Mechanistic Study on Orpiment Pigment Discoloration Induced by Reactive Oxygen Species
Abstract
1. Introduction
2. Results
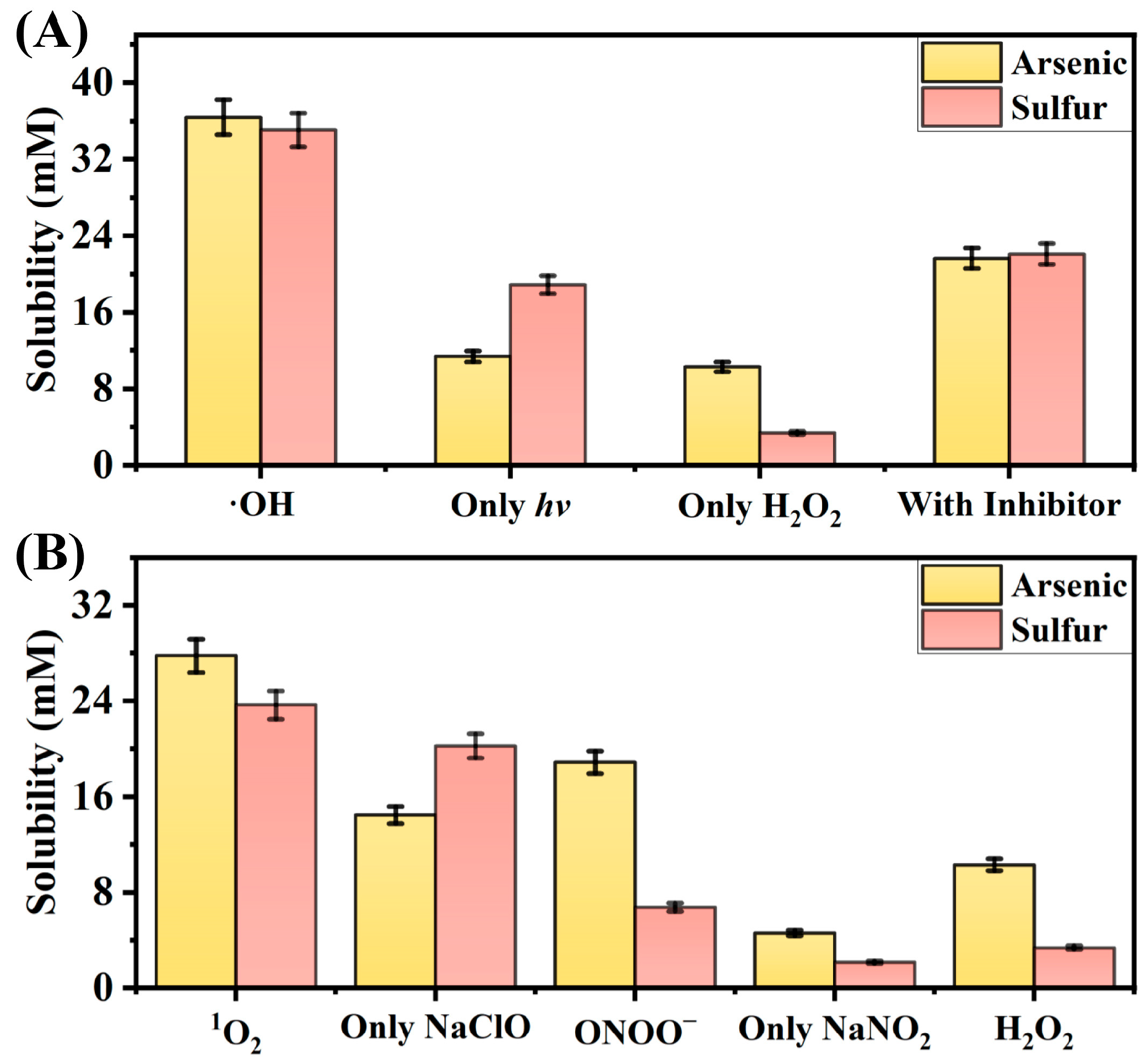
2.1. Verification of the Reactivity of Four Kinds of Reactive Oxygen Species
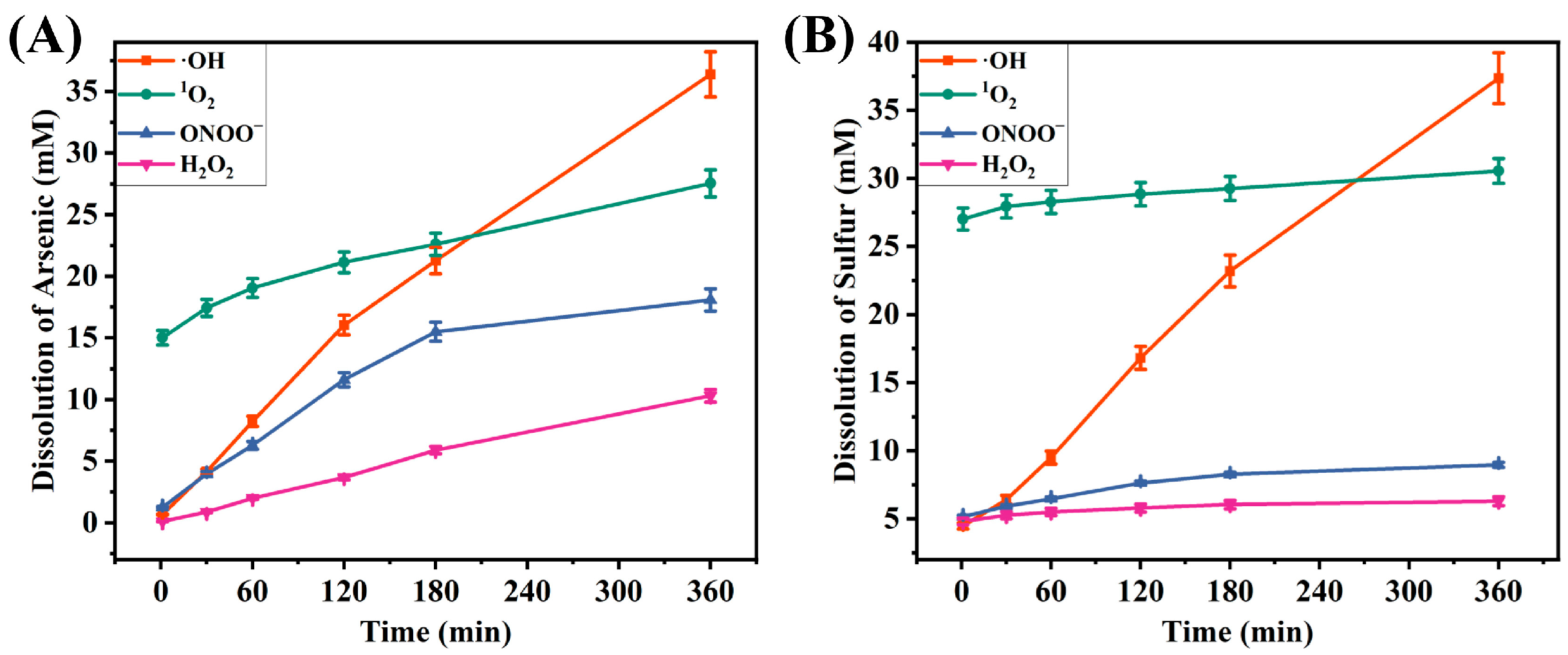
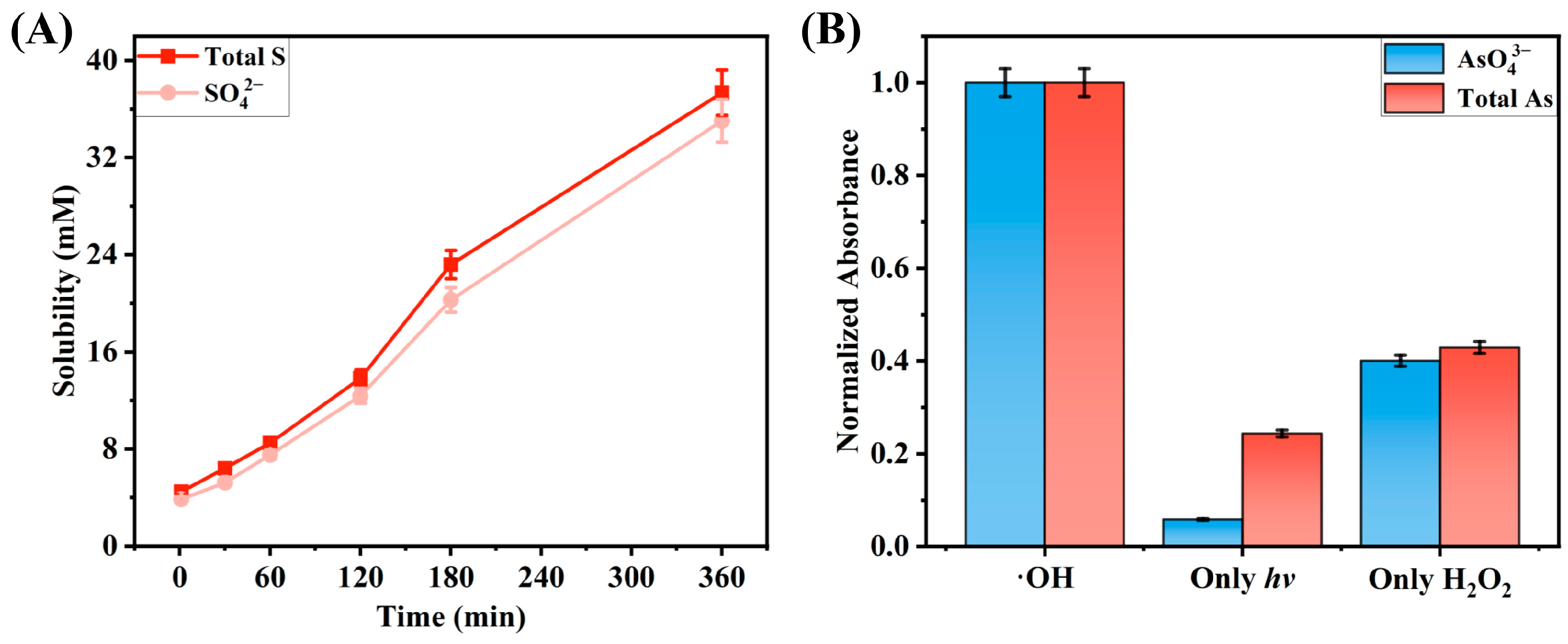
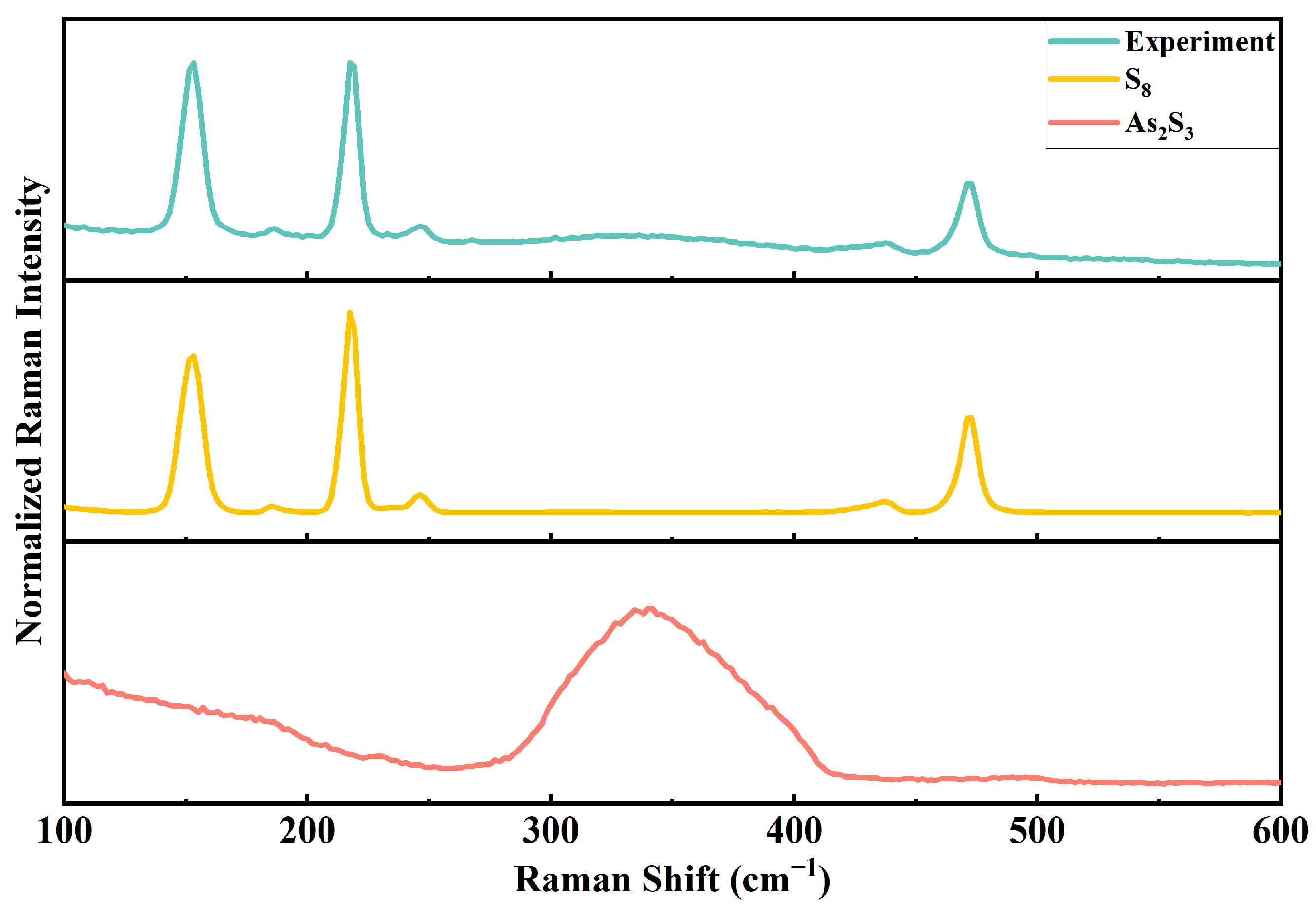
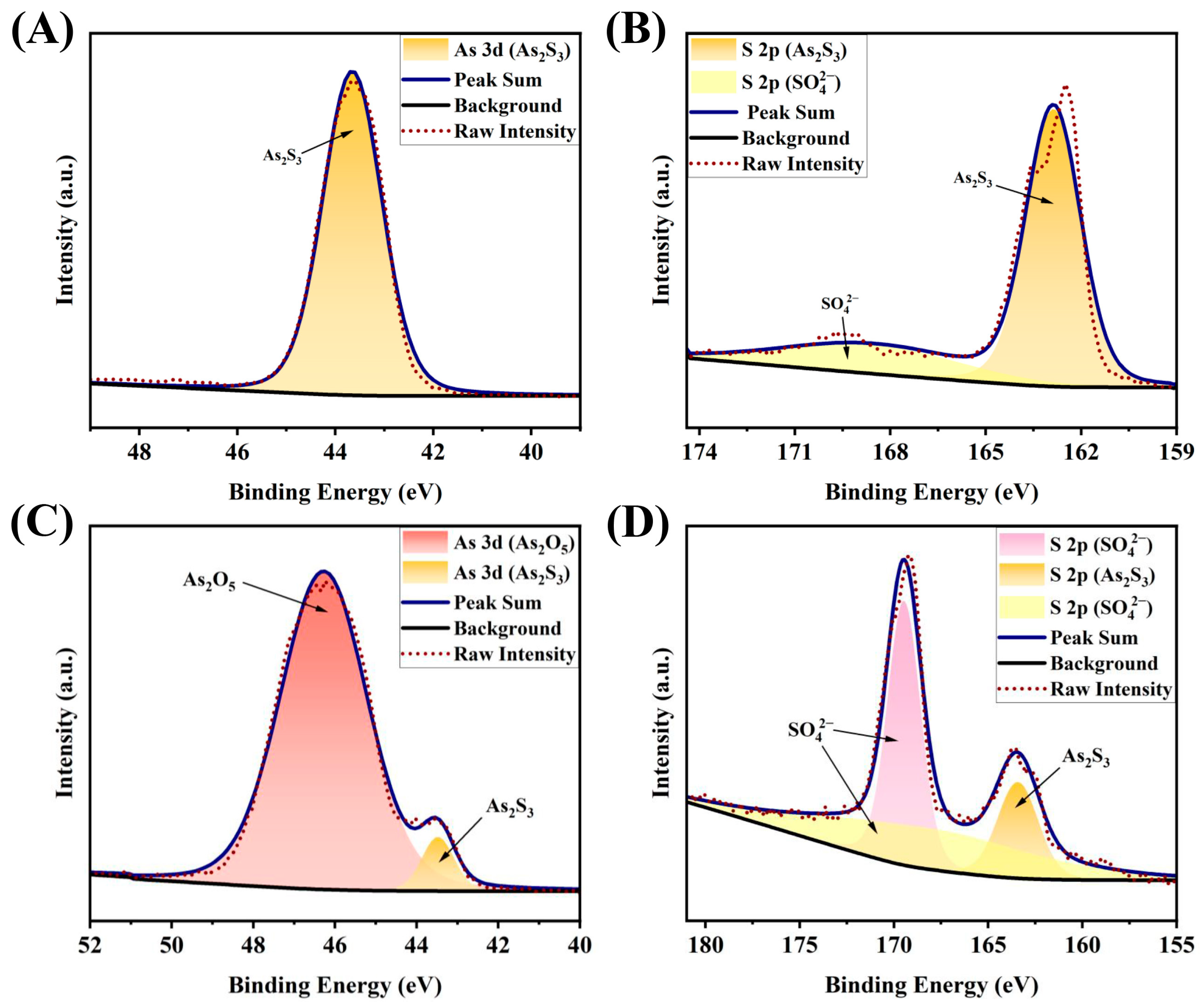
2.2. Characterization of the Reaction Results of ·OH
2.3. Characterization of the Reaction Results of the Other ROS
2.4. Based on Gibbs Free Energy Change to Analyze Reaction Equations
3. Discussion
4. Materials and Methods
4.1. Materials and Apparatus
4.2. Experimental Methods
4.2.1. Reaction of As2S3 with ·OH
4.2.2. Reaction of As2S3 with 1O2, ONOO−, or H2O2
4.3. Sample Treatment Methods
4.4. Sample Characterization
4.4.1. Inductively Coupled Plasma Optical Emission Spectrometry (ICP-OES) Test
4.4.2. Ion Chromatography (IC) Test
