Investigation on Fuel Quality and Combustion Characteristics of Blended Fuel (Biomass and Lignite) Derived from Low-Temperature Co-Upgradation
Abstract
1. Introduction
2. Results and Discussion
2.1. Analysis of Raw Material Characteristics Before and After Co-Upgradation
2.1.1. Elemental and Industrial Analysis
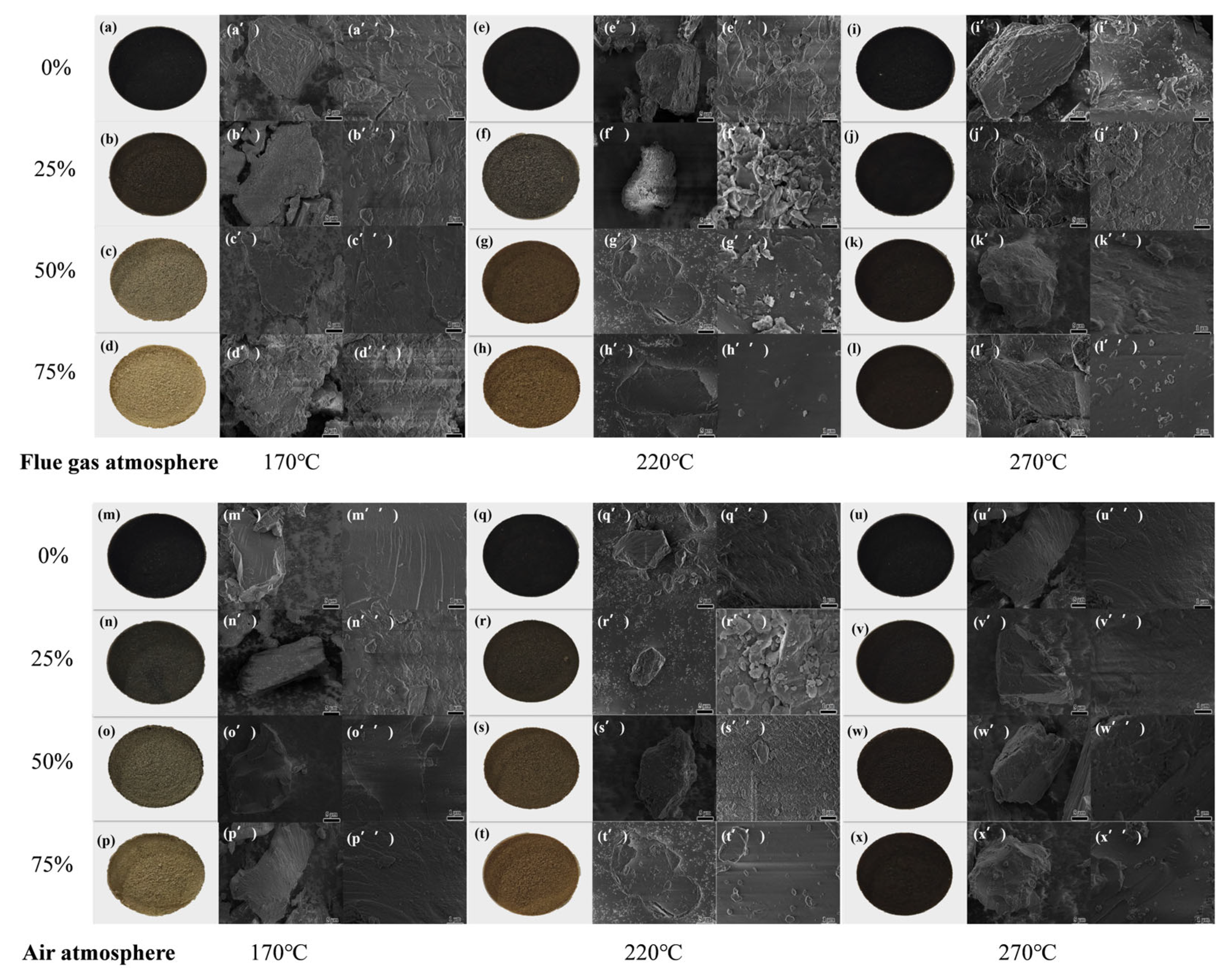
2.1.2. SEM
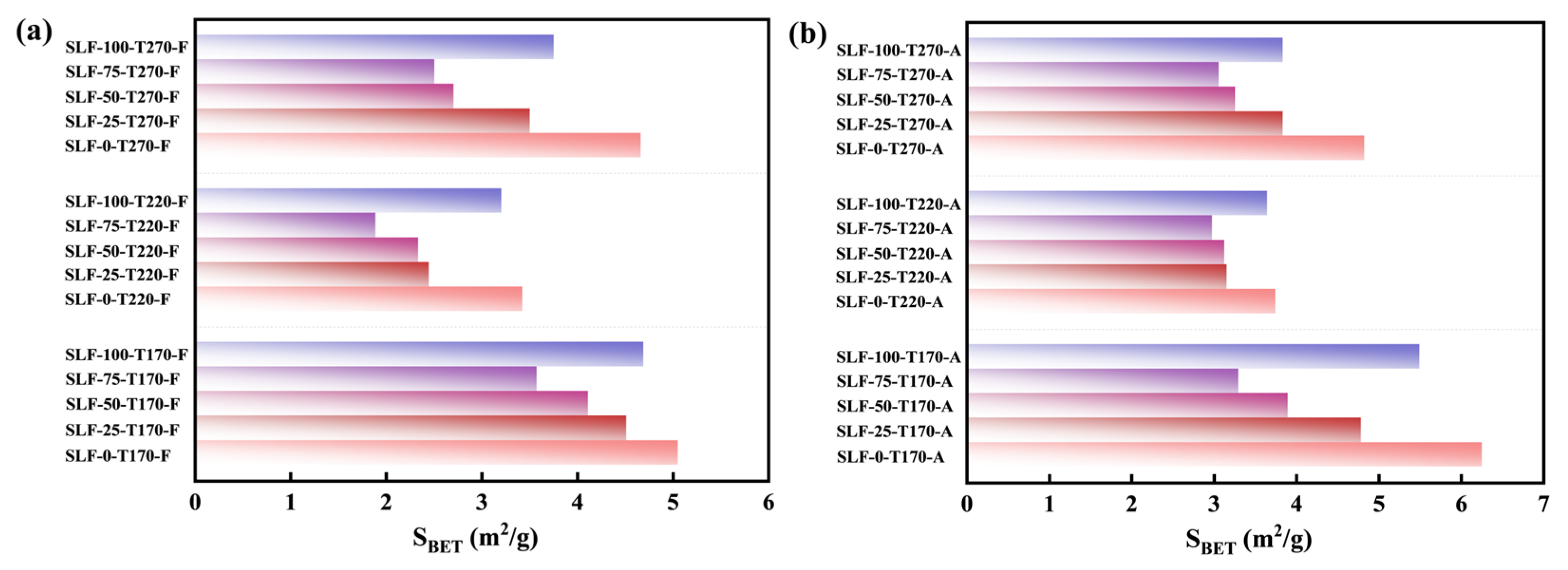
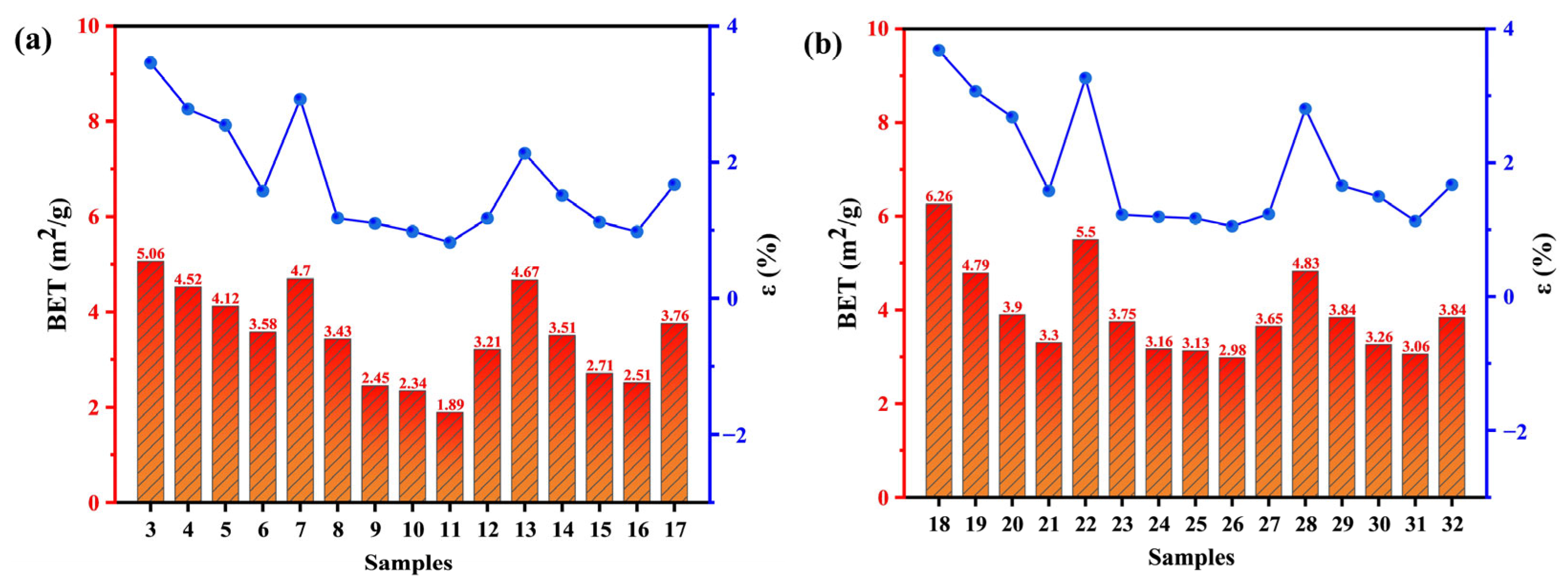
2.2. Hydrophilicity Analysis in Co-Upgraded Raw Materials
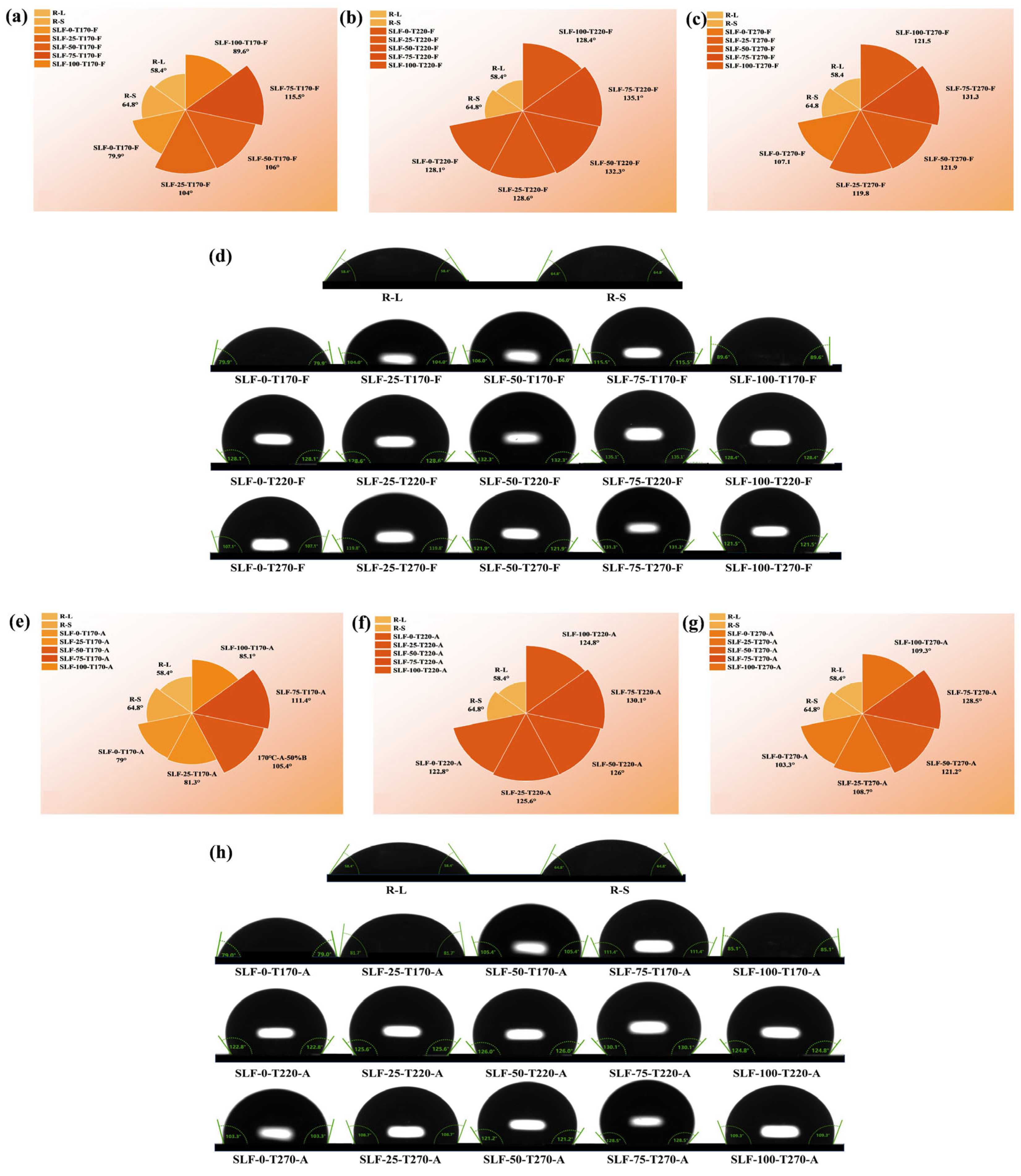
2.2.1. Contact Angle Characterization
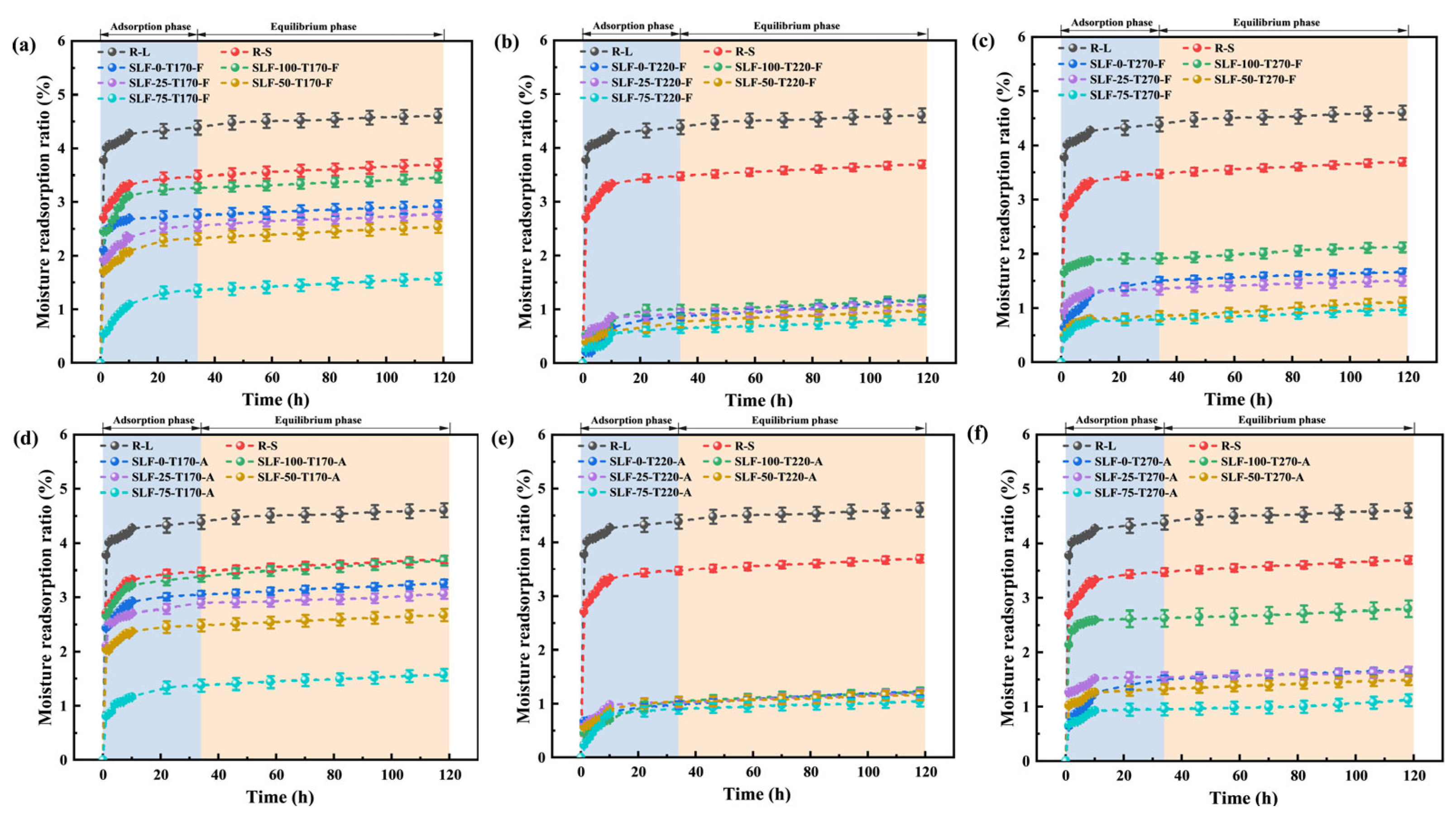
2.2.2. Water Reabsorption Experiment
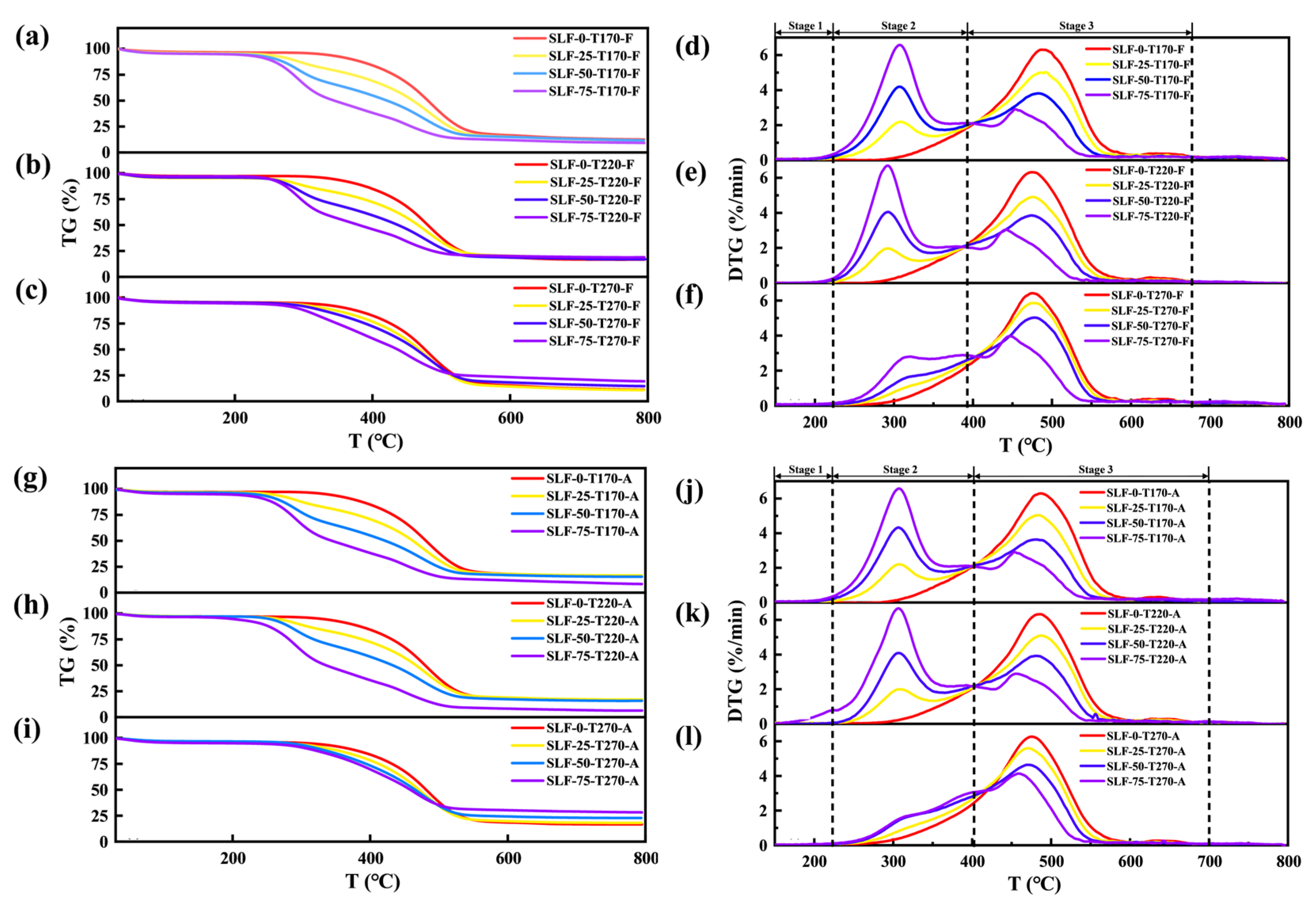
