GelMA Core–Shell Microgel Preparation Based on a Droplet Microfluidic Device for Three-Dimensional Tumor Ball Culture and Its Drug Testing
Abstract
1. Introduction
2. Results and Discussion
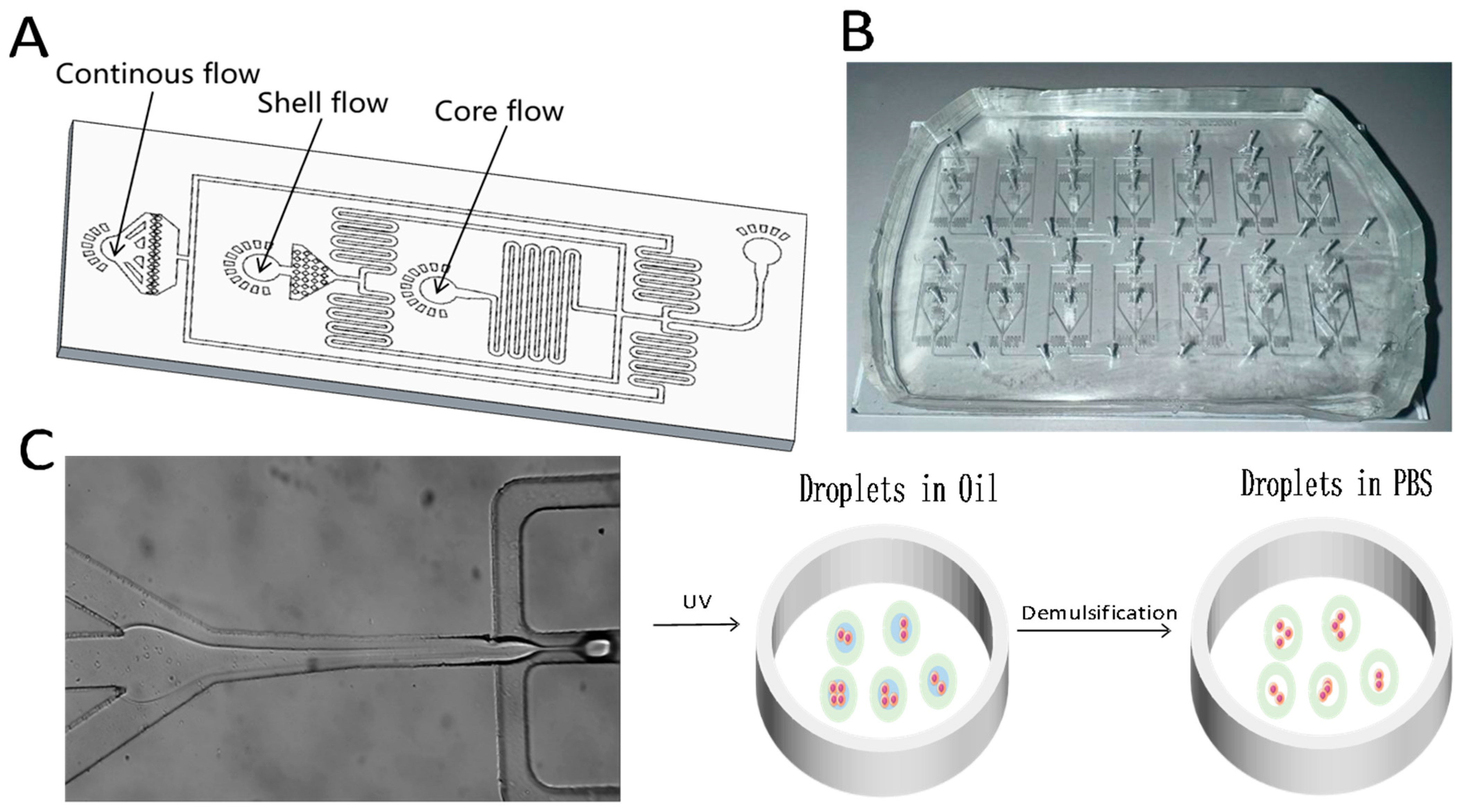
2.1. Design and Fabrication of Droplet Microfluidic Chips
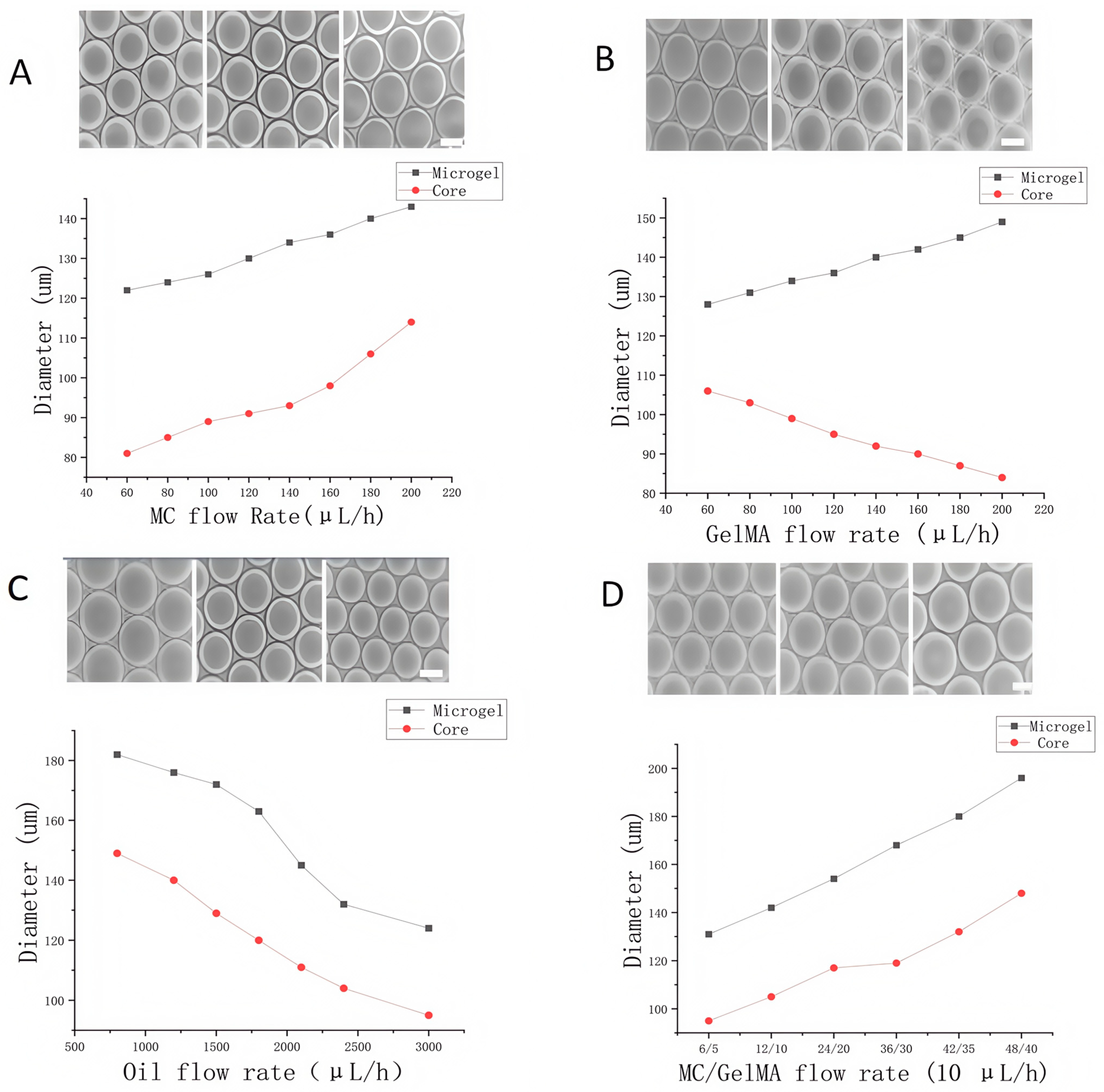
2.2. Optimization of Flow Rates in Droplet Microfluidic Chips
3. Performance Validation of a Three-Dimensional Cell Culture System Based on a Droplet Microfluidic Chip
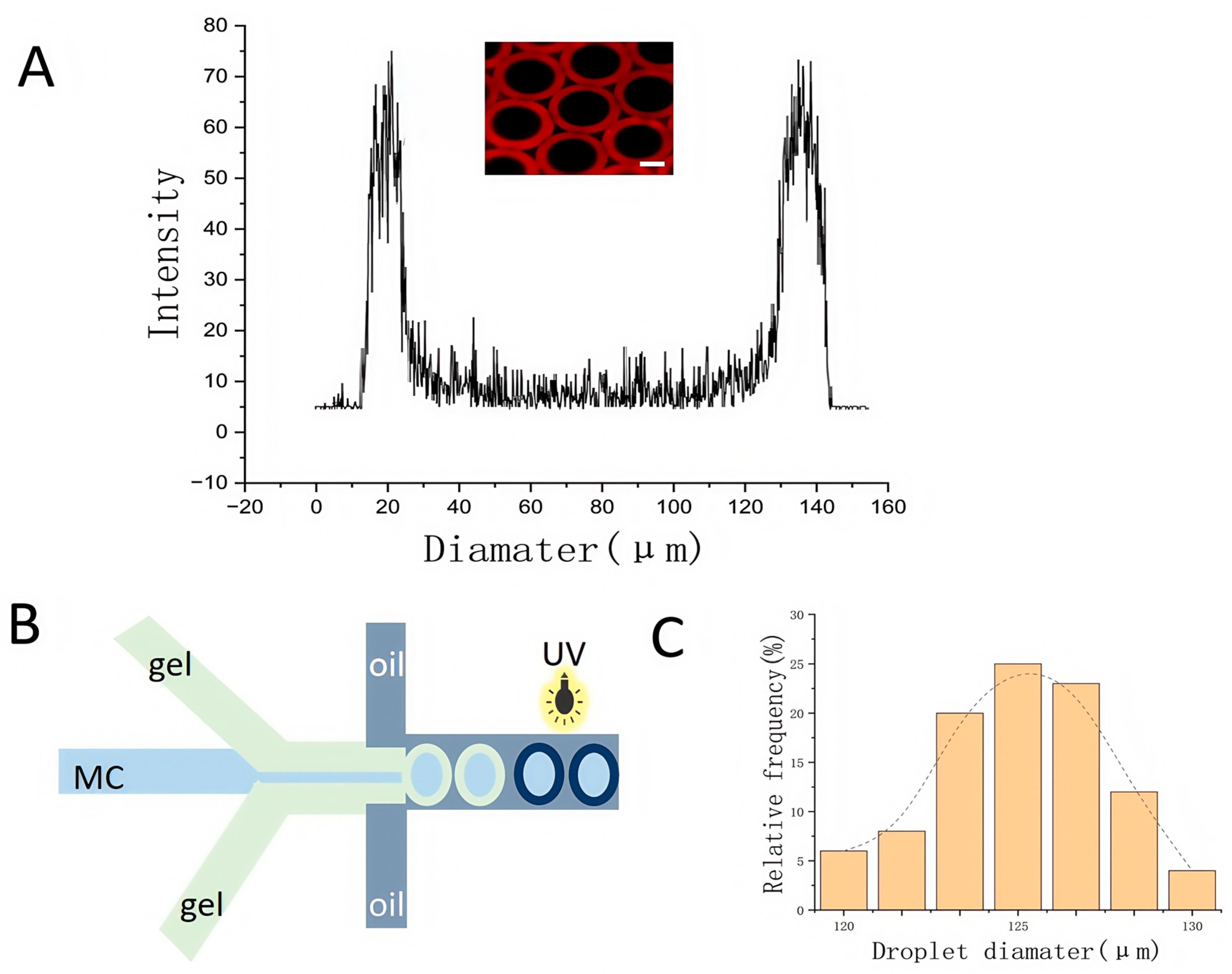
3.1. Permeability Testing of GelMA Core–Shell Microgels
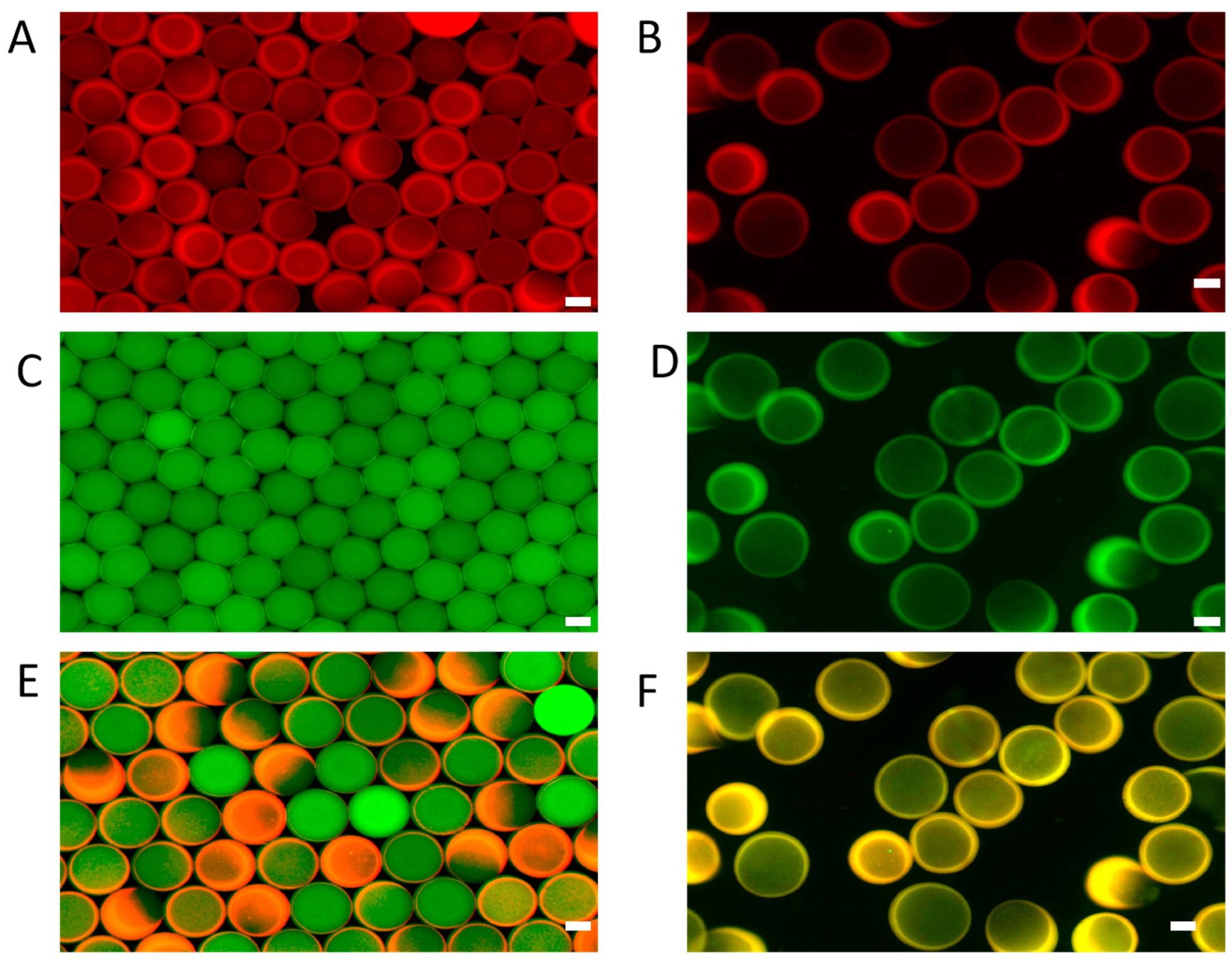
3.2. Morphological Characterization of GelMA Core–Shell Microgels
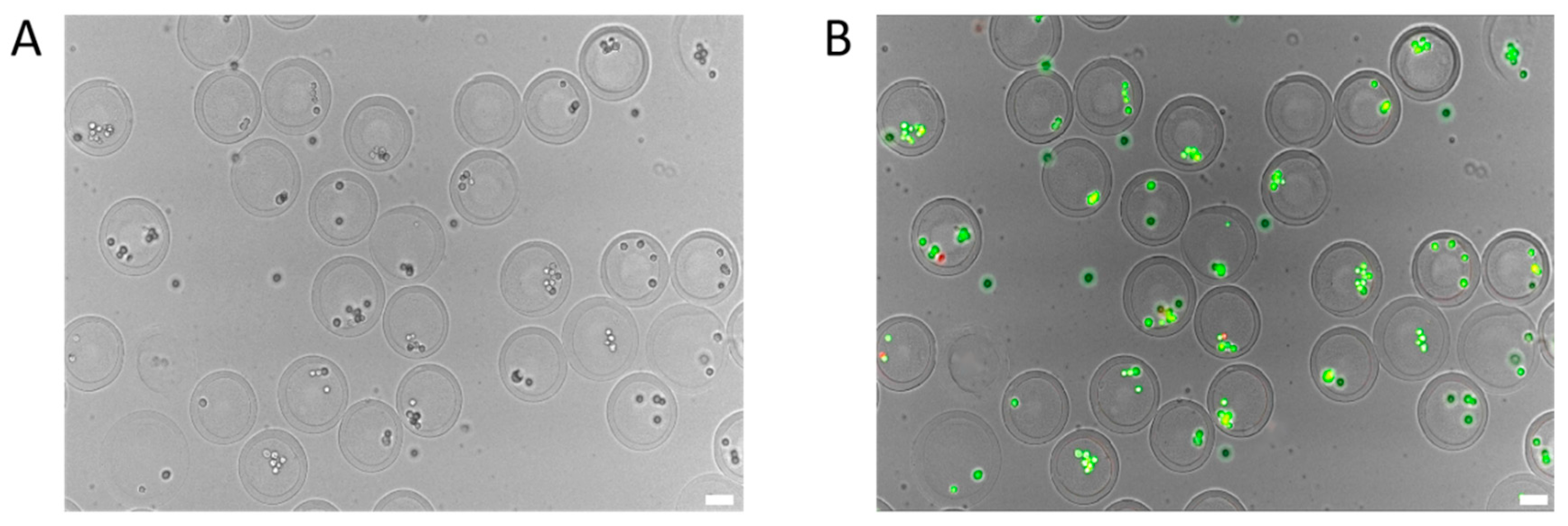
3.3. Testing the Effect of Cell Encapsulation Process on Cell Activity Based on Droplet Microfluidic Chip
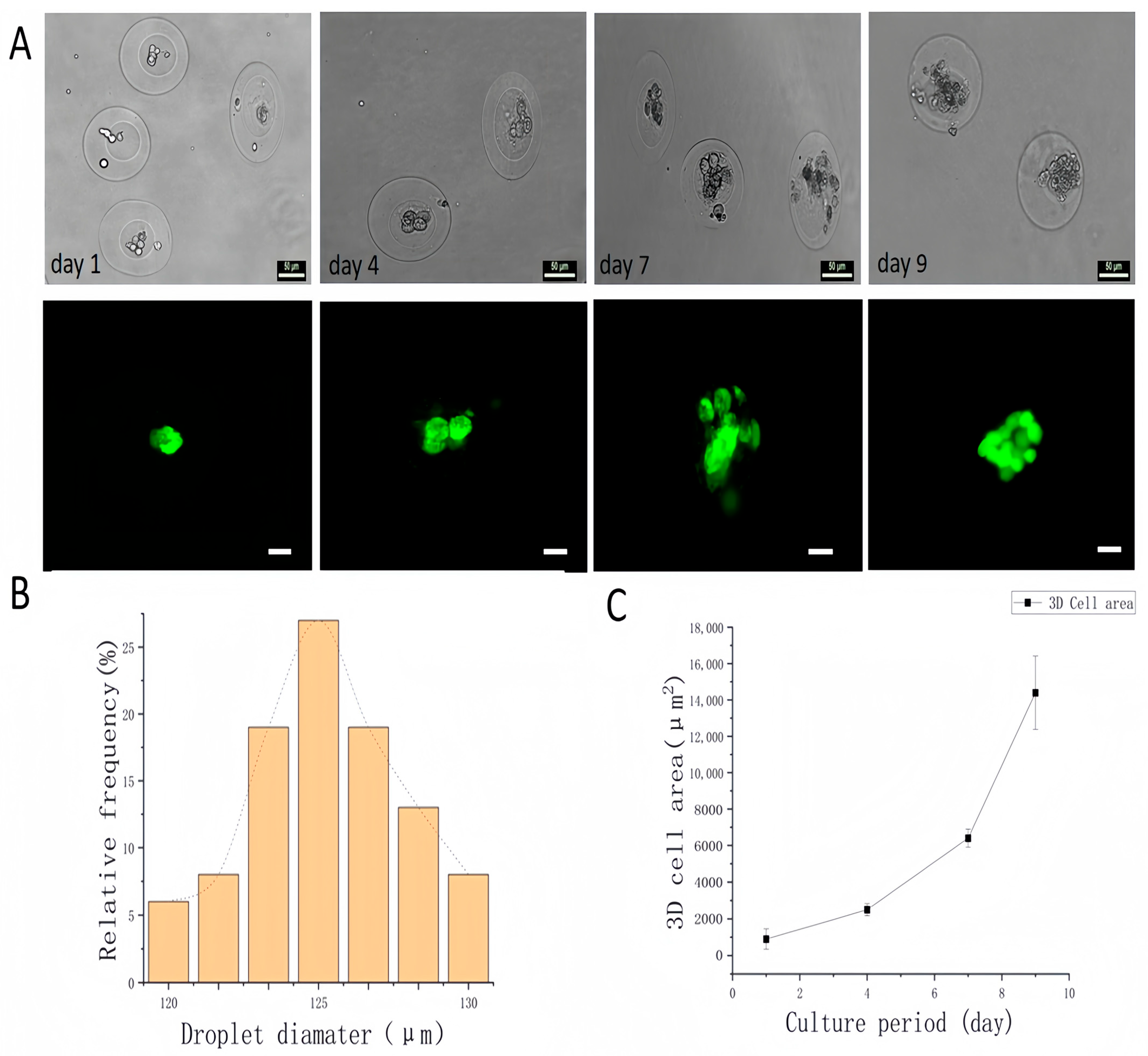
4. Core–Shell Microgels as Carriers for Three-Dimensional Cell Culture
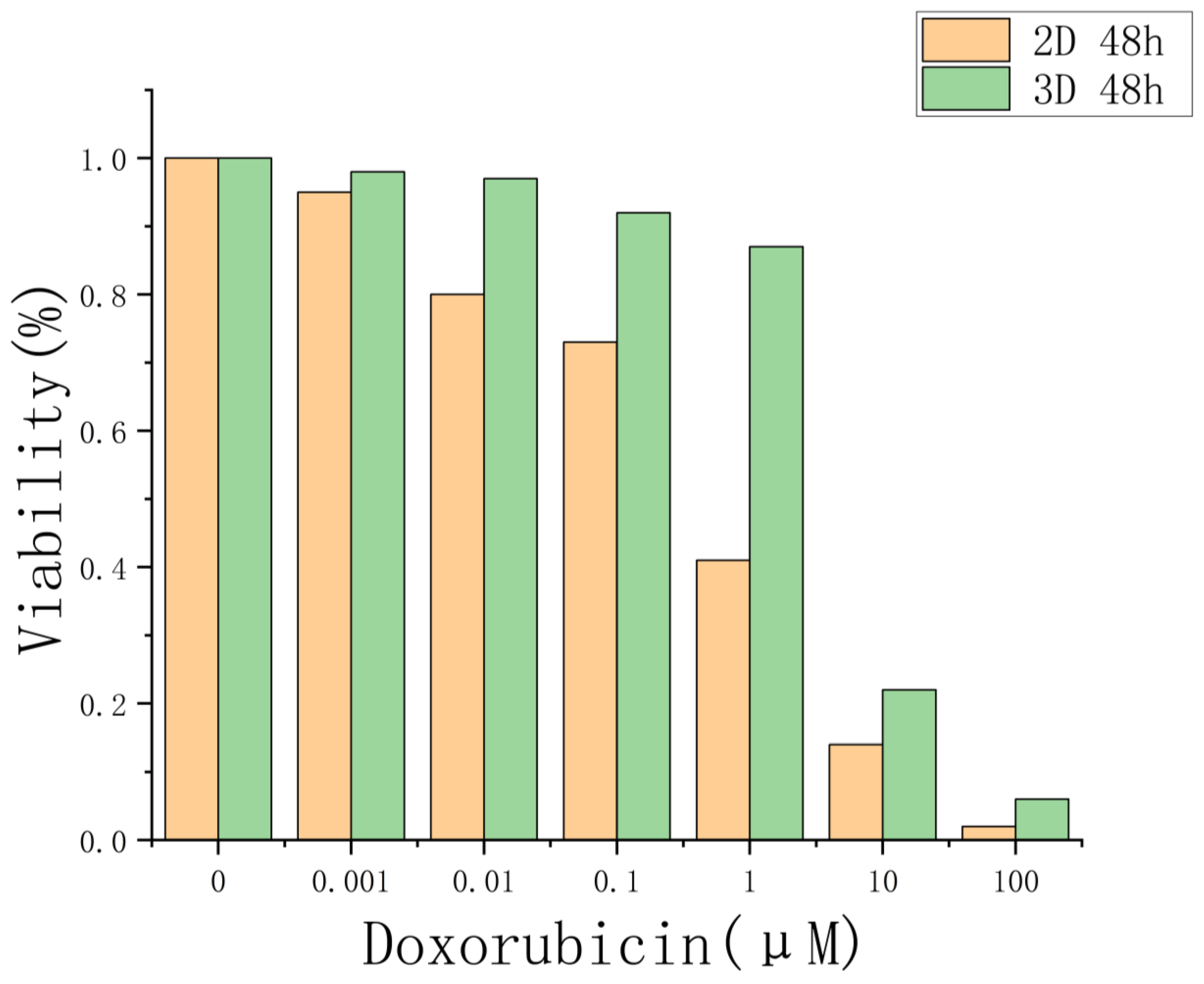
5. Drug Evaluation Based on Three-Dimensional Cells of Core–Shell Microgels
6. Experimental Section
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, Y.; Kankala, R.K.; Zhu, K.; Wang, S.B.; Chen, A.Z. Coaxial extrusion of tubular tissue constructs using a gelatin/GelMA blend bioink. ACS Biomater. Sci. Eng. 2019, 5, 5514–5524. [Google Scholar] [CrossRef]
- Augustine, R.; Zahid, A.A.; Hasan, A.; Dalvi, Y.B.; Jacob, J. Cerium oxide nanoparticle-loaded gelatin methacryloyl hydrogel wound-healing patch with free radical scavenging activity. ACS Biomater. Sci. Eng. 2021, 7, 279–290. [Google Scholar]
- Lv, B.; Lu, L.; Hu, L.; Cheng, P.; Hu, Y.; Xie, X.; Liu, G. Recent advances in GelMA hydrogel transplantation for musculoskeletal disorders and related disease treatment. Theranostics 2023, 13, 2015–2039. [Google Scholar] [CrossRef] [PubMed]
- Rastin, H.; Ormsby, R.T.; Atkins, G.J.; Losic, D. 3D bioprinting of methylcellulose/gelatin-methacryloyl (MC/GelMA) bioink with high shape integrity. ACS Appl. Bio Mater. 2020, 3, 1815–1826. [Google Scholar] [CrossRef] [PubMed]
