The Spectroscopic Characterization and Photophysical Properties of a Hydrated Lanthanum Ion Complex with a Triazole Ligand by Several DFT Methods
Abstract
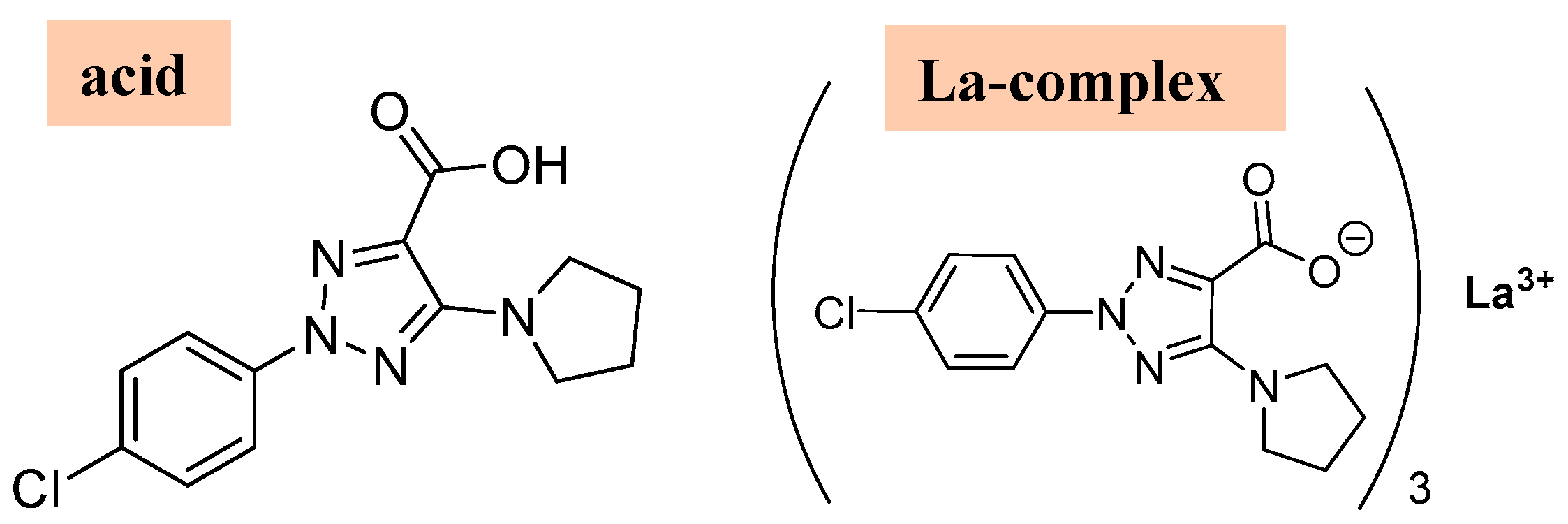
1. Introduction
2. Results and Discussion
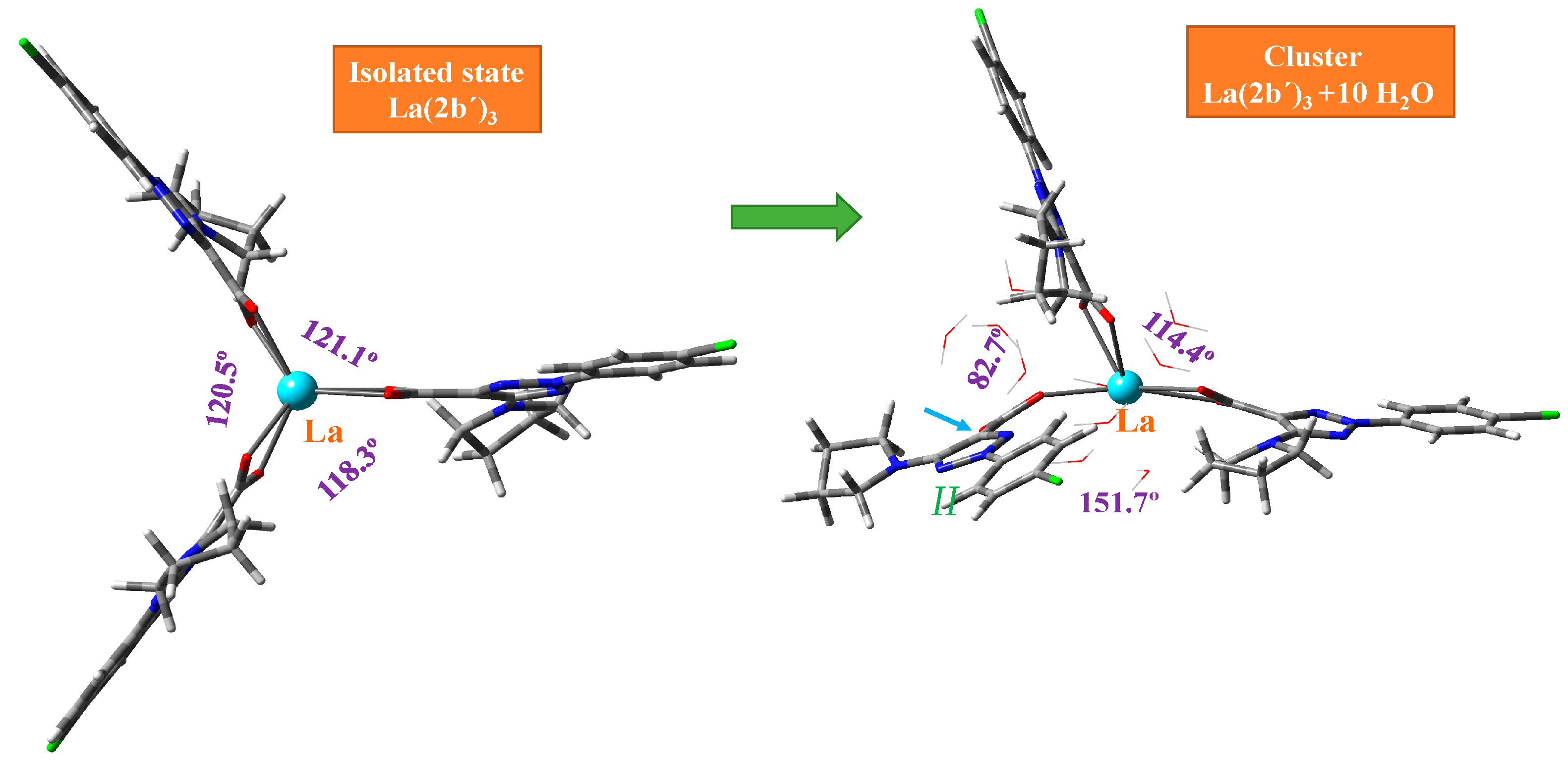
2.1. Calculated Molecular Structure in the Isolated State

2.2. Calculations of the Hydrated Molecular Structure
2.3. Calculation of Atomic Charges and Molecular Properties
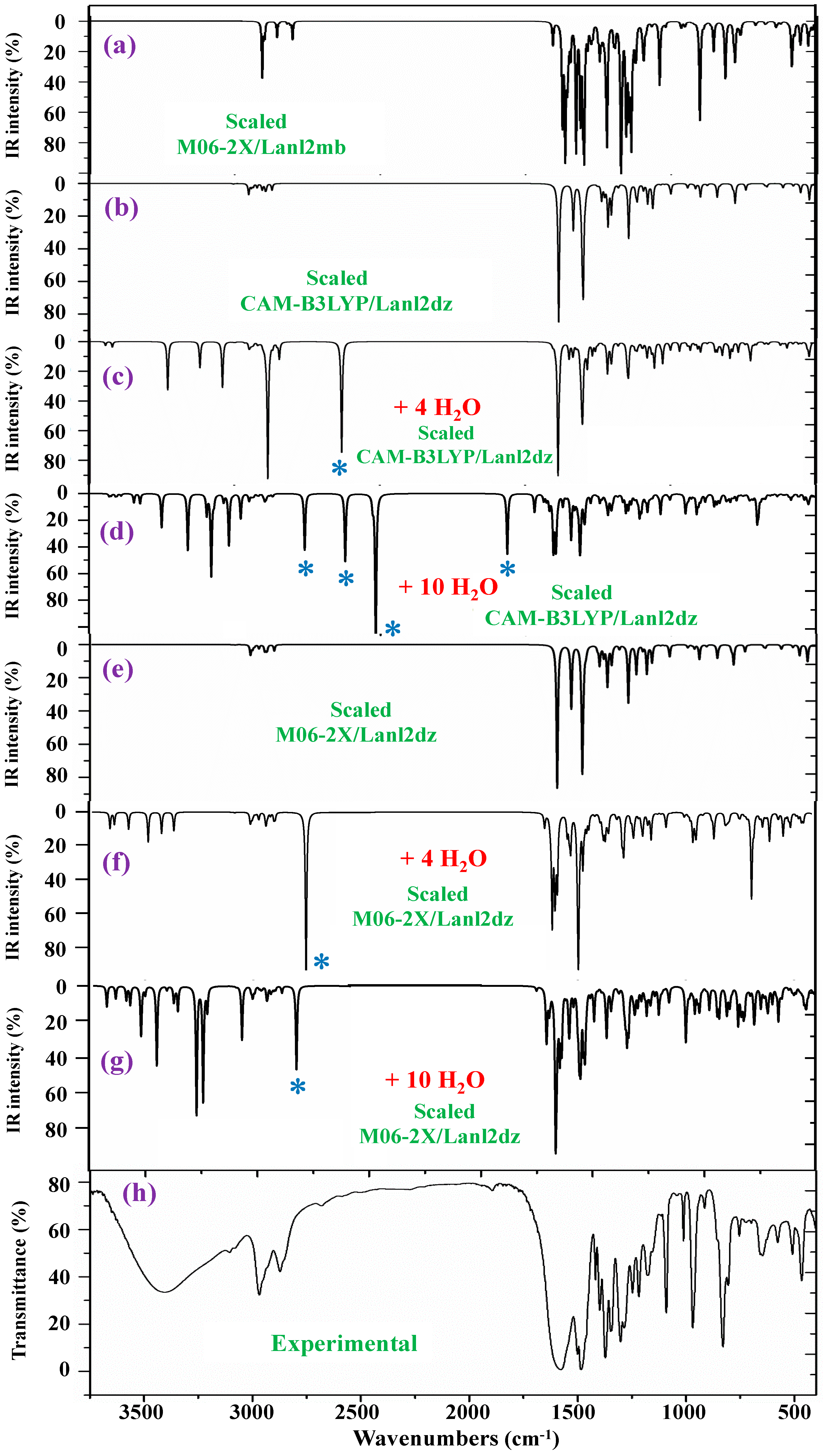
2.4. Vibrational Analysis
- A very broad band, centered at ca. 3400 cm−1, is observed in the experimental IR spectrum, which can only correspond to the O-H stretching ν(O-H) mode of hydration water strongly H-bonded to the ligands in the La(2b′)3 complex system. Due to the spatial arrangement of the ligands in the complex, many holes appear in the structure, which can be occupied by water molecules, associated with the synthesis of the complex. Due to the large negative charge around the three carboxylate groups, water molecules can be H-bonded through their hydrogen atoms. Moreover, this band does not appear in the Raman spectrum, as expected.