2.3. Analysis of Combustion Characteristics in Co-Upgraded Raw Materials
2.3.1. Co-Combustion Performance
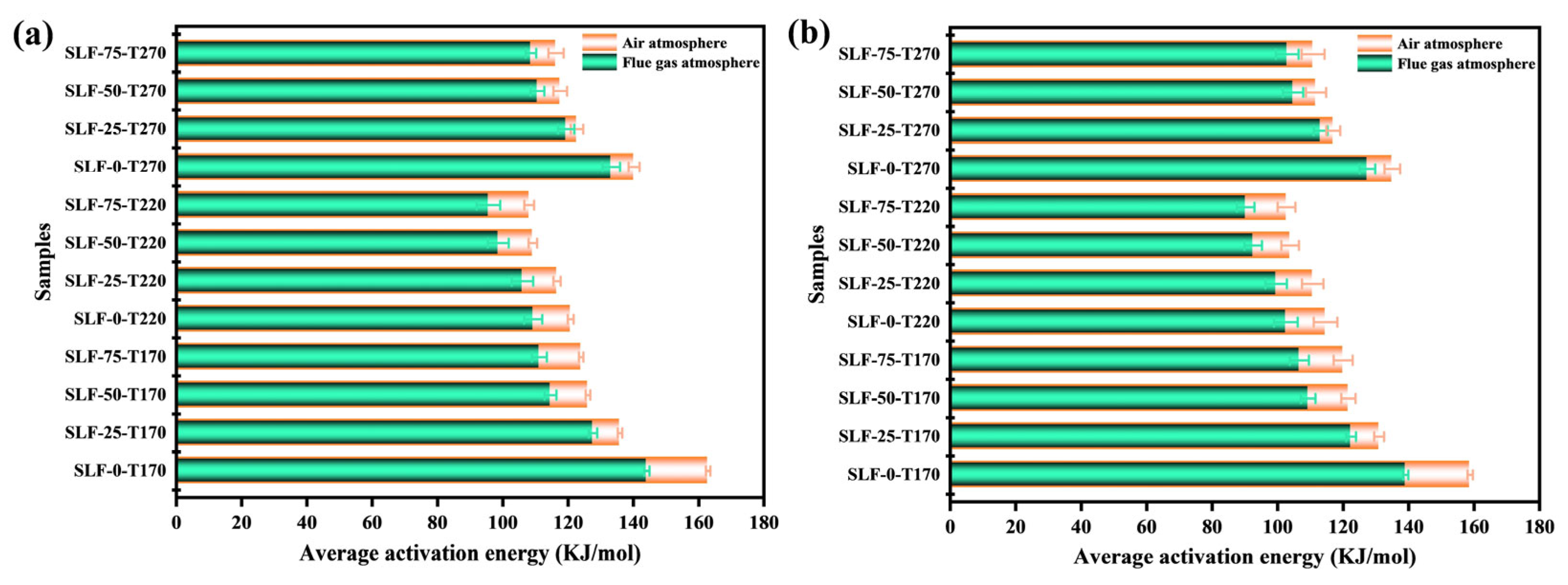
2.3.2. Combustion Kinetics
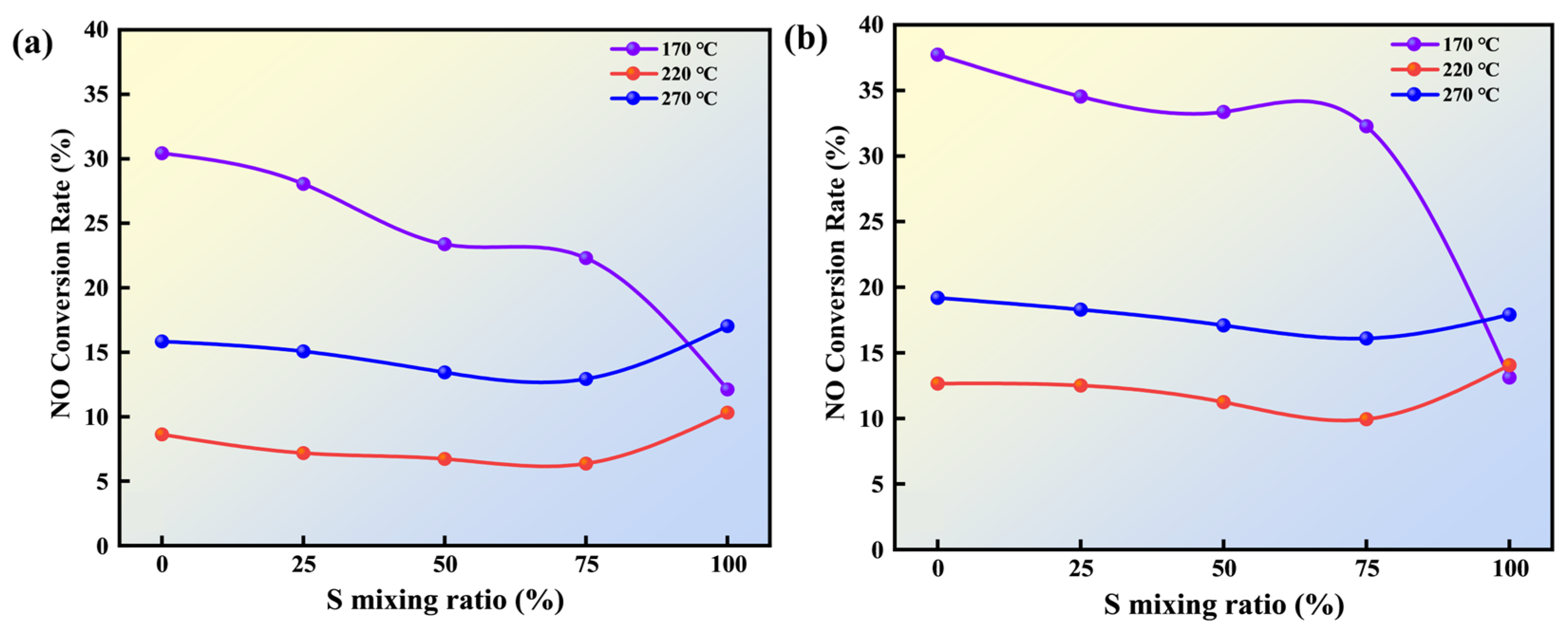
2.3.3. NO Release Analysis
2.4. Reactivity Characteristics of Pre-Oxidized Semi-Coke from Co-Upgraded Raw Materials
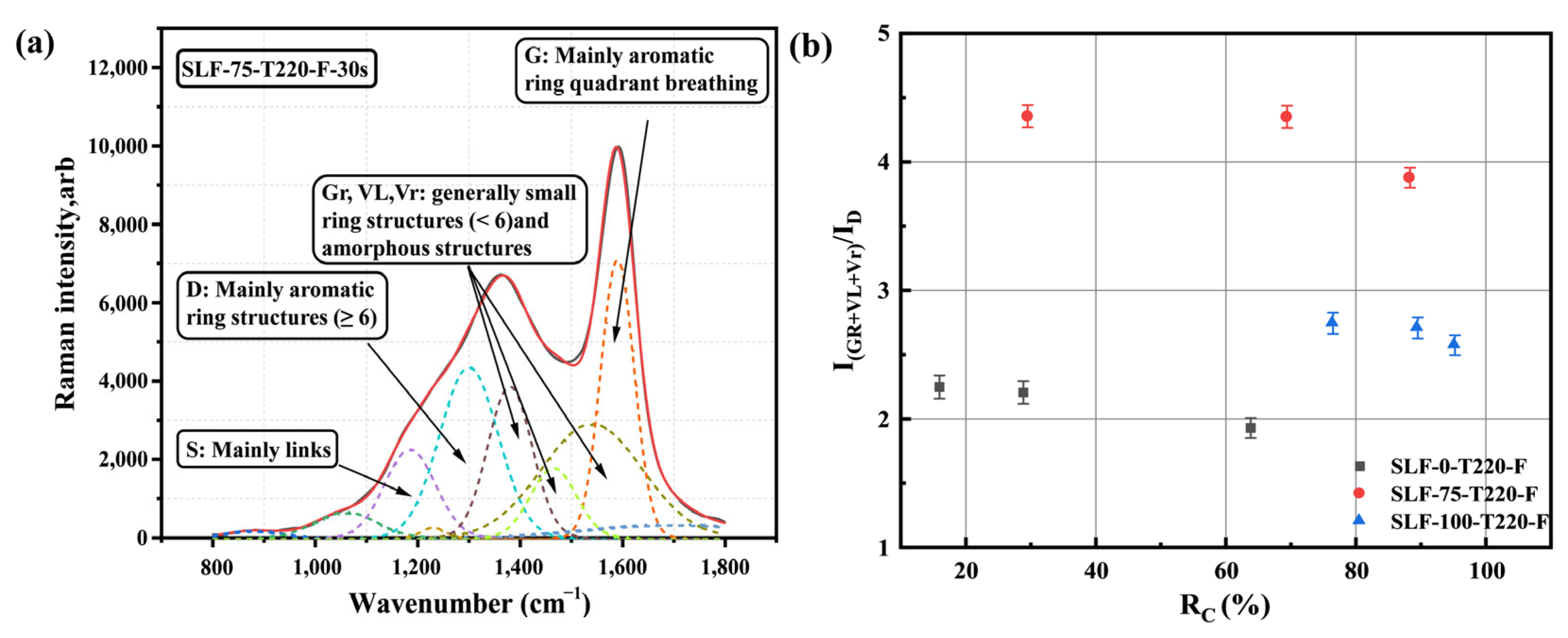
2.4.1. Raman Analysis
2.4.2. XPS Analysis
3. Experimental
3.1. Sample Preparation
3.2. Elemental Analysis and Industrial Analysis
3.3. Combustion Characteristic Determination
3.4. Kinetic Analysis
3.5. Physical Property Characterization
3.5.1. Pore Structure Characterization
3.5.2. Topography Characterization
3.6. Isothermal Combustion Experiment
3.7. Co-Upgraded Fuel Semi-Coke Raman Spectroscopy Analysis
3.8. Co-Upgraded Fuel Semi-Coke XPS Analysis
3.9. Hydrophilicity Analysis
3.9.1. Contact Angle Characterization
3.9.2. Moisture Re-Absorption Test
4. Conclusions
- (1)
- Under flue gas conditions, the calorific value of the co-upgraded samples exhibits significant enhancement. When the reaction temperature reaches 220 °C, the most pronounced improvement in fuel quality is observed, effectively enhancing the combustion reactivity of the modified fuel. However, further temperature elevation results in substantial depletion of combustible components with radical activity, consequently diminishing the combustion reactivity of the modified fuel.
