Research and Developments of Heterogeneous Catalytic Technologies †
Abstract
1. Introduction
- Homogeneous: The catalyst and reactants are in the same phase, typically liquid. Gas-phase homogeneous catalysis is rare but exemplified by the oxidation of SO2 to SO3 using nitrogen oxides.
- Heterogeneous: The catalyst and reactants are in different phases, usually involving solid catalysts interacting with gaseous, vapor, and/or liquid reactants.
- Biocatalysis: Catalysis mediated by whole microorganisms or isolated enzymes, typically in the liquid phase. While classical biocatalytic processes include alcohols [15,16] and citric acid production [17,18,19,20], more recent developments involve engineered enzymes for specialty chemical [21,22,23,24,25,26] and pharmaceutical synthesis, including enantioselective transformations and active pharmaceutical ingredient (API) production [27,28,29,30].
2. Properties of Catalysts
- (i)
- Chemical composition and crystallographic structure.
- (ii)
- Texture and physical–chemical properties.
- (iii)
- Temperature and chemical stability.
- (iv)
- Mechanical stability.
- (v)
- Mass, heat, and electrical transport properties.
- (vi)
- Catalytic performance.
2.1. Chemical Composition and Crystallographic Structure
- Chemical analysis and determination of the molecular structure, typically achieved through classical spectroscopic and analytical techniques.
- Chemical analysis of both the support material and the active catalyst with deposited metal, often involving decomposition in acidic media, followed by atomic absorption (AAS) or emission spectrophotometry—Inductively Coupled Plasma Optical Emission Spectroscopy and Mass Spectroscopy (ICP OES, ICP MS).
- Nuclear Magnetic Resonance (NMR) performed in both solution and solid state to elucidate the structural features of the support, including surface functional groups.
- X-ray reflection spectroscopy (XRF) to characterize surface composition.
- X-ray Powder Diffraction (XRPD), used for phase identification and crystallographic analysis; also applicable for estimating average crystallite size via the Scherrer equation.
- Electron Diffraction X-ray Analysis (EDX), often coupled with electron microscopy, to determine surface composition and the spatial distribution of metal species.
- Wavelength-dispersive X-ray spectroscopy (WDS, WDX), offering enhanced sensitivity over EDX for surface elemental analysis.
- Fourier Transform Infrared Spectroscopy (FTIR), utilized for the identification of surface functional groups and chemical bonding environments.
- Raman spectroscopy for characterizing molecular and surface species.
- X-ray photoelectron spectroscopy (XPS), critical for determining the valence state of metal particles on the surface.
2.2. Texture and Physical–Chemical Properties
- Particle size distribution measurements in the range of 10 nm to 5 mm.
- Optical microscopy for determining particle size, shape, and surface texture (resolution: 250 nm; high-quality laser scanning confocal microscopy (LSCM): resolution down to 0.5 nm).
- Raman spectroscopy for identifying surface phases and irregularities, e.g., quantification of graphite and disordered carbon content on activated carbon [69].
- Mercury porosimetry (applicable only to mechanically stable materials) for pore size distribution ranging from 7.5 nm at 200 MPa to 15 μm at atmospheric pressure.
- Transmission electron microscopy (TEM), including High-Resolution TEM (HR-TEM) and Scanning TEM (STEM), for determining the particle size distribution of metal crystallites, atomic arrangement, and crystallographic phases (resolution: 0.1 nm).
- Chemisorption of H2 or CO for evaluating the specific surface area of metallic crystallites or agglomerates (applicable when internal volume is accessible—see [49]).
- Titration with basic components, e.g., NaOH solution, to determine acidity.
- Adsorption measurements of basic components (e.g., NH3, organic amines) to evaluate acidity; FTIR spectroscopy is used to identify Lewis and Brønsted acid sites.
- Titration with acidic components, e.g., HCl, to determine alkalinity.
- Adsorption measurements of acidic components, e.g., CO2, to determine alkalinity.
- Contact angle (CA)—Water is used as the reference hydrophilic liquid. A CA value less than 90° indicates a hydrophilic surface, while a value greater than 90° indicates a hydrophobic surface. This is a static measurement method. Powder samples must be compacted into larger particles with a “flat surface” (e.g., tablets) to enable accurate measurements.
- Washburn dynamic method (WDM)—A liquid with defined polarity rises through a porous medium in a cylindrical setup; both the rate of wetting and the maximum height achieved are recorded [74]. The referenced study also provides useful correlations between CA and WDM data.
- Adsorption of adsorptives with a different polarity—This method evaluates surface polarity based on the adsorption behavior of molecules with varying polarities. Surface roughness significantly affects the results [75].
- Zeta Potential (ζ-Potential)—Values close to zero suggest low surface polarity and are typically associated with hydrophobic surfaces. However, establishing a quantitative correlation between zeta potential and hydrophilicity is complex and not straightforward [76].
2.3. Temperature and Chemical Stability
- Thermogravimetric analysis (TGA)—Measures the extent of decomposition of the carrier or catalyst as temperature increases. TGA can be performed in an oxidative atmosphere (Temperature Programmed Oxidation—TPO) or a reductive atmosphere (Temperature Programmed Reduction—TPR).
- Differential scanning calorimetry (DSC)—Measures decomposition and thermal effects, such as the release of water from the crystalline lattice or pyrolysis effects in organic polymer carriers. DSC is often followed by analysis of degradation products (e.g., using GC or GC-MS).
- (Micro)Pyrolysis combined with pyrolysis product analysis—Similar to DSC with follow-up analysis, but carried out under different conditions to study pyrolysis products in more detail.
- Hydrolytic, Acidic, Basic, Aminolytic, Alcoholytic, and Other Stability Tests—These tests are particularly important for evaluating the stability of polymer-based catalysts.
2.4. Mechanical Stability
- Crush Strength;
- Young’s Modulus of Elasticity;
- Breakage by Collision;
- Breakage by Stress in a Fixed Bed;
- Breakage in Contiguous Equipment.
2.5. Mass, Heat, and Electrical Transport Properties
- Diffusivity in pores—Includes bulk diffusion, Knudsen diffusion, wall effects, and the impact of tortuosity.
- Thermal conductivity—Critical for heat management in exothermic or endothermic reactions.
- Hydrodynamic resistance—Resistance of the catalyst bed or layer to the flow of gas/vapor or gas–vapor–liquid mixtures.
- Electrical conductivity—Particularly important for catalyst supports, as it strongly influences metal–support interactions.
- Character of a semiconductor’s surface—Especially relevant for metal oxides (e.g., ZnO, NiO, Fe2O3, V2O5, Cr2O3). These materials can exhibit n-type behavior (donating electrons to chemisorbed molecules) or p-type behavior (withdrawing electrons) [8,79]. This distinction is important for quantifying Van der Waals interactions and their influence on HOMO and LUMO energy levels, see, e.g., [80]. Measurements of isoelectric points provide an initial estimation of surface electronic properties [81].
2.6. Catalytic Performance
- Katal, kat—SI coherent derived unit for catalytic activity, defined as the amount of reactant (in moles) converted per second: (molreactant·s−1).
- Molar Catalytic Activity (kat/mol)—Catalytic activity per mole of catalyst: (molreactant·s−1·molcatalyst−1).
- Mass Catalytic Activity (kat/mcatalyst)—Catalytic activity per unit mass of catalyst: (molreactant·s−1·kgcatalyst−1).
- Turnover Frequency (TOF) [83]—Also expressed as (molreactant·s−1·molcatalyst−1), TOF is functionally identical to kat/mol but predates it and remains widely used, especially in heterogeneous catalysis. TOF refers to the number of reactant molecules converted per active site per second. It is crucial to specify how the number of active sites is determined, e.g., by acidity, accessible metal atoms via chemisorption, etc. Additionally, temperature, pressure, and reactant concentrations must be reported to fully specify TOF.
- Turnover Number (TON)—Defined as the number of moles of reactant converted per mole of catalyst during its lifetime: (molreactant·molcatalyst−1). TON is useful for describing catalyst durability in non-regenerated systems (e.g., in continuous reactors over time on stream). However, it is generally discouraged, especially when estimated from batch reactors, as it can be misleading [83].
- Catalyst productivity (CatProd)—Expressed in the same units as TON (molreactant·molcatalyst−1), this parameter describes the total productivity of a catalyst under practical conditions. While not an official IUPAC term, CatProd is useful in process design and techno-economic evaluations. It requires well-defined experiments, typically continued to the point at which the catalyst is no longer economically viable or is fully consumed, as in some polymerization catalysts [84]. Like TOF, full specification includes temperature, pressure, and reactant concentrations.
- Selectivity to a desired product (Sdes_pr)—The number of moles of the desired product relative to the theoretical maximum according to stoichiometry, at a given conversion of a key reactant.
- Catalyst lifetime (CLT)—The total time the catalyst remains active in the reactor (typically in days or years), from its introduction until deactivation to an unacceptable level.
- Catalyst life cycle (CLC)—The full period covering catalyst use, deactivation, regeneration, and eventual replacement. It is also expressed in days or years.
- 0.
- Transport of reactants from the bulk phase to the vicinity of the catalyst (often neglected due to intensive mixing);
- 1.
- Transport of reactants through the external surface layer surrounding the catalyst;
- 2.
- Diffusion of reactants into the catalyst pores toward active sites;
- 3.
- Chemisorption of at least one reactant onto the active sites;
- 4.
- Surface catalytic reaction;
- 5.
- Desorption of products from the catalyst surface;
- 6.
- Diffusion of products out of the catalyst pores;
- 7.
- Transport of products through the external surface layer;
- 8.
- Transport of products into the bulk phase (often neglected due to intensive mixing).
- Apparent Activation Energy (Ea) as an indicator:
- (a)
- If transport limitations dominate, Ea ≈ 4–12 kJ·mol−1.
- (b)
- For catalytic reactions, Ea typically ranges from 30 to 120 kJ·mol−1.
- (c)
- Non-catalyzed reactions tend to have Ea > 120 kJ·mol−1.
- This implies that performing experiments at different temperatures and calculating Ea can help identify whether the regime is kinetic or transport-limited. However, caution is needed; at high temperatures, the reaction rate may exceed the transport rate, resulting in a drop in apparent activation energy, e.g., hexene combustion over platinum gauze at >400 °C [56].
- Effect of Particle Size:Transport limitations become more significant with larger catalyst particles (>1 mm). If active sites are uniformly distributed, decreasing particle size reduces diffusion limitations until a threshold is reached, beyond which mass-specific activity remains constant.
2.6.1. Kinetic Regime
- Statistical, experimental, usually in the form of power-law equations.
- Mechanistic, considering steps of chemical transformation. Commonly, three basic types of chemical reactions are considered:
- a.
- Reactants are chemisorbed on catalytic centers (Langmuir–Houghen–Hinshelwood–Watson models—LHHW);
- b.
- One reactant is chemisorbed, and another one comes as not-chemisorbed (Elley– Rideal models—ER);
- c.
- One of the reactants (e.g., oxygen) is exchanging positions in the crystal framework of the catalysts (Mars van Krevelen models—MarKre).
2.6.2. Mass and Heat Transport
3. Heterogeneous Catalysts
- Unsupported catalysts—Catalytic centers are relatively uniformly distributed throughout the entire catalyst volume. All components are typically mixed before the final formulation of the catalyst.
- Supported catalysts—Catalytic centers are located on the surface of a support, including within micropores. This includes surface functionalization of a pre-formed support and/or the deposition or generation of metal nanoparticles on the support.
3.1. Unsupported Catalysts
3.2. Supported Catalysts
- Preparation of relatively large catalyst particles (up to 20 mm), which helps minimize resistance to fluid flow (gas and/or liquid).
- Increase in mechanical strength compared to unsupported catalysts.
- Reduced amounts of catalytically active components, particularly expensive metals and their compounds.
- Favorable interactions with the support, including reactant–support, product–support, solvent–support, catalytic moiety–support, e.g., metal (nano)particles, which influence chemisorption of both reactants and products.
- Stabilization of catalytic moieties on the surface of support (e.g., minimization of sintration by embedding particles in pores of a support, or by interactions with functional groups of the support).
- Enhanced control over reaction temperature and contact time by adjusting the amount and distribution of catalytic moieties (e.g., Pd on alumina for selective hydrogenation of acetylene in ethylene production).
- Inorganic (e.g., elemental carbon or metals, oxides, carbonates);
- Organic (various types of polymers);
- Hybrid (inorganic–organic composites).
- Natural polymers (e.g., celluloses, lignocellulose, chitin, starch);
- Synthetic polymers, primarily those containing heteroatoms (O, N, P, S, etc.);
- Covalent Organic Frameworks (COFs) and Metal–Organic Frameworks (MOFs)
- Interactions with Atoms and Surface Defects of the Support Framework: This type is especially relevant for inorganic semiconductor-type supports, and has been recognized since the early systematic studies of catalysis (pre-1960) [8,10]. The electronic nature of atoms in the support—whether electron-donating or electron-withdrawing—significantly influences catalytic performance. One way to evaluate these effects is by comparing electronegativities (χ). For example, effect of gold (χ = 2.54) as electron attracting atom with respect to Fe (χ = 1.83) exhibited positive effect on lowering the activation energy (EA) in oxidation of CO over the Fe2O3/Al2O3 catalyst, and manganese (χ = 1.55) as electron repelling with respect to Fe increased EA [117]. A peculiar position belongs to cerium (χ = 1.12) mainly as CeO2. Due to low electronegativity, a strong electron repelling effect helps in reactions where activation starts with the addition of an electron to a reactant, e.g., in oxidations, the best-known examples are convertors for oxidation abatement of flue gases [79]. Low electronegativities are also exhibited by lantanoids (χ = 1.1–1.3); however, industrial applications of these metals and their compounds as promoters are limited due to their relatively high prices. Furthermore, metal particles supported on oxides or other substrates can exhibit Strong Metal–Support Interactions (SMSI), a phenomenon studied extensively since the 1970s [118]. Notable support materials involved in SMSI include ZnO, TiO2, CeO2, MgO, and nitrides, especially in combination with transition non-noble or noble metals. These interactions are now well characterized using advanced analytical techniques (see Figure 6).
- Interactions with Functional Groups: Functional groups on the support—especially those containing lone-pair donating atoms like nitrogen (e.g., –NH2, –NH–CO– in polyamides, polyurethanes, polyureas, and porphyrins) or phosphorus—can significantly influence the behavior of supported catalysts [49]. In the case of metal particles, these can form Covalent Metal–Support Interactions (CMSI), enhancing stability and tuning reactivity.
- Chemical modification of a support:
- Oxidation—to introduce functional groups such as carboxyl, keto, aldehyde, and hydroxyl;
- Amination—addition of amine, substituted amine, or tetraalkylamine groups;
- Sulfonation—formation of –SO3H groups;
- Covalent binding of organocatalysts—e.g., nitrogen- or phosphorus-based moieties, metal–organic catalytic complexes;
- Immobilization of enzymes or enzyme-like catalytic moieties.
- These methods are used to prepare heterogenized catalysts—homogeneous catalysts made heterogeneous by anchoring onto supports.
- Introduction of metal (nano)particles:
- Fixation of pre-synthesized nanoparticles;
- Adsorption of metal precursors followed by decomposition or reduction, through techniques such as:
- Wetting processes;
- Adsorption, ion-exchange procedure;
- Chemical vapor deposition;
- Electrochemical deposition, especially effective for single-atom catalysts (SACs).
3.3. Composite Catalysts
3.4. Promoters
- Promoters Permanently Incorporated into the Catalyst Structure.
- These modifiers are embedded into the catalyst and influence performance through various mechanisms.
- Structural Promoters: Prevent undesirable structural changes, such as the sintering of metal nanoparticles, thereby preserving active surface area.
- Electronic Promoters: Modify electron density at active sites. For instance, alkali metals increase the electron density on iron catalyst surfaces, enhancing nitrogen adsorption and activation in ammonia synthesis [6].
- Surface modifiers: Influence surface chemistry and physical properties. Examples include sulfur-induced poisoning of certain sites on Fischer–Tropsch catalysts to improve olefin selectivity [130], conversion of hydrophilic to hydrophobic surfaces via tethering of polytetrafluoroethylene oligomers [131], ionic liquids [132], or anchoring of stereospecific ligands (heterogenized) to improve enantioselectivity. For example, over 90% selectivity to (R)-1-phenyl-1-propanol was achieved using PS-supported hydroxyamides in the enantioselective ethylation of benzaldehyde [133].
- 2.
- Promoters Introduced into the Reaction Medium: These act dynamically, affecting surface interactions and reaction pathways. They may compete for adsorption sites, modify the reaction mechanism, or create transient intermediates. For instance, chlorine-containing species formed in situ during ethylene epoxidation facilitate oxygen activation over silver catalysts [129], and water vapor used to suppress carbon deposition in the dehydrogenation of ethylbenzene to styrene over iron catalysts [134]. Numerous additional cases highlight the use of in situ promoters to alter surface behavior and enhance catalyst performance [79].
3.5. Structured Catalysts
- Structures formed as a result of standard preparation processes:
- Fibers and meshes:
- Skeletal catalysts:
- Corrugated supports prepared from inorganic or organic sheets:
- Extruded and 3D printed:
- Inorganic: Skeletons are typically made of silica, spinels (e.g., MgAl2O4), or cordierite ((Mg,Fe)2[Si5Al4O18]·nH2O), using extrusion. Additives like aluminum hydroxide or zinc salts improve plasticity and structural stability [96]. After calcination, monoliths can withstand up to 1200 °C. Porosity is improved via a washcoat layer (e.g., Al2O3) with anchored catalytic nanoparticles [148]. For more complex architectures, 3D printing is employed, enabling vertical and horizontal channel formation [154]. Additive Manufacturing (AM 3D) methods can include zeolites, MOFs, COFs, or burnable organic additives to increase porosity during calcination.
- Hierarchical catalysts (HiCat): Figure 2b refers to the multi-level porous architecture (micro-, meso-, and macropores) that facilitates enhanced diffusion and accessibility to active sites. These catalysts are particularly relevant for zeolites [123,140,158,159,160]. Conventional zeolites are microporous (0.4–0.8 nm). Post-treatment (e.g., desilication with alkaline hydroxides) introduces mesoporosity, improving performance by reducing transport limitations. Hierarchical structuring can also be achieved using bulky templates (alcohols, amines), polymer nanoparticles, or carbon black, followed by calcination. In this context, the term hierarchy factor (HF) is important; HF = (Vmicrop/Vp) × (Smesop/SBET) with V = volume, S = surface. Typical HiCats of zeolite type exhibit HFs from 0.15 to 0.2. HiCats’ zeolite type extended potential for their application [160,161,162]. In addition to zeolites, some other HiCats were prepared and tested [139,163,164,165]. OMOPs, including MOFs and COFs StrCats with regular texture, are also considered as HiCats [166].
- Encapsulated catalysts (EnCat): Encapsulation of catalytic active moieties (enzymes, metal–organic complexes, metal nanoparticles) is an effective method for protecting catalysts from deactivation. The active species are enclosed within a capsule with permeable walls that allow the passage of reactants and products. Encapsulation prevents the formation of agglomerates (e.g., sintering in the case of inorganic materials), while diffusion through the surrounding layer can regulate selectivity. Various techniques for preparing EnCats, including the encapsulation of magnetic particles for easy catalyst separation, are summarized in [167]. EnCats are also used industrially, for example, palladium encapsulated in polyureas [168,169]. EnCats based on polymeric organic films are typically suited for mild reaction conditions, with operational temperatures not exceeding 150 °C [167,170,171,172]. When enzymes are encapsulated, the working temperature is even lower (around 75 °C), although this is still higher than the operational limit for free enzymes [173]. Catalytic species can also be encapsulated within porous inorganic materials, such as zeolites, which allow much higher working temperatures—up to 400 °C [174,175]. In this context, it is worth comparing encapsulated catalysts with metal catalysts anchored to supports containing strong chelating groups (e.g., nitrogen-based moieties). Recent studies have demonstrated that palladium supported on crosslinked polyureas exhibits higher stability than encapsulated variants [122]. This finding highlights the importance of considering both approaches during catalyst design, with attention to both reaction rate and catalyst lifetime.
3.6. Electrocatalysts
- Faradaic efficiency:
- Energetic efficiency
3.7. Photocatalysts
3.8. Deactivation
- Poisoning by impurities, e.g., reaction of sulfur compounds with metals in hydrogenation processes.
- Formation of side products that adhere to the surface and block reactants from accessing catalytic sites (e.g., tar formation on cracking catalysts).
- Detachment of anchored functional groups from the surface of heterogenized catalysts (acidic groups, basic groups, metal–organic groups, etc.).
- Transformation of catalytic centers into soluble or vaporizable compounds and subsequent leaching into the reaction environment (common in reductions involving oxidizing reactants such as nitro- and nitroso-compounds).
- Growth of metal (nano)particles into larger ones (sintering), resulting in loss of activity due to decreased specific surface area.
- Abrasion or breakage of catalyst particles, mainly occurring in fluid and suspension reactor systems, although catalysts in fixed-bed reactors also experience this slowly over time.
- Use catalysts that are chemically stable and operate within appropriate temperature ranges; for example, observe the limited working temperature of OMOP catalysts.
- Operate at the lowest possible temperature that still ensures good catalyst performance.
- Utilize supported catalysts, preferably structured catalysts (StrCats) when possible.
- Choose suitable chelating groups for supported catalysts.
- Minimize the presence of chemical compounds that can attack or poison the catalyst.
- Ensure proper flow conditions around the catalyst surface—adequate for efficient transport of reactants and products but not so intense as to cause catalyst abrasion; this also applies to mixing intensity.
4. Catalytic Technologies
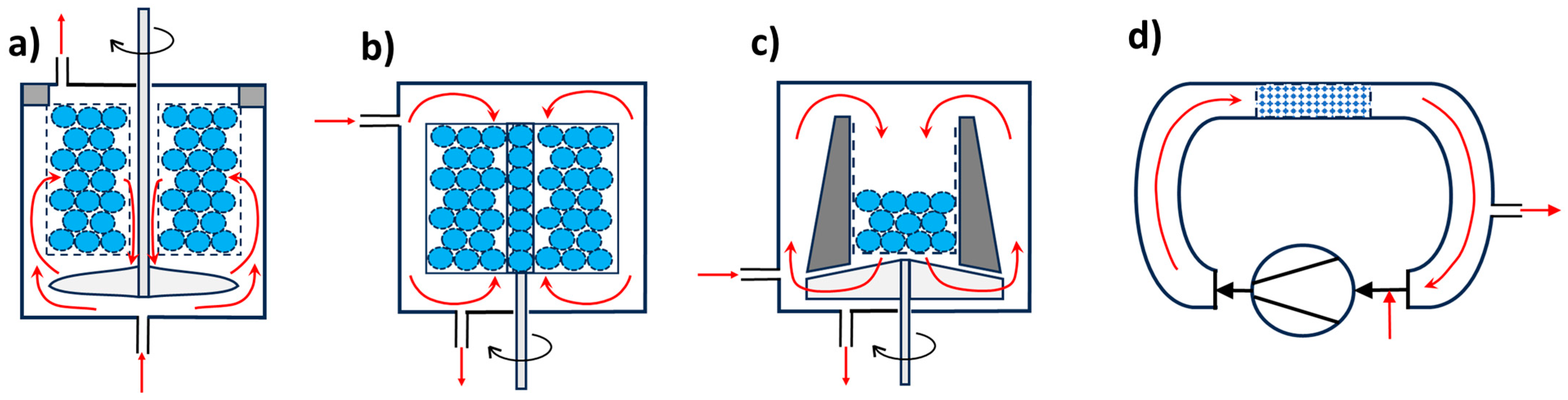
4.1. Gas-Phase Reactors (G-S)
4.2. Liquid-Phase Reactors (L-S)
4.3. Gas Liquid Phase Reactors (G-L-S)
- G-L:
- L-S (Equation (10));
- Internal diffusion (Equation (5)).
