Lectins as Natural Antibiofilm Agents in the Fight Against Antibiotic Resistance: A Review
Abstract
1. Introduction
2. Biofilms
3. The Antibiofilm Activities of Lectins
3.1. Inhibitory Effect of Lectins on Biofilm Formation
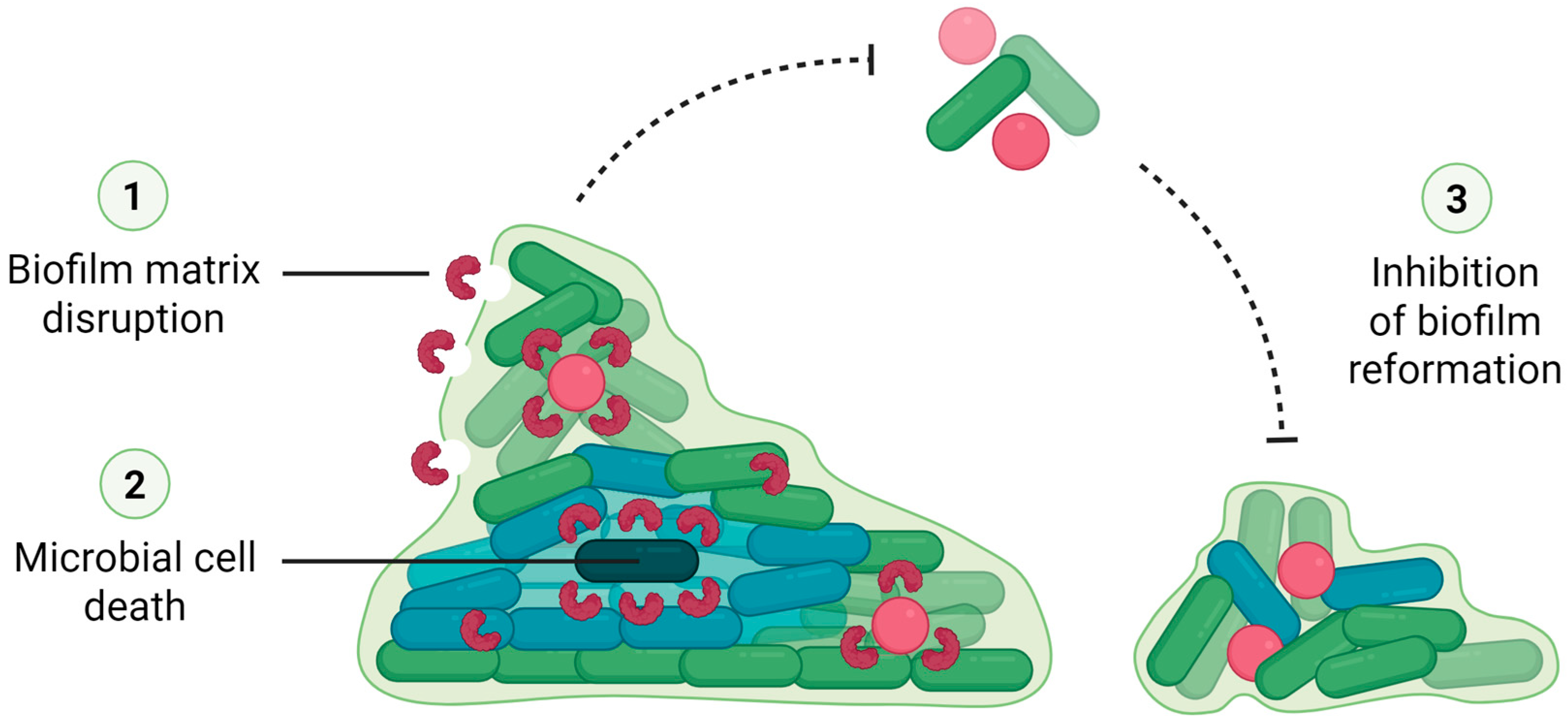
3.2. Lectins Can Also Eradicate Preformed Biofilms
3.3. Dual Role of Lectins in Biofilm Modulation: Inhibition vs. Promotion
3.4. Lectin-Drug Synergism and Delivery Approaches
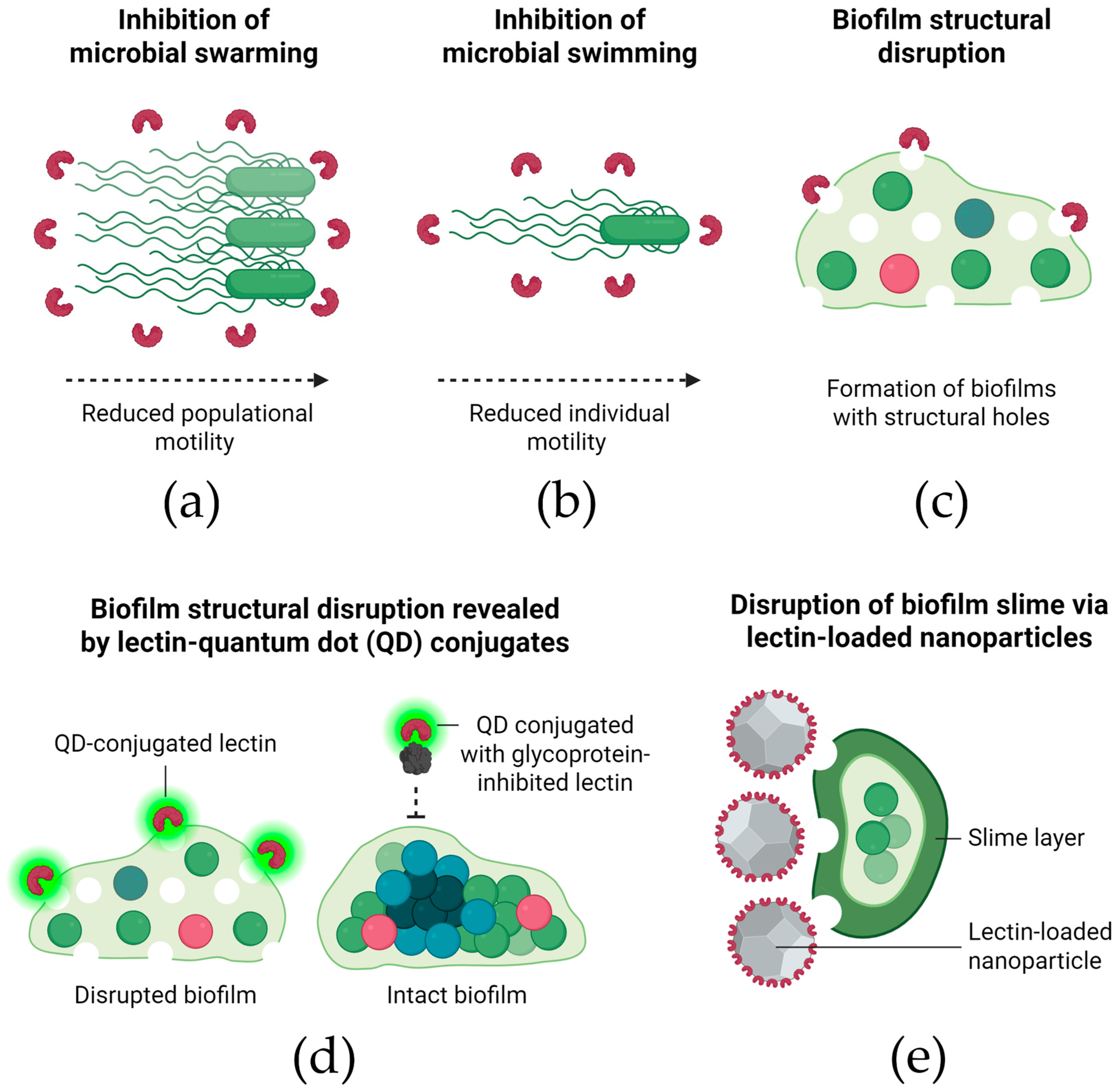
3.5. Mechanisms of Biofilm Inhibition by Lectins
3.6. Challenges and Knowledge Gaps
4. Final Considerations
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Amsellem, L.; Brouat, C.; Duron, O.; Porter, S.S.; Vilcinskas, A.; Facon, B. Importance of microorganisms to macroorganisms invasions: Is the essential invisible to the eye? (The Little Prince, A. de Saint-Exupéry, 1943). Adv. Ecol. Res. 2017, 57, 99–146. [Google Scholar]
- Pessôa, M.G.; Vespermann, K.A.C.; Paulino, B.N.; Barcelos, M.C.S.; Pastore, G.M.; Molina, G. Newly isolated microorganisms with potential application in biotechnology. Biotechnol. Adv. 2019, 37, 319–339. [Google Scholar] [CrossRef]
- GBD 2021 Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance 1990-2021: A systematic analysis with forecasts to 2050. Lancet 2024, 404, 1199–1226. [Google Scholar] [CrossRef]
- Durand, G.A.; Raoult, D.; Dubourg, G. Antibiotic discovery: History, methods and perspectives. Int. J. Antimicrob. Agents 2019, 53, 371–382. [Google Scholar] [CrossRef] [PubMed]
- Horváth, G.; Bencsik, T.; Ács, K.; Kocsis, B. Sensitivity of ESBL-Producing Gram-Negative Bacteria to Essential Oils, Plant Extracts, and Their Isolated Compounds. In Antibiotic Resistance-Mechanisms and New Antimicrobial Approaches, 1st ed.; Kon, K., Rai, M., Eds.; Academic Press: New York, NY, USA, 2016; pp. 239–269. [Google Scholar]
- Orji, F.A.; Ugbogu, O.C.; Ugbogu, E.A.; Barbabosa-Pliego, A.; Monroy, J.C.; Elghandour, M.M.M.Y.; Salem, A.Z.M. Pathogenic flora composition and overview of the trends used for bacterial pathogenicity identifications. Microb. Pathog. 2018, 121, 139–146. [Google Scholar] [CrossRef]
- Defoirdt, T. Quorum-Sensing Systems as Targets for Antivirulence Therapy. Trends Microbiol. 2018, 26, 313–328. [Google Scholar] [CrossRef]
- Kalia, V.C.; Patel, S.K.S.; Kang, Y.C.; Lee, J.K. Quorum sensing inhibitors as antipathogens: Biotechnological applications. Biotechnol. Adv. 2019, 37, 68–90. [Google Scholar] [CrossRef] [PubMed]
- Dale, J.L.; Nilson, J.L.; Barnes, A.M.T.; Dunny, G.M. Restructuring of Enterococcus faecalis biofilm architecture in response to antibiotic-induced stress. npj Biofilms Microbiomes 2017, 3, 15. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Yang, L.; Chen, G.; Li, B.; Chen, D.; Li, L.; Xu, Z. Pathogenic features and characteristics of food borne pathogens biofilm: Biomass, viability and matrix. Microb. Pathog. 2017, 111, 285–291. [Google Scholar] [CrossRef]
- Moura, M.C.; Trentin, D.S.; Napoleão, T.H.; Primon-Barros, M.; Xavier, A.S.; Carneiro, N.P.; Paiva, P.M.G.; Macedo, A.J.; Coelho, L.C.B.B. Multi-effect of the water-soluble Moringa oleifera lectin against Serratia marcescens and Bacillus sp.: Antibacterial, antibiofilm and anti-adhesive properties. J. Appl. Microbiol. 2017, 123, 861–874. [Google Scholar] [CrossRef]
