CO2 Capture Performance and Preliminary Mechanistic Analysis of a Phase Change Absorbent
Abstract
1. Introduction
2. Results and Discussion
2.1. CO2 Absorption Performance of [TEPAH][Im]/NPA/H2O
2.1.1. Optimization of the Component Ratio for CO2 Absorption by [TEPAH][Im]/NPA/H2O
2.1.2. The Influence of Temperature on the CO2 Absorption Characteristics of [TEPAH][Im]/NPA/H2O
2.2. Regeneration Performance of [TEPAH][Im]/NPA/H2O
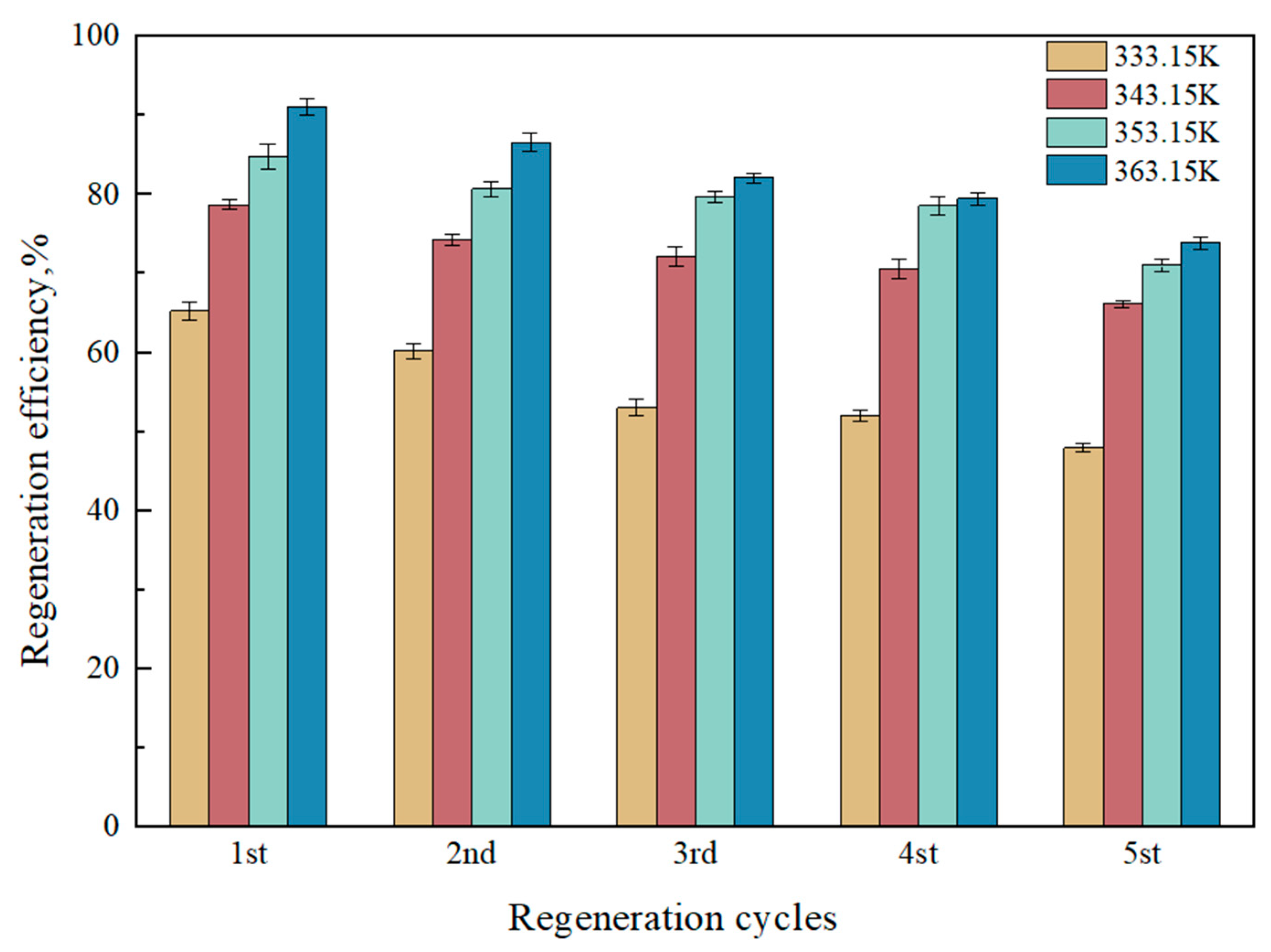
2.2.1. Regeneration Efficiency of [TEPAH][Im]/NPA/H2O
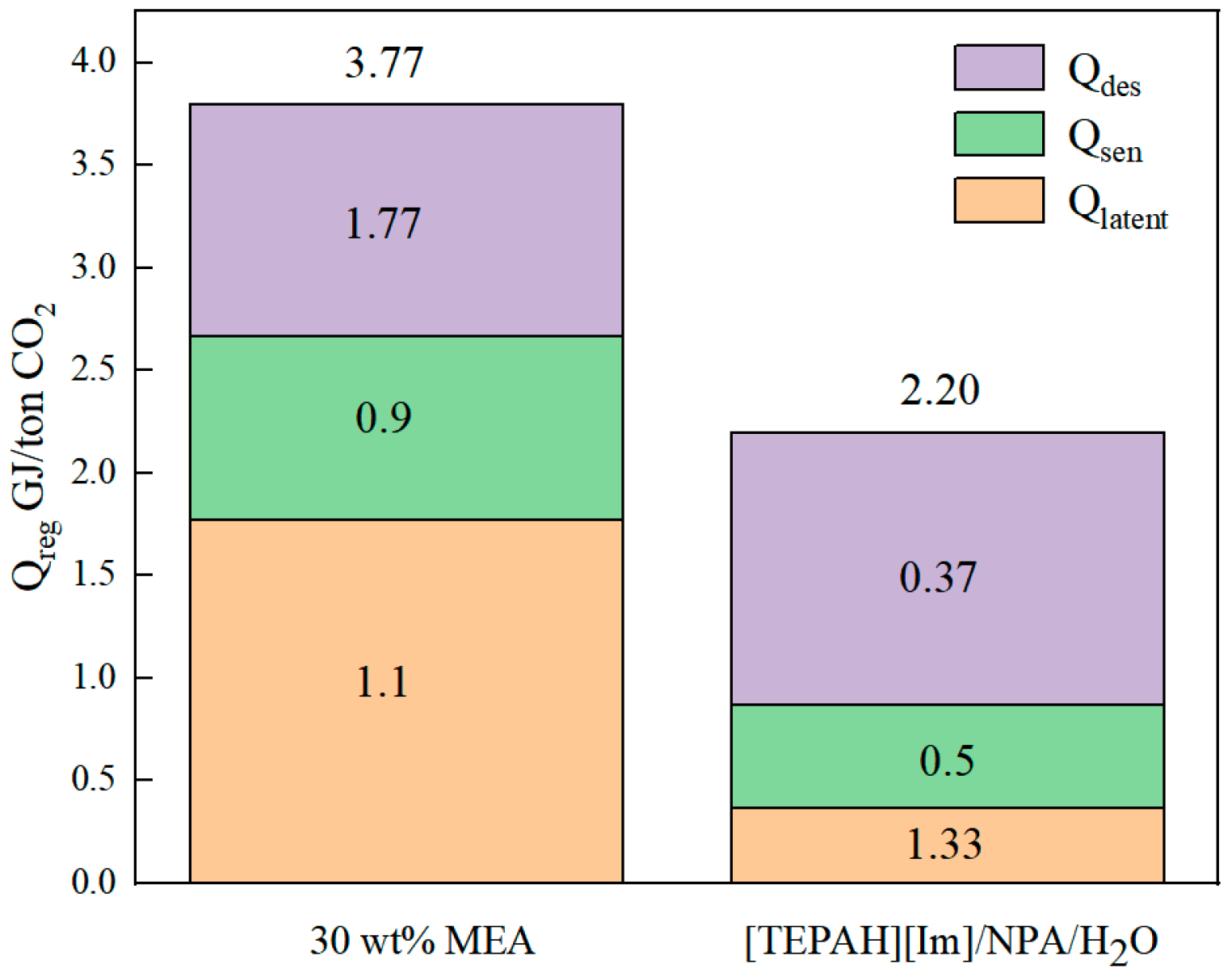
2.2.2. Heat Duty for Regeneration of [TEPAH][Im]/NPA/H2O
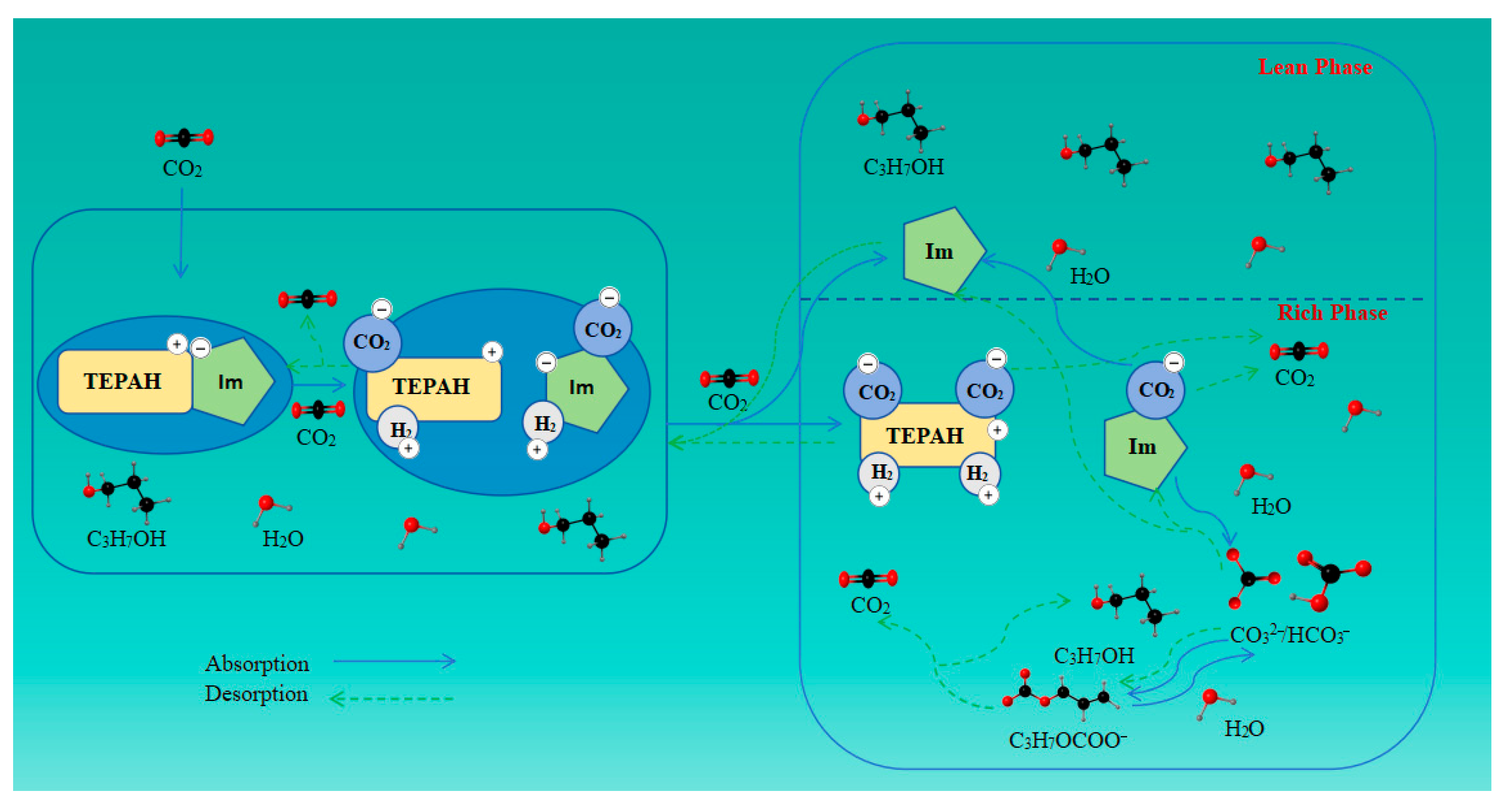
2.3. Proposed Mechanism of CO2 Capture by [TEPAH][Im]/NPA/H2O
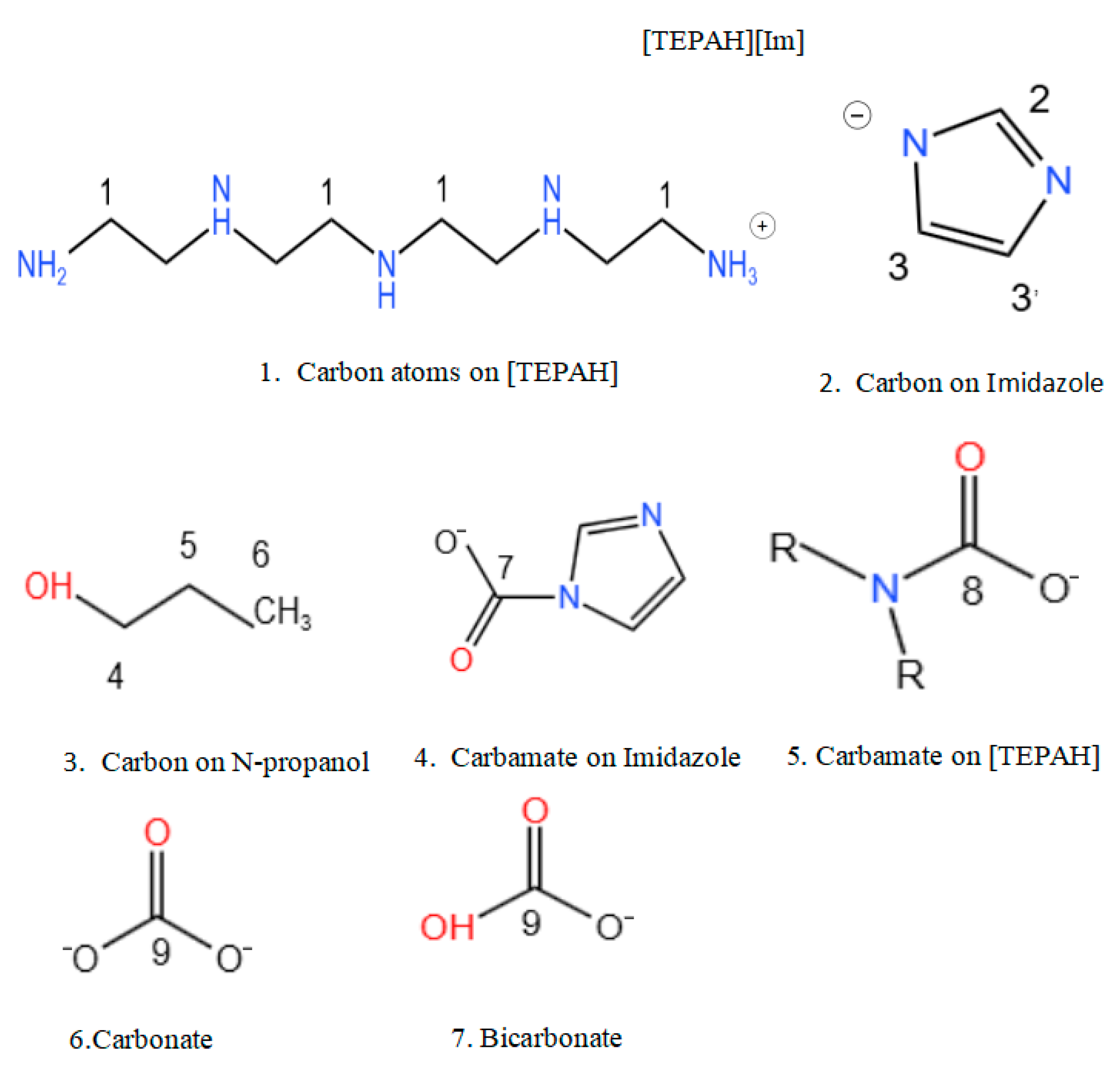
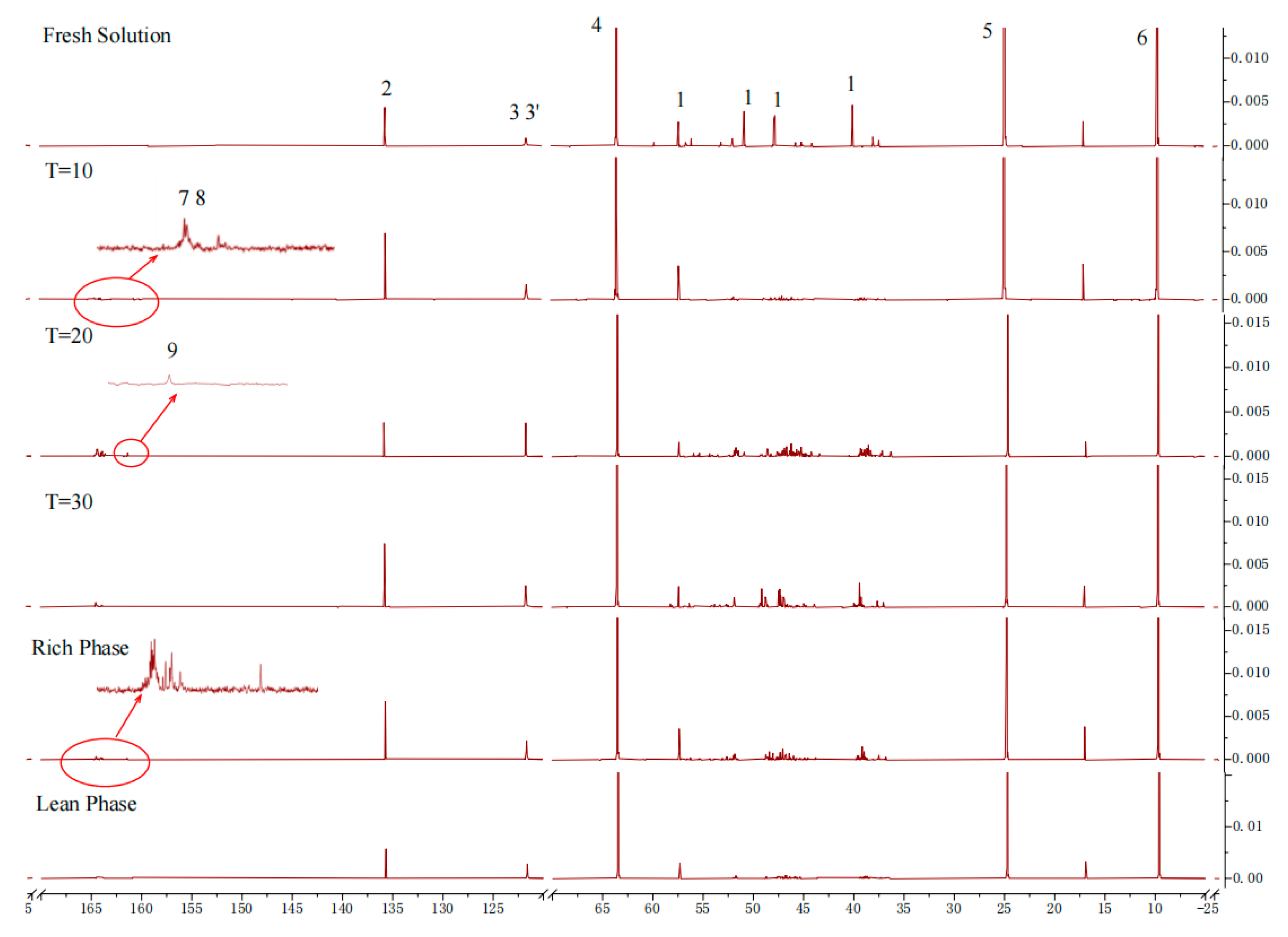
2.3.1. Characteristic of CO2 Absorption
2.3.2. Characteristic of CO2 Desorption
2.3.3. Proposed Mechanism of the CO2 Absorption–Desorption Reaction
3. Materials and Methods
3.1. Materials
3.2. Preparation of [TEPAH][Im]/NPA/H2O
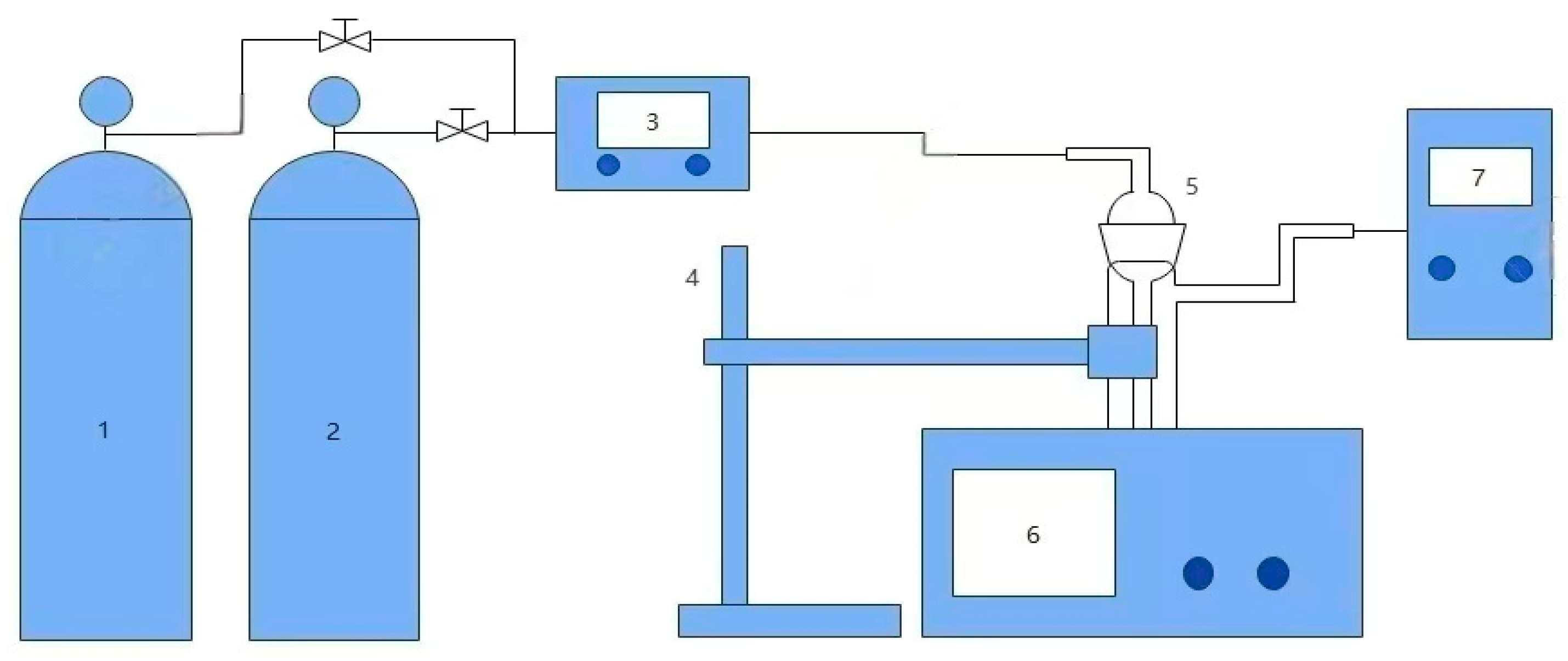
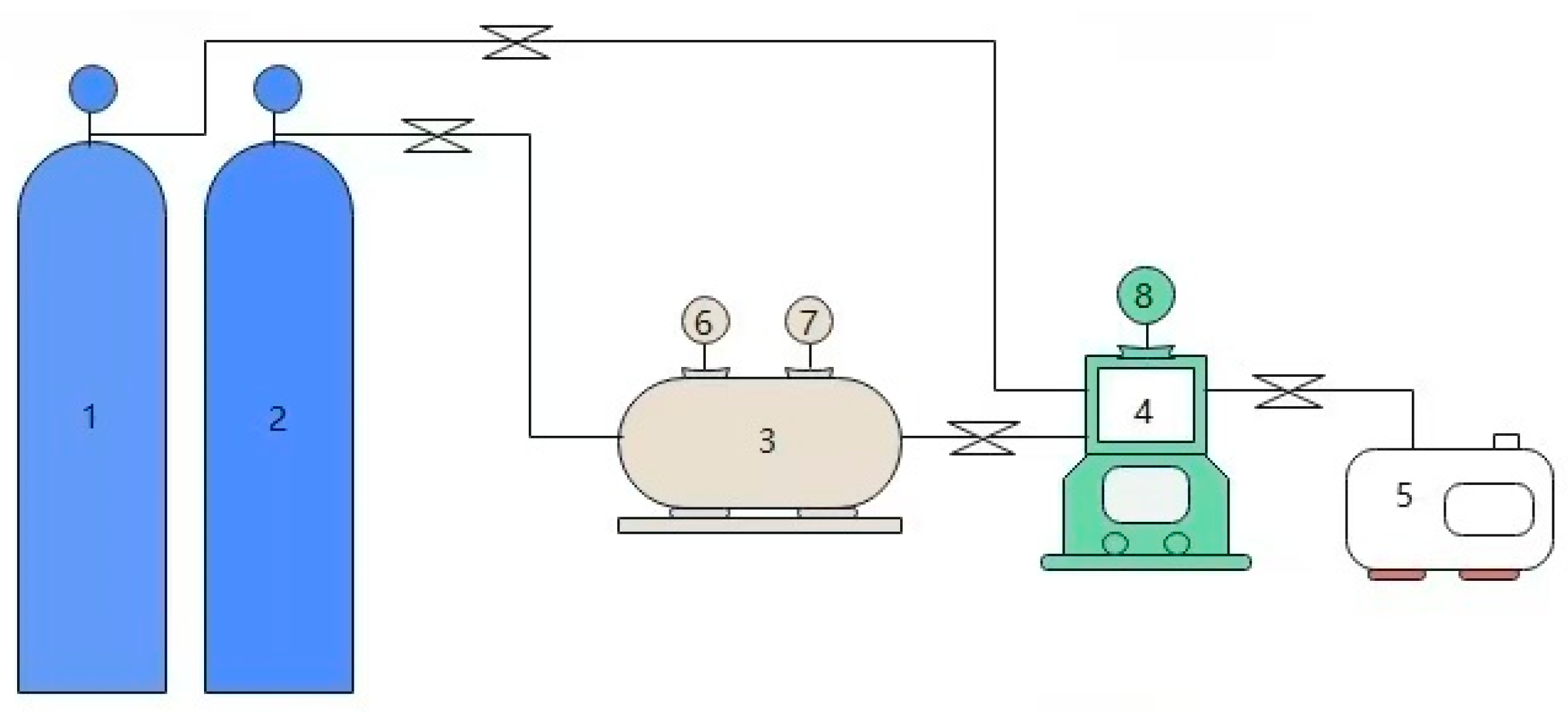
3.3. CO2 Absorption–Desorption Experiments
3.4. Viscosity Analysis
3.5. Nuclear Magnetic Resonance Experiments
3.6. Heat Duty
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Akitt, J.W. Some observations on the greenhouse effect at the Earth’s surface. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 188, 127–134. [Google Scholar] [CrossRef]
- Tao, C.; Sun, H.; Qiao, J.; Zhu, L.; Zhang, F.; Tang, Z.; Wei, X.; Yang, J.; Yuan, Q.; Wang, W.; et al. Cell-free chemoenzymatic starch synthesis from carbon dioxide. Science 2021, 373, 1523–1527. [Google Scholar] [CrossRef]
