Exploitation of Apulian Salicornia europaea L. via NADES-UAE: Extraction, Antioxidant Activity and Antimicrobial Potential
Abstract
1. Introduction
2. Results and Discussion
2.1. NADES Screening Using COSMOtherm
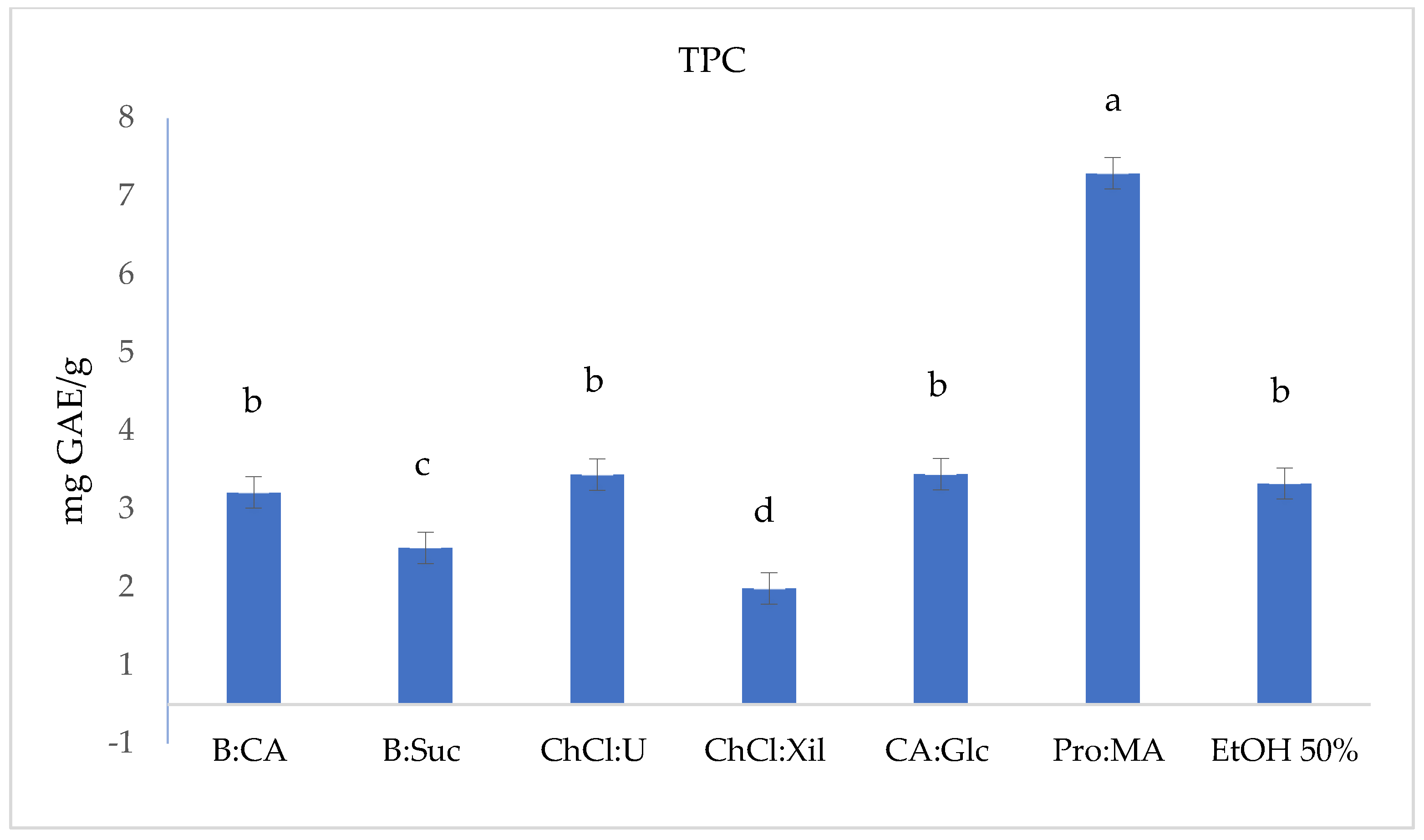
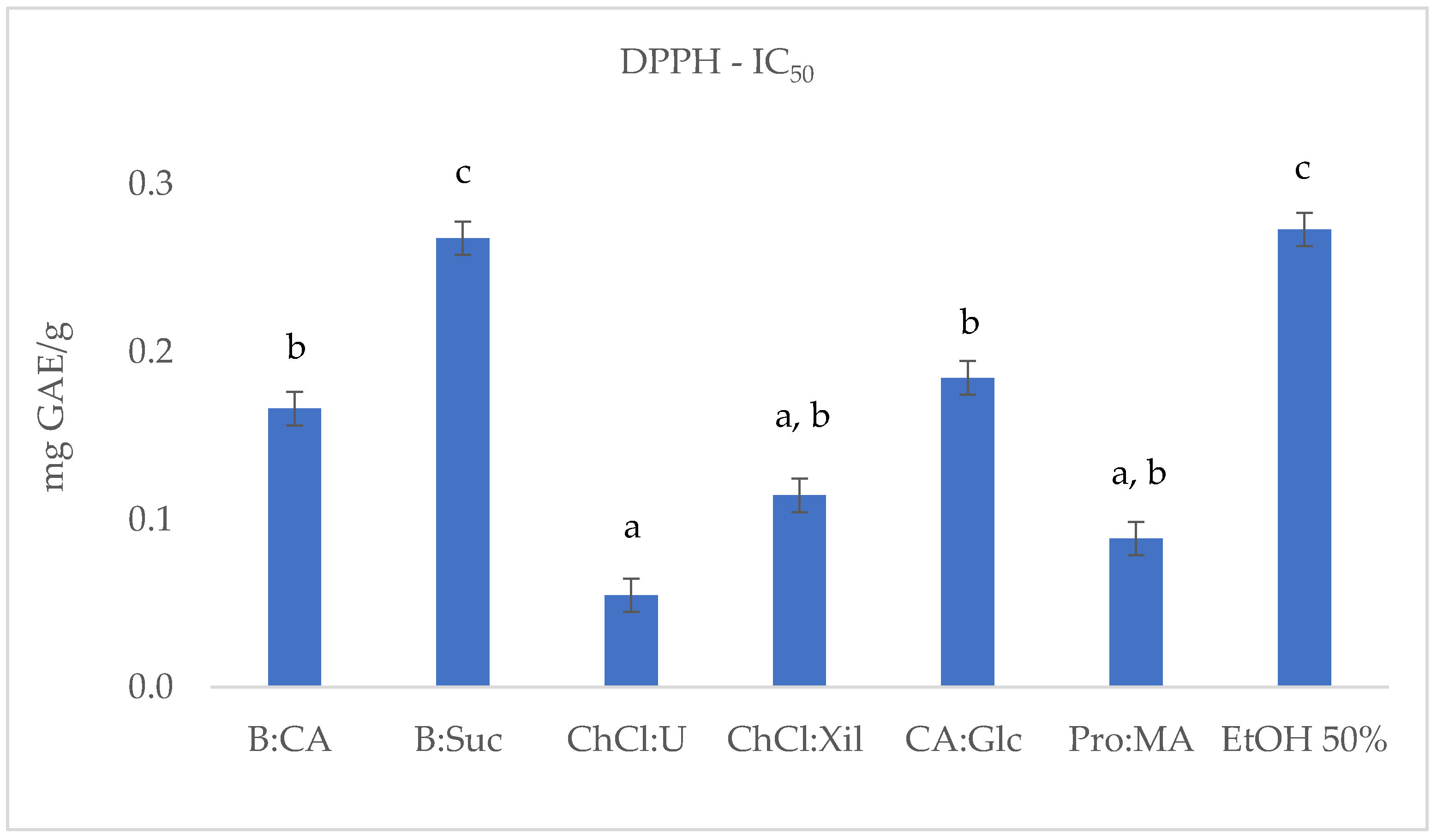
2.2. TPC, Antioxidant and Antiradical Assays
2.3. Antibacterial Studies
2.4. TPC Stability Over Time (Extracts)
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Plant Material
3.3. Bacterial Strains
3.4. COSMO Simulation
3.5. NADES Preparation
3.6. NADES-UAE Extraction
3.7. Determination of Total Polyphenol Content (TPC)
3.8. Determination of Radical Scavenging Activity (DPPH Assay)
3.9. Determination of Antioxidant Activity (ABTS Assay)
3.10. Determination of Ferric Reducing Antioxidant Power (FRAP Assay)
3.11. Stability of the Extracts
3.12. Antibacterial Activity
3.13. Data Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Limongelli, F.; Crupi, P.; Clodoveo, M.L.; Corbo, F.; Muraglia, M. Overview of the Polyphenols in Salicornia: From Recovery to Health-Promoting Effect. Molecules 2022, 27, 7954. [Google Scholar] [CrossRef]
- Ekanayake, S.; Egodawatta, C.; Attanayake, R.N.; Perera, D. From salt pan to saucepan: Salicornia, a halophytic vegetable with an array of potential health benefits. Food Front. 2023, 4, 641–676. [Google Scholar] [CrossRef]
- Mudie, P.J.; Greer, S.; Brakel, J.; Dickson, J.H.; Schinkel, C.; Peterson Welsh, R.; Stevens, M.; Turner, N.J.; Shadow, M.; Washington, R. Forensic palynology and ethnobotany of Salicornia species (Chenopodiaceae) in northwest Canada and Alaska. Can. J. Bot. 2005, 83, 111–123. [Google Scholar] [CrossRef]
- Rhee, M.H.; Park, H.J.; Cho, J.Y. Salicornia herbacea: Botanical, chemical, and pharmacological review of halophyte marsh plant. J. Med. Plant Res. 2009, 3, 548–555. [Google Scholar]
- Hulkko, L.S.S.; Rocha, R.M.; Trentin, R.; Fredsgaard, M.; Chaturvedi, T.; Custódio, L.; Thomsen, M.H. Bioactive Extracts from Salicornia ramosissima J. Woods Biorefinery as a Source of Ingredients for High-Value Industries. Plants 2023, 12, 1251. [Google Scholar] [CrossRef] [PubMed]
- Ksouri, R.; Ksouri, W.M.; Jallali, I.; Debez, A.; Magné, C.; Hiroko, I.; Abdelly, C. Medicinal halophytes: Potent source of health promoting biomolecules with medicinal, nutraceuticals and food applications. Crit. Rev. Biotechnol. 2012, 32, 289–326. [Google Scholar] [CrossRef]
- Gouda, M.S.; Elsebaie, E.M. Glasswort (Salicornia spp.) as a source of bioactive compounds and its health benefits: A review. Alex. J. Sci. Technol. 2016, 13, 1–7. [Google Scholar]
- Cárdenas-Pérez, S.; Piernik, A.; Chanona-Pérez, J.J.; Grigore, M.N.; Perea-Flores, M.J. An overview of the emerging trends of the Salicornia L. genus as a sustainable crop. Environ. Exp. Bot. 2021, 191, 104606. [Google Scholar] [CrossRef]