- Ning, L.; Mehta, R.; Cao, C.; Andrea, T.; Martin, T.; Ning, Z.; Weeks, E.R.; Bauser-Heaton, H.; Vahid, S. Embedded 3D bioprinting of gelatin methacryloyl-based constructs with highly tunable structural fidelity. ACS Appl. Mater. Interfaces 2020, 12, 44563–44577. [Google Scholar] [CrossRef]
- Song, C.; Wang, L.; Ye, G.; Song, X.; He, Y.; Qiu, X. Residual ammonium persulfate in nanoparticles has cytotoxic effects on cells through epithelial-mesenchymal transition. Sci. Rep. 2017, 7, 11769. [Google Scholar] [CrossRef]
- Guermani, H.; Shaki, S.; Mohanty, M.; Mehrali, A.; Arpanaei, A.K.; Gaharwar, A. Dolatshahi-Pirouz, Engineering complex tissue-like microgel arrays for evaluating stem cell differentiation. Sci. Rep. 2016, 6, 30445. [Google Scholar] [CrossRef]
- Mũnoz, Z.; Shih, H.; Lin, C.-C. Gelatin hydrogels formed by orthogonal thiol–norbornene photochemistry for cell encapsulation. Biomater. Sci. 2014, 2, 1063–1072. [Google Scholar] [CrossRef]
- Mahadik, B.P.; Pedron Haba, S.; Skertich, L.J.; Harley, B.A.C. The use of covalently immobilized stem cell factor to selectively affect hematopoietic stem cell activity within a gelatin hydrogel. Biomaterials 2015, 67, 297–307. [Google Scholar] [CrossRef]
- Lin, R.Z.; Chen, Y.C.; Moreno Luna, R.; Khademhosseini, A.; Melero-Martin, J.M. Transdermal regulation of vascular network bioengineering using a photopolymerizable methacrylated gelatin hydrogel. Biomaterials 2013, 34, 6785–6796. [Google Scholar] [CrossRef]
- Agrawal, G.; Agrawal, R. Functional Microgels: Recent Advances in Their Biomedical Applications. Small 2018, 14, e1801724. [Google Scholar] [CrossRef]
- Wang, H.; Liu, H.; Liu, H.; Su, W.; Chen, W.; Qin, J. One-Step Generation of Core–Shell Gelatin Methacrylate (GelMA) Microgels Using a Droplet Microfluidic System. Adv. Mater. Technol. 2019, 4, 1800632. [Google Scholar] [CrossRef]
