Photochemically Assisted Synthesis of Thienobenzotriazole-Based Dual Cholinesterase Inhibitors
Abstract
1. Introduction
2. Results and Discussion
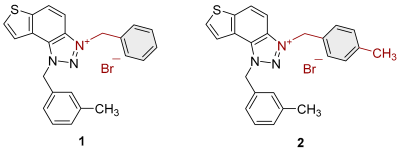
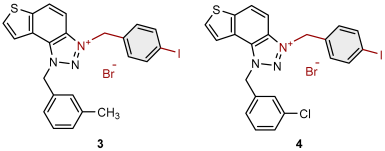
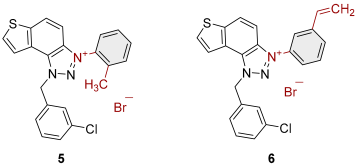
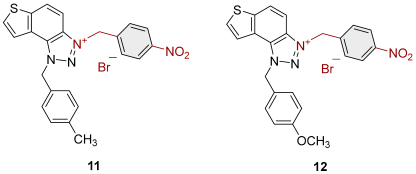
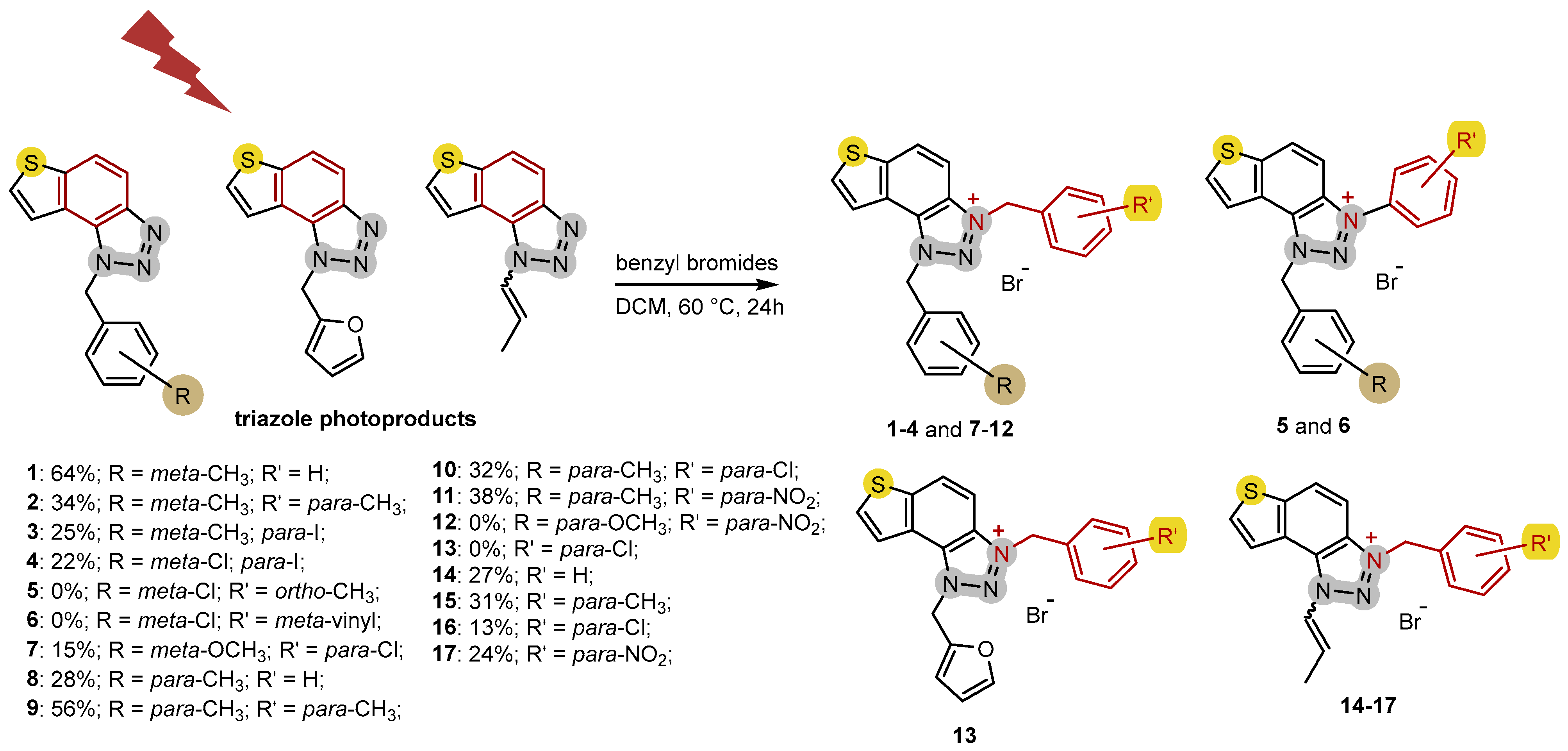