- Wang, H.; Xu, Z.; Li, X.; Sun, J.; Yao, D.; Jiang, H.; Zhou, T.; Liu, Y.; Li, J.; Wang, C.; et al. Extraction, preliminary characterization and antioxidant properties of polysaccharides from the test a of Salicornia herbacea. Carbohydr. Polym. 2017, 176, 99–106. [Google Scholar] [CrossRef]
- Wang, X.Y.; Feng, X.; Wang, M.; Chen, Y.; Dong, Y.F.; Zhao, Y.Y.; Sun, H. Studies on the chemical constituents of Salicornia europaea. J. Chin. Med. Mater. 2011, 34, 67–69. [Google Scholar]
- Parailloux, M.; Godin, S.; Lobinski, R. Nontargeted Screening for Flavonoids in Salicornia Plant by Reversed-Phase Liquid Chromatography–Electrospray Orbitrap Data-Dependent MS2/MS3. Molecules 2023, 28, 3022. [Google Scholar] [CrossRef]
- Lee, S.J.; Jeong, E.M.; Ki, A.Y.; Oh, K.S.; Kwon, J.; Jeong, J.H.; Chung, N.J. Oxidative defense metabolites induced by salinity stress in roots of Salicornia herbacea. J. Plant Physiol. 2016, 206, 133–142. [Google Scholar] [CrossRef]
- Wang, X.H.; Wang, J.P. Ultrasonic-assisted extraction and enrichment of the flavonoids from Salicornia europaea leaves using macroporous resins and response surface methodology. Chem. Pap. 2023, 77, 2769–2781. [Google Scholar] [CrossRef]
- Karthivashan, G.; Kweon, M.H.; Park, S.Y.; Kim, J.S.; Kim, D.H.; Ganesan, P.; Choi, D.K. Cognitive-enhancing and ameliorative effects of acanthoside B in a scopolamine-induced amnesic mouse model through regulation of oxidative/inflammatory/cholinergic systems and activation of the TrkB/CREB/BDNF pathway. Food Chem. Toxicol. 2019, 129, 444–457. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, C.; Colombo, F.; Biella, S.; Stockley, C.; Restani, P. Polyphenols and human health: The role of bioavailability. Nutrients 2021, 13, 273. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Goel, N. Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechnol. Rep. 2019, 24, e00370. [Google Scholar] [CrossRef] [PubMed]
- Ullah, A.; Munir, S.; Badshah, S.L.; Khan, N.; Ghani, L.; Poulson, B.G.; Jaremko, M. Important flavonoids and their role as a therapeutic agent. Molecules 2020, 25, 5243. [Google Scholar] [CrossRef] [PubMed]
- Maleki, S.J.; Crespo, J.F.; Cabanillas, B. Anti-inflammatory effects of flavonoids. Food Chem. 2019, 299, 125124. [Google Scholar] [CrossRef]
- Pospíšil, J. Lignans and Neolignans: Plant secondary metabolites as a reservoir of biologically active substances. Pharmacol. Res. 2019, 146, 104284. [Google Scholar] [CrossRef]
- Urbano, M.; Tomaselli, V.; Bisignano, V.; Veronico, G.; Hammer, K.; Laghetti, G. Salicornia patula Duval-Jouve: From gathering of wild plants to some attempts of cultivation in Apulia region (southern Italy). Genet. Resour. Crop Evol. 2017, 64, 1465–1472. [Google Scholar] [CrossRef]
- Won, K.J.; Lee, K.P.; Baek, S.; Cui, L.; Kweon, M.H.; Jung, S.H.; Kim, B. Desalted Salicornia europaea extract attenuated vascular neointima formation by inhibiting the MAPK pathway-mediated migration and proliferation in vascular smooth muscle cells. Biomed. Pharmacother. 2017, 94, 430–438. [Google Scholar] [CrossRef]
