Chemical Versus Biological Approaches to the Synthesis of Lactobionic Acid: A Review
Abstract
1. Introduction
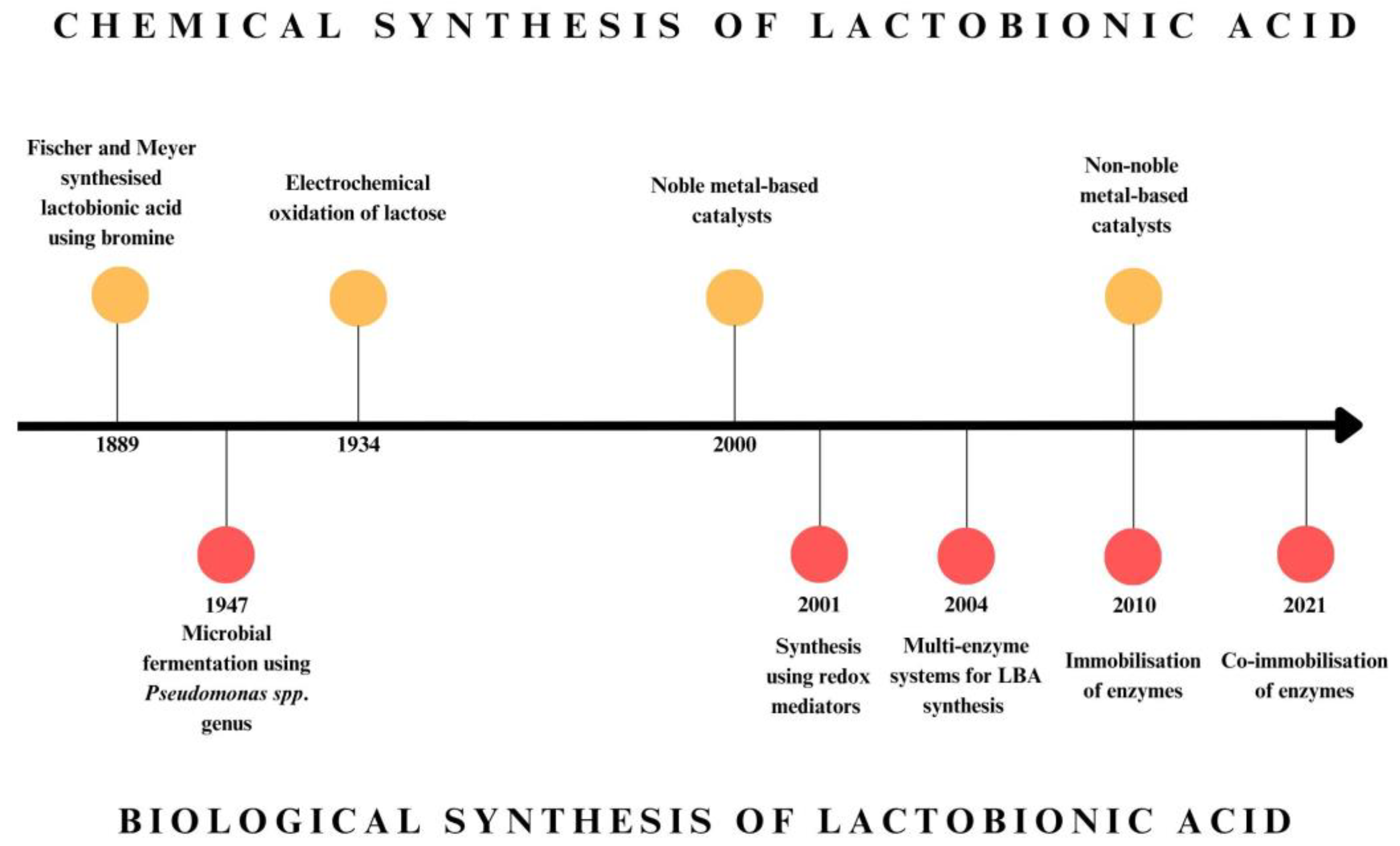
2. Development of Lactobionic Acid Production Methods
3. Chemical Methods of Lactobionic Acid Production
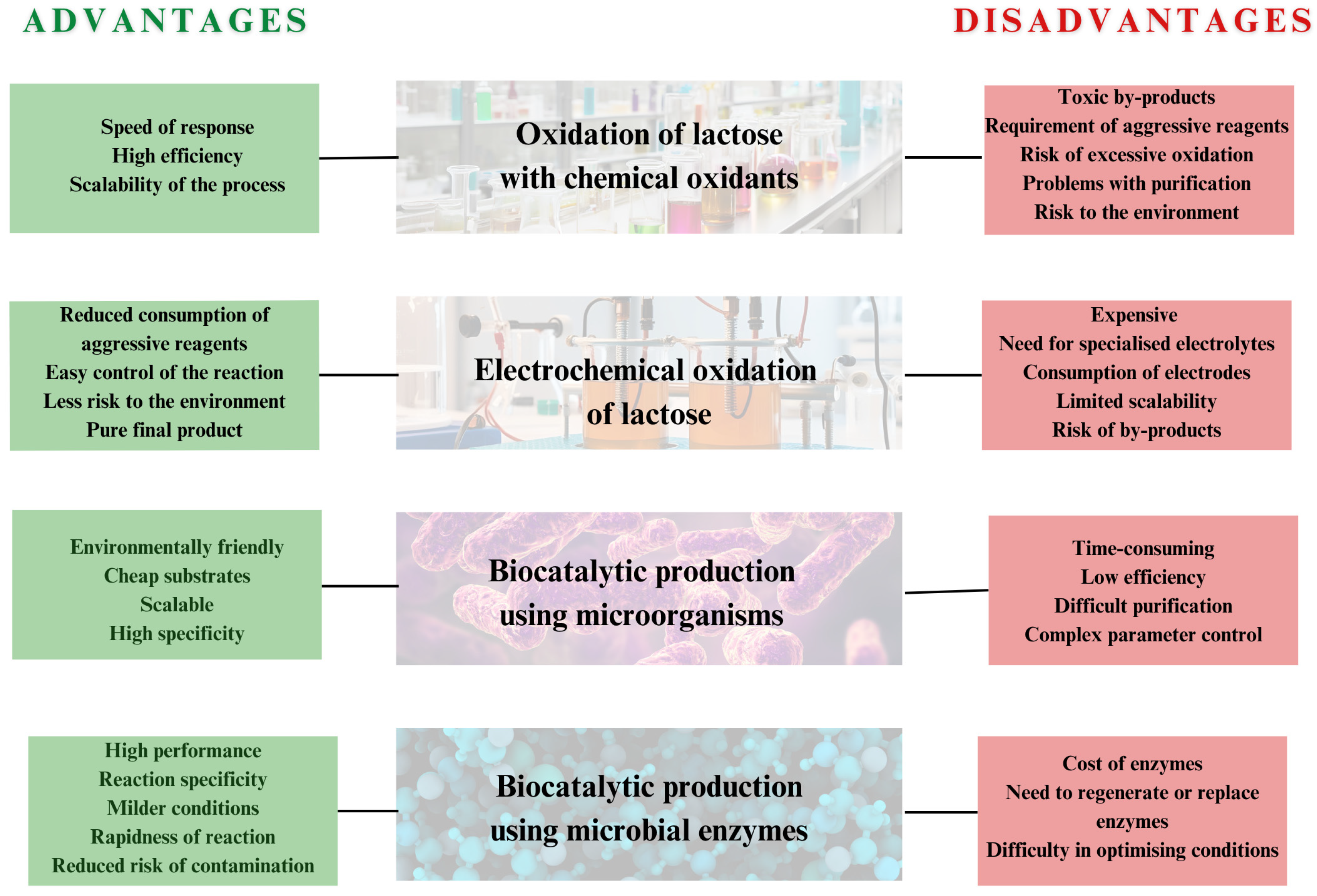
3.1. Heterogeneous Catalytic Oxidation
3.2. Electrochemical Oxidation
4. Biological Methods of Lactobionic Acid Production
4.1. Microbial Production of Lactobionic Acid
4.2. Enzymatic Synthesis of Lactobionic Acid
| Lactose-Oxidising Enzyme | Regenerative Enzyme | Redox Mediator | Substrate | Yield (%) | Productivity (g/L/h) | References |
|---|---|---|---|---|---|---|
| GFOR/GL complex Zymomonas mobilis | n.d. | n.d. | Lactose | 100 | 5.80 | [90] |
| GFOR/GL complex Zymomonas mobilis | n.d. | n.d. | Lactose/ fructose | 78 | 7.6 | [91] |
| CDH from Athelia (Sclerotium) rolfsii | Trametes pubescens laccase | ABTS | Lactose | 100 | 18 | [27] |
| CDH from Athelia (Sclerotium) rolfsii | Trametes pubescens laccase | DCIP | Lactose | 100 | 7 | [97] |
| CDH from Athelia (Sclerotium) rolfsii | Trametes pubescens laccase | ABTS | Lactose | 100 | 19.3 | [97] |
| CDH from Termitomyces clypeatu | n.d. | n.d. | Lactose | 100 | 0.01 | [111] |
| CDH from Myriococcum thermophilum | n.d. | n.d. | Lactose | 100 | 3.3 | [112] |
| CDH from Pycnoporus sp. | Laccase | 3-Hydroxyanthranilic acid 3-HAA | Lactose | 96 | 3.1 | [99] |
| CDH from Phanerochaete chrysosporium | Cerrena unicolor laccase | ABTS | Lactose | 100 | n.d. | [100] |
| CDH from Aspergillus fumigatus | Trametes sp. laccase | ABTS | Lactose | 100 | 7.14 | [28] |
| Carbohydrate oxidase from Microdochium nivale | Catalase | - | Lactose | 98 | 4.9 | [113] |
| Lactose oxidase (LOX) | Catalase | - | Lactose and whey permeate | 98 | n.d. | [114] |
| Acremonium strictum glucooligosaccharide oxidase | Catalase | - | Lactose | 98 | 6.7 | [115] |
| Carbohydrate acceptor oxidoreductase from Paraconiothyrium sp. | - | - | Lactose | 100 | 14.3 | [116] |
| LactoYIELD | Catazyme | - | Lactose | 42–79 | 7.6–13.2 | [17] |
| LactoYIELD | Catazyme | - | Whey permeate | 23–100 | 11.6–17.5 | [17] |
| LactoYIELD | Catazyme/ laccase | ABTS | Lactose | 28.3–100 | 14.4–21.1 | [17] |
| Malate: quinone oxidoreductase MQO, EC 1.1.5.4 | - | - | Lactose | 100 | 0.62 | [76] |
5. Systematic Comparison of Chemical and Biological Approaches
5.1. Productivity and Efficiency
5.2. Costs, Quality, and Purification in the LBA Production Process
5.3. Environmental Impact
- (a)
- The use of non-renewable resources, as many chemical reactants utilised in this process are derived from fossil fuel-based materials [132].
- (b)
- Production of chemical waste—in chemical processes, toxic wastes are produced as by-products; such wastes may need neutralisation prior to disposal [129].
- (c)
- High energy consumption—the high temperatures and pressures needed in the synthesis activity bring about a rise in greenhouse gas emissions, particularly when this energy comes from fossil fuels [45].
- (d)
- The toxicity of reactants—substances used in the process, such as bromine—can pose a risk to ecosystems, especially when waste is improperly handled [117].
- (a)
- The use of renewable raw materials—the main raw material is lactose, which can be obtained from waste from the dairy industry so as to promote a closed-loop economy [11].
- (b)
- (c)
- Low energy consumption—biological reactions occur in mild temperature conditions and low pressure, which results in a decreased carbon footprint [16].
- (d)
- No toxicity—the lack of the need to use toxic chemicals reduces the risk of environmental contamination and adverse effects on human health.
6. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kornhauser, A.; Coelho, S.G.; Hearing, V.J. Applications of hydroxy acids: Classification, mechanisms, and photoactivity. Clin. Cosmet. Investig. Dermatol. 2010, 3, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Date, A.; Patravale, V.; Korting, H.C.; Roeder, A.; Weindl, G. Retinoids in the treatment of skin aging: An overview of clinical efficacy and safety. Clin. Interv. Aging 2006, 1, 327–348. [Google Scholar] [CrossRef]
- Green, B.A.; Ruey, J.Y.; Van Scott, E.J. Clinical and cosmeceutical uses of hydroxyacids. Clin. Dermatol. 2009, 27, 495–501. [Google Scholar] [CrossRef]
- Bhalla, T.C.; Kumar, V.; Bhatia, S.K. Hydroxy acids: Production and applications. In Advances in Industrial Biotechnology; I K Internationl Publishing House pvt Ltd.: New Delhi, India, 2014; pp. 56–76. [Google Scholar]
- Grimes, P.E.; Green, B.A.; Wildnauer, R.H.; Edison, B.L. The use of polyhydroxy acids (PHAs) in photoaged skin. Cutis 2004, 73, 3–13. [Google Scholar]
- Egli, C.; Min, M.; Afzal, N.; Sivamani, R.K. The hydroxy acids: Where have we been and what’s new? Dermatol. Rev. 2023, 4, 260–267. [Google Scholar] [CrossRef]
- Ramos-e-Silva, M.; Hexsel, D.M.; Rutowitsch, M.S.; Zechmeister, M. Hydroxy acids and retinoids in cosmetics. Clin. Dermatol. 2001, 19, 460–466. [Google Scholar] [CrossRef]
- Johnson, A.W. Hydroxyacids. In Skin Moisturization; CRC Press: Boca Raton, FL, USA, 2002; pp. 347–376. [Google Scholar]
