A Portable and Thermally Degradable Hydrogel Sensor Based on Eu-Doped Carbon Dots for Visual and Ultrasensitive Detection of Ferric Ion
Abstract
1. Introduction
2. Results
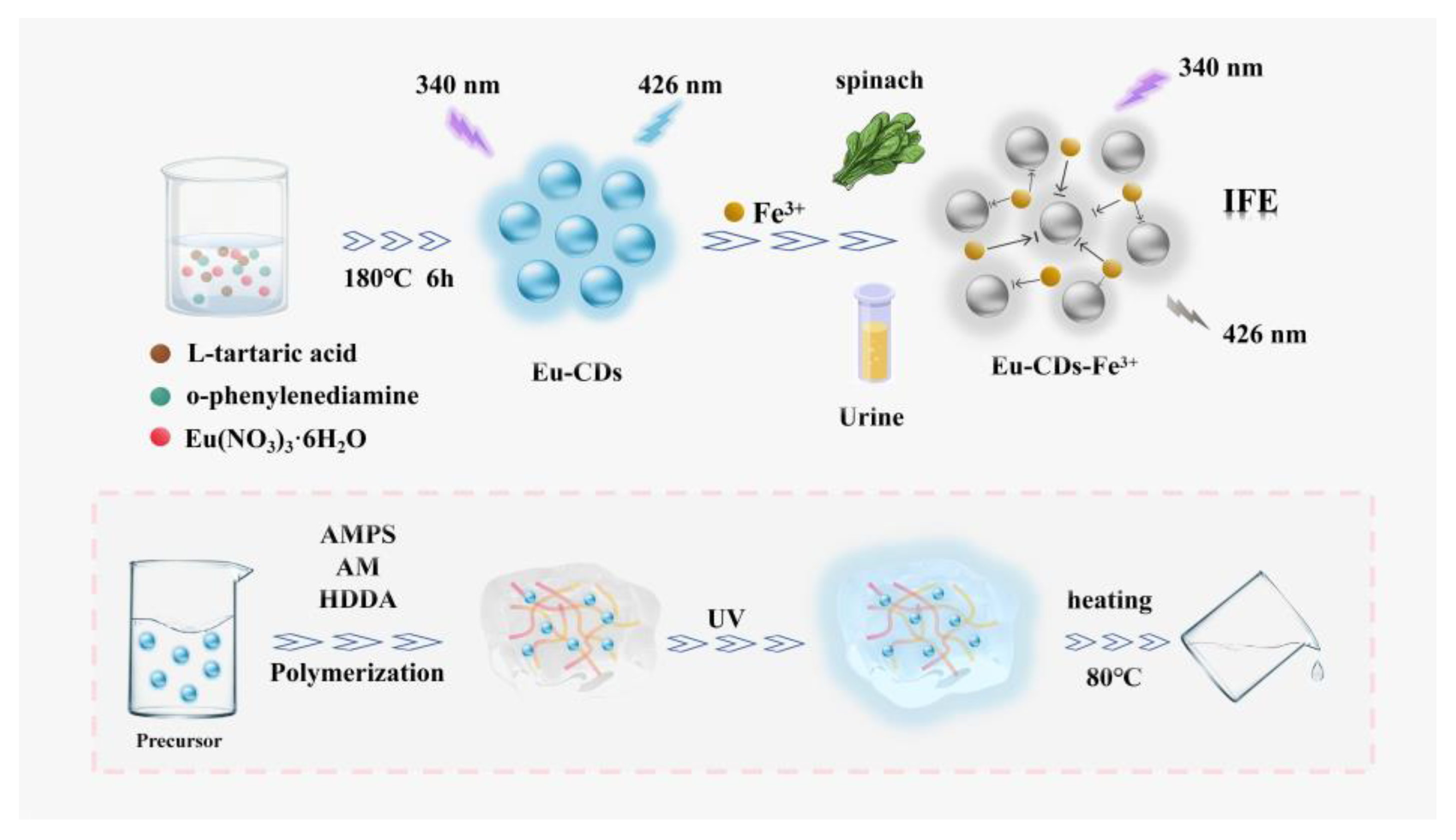
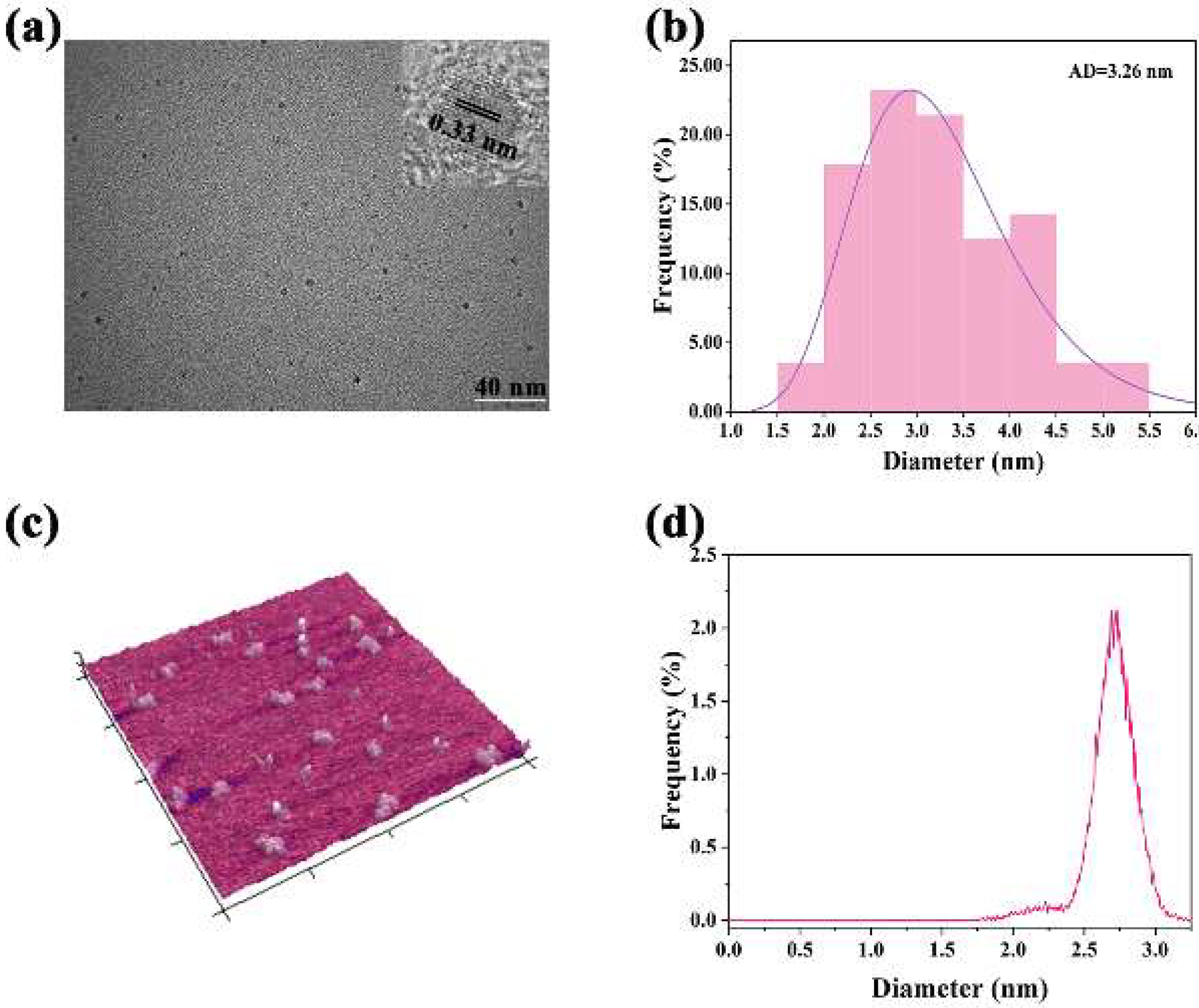
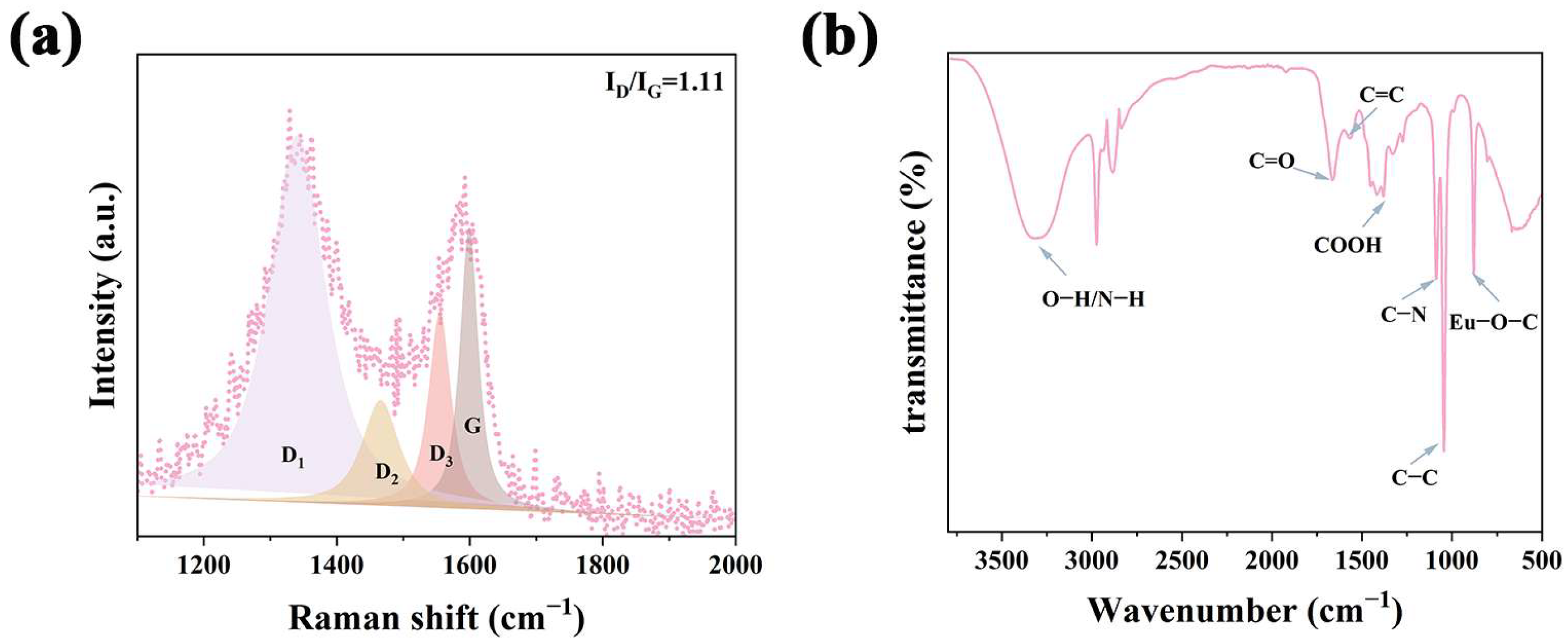
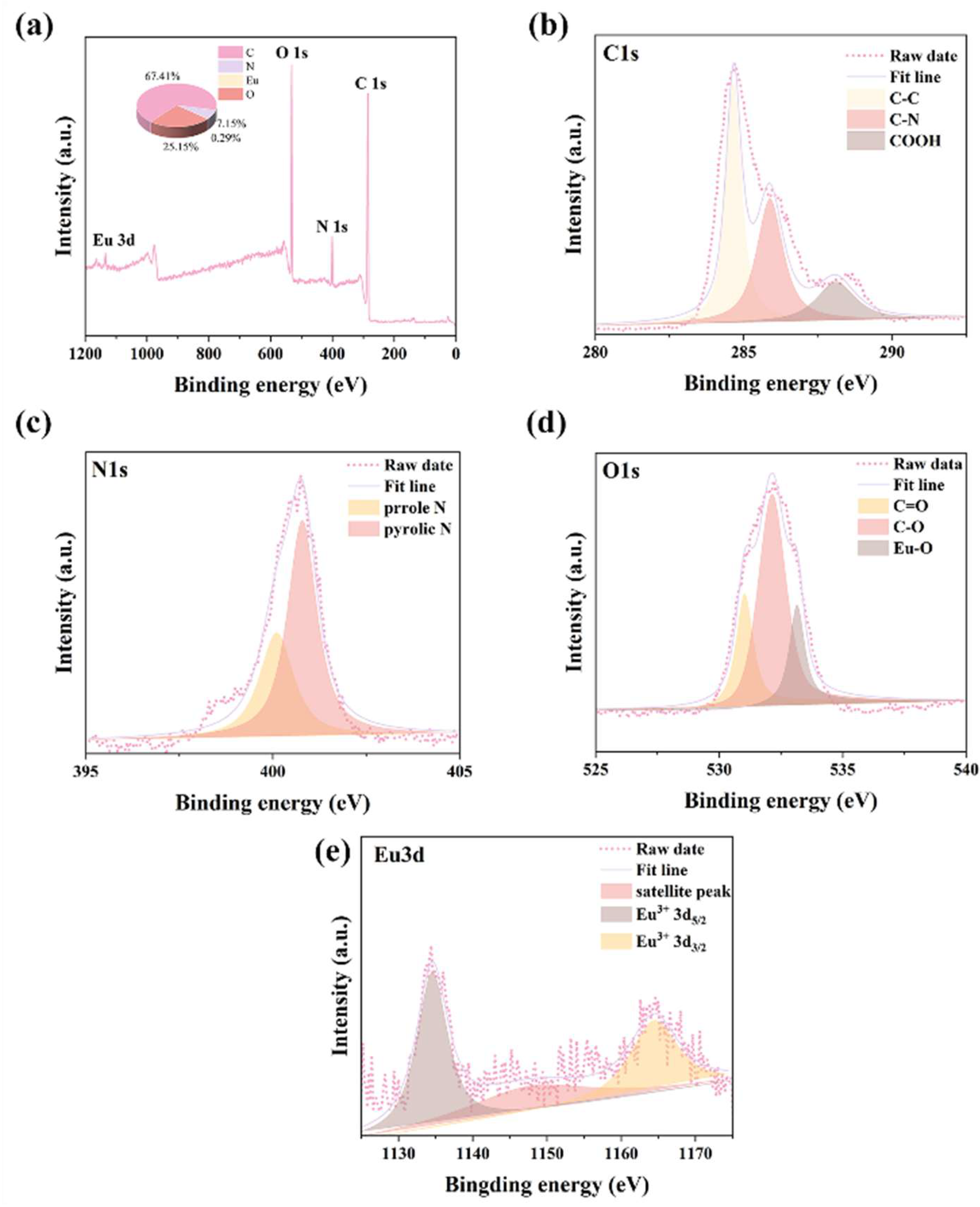
2.1. Synthesis and Characterization of Eu-CDs
2.2. Optical Properties of Eu-CDs
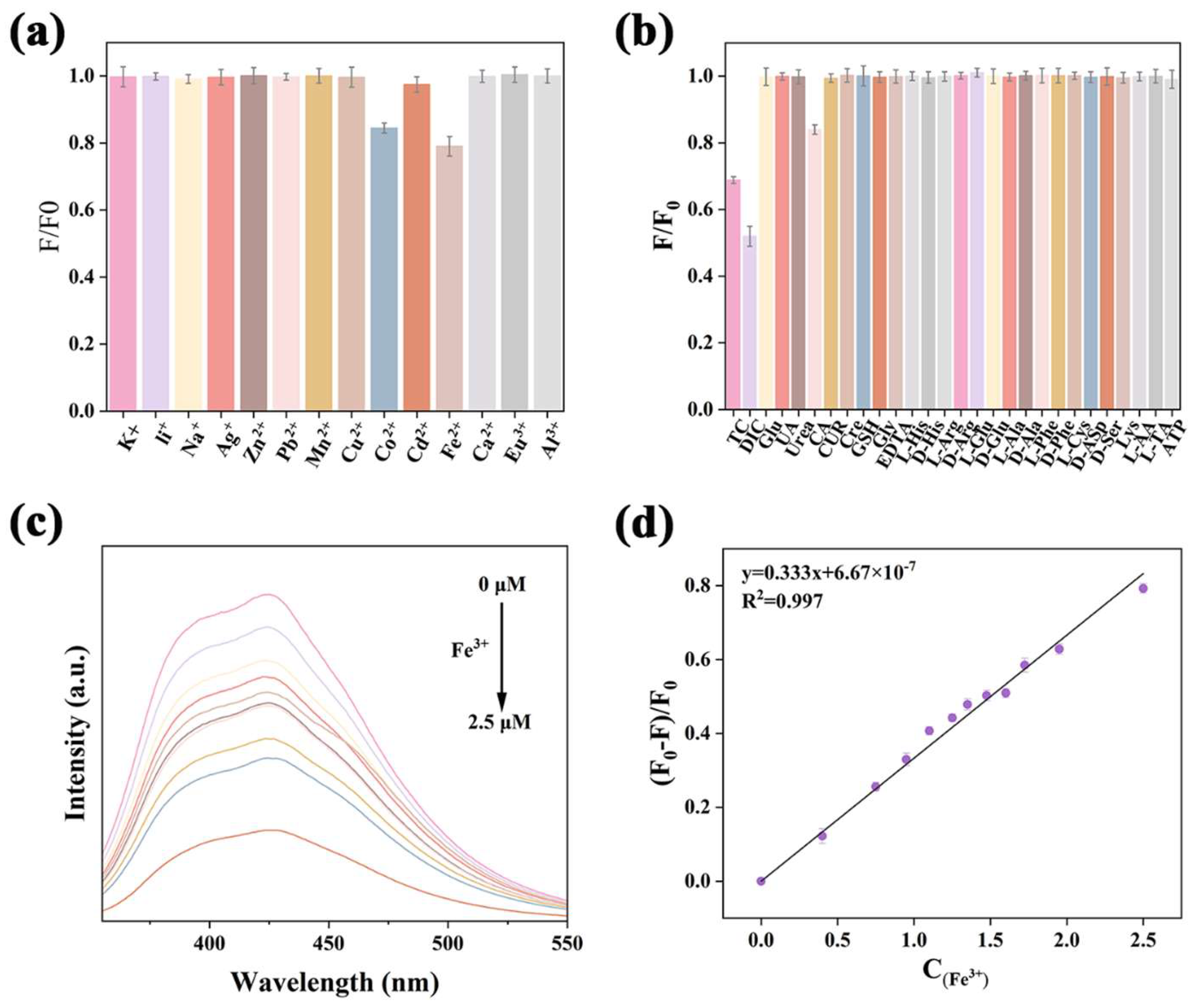
2.3. Detection of Fe3+
2.4. Proposed Sensing Mechanism
2.5. Real Sample Analysis
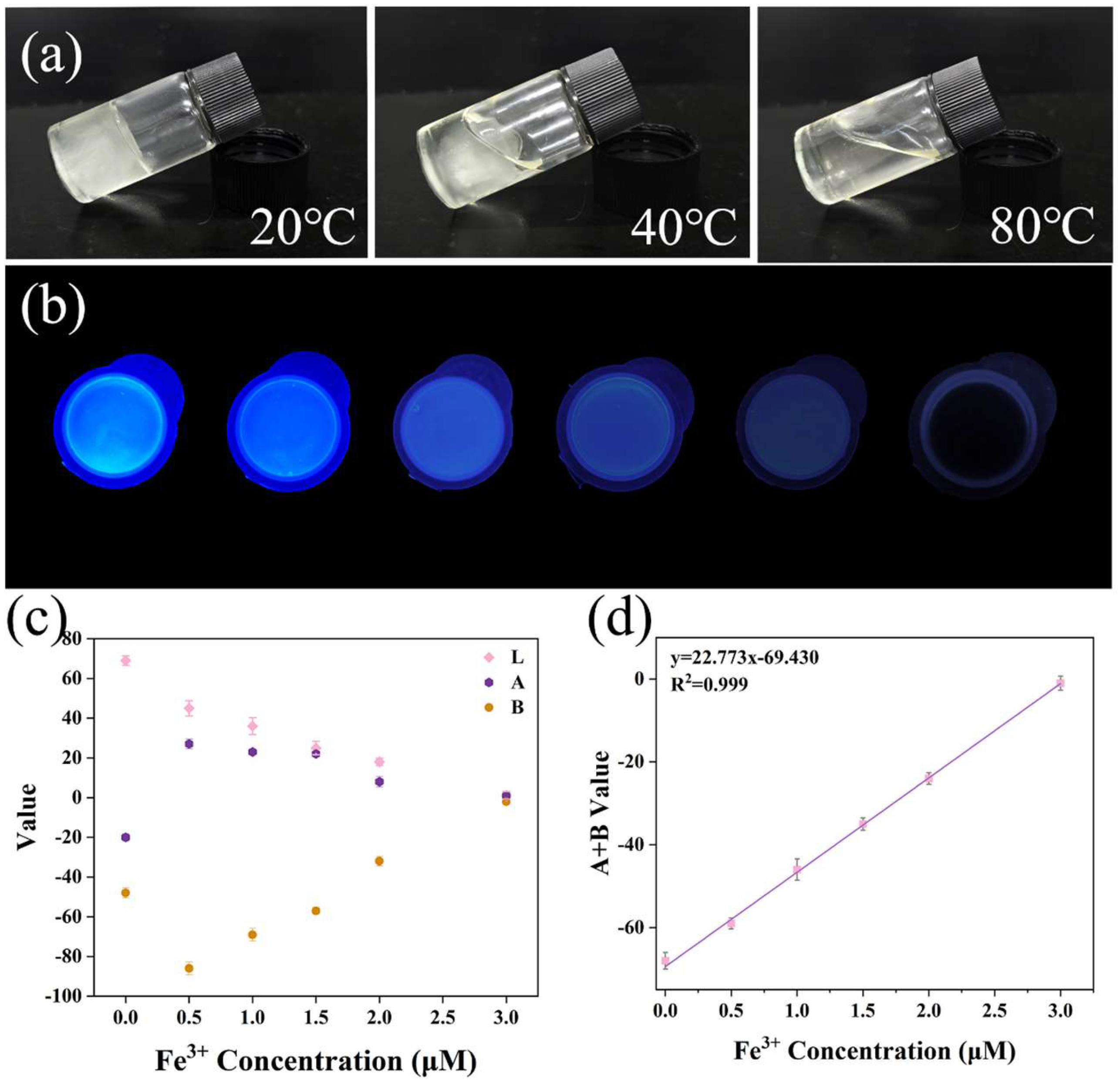
2.6. Portable and Self-Degradable Eu-CDs@DPPG Sensor
3. Materials and Methods
3.1. Chemicals and Materials
3.2. Instrumentation
3.3. Synthesis of Eu-CDs
3.4. Stability Evaluation of Eu-CDs
3.5. Detection of Fe3+
3.6. Fabrication of Eu-CDs@DPPG Hydrogel
3.7. Pretreatment of Real Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Qiu, D.; Deng, Y.; Wen, Y.; Yin, J.; Feng, J.; Huang, J.; Song, M.; Zhang, G.; Chen, C.; Xia, J. Iron corroded granules inhibiting vascular smooth muscle cell proliferation. Mater. Today Bio 2022, 16, 100420. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Meng, X.; Meng, L.; Guo, Y.; Li, Y.; Yang, C.; Pei, Z.; Li, J.; Wang, F. Joint efficacy of the three biomarkers SNCA, GYPB and HBG1 for atrial fibrillation and stroke: Analysis via the support vector machine neural network. J. Cell. Mol. Med. 2022, 26, 2010–2022. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Liang, R.; Dai, T.; Chen, J.; Shuai, X.; Liu, C. Pectin-based adsorbents for heavy metal ions: A review. Trends Food Sci. Technol. 2019, 91, 319–329. [Google Scholar] [CrossRef]
