From Binding to Building: A Squaramide-Based Ion Pair Receptor as an Iniferter for Functional Polymer Synthesis
Abstract
1. Introduction
2. Results and Discussion
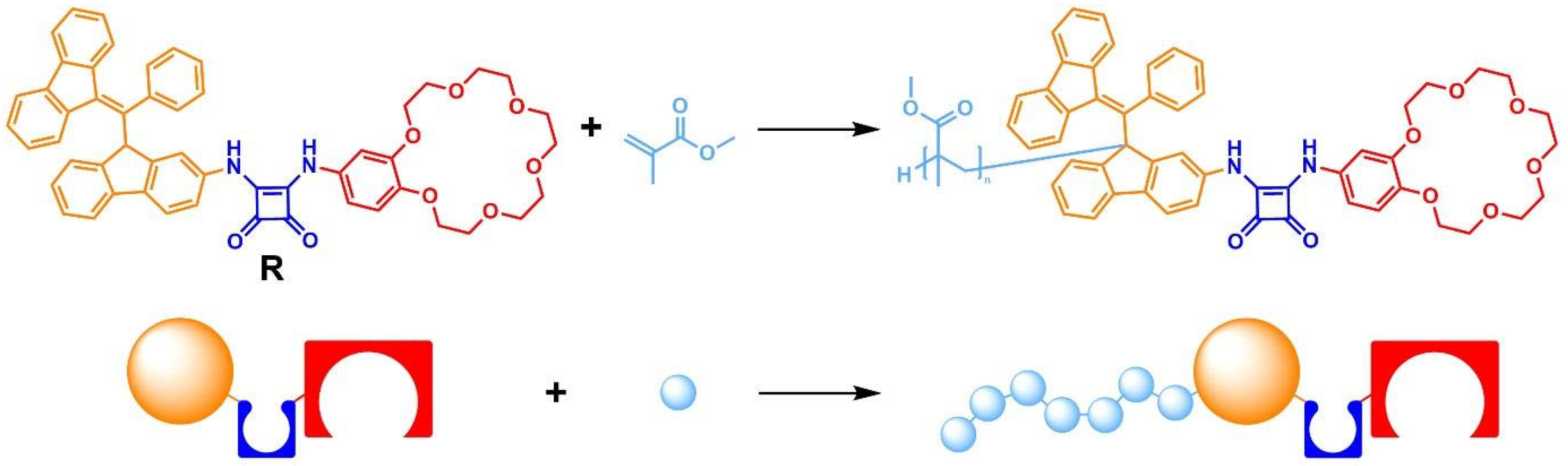
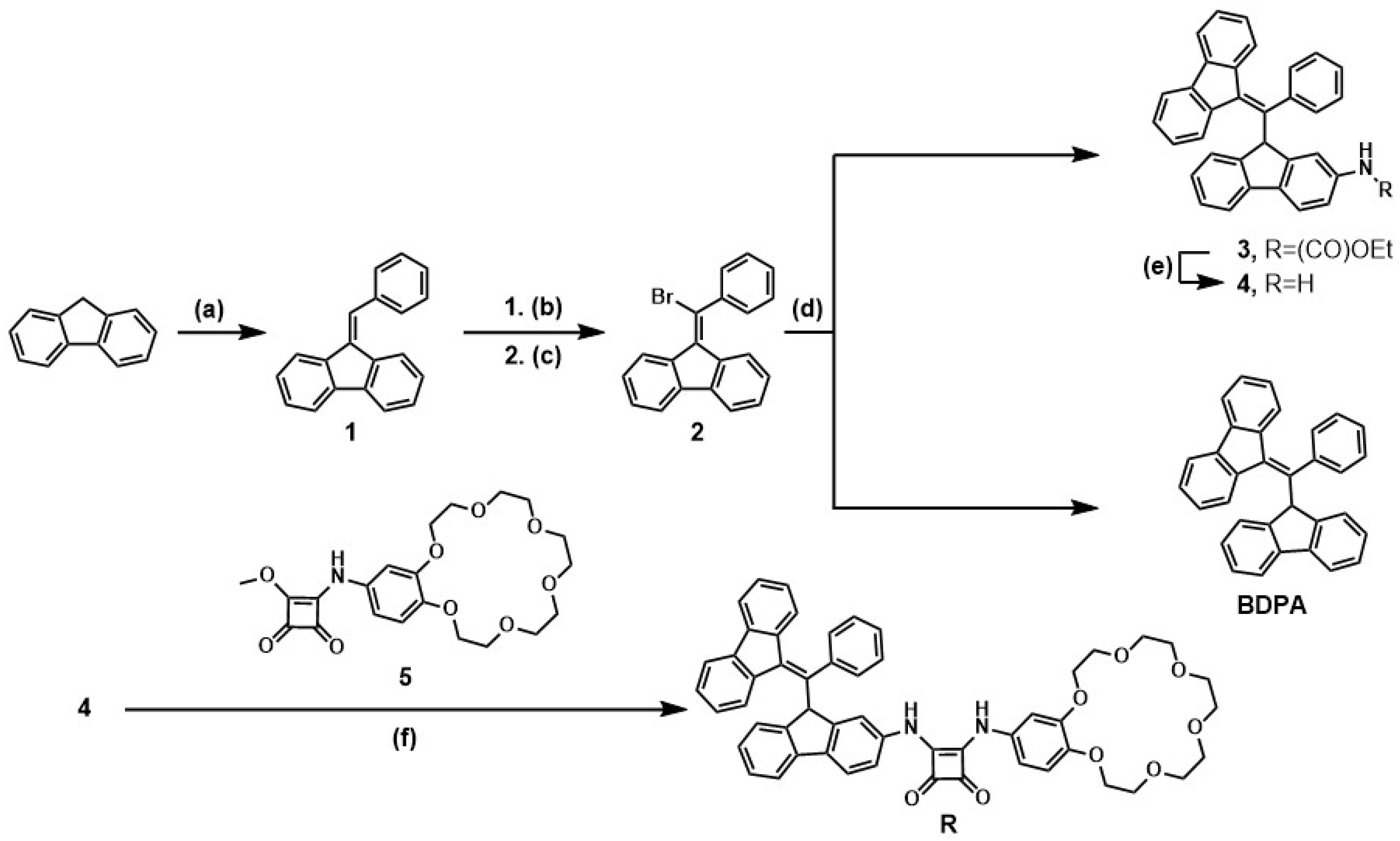
2.1. Design and Synthesis
2.2. Binding Studies
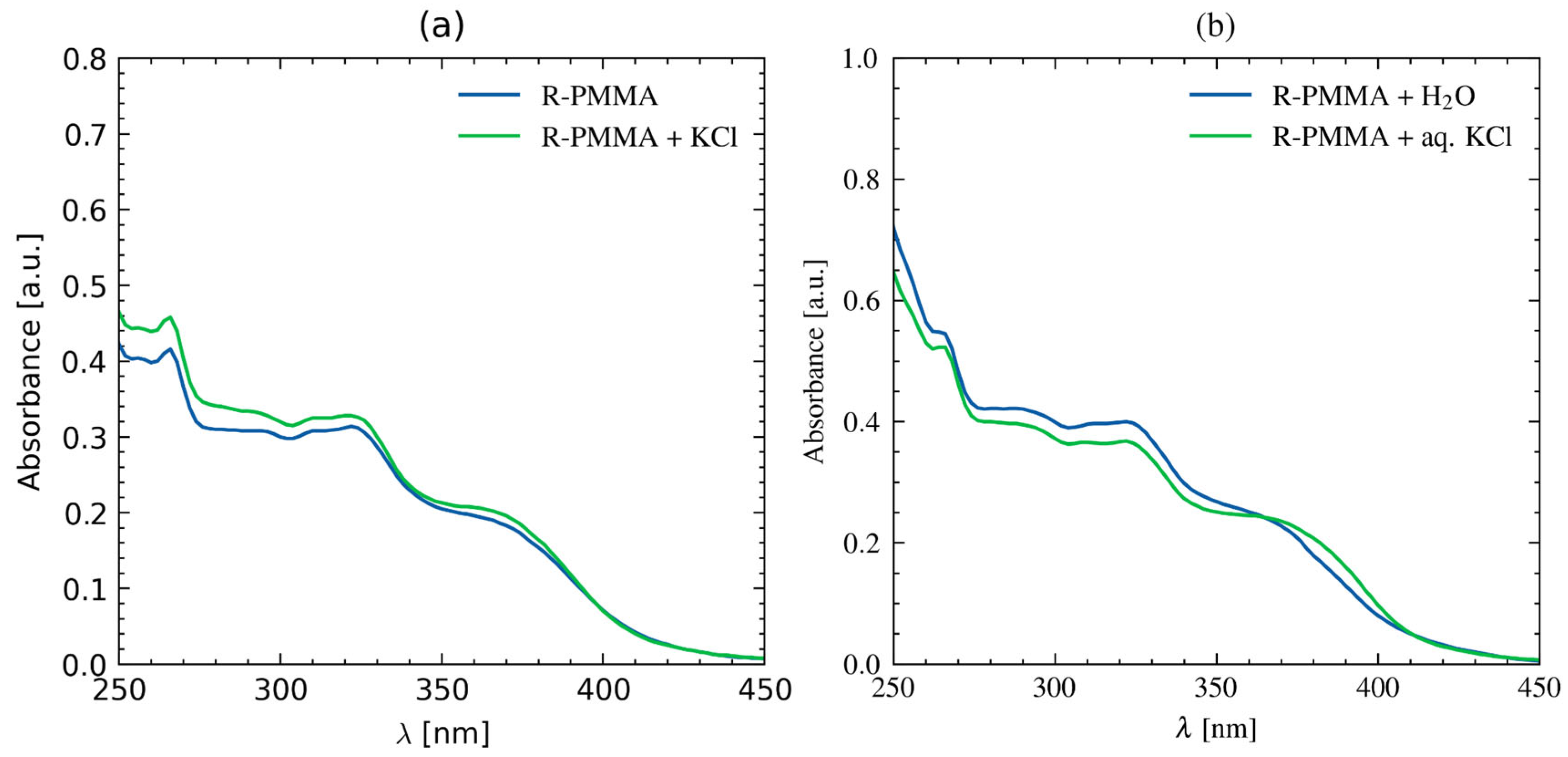
2.3. Polymer Studies
3. Materials and Methods
3.1. Materials and Reagents
3.2. Synthetic Details
3.2.1. Preparation of Compound 1
3.2.2. Preparation of 9-Bromo-9-[bromo-(phenyl)methyl]-9H-fluorene
3.2.3. Preparation of Compound 2
3.2.4. Preparation of Ethyl N-(Fluorenyl)carbamate
3.2.5. Preparation of BDPA
3.2.6. Preparation of Compound 3
3.2.7. Preparation of Compound 4
3.2.8. Preparation of Compound 5
3.2.9. Preparation of Receptor R
3.2.10. Preparation of Radical R˙
3.2.11. Preparation of BDPA–PMMA
3.2.12. Preparation of R–PMMA
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Koelsch, C. Syntheses with Triarylvinylmagnesium Bromides. α,γ-Bisdiphenylene-β-phenylallyl, a Stable Free Radical. J. Am. Chem. Soc. 1957, 79, 4439–4441. [Google Scholar] [CrossRef]
- Kreevoy, M. Resonance energies of two unusually stable hydrocarbon free radicals. Tetrahedron 1958, 2, 354–355. [Google Scholar] [CrossRef]
- Kuhn, R.; Neugebauer, A. Uber substituierte Bis-biphenylen-allyl-Radikale. Monatsh. Chem. 1964, 95, 3–23. [Google Scholar] [CrossRef]
- Bennati, M.; Farrar, C.T.; Bryant, J.A.; Inati, S.J.; Weis, V.; Gerfen, G.J.; Riggs-Gelasco, P.; Stubbe, J.; Griffin, R.G. Pulsed Electron-Nuclear Double Resonance (ENDOR) at 140 GHz. J. Magn. Reson. 1999, 138, 232–243. [Google Scholar] [CrossRef]