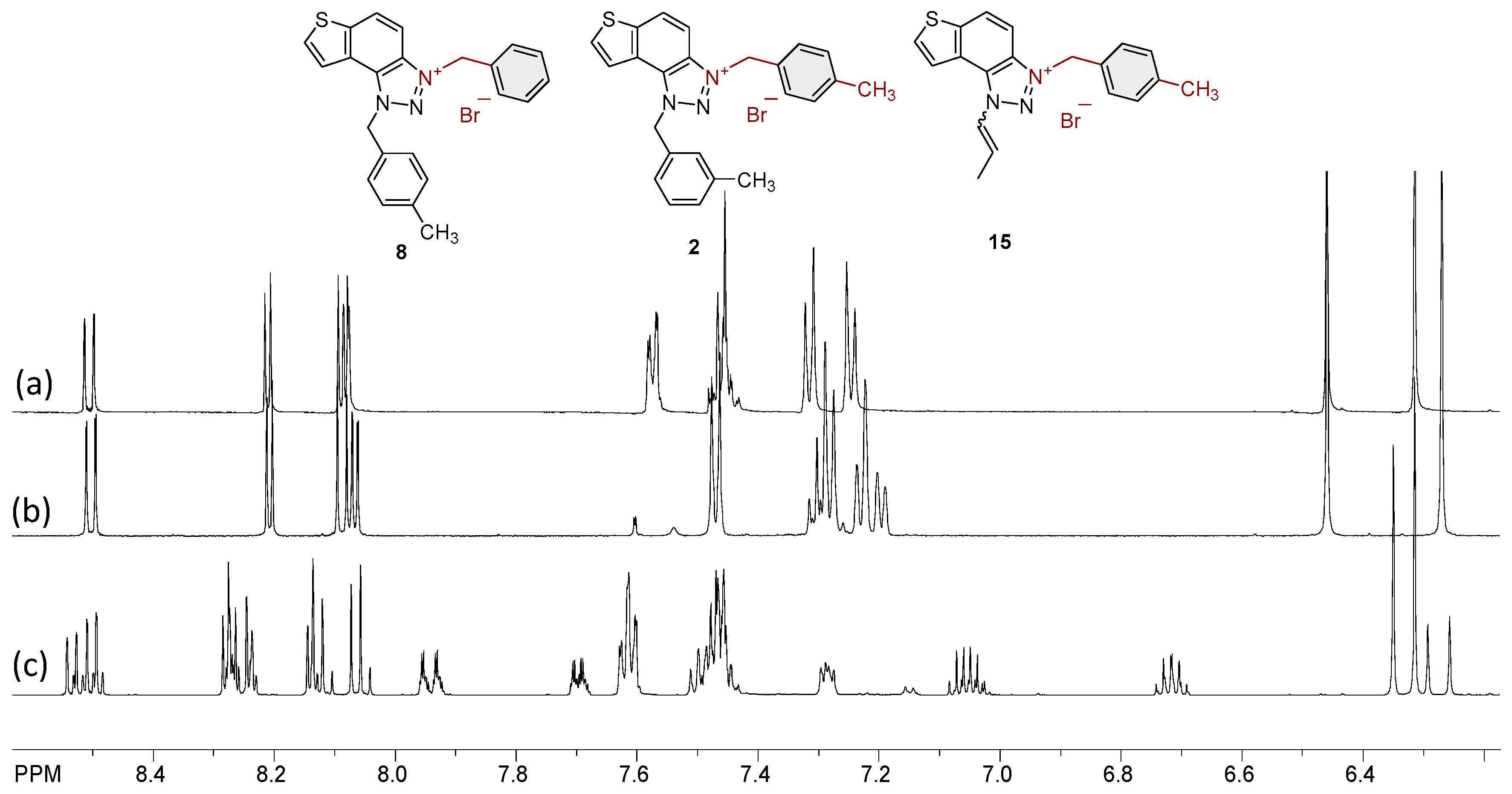
2.1. Photochemically Assisted Synthesis of Charged Thienobenzo-1,2,3-Triazoles 1–17
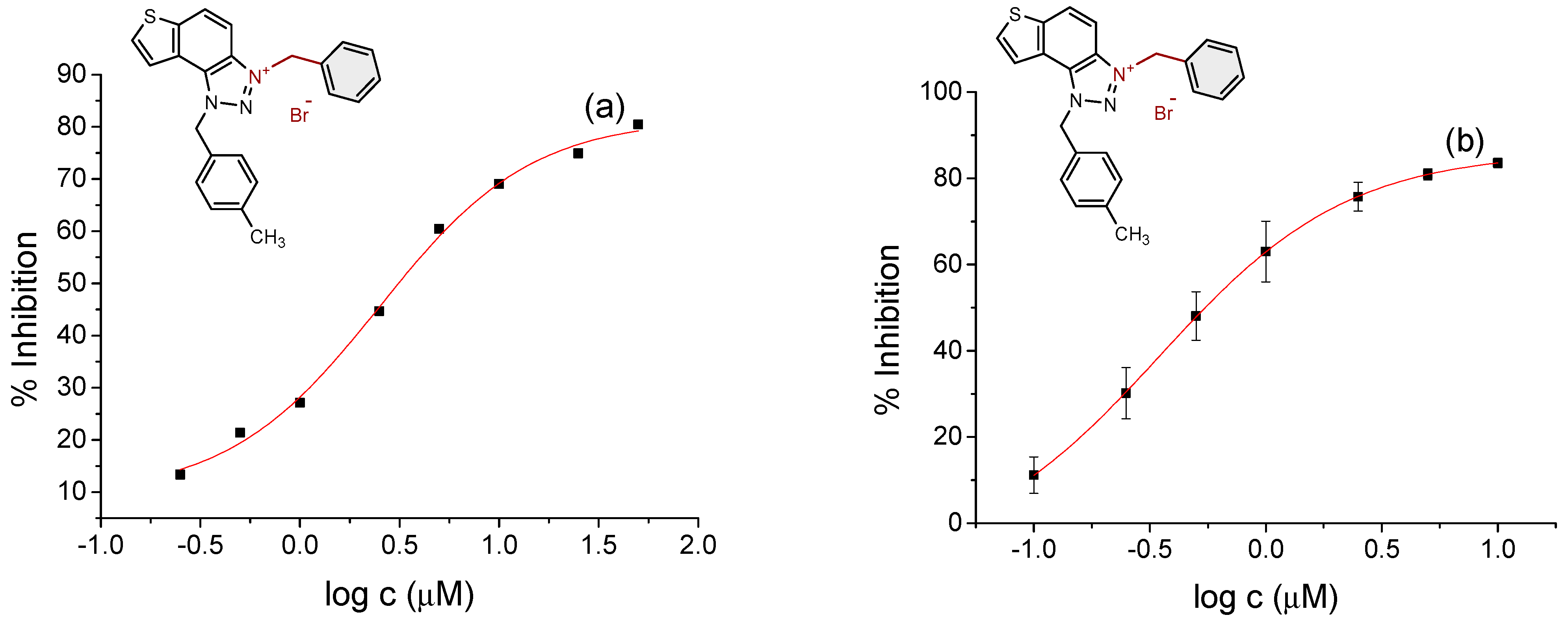
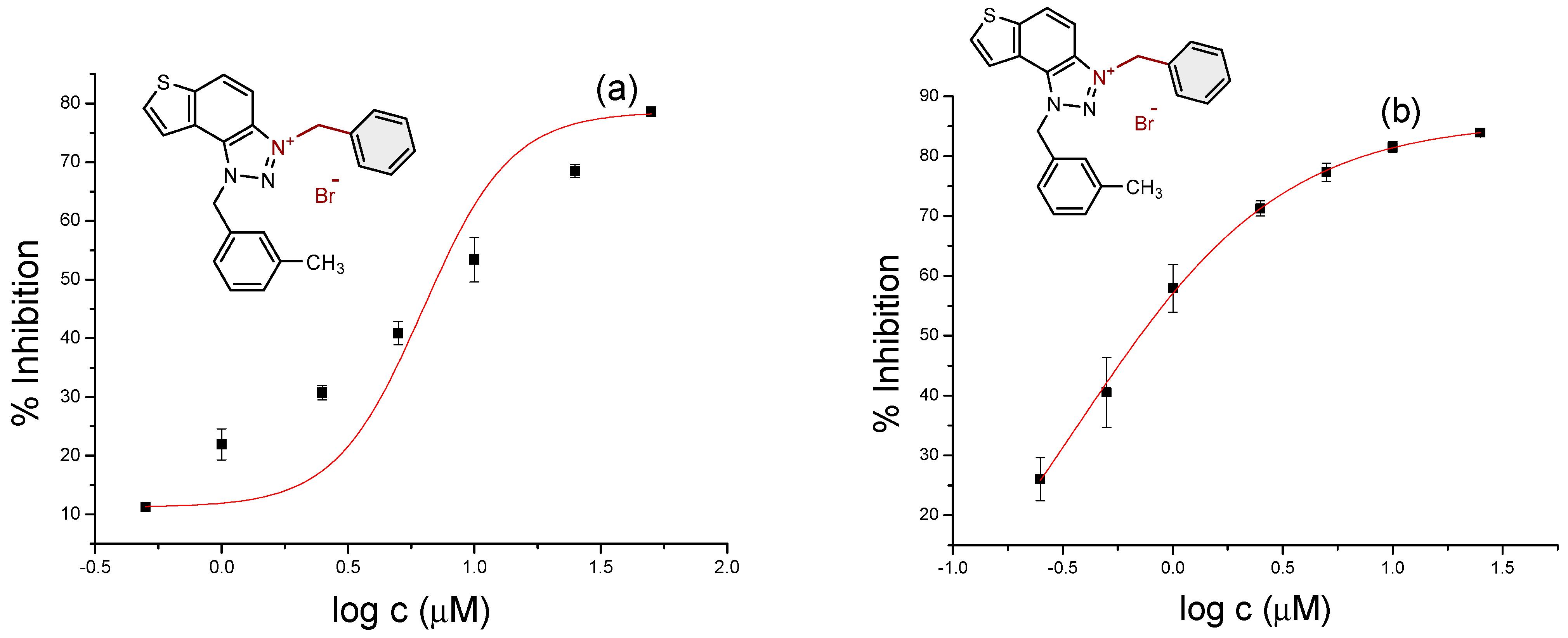
2.2. Cholinesterase Inhibition Activity of Triazolinium Salts 1–17
2.3. Anti-Inflammatory Activity of Triazolinium Salts 1–17
3. Materials and Methods
3.1. General Remarks
3.2. Synthesis of Bromide Salts 1–17



3.3. In Vitro Cholinesterase Inhibition Activity Measurements of Bromide Salts 1–17
3.4. Anti-Inflammatory Activity of 1–17
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pohanka, M. Inhibitors of Acetylcholinesterase and Butyrylcholinesterase Meet Immunity. Int. J. Mol. Sci. 2014, 15, 9809–9825. [Google Scholar] [CrossRef]
- Bajda, M.; Więckowska, A.; Hebda, M.; Guzior, N.; Sotriffer, C.A.; Malawska, B. Structure-Based Search for New Inhibitors of Cholinesterases. Int. J. Mol. Sci. 2013, 14, 5608–5632. [Google Scholar] [CrossRef]
- Trang, A.; Khandhar, P.B. Physiology, Acetylcholinesterase. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK539735/ (accessed on 11 June 2025).