- Adegoke, K.A.; Maxakato, N.W. Efficient strategies for boosting the performance of 2D graphitic carbon nitride nanomaterials during photoreduction of carbon dioxide to energy-rich chemicals. Mater. Today Chem. 2022, 23, 100605. [Google Scholar] [CrossRef]
- Raza, A.; Gholami, R.; Rezaee, R.; Rasouli, V.; Rabiei, M. Significant aspects of carbon capture and storage—A review. Petroleum 2019, 54, 335–340. [Google Scholar] [CrossRef]
- Ahmed, S.; Khan, M.K.; Kim, J. Revolutionary advancements in carbon dioxide valorization via metal-organic framework-based strategies. Carbon Capture Sci. Technol. 2025, 15, 100405. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, J.B. Intermolecular interaction of diamine-diol binary system: A mini-review. Adv. Colloid Interface Sci. 2022, 304, 102662. [Google Scholar] [CrossRef]
- Li, X.L.; Zhou, X.B.; Wei, J.W.; Fan, Y.; Liao, L.; Wang, H. Reducing the energy penalty and corrosion of carbon dioxide capture using a novel nonaqueous monoethanolamine-based biphasic solvent. Sep. Purif. Technol. 2021, 265, 118481. [Google Scholar] [CrossRef]
- Li, Q.W.; Gao, G.; Wang, R.J.; Zhang, S.; An, S.; Wang, L. Role of 1-methylimidazole in regulating the CO2 capture performance of triethylenetetramine-based biphasic solvents. Int. J. Greenh. Gas Control 2021, 108, 103330. [Google Scholar] [CrossRef]
- Li, H.; Guo, H.; Shen, S. Low-Energy-Consumption CO2 Capture by Liquid–Solid Phase Change Absorption Using Water-Lean Blends of Amino Acid Salts and 2-Alkoxyethanols. ACS Sustain. Chem. Eng. 2020, 8, 12956–12967. [Google Scholar] [CrossRef]
- Jiang, W.F.; Wu, F.; Gao, G.; Li, X.; Zhang, L.; Luo, C. Absorption performance and reaction mechanism study on a novel anhydrous phase change absorbent for CO2 capture. Chem. Eng. J. 2021, 420, 129897. [Google Scholar] [CrossRef]