- Goldfarb, D.; Lipkin, Y.; Potapov, A.; Gorodetsky, Y.; Epel, B.; Raitsimring, A.M.; Radoul, M.; Kaminker, I. HYSCORE and DEER with an upgraded 95 GHz pulse EPR spectrometer. J. Magn. Reson. 2008, 194, 8–15. [Google Scholar] [CrossRef]
- Lumata, L.; Ratnakar, S.J.; Jindal, A.; Merritt, M.; Comment, A.; Malloy, C.; Sherry, A.D.; Kovacs, Z. BDPA: An Efficient Polarizing Agent for Fast Dissolution Dynamic Nuclear Polarization NMR Spectroscopy. Chem. Eur. J. 2011, 17, 10825–10827. [Google Scholar] [CrossRef]
- Mandal, S.; Sigurdsson, S. On the Limited Stability of BDPA Radicals. Chem. Eur. J. 2020, 26, 7486–7491. [Google Scholar] [CrossRef] [PubMed]
- Haze, O.; Corzilius, B.; Smith, A.A.; Griffin, R.G.; Swager, T.M. Water-soluble narrow-line radicals for dynamic nuclear polarization. J. Am. Chem. Soc. 2012, 134, 14287–14290. [Google Scholar] [CrossRef] [PubMed]
- Dane, E.; Swager, T. Synthesis of a water-soluble 1,3-Bis(diphenylene)-2-phenylallyl Radical. J. Org. Chem. 2010, 75, 3533–3536. [Google Scholar] [CrossRef] [PubMed]
- Dane, E.L.; Maly, T.; Debelouchina, G.T.; Griffin, R.G.; Swager, T.M. Synthesis of a BDPA-TEMPO biradical. Org. Lett. 2009, 11, 1871–1874. [Google Scholar] [CrossRef]
- Wisser, D.; Karthikeyan, G.; Lund, A.; Casano, G.; Karoui, H.; Yulikov, M.; Menzildjian, G.; Pinon, A.C.; Purea, A.; Engelke, F.; et al. BDPA-Nitroxide Biradicals Tailored for Efficient Dynamic Nuclear Polarization Enhanced Solid-State NMR at Magnetic Fields up to 21.1 T. J. Am. Chem. Soc. 2018, 140, 13340–13349. [Google Scholar] [CrossRef]
- Mandal, S.; Sigurdsson, S. Water-soluble BDPA radicals with improved persistence. Chem. Commun. 2020, 56, 13121–13124. [Google Scholar] [CrossRef]