- (2)
- Samples subjected to low-temperature treatments and high-ratio blending exhibit a diminished specific surface area and demonstrate a smoother surface morphology, thereby significantly weakening the moisture re-adsorption capacity of the modified fuels. Through the combined action of physical restructuring and chemical transformations, the hydrophobicity of the modified fuels achieves remarkable enhancement.
- (3)
- High-ratio biomass blending facilitates the in situ migration and adsorption of organic constituents onto lignite particle surfaces. These organic components undergo pyrolytic decomposition during combustion to generate reductive gaseous products, which actively participate in the homogeneous reduction reaction of NO, thereby effectively suppressing NO formation during the combustion process.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Nomenclature
| R-L | Untreated lignite |
| R-S | Untreated straw |
| SLF-0-T170-F | Upgraded fuel (straw 0%) obtained from flue gas atmosphere at 170 °C |
| SLF-25-T170-F | Upgraded fuel (straw 25%) obtained from flue gas atmosphere at 170 °C |
| SLF-50-T170-F | Upgraded fuel (straw 50%) obtained from flue gas atmosphere at 170 °C |
| SLF-75-T170-F | Upgraded fuel (straw 75%) obtained from flue gas atmosphere at 170 °C |
| SLF-100-T170-F | Upgraded fuel (straw 100%) obtained from flue gas atmosphere at 170 °C |
| SLF-0-T220-F | Upgraded fuel (straw 0%) obtained from flue gas atmosphere at 220 °C |
| SLF-25-T220-F | Upgraded fuel (straw 25%) obtained from flue gas atmosphere at 220 °C |
| SLF-50-T220-F | Upgraded fuel (straw 50%) obtained from flue gas atmosphere at 220 °C |
| SLF-75-T220-F | Upgraded fuel (straw 75%) obtained from flue gas atmosphere at 220 °C |
| SLF-100-T220-F | Upgraded fuel (straw 100%) obtained from flue gas atmosphere at 220 °C |
| SLF-0-T270-F | Upgraded fuel (straw 0%) obtained from flue gas atmosphere at 270 °C |
| SLF-25-T270-F | Upgraded fuel (straw 25%) obtained from flue gas atmosphere at 270 °C |
| SLF-50-T270-F | Upgraded fuel (straw 50%) obtained from flue gas atmosphere at 270 °C |
| SLF-75-T270-F | Upgraded fuel (straw 75%) obtained from flue gas atmosphere at 270 °C |
| SLF-100-T270-F | Upgraded fuel (straw 100%) obtained from flue gas atmosphere at 270 °C |
| SLF-0-T170-A | Upgraded fuel (straw 0%) obtained from air gas atmosphere at 170 °C |
| SLF-25-T170-A | Upgraded fuel (straw 25%) obtained from air gas atmosphere at 170 °C |
| SLF-50-T170-A | Upgraded fuel (straw 50%) obtained from air gas atmosphere at 170 °C |
| SLF-75-T170-A | Upgraded fuel (straw 75%) obtained from air gas atmosphere at 170 °C |
| SLF-100-T170-A | Upgraded fuel (straw 100%) obtained from air gas atmosphere at 170 °C |
| SLF-0-T220-A | Upgraded fuel (straw 0%) obtained from air gas atmosphere at 220 °C |
| SLF-25-T220-A | Upgraded fuel (straw 25%) obtained from air gas atmosphere at 220 °C |
| SLF-50-T220-A | Upgraded fuel (straw 50%) obtained from air gas atmosphere at 220 °C |
| SLF-75-T220-A | Upgraded fuel (straw 75%) obtained from air gas atmosphere at 220 °C |
| SLF-100-T220-A | Upgraded fuel (straw 100%) obtained from air gas atmosphere at 220 °C |
| SLF-0-T270-A | Upgraded fuel (straw 0%) obtained from air gas atmosphere at 270 °C |
| SLF-25-T270-A | Upgraded fuel (straw 25%) obtained from air gas atmosphere at 270 °C |
| SLF-50-T270-A | Upgraded fuel (straw 50%) obtained from air gas atmosphere at 270 °C |
| SLF-75-T270-A | Upgraded fuel (straw 75%) obtained from air gas atmosphere at 270 °C |
| SLF-100-T270-A | Upgraded fuel (straw 100%) obtained from air gas atmosphere at 270 °C |
| XPS | X-ray photoelectron spectroscopy |
| Raman | Raman spectra |
| RC | Conversion rate of carbon content after pretreatment |
| ε | Amount of water reabsorption |
References
- Meng, L.; Zhang, X.; Li, N.; Lu, W.; He, Z.; Gong, H.; Liao, A.; Yuan, S. Study on Sulfur Transformation during the Drying of Lignite and Sulfur Distribution in Pyrolysis. J. Anal. Appl. Pyrolysis 2024, 180, 106535. [Google Scholar] [CrossRef]
- Horbach, J.; Chen, Q.; Rennings, K.; Vögele, S. Do Lead Markets for Clean Coal Technology Follow Market Demand? A Case Study for China, Germany, Japan and the US. Environ. Innov. Soc. Transit. 2014, 10, 42–58. [Google Scholar] [CrossRef]
- Han, X.; Liu, M.; Yan, J.; Karellas, S.; Wang, J.; Xiao, F. Thermodynamic Analysis of an Improved Flue Gas Pre-Dried Lignite-Fired Power System Integrated with Water Recovery and Drying Exhaust Gas Recirculation. Dry. Technol. 2020, 38, 1971–1987. [Google Scholar] [CrossRef]
- Yang, Q.; Zhou, H.; Bartocci, P.; Fantozzi, F.; Masek, O.; Agblevor, F.A.; Wei, Z.; Yang, H.; Chen, H.; Lu, X.; et al. Prospective Contributions of Biomass Pyrolysis to China’s 2050 Carbon Reduction and Renewable Energy Goals. Nat. Commun. 2021, 12, 1698. [Google Scholar] [CrossRef]
- Savolainen, K. Co-Firing of Biomass in Coal-Fired Utility Boilers. Appl. Energy 2003, 74, 369–381. [Google Scholar] [CrossRef]
- Guo, F.; He, Y.; Hassanpour, A.; Gardy, J.; Zhong, Z. Thermogravimetric Analysis on the Co-Combustion of Biomass Pellets with Lignite and Bituminous Coal. Energy 2020, 197, 117147. [Google Scholar] [CrossRef]
- Zheng, Y.; Jensen, P.A.; Jensen, A.D.; Sander, B.; Junker, H. Ash Transformation during Co-Firing Coal and Straw. Fuel 2007, 86, 1008–1020. [Google Scholar] [CrossRef]
- Liao, X.; Zhang, S.; Wang, X.; Shao, J.; Zhang, X.; Wang, X.; Yang, H.; Chen, H. Co-Combustion of Wheat Straw and Camphor Wood with Coal Slime: Thermal Behaviour, Kinetics, and Gaseous Pollutant Emission Characteristics. Energy 2021, 234, 121292. [Google Scholar] [CrossRef]
- Dong, L.; Alexiadis, A. Simulation of Char Burnout Characteristics of Biomass/Coal Blend with a Simplified Single Particle Reaction Model. Energy 2023, 264, 126075. [Google Scholar] [CrossRef]
- Gao, M.; Wan, K.; Miao, Z.; He, Q.; Xue, S.; Dong, X. Hot-Air Drying Shrinkage Process of Lignite and Its Cracking Mechanism. Fuel 2022, 316, 123187. [Google Scholar] [CrossRef]
- Liu, S.; Zheng, Y.; Zhu, K.; Zhao, H.; Feng, L.; Weng, X.; Jiaoyang, Y.; Chen, H.; Zhang, J. Investigation on the Structure Evolution and Thermochemical Conversion Characteristics of Lignite after Different Pretreatment. Fuel 2023, 339, 126963. [Google Scholar] [CrossRef]