- Lv, B.H.; Yang, K.X.; Zhou, X.B.; Zhou, Z.; Jing, Z. 2-Amino-2-methyl-1-propanol based non-aqueous absorbent for energy-efficient and non-corrosive carbon dioxide capture. Appl. Energy 2020, 264, 114703. [Google Scholar]
- Zhou, X.B.; Li, X.L.; Wei, J.W.; Fan, Y.; Liao, L.; Wang, H. Novel Nonaqueous Liquid-Liquid Biphasic Solvent for Energy-Efficient Carbon Dioxide Capture with Low Corrosivity. Environ. Sci. Technol. 2020, 54, 16138–16146. [Google Scholar] [CrossRef]
- Karunarathne, S.S.; Eimer, D.A.; Qi, L.E. Density, viscosity and free energy of activation for viscous flow of CO2 loaded 2-amino-2-methyl-1-propanol (AMP), monoethanol amine (MEA) and H2O mixtures. J. Mol. Liq. 2020, 311, 113286. [Google Scholar] [CrossRef]
- Moore, T.; Rim, G.; Park, A.H.-A.; Mumford, K.A.; Stevens, G.W.; Webley, P.A. Encapsulation of highly viscous CO2 capture solvents for enhanced capture kinetics: Modeling investigation of mass transfer mechanisms. Chem. Eng. J. 2022, 428, 131603. [Google Scholar] [CrossRef]
- Shen, L.; Liu, F.; Shen, Y.; Sun, C.; Zhang, Y.; Wang, Q.; Li, S.; Li, W. Novel biphasic solvent of AEP/1-propanol/H2O for CO2 capture with efficient regeneration performance and low energy consumption. Sep. Purif. Technol. 2021, 270, 118700. [Google Scholar] [CrossRef]
- Wang, L.D.; Zhang, Y.F.; Wang, R.J.; Li, Q.; Zhang, S.; Li, M.; Liu, J.; Chen, B. Advanced Monoethanolamine Absorption Using Sulfolane as a Phase Splitter for CO2 Capture. Environ. Sci. Technol. 2018, 52, 14556–14563. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.F.; Liu, S.D.; Sun, K.; Jiang, J.C.; Wang, D.; Yan, Z.H.; Ji, X.Y. Kinetics study and performance evaluation of a hybrid choline-glycine/polyethylene glycol/water absorbent for CO2 separation. Sep. Purif. Technol. 2023, 304, 122410. [Google Scholar] [CrossRef]
