Evaluation of the Cytotoxic Activity of Nanostructured Lipid Carrier Systems for Fatty Acid Amides and Silk Fibroins in Breast Cancer Cell Lines
Abstract
1. Introduction
2. Results
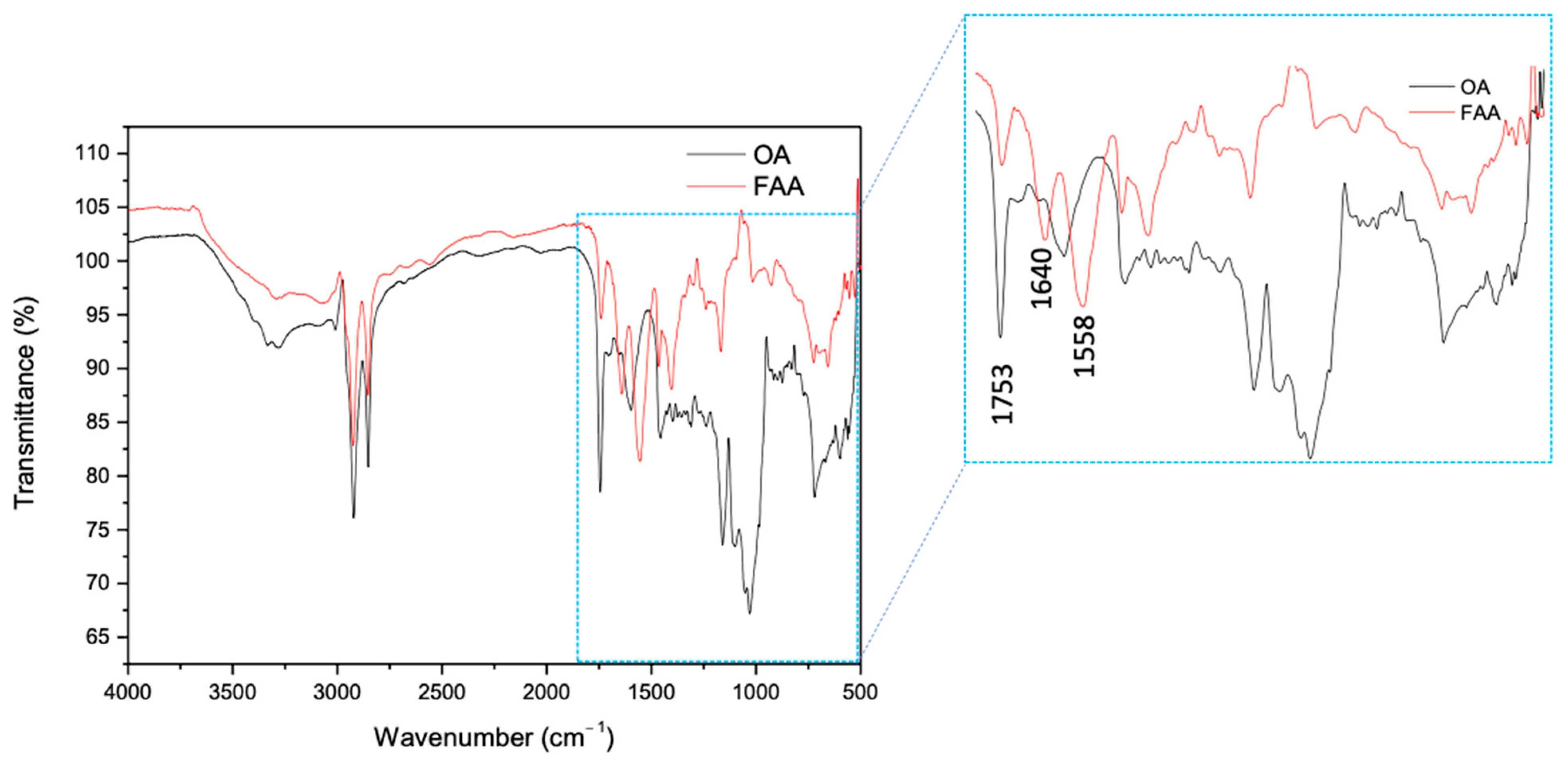
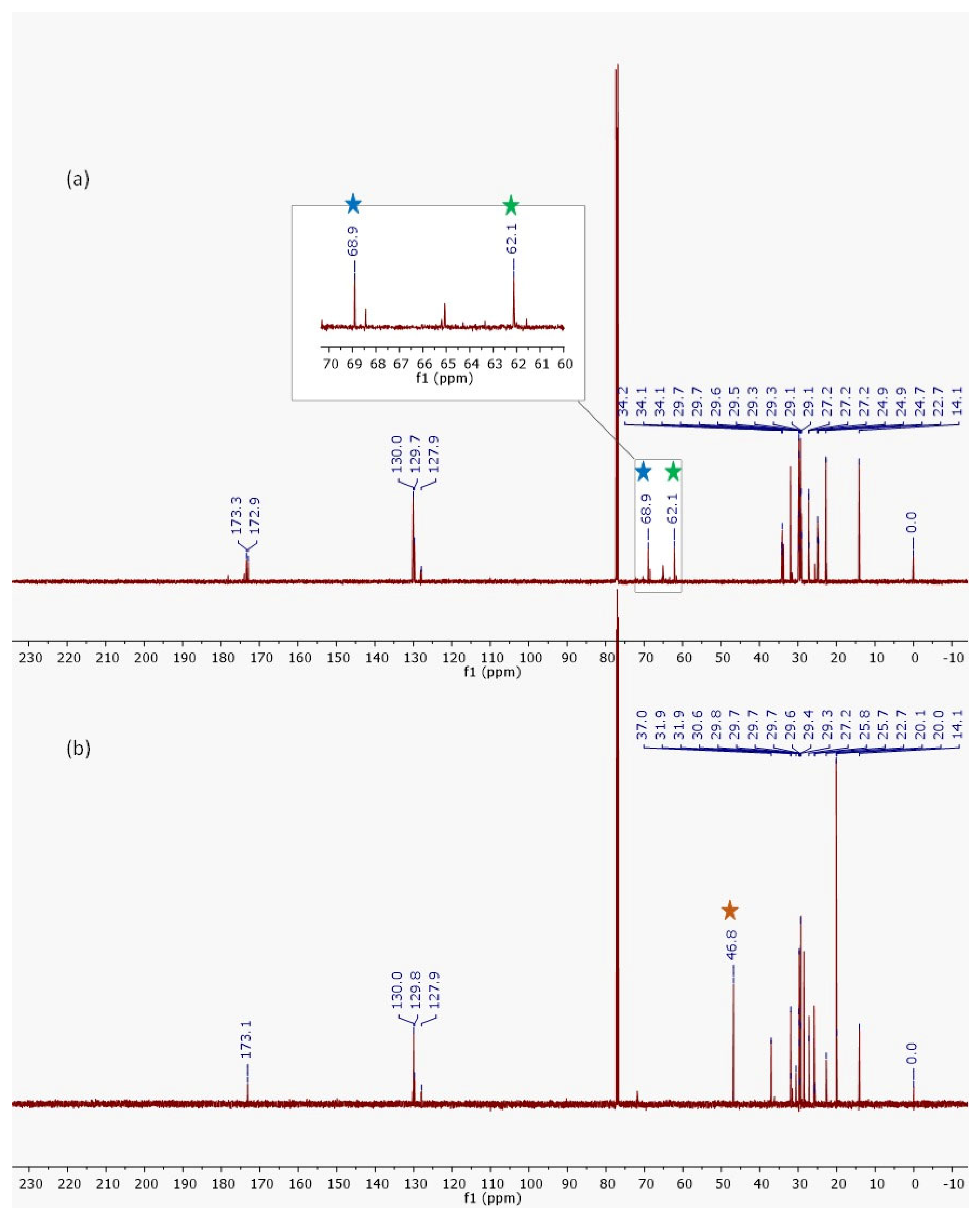
2.1. Evaluation of Semi-Synthesis and Spectroscopic Characterizations
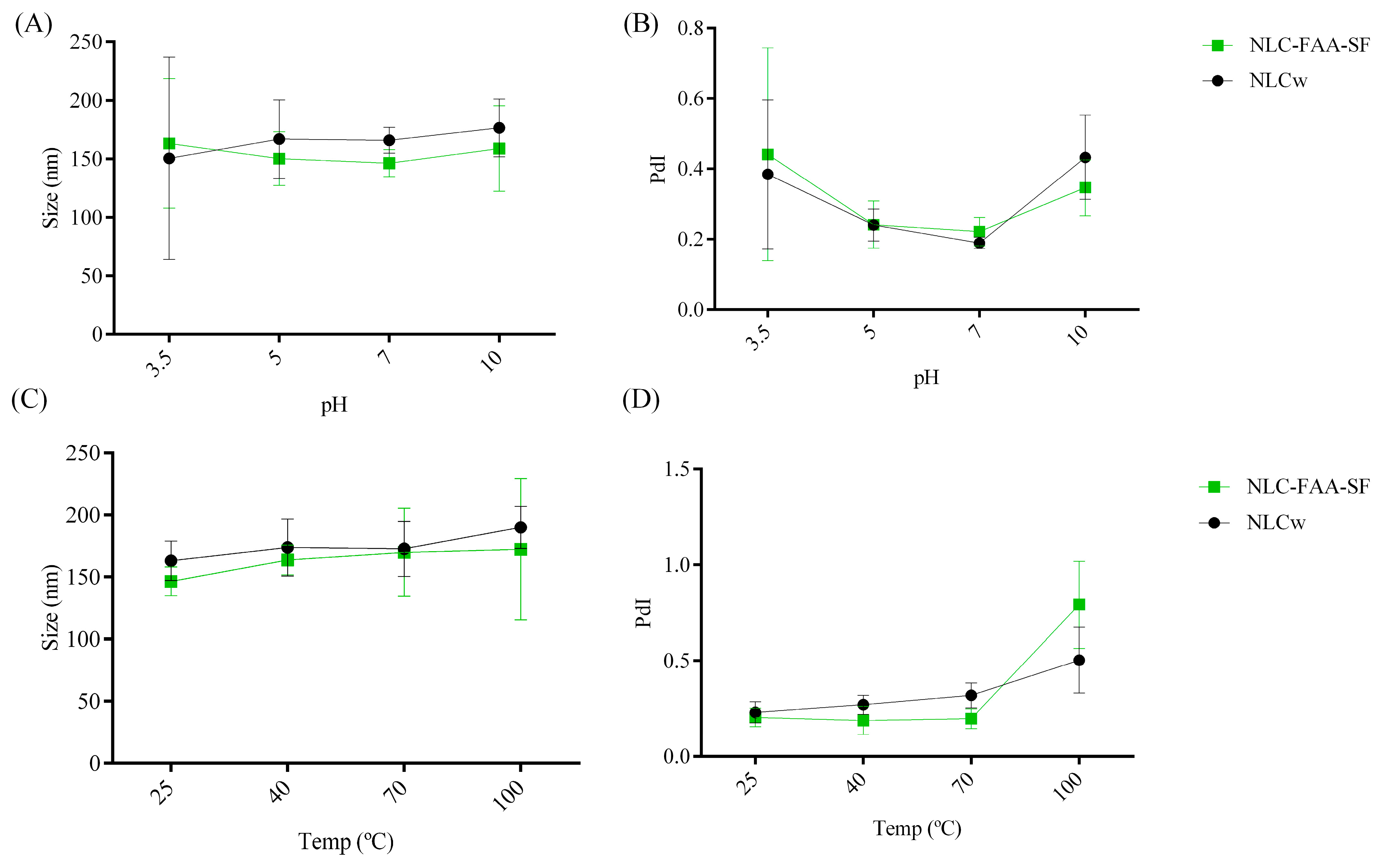
2.2. Characterization and Evaluation of the Physicochemical Stability of NLCw and NLC-FAA/SF
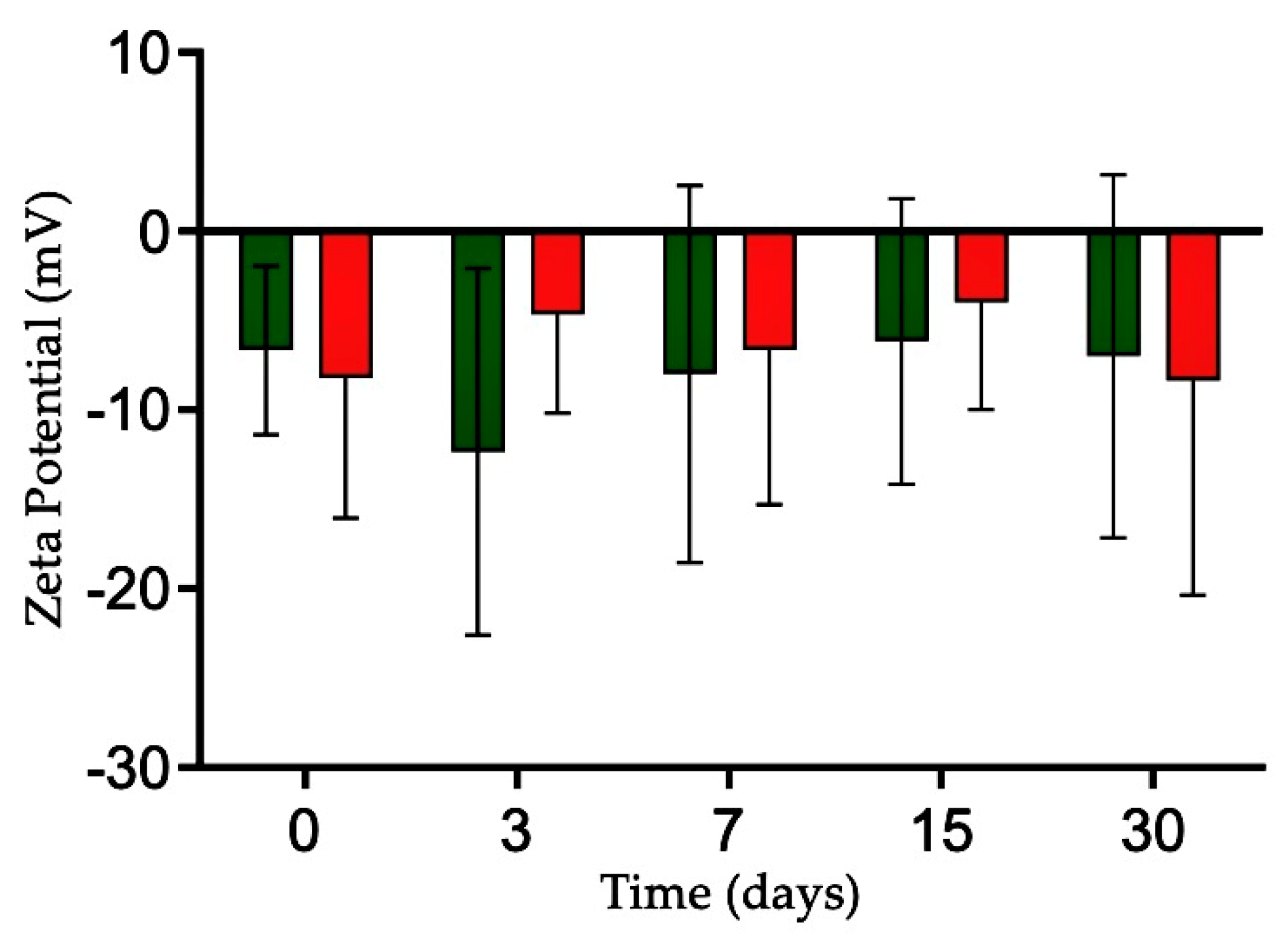
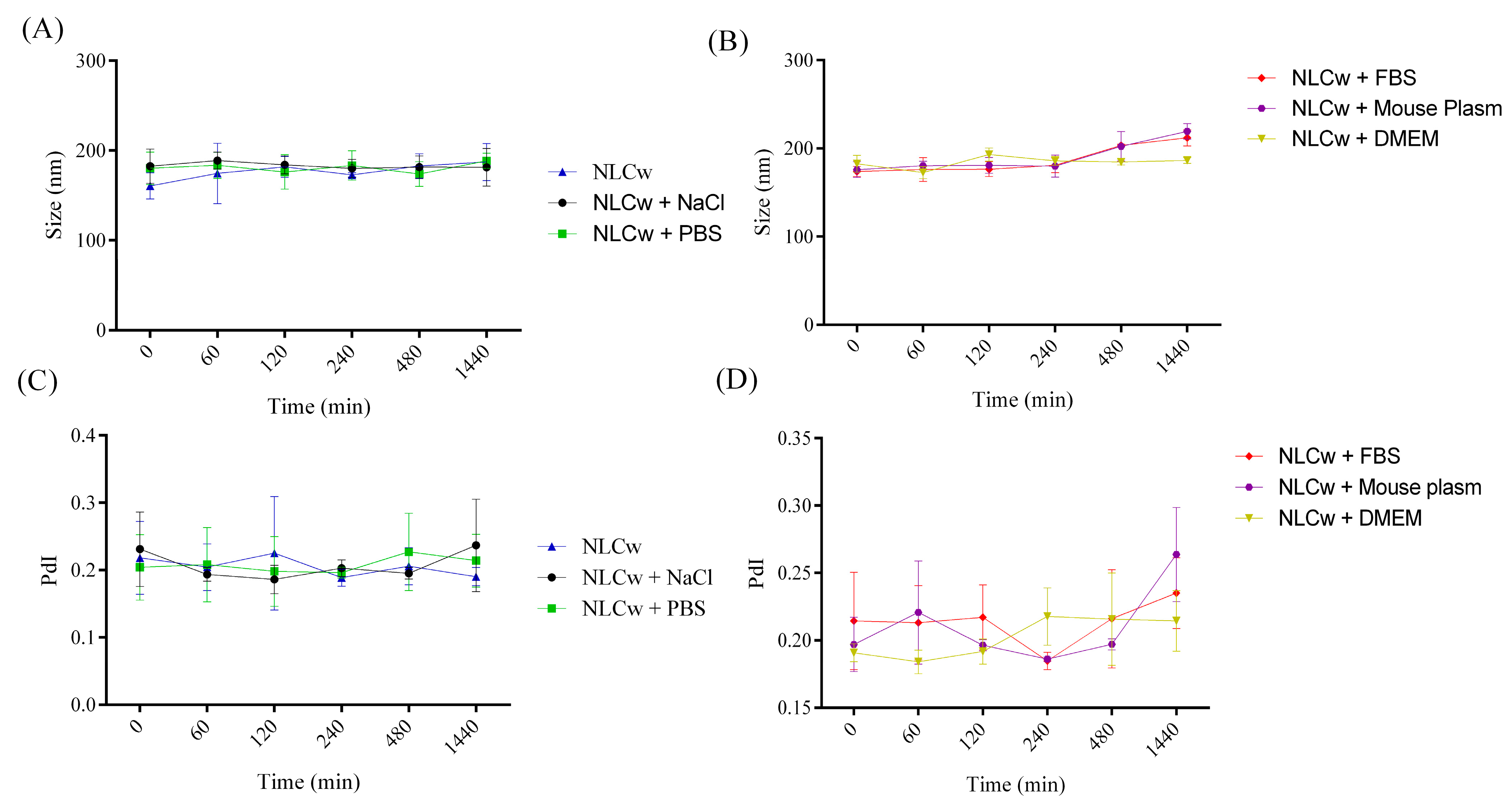
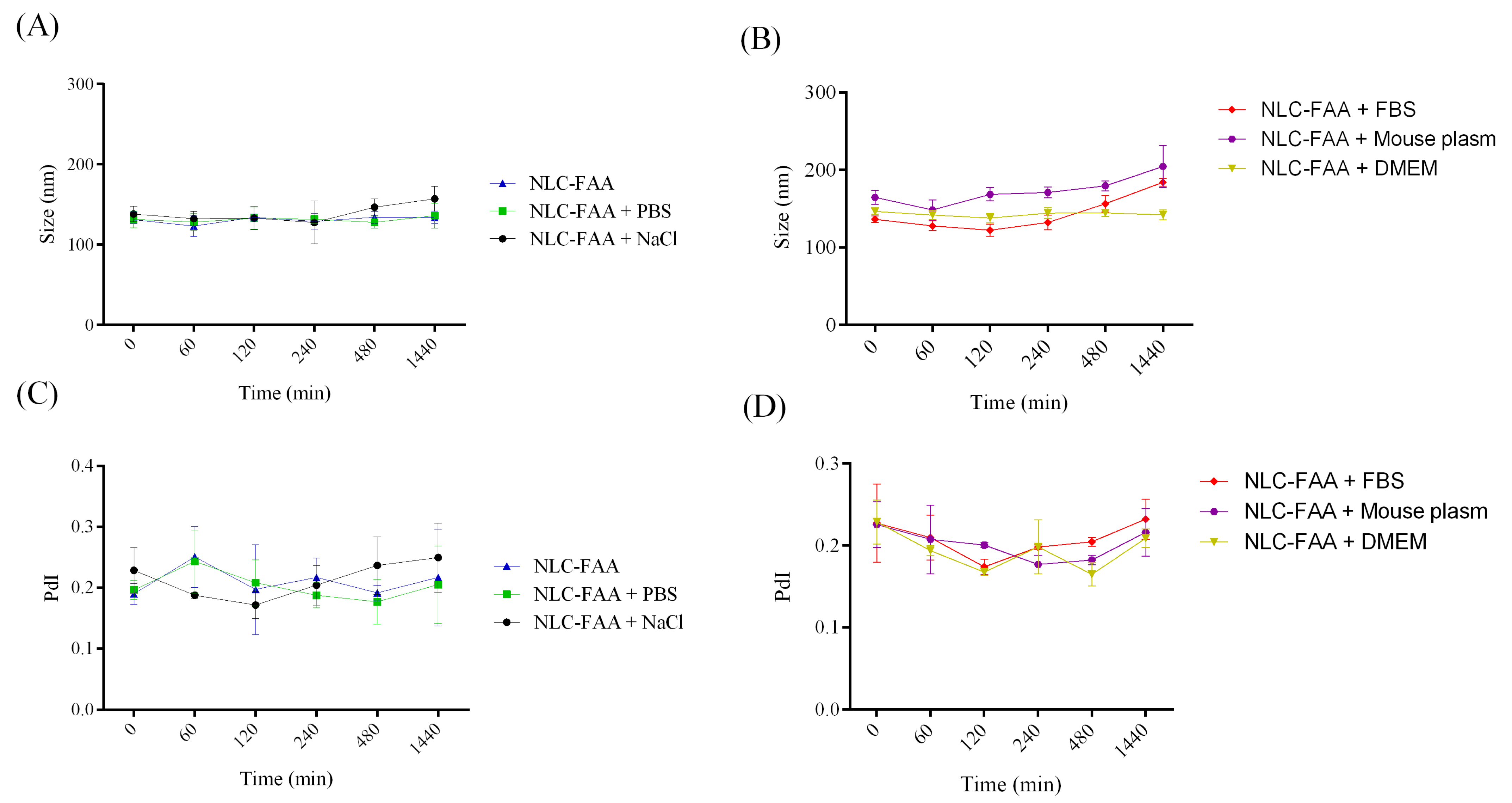
2.3. Evaluation of Colloidal Stability in Biological Media
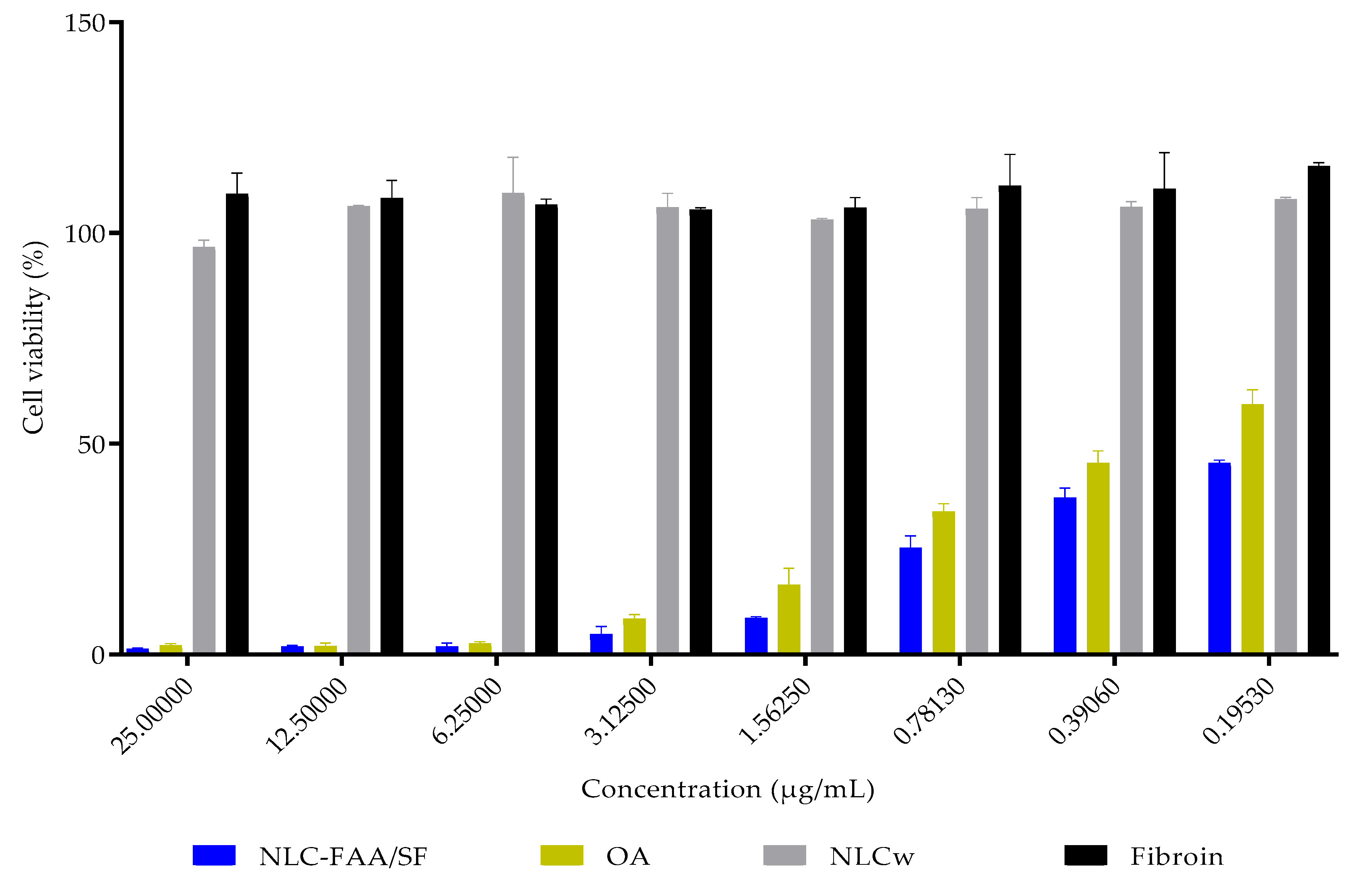
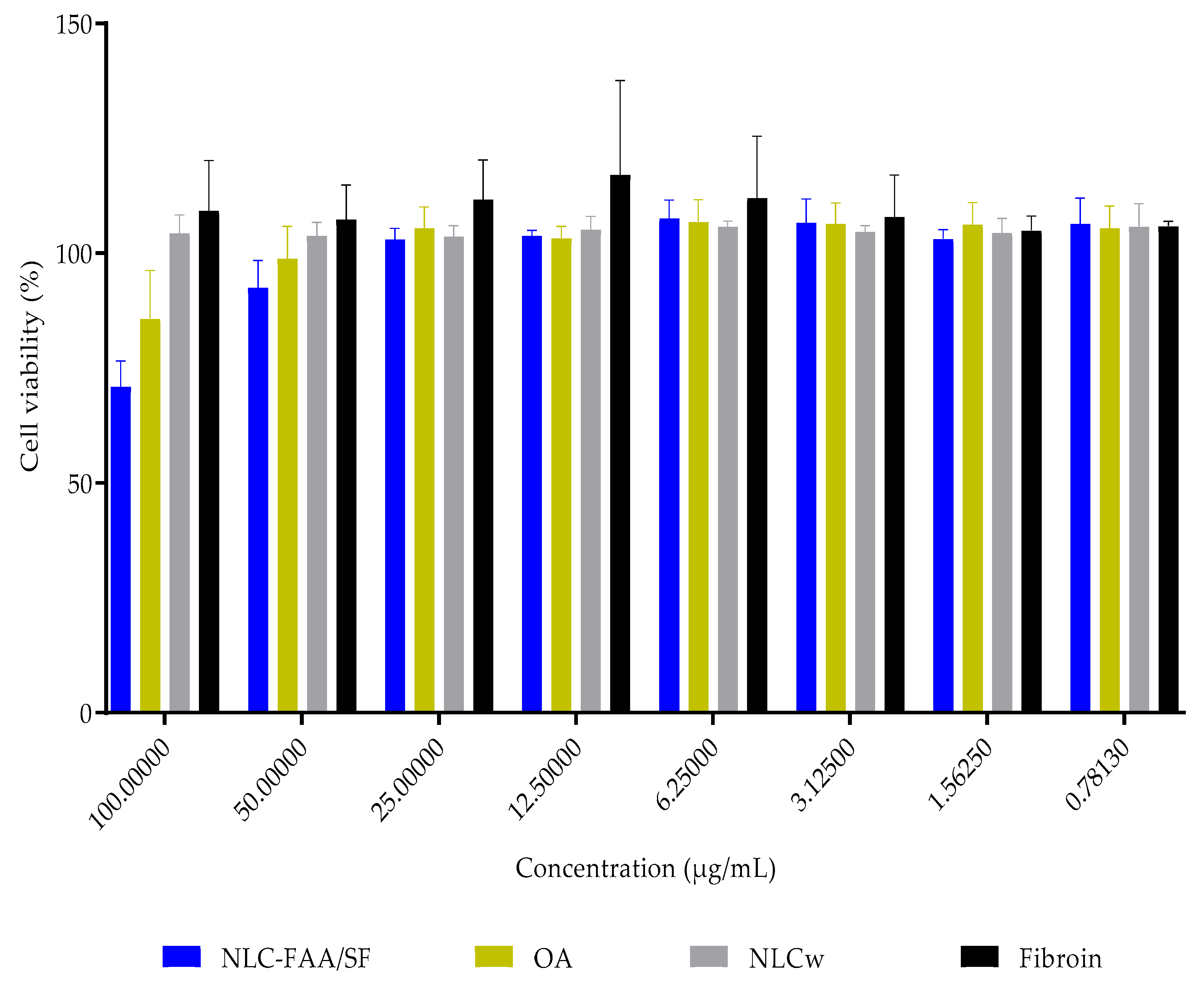
2.4. In Vitro Cytotoxicity
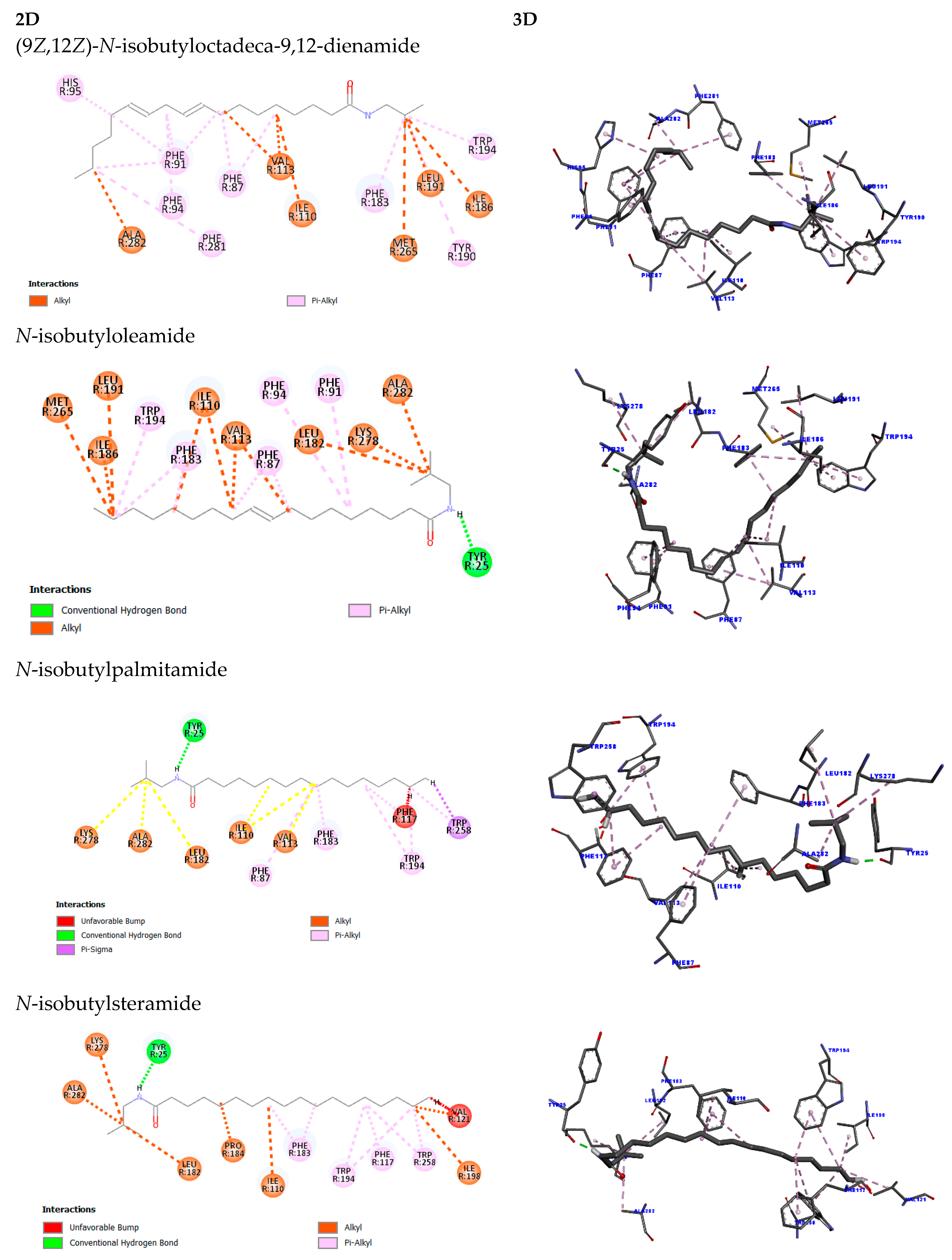
2.5. Molecular Docking
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Direct Amidation Reaction to Obtain Fatty Amides
4.3. Silk Fibroin (SF) Solution
4.4. Preparation of the Nanostructured Lipid Carrier Containing FAA and SF (NLC-FAA/SF)
4.5. Gas Chromatography with Mass Spectrometry Detection (GC-MS)
4.6. Fourier Transform Infrared Analysis (FTIR)
4.7. Nuclear Magnetic Resonance (13C NMR)
4.8. Particle Size, Polydispersity Index (PdI), Zeta Potential, and pH
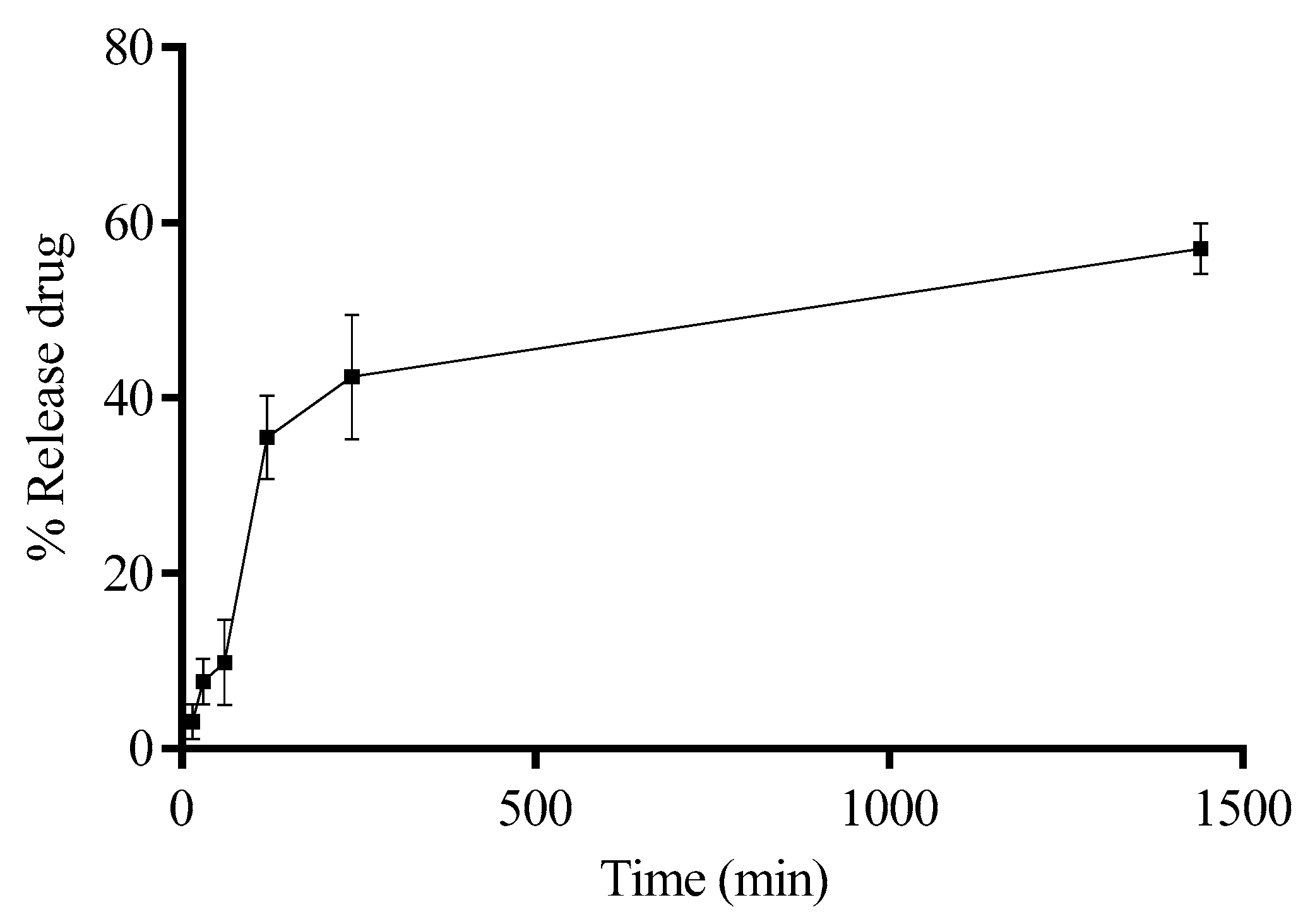
4.9. Drug Release
4.10. Colloidal Stability
4.11. Cell Culture
4.12. Cell Viability
4.13. Obtaining Molecular Docking
4.14. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Brown, J.S.; Amend, S.R.; Austin, R.H.; Gatenby, R.A.; Hammarlund, E.U.; Pienta, K.J. Updating the Definition of Cancer. Mol. Cancer Res. 2023, 21, 1142–1147. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Arnold, M.; Morgan, E.; Rumgay, H.; Mafra, A.; Singh, D.; Laversanne, M.; Vignat, J.; Gralow, J.R.; Cardoso, F.; Siesling, S.; et al. Current and future burden of breast cancer: Global statistics for 2020 and 2040. Breast 2022, 66, 15–23. [Google Scholar] [CrossRef]
- Xu, Y.; Gong, M.; Wang, Y.; Yang, Y.; Liu, S.; Zeng, Q. Global trends and forecasts of breast cancer incidence and deaths. Sci. Data 2023, 10, 334. [Google Scholar] [CrossRef] [PubMed]
- de Bessa, F.J. Breast imaging hindered during COVID-19 pandemic, in Brazil. Rev. Saude Publica 2021, 55, 8. [Google Scholar] [CrossRef] [PubMed]
- Kefayat, A.; Ghahremani, F.; Safavi, A.; Hajiaghababa, A.; Moshtaghian, J. Spirulina extract enriched for Braun-type lipoprotein (Immulina®) for inhibition of 4T1 breast tumors growth and metastasis. Phytother. Res. 2020, 34, 368–378. [Google Scholar] [CrossRef] [PubMed]
- Lopes, C.R. Synthesis of new fatty acids amides from aminolysis of fames. Quím. Nova 2010, 33, 1335–1341. [Google Scholar] [CrossRef]
- Araújo, P.H.F.; Barata, P.H.D.S.; Araújo, I.F.; Curti, J.M.; Amaral, R.R.; Bereau, D.; Carvalho, J.C.T.; Ferreira, I.M. Direct and Solvent-Free Aminolysis of Triglyceride from Oenocarpus bataua (Patawa) Oil Catalyzed by Al2O3. Catal. Lett. 2018, 148, 843–851. [Google Scholar] [CrossRef]
- D’Oca, C.D.R.M.; Coelho, T.; Marinho, T.G.; Hack, C.R.L.; da Costa Duarte, R.; da Silva, P.A.; D’Oca, M.G.M. Synthesis and antituberculosis activity of new fatty acid amides. Bioorg. Med. Chem. Lett. 2010, 20, 5255–5257. [Google Scholar] [CrossRef]
- Mata-Santos, T.; D’Oca, C.D.R.M.; Mata-Santos, H.A.; Fenalti, J.; Pinto, N.; Coelho, T.; Berne, M.E.; da Silva, P.E.A.; D’Oca, M.G.M.; Scaini, C.J. Toxocara canis: Larvicidal activity of fatty acid amides. Bioorg. Med. Chem. Lett. 2016, 26, 739–741. [Google Scholar] [CrossRef]
