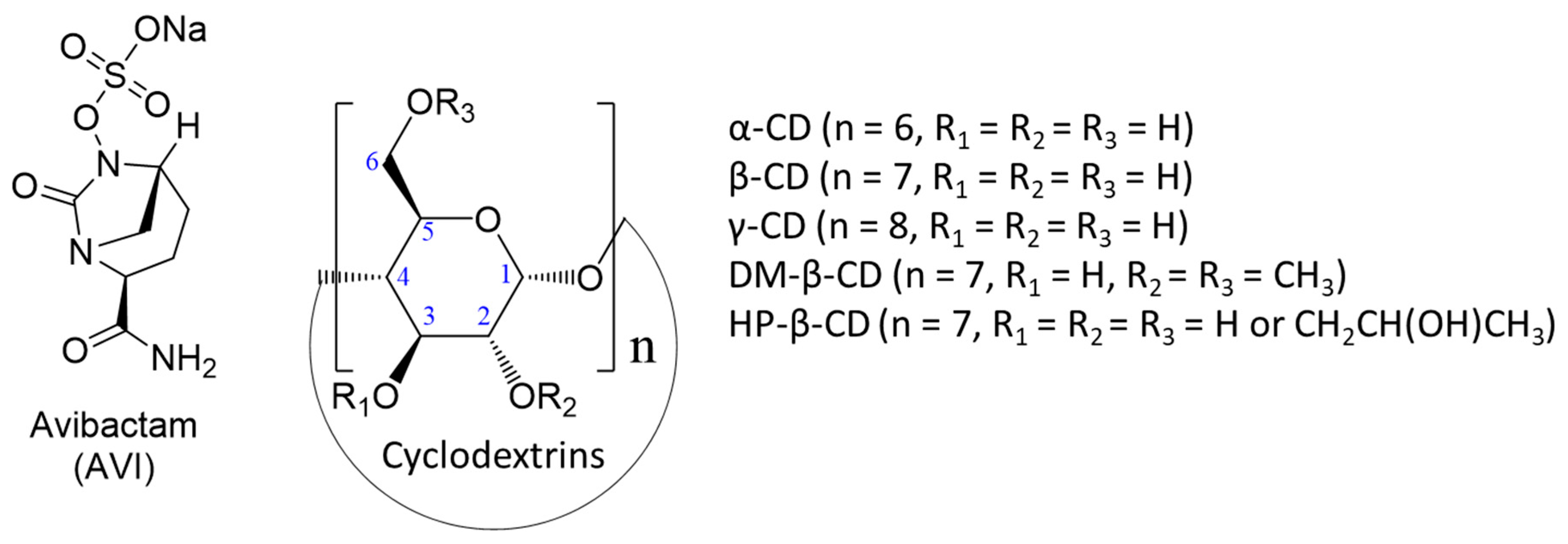
Avibactam–Cyclodextrin Inclusion Complexes: Computational and Thermodynamic Insights for Drug Delivery, Detection, and Environmental Scavenging
Abstract
1. Introduction
2. Results and Discussion
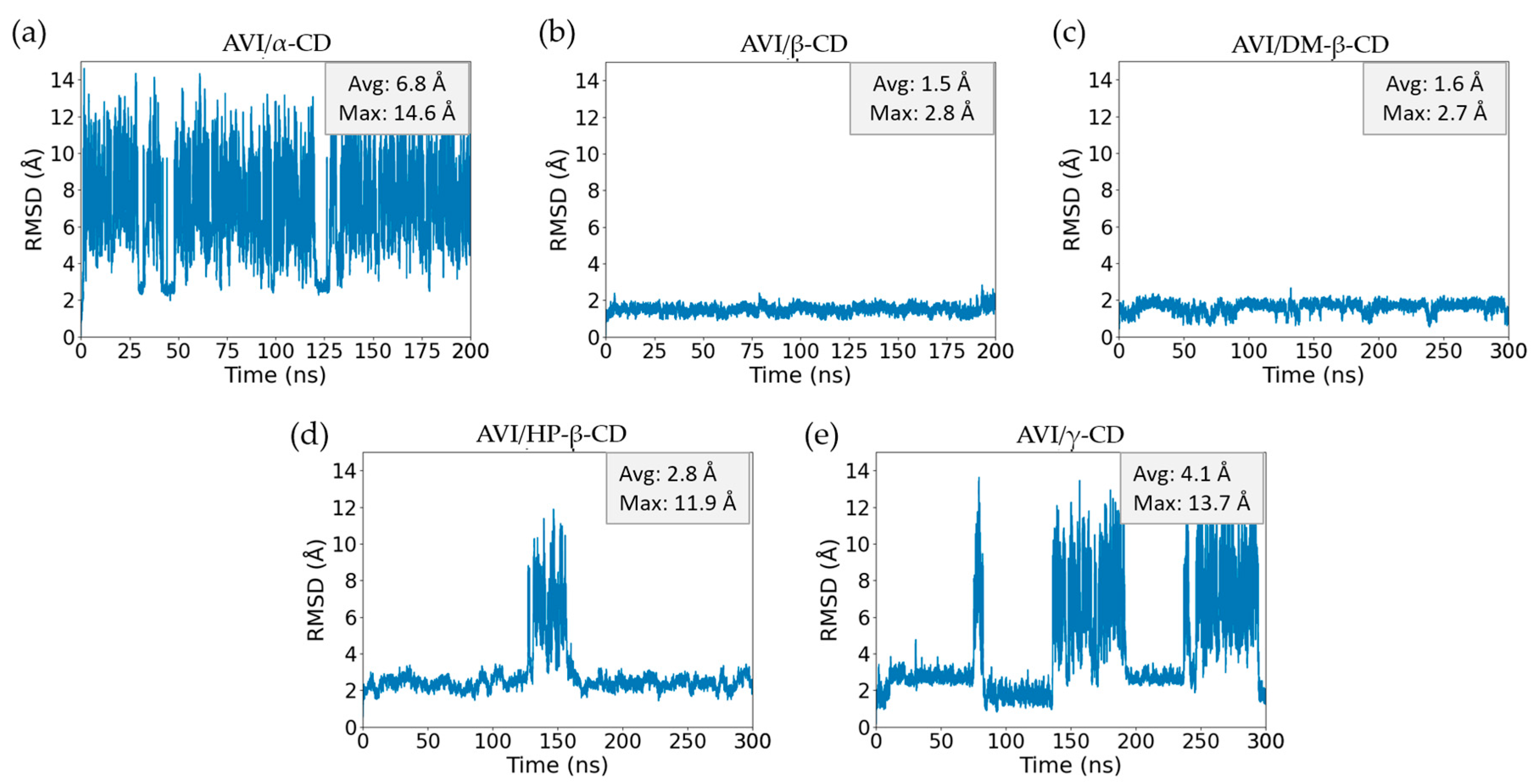
2.1. Molecular Dynamic
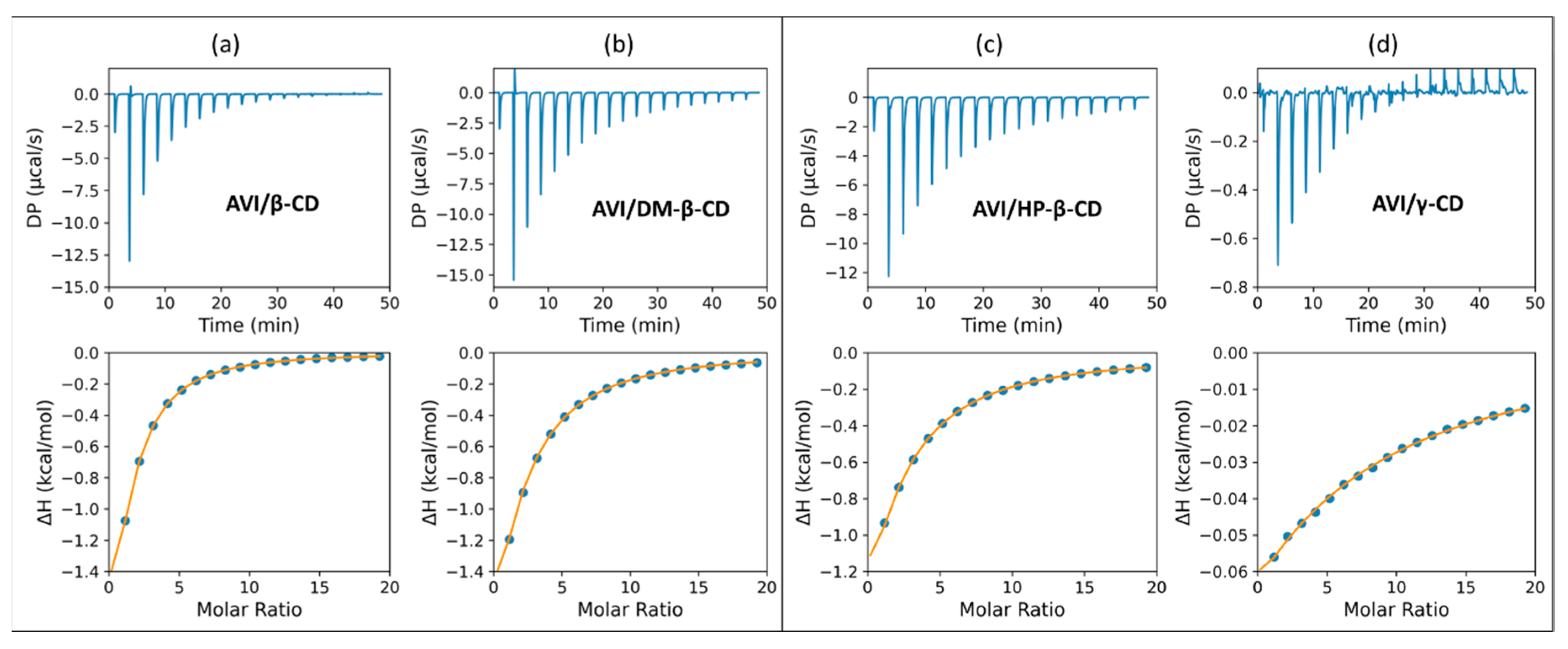
2.2. Isothermal Titration Calorimetry
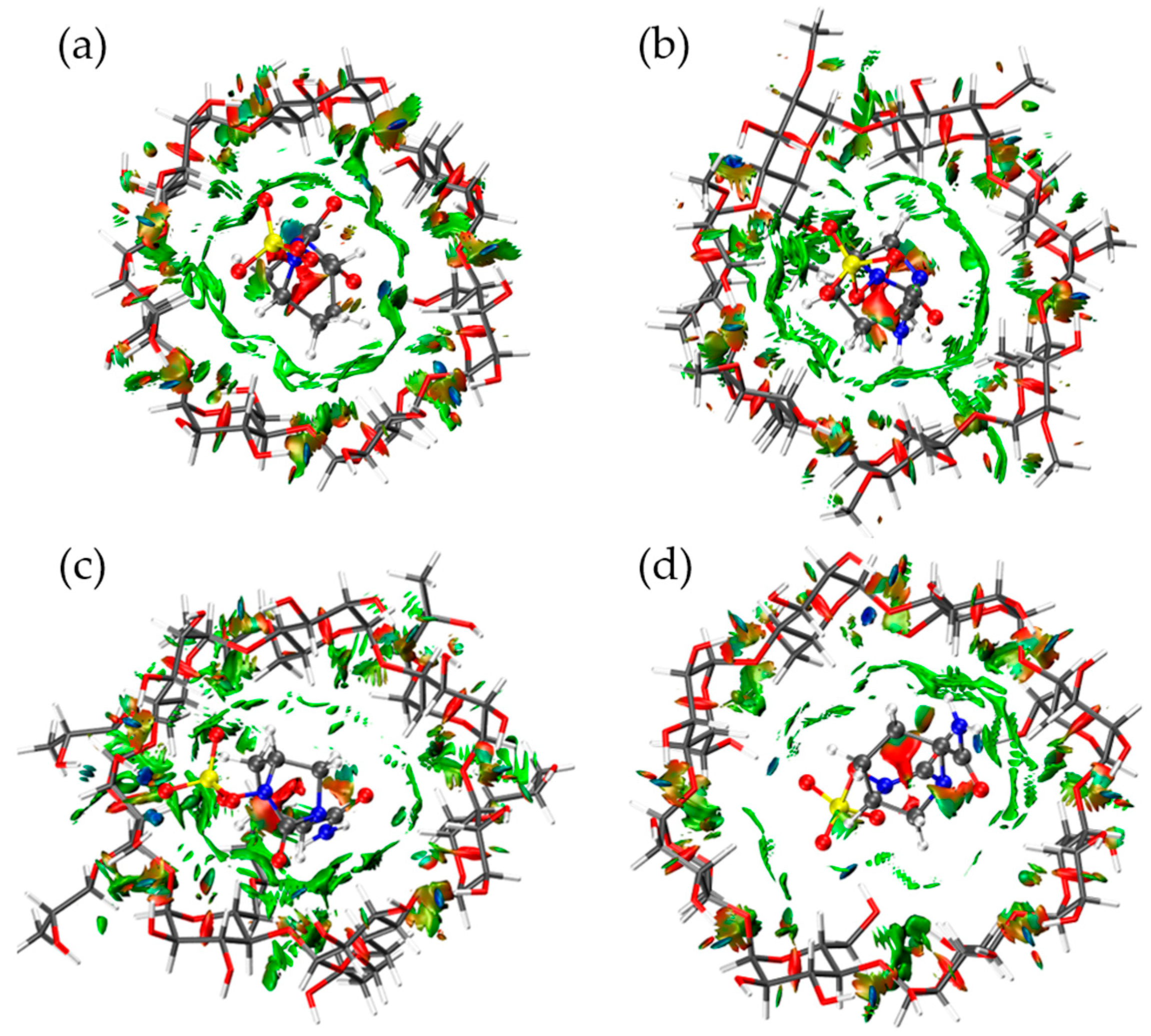
2.3. Non-Covalent Interactions
3. Materials and Methods
3.1. Molecular Dynamics (MD) Calculation
3.2. Isothermal Titration Calorimetry (ITC) Analysis
3.3. Non-Covalent Interaction (NCI) Analysis by DFT Method
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vincent, J.L.; Sakr, Y.; Singer, M.; Martin-Loeches, I.; MacHado, F.R.; Marshall, J.C.; Finfer, S.; Pelosi, P.; Brazzi, L.; Aditianingsih, D.; et al. Prevalence and Outcomes of Infection among Patients in Intensive Care Units in 2017. JAMA—J. Am. Med. Assoc. 2020, 323, 1478–1487. [Google Scholar] [CrossRef]
- Xu, L.; Sun, X.; Ma, X. Systematic review and meta-analysis of mortality of patients infected with carbapenem-resistant Klebsiella pneumoniae. Ann. Clin. Microbiol. Antimicrob. 2017, 16, 1–12. [Google Scholar] [CrossRef]
- Naghavi, M.; Vollset, S.E.; Ikuta, K.S.; Swetschinski, L.R.; Gray, A.P.; Wool, E.E.; Aguilar, G.R.; Mestrovic, T.; Smith, G.; Han, C.; et al. Global burden of bacterial antimicrobial resistance 1990–2021: A systematic analysis with forecasts to 2050. Lancet 2024, 404, 1199–1226. [Google Scholar] [CrossRef]
- La Rosa, M.C.; Maugeri, A.; Favara, G.; La Mastra, C.; Magnano San Lio, R.; Barchitta, M.; Agodi, A. The Impact of Wastewater on Antimicrobial Resistance: A Scoping Review of Transmission Pathways and Contributing Factors. Antibiotics 2025, 14, 131. [Google Scholar] [CrossRef]
- Yao, J.; Zou, P.; Cui, Y.; Quan, L.; Gao, C.; Li, Z.; Gong, W.; Yang, M. Recent Advances in Strategies to Combat Bacterial Drug Resistance: Antimicrobial Materials and Drug Delivery Systems. Pharmaceutics 2023, 15, 1188. [Google Scholar] [CrossRef]
- Lu, Z.Y.; Ma, Y.L.; Zhang, J.T.; Fan, N.S.; Huang, B.C.; Jin, R.C. A critical review of antibiotic removal strategies: Performance and mechanisms. J. Water Process Eng. 2020, 38, 101681. [Google Scholar] [CrossRef]