- Silva, A.M.; Lago, J.P.; Pinto, D.; Moreira, M.M.; Grosso, C.; Cruz Fernandes, V.; Rodrigues, F. Salicornia ramosissima bioactive composition and safety: Eco-friendly extractions approach (microwave-assisted extraction vs. conventional maceration). Appl. Sci. 2021, 11, 4744. [Google Scholar] [CrossRef]
- Noh, E.J.; Lee, J.Y.; Park, S.Y.; Park, J.H.; Cho, J.Y.; Kim, Y.M.; Lee, S.K. Salicornia herbacea Aqueous Extracts Regulate NLRP3 Inflammasome Activation in Macrophages and Trophoblasts. J. Med. Food 2022, 25, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, R.; Arbi, K.E.; Aydi, S.S.; Hzami, A.; Tlahig, S.; Najar, R.; Debouba, M. Biochemical composition and biological activities of Salicornia europaea L. from southern Tunisia. J. Food Meas. Charact. 2022, 16, 4833–4846. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, M.; Zhao, Y.; Wang, H.; Liu, T.; Xin, Z. Pentadecyl ferulate, a potent antioxidant and antiproliferative agent from the halophyte Salicornia herbacea. Food Chem. 2013, 141, 2066–2074. [Google Scholar] [CrossRef]
- Kim, H.J.; Lee, J.H. Optimization of ultrasound-assisted extraction of phenolic compounds from Salicornia herbacea powder. Prev. Nutr. Food Sci. 2009, 14, 129–133. [Google Scholar] [CrossRef][Green Version]
- Cristina, C.; Lucia, P.; Sara, S.; Francesco, S.; Nobile Matteo Alessandro, D.; Amalia, C. Study of the Efficacy of Two Extraction Techniques from Crithmum maritimum and Salicornia europaea. J. Food Nutr. Res. 2018, 6, 456. [Google Scholar] [CrossRef]
- Faria, G.Y.Y.; Souza, M.M.; Oliveira, J.R.M.; Costa, C.S.B.; Collares, M.P.; Prentice, C. Effect of ultrasound-assisted cold plasma pretreatment to obtain sea asparagus extract and its application in Italian salami. Food Res. Int. 2020, 137, 109435. [Google Scholar] [CrossRef]
- Oliveira-Alves, S.C.; Andrade, F.; Prazeres, I.; Silva, A.B.; Capelo, J.; Duarte, B.; Bronze, M.R. Impact of drying processes on the nutritional composition, volatile profile, phytochemical content and bioactivity of Salicornia ramosissima. J. woods. Antioxidants 2021, 10, 1312. [Google Scholar] [CrossRef]
- Vinatoru, M.; Mason, T.J.; Calinescu, I. Ultrasonically assisted extraction (UAE) and microwave assisted extraction (MAE) of functional compounds from plant materials. Trends Anal. Chem. 2017, 97, 159–178. [Google Scholar] [CrossRef]
- Oh, J.H.; Kim, E.O.; Lee, S.K.; Woo, M.H.; Choi, S.W. Antioxidant activities of the ethanol extract of hamcho (Salicornia herbacea L.) cake prepared by enzymatic treatment. Food Sci. Biotechnol. 2007, 16, 90–98. [Google Scholar]
- Panth, N.; Park, S.H.; Kim, H.J.; Kim, D.H.; Oak, M.H. Protective effect of Salicornia europaea extracts on high salt intake-induced vascular dysfunction and hypertension. Int. J. Mol. Sci. 2016, 17, 1176. [Google Scholar] [CrossRef]