- Guo, L.; Zhang, C.; Chen, G.; Wu, M.; Liu, W.; Ding, C.; Dong, Q.; Fan, E.; Liu, Q. Reactive oxygen species inhibit biofilm formation of Listeria monocytogenes. Microb. Pathog. 2019, 127, 183–189. [Google Scholar] [CrossRef]
- Liu, H.Y.; Prentice, E.L.; Webber, M.A. Mechanisms of antimicrobial resistance in biofilms. npj Antimicrob. Resist. 2024, 2, 27. [Google Scholar] [CrossRef]
- Silva, D.P.; Matwichuk, M.L.; Townsend, D.O.; Reichhardt, C.; Lamba, D.; Wozniak, D.J.; Parsek, M.R. The Pseudomonas aeruginosa lectin LecB binds to the exopolysaccharide Psl and stabilizes the biofilm matrix. Nat. Commun. 2019, 10, 2183. [Google Scholar] [CrossRef]
- Azeredo, J.; Azevedo, N.F.; Briandet, R.; Cerca, N.; Coenye, T.; Costa, A.R.; Desvaux, M.; Di Bonaventura, G.; Hebraud, M.; Jaglic, Z.; et al. Critical review on biofilm methods. Crit. Rev. Microbiol. 2017, 43, 313–351. [Google Scholar] [CrossRef]
- Jamal, M.; Ahmad, W.; Andleeb, S.; Jalil, F.; Imran, M.; Nawaz, M.A.; Hussain, T.; Ali, M.; Rafiq, M.; Kamil, M.A. Bacterial biofilm and associated infections. J. Chin. Med. Assoc. 2018, 81, 7–11. [Google Scholar] [CrossRef]
- Flemming, H.; van Hullebusch, E.D.; Neu, T.R.; Nielsen, P.H.; Seviour, T.; Stoodley, P.; Wingender, J.; Wuertz, S. The biofilm matrix: Multitasking in a shared space. Nat. Rev. Microbiol. 2023, 21, 70–86. [Google Scholar] [CrossRef]
- Vasudevan, R. Biofilms: Microbial cities of scientific significance. J. Microbiol. Exp. 2014, 1, 84–98. [Google Scholar] [CrossRef]
- Flemming, H.C.; Wingender, J.; Szewzyk, U.; Steinberg, P.; Rice, S.A.; Kjelleberg, S. Biofilms: An emergent form of bacterial life. Nat. Rev. Microbiol. 2016, 14, 563–575. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.; Wingender, J. The biofilm matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Melville, S.; Craig, L. Type IV Pili in Gram-Positive Bacteria. Microbiol. Mol. Biol. Rev. 2013, 77, 323–341. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Hurley, A.; Hu, W.; Warrick, J.W.; Lozano, G.L.; Ayuso, J.M.; Pan, W.; Handelsman, J.; Beebe, D.J. Social motility of biofilm-like microcolonies in a gliding bacterium. Nat. Commun. 2021, 12, 5700. [Google Scholar] [CrossRef]
- Percival, S.L. Importance of biofilm formation in surgical infection. Brit. J. Surg. 2017, 104, e85–e94. [Google Scholar] [CrossRef]
- Grebeyohannes, G.; Nyerere, A.; Bii, C.; Sbhatu, D.B. Challenges of intervention, treatment, and antibiotic resistance of biofilm-forming microorganisms. Heliyon 2019, 5, e02192. [Google Scholar] [CrossRef] [PubMed]
- Ch’ng, J.H.; Chong, K.K.L.; Lam, L.N.; Wong, J.J.; Kline, K.A. Biofilm-associated infection by enterococci. Nat. Rev. Microbiol. 2019, 17, 82–94. [Google Scholar] [CrossRef]
- Romling, U.; Balsalobre, C. Biofilm infections, their resilience to therapy and innovative treatment strategies. J. Intern. Med. 2012, 272, 541–561. [Google Scholar] [CrossRef]
- Le, K.Y.; Park, M.D.; Otto, M. Immune evasion mechanisms of Staphylococcus epidermidis biofilm infection. Front. Microbiol. 2018, 9, 359. [Google Scholar] [CrossRef] [PubMed]
- Kvich, L.; Burmølle, M.; Bjarnsholt, T.; Lichtenberg, M. Do mixed-species biofilms dominate in chronic infections? Need for in situ visualization of bacterial organization. Front. Cell. Infect. Microbiol. 2020, 10, 396. [Google Scholar] [CrossRef]
