Molecular Structure, Matrix-Isolation IR Spectrum and UV-Induced Transformations of 2-Amino-5-(4-Methoxyphenyl)-1,3,4-Oxadiazole
Simple Summary
Abstract
1. Introduction
2. Results and Discussion
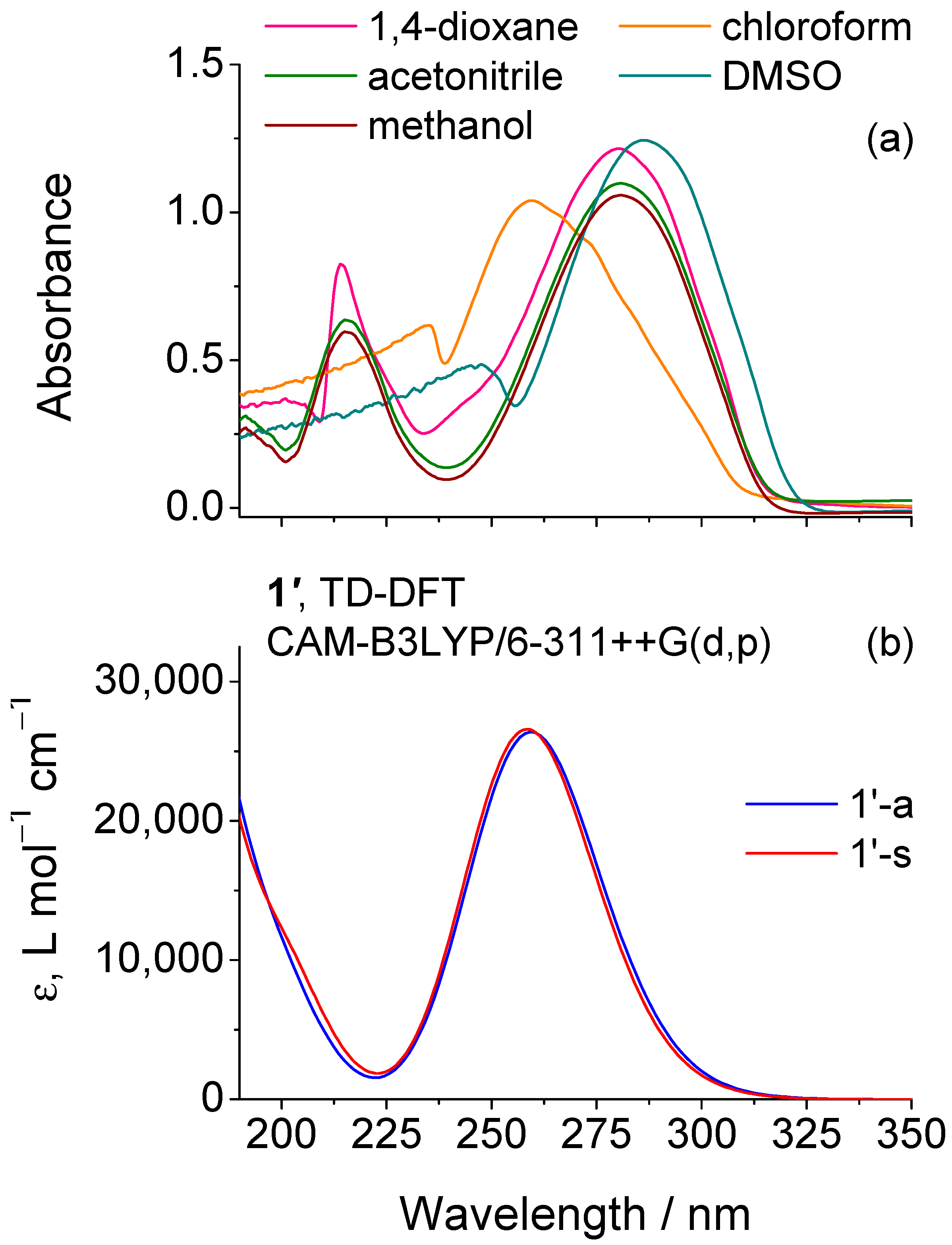
2.1. Isomeric Forms of 2-Amino-5-(4-Methoxyphenyl)-1,3,4-Oxadiazole 1
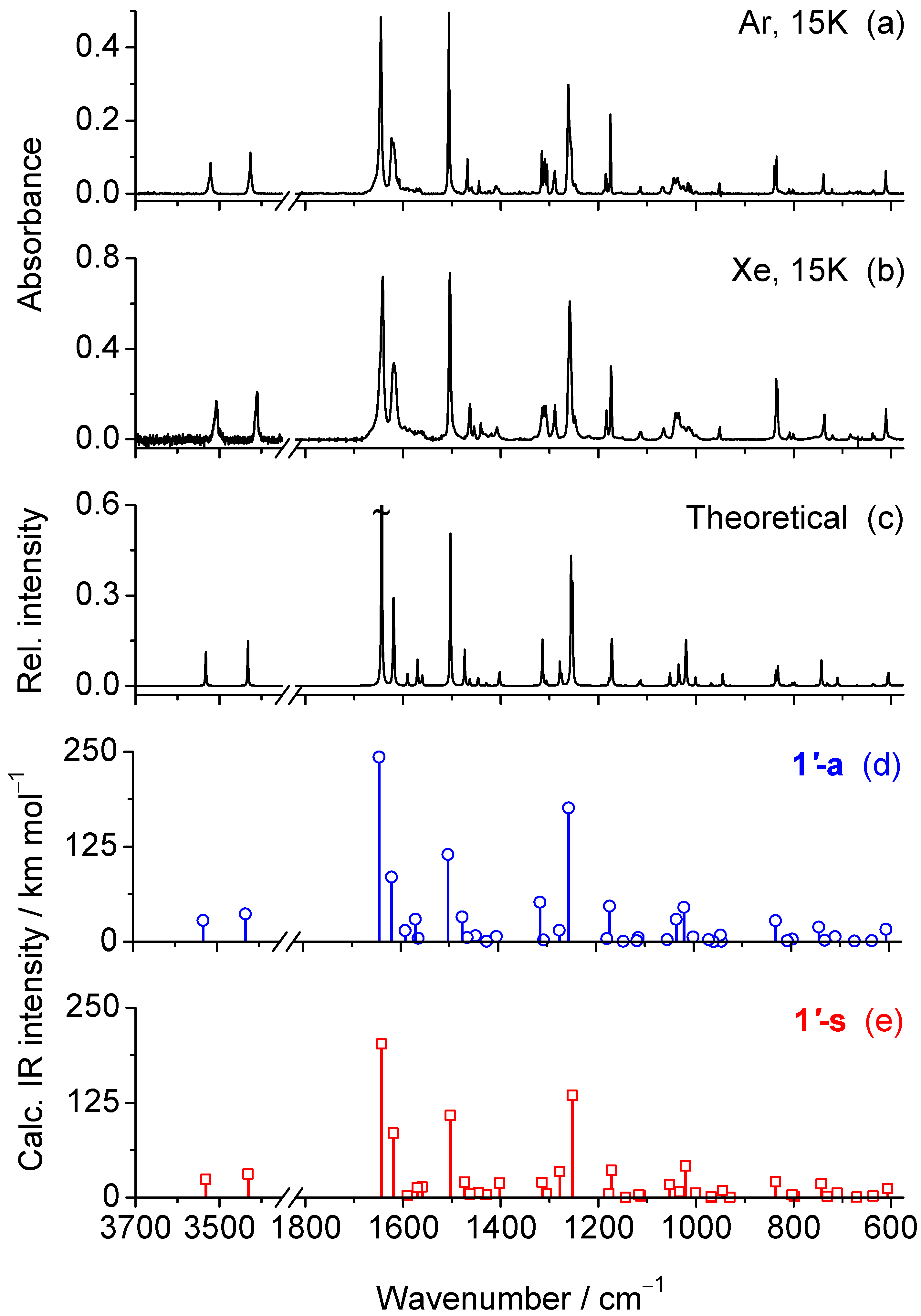
2.2. Matrix Isolation Spectrum of the Freshly Deposited Matrices
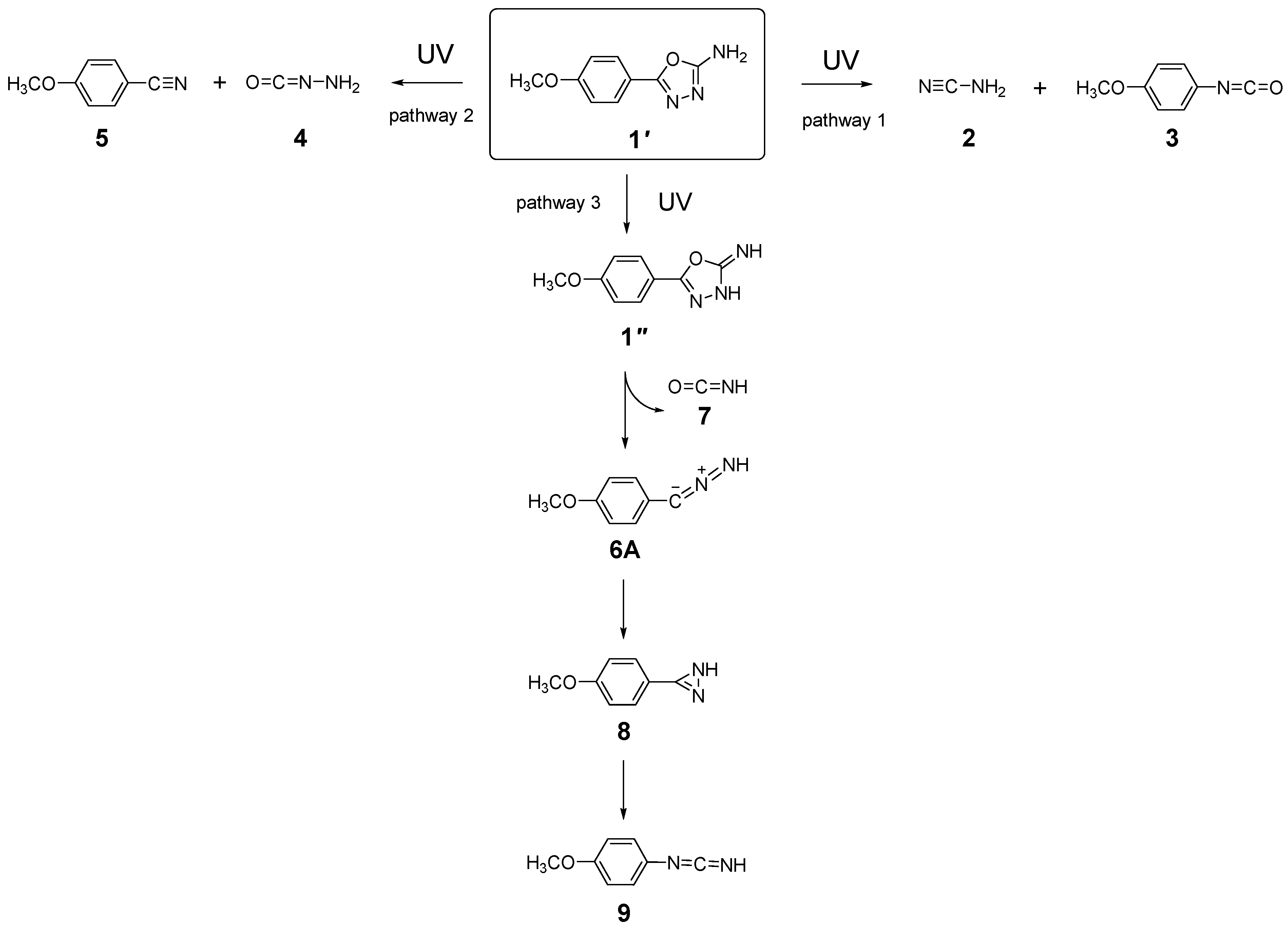
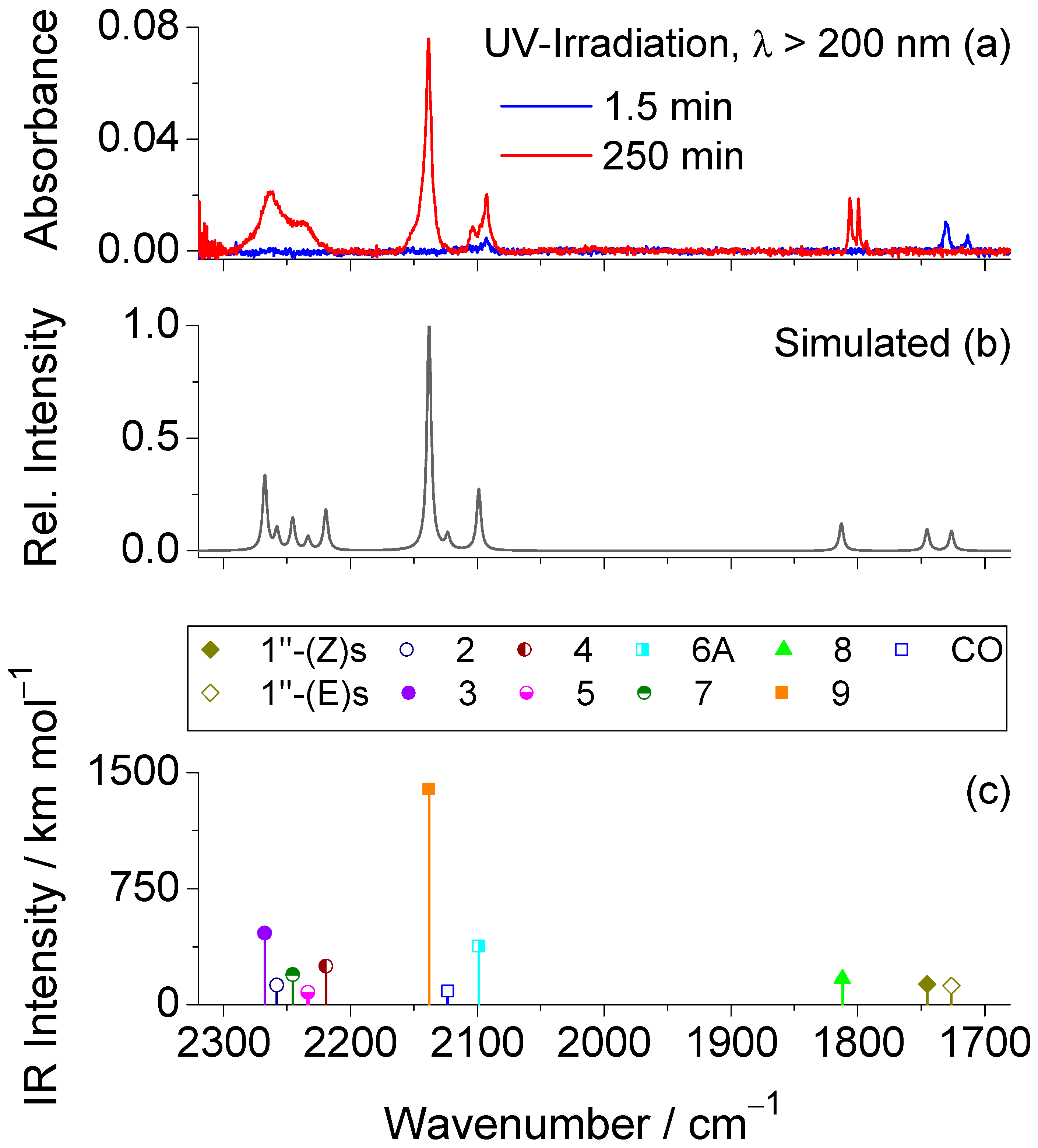
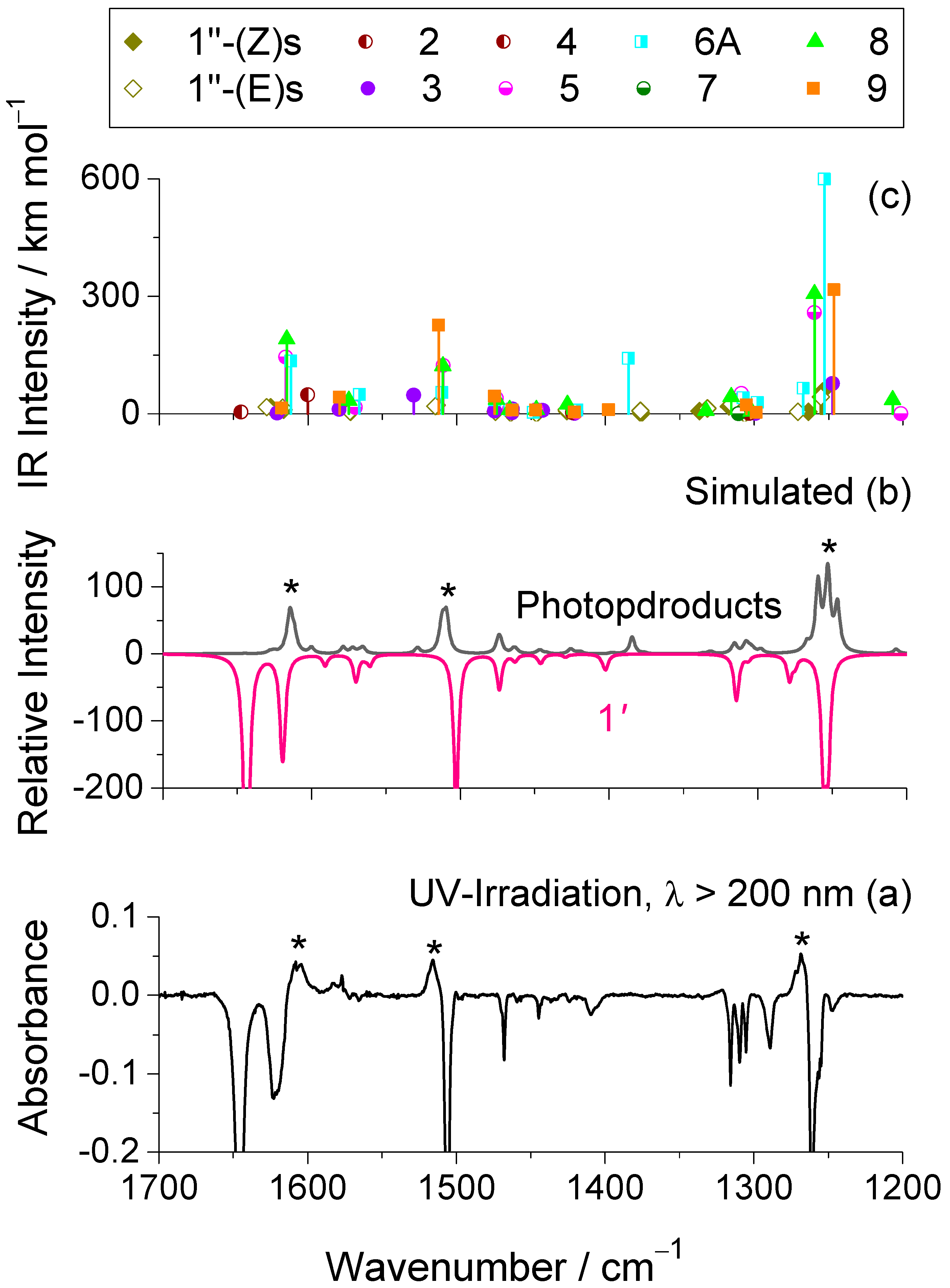
2.3. UV-Induced Photochemistry of Matrix-Isolated 1
3. Methods
3.1. Experimental Methods
3.2. Computational Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Glomb, T.; Świątek, P. Antimicrobial Activity of 1,3,4-Oxadiazole Derivatives. Int. J. Mol. Sci. 2021, 22, 6979. [Google Scholar] [CrossRef]
- Boström, J.; Hogner, A.; Llinàs, A.; Wellner, E.; Plowright, A.T. Oxadiazoles in Medicinal Chemistry. J. Med. Chem. 2012, 55, 1817–1830. [Google Scholar] [CrossRef]
- Khalilullah, H.; Ahsan, M.J.; Hedaitullah, M.; Khan, S.; Ahmed, B. 1,3,4-Oxadiazole: A Biologically Active Scaffold. Mini-Rev. Med. Chem. 2012, 12, 789–801. [Google Scholar] [CrossRef]
- De Oliveira, C.S.; Lira, B.F.; Barbosa-Filho, J.M.; Lorenzo, J.G.F.; De Athayde-Filho, P.F. Synthetic Approaches and Pharmacological Activity of 1,3,4-Oxadiazoles: A Review of the Literature from 2000–2012. Molecules 2012, 17, 10192–10231. [Google Scholar] [CrossRef]
- Salahuddin; Mazumder, A.; Yar, M.S.; Mazumder, R.; Chakraborthy, G.S.; Ahsan, M.J.; Rahman, M.U. Updates on synthesis and biological activities of 1,3,4-oxadiazole: A review. Synth. Commun. 2017, 47, 1805–1847. [Google Scholar] [CrossRef]
- Verma, G.; Khan, M.F.; Akhtar, W.; Alam, M.M.; Akhter, M.; Shaquiquzzaman, M. A Review Exploring Therapeutic Worth of 1,3,4-Oxadiazole Tailored Compounds. Mini-Rev. Med. Chem. 2019, 19, 477–509. [Google Scholar] [CrossRef] [PubMed]
- Siwach, A.; Verma, P.K. Therapeutic potential of oxadiazole or furadiazole containing compounds. BMC Chem. 2020, 14, 70. [Google Scholar] [CrossRef]
- Vaidya, A.; Pathak, D.; Shah, K. 1,3,4-oxadiazole and its derivatives: A review on recent progress in anticancer activities. Chem. Biol. Drug Des. 2021, 97, 572–591. [Google Scholar] [CrossRef] [PubMed]
- Luczynski, M.; Kudelko, A. Synthesis and Biological Activity of 1,3,4-Oxadiazoles Used in Medicine and Agriculture. Appl. Sci. 2022, 12, 3756. [Google Scholar] [CrossRef]
