Structure-Activity Relationships in Alkoxylated Resorcinarenes: Synthesis, Structural Features, and Bacterial Biofilm-Modulating Properties
Abstract
1. Introduction
2. Results
2.1. Synthesis
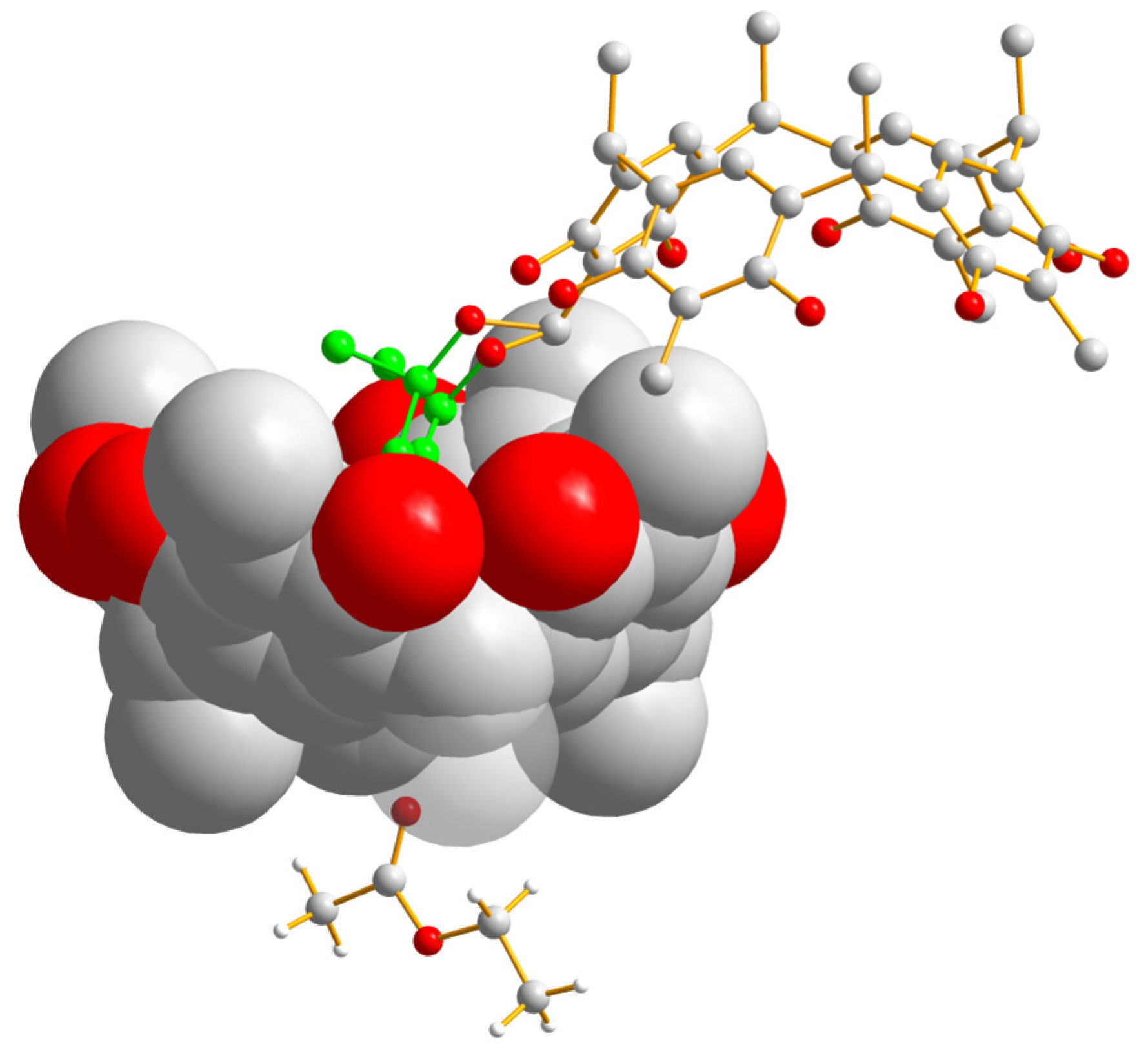
2.2. Crystallographic Studies
2.3. Biological Properties
2.3.1. Biofilm and Its Role in Pathogenesis
2.3.2. Mechanism of Action and Antimicrobial Properties of Secondary and Tertiary Alcohols
2.3.3. Experimental Results and Analysis
2.3.4. Hypothetical Molecular Mechanisms
3. Discussion
4. Materials and Methods
4.1. General Procedure for the Synthesis
4.1.1. Tetra Iso-Propoxy Resorcinarene (2a)
4.1.2. Tetra Sec-Butoxy Resorcinarene (2b)
4.1.3. Tetra Sec-Pentoxy Resorcinarene (2c)
4.1.4. Tetra Sec-Hexoxy Resorcinarene (2d)
4.1.5. Tetra Cyclo-Hexoxy Resorcinarene (2e)
4.1.6. Tetra Tert-Butyloxy Resorcinarene (2f)
4.1.7. Tetra Tert-Amyloxy Resorcinarene (2g)
4.2. Model Organisms and Experimental Design
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Han, X.N.; Han, Y.; Chen, C.F. Recent advances in the synthesis and applications of macrocyclic arenes. Chem. Soc. Rev. 2023, 52, 3265–3298. [Google Scholar] [CrossRef]
- Li, L.; Tang, J.; Liu, H.; Qian, Y. Highly Selective Potentiometric Sensing of Biologically Relevant Pyrophosphate and Lysophosphatidic Acid Using N-Alkyl/Aryl Ammonium Resorcinarenes/Extended-Resorcinarenes as Ionophores. Anal. Chem. 2022, 94, 14854–14860. [Google Scholar] [CrossRef] [PubMed]
- Quaglio, D.; Mangiardi, L.; Venditti, G.; Del Plato, C.; Polli, F.; Ghirga, F.; Favero, G.; Pierini, M.; Botta, B.; Mazzei, F. Site-Directed Antibody Immobilization by Resorc[4]arene-Based Immunosensors. Chem. Eur. J. 2020, 26, 8400–8406. [Google Scholar] [CrossRef] [PubMed]