- Wei, X.; Zhu, T.; Ma, Y.; Sun, J.; Zheng, G.; Ma, T.; Yang, X.; Song, Z.; Lv, Y.; Zhang, J.; et al. Monitoring acetylcholinesterase level changes under oxidative stress through ESIPT-ICT-based near-infrared fluorescent probe. Sens. Actuators B Chem. 2023, 380, 133392. [Google Scholar] [CrossRef]
- Tambutté, E.; Venn, A.A.; Holcomb, M.; Segonds, N.; Techer, N.; Zoccola, D.; Allemand, D.; Tambutté, S. Morphological plasticity of the coral skeleton under CO2-driven seawater acidification. Nat. Commun. 2015, 6, 7368. [Google Scholar] [CrossRef]
- Nayeem, A.; Ali, M.F.; Shariffuddin, J.H. Recovery of waste cooking palm oil as a crosslinker for inverse vulcanized adsorbent to remove iron (Fe3+) ions. J. Environ. Chem. Eng. 2024, 12, 111853. [Google Scholar] [CrossRef]
- Liu, L.-L.; Zhang, H.-W.; Ren, J.-Y.; Wang, L.; Zhang, Y. A molecular sensor for selective recognition of Fe3+ by a functional seven-nuclear Zn(II) cluster compound formed from a quinoline-modified half-salamo-type Precursor. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2025, 331, 125762. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, J.; Wu, W.; Han, C.; Li, M. Graphene oxide supported MOFs-nanofiber carbon aerogel/SPCE for simultaneous detection of Cd2+ and Pb2+ in seafood. Food Chem. 2024, 470, 142643. [Google Scholar] [CrossRef]
- Yan, Z.; Hu, L.; You, J. Sensing materials developed and applied for bio-active Fe3+ recognition in water environment. Anal. Methods 2016, 8, 5738–5754. [Google Scholar] [CrossRef]