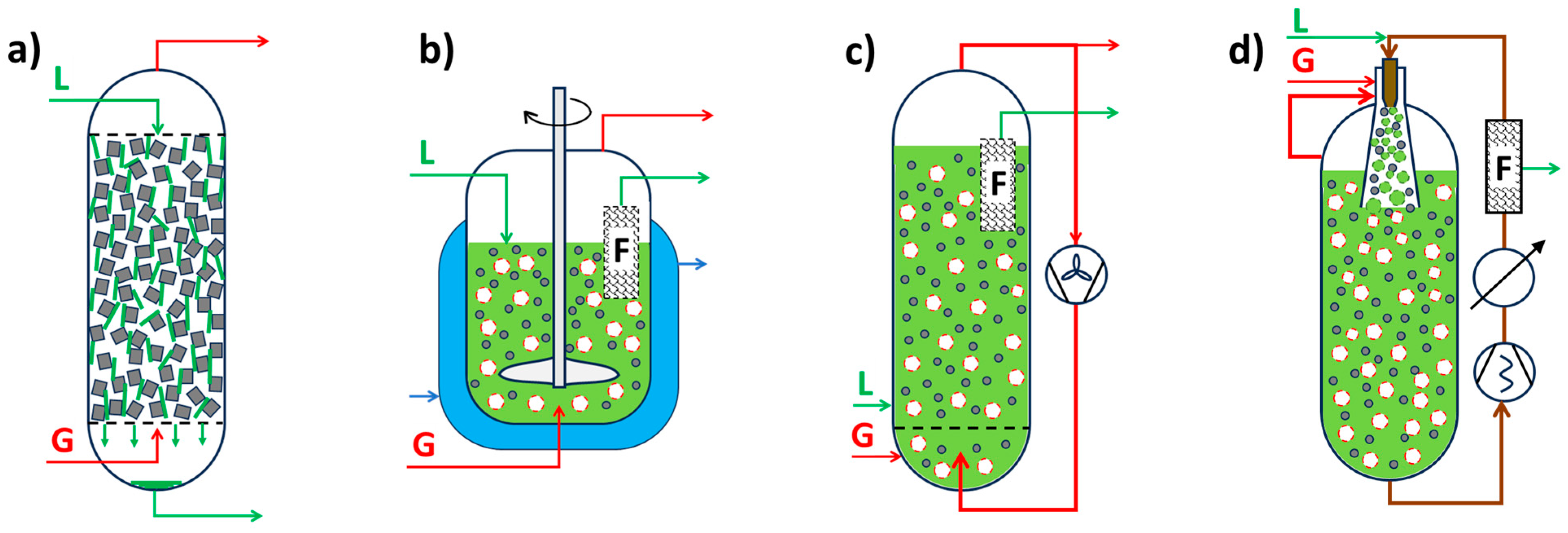
4.4. Special Chemical Catalytic Reactors
4.5. Electrocatalytic Processes and Reactors
- Electron transfer;
- Reorganization of intramolecular bonds;
- Reorganization of the solvation shell.
4.6. Heterogeneous Photocatalytic Processes and Reactors
- Light source:
- Natural or Artificial;
- Light concentrators (mirrors, lens):
- Without Light Concentrators;
- With Light Concentrators;
- Wavelength:
- Visible (400–780 nm);
- UV (190–400 nm);
- Type:
- Conventional Lamps;
- Light Emitting Diodes (LED);
- Catalyst:
- TiO2 or not TiO2 based;
- Size:
- Micron;
- Nanometer;
- Catalyst movement:
- Immobilized (On the wall of the reactor, Bed, Fibers, Membrane, or Disk);
- Suspended;
- Reactor:
- Batch or Continuous;
- Hydrodynamic behavior:
- Continuously Stirred (CSTR);
- Plug Flow (PFR).
- C1 chemistry:
- Waste treatment:
- Special chemicals:
4.7. Microreactors (MRs)
- Silicon/ceramic materials (well-characterized, high precision but expensive fabrication, high-temperature resistance up to 1300 K);
- Glass (allows visualization of reaction and flow, electroosmotic flow (EOF) possible, withstands high operating pressures up to 50 bar);
- Polymers (low cost, various fabrication techniques, tunable properties, disposable microreactors possible, low chemical and temperature resistance—max 500 K);
- Metals (durable, well-established fabrication techniques, sensitivity to reaction environment, suitable for higher temperature—up to 1100 K—and pressure—up to 50 bar—regimes).
- High intensity of mixing; enhanced mass and heat transport;
- Increased chemical reaction rates;
- Efficient application of external energy sources (e.g., light, microwaves, ultrasound);
- Potential integration with membrane technologies (especially “tube-in-tube” reactors);
- Precise temperature control;
- High operational pressure tolerance;
- High selectivity;
- High volumetric productivity.
- Fouling and clogging;
- High pressure drop;
- Requirement for high-purity reactants;
- Catalyst deactivation (due to abrasion or leaching);
- Not suitable for large-scale production (despite high volume-specific productivity).
5. Design of Catalytic Technologies
- Basic Research (BaR, also known as fundamental or pure research)The focus is on expanding knowledge and understanding the essence of phenomena, without aiming at practical applications. In chemistry, this includes hypothesizing, experimentation, analysis, and synthesis of data related to phenomena at the picometric scale (10−12 m) and down to the attosecond scale (10−18 s), i.e., interactions of atoms, electrons, subatomic particles, and electromagnetic radiation.
- Applied Research (ApR)The goal is to solve practical, real-world problems. ApR uses insights from BaR and solutions derived by analogy to previously solved issues. In chemistry, ApR involves the characterization of reaction systems (e.g., reaction kinetics, mass and heat transfer, catalysts—see section on catalyst properties), and the study of physical–chemical properties such as enthalpy, rheological behavior, melting/boiling points, electrical conductivity, magnetic susceptibility, and polarizability. It also quantifies interactions among reactants (e.g., deviations from ideal behavior, miscibility). Both experimental and theoretical approaches are used to achieve desired values of relevant parameters.
- Technological Research (TeR)The aim is to develop new products that meet emerging societal needs or to improve existing production routes. Objectives include enhancing product quality (e.g., enantiomeric purity in APIs), reducing raw material and energy consumption, improving process safety, and minimizing environmental impact. These improvements depend on factors such as catalyst performance (activity, selectivity, productivity, lifetime, regenerability), reactor type, solvent choice, process conditions (pressure, temperature, mixing mode, batch vs. continuous operation), separation units, waste treatment, and synergistic effects (Figure 14). TeR makes full use of BaR and ApR findings but can also stimulate further BaR or require complementary ApR. It commonly involves experimental and mathematical modeling and includes application testing, which can span several years. For example, testing a new fertilizer typically requires at least four years of field trials (excluding the “worst year”) to evaluate efficacy, consistency, and economic viability. For biologically active substances or APIs, the evaluation period can be even longer. TeR must fully adhere to green and sustainable design principles [63,64,66].
- Large-Scale Technologies (Bulk Chemical Production)
- Inorganic (NH3, HNO3, H2SO4, etc.);
- Organic (components for fuels, monomers, polymers, etc.);
- Biotechnologies (fermentation, enzymatic hydrolysis, etc.).
- Chemical specialties:
- Inorganic (components for production of catalysts, pure chemicals for electronics, special technical products—glasses, etc.);
- Organic and organic–inorganic mixed (additives to polymers, antidegradants, explosives, dyes, biologically active substances);
- Biotechnologies (production of API and their precursors, e.g., penicillin, etc.).
- Waste Treatment (liquid, gas, soil).
- A relatively inexpensive catalyst with average activity and selectivity, combined with a simple separation system.
- A more expensive, highly active, and selective catalyst, which may be sensitive to impurities, temperature, and pressure fluctuations. This choice would necessitate high-purity inputs and sophisticated process control systems, increasing costs.
- TeR.
- Feasibility Study—Includes process principles, process flow diagrams (PFDs), raw material and energy requirements, waste treatment strategies, a preliminary layout, economic evaluation (CAPEX and OPEX), safety and environmental assessments, and comparison with Best Available Technology (BAT).
- Basic Design (BD)—Covers process principles, PFDs, material and energy balances, equipment selection and integration, control systems, Piping and Instrumentation Diagrams (P&IDs), utility requirements (cooling, water supply, energy), analytics, packaging and transportation of products, waste treatment, preliminary economic and ecological assessments, HAZOP (Hazard and Operability Study), and identification of unresolved issues.
- Legislation—Involves Environmental Impact Assessment (EIA), REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals), IPPC (Integrated Pollution Prevention and Control), and other applicable regulatory requirements, including those related to hazardous substances and construction permits.
- Detailed Design (DD)—Provides a more detailed version of all BD elements.
- Realization—Physical construction and implementation of the technology.
- Testing—Includes commissioning and validation of the process.
- Update of DD Documentation—Reflects changes based on real-world implementation.
- Routine operation.
- Optimization.
- Retrofit or Decommissioning of Technology.
- New technology development (novel catalyst, reactor, separation system).
- Retrofit (existing or new product, with a new catalyst and adaptation of existing equipment). Retrofits are more cost-effective and quicker, but come with limitations—material, volume, mechanical strength (e.g., maximum pressure and temperature), and constraints related to heating/cooling, mixing, and separation units [228,443,444].
- Battery limits investment (BL, cost_basis = 1)
- 2.
- Utility investment (not all items are necessary for every technology) (ca. 0.5*cost_basis)
- 3.
- Off-site investment (not all items are necessary for every technology) (ca. 0.2*cost_basis)
- 4.
- Working capital (ca. 0.7*cost_basis)
- Raw materials;
- Catalysts and chemicals consumed in manufacturing (other than raw materials);
- Utility costs: fuel, electricity, steam, cooling water, refrigeration, compressed air, inert gas;
- Labor (about 5% of total expenses for batch processes, about 1% for continuous processes);
- Maintenance (usually ~6% of CAPEX).
- Capital cost repayments (depreciation; typical lifetimes for new technology are 8, 12, or 15 years, used for linear, degressive, or progressive depreciation calculations);
- Routine maintenance;
- Overheads (e.g., safety, laboratories, personnel facilities, administration);
- Quality control;
- Local taxes;
- Labor unrelated to production (e.g., security);
- Insurance.
- Payback Time (PaybTi): The time needed to recover the capital investment from average annual cash flow. For retrofits, it is the time needed to recover the retrofit cost through improved operating savings. PaybTi should be significantly shorter than the technology’s planned lifetime (usually not exceeding 80% of it).
- Return on Investment (ROI): The ratio of average annual income over the productive life of the project to the total initial investment, expressed as a percentage.
- Brief description of technology;
- Block flow diagram (BFD), i.e., definition of main technological units linked with streams, including recycles;
- If possible, comparison with best available technology (BAT);
- Raw materials and energy demands;
- Catalyst and type of reactor (see above);
- Kinetics;
- Lifetime, regeneration, and disposal of deactivated catalyst;
- Preliminary calculation of reactor volume;
- Range of temperature and pressure for operation;
- Recommendations for separation units;
- Physical–chemical, explosive, and hazardous/toxic properties of individual components and their mixture;
- Characterization of wastes;
- Preliminary economic, safety, ecological, and technological risk analysis.
6. Examples of Technologies
6.1. Ammonia
6.2. Fluid Catalytic Cracking (FCC)
6.3. Methanol
6.4. Alkyl Tert-Butyl Ethers (ATBE)
6.5. Aniline (AN)
- Fermentation of sugars in the presence of ammonia to form ammonium 2-aminobenzoate (NH4–OAB);
- Thermal and/or catalytic decarboxylation NH4-OAB to aniline;
- Extraction of the resulting aniline using a suitable solvent (e.g., dodecanol);
- Separation of aniline via rectification and solvent recycling;
- Separation and recycling of ammonia.
6.6. Photocatalytic Water Splitting
6.7. Catalysts in Treatment of Waste Plastics
7. Conclusions
- Prioritize biotechnological methods (see examples in [522]).
- Utilize electrochemical processes when affordable electricity is available.
- Apply photocatalytic techniques for waste treatment and the production of chemical specialties.
- When opting for a heterogeneous catalyst, verify its availability from commercial suppliers.
- Catalyst development should follow thorough physicochemical analysis, including molecular modeling (using appropriate software), diffusion properties, thermal and chemical stability, toxicity, cost of preparation chemicals, ecological impact, regeneration potential, disposal methods, and reproducibility. Adhere strictly to principles of green and sustainable chemistry [63,66,523].
- Use commercial laboratory reactors or design custom ones for catalytic testing. Conduct a sufficient number of experiments to evaluate activity, selectivity, temperature effects on performance, and catalyst lifetime.
- Develop a robust physicochemical model of the reactor suitable for scale-up, employing appropriate software tools.
- Favor continuous reactors over batch reactors whenever feasible.
- For low-scale production (up to 1 kg/h), consider the use of flow microreactors.
- In exothermic reactions, design for the highest possible operating temperature to efficiently utilize generated heat while accounting for material stability and product selectivity.
- Address the trade-off between reaction rate and equilibrium composition by optimizing temperature regimes and feed locations. Consider autothermal reactors (e.g., those used in ammonia synthesis [6]).
- Compile data on the physicochemical hazards and toxicities of individual components and mixtures (e.g., explosion limits).
- If application tests are required (for new polymers/composites, biologically active compounds, polymer additives, fertilizers, etc.), propose test protocols and estimate the necessary sample quantities.
- Prepare detailed Block Flow Diagrams.
- Estimate raw material and energy consumption, along with waste generation.
- Assess economic feasibility.
- Identify and evaluate risks: economic, safety, ecological, and technological.
- Define open questions and uncertainties.
- Recommend pilot plant implementation when necessary to resolve open questions and minimize risks.
- Collaborate on feasibility studies and basic design phases.
- Participate in large-scale technology testing.
- Engage in the optimization of large-scale processes.
- Collect and maintain data for the future development of similar technologies.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jess, A.; Wasserscheid, P. Chemical Technology: From Principles to Products, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA; SPi Global: Chennai, India, 2020; pp. 1–912. [Google Scholar]
- Murzin, D.Y. Chemical Product Technology; De Gruyter: Berlin, Germany, 2018; pp. 1–280. [Google Scholar]
- Rothenberg, G. Catalysis: Concepts and Green Applications; John Wiley and Sons: Hoboken, NJ, USA, 2008; pp. 1–279. [Google Scholar] [CrossRef]
- Benvenuto, M.A. Industrial Biotechnology, 2nd ed.; De Gruyter, Incorporated: Berlin, Germany, 2024; pp. 1–151. [Google Scholar]
- De Gonzalo, G.; Lavandera, I. Biocatalysis for Practitioners: Techniques, Reactions and Applications; Wiley: Hoboken, NJ, USA, 2021; pp. 1–509. [Google Scholar] [CrossRef]
- Twigg, M.V. Catalyst Handbook, 2nd ed.; Manson Publishing Ltd: Frome, UK, 2016; pp. 1–608. [Google Scholar]
- Chorkendorff, I.; Niemantsverdriet, J.W. Concepts of Modern Catalysis and Kinetics: Third, Completely Revised and Enlarged Edition; Wiley-VCH Verlag: Weinheim, Germany, 2017; pp. 1–526. [Google Scholar]
- Germain, J.E. Catalyse Hétérogène; Dunod: Paris, France, 1959; pp. 1–230. [Google Scholar]
- Stratton, S.M.; Zhang, S.; Montemore, M.M. Addressing complexity in catalyst design: From volcanos and scaling to more sophisticated design strategies. Surf. Sci. Rep. 2023, 78, 100597. [Google Scholar] [CrossRef]
- Berkman, S.; Morell, J.C.; Egloff, G. Catalysis, Inorganic and Organic; Reinhold Publishing Corporation: New York, NY, USA, 1940; pp. 1–1130. [Google Scholar]
- Smallman, R.E.; Ngan, A.H.W. Surfaces, Grain Boundaries and Interfaces. In Modern Physical Metallurgy, 8th ed.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 415–442. [Google Scholar] [CrossRef]
- Xue, S.K.; Huang, W.Q.; Lin, W.; Xing, W.D.; Shen, M.; Ye, X.Y.; Liang, X.C.; Yang, C.; Hou, Y.D.; Yu, Z.Y.; et al. Interfacial engineering of lattice coherency at ZnO-ZnS photocatalytic heterojunctions. Chem. Catal. 2022, 2, 125–139. [Google Scholar] [CrossRef]
- García-Urdiales, E.; Lavandera, I.; Gotor, V. Concepts in Biocatalysis. In Enzyme Catalysis in Organic Synthesis, 3rd ed.; Wiley-VCH: Weinheim, Germany, 2012; Volume 1, pp. 43–66. [Google Scholar]
- Hanefeld, U.E.; Lefferts, L.E. Catalysis: An Integrated Textbook for Students; Wiley-VCH: Weinheim, Germany; SPi Global: Chennai, India, 2018. [Google Scholar]
- Dhagat, S.; Jujjavarapu, S.E.; Sampath Kumar, N.S.; Mahapatra, C. Recent Advances in Bioprocess Engineering and Bioreactor Design; Springer Nature: Singapore, 2024; pp. 1–298. [Google Scholar] [CrossRef]
- Cardona Alzate, C.A.; Solarte Toro, J.C.; Peña, Á.G. Fermentation, thermochemical and catalytic processes in the transformation of biomass through efficient biorefineries. Catal. Today 2018, 302, 61–72. [Google Scholar] [CrossRef]
- Ciriminna, R.; Meneguzzo, F.; Delisi, R.; Pagliaro, M. Citric acid: Emerging applications of key biotechnology industrial product. Chem. Cent. J. 2017, 11, 2222. [Google Scholar] [CrossRef] [PubMed]
- Książek, E. Citric Acid: Properties, Microbial Production, and Applications in Industries. Molecules 2024, 29, 22. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, G.S.; Brar, S.K.; Verma, M.; Tyagi, R.D. Recent Advances in Citric Acid Bio-production and Recovery. Food. Bioprocess Technol. 2011, 4, 505–529. [Google Scholar] [CrossRef]
- Show, P.L.; Oladele, K.O.; Siew, Q.Y.; Aziz Zakry, F.A.; Lan, J.C.W.; Ling, T.C. Overview of citric acid production from Aspergillus niger. Front. Life Sci. 2015, 8, 271–283. [Google Scholar] [CrossRef]
- Reetz, M.T. Biocatalysis in organic chemistry and biotechnology: Past, present, and future. J. Am. Chem. Soc. 2013, 135, 12480–12496. [Google Scholar] [CrossRef]
- Boukid, F.; Ganeshan, S.; Wang, Y.; Tülbek, M.Ç.; Nickerson, M.T. Bioengineered Enzymes and Precision Fermentation in the Food Industry. Int. J. Mol. Sci. 2023, 24, 10156. [Google Scholar] [CrossRef]
- Ingram, A.A.; Oike, K. Artificial Biocatalysis: Quo Vadis? ChemCatChem 2024, 16, e202301759. [Google Scholar] [CrossRef]
- Rudroff, F.; Mihovilovic, M.D.; Gröger, H.; Snajdrova, R.; Iding, H.; Bornscheuer, U.T. Opportunities and challenges for combining chemo- and biocatalysis. Nat. Catal. 2018, 1, 12–22. [Google Scholar] [CrossRef]
- De Kok, N.A.W.; Schmidt, S. Tapping into abiological reaction chemistries in biocatalysis. Chem. Catal. 2023, 3, 100493. [Google Scholar] [CrossRef]
- Jain, S.; Ospina, F.; Hammer, S.C. A New Age of Biocatalysis Enabled by Generic Activation Modes. JACS Au 2024, 4, 2068–2080. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Mattana, A.; Alam, R.; Montgomery, S.L.; Pandya, A.; Manetti, F.; Dominguez, B.; Castagnolo, D. Cooperative chemoenzymatic and biocatalytic cascades to access chiral sulfur compounds bearing C(sp3)–S stereocentres. Nat. Commun. 2024, 15, 8332. [Google Scholar] [CrossRef]
- France, S.P.; Lewis, R.D.; Martinez, C.A. The Evolving Nature of Biocatalysis in Pharmaceutical Research and Development. JACS Au 2023, 3, 715–735. [Google Scholar] [CrossRef]
- Zhang, N.; Domínguez de María, P.; Kara, S. Biocatalysis for the Synthesis of Active Pharmaceutical Ingredients in Deep Eutectic Solvents: State-of-the-Art and Prospects. Catalysts 2024, 14, 84. [Google Scholar] [CrossRef]
- Santi, M.; Sancineto, L.; Nascimento, V.; Azeredo, J.B.; Orozco, E.V.M.; Andrade, L.H.; Gröger, H.; Santi, C. Flow biocatalysis: A challenging alternative for the synthesis of APIs and natural compounds. Int. J. Mol. Sci. 2021, 22, 990. [Google Scholar] [CrossRef]
- Dalko, P.I.; Moisan, L. Enantioselective organocatalysis. Angew. Chem. Int. Ed. 2001, 40, 3726–3748. [Google Scholar] [CrossRef]
- Cmelová, P.; Vargová, D.; Sebesta, R. Hybrid Peptide-Thiourea Catalyst for Asymmetric Michael Additions of Aldehydes to Heterocyclic Nitroalkenes. J. Org. Chem. 2021, 86, 581–592. [Google Scholar] [CrossRef]
- Yang, X.F.; Wang, A.; Qiao, B.; Li, J.; Liu, J.; Zhang, T. Single-atom catalysts: A new frontier in heterogeneous catalysis. Acc. Chem. Res. 2013, 46, 1740–1748. [Google Scholar] [CrossRef]
- Yang, J.; Huang, Y.; Qi, H.; Zeng, C.; Jiang, Q.; Cui, Y.; Su, Y.; Du, X.; Pan, X.; Liu, X.; et al. Modulating the strong metal-support interaction of single-atom catalysts via vicinal structure decoration. Nat. Commun. 2022, 13, 4244. [Google Scholar] [CrossRef] [PubMed]
- Bard, A.J.; Faulkner, L.R.; White, H.S. Electrochemical Methods, Fundamentals and Applications, 3rd ed.; John Wiley & Sons, Ltd: Hoboken, NJ, USA, 2022; pp. 1–1104. [Google Scholar]
- Palma, V.; Barba, D.; Cortese, M.; Martino, M.; Renda, S.; Meloni, E. Microwaves and heterogeneous catalysis: A review on selected catalytic processes. Catalysts 2020, 10, 246. [Google Scholar] [CrossRef]
- Toukoniitty, B.; Mikkola, J.P.; Murzin, D.Y.; Salmi, T. Utilization of electromagnetic and acoustic irradiation in enhancing heterogeneous catalytic reactions. Appl. Catal. A Gen. 2005, 279, 1–22. [Google Scholar] [CrossRef]
- Swiegers, G.F. Mechanical Catalysis: Methods of Enzymatic, Homogeneous, and Heterogeneous Catalysis; John Wiley and Sons: Hoboken, NJ, USA, 2008; pp. 1–351. [Google Scholar] [CrossRef]
- Liu, D.; Huang, Y.; Hu, J.; Wang, B.; Lu, Y. Multiscale Catalysis Under Magnetic Fields: Methodologies, Advances, and Trends. ChemCatChem 2022, 14, e202200889. [Google Scholar] [CrossRef]
- Khan, M.M. Principles and mechanisms of photocatalysis. In Photocatalytic Systems by Design: Materials, Mechanisms and Applications; Elsevier: Amsterdam, The Netherlands, 2021; pp. 1–22. [Google Scholar] [CrossRef]
- Araujo, T.P.; Quiroz, J.; Barbosa, E.C.M.; Camargo, P.H.C. Understanding plasmonic catalysis with controlled nanomaterials based on catalytic and plasmonic metals. Curr. Opin. Colloid Interface Sci. 2019, 39, 110–122. [Google Scholar] [CrossRef]
- Bößl, F.; Tudela, I. Piezocatalysis: Can catalysts really dance? Curr. Opin. Green Sustain. Chem. 2021, 32, 100537. [Google Scholar] [CrossRef]
- Meng, N.; Liu, W.; Jiang, R.; Zhang, Y.; Dunn, S.; Wu, J.; Yan, H. Fundamentals, advances and perspectives of piezocatalysis: A marriage of solid-state physics and catalytic chemistry. Prog. Mater. Sci. 2023, 138, 101161. [Google Scholar] [CrossRef]
- He, J.; Dong, C.; Chen, X.; Cai, H.; Chen, X.; Jiang, X.; Zhang, Y.; Peng, A.; Badsha, M.A.H. Review of Piezocatalysis and Piezo-Assisted Photocatalysis in Environmental Engineering. Crystals 2023, 13, 1382. [Google Scholar] [CrossRef]
- Fojt, J.; Erhart, P.; Schäfer, C. Controlling Plasmonic Catalysis via Strong Coupling with Electromagnetic Resonators. Nano Lett. 2024, 24, 11913–11920. [Google Scholar] [CrossRef]
- Vensaus, P.; Liang, Y.; Ansermet, J.P.; Soler-Illia, G.J.A.A.; Lingenfelder, M. Enhancement of electrocatalysis through magnetic field effects on mass transport. Nat. Commun. 2024, 15, 2867. [Google Scholar] [CrossRef]
- Song, X.; Shan, X.; Xue, H.; Li, X.; Liu, R.; Kong, J.; Zuo, Z.; Su, X.; Zhang, Q.; Yin, Y.; et al. Advances in Photothermal Catalysis: Mechanisms, Materials, and Environmental Applications. ACS Appl. Nano Mater. 2024, 7, 26489–26514. [Google Scholar] [CrossRef]
- Jadhav, H.; Gogate, P.; Annapure, U. Intensification of synthesis of triglyceride of Decanoic acid in the presence of amberlyst 15 as catalyst based on the use of ultrasound and microwave irradiations. Chem. Eng. Process. Process Intensif. 2021, 165, 108424. [Google Scholar] [CrossRef]
- Králik, M.; Koóš, P.; Markovič, M.; Lopatka, P. Organic and Metal–Organic Polymer-Based Catalysts—Enfant Terrible Companions or Good Assistants? Molecules 2024, 29, 4623. [Google Scholar] [CrossRef]
- Macho, V.; Králik, M.; Jurecekova, E.; Hudec, J.; Jurecek, L. Dehydration of C4 alkanols conjugated with a positional and skeletal isomerisation of the formed C4 alkenes. Appl. Catal. A Gen. 2001, 214, 251–257. [Google Scholar] [CrossRef]
- Cui, X.; Li, W.; Ryabchuk, P.; Junge, K.; Beller, M. Bridging homogeneous and heterogeneous catalysis by heterogeneous single-metal-site catalysts. Nat. Catal. 2018, 1, 385–397. [Google Scholar] [CrossRef]
- Astruc, D.; Lu, F.; Aranzaes, J.R. Nanoparticles as recyclable catalysts: The frontier between homogeneous and heterogeneous catalysis. Angew. Chem. Int. Ed. 2005, 44, 7852–7872. [Google Scholar] [CrossRef]
- Cozzi, F. Immobilization of organic catalysts: When, why, and how. Adv. Synth. Catal. 2006, 348, 1367–1390. [Google Scholar] [CrossRef]
- Alács, B.; Zrinyi, A.; Hornyánszky, G.; Poppe, L.; Bell, E. Upgrading Epoxy Supports for Enzyme Immobilization by Affinity Function Doping—A Case Study with Phenylalanine Ammonia-Lyase from Petroselinum crispum. Catalysts 2024, 14, 14. [Google Scholar] [CrossRef]