- Twum, K.; Bhattacharjee, A.; Laryea, E.T.; Esposto, J.; Omolloh, G.; Mortensen, S.; Jaradi, M.; Stock, N.L.; Schileru, N.; Elias, B.; et al. Functionalized resorcinarenes effectively disrupt the aggregation of αA66-80 crystallin peptide related to cataracts. RSC Med. Chem. 2021, 12, 2022–2030. [Google Scholar] [CrossRef]
- Galindres, D.M.; Cifuentes, D.; Tinoco, L.E.; Murillo-Acevedo, Y.; Rodrigo, M.M.; Ribeiro, A.C.F.; Esteso, M.A. A Review of the Application of Resorcinarenes and SBA-15 in Drug Delivery. Processes 2022, 10, 684. [Google Scholar] [CrossRef]
- Fahmy, S.A.; Ponte, F.; Abd El-Rahman, M.K.; Russo, N.; Sicilia, E.; Shoeib, T. Investigation of the host-guest complexation between 4-sulfocalix[4]arene and nedaplatin for potential use in drug delivery. Spectrochim. Acta A 2018, 193, 528–536. [Google Scholar] [CrossRef]
- Patel, M.B.; Valand, N.N.; Modi, N.R.; Joshi, K.V.; Harikrishnan, U.; Kumar, S.P.; Jasraib, Y.T.; Menon, S.K. Effect of p-sulfonatocalix[4]resorcinarene (PSC[4]R) on the solubility and bioavailability of a poorly water soluble druglamotrigine (LMN) and computational investigation. RSC Adv. 2013, 3, 15971–15981. [Google Scholar] [CrossRef]
- Kashapov, R.; Razuvayeva, Y.; Kashapova, N.; Ziganshina, A.; Salnikov, V.; Sapunova, A.; Voloshina, A.; Zakharova, L. Emergence of Nanoscale Drug Carriers through Supramolecular Self-Assembly of RNA with Calixarene. Int. J. Mol. Sci. 2023, 24, 7911. [Google Scholar] [CrossRef]
- Casas, J.; Ibarguren, M.; Álvarez, R.; Terés, S.; Llado, V.; Piotto, S.P.; Concilio, S.; Busquets, X.; López, D.J.; Escribá, P.V. G protein-membrane interactions II: Effect of G protein-linked lipids on membrane structure and G protein-membrane interactions. Biochim. Biophys. Acta Biomembr. 2017, 1859, 1526–1535. [Google Scholar] [CrossRef]
- Rifici, S.; D’Angelo, G.; Crupi, C.; Branca, C.; Conti Nibali, V.; Corsaro, C.; Wanderlingh, U. Influence of Alcohols on the Lateral Diffusion in Phospholipid Membranes. J. Phys. Chem. B 2016, 120, 1285–1290. [Google Scholar] [CrossRef]