- Xia, P.; Zhang, K.; Gong, Y.; Li, G.; Yan, S.; Yin, J. Injectable stem cell laden open porous microgels that favor adipogenesis: In vitro and in vivo evaluation. ACS Appl. Mater. Interfaces 2017, 9, 34751–34761. [Google Scholar] [CrossRef] [PubMed]
- Lesher-Pérez, S.C.; Kim, G.-A.; Kuo, C.; Leung, B.M.; Mong, S.; Kojima, T.; Takayama, S. Dispersible oxygen microsensors map oxygen gradients in three-dimensional cell cultures. Biomater. Sci. 2017, 5, 2106–2113. [Google Scholar] [CrossRef] [PubMed]
- Faulkner-Jones, A.; Greenhough, S.; King, J.A.; Gardner, J.; Courtney, A.; Shu, W. Development of a valve-based cell printer for the formation of human embryonic stem cell spheroid aggregates. Biofabrication 2013, 5, 015013. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, S.; Yildirimer, L.; Zhao, H.; Ding, R.; Wang, H.; Weitz, D. Injectable stem cell-laden photocrosslinkable microspheres fabricated using microfluidics for rapid generation of osteogenic tissue constructs. Adv. Funct. Mater. 2016, 26, 2809–2819. [Google Scholar] [CrossRef]
- Cha, C.; Oh, J.; Kim, K.; Qiu, Y.; Joh, M.; Shin, S.R.; Khademhosseini, A. Microfluidics-assisted fabrication of gelatin-silica core–shell microgels for injectable tissue constructs. Biomacromolecules 2014, 15, 283–290. [Google Scholar] [CrossRef]
- Mohamed, M.G.A.; Kheiri, S.; Islam, S.; Kumar, H.; Yang, A.; Kim, K. An integrated microfluidic flow-focusing platform for on-chip fabrication and filtration of cell-laden microgels. Lab A Chip 2019, 19, 1621–1632. [Google Scholar] [CrossRef]
- Ou, Y.; Cao, S.; Zhang, Y.; Zhu, H.; Guo, C.; Yan, W.; Xin, F.; Dong, W.; Zhang, Y.; Narita, M.; et al. Bioprinting microporous functional living materials from protein-based core-shell microgels. Nat. Commun. 2023, 14, 322. [Google Scholar] [CrossRef]
- Kim, J.Y.; Kim, G.H. Formation of various cell-aggregated structures in the core of hydrogel filament using a microfluidic device and its application as an in vitro neuromuscular junction model. Chem. Eng. J. 2023, 472, 144979. [Google Scholar] [CrossRef]