- Quitian-Useche, Y.F.; Sánchez-Ortiz, B.L.; Borges, S.F.; Ramos, B.; de Souza, G.C.; Batista, M.A.; da Silva Hage Melim, L.I.; Ferreira, I.M.; Carvalho, J.C.T.; Borges, R.S. Fatty ethanolamide of Bertholletia excelsa triglycerides (Brazil nuts): Anti-inflammatory action and acute toxicity evaluation in Zebrafish (Danio rerio). Inflammopharmacology 2021, 29, 1519–1537. [Google Scholar] [CrossRef]
- Çetin, İ.; Topçul, M. Investigation of the Effects of the Endogenous Cannabinoid Anandamide on Luminal A Breast Cancer Cell Line MCF-7. Cell. Mol. Biol. 2022, 68, 129–133. [Google Scholar] [CrossRef]
- Di Marzo, V.; Melck, D.; Orlando, P.; Bisogno, T.; Zagoory, O.; Bifulco, M.; Vogel, Z.; de Petrocellis, L. Palmitoylethanolamide inhibits the expression of fatty acid amide hydrolase and enhances the anti-proliferative effect of anandamide in human breast cancer cells. Biochem. J. 2001, 358, 249. [Google Scholar] [CrossRef]
- Rai, N.; Gupta, P.; Verma, A.; Singh, S.K.; Gautam, V. Isolation and characterization of N-(2-Hydroxyethyl)hexadecanamide from Colletotrichum gloeosporioides with apoptosis-inducing potential in breast cancer cells. BioFactors 2023, 49, 663–683. [Google Scholar] [CrossRef]
- Morales, P.; Jagerovic, N. Antitumor Cannabinoid Chemotypes: Structural Insights. Front. Pharmacol. 2019, 10, 621. [Google Scholar] [CrossRef]
- Schwarz, R.; Ramer, R.; Hinz, B. Targeting the endocannabinoid system as a potential anticancer approach. Drug Metab. Rev. 2018, 50, 26–53. [Google Scholar] [CrossRef] [PubMed]
- Grosso, A.F. Cannabis: From plant condemned by prejudice to one of the greatest therapeutic options of the century. J. Hum. Growth Dev. 2020, 30, 94–97. [Google Scholar] [CrossRef]
- Barata, P.; Sarquis, Í.R.; Carvalho, H.O.; Barros, A.S.; Rodrigues, A.B.; Galue-Parra, A.J.; Silva, E.O.; Carvalho, J.C.T.; Ferreira, I.M. Chemoenzymatic Synthesis and Anti-Inflammatory Activity of Fatty Acid Amides Prepared from Bertholletia excelsa (Brazil Nut) Triglycerides. J. Braz. Chem. Soc. 2020, 31, 1557–1565. [Google Scholar] [CrossRef]
- Lessa, M.A.; Cavalcanti, I.L.; Figueiredo, N.V. Cannabinoid derivatives and the pharmacological management of pain. Rev. Dor 2016, 17, 47–51. [Google Scholar] [CrossRef]
- De Moura, P.R.; Augusto, F.; Vidal, P. Signal transduction: A review about G protein. Sci. Medica 2011, 21, 31–36. [Google Scholar]
- Kleuss, C. Type III Adenylate Cyclase. In xPharm: The Comprehensive Pharmacology Reference; Elsevier: Amsterdam, The Netherlands, 2007; pp. 1–4. [Google Scholar] [CrossRef]
- Santos, D.B.D.; Lemos, J.A.; Miranda, S.E.; Di Filippo, L.D.; Duarte, J.L.; Ferreira, L.A.; Barros, A.L.; Oliveira, A.E. Current Applications of Plant-Based Drug Delivery Nano Systems for Leishmaniasis Treatment. Pharmaceutics 2022, 14, 2339. [Google Scholar] [CrossRef]
- Cola, D.; Pasquoto, T.; Guilger, M.; Lima, R.; Silva, C.; Fraceto, L. Lipid nanostructured carriers systems for ivermectin and methoprene aiming parasite control. Quim. Nova 2016, 39, 1034–1043. [Google Scholar] [CrossRef]
- Zhu, S.; Li, X.; Lansakara-P, D.S.P.; Kumar, A.; Cui, Z. A nanoparticle depot formulation of 4-(N)-stearoyl gemcitabine shows a strong anti-tumour activity. J. Pharm. Pharmacol. 2013, 65, 236–242. [Google Scholar] [CrossRef]
- Holanda, F.H.; Pereira, R.R.; Marinho, V.H.S.; Jimenez, D.E.; Ferreira, L.M.C.; Ribeiro-Costa, R.M.; de Sousa, F.F.O.; Ferreira, I.M. Development of nanostructured formulation from naringenin and silk fibroin and application for inhibition of lipoxygenase (LOX). RSC Adv. 2023, 13, 23063–23075. [Google Scholar] [CrossRef]
- Queiroz-Souza, P.; Galue-Parra, A.; Silveira Moraes, L.; Macedo, C.G.; Rodrigues, A.P.D.; Marinho, V.H.S.; Holanda, F.H.; Ferreira, I.M.; Oliveira da Silva, E. Polymeric nanoparticles containing kojic acid induce structural alterations and apoptosis-like death in Leishmania (Leishmania) amazonensis. Front. Pharmacol. 2024, 15, 1331240. [Google Scholar] [CrossRef]