- Malajczuk, C.J.; Armstrong, B.I.; Stachura, S.S.; Mancera, R.L. Mechanisms of Interaction of Small Hydroxylated Cryosolvents with Dehydrated Model Cell Membranes: Stabilization vs Destruction. J. Phys. Chem. B 2022, 126, 197–216. [Google Scholar] [CrossRef]
- Shurpik, D.N.; Padnya, P.L.; Stoikov, I.I.; Cragg, P.J. Antimicrobial Activity of Calixarenes and Related Macrocycles. Molecules 2020, 25, 5145. [Google Scholar] [CrossRef]
- Mourer, M.; Regnouf-de-Vains, J.-B.; Duval, R.E. Functionalized Calixarenes as Promising Antibacterial Drugs to Face Antimicrobial Resistance. Molecules 2023, 28, 6954. [Google Scholar] [CrossRef]
- Pan, Y.C.; Hu, X.Y.; Guo, D.S. Biomedical Applications of Calixarenes: State of the Art and Perspectives. Angew. Chem. Int. Ed. 2021, 60, 2768–2794. [Google Scholar] [CrossRef] [PubMed]
- Urbaniak, M.; Iwanek, W. Synthesis of alkoxymethyl derivatives of resorcinarene via the Mannich reaction catalysed with iminodiacetic acid. Tetrahedron 2006, 62, 1508–1511. [Google Scholar] [CrossRef]
- Urbaniak, M.; Mattay, J.; Iwanek, W. Synthesis of Resorcinarene Derivatives by the Catalyzed Mannich Reaction, Part 2: Resorcinarene Derivatives with Unsaturated Bonds. Synth. Commun. 2008, 38, 4345–4351. [Google Scholar] [CrossRef]
- Urbaniak, M.; Mattay, J.; Iwanek, W. Synthesis of Resorcinarene Derivatives via the Catalyzed Mannich Reaction, Part 3: Glycol Derivatives of Resorcinarene. Synth. Commun. 2011, 41, 670–676. [Google Scholar] [CrossRef]
- Urbaniak, M.; Gawdzik, B.; Wzorek, A.; Kaca, W.; Lechowicz, Ł. Synthesis and complexing properties of diglycol resorcinarene podands. J. Incl. Phenom. Macrocycl. Chem. 2015, 81, 357–365. [Google Scholar] [CrossRef]