- A broad and very strong band centered at 1577.8 cm−1 in the experimental IR spectrum, in which the in-plane bending δ(O-H) mode of these hydrated water molecules contributes to its broadness.
- Very obvious similarity between the scaled spectra in the isolated state at the M06-2X/Lanl2dz and CAM-B3LYP/Lanl2dz levels was observed, which could be due to the symmetric and better optimized structure obtained at these levels. However, the spectrum at the M06-2X/Lanl2mb level has the best correlation with the experimental one, perhaps due to its deformed
- The coordination of the 2b′ ligands to the lanthanum ion noticeably changes the IR and Raman spectra, and therefore, they seem different from those obtained with the 2b′ ligand molecule alone in its anionic form [29].
- The scaled spectra with four hydration water molecules do not appropriately reproduce the broad band centered at 3401.6 cm−1 (Table 4) observed in the experimental spectrum. In addition, a very strong band, with the highest IR intensity of the spectrum appears at 2927 cm−1 by CAM-B3LYP without correspondence to the experimental spectrum. This very strong band, assigned as νas(O-H), corresponds to a water molecule directly bonded to the La ion, but it seems that it does not occur in the synthesized solid-state sample.
- The scaled spectra with 10 hydration water molecules at the M06-2X/Lanl2dz and CAM-B3LYP/Lanl2dz levels somewhat reproduce the broad experimental band centered at 3401.6 cm−1, although it is reproduced with an IR intensity slightly higher than that observed in the experimental spectrum and at ca. 100–200 cm−1 to lower wavenumbers. For clarity, this broad experimental band was reproduced theoretically with dotted lines involving the scaled ν(O-H) stretching bands of the water molecules; see Figure 8. It was reproduced slightly better by the M06-2X method than by CAM-B3LYP, with the band center being at a higher wavenumber (ca. 100 cm−1) than by CAM-B3LYP and closer to the experimental band. The scaled spectra with seven or eight hydration water molecules appear the best. The M06-2X/Lanl2mb level dramatically fails in the calculation of the hydrated form cluster, and its spectrum shows very strong ν(O-H) stretching bands corresponding to the water molecules and with remarkably higher intensity compared to the remaining bands of the spectrum; therefore, it was included in Figure S8b.
- In our hydration model, water molecules are directly H-bonded to the carboxylate group or strongly interacting with the La ion. This causes them to be slightly deformed, with O-H bond lengthening, and therefore, the ν(O-H) stretching vibrations strongly shift to lower wavenumbers. The calculated bands of these water molecules are indicated by a blue (*) symbol in Figure 7. Since they do not have correspondence to the experimental spectra, it means that these water molecules should be present around other active atoms of the complex with weaker H-bonds. This explanation is also supported by the fact that the experimental broad band, corresponding to ν(O-H)H2O, appears centered at 3406 cm−1, while the scaled main bands of this mode appear around 3250 cm−1 in the 10-hydration-water-molecule spectra.
- The scaled spectrum with 10 water molecules by CAM-B3LYP/Lanl2dz shows much more water vibrational bands than that by M06-2X/Lanl2dz, which is due to a noticeably larger effect of the water molecules by CAM-B3LYP than by M06-2X on the COO group, with the breaking of two O-La bonds, as described in Section 2.2.
| Calculated at M06-2X/Lanl2dz | Scaled | Experimental | Characterization | |||||
| ν | A | S | DP | DU | IR | Raman | ||
| 3401.6 br, s | ν(O-H) H2O bonded | |||||||
| 3188, 3187, 3187 | 6 | 3 | 0.72 | 0.84 | 2973 | 2968.1 s | νas(C-H) in C16H2, C17H2 (100) | |
| 3116, 3116, 3114 | 4 | 4 | 0.03 | 0.06 | 2907 | 2872.2 m | νs(C-H) in C18H2 in pyrrolidine (100) | |
| 1687, 1687, 1686 | 0 | 55 | 0.39 | 0.56 | 1600 | 1597 vs | 8a, ν(C=C) in aryl (89) | |