- Glomb, T.; Wiatrak, B.; Gębczak, K.; Gębarowski, T.; Bodetko, D.; Czyżnikowska, Ż.; Świątek, P. New 1,3,4-Oxadiazole Derivatives of Pyridothiazine-1,1-Dioxide with Anti-Inflammatory Activity. Int. J. Mol. Sci. 2020, 21, 9122. [Google Scholar] [CrossRef]
- Desai, N.; Monapara, J.; Jethawa, A.; Khedkar, V.; Shingate, B. Oxadiazole: A highly versatile scaffold in drug discovery. Arch. Pharm. 2022, 355, 2200123. [Google Scholar] [CrossRef]
- Kumari, S.; Carmona, A.V.; Tiwari, A.K.; Trippier, P.C. Amide Bond Bioisosteres: Strategies, Synthesis, and Successes. J. Med. Chem. 2020, 63, 12290–12358. [Google Scholar] [CrossRef]
- Tokumaru, K.; Johnston, J.N. A convergent synthesis of 1,3,4-oxadiazoles from acyl hydrazides under semiaqueous conditions. Chem. Sci. 2017, 8, 3187–3191. [Google Scholar] [CrossRef]
- Goldberg, K.; Groombridge, S.; Hudson, J.; Leach, A.G.; MacFaul, P.A.; Pickup, A.; Poultney, R.; Scott, J.S.; Svensson, P.H.; Sweeney, J. Oxadiazole isomers: All bioisosteres are not created equal. Med. Chem. Commun. 2012, 3, 600–604. [Google Scholar] [CrossRef]
- Patani, G.A.; LaVoie, E.J. Bioisosterism: A Rational Approach in Drug Design. Chem. Rev. 1996, 96, 3147–3176. [Google Scholar] [CrossRef]
- Steeneck, C.; Gege, C.; Kinzel, O.; Albers, M.; Kleymann, G.; Schlüter, T.; Schulz, A.; Xue, X.; Cummings, M.D.; Fourie, A.M.; et al. Discovery and optimization of new oxadiazole substituted thiazole RORγt inverse agonists through a bioisosteric amide replacement approach. Bioorg. Med. Chem. Lett. 2020, 30, 127174. [Google Scholar] [CrossRef] [PubMed]
- Alburquerque-González, B.; Bernabé-García, Á.; Bernabé-García, M.; Ruiz-Sanz, J.; López-Calderón, F.F.; Gonnelli, L.; Banci, L.; Peña-García, J.; Luque, I.; Nicolás, F.J.; et al. The FDA-Approved Antiviral Raltegravir Inhibits Fascin1-Dependent Invasion of Colorectal Tumor Cells In Vitro and In Vivo. Cancers 2021, 13, 861. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, H.; Shen, W.; He, P.; Zhou, Z. Effectiveness and tolerability of targeted drugs for the treatment of metastatic castration-resistant prostate cancer: A network meta-analysis of randomized controlled trials. J. Cancer Res. Clin. Oncol. 2018, 144, 1751–1768. [Google Scholar] [CrossRef]