- Nan, L.; Yang, Z.; Lyu, H.; Lau, K.Y.Y.; Shum, H.C. A microfluidic system for one-chip harvesting of single-cell-laden hydrogels in culture medium. Adv. Biosyst. 2019, 3, 1900076. [Google Scholar] [CrossRef]
- Tang, H.; Bi, F.; Chen, G.; Zhang, S.; Huang, Y.; Chen, J.; Xie, L.; Qiao, X.; Guo, W. 3D-bioprinted Recombination Structure of Hertwig’s Epithelial Root Sheath Cells and Dental Papilla Cells for Alveolar Bone Regeneration. Int. J. Bioprint. 2022, 8, 512. [Google Scholar] [CrossRef]
- Durruthy-Durruthy, R.; Gottlieb, A.; Heller, S. 3D computational reconstruction of tissues with hollow spherical morphologies using single-cell gene expression data. Nat. Protoc. 2015, 10, 459–474. [Google Scholar] [CrossRef]
- Alsina, B.; Whitfield, T.T. Sculpting the labyrinth: Morphogenesis of the developing inner ear. Semin. Cell Dev. Biol. 2017, 65, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Chamberlain, C.G.; McAvoy, J. Induction of lens fibre differentiation by acidic and basic fibroblast growth factor (FGF). Growth Factors 1989, 1, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Basil, M.C.; Cardenas-Diaz, F.L.; Kathiriya, J.J.; Morley, M.P.; Carl, J.; Brumwell, A.N.; Katzen, J.; Slovik, K.J.; Babu, A.; Zhou, S.; et al. Human distal airways contain a multipotent secretory cell that can regenerate alveoli. Nature 2022, 604, 120–126. [Google Scholar] [CrossRef]
- McAvoy, J.W.; Chamberlain, C.G.; de Iongh, R.U.; Hales, A.M.; Lovicu, F.J. Lens development. Eye 1999, 13 Pt 3b, 425–437. [Google Scholar] [CrossRef]
- Koehler, K.R.; Mikosz, A.M.; Molosh, A.I.; Patel, D.; Hashino, E. Generation of inner ear sensory epithelia from pluripotent stem cells in 3D culture. Nature 2013, 500, 217–221. [Google Scholar] [CrossRef]
- Hofbauer, P.; Jahnel, S.; Papai, N.; Giesshammer, M.; Mendjan, S. Cardioids Reveal Self-Organizing Principles of Human Cardiogenesis. Cold Spring Harb. Lab. 2020, 184, 3299–3317. [Google Scholar] [CrossRef]