- Wei, F.; Liao, J.; Chang, L.; Han, Y.; Bao, W. Transformation of Functional Groups during Lignite Heat-Treatment and Its Effects on Moisture Re-Adsorption Properties. Fuel Process. Technol. 2019, 192, 210–219. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Zhang, S. Effect of Drying Conditions on Moisture Re-Adsorption and Particulate Matter Emissions during the Classification Drying of Coking Coal. Fuel Process. Technol. 2019, 192, 65–74. [Google Scholar] [CrossRef]
- Liu, R.; Liu, M.; Han, X.; Yan, J. Drying Characteristics and Kinetics Analyses for Yimin Lignite at Various Temperatures. Dry. Technol. 2021, 39, 912–924. [Google Scholar] [CrossRef]
- Choi, H.; Thiruppathiraja, C.; Kim, S.; Rhim, Y.; Lim, J.; Lee, S. Moisture Readsorption and Low Temperature Oxidation Characteristics of Upgraded Low Rank Coal. Fuel Process. Technol. 2011, 92, 2005–2010. [Google Scholar] [CrossRef]
- Zhao, H.; Li, Y.; Song, Q.; Wang, X.; Shu, X. Drying, Re-Adsorption Characteristics, and Combustion Kinetics of Xilingol Lignite in Different Atmospheres. Fuel 2017, 210, 592–604. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, C.; Qiu, Y.; Chen, L.; Tan, P.; Chen, G. Evaluation of Moisture Readsorption and Combustion Characteristics of a Lignite Thermally Upgraded with the Addition of Asphalt. Energy Fuels 2014, 28, 7680–7688. [Google Scholar] [CrossRef]
- Ren, Y.; Zheng, J.; Xu, Z.; Zhang, Y.; Cao, Z. Effect of Petcoke on the Physical-Chemical Properties of Lignite under Microwave Pyrolysis and Its Moisture Re-Adsorption Capacity. Fuel 2019, 250, 1–7. [Google Scholar] [CrossRef]
- Cahyanti, M.N.; Doddapaneni, T.R.K.C.; Kikas, T. Biomass Torrefaction: An Overview on Process Parameters, Economic and Environmental Aspects and Recent Advancements. Bioresour. Technol. 2020, 301, 122737. [Google Scholar] [CrossRef]
- Wang, T.; Liu, H.; Toan, S.; Sun, Z.; Sun, Z. Deoxygenated Pyrolysis-Gasification of Biomass for Intensified Bio-Oil and Syngas Co-Production with Tar Abatement. Fuel 2024, 371, 131883. [Google Scholar] [CrossRef]
- Jia, J.; Zhao, L.; Liu, Z.; Hao, X.; Huo, L.; Zhao, Y. Spray Atomization Characteristics of Biomass Pyrolysis Tar: Influence of Methanol Addition, Temperature, and Atomization Pressure. Energy 2022, 242, 122534. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, G.; Sun, R.; Wang, Z.; Liu, H. Effects of Lignite Dewatering Treatment on the Surface Behaviour and NO Emission Characteristics during the Combustion Process. Can. J. Chem. Eng. 2019, 97, 1418–1428. [Google Scholar] [CrossRef]
- Wang, C.; Liu, F.; Zhu, L.; Wang, X.; Zhang, C.; Zhang, M.; Han, Z.; Jia, X.; Guan, G.; Zeng, X.; et al. Two-Stage Fluidized Bed Gasification Enhanced with Oxidative Pyrolysis for Low-Tar Producer Gas from Biomass. Fuel 2024, 363, 130839. [Google Scholar] [CrossRef]
- Zhao, Z.; Feng, S.; Zhao, Y.; Wang, Z.; Ma, J.; Xu, L.; Yang, J.; Shen, B. Investigation on the Fuel Quality and Hydrophobicity of Upgraded Rice Husk Derived from Various Inert and Oxidative Torrefaction Conditions. Renew. Energy 2022, 189, 1234–1248. [Google Scholar] [CrossRef]
- Ohm, T.-I.; Chae, J.-S.; Lim, J.-H.; Moon, S.-H. Evaluation of a Hot Oil Immersion Drying Method for the Upgrading of Crushed Low-Rank Coal. J. Mech. Sci. Technol. 2012, 26, 1299–1303. [Google Scholar] [CrossRef]
- Guo, X.; He, Y.; Wang, J.; Zhou, R. Microscopic Adsorption Properties of Methyl Acrylate on Lignite Surface: Experiment and Molecular Simulation Study. Colloids Surf. A Physicochem. Eng. Asp. 2022, 641, 128468. [Google Scholar] [CrossRef]
- Zhuo, F.; Cheng, J.; Liu, J.; Zhou, J.; Cen, K. Improving Physicochemical Properties of Upgraded Indonesian Lignite through Microwave Irradiation with Char Adsorbent. Fuel 2018, 218, 275–281. [Google Scholar] [CrossRef]
- Ciolkosz, D.; Wallace, R. A Review of Torrefaction for Bioenergy Feedstock Production. Biofuels Bioprod. Biorefining 2011, 5, 317–329. [Google Scholar] [CrossRef]
- Murray, J.B.; Evans, D.G. The Brown-Coal/Water System: Part 3. Thermal Dewatering of Brown Coal. Fuel 1972, 51, 290–296. [Google Scholar] [CrossRef]
- Wang, H.; Wang, L.; Zheng, S.; Sun, Y.; Shen, S.; Zhang, X. Research on Coal Matrix Pore Structure Evolution and Adsorption Behavior Characteristics under Different Thermal Stimulation. Energy 2024, 287, 129677. [Google Scholar] [CrossRef]
- Švábová, M.; Weishauptová, Z.; Přibyl, O. Water Vapour Adsorption on Coal. Fuel 2011, 90, 1892–1899. [Google Scholar] [CrossRef]
- Li, J.; Li, Q.; Xu, Z.; Yang, X. Molecular Simulation of Adsorption Behavior for Phenol Aqueous Solution into Layered Graphene Oxides. Sep. Purif. Technol. 2024, 335, 126215. [Google Scholar] [CrossRef]
- Umar, D.F.; Usui, H.; Daulay, B. Change of Combustion Characteristics of Indonesian Low Rank Coal Due to Upgraded Brown Coal Process. Fuel Process. Technol. 2006, 87, 1007–1011. [Google Scholar] [CrossRef]
- Wang, R.; Liu, Z.; Song, X.; Liu, S. Co-Combustion of Pulverized Coal and Walnut Shells in Air and Oxy-Fuel Atmospheres: Thermal Behavior, Synergistic Effect and Kinetics. J. Energy Inst. 2023, 108, 101243. [Google Scholar] [CrossRef]
- Yi, B.; Zhang, L.; Huang, F.; Mao, Z.; Zheng, C. Effect of H2O on the Combustion Characteristics of Pulverized Coal in O2/CO2 Atmosphere. Appl. Energy 2014, 132, 349–357. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, X.; Yang, S.; He, X.; Chen, Z.; Zhang, S. Effect of Steam Concentration on Char Reactivity and Structure in the Presence/Absence of Oxygen Using Shengli Brown Coal. Fuel Process. Technol. 2015, 135, 174–179. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, L.; Zhao, Y.; Feng, S.; Ma, J.; Kong, W.; Shen, B.; Sun, R. Experimental Investigation on the Evolution Characteristics of Anthracite-N and Semi-Coke Reactivity under Various O2/H2O Pre-Oxidation Atmospheres. Fuel Process. Technol. 2021, 216, 106725. [Google Scholar] [CrossRef]
- Li, X.; Hayashi, J.; Li, C.-Z. FT-Raman Spectroscopic Study of the Evolution of Char Structure during the Pyrolysis of a Victorian Brown Coal. Fuel 2006, 85, 1700–1707. [Google Scholar] [CrossRef]