4.4.3. Molybdenum Blue Spectrophotometry Test
4.4.4. Raman Spectroscopy Test
4.4.5. X-Ray Photoelectron Spectroscopy (XPS) Test
4.4.6. Quantum Chemical Computational Methods and Conditions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ROS | Reactive Oxygen Species |
| ICP-OES | Inductively Coupled Plasma Optical Emission Spectrometry |
| IC | Ion Chromatography |
| XPS | X-ray Photoelectron Spectroscopy |
References
- Gliozzo, E.; Burgio, L. Pigments—Arsenic-based yellows and reds. Archaeol. Anthropol. Sci. 2021, 14, 4. [Google Scholar] [CrossRef]
- Dang, R.; Yuan, Y.; Liu, G.; Liu, J. Chromaticity changes of inorganic pigments in Chinese traditional paintings due to the illumination of frequently-used light sources in museum. Color Res. Appl. 2018, 43, 596–605. [Google Scholar] [CrossRef]
- Trentelman, K.; Stodulski, L.; Pavlosky, M. Characterization of Pararealgar and Other Light-Induced Transformation Products from Realgar by Raman Microspectroscopy. Anal. Chem. 1996, 68, 1755–1761. [Google Scholar] [CrossRef] [PubMed]
- Vermeulen, M.; Nuyts, G.; Sanyova, J.; Vila, A.; Buti, D.; Suuronen, J.-P.; Janssens, K. Visualization of As(iii) and As(v) distributions in degraded paint micro-samples from Baroque- and Rococo-era paintings. J. Anal. At. Spectrom. 2016, 31, 1913–1921. [Google Scholar] [CrossRef]
- Keune, K.; Mass, J.; Meirer, F.; Pottasch, C.; van Loon, A.; Hull, A.; Church, J.; Pouyet, E.; Cotte, M.; Mehta, A. Tracking the transformation and transport of arsenic sulfide pigments in paints: Synchrotron-based X-ray micro-analyses. J. Anal. At. Spectrom. 2015, 30, 813–827. [Google Scholar] [CrossRef]
- Simoen, J.; De Meyer, S.; Vanmeert, F.; de Keyser, N.; Avranovich, E.; Van der Snickt, G.; Van Loon, A.; Keune, K.; Janssens, K. Combined Micro- and Macro scale X-ray powder diffraction mapping of degraded Orpiment paint in a 17th century still life painting by Martinus Nellius. Herit. Sci. 2019, 7, 83. [Google Scholar] [CrossRef]