- Matsui, Y.; Shigemori, M.; Endo, T.; Ogaki, T.; Ohta, E.; Mizuno, K.; Naito, H.; Ikeda, H. Spectroscopic and electrical characterization of α,γ-Bisdiphenylene-β-phenylallyl radical as an organic semiconductor. Res. Chem. Intermed. 2018, 44, 4765–4774. [Google Scholar] [CrossRef]
- Wang, P.; Lin, S.; Lin, Z.; Peeks, M.D.; Van Voorhis, T.; Swager, T.M. A Semiconducting Conjugated Radical Polymer: Ambipolar Redox Activity and Faraday Effect. J. Am. Chem. Soc. 2018, 140, 10881–10889. [Google Scholar] [CrossRef] [PubMed]
- Plater, M.; Kemp, S.; Lattmann, E. Heterocyclic free radicals. Part 1. 4,5-Diazafluorene derivatives of Koelsch’s free radical: An EPR and metal-ion complexation study. J. Chem. Soc. Perkin Trans. 1 2000, 971–979. [Google Scholar] [CrossRef]
- Otsu, T.; Yoshida, M.; Tazaki, T. A model for living radical polymerization. Makromol. Rapid Commun. 1982, 3, 133–140. [Google Scholar] [CrossRef]
- Otsu, T. Iniferter concept and living radical polymerization. J. Polym. Sci. Part A Polym. Chem. 2000, 38, 2121–2136. [Google Scholar] [CrossRef]
- Otsu, T.; Yoshida, M. Role of initiator-transfer agent-terminator (iniferter) in radical polymerizations: Polymer design by organic disulfides as iniferters. Makromol. Rapid Commun. 1982, 3, 127–132. [Google Scholar] [CrossRef]
- Xu, Y.; Lu, J.; Li, N.; Yan, F.; Xia, X.; Xu, Q. Pseudo-living radical polymerization using triarylmethane as the thermal iniferter. Eur. Polym. J. 2008, 44, 2404–2411. [Google Scholar] [CrossRef]
- Xu, Y.; Fang, X.; Ding, T.; Ren, Y. Living Radical Polymerizations of Methyl Methacrylate Mediated by Tris-(4-Carboxyphenyl) Methane. Adv. Mater. Res. 2013, 631–632, 3–8. [Google Scholar] [CrossRef]