- de Oliveira, F.R.; Eleuterio Rodrigues, K.; Hamoy, M.; Rodrigues Sarquis, Í.; Otake Hamoy, A.; Elena Crespo Lopez, M.; Maciel Ferreira, I.; de Matos Macchi, B.; Luiz Martins do Nascimento, J. Fatty Acid Amides Synthesized from Andiroba Oil (Carapa guianensis Aublet.) Exhibit Anticonvulsant Action with Modulation on GABA-A Receptor in Mice: A Putative Therapeutic Option. Pharmaceuticals 2020, 13, 43. [Google Scholar] [CrossRef]
- Threlfall, T. The infrared spectra of amides. Part 1. The stretching vibrations of primary carboxamides. Vib. Spectrosc. 2022, 121, 103386. [Google Scholar] [CrossRef]
- Bigaj-Józefowska, M.J.; Grześkowiak, B.F. Polymeric nanoparticles wrapped in biological membranes for targeted anticancer treatment. Eur. Polym. J. 2022, 176, 111427. [Google Scholar] [CrossRef]
- da Ribeiro, C.A.; da Costa, M.; Santos, C.R.D.; Bonadio, T.G.M.; Tominaga, T.T. Obtaintion of magnetic nanoparticles using everyday materials: Synthesis, characterization and didactic approach for high school. Rev. Bras. Ensino Física. 2021, 43, e20200374. [Google Scholar] [CrossRef]
- Safwat, S.; Ishak, R.A.H.; Hathout, R.M.; Mortada, N.D. Nanostructured lipid carriers loaded with simvastatin: Effect of PEG/glycerides on characterization, stability, cellular uptake efficiency and in vitro cytotoxicity. Drug Dev. Ind. Pharm. 2017, 43, 1112–1125. [Google Scholar] [CrossRef] [PubMed]
- Echeverría, J.; Albuquerque, R. Nanoemulsions of Essential Oils: New Tool for Control of Vector-Borne Diseases and In Vitro Effects on Some Parasitic Agents. Medicines 2019, 6, 42. [Google Scholar] [CrossRef]
- Ceylani, T.; Teker, H.T.; Samgane, G.; Gurbanov, R. Intermittent fasting-induced biomolecular modifications in rat tissues detected by ATR-FTIR spectroscopy and machine learning algorithms. Anal. Biochem. 2022, 654, 114825. [Google Scholar] [CrossRef] [PubMed]
- Stier, H.; Schlosshauer, B. Axonal guidance in the chicken retina. Development 1995, 121, 1443–1454. [Google Scholar] [CrossRef]
- Mercier-Letondal, P.; Marton, C.; Godet, Y.; Galaine, J. Validation of a method evaluating T cell metabolic potential in compliance with ICH Q2 (R1). J. Transl. Med. 2021, 19, 21. [Google Scholar] [CrossRef]
- Zhu, Y.-A.; Deng, Y.; Sun, P.; Cao, Y.; Kong, B.; Liu, Q.; Wang, H.; Chen, Q. Physicochemical and digestive properties of curcumin stabilized by quaternized chitosan/sodium alginate nanoemulsion coatings. Food Chem. 2025, 464, 141551. [Google Scholar] [CrossRef] [PubMed]
- Maitz, M.F.; Sperling, C.; Wongpinyochit, T.; Herklotz, M.; Werner, C.; Seib, F.P. Biocompatibility assessment of silk nanoparticles: Hemocompatibility and internalization by human blood cells. Nanomedicine 2017, 13, 2633–2642. [Google Scholar] [CrossRef] [PubMed]
- Pham, D.T.; Tiyaboonchai, W. Fibroin nanoparticles: A promising drug delivery system. Drug Deliv. 2020, 27, 431–448. [Google Scholar] [CrossRef]
- van der Lubbe, S.C.C.; Fonseca, C. The Nature of Hydrogen Bonds: A Delineation of the Role of Different Energy Components on Hydrogen Bond Strengths and Lengths. Chem. Asian J. 2019, 14, 2760–2769. [Google Scholar] [CrossRef]
- Hua, T.; Vemuri, K.; Nikas, S.P.; Laprairie, R.B.; Wu, Y.; Qu, L.; Pu, M.; Korde, A.; Jiang, S.; Ho, J.-H.; et al. Crystal structures of agonist-bound human cannabinoid receptor CB1. Nature 2017, 547, 468–471. [Google Scholar] [CrossRef]
- Pawar, H.D.; Mahajan, U.B.; Nakhate, K.T.; Agrawal, Y.O.; Patil, C.R.; Meeran, M.N.; Sharma, C.; Ojha, S.; Goyal, S.N. Curcumin Protects Diabetic Mice against Isoproterenol-Induced Myocardial Infarction by Modulating CB2 Cannabinoid Receptors. Life 2022, 12, 624. [Google Scholar] [CrossRef]