- Moulijn, J.; Makkee, M.; Van Diepen, A.E. Chemical Process Technology, 2nd ed.; Wiley: New Delhi, India, 2013; pp. 1–566. [Google Scholar]
- Satterfield, C.N. Heterogeneous Catalysis in Industrial Practice; McGraw-Hill: New York, NY, USA, 1996; pp. 1–564. [Google Scholar]
- Deutschmann, O.; Knozinger, H.; Kochloefl, K.; Turek, T. Heterogeneous Catalysis and Solid Catalysts. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag: Weinheim, Germany, 2009; pp. 1–110. [Google Scholar] [CrossRef]
- Cole-Hamilton, D.J. Homogeneous catalysis—New approaches to catalyst separation, recovery, and recycling. Science 2003, 299, 1702–1706. [Google Scholar] [CrossRef]
- Fassbach, T.A.; Ji, J.M.; Vorholt, A.J.; Leitner, W. Recycling of Homogeneous Catalysts—Basic Principles, Industrial Practice, and Guidelines for Experiments and Evaluation. ACS Catal. 2024, 14, 7289–7298. [Google Scholar] [CrossRef]
- Salmi, T.; Murzin, D.Y.; Wärn, J.; Kangas, M.; Toukoniitty, E.; Nieminen, V. An integrated approach to modelling of chemical transformations in chemical reactors. Comput. Aided Chem. Eng. 2005, 20, 1531–1536. [Google Scholar] [CrossRef]
- Murzin, D.Y.; Salmi, T. Catalytic Kinetics: Chemistry and Engineering, 2nd ed.; Elsevier Inc.: Amsterdam, Netherlands, 2016; pp. 1–740. [Google Scholar] [CrossRef]
- Murzin, D.Y. Chemical Reaction Technology, 2nd ed.; De Gruyter: Berlin, Germany, 2022; pp. 1–625. [Google Scholar] [CrossRef]
- Anastas, P.; Eghbali, N. Green Chemistry: Principles and Practice. Chem. Soc. Rev. 2010, 39, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Amoneit, M.; Weckowska, D.; Spahr, S.; Wagner, O.; Adeli, M.; Mai, I.; Haag, R. Green chemistry and responsible research and innovation: Moving beyond the 12 principles. J. Clean. Prod. 2024, 484, 144011. [Google Scholar] [CrossRef]
- Kaur, A.; Goyal, A.; Kumari, S.; Garg, M.; Sharma, S.; Akram, M.; Laila, U.; Khalil, M.T.; Kaur, P.; Sahu, S.K. Green chemistry and catalysis: An emerging sustainable approach in synthetic chemistry. BIO Web Conf. 2024, 86, 01027. [Google Scholar] [CrossRef]
- Kurul, F.; Doruk, B.; Topkaya, S.N. Principles of green chemistry: Building a sustainable future. Discov. Chem. 2025, 2, 68. [Google Scholar] [CrossRef]
- Bond, J.Q.; Stangland, E.E.; Cybulskis, V.J. Best practices in the characterization of bulk catalyst properties. J. Catal. 2024, 433, 115487. [Google Scholar] [CrossRef]
- Che, M.; Védrine, J.C. Characterization of Solid Materials and Heterogeneous Catalysts: From Structure to Surface Reactivity; Wiley-VCH: Weinheim, Germany, 2012; Volume 1–2, pp. 1–1224. [Google Scholar]
- Gašparovičová, D.; Králik, M.; Horváth, B.; Soták, T.; Hudec, P. Liquid phase oxidation of cyclopentanone over metal-free carbon catalysts. Chem. Pap. 2024, 78, 5943–5960. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Králik, M. Adsorption, chemisorption, and catalysis. Chem. Pap. 2014, 68, 1625–1638. [Google Scholar] [CrossRef]
- Biffis, A.; Corain, B.; Cvengrošová, Z.; Hronec, M.; Jeřábek, K.; Králik, M. Relationships between physico-chemical properties and catalytic activity of polymer-supported palladium catalysts, Part I. Experimental investigations. Appl. Catal. A Gen. 1995, 124, 355–365. [Google Scholar] [CrossRef]
- Králik, M.; Corain, B.; Zecca, M. Catalysis by metal nanoparticles supported on functionalized polymers. Chem. Pap. 2000, 54, 254–264. [Google Scholar]
- Galet, L.; Patry, S.; Dodds, J. Determination of the wettability of powders by the Washburn capillary rise method with bed preparation by a centrifugal packing technique. J. Colloid Interface Sci. 2010, 346, 470–475. [Google Scholar] [CrossRef]
- Saidi, M.; Bihl, F.; Gimello, O.; Louis, B.; Roger, A.C.; Trens, P.; Salles, F. Evaluation of the Hydrophilic/Hydrophobic Balance of 13X Zeolite by Adsorption of Water, Methanol, and Cyclohexane as Pure Vapors or as Mixtures. Nanomaterials 2024, 14, 213. [Google Scholar] [CrossRef]
- Lopes, M.A.; Monteiro, F.J.; Santos, J.D.; Serro, A.P.; Saramago, B. Hydrophobicity, surface tension, and zeta potential measurements of glass-reinforced hydroxyapatite composites. J. Biomed. Mater. Res. 1999, 45, 370–375. [Google Scholar] [CrossRef]
- Jablonský, M.; Botková, M.; Šutý, S.; Šmatko, L.; Šima, J. Accelerated ageing of newsprint paper: Changes in swelling ability, WRV and electrokinetic properties of fibres. Fibres Text. East. Eur. 2014, 104, 108–113. [Google Scholar]
- Beeckman, J.W.L. Catalyst Engineering Technology. Fundamentals and Applications; Wiley: Pondicherry, India, 2020; pp. 1–301. [Google Scholar]
- Ertl, G.; Knözinger, H.; Schüth, F.J.W. (Eds.) Handbook of Heterogeneous Catalysis, 2nd ed.; Wiley-VCH: Weinheim, Germany, 2008; p. 3865. [Google Scholar]
- Luna-Valenzuela, A.; Cabellos, J.L.; Alonso, J.A.; Posada-Amarillas, A. Effects of van der Waals interactions on the structure and stability of Cu8-xPdx (x = 0, 4, 8) cluster isomers. Mater. Today Commun. 2021, 26, 102024. [Google Scholar] [CrossRef]
- Parks, G.A. The Isoelectric Points of Solid Oxides, Solid Hydroxides, and Aqueous Hydroxo Complex Systems. Chem. Rev. 1965, 65, 177–198. [Google Scholar] [CrossRef]
- Dybkaer, R. Unit “katal” for catalytic activity (IUPAC Technical Report). Pure Appl. Chem. 2001, 73, 927–931. [Google Scholar] [CrossRef]
- Haber, J. Manual on catalyst characterization. Pure Appl. Chem. 1991, 63, 1227–1246. [Google Scholar] [CrossRef]
- Chang, M.; Liu, X.; Nelson, P.J.; Munzing, G.R.; Gegan, T.A.; Kissin, Y.V. Ziegler-Natta catalysts for propylene polymerization: Morphology and crystal structure of a fourth-generation catalyst. J. Catal. 2006, 239, 347–353. [Google Scholar] [CrossRef]
- Ountaksinkul, K.; Sripinun, S.; Bumphenkiattikul, P.; Vongachariya, A.; Praserthdam, P.; Assabumrungrat, S. A Review of Packed Bed Reactor and Gradient-less Recycle Reactor for Determination of Intrinsic Reaction Kinetics. Eng. J. 2022, 26, 17–42. [Google Scholar] [CrossRef]
- Carberry, J.J. Chemical and Catalytic Reaction Engineering; Courier Corporation, General Publishing Company: Chelmsford, MA, USA, 2001; pp. 1–642. [Google Scholar]
- Laboratory Systems. Available online: https://www.hte-company.com/en/laboratory-systems/ (accessed on 15 February 2025).
- Čejka, J.; Morris, R.E.; Nachtigall, P. (Eds.) Zeolites in Catalysis: Properties and Applications; RSC: London, UK, 2017; pp. 1–546. [Google Scholar] [CrossRef]
- Hou, Q.; Zheng, B.; Bi, C.; Luan, J.; Zhao, Z.; Guo, H.; Wang, G.; Li, Z. Liquid-phase cascade acylation/dehydration over various zeolite catalysts to synthesize 2-methylanthraquinone through an efficient one-pot strategy. J. Catal. 2009, 268, 376–383. [Google Scholar] [CrossRef]
- Murzin, D.Y. Rate Equations of Structure-Sensitive Catalytic Reactions with Arbitrary Kinetics. Chem. Eng. 2023, 7, 12. [Google Scholar] [CrossRef]
- Lu, L.; Zou, S.; Fang, B. The Critical Impacts of Ligands on Heterogeneous Nanocatalysis: A Review. ACS Catal. 2021, 11, 6020–6058. [Google Scholar] [CrossRef]
- Hao, L.; Zhao, Y.; Yu, B.; Zhang, H.; Xu, H.; Xu, J.; Liu, Z. Polyurea-supported metal nanocatalysts: Synthesis, characterization and application in selective hydrogenation of o-chloronitrobenzene. J. Colloid Interface Sci. 2014, 424, 44–48. [Google Scholar] [CrossRef]
- Mahdavi, H.; Sahraei, R. Synthesis and Application of Hyperbranched Polyester-Grafted Polyethylene (HBPE-g-PE) Containing Palladium Nanoparticles as Efficient Nanocatalyst. Catal. Lett. 2016, 146, 977–990. [Google Scholar] [CrossRef]
- Murzin, D.Y. Kinetics of enantioselective heterogeneous nanocatalysis with limited cluster occupancy: Beyond the coverage concept. Chem. Eng. J. 2024, 497, 154570. [Google Scholar] [CrossRef]
- Stiles, A.B.; Koch, T.A. Catalyst Manufacture, 2nd ed.; CRC Press: Boca Raton, FL, USA, 1995; pp. 1–312. [Google Scholar]
- Smith, A.J.; Trimm, D.L. The preparation of skeletal catalysts. Annu. Rev. Mater. Res. 2005, 35, 127–142. [Google Scholar] [CrossRef]
- Bendová, H.; Kamenická, B.; Weidlich, T.; Beneš, L.; Vlček, M.; Lacina, P.; Švec, P. Application of Raney Al-Ni Alloy for Simple Hydrodehalogenation of Diclofenac and Other Halogenated Biocidal Contaminants in Alkaline Aqueous Solution under Ambient Conditions. Materials 2022, 15, 3939. [Google Scholar] [CrossRef]
- Salanov, A.N.; Serkova, A.N.; Chesnokova, N.M.; Isupova, L.A.; Parmon, V.N. Characterization of platinum alloy used in ammonia oxidation. Morphology and microstructure of Pt–Pd–Rh–Ru gauzes after the oxidation of NH3 with air at 1133 K. Mater. Chem. Phys. 2021, 273, 125138. [Google Scholar] [CrossRef]
- Wittstock, A.; Bäumer, M. Catalysis by unsupported skeletal gold catalysts. Acc. Chem. Res. 2014, 47, 731–739. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, L. Handbook of Industrial Catalysts; Springer: New York, NY, USA, 2011; pp. 1–490. [Google Scholar] [CrossRef]
- Shigapov, A.N.; Graham, G.W.; McCabe, R.W.; Peck, M.P.; Plummer, H.K., Jr. The preparation of high-surface-area cordierite monolith by acid treatment. Appl. Catal. A Gen. 1999, 182, 137–146. [Google Scholar] [CrossRef]
- Zhu, Y.; Lv, G.; Song, C.; Li, B.; Zhu, Y.; Liu, Y.; Zhang, W.; Wang, Y. Optimization of the washcoat slurry for hydrotalcite-based LNT catalyst. Catalysts 2019, 9, 696. [Google Scholar] [CrossRef]
- Lam, E.; Luong, J.H.T. Carbon materials as catalyst supports and catalysts in the transformation of biomass to fuels and chemicals. ACS Catal. 2014, 4, 3393–3410. [Google Scholar] [CrossRef]
- Ma, A.Z.; Muhler, M.; Grünert, W. Selective catalytic reduction of NO by ammonia over Raney-Ni supported Cu-ZSM-5. II. Interactions between support and supported Cu-ZSM-5. Appl. Catal. B Environ. 2000, 27, 37–47. [Google Scholar] [CrossRef]
- Niculescu, A.G.; Tudorache, D.I.; Bocioagă, M.; Mihaiescu, D.E.; Hadibarata, T.; Grumezescu, A.M. An Updated Overview of Silica Aerogel-Based Nanomaterials. Nanomaterials 2024, 14, 469. [Google Scholar] [CrossRef]
- Dunn, B.C.; Cole, P.; Covington, D.; Webster, M.C.; Pugmire, R.J.; Ernst, R.D.; Eyring, E.M.; Shah, N.; Huffman, G.P. Silica aerogel supported catalysts for Fischer-Tropsch synthesis. Appl. Catal. A Gen. 2005, 278, 233–238. [Google Scholar] [CrossRef]
- Bang, J.H.; Jang, Y.N.; Kim, W.; Song, K.S.; Jeon, C.W.; Chae, S.C.; Lee, S.W.; Park, S.J.; Lee, M.G. Specific surface area and particle size of calcium carbonate precipitated by carbon dioxide microbubbles. Chem. Eng. J. 2012, 198–199, 254–260. [Google Scholar] [CrossRef]
- Wang, X.; Fang, Q.; Wang, J.; Gui, K.; Thomas, H.R. Effect of CaCO3 on catalytic activity of Fe-Ce/Ti catalysts for NH3-SCR reaction. RSC Adv. 2020, 10, 44876–44883. [Google Scholar] [CrossRef]
- Diamond, S.; Kinter, E.B. Surface Areas of Clay Minerals as Derived from Measurements of Glycerol Retention. Clays Clay Miner. 1956, 5, 334–337. [Google Scholar] [CrossRef]
- Trujillano, R.; González-García, I.; Morato, A.; Rives, V. Controlling the synthesis conditions for tuning the properties of hydrotalcite-like materials at the nano scale. Chem. Eng. 2018, 2, 31. [Google Scholar] [CrossRef]
- Fierro, J.L.G. Structure and composition of perovskite surface in relation to adsorption and catalytic properties. Catal. Today 1990, 8, 153–174. [Google Scholar] [CrossRef]
- Shi, L.; Chang, D.; Ji, X.; Lu, W. Using Data Mining to Search for Perovskite Materials with Higher Specific Surface Area. J. Chem. Inf. Model. 2018, 58, 2420–2427. [Google Scholar] [CrossRef]
- Elhage, A.; Wang, B.; Marina, N.; Marin, M.L.; Cruz, M.; Lanterna, A.E.; Scaiano, J.C. Glass wool: A novel support for heterogeneous catalysis. Chem. Sci. 2018, 9, 6844–6852. [Google Scholar] [CrossRef]
- Králik, M.; Biffis, A. Catalysis by metal nanoparticles supported on functional organic polymers. J. Mol. Catal. A Chem. 2001, 177, 113–138. [Google Scholar] [CrossRef]
- Hronec, M.; Cvengrošová, Z.; Králik, M.; Palma, G.; Corain, B. Hydrogenation of benzene to cyclohexene over polymer-supported ruthenium catalysts. J. Mol. Catal. A Chem. 1996, 105, 25–30. [Google Scholar] [CrossRef]
- Cui, H.; Li, P.; Chen, Y.; Ai, W.; Liu, Y.; Zou, J.; Jiao, M.; Shi, X.L. Practical Applications of Fiber-Supported Catalysts in Organic Transformations. Chem. Sel. 2024, 9, e202402382. [Google Scholar] [CrossRef]
- Radwan, N.R.E.; El-Sharkawy, E.A.; Youssef, A.M. Influence of gold and manganese as promoters on surface and catalytic performance of Fe2O3/Al2O3 system. Appl. Catal. A Gen. 2005, 281, 93–106. [Google Scholar] [CrossRef]
- Luo, Z.; Zhao, G.; Pan, H.; Sun, W. Strong Metal–Support Interaction in Heterogeneous Catalysts. Adv. Energy Mater. 2022, 12, 2201395. [Google Scholar] [CrossRef]
- Poelman, H.; Eufinger, K.; Depla, D.; Poelman, D.; De Gryse, R.; Sels, B.F.; Marin, G.B. Magnetron sputter deposition for catalyst synthesis. Appl. Catal. A Gen. 2007, 325, 213–219. [Google Scholar] [CrossRef]
- Rossnagel, S.M. Magnetron sputtering. J. Vac. Sci. Technol. A 2020, 38, 060805. [Google Scholar] [CrossRef]
- Králik, M.; Hronec, M.; Lora, S.; Palma, G.; Zecca, M.; Biffis, A.; Corain, B. Microporous poly-N,N-dimethylacrylamide-p-styrylsulfonate-methylene bis(acrylamide): A promising support for metal catalysis. J. Mol. Catal. A Chem. 1995, 97, 145–155. [Google Scholar] [CrossRef]
- Markovič, M.; Lopatka, P.; Koóš, P.; Soták, T.; Ház, A.; Gracza, T.; Ley, S.V.; Králik, M. Palladium catalysts supported on biodegradable urea-based polymers in synthesis with CO—Part B. Catal. Today 2024, 440, 114831. [Google Scholar] [CrossRef]
- Cornils, B.; Herrmann, W.A.; Zanthoff, H.-W.; Wong, C.-H. Catalysis from A to Z: A Concise Encyclopedia; Wiley: Singapore, 2013. [Google Scholar] [CrossRef]
- Hutchings, G.J. Promotion in heterogeneous catalysis: A topic requiring a new approach? Catal. Lett. 2001, 75, 1–12. [Google Scholar] [CrossRef]
- Brosda, S.; Vayenas, C.G.; Wei, J. Rules of chemical promotion. Appl. Catal. B Environ. 2006, 68, 109–124. [Google Scholar] [CrossRef]
- Constable, F.H. On the present position of the Theory of Centres of Activity in Heterogeneous Catalysis. Math. Proc. Camb. Philos. Soc. 1928, 24, 291–306. [Google Scholar] [CrossRef]
- Cao, A.; Bukas, V.J.; Shadravan, V.; Wang, Z.; Li, H.; Kibsgaard, J.; Chorkendorff, I.; Nørskov, J.K. A spin promotion effect in catalytic ammonia synthesis. Nat. Commun. 2022, 13, 2382. [Google Scholar] [CrossRef] [PubMed]
- Rocha, T.C.R.; Hävecker, M.; Knop-Gericke, A.; Schlögl, R. Promoters in heterogeneous catalysis: The role of Cl on ethylene epoxidation over Ag. J. Catal. 2014, 312, 12–16. [Google Scholar] [CrossRef]
- Santos, V.P.; Plauck, A.; Gold, J.; Majumdar, P.; McAdon, M.H.; Calverley, T. The Complex Chlorination Effects on High Selectivity Industrial EO Catalysts: Dynamic Interplay between Catalyst Composition and Process Conditions. ACS Catal. 2024, 14, 10839–10852. [Google Scholar] [CrossRef]
- McCue, A.J.; Anderson, J.A. Sulfur as a catalyst promoter or selectivity modifier in heterogeneous catalysis. Catal. Sci. Technol. 2014, 4, 272–294. [Google Scholar] [CrossRef]
- Yamada, H.; Saito, K.; Goto, S.; Tagawa, T. Effect of Teflon powder deposition on the catalyst in a four-phase trickle-bed reactor. Ind. Eng. Chem. Res. 2005, 44, 6403–6405. [Google Scholar] [CrossRef]
- Bartlewicz, O.; Dąbek, I.; Szymańska, A.; Maciejewski, H. Heterogeneous catalysis with the participation of ionic liquids. Catalysts 2020, 10, 1227. [Google Scholar] [CrossRef]
- Sánchez-Carnerero, E.M.; Sandoval-Torrientes, R.; Urieta-Mora, J.; Moreno, F.; Maroto, B.L.; de la Moya, S. Speeding up heterogeneous catalysis with an improved highly reusable catalyst for the preparation of enantioenriched secondary alcohols. React. Funct. Polym. 2017, 113, 23–30. [Google Scholar] [CrossRef]
- Zha, K.; Zeng, T.; Zhu, M.; Wei, C.; Song, L.; Miao, C. Insights into promotional effects for ethylbenzene dehydrogenation to styrene with steam over Fe-K, Fe-K-Ce and Fe-K-Ce-Mo mixed oxide catalysts. Appl. Catal. A Gen. 2023, 666, 119372. [Google Scholar] [CrossRef]
- Morbidelli, M.; Gavriilidis, A.; Varma, A. Catalyst Design: Optimal Distribution of Catalyst in Pellets, Reactors, and Membranes; Cambridge University Press: Cambridge, UK, 2001; pp. 1–240. [Google Scholar]
- Basile, A.E.; Di Paola, L.E.; Hai, F.I.E.; Piemonte, V.E. Membrane Reactors for Energy Applications and Basic Chemical Production; Elsevier Inc.: Amsterdam, The Netherlands, 2015; pp. 1–673. [Google Scholar] [CrossRef]
- Tan, L.; Tan, B. Hypercrosslinked porous polymer materials: Design, synthesis, and applications. Chem. Soc. Rev. 2017, 46, 3322–3356. [Google Scholar] [CrossRef]
- Manaenkov, O.; Nikoshvili, L.; Bykov, A.; Kislitsa, O.; Grigoriev, M.; Sulman, M.; Matveeva, V.; Kiwi-Minsker, L. An Overview of Heterogeneous Catalysts Based on Hypercrosslinked Polystyrene for the Synthesis and Transformation of Platform Chemicals Derived from Biomass. Molecules 2023, 28, 8126. [Google Scholar] [CrossRef]
- Sun, M.; Chen, C.; Chen, L.; Su, B. Hierarchically porous materials: Synthesis strategies and emerging applications. Front. Chem. Sci. Eng. 2016, 10, 301–347. [Google Scholar] [CrossRef]
- Venezia, V.; Pota, G.; Silvestri, B.; Costantini, A.; Vitiello, G.; Luciani, G. Tailoring Structure: Current Design Strategies and Emerging Trends to Hierarchical Catalysts. Catalysts 2022, 12, 1152. [Google Scholar] [CrossRef]
- Yang, M.; Mao, Y.; Wang, B.; Lin, L.; Wang, Y.; Zhang, L.; Jiang, Y.; Zhao, M.; Chen, H.; Zhang, Y. Heterometallic Mg@Fe-MIL-101/TpPa-1-COF grown on stainless steel mesh: Enhancing photo-degradation, fluorescent detection and toxicity assessment for tetracycline hydrochloride. Colloids Surf. A Physicochem. Eng. Asp. 2021, 631, 127725. [Google Scholar] [CrossRef]
- Yang, L.; Li, J.; Liu, B. Recent Advances of Monolithic Metal Mesh-Based Catalysts for CO Oxidation. ChemCatChem 2024, 16, e202401122. [Google Scholar] [CrossRef]
- Gheorghiu, C.C.; García-Bordejé, E.; Job, N.; Román-Martínez, M.C. Structured carbons as supports for hydrogenation hybrid catalysts prepared by the immobilization of a Rh diamine complex. Chem. Eng. J. 2016, 291, 47–54. [Google Scholar] [CrossRef]
- Jarrah, N.A.; Li, F.; Van Ommen, J.G.; Lefferts, L. Immobilization of a layer of carbon nanofibres (CNFs) on Ni foam: A new structured catalyst support. J. Mater. Chem. 2005, 15, 1946–1953. [Google Scholar] [CrossRef]
- Lopatin, S.; Mikenin, P.; Elyshev, A.; Udovichenko, S.; Zagoruiko, A. Catalytic device on the base of glass-fiber catalyst for environmentally safe combustion of fuels and utilization of toxic wastes. Chem. Eng. J. 2019, 373, 406–412. [Google Scholar] [CrossRef]
- Colacot, T.J. Palladium based FibreCat and SMOPEX® as supported homogenous catalyst systems for simple to challenging carbon-carbon coupling reactions. Top. Catal. 2008, 48, 91–98. [Google Scholar] [CrossRef]
- Jiang, N.; Liu, H.; Zhao, G.; Li, H.; Yang, S.; Xu, X.; Zhuang, X.; Cheng, B. Aramid nanofibers supported metal-organic framework aerogel for protection of chemical warfare agent. J. Colloid Interface Sci. 2023, 640, 192–198. [Google Scholar] [CrossRef]
- Baharudin, L.; Watson, M.J. Monolithic substrate support catalyst design considerations for steam methane reforming operation. Rev. Chem. Eng. 2018, 34, 481–501. [Google Scholar] [CrossRef]
- Pérez, N.C.; Miró, E.E.; Zamaro, J.M. Cu, Ce/mordenite coatings on FeCrAl-alloy corrugated foils employed as catalytic microreactors for CO oxidation. Catal. Today 2013, 213, 183–191. [Google Scholar] [CrossRef]
- Barbero, B.P.; Costa-Almeida, L.; Sanz, O.; Morales, M.R.; Cadus, L.E.; Montes, M. Washcoating of metallic monoliths with a MnCu catalyst for catalytic combustion of volatile organic compounds. Chem. Eng. J. 2008, 139, 430–435. [Google Scholar] [CrossRef]
- Koga, H.; Kitaoka, T.; Isogai, A. Chemically-modified cellulose paper as a microstructured catalytic reactor. Molecules 2015, 20, 1495–1508. [Google Scholar] [CrossRef]
- Yan, W.Z.; Liu, J.; Zheng, X.J.; Zhang, J.; Tang, K.Y. Wood-derived high-performance cellulose structural materials. E-Polymers 2023, 23, 20230010. [Google Scholar] [CrossRef]
- Krupšová, S.; Almáši, M. Cellulose–Amine Porous Materials: The Effect of Activation Method on Structure, Textural Properties, CO2 Capture, and Recyclability. Molecules 2024, 29, 1158. [Google Scholar] [CrossRef]
- Soliman, A.; Alamoodi, N.; Karanikolos, G.N.; Doumanidis, C.C.; Polychronopoulou, K. A review on new 3-d printed materials’ geometries for catalysis and adsorption: Paradigms from reforming reactions and CO2 capture. Nanomaterials 2020, 10, 2198. [Google Scholar] [CrossRef]
- Ma, T.; Zhang, Y.; Ruan, K.; Guo, H.; He, M.; Shi, X.; Guo, Y.; Kong, J.; Gu, J. Advances in 3D printing for polymer composites: A review. InfoMat 2024, 6, e12568. [Google Scholar] [CrossRef]
- Bagheri, A.; Jin, J. Photopolymerization in 3D Printing. ACS Appl. Polymer Mat. 2019, 1, 593–611. [Google Scholar] [CrossRef]
- He, T.; Yip, W.S.; Yan, E.H.; Tang, J.; Rehan, M.; Teng, L.; Wong, C.H.; Sun, L.; Zhang, B.; Guo, F.; et al. 3D printing for ultra-precision machining: Current status, opportunities, and future perspectives. Front. Mech. Eng. 2024, 19, 23. [Google Scholar] [CrossRef]
- Oshimura, H.; Tanaka, S.; Nagata, S.; Matsuura, S.; Hashimoto, T.; Ishihara, A. Preparation of Novel Hierarchical Catalysts by Simultaneous Generation of β-Zeolite and Mesoporous Silica for Catalytic Cracking. ChemPlusChem 2024, 89, e202400447. [Google Scholar] [CrossRef]
- Hartmann, M.; Thommes, M.; Schwieger, W. Hierarchically-Ordered Zeolites: A Critical Assessment. Adv. Mater. Interfaces 2021, 8, 2001841. [Google Scholar] [CrossRef]
- Chen, L.H.; Sun, M.H.; Wang, Z.; Yang, W.; Xie, Z.; Su, B.L. Hierarchically structured zeolites: From design to application. Chem. Rev. 2020, 120, 11194–11294. [Google Scholar] [CrossRef]
- Rutkowska, M.; Chmielarz, L. Application of Mesoporous/Hierarchical Zeolites as Catalysts for the Conversion of Nitrogen Pollutants: A Review. Catalysts 2024, 14, 290. [Google Scholar] [CrossRef]
- Xiang, H.; Zainal, S.; Jones, H.; Ou, X.; D’Agostino, C.; Esteban, J.; Parlett, C.M.A.; Fan, X. Hierarchical zeolite catalysed fructose dehydration to 5-hydroxymethylfurfural within a biphasic solvent system under microwave irradiation. RSC Sustain. 2023, 1, 1530–1539. [Google Scholar] [CrossRef]
- Yuan, Z.; Liu, L.; Ru, W.; Zhou, D.; Kuang, Y.; Feng, J.; Liu, B.; Sun, X. 3D printed hierarchical spinel monolithic catalysts for highly efficient semi-hydrogenation of acetylene. Nano Res. 2022, 15, 6010–6018. [Google Scholar] [CrossRef]
- Tian, Z.; Deng, X.; He, P.; Wang, G.H. Atomic Pt anchored on hierarchically porous monolithic carbon nanowires as high-performance catalyst for liquid hydrogenation. Nano Res. 2023, 16, 5880–5886. [Google Scholar] [CrossRef]
- Huo, C.; Tian, X.; Nan, Y.; Qiu, Z.; Zhong, Q.; Huang, X.; Li, D. 3D printing of hierarchically porous monolithic TS-1 catalyst for one-pot synthesis of ethylene glycol. Chem. Eng. J. 2022, 450, 138259. [Google Scholar] [CrossRef]
- Tan, L.; Tan, B. Functionalized hierarchical porous polymeric monoliths as versatile platforms to support uniform and ultrafine metal nanoparticles for heterogeneous catalysis. Chem. Eng. J. 2020, 390, 124485. [Google Scholar] [CrossRef]
- Li, M.; Yang, Y.; Yu, D.; Li, W.; Ning, X.; Wan, R.; Zhu, H.; Mao, J. Recent advances on the construction of encapsulated catalyst for catalytic applications. Nano Res. 2023, 16, 3451–3474. [Google Scholar] [CrossRef]
- EnCat Catalysts. Available online: http://reaxa.com/ (accessed on 13 July 2024).