- Jaramillo-Sánchez, R.; Alcaraz-Zapata, W. Limitations on production methods for PHAs obtention: A systematic review. Dyna 2020, 87, 193–203. [Google Scholar] [CrossRef]
- Cardoso, T.; Marques, C.; Sotiles, A.R.; Dagostin, J.L.A.; Masson, M.L. Characterization of lactobionic acid evidencing its potential for food industry application. J. Food Process Eng. 2019, 42, e13277. [Google Scholar] [CrossRef]
- Alonso, S.; Rendueles, M.; Díaz, M. Bio-production of lactobionic acid: Current status, applications and future prospects. Biotechnol. Adv. 2013, 31, 1275–1291. [Google Scholar] [CrossRef] [PubMed]
- Hossain, S.; Khetra, Y.; Dularia, C. Biosynthesis of lactobionic acid: A systematic review. Crit. Rev. Food Sci. Nutr. 2025, 65, 353–366. [Google Scholar] [CrossRef] [PubMed]
- Algiert-Zielińska, B.; Mucha, P.; Rotsztejn, H. Lactic and lactobionic acids as typically moisturizing compounds. Int. J. Dermatol. 2019, 58, 374–379. [Google Scholar] [CrossRef]
- Wojciechowska, A.; Klewicki, R.; Klewicka, E. The potential of new bionic acids as prebiotics and antimicrobials. LWT 2020, 125, 109246. [Google Scholar] [CrossRef]
- Gutiérrez, L.-F.; Hamoudi, S.; Belkacemi, K. Lactobionic acid: A high value-added lactose derivative for food and pharmaceutical applications. Int. Dairy J. 2012, 26, 103–111. [Google Scholar] [CrossRef]
- Sarenkova, I.; Ciprovica, I. The current status and future perspectives of lactobionic acid production: A review. Res. Rural Dev. 2018, 1, 233–239. [Google Scholar] [CrossRef]
- Chidar, E.; Liu, L.; Karboune, S. Investigation of the efficiency of different biocatalytic systems for the bioconversion of lactose and dairy by-products into lactobionic acid. LWT 2022, 166, 113781. [Google Scholar] [CrossRef]
- Fischer, E.; Meyer, J. Oxydation des milchzuckers. Berichte Der Dtsch. Chem. Ges. 1889, 22, 361–364. [Google Scholar] [CrossRef]
- Magariello, E.R. Production of Lactobionic Acid and Its Delta-Lactone. U.S. Patent 2746916A, 22 May 1956. [Google Scholar]
- Isbell, H.S. Process for the Preparation of Calcium Lactobionate. U.S. Patent 1980996A, 20 November 1934. [Google Scholar]
- Bieringer, E.; García Vázquez, U.; Klein, L.; Moretó Bravo, N.; Tobler, M.; Weuster-Botz, D. Bioproduction and applications of aldobionic acids with a focus on maltobionic and cellobionic acid. Bioprocess Biosyst. Eng. 2023, 46, 921–940. [Google Scholar] [CrossRef]
- Karski, S. Activity and selectivity of Pd–Bi/SiO2 catalysts in the light of mutual interaction between Pd and Bi. J. Mol. Catal. A Chem. 2006, 253, 147–154. [Google Scholar] [CrossRef]
- Chia, Y.N.; Latusek, M.P.; Holles, J.H. Catalytic wet oxidation of lactose. Ind. Eng. Chem. Res. 2008, 47, 4049–4055. [Google Scholar] [CrossRef]
- Stodola, F.H.; Lockwood, L.B. The oxidation of lactose and maltose to bionic acids by Pseudomonas. J. Biol. Chem. 1947, 171, 213–221. [Google Scholar] [CrossRef]
- Bean, R.; Hassid, W. Carbohydrate oxidase from a red alga, Iridophycus flaccidum. J. Biol. Chem. 1956, 218, 425–436. [Google Scholar] [CrossRef] [PubMed]
- Canevascini, G.; Etienne, K.; Meier, H. A direct enzymatic lactose assay using cellobiose-(lactose-)dehydrogenase from sporotrichum thermophile. Z. Für Lebensm.-Unters. Und Forsch. 1982, 175, 125–129. [Google Scholar] [CrossRef]
- Baminger, U.; Ludwig, R.; Galhaup, C.; Leitner, C.; Kulbe, K.D.; Haltrich, D. Continuous enzymatic regeneration of redox mediators used in biotransformation reactions employing flavoproteins. J. Mol. Catal. B Enzym. 2001, 11, 541–550. [Google Scholar] [CrossRef]
- Yang, J.; Xu, P.; Long, L.; Ding, S. Production of lactobionic acid using an immobilized cellobiose dehydrogenase/laccase system on magnetic chitosan spheres. Process Biochem. 2021, 100, 1–9. [Google Scholar] [CrossRef]
- Piątek-Gołda, W.; Sulej, J.; Grąz, M.; Waśko, P.; Janik-Zabrotowicz, E.; Osińska-Jaroszuk, M. Multi-Enzymatic Synthesis of Lactobionic Acid Using Wood-Degrading Enzymes Produced by White Rot Fungi. Metabolites 2023, 13, 469. [Google Scholar] [CrossRef] [PubMed]
- Mirescu, A.; Prüße, U. A new environmental friendly method for the preparation of sugar acids via catalytic oxidation on gold catalysts. Appl. Catal. B Environ. 2007, 70, 644–652. [Google Scholar] [CrossRef]
- Zhang, Q.; Wan, Z.; Iris, K.; Tsang, D.C. Sustainable production of high-value gluconic acid and glucaric acid through oxidation of biomass-derived glucose: A critical review. J. Clean. Prod. 2021, 312, 127745. [Google Scholar] [CrossRef]
- Shinde, P.S.; Suryawanshi, P.S.; Patil, K.K.; Belekar, V.M.; Sankpal, S.A.; Delekar, S.D.; Jadhav, S.A. A brief overview of recent progress in porous silica as catalyst supports. J. Compos. Sci. 2021, 5, 75. [Google Scholar] [CrossRef]
- van den Berg, R.; Peters, J.A.; van Bekkum, H. Selective alkaline oxidative degradation of mono-and di-saccharides by hydrogen peroxide using borate as catalyst and protecting group. Carbohydr. Res. 1995, 267, 65–77. [Google Scholar] [CrossRef]