- Karthivashan, G.; Park, S.Y.; Kweon, M.H.; Kim, J.; Haque, M.; Cho, D.Y.; Choi, D.K. Ameliorative potential of desalted Salicornia europaea L. extract in multifaceted Alzheimer’s-like scopolamine-induced amnesic mice model. Sci. Rep. 2018, 8, 7174. [Google Scholar] [CrossRef] [PubMed]
- Folayan, A.J.; Anawe, P.A.L.; Ayeni, A.O. Synthesis and characterization of Salicornia bigelovii and Salicornia brachiata halophytic plants oil extracted by supercritical CO2 modified with ethanol for biodiesel production via enzymatic transesterification reaction using immobilized Candida antarctica lipase catalyst in tert-butyl alcohol (TBA) solvent. Cogent Eng. 2019, 6, 1625847. [Google Scholar]
- Uwineza, P.A.; Wa´skiewicz, A. Recent advances in supercritical fluid extraction of natural bioactive compounds from natural plant materials. Molecules 2020, 25, 3847. [Google Scholar] [CrossRef] [PubMed]
- Didion, Y.P.; Tjalsma, T.G.; Su, Z.; Malankowska, M.; Pinelo, M. What is next? The greener future of solid liquid extraction of biobased compounds: Novel techniques and solvents overpower traditional ones. Sep. Purif. Technol. 2023, 320, 124147. [Google Scholar] [CrossRef]
- Bashir, I.; Dar, A.H.; Dash, K.K.; Pandey, V.K.; Fayaz, U.; Shams, R.; Srivastava, S.; Singh, R. Deep eutectic solvents for extraction of functional components from plant-based products: A promising approach. Sustain. Chem. Pharm. 2023, 33, 101102. [Google Scholar] [CrossRef]
- Hashemi, B.; Shiri, F.; Švec, F.; Nováková, L. Green solvents and approaches recently applied for extraction of natural bioactive compounds. TRAC-Trend. Anal. Chem. 2022, 157, 116732. [Google Scholar] [CrossRef]
- Mansur, A.R.; Song, N.E.; Jang, H.W.; Lim, T.G.; Yoo, M.; Nam, T.G. Optimizing the ultrasound-assisted deep eutectic solvent extraction of flavonoids in common buckwheat sprouts. Food Chem. 2019, 293, 438–445. [Google Scholar] [CrossRef]
- Jovanović, M.S.; Krgović, N.; Radan, M.; Ćujić-Nikolić, N.; Mudrić, J.; Lazarević, Z.; Šavikin, K. Natural deep eutectic solvents combined with cyclodextrins: A novel strategy for chokeberry anthocyanins extraction. Food Chem. 2023, 405, 134816. [Google Scholar] [CrossRef]
- Rente, D.; Bubalo, M.C.; Panić, M.; Paiva, A.; Caprin, B.; Redovniković, I.R.; Duart, A.R.C. Review of deep eutectic systems from laboratory to industry, taking the application in the cosmetics industry as an example. J. Clean. Prod. 2022, 380, 135147. [Google Scholar] [CrossRef]
- Jurić, T.; Mićić, N.; Potkonjak, A.; Milanov, D.; Dodić, J.; Trivunović, Z.; Popović, B.M. The evaluation of phenolic content, in vitro antioxidant and antibacterial activity of Mentha piperita extracts obtained by natural deep eutectic solvents. Food Chem. 2021, 362, 130226. [Google Scholar] [CrossRef]