- Gawdzik, B.; Wzorek, A.; Lechowicz, Ł.; Urbaniak, M. Synthesis of New Arylmethylene Derivatives of Resorcinarene via the Catalyzed Mannich Reaction. Synlett 2016, 27, 249–253. [Google Scholar] [CrossRef]
- Tong, S.Y.C.; Davis, J.S.; Eichenberger, E.; Holland, T.L.; Fowler, V.G. Staphylococcus aureus Infections: Epidemiology, Pathophysiology, Clinical Manifestations, and Management. Clin. Microbiol. Rev. 2015, 28, 603–661. [Google Scholar] [CrossRef]
- Geurtsen, J.; De Been, M.; Weerdenburg, E.; Zomer, A.; McNally, A.; Poolman, J. Genomics and pathotypes of the many faces of Escherichia coli. FEMS Microbiol. Rev. 2022, 46, fuac031. [Google Scholar] [CrossRef]
- Qin, S.; Xiao, W.; Zhou, C.; Pu, Q.; Deng, X.; Lan, L.; Liang, H.; Song, X.; Wu, M. Pseudomonas aeruginosa: Pathogenesis, virulence factors, antibiotic resistance, interaction with host, technology advances and emerging therapeutics. Sig Transduct. Target. Ther. 2022, 7, 199. [Google Scholar] [CrossRef]
- Su, Y.; Liu, C.; Fang, H.; Zhang, D. Bacillus subtilis: A universal cell factory for industry, agriculture, biomaterials and medicine. Microb. Cell Fact. 2020, 19, 173. [Google Scholar] [CrossRef]
- Damyanova, T.; Paunova-Krasteva, T. What We Still Don’t Know About Biofilms—Current Overview and Key Research Information. Microbiol. Res. 2025, 16, 46. [Google Scholar] [CrossRef]
- Sousa, M.; Afonso, A.C.; Teixeira, L.S.; Borges, A.; Saavedra, M.J.; Simões, L.C.; Simões, M. Hydrocinnamic Acid and Perillyl Alcohol Potentiate the Action of Antibiotics against Escherichia coli. Antibiotics 2023, 12, 360. [Google Scholar] [CrossRef]
- Nassarawa, S.S.; Nayik, G.A.; Gupta, S.D.; Areche, F.O.; Jagdale, Y.D.; Ansari, M.J.; Hemeg, H.A.; Al-Farga, A.; Alotaibi, S.S. Chemical aspects of polyphenol-protein interactions and their antibacterial activity. Crit. Rev. Food Sci. Nutr. 2023, 63, 9482–9505. [Google Scholar] [CrossRef]