- Carcione, D.; Siracusa, C.; Sulejmani, A.; Leoni, V.; Intra, J. Old and new beta-lactamase inhibitors: Molecular structure, mechanism of action and clinical use. Antibiotics 2021, 10, 995. [Google Scholar] [CrossRef] [PubMed]
- Shirley, M. Ceftazidime-Avibactam: A Review in the Treatment of Serious Gram-Negative Bacterial Infections. Drugs 2018, 78, 675–692. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, P. Complementary supramolecular drug associates in perfecting the multidrug therapy against multidrug resistant bacteria. Front. Immunol. 2024, 15, 1352483. [Google Scholar] [CrossRef] [PubMed]
- Alfei, S.; Schito, A.M. β-Lactam Antibiotics and β-Lactamase Enzymes Inhibitors, Part 2: Our Limited Resources. Pharmaceuticals 2022, 15, 476. [Google Scholar] [CrossRef]
- Stachyra, T.; Levasseur, P.; Péchereau, M.C.; Girard, A.M.; Claudon, M.; Miossec, C.; Black, M.T. In vitro activity of the β-lactamase inhibitor NXL104 against KPC-2 carbapenemase and Enterobacteriaceae expressing KPC carbapenemases. J. Antimicrob. Chemother. 2009, 64, 326–329. [Google Scholar] [CrossRef]
- Coleman, K. Diazabicyclooctanes (DBOs): A potent new class of non-β-lactam β-lactamase inhibitors. Curr. Opin. Microbiol. 2011, 14, 550–555. [Google Scholar] [CrossRef]
- Stachyra, T.; Péchereau, M.C.; Bruneau, J.M.; Claudon, M.; Frère, J.M.; Miossec, C.; Coleman, K.; Black, M.T. Mechanistic studies of the inactivation of TEM-1 and P99 by NXL104, a novel non-β-lactam β-lactamase inhibitor. Antimicrob. Agents Chemother. 2010, 54, 5132–5138. [Google Scholar] [CrossRef] [PubMed]
- Leonard, D.A.; Bonomo, R.A.; Powers, R.A. Class D β-lactamases: A reappraisal after five decades. Acc. Chem. Res. 2013, 46, 2407–2415. [Google Scholar] [CrossRef]
- Zasowski, E.J.; Rybak, J.M.; Rybak, M.J. The β-Lactams Strike Back: Ceftazidime-Avibactam. Pharmacotherapy 2015, 35, 755–770. [Google Scholar] [CrossRef] [PubMed]
- Ehmann, D.E.; Jahić, H.; Ross, P.L.; Gu, R.F.; Hu, J.; Kern, G.; Walkup, G.K.; Fisher, S.L. Avibactam is a covalent, reversible, non-β-lactam β-lactamase inhibitor. Proc. Natl. Acad. Sci. USA 2012, 109, 11663–11668. [Google Scholar] [CrossRef]