- Bai, L.J.; Lu, S.J.; Zhao, Q.Z.; Chen, L.L.; Jiang, Y.J.; Jia, C.X.; Chen, S.M. Low–energy–consuming CO2 capture by liquid–liquid biphasic absorbents of EMEA/DEEA/PX. Chem. Eng. J. 2022, 450, 138490. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, X.X.; Ruan, H.B.; Li, J.L.; Yang, G.H. Study on the CO2 Absorption Performance of an AEP + DEGDME + H2O Phase-Change Absorbent. Energy Fuels 2024, 39, 592–603. [Google Scholar] [CrossRef]
- Hong, S.H.; Li, T.; Xiao, M.; Sema, T.; Gao, H.X.; Liang, Z.W. A low energy-consuming phase change absorbent of MAE/DGM/H2O for CO2 capture. Chem. Eng. J. 2024, 480, 148079. [Google Scholar] [CrossRef]
- Zalewski, M.; Krawczyk, T.; Siewniak, A.; Sobolewski, A. Carbon dioxide capture using water-imidazolium ionic liquids-amines ternary systems. Int. J. Greenh. Gas Control 2021, 105, 103210. [Google Scholar] [CrossRef]
- Wang, R.J.; Zhao, H.J.; Qi, C.; Yang, X.T.; Zhang, S.H.; Li, M.; Wang, L.D. Novel tertiary amine-based biphasic solvent for energy-efficient CO2 capture with low corrosivity. Energy 2022, 260, 125045. [Google Scholar] [CrossRef]
- Li, X.S.; Liu, J.; Jiang, W.F.; Gao, G.; Wu, F.; Luo, C.; Zhang, L.Q. Low energy-consuming CO2 capture by phase change absorbents of amine/alcohol/H2O. Sep. Purif. Technol. 2021, 275, 119181. [Google Scholar] [CrossRef]
- Chen, X.Y.; Lijin, Y.Z.; Chen, Y.L.; Jing, G.; Lv, B.; Dong, J.; Zhou, Z. Regulation mechanism of CO2 capture into the novel non-aqueous biphasic solvent: Solid-liquid phase change controllable. Sep. Purif. Technol. 2024, 340, 126751. [Google Scholar] [CrossRef]
- Zheng, Y.M.; Zhang, S.Y.; Liu, Y.C.; Wang, C.; Lv, B.; Jing, G.; Zhou, Z. A novel binary solid-liquid biphasic functionalized ionic liquids for efficient CO2 capture: Reversible polarity and low energy penalty. Purif. Technol. 2023, 313, 123486. [Google Scholar] [CrossRef]