- Ley, S.V.; Ramarao, C.; Gordon, R.S.; Holmes, A.B.; Morrison, A.J.; McConvey, I.F.; Shirley, I.M.; Smith, S.C.; Smith, M.D. Polyurea-encapsulated palladium(II) acetate: A robust and recyclable catalyst for use in conventional and supercritical media. Chem. Commun. 2002, 2, 1134–1135. [Google Scholar] [CrossRef]
- Gao, C.; Lyu, F.; Yin, Y. Encapsulated Metal Nanoparticles for Catalysis. Chem. Rev. 2021, 121, 834–881. [Google Scholar] [CrossRef]
- Sebati, W.; Ray, S.S. Advances in nanostructured metal-encapsulated porous organic-polymer composites for catalyzed organic chemical synthesis. Catalysts 2018, 8, 492. [Google Scholar] [CrossRef]
- Begum, R.; Farooqi, Z.H.; Xiao, J.; Ahmed, E.; Sharif, A.; Irfan, A. Crosslinked polymer encapsulated palladium nanoparticles for catalytic reduction and Suzuki reactions in aqueous medium. J. Mol. Liq. 2021, 338, 116780. [Google Scholar] [CrossRef]
- Naseem, K.; Arif, M.; Ahmad Haral, A.; Tahir, M.H.; Khurshid, A.; Ahmed, K.; Majeed, H.; Haider, S.; Khan, S.U.D.; Nazar, M.F.; et al. Enzymes encapsulated smart polymer micro assemblies and their tuned multi-functionalities: A critical review. Int. J. Polym. Mater. Polym. Biomater. 2024, 73, 785–816. [Google Scholar] [CrossRef]
- Yan, P.; Li, K. Readily scalable and controllable micro-structured catalyst with encapsulated Pd@Silicate-1 towards sustainable catalytic emission control. Chem. Eng. Sci. 2024, 293, 120037. [Google Scholar] [CrossRef]
- Kruse, N.; Machoke, A.G.; Schwieger, W.; Güttel, R. Nanostructured encapsulated catalysts for combination of Fischer-Tropsch synthesis and hydroprocessing. ChemCatChem 2015, 7, 1018–1022. [Google Scholar] [CrossRef]
- Chatenet, M.; Pollet, B.G.; Dekel, D.R.; Dionigi, F.; Deseure, J.; Millet, P.; Braatz, R.D.; Bazant, M.Z.; Eikerling, M.; Staffell, I.; et al. Water electrolysis: From textbook knowledge to the latest scientific strategies and industrial developments. Chem. Soc. Rev. 2022, 51, 4583–4762. [Google Scholar] [CrossRef]
- Franco, A.; Giovannini, C. Recent and Future Advances in Water Electrolysis for Green Hydrogen Generation: Critical Analysis and Perspectives. Sustainability 2023, 15, 16917. [Google Scholar] [CrossRef]
- Devi, Y.; Huang, P.J.; Chen, W.T.; Jhang, R.H.; Chen, C.H. Roll-to-Roll Production of Electrocatalysts Achieving High-Current Alkaline Water Splitting. ACS Appl. Mater. Interfaces 2023, 15, 9231–9239. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Shao, G.; Zheng, X.; Jia, Y.; Xia, Y.; Dou, Y.; Huang, M.; Gu, C.; Shi, J.; Zheng, J.; et al. Cutting-edge advances in pressurized electrocatalytic reactors. eScience 2025, 5, 100369. [Google Scholar] [CrossRef]
- Trasatti, S. Work function, electronegativity, and electrochemical behaviour of metals. III. Electrolytic hydrogen evolution in acid solutions. J. Electroanal. Chem. 1972, 39, 163–184. [Google Scholar] [CrossRef]
- Kempler, P.A.; Nielander, A.C. Reliable reporting of Faradaic efficiencies for electrocatalysis research. Nat. Commun. 2023, 14, 1158. [Google Scholar] [CrossRef]
- Lamy, C.; Millet, P. A critical review on the definitions used to calculate the energy efficiency coefficients of water electrolysis cells working under near ambient temperature conditions. J. Power Sources 2020, 447, 227350. [Google Scholar] [CrossRef]
- Godula-Jopek, A. Hydrogen Production: By Electrolysis; Wiley: Hoboken, NJ, USA, 2015; pp. 1–402. [Google Scholar] [CrossRef]
- Zhang, H.; Lin, G.; Chen, J. Evaluation and calculation on the efficiency of a water electrolysis system for hydrogen production. Int. J. Hydrogen Energy 2010, 35, 10851–10858. [Google Scholar] [CrossRef]
- Malkow, T.; Pilenga, A. EU Harmonised Testing Procedure: Determination of Water Electrolyser Energy Performance; Publications Office of the European Union: Luxembourg, 2023; pp. 1–73. [Google Scholar] [CrossRef]
- Seo, J.M.; Noh, H.J.; Jeon, J.P.; Kim, H.; Han, G.F.; Kwak, S.K.; Jeong, H.Y.; Wang, L.; Li, F.; Baek, J.B. Conductive and Ultrastable Covalent Organic Framework/Carbon Hybrid as an Ideal Electrocatalytic Platform. J. Am. Chem. Soc. 2022, 144, 19973–19980. [Google Scholar] [CrossRef]
- Gallagher, C.; Kothakonda, M.; Zhao, Q. Graphene-based single-atom catalysts for electrochemical CO2 reduction: Unraveling the roles of metals and dopants in tuning activity. Phys. Chem. Chem. Phys. 2025, 27, 13. [Google Scholar] [CrossRef]
- Zhou, J.; Ming, F.; Liang, H. Application of functional coatings in water electrolyzers and fuel cells. Nanoscale 2025, 17, 8289–8300. [Google Scholar] [CrossRef] [PubMed]
- Linnemann, J.; Kanokkanchana, K.; Tschulik, K. Design Strategies for Electrocatalysts from an Electrochemist’s Perspective. ACS Catal. 2021, 11, 5318–5346. [Google Scholar] [CrossRef]
- Chen, Z.; Ma, T.; Wei, W.; Wong, W.Y.; Zhao, C.; Ni, B.J. Work Function-Guided Electrocatalyst Design. Adv. Mater. 2024, 36, 2401568. [Google Scholar] [CrossRef] [PubMed]
- Morab, S.; Sundaram, M.M.; Pivrikas, A. Review on Charge Carrier Transport in Inorganic and Organic Semiconductors. Coatings 2023, 13, 1657. [Google Scholar] [CrossRef]
- Májek, M. Modern Organic Photochemistry and Electrochemistry; Book, Publishing House of Comenius University in Bratislava: Bratislava, Slovakia, 2025; pp. 1–99. [Google Scholar]
- Chu, X.Z.; Sathish, C.I.; Yang, J.H.; Li, W.; Qi, D.C.; Guan, X.W.; Yu, X.J.; Breese, M.B.H.; Qiao, L.; Yi, J.B. Sodium Chloride-Assisted Crystalline Graphitic Carbon Nitride for Efficient Photocatalytic Hydrogen Evolution. Electron 2025, 3, e70000. [Google Scholar] [CrossRef]
- Parrino, F.; Bellardita, M.; García-López, E.I.; Marcì, G.; Loddo, V.; Palmisano, L. Heterogeneous Photocatalysis for Selective Formation of High-Value-Added Molecules: Some Chemical and Engineering Aspects. ACS Catal. 2018, 8, 11191–11225. [Google Scholar] [CrossRef]
- Low, J.; Yu, J.; Jaroniec, M.; Wageh, S.; Al-Ghamdi, A.A. Heterojunction Photocatalysts. Adv. Mater. 2017, 29, 1601694. [Google Scholar] [CrossRef]
- Xu, Q.; Zhang, L.; Cheng, B.; Fan, J.; Yu, J. S-Scheme Heterojunction Photocatalyst. Chem 2020, 6, 1543–1559. [Google Scholar] [CrossRef]
- Zhao, Y.; Linghu, X.; Shu, Y.; Zhang, J.; Chen, Z.; Wu, Y.; Shan, D.; Wang, B. Classification and catalytic mechanisms of heterojunction photocatalysts and the application of titanium dioxide (TiO2)-based heterojunctions in environmental remediation. J. Environ. Chem. Eng. 2022, 10, 108077. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, J.; Wu, R.; Qiu, P.; Yao, Y.; Liao, X.; Jiang, Y.; Shi, J.; Chen, Y.; Lu, S. An S-scheme α-Fe2O3/g-C3N4 heterojunction nanostructure with superior visible-light photocatalytic activity for the aza-Henry reaction. J. Mater. Chem. C 2022, 10, 17075–17083. [Google Scholar] [CrossRef]
- Marcolongo, D.M.S.; Nocito, F.; Ditaranto, N.; Comparelli, R.; Aresta, M.; Dibenedetto, A. Opto-Electronic Characterization of Photocatalysts Based on p,n-Junction Ternary and Quaternary Mixed Oxides Semiconductors (Cu2O-In2O3 and Cu2O-In2O3-TiO2). Catalysts 2022, 12, 153. [Google Scholar] [CrossRef]
- Schumacher, L.; Marschall, R. Recent Advances in Semiconductor Heterojunctions and Z-Schemes for Photocatalytic Hydrogen Generation. Top. Curr. Chem. 2022, 380, 53. [Google Scholar] [CrossRef]
- Li, C.; Ding, G.; Liu, X.; Huo, P.; Yan, Y.; Yan, Y.; Liao, G. Photocatalysis over NH2-UiO-66/CoFe2O4/CdIn2S4 double p-n junction: Significantly promoting photocatalytic performance by double internal electric fields. Chem. Eng. J. 2022, 435, 134740. [Google Scholar] [CrossRef]
- Sasikumar, K.; Rajamanikandan, R.; Ju, H. Construction of Z-Scheme ZIF67/NiMoO4 Heterojunction for Enhanced Photocatalytic Degradation of Antibiotic Pollutants. Materials 2024, 17, 6225. [Google Scholar] [CrossRef]
- Xie, J.; Fu, C.; Quesne, M.G.; Guo, J.; Wang, C.; Xiong, L.; Windle, C.D.; Gadipelli, S.; Guo, Z.X.; Huang, W.; et al. Methane oxidation to ethanol by a molecular junction photocatalyst. Nature 2025, 639, 368–374. [Google Scholar] [CrossRef]
- Li, J.; Li, Z.; Sun, Q.; Wang, Y.; Li, Y.; Peng, Y.K.; Li, Y.; Zhang, C.; Liu, B.; Zhao, Y. Recent Advances in the Large-Scale Production of Photo/Electrocatalysts for Energy Conversion and beyond. Adv. Energy Mater. 2024, 14, 2402441. [Google Scholar] [CrossRef]
- Behul, M.; Marton, M.; Chvála, A.; Michniak, P.; Pifko, M.; Honig, H.; Scheler, T.; Kurniawan, M.; Vojs, M. Nanoscale Engineering of Si/BDD/TiO2 heterostructure interfaces to enhance photoelectrochemical performance. Appl. Surf. Sci. Adv. 2025, 27, 100757. [Google Scholar] [CrossRef]
- Sun, S.; He, L.; Yang, M.; Cui, J.; Liang, S. Facet Junction Engineering for Photocatalysis: A Comprehensive Review on Elementary Knowledge, Facet-Synergistic Mechanisms, Functional Modifications, and Future Perspectives. Adv. Funct. Mater. 2022, 32, 2106982. [Google Scholar] [CrossRef]
- Bogaerts, A.; Centi, G.; Hessel, V.; Rebrov, E. Challenges in unconventional catalysis. Catal. Today 2023, 420, 114180. [Google Scholar] [CrossRef]
- Martirez, J.M.P.; Bao, J.L.; Carter, E.A. First-Principles Insights into Plasmon-Induced Catalysis. Annu. Rev. Phys. Chem. 2020, 72, 99–119. [Google Scholar] [CrossRef]
- Zhang, Z. Plasmonic Photocatalysis, Principles and Applications; Springer: Singapore, 2022; pp. 1–91. [Google Scholar] [CrossRef]
- Xu, M.; Wang, H.; Yan, C.; Wang, H.; Li, B.; Song, X.; Liu, X.; Zhang, J.; Yang, Y.; Zhou, W.; et al. Enhancing selective photoreduction of CO2 via bimetallic site directed electric fields in synergy with plasmonic near-field effects. Appl. Catal. B Environ. 2025, 366, 124983. [Google Scholar] [CrossRef]
- Cai, J.; Peng, Y.; Jiang, Y.; Li, L.; Wang, H.; Li, K. Application of Fe-MOFs in Photodegradation and Removal of Air and Water Pollutants: A Review. Molecules 2023, 28, 7121. [Google Scholar] [CrossRef] [PubMed]
- An, X.; Kays, J.C.; Lightcap, I.V.; Ouyang, T.; Dennis, A.M.; Reinhard, B.M. Wavelength-Dependent Bifunctional Plasmonic Photocatalysis in Au/Chalcopyrite Hybrid Nanostructures. ACS Nano 2022, 16, 6813–6824. [Google Scholar] [CrossRef] [PubMed]
- De Barros, H.R.; García, I.; Kuttner, C.; Zeballos, N.; Camargo, P.H.C.; De Torresi, S.I.C.; López-Gallego, F.; Liz-Marzán, L.M. Mechanistic Insights into the Light-Driven Catalysis of an Immobilized Lipase on Plasmonic Nanomaterials. ACS Catal. 2021, 11, 414–423. [Google Scholar] [CrossRef]
- Huang, H.J.; Wu, J.C.S.; Chiang, H.P.; Chau, Y.F.C.; Lin, Y.S.; Wang, Y.H.; Chen, P.J. Review of experimental setups for plasmonic photocatalytic reactions. Catalysts 2020, 10, 46. [Google Scholar] [CrossRef]
- Pei, L.; Li, T.; Yuan, Y.; Yang, T.; Zhong, J.; Ji, Z.; Yan, S.; Zou, Z. Schottky junction effect enhanced plasmonic photocatalysis by TaON@Ni NP heterostructures. Chem. Commun. 2019, 55, 11754–11757. [Google Scholar] [CrossRef]
- Rogolino, A.; Claes, N.; Cizaurre, J.; Marauri, A.; Jumbo-Nogales, A.; Lawera, Z.; Kruse, J.; Sanromán-Iglesias, M.; Zarketa, I.; Calvo, U.; et al. Metal-Polymer Heterojunction in Colloidal-Phase Plasmonic Catalysis. J. Phys. Chem. Lett. 2022, 13, 2264–2272. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, T.; Wang, J.; Liu, H.; Dao, T.D.; Li, M.; Liu, G.; Meng, X.; Chang, K.; Shi, L.; et al. Surface-Plasmon-Enhanced Photodriven CO2 Reduction Catalyzed by Metal-Organic-Framework-Derived Iron Nanoparticles Encapsulated by Ultrathin Carbon Layers. Adv. Mater. 2016, 28, 3703–3710. [Google Scholar] [CrossRef]
- Braslavsky, S.E.; Braun, A.M.; Cassano, A.E.; Emeline, A.V.; Litter, M.I.; Palmisano, L.; Parmon, V.N.; Serpone, N. Glossary of terms used in photocatalysis and radiation catalysis (IUPAC recommendations 2011). Pure Appl. Chem. 2011, 83, 931–1014. [Google Scholar] [CrossRef]
- Schneider, J.E.; Bahnemann, D.E.; Ye, J.E.; Puma, G.-L.E.; Dionysiou, D.-D.E. Photocatalysis: Fundamentals and Perspectives; Royal Soc Chemistry: Cambridge, UK, 2016; pp. 1–436. [Google Scholar] [CrossRef]
- De Lasa, H.; Serrano, B.; Salaices, M. Photocatalytic Reaction Engineering; Springer: New York, NY, USA, 2005; pp. 1–187. [Google Scholar] [CrossRef]
- Cao, S.; Piao, L. Considerations for a More Accurate Evaluation Method for Photocatalytic Water Splitting. Angew. Chem. Int. Ed. 2020, 59, 18312–18320. [Google Scholar] [CrossRef]
- Serrano, B.; Ortíz, A.; Moreira, J.; De Lasa, H.I. Photocatalytic thermodynamic efficiency factors. Practical limits in photocatalytic reactors. Ind. Eng. Chem. Res. 2010, 49, 6824–6833. [Google Scholar] [CrossRef]
- Lugo-Vega, C.S.; Serrano-Rosales, B.; de Lasa, H. Energy efficiency limits in Photo-CREC-Air photocatalytic reactors. Chem. Eng. Sci. 2016, 156, 77–88. [Google Scholar] [CrossRef]
- Sayago-Carro, R.; Jiménez-Chavarriga, L.J.; Fernández-García, E.; Kubacka, A.; Fernández-García, M. Efficiency in photocatalytic production of hydrogen: Energetic and sustainability implications. Energy Adv. 2024, 3, 2738–2757. [Google Scholar] [CrossRef]
- Forzatti, P.; Lietti, L. Catalyst deactivation. Catal. Today 1999, 52, 165–181. [Google Scholar] [CrossRef]
- Argyle, M.D.; Bartholomew, C.H. Heterogeneous catalyst deactivation and regeneration: A review. Catalysts 2015, 5, 145–269. [Google Scholar] [CrossRef]
- Walas, S.M. Chemical Process Equipment: Selection and Design; Butterworth-Heinemann: Newton, MA, USA, 1990; p. 755. [Google Scholar]
- Smith, R. Chemical Process Design and Integration, 2nd ed.; Wiley: Noida, India, 2016; p. 928. [Google Scholar]
- Semmel, M.; Steiner, L.; Bontrup, M.; Sauer, J.; Salem, O. Catalyst screening and reaction kinetics of liquid phase DME synthesis under reactive distillation conditions. Chem. Eng. J. 2023, 455, 140525. [Google Scholar] [CrossRef]
- Kiss, A.A.; Muthia, R.; Pazmiño-Mayorga, I.; Harmsen, J.; Jobson, M.; Gao, X. Conceptual methods for synthesis of reactive distillation processes: Recent developments and perspectives. J. Chem. Technol. Biotechnol. 2024, 99, 1263–1290. [Google Scholar] [CrossRef]
- Kudaibergenov, S.E.; Dzhardimalieva, G.I. Flow-through catalytic reactors based on metal nanoparticles immobilized within porous polymeric gels and surfaces/hollows of polymeric membranes. Polymers 2020, 12, 572. [Google Scholar] [CrossRef]
- Jun, B.M.; Al-Hamadani, Y.A.J.; Son, A.; Park, C.M.; Jang, M.; Jang, A.; Kim, N.C.; Yoon, Y. Applications of metal-organic framework based membranes in water purification: A review. Sep. Purif. Technol. 2020, 247, 116947. [Google Scholar] [CrossRef]
- Amin, A. Review of autothermal reactors: Catalysis, reactor design, and processes. Int. J. Hydrogen Energy 2024, 65, 271–291. [Google Scholar] [CrossRef]
- Paul, E.L.; Atiemo-Obeng, V.A.; Kresta, S.M. Handbook of Industrial Mixing: Science and Practice; Wiley: Hoboken, NJ, USA, 2004; pp. 1–1433. [Google Scholar] [CrossRef]
- Hill, J.C. Homogeneous Turbulent Mixing with Chemical-Reaction. Annu. Rev. Fluid Mech. 1976, 8, 135–161. [Google Scholar] [CrossRef]
- Bourne, J.R. Mixing and the selectivity of chemical reactions. Org. Process Res. Dev. 2003, 7, 471–508. [Google Scholar] [CrossRef]
- Danckwerts, P.V. The definition and measurement of some characteristics of mixtures. Appl. Sci. Res. 1952, 3, 279–296. [Google Scholar] [CrossRef]
- Gobert, S.R.L.; Kuhn, S.; Braeken, L.; Thomassen, L.C.J. Characterization of Milli- and Microflow Reactors: Mixing Efficiency and Residence Time Distribution. Org. Process Res. Dev. 2017, 21, 531–542. [Google Scholar] [CrossRef]
- Silverstein, J.; Shinnar, R. Design of Fixed Bed Catalytic Microreactors. Ind. Eng. Chem. Process. Des. Dev. 1975, 14, 127–137. [Google Scholar] [CrossRef]
- COMSOL Multiphysics®. Available online: https://www.comsol.com/ (accessed on 2 March 2025).