- Omar, A.M.; Aljahdali, A.S.; Safo, M.K.; Mohamed, G.A.; Ibrahim, S.R.M. Docking and Molecular Dynamic Investigations of Phenylspirodrimanes as Cannabinoid Receptor-2 Agonists. Molecules 2022, 28, 44. [Google Scholar] [CrossRef]
- Aviz-Amador, A.; Contreras-Puentes, N.; Mercado-Camargo, J. Virtual screening using docking and molecular dynamics of cannabinoid analogs against CB1 and CB2 receptors. Comput. Biol. Chem. 2021, 95, 107590. [Google Scholar] [CrossRef]
- Godase, S.S.; Kulkarni, N.S.; Dhole, S.N. A comprehensive review on Novel Lipid- Based nano drug delivery. Adv. Pharm. Bull. 2023, 14, 34. [Google Scholar] [CrossRef]
- Khosa, A.; Reddi, S.; Saha, R.N. Nanostructured lipid carriers for site-specific drug delivery. Biomed. Pharmacother. 2018, 103, 598–613. [Google Scholar] [CrossRef]
- Liu, K.; Chen, Y.-Y.; Pan, L.-H.; Li, Q.-M.; Luo, J.-P.; Zha, X.-Q. Co-encapsulation systems for delivery of bioactive ingredients. Food Res. Int. 2022, 155, 111073. [Google Scholar] [CrossRef]
- Kumar, D.; Ali, A. Direct synthesis of fatty acid alkanolamides and fatty acid alkyl esters from high free fatty acid containing triglycerides as lubricity improvers using heterogeneous catalyst. Fuel 2015, 159, 845–853. [Google Scholar] [CrossRef]
- Citoler, J.; Finnigan, W.; Bevinakatti, H.; Turner, N.J. Direct Enzymatic Synthesis of Fatty Amines from Renewable Triglycerides and Oils. ChemBioChem 2022, 23, e202100578. [Google Scholar] [CrossRef] [PubMed]
- Jamil, M.A.R.; Siddiki, S.H.; Touchy, A.S.; Rashed, M.N.; Poly, S.S.; Jing, Y.; Ting, K.W.; Toyao, T.; Maeno, Z.; Shimizu, K.I. Selective Transformations of Triglycerides into Fatty Amines, Amides, and Nitriles by using Heterogeneous Catalysis. ChemSusChem 2019, 12, 3115–3125. [Google Scholar] [CrossRef] [PubMed]
- Shinoda, K.; Arai, H. The Correlation between Phase Inversion Temperature In Emulsion and Cloud Point in Solution of Nonionic Emulsifier. J. Phys. Chem. 1964, 68, 3485–3490. [Google Scholar] [CrossRef]
- McClements, D.J.; Li, Y. Structured emulsion-based delivery systems: Controlling the digestion and release of lipophilic food components. Adv. Colloid Interface Sci. 2010, 159, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Cong, Y.; Wang, B.; Zhang, N. Applications of Fourier transform infrared spectroscopy to pharmaceutical preparations. Expert Opin. Drug Deliv. 2020, 17, 551–571. [Google Scholar] [CrossRef]
- Sarquis, I.R.; Sarquis, R.S.; Marinho, V.H.; Neves, F.B.; Araújo, I.F.; Damasceno, L.F.; Ferreira, R.M.; Souto, R.N.; Carvalho, J.C.; Ferreira, I.M. Carapa guianensis Aubl. (Meliaceae) oil associated with silk fibroin, as alternative to traditional surfactants, and active against larvae of the vector Aedes aegypti. Ind. Crops Prod. 2020, 157, 112931. [Google Scholar] [CrossRef]
- Marinho, V.H.S.; Holanda, F.H.; Araújo, I.F.; Jimenez, D.E.; Pereira, R.R.; Porto, A.L.; Ferreira, A.M.; Carvalho, J.C.; de Freitas, A.C.A.; Fernandes, C.P.; et al. Nanoparticles from silk fibroin and Amazon oils: Potential larvicidal activity and oviposition deterrence against Aedes aegypti. Ind. Crops Prod. 2023, 203, 117133. [Google Scholar] [CrossRef]
- Ramalingam, P.; Yoo, S.W.; Ko, Y.T. Nanodelivery systems based on mucoadhesive polymer coated solid lipid nanoparticles to improve the oral intake of food curcumin. Food Res. Int. 2016, 84, 113–119. [Google Scholar] [CrossRef]
- Helgason, T.; Awad, T.S.; Kristbergsson, K.; McClements, D.J.; Weiss, J. Effect of surfactant surface coverage on formation of solid lipid nanoparticles (SLN). J. Colloid Interface Sci. 2009, 334, 75–81. [Google Scholar] [CrossRef]