- Qi, P.; Xie, J.; Xia, G.; Wang, Y.; Xin, J.H. Advanced Bionic Textile Materials: From Principles to Functional Applications. Adv. Mater. 2025, e02118. [Google Scholar] [CrossRef]
- Zhou, J.; Chizhik, A.I.; Chu, S.; Jin, D. Single-particle spectroscopy for functional nanomaterials. Nature 2020, 579, 41–50. [Google Scholar] [CrossRef]
- Miao, J.; Lang, Z.; Xue, T.; Li, Y.; Li, Y.; Cheng, J.; Zhang, H.; Tang, Z. Revival of Zeolite-Templated Nanocarbon Materials: Recent Advances in Energy Storage and Conversion. Adv. Sci. 2020, 7, 2001335. [Google Scholar] [CrossRef]
- Chan, K.M.; Xu, W.; Kwon, H.; Kietrys, A.M.; Kool, E.T. Luminescent Carbon Dot Mimics Assembled on DNA. J Am Chem Soc. 2017, 139, 13147–13155. [Google Scholar] [CrossRef]
- Tohamy, H.-A.S. Novel intelligent naked-eye food packaging pH-sensitive and fluorescent sulfur, nitrogen-carbon dots biosensors for tomato spoilage detection including DFT and molecular docking characterization. Int. J. Biol. Macromol. 2025, 310, 143330. [Google Scholar] [CrossRef]
- Zhang, S.; Li, X.; Yan, X.; McClements, D.J.; Ma, C.; Liu, X.; Liu, F. Ultrasound-assisted preparation of lactoferrin-EGCG conjugates and their application in forming and stabilizing algae oil emulsions. Ultrason. Sonochemistry 2022, 89, 106110. [Google Scholar] [CrossRef] [PubMed]
- Hao, B.; Wang, J.; Wang, C.; Xue, K.; Xiao, M.; Lv, S.; Zhu, C. Bridging D–A type photosensitizers with the azo group to boost intersystem crossing for efficient photodynamic therapy. Chem. Sci. 2022, 13, 4139–4149. [Google Scholar] [CrossRef] [PubMed]