- Abbadi, A.; Gotlieb, K.; Meiberg, J.; Van Bekkum, H. Selective chemo-catalytic oxidation of lactose and/of lactobionic acid towards 1-carboxylactulose (2-keto-lactobionic acid). Appl. Catal. A Gen. 1997, 156, 105–115. [Google Scholar] [CrossRef]
- Suresh, R.; Rajoo, B.; Chenniappan, M.; Palanichamy, M. Experimental analysis on the synergistic effect of combined use of ozone and UV radiation for the treatment of dairy industry wastewater. Environ. Eng. Res. 2021, 26, 200375. [Google Scholar] [CrossRef]
- Parangi, T. Heterogeneous catalysis: An alternative approach for energy and environment. Rev. Inorg. Chem. 2025. [Google Scholar] [CrossRef]
- Wacławek, S.; Padil, V.V.; Černík, M. Major advances and challenges in heterogeneous catalysis for environmental applications: A review. Ecol. Chem. Eng. 2018, 25, 9–34. [Google Scholar] [CrossRef]
- Poovan, F.; Chandrashekhar, V.G.; Natte, K.; Jagadeesh, R.V. Synergy between homogeneous and heterogeneous catalysis. Catal. Sci. Technol. 2022, 12, 6623–6649. [Google Scholar] [CrossRef]
- Climent, M.J.; Corma, A.; Iborra, S. Converting carbohydrates to bulk chemicals and fine chemicals over heterogeneous catalysts. Green Chem. 2011, 13, 520–540. [Google Scholar] [CrossRef]
- Corma, A.; Iborra, S.; Velty, A. Chemical Routes for the Transformation of Biomass into Chemicals. Chem. Rev. 2007, 107, 2411–2502. [Google Scholar] [CrossRef]
- Besson, M.; Gallezot, P.; Pinel, C. Conversion of Biomass into Chemicals over Metal Catalysts. Chem. Rev. 2014, 114, 1827–1870. [Google Scholar] [CrossRef]
- Kusema, B.; Murzin, D. Catalytic oxidation of rare sugars over gold catalysts. Catal. Sci. Technol. 2013, 3, 297–307. [Google Scholar] [CrossRef]
- Chatterjee, C.; Pong, F.; Sen, A. Chemical Conversion Pathways for Carbohydrates. Green Chem. 2014, 17, 40–71. [Google Scholar] [CrossRef]
- Hendriks, H.E.; Kuster, B.F.; Marin, G.B. The effect of bismuth on the selective oxidation of lactose on supported palladium catalysts. Carbohydr. Res. 1990, 204, 121–129. [Google Scholar] [CrossRef]
- Kuusisto, J.; Mikkola, J.-P.; Sparv, M.; Wärnå, J.; Karhu, H.; Salmi, T. Kinetics of the catalytic hydrogenation of D-lactose on a carbon supported ruthenium catalyst. Chem. Eng. J. 2008, 139, 69–77. [Google Scholar] [CrossRef]
- Vlad-Cristea, M.S. Production of bioactive lactobionic acid using a novel catalytic method. In Catalysis; Université Laval: Québec, QC, Canada, 2007; pp. 1–102. [Google Scholar]
- Belkacemi, K.; Hamoudi, S. Chemocatalytic oxidation of lactose to lactobionic acid over Pd− Bi/SBA-15: Reaction kinetics and modeling. Ind. Eng. Chem. Res. 2010, 49, 6878–6889. [Google Scholar] [CrossRef]
- Kuusisto, J.; Tokarev, A.V.; Murzina, E.V.; Roslund, M.U.; Mikkola, J.-P.; Murzin, D.Y.; Salmi, T. From renewable raw materials to high value-added fine chemicals—catalytic hydrogenation and oxidation of D-lactose. Catal. Today 2007, 121, 92–99. [Google Scholar] [CrossRef]
- Mäki-Arvela, P.; Murzina, E.V.; Campo, B.; Heikkilä, T.; Leino, A.-R.; Kordas, K.; Wolf, D.; Tokarev, A.V.; Murzin, D.Y. The effect of palladium dispersion and promoters on lactose oxidation kinetics. Res. Chem. Intermed. 2010, 36, 423–442. [Google Scholar] [CrossRef]
- Tokarev, A.V.; Murzina, E.V.; Seelam, P.K.; Kumar, N.; Murzin, D.Y. Influence of surface acidity in lactose oxidation over supported Pd catalysts. Microporous Mesoporous Mater. 2008, 113, 122–131. [Google Scholar] [CrossRef]
- Tokarev, A.V.; Murzina, E.V.; Kuusisto, J.; Mikkola, J.-P.; Eränen, K.; Murzin, D.Y. Kinetic behaviour of electrochemical potential in three-phase heterogeneous catalytic oxidation reactions. J. Mol. Catal. A Chem. 2006, 255, 199–208. [Google Scholar] [CrossRef]
- Mäki-Arvela, P.; Tokarev, A.V.; Murzina, E.V.; Campo, B.; Heikkilä, T.; Brozinski, J.-M.; Wolf, D.; Murzin, D.Y. Kinetics of lactose and rhamnose oxidation over supported metal catalysts. Phys. Chem. Chem. Phys. 2011, 13, 9268–9280. [Google Scholar] [CrossRef]
- Alonso, S.; Rendueles, M.; Díaz, M. Efficient lactobionic acid production from whey by Pseudomonas taetrolens under pH-shift conditions. Bioresour. Technol. 2011, 102, 9730–9736. [Google Scholar] [CrossRef]
- Meyer, C.I.; Regenhardt, S.A.; Zelin, J.; Sebastian, V.; Marchi, A.J.; Garetto, T.F. A kinetic modeling of the liquid-phase oxidation of lactose over Pt-and Au-supported catalysts. Top. Catal. 2016, 59, 168–177. [Google Scholar] [CrossRef]
- Meyer, C.I.; Regenhardt, S.A.; Duarte, H.A.; Zelin, J.; Sebastian, V.; Garetto, T.F.; Marchi, A.J. Selective lactose oxidation in aqueous-phase over Ag-Au bimetallic nanoparticles supported on Al2O3 under mild reaction conditions. Mol. Catal. 2020, 481, 110249. [Google Scholar] [CrossRef]
- Vedovato, V.; Vanbroekhoven, K.; Pant, D.; Helsen, J. Electrosynthesis of biobased chemicals using carbohydrates as a feedstock. Molecules 2020, 25, 3712. [Google Scholar] [CrossRef]
- Pleissner, D.; Dietz, D.; van Duuren, J.B.J.H.; Wittmann, C.; Yang, X.; Lin, C.S.K.; Venus, J. Biotechnological production of organic acids from renewable resources. Adv. Biochem. Eng. Biotechnol. 2019, 166, 373–410. [Google Scholar] [CrossRef]
- Saji, V.S.; Pillai, V.K. Multi-Functional Electrocatalysts: Fundamentals and Applications; Royal Society of Chemistry: London, UK, 2024; Volume 46. [Google Scholar] [CrossRef]