- Tao, Y.; Wang, Q.; Shang, Y.; Yang, C.; Ao, L.; Qin, J.; Ma, D.; Shuai, Z. Multifunctional bipolar triphenylamine/oxadiazole derivatives: Highly efficient blue fluorescence, red phosphorescence host and two-color based white OLEDs. Chem. Commun. 2009, 1, 77–79. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Wang, Q.; Yang, C.; Wang, Q.; Zhang, Z.; Zou, T.; Qin, J.; Ma, D. A Simple Carbazole/Oxadiazole Hybrid Molecule: An Excellent Bipolar Host for Green and Red Phosphorescent OLEDs. Angew. Chem. 2008, 47, 8104–8107. [Google Scholar] [CrossRef]
- Paun, A.; Hadade, N.D.; Paraschivescu, C.C.; Matache, M. 1,3,4-Oxadiazoles as luminescent materials for organic light emitting diodes via cross-coupling reactions. J. Mater. Chem. C 2016, 4, 8596–8610. [Google Scholar] [CrossRef]
- Tao, Y.; Yang, C.; Qin, J. Organic host materials for phosphorescent organic light-emitting diodes. Chem. Soc. Rev. 2011, 40, 2943–2970. [Google Scholar] [CrossRef]
- Green, L.; Livingstone, K.; Bertrand, S.; Peace, S.; Jamieson, C. UV-induced 1, 3, 4-oxadiazole formation from 5-substituted tetrazoles and carboxylic acids in flow. Chem. Eur. J. 2020, 26, 14866–14870. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.D.; Prajapati, S.M.; Panchal, S.N.; Patel, H.D. Review of Synthesis of 1,3,4-Oxadiazole Derivatives. Synth. Commun. 2014, 44, 1859–1875. [Google Scholar] [CrossRef]
- Tsuge, O.; Oe, K.; Tashiro, M. Photolysis of 1,3,4-oxadiazoles in alcohols. Chem. Lett. 1977, 6, 1207–1210. [Google Scholar] [CrossRef]
- Keresztes, B.; Csonka, I.P.; Lajgút, G.H.H.G.; Bazsó, G.; Tarczay, G. Matrix isolation study on the 1: 1 hydrogen-bonded complexes [H, C, N]…[H, C, N, O]. J. Mol. Struct. 2020, 1219, 128535. [Google Scholar] [CrossRef]
- Sıdır, İ.; Lopes, S.; Paixão, J.A.; Nikitin, T.; Jesus, A.J.L.; Fausto, R. Structure, IR spectrum and UV-induced photochemistry of a pyridyl-substituted 1,3,4-oxadiazole-thione in Ar matrix and intermolecular interactions in the neat crystalline phase. J. Photochem. Photobiol. A 2026, 470, 116610. [Google Scholar] [CrossRef]
- Lopes Jesus, A.J.; Rosado, M.T.S.; Reva, I.; Fausto, R.; Eusébio, M.E.; Redinha, J.S. Conformational Study of Monomeric 2,3-Butanediols by Matrix-Isolation Infrared Spectroscopy and DFT Calculations. J. Phys. Chem. A 2006, 110, 4169–4179. [Google Scholar] [CrossRef]
- Lopes Jesus, A.J.; Rosado, M.T.S.; Reva, I.; Fausto, R.; Eusébio, M.E.S.; Redinha, J.S. Structure of Isolated 1,4-Butanediol: Combination of MP2 Calculations, NBO Analysis, and Matrix-Isolation Infrared Spectroscopy. J. Phys. Chem. A 2008, 112, 4669–4678. [Google Scholar] [CrossRef] [PubMed]