- Masson, P.; Shaihutdinova, Z.; Lockridge, O. Drug and Pro-Drug Substrates and Pseudo-Substrates of Human Butyrylcholinesterase. Biochem. Pharmacol. 2023, 218, 115910. [Google Scholar] [CrossRef] [PubMed]
- Mesulam, M.M.; Guillozet, A.; Shaw, P.; Levey, A.; Duysen, E.G.; Lockridge, O. Acetylcholinesterase Knockouts establish Central Cholinergic Pathways and can use Butyrylcholinesterase to Hydrolyze Acetylcholine. Neuroscience 2002, 110, 627–639. [Google Scholar] [CrossRef] [PubMed]
- Guillozet, A.L.; Smiley, J.F.; Mash, D.C. Butyrylcholinesterase in the Life Cvcle of Amyloid Plaques. Ann. Neurol. 1997, 42, 909–918. [Google Scholar] [CrossRef] [PubMed]
- Mesulam, M.M.; Geula, C. Butyrylcholinesterase Reactivity Dfierentiates the Amyloid Plaques of Aging from Those of Dementia. Ann. Neurol. 1994, 36, 722–727. [Google Scholar] [CrossRef]
- Darvesh, S.; Walsh, R.; Kumar, R.; Caines, A.; Roberts, S.; Magee, D.; Rockwood, K.; Martin, E. Inhibition of Human Cholinesterases by Drugs Used to Treat Alzheimer Disease. Alzheimer Dis. Assoc. Disord. 2003, 17, 117–126. [Google Scholar] [CrossRef]
- Cacabelos, R.; Martínez-Iglesias, O.; Cacabelos, N.; Carrera, I.; Corzo, L.; Naidoo, V. Therapeutic Options in Alzheimer’s Disease: From Classic Acetylcholinesterase Inhibitors to Multi-Target Drugs with Pleiotropic Activity. Life 2024, 14, 1555. [Google Scholar] [CrossRef]
- Anand, P.; Singh, B. A Review on Cholinesterase Inhibitors for Alzheimer’s Disease. Arch. Pharmacal Res. 2013, 36, 375–399. [Google Scholar] [CrossRef]
- Talesa, V.N. Acetylcholinesterase in Alzheimer’s disease. Curr. Drug Targets 2001, 2, 363–373. [Google Scholar] [CrossRef]
- Greig, N.H.; Utsuki, T.; Yu, Q.S.; Holloway, H.W. A New Therapeutic Target in Alzheimer’s Disease Treatment: Selective Butyrylcholinesterase Inhibition. Curr. Med. Chem. 2005, 12, 237–243. [Google Scholar]
- Mesulam, M.M.; Guillozet, A.; Shaw, P.; Levey, A. Acetylcholinesterase knockouts establish centrality of cholinergic networks. Ann. Neurol. 2002, 52, 253–256. [Google Scholar]
- Lane, R.M.; Potkin, S.G.; Enz, A. Targeting Acetylcholinesterase and Butyrylcholinesterase in Dementia. Int. J. Neuropsychopharmacol. 2006, 1, 101–124. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Kaur, M.; Kukreja, H.; Chugh, R.; Silakari, O.; Singh, D. Acetylcholinesterase Inhibitors as Alzheimer therapy: From Nerve Toxins to Neuroprotection. Eur. J. Med. Chem. 2013, 70, 165–188. [Google Scholar] [CrossRef]
- Roth, B.L.; Driscol, J.; Glennon, R.A. Drugs with Anticholinergic Properties: Functional Roles and Side Effects. Pharmacol. Rev. 2002, 54, 364–385. [Google Scholar]
- Bosak, A.; Ramić, A.; Šmidlehner, T.; Hrenar, T.; Primožič, I.; Kovarik, Z. Design and Evaluation of Selective Butyrylcholinesterase Inhibitors based on Cinchona Alkaloid Scaffold. PLoS ONE 2018, 13, e0205193. [Google Scholar] [CrossRef]
- Bosak, A.; Opsenica, D.M.; Šinko, G.; Zlatar, M.; Kovarik, Z. Structural Aspects of 4-Aminoquinolines as Reversible Inhibitors of Human Acetylcholinesterase and Butyrylcholinesterase. Chem. Biol. Interact. 2019, 308, 101–109. [Google Scholar] [CrossRef]