- Cheng, S.; Zhong, H.; Jin, F. A mini review of electrocatalytic upgrading of carbohydrate biomass—System, path, and optimization. Energy Sci. Eng. 2023, 11, 2944–2965. [Google Scholar] [CrossRef]
- Leech, M.C.; Garcia, A.D.; Petti, A.; Dobbs, A.P.; Lam, K. Organic electrosynthesis: From academia to industry. React. Chem. Eng. 2020, 5, 977–990. [Google Scholar] [CrossRef]
- Druliolle, H.; Kokoh, K.; Beden, B. Electro-oxidation of lactose on platinum and on modified platinum electrodes in alkaline medium. Electrochim. Acta 1994, 39, 2577–2584. [Google Scholar] [CrossRef]
- Druliolle, H.; Kokoh, K.; Beden, B. Selective oxidation of lactose to lactobionic acid on lead-adatoms modified platinum electrodes in Na2CO3 + NaHCO3 buffered medium. J. Electroanal. Chem. 1995, 385, 77–83. [Google Scholar] [CrossRef]
- Druliolle, H.; Kokoh, K.B.; Hahn, F.; Lamy, C.; Beden, B. On some mechanistic aspects of the electrochemical oxidation of lactose at platinum and gold electrodes in alkaline medium. J. Electroanal. Chem. 1997, 426, 103–115. [Google Scholar] [CrossRef]
- Kokoh, K.B.; Alonso-Vante, N. Electrocatalytic oxidation of lactose on gold nanoparticle modified carbon in carbonate buffer. J. Appl. Electrochem. 2006, 36, 147–151. [Google Scholar] [CrossRef]
- Van Hecke, W.; Haltrich, D.; Frahm, B.; Brod, H.; Dewulf, J.; Van Langenhove, H.; Ludwig, R. A biocatalytic cascade reaction sensitive to the gas–liquid interface: Modeling and upscaling in a dynamic membrane aeration reactor. J. Mol. Catal. B Enzym. 2011, 68, 154–161. [Google Scholar] [CrossRef]
- Nakano, H.; Kiryu, T.; Kiso, T.; Murakami, H. Biocatalytic production of lactobionic acid. In Biocatalysis and Biomolecular Engineering; John Wiley & Sons: Hoboken, NJ, USA, 2010; pp. 391–404. [Google Scholar]
- Sarenkova, I.; Orviz, S.S.; Ciprovica, I.; Rendueles, M.; Diaz, M. Lactobionic acid production from acid whey under different fermentative conditions. J. Adv. Agric. Technol. 2021, 8. [Google Scholar] [CrossRef]
- Goderska, K.; Juzwa, W.; Karpiński, T.M. Quantitative Analysis of Lactobionic Acid in Bioreactor Cultures and Selected Biological Activities. Molecules 2024, 29, 5400. [Google Scholar] [CrossRef] [PubMed]
- Shu, C.; Tseng, K.; Jaiswal, R. Effects of light intensity and wavelength on the production of lactobionic acid from whey by Pseudomonas taetrolens in batch cultures. J. Chem. Technol. Biotechnol. 2018, 93, 1595–1600. [Google Scholar] [CrossRef]
- Alonso, S.; Rendueles, M.; Díaz, M. Tunable decoupled overproduction of lactobionic acid in Pseudomonas taetrolens through temperature-control strategies. Process Biochem. 2017, 58, 9–16. [Google Scholar] [CrossRef]
- Murakami, H.; Seko, A.; Azumi, M.; Ueshima, N.; Yoshizumi, H.; Nakano, H.; Kitahata, S. Fermentative production of lactobionic acid by Burkholderia cepacia. J. Appl. Glycosci. 2003, 50, 117–120. [Google Scholar] [CrossRef]
- Kiryu, T.; Yamauchi, K.; Masuyama, A.; Ooe, K.; Kimura, T.; Kiso, T.; Nakano, H.; Murakami, H. Optimization of lactobionic acid production by Acetobacter orientalis isolated from Caucasian fermented milk, “Caspian Sea yogurt”. Biosci. Biotechnol. Biochem. 2012, 76, 361–363. [Google Scholar] [CrossRef]
- Han, H.J.; Oh, Y.-R.; Eom, G.T. Isolation and characterization of a new superior lactobionic acid-producing bacterium, Enterobacter cloacae KRICT-1, from environmental soil samples. ACS Food Sci. Technol. 2021, 2, 66–74. [Google Scholar] [CrossRef]
- Lee, S.S.; Oh, Y.-R.; Jeong, B.-Y.; Eom, G.T. Isolation of new lactobionic acid-producing microorganisms and improvement of their production ability by heterologous expression of glucose dehydrogenase from Pseudomonas taetrolens. Enzym. Microb. Technol. 2022, 153, 109954. [Google Scholar] [CrossRef]
- Bucek, W.; Connors, W.M.; Cort, W.M.; Robert, H.R. Evidence for the formation and utilization of lactobionic acid by Penicillium chrysogenum. Arch. Biochem. Biophys. 1956, 63, 477–478. [Google Scholar] [CrossRef]
- Oh, Y.-R.; Jang, Y.-A.; Hong, S.H.; Eom, G.T. Purification and characterization of a malate: Quinone oxidoreductase from Pseudomonas taetrolens capable of producing valuable lactobionic acid. J. Agric. Food Chem. 2020, 68, 13770–13778. [Google Scholar] [CrossRef]
- Oh, Y.-R.; Jang, Y.-A.; Hong, S.H.; Han, J.J.; Eom, G.T. Efficient production of lactobionic acid using genetically engineered Pseudomonas taetrolens as a whole-cell biocatalyst. Enzym. Microb. Technol. 2020, 141, 109668. [Google Scholar] [CrossRef]
- Kiryu, T.; Kiso, T.; Koma, D.; Tanaka, S.; Murakami, H. Identifying membrane-bound quinoprotein glucose dehydrogenase from acetic acid bacteria that produce lactobionic and cellobionic acids. Biosci. Biotechnol. Biochem. 2019, 83, 1171–1179. [Google Scholar] [CrossRef] [PubMed]
- García, C.; Rendueles, M.; Díaz, M. Synbiotic fermentation for the Co-production of lactic and lactobionic acids from residual dairy whey. Biotechnol. Prog. 2017, 33, 1250–1256. [Google Scholar] [CrossRef]