- Rosado, M.T.S.; Lopes Jesus, A.J.; Reva, I.D.; Fausto, R.; Redinha, J.S. Conformational Cooling Dynamics in Matrix-Isolated 1,3-Butanediol. J. Phys. Chem. A 2009, 113, 7499–7507. [Google Scholar] [CrossRef]
- Reva, I.D.; Stepanian, S.G.; Adamowicz, L.; Fausto, R. Missing conformers. Comparative study of conformational cooling in cyanoacetic acid and methyl cyanoacetate isolated in low temperature inert gas matrixes. Chem. Phys. Lett. 2003, 374, 631–638. [Google Scholar] [CrossRef]
- Lopes Jesus, A.J.; Reva, I.; Araujo-Andrade, C.; Fausto, R. Conformational changes in matrix-isolated 6-methoxyindole: Effects of the thermal and infrared light excitations. J. Chem. Phys. 2016, 144, 124306. [Google Scholar] [CrossRef]
- Lopes Jesus, A.J.; Fausto, R.; Reva, I. Conformational Changes in 5-Methoxyindole: Effects of Thermal, Vibrational, and Electronic Excitations. J. Phys. Chem. A 2017, 121, 3372–3382. [Google Scholar] [CrossRef]
- Kuş, N.; Sharma, A.; Reva, I.; Lapinski, L.; Fausto, R. Thermal and Photoinduced Control of Relative Populations of 4-Methoxybenzaldehyde (p-Anisaldehyde) Conformers. J. Phys. Chem. A 2010, 114, 7716–7724. [Google Scholar] [CrossRef]
- Reva, I.D.; Jesus, A.J.L.; Rosado, M.T.S.; Fausto, R.; Eusebio, M.E.; Redinha, J.S. Stepwise conformational cooling towards a single isomeric state in the four internal rotors system 1, 2-butanediol. Phys. Chem. Chem. Phys. 2006, 8, 5339–5349. [Google Scholar] [CrossRef]
- Barnes, A.J. Matrix isolation vibrational spectroscopy as a tool for studying conformational isomerism. J. Mol. Struct. 1984, 113, 161–174. [Google Scholar] [CrossRef]
- Lopes Jesus, A.J.; Nunes, C.M.; Ferreira, G.A.; Keyvan, K.; Fausto, R. Photochemical Generation and Characterization of C-Aminophenyl-Nitrilimines: Insights on Their Bond-Shift Isomers by Matrix-Isolation IR Spectroscopy and Density Functional Theory Calculations. Molecules 2024, 29, 3497. [Google Scholar] [CrossRef]
- Ferreira, G.A.; Nunes, C.M.; Lopes Jesus, A.J.; Fausto, R. The meta and para OH Substitution Effect on C-Phenyl-Nitrilimine Bond-Shift Isomers. Eur. J. Org. Chem. 2023, 26, e202300310. [Google Scholar] [CrossRef]
- Reva, I.; Lapinski, L.; Fausto, R. Infrared spectra of methyl isocyanate isolated in Ar, Xe and N2 matrices. J. Mol. Struct. 2010, 976, 333–341. [Google Scholar] [CrossRef]