- Ruggiero, M.; Papp-Wallace, K.M.; Taracila, M.A.; Mojica, M.F.; Bethel, C.R.; Rudin, S.D.; Zeiser, E.T.; Gutkind, G.; Bonomo, R.A.; Power, P. Exploring the landscape of diazabicyclooctane (DBO) inhibition: Avibactam inactivation of PER-2 β-Lactamase. Antimicrob. Agents Chemother. 2017, 61, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- O’Jeanson, A.; Ioannidis, K.; Nielsen, E.I.; Galani, L.; Ginosyan, A.; Paskalis, H.; Loryan, I.; Giamarellou, H.; Friberg, L.E.; Karaiskos, I. Ceftazidime-avibactam (CAZ-AVI) pharmacokinetics in critically ill patients undergoing continuous venovenous hemodiafiltration (CVVHDF). Int. J. Antimicrob. Agents 2025, 65, 107394. [Google Scholar] [CrossRef]
- Gatti, M.; Cojutti, P.G.; Pea, F. Impact of attaining aggressive vs. conservative PK/PD target on the clinical efficacy of beta-lactams for the treatment of Gram-negative infections in the critically ill patients: A systematic review and meta-analysis. Crit. Care 2024, 28, 1–18. [Google Scholar] [CrossRef]
- Rasheed, A.; Kumar, C.K.A.; Sravanthi, V.V.N.S.S. Cyclodextrins as drug carrier molecule: A review. Sci. Pharm. 2008, 76, 567–598. [Google Scholar] [CrossRef]
- Sillén, H.; Mitchell, R.; Sleigh, R.; Mainwaring, G.; Catton, K.; Houghton, R.; Glendining, K. Determination of avibactam and ceftazidime in human plasma samples by LC-MS. Bioanalysis 2015, 7, 1423–1434. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Chen, M.; Zhang, B.; Lin, H.; Li, X.; Cai, Y.; Zhang, H.; Que, W.; Liu, M.; Qiu, H. Rapid, simple, and economical LC–MS/MS method for simultaneous determination of ceftazidime and avibactam in human plasma and its application in therapeutic drug monitoring. J. Clin. Pharm. Ther. 2022, 47, 1426–1437. [Google Scholar] [CrossRef]
- Magréault, S.; Jaureguy, F.; Zahar, J.R.; Méchaï, F.; Toinon, D.; Cohen, Y.; Carbonnelle, E.; Jullien, V. Automated HPLC-MS/MS assay for the simultaneous determination of ten plasma antibiotic concentrations. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2022, 1211, 123496. [Google Scholar] [CrossRef]
