The Influence of Dietary Fiber (β-Glucan) on the Beneficial Effects of Phenolic Compounds from Chokeberry After Simulated Digestion In Vitro
Abstract
1. Introduction
2. Results
2.1. Identification of Phenolic Compounds in Chokeberry
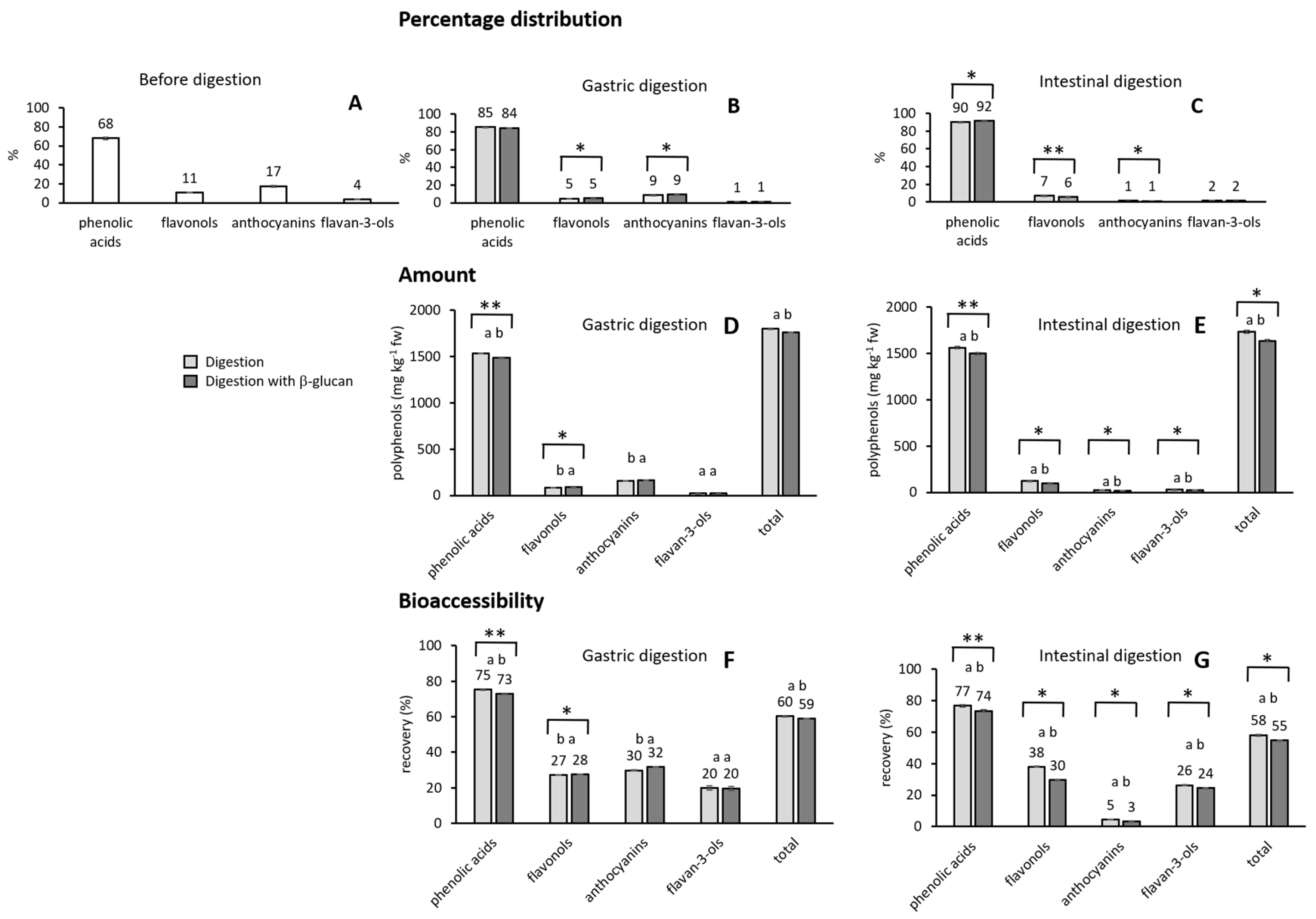
2.2. Amounts and Distribution of Phenolic Compounds in Digestion
2.3. The Influence of β-Glucan on the Release of Phenolic Compounds
2.4. Antiradical Activity of Phenolic Compounds in Digestion
2.5. The Inhibition of α-Amylase and α-Glucosidase
2.6. The Influence of β-Glucan on Bioactivities in the Digestive Tract
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Preparation of Solutions for the Simulated Digestion
4.3. Chokeberry Sample
4.4. The Extraction of Phenolic Compounds from Chokeberry
4.5. Simulated Digestion
4.6. RP-HPLC Analysis of Phenolic Compounds
4.7. Antiradical Activity
4.8. α-Amylase Inhibition
4.9. α-Glucosidase Inhibition
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huo, J.; Ni, Y.; Li, D.; Qiao, J.; Huang, D.; Sui, X.; Zhang, Y. Comprehensive structural analysis of polyphenols and their enzymatic inhibition activities and antioxidant capacity of black mulberry (Morus nigra L.). Food Chem. 2023, 427, 136605. [Google Scholar] [CrossRef]
- Wojdyło, A.; Nowicka, P.; Turkiewicz, I.P.; Tkacz, K. Profiling of polyphenols by LC-QTOF/ESI-MS, characteristics of nutritional compounds and in vitro effect on pancreatic lipase, α-glucosidase, α-amylase, cholinesterase and cyclooxygenase activities of sweet (Prunus avium) and sour (P. cerasus) cherries leaves and fruits. Ind. Crop. Prod. 2021, 174, 114214. [Google Scholar] [CrossRef]
- Worsztynowicz, P.; Napierała, M.; Białas, W.; Grajek, W.; Olkowicz, M. Pancreatic α-amylase and lipase inhibitory activity of polyphenolic compounds present in the extract of black chokeberry (Aronia melanocarpa L.). Process Biochem. 2014, 49, 1457–1463. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Y.; Zhang, X.; Lu, Y.; Chen, H. New insights in intestinal oxidative stress damage and the health intervention effects of nutrients: A review. J. Funct. Food 2020, 75, 104248. [Google Scholar] [CrossRef]
- Kanner, J.; Selhub, J.; Shpaizer, A.; Rabkin, B.; Shacham, I.; Tirosh, O. Redox homeostasis in stomach medium by foods: The Postprandial Oxidative Stress Index (POSI) for balancing nutrition and human health. Redox Biol. 2017, 12, 929–936. [Google Scholar] [CrossRef] [PubMed]