| 1666, 1663, 1662 | 100 | 24 | 0.06 | 0.12 | 1581, 1578 | 1577.8 br, vs | ν(C8-N14) (73) + νs(N7CC) (15) | |
| 1607, 1596, 1596 | 46 | 100 | 0.08 | 0.15 | 1527, 1516 | 1500.2 s | 1504 s | νas(COO) (49) + ν(C9-C11) |
| 1549, 1544, 1543 | 83 | 5 | 0.75 | 0.86 | 1474, 1470 | 1484.5 vs | 19a, ν(CC)(35) + δ(CH) in pyrrolidine (15) | |
| 1461, 1455, 1454 | 28 | 0 | 0.75 | 0.86 | 1393, 1388 | 1398.1 m | νas(CCOO) + νs(NNN) + ν(C-N) | |
| 1441, 1440, 1440 | 8 | 21 | 0.32 | 0.48 | 1375 | 1372.3 vs | 1375 vs | νs(COO) + νs(NNN) + δs(CC,CH) |
| 1404, 1402, 1401 | 14 | 2 | 0.75 | 0.85 | 1341, 1340 | 1347.5 s | δs(C-H) in pyrrolidine (30) | |
| 1363, 1363, 1363 | 2 | 0 | 0.38 | 0.55 | 1304 | 1302.0 vs | Γ((pyrrolidine) | |
| 1326, 1322, 1321 | 42 | 12 | 0.75 | 0.86 | 1270, 1267 | 1285.5 s | ν(NN,CN) + γas(CC,CH) in pyrrolidine | |
| 1291, 1291, 1290 | 2 | 4 | 0.65 | 0.79 | 1238 | 1246.8 m | νas(NN,CN)+3,δ(CH) in aryl+δ(pyrrolidine) | |
| 1285, 1283, 1283 | 22 | 2 | 0.75 | 0.86 | 1232, 1230 | 1218.0 m | γas(C-H) out-of-phase in pyrrolidine | |
| 1235, 1233, 1232 | 22 | 0 | 0.75 | 0.86 | 1186, 1184 | 1178.2 m | γas(C-H) in pyrrolidine | |
| 1123, 1123, 1123 | 8 | 5 | 0.02 | 0.04 | 1084 | 1091.2 vs | 1091 m | 12, δ(CC,CH) in aryl (96) |
| 1037, 1037, 1036 | 2 | 1 | 0.22 | 0.36 | 1005 | 1011.8 m | 1013 w | 18a, δ(CC, CH) in aryl (98) |
| 981, 981, 981 | 10 | 17 | 0.07 | 0.14 | 954 | 969.0 vs | 970 s | νas(NNN) + 18a, δ(CC,CH) in aryl |
| 830, 828, 827 | 4 | 3 | 0.04 | 0.07 | 816, 814 | 829.7 vs | γs(COO) + γ(triazol) | |
| 826, 819, 818 | 16 | 3 | 0.04 | 0.07 | 812, 805 | 805.4 m | δas(COO) + γ(C9-C11) | |
| 675, 675, 674 | 2 | 0 | 0.73 | 0.85 | 674 | 654 m | γs(NNN) + δas(COO) | |
| 668, 668, 667 | 2 | 0 | 0.73 | 0.84 | 668 | 647.1 m | ((triazol) (65) + δas(COO) (18) | |
| 511, 507, 507 | 8 | 0 | 0.74 | 0.85 | 524, 520 | 508.9 m | δas(NNN) +δ(CCL) +((CC) aryl +δas(COO) | |
| 472, 469, 468 | 12 | 1 | 0.34 | 0.50 | 489, 486 | 466.5 m | δas(COO) in phase + 6b, δ(CCC) in aryl +La | |
| Calculated at M06-2X/Lanl2MB | Scaled | Experimental | Characterization | |||||
| ν | A | S | IR | Raman | ||||
| -- | -- | 3401.6 br, s | ν(O-H) H2O bonded | |||||
| 3553, 3553, 3553 | 26 | 3 | 2956 | 2968.1 s | 20b, ν(C6-H) in aryl (100) | |||
| 3488, 3468, 3453 | 14 | 16 | 2911, 2892 | 2872.2 m | νas(C-H) in C15H2 pyrrolidine (100) | |||
| 1769, 1769, 1769 | 9 | 97 | 1617 | 1597 vs | 8a, ν(C=C) in aryl (93) | |||
| 1716, 1701, 1700 | 83 | 22 | 1574, 1560 | 1577.8 br, vs | ν(C8-N14) + νs(N7CC) | |||
| 1640, 1640, 1639 | 55 | 100 | 1511 | 1500.2 s | 1504 s | 19a, ν(CC,CH) + ν(C4-N4) +ν(C9-C11) | ||
| 1616, 1602, 1595 | 100 | 80 | 1491, 1477, 1472 | 1484.5 vs | νas(COO) + νs(CCN)triazol+ν(C9-C11) + 19a,ν(CC) | |||
| 1501, 1500, 1498 | 7 | 16 | 1395, 1392 | 1398.1 m | δs(C-H) in pyrrolidine + νs(NNN) | |||
| 1484, 1477, 1475 | 19 | 33 | 1380, 1375, 1373 | 1375 vs | νs(NNN) + νs(COO) + γs(C-H) in pyrrolidine | |||
| 1474, 1469, 1468 | 71 | 18 | 1372, 1368 | 1372.3 vs | νs(COO) + νs(NNN) + δs(CC,CH) + 19a, ν(CC) | |||
| 1433, 1425, 1424 | 15 | 1 | 1337, 1329 | 1347.5 s | Γ((C-H) in pyrrolidine | |||
| 1399, 1394, 1390 | 94 | 16 | 1309, 1304, 1301 | 1302.0 vs | νas(NNN)(36) + δ(C-H) in pyrrolidine (30) | |||
| 1367, 1365, 1362 | 50 | 16 | 1281, 1279, 1276 | 1285.5 s | δas(C-H) in pyrrolidine + ν(triazol) | |||
| 1339, 1337, 1336 | 82 | 8 | 1258, 1256 | 1246.8 m | ν(triazol) + 3, δ(C-H) + δ(C-H) in pyrrolidine | |||
| 1312, 1311, 1311 | 17 | 0 | 1234 | 1218.0 m | 14,ν(CC) in aryl + νas(NN) + δ(C-H) pyrrolidine | |||
| 1269, 1259, 1256 | 22 | 1 | 1198, 1189, 1186 | 1178.2 m | ν(triazol) + γas(C-H) in pyrrolidine + νs(COO) | |||
| 1155, 1154, 1153 | 3 | 1 | 1099, 1097 | 1091.2 vs | 1091 m | νs(NNN) + 18a, δ(C-H) in aryl | ||
| 1054, 1050, 1050 | 3 | 1 | 1011, 1007 | 1011.8 m | 1013 w | νas(triazol) + δ(CC,CH) pyrrolidine + δs(COO) | ||