- Marinho, V.H.S.; Neves, F.B.; Jimenez, D.E.; Oliveira, F.R.; Santos, A.V.T.; Ferreira, R.M.; Souto, R.N.; Carvalho, J.C.; Yoshioka, S.A.; Ferreira, I.M. Development of an environmentally friendly formulation of silk fibroin combined with fatty acid from Astrocaryum murumuru Mart. effective against Aedes aegypti larvae. J. Drug Deliv. Sci. Technol. 2022, 75, 103626. [Google Scholar] [CrossRef]
- Shi, X.; Cao, Y.; Li, N.; Zhu, N.; Chen, Y.; Ma, B. Composition, physicochemical properties, preparation methods and application research status on Functional oils and fats of nanoemulsion: A comprehensive review. IOP Conf. Ser. Earth Environ. Sci. 2021, 792, 012021. [Google Scholar] [CrossRef]
- Wlastra, P. Encyclopedia of Emulsion Technology; Dekker, M., Ed.; CRC: Boca Raton, FL, USA, 1996; Volume 4. [Google Scholar]
- Song, D.; Forciniti, D. Effects of Cosolvents and pH on Protein Adsorption on Polystyrene Latex: A Dynamic Light Scattering Study. J. Colloid Interface Sci. 2000, 221, 25–37. [Google Scholar] [CrossRef]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, Y.M. Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef]
- McNeil, S.E. Nanotechnology for the biologist. J. Leukoc. Biol. 2005, 78, 585–594. [Google Scholar] [CrossRef]
- Chenthamara, D.; Subramaniam, S.; Ramakrishnan, S.G.; Krishnaswamy, S.; Essa, M.M.; Lin, F.H.; Qoronfleh, M.W. Therapeutic efficacy of nanoparticles and routes of administration. Biomater. Res. 2019, 23, 20. [Google Scholar] [CrossRef]
- Qu, W.; Ji, P.; Han, X.; Wang, X.; Li, Y.; Liu, J. Highly Biocompatible Apigenin-Loaded Silk Fibroin Nanospheres: Preparation, Characterization, and Anti-Breast-Cancer Activity. Polymers 2022, 15, 23. [Google Scholar] [CrossRef]
- Melo, M.A., Jr.; Santos, L.S.S.; Gonçalves, M.D.C.; Nogueira, A.F. Preparation of silver and gold nanoparticles: A simple method to introduce nanotechnology into teaching laboratories. Quim. Nova 2012, 35, 1872–1878. [Google Scholar] [CrossRef]
- de Carvalho, F.V.; Ribeiro, L.N.D.M.; Moura, L.D.D.; Rodrigues da Silva, G.H.; Mitsutake, H.; Mendonça, T.C.; Geronimo, G.; Breitkreitz, M.C.; de Paula, E. Docetaxel Loaded in Copaiba Oil-Nanostructured Lipid Carriers as a Promising DDS for Breast Cancer Treatment. Molecules 2022, 27, 8838. [Google Scholar] [CrossRef]
- Rizwanullah, M.; Ahmad, J.; Amin, S. Nanostructured Lipid Carriers: A Novel Platform for Chemotherapeutics. Curr. Drug Deliv. 2016, 13, 4–26. [Google Scholar] [CrossRef]
- Kanugo, A.; Gautam, R.K.; Kamal, M.A. Recent Advances of Nanotechnology in the Diagnosis and Therapy of Triple- Negative Breast Cancer (TNBC). Curr. Pharm. Biotechnol. 2022, 23, 1581–1595. [Google Scholar] [CrossRef]
- Subramaniam, B.; Arshad, N.M.; Malagobadan, S.; Misran, M.; Nyamathulla, S.; Mun, K.S.; Nagoor, N.H. Development and Evaluation of 1′-Acetoxychavicol Acetate (ACA)-Loaded Nanostructured Lipid Carriers for Prostate Cancer Therapy. Pharmaceutics 2021, 13, 439. [Google Scholar] [CrossRef] [PubMed]
- Miranda, S.E.M.; de Alcantara Lemos, J.; Ottoni, F.M.; Cassali, G.D.; Townsend, D.M.; de Aguiar Ferreira, C.; Alves, R.J.; Ferreira, L.A.M.; de Barros, A.L.B. Preclinical evaluation of L-fucoside from lapachol-loaded nanoemulsion as a strategy to breast cancer treatment. Biomed. Pharmacother. 2024, 170, 116054. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; He, Q. The interaction of nanoparticles with plasma proteins and the consequent influence on nanoparticles behavior. Expert Opin. Drug Deliv. 2014, 11, 409–420. [Google Scholar] [CrossRef]
- United States Pharmacopeial Convention. Globule Size Distribution in Lipid Injectable Emulsions—Usp43-Nf38; United States Pharmacopeial Convention: Rockville, MD, USA, 2020. [Google Scholar]