- Tay, H.-L.; Kajitani, S.; Wang, S.; Li, C.-Z. A Preliminary Raman Spectroscopic Perspective for the Roles of Catalysts during Char Gasification. Fuel 2014, 121, 165–172. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, J.; Sun, R.; Zhao, Y.; Li, Y.; Ismail, T.M. Investigation of the NO Reduction Characteristics of Coal Char at Different Conversion Degrees under an NO Atmosphere. Energy Fuels 2017, 31, 8722–8732. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, Y.; Sun, R.; Li, Y.; Ren, X.; Xu, J. Effect of Reaction Conditions on the Evolution of Surface Functional Groups in O2/H2O Combustion Process of Demineralized Coal Char. Fuel Process. Technol. 2019, 195, 106144. [Google Scholar] [CrossRef]
- Xu, J.; Su, S.; Sun, Z.; Qing, M.; Xiong, Z.; Wang, Y.; Jiang, L.; Hu, S.; Xiang, J. Effects of Steam and CO2 on the Characteristics of Chars during Devolatilization in Oxy-Steam Combustion Process. Appl. Energy 2016, 182, 20–28. [Google Scholar] [CrossRef]
- Cui, X.; Li, X.; Li, Y.; Li, S. Evolution Mechanism of Oxygen Functional Groups during Pyrolysis of Datong Coal. J. Therm. Anal. Calorim. 2017, 129, 1169–1180. [Google Scholar] [CrossRef]
- Kang, Z.; Huang, D.; Zhao, J.; Fan, S.; Yang, D.; Lu, Y. Hydrogen Transfer and Reaction Mechanism during In-Situ Pyrolysis of Fushun Oil Shale with Steam Injection. Fuel 2025, 381, 133583. [Google Scholar] [CrossRef]
- Li, Y.; Sun, R.; Wu, J.; Wang, Z.; Wang, M.; Song, Z. Effect of H2O on Char-Nitrogen Conversion during Char-O2/H2O Combustion under High-Temperature Entrained Flow Conditions. Combust. Flame 2019, 207, 391–405. [Google Scholar] [CrossRef]
- Duan, C.; Yan, X.; Zhang, W.; Zhang, Y.; Ji, X.; Huang, Z.; Chu, H. A Review on Nitrogen Transformation Mechanism during Biomass Pyrolysis. J. Anal. Appl. Pyrolysis 2024, 184, 106863. [Google Scholar] [CrossRef]
- Zhu, H.; Cheng, M.; Xu, J.; Chen, S.; Niu, F.; Yu, D. Nitrogen Migration and Transformation from Ammonia to Char during Ammonia-Coal/Char Co-Pyrolysis. Int. J. Hydrogen Energy 2024, 49, 137–148. [Google Scholar] [CrossRef]
- Troiano, M.; Ianzito, V.; Solimene, R.; Ganda, E.T.; Salatino, P. Fluidized Bed Pyrolysis of Biomass: A Model-Based Assessment of the Relevance of Heterogeneous Secondary Reactions and Char Loading. Energy Fuels 2022, 36, 9660–9671. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Z.; Ma, J.; Kong, W.; Yuan, P.; Sun, R.; Shen, B. Analysis of Functionality Distribution and Microstructural Characteristics of Upgraded Rice Husk after Undergoing Non-Oxidative and Oxidative Torrefaction. Fuel 2022, 310, 122477. [Google Scholar] [CrossRef]
- Zhang, S.; Min, G.; Wang, J.; Zhu, S.; Zhang, H.; Liu, X. Release Characteristics of Potassium and Chlorine for Torrefied Wheat Straw during a Combined Pyrolysis-Combustion System. Bioresour. Technol. 2020, 312, 123591. [Google Scholar] [CrossRef]
- Liang, W.; Jiang, C.; Wang, G.; Ning, X.; Zhang, J.; Guo, X.; Xu, R.; Wang, P.; Ye, L.; Li, J.; et al. Research on the Co-Combustion Characteristics and Kinetics of Agricultural Waste Hydrochar and Anthracite. Renew. Energy 2022, 194, 1119–1130. [Google Scholar] [CrossRef]
- Vamvuka, D.; Loukakou, E.; Sfakiotakis, S.; Petrakis, E. The Impact of a Combined Pre-Treatment on the Combustion Performance of Various Biomass Wastes and Their Blends with Lignite. Thermochim. Acta 2020, 688, 178599. [Google Scholar] [CrossRef]
- Patidar, K.; Singathia, A.; Vashishtha, M.; Kumar Sangal, V.; Upadhyaya, S. Investigation of Kinetic and Thermodynamic Parameters Approaches to Non-Isothermal Pyrolysis of Mustard Stalk Using Model-Free and Master Plots Methods. Mater. Sci. Energy Technol. 2022, 5, 6–14. [Google Scholar] [CrossRef]
- Li, T.; Wu, C. The Grain Size Effect on Pores Structure Characteristics of High-Rank Coal before and after the Methane Adsorption. J. Nat. Gas Geosci. 2021, 6, 111–120. [Google Scholar] [CrossRef]
- Bardestani, R.; Patience, G.S.; Kaliaguine, S. Experimental Methods in Chemical Engineering: Specific Surface Area and Pore Size Distribution Measurements—BET, BJH, and DFT. Can. J. Chem. Eng. 2019, 97, 2781–2791. [Google Scholar] [CrossRef]
- Jin, M.; Cheng, L.; Zheng, W.; Ding, Y.; Zhu, Y.; Jia, L.; Huang, F. Raman Tensor of Graphite: Symmetry of G, D and D′ Phonons. Sci. China Mater. 2022, 65, 268–272. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, H.; Song, Q.; Wang, X.; Shu, X. Influence of Critical Moisture Content in Lignite Dried by Two Methods on Its Physicochemical Properties during Oxidation at Low Temperature. Fuel 2018, 211, 27–37. [Google Scholar] [CrossRef]



| Samples | Ultimate Analysis (daf) (wt.%) | Proximate Analysis (ar) (wt.%) | HHV (MJ/kg) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| C | H | N | O | Mar | Ash | Volatile | FC | ||
| 1. R-L | 56.23 | 4.69 | 1.48 | 37.60 | 6.84 | 17.64 | 17.32 | 58.20 | 23.65 |
| 2. R-S | 35.75 | 5.56 | 2.04 | 56.64 | 7.49 | 14.38 | 42.78 | 35.35 | 17.40 |
| 3. SLF-0-T170-F | 67.00 | 3.97 | 0.87 | 28.16 | 3.56 | 16.77 | 17.84 | 61.83 | 26.72 |
| 4. SLF-25-T170-F | 65.50 | 3.76 | 0.83 | 29.92 | 3.66 | 15.84 | 36.45 | 44.05 | 25.95 |
| 5. SLF-50-T170-F | 64.84 | 3.63 | 0.81 | 30.73 | 3.27 | 15.44 | 40.46 | 40.83 | 25.57 |
| 6. SLF-75-T170-F | 64.24 | 3.42 | 0.76 | 31.58 | 2.52 | 14.99 | 42.23 | 40.26 | 25.13 |
| 7. SLF-100-T170-F | 50.87 | 6.93 | 0.99 | 41.21 | 3.46 | 20.67 | 35.41 | 40.46 | 24.39 |
| 8. SLF-0-T220-F | 77.94 | 3.63 | 0.77 | 17.67 | 3.83 | 18.18 | 17.78 | 60.21 | 30.19 |
| 9. SLF-25-T220-F | 75.59 | 2.99 | 0.74 | 20.67 | 5.70 | 18.77 | 25.25 | 50.28 | 28.59 |
| 10. SLF-50-T220-F | 74.00 | 2.65 | 0.75 | 22.60 | 5.17 | 19.98 | 23.25 | 51.60 | 27.59 |
| 11. SLF-75-T220-F | 71.93 | 2.48 | 0.72 | 24.87 | 5.14 | 20.48 | 16.99 | 57.39 | 26.63 |
| 12. SLF-100-T220-F | 53.91 | 6.49 | 0.47 | 39.14 | 4.17 | 21.49 | 29.45 | 44.89 | 24.98 |
| 13. SLF-0-T270-F | 62.27 | 3.15 | 0.62 | 33.96 | 4.65 | 13.26 | 18.33 | 63.76 | 24.15 |
| 14. SLF-25-T270-F | 62.36 | 2.75 | 0.53 | 34.36 | 4.99 | 13.99 | 27.07 | 53.95 | 23.70 |
| 15. SLF-50-T270-F | 64.17 | 2.94 | 0.49 | 32.41 | 4.89 | 17.66 | 22.69 | 54.76 | 24.49 |
| 16. SLF-75-T270-F | 66.04 | 2.68 | 0.42 | 30.86 | 5.79 | 20.67 | 18.35 | 55.19 | 24.79 |
