Silane-Coupled Silica Nanoparticles Encapsulating Emitting Quantum Dots: Advancing Robust Phosphors for Displays and Beyond
Abstract
1. Introduction
2. Background of Related Fields and Article Outline
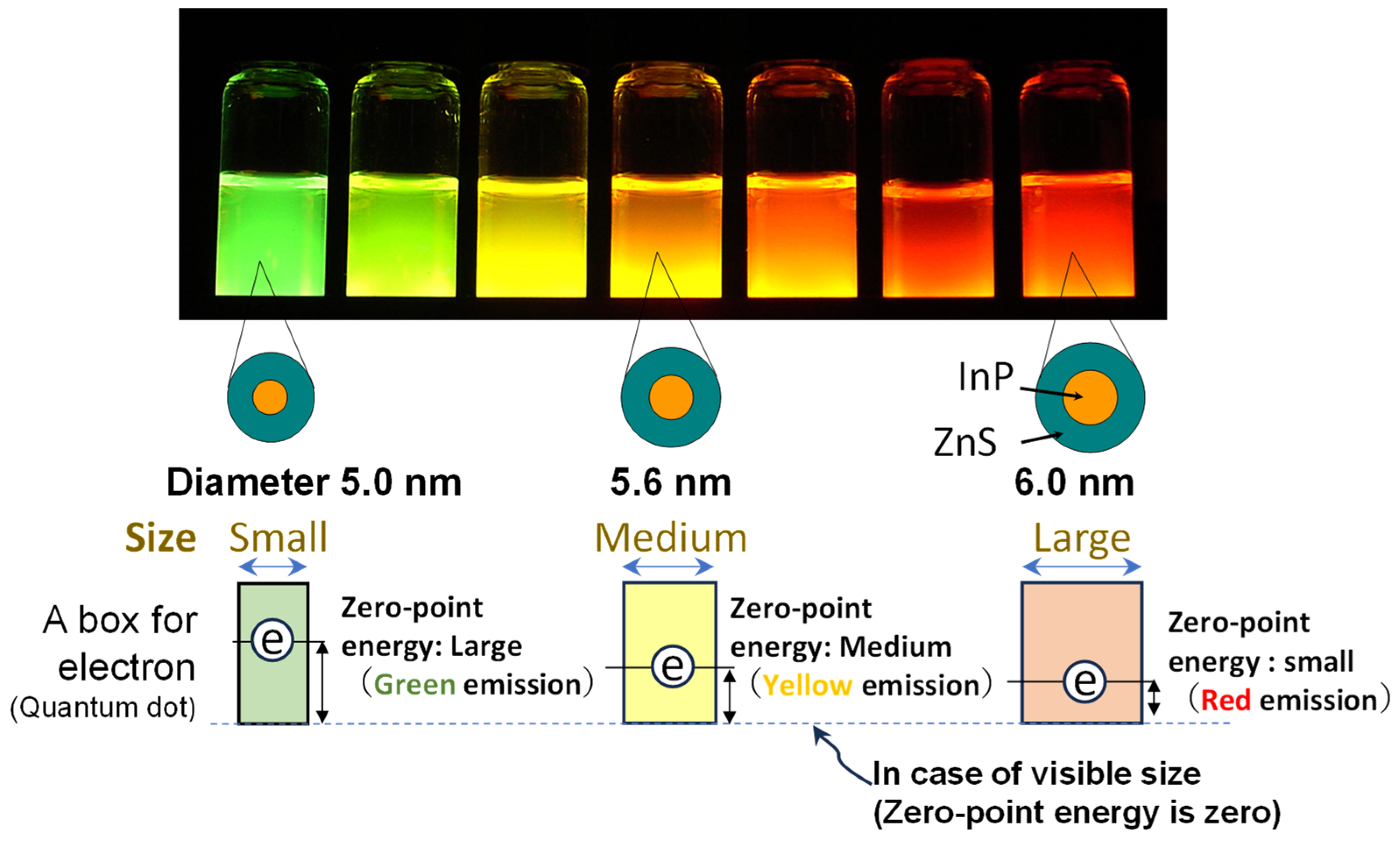
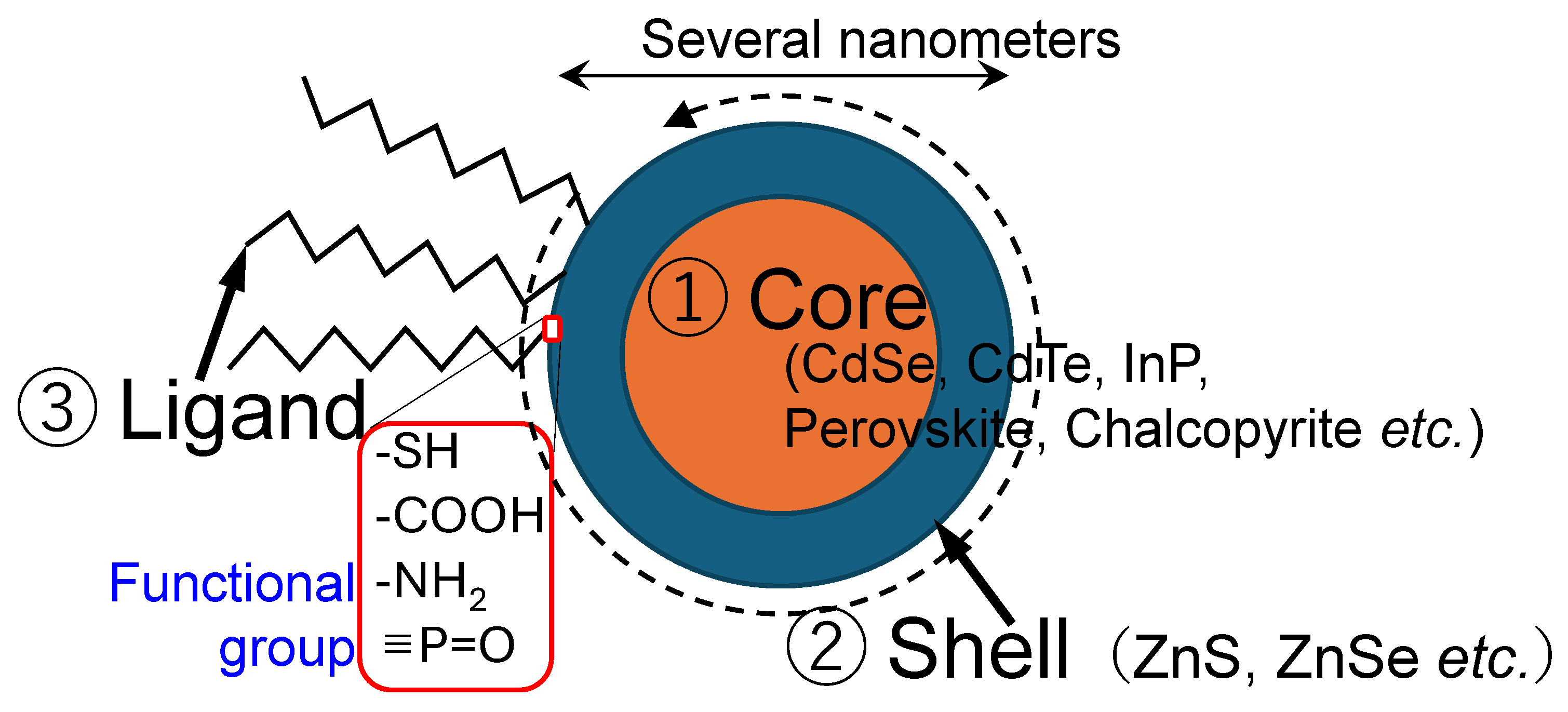
2.1. Colloidal Quantum Dots
2.2. Matrices for QD Encapsulation
2.3. Outline of the Article
2.4. Silica Encapsulation of Ternary QDs
| Category No. | Quantum Dot | Interaction with Water | Silane-Coupling Agent | Method | Morphology of Silica Glass | Comments | This Article | Main Year | References |
|---|---|---|---|---|---|---|---|---|---|
| A | 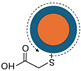 CdTe, ZnSe, & InP-based | Hydrophilic | — | Reverse micelle |  Nanoparticle | Not incorporated into glass spheres. | Section 4.1 | 2004 | [51,52,53] |
| B | APS | (Normal sol-gel) |  Bulk | First successful incorporation into glass with high PLQY; however, the glass dissolved upon exposure to hot water. | Section 3.1 | 2004 | [54,55,56,57,58] | ||