- Gorelik, S.; Ligumsky, M.; Kohen, R.; Kanner, J. The stomach as a “bioreactor”: When red meat meets red wine. J. Agric. Food Chem. 2008, 56, 5002–5007. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Kim, E.H.; Hahm, K.B. Oxidative stress in inflammation-based gastrointestinal tract diseases: Challenges and opportunities. J. Gastroenterol. Hepatol. 2012, 27, 1004–1010. [Google Scholar] [CrossRef] [PubMed]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef]
- Kanner, J.; Lapidot, T. The stomach as a bioreactor: Dietary lipid peroxidation in the gastric fluid and the effects of plant-derived antioxidants. Free Radic. Biol. Med. 2001, 31, 1388–1395. [Google Scholar] [CrossRef]
- Kuffa, M.; Priesbe, T.J.; Krueger, C.G.; Reed, J.D.; Richards, M.P. Ability of dietary antioxidants to affect lipid oxidation of cooked turkey meat in a simulated stomach and blood lipids after a meal. J. Funct. Food 2009, 1, 208–216. [Google Scholar] [CrossRef]
- Hao, J.; Wang, Y.; Xiao, Y.; He, S.; Chen, M.; Mao, X.; Huang, Q.; Su, Z.; Lu, Q.; Guo, H. Proanthocyanidin polymers (condensed tannins) from lychee seeds exhibit antioxidant, anticancer, anti-α-amylase, and anti-tyrosinase activities. Int. J. Biol. Macromol. 2025, 308, 142641. [Google Scholar] [CrossRef]
- Denev, P.; Čiž, M.; Kratchanova, M.; Blazheva, D. Black chokeberry (Aronia melanocarpa) polyphenols reveal different antioxidant, antimicrobial and neutrophil-modulating activities. Food Chem. 2019, 284, 108–117. [Google Scholar] [CrossRef]
- Gao, N.; Si, X.; Han, W.; Gong, E.; Shu, C.; Tian, J.; Wang, Y.; Zhang, J.; Li, B.; Li, B. The contribution of different polyphenol compositions from chokeberry produced in China to cellular antioxidant and antiproliferative activities. Food Sci. Hum. Well 2023, 12, 1590–1600. [Google Scholar] [CrossRef]
- Gao, N.; Shu, C.; Wang, Y.; Tian, J.; Lang, Y.; Jin, C.; Cui, X.; Jiang, H.; Liu, S.; Li, Z.; et al. Polyphenol components in black chokeberry (Aronia melanocarpa) as clinically proven diseases control factors—An overview. Food Sci. Hum. Well 2024, 13, 1152–1167. [Google Scholar] [CrossRef]
- Sasmaz, H.K.; Kilic-Buyukkurt, O.; Selli, S.; Bouaziz, M.; Kelebek, H. Antioxidant capacity, sugar content, and tandem HPLC−DAD−ESI/MS profiling of phenolic compounds from Aronia melanocarpa fruits and leaves (Nero and Viking Cultivars). ACS Omega 2024, 9, 14963–14976. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.E.; Kim, G.S.; Park, S.; Kim, Y.H.; Kim, M.B.; Lee, W.S.; Jeong, S.W.; Lee, S.J.; Jin, J.S.; Shin, S.C. Determination of chokeberry (Aronia melanocarpa) polyphenol components using liquid chromatography–tandem mass spectrometry: Overall contribution to antioxidant activity. Food Chem. 2014, 146, 1–5. [Google Scholar] [CrossRef]