- Shen, Y.; Jiang, C.K.; Zhang, S.H.; Chen, J.; Wang, L.D.; Chen, J.M. Biphasic solvent for CO2 capture: Amine property-performance and heat duty relationship. Chem. Eng. 2020, 838, 14493–14503. [Google Scholar] [CrossRef]
- Liu, C.; Jing, G.H.; Zhu, Z.P.; Fan, Y.M.; Mo, S.P.; Zhang, Y.N.; Wang, D.Q.; Lv, B.H.; Fu, M.M.; Zhou, X.B. Inhibiting effect of 2-amino-2-methyl-1-propanol on gelatinous product formation in non-aqueous CO2 absorbents: Experimental study and molecular understanding. Chem. Eng. Sci. 2024, 481, 148545. [Google Scholar] [CrossRef]
- Zhou, X.B.; Liu, C.; Fan, Y.M.; Zhang, L.H.; Tang, S.; Mo, S.P.; Zhu, Y.N.; Zhu, Z.Q. Energy-efficient carbon dioxide capture using a novel low-viscous secondary amine-based nonaqueous biphasic solvent: Performance, mechanism, and thermodynamics. Energy 2022, 255, 124570. [Google Scholar] [CrossRef]
- Han, J.C.; Qiu, Z.H.; Chen, Y.Y.; Gui, X.; Chen, X. A Novel Phase Change Absorbent for CO2 Capture with Low Viscosity and Effective Absorption–Desorption Properties. Energy Technol. 2024, 12, 2400114. [Google Scholar] [CrossRef]
- Zhang, W.D.; Jin, X.H.; Tu, W.W.; Ma, Q.; Mao, M.L.; Cui, C.H. Development of MEA-based CO2 phase change absorbent. Appl. Energy 2017, 195, 316–323. [Google Scholar] [CrossRef]
- Liu, F.; Fang, M.X.; Dong, W.F.; Wang, T.; Xia, Z.; Wang, Q.; Luo, Z. Carbon dioxide absorption in aqueous alkanolamine blends for biphasic solvents screening and evaluation. Appl. Energy 2019, 233–234, 468–477. [Google Scholar] [CrossRef]
- Ye, Q.; Wang, X.L.; Lu, Y.Q. Screening and evaluation of novel biphasic solvents for energy-efficient post-combustion CO2 capture. Int. J. Greenh. Gas Control 2015, 39, 205–214. [Google Scholar] [CrossRef]