| C | APS | Layer-by-layer |  Thin film | High QD loading achieved; however, the resulting film was fragile. | Section 3.2 | 2005 | [59] | ||
| D | MPS | Stöber | 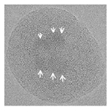 Nanoparticle | Porosity was characterized; QDs exhibited a broad FWHM. | Section 4.2 | 2010, 2020 | [60,61,62,63] | ||
| E |  CdSe, & InP-based | Hydrophobic | — | Reverse micelle | 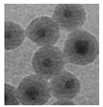 Nanoparticle | Single QD per silica particle achieved; however, dispersion concentration remained insufficient. | Section 4.3 | 2006 | [64,65,66,67,68,69] |
| F | MPS | Modified Stöber | 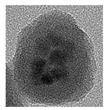 Nanoparticle | Multiple QDs per silica particle; extensively investigated due to several applications. | Section 4.4, and Section 5 and Section 6 | 2010–2025 | [70,71,72,73,74,75,76,77] |
3. Incorporation of QDs in Bulk and Thin-Film Silica Derived from Silane Coupling Agents
3.1. Bulk Incorporation of QDs Using APS
3.2. Layer-by-Layer (LbL) Incorporation of QDs Using APS
4. Incorporation of QDs into Sol–Gel-Derived Silica Nanoparticles
4.1. Hydrophilic QDs Incorporated into Silica Nanoparticles via Reverse Micelle Method
4.2. Hydrophilic QDs Incorporated into Silica Nanoparticles via the Stöber Method
4.2.1. Nanoparticle Morphology and PL Properties
4.2.2. Hybrid Structure and Porous Properties
4.3. Hydrophobic QDs Incorporated via Reverse Micelle Method
4.4. Hydrophobic QDs Incorporated via the Stöber Method
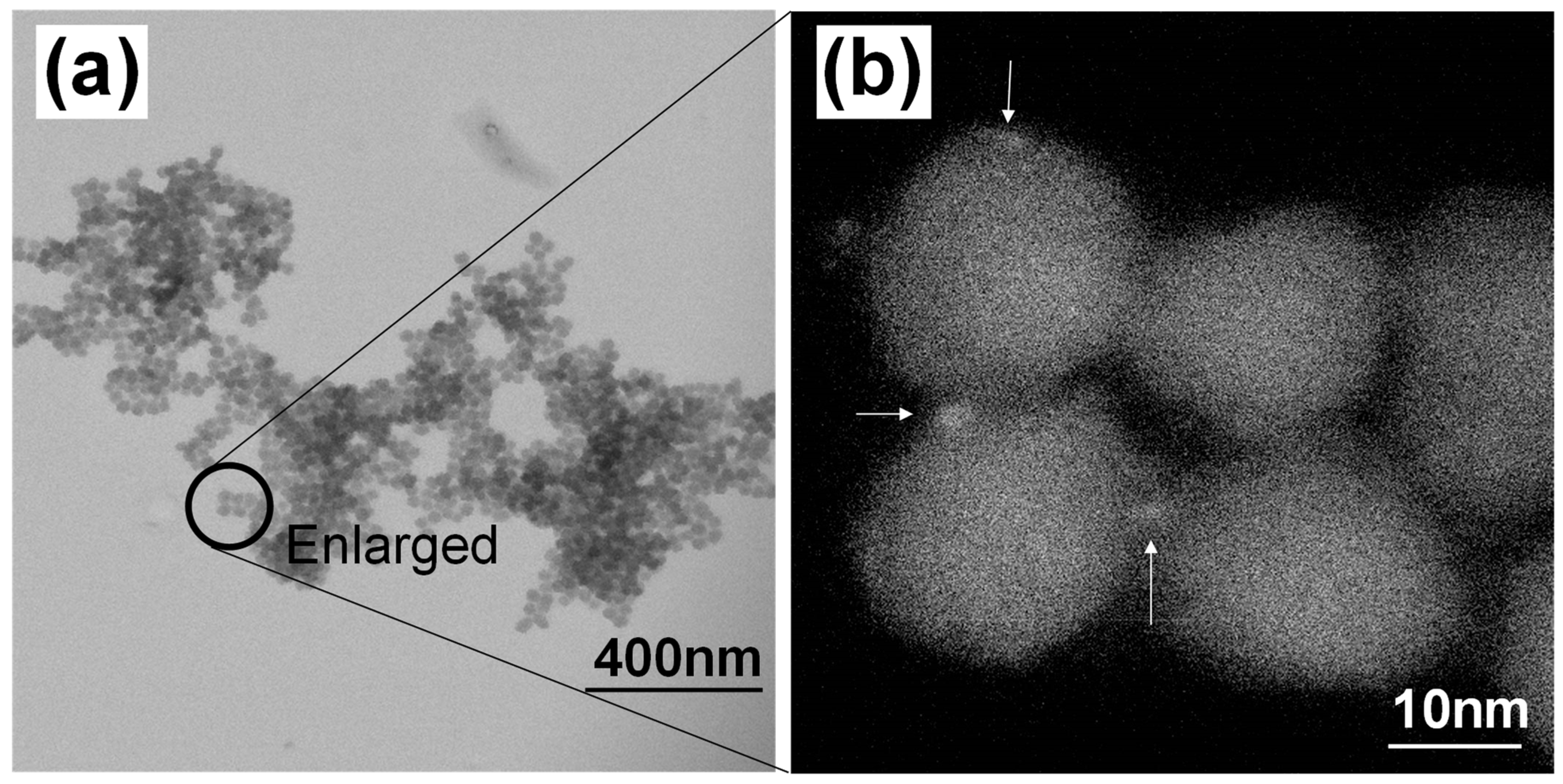
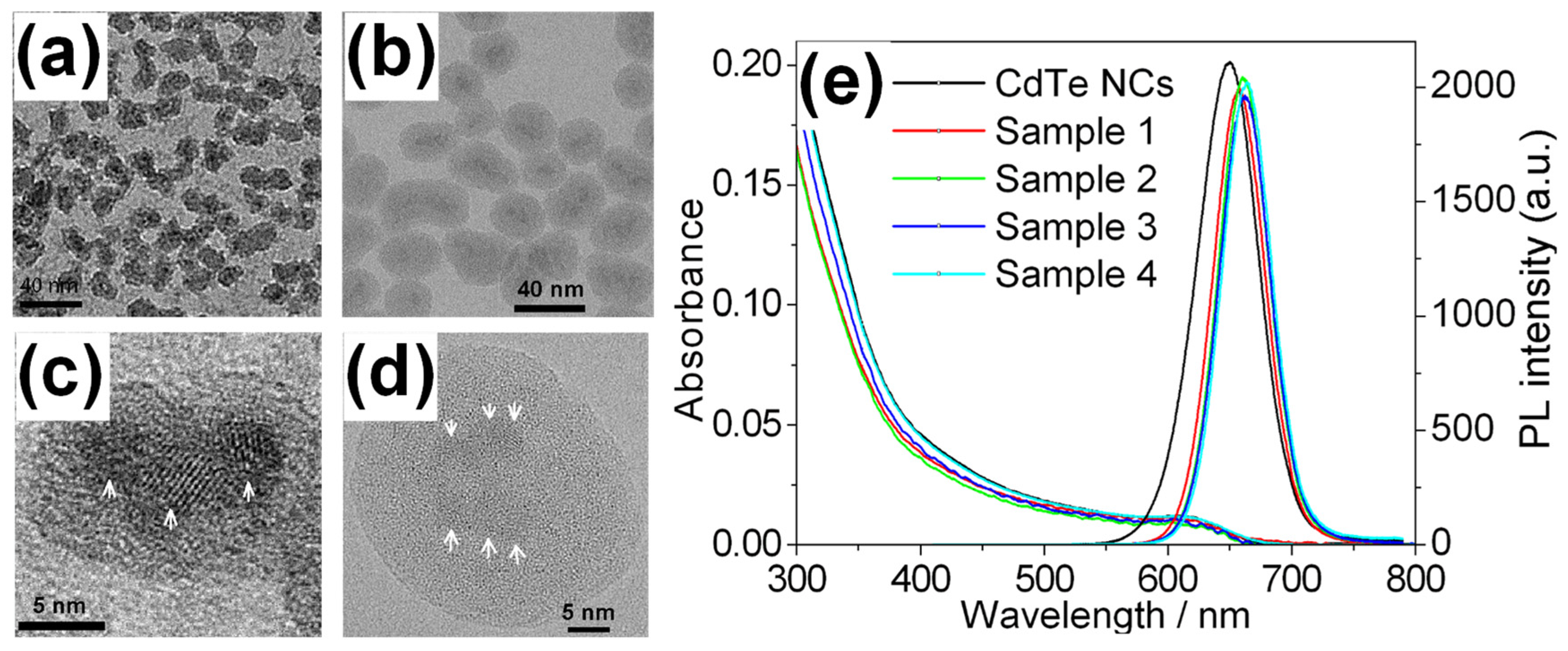
5. Silica Nanoparticles with Encapsulated Dozens of Hydrophobic CdSe-Based QDs
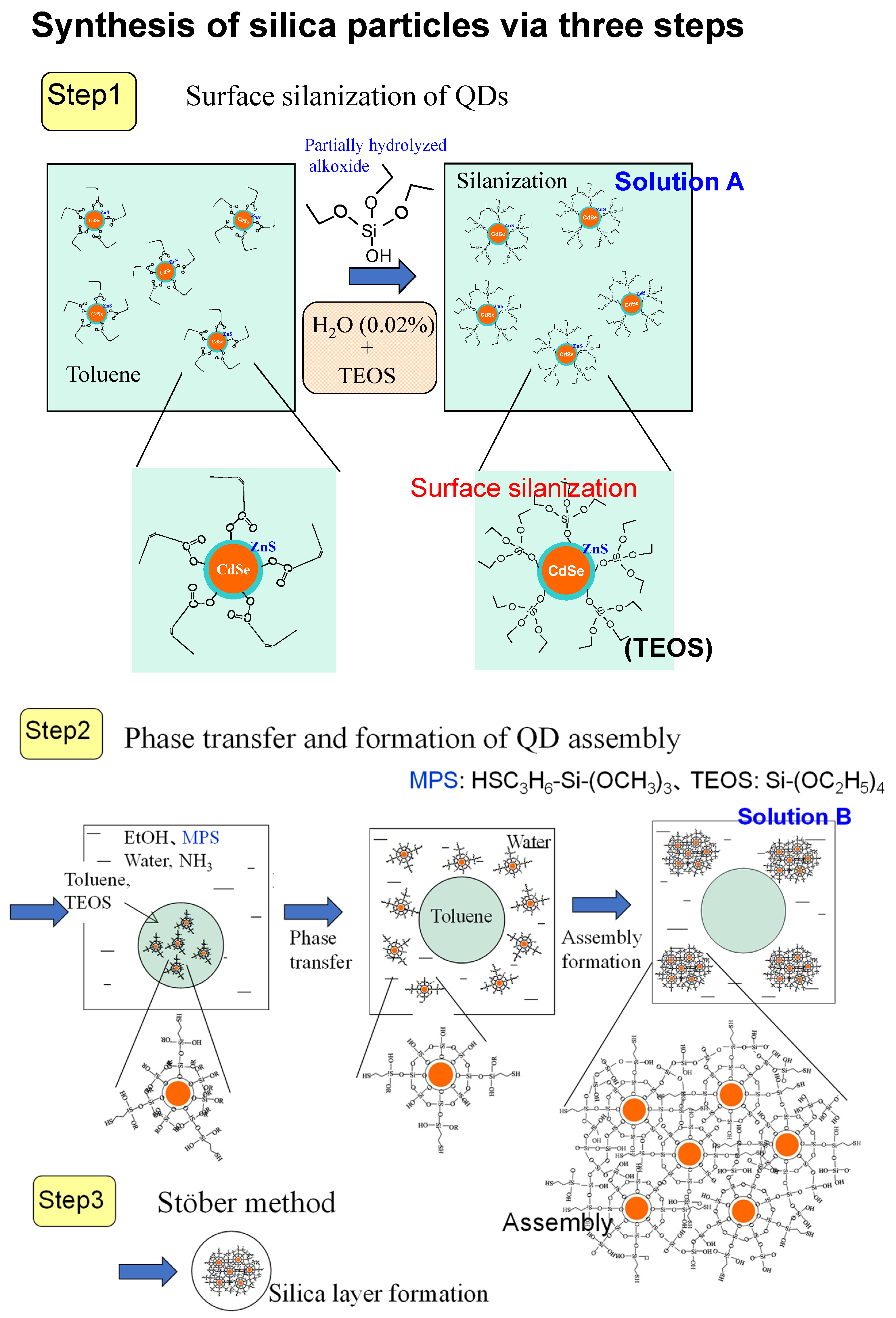
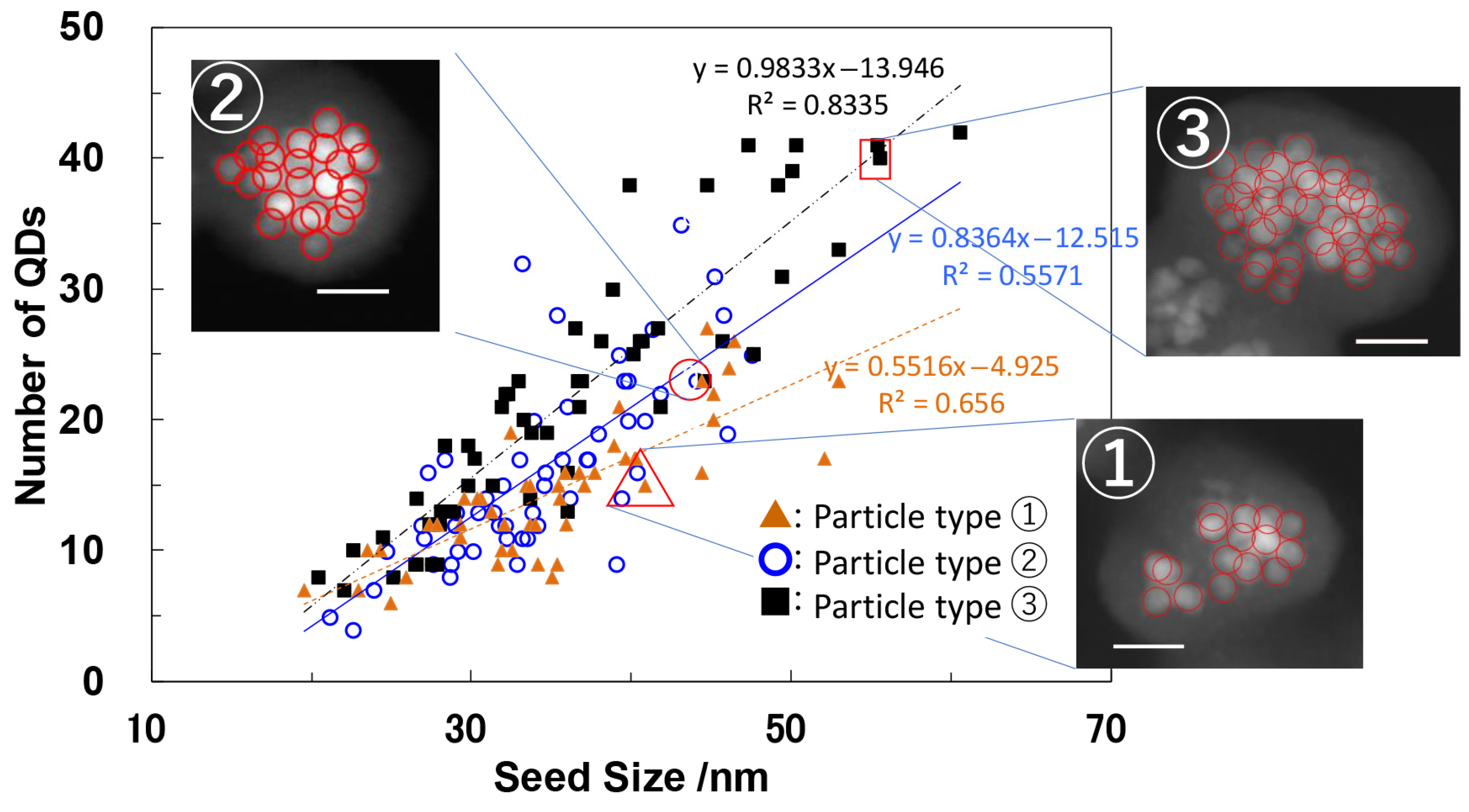
5.1. Preparation and Formation Mechanism
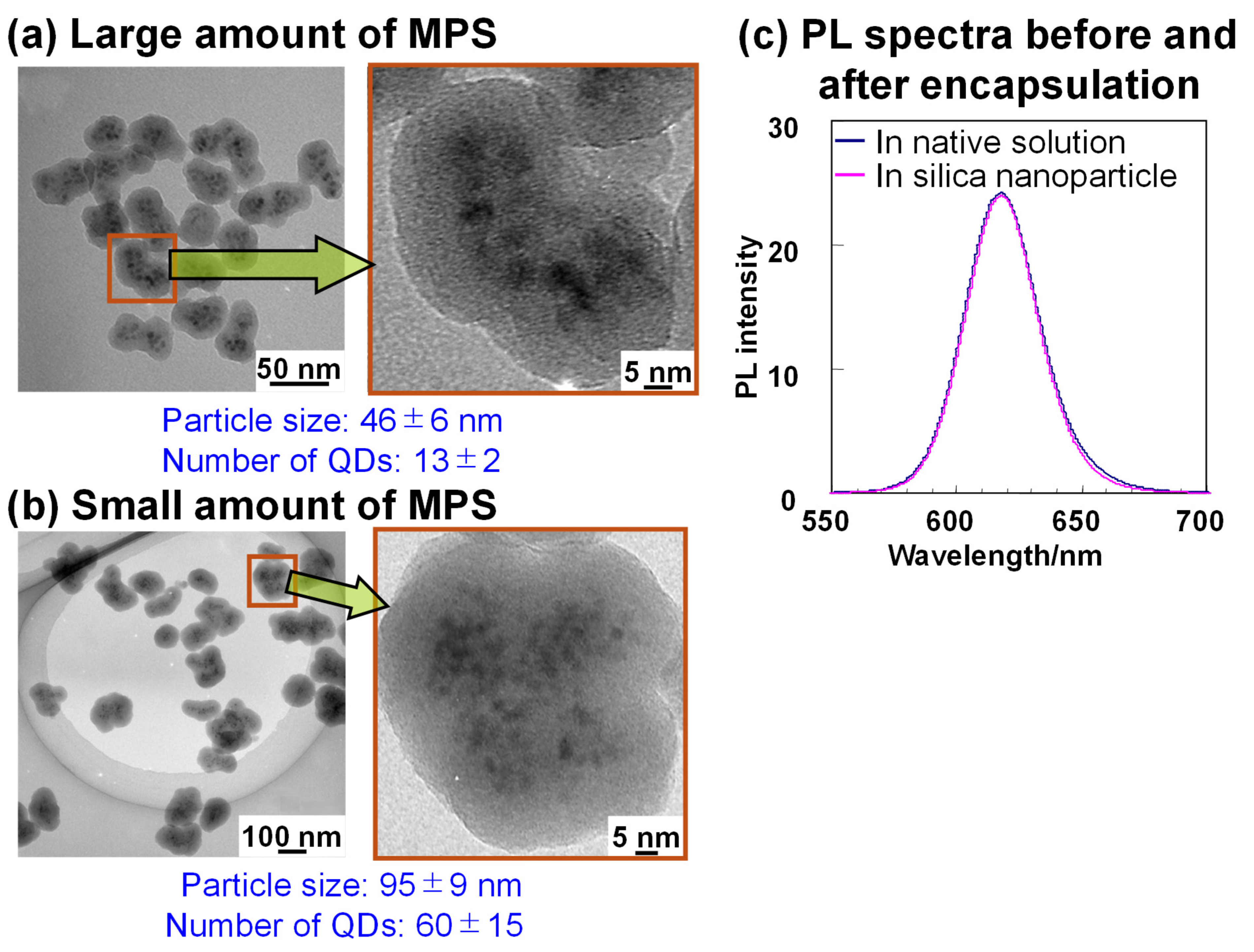
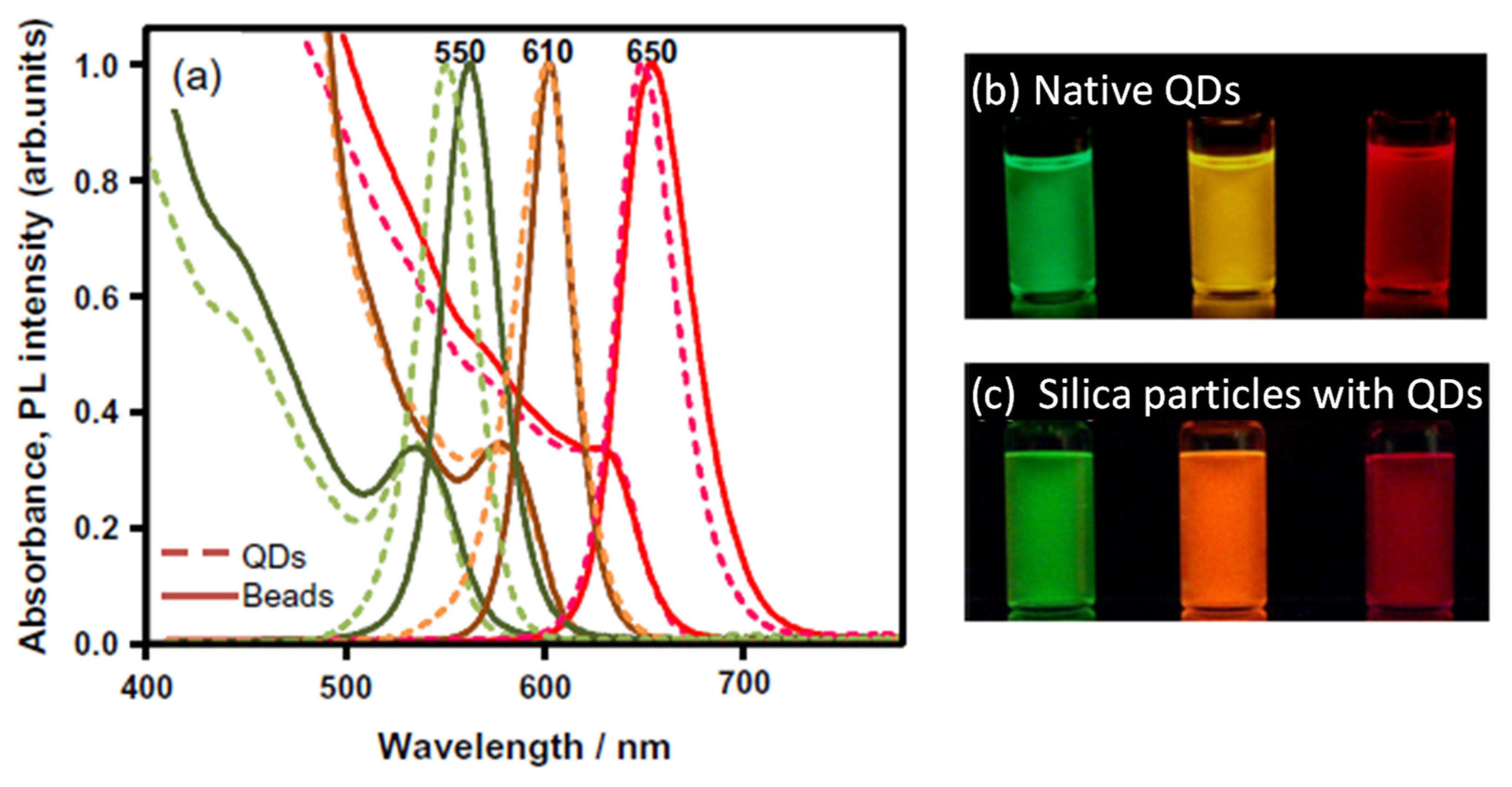
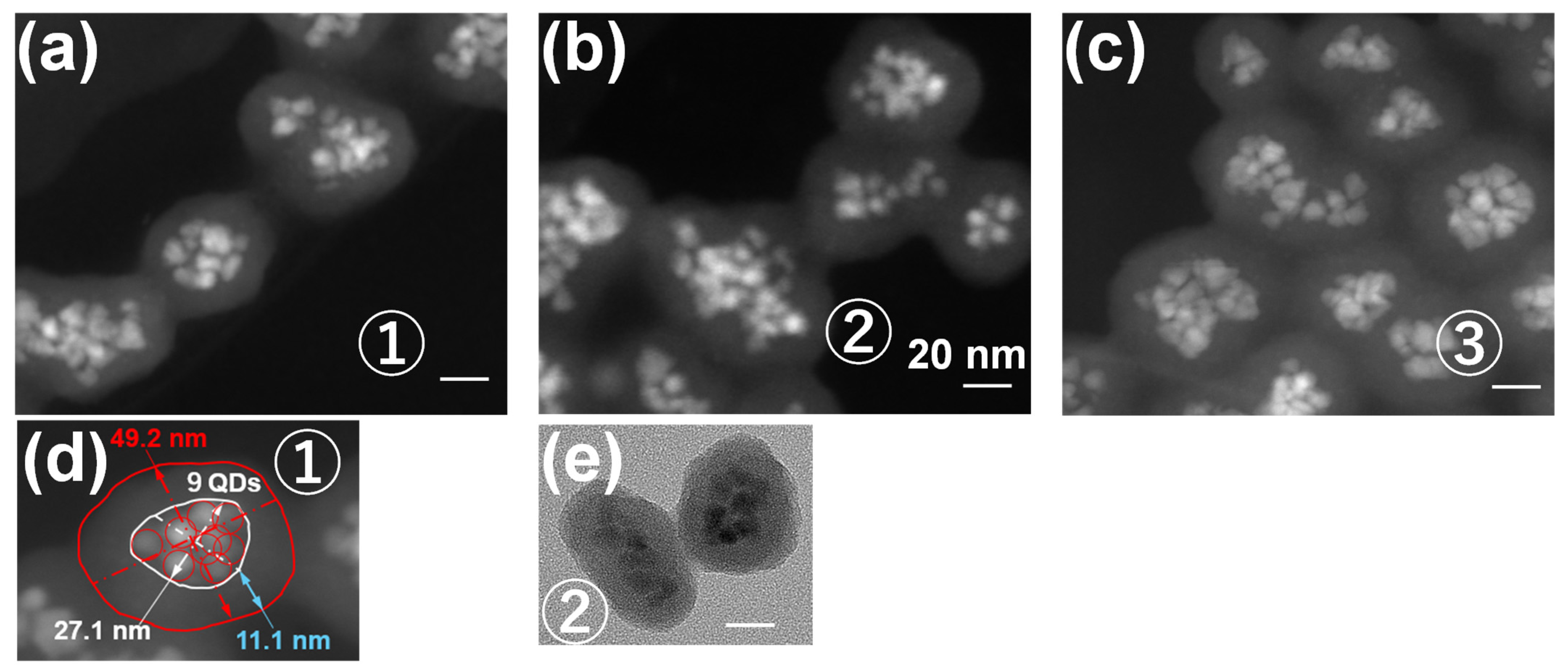