- Martens-Lobenhoffer, J.; Angermair, S.; Bode-Böger, S.M. Quantification of ceftazidime/avibactam in human plasma and dried blood spots: Implications on stability and sample transport. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2022, 1193, 123164. [Google Scholar] [CrossRef]
- Pinder, N.; Brenner, T.; Swoboda, S.; Weigand, M.A.; Hoppe-Tichy, T. Therapeutic drug monitoring of beta-lactam antibiotics—Influence of sample stability on the analysis of piperacillin, meropenem, ceftazidime and flucloxacillin by HPLC-UV. J. Pharm. Biomed. Anal. 2017, 143, 86–93. [Google Scholar] [CrossRef]
- Gong, Z.; Zhang, Z. Determination of tetracyclines with a modified β-cyclodextrin based fluorosensor. Anal. Chim. Acta 1997, 351, 205–210. [Google Scholar] [CrossRef]
- Garrido, J.M.P.J.; Melle-Franco, M.; Strutyński, K.; Borges, F.; Brett, C.M.A.; Garrido, E.M.P.J. β–Cyclodextrin carbon nanotube-enhanced sensor for ciprofloxacin detection. J. Environ. Sci. Heal.—Part A Toxic/Hazardous Subst. Environ. Eng. 2017, 52, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Markina, N.E.; Markin, A.V.; Cialla-May, D. Cyclodextrin-assisted SERS determination of fluoroquinolone antibiotics in urine and blood plasma. Talanta 2023, 254, 124083. [Google Scholar] [CrossRef]
- Ouyang, L.; Zhu, L.; Ruan, Y.; Tang, H. Preparation of a native β-cyclodextrin modified plasmonic hydrogel substrate and its use as a surface-enhanced Raman scattering scaffold for antibiotics identification. J. Mater. Chem. C 2015, 3, 7575–7582. [Google Scholar] [CrossRef]
- Ruiz-Palomero, C.; Soriano, M.L.; Valcárcel, M. β-Cyclodextrin decorated nanocellulose: A smart approach towards the selective fluorimetric determination of danofloxacin in milk samples. Analyst 2015, 140, 3431–3438. [Google Scholar] [CrossRef]
- Boczar, D.; Michalska, K. Cyclodextrin Inclusion Complexes with Antibiotics and Antibacterial Agents as Drug-Delivery Systems—A Pharmaceutical Perspective. Pharmaceutics 2022, 14, 1389. [Google Scholar] [CrossRef]
- Pardeshi, C.V.; Kothawade, R.V.; Markad, A.R.; Pardeshi, S.R.; Kulkarni, A.D.; Chaudhari, P.J.; Longhi, M.R.; Dhas, N.; Naik, J.B.; Surana, S.J.; et al. Sulfobutylether-β-cyclodextrin: A functional biopolymer for drug delivery applications. Carbohydr. Polym. 2023, 301, 120347. [Google Scholar] [CrossRef] [PubMed]