| 974, 973, 973 | 36 | 24 | 941 | 969.0 vs | 970 s | νas(NNN, CC) in triazol + 18a, δ(C-H) in aryl | ||
| 846, 842, 840 | 36 | 3 | 827, 824, 822 | 829.7 vs | δas(COO) + γas(C-H) pyrrolidine + ν(triazol) | |||
| 799, 792, 789 | 26 | 1 | 785, 779, 777 | 805.4 m | δas(COO) + γas(C-H) pyrrolidine + ν(triazol) | |||
| 658, 658, 658 | 3 | 1 | 658 | 654 m | 6a, δ(CC) in aryl | |||
| 638, 636, 633 | 2 | 0 | 640, 638, 635 | 647.1 m | Γ((triazol) + γas(COO) + γ(C-H) pyrrolidine | |||
| 504, 500, 489 | 32 | 3 | 518, 514, 503 | 508.9 m | δas(COO)phase + δas(triazol) + ν(CCL) + 6b,δ(CCC) | |||
| 462, 460, 457 | 13 | 1 | 479, 477, 474 | 466.5 m | ν(CCL) + δas(COO) + δ(NNN) + 6b,δ(CCC) | |||
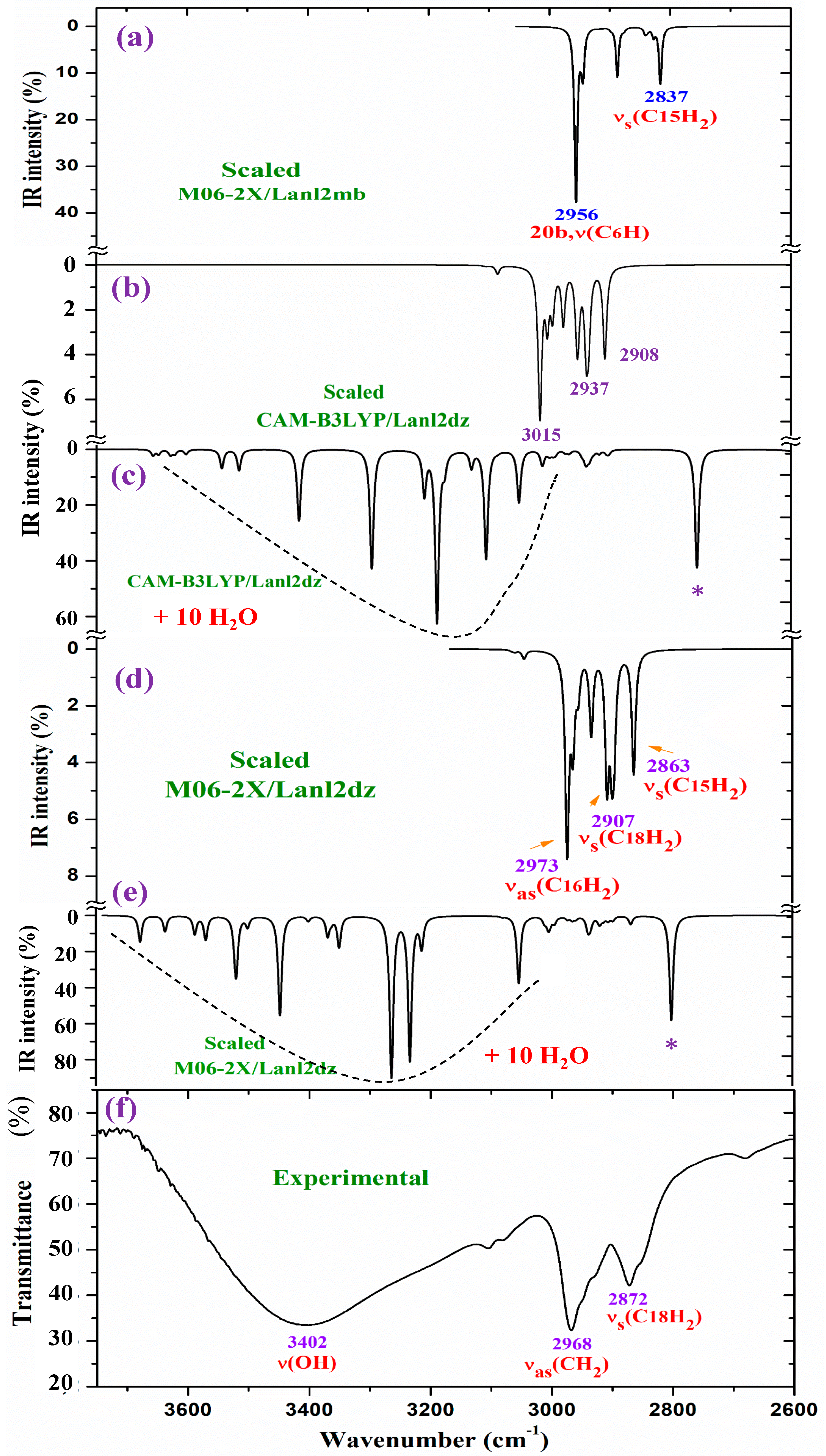
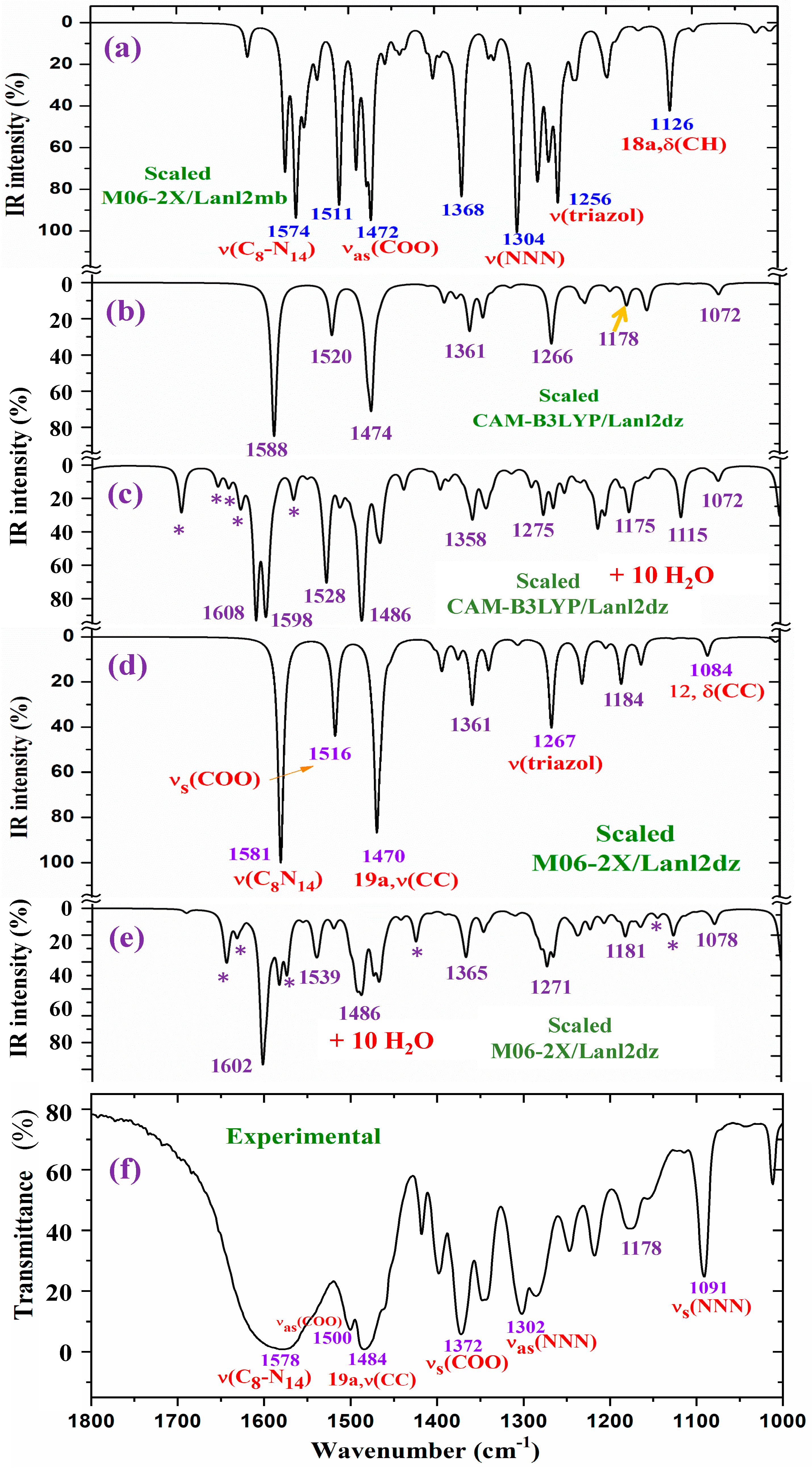
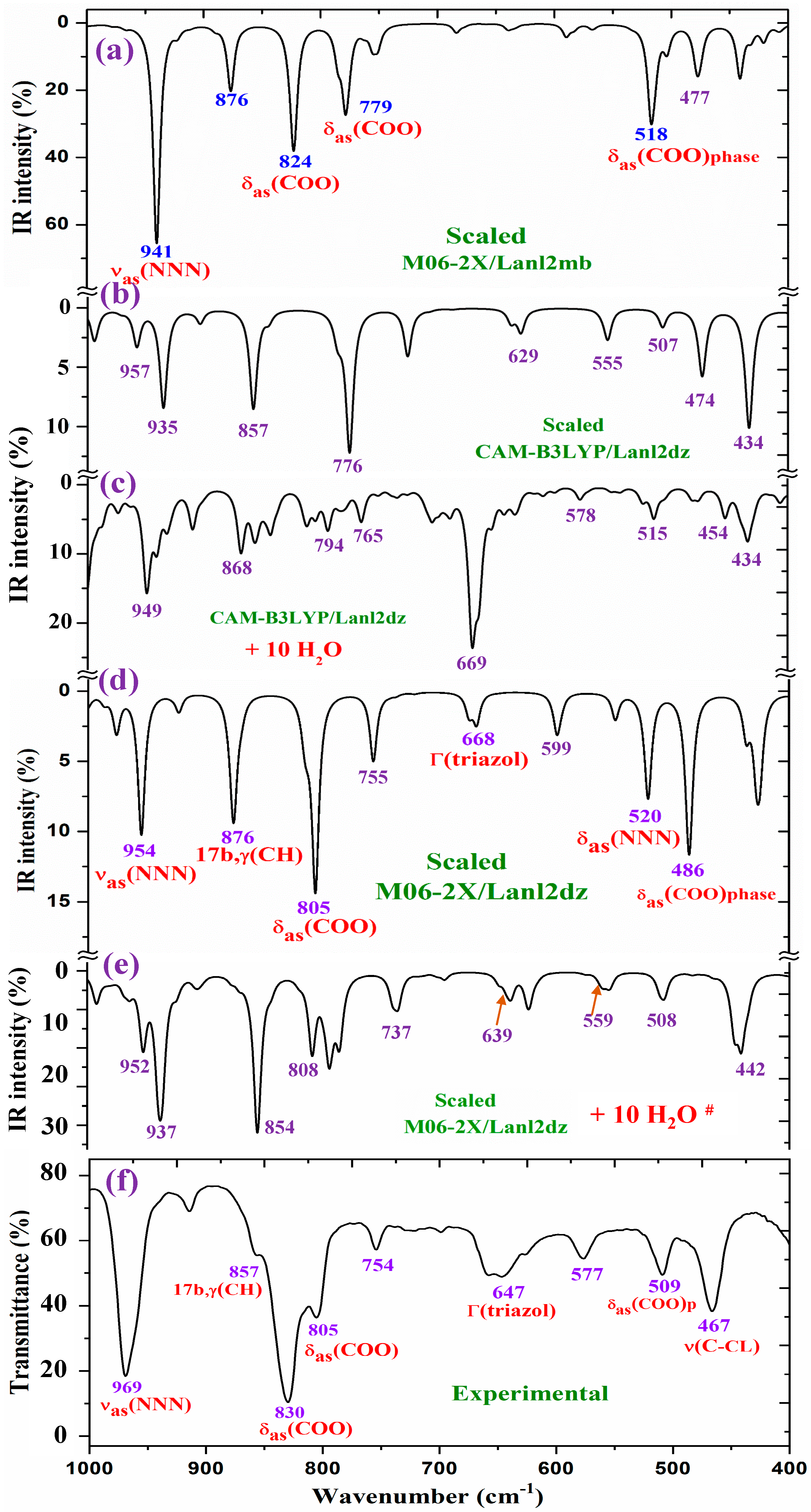
2.4.1. The Carboxylate COO Group Modes
2.4.2. The Triazole Ring Modes
2.4.3. The Aryl Ring Modes
2.4.4. Comparison with Other Lanthanoid Ln(2b′)3 Complexes
2.5. Photophysical Properties
2.5.1. Photophysical Properties of the 2b′ Free Ligand and in Its La Complex
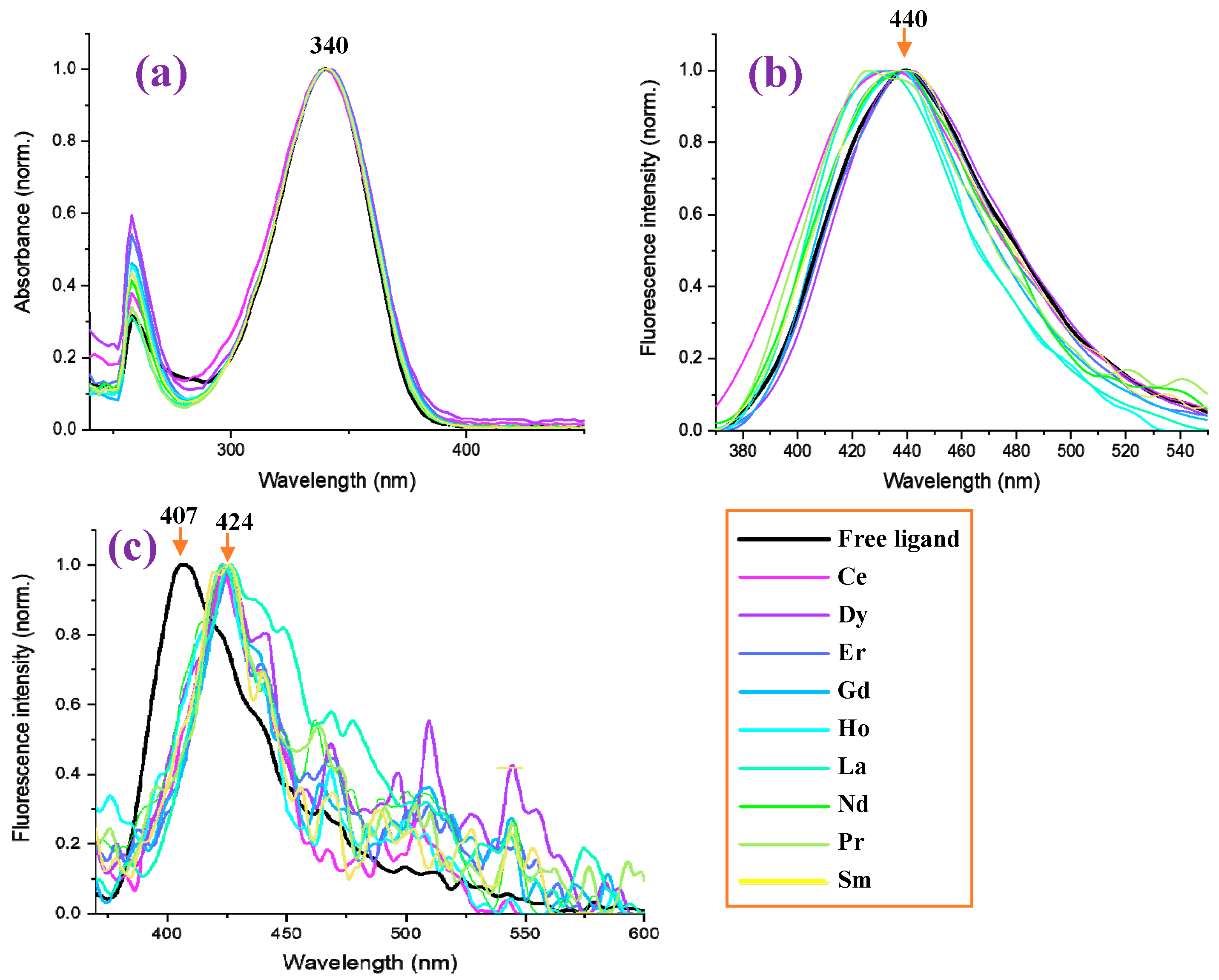
2.5.2. Photophysical Properties of Lanthanide Complexes
3. Materials and Methods
3.1. Experimental Details
3.2. Computational Details
Scaling the Wavenumbers
4. Summary and Conclusions
- The optimized La(III) complex at the CAM-B3LYP/Lanl2dz and M06-2X/Lanl2dz levels show a symmetrical arrangement of the ligands, while at B3LYP/Cep-4g and especially at M06-2X/Lanl2mb, a deformed structure with packing interaction of two ligands was obtained.
- The hydration water molecules present in the experimental sample were considered under the DM model with different numbers of explicit water molecules around the La(III) ion and the carboxylate groups and in different positions. The theoretical spectra obtained at different DFT levels were compared to the experimental one. The optimized cluster at the M06-2X/Lanl2dz level appears less deformed and has an IR spectrum better resembling the experimental one, compared to those at the CAM-B3LYP/Lanl2dz and M06-2X/Lanl2mb levels.
- By comparing the scaled spectrum at the M06-2X/Lanl2dz level with the experimental broad band intensity at 3401.6 cm−1, the number of hydration water molecules was predicted to be seven or eight. The distribution of these water molecules appears to be around the active centers of the complex, not only the La(III) ion or the carboxylate groups, but also the nitrogen atoms. These H-bonds/interactions should not be very strong because, in the experimental spectrum, bands corresponding to strongly H-bonded water molecules do not appear.
- Several new scaling equations were used to improve the calculated spectra. In the isolated state, the scaled spectra at the M06-2X/Lanl2mb level appear closest to the experimental one due to its packing form. However, this level dramatically fails in the calculation of the hydrated form spectrum. With the scaling, the worst fit with experimental data was observed at the B3LYP/Cep-4g level, and therefore, the results achieved with this level were not considered.
- The scaled wavenumbers of the most intense IR and Raman vibrations in the spectrum at M06-2X/Lanl2dz level appear to show the best adherence, based on both frequency and intensity, to the most intense experimental IR and Raman bands. Therefore, the spatial arrangement of the ligands in the synthesized La(III) complex was confirmed, as well as the assignments of the bands.
- The O-H bending of the water molecules contributes to the broadening of the experimental IR band at 1577.8 cm−1.
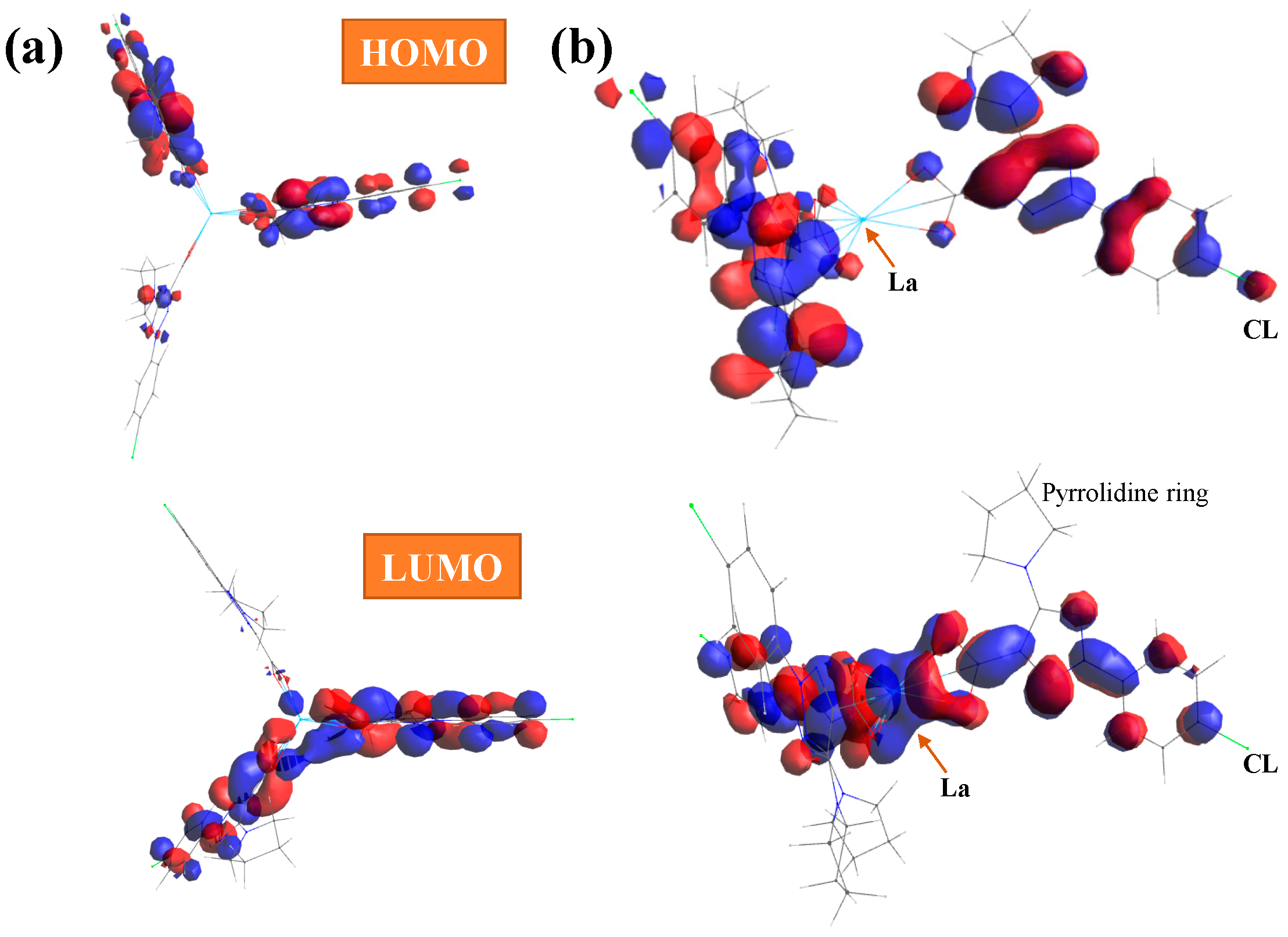
- The HOMO orbital shows the charge density localized over the triazole ring and the neighboring carbon atoms, while in the LUMO orbital, the charge distribution appears mainly on the oxygen and lanthanum ion. The hydration water affects the orbital energies very little, and only a slight reduction in negative HOMO energy was observed.