5.2. Structural and Optical Properties
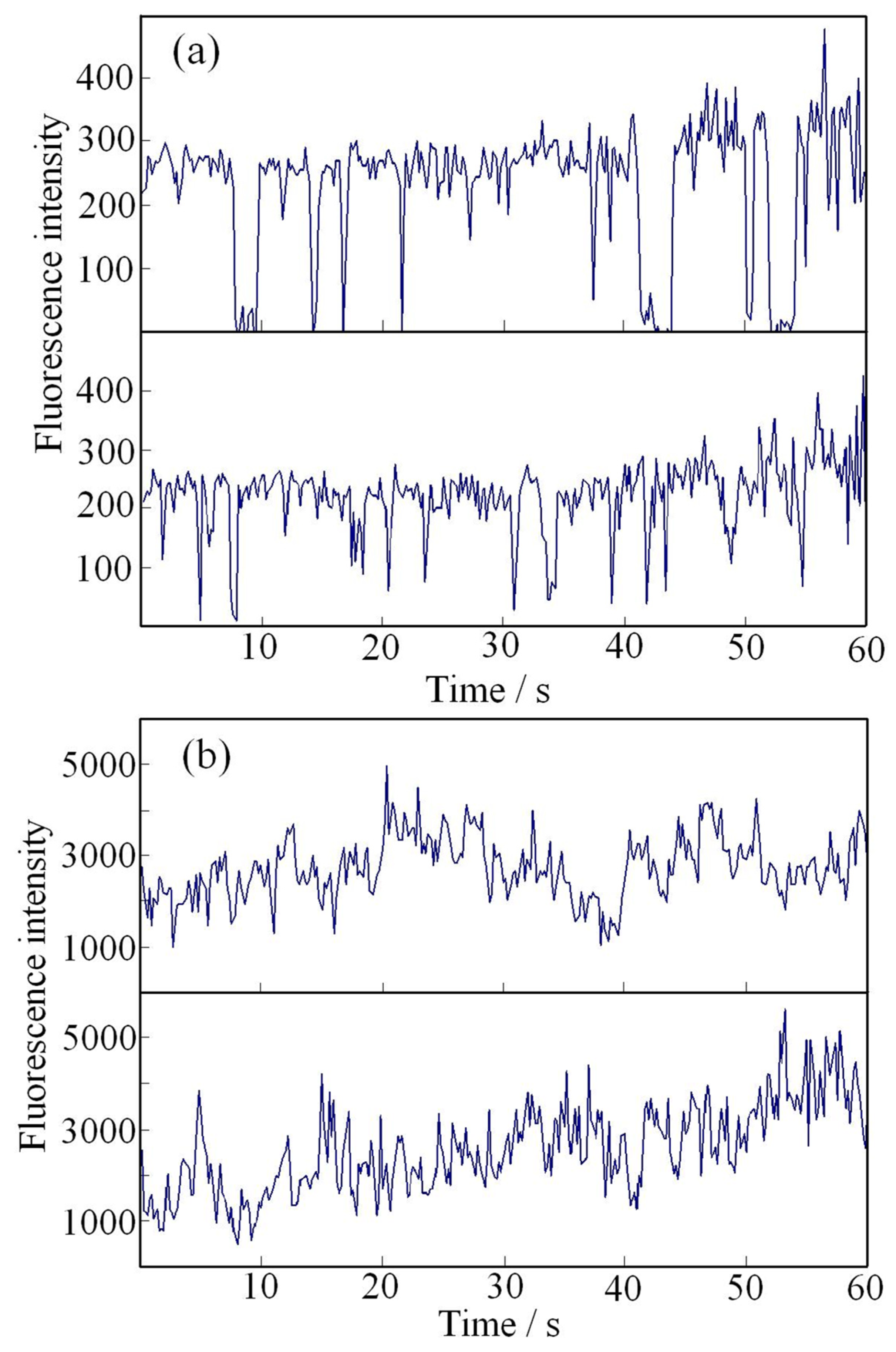
5.3. PL Properties at the Single-Particle Level
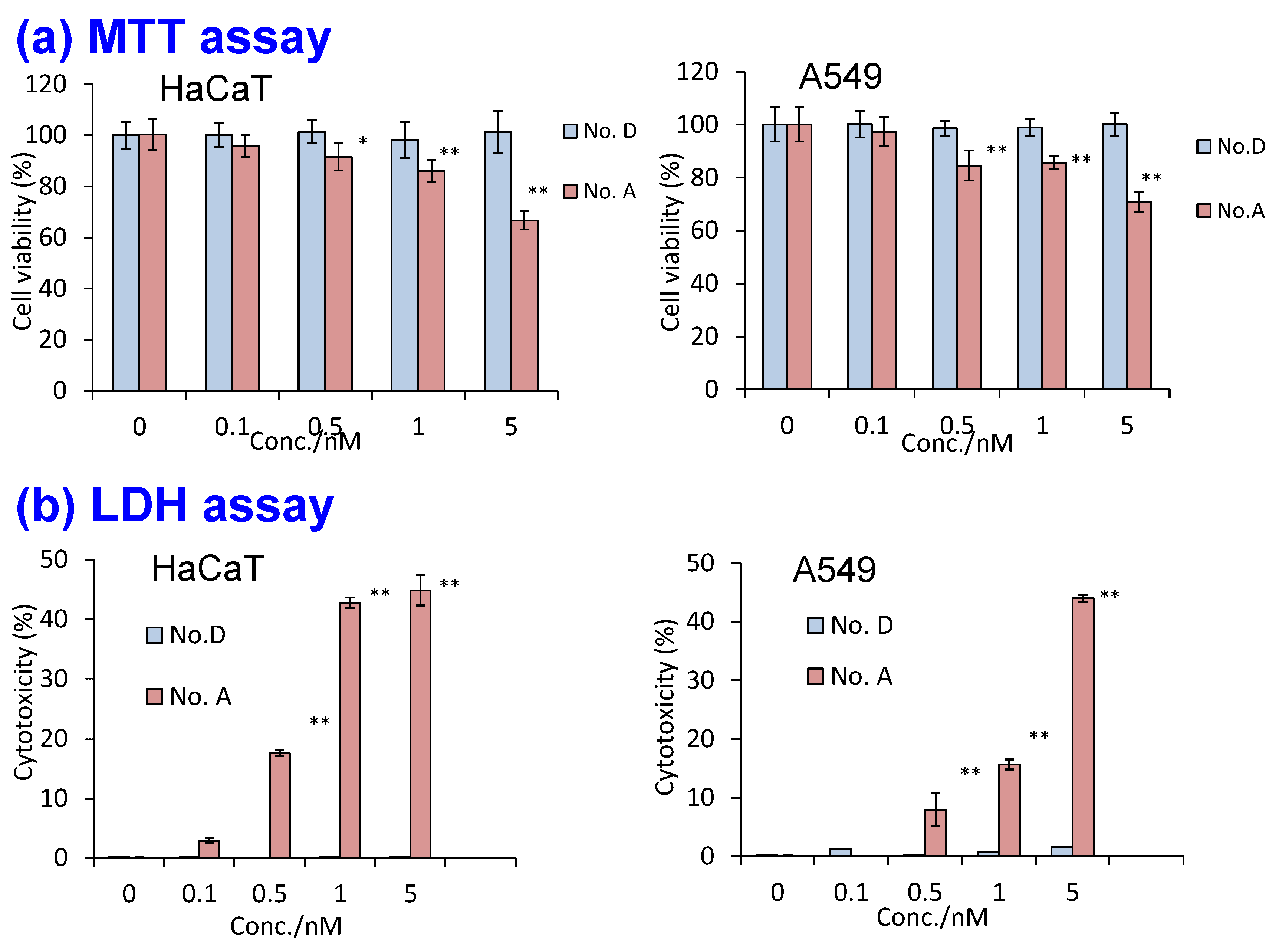
5.4. Elusion of Heavy Metal Ions and Cytotoxicity of Silica Nanoparticles
6. Recent Progress and Applications
6.1. Adapting Earlier Approaches for Silica Nanoparticle Encapsulation with InP-Based QDs
6.2. Phosphors for Display Technologies and Mass Production Strategies
7. Concluding Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chang, C.Y.; Sze, S.M. Semiconductor Devices: Physics and Technology, International Student Version, 3rd ed.; Wiley: Berlin, Germany, 2012. [Google Scholar]
- Chen, J.; Hardev, V.; Yurek, J. Quantum-dot displays: Giving LCDs a competitive edge through color. Inf. Disp. 2013, 29, 12–17. [Google Scholar] [CrossRef]
- Quantum Dot Display Market Size & Trends. Available online: https://www.grandviewresearch.com/industry-analysis/quantum-dot-qd-display-market (accessed on 21 June 2025).