| 17. SLF-100-T270-F | 53.00 | 6.80 | 0.51 | 39.70 | 4.57 | 23.73 | 24.41 | 47.29 | 24.97 |
| 18. SLF-0-T170-A | 62.87 | 6.00 | 1.53 | 29.61 | 2.94 | 20.96 | 17.61 | 58.49 | 27.51 |
| 19. SLF-25-T170-A | 60.61 | 5.55 | 1.40 | 32.44 | 3.38 | 19.58 | 40.99 | 36.05 | 26.22 |
| 20. SLF-50-T170-A | 59.23 | 5.39 | 1.32 | 34.07 | 2.93 | 18.35 | 42.11 | 36.61 | 25.56 |
| 21. SLF-75-T170-A | 53.25 | 4.61 | 1.17 | 40.97 | 2.37 | 10.06 | 45.56 | 42.01 | 22.69 |
| 22. SLF-100-T170-A | 44.48 | 6.89 | 0.87 | 47.76 | 2.66 | 21.11 | 32.16 | 44.07 | 22.07 |
| 23. SLF-0-T220-A | 61.49 | 4.58 | 1.21 | 32.72 | 2.97 | 16.84 | 18.15 | 62.04 | 25.44 |
| 24. SLF-25-T220-A | 60.46 | 4.42 | 1.16 | 33.96 | 3.10 | 16.07 | 41.57 | 39.26 | 24.91 |
| 25. SLF-50-T220-A | 59.02 | 4.22 | 1.09 | 35.67 | 3.03 | 15.13 | 42.56 | 39.28 | 24.18 |
| 26. SLF-75-T220-A | 54.00 | 3.77 | 0.98 | 41.25 | 4.01 | 7.76 | 48.09 | 40.14 | 22.02 |
| 27. SLF-100-T220-A | 54.74 | 4.04 | 0.45 | 40.77 | 3.41 | 22.90 | 27.81 | 45.88 | 22.33 |
| 28. SLF-0-T270-A | 55.72 | 5.18 | 1.08 | 38.02 | 3.86 | 17.63 | 17.43 | 61.08 | 24.09 |
| 29. SLF-25-T270-A | 57.21 | 4.99 | 1.03 | 36.78 | 4.28 | 19.11 | 23.16 | 53.45 | 24.37 |
| 30. SLF-50-T270-A | 60.13 | 4.98 | 1.05 | 33.85 | 5.43 | 23.77 | 29.18 | 41.62 | 25.31 |
| 31. SLF-75-T270-A | 60.74 | 4.71 | 1.01 | 33.55 | 5.20 | 25.24 | 31.30 | 38.26 | 25.17 |
| 32. SLF-100-T270-A | 58.39 | 2.53 | 0.46 | 38.63 | 3.77 | 24.32 | 22.16 | 49.75 | 21.80 |
| Samples | Ta (°C) | Tb (°C) | DTGa (%/min) | DTGm (%/min) | Samples | Ta (°C) | Tb (°C) | DTGa (%/min) | DTGm (%/min) |
|---|---|---|---|---|---|---|---|---|---|
| SLF-0-T170-F | 396.54 | 647.88 | 1.14 | 9.90 | SLF-0-T170-A | 395.28 | 734.11 | 1.09 | 6.31 |
| SLF-25-T170-F | 366.67 | 638.00 | 1.15 | 5.13 | SLF-25-T170-A | 249.59 | 732.95 | 1.08 | 5.05 |
| SLF-50-T170-F | 246.21 | 641.34 | 1.15 | 4.21 | SLF-50-T170-A | 248.61 | 726.92 | 1.09 | 4.33 |
| SLF-75-T170-F | 250.87 | 630.29 | 1.19 | 6.59 | SLF-75-T170-A | 254.55 | 719.68 | 1.06 | 8.84 |
| SLF-0-T220-F | 400.14 | 644.20 | 1.07 | 6.33 | SLF-0-T220-A | 400.22 | 656.27 | 1.08 | 6.33 |
| SLF-25-T220-F | 250.83 | 639.39 | 1.05 | 4.91 | SLF-25-T220-A | 251.87 | 651.66 | 1.06 | 5.12 |
| SLF-50-T220-F | 247.19 | 636.33 | 1.07 | 4.05 | SLF-50-T220-A | 250.87 | 650.34 | 1.08 | 4.09 |
| SLF-75-T220-F | 244.89 | 634.85 | 1.05 | 6.70 | SLF-75-T220-A | 245.59 | 643.57 | 1.20 | 6.67 |
| SLF-0-T270-F | 400.18 | 647.64 | 1.16 | 6.43 | SLF-0-T270-A | 401.52 | 729.97 | 1.07 | 6.27 |
| SLF-25-T270-F | 382.90 | 645.95 | 1.16 | 5.87 | SLF-25-T270-A | 386.58 | 726.28 | 1.05 | 5.61 |
| SLF-50-T270-F | 365.37 | 641.31 | 1.11 | 5.04 | SLF-50-T270-A | 371.65 | 722.69 | 1.00 | 4.64 |
| SLF-75-T270-F | 294.37 | 643.59 | 1.05 | 3.98 | SLF-75-T270-A | 343.94 | 718.12 | 0.92 | 4.17 |
| Conversion Ratio β | FWO | KAS | Conversion Ratio β | FWO | KAS | ||||
|---|---|---|---|---|---|---|---|---|---|
| Ea (kJ/mol) | R2 | Ea (kJ/mol) | R2 | Ea (kJ/mol) | R2 | Ea (kJ/mol) | R2 | ||
| SLF-0-T170-F | SLF-25-T170-F | ||||||||
| 0.2 | 154.2 | 0.9957 | 150.7 | 0.9950 | 0.2 | 128.3 | 0.9999 | 124.9 | 0.9999 |
| 0.3 | 147.0 | 0.9990 | 142.7 | 0.9988 | 0.3 | 126.4 | 0.9989 | 121.9 | 0.9988 |
| 0.4 | 145.8 | 0.9983 | 141.1 | 0.9979 | 0.4 | 126.3 | 0.9963 | 121.2 | 0.9956 |
| 0.5 | 143.7 | 0.9978 | 138.6 | 0.9973 | 0.5 | 128.8 | 0.9774 | 123.3 | 0.9730 |
| 0.6 | 140.9 | 0.9978 | 135.4 | 0.9972 | 0.6 | 127.7 | 0.9930 | 121.9 | 0.9916 |
| 0.7 | 138.4 | 0.9971 | 132.5 | 0.9964 | 0.7 | 127.4 | 0.9962 | 121.2 | 0.9955 |
| 0.8 | 138.7 | 0.9978 | 132.6 | 0.9972 | 0.8 | 128.6 | 0.9979 | 122.2 | 0.9974 |
| Average | 144.1 | 139.1 | Average | 127.6 | 122.4 | ||||
| SLF-50-T170-F | SLF-75-T170-F | ||||||||
| 0.2 | 118.3 | 0.9705 | 115.0 | 0.9657 | 0.2 | 114.1 | 0.9844 | 110.9 | 0.9817 |
| 0.3 | 113.8 | 0.9980 | 109.9 | 0.9976 | 0.3 | 111.0 | 0.9875 | 107.3 | 0.9851 |
| 0.4 | 106.7 | 0.9985 | 101.6 | 0.9982 | 0.4 | 105.7 | 0.9999 | 101.5 | 0.9999 |
| 0.5 | 109.7 | 0.9733 | 104.0 | 0.9672 | 0.5 | 108.6 | 0.9972 | 104.1 | 0.9967 |
| 0.6 | 119.0 | 0.9736 | 113.2 | 0.9677 | 0.6 | 114.3 | 0.9911 | 109.4 | 0.9893 |
| 0.7 | 118.2 | 0.9903 | 111.9 | 0.9879 | 0.7 | 113.7 | 0.9937 | 108.1 | 0.9923 |
| 0.8 | 116.2 | 0.9852 | 109.5 | 0.9815 | 0.8 | 111.1 | 0.9943 | 104.6 | 0.9928 |
| Average | 114.6 | 109.3 | Average | 111.2 | 106.6 | ||||
| SLF-0-T220-F | SLF-25-T220-F | ||||||||
| 0.2 | 107.7 | 0.9960 | 101.8 | 0.9951 | 0.2 | 106.2 | 0.9810 | 101.5 | 0.9772 |
| 0.3 | 109.3 | 0.9999 | 103.0 | 0.9999 | 0.3 | 101.3 | 0.9899 | 95.5 | 0.9876 |
| 0.4 | 110.1 | 0.9982 | 103.5 | 0.9977 | 0.4 | 95.4 | 0.9658 | 88.8 | 0.9569 |
| 0.5 | 109.8 | 0.9952 | 103.0 | 0.9938 | 0.5 | 116.3 | 0.9641 | 110.3 | 0.9563 |
| 0.6 | 108.5 | 0.9933 | 101.4 | 0.9913 | 0.6 | 107.3 | 0.9967 | 100.3 | 0.9960 |
| 0.7 | 108.4 | 0.9926 | 101.1 | 0.9904 | 0.7 | 108.9 | 0.9986 | 101.6 | 0.9981 |
| 0.8 | 111.5 | 0.9945 | 104.0 | 0.9929 | 0.8 | 106.7 | 0.9793 | 98.7 | 0.9736 |
| Average | 109.3 | 102.5 | Average | 106.0 | 99.5 | ||||
| SLF-50-T220-F | SLF-75-T220-F | ||||||||
| 0.2 | 95.6 | 0.9823 | 91.1 | 0.9786 | 0.2 | 98.4 | 0.9989 | 94.4 | 0.9987 |
| 0.3 | 97.3 | 0.9815 | 92.4 | 0.9775 | 0.3 | 94.7 | 0.9950 | 90.2 | 0.9940 |
| 0.4 | 103.5 | 0.9813 | 98.1 | 0.9773 | 0.4 | 96.6 | 0.9985 | 91.8 | 0.9982 |
| 0.5 | 96.3 | 0.9869 | 89.9 | 0.9837 | 0.5 | 95.7 | 0.9729 | 90.5 | 0.9669 |
| 0.6 | 101.7 | 0.9980 | 95.1 | 0.9975 | 0.6 | 97.0 | 0.9673 | 91.3 | 0.9596 |
| 0.7 | 107.5 | 0.9623 | 100.8 | 0.9532 | 0.7 | 94.5 | 0.9753 | 87.9 | 0.9688 |
| 0.8 | 88.2 | 0.9654 | 79.9 | 0.9535 | 0.8 | 92.5 | 0.9914 | 85.2 | 0.9890 |
| Average | 98.6 | 92.5 | Average | 95.6 | 90.2 | ||||
| SLF-0-T270-F | SLF-25-T270-F | ||||||||
| 0.2 | 134.0 | 0.9994 | 129.6 | 0.9993 | 0.2 | 126.9 | 0.9960 | 122.5 | 0.9953 |
| 0.3 | 137.0 | 0.9783 | 132.3 | 0.9744 | 0.3 | 118.4 | 0.9991 | 112.9 | 0.9990 |
| 0.4 | 133.1 | 0.9951 | 127.8 | 0.9941 | 0.4 | 112.0 | 0.9999 | 105.7 | 0.9999 |
| 0.5 | 138.7 | 0.9774 | 133.3 | 0.9729 | 0.5 | 118.5 | 0.9667 | 112.2 | 0.9590 |
| 0.6 | 125.5 | 0.9879 | 119.0 | 0.9851 | 0.6 | 99.2 | 0.9841 | 91.2 | 0.9791 |
| 0.7 | 138.0 | 0.9901 | 131.6 | 0.9879 | 0.7 | 129.1 | 0.9718 | 122.2 | 0.9652 |