- Joshi, R.V.; Gunawan, C.; Mann, R. We Are One: Multispecies Metabolism of a Biofilm Consortium and Their Treatment Strategies. Front. Microbiol. 2021, 12, 635432. [Google Scholar] [CrossRef] [PubMed]
- Norsworthy, A.N.; Pearson, M.M. From catheter to kidney stone: The uropathogenic lifestyle of Proteus mirabilis. Trends Microbiol. 2017, 25, 304–315. [Google Scholar] [CrossRef]
- Peumans, W.J.; Van Damme, E.J.M. Lectins as plant defense proteins. Plant Physiol. 1995, 109, 347–352. [Google Scholar] [CrossRef]
- Tsaneva, M.; Van Damme, E.J.S. 130 years of Plant Lectin Research. Glycoconj. J. 2020, 37, 533–551. [Google Scholar] [CrossRef] [PubMed]
- Siddesh, B.M.; Kiran, B.K.; Sherapura, A.; Banumathi; Mahmood, R.; Kumari, N.S.; Prabhakar, B.T. Immunomodulatory Lectin from Cordia myxa targets PI3K/AKT signaling mediated apoptosis to regress both in-vitro and in-vivo tumour. Int. J. Biol. Macromol. 2025, 294, 139433. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, M.L.S.; Alves, R.R.V.; Napoleão, T.H.; Paiva, P.M.G.; Coelho, L.C.B.B.; Bezerra, A.C.D.S.; Silva, M.D.C. Anthelmintic effect of a water soluble Moringa oleifera lectin in rodents experimentally infected with Haemonchus contortus. Parasitol. Int. 2023, 92, 102656. [Google Scholar] [CrossRef]
- Gupta, A.; Yadav, K.; Yadav, A.; Ahmad, R.; Srivastava, A.; Kumar, D.; Khan, M.A.; Dwivedi, U.N. Mannose-specific plant and microbial lectins as antiviral agents: A review. Glycoconj. J. 2024, 41, 1–33. [Google Scholar] [CrossRef]
- Santos, N.D.L.; Santos, P.E.M.; Lira, T.L.S.; Santos, A.R.S.; Silva, J.N.O.; Santos, A.N.S.; Amorim, M.M.R.; Barros, M.R.; Coelho, L.C.B.B.; Paiva, P.M.G.; et al. Insecticidal Activity of Lectin Preparations from Moringa oleifera Lam. (Moringaceae) Seeds against Alphitobius diaperinus (Panzer) (Coleoptera: Tenebrionidae). Plants 2025, 14, 511. [Google Scholar] [CrossRef]
- Brito, J.S.; Marinho, A.O.; Coelho, L.C.B.B.; Oliveira, A.M.; Paiva, P.M.G.; Patriota, L.L.S.; Napoleão, T.H. Toxicity and antitumor activity of the water-soluble lectin from Moringa oleifera Lam. seeds (WSMoL) in sarcoma 180-bearing mice. Toxicon 2023, 234, 107306. [Google Scholar] [CrossRef] [PubMed]
- Marinho, A.O.; Costa, J.A.; Santos, A.N.S.; Barros, M.C.; Pimentel, C.N.; Silva, A.A.; Paiva, P.M.G.; Napoleão, T.H.; Patriota, L.L.S. Assessment of acute toxicity, genotoxicity, and anti-inflammatory activity of SteLL, a lectin from Schinus terebinthifolia Raddi. leaves, in mice. J. Ethnopharmacol. 2024, 333, 118496. [Google Scholar] [CrossRef]
- Procópio, T.F.; Patriota, L.L.S.; Moura, M.C.; Silva, P.M.; Oliveira, A.P.S.; Carvalho, L.V.N.; Lima, T.A.; Soares, T.; Silva, T.D.; Coelho, L.C.B.B.; et al. CasuL: A new lectin isolated from Calliandra surinamensis leaf pinnulae with cytotoxicity to cancer cells, antimicrobial activity and antibiofilm effect. Int. J. Biol. Macromol. 2017, 98, 419–429. [Google Scholar] [CrossRef]
- Procópio, T.F.; Moura, M.C.; Bento, E.F.L.; Soares, T.; Coelho, L.C.B.B.; Bezerra, R.P.; Mota, R.A.; Porto, A.L.F.; Paiva, P.M.G.; Napoleão, T.H. Looking for alternative treatments for bovine and caprine mastitis: Evaluation of the potential of Calliandra surinamensis leaf pinnulae lectin (CasuL), both alone and in combination with antibiotics. MicrobiologyOpen 2019, 8, e869. [Google Scholar] [CrossRef]