- Medintz, I.; Uyeda, H.; Goldman, E.; Mattoussi, H. Quantum dot bioconjugates for imaging, labelling and sensing. Nat. Mater. 2005, 4, 435–446. [Google Scholar] [CrossRef]
- Kairdolf, B.; Smith, A.; Stokes, T.; Wang, M.; Young, A.; Nie, S. Semiconductor Quantum Dots for Bioimaging and Biodiagnostic Applications. Annu. Rev. Anal. Chem. 2013, 6, 143–162. [Google Scholar] [CrossRef]
- Xu, G.; Zeng, S.; Zhang, B.; Swihart, M.; Yong, K.; Prasad, P. New Generation Cadmium-Free Quantum Dots for Biophotonics and Nanomedicine. Chem. Rev. 2016, 116, 12234–12327. [Google Scholar] [CrossRef]
- What is the “Quantum Dots” That Won the Nobel Prize in Chemistry in 2023? Available online: https://www.aist.go.jp/aist_j/magazine/20231227.html (accessed on 21 June 2025).
- Ekimov, A.I.; Onushchenko, A.A. Quantum size effect in the optical-spectra of semiconductor micro-crystals. Sov. Phys. Semicond. 1982, 16, 775–778. [Google Scholar]
- Rossetti, R.; Nakahara, S.; Brus, L.E. Quantum size effects in the redox potentials, resonance Raman spectra, and electronic spectra of CdS crystallites in aqueous solution. J. Chem. Phys. 1983, 79, 1086–1088. [Google Scholar] [CrossRef]
- Murase, N.; Gaponik, N.; Weller, H. Effect of chemical composition on luminescence of thiol-stabilized CdTe nanocrystals. Nanoscale Res. Lett. 2007, 2, 230–234. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tang, X.; Yang, J.; Li, S.; Liu, Z.; Hu, Z.; Hao, J.; Du, J.; Leng, Y.; Qin, H.; Lin, X.; et al. Single Halide Perovskite/Semiconductor Core/Shell Quantum Dots with Ultrastability and Nonblinking Properties. Adv. Sci. 2019, 6, 1900412. [Google Scholar] [CrossRef] [PubMed]
- Gaponik, N.; Talapin, D.; Rogach, A.; Hoppe, K.; Shevchenko, E.; Kornowski, A.; Eychmüller, A.; Weller, H. Thiol-capping of CdTe nanocrystals: An alternative to organometallic synthetic routes. J. Phys. Chem. B 2002, 106, 7177–7185. [Google Scholar] [CrossRef]
- Murray, C.B.; Norris, D.J.; Bawendi, M.G. Synthesis and characterization of nearly monodisperse CdE (E = sulfur, selenium, tellurium) semiconductor nanocrystallites. J. Am. Chem. Soc. 1993, 115, 8706–8715. [Google Scholar] [CrossRef]
- Directive 2011/65/EU of the European Parliament and of the Council of 8 June 2011 on the Restriction of the Use of Certain Hazardous Substances in Electrical and Electronic Equipment (Recast) Text with EEA Relevance. Available online: https://eur-lex.europa.eu/eli/dir/2011/65/oj (accessed on 21 June 2025).
- Murase, N. Quantum Dot-Core Silica Glass-Shell Nanomaterials: Synthesis, Characterization, and Potential Biomedical Applications. In Nanotechnologies for the Life Sciences; Wiley-VCH Verlag GmbH: Berlin, Germany, 2011; pp. 393–427. [Google Scholar]
- Mićić, O.I.; Sprague, J.; Lu, Z.; Nozik, A.J. Highly efficient band-edge emission from InP quantum dots. Appl. Phys. Lett. 1996, 68, 3150–3152. [Google Scholar] [CrossRef]
- Talapin, D.; Gaponik, N.; Borchert, H.; Rogach, A.; Haase, M.; Weller, H. Etching of colloidal InP nanocrystals with fluorides: Photochemical nature of the process resulting in high photoluminescence efficiency. J. Phys. Chem. B 2002, 106, 12659–12663. [Google Scholar] [CrossRef]
- Preparation of Semiconductor Nanocrystals with High Photoluminescence Efficiency for Labeling in Biology. Available online: https://www.aist.go.jp/aist_e/list/latest_research/2009/20090325/20090325.html (accessed on 21 June 2025).
- Li, Y.; Hou, X.; Dai, X.; Yao, Z.; Lv, L.; Jin, Y.; Peng, X. Stoichiometry-Controlled InP-Based Quantum Dots: Synthesis, Photoluminescence, and Electroluminescence. J. Am. Chem. Soc. 2019, 141, 6448–6452. [Google Scholar] [CrossRef] [PubMed]
- Won, Y.; Cho, O.; Kim, T.; Chung, D.; Kim, T.; Chung, H.; Jang, H.; Lee, J.; Kim, D.; Jang, E. Highly efficient and stable InP/ZnSe/ZnS quantum dot light-emitting diodes. Nature 2019, 575, 634–638. [Google Scholar] [CrossRef]
- Nakamura, H.; Kato, W.; Uehara, M.; Nose, K.; Omata, T.; Otsuka-Yao-Matsuo, S.; Miyazaki, M.; Maeda, H. Tunable photoluminescence wavelength of chalcopyrite CuInS2-based semiconductor nanocrystals synthesized in a colloidal system. Chem. Mater. 2018, 18, 3330–3335. [Google Scholar] [CrossRef]
- Uematsu, T.; Wajima, K.; Sharma, D.; Hirata, S.; Yamamoto, T.; Kameyama, T.; Vacha, M.; Torimoto, T.; Kuwabata, S. Narrow band-edge photoluminescence from AglnS2 semiconductor nanoparticles by the formation of amorphous III-VI semiconductor shells. NPG Asia Mater. 2018, 10, 713–726. [Google Scholar] [CrossRef]
- Uematsu, T.; Tepakidareekul, M.; Hirano, T.; Torimoto, T.; Kuwabata, S. Facile High-Yield Synthesis of Ag-In-Ga-S Quaternary Quantum Dots and Coating with Gallium Sulfide Shells for Narrow Band-Edge Emission. Chem. Mater. 2023, 35, 1094–1106. [Google Scholar] [CrossRef]
- Huang, H.; Bodnarchuk, M.; Kershaw, S.; Kovalenko, M.; Rogach, A. Lead Halide Perovskite Nanocrystals in the Research Spotlight: Stability and Defect Tolerance. ACS Energy Lett. 2017, 2, 2071–2083. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, R.; Feng, B.; Zhong, X.; Ostrikov, K. Photoluminescence mechanism of carbon dots: Triggering high-color-purity red fluorescence emission through edge amino protonation. Nat. Commun. 2021, 12, 6856. [Google Scholar] [CrossRef]
- Saitow, K. Bright silicon quantum dot synthesis and LED design: Insights into size-ligand-property relationships from slow- and fast-band engineering. Bull. Chem. Soc. Jpn. 2024, 97, uoad002. [Google Scholar] [CrossRef]
- Kim, J.; Shim, K.; Han, J.; Joo, J.; Heo, N.; Seff, K. Quantum Dots of [Na4Cs6PbBr4]8+, Water Stable in Zeolite X, Luminesce Sharply in the Green. Adv. Mater. 2020, 32, 2001868. [Google Scholar] [CrossRef]
- Qu, L.; Miller, G. Passivated Nanoparticles. U.S. Patent 9,425,253B2, 23 September 2010. [Google Scholar]
- Li, Z.; Yao, W.; Kong, L.; Zhao, Y.; Li, L. General Method for the Synthesis of Ultrastable Core/Shell Quantum Dots by Aluminum Doping. J. Am. Chem. Soc. 2015, 137, 12430–12433. [Google Scholar] [CrossRef]
- Yin, B.; Sadtler, B.; Berezin, M.; Thimsen, E. Quantum dots protected from oxidative attack using alumina shells synthesized by atomic layer deposition. Chem. Commun. 2016, 52, 11127–11130. [Google Scholar] [CrossRef]
- Zhang, Y.; Ni, H. Diffusion of H, C, and O Components in Silicate Melts. In Diffusion in Minerals and Melts; Zhang, Y., Cherniak, D.J., Eds.; De Gruyter Brill: Beijing, China, 2010; Volume 72, pp. 171–225. [Google Scholar]
- Song, W.; Kim, J.; Yang, H. Silica-embedded quantum dots as downconverters of light-emitting diode and effect of silica on device operational stability. Mater. Lett. 2013, 111, 104–107. [Google Scholar] [CrossRef]
- Lin, Z.; Fei, X.; Ma, Q.; Gao, X.; Su, X. CuInS2 quantum dots@silica near-infrared fluorescent nanoprobe for cell imaging. New J. Chem. 2014, 38, 90–96. [Google Scholar] [CrossRef]
- Wada, C.; Iso, Y.; Isobe, T.; Sasaki, H. Preparation and photoluminescence properties of yellow-emitting CuInS2/ZnS quantum dots embedded in TMAS-derived silica. RSC Adv. 2017, 7, 7936–7943. [Google Scholar] [CrossRef]
- Foda, M.; Huang, L.; Shao, F.; Han, H. Biocompatible and Highly Luminescent Near-Infrared CuInS2/ZnS Quantum Dots Embedded Silica Beads for Cancer Cell Imaging. ACS Appl. Mater. Interfaces 2014, 6, 2011–2017. [Google Scholar] [CrossRef]
- Kim, H.; Jang, H.; Kwon, B.; Suh, M.; Kim, Y.; Cheong, S.; Jeon, D. In Situ Synthesis of Thiol-Capped CuInS2-ZnS Quantum Dots Embedded in Silica Powder by Sequential Ligand-Exchange and Silanization. Electrochem. Solid State Lett. 2012, 15, K16–K18. [Google Scholar] [CrossRef]
- Yin, D.; Pei, L.; Liu, Z.; Yang, X.; Xiang, W.; Zhang, X. Synthesis, Characterization, and Photoluminescence on the Glass Doped with AgInS2 Nanocrystals. Adv. Condens. Matter Phys. 2015, 2015, 141056. [Google Scholar] [CrossRef]
- Yang, M.; Peng, H.; Zeng, F.; Teng, F.; Qu, Z.; Yang, D.; Wang, Y.; Chen, G.; Wang, D. In situ silica coating-directed synthesis of orthorhombic methylammonium lead bromide perovskite quantum dots with high stability. J. Colloid Interface Sci. 2018, 509, 32–38. [Google Scholar] [CrossRef]
- Chen, J.; Wu, H.; Zhang, W.; Huang, Y.; Zheng, J.; Shao, J.; Chi, Y. Silica coated perovskite quantum dots with single-particle level for dual-mode and smartphone-assisted detection of glutathione. Sens. Actuator B-Chem. 2025, 432, 137489. [Google Scholar] [CrossRef]
- Kim, J.; Kim, B.; Kim, M.; Jang, W.; Wang, D. One-step formation of core/shell structure based on hydrophobic silane ligands for enhanced luminescent perovskite quantum dots. J. Alloys Compd. 2021, 886, 161347. [Google Scholar] [CrossRef]
- Liu, Z.; Hu, Z.; Shi, T.; Du, J.; Yang, J.; Zhang, Z.; Tang, X.; Leng, Y. Stable and enhanced frequency up-converted lasing from CsPbBr3 quantum dots embedded in silica sphere. Opt. Express 2019, 27, 9459–9466. [Google Scholar] [CrossRef]
- Zhong, Q.; Cao, M.; Hu, H.; Yang, D.; Chen, M.; Li, P.; Wu, L.; Zhang, Q. One-Pot Synthesis of Highly Stable CsPbBr3@SiO2 Core-Shell Nanoparticles. ACS Nano 2018, 12, 8579–8587. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Li, Z.; Kong, L.; Zhu, N.; Shan, A.; Li, L. Enhancing the Stability of CH3NH3PbBr3 Quantum Dots by Embedding in Silica Spheres Derived from Tetramethyl Orthosilicate in “Waterless” Toluene. J. Am. Chem. Soc. 2016, 138, 5749–5752. [Google Scholar] [CrossRef]