- Xu, Z.C.; Wang, S.J.; Chen, C.H. CO2 absorption by biphasic solvents: Mixtures of 1,4-Butanediamine and 2-(Diethylamino)-ethanol. Ind. Eng. Chem. Res. 2013, 16, 107–115. [Google Scholar] [CrossRef]
- Zhou, X.B.; Liu, F.; Lv, B.H.; Zhou, Z.M.; Jing, G.H. Evaluation of the novel biphasic solvents for CO2 capture: Performance and mechanism. Int. J. Greenh. Gas Control 2017, 60, 120–128. [Google Scholar] [CrossRef]
- Zhou, X.B.; Jing, G.H.; Lv, B.H.; Liu, F.; Zhou, Z. Low-viscosity and efficient regeneration of carbon dioxide capture using a biphasic solvent regulated by 2-amino-2-methyl-1-propanol. Appl. Energy 2019, 235, 379–390. [Google Scholar] [CrossRef]
- Jin, L.J.; Hou, X.Y.; Zhan, L.X.; Hou, D.; Gu, L.; Zhang, D.; Shen, J.; Zheng, Z.; Lv, C.; Liu, S.; et al. Tuning and optimization of two-phase absorbents (DEEA/AEEA/H2O) with hybrid phase splitter (n-butanol/DEEA) for several properties: Carbon capture, phase separation, physical properties. Energy 2024, 288, 129802. [Google Scholar] [CrossRef]
- Shen, K.P.; Li, M.H. Solubility of CO2 in aqueous mixtures of monoethanolamine and dicyanamide-based ionic liquids. J. Chem. Eng. Data 1992, 371, 96–100. [Google Scholar] [CrossRef]
- Wang, L.D.; Liu, S.S.; Wang, R.J.; Li, Q.; Zhang, S. Regulating Phase Separation Behavior of a DEEA-TETA Biphasic Solvent Using Sulfolane for Energy-Saving CO2 Capture. Environ. Sci. Technol. 2019, 53, 12873–12881. [Google Scholar] [CrossRef]
- Wu, Z.Y.; Liu, S.; Gao, H.X.; Yin, Q.; Liang, Z. A study of structure-activity relationships of aqueous diamine solutions with low heat of regeneration for post-combustion CO2 capture. Energy 2019, 167, 359–368. [Google Scholar] [CrossRef]
- Lee, J.I.; Otto, F.D. Equilibrium between carbon dioxide and aqueous monoethanolamine solutions. J. Chem. Technol. Biotechnol. 2007, 261, 541–549. [Google Scholar] [CrossRef]