- Ferreira, G.R.S.; Brito, J.S.; Procópio, T.F.; Santos, N.D.L.; Lima, B.J.R.C.; Coelho, L.C.B.B.; Navarro, D.M.A.F.; Paiva, P.M.G.; Soares, T.; Moura, M.C.; et al. Antimicrobial potential of Alpinia purpurata lectin (ApuL): Growth inhibitory action, synergistic effects in combination with antibiotics, and antibiofilm activity. Microb. Pathog. 2018, 124, 152–162. [Google Scholar] [CrossRef]
- Campos, L.A.A.; Souza, Z.N.; Costa Junior, S.D.; Silva, C.E.S.; Santos, J.V.O.; Cristóvão-Silva, A.C.; Brelaz-de-Castro, M.C.A.; Paiva, P.M.G.; Pereira, V.R.A.; Correia, M.T.S.; et al. Anti-staphylococcal, antibiofilm and trypanocidal activities of CrataBL encapsulated into liposomes: Lectin with potential against infectious diseases. Microb. Pathog. 2024, 196, 107007. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, R.F.; Duarte, P.L.; Chaves, R.P.; Silva, S.R.; Feitosa, R.R.; Sousa, B.L.; Alves, A.W.; Vasconcelos, M.A.; Rocha, B.A.M.; Teixeira, E.H.; et al. New lectins from Codium isthmocladum Vickers show unique amino acid sequence and antibiofilm effect on pathogenic bacteria. J. Appl. Phycol. 2020, 32, 4263–4276. [Google Scholar] [CrossRef]
- Vasconcelos, M.A.; Arruda, F.V.S.; Carneiro, V.A.; Silva, H.C.; Nascimento, K.S.; Sampaio, A.H.; Cavada, B.; Teixeira, E.H.; Henriques, M.; Pereira, M.O. Effect of Algae and Plant Lectins on Planktonic Growth and Biofilm Formation in Clinically Relevant Bacteria and Yeasts. Biomed Res. Int. 2014, 2014, 365272. [Google Scholar] [CrossRef]
- Silva, P.M.; Baldry, M.; Peng, P.; Silva, J.N.O.; Soares, T.; Brayner, F.A.; Alves, L.C.; Feitosa, A.P.S.; Paiva, P.M.G.; Ingmer, H.; et al. Punica granatum sarcotesta lectin (PgTeL) impairs growth, structure, viability, aggregation, and biofilm formation ability of Staphylococcus aureus clinical isolates. Int. J. Biol. Macromol. 2019, 123, 600–608. [Google Scholar] [CrossRef]
- Moura, M.C.; Procópio, T.F.; Ferreira, G.R.S.; Alves, R.R.V.; Sá, R.A.; Paiva, P.M.G.; Ingmer, H.; Coelho, L.C.B.B.; Napoleão, T.H. Anti-staphylococcal effects of Myracrodruon urundeuva lectins on nonresistant and multidrug resistant isolates. J. Appl. Microbiol. 2021, 130, 745–754. [Google Scholar] [CrossRef]
- Klein, R.C.; Fabres-Klein, M.H.; Oliveira, L.L.; Feio, R.N.; Malouin, F.; Ribon, A.O.B. A C-Type Lectin from Bothrops jararacussu Venom Disrupts Staphylococcal Biofilms. PLoS ONE 2015, 10, e0120514. [Google Scholar] [CrossRef] [PubMed]
- Andrade, F.R.N.; Tabosa, P.A.S.; Torres, R.C.F.; Carneiro, R.F.; Vasconcelos, M.A.; Andrade, A.L.; Nascimento, E.; Pinheiro, U.; Teixeira, E.H.; Nagano, C.S.; et al. New lectin isolated from the tropical sponge Haliclona (Reniera) implexiformis (Hechtel, 1965) shows antibiofilm effect. An. Acad. Bras. Cienc. 2023, 95, e20220379. [Google Scholar] [CrossRef] [PubMed]
- Belfiori, B.; Riccioni, C.; Pietrella, D.; Pupilli, F.; Bellucci, M.; De Marchis, F. Vicia ervilia lectin (VEA) has an antibiofilm effect on both Gram-positive and Gram-negative pathogenic bacteria. Arch. Microbiol. 2024, 206, 371. [Google Scholar] [CrossRef]
- Almeida, A.S.; Mendonça, D.N.M.; Carneiro, R.F.; Pinheiro, U.; Nascimento, E.F.; Andrade, A.L.; Vasconcelos, M.A.; Teixeira, E.H.; Nagano, C.S.; Sampaio, A.H. Purification, biochemical characterization of a lectin from marine sponge Ircinia strobilina and its effect on the inhibition of bacterial biofilms. An. Acad. Bras. Cienc. 2023, 95, e20220619. [Google Scholar] [CrossRef]