- Oh, Y.-R.; Eom, G.T. Identification of a lactose-oxidizing enzyme in Escherichia coli and improvement of lactobionic acid production by recombinant expression of a quinoprotein glucose dehydrogenase from Pseudomonas taetrolens. Enzym. Microb. Technol. 2021, 148, 109828. [Google Scholar] [CrossRef]
- De Mello, A.F.M.; Vandenberghe, L.P.d.S.; Herrmann, L.W.; Letti, L.A.J.; Burgos, W.J.M.; Scapini, T.; Manzoki, M.C.; De Oliveira, P.Z.; Soccol, C.R. Strategies and engineering aspects on the scale-up of bioreactors for different bioprocesses. Syst. Microbiol. Biomanuf. 2023, 4, 365–385. [Google Scholar] [CrossRef]
- Alonso, S.; Rendueles, M.; Díaz, M. Feeding strategies for enhanced lactobionic acid production from whey by Pseudomonas taetrolens. Bioresour. Technol. 2013, 134, 134–142. [Google Scholar] [CrossRef]
- Mao, S.; Liu, Y.; Hou, Y.; Ma, X.; Yang, J.; Han, H.; Wu, J.; Jia, L.; Qin, H.; Lu, F. Efficient production of sugar-derived aldonic acids by Pseudomonas fragi TCCC11892. RSC Adv. 2018, 8, 39897–39901. [Google Scholar] [CrossRef]
- Wu, J.; Liu, P.; Zheng, Z.; Ouyang, J. Valorization of cheese whey to lactobionic acid by a novel strain Pseudomonas fragi and identification of enzyme involved in lactose oxidation. Microb. Cell Factories 2022, 21, 184. [Google Scholar] [CrossRef]
- Liu, P.; Hong, L.; Fu, P.; Wu, J.; Zheng, Z. Co-production of galactonic acid and lactobionic acid from cheese whey using recyclable engineered Pseudomonas putida. Int. J. Dairy Technol. 2025, 78, e70001. [Google Scholar] [CrossRef]
- Han, H.J.; Eom, G.T. Production of lactobionic acid at high salt concentrations by Acinetobacter halotolerans isolated from seaside soil. Bioprocess Biosyst. Eng. 2022, 45, 1683–1691. [Google Scholar] [CrossRef] [PubMed]
- Sarenkova, I.; Sáez-Orviz, S.; Ciprovica, I.; Rendueles, M.; Díaz, M. Lactobionic acid production by Pseudomonas taetrolens in a fed-batch bioreactor using acid whey as substrate. Int. J. Dairy Technol. 2022, 75, 361–371. [Google Scholar] [CrossRef]
- Romano, R.; Alberini, F.; Raddadi, N.; Fava, F.; Paglianti, A. Inline monitoring of lactobionic acid production from cheese whey by Pseudomonas taetrolens in a stirred bioreactor using electrical conductivity. Can. J. Chem. Eng. 2025, 103, 373–384. [Google Scholar] [CrossRef]
- Lin, S.-F.; Hu, H.-M.; Inukal, T.; Tsai, Y.-C. Production of novel oligosaccharide oxidase by wheat bran solid-state fermentation. Biotechnol. Adv. 1993, 11, 417–427. [Google Scholar] [CrossRef]
- Pedruzzi, I.; da Silva, E.A.B.; Rodrigues, A.E. Production of lactobionic acid and sorbitol from lactose/fructose substrate using GFOR/GL enzymes from Zymomonas mobilis cells: A kinetic study. Enzym. Microb. Technol. 2011, 49, 183–191. [Google Scholar] [CrossRef]
- Malvessi, E.; Carra, S.; Pasquali, F.C.; Kern, D.B.; Da Silveira, M.M.; Ayub, M.A.Z. Production of organic acids by periplasmic enzymes present in free and immobilized cells of Zymomonas mobilis. J. Ind. Microbiol. Biotechnol. 2013, 40, 1–10. [Google Scholar] [CrossRef]
- Satory, M.; Fürlinger, M.; Haltrich, D.; Kulbe, K.; Pittner, F.; Nidetzky, B. Continuous enzymatic production of lactobionic acid using glucose-fructose oxidoreductase in an ultrafiltration membrane reactor. Biotechnol. Lett. 1997, 19, 1205–1208. [Google Scholar] [CrossRef]
- Henriksson, G.; Johansson, G.; Pettersson, G. A critical review of cellobiose dehydrogenases. J. Biotechnol. 2000, 78, 93–113. [Google Scholar] [CrossRef] [PubMed]
- Sulej, J.; Jaszek, M.; Osińska-Jaroszuk, M.; Matuszewska, A.; Bancerz, R.; Janczarek, M. Natural microbial polysaccharides as effective factors for modification of the catalytic properties of fungal cellobiose dehydrogenase. Arch. Microbiol. 2021, 203, 4433–4448. [Google Scholar] [CrossRef]
- Oh, Y.-R.; Jang, Y.-A.; Lee, S.S.; Kim, J.-H.; Hong, S.H.; Han, J.J.; Eom, G.T. Enhancement of lactobionic acid productivity by homologous expression of quinoprotein glucose dehydrogenase in Pseudomonas taetrolens. J. Agric. Food Chem. 2020, 68, 12336–12344. [Google Scholar] [CrossRef]
- Splechtna, B.; Petzelbauer, I.; Baminger, U.; Haltrich, D.; Kulbe, K.D.; Nidetzky, B. Production of a lactose-free galacto-oligosaccharide mixture by using selective enzymatic oxidation of lactose into lactobionic acid. Enzym. Microb. Technol. 2001, 29, 434–440. [Google Scholar] [CrossRef]
- Ludwig, R.; Ozga, M.; Zámocky, M.; Peterbauer, C.; Kulbe, K.D.; Haltrich, D. Continuous enzymatic regeneration of electron acceptors used by flavoenzymes: Cellobiose dehydrogenase-catalyzed production of lactobionic acid as an example. Biocatal. Biotransform. 2004, 22, 97–104. [Google Scholar] [CrossRef]
- Elgahwash, R.G.A.; Blažić, M.; Balaž, A.M.; Prodanović, R. Lactobionic acid production via mutant cellobiose dehydrogenase/laccase continuous enzymatic regeneration of electron acceptors. Biocatal. Biotransform. 2024, 42, 316–323. [Google Scholar] [CrossRef]
- Tian, Q.; Feng, Y.; Huang, H.; Zhang, J.; Yu, Y.; Guan, Z.; Cai, Y.; Liao, X. Production of lactobionic acid from lactose using the cellobiose dehydrogenase-3-HAA-laccase system from Pycnoporus sp. SYBC-L10. Lett. Appl. Microbiol. 2018, 67, 589–597. [Google Scholar] [CrossRef] [PubMed]