- Liu, X.; Martin, D.A.; Valdez, J.C.; Sudakaran, S.; Rey, F.; Bolling, B.W. Aronia berry polyphenols have matrix-dependent effects on the gut microbiota. Food Chem. 2021, 359, 129831. [Google Scholar] [CrossRef]
- Xie, L.; Vance, T.; Kim, B.; Lee, S.G.; Caceres, C.; Wang, Y.; Hubert, P.A.; Lee, J.Y.; Chun, O.K.; Bolling, B.W. Aronia berry polyphenol consumption reduces plasma total and low-density lipoprotein cholesterol in former smokers without lowering biomarkers of inflammation and oxidative stress: A randomized controlled trial. Nutr. Res. 2017, 37, 67–77. [Google Scholar] [CrossRef]
- Zdunić, G.; Alimpić Aradski, A.; Gođevac, D.; Živković, J.; Duletić Laušević, S.; Krstić Milošević, D.; Šavikin, K. In vitro hypoglycemic, antioxidant and antineurodegenerative activity of chokeberry (Aronia melanocarpa) leaves. Ind. Crop Prod. 2020, 148, 112328. [Google Scholar] [CrossRef]
- Kim, I.; Moon, J.K.; Hur, S.J.; Lee, J. Structural changes in mulberry (Morus Microphylla. Buckl) and chokeberry (Aronia melanocarpa) anthocyanins during simulated in vitro human digestion. Food Chem. 2020, 318, 126449. [Google Scholar] [CrossRef]
- Jang, Y.; Koh, E. Effect of encapsulation on stability of anthocyanins and chlorogenic acid isomers in aronia during in vitro digestion and their transformation in a model system. Food Chem. 2024, 434, 137443. [Google Scholar] [CrossRef]
- Saracila, M.; Untea, A.E.; Oancea, A.G.; Varzaru, J.; Vlaicu, P.A. Comparative analysis of black chokeberry (Aronia melanocarpa L.) fruit, leaves, and pomace for their phytochemical composition, antioxidant potential, and polyphenol bioaccessibility. Foods 2024, 13, 1856. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.S.; Nadeem, M.; Khalid, W.; Ainee, A.; Roheen, T.; Javaria, S.; Ahmed, A.; Fatima, H.; Riaz, M.N.; Khalid, M.Z.; et al. Optimization of ultrasound assisted extraction and characterization of functional properties of dietary fiber from oat cultivar S2000. Food Sci. Technol. LWT 2024, 197, 115875. [Google Scholar] [CrossRef]
- Du, B.; Meenu, M.; Liu, H.; Xu, B. A concise review on the molecular structure and function relationship of β-glucan. Int. J. Mol. Sci. 2019, 20, 4032. [Google Scholar] [CrossRef] [PubMed]
- Köpsel, M.; Ozkan, G.; Esatbeyoglu, T. Metabolic fate of chokeberry (Aronia melanocarpa) phenolics in different food matrices. Curr. Res. Food Sci. 2025, 10, 100967. [Google Scholar] [CrossRef]