- Mamedova, G.; Mahmudova, A.; Mamedov, S.; Erden, Y.; Taslimi, P.; Tüzün, B.; Tas, R.; Farzaliyev, V.; Sujayev, A.; Alwasel, S.H.; et al. Novel Tribenzylaminobenzolsulphonylimine based on their Pyrazine and Pyridazines: Synthesis, Characterization, Antidiabetic, Anticancer, Anticholinergic, and Molecular Docking Studies. Bioorg. Chem. 2019, 93, 103313. [Google Scholar] [CrossRef]
- El-Sayed, N.A.E.; El-Said Farag, A.; Ezzat, M.A.F.; Akincioglu, H.; Gulçin, I.; Abou-Seri, S.M. Design, Synthesis, In Vitro and In Vivo Evaluation of Novel Pyrrolizine-based Compounds with Potential Activity as Cholinesterase Inhibitors and Anti-Alzheimer’s Agents. Bioorg. Chem. 2019, 93, 103312. [Google Scholar] [CrossRef]
- Taslimi, P.; Türkan, F.; Cetin, A.; Burhan, H.; Karaman, M.; Bildirici, I.; Gulçin, I.; Şen, F. Pyrazole[3,4-d]pyridazine Derivatives: Molecular docking and Explore of Acetylcholinesterase and Carbonic Anhydrase Enzymes Inhibitors as Anticholinergics Potentials. Bioorg. Chem. 2019, 92, 103213. [Google Scholar] [CrossRef]
- Katalinić, M.; Bosak, A.; Kovarik, Z. Flavonoids as Inhibitors of Human Butyrylcholinesterase Variants. Food Technol. Biotechnol. 2014, 52, 64–67. [Google Scholar]
- Mlakić, M.; Odak, I.; Faraho, I.; Talić, S.; Bosnar, M.; Lasić, K.; Barić, D.; Škorić, I. New Naphtho/thienobenzo-triazoles with Interconnected Anti-inflammatory and Cholinesterase Inhibitory Activity. Eur. J. Med. Chem. 2022, 241, 114616. [Google Scholar] [CrossRef] [PubMed]
- Mlakić, M.; Selec, I.; Ćaleta, I.; Odak, I.; Barić, D.; Ratković, A.; Molčanov, K.; Škorić, I. New Thienobenzo/Naphtho-Triazoles as Butyrylcholinesterase Inhibitors: Design, Synthesis and Computational Study. Int. J. Mol. Sci. 2023, 24, 5879. [Google Scholar] [CrossRef] [PubMed]
- Mlakić, M.; Faraho, I.; Odak, I.; Kovačević, B.; Raspudić, A.; Šagud, I.; Bosnar, M.; Škorić, I.; Barić, D. Cholinesterase Inhibitory and Anti-Inflammatory Activity of the Naphtho- and Thienobenzo-Triazole Photoproducts: Experimental and Computational Study. Int. J. Mol. Sci. 2023, 24, 14676. [Google Scholar] [CrossRef] [PubMed]
- Mlakić, M.; Faraho, I.; Odak, I.; Talić, S.; Vukovinski, A.; Raspudić, A.; Bosnar, M.; Zadravec, R.; Ratković, A.; Lasić, K.; et al. Synthesis, Photochemistry and Computational Study of novel 1,2,3-Triazole Heterostilbenes: Expressed Biological Activity of their Electrocyclization Photoproducts. Bioorg. Chem. 2022, 121, 105701. [Google Scholar] [CrossRef]
- Mlakić, M.; Barić, D.; Ratković, A.; Šagud, I.; Čipor, I.; Piantanida, I.; Odak, I.; Škorić, I. New Charged Cholinesterase Inhibitors: Design, Synthesis, and Characterization. Molecules 2024, 29, 1622. [Google Scholar] [CrossRef]
- Krátký, M.; Vinšová, J.; Buchta, V.; Stolaříková, J. Quaternary Ammonium-based Cholinesterase Inhibitors: Synthesis and Biological Evaluation. Bioorganic Med. Chem. Lett. 2016, 26, 1125–1130. [Google Scholar]
- Mlakić, M.; Sviben, M.; Ratković, A.; Raspudić, A.; Barić, D.; Šagud, I.; Lasić, Z.; Odak, I.; Škorić, I. Efficient Access to New Thienobenzo-1,2,3-Triazolium Salts as Preferred Dual Cholinesterase Inhibitors. Biomolecules 2024, 14, 1391. [Google Scholar] [CrossRef]