- Sulej, J.; Piątek-Gołda, W.; Grąz, M.; Szałapata, K.; Waśko, P.; Janik-Zabrotowicz, E.; Osińska-Jaroszuk, M. Immobilisation of cellobiose dehydrogenase and laccase on chitosan particles as a multi-enzymatic system for the synthesis of lactobionic acid. J. Funct. Biomater. 2023, 14, 383. [Google Scholar] [CrossRef]
- Sheldon, R.A. Enzyme immobilization: The quest for optimum performance. Adv. Synth. Catal. 2007, 349, 1289–1307. [Google Scholar] [CrossRef]
- Homaei, A.A.; Sariri, R.; Vianello, F.; Stevanato, R. Enzyme immobilization: An update. J. Chem. Biol. 2013, 6, 185–205. [Google Scholar] [CrossRef] [PubMed]
- Datta, S.; Christena, L.R.; Rajaram, Y.R.S. Enzyme immobilization: An overview on techniques and support materials. 3 Biotech 2013, 3, 1–9. [Google Scholar] [CrossRef]
- Ren, S.; Li, C.; Jiao, X.; Jia, S.; Jiang, Y.; Bilal, M.; Cui, J. Recent progress in multienzymes co-immobilization and multienzyme system applications. Chem. Eng. J. 2019, 373, 1254–1278. [Google Scholar] [CrossRef]
- Luo, Z.; Qiao, L.; Chen, H.; Mao, Z.; Wu, S.; Ma, B.; Xie, T.; Wang, A.; Pei, X.; Sheldon, R.A. Precision Engineering of the Co-Immobilization of Enzymes for Cascade Biocatalysis. Angew. Chem. 2024, 136, e202403539. [Google Scholar] [CrossRef]
- Sulej, J.; Osińska-Jaroszuk, M.; Jaszek, M.; Olszewska, A.; Belcarz, A.; Piątek-Gołda, W. Chitosan as a promising support of a CDH activity preservation system for biomedical and industrial applications. Int. J. Mol. Sci. 2023, 24, 4535. [Google Scholar] [CrossRef]
- Cardoso, T.; Marques, C.; Dagostin, J.L.A.; Masson, M.L. Lactobionic acid as a potential food ingredient: Recent studies and applications. J. Food Sci. 2019, 84, 1672–1681. [Google Scholar] [CrossRef]
- Savary, B.J.; Hicks, K.B.; O’Connor, J.V. Hexose oxidase from Chondrus crispus: Improved purification using perfusion chromatography. Enzym. Microb. Technol. 2001, 29, 42–51. [Google Scholar] [CrossRef]
- Lin, S.; Li, C.; Chung, Y. Identification of a novel lactose oxidase in Myrmecridium flexuosum NUK-21. FEBS Open Bio 2019, 9, 364–373. [Google Scholar] [CrossRef]
- Staudigl, P.; Krondorfer, I.; Haltrich, D.; Peterbauer, C.K. Pyranose dehydrogenase from Agaricus campestris and Agaricus xanthoderma: Characterization and applications in carbohydrate conversions. Biomolecules 2013, 3, 535–552. [Google Scholar] [CrossRef]
- Saha, T.; Ghosh, D.; Mukherjee, S.; Bose, S.; Mukherjee, M. Cellobiose dehydrogenase production by the mycelial culture of the mushroom Termitomyces clypeatus. Process Biochem. 2008, 43, 634–641. [Google Scholar] [CrossRef]
- Pricelius, S.; Ludwig, R.; Lant, N.; Haltrich, D.; Guebitz, G. Substrate specificity of Myriococcum thermophilum cellobiose dehydrogenase on mono-, oligo-, and polysaccharides related to in situ production of H2O2. Appl. Microbiol. Biotechnol. 2009, 85, 75–83. [Google Scholar] [CrossRef]
- Nordkvist, M.; Nielsen, P.M.; Villadsen, J. Oxidation of lactose to lactobionic acid by a Microdochium nivale carbohydrate oxidase: Kinetics and operational stability. Biotechnol. Bioeng. 2007, 97, 694–707. [Google Scholar] [CrossRef]
- Hua, L.; Nordkvist, M.; Nielsen, P.M.; Villadsen, J. Scale-up of enzymatic production of lactobionic acid using the rotary jet head system. Biotechnol. Bioeng. 2007, 97, 842–849. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.-F.; Yang, T.-Y.; Inukai, T.; Yamasaki, M.; Tsai, Y.-C. Purification and characterization of a novel glucooligosaccharide oxidase from Acremonium strictum T1. Biochim. Et Biophys. Acta (BBA)-Protein Struct. Mol. Enzymol. 1991, 1118, 41–47. [Google Scholar] [CrossRef]
- Kiryu, T.; Nakano, H.; Kiso, T.; Murakami, H. Purification and characterization of a carbohydrate: Acceptor oxidoreductase from Paraconiothyrium sp. that produces lactobionic acid efficiently. Biosci. Biotechnol. Biochem. 2008, 72, 833–841. [Google Scholar] [CrossRef]
- Yang, B.Y.; Montgomery, R. Oxidation of lactose with bromine. Carbohydr. Res. 2005, 340, 2698–2705. [Google Scholar] [CrossRef] [PubMed]
- Kambo, N.; Upadhyay, S.K. Kinetics and mechanism of the ruthenium (III) catalysed oxidation of reducing sugars by chloramine-T in alkaline medium. Transit. Met. Chem. 2000, 25, 461–464. [Google Scholar] [CrossRef]
- Kim, J.-H.; Jang, Y.-A.; Seong, S.-B.; Jang, S.A.; Hong, S.H.; Song, J.K.; Eom, G.T. High-level production and high-yield recovery of lactobionic acid by the control of pH and temperature in fermentation of Pseudomonas taetrolens. Bioprocess Biosyst. Eng. 2020, 43, 937–944. [Google Scholar] [CrossRef] [PubMed]
- Maghraby, Y.R.; El-Shabasy, R.M.; Ibrahim, A.H.; Azzazy, H.M.E.-S. Enzyme Immobilization Technologies and Industrial Applications. ACS Omega 2023, 8, 5184–5196. [Google Scholar] [CrossRef]
- Robescu, M.S.; Bavaro, T. A Comprehensive Guide to Enzyme Immobilization: All You Need to Know. Molecules 2025, 30, 939. [Google Scholar] [CrossRef]
- Global Lactobionic Acid Sales Market Report, Competitive Analysis and Regional Opportunities 2025–2031. Available online: https://reports.valuates.com/market-reports/QYRE-Auto-33M55/global-lactobionic-acid (accessed on 4 March 2025).