- Ye, L.; Hong, S.; Lu, C.; Zhao, Q. Stable random laser of perovskite quantum dots based on SiO2-QDs-SiO2 composite nanostructure. J. Lumin. 2025, 277, 120946. [Google Scholar] [CrossRef]
- Hai, O.; Li, T.; Li, J.; Qin, B.; Ren, Q.; Wu, X.; Wu, Y. Preparation and properties of highly stabilized CsPbX3 (X = Cl, Br, I) by liquid phase molten salt method. Opt. Mater. 2025, 161, 116824. [Google Scholar] [CrossRef]
- Wang, H.; Lin, S.; Tang, A.; Singh, B.; Tong, H.; Chen, C.; Lee, Y.; Tsai, T.; Liu, R. Mesoporous Silica Particles Integrated with All-Inorganic CsPbBr3 Perovskite Quantum-Dot Nanocomposites (MP-PQDs) with High Stability and Wide Color Gamut Used for Backlight Display. Angew. Chem.-Int. Edit. 2016, 55, 7924–7929. [Google Scholar] [CrossRef]
- Di, X.; Jiang, J.; Hu, Z.; Zhou, L.; Li, P.; Liu, S.; Xiang, W.; Liang, X. Stable and brightly luminescent all-inorganic cesium lead halide perovskite quantum dots coated with mesoporous silica for warm WLED. Dyes Pigment. 2017, 146, 361–367. [Google Scholar] [CrossRef]
- Di, X.; Hu, Z.; Jiang, J.; He, M.; Zhou, L.; Xiang, W.; Liang, X. Use of long-term stable CsPbBr3 perovskite quantum dots in phospho-silicate glass for highly efficient white LEDs. Chem. Commun. 2017, 53, 11068–11071. [Google Scholar] [CrossRef]
- Ekimov, A. Growth and optical properties of semiconductor nanocrystals in a glass matrix. J. Lumin. 1996, 70, 1–20. [Google Scholar] [CrossRef]
- Murase, N.; Yazawa, T. Partially Reduced Cuprous Oxide Nanoparticles Formed in Porous Glass Reaction Fields. J. Am. Ceram. Soc. 2001, 84, 2269–2273. [Google Scholar] [CrossRef]
- Selvan, S.; Li, C.; Ando, M.; Murase, N. Formation of luminescent CdTe-silica nanoparticles through an inverse microemulsion technique. Chem. Lett. 2004, 33, 434–435. [Google Scholar] [CrossRef]
- Yang, P.; Ando, M.; Murase, N. Encapsulation of emitting CdTe QDs within silica beads to retain initial photoluminescence efficiency. J. Colloid Interface Sci. 2007, 316, 420–427. [Google Scholar] [CrossRef]
- Teng, F.; Tian, Z.; Xiong, G.; Xu, Z. Preparation of CdS-SiO2 core-shell particles and hollow SiO2 spheres ranging from nanometers to microns in the nonionic reverse microemulsions. Catal. Today 2004, 93–95, 651–657. [Google Scholar] [CrossRef]
- Selvan, S.; Bullen, C.; Ashokkumar, M.; Mulvaney, P. Synthesis of tunable, highly luminescent QD-glasses through sol-gel processing. Adv. Mater. 2001, 13, 985–988. [Google Scholar] [CrossRef]
- Li, C.; Murase, N. Synthesis of highly luminescent glasses incorporating CdTe nanocrystals through sol-gel processing. Langmuir 2004, 20, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Lee, H.; Kang, S.; Bae, B. Two-Step-Enhanced Stability of Quantum Dots via Silica and Siloxane Encapsulation for the Long-Term Operation of Light Emitting Diodes. ACS Appl. Mater. Interfaces 2019, 11, 22801–22808. [Google Scholar] [CrossRef]
- Watanabe, T.; Wada, C.; Iso, Y.; Isobe, T.; Sasaki, H. Preparation of Photostable Fluorescent InP/ZnS Quantum Dots Embedded in TMAS-Derived Silica. ECS J. Solid State Sci. Technol. 2017, 6, R75–R80. [Google Scholar] [CrossRef]
- Watanabe, T.; Iso, Y.; Isobe, T.; Sasaki, H. Photoluminescence color stability of green-emitting InP/ZnS core/shell quantum dots embedded in silica prepared via hydrophobic routes. RSC Adv. 2018, 8, 25526–25533. [Google Scholar] [CrossRef]
- Yang, P.; Li, C.; Murase, N. Highly photoluminescent multilayer QD-glass films prepared by LbL self-assembly. Langmuir 2005, 21, 8913–8917. [Google Scholar] [CrossRef]
- Rogach, A.; Nagesha, D.; Ostrander, J.; Giersig, M.; Kotov, N. “Raisin bun”-type composite spheres of silica and semiconductor nanocrystals. Chem. Mater. 2000, 12, 2676–2685. [Google Scholar] [CrossRef]
- Yang, P.; Murase, N. Size-Tunable Highly Luminescent SiO2 Particles Impregnated with Number-Adjusted CdTe Nanocrystals. ChemPhysChem 2010, 11, 815–821. [Google Scholar] [CrossRef]
- Brinker, C.J.; Scherer, G.W. Theory of Deformation and Flow in Gels. In Sol-Gel Science, 2nd ed.; Academic Press: Pittsburgh, PA, USA, 1990; pp. 406–451. [Google Scholar]
- Li, C.; Hosokawa, C.; Suzuki, M.; Taguchi, T.; Murase, N. Preparation and biomedical applications of bright robust silica nanocapsules with multiple incorporated InP/ZnS quantum dots. New J. Chem. 2018, 42, 18951–18960. [Google Scholar] [CrossRef]
- Darbandi, M.; Thomann, R.; Nann, T. Single quantum dots in silica spheres by microemulsion synthesis. Chem. Mater. 2005, 17, 5720–5725. [Google Scholar] [CrossRef]
- Selvan, S.; Tan, T.; Ying, J. Robust, non-cytotoxic, silica-coated CdSe quantum dots with efficient photoluminescence. Adv. Mater. 2005, 17, 1620–1625. [Google Scholar] [CrossRef]
- Koole, R.; van Schooneveld, M.; Hilhorst, J.; Donegá, C.; Hart, D.; van Blaaderen, A.; Vanmaekelbergh, D.; Meijerink, A. On the incorporation mechanism of hydrophobic quantum dots in silica spheres by a reverse microemulsion method. Chem. Mater. 2008, 20, 2503–2512. [Google Scholar] [CrossRef]
- Drozd, D.; Zhang, H.; Goryacheva, I.; De Saeger, S.; Beloglazova, N. Silanization of quantum dots: Challenges and perspectives. Talanta 2019, 205, 120164. [Google Scholar] [CrossRef]
- Yang, P.; Ando, M.; Murase, N. Highly Luminescent CdSe/CdxZn1-xS Quantum Dots Coated with Thickness-Controlled SiO2 Shell through Silanization. Langmuir 2011, 27, 9535–9540. [Google Scholar] [CrossRef] [PubMed]
- Murase, N.; Li, C.; Gunshi, T. Improved photostability of silica bead impregnated with CdSe-based quantum dots prepared through proper surface silanization. Colloid Surf. A 2020, 600, 124811. [Google Scholar] [CrossRef]
- Nann, T.; Mulvaney, P. Single quantum dots in spherical silica particles. Angew. Chem.-Int. Edit. 2004, 43, 5393–5396. [Google Scholar] [CrossRef]
- Chan, Y.; Zimmer, J.; Stroh, M.; Steckel, J.; Jain, R.; Bawendi, M. Incorporation of luminescent nanocrystals into monodisperse core-shell silica microspheres. Adv. Mater. 2004, 16, 2092–2097. [Google Scholar] [CrossRef]
- Yang, P.; Murase, N.; Suzuki, M.; Hosokawa, C.; Kawasaki, K.; Kato, T.; Taguchi, T. Bright, non-blinking, and less-cytotoxic SiO2 beads with multiple CdSe/ZnS nanocrystals. Chem. Commun. 2010, 46, 4595–4597. [Google Scholar] [CrossRef] [PubMed]
- Fabrication of Photoluminescent Small Glass Capsules Containing Quantum Dots at High Concentration. Available online: https://www.aist.go.jp/aist_e/list/latest_research/2010/20100723/20100723.html (accessed on 21 June 2025).
- Li, C.; Murase, N. Formation mechanism of highly luminescent silica capsules incorporating multiple hydrophobic quantum dots with various emission wavelengths. J. Colloid Interface Sci. 2013, 411, 82–91. [Google Scholar] [CrossRef]
- Murase, N.; Horie, M.; Sawai, T.; Kawasaki, K. Silica layer-dependent leakage of cadmium from CdSe/ZnS quantum dots and comparison of cytotoxicity with polymer-coated analogues. J. Nanopart. Res. 2019, 21, 10. [Google Scholar] [CrossRef]
- Murase, N.; Hosokawa, C.; Li, C.; Onishi, M.; Sawai, T. Drastic photostability improvement of silica particles impregnated with multiple emitting CdSe quantum dots prepared through efficient surface silanization. Colloid Surf. A 2022, 654, 130084. [Google Scholar] [CrossRef]
- Murase, N.; Sawai, T.; Mori, R.; Inada, K.; Eguchi, D.; Tamai, N. Preparation of green-emitting InP-based quantum dots with controlled shell thickness and their photoluminescence quantum yield upon silica encapsulation. J. Nanopart. Res. 2025, 27, 75. [Google Scholar] [CrossRef]
- Dabbousi, B.O.; Rodriguez-Viejo, J.; Mikulec, F.V.; Heine, J.R.; Mattoussi, H.; Ober, R.; Jensen, K.F.; Bawendi, M.G. (CdSe)ZnS Core–Shell Quantum Dots: Synthesis and Characterization of a Size Series of Highly Luminescent Nanocrystallites. J. Phys. Chem. B 1997, 101, 9463–9475. [Google Scholar] [CrossRef]
- Lee, J.; Sundar, V.; Heine, J.; Bawendi, M.; Jensen, K. Full color emission from II-VI semiconductor quantum dot-polymer composites. Adv. Mater. 2000, 12, 1102–1105. [Google Scholar] [CrossRef]
- Talapin, D.; Rogach, A.; Haase, M.; Weller, H. Evolution of an ensemble of nanoparticles in a colloidal solution: Theoretical study. J. Phys. Chem. B 2001, 105, 12278–12285. [Google Scholar] [CrossRef]
- Yu, W.; Qu, L.; Guo, W.; Peng, X. Experimental determination of the extinction coefficient of CdTe, CdSe, and CdS nanocrystals. Chem. Mater. 2003, 15, 2854–2860. [Google Scholar] [CrossRef]
- Han, J.; Liu, X.; Tan, J.; He, Z.; Li, C. Facile Method of Preparing Highly Luminescent Silica Gel Glass Incorporating Hydrophobic Semiconductor Quantum Dots. Chem. Lett. 2015, 44, 1434–1436. [Google Scholar] [CrossRef]
- Liu, X.; Han, J.; Wu, W.; Shi, Q.; Li, W.; Li, C. Highly Luminescent Glass Films Incorporating Hydrophobic Quantum Dots Prepared by Layer-by-layer Self-assembly Method. Chem. Lett. 2016, 45, 10–12. [Google Scholar] [CrossRef]
- Gerion, D.; Pinaud, F.; Williams, S.; Parak, W.; Zanchet, D.; Weiss, S.; Alivisatos, A. Synthesis and properties of biocompatible water-soluble silica-coated CdSe/ZnS semiconductor quantum dots. J. Phys. Chem. B 2001, 105, 8861–8871. [Google Scholar] [CrossRef]
- Jana, N.; Earhart, C.; Ying, J. Synthesis of water-soluble and functionalized nanoparticles by silica coating. Chem. Mater. 2007, 19, 5074–5082. [Google Scholar] [CrossRef]
- In Vivo Nano-Imaging of Movement of Molecules in Mice. Available online: https://www.jst.go.jp/kisoken/crest/en/area02/3-02.html (accessed on 21 June 2025).