- Spiridon, I.; Anghel, N. Cyclodextrins as Multifunctional Platforms in Drug Delivery and Beyond: Structural Features, Functional Applications, and Future Trends. Molecules 2025, 30, 3044. [Google Scholar] [CrossRef]
- Syeda, S.E.Z.; Nowacka, D.; Khan, M.S.; Skwierawska, A.M. Recent Advancements in Cyclodextrin-Based Adsorbents for the Removal of Hazardous Pollutants from Waters. Polymers 2022, 14, 2341. [Google Scholar] [CrossRef]
- Alsbaiee, A.; Smith, B.J.; Xiao, L.; Ling, Y.; Helbling, D.E.; Dichtel, W.R. Rapid removal of organic micropollutants from water by a porous β-cyclodextrin polymer. Nature 2016, 529, 190–194. [Google Scholar] [CrossRef]
- Scriba, G.K.E.; Konjaria, M.L.; Krait, S. Cyclodextrins. In Chiral Separations and Stereochemical Elucidation: Fundamentals, Methods, and Applications; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2023; pp. 273–323. ISBN 9781119802280. [Google Scholar]
- Poulson, B.G.; Alsulami, Q.A.; Sharfalddin, A.; El Agammy, E.F.; Mouffouk, F.; Emwas, A.-H.; Jaremko, L.; Jaremko, M. Cyclodextrins: Structural, Chemical, and Physical Properties, and Applications. Polysaccharides 2021, 3, 1–31. [Google Scholar] [CrossRef]
- Geue, N.; Alcázar, J.J.; Campodónico, P.R. Influence of β-Cyclodextrin Methylation on Host-Guest Complex Stability: A Theoretical Study of Intra- and Intermolecular Interactions as Well as Host Dimer Formation. Molecules 2023, 28, 2625. [Google Scholar] [CrossRef]
- Jansook, P.; Ogawa, N.; Loftsson, T. Cyclodextrins: Structure, physicochemical properties and pharmaceutical applications. Int. J. Pharm. 2018, 535, 272–284. [Google Scholar] [CrossRef] [PubMed]
- Dhiman, P.; Bhatia, M. Pharmaceutical applications of cyclodextrins and their derivatives. J. Incl. Phenom. Macrocycl. Chem. 2020, 98, 171–186. [Google Scholar] [CrossRef]
- Vikas, Y.; Sandeep, K.; Braham, D.; Manjusha, C.; Budhwar, V. Cyclodextrin complexes: An approach to improve the physicochemical properties of drugs and applications of cyclodextrin complexes. Asian J. Pharm. 2018, 12, S394–S409. [Google Scholar] [CrossRef]