- Silva, W.S.F.L.; Amorim, P.K.; Ferreira, G.R.S.; Alves, R.R.V.; Silva, C.E.S.; Santos, P.E.M.; Paiva, P.M.G.; Ingmer, H.; Silva, S.P.; Silva, P.M.; et al. Moringa oleifera seed preparations containing the lectin WSMoL inhibit growth, cell aggregation, and biofilm production of Listeria monocytogenes. Microb. Pathog. 2025, 204, 107600. [Google Scholar] [CrossRef]
- Silva, P.M.; Silva, J.N.O.; Silva, B.R.; Ferreira, G.R.S.; Gaião, W.D.C.; Recio, M.V.; Gonçalves, G.G.A.; Rodrigues, C.G.; Medeiros, P.L.; Brayner, F.A.; et al. Antibacterial effects of the lectin from pomegranate sarcotesta (PgTeL) against Listeria monocytogenes. J. Appl. Microbiol. 2021, 131, 671–681. [Google Scholar] [CrossRef]
- Jin, X.; Lee, Y.J.; Hong, S.H. Canavalia ensiformis-derived lectin inhibits biofilm formation of enterohemorrhagic Escherichia coli and Listeria monocytogenes. J. Appl. Microbiol. 2018, 126, 300–310. [Google Scholar] [CrossRef]
- Janež, N.; Zupan, T.; Ladányi, M.; Sterniša, M.; Jug, B.; Peternel, T.; Sebastijanović, A.; Nanut, M.P.; Karničar, K.; Taler-Verčič, A.; et al. Exposure to specific fungal lectins during adhesion impairs biofilm formation of Listeria on polystyrene. Microb. Biotechnol. 2024, 17, e70040. [Google Scholar] [CrossRef]
- Arfin, N.; Podder, M.K.; Kabir, S.R.; Asaduzzaman, A.K.M.; Hasan, I. Antibacterial, antifungal and in vivo anticancer activities of chitin-binding lectins from Tomato (Solanum lycopersicum) fruits. Arab. J. Chem. 2022, 15, 104001. [Google Scholar] [CrossRef]
- Chaves, R.P.; Santos, A.K.B.; Andrade, A.L.; Pinheiro, A.A.; Silva, J.M.S.; Silva, F.M.S.; Sousa, J.P.; Neto, I.L.B.; Bezerra, E.H.S.; Abreu, J.O.; et al. Structural study and antimicrobial and wound healing effects of lectin from Solieria filiformis (Kützing) P.W.Gabrielson. Biochimie 2023, 214, 61–76. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.A.; Hossain, M.M.; Kabir, S.R.; Ozeki, Y.; Khanam, A.; Fujii, Y.; Asaduzzaman, A.K.M.; Hasan, I. Carbohydrate-Binding Properties and Antimicrobial and Anticancer Potential of a New Lectin from the Phloem Sap of Cucurbita pepo. Molecules 2024, 29, 2531. [Google Scholar] [CrossRef]
- Podder, M.K.; Hossain, M.M.; Kabir, S.R.; Asaduzzaman, A.K.M.; Hasan, I. Antimicrobial, antioxidant and antiproliferative activities of a galactose-binding seed lectin from Manilkara zapota. Heliyon 2024, 10, e24592. [Google Scholar] [CrossRef]
- Petrova, M.I.; Imholz, N.C.E.; Verhoeven, T.L.A.; Balzarini, J.; Van Damme, E.J.M.; Schols, D.; Vanderleyden, J.; Lebeer, S. Lectin-Like Molecules of Lactobacillus rhamnosus GG Inhibit Pathogenic Escherichia coli and Salmonella Biofilm Formation. PLoS ONE 2016, 11, e0161337. [Google Scholar] [CrossRef] [PubMed]
- Silva, P.M.; Silva, B.R.; Silva, J.N.O.; Moura, M.C.; Soares, T.; Feitosa, A.P.S.; Brayner, F.A.; Alves, L.C.; Paiva, P.M.G.; Damborg, P.; et al. Punica granatum sarcotesta lectin (PgTeL) has antibacterial activity and syn ergistic effects with antibiotics against β-lactamase-producing Escherichia coli. Int. J. Biol. Macromol. 2019, 135, 931–939. [Google Scholar] [CrossRef]