- Hermanson, G.T. Bioconjugate Techniques, 3rd ed.; Elsevier: Waltham, MA, USA, 2013; p. 02451. [Google Scholar]
- Oh, E.; Liu, R.; Nel, A.; Gemill, K.; Bilal, M.; Cohen, Y.; Medintz, I. Meta-analysis of cellular toxicity for cadmium-containing quantum dots. Nat. Nanotechnol. 2016, 11, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Vafaie, M.; Fan, J.Z.; Najarian, A.M.; Ouellette, O.; Sagar, L.K.; Bertens, K.; Sun, B.; Arquer, F.P.G.; Sargent, E.H. Colloidal quantum dot photodetectors with 10-ns response time and 80% quantum efficiency at 1,550 nm. Matter 2021, 4, 1042–1053. [Google Scholar] [CrossRef]
- Quantum Materials Technology. Available online: https://www.quantum-mater-tech.com/ (accessed on 21 June 2025).




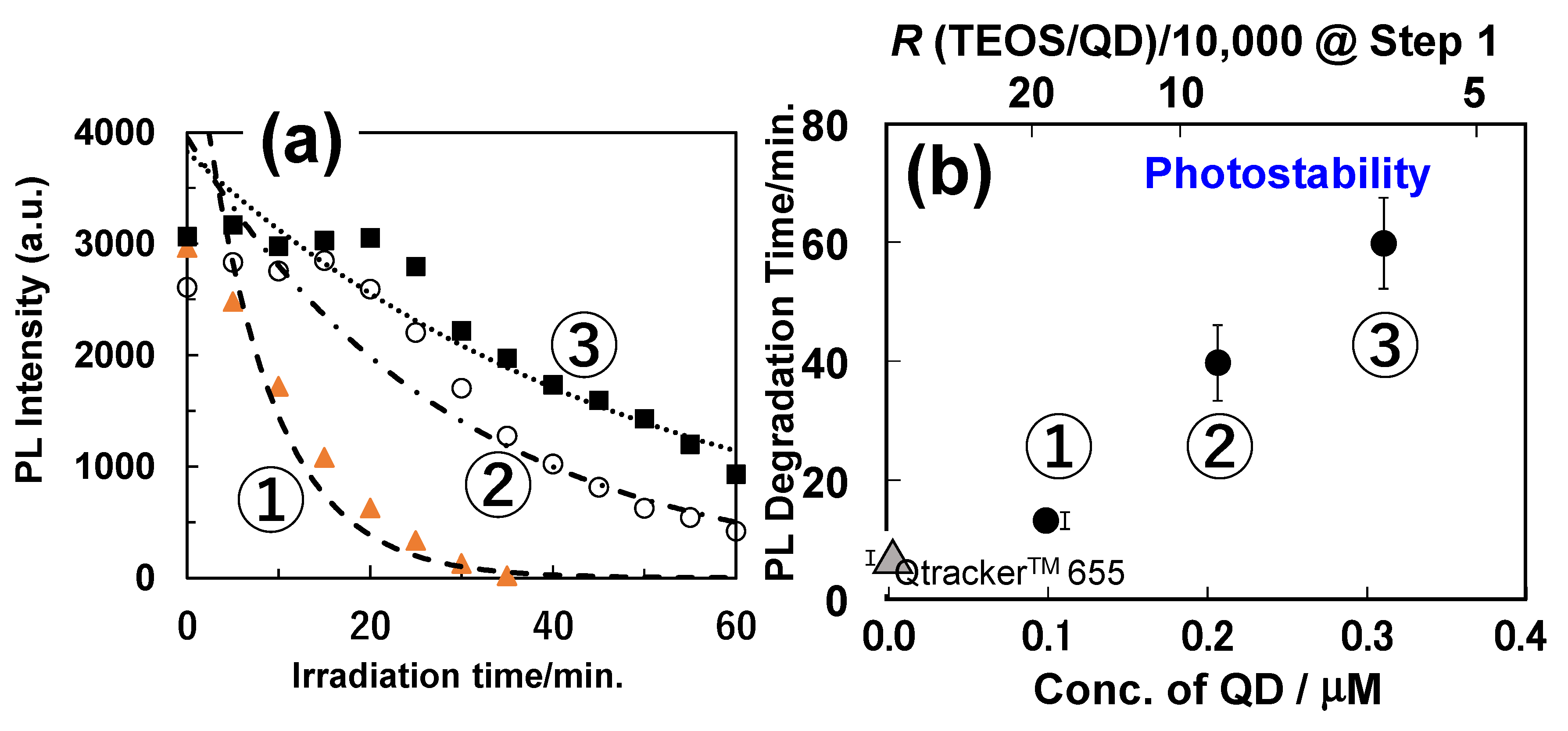
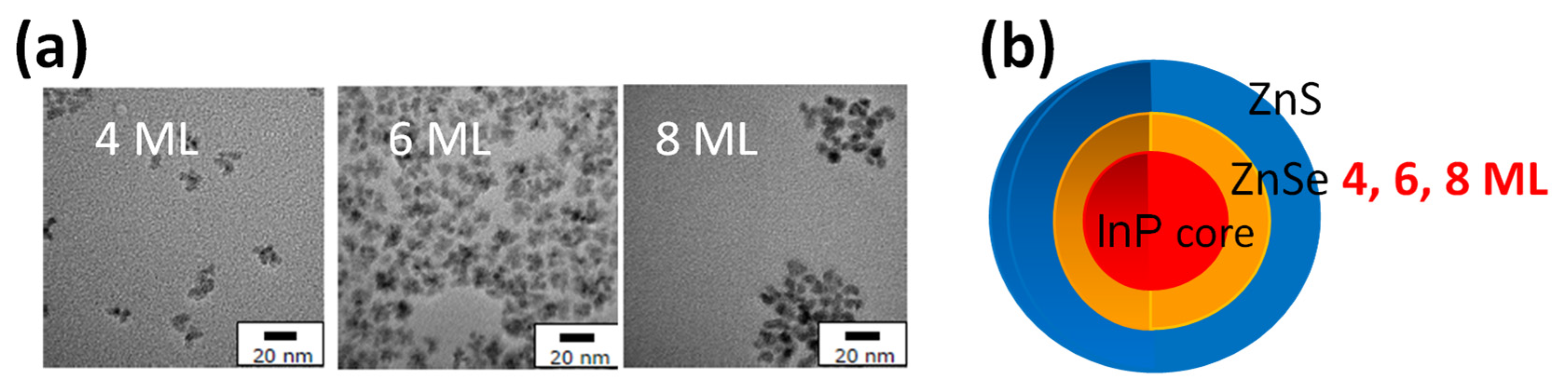

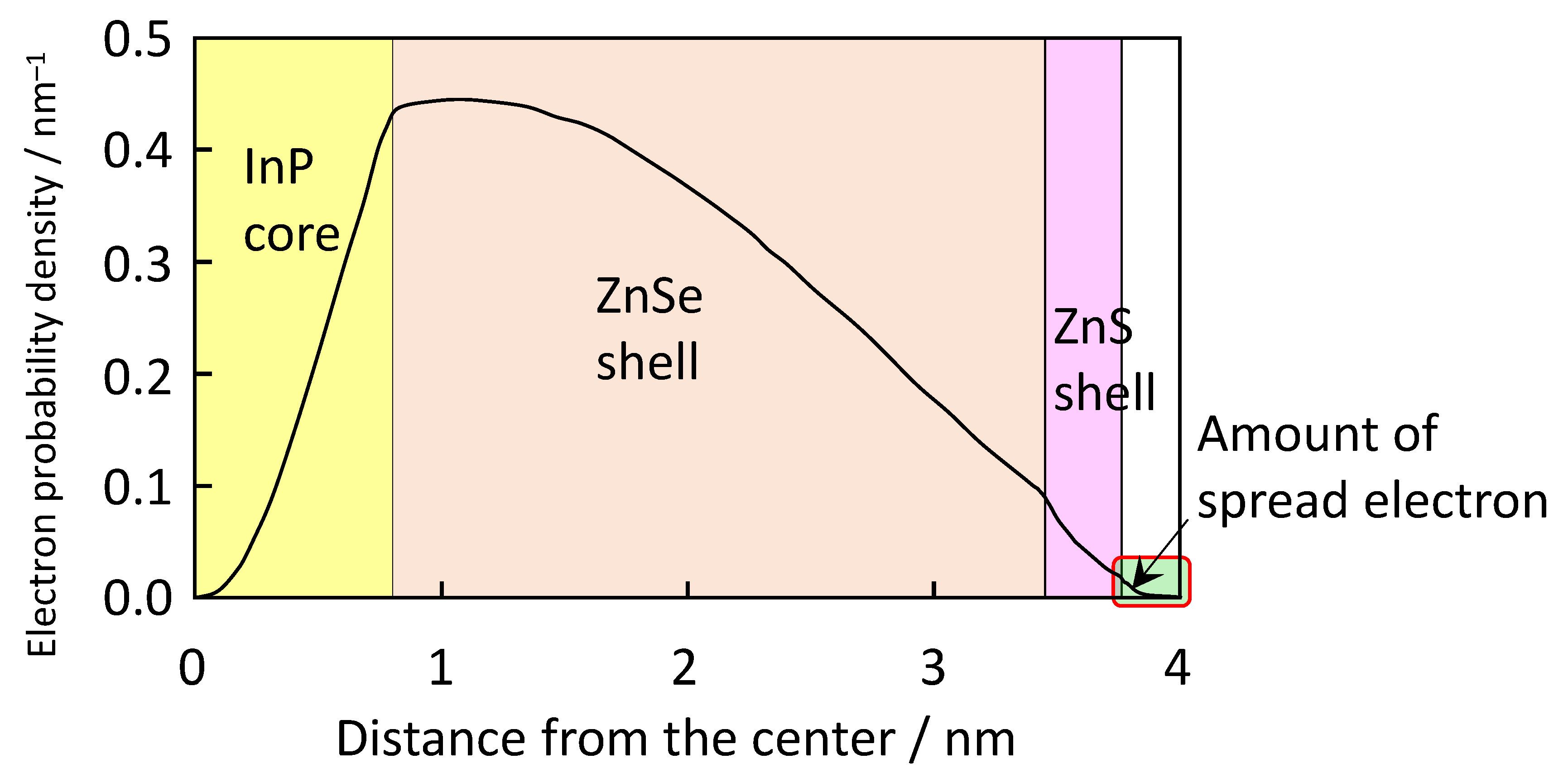
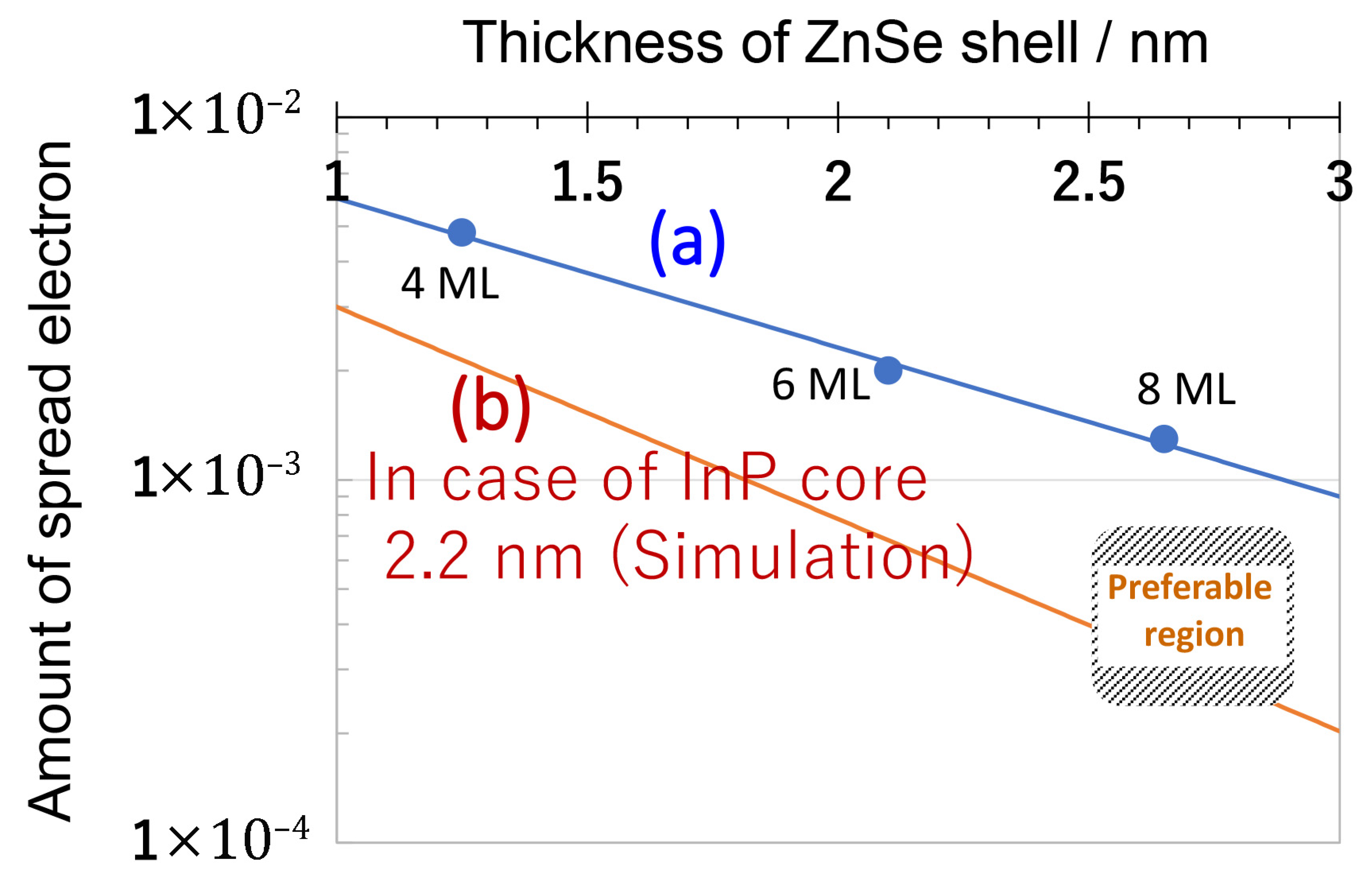
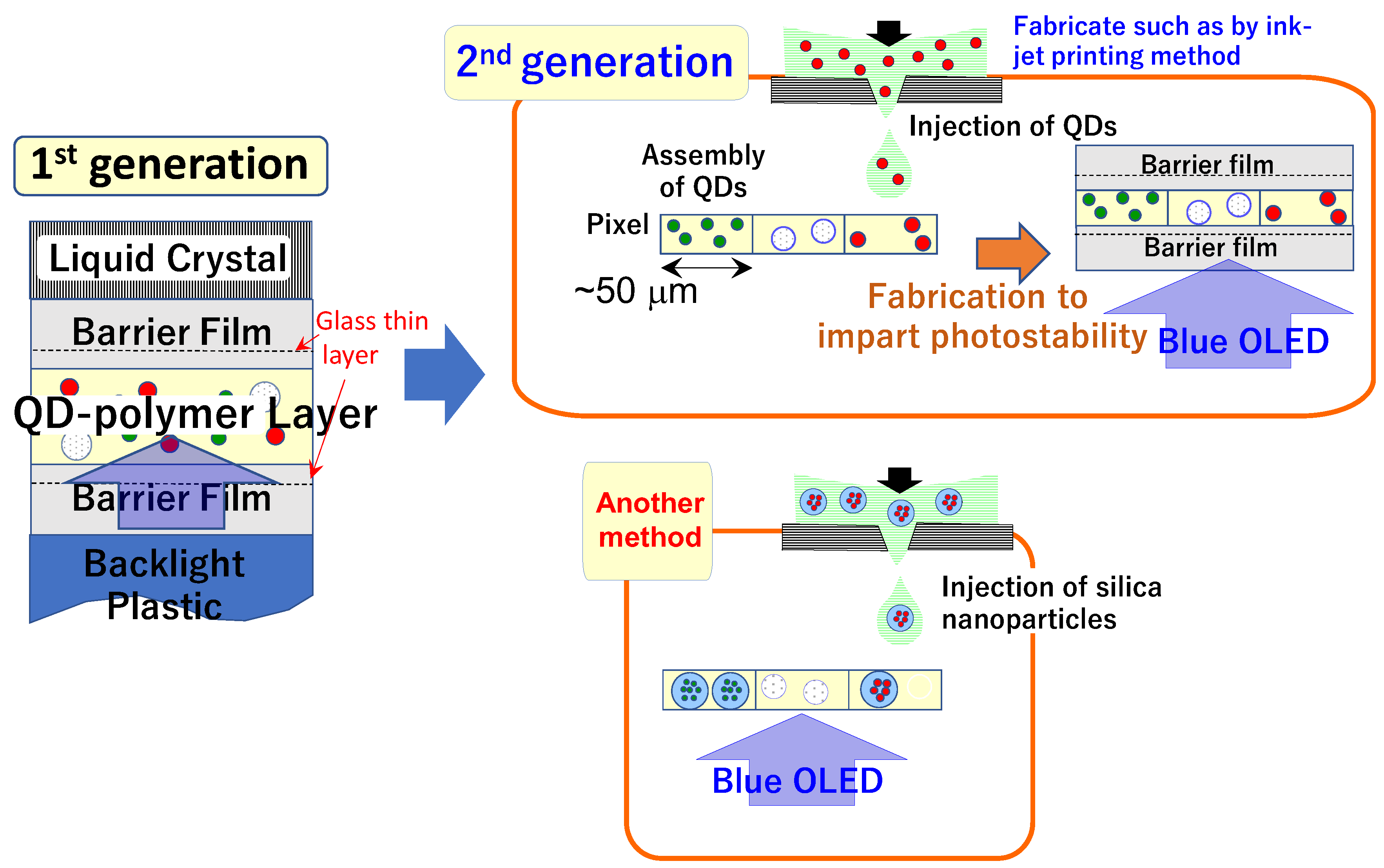
| Property | Shielding Ability (Photostability) | PLQY | Main Producer | ||
|---|---|---|---|---|---|
| Matrix | |||||
| Organic | Amorphous | Polymer | - | + | General |
| Inorganic | Silica(b) | + | +/- | The authors | |
| Crystal | Zeolite | + | - | Korean group [27] | |
| Alumina | + | - | Crystalplex Inc., USA [28], Related papers [29,30] |
| Sample | PL Peak /nm | PLQY (%) | FWHM /nm | Mean Size /nm (b) | Average QDs No. in Each Nanoparticle (b) |
|---|---|---|---|---|---|
| 1 | 658.4 | 34 | 49.2 | 12.5 ± 1.7 | 1.6 ± 0.7 |
| 2 | 660.8 | 40 | 48.0 | 15.9 ± 1.6 | 2.9 ± 0.9 |