- Brewster, M.E.; Loftsson, T. Cyclodextrins as pharmaceutical solubilizers. Adv. Drug Deliv. Rev. 2007, 59, 645–666. [Google Scholar] [CrossRef]
- Jones, D.S.; Dressman, J.B.; Loftsson, T.; Moya-Ortega, M.D.; Alvarez-Lorenzo, C.; Concheiro, A. Pharmacokinetics of cyclodextrins and drugs after oral and parenteral administration of drug/cyclodextrin complexes. J. Pharm. Pharmacol. 2016, 68, 544–555. [Google Scholar] [CrossRef]
- Hennig, A.; Schwarzlose, T.; Nau, W.M. Bridgehead carboxy-substituted 2, 3-diazabicyclo [2.2. 2] oct-2-enes: Synthesis, fluorescent properties, and host-guest complexation. Arkivoc 2007, 8, 341–357. [Google Scholar] [CrossRef]
- Liu, Y.; You, C.C. Inclusion complexation of β-cyclodextrin and 6-O-α-maltosyl- and 2-O-(2-hydroxypropyl)-β-cyclodextrins with some fluorescent dyes. J. Phys. Org. Chem. 2001, 14, 11–16. [Google Scholar] [CrossRef]
- Wagner, B.D.; Fitzpatrick, S.J. A comparison of the host-guest inclusion complexes of 1,8-ANS and 2,6-ANS in parent and modified cyclodextrins. J. Incl. Phenom. 2000, 38, 467–478. [Google Scholar] [CrossRef]
- Hanwell, M.D.; Curtis, D.E.; Lonie, D.C.; Vandermeerschd, T.; Zurek, E.; Hutchison, G.R. Avogadro: An advanced semantic chemical editor, visualization, and analysis platform. J. Cheminform. 2012, 4, 1–17. [Google Scholar] [CrossRef]
- Phillips, J.C.; Braun, R.; Wang, W.; Gumbart, J.; Tajkhorshid, E.; Villa, E.; Chipot, C.; Skeel, R.D.; Kalé, L.; Schulten, K. Scalable molecular dynamics with NAMD. J. Comput. Chem. 2005, 26, 1781–1802. [Google Scholar] [CrossRef]
- Jo, S.; Kim, T.; Iyer, V.G.; Im, W. CHARMM-GUI: A web-based graphical user interface for CHARMM. J. Comput. Chem. 2008, 29, 1859–1865. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Lee, J.; Jo, S.; Brooks, C.L.; Lee, H.S.; Im, W. CHARMM-GUI ligand reader and modeler for CHARMM force field generation of small molecules. J. Comput. Chem. 2017, 38, 1879–1886. [Google Scholar] [CrossRef]