- Wu, Z.; Ming, J.; Gao, R.; Wang, Y.; Liang, Q.; Yu, H.; Zhao, G. Characterization and antioxidant activity of the complex of tea polyphenols and oat β-glucan. J. Agric. Food Chem. 2011, 59, 10737–10746. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, J.; Chen, F.; Zhao, G. Effects of molecular structure of polyphenols on their noncovalent interactions with oat β-glucan. J. Agric. Food Chem. 2013, 61, 4533–4538. [Google Scholar] [CrossRef]
- Jakobek, L.; Matić, P.; Ištuk, J.; Barron, A. Study of interactions between individual phenolics of Aronia with barley β-Glucan. Pol. J. Food Nutr. Sci. 2021, 71, 187–196. [Google Scholar] [CrossRef]
- Jakobek, L.; Ištuk, J.; Tomac, I.; Matić, P. β-Glucan and Aronia (Aronia melanocarpa) phenolics: Interactions during in vitro simulated gastrointestinal digestion and adsorption. Pol. J. Food Nutr. Sci. 2022, 72, 371–380. [Google Scholar] [CrossRef]
- Jakobek, L.; Strelec, I.; Kenjerić, D.; Šoher, L.; Tomac, I.; Matić, P. Simulated gastric and intestinal fluid electrolyte solutions as an environment for the adsorption of apple polyphenols onto β-Glucan. Molecules 2022, 27, 6683. [Google Scholar] [CrossRef]
- Jakobek, L.; Ištuk, J.; Matić, P.; Skendrović Babojelić, M. Interactions of polyphenols from traditional apple varieties ‘Bobovac’, ‘Ljepocvjetka’ and ‘Crvenka’ with β-Glucan during in vitro simulated digestion. Food Chem. 2021, 363, 130283. [Google Scholar] [CrossRef] [PubMed]
- Matić, P.; Ukić, Š.; Jakobek, L. Adsorption of procyanidin B1 and B2 onto β-glucan: Adsorption isotherm and thermodynamics. Adsorption 2024, 30, 1303–1313. [Google Scholar] [CrossRef]
- Matić, P.; Ukić, Š.; Jakobek, L. The study of adsorption kinetics of flavan-3-ols, dihydrochalcones and anthocyanins onto barley β-glucan. Croat. Chem. Acta 2022, 95, 7–13. [Google Scholar] [CrossRef]
- Matić, P.; Ukić, Š.; Jakobek, L. Interactions of phenolic acids and β-glucan: Studies of adsorption isotherms and thermodynamics. Chem. Biochem. Eng. Q. 2021, 35, 177–187. [Google Scholar] [CrossRef]
- Yu, M.; Zhu, S.; Huang, D.; Tao, X.; Li, Y. Inhibition of starch digestion by phenolic acids with a cinnamic acid backbone: Structural requirements for the inhibition of α-amylase and α-glucosidase. Food Chem. 2024, 435, 137499. [Google Scholar] [CrossRef]
- Aleixandre, A.; Gil, J.V.; Sineiro, J.; Rosell, C.M. Understanding phenolic acids inhibition of α-amylase and α-glucosidase and influence of reaction conditions. Food Chem. 2022, 372, 131231. [Google Scholar] [CrossRef]
- Li, F.; Chen, Z.; Chang, M.; Zhang, X.; Liu, X.; Wang, L. Three anthocyanin-rich berry extracts regulate the in vitro digestibility of corn starch: Physicochemical properties, structure and α-amylase. Int. J. Biol. Macromol. 2023, 253, 127484. [Google Scholar] [CrossRef]