- Bartholomew, C.H.; Farrauto, R.J. Fundamentals of Industrial Catalytic Processes, 2nd ed.; John Wiley and Sons: Hoboken, NJ, USA, 2010; pp. 1–966. [Google Scholar] [CrossRef]
- Lachman, I.M.; Williams, J.L. Extruded monolithic catalyst supports. Catal. Today 1992, 14, 317–329. [Google Scholar] [CrossRef]
- Erfani, N.; Symons, D.; Fee, C.; James Watson, M. A novel method to design monolithic catalysts for non-isothermal packed-bed reactors using topology optimisation. Chem. Eng. Sci. 2023, 267, 118347. [Google Scholar] [CrossRef]
- Boger, T.; Heibel, A.K.; Sorensen, C.M. Monolithic catalysts for the chemical industry. Ind. Eng. Chem. Res. 2004, 43, 4602–4611. [Google Scholar] [CrossRef]
- Yates, J.G.; Paola Lettieri, P. Fluidized-Bed Reactors: Processes and Operating Conditions; Springer: Cham, Switzerland, 2016; pp. 1–214. [Google Scholar] [CrossRef]
- Shirzad, M.; Karimi, M.; Silva, J.A.C.; Rodrigues, A.E. Moving Bed Reactors: Challenges and Progress of Experimental and Theoretical Studies in a Century of Research. Ind. Eng. Chem. Res. 2019, 58, 9179–9198. [Google Scholar] [CrossRef]
- Haynes, T.N.; Caram, H.S. The simulated moving bed chemical reactor. Chem. Eng. Sci. 1994, 49, 5465–5472. [Google Scholar] [CrossRef]
- Magyarová, Z.; Králik, M.; Soták, T. Utilization of zeolite catalysts in biomass exploitation: A mini review. Monatsh. Chem. 2023, 154, 815–835. [Google Scholar] [CrossRef]
- Kouser, S.; Hezam, A.; Khadri, M.J.N.; Khanum, S.A. A review on zeolite imidazole frameworks: Synthesis, properties, and applications. J. Porous Mater. 2022, 29, 663–681. [Google Scholar] [CrossRef]
- Sklenár, M.; Danielik, V.; Hudec, P. Alkylation of diphenylamine with alkenes catalyzed by acidic catalysts based on bentonite. Pet. Coal 2016, 58, 597–604. [Google Scholar]
- Sklenár, M.; Danielik, V.; Hudec, P. Discontinuous alkylation of diphenylamine with nonene for maximum catalyst utilization. Chem. Pap. 2017, 71, 1453–1461. [Google Scholar] [CrossRef]
- Hájek, M.; Tomášová, A.; Kocík, J.; Podzemná, V. Statistical evaluation of the mutual relations of properties of Mg/Fe hydrotalcites and mixed oxides as transesterification catalysts. Appl. Clay Sci. 2018, 154, 28–35. [Google Scholar] [CrossRef]
- Mališová, M.; Horňáček, M.; Mikulec, J.; Hudec, P.; Hájek, M.; Peller, A.; Jorík, V.; Frolich, K.; Hadvinová, M.; Hájeková, E. Transesterification of Camelina sativa Oil Catalyzed by Mg/Al Mixed Oxides with Added Divalent Metals. ACS Omega 2020, 5, 32040–32050. [Google Scholar] [CrossRef]
- Mück, J.; Kocík, J.; Frolich, K.; Šimek, J.; Michálková, M.; Hájek, M. Transition Metal-Promoted Mg-Fe Mixed Oxides for Conversion of Ethanol to Valuable Products. ACS Omega 2023, 8, 19374–19384. [Google Scholar] [CrossRef]
- Rahmani, E.; Rahmani, M. Al-Based MIL-53 Metal Organic Framework (MOF) as the New Catalyst for Friedel-Crafts Alkylation of Benzene. Ind. Eng. Chem. Res. 2018, 57, 169–178. [Google Scholar] [CrossRef]
- Xiang, B.L.; Fu, L.; Li, Y.; Liu, Y. Preparation of Fe(II)/MOF-5 Catalyst for Highly Selective Catalytic Hydroxylation of Phenol by Equivalent Loading at Room Temperature. J. Chem. 2019, 2019, 8950630. [Google Scholar] [CrossRef]
- Greifenstein, R.; Ballweg, T.; Hashem, T.; Gottwald, E.; Achauer, D.; Kirschhöfer, F.; Nusser, M.; Brenner-Weiß, G.; Sedghamiz, E.; Wenzel, W.; et al. MOF-Hosted Enzymes for Continuous Flow Catalysis in Aqueous and Organic Solvents. Angew. Chem. Int. Ed. 2022, 61, e202117144. [Google Scholar] [CrossRef]
- Wu, J.; Jiang, X.; Wheatley, A. Characterizing activated sludge process effluent by particle size distribution, respirometry and modelling. Desalination 2009, 249, 969–975. [Google Scholar] [CrossRef]
- Kuśnierz, M. Scale of Small Particle Population in Activated Sludge Flocs. Water Air Soil Pollut. 2018, 229, 327. [Google Scholar] [CrossRef] [PubMed]
- Shabestary, K.; Klamt, S.; Link, H.; Mahadevan, R.; Steuer, R.; Hudson, E.P. Design of microbial catalysts for two-stage processes. Nat. Rev. Bioeng. 2024, 2, 1039–1055. [Google Scholar] [CrossRef]
- Hamawand, I. Energy Consumption in Water/Wastewater Treatment Industry—Optimisation Potentials. Energies 2023, 16, 2433. [Google Scholar] [CrossRef]
- Yeoh, G.H.E.; Joshi, J.B.E. Handbook of Multiphase Flow Science and Technology; Springer Nature: Singapore, 2023; pp. 1–1634. [Google Scholar] [CrossRef]
- Zhang, H.; Song, N.; Yu, T.; Qu, C. Process Intensification in Gas–Liquid Mass Transfer by Modification of Reactor Design: A Review. Energy Technol. 2023, 11, 2201495. [Google Scholar] [CrossRef]
- Gates, B.C. Catalytic Chemistry; Wiley: Hoboken, NJ, USA, 1992; p. 480. [Google Scholar]
- Speight, J.G. Handbook of Petroleum Refining, 1st ed.; CRC Press: Boca Raton, FL, USA, 2016; p. 789. [Google Scholar] [CrossRef]
- Pjontek, D.; McKnight, C.A.; Wiens, J.; Macchi, A. Ebullated bed fluid dynamics relevant to industrial hydroprocessing. Chem. Eng. Sci. 2015, 126, 730–744. [Google Scholar] [CrossRef]
- Martinez, J.; Sanchez, J.L.; Ancheyta, J.; Ruiz, R.S. A review of process aspects and modeling of ebullated bed reactors for hydrocracking of heavy oils. Catal. Rev. Sci. Eng. 2010, 52, 60–105. [Google Scholar] [CrossRef]
- Chaudhari, R.V.; Shah, Y.T.; Foster, N.R. Novel Gas-Liquid-Solid Reactors. Catal. Rev. 1986, 28, 431–518. [Google Scholar] [CrossRef]
- Dautzenberg, F.M.; Mukherjee, M. Process intensification using multifunctional reactors. Chem. Eng. Sci. 2001, 56, 251–267. [Google Scholar] [CrossRef]
- The BUSS-Loop® Reactor Explained. Available online: https://www.buss-ct.com/gas-liquid-reactions/buss-loop-reactor/ (accessed on 2 April 2025).
- Li, W.; Sun, Z.; Ye, X.; He, Y. Ultra-thin PPS mesh-based PSF-ZrO2 composite diaphragm for efficient electrolysis of water for hydrogen production. Int. J. Hydrogen Energy 2024, 81, 918–926. [Google Scholar] [CrossRef]
- Wang, S.; Lu, A.; Zhong, C.J. Hydrogen production from water electrolysis: Role of catalysts. Nano Converg. 2021, 8, 4. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Wang, J.; Sun, L.; Li, Y.; Xia, H.; He, W. Optimal electrode configuration and system design of compactly-assembled industrial alkaline water electrolyzer. Energy Convers. Manag. 2024, 299, 117875. [Google Scholar] [CrossRef]
- Rizwan, M.; Alstad, V.; Jäschke, J. Design considerations for industrial water electrolyzer plants. Int. J. Hydrogen Energy 2021, 46, 37120–37136. [Google Scholar] [CrossRef]
- Şahin, M.E. An Overview of Different Water Electrolyzer Types for Hydrogen Production. Energies 2024, 17, 4944. [Google Scholar] [CrossRef]
- Jang, I.; Carneiro, J.S.A.; Crawford, J.O.; Cho, Y.J.; Parvin, S.; Gonzalez-Casamachin, D.A.; Baltrusaitis, J.; Lively, R.P.; Nikolla, E. Electrocatalysis in Solid Oxide Fuel Cells and Electrolyzers. Chem. Rev. 2024, 124, 8233–8306. [Google Scholar] [CrossRef]
- Van ’t Noordende, H.; Ripson, P. A One-GigaWatt Green-Hydrogen Plant. Advanced Design and Total Installed-Capital Costs; ISPT: Amersfoort, The Netherlands, 2023; pp. 1–45. [Google Scholar]
- Ouimet, R.J.; Glenn, J.R.; De Porcellinis, D.; Motz, A.R.; Carmo, M.; Ayers, K.E. The Role of Electrocatalysts in the Development of Gigawatt-Scale PEM Electrolyzers. ACS Catal. 2022, 12, 6159–6171. [Google Scholar] [CrossRef]
- Duca, M.; Koper, M.T.M. Fundamental Aspects of Electrocatalysis. In Surface and Interface Science, Volume 8: Interfacial Electrochemistry; Wiley: Hoboken, NJ, USA, 2018; Volume 8, pp. 773–890. [Google Scholar]
- Zeng, Y.; Zhao, M.; Zeng, H.; Jiang, Q.; Ming, F.; Xi, K.; Wang, Z.; Liang, H. Recent progress in advanced catalysts for electrocatalytic hydrogenation of organics in aqueous conditions. eScience 2023, 3, 100156. [Google Scholar] [CrossRef]
- Kundu, B.K.; Sun, Y. Electricity-driven organic hydrogenation using water as the hydrogen source. Chem. Sci. 2024, 15, 16424–16435. [Google Scholar] [CrossRef]
- Lee, K.M.; Lee, H.J.; Seo, J.; Lee, T.; Yoon, J.; Kim, C.; Lee, C. Electrochemical Oxidation Processes for the Treatment of Organic Pollutants in Water: Performance Evaluation Using Different Figures of Merit. ACS ES&T Eng. 2022, 2, 1797–1824. [Google Scholar] [CrossRef]
- Mitsudo, K.; Osaki, A.; Inoue, H.; Sato, E.; Shida, N.; Atobe, M.; Suga, S. Electrocatalytic hydrogenation of cyanoarenes, nitroarenes, quinolines, and pyridines under mild conditions with a proton-exchange membrane reactor. Beilstein J. Org. Chem. 2024, 20, 1560–1571. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Li, W.; Lan, J.; Zhu, T. Electroredox carbene organocatalysis with iodide as promoter. Nat. Commun. 2022, 13, 3827. [Google Scholar] [CrossRef] [PubMed]
- Jensen, M.T.; Rønne, M.H.; Ravn, A.K.; Juhl, R.W.; Nielsen, D.U.; Hu, X.M.; Pedersen, S.U.; Daasbjerg, K.; Skrydstrup, T. Scalable carbon dioxide electroreduction coupled to carbonylation chemistry. Nat. Commun. 2017, 8, 489. [Google Scholar] [CrossRef] [PubMed]
- Kusama, S.; Saito, T.; Hashiba, H.; Sakai, A.; Yotsuhashi, S. Crystalline Copper(II) Phthalocyanine Catalysts for Electrochemical Reduction of Carbon Dioxide in Aqueous Media. ACS Catal. 2017, 7, 8382–8385. [Google Scholar] [CrossRef]
- Zhu, H.J.; Lu, M.; Wang, Y.R.; Yao, S.J.; Zhang, M.; Kan, Y.H.; Liu, J.; Chen, Y.; Li, S.L.; Lan, Y.Q. Efficient electron transmission in covalent organic framework nanosheets for highly active electrocatalytic carbon dioxide reduction. Nat. Commun. 2020, 11, 497. [Google Scholar] [CrossRef]
- Park, S.; Wijaya, D.T.; Na, J.; Lee, C.W. Towards the large-scale electrochemical reduction of carbon dioxide. Catalysts 2021, 11, 253. [Google Scholar] [CrossRef]
- Perry, S.C. Electrochemical Carbon Dioxide Reduction; De Gruyter: Berlin, Germany, 2021; pp. 1–152. [Google Scholar] [CrossRef]
- Chen, T.W.; Chen, S.M.; Anushya, G.; Kannan, R.; Al-Sehemi, A.G.; Alargarsamy, S.; Gajendran, P.; Ramachandran, R. Development of Different Kinds of Electrocatalyst for the Electrochemical Reduction of Carbon Dioxide Reactions: An Overview. Molecules 2023, 28, 7016. [Google Scholar] [CrossRef]
- Peng, L.; Zhang, Y.; He, R.; Xu, N.; Qiao, J. Research Advances in Electrocatalysts, Electrolytes, Reactors and Membranes for the Electrocatalytic Carbon Dioxide Reduction Reaction. Acta Phys. Chim. Sin. 2023, 39, 2302037. [Google Scholar] [CrossRef]
- Chen, Z.; Li, N.; Zhang, Q. Covalent Organic Frameworks as Promising Platforms for Efficient Electrochemical Reduction of Carbon Dioxide: A Review. Small Struct. 2024, 5, 2300495. [Google Scholar] [CrossRef]
- Varhade, S.; Guruji, A.; Singh, C.; Cicero, G.; García-Melchor, M.; Helsen, J.; Pant, D. Electrochemical CO2 Reduction: Commercial Innovations and Prospects. ChemElectroChem 2025, 12, e202400512. [Google Scholar] [CrossRef]
- Pourebrahimi, S.; Pirooz, M.; Ahmadi, S.; Kazemeini, M.; Vafajoo, L. Nanoengineering of metal-based electrocatalysts for carbon dioxide (CO2) reduction: A critical review. Mater. Today Phys. 2023, 38, 101250. [Google Scholar] [CrossRef]
- Ma, S.; Sadakiyo, M.; Luo, R.; Heima, M.; Yamauchi, M.; Kenis, P.J.A. One-step electrosynthesis of ethylene and ethanol from CO2 in an alkaline electrolyzer. J. Power Sources 2016, 301, 219–228. [Google Scholar] [CrossRef]
- Gao, D.; Li, W.; Wang, H.; Wang, G.; Cai, R. Heterogeneous Catalysis for CO2 Conversion into Chemicals and Fuels. Trans. Tianjin Univ. 2022, 28, 245–264. [Google Scholar] [CrossRef]
- Díaz-Sainz, G.; Alvarez-Guerra, M.; Irabien, A. Continuous electroreduction of CO2 towards formate in gas-phase operation at high current densities with an anion exchange membrane. J. CO2 Util. 2022, 56, 101822. [Google Scholar] [CrossRef]
- Huang, G.; Huang, Y.J.; Ali, A.; Chen, Z.J.; Shen, P.K.; Ni, B.J.; Zhu, J.L. Phase-controllable cobalt phosphide heterostructure for efficient electrocatalytic hydrogen evolution in water and seawater. Electron 2024, 2, e58. [Google Scholar] [CrossRef]
- Si, D.; Teng, X.; Xiong, B.; Chen, L.; Shi, J. Electrocatalytic functional group conversion-based carbon resource upgrading. Chem. Sci. 2024, 15, 6269–6284. [Google Scholar] [CrossRef]
- Novaes, L.F.T.; Liu, J.; Shen, Y.; Lu, L.; Meinhardt, J.M.; Lin, S. Electrocatalysis as an enabling technology for organic synthesis. Chem. Soc. Rev. 2021, 50, 7941–8002. [Google Scholar] [CrossRef]
- Gao, Y.; Ge, L.; Xu, H.; Davey, K.; Zheng, Y.; Qiao, S.Z. Electrocatalytic Refinery of Biomass-Based 5-Hydroxymethylfurfural to Fine Chemicals. ACS Catal. 2023, 13, 11204–11231. [Google Scholar] [CrossRef]
- Tang, C.; Wei, C.; Fang, Y.; Liu, B.; Song, X.; Bian, Z.; Yin, X.; Wang, H.; Liu, Z.; Wang, G.; et al. Electrocatalytic hydrogenation of acetonitrile to ethylamine in acid. Nat. Commun. 2024, 15, 3233. [Google Scholar] [CrossRef]
- Zhu, Y.; Wu, D.; Tang, J.; Braaten, D.; Liu, B.; Peng, Z. Advances in electrocatalytic dehydrogenation of ethylamine to acetonitrile. Chem. Commun. 2024, 60, 9007–9021. [Google Scholar] [CrossRef]
- Sen, R.; Das, S.; Nath, A.; Maharana, P.; Kar, P.; Verpoort, F.; Liang, P.; Roy, S. Electrocatalytic Water Oxidation: An Overview with an Example of Translation from Lab to Market. Front. Chem. 2022, 10, 861604. [Google Scholar] [CrossRef]
- Guan, M.H.; Wu, T.; Lu, A.H. Tandem Synthesis of Valuable Chemicals via Electrocatalysis. ChemCatChem 2023, 15, e202301311. [Google Scholar] [CrossRef]
- Dong, L.-Y.; Guan, M.-H.; Ren, Y.-Q.; Hao, G.-P.; Lu, A.-H. Mesoporous Fe-Doped Carbon Electrocatalysts with Highly Exposed Active Sites for Efficient Synthesis of Hydrogen Peroxide and Tandem Epoxidation of Propene. Renewables 2023, 1, 562–571. [Google Scholar] [CrossRef]
- Guan, M.H.; Dong, L.Y.; Wu, T.; Li, W.C.; Hao, G.P.; Lu, A.H. Boosting Selective Oxidation of Ethylene to Ethylene Glycol Assisted by In situ Generated H2O2 from O2 Electroreduction. Angew. Chem. Int. Ed. 2023, 62, e202302466. [Google Scholar] [CrossRef] [PubMed]
- Lazaridis, T.; Stühmeier, B.M.; Gasteiger, H.A.; El-Sayed, H.A. Capabilities and limitations of rotating disk electrodes versus membrane electrode assemblies in the investigation of electrocatalysts. Nat. Catal. 2022, 5, 363–373. [Google Scholar] [CrossRef]
- Dong, Q.; Li, T.; Yao, Y.; Wang, X.; He, S.; Li, J.; Luo, J.; Zhang, H.; Pei, Y.; Zheng, C.; et al. A General Method for Regenerating Catalytic Electrodes. Joule 2020, 4, 2374–2386. [Google Scholar] [CrossRef]
- Bisztyga-Szklarz, M.; Mech, K.; Marzec, M.; Kalendarev, R.; Szaciłowski, K. In situ regeneration of copper-coated gas diffusion electrodes for electroreduction of CO2 to ethylene. Materials 2021, 14, 3171. [Google Scholar] [CrossRef]
- Sundar, K.P.; Kanmani, S. Progression of Photocatalytic reactors and it’s comparison: A Review. Chem. Eng. Res. Des. 2020, 154, 135–150. [Google Scholar] [CrossRef]
- Kant, P.; Liang, S.; Rubin, M.; Ozin, G.A.; Dittmeyer, R. Low-cost photoreactors for highly photon/energy-efficient solar-driven synthesis. Joule 2023, 7, 1347–1362. [Google Scholar] [CrossRef]
- Meng, T.; Su, X.; Sun, P.; Sun, W.; Santoro, D.; Yao, H.; Wang, H. UV-based advanced oxidation processes in photoreactors with reflective sleeves. J. Clean. Prod. 2023, 416, 137945. [Google Scholar] [CrossRef]
- Cabrera-Reina, A.; Salazar-González, R.; Marugán, J.; Sánchez Pérez, J.A.; Miralles-Cuevas, S. Comprehensive evaluation of a novel pilot-scale UVA-LED photoreactor for water treatment applications: Characterization, catalytic efficiency, energy performance and economic viability. J. Water Process Eng. 2024, 63, 105483. [Google Scholar] [CrossRef]
- Photoreactors—Lab, Pilot and Industrial Scale. Available online: https://photoreactors.com/ (accessed on 20 May 2025).