- Chen, J.; Liu, Z.; Wang, Z.; Zhang, X.; Zhang, Y.; Zhan, Z.; Xu, T. One-step biofabrication of liquid core—GelMA shell microbeads for in situ hollow cell ball self-assembly. Regen. Biomater. 2024, 11, rbae021. [Google Scholar] [CrossRef]
- Updegraff, D.M. Semimicro Determination of Cellulose in Biological Materials. Anal. Biochem. 1969, 32, 420. [Google Scholar] [CrossRef]
- Maritan, S.M.; Lian, E.Y.; Mulligan, L.M. An Efficient and Flexible Cell Aggregation Method for 3D Spheroid Production. J. Vis. Exp. 2017, 121, 55544. [Google Scholar]
- Agarwal, P.; Zhao, S.; Bielecki, P.; Rao, W.; Choi, J.K.; Zhao, Y.; He, X. One-Step Microfluidic Generation of Pre-Hatching Embryo-like Core–Shell Microcapsules for Miniaturized 3D Culture of Pluripotent Stem Cells. Lab A Chip 2013, 13, 4525–4533. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Wei, W.; Wang, Y.; Xu, C.; Guo, Y.; Qin, J. Simple Spinning of Heterogeneous Hollow Microfibers on Chip. Adv. Mater. 2016, 28, 6649–6655. [Google Scholar] [CrossRef]
- Xia, Z.; Villa, M.M.; Wei, M. A Biomimetic Collagen-Apatite Scaffold with a Multi-Level Lamellar Structure for Bone Tissue Engineering. J. Mater. Chem. B 2014, 2, 1998–2007. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Zhang, D.; Alexander, P.G.; Yang, G.; Tan, J.; Cheng, A.W.-M.; Tuan, R.S. Application of visible light-based projection stereolithography for live cell-scaffold fabrication with designed architecture. Biomaterials 2013, 34, 331–339. [Google Scholar] [CrossRef]
- Shikanov, A.; Smith, R.M.; Xu, M.; Woodruff, T.K.; Shea, L.D. Hydrogel network design using multifunctional macromers to coordinate tissue maturation in ovarian follicle culture. Biomaterials 2011, 32, 2524–2531. [Google Scholar] [CrossRef]
- Jiang, Z.; Xia, B.; McBride, R.; Oakey, J. A microfluidic-based cell encapsulation platform to achieve high long-term cell viability in photopolymerized PEGNB hydrogel microspheres. J. Mater. Chem. B 2017, 5, 173–180. [Google Scholar] [CrossRef]