- Mayne, C.G.; Saam, J.; Schulten, K.; Tajkhorshid, E.; Gumbart, J.C. Rapid parameterization of small molecules using the force field toolkit. J. Comput. Chem. 2013, 34, 2757–2770. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Hehre, W.J.; Ditchfield, K.; Pople, J.A. Self-consistent molecular orbital methods. XII. Further extensions of gaussian-type basis sets for use in molecular orbital studies of organic molecules. J. Chem. Phys. 1972, 56, 2257–2261. [Google Scholar] [CrossRef]
- Francl, M.M.; Pietro, W.J.; Hehre, W.J.; Binkley, J.S.; Gordon, M.S.; DeFrees, D.J.; Pople, J.A. Self-consistent molecular orbital methods. XXIII. A polarization-type basis set for second-row elements. J. Chem. Phys. 1982, 77, 3654–3665. [Google Scholar] [CrossRef]
- Møller, C.; Plesset, M.S. Note on an approximation treatment for many-electron systems. Phys. Rev. 1934, 46, 618–622. [Google Scholar] [CrossRef]
- Neese, F. Software update: The ORCA program system, version 4.0. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2018, 8, e1327. [Google Scholar] [CrossRef]
- Steffen, C.; Thomas, K.; Huniar, U.; Hellweg, A.; Rubner, O.; Schroer, A. AutoDock4 and AutoDockTools4: Automated Docking with Selective Receptor Flexibility. J. Comput. Chem. 2010, 31, 2967–2970. [Google Scholar] [CrossRef]
- Kollman, P.A.; Merz, K.M. Computer Modeling of the Interactions of Complex Molecules. Acc. Chem. Res. 1990, 23, 246–252. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Brandenburg, J.G.; Bannwarth, C.; Hansen, A.; Grimme, S. B97-3c: A revised low-cost variant of the B97-D density functional method. J. Chem. Phys. 2018, 148, 064104. [Google Scholar] [CrossRef]
- Neese, F. Software update: The ORCA program system—Version 5.0. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2022, 12, e1606. [Google Scholar] [CrossRef]
- Neese, F.; Wennmohs, F.; Becker, U.; Riplinger, C. The ORCA quantum chemistry program package. J. Chem. Phys. 2020, 152, 224108. [Google Scholar] [CrossRef] [PubMed]
- Barone, V.; Cossi, M. Quantum calculation of molecular energies and energy gradients in solution by a conductor solvent model. J. Phys. Chem. A 1998, 102, 1995–2001. [Google Scholar] [CrossRef]
- Alcázar, J.J.; García-Río, L.; Robles, A.I.; Dinamarca-Villarroel, L.; Fierro, A.; Santos, J.G.; Aliaga, M.E. Linear relationship between emission quantum yield and Stokes shift in 3-styryl aza-coumarin based dyes in the presence of cyclodextrins. J. Mol. Liq. 2023, 381, 121790. [Google Scholar] [CrossRef]