- The calculated molecular properties and global chemical descriptors of this complex indicate large chemical reactivity and low excitation energies for the excited states.
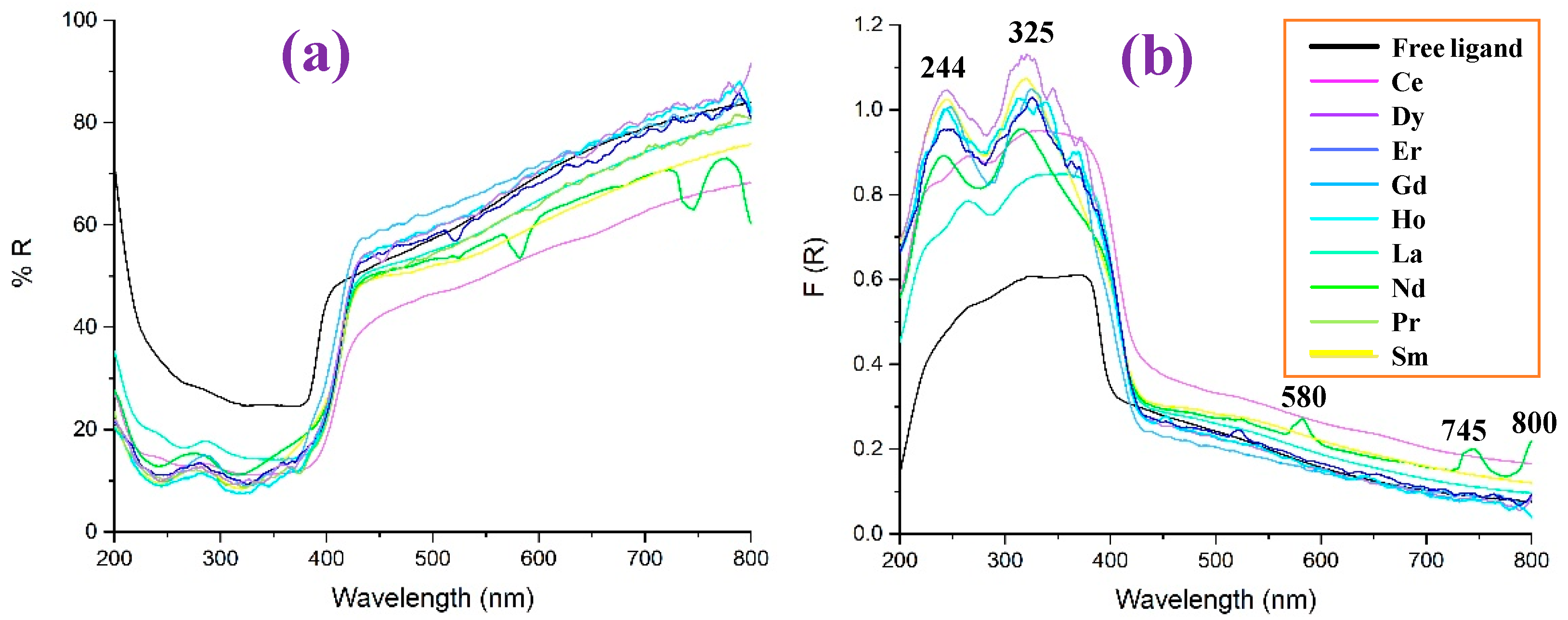
- Large similarity was observed in the comparison of the IR and Raman spectra of La(2b′)3 with that obtained with other lanthanide ions. Only small shifts appear in the very broad IR band corresponding to the ν(O-H) stretching mode, due to the different arrangements of the hydration water molecules.
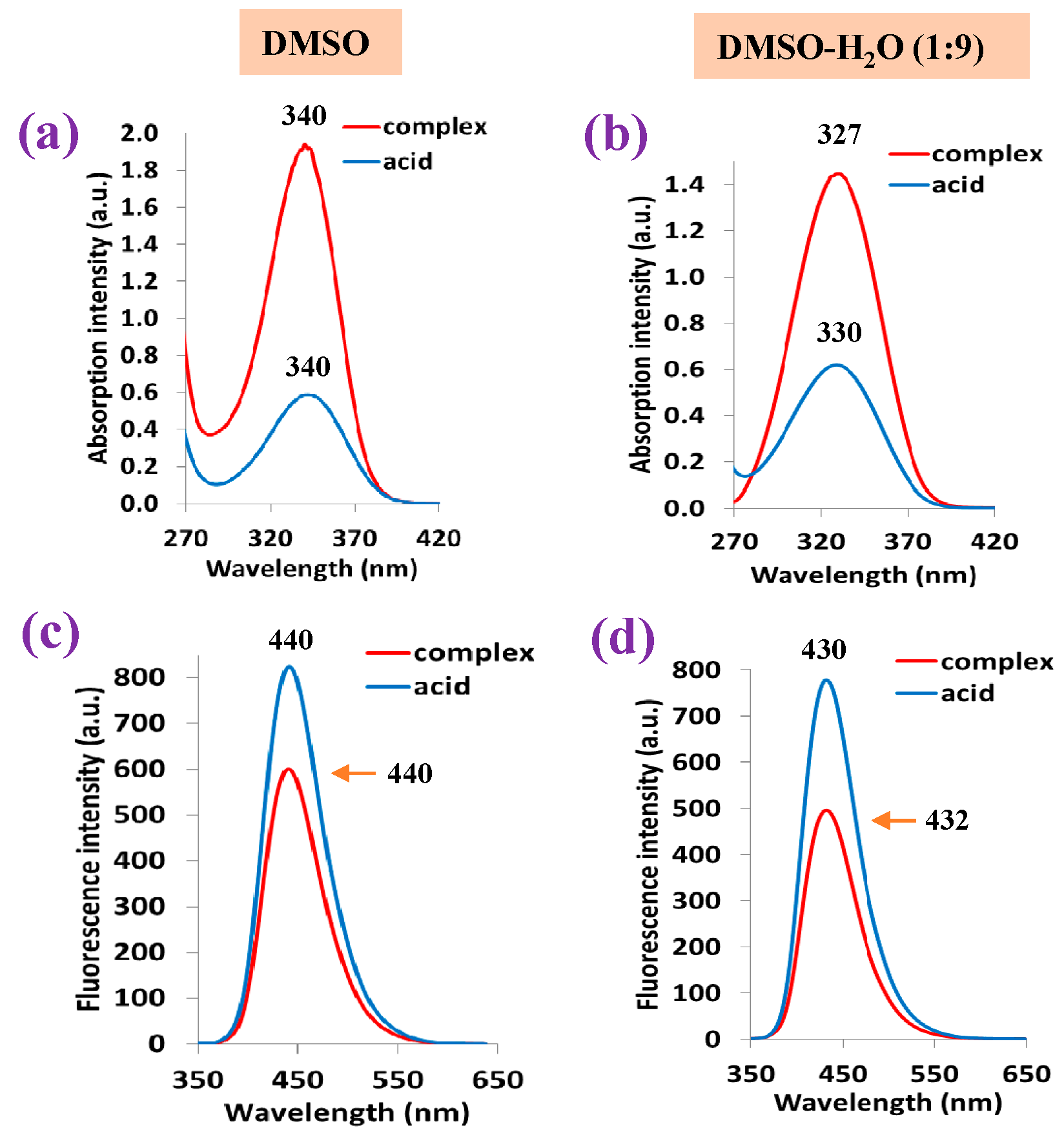
- Photophysical properties were also investigated. The absorbance of the free ligand in DMSO solution is similar to that obtained in all Ln complexes, with the same absorbance maximum at 340 nm. However, a blue shift in the fluorescence spectra in DMSO solution is observed in the complexes compared to the free ligand. In addition, a general blue shift in the fluorescence is observed in the solid-state sample with respect to that in solution, probably due to the appearance of H-aggregates.