- Meng, X.; Liu, R.; Xie, J.; Li, L.; Yu, K.; Liu, J.; Zhang, Y.; Wang, H. Valuation of the significant hypoglycemic activity of black currant anthocyanin extract by both starch structure transformation and glycosidase activity inhibition. Int. J. Biol. Macromol. 2024, 269, 132112. [Google Scholar] [CrossRef]
- Siegień, J.; Buchholz, T.; Popowski, D.; Granica, S.; Osińska, E.; Melzig, M.F.; Czerwińska, M.E. Pancreatic lipase and α-amylase inhibitory activity of extracts from selected plant materials after gastrointestinal digestion in vitro. Food Chem. 2021, 355, 129414. [Google Scholar] [CrossRef]
- Song, Y.; Li, W.; Yang, H.; Peng, X.; Yang, X.; Liu, X.; Sun, L. Caffeoyl substitution decreased the binding and inhibitory activity of quinic acid against α-amylase: The reason why chlorogenic acid is a relatively weak enzyme inhibitor. Food Chem. 2022, 371, 131278. [Google Scholar] [CrossRef]
- Zheng, X.; Yang, W.; Sun, W.; Chen, S.; Liu, D.; Kong, X.; Tian, J.; Ye, X. Inhibition of porcine pancreatic α-amylase activity by chlorogenic acid. J. Funct. Food 2020, 64, 103587. [Google Scholar] [CrossRef]
- Nyambe-Silavwe, H.; Williamson, G. Chlorogenic and phenolic acids are only very weak inhibitors of human salivary α-amylase and rat intestinal maltase activities. Food Res. Int. 2018, 113, 452–455. [Google Scholar] [CrossRef] [PubMed]
- Sui, X.; Zhang, Y.; Zhou, W. In vitro and in silico studies of the inhibition activity of anthocyanins against porcine pancreatic α-amylase. J. Funct. Food 2016, 21, 50–57. [Google Scholar] [CrossRef]
- Cásedas, G.; Les, F.; González-Burgos, E.; Gómez-Serranillos, M.P.; Smith, C.; López, V. Cyanidin-3-O-glucoside inhibits different enzymes involved in central nervous system pathologies and type-2 diabetes. S. Afr. J. Bot. 2019, 120, 241–246. [Google Scholar] [CrossRef]
- Visvanathan, R.; Houghton, M.J.; Barber, E.; Williamson, G. Structure-function relationships in (poly) phenol-enzyme binding: Direct inhibition of human salivary and pancreatic α-amylases. Food Res. Int. 2024, 188, 114504. [Google Scholar] [CrossRef]
- Huang, M.; Xiao, Q.; Li, Y.; Ahmad, M.; Tang, J.; Liao, Q.; Tan, C. Inhibition of α-amylase activity by quercetin via multi-spectroscopic and molecular docking approaches. Food Biosci. 2024, 61, 104951. [Google Scholar] [CrossRef]
- Jakobek, L.; Ištuk, J.; Barron, A.R.; Matić, P. Bioactive phenolic compounds from apples during simulated in vitro gastrointestinal digestion; Kinetics of their release. Appl. Sci. 2023, 13, 8434. [Google Scholar] [CrossRef]