- Klimecka-Tatar, D. The costs of quality in production engineering: Business development or growth inhibition? Syst. Saf. Hum.-Tech. Facil.-Environ. 2024, 6, 260–269. [Google Scholar] [CrossRef]
- Sarenkova, I.; Sáez-Orviz, S.; Rendueles, M.; Ciprovica, I.; Zagorska, J.; Díaz, M. Downstream approach routes for the purification and recovery of lactobionic acid. Foods 2022, 11, 583. [Google Scholar] [CrossRef]
- El Ghoul, M.; Escoula, B.; Rico, I.; Lattes, A. Synthesis of new fluorinated non-ionic surfactants derived from lactose: The N-[2-(F-alkyl) ethyl]-lactosylamines and-lactobionamides. J. Fluor. Chem. 1992, 59, 107–112. [Google Scholar] [CrossRef]
- Timmermans, E. Lactose: Its manufacture and physico-chemical properties. In Carbohydrates as Organic Raw Materials III; John Wiley & Sons: Hoboken, NJ, USA, 1996; pp. 93–113. [Google Scholar] [CrossRef]
- Lactobionic Acid Market Size and Share Analysis–Growth Trends and Forecasts (2025–2030). Available online: https://www.mordorintelligence.com/industry-reports/lactobionic-acid-market (accessed on 4 March 2025).
- Lactobionic Acid Market Research Report by Application (Personal Care, Food and Beverage, Pharmaceuticals, Diagnostics), by Source (Lactose, Cellulose, Whey Protein), by Purity (99%, 98%, 97%) and by Regional (North America, Europe, South America, Asia Pacific, Middle East and Africa)–Forecast to 2032. Available online: https://www.marketresearchfuture.com/reports/lactobionic-acid-market-30547 (accessed on 4 March 2025).
- Marques, C.; Wojeicchowski, J.P.; Cardoso, T.; Mafra, M.R.; Mitterer-Daltoé, M.L.; Masson, M.L. Lactobionic acid as a suitable food preservative for yacon juice. Innov. Food Sci. Emerg. Technol. 2020, 64, 102400. [Google Scholar] [CrossRef]
- Carra, S.; Rodrigues, D.C.; Beraldo, N.M.C.; Folle, A.B.; Delagustin, M.G.; de Souza, B.C.; Reginatto, C.; Polidoro, T.A.; da Silveira, M.M.; Bassani, V.L.; et al. High lactobionic acid production by immobilized Zymomonas mobilis cells: A great step for large-scale process. Bioprocess Biosyst. Eng. 2020, 43, 1265–1276. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, R.A.; Woodley, J.M. Role of biocatalysis in sustainable chemistry. Chem. Rev. 2018, 118, 801–838. [Google Scholar] [CrossRef] [PubMed]
- Haworth, W.N.; Long, C.W. LXXXI.—The constitution of the disaccharides. Part XII. Lactose. J. Chem. Soc. (Resumed) 1927, 544–548. [Google Scholar] [CrossRef]






| Catalyst | Oxidant | Reaction Conditions | Conversion Yield (%) | Selectivity (%) | Reaction Time (min) | References |
|---|---|---|---|---|---|---|
| Pd/C | O2 | pH 8, 70 °C | 29–97 | 46–95.8 | 200 | [44,48,49,50] |
| Pd/Al2O3 | O2 | pH 9, 100–170 °C | 54–99 | 63–97 | 200 | [51] |
| Pd/Al2O3 | O2 | pH 9, 40 °C, flow rate 500 mL min−1 | 100 | ≥99.5 | n.d. | [30] |
| Pd/SiO2 | O2 | pH = 9, 70 °C, stirred at 1300 rpm | 16.3–27 | 30–80 | 120–200 | [22] |
| Pd-Bi/C | O2 | pH 7–10, 60 °C | 95% | 100 | 60 | [44] |
| Pd-Pb/CaCO3 | O2 | pH 8, 70 °C | 92 | 83 | 200 | [49] |
| Pd-Pt-Bi/C | O2 | pH 8, 70 °C | 93 | 83 | 200 | [49] |
| Pt/C | O2 | pH 8, 70 °C, oxygen flow 3.5 mL min−1 | 87–94 | 54–75 | 150 | [52] |
| Pt/Al2O3 | O2 | pH 9, 100–170 °C, pressures of 100 psig | 67–100 | 0–44 | 150–300 | [23,52] |
| Pt-Bi/C | O2 | pH 7, 170 °C | 82 | 71 | 80–300 | [52] |
| Pt/CeMn | O2 | pH 9, 100–170 °C | 100 | 95 | 150–300 | [23] |
| Ru/C | O2 | pH 8, 70 °C | 33–34 | 17–38 | 200 | [52] |
| Au/Al2O3 Au/TiO2 | O2 | pH 9, 40 °C, flow rate 500 mL min−1 | 100 | ≥99.5 | n.d. | [30] |
| Au/Al2O3 | O2 | pH 9, 65 °C | 85–97 | 100 | 150–180 | [53,54] |
| Au-Ag/Al2O3 | O2 | pH 8–11, 65 °C | ≥90 | 100 | 20–120 | [55] |
| Mechanism of Action | Catalyst | Electrode | Reaction Conditions | Conversion Yield (%) | Selectivity (%) | References |
|---|---|---|---|---|---|---|
| Bromine-mediated electrochemical oxidation | Br2 (mediator) | Graphite electrodes | pH > 5.2, ≤25 °C, CaCO3/NaHCO3 buffer | ~98 | ~100 | [19,20] |
| Direct oxidation of primary alcohol groups | Pt | Pt electrodes | Alkaline media, pH 8–11, 25–60 °C | >90 | ~100 | [61,62,63] |
| Direct oxidation of primary alcohol groups | Au | Au electrodes | Alkaline media, pH 8–11, 25–60 °C | >90 | ~100 | [61,62,63] |
| Indirect oxidation via hypobromite generation | Electrochemically regenerated Br2 | Graphite electrodes | pH 5–7, 25–30 °C, electrolysis | 80–90 | 80–90 | [65] |
| Direct electro-oxidation in an aqueous medium (flow reactor) on carbon modified by gold nanoparticles | Au-NMC | Carbon felt electrode with immobilised Au-colloids | Carbonated buffer, pH 10.2 | 91 | 98 | [56,64] |
| Indirect electrochemical oxidation | Pt with TEMPO mediator | Glassy carbon anode | Alkaline media NaHCO3/Na2CO3 | n.d. | n.d. | [56] |
| Microorganism | Production Mode/Culture Conditions | Biocatalyst | Substrate | Yield (%) | Productivity (g/L/h) | References |
|---|---|---|---|---|---|---|
| Burkholderia cepacia | Shaking flask/110 rpm, 40 °C, 24–48 h | Resting cells | Lactose | 100 | 5.55 | [71] |
| Pseudomonas taetrolens | Batch cultivations in stirred tank bioreactor/350 rpm, 30 °C, pH 6.5, 60 h | Whole cell | Whey | 100 | 1.27 | [11,53] |