- Ingram, L.O. Ethanol tolerance in bacteria. Crit. Rev. Biotechnol. 1990, 9, 305–319. [Google Scholar] [CrossRef]
- Huffer, S.; Clark, M.E.; Ning, J.C.; Blanch, H.W.; Clark, D.S. Role of Alcohols in Growth, Lipid Composition, and Membrane Fluidity of Yeasts, Bacteria, and Archaea. Appl. Envion. Microbiol. 2011, 77, 6400–6408. [Google Scholar] [CrossRef] [PubMed]
- Shobhna; Dutta, A.; Kumari, P.; Kashyap, H.K. Stability of Cytoplasmic Membrane of Escherichia coli Bacteria in Aqueous and Ethanolic Environment. Langmuir 2024, 40, 2893–2906. [Google Scholar] [CrossRef] [PubMed]
- Da Silveira, M.G.; Golovina, E.A.; Hoekstra, F.A.; Rombouts, F.M.; Abee, T. Membrane Fluidity Adjustments in Ethanol-Stressed Oenococcus oeni Cells. Appl. Envion. Microbiol. 2003, 69, 5826–5832. [Google Scholar] [CrossRef]
- Zhang, K.; Yang, X.; Yang, J.; Qiao, X.; Li, F.; Liu, X.; Wei, J.; Wang, L. Alcohol dehydrogenase modulates quorum sensing in biofilm formations of Acinetobacter baumannii. Microb. Pathog. 2020, 148, 104451. [Google Scholar] [CrossRef]
- He, S.; Zhan, Z.; Shi, C.; Wang, S.; Shi, X. Ethanol at Subinhibitory Concentrations Enhances Biofilm Formation in Salmonella Enteritidis. Foods 2022, 11, 2237. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, F.; Yu, Y.; Ding, S.; Chen, T.; Sun, W.; Liang, C.; Yu, B.; Ying, H.; Liu, D.; et al. Effect of quorum-sensing molecule 2-phenylethanol and ARO genes on Saccharomyces cerevisiae biofilm. Appl. Microbiol. Biotechnol. 2021, 105, 3635–3648. [Google Scholar] [CrossRef]
- Wagner, M.; Bentley, K.; Philpot, C.; Farnsworth, C.; Stoll, M.; Jimenez, V.M. Ethanol Induces a Persister Cell Phenotype in E. coli. and S. epidermidis. J. Infect. Dis. Case Rep. 2025, 6, 1–5. [Google Scholar] [CrossRef]
- Yao, S.; Zhou, R.; Jin, Y.; Huang, J.; Qin, J.; Wu, C. Formation of biofilm changed the responses of Tetragenococcus halophilus to ethanol stress revealed by transcriptomic and proteomic analyses. Food Res. Int. 2022, 161, 111817. [Google Scholar] [CrossRef]