- Melck, D.; Rueda, D.; Galve-Roperh, I.; De Petrocellis, L.; Guzmán, M.; Di Marzo, V. Involvement of the cAMP/protein kinase A pathway and of mitogen-activated protein kinase in the anti-proliferative effects of anandamide in human breast cancer cells. FEBS Lett. 1999, 463, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Maccarrone, M.; Finazzi-Agró, A. The endocannabinoid system, anandamide and the regulation of mammalian cell apoptosis. Cell Death Differ. 2003, 10, 946–955. [Google Scholar] [CrossRef] [PubMed]
- Melck, D.; De Petrocellis, L.; Orlando, P.; Bisogno, T.; Laezza, C.; Bifulco, M.; Di Marzo, V. Suppression of Nerve Growth Factor Trk Receptors and Prolactin Receptors by Endocannabinoids Leads to Inhibition of Human Breast and Prostate Cancer Cell Proliferation. Endocrinology 2000, 141, 118–126. [Google Scholar] [CrossRef]
- Dobovišek, L.; Krstanović, F.; Borštnar, S.; Debeljak, N. Cannabinoids and Hormone Receptor-Positive Breast Cancer Treatment. Cancers 2020, 12, 525. [Google Scholar] [CrossRef]
- Terry, M.B.; Colditz, G.A. Epidemiology and Risk Factors for Breast Cancer: 21st Century Advances, Gaps to Address through Interdisciplinary Science. Cold Spring Harb. Perspect. Med. 2023, 13, a041317. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, J.D.B.; Morgan, E.; de Luna Aguilar, A.; Mafra, A.; Shah, R.; Giusti, F.; Vignat, J.; Znaor, A.; Musetti, C.; Yip, C.H.; et al. Global Stage Distribution of Breast Cancer at Diagnosis. JAMA Oncol. 2024, 10, 71. [Google Scholar] [CrossRef]
- Arroyo-Crespo, J.J.; Armiñán, A.; Charbonnier, D.; Deladriere, C.; Palomino-Schätzlein, M.; Lamas-Domingo, R.; Forteza, J.; Pineda-Lucena, A.; Vicent, M.J. Characterization of triple-negative breast cancer preclinical models provides functional evidence of metastatic progression. Int. J. Cancer 2019, 145, 2267–2281. [Google Scholar] [CrossRef]
- Karim, A.M.; Kwon, J.E.; Ali, T.; Jang, J.; Ullah, I.; Lee, Y.G.; Park, D.W.; Park, J.; Jeang, J.W.; Kang, S.C. Triple-negative breast cancer: Epidemiology, molecular mechanisms, and modern vaccine-based treatment strategies. Biochem. Pharmacol. 2023, 212, 115545. [Google Scholar] [CrossRef] [PubMed]
- Slivicki, R.A.; Xu, Z.; Mali, S.S.; Hohmann, A.G. Brain permeant and impermeant inhibitors of fatty-acid amide hydrolase suppress the development and maintenance of paclitaxel-induced neuropathic pain without producing tolerance or physical dependence in vivo and synergize with paclitaxel to reduce tumor cell line viability in vitro. Pharmacol. Res. 2019, 142, 267–282. [Google Scholar] [CrossRef]
- Montero, C.; Campillo, N.E.; Goya, P.; Páez, J.A. Homology models of the cannabinoid CB1 and CB2 receptors. A docking analysis study. Eur. J. Med. Chem. 2005, 40, 75–83. [Google Scholar] [CrossRef]
- Shao, Z.; Yin, J.; Chapman, K.; Grzemska, M.; Clark, L.; Wang, J.; Rosenbaum, D.M. High-resolution crystal structure of the human CB1 cannabinoid receptor. Nature 2016, 540, 602–606. [Google Scholar] [CrossRef]
- Stasiulewicz, A.; Lesniak, A.; Bujalska-Zadrożny, M.; Pawiński, T.; Sulkowska, J.I. Identification of Novel CB2 Ligands through Virtual Screening and In Vitro Evaluation. J. Chem. Inf. Model. 2023, 63, 1012–1027. [Google Scholar] [CrossRef]
- Kumar, K.K.; Robertson, M.J.; Thadhani, E.; Wang, H.; Suomivuori, C.M.; Powers, A.S.; Ji, L.; Nikas, S.P.; Dror, R.O.; Inoue, A.; et al. Structural basis for activation of CB1 by an endocannabinoid analog. Nat. Commun. 2023, 14, 2672. [Google Scholar] [CrossRef]