- Lanthanide complexes exhibit two distinct absorption peaks in the diffuse reflectance F(R) spectra, while the free ligand shows remarkably higher reflectance values, UV-Vis values, and a lower F(R) value in the 200–400 nm range.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kostova, I.; Soni, R. Bioinorganic Chemistry; Shree Publishers & Distributors: New Delhi, India, 2011; ISBN 978-81-8329-420-1. [Google Scholar]
- Goswami, A.K.; Kostova, I. Medicinal and Biological Inorganic Chemistry; Walter de Gruyter, Inc.: Boston, MA, USA, 2022; ISBN 1501516116/9781501516115. [Google Scholar]
- Omodara, L.; Pitkäaho, S.; Turpeinen, E.-M.; Saavalainen, P.; Oravisjärvi, K.; Keiski, R.L. Recycling and substitution of light rare earth elements, cerium, lanthanum, neodymium, and praseodymium from end-of-life applications-A review. J. Clean. Prod. 2019, 236, 117573. [Google Scholar] [CrossRef]
- Li, L.; Yan, M. Recent progresses in exploring the rare earth based intermetallic compounds for cryogenic magnetic refrigeration. J. Alloys Compd. 2020, 823, 153810. [Google Scholar] [CrossRef]
- Ascenzi, P.; Bettinelli, M.; Boffi, A.; Botta, M.; De Simone, G.; Luchinat, C.; Marengo, E.; Mei, H.; Aime, S. Rare earth elements (REE) in biology and medicine. Rend. Lince-Sci. Fis. Nat. 2020, 31, 821–833. [Google Scholar] [CrossRef]
- Wang, J.; Li, S. Applications of rare earth elements in cancer: Evidence mapping and scientometric analysis. Front. Med. 2022, 9, 946100. [Google Scholar] [CrossRef]
- Patyal, M.; Kaur, K.; Bala, N.; Gupta, N.; Malik, A.K. Innovative Lanthanide Complexes: Shaping the future of cancer/tumor Chemotherapy. J. Trace Elements Med. Biol. 2023, 80, 127277. [Google Scholar] [CrossRef]
- Fouad, R. Synthesis and characterization of lanthanide complexes as potential therapeutic agents. J. Coord. Chem. 2020, 73, 2015–2028. [Google Scholar] [CrossRef]
- Bao, G. Lanthanide complexes for drug delivery and therapeutics. J. Lumin. 2020, 228, 117622. [Google Scholar] [CrossRef]
- Peica, N.; Kostova, I.; Kiefer, W. Theoretical and experimental studies on binding mode of 3,5-pyrazoledicarboxylic acid in its new La (III) complex. Chem. Phys. 2006, 325, 411–421. [Google Scholar] [CrossRef]
- Dong, G.; Jiang, Y.; Zhang, F.; Zhu, F.; Liu, J.; Xu, Z. Recent updates on 1,2,3-, 1,2,4-, and 1,3,5-triazine hybrids (2017–present): The anticancer activity, structure–activity relationships, and mechanisms of action. Arch. Pharm. 2023, 356, 2200479. [Google Scholar] [CrossRef]
- Le, D.A.; Truong, N.H.; Vu, V.D.; Doan, T.H.; Le, M.T.; Nguyen, T.H.; Nguyen, M.C.; Do, H.N.; Ninh, D.B.; Le, P.; et al. Design, Synthesis, and Biological Activity Evaluation of Novel AZT and Adenosine-Derived 1,2,3-Triazoles. J. Chem. 2023, 2023, 1–17. [Google Scholar] [CrossRef]
- Bozorov, K.; Zhao, J.; Aisa, H.A. 1,2,3-Triazole-containing hybrids as leads in medicinal chemistry: A recent overview. Bioorg. Med. Chem. 2019, 27, 3511–3531. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.M. 1,2,3-Triazole hybrids as anticancer agents: A review. Arch. Pharm. 2022, 355, e2100158. [Google Scholar] [CrossRef]
- Ihmaid, S.K.; Alraqa, S.Y.; Aouad, M.R.; Aljuhani, A.; Elbadawy, H.M.; Salama, S.A.; Rezki, N.; Ahmed, H.E.A. Design of molecular hybrids of phthalimide-triazole agents with potent selective MCF-7/HepG2 cytotoxicity: Synthesis, EGFR inhibitory effect, and metabolic stability. Bioorg. Chem. 2021, 111, 104835. [Google Scholar] [CrossRef] [PubMed]
- Liang, T.; Sun, X.; Li, W.; Hou, G.; Gao, F. 1,2,3-Triazole-containing compounds as anti–lung cancer agents: Current developments, mechanisms of action, and structure–activity relationship. Front. Pharmacol. 2021, 12, 661173. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Wang, J.; Xia, S.; Cheng, S.; Shi, Y. 1,2,3-Triazole Derivatives with Anti-breast Cancer Potential. Curr. Top. Med. Chem. 2022, 22, 1406–1425. [Google Scholar] [CrossRef]
- Çelik, F.; Ustabaş, R.; Süleymanoğlu, N.; Direkel, Ş.; Güler, H.İ.; Ünver, Y. 1,2,3-triazole derivative: Synthesis, characterization, DFT, molecular docking study and antibacterial-antileishmanial activities. J. Indian Chem. Soc. 2021, 98, 100105. [Google Scholar] [CrossRef]
- Tariq, S.; Kamboj, P.; Alam, O.; Amir, M. 1,2,4-Triazole-based benzothiazole/benzoxazole derivatives: Design, synthesis, p38α MAP kinase inhibition, anti-inflammatory activity and molecular docking studies. Bioorganic Chem. 2018, 81, 630–641. [Google Scholar] [CrossRef]
- Danne, A.B.; Deshpande, M.V.; Sangshetti, J.N.; Khedkar, V.M.; Shingate, B.B. New 1,2,3-triazole-appended bis-pyrazoles: Synthesis, bioevaluation, and molecular docking. ACS Omega 2021, 6, 24879–24890. [Google Scholar] [CrossRef]
- Poonia, N.; Kumar, A.; Kumar, V.; Yadav, M.; Lal, K. Recent progress in 1H-1,2,3-triazoles as potential antifungal agents. Curr. Topics Med. Chem. 2021, 21, 2109–2133. [Google Scholar] [CrossRef]
- Aouad, M.R.; Khan, D.J.O.; Said, M.A.; Al-Kaff, N.S.; Rezki, N.; Ali, A.A.; Bouqellah, N.; Hagar, M. Novel 1,2,3-triazole derivatives as potential inhibitors against covid-19 main protease: Synthesis, characterization, molecular docking and DFT studies. Chem. Select. 2021, 6, 3468–3486. [Google Scholar] [CrossRef]
- Bonandi, E.; Christodoulou, M.S.; Fumagalli, G.; Perdicchia, D.; Rastelli, G.; Passarella, D. The 1,2,3-triazole ring as a bioisostere in medicinal chemistry. Drug Disc. Today 2017, 22, 1572–1581. [Google Scholar] [CrossRef]
- Chatterjee, S.; Kumar, N.; Sehrawat, H.; Yadav, N.; Mishra, V. Click triazole as a linker for drug repurposing against SARs-CoV-2: A greener approach in race to find COVID-19 therapeutic. Curr. Res. Green Sustain. Chem. 2021, 4, 100064. [Google Scholar] [CrossRef]
- Al-Humaidi, J.Y.; Shaaban, M.M.; Rezki, N.; Aouad, M.R.; Zakaria, M.; Jaremko, M.; Hagar, M.; Elwakil, B.H. 1,2,3-Triazole-Benzofused Molecular Conjugates as Potential Antiviral Agents against SARS-CoV-2 Virus Variants. Life 2022, 12, 1341. [Google Scholar] [CrossRef]
- Sur, V.P.; Sen, M.K.; Komrskova, K. In silico identification and validation of organic triazole based ligands as potential inhibitory drug compounds of SARS-CoV-2 main protease. Molecules 2021, 26, 6199. [Google Scholar] [CrossRef]
- Mohammadsaleh, F.; Jahromi, M.D.; Hajipour, A.R.; Hosseini, S.M.; Niknam, K. 1,2,3-Triazole framework: A strategic structure for C-H-X hydrogen bonding and practical design of an effective Pd-catalyst for carbonylation and carbon–carbon bond formation. RSC Adv. 2021, 11, 20812–20823. [Google Scholar] [CrossRef] [PubMed]
- Lauria, A.; Delisi, R.; Mingoia, F.; Terenzi, A.; Martorana, A.; Barone, G.; Almerico, A.M. 1,2,3-Triazole in heterocyclic compounds, endowed with biological activity, through 1,3-dipolar cycloadditions: 1,2,3-triazole units in biologically active heterocycles. Eur. J. Org. Chem. 2014, 2014, 3289–3306. [Google Scholar] [CrossRef]
- Palafox, M.A.; Belskaya, N.P.; Kostova, I.P. Study of the Molecular Architectures of 2-(4-Chlorophenyl)-5-(pyrrolidin-1-yl)-2H-1,2,3-triazole-4-carboxylic Acid Using Their Vibrational Spectra, Quantum Chemical Calculations and Molecular Docking with MMP-2 Receptor. Pharmaceutics 2023, 15, 2686. [Google Scholar] [CrossRef] [PubMed]
- Safronov, N.E.; Kostova, I.P.; Palafox, M.A.; Belskaya, N.P. Combined NMR Spectroscopy and Quantum-Chemical Calculations in Fluorescent 1,2,3-Triazole-4-carboxylic Acids Fine Structures Analysis. Int. J. Mol. Sci. 2023, 24, 8947. [Google Scholar] [CrossRef]
- Taha, Z.A.; Hijazi, A.K.; Al Momani, W.M. Lanthanide complexes of the tridentate Schiff base ligand salicylaldehyde-2-picolinoylhydrazone: Synthesis, characterization, photophysical properties, biological activities and catalytic oxidation of aniline. J. Mol. Struct. 2020, 1220, 128712. [Google Scholar] [CrossRef]