- Wareing, T.C.; Gentile, P.; Phan, A.N. Biomass-Based Carbon Dots: Current Development and Future Perspectives. ACS Nano 2021, 15, 15471–15501. [Google Scholar] [CrossRef] [PubMed]
- Gan, J.; Chen, L.; Chen, Z.; Zhang, J.; Yu, W.; Huang, C.; Wu, Y.; Zhang, K. Lignocellulosic Biomass-Based Carbon Dots: Synthesis Processes, Properties, and Applications. Small 2023, 19, 2304066. [Google Scholar] [CrossRef]
- Omidian, H.; Wilson, R.L. Enhancing Hydrogels with Quantum Dots. J. Compos. Sci. 2024, 8, 203. [Google Scholar] [CrossRef]
- Sun, X.; Agate, S.; Salem, K.S.; Lucia, L.; Pal, L. Hydrogel-Based Sensor Networks: Compositions, Properties, and Applications—A Review. ACS Appl. Bio Mater. 2020, 4, 140–162. [Google Scholar] [CrossRef]
- Zhang, S.; Zhong, R.; Younis, M.R.; He, H.; Xu, H.; Li, G.; Yang, R.; Lui, S.; Wang, Y.; Wu, M. Hydrogel Applications in the Diagnosis and Treatment of Glioblastoma. ACS Appl. Mater. Interfaces 2024, 16, 65754–65778. [Google Scholar] [CrossRef] [PubMed]
- Das, P.; Ganguly, S.; Marvi, P.K.; Sherazee, M.; Tang, X.; Srinivasan, S.; Rajabzadeh, A.R. Carbon Dots Infused 3D Printed Cephalopod Mimetic Bactericidal and Antioxidant Hydrogel for Uniaxial Mechano-Fluorescent Tactile Sensor. Adv. Mater. 2024, 36, 2409819. [Google Scholar] [CrossRef] [PubMed]
- Sargazi, S.; Fatima, I.; Kiani, M.H.; Mohammadzadeh, V.; Arshad, R.; Bilal, M.; Rahdar, A.; Díez-Pascual, A.M.; Behzadmehr, R. Fluorescent-based nanosensors for selective detection of a wide range of biological macromolecules: A comprehensive review. Int. J. Biol. Macromol. 2022, 206, 115–147. [Google Scholar] [CrossRef]