| Acetobacter orientalis strain KYG22 | Shaking flasks/120 rpm, 28 °C, 72 h | Resting cells | Lactose | 99.7 | n.d. | [72] |
| Pseudomonas taetrolens (formerly known as P. graveolens) | Shaking flasks/25 °C, 165 h | Whole cell | Lactose | 75 | n.d. | [24] |
| Pseudomonas fragi TCCC11892 | Bioreactor/350 rpm, 37 °C, pH 6.5, 36 h | Whole cell | Cheese whey | 97 | 3.3 | [83] |
| Pseudomonas fragi NL20W | Bioreactor/200 rpm, 30 °C, pH 6.0, 102 h | Whole cell | Whey powder | 100 | 3.09 | [84] |
| Pseudomonas putida KT2440 (pBB-GDH1) recombinant strain | Bioreactor/ 25 °C to 40 °C, pH 7.0, 84 h | Whole cell | Cheese whey powder | 98.73 | 2.42 | [85] |
| Recombinant E. coli strain with homologous expression of malate quinone oxidoreductase | Batch in flask/PQQ as an electron acceptor, 37 h | Growing cell | Concentrate whey | 100 | 0.62 | [77] |
| Acinetobacter halotolerans strain KRICT-1 | Bioreactor/30 °C, 7% NaCl | Growing cell | Lactose | n.d. | 0.22 | [86] |
| Komagataeibacter medellinensis (Gluconacetobacter xylinus) NBRC3288 | Batch in a flask | Resting cell | Lactose | n.d. | n.d. | [78] |
| Pseudomonas taetrolens LMG 2336 | Stirred tank bioreactor/350 rpm, 30 °C, pH 6.5, 32 h | Growing cell | Sweet whey | 100 | 1.12 | [53] |
| Pseudomonas taetrolens LMG 2336 | Bioreactor/30 °C, pH 6.5, 48 h | Growing cell | Acid whey | 100 | 1.12 | [87] |
| Pseudomonas taetrolens DSM 21104 | Bioreactor | Growing cell | Various cheese whey | 85.8 | 26.53 | [88] |
| Acetobacter orientalis | Bioreactor | Growing cell | Lactose, yeast extract, polypeptone, D-glucose, salts. | 98 | 0.54 | [72] |
| Enterobacter cloacae KRICT-1 | Batch in flask, 180 rpm, 35 °C, 27 h | Growing cell | Lactose | 100 | 8.72 | [73] |
| Enterobacter cloacae KRICT-1 | Batch in bioreactor, 40 °C, 27 h | Growing cell | Lactose | 100 | 9.97 | [73] |
| Pseudomonas taetrolens LMG 2336 and Lactobacillus casei CECT 475 | Flask/simultaneous fermentation, 250 rpm, 30 °C | Growing cell | Sweet whey | n.d. | 0.02 | [79] |
| Penicillium chrysogenum | Shake flask cultures, 120 h | Growing cell | Lactose | 50 | 0.004–0.007 | [75] |
| Acinetobacter sp. | Batch in a flask | Growing cell | Lactose | 69.5 | 0.72 | [74] |
| Acinetobacter sp. recombinant strain with heterologous expression of quinoprotein glucose dehydrogenase from Pseudomonas taetrolens | Batch in a flask | Growing cell | Lactose | 97.5 | 0.94 | [74] |
| Psychrobacter sp. | Batch in a flask | Growing cell | Lactose | 96.5 | 1.15 | [74] |
| Psychrobacter sp. recombinant strain with heterologous expression of quinoprotein glucose dehydrogenase from Pseudomonas taetrolens | Batch in a flask | Growing cell | Lactose | 97.3 | 2.83 | [74] |
| Manufacturer/Supplier | Product Description | Cost/Price |
|---|---|---|
| Science research | ||
| Thermo Scientific Chemicals (Alfa Aesar), Waltham, MA, USA | Purity: 97%, water content: 5.0% max | USD 244 per 100 g |
| Chem-Impex International, Wood Dale, IL, USA | Purity: ≥98% | USD 60 per 100 g |
| RPI Research Products International, Mount Prospect, IL, USA | Assay: 97.0–103.0%, water content: ≤5.0%, material source: cow milk | USD 67 per 100 g |
| Sigma-Aldrich, Merck KGaA, Darmstadt, Germany | Assay: 97% (TLC), solubility 5%, biological source: synthetic | EUR 372 per 100 g |
| AG Scientific Holdings, LLC, San Diego, CA, USA | Assay: 97% | USD 239–288 per 100 g |
| BIOSYNTH International, Inc., Gardner, MA, USA | n.d. | USD 302 per 100 g |
| BOC Sciences Bio-Fermen, Inc., Shirley, NY, USA | Purity: 98% | USD 299 per 500 g |
| Glentham Life Sciences Ltd., Corsham, UK | Assay: 97.0–103.0%, water content: ≤5.0% | EUR 118 per 100 g |
| Cosmetics and Pharmaceutical Intermediates | ||
| M.C. Biotec Inc., Nantong, China | Assay: 98.0–102.0%, water content: ≤5.0% | USD 40–45 per 100 g |
| Cavin Resources Malaysia, Miri, Sarawak, Malaysia | Purity: ≥98%, cosmetic/pharmaceutical grade | USD 1000 per 50 kg |
| Food Industry | ||
| Allright GC (Jinan) Biotechnology Ltd., Yiwu, China | Assay: 98.0–102.0%, water content: 1.0% max, medicine grade | USD 80–100 per 25 kg |
| Shandong WorldSun Biological Technology Co., Ltd., Jinan, China | Purity: 99.9%, food grade | USD 150–200 per 50 kg |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piątek-Gołda, W.; Osińska-Jaroszuk, M.; Pawlik, A.; Komoń-Janczara, E.; Sulej, J. Chemical Versus Biological Approaches to the Synthesis of Lactobionic Acid: A Review. Molecules 2025, 30, 3330. https://doi.org/10.3390/molecules30163330
Piątek-Gołda W, Osińska-Jaroszuk M, Pawlik A, Komoń-Janczara E, Sulej J. Chemical Versus Biological Approaches to the Synthesis of Lactobionic Acid: A Review. Molecules. 2025; 30(16):3330. https://doi.org/10.3390/molecules30163330
Chicago/Turabian StylePiątek-Gołda, Wiktoria, Monika Osińska-Jaroszuk, Anna Pawlik, Elwira Komoń-Janczara, and Justyna Sulej. 2025. "Chemical Versus Biological Approaches to the Synthesis of Lactobionic Acid: A Review" Molecules 30, no. 16: 3330. https://doi.org/10.3390/molecules30163330
APA StylePiątek-Gołda, W., Osińska-Jaroszuk, M., Pawlik, A., Komoń-Janczara, E., & Sulej, J. (2025). Chemical Versus Biological Approaches to the Synthesis of Lactobionic Acid: A Review. Molecules, 30(16), 3330. https://doi.org/10.3390/molecules30163330