- Xu, Y.; Ding, T.; Fang, X.; Xu, H.; Ren, Y. Living Radical Polymerization of Methyl Methacrylate Mediated by Tris-(4-Acetyphenyl)Methane. Adv. Mater. Res. 2014, 933, 91–96. [Google Scholar] [CrossRef]
- Aydogan, A.; Coady, D.; Kim, S.; Akar, A.; Bielawski, C.; Marquez, M.; Sessler, J. Poly(methyl methacrylate)s with pendant calixpyrroles and crown ethers: Polymeric extractants for potassium halides. Angew. Chem. Int. Ed. 2008, 47, 9648–9652. [Google Scholar] [CrossRef] [PubMed]
- Romanski, J.; Piatek, P. Tuning the binding properties of a new heteroditopic salt receptor through embedding in a polymeric system. Chem. Commun. 2012, 48, 11346–11348. [Google Scholar] [CrossRef] [PubMed]
- Chi, X.; Peters, G.M.; Brockman, C.; Lynch, V.M.; Sessler, J.L. Controlling Structure Beyond the Initial Coordination Sphere: Complexation-Induced Reversed Micelle Formation in Calix[4]-pyrrole-Containing Diblock Copolymers. J. Am. Chem. Soc. 2018, 140, 13219–13222. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Guo, C.; Chen, W.; Long, L.; Zhang, G.; Khashab, N.M.; Sessler, J.L. Removal of Anions from Aqueous Media by Means of a Thermoresponsive Calix[4]pyrrole Amphiphilic Polymer. Chem. Eur. J. 2018, 24, 15791–15795. [Google Scholar] [CrossRef]
- Picci, G.; Montis, R.; Lippolis, V.; Caltagirone, C. Squaramide-based receptors in anion supramolecular chemistry: Insights into anion binding, sensing, transport and extraction. Chem. Soc. Rev. 2024, 53, 3952–3975. [Google Scholar] [CrossRef]
- Zaleskaya-Hernik, M.; Megiel, E.; Romański, J. Utilizing a polymer containing squaramide-based ion pair receptors for salt extraction. J. Mol. Liq. 2022, 361, 119600. [Google Scholar] [CrossRef]