- Pritchina, E.A.; Gritsan, N.P.; Maltsev, A.; Bally, T.; Autrey, T.; Liu, Y.; Wang, Y.; Toscano, J.P. Matrix isolation, time-resolved IR, and computational study of the photochemistry of benzoyl azide. Phys. Chem. Chem. Phys. 2003, 5, 1010–1018. [Google Scholar] [CrossRef]
- Gómez-Zavaglia, A.; Reva, I.D.; Frija, L.; Cristiano, M.L.; Fausto, R. Photochemistry of 1-phenyl-tetrazolone isolated in solid argon. J. Photochem. Photobiol. A 2006, 179, 243–255. [Google Scholar] [CrossRef]
- Nunes, C.M.; Reva, I.; Rosado, M.T.S.; Fausto, R. The Quest for Carbenic Nitrile Imines: Experimental and Computational Characterization of C-Amino Nitrile Imine. Eur. J. Org. Chem. 2015, 2015, 7484–7493. [Google Scholar] [CrossRef]
- Bégué, D.; Wentrup, C. Carbenic Nitrile Imines: Properties and Reactivity. J. Org. Chem. 2014, 79, 1418–1426. [Google Scholar] [CrossRef]
- Bertrand, G.; Wentrup, C. Nitrile Imines: From Matrix Characterization to Stable Compounds. Angew. Chem., Int. Ed. Engl. 1994, 33, 527–545. [Google Scholar] [CrossRef]
- Bégué, D.; Qiao, G.G.; Wentrup, C. Nitrile Imines: Matrix Isolation, IR Spectra, Structures, and Rearrangement to Carbodiimides. J. Am. Chem. Soc. 2012, 134, 5339–5350. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.W.; Wentrup, C. Structure of nitrilimine: Allenic or propargylic? J. Am. Chem. Soc. 1993, 115, 7743–7746. [Google Scholar] [CrossRef]
- Nunes, C.M.; Reva, I.; Fausto, R.; Bégué, D.; Wentrup, C. Bond-shift isomers: The co-existence of allenic and propargylic phenylnitrile imines. Chem. Commun. 2015, 51, 14712–14715. [Google Scholar] [CrossRef]
- Bégué, D.; Santos-Silva, H.; Dargelos, A.; Wentrup, C. Imidoylnitrenes R′C(=NR)–N, Nitrile Imines, 1H-Diazirines, and Carbodiimides: Interconversions and Rearrangements, Structures, and Energies at DFT and CASPT2 Levels of Theory. J. Phys. Chem. A 2017, 121, 8227–8235. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, Q.; Feng, R.; Zeng, X.; Wentrup, C. Photolysis and Pyrolysis of Phenyltetrazoles: Formation of Phenylcarbodiimide, N-Phenylnitrile Imine, Phenylnitrene, Indazole, and Fulvenallene. Eur. J. Org. Chem. 2019, 2019, 6945–6950. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 09; Revision A.02; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16; Revision B.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Vosko, S.H.; Wilk, L.; Nusair, M. Accurate spin-dependent electron liquid correlation energies for local spin density calculations: A critical analysis. Can. J. Phys. 1980, 58, 1200–1211. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785. [Google Scholar] [CrossRef]