- Monico, L.; Prati, S.; Sciutto, G.; Catelli, E.; Romani, A.; Balbas, D.Q.; Li, Z.; De Meyer, S.; Nuyts, G.; Janssens, K.; et al. Development of a multi-method analytical approach based on the combination of synchrotron radiation X-ray micro-analytical techniques and vibrational micro-spectroscopy methods to unveil the causes and mechanism of darkening of “fake-gilded” decorations in a Cimabue painting. J. Anal. At. Spectrom. 2021, 37, 114–129. [Google Scholar] [CrossRef]
- De Keyser, N.; Broers, F.; Vanmeert, F.; De Meyer, S.; Gabrieli, F.; Hermens, E.; Van der Snickt, G.; Janssens, K.; Keune, K. Reviving degraded colors of yellow flowers in 17th century still life paintings with macro- and microscale chemical imaging. Sci. Adv. 2022, 8, eabn6344. [Google Scholar] [CrossRef] [PubMed]
- Keune, K.; Mass, J.; Mehta, A.; Church, J.; Meirer, F. Analytical imaging studies of the migration of degraded orpiment, realgar, and emerald green pigments in historic paintings and related conservation issues. Herit. Sci. 2016, 4, 10. [Google Scholar] [CrossRef]
- Broers, F.T.H.; Janssens, K.; Nelson Weker, J.; Webb, S.M.; Mehta, A.; Meirer, F.; Keune, K. Two Pathways for the Degradation of Orpiment Pigment (As2S3) Found in Paintings. J. Am. Chem. Society 2023, 145, 8847–8859. [Google Scholar] [CrossRef] [PubMed]
- Vermeulen, M.; Sanyova, J.; Janssens, K.; Nuyts, G.; De Meyer, S.; De Wael, K. The darkening of copper- or lead-based pigments explained by a structural modification of natural orpiment: A spectroscopic and electrochemical study. J. Anal. At. Spectrom. 2017, 32, 1331–1341. [Google Scholar] [CrossRef]
- Monks, P.S. Gas-phase radical chemistry in the troposphere. Chem. Soc. Rev. 2005, 34, 376–395. [Google Scholar] [CrossRef] [PubMed]
- Platt, U. Spectroscopic measurement of free radicals (OH, NO3) in the atmosphere. Fresenius’ J. Anal. Chem. 1991, 340, 633–637. [Google Scholar] [CrossRef]