- Roselli, V.; Leuci, R.; Pugliese, G.; Barbarossa, A.; Laghezza, A.; Paparella, M.; Carocci, A.; Tufarelli, V.; Gambacorta, L.; Piemontese, L. Deep Eutectic Solvents (DESs) as Alternative Sustainable Media for the Extraction and Characterization of Bioactive Compounds from Winemaking Industry Wastes. Molecules 2025, 30, 1855. [Google Scholar] [CrossRef]
- Sateriale, D.; Forgione, G.; Di Rosario, M.; Pagliuca, C.; Colicchio, R.; Salvatore, P.; Paolucci, M.; Pagliarulo, C. Vine-Winery Byproducts as Precious Resource of Natural Antimicrobials: In Vitro Antibacterial and Antibiofilm Activity of Grape Pomace Extracts against Foodborne Pathogens. Microorganisms 2024, 12, 437. [Google Scholar] [CrossRef]
- Castagna, A.; Mariottini, G.; Gabriele, M.; Longo, V.; Souid, A.; Dauvergne, X.; Ranieri, A. Nutritional composition and bioactivity of Salicornia europaea L. plants grown in monoculture or intercropped with tomato plants in salt-affected soils. Horticulturae 2022, 8, 828. [Google Scholar] [CrossRef]
- Kim, S.; Lee, E.Y.; Hillman, P.F.; Ko, J.; Yang, I.; Nam, S.J. Chemical structure and biological activities of secondary metabolites from Salicornia europaea L. Molecules 2021, 26, 2252. Molecules 2021, 26, 2252. [Google Scholar] [CrossRef]
- Elatif, R.A.; Shabana, M.; Mansour, R.; Awad, H. Chemical composition and biological activity of Salicornia fruticosa L. Egypt. J. Chem. 2020, 63, 1713–1721. [Google Scholar]
- Lee, J.H.; Lee, S.; Park, J.Y.; Park, I.-H.; Kang, K.S.; Shin, M.-S. The Beneficial Effect of Salicornia herbacea Extract and Isorhamnetin-3-O-glucoside on Obesity. Processes 2023, 11, 977. [Google Scholar] [CrossRef]
- Panić, M.; Gunjević, V.; Cravotto, G.; Radojčić Redovniković, I. Enabling technologies for the extraction of grape-pomace anthocyanins using natural deep eutectic solvents in up-to-half-litre batches. Food Chem. 2019, 300, 125185. [Google Scholar] [CrossRef]
- Viñas-Ospino, A.; Panić, M.; Bagović, M.; Radošević, K.; Esteve, M.J.; Redovniković, I.R. Green approach to extract bioactive compounds from orange peel employing hydrophilic and hydrophobic deep eutectic solvents. Sustain. Chem. Pharm. 2023, 31, 100942. [Google Scholar] [CrossRef]
- Panić, M.; Radović, M.; Cvjetko Bubalo, M.; Radošević, K.; Rogošić, M.; Coutinho, J.A.P.; Radojčić Redovniković, I.; Jedinak Tušek, A. Prediction of pH Value of Aqueous Acidic and Basic Deep Eutectic Solvent Using COSMO-RS σ Profiles’ Molecular Descriptors. Molecules 2022, 27, 4489. [Google Scholar] [CrossRef]
- Ivanović, M.; Grujić, D.; Cerar, J.; Islamčević Razboršek, M.; Topalić-Trivunović, L.; Savić, A.; Kolar, M. Extraction of bioactive metabolites from Achillea millefolium L. with choline chloride based natural deep eutectic solvents: A study of the antioxidant and antimicrobial activity. Antioxidants 2022, 11, 724. [Google Scholar] [CrossRef]
- Hayyan, M.; Mbous, Y.P.; Looi, C.Y.; Wong, W.F.; Hayyan, A.; Salleh, M.Z.M.; Mohd-Ali, O. Natural deep eutectic solvents: Cytotoxic profile. SpringerPlus 2016, 5, 913. [Google Scholar] [CrossRef]