| 3 | 662.6 | 31 | 48.4 | 29.1 ± 3.8 | 4.0 ± 1.6 |
| 4 | 663.8 | 32 | 47.2 | 35.9 ± 4.4 | 5.7 ± 2.3 |
| CdTe (a) | 650.2 | 46 | 58.0 | 3.9 ± 0.2 | — |
| No. | Sample | Concentration Ratio of Dissolved Cd2+ in HEPES Solution (a) | |
|---|---|---|---|
| A | Polymer-coated QDs (-COOH surface) | 1 |  |
| B | Silica nanoparticle with QDs (-OH surface, prepared at room temperature) | 0.26 |  |
| C | Silica nanoparticle with QDs (-OH surface, prepared at 40 °C) | 0.06 |  |
| D | Silica nanoparticle with QDs (-COOH surface, prepared at room temperature) | 0.009 |  |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murase, N.; Li, C. Silane-Coupled Silica Nanoparticles Encapsulating Emitting Quantum Dots: Advancing Robust Phosphors for Displays and Beyond. Molecules 2025, 30, 3369. https://doi.org/10.3390/molecules30163369
Murase N, Li C. Silane-Coupled Silica Nanoparticles Encapsulating Emitting Quantum Dots: Advancing Robust Phosphors for Displays and Beyond. Molecules. 2025; 30(16):3369. https://doi.org/10.3390/molecules30163369
Chicago/Turabian StyleMurase, Norio, and Chunliang Li. 2025. "Silane-Coupled Silica Nanoparticles Encapsulating Emitting Quantum Dots: Advancing Robust Phosphors for Displays and Beyond" Molecules 30, no. 16: 3369. https://doi.org/10.3390/molecules30163369
APA StyleMurase, N., & Li, C. (2025). Silane-Coupled Silica Nanoparticles Encapsulating Emitting Quantum Dots: Advancing Robust Phosphors for Displays and Beyond. Molecules, 30(16), 3369. https://doi.org/10.3390/molecules30163369