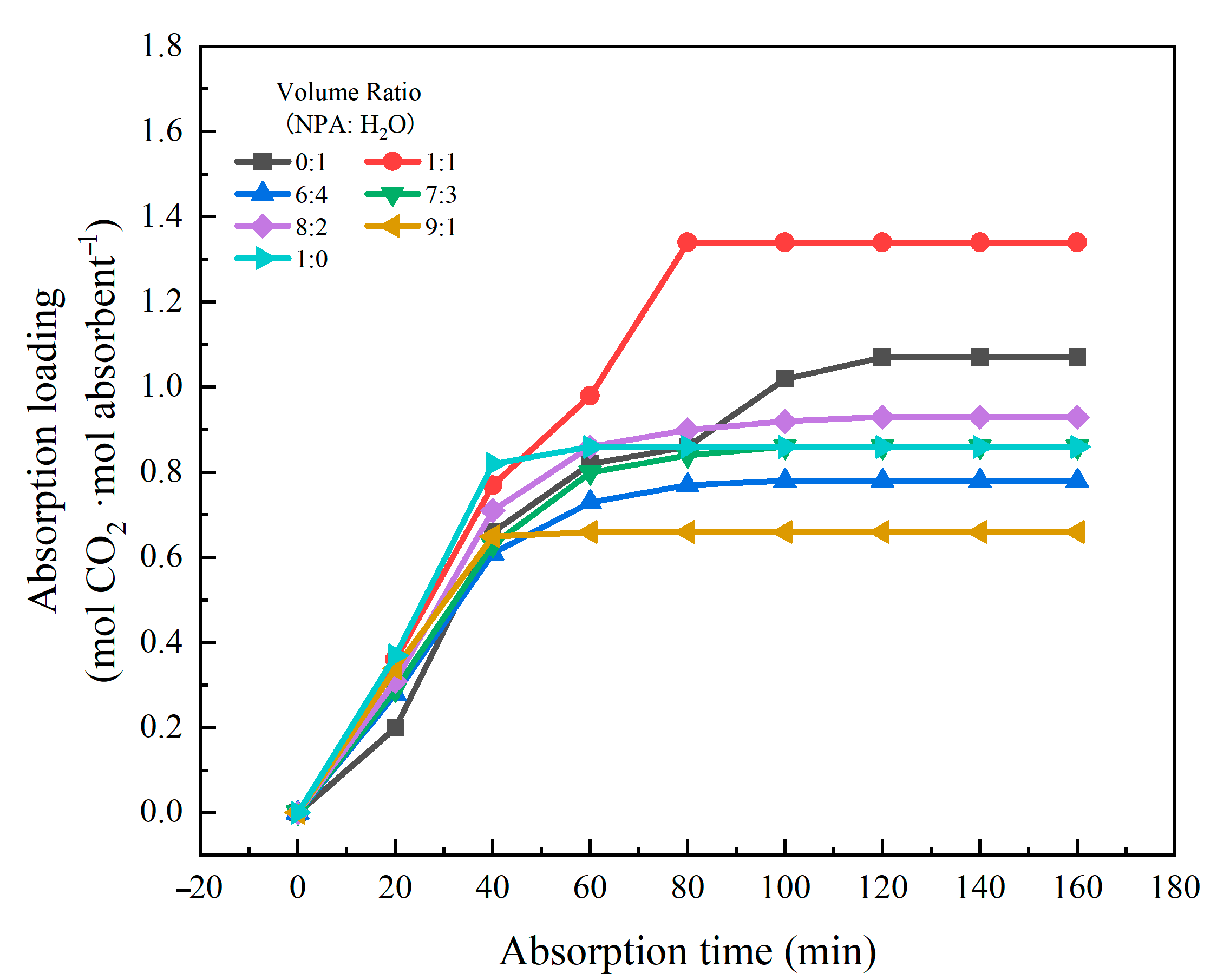

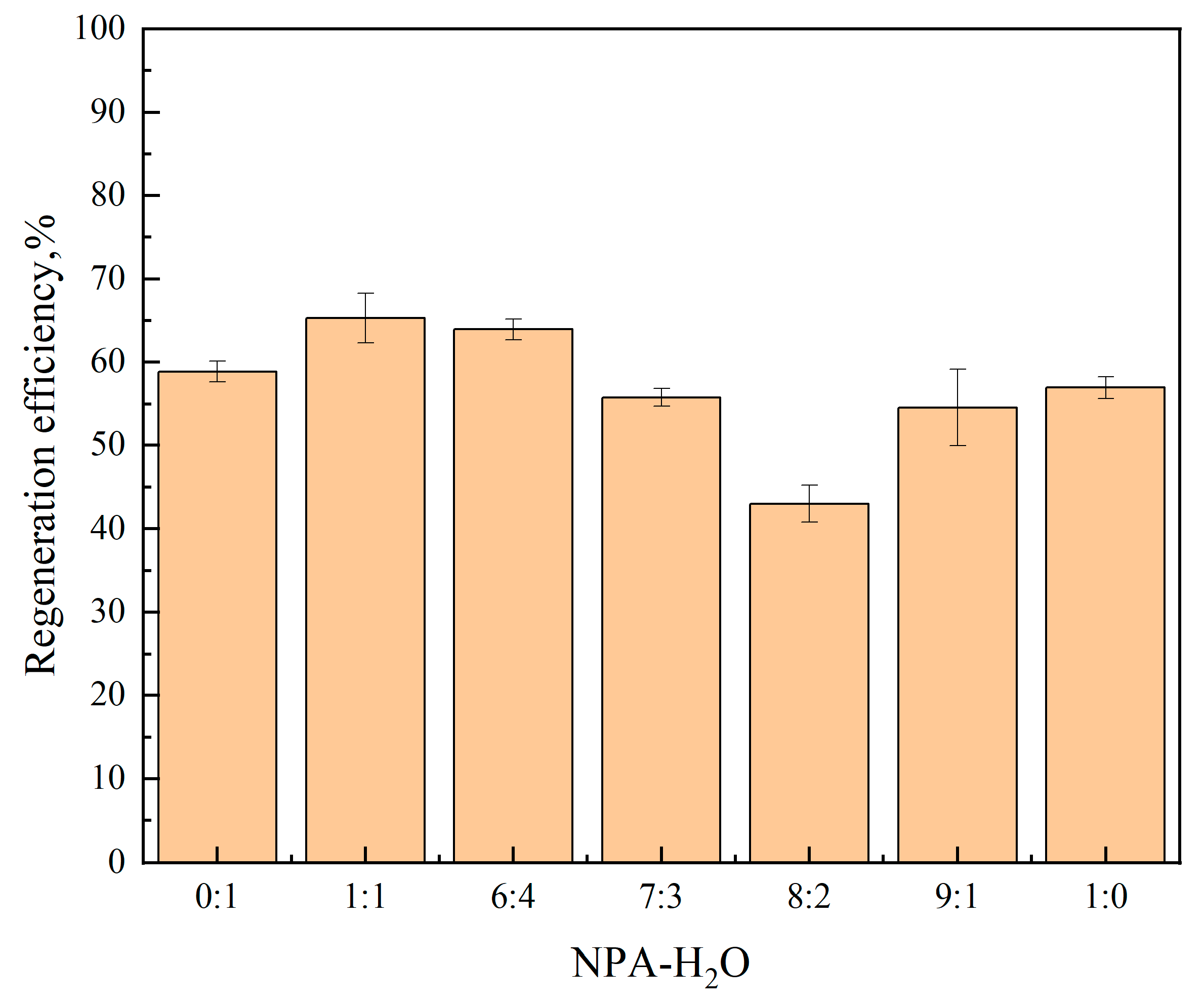


| VNPA:VH2O | Phase Separation | Viscosity (mPa·s) | Loading (mol·mol−1) | ||
|---|---|---|---|---|---|
| Before Absorption | After Absorption | Before Absorption | After Absorption | ||
| 0:1 | N | N | 1.23 ± 0.036 | 1.44 ± 0.029 | 1.07 ± 0.068 |
| 1:9 | N | N | 1.44 ± 0.056 | 1.48 ± 0.034 | 1.03 ± 0.083 |
| 2:8 | N | N | 1.84 ± 0.066 | 1.88 ± 0.057 | 1.15 ± 0.069 |
| 3:7 | N | N | 1.91 ± 0.055 | 1.92 ± 0.044 | 1.12 ± 0.071 |
| 4:6 | N | N | 2.22 ± 0.039 | 2.27 ± 0.052 | 0.95 ± 0.075 |
| 1:1 | N | Y | 2.80 ± 0.048 | 2.85 ± 0.047 (upper) | 1.34 ± 0.094 |
| 3.58 ± 0.044 (lower) | |||||
| 6:4 | N | Y | 3.17 ± 0.034 | 2.81 ± 0.032 (upper) | |
| 3.70 ± 0.029 (lower) | 0.78 ± 0.112 | ||||
| 7:3 | N | Y | 3.28 ± 0.033 | 2.77 ± 0.027 (upper) | |
| 3.62 ± 0.031 (lower) | 0.86 ± 0.136 | ||||
| 8:2 | N | Y | 3.51 ± 0.042 | 2.72 ± 0.033 (upper) | |
| 3.55 ± 0.041(lower) | 0.93 ± 0.083 | ||||
| 9:1 | N | Y | 3.63 ± 0.046 | 2.61 ± 0.044 (upper) | |
| 3.77 ± 0.046 (lower) | 0.66 ± 0.141 | ||||
| 1:0 | N | Y | 4.93 ± 0.035 | 2.45 ± 0.047 (upper) | |
| 4.53 ± 0.049 (lower) | 0.86 ± 0.106 | ||||
| Absorbents | Absorption Loading (mol·mol−1) | Saturated Viscosity (mPa·s) | Absorption Temperature (K) | Ref. |
|---|---|---|---|---|
| EDA/AMP/NMF | 1.07 | - | 303.15 | [24] |
| [IPDAH][Im]/H2O | 1.26 | 1.35 | 313.15 | [25] |
| AMP/PZ/DME | 0.87 | - | 313.15 | [26] |
| AMP/AEEA/NMP | 0.74 | - | 313.15 | [27] |
| MAE/DMSO/PMDETA | 0.84 | 8.87 | 313.15 | [28] |
| AEP/DEGDME/H2O | 1.23 | 6.20 | 313.15 | [29] |
| [TEPAH][Im]/NPA/H2O | 1.34 | 3.58 | 303.15 | This work |
| Temperature, K | Phase | Viscosity, mPa·s | Loading, mol·mol−1 |