- Carneiro, R.F.; Viana, J.T.; Torres, R.C.F.; Silva, L.T.; Andrade, A.L.; Vasconcelos, M.A.; Pinheiro, U.; Teixeira, E.H.; Nagano, C.S.; Sampaio, A.H. A new mucin-binding lectin from the marine sponge Aplysina fulva (AFL) exhibits antibiofilm effects. Arch. Biochem. Biophys. 2019, 662, 169–176. [Google Scholar] [CrossRef]
- Carneiro, R.F.; Lima, P.H.P., Jr.; Chaves, R.P.; Pereira, R.; Pereira, A.L.; Vasconcelos, M.A.; Pinheiro, U.; Teixeira, E.H.; Nagano, C.S.; Sampaio, A.H. Isolation, biochemical characterization and antibiofilm effect of a lectin from the marine sponge Aplysina lactuca. Int. J. Biol. Macromol. 2017, 99, 213–222. [Google Scholar] [CrossRef]
- Bose, P.P.; Bhattacharjee, S.; Singh, S.; Mandal, S.; Mondal, G.; Gupta, P.; Chatterjee, B.P. A glucose/mannose binding lectin from litchi (Litchi chinensis) seeds: Biochemical and biophysical characterizations. Biochem. Biophys. Rep. 2016, 6, 242–252. [Google Scholar] [CrossRef]
- Silva, P.M.; Ferreira, G.R.S.; Silva, A.R.; Oliveira, W.F.; Correia, M.T.S.; Cabral Filho, P.E.; Fontes, A.; Napoleão, T.H.; Paiva, P.M.G. Punica granatum sarcotesta lectin (PgTeL) inhibits Pseudomonas aeruginosa replication, viability, aggregation, and biofilms. S. Afr. J. Bot. 2024, 165, 264–274. [Google Scholar] [CrossRef]
- Fu, T.; Ng, S.; Chen, Y.; Lee, Y.; Demeter, F.; Herczeg, M.; Borbás, A.; Chiu, C.; Lan, C.; Chen, C.; et al. Rhamnose Binding Protein as an Anti-Bacterial Agent—Targeting Biofilm of Pseudomonas aeruginosa. Mar. Drugs 2019, 17, 355. [Google Scholar] [CrossRef]
- Alyousef, A.A.; Alqasim, A.; Aloahd, M.S. Isolation and characterization of lectin with antibacterial, antibiofilm and antiproliferative activities from Acinetobacter baumannii of environmental origin. J. Appl. Microbiol. 2018, 124, 1139–1146. [Google Scholar] [CrossRef]
- Klafke, G.B.; Borsuk, S.; Goncales, R.A.; Arruda, F.V.S.; Carneiro, V.A.; Teixeira, E.H.; Silva, A.L.C.; Cavada, B.S.; Dellagostin, O.A.; Pinto, L.S. Inhibition of initial adhesion of oral bacteria through a lectin from Bauhinia variegata L. var. variegata expressed in Escherichia coli. J. Appl. Microbiol. 2013, 115, 1222–1230. [Google Scholar] [CrossRef]
- Ghequire, M.G.K.; De Canck, E.; Wattiau, P.; Van Winge, I.; Loris, R.; Coenye, T.; De Mot, R. Antibacterial activity of a lectin-like Burkholderia cenocepacia protein. MicrobiologyOpen 2013, 2, 566–575. [Google Scholar] [CrossRef] [PubMed]
- Jayanthi, S.; Ishwarya, R.; Anjugam, M.; Iswarya, A.; Karthikeyan, S.; Vaseeharan, B. Purification, characterization and functional analysis of the immune molecule lectin from the haemolymph of blue swimmer crab Portunus pelagicus and their antibiofilm properties. Fish Shellfish Immunol. 2017, 62, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Preetham, E.; Rubeen, A.S.; Vaseeharan, B.; Chaurasia, M.K.; Arockiaraj, J.; Olsen, R.E. Anti-biofilm properties and immunological response of an immune molecule lectin isolated from shrimp Metapenaeus monoceros. Fish Shellfish Immunol. 2019, 94, 896–906. [Google Scholar] [CrossRef] [PubMed]
- Preetham, E.; Arockiaraj, J.; Lakshmi, S.; Olsen, R.F.; Wongpanya, R.; Vaseeharan, B. Antibiofilm and immunological properties of lectin purified from shrimp Penaeus semisulcatus. Fish Shellfish Immunol. 2020, 106, 776–782. [Google Scholar] [CrossRef]
- Kaplan, J.B. Antibiotic-induced biofilm formation. Int. J. Artif. Organs 2011, 9, 737–751. [Google Scholar] [CrossRef] [PubMed]