- Jiang, C.; Kuang, L.; Merkel, M.P.; Yue, F.; Cano-Vega, M.A.; Narayanan, N.; Kuang, S.; Deng, M. Biodegradable Polymeric Microsphere-Based Drug Delivery for Inductive Browning of Fat. Front. Endocrinol. 2015, 6, 169. [Google Scholar] [CrossRef]
- Luther, J.; Garber, J.J.; Khalili, H.; Dave, M.; Bale, S.S.; Jindal, R.; Motola, D.L.; Luther, S.; Bohr, S.; Jeoung, S.W.; et al. Hepatic Injury in Nonalcoholic Steatohepatitis Contributes to Altered Intestinal Permeability. Cell Mol. Gastroenterol. Hepatol. 2015, 1, 222–232. [Google Scholar] [CrossRef]
- Natarajan, R.; Northrop, N.; Yamamoto, B. Fluorescein Isothiocyanate (FITC)-Dextran Extravasation as a Measure of Blood-Brain Barrier Permeability. Curr. Protoc. Neurosci. 2017, 79, 9.58.1–9.58.15. [Google Scholar] [CrossRef] [PubMed]
- Shuichi, M.; Shimono, K. Molecular Modeling to Estimate the Diffusion Coefficients of Drugs and Other Small Molecules. Molecules 2020, 25, 5340. [Google Scholar] [CrossRef] [PubMed]
- Kaemmerer, E.; Melchels, F.P.; Holzapfel, B.M.; Meckel, T.; Hutmacher, D.W.; Loessner, D. Gelatine Methacrylamide-Based Hydrogels: An Alternative Three-Dimensional Cancer Cell Culture System. Acta Biomater. 2014, 10, 2551–2562. [Google Scholar] [CrossRef] [PubMed]
- Aung, A.; Theprungsirikul, J.; Lim, H.L.; Varghese, S. Chemotaxis-Driven Assembly of Endothelial Barrier in a Tumor-on-a-Chip Platform. Lab A Chip 2016, 16, 1886–1898. [Google Scholar] [CrossRef]
- Aung, A.; Kumar, V.; Theprungsirikul, J.; Davey, S.K.; Varghese, S. An Engineered Tumor-on-a-Chip Device with Breast Cancer–Immune Cell Interactions for Assessing T-cell Recruitment. Cancer Res. 2020, 80, 263–275. [Google Scholar] [CrossRef]
- Moon, Y.; Shim, M.K.; Choi, J.; Yang, S.; Kim, J.; Yun, W.S.; Cho, H.; Park, J.Y.; Kim, Y.; Seong, J.-K.; et al. Anti-PD-L1 peptide-conjugated prodrug nanoparticles for targeted cancer immunotherapy combining PD-L1 blockade with immunogenic cell death. Theranostics 2022, 12, 1999–2014. [Google Scholar] [CrossRef]