- Meinhardová, V.; Dubnová, L.; Drobná, H.; Matějová, L.; Kočí, K.; Čapek, L. Role of lamp type in conventional batch and micro-photoreactor for photocatalytic hydrogen production. Front. Chem. 2023, 11, 1271410. [Google Scholar] [CrossRef]
- Markushyna, Y.; Savateev, A. Light as a Tool in Organic Photocatalysis: Multi-Photon Excitation and Chromoselective Reactions. Eur. J. Org. Chem. 2022, 2022, e202200026. [Google Scholar] [CrossRef]
- Veréb, G.; Gyulavári, T.; Virág, O.; Alapi, T.; Hernadi, K.; Pap, Z. Wavelength Dependence of the Photocatalytic Performance of Pure and Doped TiO2 Photocatalysts—A Reflection on the Importance of UV Excitability. Catalysts 2022, 12, 1492. [Google Scholar] [CrossRef]
- Menzel, J.P.; Noble, B.B.; Blinco, J.P.; Barner-Kowollik, C. Predicting wavelength-dependent photochemical reactivity and selectivity. Nat. Commun. 2021, 12, 1691. [Google Scholar] [CrossRef]
- Watts, R.J.; Kong, S.; Lee, W. Sedimentation and reuse of titanium dioxide: Application to suspended-photocatalyst reactors. J. Environ. Eng. 1995, 121, 730–735. [Google Scholar] [CrossRef]
- Romero, R.L.; Alfano, O.M.; Cassano, A.E. Cylindrical Photocatalytic Reactors. Radiation Absorption and Scattering Effects Produced by Suspended Fine Particles in an Annular Space. Ind. Eng. Chem. Res. 1997, 36, 3094–3109. [Google Scholar] [CrossRef]
- Negishi, N.; Sano, T. Photocatalytic Solar Tower Reactor for the Elimination of a Low Concentration of VOCs. Molecules 2014, 19, 16624–16639. [Google Scholar] [CrossRef] [PubMed]
- Manassero, A.; Satuf, M.L.; Alfano, O.M. Photocatalytic degradation of an emerging pollutant by TiO2-coated glass rings: A kinetic study. Environ. Sci. Pollut. Res. 2017, 24, 6031–6039. [Google Scholar] [CrossRef]
- Šuligoj, A.; Cerc Korošec, R.; Žerjav, G.; Novak Tušar, N.; Lavrenčič Štangar, U. Solar-Driven Photocatalytic Films: Synthesis Approaches, Factors Affecting Environmental Activity, and Characterization Features. Top. Curr. Chem. 2022, 380, 51. [Google Scholar] [CrossRef]
- Chen, C.; Fei, L.; Wang, B.; Xu, J.; Li, B.; Shen, L.; Lin, H. MOF-Based Photocatalytic Membrane for Water Purification: A Review. Small 2024, 20, 2305066. [Google Scholar] [CrossRef]
- Sriramoju, J.B.; Paramesh, C.C.; Halligudra, G.; Rangappa, D.; Shivaramu, P.D. Magnetic photocatalytic systems. In Photocatalytic Systems by Design: Materials, Mechanisms and Applications; Elsevier: Amsterdam, The Netherlands, 2021; pp. 503–536. [Google Scholar] [CrossRef]
- Qian, Z.; Zhang, R.; Xiao, Y.; Huang, H.; Sun, Y.; Chen, Y.; Ma, T.; Sun, X. Trace to the Source: Self-Tuning of MOF Photocatalysts. Adv. Energy Mater. 2023, 13, 2300086. [Google Scholar] [CrossRef]
- He, S.; Yin, B.; Niu, H.; Cai, Y. Targeted synthesis of visible-light-driven covalent organic framework photocatalyst via molecular design and precise construction. Appl. Catal. B Environ. 2018, 239, 147–153. [Google Scholar] [CrossRef]
- Ruidas, S.; Chowdhury, A.; Ghosh, A.; Ghosh, A.; Mondal, S.; Wonanke, A.D.D.; Addicoat, M.; Das, A.K.; Modak, A.; Bhaumik, A. Covalent Organic Framework as a Metal-Free Photocatalyst for Dye Degradation and Radioactive Iodine Adsorption. Langmuir 2023, 39, 4071–4081. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Luo, P.; Wu, H.; Wei, C.; Hu, Y.; Qiu, G. Strategies for Improving the Performance and Application of MOFs Photocatalysts. ChemCatChem 2019, 11, 2978–2993. [Google Scholar] [CrossRef]
- Wang, S.; Sun, Q.; Chen, W.; Tang, Y.; Aguila, B.; Pan, Y.; Zheng, A.; Yang, Z.; Wojtas, L.; Ma, S.; et al. Programming Covalent Organic Frameworks for Photocatalysis: Investigation of Chemical and Structural Variations. Matter 2020, 2, 416–427. [Google Scholar] [CrossRef]
- Guo, M.; Zhang, M.; Liu, R.; Zhang, X.; Li, G. State-of-the-Art Advancements in Photocatalytic Hydrogenation: Reaction Mechanism and Recent Progress in Metal-Organic Framework (MOF)-Based Catalysts. Adv. Sci. 2022, 9, 2103361. [Google Scholar] [CrossRef]
- Mondal, I.; Ghosh, D.; Pal, U.; Mistry, S. Linker’s Functionality: A Critical Exploration of Metal-Organic Frameworks and Their Derived Materials in Photocatalysis. ChemCatChem 2025, 17, e202500022. [Google Scholar] [CrossRef]
- Binjhade, R.; Mondal, R.; Mondal, S. Continuous photocatalytic reactor: Critical review on the design and performance. J. Environ. Chem. Eng. 2022, 10, 107746. [Google Scholar] [CrossRef]
- Du, R.; Chen, Y.; Ding, Z.; Fan, C.; Wang, G.; Zhang, J.; Tang, Z. Scale-up of slurry Taylor flow microreactor for heterogeneous photocatalytic synthesis of azo-products. React. Chem. Eng. 2024, 9, 2249–2261. [Google Scholar] [CrossRef]
- Crawford, R.; Baumann, M. Continuous Flow Technology Enabling Photochemistry. Adv. Synth. Catal. 2025, 367, e202500133. [Google Scholar] [CrossRef]
- Thomson, C.G.; Lee, A.L.; Vilela, F. Heterogeneous photocatalysis in flow chemical reactors. Beilstein J. Org. Chem. 2020, 16, 1495–1549. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Meng, X. Photothermal catalysis: From principles to applications. Int. J. Hydrogen Energy 2023, 48, 34659–34676. [Google Scholar] [CrossRef]
- Gupta, A.; Likozar, B.; Jana, R.; Chanu, W.C.; Singh, M.K. A review of hydrogen production processes by photocatalytic water splitting—From atomistic catalysis design to optimal reactor engineering. Int. J. Hydrogen Energy 2022, 47, 33282–33307. [Google Scholar] [CrossRef]
- Guan, X.; Shen, S.; Mao, S.S. Harvesting the two-electron process for solar water splitting. Cell Rep. Phys. Sci. 2023, 4, 101211. [Google Scholar] [CrossRef]
- Adeola, A.O.; Ighalo, J.O.; Kyesmen, P.I.; Nomngongo, P.N. Metal-Organic Polyhedra (MOPs) as emerging class of metal-organic frameworks for CO2 photocatalytic conversions: Current trends and future outlook. J. CO2 Util. 2024, 80, 102664. [Google Scholar] [CrossRef]
- Guene Lougou, B.; Geng, B.X.; Pan, R.M.; Wang, W.; Yan, T.T.; Li, F.H.; Zhang, H.; Djandja, O.S.; Shuai, Y.; Tabatabaei, M.; et al. Solar-driven photothermal catalytic CO2 conversion: A review. Rare Met. 2024, 43, 2913–2939. [Google Scholar] [CrossRef]
- Liu, H.; Sun, R.; Yang, Y.; Zhang, C.; Zhao, G.; Zhang, K.; Liang, L.; Huang, X. Review on Microreactors for Photo-Electrocatalysis Artificial Photosynthesis Regeneration of Coenzymes. Micromachines 2024, 15, 789. [Google Scholar] [CrossRef]
- Tian, J.; Xu, W.; Gao, J.; Zhu, T.; Xu, G.; Zhong, Z.; Su, F. Electrocatalytic and Photocatalytic CO2 Methanation: From Reaction Fundamentals to Catalyst Developments. ChemCatChem 2024, 16, e202301406. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, S.; Ning, S.; Yang, C.; Li, L.; Tang, L.; Wang, J.; Liu, R.; Yin, X.; Zhu, Y.; et al. Recent advances and developments in solar-driven photothermal catalytic CO2 reduction into multicarbon (C2+) products. Chem. Sci. 2025, 16, 4568–4594. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Li, S.; Fan, X.; Tang, Z. Recent advances on aerobic photocatalytic methane conversion under mild conditions. Nano Res. 2023, 16, 12558–12571. [Google Scholar] [CrossRef]
- Xie, J.; Jiang, Y.H.; Li, S.Y.; Xu, P.; Zheng, Q.; Fan, X.Y.; Peng, H.L.; Tang, Z.Y. Stable Photocatalytic Coupling of Methane to Ethane with Water Vapor Using TiO2 Supported Ultralow Loading AuPd Nanoparticles. Acta Phys. Chim. Sin. 2023, 39, 2306037. [Google Scholar] [CrossRef]
- Bo, C.; Zhang, L.; Liu, X.; Chang, H.; Sun, Y.; Zhang, X.; Tan, T.; Piao, L. Highly selective photocatalytic oxidation of methane to methyl hydroperoxide. Nano Res. 2024, 17, 2473–2480. [Google Scholar] [CrossRef]
- Wang, P.; Shi, R.; Zhao, J.; Zhang, T. Photodriven Methane Conversion on Transition Metal Oxide Catalyst: Recent Progress and Prospects. Adv. Sci. 2024, 11, 2305471. [Google Scholar] [CrossRef]
- Bazargan, A.E. Photocatalytic Water and Wastewater Treatment; IWA Publishing: London, UK, 2022; pp. 1–207. [Google Scholar]
- Davari, N.; Falletta, E.; Bianchi, C.L.; Yargeau, V.; Rodriguez-Seco, C.; Boffito, D.C. Comparing the photocatalytic activity of suspended and floating Ag-decorated TiO2 for dye removal. Tetrahedron Green Chem. 2025, 5, 100059. [Google Scholar] [CrossRef]
- Gogoi, R.; Jena, S.K.; Singh, A.; Sharma, K.; Khanna, K.; Chowdhury, S.; Kumar, R.; Siril, P.F. Mechanically pulverized covalent organic framework as a metal-free photocatalyst for fenton-like degradation of organic pollutants and hexavalent chromium reduction. J. Environ. Chem. Eng. 2024, 12, 112006. [Google Scholar] [CrossRef]
- Chu, C.; Chen, Z.; Yao, D.; Liu, X.; Cai, M.; Mao, S. Large-Scale Continuous and In Situ Photosynthesis of Hydrogen Peroxide by Sulfur-Functionalized Polymer Catalyst for Water Treatment. Angew. Chem. Int. Ed. 2024, 63, e202317214. [Google Scholar] [CrossRef]
- Meng, J.; Huang, Y.; Wang, X.; Liao, Y.; Zhang, H.; Dai, W. Photocatalytic Production of Hydrogen Peroxide from Covalent-Organic-Framework-Based Materials: A Mini-Review. Catalysts 2024, 14, 429. [Google Scholar] [CrossRef]
- Yong, Z.; Ma, T. Solar-to-H2O2 Catalyzed by Covalent Organic Frameworks. Angew. Chem. Int. Ed. 2023, 62, e202308980. [Google Scholar] [CrossRef]
- Feng, S.; Su, R. Synthetic Chemistry in Flow: From Photolysis & Homogeneous Photocatalysis to Heterogeneous Photocatalysis. ChemSusChem 2024, 17, e202400064. [Google Scholar] [CrossRef]
- Jia, T.; Zhao, Y.; Song, W.; Zhao, J. Metallaphotocatalytic Platforms Based on Covalent Organic Frameworks for Cross-Coupling Reactions. ChemCatChem 2024, 16, e202301522. [Google Scholar] [CrossRef]
- Ghosh, I.; König, B. Chromoselective Photocatalysis: Controlled Bond Activation through Light-Color Regulation of Redox Potentials. Angew. Chem. Int. Ed. 2016, 55, 7676–7679. [Google Scholar] [CrossRef] [PubMed]
- Galushchinskiy, A.; Pulignani, C.; Szalad, H.; Reisner, E.; Albero, J.; Tarakina, N.V.; Pelicano, C.M.; García, H.; Savateev, O.; Antonietti, M. Heterostructured PHI-PTI/Li+Cl− Carbon Nitrides for Multiple Photocatalytic Applications. Sol. RRL 2023, 7, 2300077. [Google Scholar] [CrossRef]
- Ehrfeld, W.; Hessel, V.; Löwe, H. Microreactors, New Technology for Modern Chemistry; Wiley-VCH Verlag: Weinheim, Germany, 2000; pp. 1–293. [Google Scholar] [CrossRef]
- Mills, P.L.; Quiram, D.J.; Ryley, J.F. Microreactor technology and process miniaturization for catalytic reactions—A perspective on recent developments and emerging technologies. Chem. Eng. Sci. 2007, 62, 6992–7010. [Google Scholar] [CrossRef]
- Saldana, M.; Gallegos, S.; Gálvez, E.; Castillo, J.; Salinas-Rodríguez, E.; Cerecedo-Sáenz, E.; Hernández-Ávila, J.; Navarra, A.; Toro, N. The Reynolds Number: A Journey from Its Origin to Modern Applications. Fluids 2024, 9, 299. [Google Scholar] [CrossRef]
- Houzelot, J.L.; Villermaux, J. Mass transfer in annular cylindrical reactors in laminar flow. Chem. Eng. Sci. 1977, 32, 1465–1470. [Google Scholar] [CrossRef]
- Wißdorf, W.; Müller, D.; Brachthäuser, Y.; Langner, M.; Derpmann, V.; Klopotowski, S.; Polaczek, C.; Kersten, H.; Brockmann, K.; Benter, T. Gas Flow Dynamics in Inlet Capillaries: Evidence for non Laminar Conditions. J. Am. Soc. Mass Spectrom. 2016, 27, 1550–1563. [Google Scholar] [CrossRef]
- Diehm, J.; Franzreb, M. Modeling the extra column volume of a micro simulated moving bed chromatography system: Introducing the equivalent radial flow rate distribution. J. Chromatogr. A 2025, 1740, 465543. [Google Scholar] [CrossRef]
- Antony, R.; Giri Nandagopal, M.S.; Sreekumar, N.; Rangabhashiyam, S.; Selvaraju, N. Liquid-liquid slug flow in a microchannel reactor and its mass transfer properties—A review. Bull. Chem. React. Eng. Catal. 2014, 9, 207–223. [Google Scholar] [CrossRef]
- Zong, J.; Yue, J. Gas–Liquid Slug Flow Studies in Microreactors: Effect of Nanoparticle Addition on Flow Pattern and Pressure Drop. Front. Chem. Eng. 2021, 3, 788241. [Google Scholar] [CrossRef]
- Zong, J.; Yue, J. Continuous Solid Particle Flow in Microreactors for Efficient Chemical Conversion. Ind. Eng. Chem. Res. 2022, 61, 6269–6291. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, Y.; Zou, H.; Li, M.; Wang, G.; Peng, M.; Zhang, J.; Tang, Z. Tuning the gas-liquid-solid segmented flow for enhanced heterogeneous photosynthesis of Azo- compounds. Chem. Eng. J. 2021, 423, 130226. [Google Scholar] [CrossRef]
- Radhi, H.H.; Sharifzadeh, E.; Azimi, N. Intensification of Liquid-Liquid Extraction in a Microreactor Equipped with Low and High-Frequency Ultrasound Waves: A Numerical Study. Iran. J. Chem. Chem. Eng. 2024, 43, 3021–3032. [Google Scholar] [CrossRef]
- Wojnicki, M.; Yang, X.; Zabinski, P.; Mutschke, G. Enhanced Mixing in Microflow Systems Using Magnetic Fields—Experimental and Numerical Analyses. Micromachines 2025, 16, 422. [Google Scholar] [CrossRef]
- Ma, T.; Zhao, S.; Tang, W.; Zhong, W.; Liu, Y.; Feng, Y.; Fang, Z.; Qin, H.; Xu, H.; Li, Y.; et al. Microreactors with multivariate external force field used for the chemical process intensification. Chem. Eng. J. 2024, 493, 152508. [Google Scholar] [CrossRef]
- Tanimu, A.; Jaenicke, S.; Alhooshani, K. Heterogeneous catalysis in continuous flow microreactors: A review of methods and applications. Chem. Eng. J. 2017, 327, 792–821. [Google Scholar] [CrossRef]
- Suryawanshi, P.L.; Gumfekar, S.P.; Bhanvase, B.A.; Sonawane, S.H.; Pimplapure, M.S. A review on microreactors: Reactor fabrication, design, and cutting-edge applications. Chem. Eng. Sci. 2018, 189, 431–448. [Google Scholar] [CrossRef]
- Bojang, A.A.; Wu, H.S. Design, fundamental principles of fabrication and applications of microreactors. Processes 2020, 8, 891. [Google Scholar] [CrossRef]
- Bugay, C.A.; Caballas, M.C.; Mercado, S.B.; Rubio, J.F.; Serote, P.K.; Villarte, P.N.; Rubi, R.V.C. A Review of Microreactors for Process Intensification. Eng. Proc. 2024, 67, 21. [Google Scholar] [CrossRef]
- Abiev, R.S.; Makusheva, I.V. Energy Dissipation Rate and Micromixing in a Two-Step Micro-Reactor with Intensively Swirled Flows. Micromachines 2022, 13, 1859. [Google Scholar] [CrossRef] [PubMed]
- Templis, C.C.; Papayannakos, N.G. Mass and heat transfer coefficients in automotive exhaust catalytic converter channels. Catalysts 2019, 9, 507. [Google Scholar] [CrossRef]
- Yue, J. Multiphase flow processing in microreactors combined with heterogeneous catalysis for efficient and sustainable chemical synthesis. Catal. Today 2018, 308, 3–19. [Google Scholar] [CrossRef]
- Abbas, I.; Romeggio, F.; Pilarczyk, K.; Kuhn, S.; Damsgaard, C.D.; Kibsgaard, J.; Lievens, P.; Grandjean, D.; Janssens, E. High pressure microreactor for minute amounts of catalyst on planar supports: A case study of CO2 hydrogenation over Pd0.25Zn0.75Ox nanoclusters. Chem. Eng. J. 2025, 503, 158127. [Google Scholar] [CrossRef]
- Togashi, S.; Miyamoto, T.; Asano, Y.; Endo, Y. Yield improvement of chemical reactions by using a microreactor and development of a pilot plant using the numbering-up of microreactors. J. Chem. Eng. Jpn. 2009, 42, 512–519. [Google Scholar] [CrossRef]
- Frost, C.G.; Mutton, L. Heterogeneous catalytic synthesis using microreactor technology. Green Chem. 2010, 12, 1687–1703. [Google Scholar] [CrossRef]
- Liu, X.; Ünal, B.; Jensen, K.F. Heterogeneous catalysis with continuous flow microreactors. Catal. Sci. Technol. 2012, 2, 2134–2138. [Google Scholar] [CrossRef]
- Feng, H.; Zhang, Y.; Liu, J.; Liu, D. Towards Heterogeneous Catalysis: A Review on Recent Advances of Depositing Nanocatalysts in Continuous–Flow Microreactors. Molecules 2022, 27, 8052. [Google Scholar] [CrossRef]
- Zhang, L.; Yue, J. Packed Bed Microreactors for Sustainable Chemistry and Process Development. Chemistry 2025, 7, 29. [Google Scholar] [CrossRef]
- Markovič, M.; Lopatka, P.; Gracza, T.; Koóš, P. Recent Applications of Continuous Flow in Homogeneous Palladium Catalysis. Synthesis 2020, 52, 3511–3529. [Google Scholar] [CrossRef]
- Fanelli, F.; Parisi, G.; Degennaro, L.; Luisi, R. Contribution of microreactor technology and flow chemistry to the development of green and sustainable synthesis. Beilstein J. Org. Chem. 2017, 13, 520–542. [Google Scholar] [CrossRef]
- Petrusová, Z.; Slouka, Z.; Vobecká, L.; Polezhaev, P.; Hasal, P.; Přibyl, M.; Izák, P. Microreaction and membrane technologies for continuous single-enantiomer production: A review. Catal. Rev. Sci. Eng. 2023, 65, 773–821. [Google Scholar] [CrossRef]
- Hone, C.A.; Kappe, C.O. Membrane Microreactors for the On-Demand Generation, Separation, and Reaction of Gases. Chem. Eur. J. 2020, 26, 13108–13117. [Google Scholar] [CrossRef] [PubMed]
- Koybasi, H.H.; Avci, A.K. Modeling of a membrane integrated catalytic microreactor for efficient DME production from syngas with CO2. Catal. Today 2022, 383, 133–145. [Google Scholar] [CrossRef]
- Yang, L.; Jensen, K.F. Mass transport and reactions in the tube-in-tube reactor. Org. Process Res. Dev. 2013, 17, 927–933. [Google Scholar] [CrossRef]
- Alza, E.; Mannhold, R.; Buschmann, H.; Holenz, J. Flow and Microreactor Technology in Medicinal Chemistry; Wiley: Hoboken, NJ, USA, 2022; pp. 1–344. [Google Scholar] [CrossRef]
- Wirth, T. Microreactors in Organic Chemistry and Catalysis, 2nd ed.; Wiley-VCH: Weinheim, Germany, 2013; pp. 1–465. [Google Scholar] [CrossRef]
- Gross, U.; Koos, P.; O’Brien, M.; Polyzos, A.; Ley, S.V. A general continuous flow method for palladium catalysed carbonylation reactions using single and multiple tube-in-tube gas-liquid microreactors. Eur. J. Org. Chem. 2014, 2014, 6418–6430. [Google Scholar] [CrossRef]
- Díaz-Angulo, J.; Cotillas, S.; Gomes, A.I.; Miranda, S.M.; Mueses, M.; Machuca-Martínez, F.; Rodrigo, M.A.; Boaventura, R.A.R.; Vilar, V.J.P. A tube-in-tube membrane microreactor for tertiary treatment of urban wastewaters by photo-Fenton at neutral pH: A proof of concept. Chemosphere 2021, 263, 128049. [Google Scholar] [CrossRef]
- Neyt, N.C.; Riley, D.L. Application of reactor engineering concepts in continuous flow chemistry: A review. React. Chem. Eng. 2021, 6, 1295–1326. [Google Scholar] [CrossRef]
- Lomel, S.; Falk, L.; Commenge, J.M.; Houzelot, J.L.; Ramdani, K. The microreactor: A systematic and efficient tool for the transition from batch to continuous process? Chem. Eng. Res. Des. 2006, 84, 363–369. [Google Scholar] [CrossRef]
- Elvira, K.S.; i Solvas, X.C.; Wootton, R.C.R.; Demello, A.J. The past, present and potential for microfluidic reactor technology in chemical synthesis. Nat. Chem. 2013, 5, 905–915. [Google Scholar] [CrossRef]
- Xing, R.; Huang, R.; Su, R.; Kong, J.; Dickey, M.D.; Qi, W. 3D-Printing of Hierarchical Porous Copper-Based Metal-Organic-Framework Structures for Efficient Fixed-Bed Catalysts. Chem. Bio. Eng. 2024, 1, 264–273. [Google Scholar] [CrossRef] [PubMed]
- Rossetti, I. Continuous flow (micro-)reactors for heterogeneously catalyzed reactions: Main design and modelling issues. Catal. Today 2018, 308, 20–31. [Google Scholar] [CrossRef]
- Chen, X.; Fu, M.; He, J.; Lu, J.; Wang, C.; Jiang, D.; Li, Y.; Yu, C. Immobilization strategies for heterogeneous catalysts in micro flow chemistry: Key techniques for efficient and sustainable chemical transformations. Coord. Chem. Rev. 2025, 538, 216723. [Google Scholar] [CrossRef]
- An, H.; Li, A.; Sasmito, A.P.; Kurnia, J.C.; Jangam, S.V.; Mujumdar, A.S. Computational fluid dynamics (CFD) analysis of micro-reactor performance: Effect of various configurations. Chem. Eng. Sci. 2012, 75, 85–95. [Google Scholar] [CrossRef]
- Yang, Y.; Qian, J.; Fang, G.; Guan, Z.; Han, H.; Liu, Q.; Liu, H.; Wang, Y.; Li, W. Research progress on the process analysis technology for microreactors. Microchem. J. 2025, 212, 113373. [Google Scholar] [CrossRef]
- Schoenitz, M.; Grundemann, L.; Augustin, W.; Scholl, S. Fouling in microstructured devices: A review. Chem. Commun. 2015, 51, 8213–8228. [Google Scholar] [CrossRef]
- Wang, H.; Wang, X.; Ren, Y.; Zhang, L. Macrostructural Design Approach of the Monolithic Catalyst and Its Application in Nitrobenzene Hydrogenation. Ind. Eng. Chem. Res. 2024, 63, 7044–7050. [Google Scholar] [CrossRef]
- Liu, M.; Zhu, X.; Liao, Q.; Chen, R.; Ye, D.; Chen, G. Stacked Catalytic Membrane Microreactor for Nitrobenzene Hydrogenation. Ind. Eng. Chem. Res. 2020, 59, 9469–9477. [Google Scholar] [CrossRef]
- Yan, W.W.; Tsuru, T. Overview: Insight into NH3 permselective membranes and their application to a membrane reactor for efficient ammonia production. Sep. Purif. Technol. 2025, 370, 133275. [Google Scholar] [CrossRef]
- Fang, H.; Xiao, Q.; Wu, F.; Floreancig, P.E.; Weber, S.G. Rapid catalyst screening by a continuous-flow microreactor interfaced with ultra-high-pressure liquid chromatography. J. Org. Chem. 2010, 75, 5619–5626. [Google Scholar] [CrossRef]
- Nieuwelink, A.E.; Vollenbroek, J.C.; Tiggelaar, R.M.; Bomer, J.G.; van den Berg, A.; Odijk, M.; Weckhuysen, B.M. High-throughput activity screening and sorting of single catalyst particles with a droplet microreactor using dielectrophoresis. Nat. Catal. 2021, 4, 1070–1079. [Google Scholar] [CrossRef]
- Hone, C.A.; Lopatka, P.; Munday, R.; O’Kearney-McMullan, A.; Kappe, C.O. Continuous-flow Synthesis of Aryl Aldehydes by Pd-catalyzed Formylation of Aryl Bromides Using Carbon Monoxide and Hydrogen. ChemSusChem 2019, 12, 326–337. [Google Scholar] [CrossRef] [PubMed]
- Greco, R.; Goessler, W.; Cantillo, D.; Kappe, C.O. Benchmarking immobilized di- and triarylphosphine palladium catalysts for continuous-flow cross-coupling reactions: Efficiency, durability, and metal leaching studies. ACS Catal. 2015, 5, 1303–1312. [Google Scholar] [CrossRef]
- Cantillo, D.; Kappe, C.O. Immobilized transition metals as catalysts for cross-couplings in continuous flow—A critical assessment of the reaction mechanism and metal leaching. ChemCatChem 2015, 6, 3286–3305. [Google Scholar] [CrossRef]
- Hübner, S.; De Vries, J.G.; Farina, V. Why Does Industry Not Use Immobilized Transition Metal Complexes as Catalysts? Adv. Synth. Catal. 2016, 358, 3–25. [Google Scholar] [CrossRef]
- Tongtummachat, T.; Jaree, A.; Akkarawatkhoosith, N. Green synthesis of 5-hydroxymethylfurfural through non-catalytic conversion of glucose in a microreactor. Energy Convers. Manag. X 2021, 12, 100141. [Google Scholar] [CrossRef]
- Cohen, K.; Blanchard, J.; Rodriguez, P.; Kelly, K.; Dorman, J.A.; Dooley, K.M. Non-Catalytic direct partial oxidation of methane to methanol in a Wall-Coated microreactor. Chem. Eng. J. 2024, 482, 149049. [Google Scholar] [CrossRef]
- El Zanati, E.M.; Elnahas, G.M. Design and scale-up of continuous flow micro-reactor. J. Eng. Sci. Technol. 2018, 13, 2481–2494. [Google Scholar]
- Lopatka, P.; Markovič, M.; Koóš, P.; Ley, S.V.; Gracza, T. Continuous Pd-Catalyzed Carbonylative Cyclization Using Iron Pentacarbonyl as a CO Source. J. Org. Chem. 2019, 84, 14394–14406. [Google Scholar] [CrossRef]
- Lopatka, P.; Gavenda, M.; Markovič, M.; Koóš, P.; Gracza, T. Flow Pd(II)-catalysed carbonylative cyclisation in the total synthesis of Jaspine B. Catalysts 2021, 11, 1513. [Google Scholar] [CrossRef]
- Ahn, G.N.; Yu, T.; Lee, H.J.; Gyak, K.W.; Kang, J.H.; You, D.; Kim, D.P. A numbering-up metal microreactor for the high-Throughput production of a commercial drug by copper catalysis. Lab Chip 2019, 19, 3535–3542. [Google Scholar] [CrossRef]
- Ciemięga, A.; Maresz, K.; Mrowiec-Białoń, J. Bifunctionalised acid-base hierarchically structured monolithic microreactor for continuous-flow tandem catalytic process of cyanocinnamate synthesis. Sci. Rep. 2024, 14, 25332. [Google Scholar] [CrossRef]
- Poletti, L.; De Risi, C.; Ragno, D.; Di Carmine, G.; Tassoni, R.; Massi, A.; Dambruoso, P. Organocatalytic Packed-Bed Reactors for the Enantioselective Flow Synthesis of Quaternary Isotetronic Acids by Direct Aldol Reactions of Pyruvates. Molecules 2025, 30, 296. [Google Scholar] [CrossRef]
- Do, H.H.; Tran, T.K.C.; Ung, T.D.T.; Dao, N.T.; Nguyen, D.D.; Trinh, T.H.; Hoang, T.D.; Le, T.L.; Tran, T.T.H. Controllable fabrication of photocatalytic TiO2 brookite thin film by 3D-printing approach for dyes decomposition. J. Water Process Eng. 2021, 43, 102319. [Google Scholar] [CrossRef]
- Gao, Z.; Pan, C.; Choi, C.H.; Chang, C.H. Continuous-flow photocatalytic microfluidic-reactor for the treatment of aqueous contaminants, simplicity, and complexity: A mini-review. Symmetry 2021, 13, 1325. [Google Scholar] [CrossRef]
- Rao Pala, L.P.; Peela, N.R. Green Hydrogen Production in an Optofluidic Planar Microreactor via Photocatalytic Water Splitting under Visible/Simulated Sunlight Irradiation. Energy Fuels 2021, 35, 19737–19747. [Google Scholar] [CrossRef]
- Mei, J.; Gao, X.; Zou, J.; Pang, F. Research on Photocatalytic Wastewater Treatment Reactors: Design, Optimization, and Evaluation Criteria. Catalysts 2023, 13, 974. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, Y.; Liu, N.; Kang, S. Understanding the interface properties of photocatalytic reactors for rational engineering applications. Chem. Eng. J. 2023, 472, 145057. [Google Scholar] [CrossRef]
- Ge, X.H.; Wei, N.; Hu, X.; Xie, Q.; Wang, X.; Li, L.; Qiu, T. The integrated microfluidic photocatalytic planar reactor under continuous operation. Front. Chem. Eng. 2024, 6, 1375071. [Google Scholar] [CrossRef]
- Lu, J.M.; Wang, H.F.; Guo, Q.H.; Wang, J.W.; Li, T.T.; Chen, K.X.; Zhang, M.T.; Chen, J.B.; Shi, Q.N.; Huang, Y.; et al. Roboticized AI-assisted microfluidic photocatalytic synthesis and screening up to 10,000 reactions per day. Nat. Commun. 2024, 15, 8826. [Google Scholar] [CrossRef]
- Towler, G.; Sinnott, R. Chemical Engineering Design: Principles, Practice and Economics of Plant and Process Design; Elsevier: Amsterdam, The Netherlands, 2021; pp. 1–1027. [Google Scholar]
- Top 10 Industrial Catalyst Companies in the World. Available online: https://www.reportsanddata.com/blog/top-industrial-catalyst-companies-in-the-world (accessed on 6 June 2025).