| 0.8 | 125.9 | 0.9732 | 118.5 | 0.9665 | 0.8 | 132.0 | 0.9879 | 124.8 | 0.9849 |
| Average | 133.2 | 127.4 | Average | 119.4 | 113.1 | ||||
| SLF-50-T270-F | SLF-75-T270-F | ||||||||
| 0.2 | 102.0 | 0.9878 | 97.1 | 0.9852 | 0.2 | 111.2 | 0.9838 | 107.1 | 0.9808 |
| 0.3 | 91.8 | 0.9727 | 86.0 | 0.9655 | 0.3 | 104.0 | 0.9850 | 99.1 | 0.9818 |
| 0.4 | 113.7 | 0.9756 | 108.5 | 0.9703 | 0.4 | 107.3 | 0.9957 | 102.0 | 0.9948 |
| 0.5 | 110.0 | 0.9939 | 104.3 | 0.9924 | 0.5 | 105.9 | 0.9823 | 100.0 | 0.9783 |
| 0.6 | 107.5 | 0.9979 | 100.8 | 0.9972 | 0.6 | 106.2 | 0.9733 | 99.9 | 0.9671 |
| 0.7 | 123.5 | 0.9968 | 117.2 | 0.9959 | 0.7 | 112.6 | 0.9947 | 106.2 | 0.9933 |
| 0.8 | 125.8 | 0.9676 | 119.2 | 0.9605 | 0.8 | 113.0 | 0.9784 | 106.1 | 0.9729 |
| Average | 110.6 | 104.7 | Average | 108.6 | 102.9 | ||||
| Conversion Ratio β | FWO | KAS | Conversion Ratio β | FWO | KAS | ||||
|---|---|---|---|---|---|---|---|---|---|
| Ea (kJ/mol) | R2 | Ea (kJ/mol) | R2 | Ea (kJ/mol) | R2 | Ea (kJ/mol) | R2 | ||
| SLF-0-T170-A | SLF-25-T170-A | ||||||||
| 0.2 | 155.8 | 0.9825 | 152.4 | 0.9798 | 0.2 | 131.6 | 0.9844 | 128.4 | 0.9998 |
| 0.3 | 178.6 | 0.9893 | 176.0 | 0.9879 | 0.3 | 142.9 | 0.9853 | 139.3 | 0.9819 |
| 0.4 | 169.5 | 0.9787 | 166.1 | 0.9756 | 0.4 | 136.1 | 0.9992 | 131.5 | 0.9828 |
| 0.5 | 149.1 | 0.9742 | 144.3 | 0.9697 | 0.5 | 146.8 | 0.9882 | 142.4 | 0.9991 |
| 0.6 | 172.5 | 0.9706 | 168.6 | 0.9660 | 0.6 | 131.2 | 0.9998 | 125.6 | 0.9862 |
| 0.7 | 150.7 | 0.9746 | 145.3 | 0.9698 | 0.7 | 125.2 | 0.9990 | 118.8 | 0.9998 |
| 0.8 | 164.1 | 0.9892 | 159.0 | 0.9872 | 0.8 | 137.3 | 0.9932 | 131.0 | 0.9875 |
| Average | 162.9 | 158.8 | Average | 135.9 | 131.0 | ||||
| SLF-50-T170-A | SLF-75-T170-A | ||||||||
| 0.2 | 126.8 | 0.9773 | 124.0 | 0.9739 | 0.2 | 134.5 | 0.9982 | 132.2 | 0.9980 |
| 0.3 | 123.4 | 0.9921 | 120.1 | 0.9908 | 0.3 | 120.4 | 0.9920 | 117.1 | 0.9906 |
| 0.4 | 127.4 | 0.9931 | 123.7 | 0.9919 | 0.4 | 117.2 | 0.9731 | 113.4 | 0.9684 |
| 0.5 | 122.9 | 0.9829 | 118.2 | 0.9797 | 0.5 | 110.7 | 0.9954 | 106.1 | 0.9945 |
| 0.6 | 127.1 | 0.9875 | 122.0 | 0.9851 | 0.6 | 122.3 | 0.9919 | 117.8 | 0.9904 |
| 0.7 | 125.2 | 0.9853 | 119.6 | 0.9823 | 0.7 | 134.2 | 0.9734 | 129.7 | 0.9685 |
| 0.8 | 129.7 | 0.9920 | 123.8 | 0.9904 | 0.8 | 128.9 | 0.9867 | 123.7 | 0.9842 |
| Average | 126.1 | 121.6 | Average | 124.0 | 120.0 | ||||
| SLF-0-T220-A | SLF-25-T220-A | ||||||||
| 0.2 | 125.2 | 0.9875 | 120.3 | 0.9849 | 0.2 | 108.3 | 0.9868 | 103.7 | 0.9840 |
| 0.3 | 124.2 | 0.9787 | 118.7 | 0.9742 | 0.3 | 116.5 | 0.9905 | 111.4 | 0.9885 |
| 0.4 | 113.6 | 0.9882 | 107.2 | 0.9853 | 0.4 | 104.4 | 0.9879 | 98.18 | 0.9847 |
| 0.5 | 115.7 | 0.9997 | 109.1 | 0.9996 | 0.5 | 123.0 | 0.9743 | 117.3 | 0.9687 |
| 0.6 | 127.3 | 0.9907 | 121.1 | 0.9886 | 0.6 | 122.8 | 0.9893 | 116.8 | 0.9868 |
| 0.7 | 120.0 | 0.9866 | 113.2 | 0.9833 | 0.7 | 119.3 | 0.9892 | 112.8 | 0.9866 |
| 0.8 | 120.0 | 0.9680 | 112.9 | 0.9602 | 0.8 | 121.5 | 0.9934 | 114.8 | 0.9917 |
| Average | 120.8 | 114.6 | Average | 116.6 | 110.7 | ||||
| SLF-50-T220-A | SLF-75-T220-A | ||||||||
| 0.2 | 106.2 | 0.9878 | 102.2 | 0.9855 | 0.2 | 108.0 | 0.9770 | 104.6 | 0.9729 |
| 0.3 | 95.0 | 0.9933 | 90.2 | 0.9919 | 0.3 | 95.8 | 0.9980 | 91.0 | 0.9976 |
| 0.4 | 105.6 | 0.9999 | 100.6 | 0.9999 | 0.4 | 100.2 | 0.9643 | 95.3 | 0.9564 |
| 0.5 | 109.9 | 0.9995 | 104.4 | 0.9993 | 0.5 | 113.2 | 0.9987 | 108.5 | 0.9984 |
| 0.6 | 110.9 | 0.9897 | 104.8 | 0.9872 | 0.6 | 115.6 | 0.9783 | 110.4 | 0.9737 |
| 0.7 | 121.2 | 0.9897 | 115.4 | 0.9875 | 0.7 | 112.8 | 0.9834 | 107.0 | 0.9797 |
| 0.8 | 115.5 | 0.9716 | 108.9 | 0.9706 | 0.8 | 111.4 | 0.9678 | 105.0 | 0.9603 |
| Average | 109.2 | 103.8 | Average | 108.1 | 102.7 | ||||
| SLF-0-T270-A | SLF-25-T270-A | ||||||||
| 0.2 | 126.0 | 0.9994 | 121.1 | 0.9992 | 0.2 | 123.8 | 0.9983 | 119.3 | 0.9980 |
| 0.3 | 135.0 | 0.9999 | 130.2 | 0.9999 | 0.3 | 115.0 | 0.9930 | 109.4 | 0.9915 |
| 0.4 | 149.1 | 0.9783 | 144.7 | 0.9744 | 0.4 | 130.5 | 0.9960 | 125.3 | 0.9953 |
| 0.5 | 165.9 | 0.9672 | 162.0 | 0.9620 | 0.5 | 120.9 | 0.9900 | 114.9 | 0.9879 |
| 0.6 | 134.0 | 0.9958 | 128.2 | 0.9948 | 0.6 | 122.0 | 0.9898 | 115.8 | 0.9875 |
| 0.7 | 130.9 | 0.9958 | 124.8 | 0.9948 | 0.7 | 122.4 | 0.9929 | 116.0 | 0.9913 |
| 0.8 | 140.3 | 0.9958 | 134.3 | 0.9872 | 0.8 | 124.6 | 0.9950 | 118.0 | 0.9940 |
| Average | 140.2 | 135.0 | Average | 122.7 | 117.0 | ||||
| SLF-50-T270-A | SLF-75-T270-A | ||||||||
| 0.2 | 114.2 | 0.9827 | 109.6 | 0.9794 | 0.2 | 113.9 | 0.9904 | 109.6 | 0.9886 |
| 0.3 | 106.7 | 0.9731 | 101.1 | 0.9672 | 0.3 | 104.0 | 0.9916 | 98.7 | 0.9897 |
| 0.4 | 126.3 | 0.9705 | 121.3 | 0.9649 | 0.4 | 117.0 | 0.9989 | 111.9 | 0.9986 |
| 0.5 | 143.2 | 0.9707 | 138.7 | 0.9657 | 0.5 | 116.1 | 0.9722 | 110.6 | 0.9665 |
| 0.6 | 116.2 | 0.9827 | 110.0 | 0.9788 | 0.6 | 127.1 | 0.9824 | 121.7 | 0.9789 |
| 0.7 | 100.5 | 0.9780 | 92.9 | 0.9714 | 0.7 | 114.2 | 0.9936 | 107.8 | 0.9919 |
| 0.8 | 116.0 | 0.9872 | 108.6 | 0.9837 | 0.8 | 122.1 | 0.9785 | 115.4 | 0.9734 |
| Average | 117.6 | 111.7 | Average | 116.3 | 110.8 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, N.; Bai, B.; Yang, X.; Wang, Z.; Shen, B. Investigation on Fuel Quality and Combustion Characteristics of Blended Fuel (Biomass and Lignite) Derived from Low-Temperature Co-Upgradation. Molecules 2025, 30, 3435. https://doi.org/10.3390/molecules30163435
Liu N, Bai B, Yang X, Wang Z, Shen B. Investigation on Fuel Quality and Combustion Characteristics of Blended Fuel (Biomass and Lignite) Derived from Low-Temperature Co-Upgradation. Molecules. 2025; 30(16):3435. https://doi.org/10.3390/molecules30163435
Chicago/Turabian StyleLiu, Ning, Bohao Bai, Xu Yang, Zhuozhi Wang, and Boxiong Shen. 2025. "Investigation on Fuel Quality and Combustion Characteristics of Blended Fuel (Biomass and Lignite) Derived from Low-Temperature Co-Upgradation" Molecules 30, no. 16: 3435. https://doi.org/10.3390/molecules30163435
APA StyleLiu, N., Bai, B., Yang, X., Wang, Z., & Shen, B. (2025). Investigation on Fuel Quality and Combustion Characteristics of Blended Fuel (Biomass and Lignite) Derived from Low-Temperature Co-Upgradation. Molecules, 30(16), 3435. https://doi.org/10.3390/molecules30163435