- Becke, A.D. A new mixing of Hartree–Fock and local density-functional theories. J. Chem. Phys. 1993, 98, 1372. [Google Scholar] [CrossRef]
- Møller, C.; Plesset, M.S. Note on an Approximation Treatment for Many-Electron Systems. Phys. Rev. 1934, 46, 618–622. [Google Scholar] [CrossRef]
- Merrick, J.P.; Moran, D.; Radom, L. An Evaluation of Harmonic Vibrational Frequency Scale Factors. J. Phys. Chem. A 2007, 111, 11683–11700. [Google Scholar] [CrossRef]
- Lopes Jesus, A.J.; Reva, I.; Araujo-Andrade, C.; Fausto, R. Conformational Switching by Vibrational Excitation of a Remote NH Bond. J. Am. Chem. Soc. 2015, 137, 14240–14243. [Google Scholar] [CrossRef] [PubMed]
- Lopes Jesus, A.J.; Nunes, C.M.; Reva, I. Conformational Structure, Infrared Spectra and Light-Induced Transformations of Thymol Isolated in Noble Gas Cryomatrices. Photochem 2022, 2, 405–422. [Google Scholar] [CrossRef]
- Lopes Jesus, A.J.; Fausto, R.; Reva, I. Conformational Space, IR-Induced, and UV-Induced Chemistry of Carvacrol Isolated in a Low-Temperature Argon Matrix. J. Phys. Chem. A 2021, 125, 8215–8229. [Google Scholar] [CrossRef]
- Teixeira, F.; Cordeiro, M.N.D.S. Improving Vibrational Mode Interpretation Using Bayesian Regression. J. Chem. Theory Comput. 2019, 15, 456–470. [Google Scholar] [CrossRef]
- Zhurko, G.A.C. Version 1.8. 2016. Available online: http://www.chemcraftprog.com (accessed on 15 May 2025).
- Runge, E.; Gross, E.K.U. Density-Functional Theory for Time-Dependent Systems. Phys. Rev. Lett. 1984, 52, 997–1000. [Google Scholar] [CrossRef]
- Yanai, T.; Tew, D.P.; Handy, N.C. A new hybrid exchange–correlation functional using the Coulomb-attenuating method (CAM-B3LYP). Chem. Phys. Lett. 2004, 393, 51–57. [Google Scholar] [CrossRef]
- Peach, M.J.G.; Benfield, P.; Helgaker, T.; Tozer, D.J. Excitation energies in density functional theory: An evaluation and a diagnostic test. J. Chem. Phys. 2008, 128, 044118. [Google Scholar] [CrossRef] [PubMed]