- Palafox, M.A.; Belskaya, N.P.; Todorov, L.T.; Kostova, I.P. Structural Study of a La (III) Complex of a 1, 2, 3-Triazole Ligand with Antioxidant Activity. Antioxidants 2023, 12, 1872. [Google Scholar] [CrossRef]
- Palafox, M.A.; Belskaya, N.P.; Todorov, L.T.; Hristova-Avakoumova, N.G.; Kostova, I.P. Effect of Lanthanide Ions and Triazole Ligands on the Molecular Properties, Spectroscopy and Pharmacological Activity. Int. J. Mol. Sci. 2024, 25, 7964. [Google Scholar] [CrossRef]
- Williams, R.W.; Cheh, J.L.; Lowrey, A.H.; Weir, A.F. Effects of hydration on scale factors for ab initio force constants. 9. methanol. J. Phys. Chem. 1995, 99, 5299–5307. [Google Scholar] [CrossRef]
- Goyal, P.; Qian, H.-J.; Irle, S.; Lu, X.; Roston, D.; Mori, T.; Elstner, M.; Cui, Q. Molecular Simulation of Water and Hydration Effects in Different Environments: Challenges and Developments for DFTB Based Models. J. Phys. Chem. B 2014, 118, 11007–11027. [Google Scholar] [CrossRef]
- Danilov, V.I.; van Mourik, T.; Poltev, V.I. Modeling of the ‘hydration shell’ of uracil and thymine in small water clusters by DFT and MP2 methods. Chem. Phys. Lett. 2006, 429, 255–260. [Google Scholar] [CrossRef]
- van Mourik, T.; Danilov, V.I.; Gonzalez, E.; Deriabina, A.; Poltev, V.I. Indication of water droplet formation in hydrated clusters of cytosine and adenine: An electronic structure study using B3LYP, LMP2 and PM6. Chem. Phys. Lett. 2007, 445, 303–308. [Google Scholar] [CrossRef]
- Mishra, V.R.; Sekar, N. Photostability of coumarin laser dyes-a mechanistic study using global and local reactivity descriptors. J. Fluoresc. 2017, 27, 1101–1108. [Google Scholar] [CrossRef]
- Pearson, R.G. Chemical hardness and density functional theory. J. Chem. Sci. 2005, 117, 369–377. [Google Scholar] [CrossRef]
- Palafox, M.A. Scaling factors for the prediction of vibrational spectra. I. Benzene molecule. Int. J. Quantum Chem. 2000, 77, 661–684. [Google Scholar] [CrossRef]
- Palafox, M.A. DFT computations on vibrational spectra: Scaling procedures to improve the wavenumbers. Phys. Sci. Rev. 2018, 3, 20170184. [Google Scholar] [CrossRef]
- Varsányi, G. Assignment for Vibrational Spectra of Seven Hundred Benzene Derivatives. Hilder, A., Ed.; Wiley: London, UK, 1974. [Google Scholar]
- Coates, J. Interpretation of Infrared Spectra, A Practical Approach. In Encyclopedia of Analytical Chemistry; John Wiley & Sons: London, UK, 2006. [Google Scholar]
- Ning, Y.-C. Interpretation of Organic Spectra; John Wiley & Sons Singapore Pte. Ltd.: Singapore, 2011. [Google Scholar]
- Aziz, S.G.; Elroby, S.A.; Alyoubi, A.; Osman, O.I.; Hilal, R. Experimental and theoretical assignment of the vibrational spectra of triazoles and benzotriazoles. Identification of IR marker bands and electric response properties. J. Mol. Model. 2014, 20, 2078. [Google Scholar] [CrossRef]
- Törnkvist, C.; Bergman, J.; Liedberg, B. Geometry and vibrations of the 1, 2, 3-triazole anion. A theoretical and experimental study. J. Phys. Chem. 1991, 95, 3119–3123. [Google Scholar] [CrossRef]
- El-Azhary, A.A.; Suter, H.U.; Kubelka, J. Experimental and Theoretical Investigation of the Geometry and Vibrational Frequencies of 1,2,3-Triazole, 1,2,4-Triazole, and Tetrazole Anions. J. Phys. Chem. A 1998, 102, 620–629. [Google Scholar] [CrossRef]
- Palafox, M.A.; Belskaya, N.P.; Todorov, L.T.; Hristova-Avakoumova, N.G.; Kostova, I.P. Molecular properties of a triazole–Ce(III) complex with antioxidant activity: Structure, spectroscopy, and relationships with related derivatives. Influence of the ligands in the complex. Front. Chem. 2024, 12, 1450106. [Google Scholar] [CrossRef]
- Safronov, N.E.; Fomin, T.O.; Minin, A.S.; Todorov, L.; Kostova, I.; Benassi, E.; Belskaya, N.P. 5-Amino-2-aryl-1,2,3-triazol-4-carboxylic acids: Synthesis, photophysical properties, and application prospects. Dye. Pigment. 2020, 178, 108343. [Google Scholar] [CrossRef]
- Seminario, J.M.; Politzer, P. Modern Density Functional Theory: A Tool for Chemistry; Elsevier: Amsterdam, The Netherlands, 1995; Volume 2. [Google Scholar]
- Ridha, S.M.A.; Ghaleb, Z.T.; Ghaleb, A.M. The Computational Investigation of IR and UV-Vis Spectra of 2-isopropyl-5-methyl-1,4-benzoquinone Using DFT and HF Methods. East Eur. J. Phys. 2023, 1, 197–204. [Google Scholar] [CrossRef]
- Balci, K.; Akkaya, Y.; Akyuz, S.; Palavan-Unsal, N. DFT and MP2 based quantum mechanical calculations and a theoretical vibrational spectroscopic investigation on roscovitine, a potential drug to treat cancers. J. Raman Spectros 2011, 42, 719–732. [Google Scholar] [CrossRef]
- Subashchandrabose, S.; Krishnan, A.R.; Saleem, H.; Thanikachalam, V.; Manikandan, G.; Erdogdu, Y. FT-IR, FT-Raman, NMR spectral analysis and theoretical NBO, HOMO-LUMO analysis of bis(4-amino-5-mercapto-1,2,4-triazol-3-yl)ethane by ab initio HF and DFT methods. J. Mol. Struct. 2010, 981, 59–70. [Google Scholar] [CrossRef]
- Sundaraganesan, N.; Anand, B.; Meganathan, C.; Joshua, B.D. FT-IR, FT-Raman spectra and ab initio HF, DFT vibrational analysis of p-chlorobenzoic acid. Spectrochim. Acta Part A Mol. Biomol. Spectros. 2008, 69, 871–879. [Google Scholar] [CrossRef]
- Frisch, M.J.; Head-Gordon, M.; Pople, J.A. Direct MP2 gradient method. Chem. Phys. Lett. 1990, 166, 275–280. [Google Scholar] [CrossRef]
- Zhao, Y.; Truhlar, D.G. A new local density functional for main-group thermochemistry, transition metal bonding, thermo-chemical kinetics, and noncovalent interactions. J. Chem. Phys. 2006, 125, 194101–194106. [Google Scholar] [CrossRef]
- Zhao, Y.; Truhlar, D.G. Applications and validations of the Minnesota density functional. Chem. Phys. Lett. 2011, 502, 1–13. [Google Scholar] [CrossRef]
- Zhao, Y.; Truhlar, D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar] [CrossRef]
- Riley, K.E.; Hobza, P. Noncovalent Interactions in Biochemistry. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2011, 1, 3–17. [Google Scholar] [CrossRef]
- Riley, K.E.; Pitoňák, M.; Jurečka, P.; Hobza, P. Stabilization and Structure Calculations for Noncovalent Interactions in Extended Molecular Systems Based on Wave Function and Density Functional Theories. Chem. Rev. 2010, 110, 5023–5063. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional thermochemistry. 3. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the elec-tron-density. Phys. Rev. 1988, B37, 785–789. [Google Scholar] [CrossRef]
- Yanai, T.; Tew, D.P.; Handy, N.C. A new hybrid exchange–correlation functional using the Coulomb-attenuating method (CAM-B3LYP). Chem. Phys. Lett. 2004, 393, 51–57. [Google Scholar] [CrossRef]
- Frisch, M.E.; Trucks, G.; Schlegel, H.; Scuseria, G.; Robb, M.; Cheeseman, J.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, revision C. 01; Gaussian, Inc.: Wallingford, CT, USA, 2016.
- Dunning, T.H., Jr.; Hay, P.J. Modern Theoretical Chemistry; Schaefer, H.F., III, Ed.; Plenum: New York, NY, USA, 1977; Volume 3, pp. 1–28. [Google Scholar]
- Collins, J.B.; Schleyer, P.v.R.; Binkley, J.S.; Pople, J.A. Self-consistent molecular orbital methods. XVII. Geometries and binding energies of second-row molecules. A comparison of three basis sets. J. Chem. Phys. 1976, 64, 5142–5151. [Google Scholar] [CrossRef]
- Cundari, T.R.; Stevens, W.J. Effective core potential methods for the lanthanides. J. Chem. Phys. 1993, 98, 5555–5565. [Google Scholar] [CrossRef]
- Srivastav, G.; Yadav, B.; Yadav, R.K.; Yadav, R. DFT studies of molecular structures conformers and vibrational characteristics of sulfanilamide. Comp. Theor. Chem. 2019, 1167, 112588. [Google Scholar] [CrossRef]
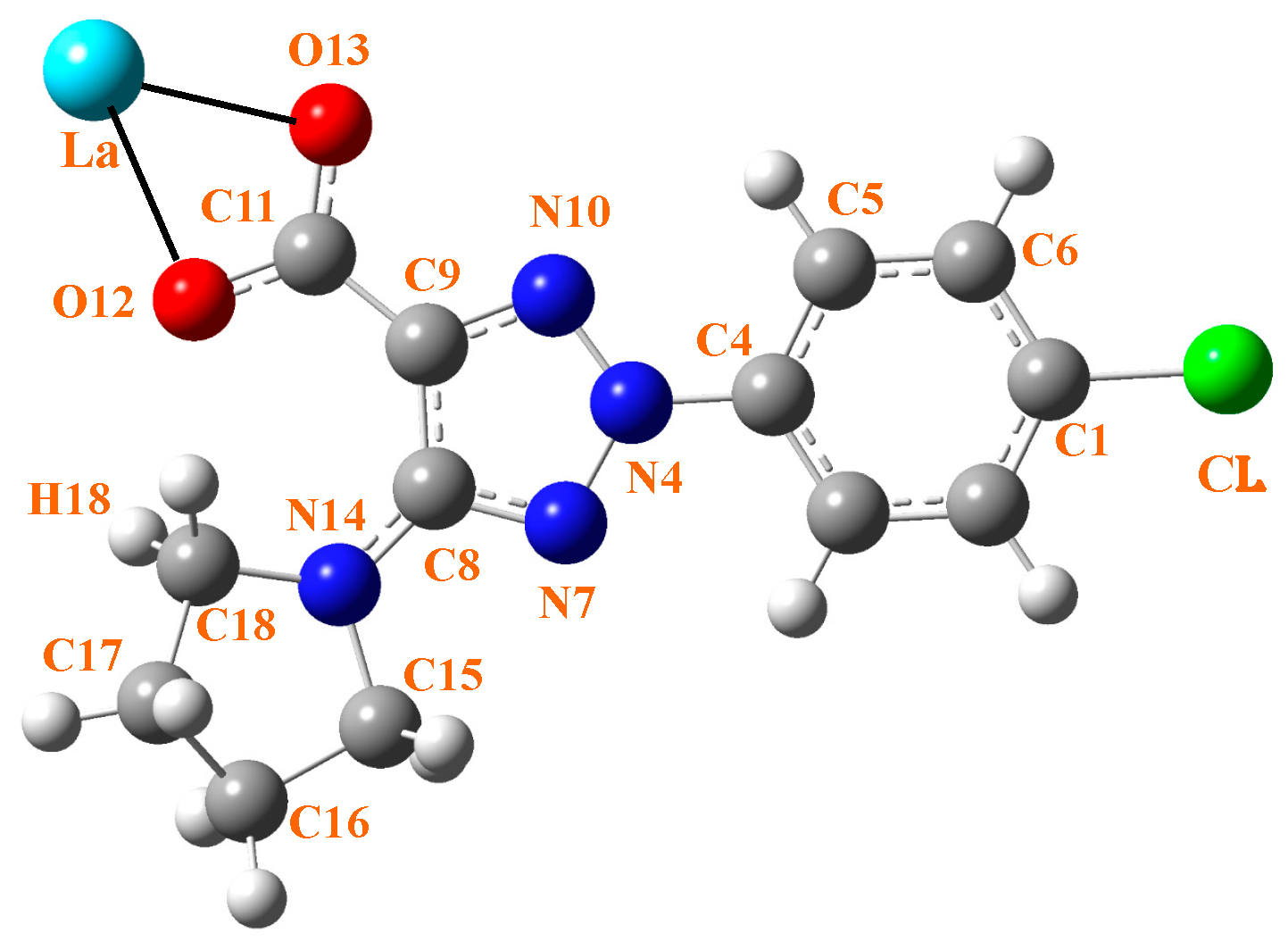