- Mbous, Y.P.; Hayyan, M.; Wong, W.F.; Looi, C.Y.; Hashim, M.A. Unraveling the Cytotoxicity and Metabolic Pathways of Binary Natural Deep Eutectic Solvent Systems. Sci. Rep. 2017, 7, 41257. [Google Scholar] [CrossRef]
- Popović, B.M.; Gligorijević, N.; Aranđelović, S.; Macedo, A.C.; Jurić, T.; Uka, D.; Mocko-Blažek, K.; Serra, A.T. Cytotoxicity profiling of choline chloride-based natural deep eutectic solvents. RSC Adv. 2023, 13, 3520–3527. [Google Scholar] [CrossRef]
- Negi, T.; Kumar, A.; Sharma, S.K.; Rawat, N.; Saini, D.; Sirohi, R.; Prakash, O.; Dubey, A.; Dutta, A.; Shahi, N.C. Deep eutectic solvents: Preparation, properties, and food applications. Heliyon 2024, 10, e28784. [Google Scholar] [CrossRef]
- Karaula, I.; Vasung, E.; Damjanović, A.; Panić, M.; Radović, M.; Radošević, K.; Radojčić Redovniković, I. Formulation of Ready-to-Use Broccoli Extracts Rich in Polyphenols and Glucosinolates Using Natural Deep Eutectic Solvents. Molecules 2024, 29, 5794. [Google Scholar] [CrossRef]
- Panić, M.; Stojković, M.R.; Kraljić, K.; Škevin, D.; Redovniković, I.R.; Srček, V.G.; Radošević, K. Ready-to-use green polyphenolic extracts from food by-products. Food Chem. 2019, 283, 628–636. [Google Scholar] [CrossRef]
- Dabetić, N.; Todorović, V.; Panić, M.; Radojčić Redovniković, I.; Šobajić, S. Impact of deep eutectic solvents on extraction of polyphenols from grape seeds and skin. Appl. Sci. 2020, 10, 4830. [Google Scholar] [CrossRef]
- Cavalluzzi, M.M.; Lamonaca, A.; Rotondo, N.P.; Miniero, D.V.; Muraglia, M.; Gabriele, P.; Lentini, G. Microwave-assisted extraction of bioactive compounds from lentil wastes: Antioxidant activity evaluation and Metabolomic characterization. Molecules 2022, 27, 7471. [Google Scholar] [CrossRef]
- Barbarossa, A.; Ceramella, J.; Carocci, A.; Iacopetta, D.; Rosato, A.; Limongelli, F.; Sinicropi, M.S. Benzothiazole-Phthalimide Hybrids as Anti-Breast Cancer and Antimicrobial Agents. Antibiotics 2023, 12, 1651. [Google Scholar] [CrossRef] [PubMed]
- Matthew, A.W.; Franklin, R.C.; William, A.C.; Micheal, N.D.; George, M.E. Clinical and Laboratory Standards Institute Document M07-A9; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2012; pp. 16–34. [Google Scholar]
- Barbarossa, A.; Rosato, A.; Carocci, A.; Arpini, S.; Bosisio, S.; Pagni, L.; Caprioli, G. Efficacy of Willow Herb (Epilobium angustifolium L. and E. parviflorum Schreb.) Crude and Purified Extracts and Oenothein B Against Prostatic Pathogens. Antibiotics 2025, 14, 117. [Google Scholar] [CrossRef] [PubMed]



| NADES | Ratio | Ln γ | NADES | Ratio | Ln γ | NADES | Ratio | Ln γ |
|---|---|---|---|---|---|---|---|---|
| B:CA | 1:1 | 0.84 | ChCl:Gly | 1:2 | 0.39 | Fru:Glc:U | 1:1:2 | 2.61 |
| B:Glc | 1:1 | −0.39 | ChCh:Ma | 1:1 | 0.81 | Glc:EG | 1:2 | 2.69 |