- Grabarek, D.; Andruniów, T. Assessment of Functionals for TDDFT Calculations of One- and Two-Photon Absorption Properties of Neutral and Anionic Fluorescent Proteins Chromophores. J. Chem. Theory Comput. 2019, 15, 490–508. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Mei, Y.; Sundholm, D.; Kaila, V.R.I. Benchmarking the Performance of Time-Dependent Density Functional Theory Methods on Biochromophores. J. Chem. Theory Comput. 2020, 16, 587–600. [Google Scholar] [CrossRef] [PubMed]

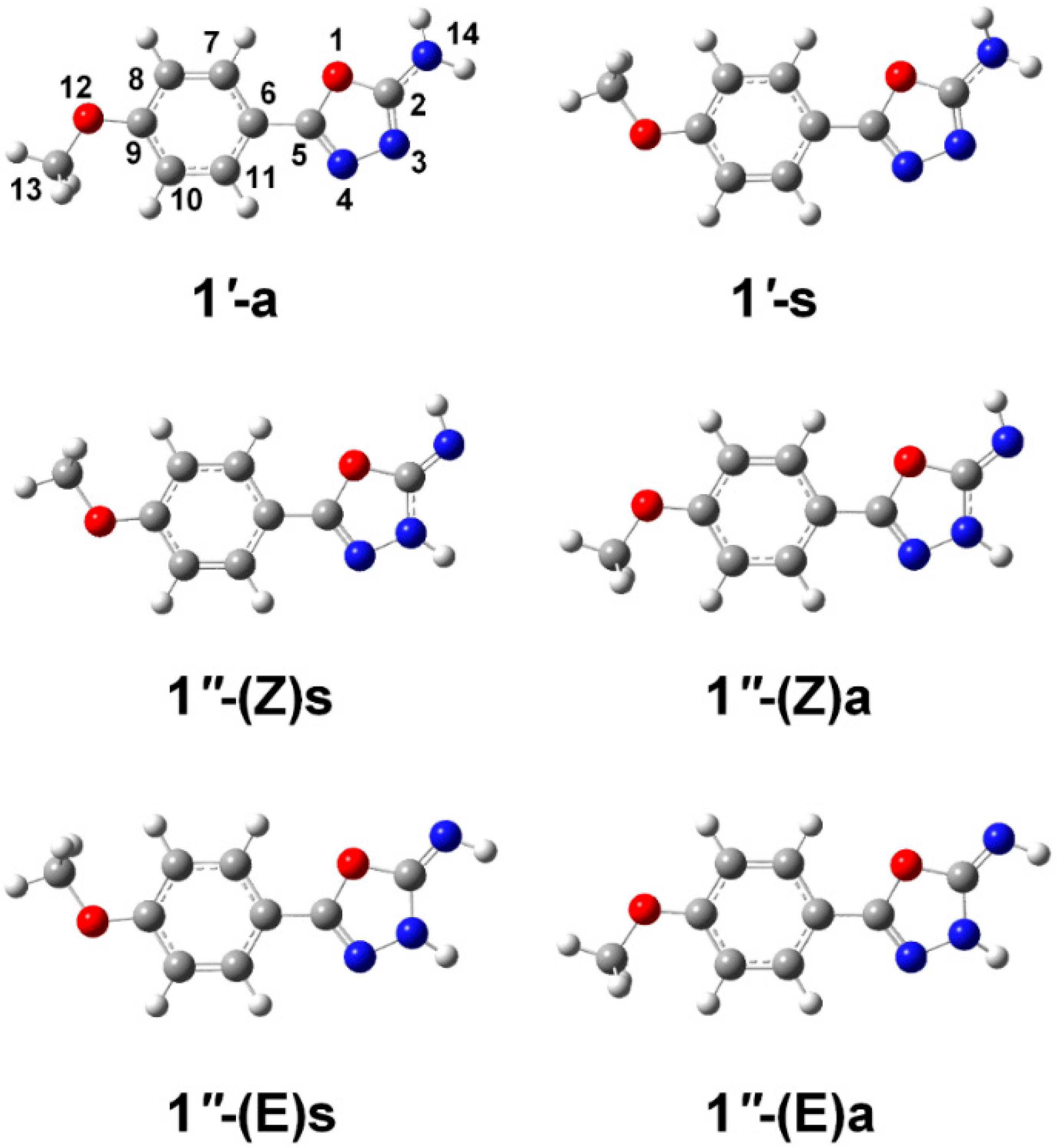
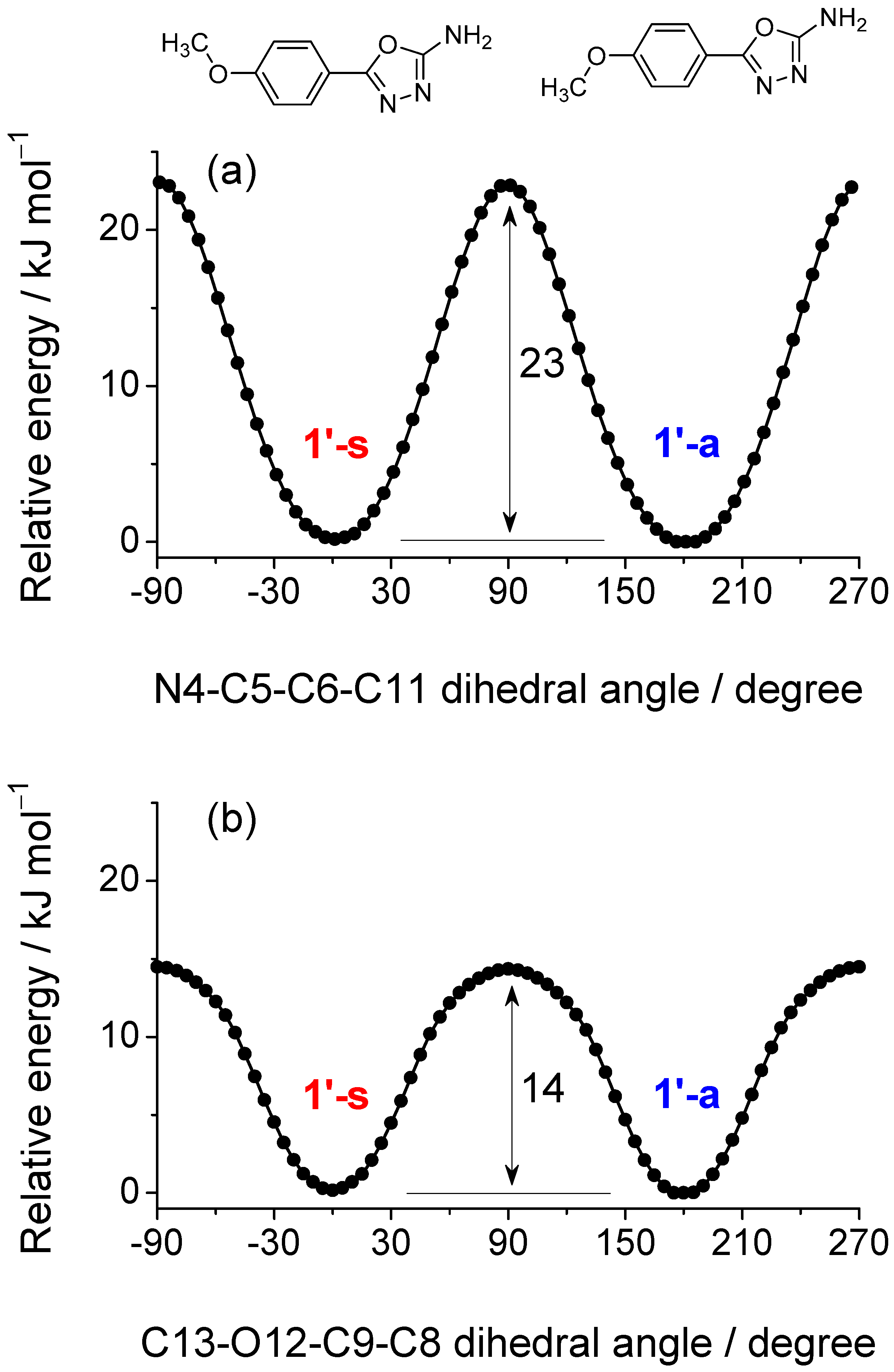
| Isomer | ΔEel | ΔEZPVE | ΔG0 | Pop (%) | μ (Debye) |
|---|---|---|---|---|---|
| B3LYP/6-311++G(d,p) | |||||
| 1′a | 0.0 | 0.0 | 0.0 | 53.44 | 3.13 |
| 1′s | 0.2 | 0.2 | 0.4 | 46.22 | 5.39 |
| 1″-(Z)s | 17.4 | 16.0 | 14.0 | 0.15 | 4.30 |
| 1″-(Z)a | 17.8 | 16.4 | 14.5 | 0.19 | 3.94 |
| 1″-(E)s | 27.7 | 27.0 | 26.9 | 0.00 | 2.34 |
| 1″-(E)a | 28.5 | 27.7 | 27.4 | 0.00 | 4.36 |
| B3LYP/aug-cc-pVTZ | |||||
| 1′-a | 0.0 | 0.0 | 0.0 | 52.5 | 3.17 |
| 1′-s | 0.1 | 0.2 | 0.3 | 47.5 | 5.25 |
| MP2/6-311++G(d,p) | |||||
| 1′-a | 0.0 | 0.4 | 0.5 | 45.5 | 3.28 |
| 1′-s | 0.2 | 0.0 | 0.0 | 54.5 | 5.45 |
| Exp. (Ar, 15 K) a | Exp. (Xe, 15K) a | Calc. 1′-a | Calc. 1′-s | Approximate Assignments c | ||
|---|---|---|---|---|---|---|
| νExp b | νExp b | νCalc. | Ath | νCalc. | Ath | |
| 3521 | 3507 | 3533 | 52.1 | 3532 | 51.9 | ν(NH2)as |
| 3426 | 3411 | 3432 | 68.1 | 3432 | 66.9 | ν(NH2)s |
| 1645 | 1642 | 1644 | 454.0 | 1644 | 435.0 | ν(C2=N3) − ν(C2=C14) |
| 1624/1620 | 1619 | 1619 | 158.9 | 1620 | 182.7 | ν(CC)ph |
| 1593/1590 | 1596/1587 | 1591 | 27.2 | 1591 | 5.1 | ν(C5=N4); ν(CC)ph; δ(NH2) |
| 1572 | 1569 | 1570 | 54.6 | 1571 | 28.4 | ν(C2=N3); δ(NH2) |
| 1566 | 1563 | 1564 | 7.7 | 1561 | 30.5 | ν(C5=N4) − ν(C5−C6) + ν(C2=N3); ν(CC)ph |
| 1505 | 1504 | 1503 | 214.4 | 1503 | 233.1 | δ(CH)ph; ν(C5=N4); ν(C9−O12); ν(CC)ph |
| 1468 | 1463 | 1474 | 60.8 | 1474 | 44.1 | δ(CH3)as |
| 1460 | 1455 | 1463 | 9.6 | 1463 | 9.6 | δ(CH3)as′ |
| 1445/1443 | 1441 | 1447 | 14.5 | 1446 | 14.3 | δ(CH3)s |
| 1424 | 1420 | 1424 | 0.8 | 1429 | 8.7 | ν(C2−O1) + ν(C5−O1); ν(CC)ph; δ(CH)ph |
| 1410/1404 (sh) | 1408 | 1404 | 12.2 | 1402 | 40.6 | ν(C2−O1) + ν(C5−O1) − ν(C2−N3) − ν(C2−N14) |
| 1316/1310 | 1315/1311 | 1314 | 96.7 | 1315 | 43.6 | ν(CC)ph |
| 1305 | 1307 | 1307 | 3.7 | 1306 | 11.2 | δ(CH) ph |
| 1289 | 1289 | 1275 | 27.9 | 1279 | 73.5 | ν(C5-O1) − ν(C5−C6) + ν(C5−N4); δ(CH)ph |
| 1261/1256 (sh) | 1258 | 1256 | 328.8 | 1253 | 289.8 | ν(C9−O12) |
| 1185 | 1183 | 1178 | 7.2 | 1178 | 11.7 | ρ(CH3) |