- Zaleskaya, M.; Jagleniec, D.; Karbarz, M.; Dobrzycki, Ł.; Romański, J. Squaramide based ion pair receptors possessing ferrocene as a signaling unit. Inorg. Chem. Front. 2020, 7, 972–983. [Google Scholar] [CrossRef]


| Ka/1000 M−1 ± Error | TBA+ | Na+ | K+ |
|---|---|---|---|
| Cl− | 20.7 ± 0.8% | 56.3 ± 1.6% | 63.2 ± 1.4% |
| Br− | 18.0 ± 2.4% | b | 29.6 ± 2.9% |
| NO3− | c | b | c |
| NO2− | 10.3 ± 1.3% | b | 14.1 ± 1.4% |
| BzO− | d | b | d |
| AcO− | d | b | d |
| SO42− | e | b | e |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prokopski, M.; Zaleskaya-Hernik, M.; Witkowski, W.; Garbacz, P.; Romański, J. From Binding to Building: A Squaramide-Based Ion Pair Receptor as an Iniferter for Functional Polymer Synthesis. Molecules 2025, 30, 3362. https://doi.org/10.3390/molecules30163362
Prokopski M, Zaleskaya-Hernik M, Witkowski W, Garbacz P, Romański J. From Binding to Building: A Squaramide-Based Ion Pair Receptor as an Iniferter for Functional Polymer Synthesis. Molecules. 2025; 30(16):3362. https://doi.org/10.3390/molecules30163362
Chicago/Turabian StyleProkopski, Mikołaj, Marta Zaleskaya-Hernik, Wojciech Witkowski, Piotr Garbacz, and Jan Romański. 2025. "From Binding to Building: A Squaramide-Based Ion Pair Receptor as an Iniferter for Functional Polymer Synthesis" Molecules 30, no. 16: 3362. https://doi.org/10.3390/molecules30163362
APA StyleProkopski, M., Zaleskaya-Hernik, M., Witkowski, W., Garbacz, P., & Romański, J. (2025). From Binding to Building: A Squaramide-Based Ion Pair Receptor as an Iniferter for Functional Polymer Synthesis. Molecules, 30(16), 3362. https://doi.org/10.3390/molecules30163362