- Brindzova, L.; Četrik, M.; Rapta, P.; Zalibera, M.; Mikulajova, A.; Takacsova, M. Antioxidant activity, b-glucan and lipid content of oats varieties. Czech J. Food Sci. 2008, 26, 163–173. [Google Scholar] [CrossRef]
- Upadhyay, T.K.; Trivedi, R.; Khan, F.; Al-Keridis, L.A.; Pandey, P.; Sharangi, A.B.; Alshammari, N.; Abdullah, N.M.; Yadav, D.K.; Saeed, M. In vitro elucidation of antioxidant, antiproliferative, and apoptotic potential of yeast-derived β-1,3-glucan particles against cervical cancer cells. Front. Oncol. 2022, 12, 942075. [Google Scholar] [CrossRef]
- Li, H.; Dhital, S. α-amylase interaction with soluble fibre: Insight from diffusion experiment using fluorescence recovery after photobleaching (FRAP) and permeation experiment using ultrafiltration membrane. Bioact. Carbohydr. Diet. Fibre 2022, 28, 100319. [Google Scholar] [CrossRef]
- Zhang, H.; Li, Z.; Xie, F.; Wang, G.; Song, Z.; Ai, L. Inhibitory mechanism of arabinoxylan and β-glucan from hull-less barley on α-amylase activity: A comparison study. Food Hydrocoll. 2024, 153, 109994. [Google Scholar] [CrossRef]
- Stanisavljević, N.; Samardžić, J.; Janković, T.; Šavikin, K.; Mojsin, M.; Topalović, V.; Stevanović, M. Antioxidant and antiproliferative activity of chokeberry juice phenolics during in vitro simulated digestion in the presence of food matrix. Food Chem. 2015, 175, 516–522. [Google Scholar] [CrossRef]
- Jurendić, T.; Ščetar, M. Aronia melanocarpa products and by-products for health and nutrition: A review. Antixidants 2021, 10, 1052. [Google Scholar] [CrossRef] [PubMed]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food—An international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef] [PubMed]


| Phenolic Compounds | Before Digestion | Gastric Digestion | Gastric with β-Glucan | Intestinal Digestion | Intestinal with β-Glucan | ||||
|---|---|---|---|---|---|---|---|---|---|
| Phenolic acids | |||||||||
| neochlorogenic acid | 1128.9 ±80.3 a | 970.3 | ±0.7 ab | 934.0 | ±0.6 b | 724.7 | ±6.3 c | 699.5 | ±4.5 c |
| chlorogenic acid | 910.8 ±68.3 a | 565.5 | ±0.2 b | 551.7 | ±0.7 b | 487.0 | ±5.9 b | 459.8 | ±7.8 b |
| cryptochlorogenic acid | 353.1 | ±2.2 a | 339.9 | ±1.0 b | |||||
| Total | 2039.7 ±148.5 a | 1535.8 | ±0.9 b | 1485.8 | ±1.3 b | 1564.8 | ±14.3 b | 1499.3 | ±13.2 b |
| Flavonols | |||||||||
| unknown | 48.9 ±4.3 a | 13.8 | ±0.0 c | 14.1 | ±0.0 c | 24.6 | ±0.1 b | 23.1 | ±0.1 bc |
| quercetin-3-rutinoside | 63.8 ±1.6 a | 28.3 | ±0.0 c | 28.4 | ±0.0 c | 32.7 | ±0.4 b | 25.4 | ±0.2 c |
| quercetin-3-galactoside | 105.1 ±1.6 a | 21.7 | ±0.0 c | 23.2 | ±0.0 c | 31.1 | ±0.4 b | 20.6 | ±0.2 c |
| quercetin-3-glucoside | 101.5 ±1.3 a | 23.1 | ±0.1 c | 22.4 | ±0.0 c | 32.9 | ±0.4 b | 25.2 | ±0.0 c |