- Rigaku Oxford Diffraction. CrysAlis PRO; Rigaku Oxford Diffraction: Yarnton, UK, 2015. [Google Scholar]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. C 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Programs for crystal structure determination. In SHELX2017; Universität Göttingen: Göttingen, Germany, 2017. [Google Scholar]
- Putz, H.; Brandenburg, K. Diamond-Crystal and Molecular Structure Visualization Crystal Impact GbR, Kreuzherrenstr. 102, D-53227 Bonn. Available online: http://ccp14.chem.ucl.ac.uk/ccp/web-mirrors/crystalimpact/diamond/forms/Bestellschein_Diamond.pdf (accessed on 23 October 2023).
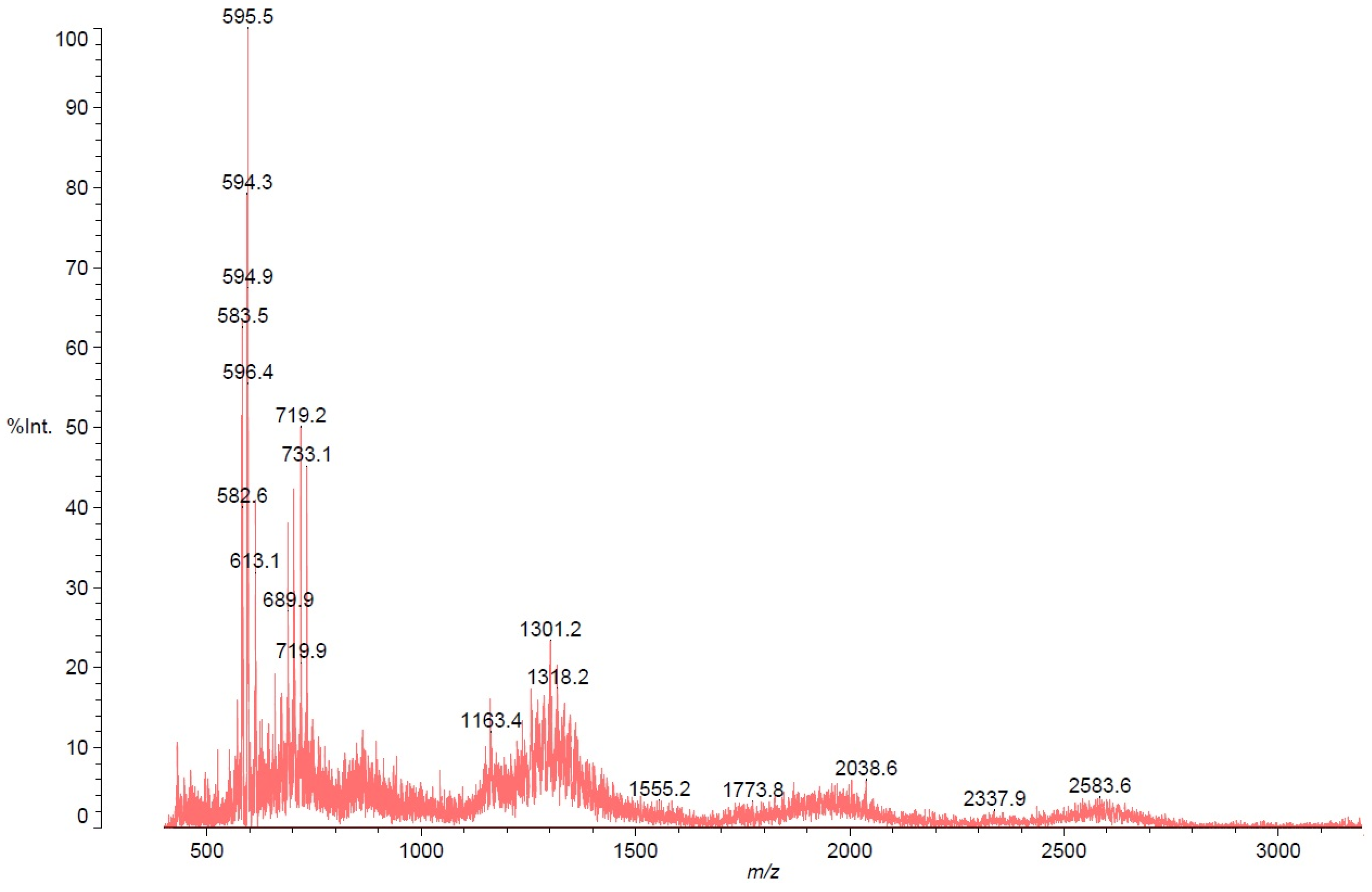
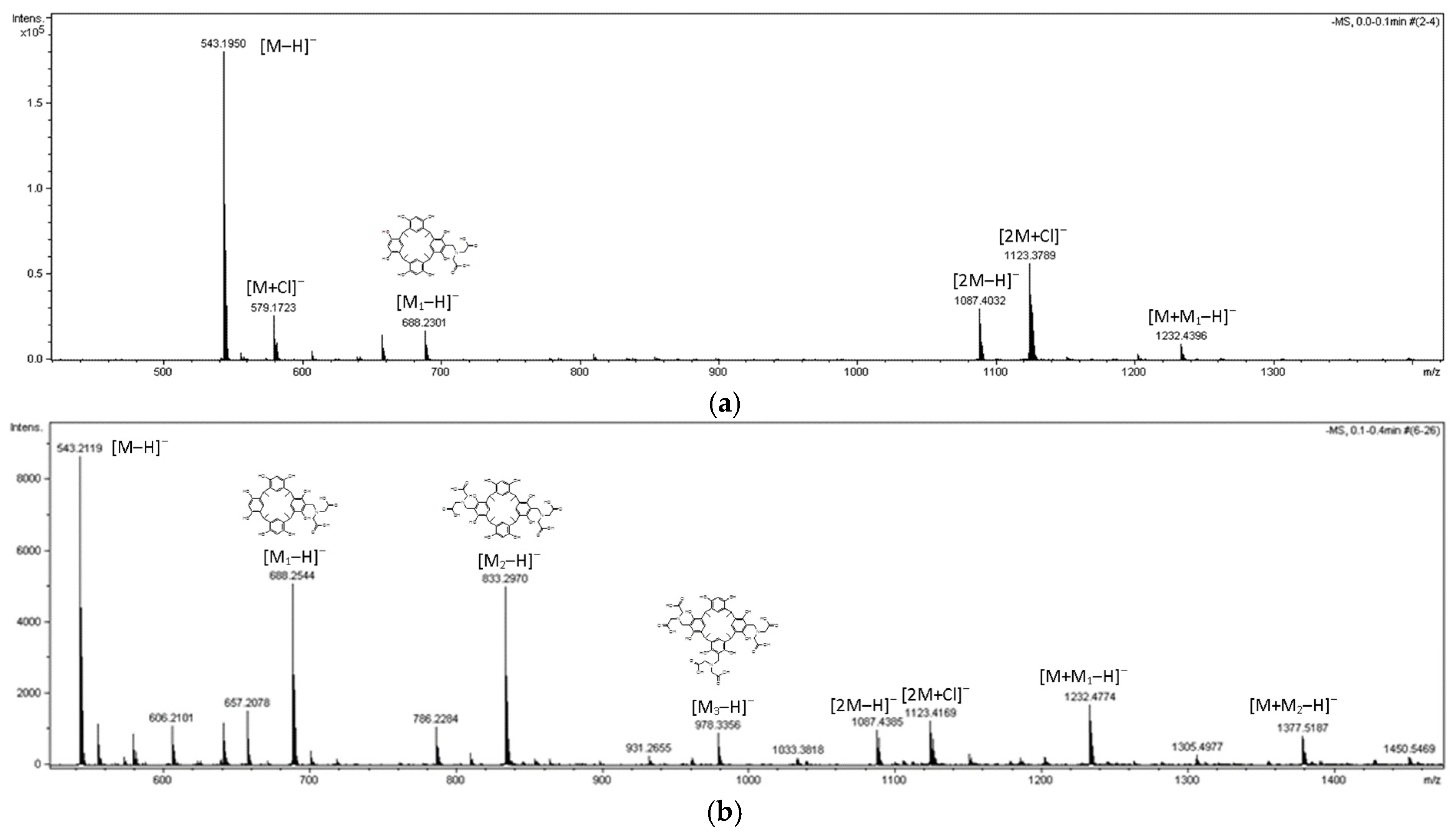

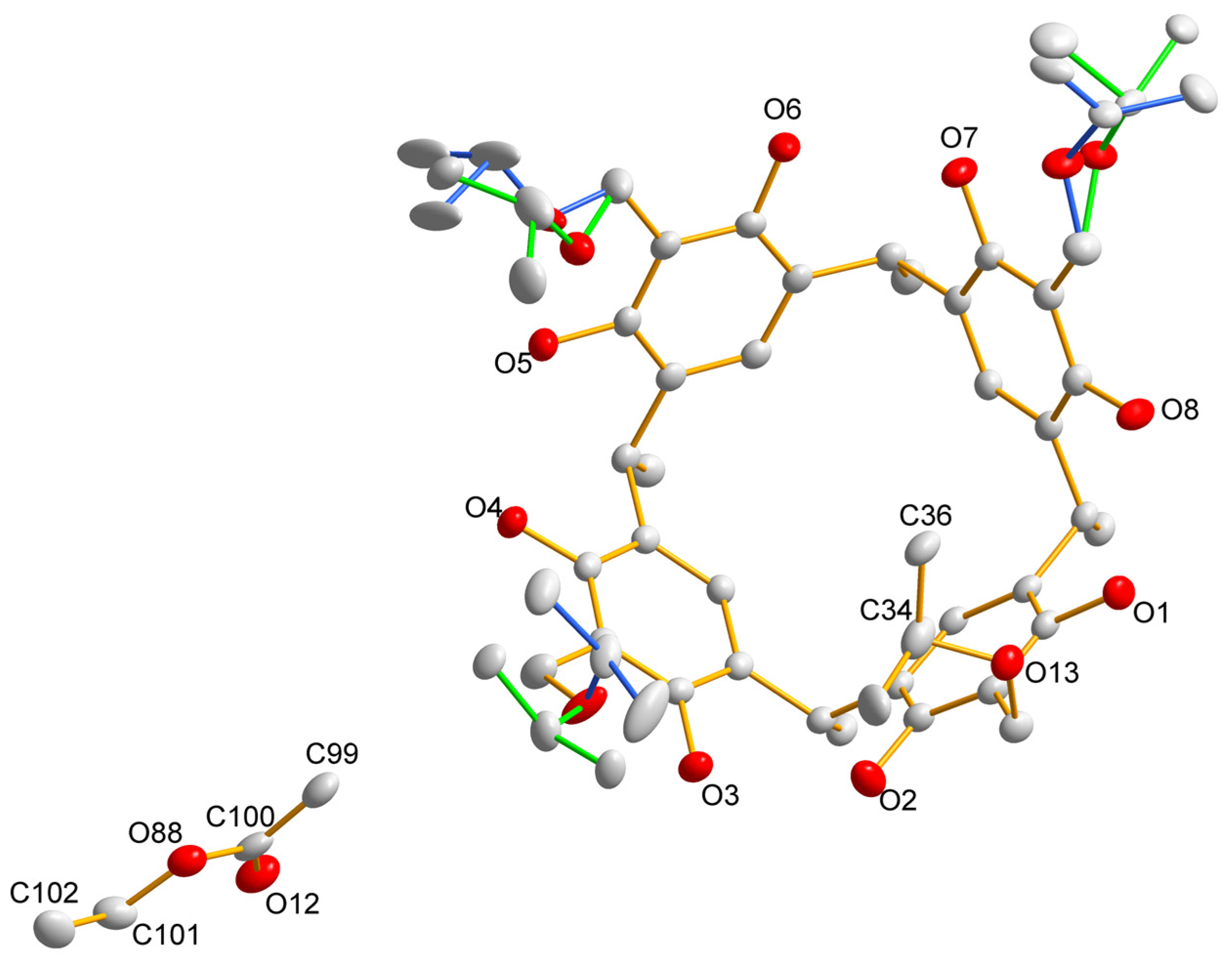
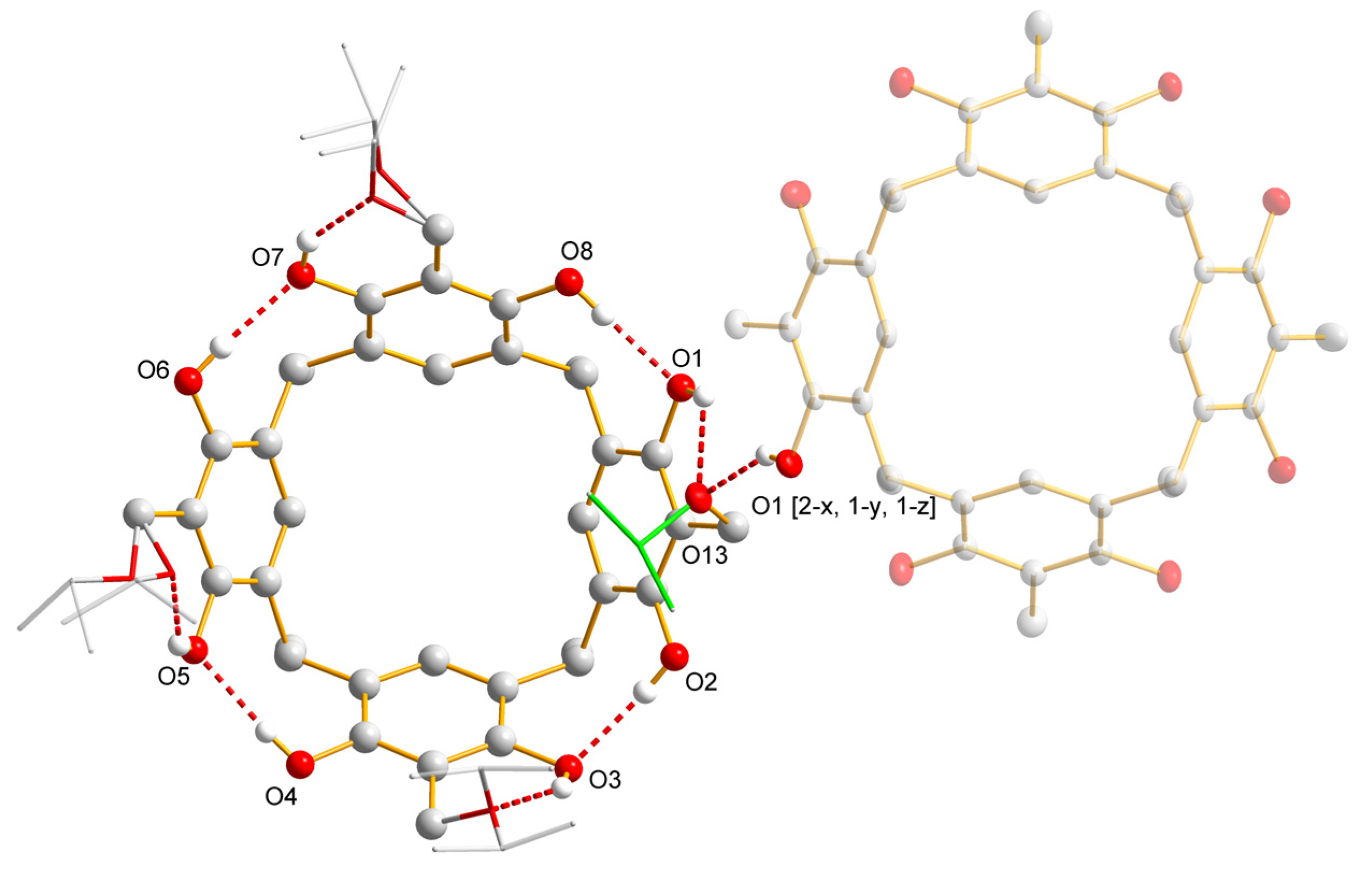
| Compound | R | Yields |
|---|---|---|
| 2a | iso-propyl | 74% |
| 2b | sec-butyl a | 73% |
| 2c | sec-pentyl | 67% |
| 2d | sec-hexyl | 63% |
| 2e | cyclohexyl | 62% |
| 2f | tert-butyl | 36% |
| 2g | tert-amyl | 29% |
| Empirical Formula | C52H72O14 |
|---|---|
| Formula weight | 921.09 |
| Temperature | 115(2) K |
| Wavelength | 0.71073 Å |
| Crystal system | Triclinic |
| Space group | P−1 |
| Unit cell dimension | a = 12.4393(4) Å α = 100.084(2)° b = 13.1667(4) Å β = 96.994(2)° c = 16.4740(4) Å γ = 108.623(3)° |
| Volume | 2471.34(13) Å3 |
| Z | 2 |
| Density (calculated) | 1.238 Mg/m3 |
| Absorption coefficient | 0.089 mm−1 |
| F(000) | 992 |
| Crystal size | 0.150 × 0.120 × 0.100 mm3 |
| Theta range for data collection | 2.558 to 30.923° |
| Index ranges | −17 ≤ h ≤ 13, −18 ≤ k ≤ 18, −22 ≤ l ≤ 22 |
| Reflections collected | 37,979 |
| Independent reflections | 12,570 [R(int) = 0.0349] |
| Completeness to theta = 25.242° | 99.7% |