- Chen, X.; Gu, J.; Sun, L.; Li, W.; Guo, L.; Gu, Z.; Wang, L.; Zhang, Y.; Zhang, W.; Han, B.; et al. Efficient drug delivery and anticancer effect of micelles based on vitamin E succinate and chitosan derivatives. Bioact. Mater. 2021, 6, 3025–3035. [Google Scholar] [CrossRef]
- Yu, J.; Wang, Y.; Zhou, S.; Li, J.; Wang, J.; Chi, D.; Wang, X.; Lin, G.; He, Z.; Wang, Y. Remote loading paclitaxel-doxorubicin prodrug into liposomes for cancer combination therapy. Acta Pharm. Sin. B 2020, 10, 1730–1740. [Google Scholar] [CrossRef]
- Sasaki, K.; Ishihara, J.; Ishihara, A.; Miura, R.; Mansurov, A.; Fukunaga, K.; Hubbell, J.A. Engineered collagen-binding serum albumin as a drug conjugate carrier for cancer therapy. Sci. Adv. 2019, 5, eaaw6081. [Google Scholar] [CrossRef]
- Musella, M.; Guarracino, A.; Manduca, N.; Galassi, C.; Ruggiero, E.; Potenza, A.; Maccafeo, E.; Manic, G.; Mattiello, L.; Rehim, S.S.A.; et al. Type I IFNs promote cancer cell stemness by triggering the epigenetic regulator KDM1B. Nat. Immunol. 2022, 23, 1379–1392. [Google Scholar] [CrossRef]
- Mishima, E.; Ito, J.; Wu, Z.; Nakamura, T.; Wahida, A.; Doll, S.; Tonnus, W.; Nepachalovich, P.; Eggenhofer, E.; Aldrovandi, M.; et al. A non-canonical vitamin K cycle is a potent ferroptosis suppressor. Nature 2022, 608, 778–783. [Google Scholar] [CrossRef]
- Lee, J.-H.; Kim, S.; Han, S.; Min, J.; Caldwell, B.; Bamford, A.-D.; Rocha, A.S.B.; Park, J.; Lee, S.; Wu, S.-H.S.; et al. p57 Kip2 imposes the reserve stem cell state of gastric chief cells. Cell Stem Cell 2022, 29, 826–839.e9. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Sabanayagam, C.R.; Harrington, D.A.; Farach-Carson, M.C.; Jia, X. A hydrogel-based tumor model for the evaluation of nanoparticle-based cancer therapeutics. Biomaterials 2014, 35, 3319–3330. [Google Scholar] [CrossRef]