| B:Gly | 1:2 | −1.2 | ChCh:Mal | 4:1 | −1.07 | Glc:Fru | 1:1 | 2.58 |
| B:OxA:Gly | 1:2:1 | 2 | ChCl:OxA | 1:1 | 1.17 | Glc:Fru:EG | 1:1:2 | 1.79 |
| B:Ma | 1:1 | −1.08 | ChCl:Pro:Ma | 1:1:1 | 0.26 | Gly:Glc | 2:1 | 1.88 |
| B:Ma:Glc | 1:1:1 | 1.01 | ChCl:Suc | 2:1 | 1.17 | Gly:Sol | 2:1 | 3.13 |
| B:Ma:Pro | 1:1:1 | −1.1 | ChCl:Sol | 1:1 | 1.37 | Ma:Fru | 1:1 | 2.51 |
| B:EG | 1:1 | −3.28 | ChCl:Sol | 2:3 | 2.21 | Ma:Fru:Gly | 1:1:1 | 2.39 |
| B:EG | 1:2 | −3.47 | ChCl:Sor | 1:1 | 0.54 | Ma:Glc | 1:1 | 2.9 |
| B:Arg | 1:1 | −2.17 | ChCl:U | 1:2 | −1.24 | Ma:Glc:Gly | 1:1:1 | 2.7 |
| B:His | 1:1 | −2.01 | ChCl:U:EG | 1:2:2 | −0.8 | Ma:Sor:Gly | 1:1:2 | 2.4 |
| B:Lys | 1:1 | −3.39 | ChCl:U:Gly | 1:2:2 | 0.45 | Ma:Suc | 2:1 | 3.82 |
| B:Xyl | 1:1 | −0.4 | ChCl:Xyl | 2:1 | −1.45 | Pro:Glc:Gly | 1:1:1 | 1.58 |
| B:Suc | 4:1 | −3.88 | ChCl:Xyol | 5:2 | −2.17 | Pro:Ma | 1:1 | 1.32 |
| ChCl:CA | 2:1 | 0.46 | CA:Fru | 1:1 | 2.95 | Suc:EG | 1:2 | 2.99 |
| ChCl:CA | 1:1 | 2.18 | CA:Fru:Gly | 1:1:1 | 2.82 | Suc:Glc:Fru | 1:1:1 | 3.67 |
| ChCl:EG | 1:2 | −2.2 | CA:Glc | 1:1 | 3.24 | Suc:Glc:U | 1:1:2 | 3.92 |
| ChCl:EG | 2:1 | −4.79 | CA:Glc:Gly | 1:1:1 | 3.07 | Sol:EG | 1:2 | 2.94 |
| ChCl:Fru | 1:1 | 0.27 | CA:Sor | 2:3 | 2.82 | Sor:EG | 1:2 | 1.07 |
| ChCl:Glc | 2:1 | −0.69 | CA:Suc | 1:1 | 4.26 | Xyl:EG | 1:2 | 1.43 |
| ChCl:Glc | 1:1 | 0.96 | Fru:EG | 1:2 | 0.94 |
| S. europaea | TPC (F-C) | DPPH | ABTS | FRAP |
|---|---|---|---|---|
| NADES | Mg GAE/g | IC50 mg GAE/g | Mg TE/g | Mg TE/g |
| B:CA | 2.71 ± 0.004 b | 0.17 ± 0.019 b | 4.48 ± 0.003 c | 1.59 ± 0.008 c,d |
| B:Suc | 2.00 ± 0.001 c | 0.27 ± 0.017 c | 4.13 ± 0.006 c | 1.03 ± 0.002 d |
| ChCl:U | 2.94 ± 0.010 b | 0.05 ± 0.009 a | 10.96 ± 0.010 a | 2.20 ± 0.008 a,b,c |
| ChCl:Xil | 1.48 ± 0.007 d | 0.11 ± 0.012 a,b | 2.63 ± 0.007 d | 0.98 ± 0.008 d |
| CA:Glc | 2.94 ± 0.006 b | 0.18 ± 0.023 b | 2.23 ± 0.011 d | 2.69 ± 0.032 a |
| Pro:MA | 6.79 ± 0.007 a | 0.09 ± 0.003 a,b | 8.12 ± 0.008 b | 2.41 ± 0.017 a,b |
| EtOH 50% | 2.82 ± 0.004 b | 0.27 ± 0.005 c | 8.65 ± 0.009 b | 1.95 ± 0.003 b,c |
| E. faecalis | S. aureus 29213 | E. coli | K. pneumoniae 13883 | |||||
|---|---|---|---|---|---|---|---|---|
| 29212 | 25922 | |||||||
| NADES Solvent | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC |
| B:CA | 1.6 | 3.1 | 1.6 | 3.1 | 1.6 | 3.1 | 25 | 50 |
| B:Suc | 50 | >50 | 25 | 50 | >50 | >50 | >50 | >50 |
| ChCl:U | 25 | 50 | 25 | 50 | >50 | >50 | >50 | >50 |
| ChCl:Xil | 12.5 | 25 | 12.5 | 25 | 25 | 50 | 25 | 50 |
| CA:Glc | 1.6 | 3.1 | 1.6 | 3.1 | 1.6 | 3.1 | 3.1 | 6.3 |
| Pro:MA | 6.3 | 12.5 | 3.1 | 6.3 | 12.5 | 25 | 25 | 50 |
| EtOH 50% | 25 | 50 | 12.5 | 25 | 25 | 50 | 25 | 50 |
| E. faecalis 29212 | S. aureus 29213 | E. coli 25922 | K. pneumoniae 13883 | |||||
|---|---|---|---|---|---|---|---|---|
| S. europaea | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC |
| NADES-UAE | ||||||||