|---|---|---|---|
| 303 | upper | 2.85 ± 0.055 | |
| lower | 3.42 ± 0.046 | 1.34 ± 0.094 | |
| 313 | upper | 2.66 ± 0.041 | |
| lower | 3.12 ± 0.051 | 1.23 ± 0.121 | |
| 323 | upper | 2.74 ± 0.039 | |
| lower | 3.05 ± 0.044 | 1.11 ± 0.142 | |
| 333 | upper | 2.71 ± 0.041 | |
| lower | 3.42 ± 0.046 | 1.05 ± 0.088 |
| Phase Change Absorbent | Regeneration Temperature (K) | Regeneration Efficiency | Desorption Time (min) | Ref. |
|---|---|---|---|---|
| [TEPAH][Im]/NPA/H2O | 363.15 | 91.1% | 60 | This work |
| MEA/NPA/H2O | 393.15 | 67.0% | 30 | [30] |
| DEEA/AEEA/H2O | 373.15 | 68.4% | 120 | [31] |
| TEEA/DEEA/H2O | 373.15 | 42.0% | 120 | [32] |
| BDA/DEEA/H2O | 353.15 | 43.6% | 90 | [33] |
| DETA/PMDETA (1:4)/H2O | 393.15 | 31.0% | 120 | [34] |
| DETA/PMDETA (2:3)/H2O | 393.15 | 40.7% | 120 | [35] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, C.; Zhang, Y.; Zhang, B.; Xu, C.; Yan, G.; Yang, N. CO2 Capture Performance and Preliminary Mechanistic Analysis of a Phase Change Absorbent. Molecules 2025, 30, 3404. https://doi.org/10.3390/molecules30163404
Zhu C, Zhang Y, Zhang B, Xu C, Yan G, Yang N. CO2 Capture Performance and Preliminary Mechanistic Analysis of a Phase Change Absorbent. Molecules. 2025; 30(16):3404. https://doi.org/10.3390/molecules30163404
Chicago/Turabian StyleZhu, Chuanyong, Yucai Zhang, Baoyue Zhang, Chongqing Xu, Guihuan Yan, and Na Yang. 2025. "CO2 Capture Performance and Preliminary Mechanistic Analysis of a Phase Change Absorbent" Molecules 30, no. 16: 3404. https://doi.org/10.3390/molecules30163404
APA StyleZhu, C., Zhang, Y., Zhang, B., Xu, C., Yan, G., & Yang, N. (2025). CO2 Capture Performance and Preliminary Mechanistic Analysis of a Phase Change Absorbent. Molecules, 30(16), 3404. https://doi.org/10.3390/molecules30163404