- Cai, J.; Hu, H.; Li, J.; Liao, J.; Gong, X. A color-changing carbon dots/hydrogel composite for human motion sensing and sweat pH detection. Nano Res. 2025, 18. [Google Scholar] [CrossRef]
- Li, T.-X.; Zhang, N.; Lan, X.-T.; Wu, F.; Xie, Y.-H.; Feng, D.; Liu, Y.; Mei, Y.; Xie, D. Enhanced detection and absorption of Fe3+ ions using robust fluorescent hydrogels incorporating carbon dots and amphiphilic polyurethane. Colloids Surf. A Physicochem. Eng. Asp. 2025, 715, 136611. [Google Scholar] [CrossRef]
- Cai, J.; Cao, M.; Bai, J.; Sun, M.; Ma, C.; Emran, M.Y.; Kotb, A.; Bo, X.; Zhou, M. Flexible epidermal wearable sensor for Athlete’s sweat biomarkers monitoring. Talanta 2024, 282, 126986. [Google Scholar] [CrossRef]
- Mazur, F.; Han, Z.; Tjandra, A.D.; Chandrawati, R. Digitalization of Colorimetric Sensor Technologies for Food Safety. Adv. Mater. 2024, 36, 2404274. [Google Scholar] [CrossRef]
- Koppel, K.; Tang, H.; Javed, I.; Parsa, M.; Mortimer, M.; Davis, T.P.; Lin, S.; Chaffee, A.L.; Ding, F.; Ke, P.C. Elevated amyloidoses of human IAPP and amyloid beta by lipopolysaccharide and their mitigation by carbon quantum dots. Nanoscale 2020, 12, 12317–12328. [Google Scholar] [CrossRef]
- Rubio-Marcos, F.; Del Campo, A.; Marchet, P.; Fernández, J.F. Ferroelectric domain wall motion induced by polarized light. Nat. Commun. 2015, 6, 6594. [Google Scholar] [CrossRef]