- Jelčić, A.; Raspudić, A.; Barić, D.; Ratković, A.; Šagud, I.; Pongrac, P.; Štefok, D.; Bosnar, M.; Roca, S.; Lasić, Z.; et al. Charged Thienobenzo-1,2,3-Triazoles as Especially Potent Non-Selective Cholinesterase Inhibitors: Design, Anti-Inflammatory Activity, and Computational Study. Pharmaceuticals 2025, 18, 1032. [Google Scholar] [CrossRef]
- Ratković, A.; Mlakić, M.; Dehaen, W.; Opsomer, T.; Barić, D.; Škorić, I. Synthesis and Photochemistry of novel 1,2,3-Triazole di-Heterostilbenes. An Experimental and Computational Study. Spectrochim. Acta. Part A Mol. Biomol. Spectrosc. 2021, 261, 120056. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtnex, K.D.; Andres, V.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]



| Compound | Structure | AChE | BChE | ||
|---|---|---|---|---|---|
| IC50/µM | Inhibition * (%) | IC50/µM | Inhibition * (%) | ||
| 1 | 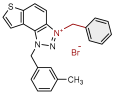 | 6.3 | 78.6 ± 0.5 (50) | 0.4 | 83.9 ± 0.2 (25) |
| 2 | 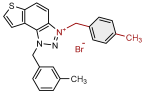 | 4.1 | 82.1 ± 4.2 (50) | 0.3 | 77.6 ± 0.3 (2.5) |
| 3 | 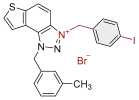 | 5.9 | 76.7 ± 0.5 (50) | 0.7 | 84.9 ± 3.6 (50) |
| 4 | 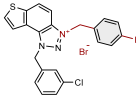 | 14.2 | 74.6 ± 5.0 (50) | 0.8 | 83.2 ± 0.9 (25) |
| 7 | 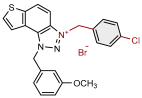 | 11.8 | 79.4 ± 4.9 (100) | 1.0 | 78.2 ± 0.4 (10) |
| 8 | 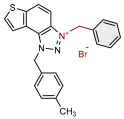 | 2.6 | 80.7 ± 2.3 (50) | 0.4 | 83.6 ± 1.0 (10) |
| 9 |  | 5.7 | 78.7 ± 0.6 (50) | 3.5 | 76.6 ± 0.4 (25) |
| 10 |  | 6.5 | 81.9 ± 0.6 (100) | 1.5 | 79.9 ± 1.1 (25) |
| 11 | 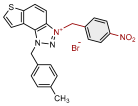 | 3.2 | 83.6 ± 2.1 (50) | 2.7 | 81.1 ± 1.7 (25) |
| 14 | 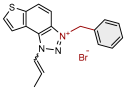 | 4.3 | 84.2 ± 0.5 (100) | 1.0 | 80.4 ± 2.9 (25) |
| 15 | 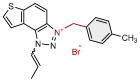 | 14.4 | 82.3 ± 0.8 (250) | 0.9 | 84.0 ± 0.7 (50) |
| 16 | 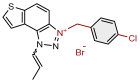 | 5.4 | 82.6 ± 1.1 (250) | 2.3 | 84.8 ± 0.8 (100) |
| 17 | 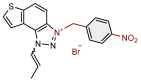 | 63.8 | 62.6 ± 5.3 (100) | 11.4 | 85.5 ± 0.5 (100) |
| Galantamine [29] | 0.15 | - | 7.9 | - | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jelčić, A.; Talić, S.; Odak, I.; Pongrac, P.; Štefok, D.; Škorić, I. Photochemically Assisted Synthesis of Thienobenzotriazole-Based Dual Cholinesterase Inhibitors. Molecules 2025, 30, 3439. https://doi.org/10.3390/molecules30163439
Jelčić A, Talić S, Odak I, Pongrac P, Štefok D, Škorić I. Photochemically Assisted Synthesis of Thienobenzotriazole-Based Dual Cholinesterase Inhibitors. Molecules. 2025; 30(16):3439. https://doi.org/10.3390/molecules30163439
Chicago/Turabian StyleJelčić, Antonija, Stanislava Talić, Ilijana Odak, Paula Pongrac, Dora Štefok, and Irena Škorić. 2025. "Photochemically Assisted Synthesis of Thienobenzotriazole-Based Dual Cholinesterase Inhibitors" Molecules 30, no. 16: 3439. https://doi.org/10.3390/molecules30163439
APA StyleJelčić, A., Talić, S., Odak, I., Pongrac, P., Štefok, D., & Škorić, I. (2025). Photochemically Assisted Synthesis of Thienobenzotriazole-Based Dual Cholinesterase Inhibitors. Molecules, 30(16), 3439. https://doi.org/10.3390/molecules30163439