- Shi, H.; Zhou, T. Computational design of heterogeneous catalysts and gas separation materials for advanced chemical processing. Front. Chem. Sci. Eng. 2021, 15, 49–59. [Google Scholar] [CrossRef]
- Hegedus, L.S.E.; Bell, A.T.; Chen, N.Y.; Haag, W.O.; Wei, J.; Aris, R.; Boudart, M.; Gates, B.C.; Somorjai, G.A. Catalyst Design: Progress and Perspectives; Wiley-Interscience: New York, NY, USA, 1987; pp. 1–288. [Google Scholar]
- Hagen, J. Industrial Catalysis: A Practical Approach, 2nd ed.; John Wiley and Sons: Hoboken, NJ, USA, 2006; pp. 1–507. [Google Scholar]
- Thomas, G.G.; Davies, C.W. lon Exchange Resins as Catalysts. Nature 1947, 159, 372. [Google Scholar] [CrossRef]
- Schmidle, C.J.; Mansfield, R.C. Catalysis by anion exchange resins. Ind. Eng. Chem. 1952, 44, 1388–1390. [Google Scholar] [CrossRef]
- Lyu, Y.; Scrimin, P. Mimicking Enzymes: The Quest for Powerful Catalysts from Simple Molecules to Nanozymes. ACS Catal. 2021, 11, 11501–11509. [Google Scholar] [CrossRef]
- Illanes, A. Enzyme Biocatalysis: Principles and Applications; Springer: Dordrecht, The Netherlands, 2008; pp. 1–391. [Google Scholar]
- Winkler, C.K.; Schrittwieser, J.H.; Kroutil, W. Power of Biocatalysis for Organic Synthesis. ACS Cent. Sci. 2021, 7, 55–71. [Google Scholar] [CrossRef]
- Albayati, S.H.; Nezhad, N.G.; Taki, A.G.; Rahman, R.N.Z.R.A. Efficient and easible biocatalysts: Strategies for enzyme improvement. A review. Int. J. Biol. Macromol. 2024, 276, 133978. [Google Scholar] [CrossRef]
- Reisenbauer, J.C.; Sicinski, K.M.; Arnold, F.H. Catalyzing the future: Recent advances in chemical synthesis using enzymes. Curr. Opin. Chem. Biol. 2024, 83, 102536. [Google Scholar] [CrossRef]
- Man, T.; Xu, C.; Liu, X.Y.; Li, D.; Tsung, C.K.; Pei, H.; Wan, Y.; Li, L. Hierarchically encapsulating enzymes with multi-shelled metal-organic frameworks for tandem biocatalytic reactions. Nat. Commun. 2022, 13, 305. [Google Scholar] [CrossRef]
- Wang, Y.; Tong, H.; Ni, S.; Huo, K.; Liu, W.; Zan, X.; Yuan, X.; Wang, S. Combining Hard Shell with Soft Core to Enhance Enzyme Activity and Resist External Disturbances. Adv. Sci. 2025, 12, e2411196. [Google Scholar] [CrossRef]
- Králik, M. Research and development of chemical technologies. In Proceedings of the 39th International Conference of Slovak Society of Chemical Engineering, Tatranské Matliare, Slovakia, 21–25 May 2012; Bratislava: Tatranské Matliare, Slovakia, 2012; pp. 451–456. [Google Scholar]
- Králik, M. Liquid phase hydrogenations over dispersed metal catalysts. In Proceedings of the 12th Pannonian Symposium on Catalysis, Třešť, Czech Republic, 16–20 September 2014; Klusoň, P., Ed.; Institute of Chemical Technology: Prague, Czech Republic, 2014; p. K1. Available online: https://pannonia2014.icpf.cas.cz/wp-content/uploads/2014/09/abstrakty_final.pdf (accessed on 30 July 2025).
- Johnson Matthey Group. Licensed Processes A Portfolio of DAVY Licensed Processes and Technologies; Johnson Matthey Group: Billingham, UK, 2021; Available online: https://matthey.com/documents/161599/440742/JM+Licensed+Processes+brochure+ML+%28c2021%29.pdf/363c5bf1-3134-dd0a-31a0-2d3a41cc0f1c?t=1653488449453 (accessed on 30 July 2025).
- Setoyama, T. Design of heterogeneous catalysts and process technologies reflecting on the relevant reaction mechanism as a methodology of research at chemical company. Res. Chem. Intermed. 2021, 47, 211–223. [Google Scholar] [CrossRef]
- Erfani, N.; Baharudin, L.; Watson, M. Recent advances and intensifications in Haber-Bosch ammonia synthesis process. Chem. Eng. Process. Process Intensif. 2024, 204, 109962. [Google Scholar] [CrossRef]
- Sebesta, R.W. Concepts of Programming Languages, 11th ed.; Pearson: London, UK, 2015; pp. 1–800. [Google Scholar]
- Brunovska, A.; Burianek, J.; Ilavsky, J.; Valtyni, J. Modelling of catalyst pellet deactivation: An irreversible chemisorption model. Adsorpt. Sci. Technol. 1986, 3, 201–215. [Google Scholar] [CrossRef]
- Ilavský, J.; Brunovská, A.; Valtýni, J.; Buriánek, J. Modeling of catalytic reactors with catalyst deactivation. I. Pseudo-homogeneous model of a plug-flow reactor. Chem. Pap. 1983, 37, 433–451. [Google Scholar]
- Markoš, J.; Brunovská, A.; Ilavský, J. Modelling of fixed bed catalytic reactors catalyst poisoning. Collect. Czech. Chem. Commun. 1985, 50, 2228–2244. [Google Scholar] [CrossRef]
- Barto, M.; Brunovská, A.; Ilavský, J. Modelling of catalytic reactors with catalyst deactivation III. Pseudohomogeneous model of reactor with axial dispersion. Chem. Pap. 1986, 40, 551–563. [Google Scholar]
- Králik, M.; Ilavský, J.; Pašek, J.; Lehocký, P. Mathematical dynamic model of the continuous bubble column slurry reactor. Chem. Eng. Proc. 1990, 28, 127–132. [Google Scholar] [CrossRef]
- Markov, P.V.; Mashkovsky, I.S.; Bragina, G.O.; Wärnå, J.; Gerasimov, E.Y.; Bukhtiyarov, V.I.; Stakheev, A.Y.; Murzin, D.Y. Particle size effect in liquid-phase hydrogenation of phenylacetylene over Pd catalysts: Experimental data and theoretical analysis. Chem. Eng. J. 2019, 358, 520–530. [Google Scholar] [CrossRef]
- Murzin, D.Y.; Wärnå, J.; Haario, H.; Salmi, T. Parameter estimation in kinetic models of complex heterogeneous catalytic reactions using Bayesian statistics. React. Kinet. Mech. Catal. 2021, 133, 1–15. [Google Scholar] [CrossRef]
- Suárez-Méndez, A.; Mendoza-Cruz, R.; Martínez-Klimov, M.E.; Wärnå, J.; Albuquerque, J.S.; Murzin, D.Y.; Klimova, T.E. NiPt supported on zeolite Y and SBA-15 composites as efficient catalysts for the hydrodeoxygenation of anisole under mild conditions. Fuel 2025, 400, 135793. [Google Scholar] [CrossRef]
- Murzin, D.Y. Chemical Reactors, Treatment of Data and Mathematical Modelling; Králik, M., Ed.; Abo-Turku: Bratislava, Slovakia, 2025. [Google Scholar]
- List of Chemical Process Simulators. Available online: https://en.wikipedia.org/wiki/List_of_chemical_process_simulators (accessed on 6 June 2025).
- Haydary, J. Chemical Process Design and Simulation. Aspen Plus and Aspen HYSYS Applications; John Wiley & Sons, Inc.: New Delhi, India, 2018; p. 418. [Google Scholar]
- De la Hera, G.; Ruiz-Gutiérrez, G.; Viguri, J.R.; Galán, B. Flexible Green Ammonia Production Plants: Small-Scale Simulations Based on Energy Aspects. Environments 2024, 11, 71. [Google Scholar] [CrossRef]
- Beale, M.H.; Hagan, M.; Demuth, H.B. Deep Learning Toolbox™ Reference, MATLAB; The MathWorks, Inc.: Natick, MA, USA, 2023; pp. 1–2706. [Google Scholar]
- Anderson, E.; Bai, Z.; Bischof, C.; Blackford, S.; Demmel, J.; Dongarra, J.; Croz, J.-D.; Greenbaum, A.; Hammarling, S.; McKenney, A.; et al. gPROMS Introductory User Guide; Process Systems Enterprise Ltd.: London, UK, 2004; pp. 1–254. [Google Scholar]
- Qayyum, H.; Cheema, I.I.; Abdullah, M.; Amin, M.; Khan, I.A.; Lee, E.J.; Lee, K.H. One-dimensional modeling of heterogeneous catalytic chemical looping steam methane reforming in an adiabatic packed bed reactor. Front. Chem. 2023, 11, 1295455. [Google Scholar] [CrossRef]
- Hachhach, M.; Russo, V.; Simakova, I.; Eränen, K.; Murzin, D.Y.; Salmi, T. Packed bed reactor technology in sugar oxidation. Chem. Eng. Sci. 2025, 311, 121580. [Google Scholar] [CrossRef]
- Nyemba, W.R. Computer Aided Design: Engineering Design and Modeling Using AutoCAD; CRC Press: Boca Raton, FL, USA, 2022; pp. 1–292. [Google Scholar]
- Schindel; Polyakova; Harding; Weinhold; Stenger; Grunewald; Bramsiepe. General approach for technology and Process Equipment Assembly (PEA) selection in process design. Chem. Eng. Process. Process Intensif. 2021, 159, 108223. [Google Scholar] [CrossRef]
- Ismaeel, H.K.; Albayati, T.M.; Dhahad, H.A.; Al-Sudani, F.T.; Salih, I.K.; Saady, N.M.C.; Zendehboudi, S. Strategies for biodiesel production with the role of reactor technologies: A comprehensive review. Chem. Eng. Process. Process Intensif. 2024, 200, 109767. [Google Scholar] [CrossRef]
- Perathoner, S.; Centi, G. Science and Technology Roadamap on Catalysis for Europe, a Path to Create a Sustainable Future; ERIC aisbl: Brussels, Belgum, 2016; pp. 1–128. [Google Scholar]
- Appl, M. Ammonia: Principles and Industrial Practice; Wiley: Hoboken, NJ, USA, 1999; pp. 1–301. [Google Scholar]
- Humphreys, J.; Lan, R.; Tao, S. Development and Recent Progress on Ammonia Synthesis Catalysts for Haber–Bosch Process. Adv. Energy Sustain. Res. 2021, 2, 2000043. [Google Scholar] [CrossRef]
- Fuller, J.; An, Q.; Fortunelli, A.; Goddard, W.A. Reaction Mechanisms, Kinetics, and Improved Catalysts for Ammonia Synthesis from Hierarchical High Throughput Catalyst Design. Acc. Chem. Res. 2022, 55, 1124–1134. [Google Scholar] [CrossRef] [PubMed]
- Nadiri, S.; Attari Moghaddam, A.; Folke, J.; Ruland, H.; Shu, B.; Fernandes, R.; Schlögl, R.; Krewer, U. Ammonia Synthesis Rate Over a Wide Operating Range: From Experiments to Validated Kinetic Models. ChemCatChem 2024, 16, e202400890. [Google Scholar] [CrossRef]
- Rouwenhorst, K.H.R.; Travis, A.S.; Lefferts, L. 1921–2021: A Century of Renewable Ammonia Synthesis. Sustain. Chem. 2022, 3, 149–171. [Google Scholar] [CrossRef]
- Varadwaj, P.R.; Marques, H.M.; Grabowski, I. Ammonia Synthesis over Transition Metal Catalysts: Reaction Mechanisms, Rate-Determining Steps, and Challenges. Int. J. Mol. Sci. 2025, 26, 4670. [Google Scholar] [CrossRef]
- Saygin, D.; Blanco, H.; Boshell, F.; Cordonnier, J.; Rouwenhorst, K.; Lathwal, P.; Gielen, D. Ammonia Production from Clean Hydrogen and the Implications for Global Natural Gas Demand. Sustainability 2023, 15, 1623. [Google Scholar] [CrossRef]
- Hansen, J.B. High Efficient Ammonia Synthesis Systems; Haldor Topsøe A/S: Lyngby, Denmark, 2019; pp. 1–23. [Google Scholar]
- Wang, B.; Li, T.; Gong, F.; Othman, M.H.D.; Xiao, R. Ammonia as a green energy carrier: Electrochemical synthesis and direct ammonia fuel cell—A comprehensive review. Fuel Process. Technol. 2022, 235, 107380. [Google Scholar] [CrossRef]
- Arabczyk, W.; Pelka, R.; Jasińska, I.; Lendzion-Bieluń, Z. Thermodynamics of Iron Ammonia Synthesis Catalyst Sintering. Crystals 2024, 14, 188. [Google Scholar] [CrossRef]
- Stull, D.R.; Westrum, E.F.; Sinke, G.C. The Chemical Thermodynamics of Organic Compounds; John Wiley and Sons: Hoboken, NJ, USA, 1969; pp. 1–865. [Google Scholar]
- Cuevas-Castillo, G.A.; Michailos, S.; Hughes, K.; Ingham, D.; Pourkashanian, M. A comprehensive process modelling, techno-economic and life cycle assessment of a power to ammonia process. Sustain. Energy Technol. Assess 2025, 76, 104278. [Google Scholar] [CrossRef]
- Bian, X.; Zhao, Y.; Waterhouse, G.I.N.; Miao, Y.; Zhou, C.; Wu, L.Z.; Zhang, T. Quantifying the Contribution of Hot Electrons in Photothermal Catalysis: A Case Study of Ammonia Synthesis over Carbon-supported Ru Catalyst. Angew. Chem. Int. Ed. 2023, 62, e202304452. [Google Scholar] [CrossRef]
- Vogt, E.T.C.; Weckhuysen, B.M. Fluid catalytic cracking: Recent developments on the grand old lady of zeolite catalysis. Chem. Soc. Rev. 2015, 44, 7342–7370. [Google Scholar] [CrossRef] [PubMed]
- Tasneem, N.; Ahmed, S.; Hossain, M.M. Enhancement of Olefins from Fluid Catalytic Cracking Processes: A Review on Effects of Catalysts and Kinetics. Arab. J. Sci. Eng. 2025, 50, 3649–3670. [Google Scholar] [CrossRef]
- Cordero-Lanzac, T.; Bilbao, J. Deactivation kinetic models for the fluid catalytic cracking (FCC). A review. Chem. Eng. J. 2025, 514, 162856. [Google Scholar] [CrossRef]
- Ferreira, J.M.M.; Sousa-Aguiar, E.F.; Aranda, D.A.G. FCC Catalyst Accessibility—A Review. Catalysts 2023, 13, 784. [Google Scholar] [CrossRef]
- Fals, J.; Garcia-Valencia, J.F.; Puello-Polo, E.; Tuler, F.; Márquez, E. Hierarchical Y Zeolite-Based Catalysts for VGO Cracking: Impact of Carbonaceous Species on Catalyst Acidity and Specific Surface Area. Molecules 2024, 29, 3085. [Google Scholar] [CrossRef]
- Tedeeva, M.A.; Kustov, A.L.; Batkin, A.M.; Garifullina, C.; Zalyatdinov, A.A.; Yang, D.; Dai, Y.; Yang, Y.; Kustov, L.M. Catalytic systems for hydrogenation of CO2 to methanol. Mol. Cat. 2024, 566, 114403. [Google Scholar] [CrossRef]
- Huang, F.; Wang, S.; Zhang, L. Design and recent advances of novel MoS2-based catalysts for methanol from carbon dioxide hydrogenation. Sep. Purif. Technol. 2025, 365, 132681. [Google Scholar] [CrossRef]
- Bozzano, G.; Manenti, F. Efficient methanol synthesis: Perspectives, technologies and optimization strategies. Prog. Energy Combust. Sci. 2016, 56, 71–105. [Google Scholar] [CrossRef]
- Sheldon, D. Methanol production—A technical history. Johns. Matthey Technol. Rev. 2017, 61, 172–182. [Google Scholar] [CrossRef]
- Atsbha, T.A.; Yoon, T.; Seongho, P.; Lee, C.J. A review on the catalytic conversion of CO2 using H2 for synthesis of CO, methanol, and hydrocarbons. J. CO2 Util. 2021, 44, 101413. [Google Scholar] [CrossRef]
- Tezel, E.; Sannes, D.K.; Svelle, S.; Szilágyi, P.I.; Olsbye, U. Direct CO2 to methanol reduction on Zr6-MOF based composite catalysts: A critical review. Mater. Adv. 2023, 4, 5479–5495. [Google Scholar] [CrossRef]
- Sahoo, K.K.; Katari, J.K.; Das, D. Recent advances in methanol production from methanotrophs. World J. Microbiol. Biotechnol. 2023, 39, 360. [Google Scholar] [CrossRef]
- Qu, T.; Wei, S.; Xiong, Z.; Zhang, J.; Zhao, Y. Progress and prospect of CO2 photocatalytic reduction to methanol. Fuel Process. Technol. 2023, 251, 107933. [Google Scholar] [CrossRef]
- Wawrzyniak, A.; Koper, M.T.M. Electrocatalytic CO2 Reduction to Methanol on Pt(111) Modified with a Pd Monolayer. ACS Catal. 2025, 15, 1514–1521. [Google Scholar] [CrossRef]
- Gielen, D.; Dolan, G. Innovation Outlook: Renewable Methanol; International Renewable Energy Agency: Abu Dhabi, United Arab Emirates, 2021; pp. 1–124. [Google Scholar]
- Khalid, M.; Dharaskar, S.A.; Sillanpää, M.; Siddiqui, H. Emerging Carbon Capture Technologies: Towards a Sustainable Future; Elsevier: Amsterdam, The Netherlands, 2022; pp. 1–479. [Google Scholar]
- Yee, K.F.; Mohamed, A.R.; Tan, S.H. A review on the evolution of ethyl tert-butyl ether (ETBE) and its future prospects. Renew. Sustain. Energy Rev. 2013, 22, 604–620. [Google Scholar] [CrossRef]
- Mahdi, H.I.; Muraza, O. Conversion of Isobutylene to Octane-Booster Compounds after Methyl tert-Butyl Ether Phaseout: The Role of Heterogeneous Catalysis. Ind. Eng. Chem. Res. 2016, 55, 11193–11210. [Google Scholar] [CrossRef]
- Králik, M.; Turáková, M.; Mačák, I.; Lehocký, P. Aniline-catalysis and chemical engineering. In Proceedings of the 39th International Conference of Slovak Society of Chemical Engineering, Tatranské Matliare, Slovakia, 21–25 May 2012; ICEE, STU in Bratislava: Tatranské Matliare, Slovakia, 2014; Volume 1, pp. 723–733. [Google Scholar]
- Graham, D.P.; Spiegler, L. Hydrogenation of Nitro Compounds to Amines and Catalyst Therefore. U.S. Patent US2823235A, 11 February 1958. [Google Scholar]
- Gelder, E.A.; Jackson, S.D.; Lok, C.M. The hydrogenation of nitrobenzene to aniline: A new mechanism. Chem. Commun. 2005, 4, 522–524. [Google Scholar] [CrossRef] [PubMed]
- Turáková, M.; Králik, M.; Lehocký, P.; Pikna, Ľ.; Smrčová, M.; Remeteiová, D.; Hudák, A. Influence of preparation method and palladium content on Pd/C catalysts activity in the liquid phase hydrogenation of nitrobenzene to aniline. Appl. Catal. A Gen. 2014, 476, 103–112. [Google Scholar] [CrossRef]
- Turáková, M.; Salmi, T.; Eränen, K.; Wärnå, J.; Murzin, D.Y.; Králik, M. Liquid phase hydrogenation of nitrobenzene. Appl. Catal. A Gen. 2015, 499, 66–76. [Google Scholar] [CrossRef]
- Bombay, C. Aniline Plant; Chematur Bombay: Mumbai, India, 2012; p. 13. [Google Scholar]
- Tang, W.; Liu, Y.; Jin, Y.; Wang, Y.; Shi, W.; Ma, P.; Niu, J.; Wang, J. Photocatalytic Reduction of Nitrobenzene to Aniline by an Intriguing {Ru(C6H6)}-Based Heteropolytungstate. Inorg. Chem. 2024, 63, 6260–6267. [Google Scholar] [CrossRef]
- Imamura, K.; Ikeuchi, K.; Sakamoto, Y.; Aono, Y.; Oto, T.; Onda, A. Photocatalytic hydrogenation of nitrobenzene to aniline over titanium(IV) oxide using various saccharides instead of hydrogen gas. RSC Adv. 2021, 11, 32300–32304. [Google Scholar] [CrossRef]
- Islam, I.U.; Zhang, Y.; Dong, B.; Iqbal, A.; Abbas, S.; Zai, J.; Ahmad Shah, S.S.; Qian, X. Highly Selective Electroreduction of Nitrobenzene to Aniline by Co-Doped 1T-MoS2. ACS Appl. Mater. Interfaces 2024, 16, 25090–25100. [Google Scholar] [CrossRef]
- Driessen, R.T.; Kamphuis, P.; Mathijssen, L.; Zhang, R.; van der Ham, L.G.J.; van den Berg, H.; Zeeuw, A.J. Industrial Process Design for the Production of Aniline by Direct Amination. Chem. Eng. Technol. 2017, 40, 838–846. [Google Scholar] [CrossRef]
- Bugosen, S.; Mantilla, I.D.; Tarazona-Vasquez, F. Techno-economic analysis of aniline production via amination of phenol. Heliyon 2020, 6, e05778. [Google Scholar] [CrossRef]
- Liu, X.; Chen, W.; Zou, J.; Ye, L.; Yuan, Y. Liquid-Phase Amination of Phenol to Aniline over the Pd/MgO Catalyst without External Hydrogen Addition. ACS Sustain. Chem. Eng. 2022, 10, 6988–6998. [Google Scholar] [CrossRef]
- Gernot, J.; Magnus, J.; Moussa, A.S. Production of Aniline via Anthranilate. U.S. Patent 10173969 B2, 8 January 2019. [Google Scholar]
- Winter, B.; Meys, R.; Bardow, A. Towards aromatics from biomass: Prospective Life Cycle Assessment of bio-based aniline. J. Clean. Prod. 2021, 290, 125818. [Google Scholar] [CrossRef]
- World’s First Pilot Plant for Bio-Based Aniline. Available online: https://www.covestro.com/press/worlds-first-pilot-plant-for-bio-based-aniline/ (accessed on 15 June 2025).