- Guo, G.; Li, T.; Liu, Z.; Luo, X.; Zhang, T.; Tang, S.; Wang, X.; Chen, D. Bell pepper derived nitrogen-doped carbon dots as a pH-modulated fluorescence switching sensor with high sensitivity for visual sensing of 4-nitrophenol. Food Chem. 2023, 432, 137232. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Wang, J.Q.; Wei, S.S.; Wang, C.Z.; Yin, X.Y.; Song, X.W.; Jiang, C.Z.; Sun, G.Y. Nitrogen-doped graphene quantum dot-based portable fluorescent sensors for the sensitive detection of Fe3+ and ATP with logic gate operation. J. Mater. Chem. B 2023, 11, 6082–6094. [Google Scholar] [CrossRef]
- Maron, L.; Perrin, L.; Eisenstein, O.; Andersen, R.A. Are the Carbon Monoxide Complexes of Cp2M (M = Ca, Eu, or Yb) Carbon or Oxygen Bonded? An Answer from DFT Calculations. J. Am. Chem. Soc. 2002, 124, 5614–5615. [Google Scholar] [CrossRef]
- Gao, Y.; Duan, J.; Zhai, X.; Guan, F.; Wang, X.; Zhang, J.; Hou, B. Photocatalytic Degradation and Antibacterial Properties of Fe3+ -Doped Alkalized Carbon Nitride. Nanomaterials 2020, 10, 1751. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, Y.; Huang, C. Development of nitrogen and sulfur-doped carbon dots for cellular imaging. J. Pharm. Anal. 2019, 9, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Kaur, M.; Huang, Z. Synthesis and optical behaviors of 6-seleno-deoxyguanosine. Sci. China Chem. 2013, 57, 314–321. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shafie, A.; Ashour, A.A.; Tayeb, F.J.; Felemban, M.F. Impacts of Excessive Iron Intake on Infant Growth and Fluorimetric and Colorimetric Detection Methods: A Comprehensive Review. J. Fluoresc. 2025, 1–21. [Google Scholar] [CrossRef]