| Refinement method | Full-matrix least-squares on F2 |
| Data/restraints/parameters | 12,570/5/713 |
| Goodness-of-fit on F2 | 1.071 |
| Final R indices [I > 2sigma(I)] | R1 = 0.0517, wR2 = 0.1272 |
| R indices (all data) | R1 = 0.0749, wR2 = 0.1388 |
| Largest diff. peak and hole | 0.619 and −0.571 e/Å3 |
| D-H⋯A | d(D-H) | d(H⋯A) | d(D⋯A) | <(DHA) |
|---|---|---|---|---|
| O(1)-H(1OO)⋯O(13) | 0.84 | 2.43 | 3.0677(15) | 133.3 |
| O(1)-H(1OO)⋯O(13)#1 | 0.84 | 1.98 | 2.7107(14) | 145.2 |
| O(3)-H(3O)⋯O(10) | 0.84 | 1.88 | 2.6194(17) | 145.9 |
| O(7)-H(7O)⋯O(12A^a) | 0.84 | 1.89 | 2.612(3) | 143.5 |
| O(7)-H(7O)⋯O(12B^b) | 0.84 | 1.77 | 2.517(3) | 146.7 |
| O(6)-H(6O)⋯O(7) | 0.84 | 1.91 | 2.7430(16) | 175.1 |
| O(2)-H(2O)⋯O(3) | 0.84 | 1.93 | 2.7496(16) | 165.9 |
| O(5)-H(5O)⋯O(11A^e) | 0.84 | 1.88 | 2.597(3) | 142.8 |
| O(5)-H(5O)⋯O(11B^f) | 0.84 | 1.82 | 2.534(5) | 142.4 |
| O(8)-H(8O)⋯O(1) | 0.84 | 1.9 | 2.7406(15) | 177.3 |
| O(4)-H(4O)⋯O(5) | 0.84 | 1.94 | 2.7721(15) | 173.4 |
| Compounds | OD600 * | SDOD600 * | CV595 * | SDCV595 * | CV595/OD600 * |
|---|---|---|---|---|---|
| S. aureus | |||||
| 2b | 0.3869 | 0.0094 | 0.1227 | 0.0113 | 0.3172 |
| 2c | 0.2817 | 0.0112 | 0.1386 | 0.0100 | 0.4921 |
| 2e | 0.3259 | 0.0230 | 0.0956 | 0.0068 | 0.2935 |
| 2f | 0.2666 | 0.0268 | 0.1100 | 0.0039 | 0.4126 |
| 2g | 0.3010 | 0.0011 | 0.0991 | 0.0014 | 0.3293 |
| DMSO 5% | 0.3179 | 0.0087 | 0.0723 | 0.0022 | 0.2275 |
| E. coli | |||||
| 2b | 0.3761 | 0.0087 | 0.1269 | 0.0037 | 0.3373 |
| 2c | 0.2844 | 0.0197 | 0.1306 | 0.0023 | 0.4591 |
| 2e | 0.3498 | 0.0219 | 0.0981 | 0.0044 | 0.2805 |
| 2f | 0.2267 | 0.0171 | 0.1162 | 0.0027 | 0.5128 |
| 2g | 0.1849 | 0.1372 | 0.1108 | 0.0054 | 0.5995 |
| DMSO 5% | 0.2693 | 0.0239 | 0.0728 | 0.0017 | 0.2702 |
| P. aeruginosa | |||||
| 2b | 0.0470 | 0.0016 | 0.3741 | 0.0230 | 7.9589 |