- Cordisco, E.; Serra, D.O. Moonlighting antibiotics: The extra job of modulating biofilm formation. Trend. Microibiol. 2025, 33, 459–471. [Google Scholar] [CrossRef]
- Hoffman, L.R.; D’Argenio, D.A.; MacCoss, M.J.; Zhang, Z.; Jones, R.A.; Miller, S.I. Aminoglycoside antibiotics induce bacterial biofilm formation. Nature 2005, 436, 1171–1175. [Google Scholar] [CrossRef]
- Oliveira, N.M.; Martinez-Garcia, E.; Xavier, J.; Durham, W.M.; Kolter, R.; Kim, W.; Foster, K.R. Biofilm Formation As a Response to Ecological Competition. PLoS Biol. 2015, 13, e1002191. [Google Scholar] [CrossRef]
- Jayasankari, S.; Vishnuvarthan, P.; Rubini, D.; Subramaniyan, S.B.; Vadivel, V.; Anbazhagan, V.; Nithyaand, P. Jacalin Hydrocolloid Nanoconjugates Mitigate Methicillin Resistant Staphylococcus aureus (MRSA) Biofilms on Meat Products. ACS Food Sci. Technol. 2021, 1, 1030–1040. [Google Scholar] [CrossRef]
- Cavalcante, T.T.A.; Rocha, B.A.M.; Carneiro, V.A.; Arruda, F.V.S.; Nascimento, A.S.F.; Sá, N.C.; Nascimento, K.S.; Cavada, B.S.; Teixeira, E.H. Effect of Lectins from Diocleinae Subtribe against Oral Streptococci. Molecules 2011, 16, 3530–3543. [Google Scholar] [CrossRef]
- Abdelhamid, A.G.; Yousef, A.E. Combating Bacterial Biofilms: Current and Emerging Antibiofilm Strategies for Treating Persistent Infections. Antibiotics 2023, 12, 1005. [Google Scholar] [CrossRef]
- Al-Madboly, L.A.; Aboulmagd, A.; El-Salam, M.A.; Kushkevych, I.; El-Morsi, R.M. Microbial Enzymes as Powerful Natural Anti-Biofilm Candidates. Microb. Cell Fact. 2024, 23, 343. [Google Scholar] [CrossRef] [PubMed]
- Roque-Borda, C.A.; Primo, L.M.D.G.; Franzyk, H.; Hansen, P.R.; Pavan, F.R. Recent Advances in the Development of Antimicrobial Peptides against ESKAPE Pathogens. Heliyon 2024, 10, e31958. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.L. Protein Denaturation and Functionality Losses. In Quality in Frozen Food, 1st ed.; Erickson, M.C., Hung, Y.C., Eds.; Springer: Boston, MA, USA, 1997; pp. 111–140. [Google Scholar]
- Butreddy, A.; Janga, K.Y.; Ajjarapu, S.; Sarabu, S.; Dudhipala, N. Instability of therapeutic proteins—An overview of stresses, stabilization mechanisms and analytical techniques involved in lyophilized proteins. Int. J. Biol. Macromol. 2021, 167, 309–325. [Google Scholar] [CrossRef]


| Lectins | Source | Antibiofilm Activity 1 |
|---|---|---|
| Algae | ||
| Bryothamnion seaforthii lectin (BSL) | B. seaforthii (red alga) | Inhibitory |
| Codium isthmocladum lectin (CiL-1 and -2) | C. isthmocladum (green alga) | Inhibitory |
| Hypnea musciformis lectin (HML) | H. musciformis (red alga) | Inhibitory |
| Solieria filiformis lectin (SfL), recombinant SfL (rSfL-1) | Solieria filiformis (red alga) | Inhibitory |
| Animals | ||
| Aplysina lactuca lectin (ALL) | A. lactuca (sponge) | Inhibitory |
| C-type lectin | Bothrops jararacussu (snake) venom | Inhibitory, Eradicating |
| Haliclona implexiformis lectin (HiL) | H. (Reniera) implexiformis (sponge) | Inhibitory |
| Ircinia strobilina lectin (IsL) | I. strobilina (sponge) | Inhibitory |
| Metapenaeus monoceros lectin (MmLec) | M. monoceros (shrimp) hemolymph | Inhibitory |
| Recombinant hemolymph plasma lectin (rHPLOE) | Tachypleus tridentatus (crab) hemolymph | Inhibitory, Eradicating |
| Semisulcatus-lectin | Penaeus semisulcatus (shrimp) hemolymph | Inhibitory |