- Filler, J.; von Krüchten, R.; Wawro, N.; Maier, L.; Lorbeer, R.; Nattenmüller, J.; Thorand, B.; Bamberg, F.; Peters, A.; Schlett, C.L.; et al. Association of Habitual Dietary Intake with Liver Iron—A Population-Based Imaging Study. Nutrients 2021, 14, 132. [Google Scholar] [CrossRef]
- Ammarellou, A.; Mozaffarian, V. The first report of iron-rich population of adapted medicinal spinach (Blitum virgatum L.) compared with cultivated spinach (Spinacia oleracea L.). Sci. Rep. 2021, 11, 1–8. [Google Scholar] [CrossRef]
- Pinto, E.; Petisca, C.; Amaro, L.F.; Pinho, O.; Ferreira, I.M.P.L.V.O. Influence of different extraction conditions and sample pretreatments on quantification of nitrate and nitrite in spinach and lettuce. J. Liq. Chromatogr. Relat. Technol. 2010, 33, 591–602. [Google Scholar] [CrossRef]
- Guo, T.; Xu, J.; Guo, Y.T.; Ning, B.; Li, J.T.; Gong, L.Z.; Lin, X.Y.; Zhuang, S.H.; Wei, Z.W. Ultrathin boron nanosheets: A novel fluorescent sensor for sensitive and selective detection of Fe3+ and ascorbic acid. Spectrochim. Acta Part A-Mol. Biomol. Spectrosc. 2025, 343, 126556. [Google Scholar] [CrossRef]
- Nuntahirun, P.; Li, C.H.; Sirisit, N.; Shashikumar, U.; Tsai, P.C.; Manjappa, K.B.; Huang, G.G.; Paoprasert, P.; Ponnusamy, V.K. Novel blue-pea flowers derived-carbon dots/iron oxide nanohybrid as sustainable “turn-off” fluorescent nanosensor for selective Fe3+ detection in food samples. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2025, 339, 126291. [Google Scholar] [CrossRef]
- Zhang, Q.K.; Dou, S.H.; Leng, H.; Shu, Y. A small molecule modified UiO series MOFs for simultaneous detection of Fe3+ and Zn2+. Talanta 2025, 286, 127483. [Google Scholar] [CrossRef]

