doi.org/10.3390/molecules30173476
Roy Chowdhury M, Mehmet M, Mukherjee J, Debnath AJ, Ražná K. Chitosan as an Elicitor in Plant Tissue Cultures: Methodological Challenges. Molecules. 2025; 30(17):3476. https://doi.org/10.3390/molecules30173476
Chicago/Turabian StyleRoy Chowdhury, Moumita, Mizgin Mehmet, Jit Mukherjee, Anirban Jyoti Debnath, and Katarína Ražná. 2025. "Chitosan as an Elicitor in Plant Tissue Cultures: Methodological Challenges" Molecules 30, no. 17: 3476. https://doi.org/10.3390/molecules30173476
APA StyleRoy Chowdhury, M., Mehmet, M., Mukherjee, J., Debnath, A. J., & Ražná, K. (2025). Chitosan as an Elicitor in Plant Tissue Cultures: Methodological Challenges. Molecules, 30(17), 3476. https://doi.org/10.3390/molecules30173476