| Portunus pelagicus lectin (Pp-Lec) | P. pelagicus (crab) hemolymph | Inhibitory |
| Microrganisms | ||
| Aleuria aurantia lectin (AAL) | A. aurantia (fungus) | Inhibitory |
| AB119 | Acinetobacter baumannii (bacteria) | Inhibitory |
| Agaricus bisporus lectin (ABL) | A. bisporus (fungus) | Inhibitory |
| Coprinus galectin 2 (CGL2) | Coprinopsis cinerea (fungus) | Inhibitory, Eradicating |
| C. cinerea mucin-binding lectin 1 (CML1) | C. cinerea (fungus) | Inhibitory |
| Clitocybe nebularis lectin (CNL) | C. nebularis (fungus) | Inhibitory |
| Lectin-like protein 1 and 2 (Llp1, Llp2) | Lactobacillus rhamnosus (bacteria) | Inhibitory |
| Lectin-like protein A (LlpA) | Burkholderia cenocepacia (bacteria) | Inhibitory, Eradicating |
| Transcript associated with perithecial development 1 (TAP1) | Sordaria macrospora (fungus) | Inhibitory |
| Plants | ||
| Alpinia purpurata lectin (ApuL) | A. purpurata inflorescences | Inhibitory |
| Bauhinia bauhinoides lectin (BBL) | B. bauhinoides seeds | Inhibitory |
| Bauhinia variegata lectin (BVL), recombinant BVL (rBVL-1) | B. variegata seeds | Inhibitory |
| Calliandra surinamensis lectin (CasuL) | C. surinamensis leaf pinnulae | Inhibitory |
| Cratylia floribunda lectin (CFL) | C. floribunda seeds | Inhibitory |
| Concanavalin A (ConA) | Canavalia ensiformis seeds | Inhibitory |
| Cucurbita pepo lectin (CPL) | C. pepo exudate phloem | Inhibitory |
| Crataeva tapia bark lectin (CrataBL) | C. tapia bark | Inhibitory, Eradicating |
| Jacalin | Artocarpus integrifolia seeds | Inhibitory, Eradicating |
| Litchi lectin | Litchi chinensis seeds | Inhibitory |
| Myracrodruon urundeuva heartwood lectin (MuHL) | M. urundeuva heartwood | Inhibitory |
| Manilkara zapota seed lectin (MZSL) | M. zapota seeds | Inhibitory |
| Punica granatum testa lectin (PgTeL) | P. granatum sarcotesta | Inhibitory, Eradicating |
| Tomato chitin-binding lectin (TCL) | Solanum lycopersicum fruit | Inhibitory |
| Vicia ervilia agglutinin (VEA) | V. ervilia seeds | Inhibitory |
| Vatairea macrocarpa lectin (VML) | V. macrocarpa seeds | Inhibitory |
| Water-soluble Moringa oleifera lectin (WSMoL) | M. oleifera seeds | Inhibitory, Eradicating |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Napoleão, T.H.; Lira, T.L.d.S.; Pontual, E.V.; Ferreira, G.R.S.; da Silva, P.M. Lectins as Natural Antibiofilm Agents in the Fight Against Antibiotic Resistance: A Review. Molecules 2025, 30, 3395. https://doi.org/10.3390/molecules30163395
Napoleão TH, Lira TLdS, Pontual EV, Ferreira GRS, da Silva PM. Lectins as Natural Antibiofilm Agents in the Fight Against Antibiotic Resistance: A Review. Molecules. 2025; 30(16):3395. https://doi.org/10.3390/molecules30163395
Chicago/Turabian StyleNapoleão, Thiago Henrique, Thiago Lucas da Silva Lira, Emmanuel Viana Pontual, Gustavo Ramos Salles Ferreira, and Pollyanna Michelle da Silva. 2025. "Lectins as Natural Antibiofilm Agents in the Fight Against Antibiotic Resistance: A Review" Molecules 30, no. 16: 3395. https://doi.org/10.3390/molecules30163395
APA StyleNapoleão, T. H., Lira, T. L. d. S., Pontual, E. V., Ferreira, G. R. S., & da Silva, P. M. (2025). Lectins as Natural Antibiofilm Agents in the Fight Against Antibiotic Resistance: A Review. Molecules, 30(16), 3395. https://doi.org/10.3390/molecules30163395