| Parameters | 2b’ | La(2b’)3 Isolated State | La(2b’)3 + 10 H2O | ||||
|---|---|---|---|---|---|---|---|
| MP2 | B3LYP | CAM-B3LYP | M06-2X | CAM-B3LYP | M06-2X | ||
| 6-31G(d,p) | Cep-4g | Lanl2dz | Lanl2dz | Lanl2mb | Lanl2dz | Lanl2dz | |
| r(C4-N4) | 1.397 | 1.538 | 1.422 | 1.421 | 1.450 | 1.422 | 1.418 |
| r(N4-N7) | 1.350 | 1.510 | 1.373 | 1.375 | 1.420 | 1.366 | 1.374 |
| r(N4-N10) | 1.352 | 1.480 | 1.330 | 1.330 | 1.391 | 1.342 | 1.341 |
| r(C8-C9) | 1.430 | 1.566 | 1.448 | 1.447 | 1.446 | 1.437 | 1.444 |
| r(C9-N10) | 1.348 | 1.487 | 1.358 | 1.359 | 1.372 | 1.350 | 1.350 |
| r(C9-C11) | 1.541 | 1.579 | 1.456 | 1.455 | 1.507 | 1.482 | 1.472 |
| r(C=O12) | 1.267 | 1.418 | 1.310 | 1.310 | 1.307 | 1.303 | 1.308 |
| r(C=O13) | 1.252 | 1.428 | 1.306 | 1.305 | 1.325 | 1.276 | 1.284 |
| r(La-O12) | - | 2.257 | 2.525 | 2.523 | 2.460 | 2.480 | 2.571 |
| r(La-O13) | - | 3.646 | 2.486 | 2.491 | 2.333 | 3.900 | 2.767 |
| O12···H18 | 2.034 | 2.257 | 2.464 | 2.443 | 1.867 | 2.323 | 2.139 |
| ∠(C4-N4-N7) | 121.9 | 120.7 | 122.0 | 121.9 | 121.6 | 122.5 | 122.2 |
| ∠(C4-N4-N10) | 122.0 | 119.8 | 122.8 | 122.8 | 121.7 | 122.8 | 122.9 |
| ∠(N-N-N) | 116.1 | 119.4 | 115.2 | 115.4 | 116.7 | 114.7 | 114.9 |
| ∠(N10-C9-C11) | 120.7 | 119.9 | 119.1 | 119.5 | 117.6 | 120.8 | 120.9 |
| ∠(N7-C8-N14) | 119.2 | 120.8 | 119.5 | 119.8 | 120.8 | 120.9 | 121.2 |
| ∠(C9-C8-N14) | 130.9 | 129.5 | 133.0 | 132.6 | 128.7 | 130.8 | 131.0 |
| ∠(C9-C11=O12) | 113.3 | 121.3 | 121.7 | 121.2 | 123.3 | 117.3 | 117.5 |
| ∠(C9-C11=O13) | 115.4 | 120.7 | 121.2 | 121.4 | 118.8 | 119.5 | 122.5 |
| ∠(O=C=O) | 131.3 | 117.9 | 117.1 | 117.4 | 117.8 | 123.1 | 120.0 |
| ∠(C=O12-La) | - | 87.9 | 94.0 | 94.0 | 87.1 | 137.3 | 98.6 |
| ∠(C=O13-La) | - | 89.0 | 95.9 | 95.7 | 92.2 | - | 90.2 |
| ∠(O12-La-O′13) | - | 95.2 | 132.0 | 132.6 | 79.8 | 120.6 | 116.7 |
| ∠(O13-La-O′12) | - | 119.2 | 121.9 | 121.8 | 132.8 | - | 115.4 |
| ∠(C5-C4-N4-N10) | −2.3 | −3.5 | 1.1 | 0.8 | 1.5 | −1.1 | −3.1 |
| ∠(C4-N4-N10-C9) | −178.8 | −177.9 | 179.0 | 178.9 | −178.6 | −179.1 | −178.1 |
| ∠(N4-N10-C9-C11) | 175.9 | 177.7 | −175.3 | −174.6 | −176.9 | 173.6 | 171.7 |
| ∠(C8-N7-N4-N10) | −1.4 | −0.6 | 0.3 | 0.3 | 0.7 | −0.3 | −0.6 |
| ∠(N10-C9-C=O12) | −145.6 | −149.9 | 167.0 | 166.8 | 160.0 | −174.4 | −151.5 |
| ∠(N10-C9-C-O13) | 33.4 | 34.0 | −11.2 | −11.2 | −16.5 | 2.8 | 26.1 |
| ∠(C8-C9-C=O12) | 29.8 | 28.9 | −8.1 | −7.5 | −16.7 | −1.6 | 19.8 |
| ∠(C11-C9-C8-N14) | 3.1 | 5.0 | −5.8 | −6.7 | -0.8 | 5.3 | 9.1 |
| ∠(C9-C8-N14-C15) | 172.2 | 167.6 | 176.3 | 176.8 | −174.7 | −172.2 | −172.5 |
| ∠(C9-C8-N14-C18) | 32.0 | 18.7 | −16.1 | −18.3 | −31.2 | 53.7 | 35.3 |
| ∠(C8-N14-C15-C16) | −162.4 | −140.6 | −177.2 | −179.4 | 159.9 | −170.9 | −166.0 |
| ∠(N14-C15-C16-C17) | −9.3 | −29.4 | −31.4 | −32.2 | 11.6 | 40.3 | −14.4 |
| ∠(C11-O12···O′12-C′11) | - | 153.5 | 11.7 | 13.2 | −169.5 | 90.5 | 3.4 |
| ∠(C9-C11···C′11-C′9) | - | −52.7 | 0.6 | 0.3 | 8.1 | 48.2 | 24.5 |
| ∠(C′9-C′11···C″11-C″9) | - | −21.1 | −4.7 | −3.2 | 6.8 | −74.9 | −66.7 |
| ∠(C11···La···C′11) | - | 145.6 | 120.6 | 121.1 | 132.2 | 96.5 | 114.4 |
| ∠(C′11···La···C″11) | 91.1 | 119.4 | 118.3 | 84.7 | 94.5 | 82.7 | |
| 2b′ | La(2b′)3 | La(2b′)3 + 10 H2O | |||||
|---|---|---|---|---|---|---|---|
| MP2 * | B3LYP | CAM-B3LYP | M06-2X | CAM-B3LYP | M06-2X | ||
| Atom | 6-31G(d,p) | Cep-4g | Lanl2dz | Lanl2dz | Lanl2mb | Lanl2dz | Lanl2dz |
| La | - | 2.884 | 3.095 | 3.164 | 2.066 | 2.979 | 2.869 |
| CL | −0.054 | −0.254 | −0.414 | −0.423 | −0.496 | −0.411 | −0.429 |
| C1 | −0.080 | 0.267 | 0.455 | 0.452 | 0.476 | 0.453 | 0.433 |
| C4 | 0.210 | 0.620 | 0.528 | 0.518 | 0.723 | 0.548 | 0.618 |
| N4 | −0.073 | −0.742 | 0.548 | −0.566 | −0.832 | −0.529 | −0.660 |
| N7 | −0.402 | −0.066 | −0.296 | −0.293 | −0.069 | −0.192 | −0.242 |
| C8 | 0.419 | 0.373 | 0.713 | 0.714 | 0.465 | 0.544 | 0.623 |
| C9 | 0.031 | −0.844 | −0.812 | −0.835 | −0.716 | −0.503 | −0.614 |
| N10 | −0.240 | 0.401 | 0.319 | 0.340 | 0.398 | 0.188 | 0.300 |
| C11 | 0.955 | 1.381 | 1.738 | 1.821 | 1.506 | 1.576 | 1.635 |
| O12 | −0.887 | −1.014 | −1.195 | −1.250 | −0.925 | −1.321 | −1.262 |
| O13 | −0.839 | −1.089 | −1.214 | −1.266 | −1.033 | −1.042 | −1.078 |
| N14 | −0.557 | −0.713 | −0.907 | −0.924 | −0.771 | −0.974 | −0.922 |
| La(2b′)3 | La(2b′)3 + 10 H2O | |||||
|---|---|---|---|---|---|---|
| Molecular Properties | B3LYP | CAM-B3LYP | M06-2X | CAM-B3LYP | M06-2X | |
| Cep-4g | Lanl2dz | Lanl2dz | Lanl2mb | Lanl2dz | Lanl2dz | |
| Rotational constants (GHz): | ||||||
| A | 0.035 | 0.019 | 0.019 | 0.040 | 0.021 | 0.023 |
| B | 0.013 | 0.017 | 0.017 | 0.015 | 0.017 | 0.016 |
| C | 0.012 | 0.012 | 0.012 | 0.014 | 0.012 | 0.012 |
| Cv (cal/mol·K) | 229.86 | 202.7 | 202.97 | 200.6 | 279.5 | 284.6 |
| S (cal/mol·K) | 376.53 | 349.2 | 348.72 | 345.3 | 424.3 | 428.0 |
| Dipole moment (Debye) | 4.377 | 4.099 | 4.085 | 3.323 | 5.807 | 5.526 |
| HOMO | −0.253 | −0.263 | −0.262 | −0.189 | −0.251 | −0.251 |
| LUMO | −0.146 | −0.039 | −0.052 | 0.009 | −0.033 | −0.052 |
| Eg | 0.107 | 0.224 | 0.210 | 0.198 | 0.218 | 0.199 |
| IP | 0.253 | 0.263 | 0.262 | 0.189 | 0.251 | 0.251 |
| EA | 0.146 | 0.039 | 0.052 | −0.009 | 0.033 | 0.052 |
| χ | 0.199 | 0.151 | 0.157 | 0.090 | 0.142 | 0.151 |
| η | 0.054 | 0.112 | 0.105 | 0.099 | 0.109 | 0.099 |
| S | 0.027 | 0.056 | 0.052 | 0.049 | 0.054 | 0.050 |
| Isolated State | +10 H2O | Experimental | Characterization | ||
|---|---|---|---|---|---|
| CAM-B3LYP | M06-2X | CAM-B3LYP | M06-2X | IR | |
| 1588 1474 1361 1266 1178 857 629 555 507 | 1581 1470 1361 1267 1184 814 668 599 520 | 1598 1486 1358 1275 1175 876 669 578 515 | 1602 1486 1365 1271 1181 854 639 559 508 | 1577.8 vs 1484.5 vs 1372.3 vs 1285.5 s 1178.2 m 829.7 vs 647.1 m 576.7 w 508.9 m | ν(C8-N14) + 8a, ν(CC) 19a, ν(CC) νs(COO) + νs(NNN) δas(C-H) in pyrrolidine ν(triazol) δas(COO) Γ(triazol) δ(NNN) δas(COO) in phase |
| Absorbance | Fluorescence | |||||
|---|---|---|---|---|---|---|
| Solvent | Compound | λmax, nm | ε, M−1 cm−1 | λem, nm | ΦF, % | Stokes Shift, nm/cm−1 |
| DMSO | Acid | 340 | 12,000 | 440 | 71.1 | 100/6684 |
| La complex | 340 | 32,200 | 440 | 41.0 | 100/6684 | |
| DMSO-H2O (1:9) | Acid | 330 | 13,200 | 430 | 44.2 | 100/6684 |
| La complex | 327 | 28,000 | 432 | 33.9 | 105/7433 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palafox, M.A.; Todorov, L.T.; Belskaya, N.P.; Álvarez-Conde, J.; Díaz-García, D.; Gómez-Ruiz, S.; Kostova, I.P. The Spectroscopic Characterization and Photophysical Properties of a Hydrated Lanthanum Ion Complex with a Triazole Ligand by Several DFT Methods. Molecules 2025, 30, 3412. https://doi.org/10.3390/molecules30163412
Palafox MA, Todorov LT, Belskaya NP, Álvarez-Conde J, Díaz-García D, Gómez-Ruiz S, Kostova IP. The Spectroscopic Characterization and Photophysical Properties of a Hydrated Lanthanum Ion Complex with a Triazole Ligand by Several DFT Methods. Molecules. 2025; 30(16):3412. https://doi.org/10.3390/molecules30163412
Chicago/Turabian StylePalafox, M. Alcolea, Lozan T. Todorov, Nataliya P. Belskaya, Javier Álvarez-Conde, Diana Díaz-García, Santiago Gómez-Ruiz, and Irena P. Kostova. 2025. "The Spectroscopic Characterization and Photophysical Properties of a Hydrated Lanthanum Ion Complex with a Triazole Ligand by Several DFT Methods" Molecules 30, no. 16: 3412. https://doi.org/10.3390/molecules30163412
APA StylePalafox, M. A., Todorov, L. T., Belskaya, N. P., Álvarez-Conde, J., Díaz-García, D., Gómez-Ruiz, S., & Kostova, I. P. (2025). The Spectroscopic Characterization and Photophysical Properties of a Hydrated Lanthanum Ion Complex with a Triazole Ligand by Several DFT Methods. Molecules, 30(16), 3412. https://doi.org/10.3390/molecules30163412