| Sample | Added (μM) | Found (μM) | Recovery (%) | RSD (%) |
|---|---|---|---|---|
| spinach | 0.5 | 0.54 | 108.00 | 1.95 |
| 1.5 | 1.53 | 101.68 | 1.05 | |
| 2.5 | 2.52 | 100.70 | 1.35 | |
| urine | 0.5 | 0.51 | 101.63 | 1.15 |
| 1.5 | 1.51 | 100.46 | 2.17 | |
| 2.5 | 2.47 | 98.70 | 1.50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Zhang, Q.; Tang, J.; Yang, H.; Ji, X.; Wang, J.; Han, C. A Portable and Thermally Degradable Hydrogel Sensor Based on Eu-Doped Carbon Dots for Visual and Ultrasensitive Detection of Ferric Ion. Molecules 2025, 30, 3280. https://doi.org/10.3390/molecules30153280
Zhang H, Zhang Q, Tang J, Yang H, Ji X, Wang J, Han C. A Portable and Thermally Degradable Hydrogel Sensor Based on Eu-Doped Carbon Dots for Visual and Ultrasensitive Detection of Ferric Ion. Molecules. 2025; 30(15):3280. https://doi.org/10.3390/molecules30153280
Chicago/Turabian StyleZhang, Hongyuan, Qian Zhang, Juan Tang, Huanxin Yang, Xiaona Ji, Jieqiong Wang, and Ce Han. 2025. "A Portable and Thermally Degradable Hydrogel Sensor Based on Eu-Doped Carbon Dots for Visual and Ultrasensitive Detection of Ferric Ion" Molecules 30, no. 15: 3280. https://doi.org/10.3390/molecules30153280
APA StyleZhang, H., Zhang, Q., Tang, J., Yang, H., Ji, X., Wang, J., & Han, C. (2025). A Portable and Thermally Degradable Hydrogel Sensor Based on Eu-Doped Carbon Dots for Visual and Ultrasensitive Detection of Ferric Ion. Molecules, 30(15), 3280. https://doi.org/10.3390/molecules30153280