- Petkov, K.; Krastev, V.; Marinova, T. XPS study of amorphous As2S3 films deposited onto chromium layers. Surf. Interface Anal. 1994, 22, 202–205. [Google Scholar] [CrossRef]
- Wren, A.G.; Phillips, R.W.; Tolentino, L.U. Surface reactions of chlorine molecules and atoms with water and sulfuric acid at low temperatures. J. Colloid Interface Sci. 1979, 70, 544–557. [Google Scholar] [CrossRef]
- Flinn, B.J.; McIntyre, N.S. Studies of the UV/Ozone oxidation of GaAs using angle-resolved x-ray photoelectron spectroscopy. Surf. Interface Anal. 1990, 15, 19–26. [Google Scholar] [CrossRef]
- Lindau, I.; Pianetta, P.; Garner, C.; Chye, P.; Gregory, P.; Spicer, W. Photoemission studies of the electronic structure of III–V semiconductor surfaces. Surf. Sci. 1977, 63, 45–55. [Google Scholar] [CrossRef]
- Zhu, X.; Zhong, Y.; Zhang, X.; Yang, D.; Zhang, L.; Wang, J.; Feng, W.; Zhang, W. Facile fabrication of BiOI/Bi2WO6 Z-scheme heterojunction composites with a three-dimensional structure for efficient degradation of pollutants. Arab. J. Chem. 2023, 16, 105286. [Google Scholar] [CrossRef]
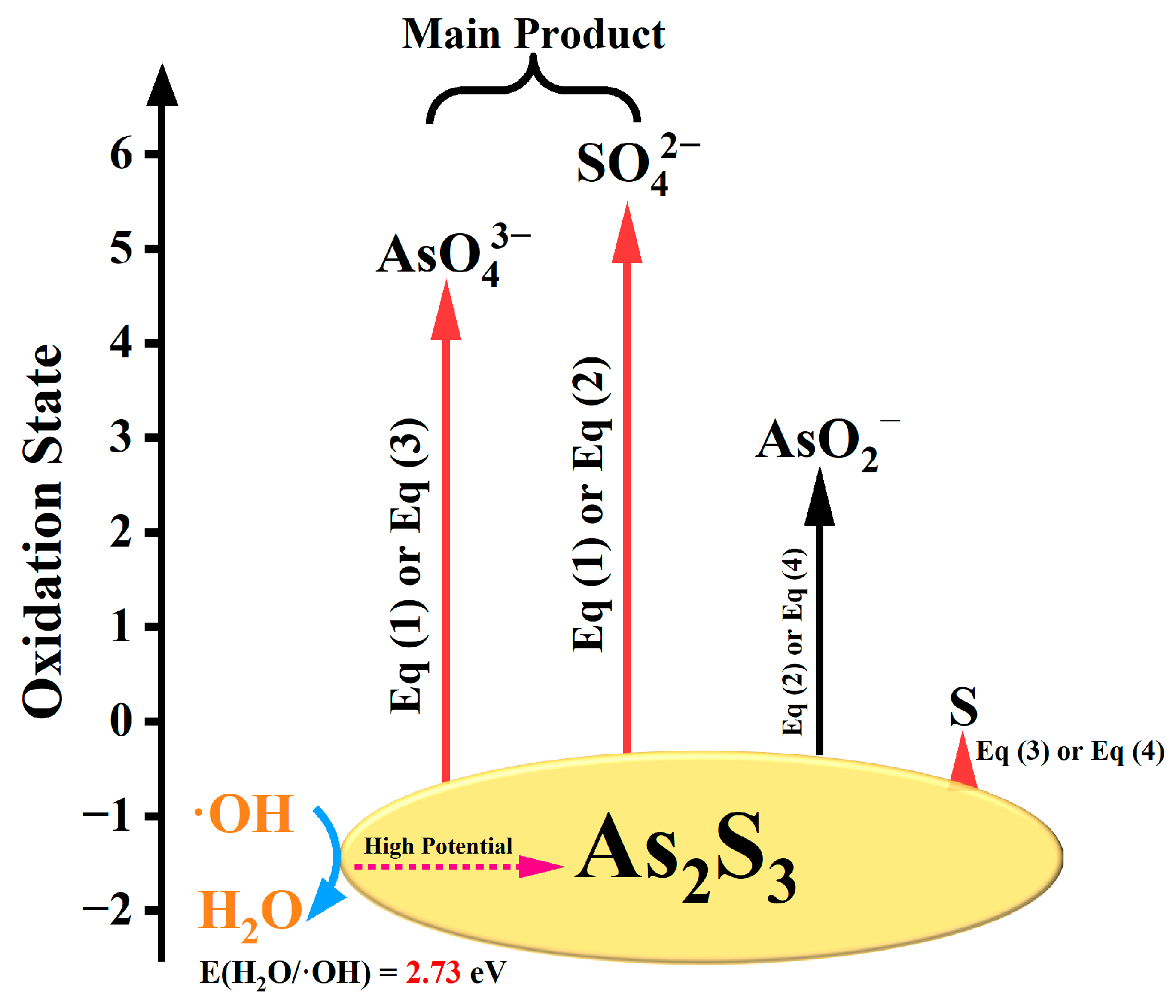
| Eqs | Reaction | ΔG/(kJ/mol) |
|---|---|---|
| (1) | As2S3 + 28·OH → 2H3AsO4 + 3H2SO4 + 8H2O | −4893 |
| (2) | As2S3 + 24·OH → 2HAsO2 + 3H2SO4 + 8H2O | −4188 |
| (3) | As2S3 + 10·OH → 2H3AsO4 + 3S + 2H2O | −785.7 |
| (4) | As2S3 + 6·OH → 2HAsO2 + 3S + 2H2O | −80.48 |
| Reagents | NaClO | NaNO2 | H2O2 | H2O | |
|---|---|---|---|---|---|
| 1O2 | Sample | 7.6 mL | - | 220 μL | 42.4 mL |
| Control | 7.6 mL | - | - | 42.4 mL | |
| ONOO− | Sample | - | 158 mg | 220 μL | 50 mL |
| Control | - | 158 mg | - | 50 mL | |
| H2O2 | Sample | - | - | 220 μL | 50 mL |
| Control | - | - | - | 50 mL | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, J.; Zhang, Z.; Chen, X.; Huang, Q.; Bian, Z.; Zhang, W.; Su, B.; Zhang, H. Mechanistic Study on Orpiment Pigment Discoloration Induced by Reactive Oxygen Species. Molecules 2025, 30, 3318. https://doi.org/10.3390/molecules30163318
Sun J, Zhang Z, Chen X, Huang Q, Bian Z, Zhang W, Su B, Zhang H. Mechanistic Study on Orpiment Pigment Discoloration Induced by Reactive Oxygen Species. Molecules. 2025; 30(16):3318. https://doi.org/10.3390/molecules30163318
Chicago/Turabian StyleSun, Jiaxing, Zhehan Zhang, Xiaofen Chen, Qin Huang, Zhilin Bian, Wenyuan Zhang, Bomin Su, and Haixia Zhang. 2025. "Mechanistic Study on Orpiment Pigment Discoloration Induced by Reactive Oxygen Species" Molecules 30, no. 16: 3318. https://doi.org/10.3390/molecules30163318
APA StyleSun, J., Zhang, Z., Chen, X., Huang, Q., Bian, Z., Zhang, W., Su, B., & Zhang, H. (2025). Mechanistic Study on Orpiment Pigment Discoloration Induced by Reactive Oxygen Species. Molecules, 30(16), 3318. https://doi.org/10.3390/molecules30163318