- Baboota, R.K.; Rawshani, A.; Bonnet, L.; Li, X.; Yang, H.; Mardinoglu, A.; Tchkonia, T.; Kirkland, J.L.; Hoffmann, A.; Dietrich, A.; et al. BMP4 and Gremlin 1 regulate hepatic cell senescence during clinical progression of NAFLD/NASH. Nat. Metab. 2022, 4, 1007–1021. [Google Scholar] [CrossRef]
- Lennaárd, A.J.; Mamand, D.R.; Wiklander, R.J.; EL Andaloussi, S.; Wiklander, O.P.B. Optimised Electroporation for Loading of Extracellular Vesicles with Doxorubicin. Pharmaceutics 2021, 14, 38. [Google Scholar] [CrossRef]
- Ouyang, L.; Armstrong, J.P.K.; Chen, Q.; Lin, Y.; Stevens, M.M. Void-free 3D Bioprinting for In-situ Endothelialization and Microfluidic Perfusion. Adv. Funct. Mater. 2020, 30, 1908349. [Google Scholar] [CrossRef]
- Bachireddy, P.; Ennis, C.; Nguyen, V.N.; Gohil, S.H.; Clement, K.; Shukla, S.A.; Forman, J.; Barkas, N.; Freeman, S.; Bavli, N.; et al. Distinct evolutionary paths in chronic lymphocytic leukemia during resistance to the graft-versus-leukemia effect. Sci. Transl. Med. 2020, 12, eabb7661. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, X.; Xu, Y.; Zhu, D.; Mi, X. GelMA Core–Shell Microgel Preparation Based on a Droplet Microfluidic Device for Three-Dimensional Tumor Ball Culture and Its Drug Testing. Molecules 2025, 30, 3305. https://doi.org/10.3390/molecules30153305
Yang X, Xu Y, Zhu D, Mi X. GelMA Core–Shell Microgel Preparation Based on a Droplet Microfluidic Device for Three-Dimensional Tumor Ball Culture and Its Drug Testing. Molecules. 2025; 30(15):3305. https://doi.org/10.3390/molecules30153305
Chicago/Turabian StyleYang, Xindong, Yi Xu, Dongchen Zhu, and Xianqiang Mi. 2025. "GelMA Core–Shell Microgel Preparation Based on a Droplet Microfluidic Device for Three-Dimensional Tumor Ball Culture and Its Drug Testing" Molecules 30, no. 15: 3305. https://doi.org/10.3390/molecules30153305
APA StyleYang, X., Xu, Y., Zhu, D., & Mi, X. (2025). GelMA Core–Shell Microgel Preparation Based on a Droplet Microfluidic Device for Three-Dimensional Tumor Ball Culture and Its Drug Testing. Molecules, 30(15), 3305. https://doi.org/10.3390/molecules30153305