| B:CA | 0.2 | 0.4 | 0.2 | 0.4 | 0.2 | 0.4 | 0.4 | 0.8 |
| B:Suc | 25 | 50 | 25 | 50 | 50 | >50 | >50 | >50 |
| ChCl:U | 25 | 50 | 12.5 | 25 | 25 | 50 | 25 | 50 |
| ChCl:Xil | 25 | 50 | 25 | 50 | 25 | 50 | >50 | >50 |
| CA:Glc | 0.2 | 0.4 | 0.2 | 0.4 | 0.4 | 0.8 | 1.6 | 3.1 |
| Pro:MA | 0.1 | 0.2 | 0.1 | 0.2 | 0.4 | 0.8 | 0.4 | 0.8 |
| EtOH 50% | 3.1 | 6.3 | 3.1 | 6.3 | 12.5 | 25 | 25 | 50 |
| Levofloxacin | 2 | - | 0.5 | - | 0.12 | - | 8 | - |
| TPC mgGAE/g | ||||
|---|---|---|---|---|
| S. europaea NADES-UAE | T0 | T1 | T2 | T3 |
| B:CA | 2.71 ± 0.004 | 2.81 ± 0.003 | 2.82 ± 0.006 | 2.77 ± 0.009 |
| B:Suc | 2.00 ± 0.001 | 1.84 ± 0.005 | 1.83 ± 0.002 | 1.29 ± 0.007 |
| ChCl:U | 2.94 ± 0.010 | 2.75 ± 0.002 | 2.19 ± 0.010 | 2.03 ± 0.008 |
| ChCl:Xil | 1.48 ± 0.007 | 1.50 ± 0.007 | 1.58 ± 0.009 | 1.48 ± 0.015 |
| CA:Glc | 2.94 ± 0.006 | 3.92 ± 0.003 | 4.38 ± 0.006 | 4.14 ± 0.012 |
| Pro:MA | 6.79 ± 0.007 | 6.82 ± 0.019 | 6.86 ± 0.023 | 6.61 ± 0.010 |
| EtOH 50% | 2.82 ± 0.004 | 2.21 ± 0.013 | 1.75 ± 0.004 | 1.80 ± 0.010 |
| Hydrogen-Bond Acceptor (HBA) | Hydrogen-Bond Donor (HBD) | Molar Ratio | Acronym |
|---|---|---|---|
| Betaine | Citric Acid | 1:1 | B:CA |
| Betaine | Saccarose | 4:1 | B:Sac |
| Choline Chloride | Urea | 1:2 | ChCl:U |
| Choline chloride | Xylitol | 5:2 | ChCl:Xil |
| Citric Acid | Glucose | 1:2 | CA:Glc |
| Proline | Malic Acid | 1:1 | Pro:MA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Limongelli, F.; Aresta, A.M.; Tardugno, R.; Clodoveo, M.L.; Barbarossa, A.; Carocci, A.; Zambonin, C.; Crupi, P.; Panić, M.; Corbo, F.; et al. Exploitation of Apulian Salicornia europaea L. via NADES-UAE: Extraction, Antioxidant Activity and Antimicrobial Potential. Molecules 2025, 30, 3367. https://doi.org/10.3390/molecules30163367
Limongelli F, Aresta AM, Tardugno R, Clodoveo ML, Barbarossa A, Carocci A, Zambonin C, Crupi P, Panić M, Corbo F, et al. Exploitation of Apulian Salicornia europaea L. via NADES-UAE: Extraction, Antioxidant Activity and Antimicrobial Potential. Molecules. 2025; 30(16):3367. https://doi.org/10.3390/molecules30163367
Chicago/Turabian StyleLimongelli, Francesco, Antonella Maria Aresta, Roberta Tardugno, Maria Lisa Clodoveo, Alexia Barbarossa, Alessia Carocci, Carlo Zambonin, Pasquale Crupi, Manuela Panić, Filomena Corbo, and et al. 2025. "Exploitation of Apulian Salicornia europaea L. via NADES-UAE: Extraction, Antioxidant Activity and Antimicrobial Potential" Molecules 30, no. 16: 3367. https://doi.org/10.3390/molecules30163367
APA StyleLimongelli, F., Aresta, A. M., Tardugno, R., Clodoveo, M. L., Barbarossa, A., Carocci, A., Zambonin, C., Crupi, P., Panić, M., Corbo, F., & Radojčić Redovniković, I. (2025). Exploitation of Apulian Salicornia europaea L. via NADES-UAE: Extraction, Antioxidant Activity and Antimicrobial Potential. Molecules, 30(16), 3367. https://doi.org/10.3390/molecules30163367