- Alcázar, J.J.; Misad Saide, A.C.; Campodónico, P.R. Reliable and accurate prediction of basic pKa values in nitrogen compounds: The pKa shift in supramolecular systems as a case study. J. Cheminform. 2023, 15, 90. [Google Scholar] [CrossRef]
- Johnson, E.R.; Keinan, S.; Mori-Sánchez, P.; Contreras-García, J.; Cohen, A.J.; Yang, W. Revealing noncovalent interactions. J. Am. Chem. Soc. 2010, 132, 6498–6506. [Google Scholar] [CrossRef]
- Bader, R.F.W. Atoms in Molecules. Acc. Chem. Res. 1985, 18, 9–15. [Google Scholar] [CrossRef]

| AVI/CDs | K1:1 | ΔG° (kcal/mol) | ΔH° (kcal/mol) | –TΔS° (kcal/mol) |
|---|---|---|---|---|
| AVI/β-CD | 467.29 ± 3.12 | –3.64 ± 0.00 | –4.57 ± 0.02 | 0.93 ± 0.03 |
| AVI/DM-β-CD | 236.41 ± 2.46 | –3.24 ± 0.01 | –7.65 ± 0.03 | 4.41 ± 0.04 |
| AVI/HP-β-CD | 191.57 ± 1.27 | –3.11 ± 0.00 | –7.08 ± 0.02 | 3.97 ± 0.03 |
| AVI/γ-CD | 44.25 ± 0.84 | –2.25 ± 0.01 | –1.42 ± 0.05 | –0.83 ± 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alcázar, J.J.; Campodónico, P.R.; López, R. Avibactam–Cyclodextrin Inclusion Complexes: Computational and Thermodynamic Insights for Drug Delivery, Detection, and Environmental Scavenging. Molecules 2025, 30, 3401. https://doi.org/10.3390/molecules30163401
Alcázar JJ, Campodónico PR, López R. Avibactam–Cyclodextrin Inclusion Complexes: Computational and Thermodynamic Insights for Drug Delivery, Detection, and Environmental Scavenging. Molecules. 2025; 30(16):3401. https://doi.org/10.3390/molecules30163401
Chicago/Turabian StyleAlcázar, Jackson J., Paola R. Campodónico, and René López. 2025. "Avibactam–Cyclodextrin Inclusion Complexes: Computational and Thermodynamic Insights for Drug Delivery, Detection, and Environmental Scavenging" Molecules 30, no. 16: 3401. https://doi.org/10.3390/molecules30163401
APA StyleAlcázar, J. J., Campodónico, P. R., & López, R. (2025). Avibactam–Cyclodextrin Inclusion Complexes: Computational and Thermodynamic Insights for Drug Delivery, Detection, and Environmental Scavenging. Molecules, 30(16), 3401. https://doi.org/10.3390/molecules30163401