- Ferreira, I.M.; Rocha, L.C.; Yoshioka, S.A.; Nitschke, M.; Jeller, A.H.; Pizzuti, L.; Seleghim, M.H.R.; Porto, A.L.M. Chemoselective reduction of chalcones by whole hyphae of marine fungus Penicillium citrinum CBMAI 1186, free and immobilized on biopolymers. Biocatal. Agric. Biotechnol. 2014, 3, 358–364. [Google Scholar] [CrossRef]
- Souza, B.S.F.; Carvalho, H.O.; Ferreira, I.M.; da Cunha, E.L.; Barros, A.S.; Taglialegna, T.; Carvalho, J.C. Effect of the treatment with Euterpe oleracea Mart. oil in rats with Triton-induced dyslipidemia. Biomed. Pharmacother. 2017, 90, 542–547. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.M.; da Sena, S.I.; Magalhães, K.F.; Oliveira, S.L.; Ferreira, I.M.; Porto, A.L.M. Amazon Oils from Andiroba (Carapa sp.) and Babassu (Orbignya sp.) for Preparation Biodiesel by Enzymatic Catalysis. Curr. Biotechnol. 2019, 7, 428–437. [Google Scholar] [CrossRef]
- Malcolm, D.W.; Varghese, J.J.; Sorrells, J.E.; Ovitt, C.E.; Benoit, D.S.W. The Effects of Biological Fluids on Colloidal Stability and siRNA Delivery of a pH-Responsive Micellar Nanoparticle Delivery System. ACS Nano 2018, 12, 187–197. [Google Scholar] [CrossRef]
- Saha, U.; Jackson, D. Analysis of moisture, oil, and fatty acid composition of olives by near-infrared spectroscopy: Development and validation calibration models. J. Sci. Food Agric. 2018, 98, 1821–1831. [Google Scholar] [CrossRef]
- Borman, P.; Elder, D. Q2(R1) Validation of Analytical Procedures. In ICH Quality Guidelines; Wiley: Hoboken, NJ, USA, 2017; pp. 127–166. [Google Scholar] [CrossRef]
- Bruschi, M.L. Drug delivery systems. In Strategies to Modify the Drug Release from Pharmaceutical Systems; Elsevier: Amsterdam, The Netherlands, 2015; pp. 87–194. [Google Scholar] [CrossRef]
- Vichai, V.; Kirtikara, K. Sulforhodamine B colorimetric assay for cytotoxicity screening. Nat. Protoc. 2006, 1, 1112–1116. [Google Scholar] [CrossRef]
- Gutowska, I.; Machoy, Z.; Machaliński, B. The role of bivalent metals in hydroxyapatite structures as revealed by molecular modeling with the HyperChem software. J. Biomed. Mater. Res. A 2005, 75A, 788–793. [Google Scholar] [CrossRef]
- Hevener, K.E.; Zhao, W.; Ball, D.M.; Babaoglu, K.; Qi, J.; White, S.W.; Lee, R.E. Validation of Molecular Docking Programs for Virtual Screening against Dihydropteroate Synthase. J. Chem. Inf. Model. 2009, 49, 444–460. [Google Scholar] [CrossRef]
- Li, X.; Chang, H.; Bouma, J.; de Paus, L.V.; Mukhopadhyay, P.; Paloczi, J.; Mustafa, M.; van der Horst, C.; Kumar, S.S.; Wu, L.; et al. Structural basis of selective cannabinoid CB2 receptor activation. Nat. Commun. 2023, 14, 1447. [Google Scholar] [CrossRef] [PubMed]



| Nanostructured Lipid Carrier | Size (nm) T = 0 | Size (nm) T = 1440 | PdI T = 0 | PdI T = 1440 |
|---|---|---|---|---|
| NLC-FAA/SF | 131.0 ± 10.15 | 130.90 ± 08.61 | 0.18 ± 0.01 | 0.21 ± 0.04 |
| NLC-FAA/SF + NaCl | 138.1 ± 9.51 | 139.20 ± 16.51 | 0.22 ± 0.03 | 0.21 ± 0.04 |
| NLC-FAA/SF + PBS | 131.6 ± 10.50 | 131.40 ± 09.77 | 0.19 ± 0.01 | 0.20 ± 0.04 |
| NLC-FAA/SF + DMEM | 146.4 ± 6.53 | 142.90 ± 08.86 | 0.22 ± 0.04 | 0.19 ± 0.03 |
| NLC-FAA/SF + Plasma | 164.6 ± 15.58 | 172.80 ± 26.71 | 0.22 ± 0.04 | 0.20 ± 0.03 |
| NLC-FAA/SF + FBS | 136.4 ± 7.03 | 141.30 ± 24.42 | 0.22 ± 0.08 | 0.20 ± 0.04 |
| NLCw | 160.7 ± 14.47 | 176.70 ± 18.07 | 0.21 ± 0.05 | 0.20 ± 0.04 |
| NLCw + NaCl | 182.6 ± 18.95 | 183.10 ± 12.50 | 0.23 ± 0.05 | 0.20 ± 0.03 |
| NLCw + PBS | 180.3 ± 18.11 | 181.00 ± 13.93 | 0.20 ± 0.04 | 0.20 ± 0.04 |
| NLCw + DEMEM | 182.7 ± 16.38 | 184.20 ± 11.05 | 0.19 ± 0.01 | 0.20 ± 0.03 |
| NLCw + Plasma | 175.9 ± 15.33 | 189.80 ± 22.60 | 0.19 ± 0.03 | 0.21 ± 0.04 |
| NLCw + FBS | 173.9 ± 10.02 | 187.20 ± 19.76 | 0.21 ± 0.06 | 0.21 ± 0.04 |
| Concentration (μg/mL) /Substances | NLC-FAA/SF | OA | NLCw | Fibroin |