| 1175 | 1174 | 1172 | 86.8 | 1173 | 77.4 | δ(CH)Ph |
| n.o. | n.o. | 1144 | 0.7 | 1144 | 0.6 | ρ(CH3)′ |
| 1116 | 1115 | 1116 | 2.2 | 1116 | 8.2 | ν(C2=N3) − ν(C2−N14); ρ(NH2); δ(CH)ph |
| 1114 | 1112 | 1113 | 10.2 | 1113 | 4.4 | δ(CH)ph; ρ(NH2) |
| 1068 | 1066 | 1054 | 4.5 | 1053 | 37.3 | ν(C5−O1) − ν(C13−O12) |
| 1045/1038 | 1042/1035 | 1035 | 54.9 | 1033 | 16.4 | ν(C13−O12) − ν(N3−N4) |
| 1026/1016/1011 | 1026/1016/1009 | 1020 | 84.1 | 1021 | 89.5 | ν(N3−N4) − ν(C2−O1) |
| 1000 | 1000 | 1001 | 11.2 | 1001 | 13.0 | δ(CCC)ph |
| 967 | 965 | 969 | 4.6 | 969 | 2.4 | ν(C2−O1) + ν(N3−N4) |
| n.o. | n.o. | 959 | 0.2 | 968 | < 0.1 | γ(C)ph; γ(CH)ph |
| 951 | 950 | 945 | 16.1 | 945 | 19.9 | δ(C5N4N3); ν(C5-O1) |
| n.o. | n.o. | 943 | 0.4 | 930 | 0.5 | γ(C)ph; γ(CH)ph |
| 839/835 | 836/833 | 832 | 51.7 | 837 | 44.8 | γ(C)ph; γ(CH)ph |
| 809 | 808 | 808 | 1.8 | 803 | 8.5 | γ(C)ph; γ(CH)ph |
| 802 | 800 | 798 | 5.6 | 798 | 4.1 | δ(CCC)ph; ν(CC)ph; δ(C9-O12) |
| 739 | 737 | 743 | 35.7 | 743 | 38.9 | γ(C)ox |
| n.o. | n.o. | 731 | 3.2 | 731 | 3.9 | δ(O1-C2-N3); ν(C2-N14) |
| 721 | 720 | 710 | 11.7 | 710 | 11.8 | γ(C)ph; γ(C)ox |
| 686 | 684 | 670 | 1.5 | 670 | 1.3 | γ(N)ox; γ(C)ox |
| 637/635 | 637/635 | 635 | 1.8 | 637 | 4.9 | δ(CCC)ph |
| 611 | 611 | 605 | 30.3 | 607 | 24.9 | δ(CCC)ph; ν(C9−O12) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sıdır, İ.; Lopes, S.; Fausto, R.; Jesus, A.J.L. Molecular Structure, Matrix-Isolation IR Spectrum and UV-Induced Transformations of 2-Amino-5-(4-Methoxyphenyl)-1,3,4-Oxadiazole. Molecules 2025, 30, 3444. https://doi.org/10.3390/molecules30163444
Sıdır İ, Lopes S, Fausto R, Jesus AJL. Molecular Structure, Matrix-Isolation IR Spectrum and UV-Induced Transformations of 2-Amino-5-(4-Methoxyphenyl)-1,3,4-Oxadiazole. Molecules. 2025; 30(16):3444. https://doi.org/10.3390/molecules30163444
Chicago/Turabian StyleSıdır, İsa, Susy Lopes, Rui Fausto, and A. J. Lopes Jesus. 2025. "Molecular Structure, Matrix-Isolation IR Spectrum and UV-Induced Transformations of 2-Amino-5-(4-Methoxyphenyl)-1,3,4-Oxadiazole" Molecules 30, no. 16: 3444. https://doi.org/10.3390/molecules30163444
APA StyleSıdır, İ., Lopes, S., Fausto, R., & Jesus, A. J. L. (2025). Molecular Structure, Matrix-Isolation IR Spectrum and UV-Induced Transformations of 2-Amino-5-(4-Methoxyphenyl)-1,3,4-Oxadiazole. Molecules, 30(16), 3444. https://doi.org/10.3390/molecules30163444