| Total | 319.3 ±8.7 a | 86.9 | ±0.1 c | 88.0 | ±0.1 c | 121.3 | ±1.2 b | 94.2 | ±0.5 c |
| Anthocyanins | |||||||||
| cyanidin-3-galactoside | 225.9 ±18.4 a | 69.1 | ±0.1 b | 80.9 | ±0.1 b | 19.1 | ±0.5 c | 15.9 | ±0.1 c |
| cyanidin-3-glucoside | 54.9 ±3.0 a | 19.4 | ±0.9 b | 16.6 | ±0.0 b | 0.6 | ±0.1 c | 0.3 | ±0.1 c |
| cyanidin-3-arabinoside * | 198.0 ±17.8 a | 54.2 | ±2.8 b | 56.7 | ±0.1 b | 3.4 | ±0.5 c | 0.8 | ±0.1 c |
| cyanidin-3-xyloside * | 43.9 ±3.3 a | 13.1 | ±0.0 b | 11.6 | ±0.1 b | 0.4 | ±0.4 c | 0.0 | ±0.0 c |
| Total | 522.7 ±42.5 a | 155.8 | ±3.9 b | 165.8 | ±0.2 b | 23.6 | ±1.5 c | 17.1 | ±0.2 c |
| Flavan-3-ols | |||||||||
| (−)-epicatechin | 108.6 ±8.7 a | 21.6 | ±1.3 b | 21.3 | ±1.3 b | 28.5 | ±0.5 b | 26.6 | ±0.4 b |
| Total | 108.6 ±8.7 a | 21.6 | ±1.3 b | 21.3 | ±1.3 b | 28.5 | ±0.5 b | 26.6 | ±0.4 b |
| TOTAL | 2990.3 ±208.4 a | 1800.2 | ±6.2 b | 1760.9 | ±3.0 bc | 1738.2 | ±17.6 bc | 1637.2 | ±14.4 c |
| Antiradical Activity | Enzyme Inhibition | |||||
|---|---|---|---|---|---|---|
| EC50 | IC50 (α-Amylase) | IC50 (α-Glucosidase) | ||||
| (μmol Polyphenols) | (μmol of Polyphenols) | (μmol of Polyphenols) | ||||
| Gastric | 0.0594 | ±0.0040 bA | ||||
| gastric + β-glucan | 0.0695 | ±0.0003 aA | ||||
| Intestinal | 0.0169 | ±0.0002 cA | 0.0378 | ±0.0014 a | 0.0177 | ±0.0002 bA |
| intestinal + β-glucan | 0.0165 | ±0.0014 cA | 0.0415 | ±0.0027 a | 0.0225 | ±0.0009 bA |
| Acarbose | 0.3690 | ±0.0133 a | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jakobek, L.; Strelec, I.; Matić, P. The Influence of Dietary Fiber (β-Glucan) on the Beneficial Effects of Phenolic Compounds from Chokeberry After Simulated Digestion In Vitro. Molecules 2025, 30, 3356. https://doi.org/10.3390/molecules30163356
Jakobek L, Strelec I, Matić P. The Influence of Dietary Fiber (β-Glucan) on the Beneficial Effects of Phenolic Compounds from Chokeberry After Simulated Digestion In Vitro. Molecules. 2025; 30(16):3356. https://doi.org/10.3390/molecules30163356
Chicago/Turabian StyleJakobek, Lidija, Ivica Strelec, and Petra Matić. 2025. "The Influence of Dietary Fiber (β-Glucan) on the Beneficial Effects of Phenolic Compounds from Chokeberry After Simulated Digestion In Vitro" Molecules 30, no. 16: 3356. https://doi.org/10.3390/molecules30163356
APA StyleJakobek, L., Strelec, I., & Matić, P. (2025). The Influence of Dietary Fiber (β-Glucan) on the Beneficial Effects of Phenolic Compounds from Chokeberry After Simulated Digestion In Vitro. Molecules, 30(16), 3356. https://doi.org/10.3390/molecules30163356