|---|---|---|---|---|
| 25.0 | 1.330 ± 0.262 | 2.135 ± 0.437 | 96.724 ± 1.559 | 109.255 ± 4.965 |
| 12.5 | 1.898 ± 0.247 | 1.973 ± 0.769 | 106.323 ± 0.281 | 108.237 ± 4.256 |
| 6.25 | 1.955 ± 0.753 | 2.631 ± 0.396 | 109.390 ± 8.625 | 106.702 ± 1.354 |
| 3.125 | 4.820 ± 1.827 | 8.440 ± 1.042 | 106.044 ± 3.366 | 105.547 ± 0.451 |
| 1.5625 | 8.644 ± 0.321 | 16.520 ± 3.957 | 103.183 ± 0.274 | 105.973 ± 2.386 |
| 0.7813 | 25.354 ± 2.797 | 33.934 ± 1.835 | 105.708 ± 2.689 | 111.170 ± 7.501 |
| 0.3906 | 37.168 ± 2.365 | 45.361 ± 2.941 | 106.116 ± 1.348 | 110.426 ± 8.683 |
| 0.1953 | 45.357 ± 0.751 | 59.349 ± 3.495 | 107.969 ± 0.509 | 115.897 ± 0.817 |
| Concentration (μg/mL) /Substances | NLC-FAA/SF | OA | NLCw | Fibroin |
|---|---|---|---|---|
| 100.00 | 70.999 ± 5.551 | 85.790 ± 10.477 | 104.389 ± 3.945 | 109.244 ± 10.903 |
| 50.00 | 92.550 ± 5.853 | 98.849 ± 6.986 | 103.831 ± 2.879 | 107.345 ± 7.494 |
| 25.00 | 102.990 ± 2.412 | 105.487 ± 4.603 | 103.692 ± 2.321 | 111.721 ± 8.619 |
| 12.5 | 103.823 ± 1.183 | 103.299 ± 2.527 | 105.190 ± 2.814 | 117.083 ± 20.543 |
| 6.25 | 107.583 ± 4.026 | 106.886 ± 4.813 | 105.769 ± 1.269 | 112.055 ± 13.431 |
| 3.125 | 106.678 ± 5.107 | 106.411 ± 4.559 | 104.723 ± 1.240 | 107.951 ± 9.075 |
| 1.5625 | 103.173 ± 1.963 | 106.296 ± 4.720 | 104.487 ± 3.082 | 104.999 ± 3.090 |
| 0.7813 | 106.412 ± 5.644 | 105.472 ± 4.813 | 105.821 ± 4.968 | 105.911 ± 1.045 |
| NLC-FAA/SF | |||
|---|---|---|---|
| Additive | Quantities (g) | Percentage (%) | Phase |
| Distilled water | 4.835 | 96.5 | Aqueous |
| SF solution (2%) | 0.04 | 1 | Aqueous |
| Tween 80 | 0.05 | 1 | Aqueous |
| FAA | 0.05 | 1 | Oily |
| Myristic acid | 0.025 | 0.5 | Oily |
| Total q.s.p. | 5 | 100 | O/A |
| NLCw | |||
| Additive | Quantities (g) | Percentage (%) | Phase |
| Distilled water | 4.885 | 97.5 | Aqueous |
| SF solution (2%) | 0.04 | 1 | Aqueous |
| Tween 80 | 0.05 | 1 | Aqueous |
| Myristic acid | 0.025 | 0,5 | Oily |
| Total q.s.p. | 5 | 100 | O/A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borges, S.d.S.; Miranda, S.E.M.; Marinho, V.H.d.S.; Barros, A.L.B.d.; Yoshioka, S.; Hage-Melim, L.I.d.S.; Silva, A.C.d.J.; Ferreira, I.M.; Oliveira, A.E.M.d.F.M. Evaluation of the Cytotoxic Activity of Nanostructured Lipid Carrier Systems for Fatty Acid Amides and Silk Fibroins in Breast Cancer Cell Lines. Molecules 2025, 30, 3337. https://doi.org/10.3390/molecules30163337
Borges SdS, Miranda SEM, Marinho VHdS, Barros ALBd, Yoshioka S, Hage-Melim LIdS, Silva ACdJ, Ferreira IM, Oliveira AEMdFM. Evaluation of the Cytotoxic Activity of Nanostructured Lipid Carrier Systems for Fatty Acid Amides and Silk Fibroins in Breast Cancer Cell Lines. Molecules. 2025; 30(16):3337. https://doi.org/10.3390/molecules30163337
Chicago/Turabian StyleBorges, Sandro da Silva, Sued Eustáquio Mendes Miranda, Victor Hugo de Souza Marinho, André Luís Branco de Barros, Sergio Yoshioka, Lorane Izabel da Silva Hage-Melim, Ana Carolina de Jesus Silva, Irlon Maciel Ferreira, and Anna Eliza Maciel de Faria Mota Oliveira. 2025. "Evaluation of the Cytotoxic Activity of Nanostructured Lipid Carrier Systems for Fatty Acid Amides and Silk Fibroins in Breast Cancer Cell Lines" Molecules 30, no. 16: 3337. https://doi.org/10.3390/molecules30163337
APA StyleBorges, S. d. S., Miranda, S. E. M., Marinho, V. H. d. S., Barros, A. L. B. d., Yoshioka, S., Hage-Melim, L. I. d. S., Silva, A. C. d. J., Ferreira, I. M., & Oliveira, A. E. M. d. F. M. (2025). Evaluation of the Cytotoxic Activity of Nanostructured Lipid Carrier Systems for Fatty Acid Amides and Silk Fibroins in Breast Cancer Cell Lines. Molecules, 30(16), 3337. https://doi.org/10.3390/molecules30163337