| 2c | 0.0428 | 0.0011 | 0.4513 | 0.0139 | 10.5354 |
| 2e | 0.0256 | 0.0040 | 0.2281 | 0.0574 | 8.9218 |
| 2f | 0.0220 | 0.0124 | 0.1759 | 0.0112 | 8.0076 |
| 2g | 0.0236 | 0.0019 | 0.1312 | 0.0042 | 5.5672 |
| DMSO 5% | 0.0267 | 0.0035 | 0.5578 | 0.0090 | 20.8926 |
| B. subtilis | |||||
| 2b | 0.2441 | 0.0192 | 0.1268 | 0.0041 | 0.5196 |
| 2c | 0.1895 | 0.0089 | 0.1356 | 0.0036 | 0.7154 |
| 2e | 0.2350 | 0.0119 | 0.1093 | 0.0076 | 0.4649 |
| 2f | 0.1775 | 0.0214 | 0.1033 | 0.0043 | 0.5821 |
| 2g | 0.2805 | 0.0178 | 0.1165 | 0.0027 | 0.4152 |
| DMSO 5% | 0.2612 | 0.0081 | 0.0788 | 0.0022 | 0.3016 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Urbaniak, M.; Lechowicz, Ł.; Gawdzik, B.; Hodorowicz, M.; Wielgus, E. Structure-Activity Relationships in Alkoxylated Resorcinarenes: Synthesis, Structural Features, and Bacterial Biofilm-Modulating Properties. Molecules 2025, 30, 3304. https://doi.org/10.3390/molecules30153304
Urbaniak M, Lechowicz Ł, Gawdzik B, Hodorowicz M, Wielgus E. Structure-Activity Relationships in Alkoxylated Resorcinarenes: Synthesis, Structural Features, and Bacterial Biofilm-Modulating Properties. Molecules. 2025; 30(15):3304. https://doi.org/10.3390/molecules30153304
Chicago/Turabian StyleUrbaniak, Mariusz, Łukasz Lechowicz, Barbara Gawdzik, Maciej Hodorowicz, and Ewelina Wielgus. 2025. "Structure-Activity Relationships in Alkoxylated Resorcinarenes: Synthesis, Structural Features, and Bacterial Biofilm-Modulating Properties" Molecules 30, no. 15: 3304. https://doi.org/10.3390/molecules30153304
APA StyleUrbaniak, M., Lechowicz, Ł., Gawdzik, B., Hodorowicz, M., & Wielgus, E. (2025). Structure-Activity Relationships in Alkoxylated Resorcinarenes: Synthesis, Structural Features, and Bacterial Biofilm-Modulating Properties. Molecules, 30(15), 3304. https://doi.org/10.3390/molecules30153304