- Hisatomi, T.; Yamada, T.; Nishiyama, H.; Takata, T.; Domen, K. Materials and systems for large-scale photocatalytic water splitting. Nat. Rev. Mater. 2025, 14. [Google Scholar] [CrossRef]
- Sheldon, R.A. Green chemistry, catalysis and valorization of waste biomass. J. Mol. Catal. A Chem. 2016, 422, 3–12. [Google Scholar] [CrossRef]
- Wink, K.; Hartmann, I. Recent Progress in Turning Waste into Catalysts for Green Syntheses. Sustain. Chem. 2024, 5, 27–39. [Google Scholar] [CrossRef]
- Iaquaniello, G.; Centi, G.; Salladini, A.; Palo, E.; Perathoner, S. Waste to Chemicals for a Circular Economy. Chem. Eur. J. 2018, 24, 11831–11839. [Google Scholar] [CrossRef]
- Bushra, R.; Khayal, A.; Ahmad, M.; Song, J.; Jin, Y.; Xiao, H. Catalytic conversion of polymer waste into high-value products for advancing circular economy and eco-sustainability. J. Anal. Appl. Pyrolysis 2025, 189, 107052. [Google Scholar] [CrossRef]
- Khopade, K.V.; Chikkali, S.H.; Barsu, N. Metal-catalyzed plastic depolymerization. Cell Rep. Phys. Sci. 2023, 4, 43101341. [Google Scholar] [CrossRef]
- Schröter, S.; Rothgänger, T.; Heymel, D.; Seitz, M. Concept of Catalytic Depolymerization of Polyolefinic Plastic Waste to High Value Chemicals. Chem. Ing. Tech. 2023, 95, 1297–1304. [Google Scholar] [CrossRef]
- Koch, W. Pathway Design for Industrial Fermentation; Wiley: Hoboken, NJ, USA, 2024; pp. 1–496. [Google Scholar]
- Török, B.; Schäfer, C.; Kokel, A. Heterogeneous Catalysis in Sustainable Synthesis; Elsevier: Amsterdam, The Netherlands, 2021; pp. 1–664. [Google Scholar]
- McCusker, L.B.; Olson, D.H.; Baerlocher, C. Atlas of Zeolite Framework Types; Elsevier: Amsterdam, The Netherlands, 2007; pp. 1–398. [Google Scholar]
- Lai, N.S.; Tew, Y.S.; Zhong, X.; Yin, J.; Li, J.; Yan, B.; Wang, X. Artificial Intelligence (AI) Workflow for Catalyst Design and Optimization. Ind. Eng. Chem. Res. 2023, 62, 17835–17848. [Google Scholar] [CrossRef]
- McCullough, K.E.; King, D.S.; Chheda, S.P.; Ferrandon, M.S.; Goetjen, T.A.; Syed, Z.H.; Graham, T.R.; Washton, N.M.; Farha, O.K.; Gagliardi, L.; et al. High-Throughput Experimentation, Theoretical Modeling, and Human Intuition: Lessons Learned in Metal-Organic-Framework-Supported Catalyst Design. ACS Cent. Sci. 2022, 9, 266–276. [Google Scholar] [CrossRef]
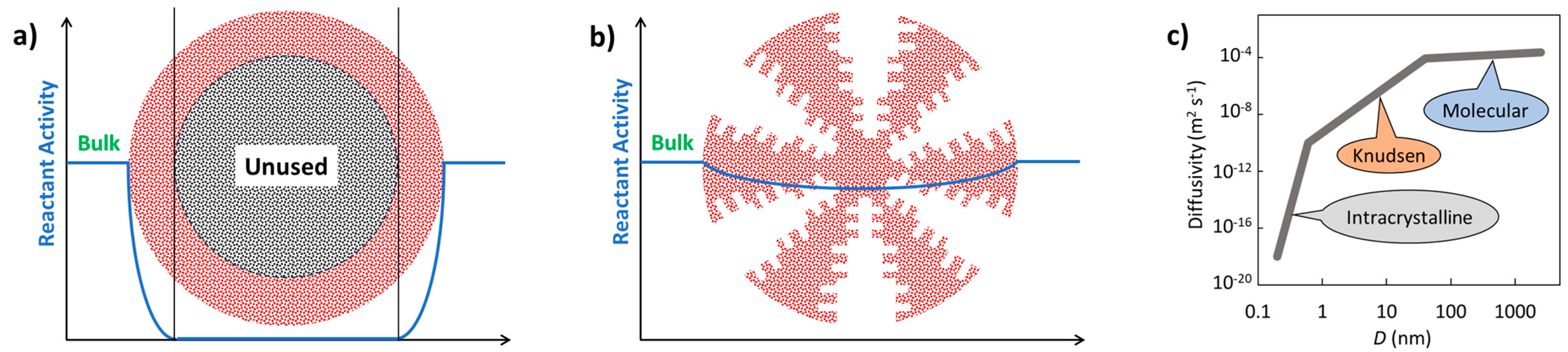


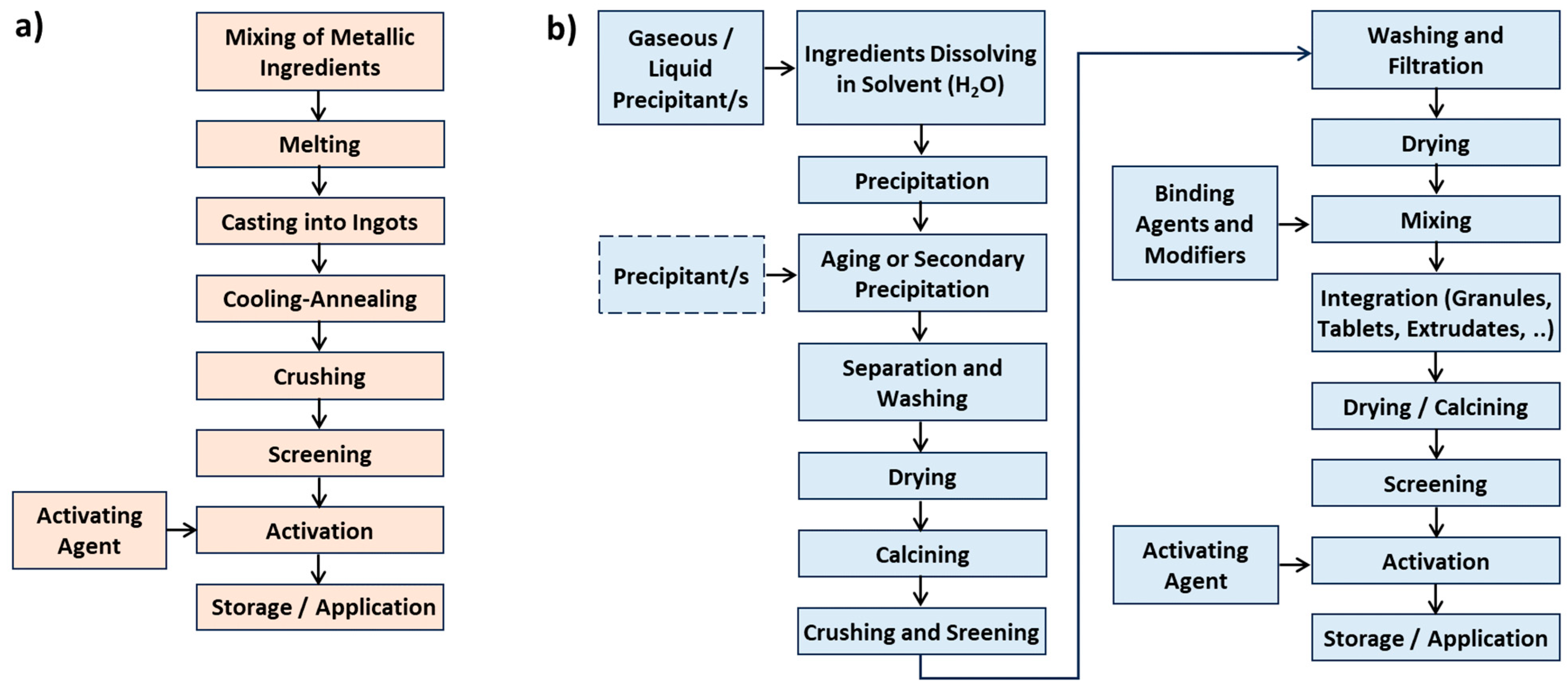
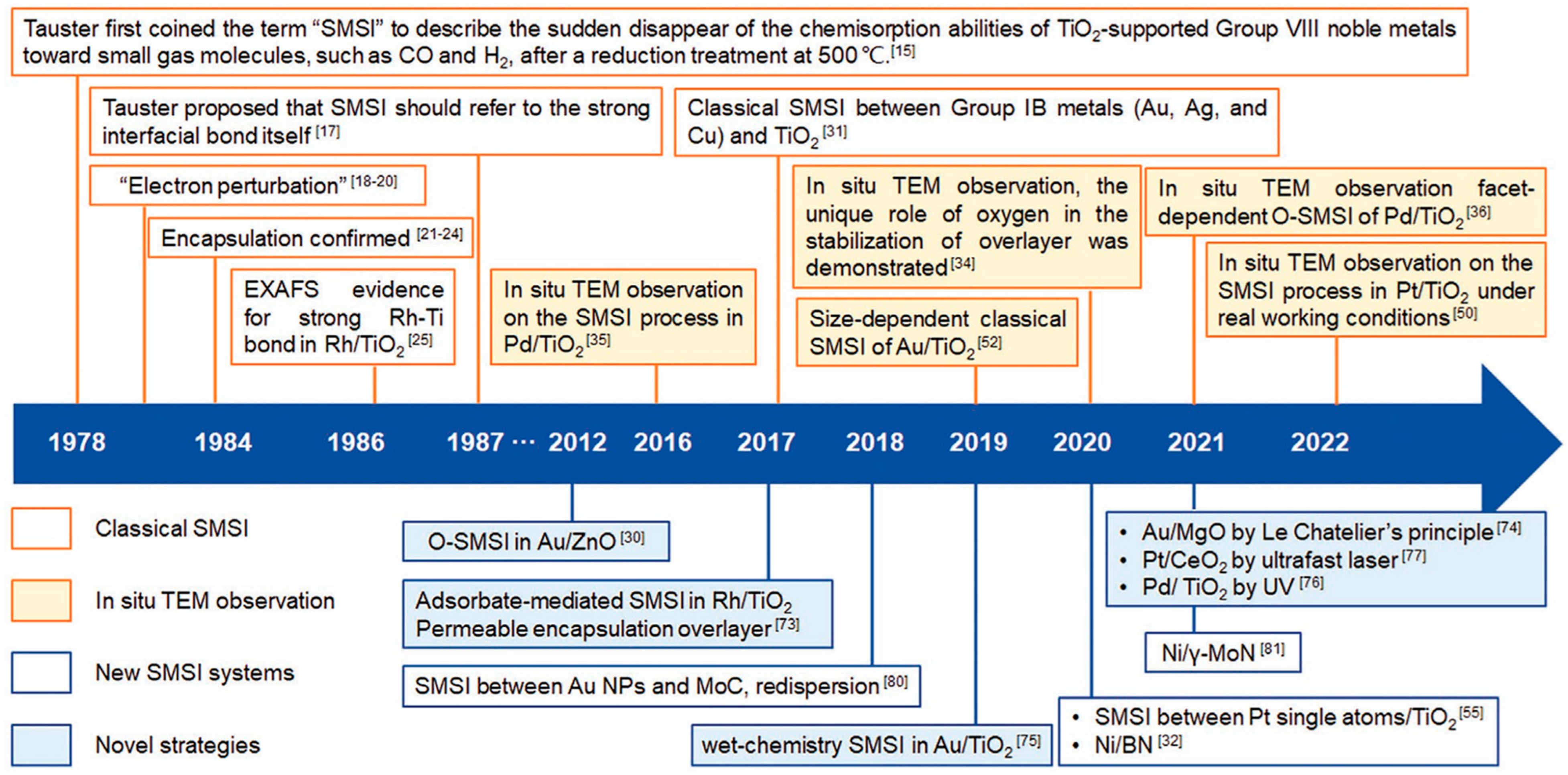
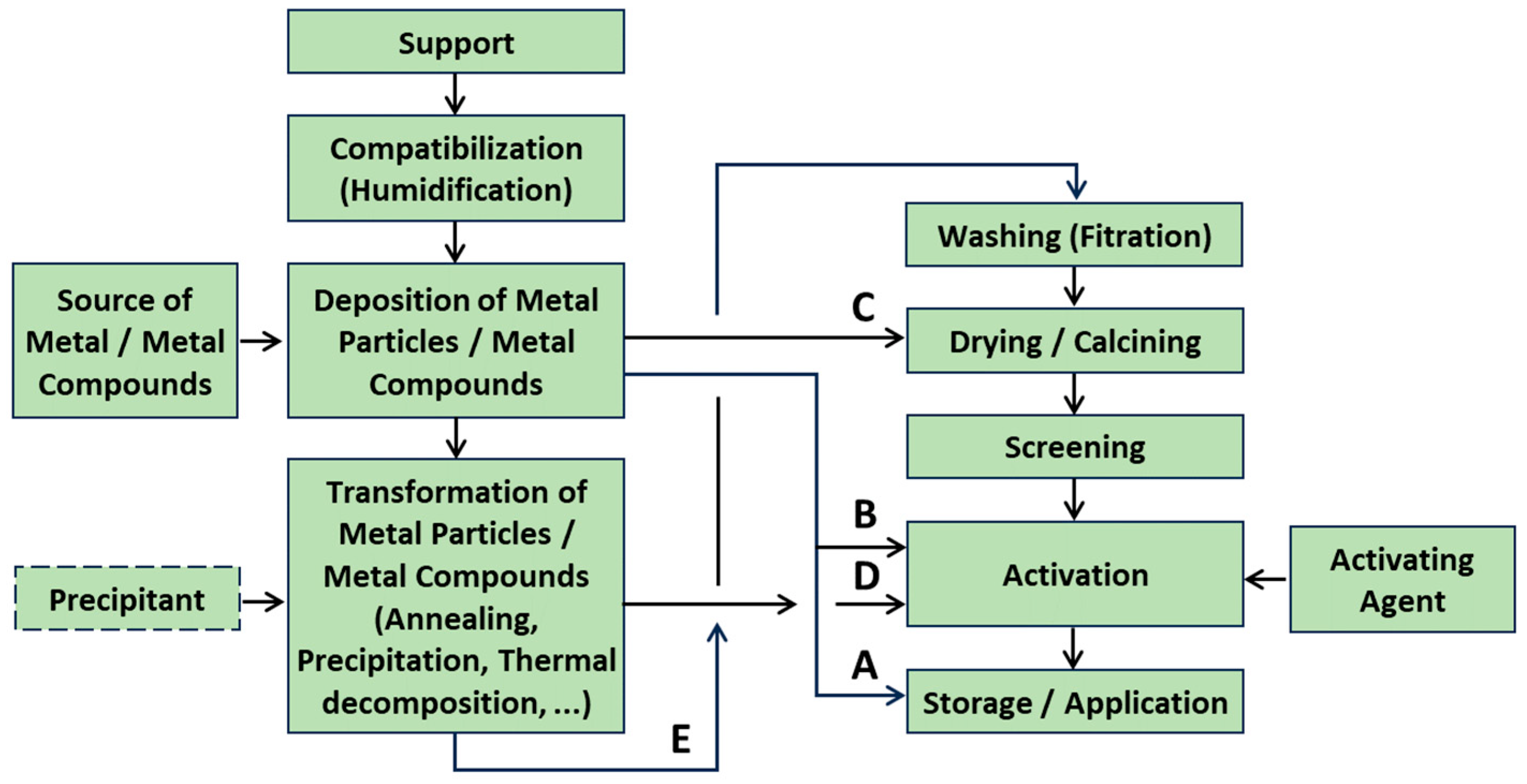
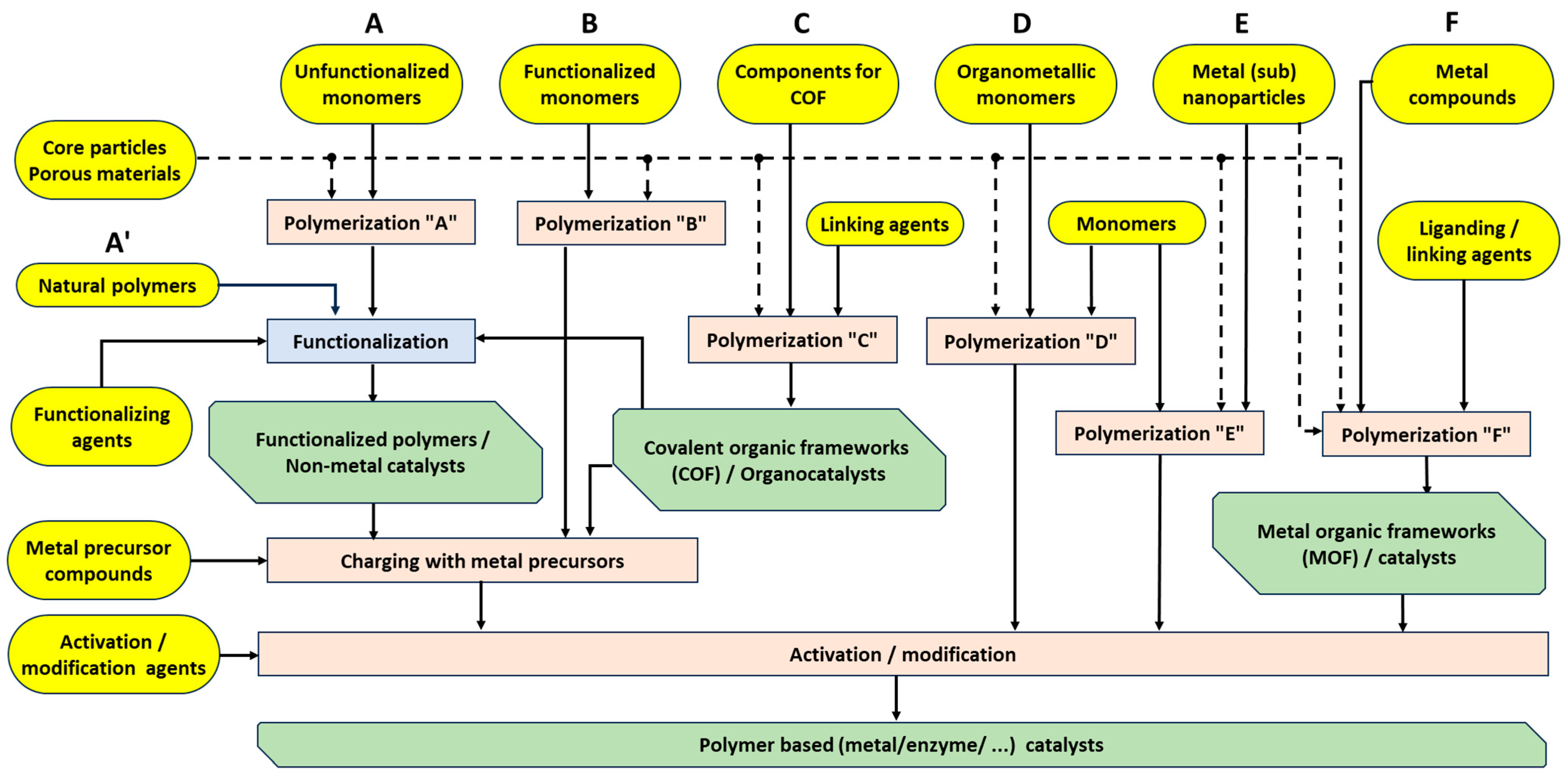
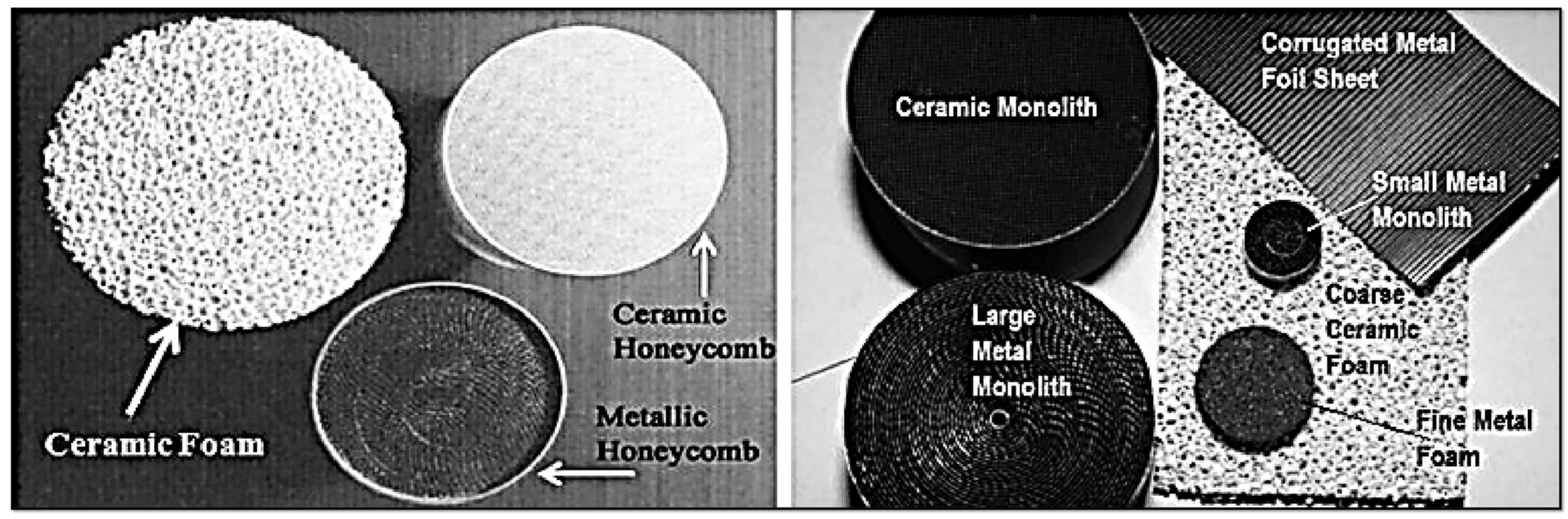

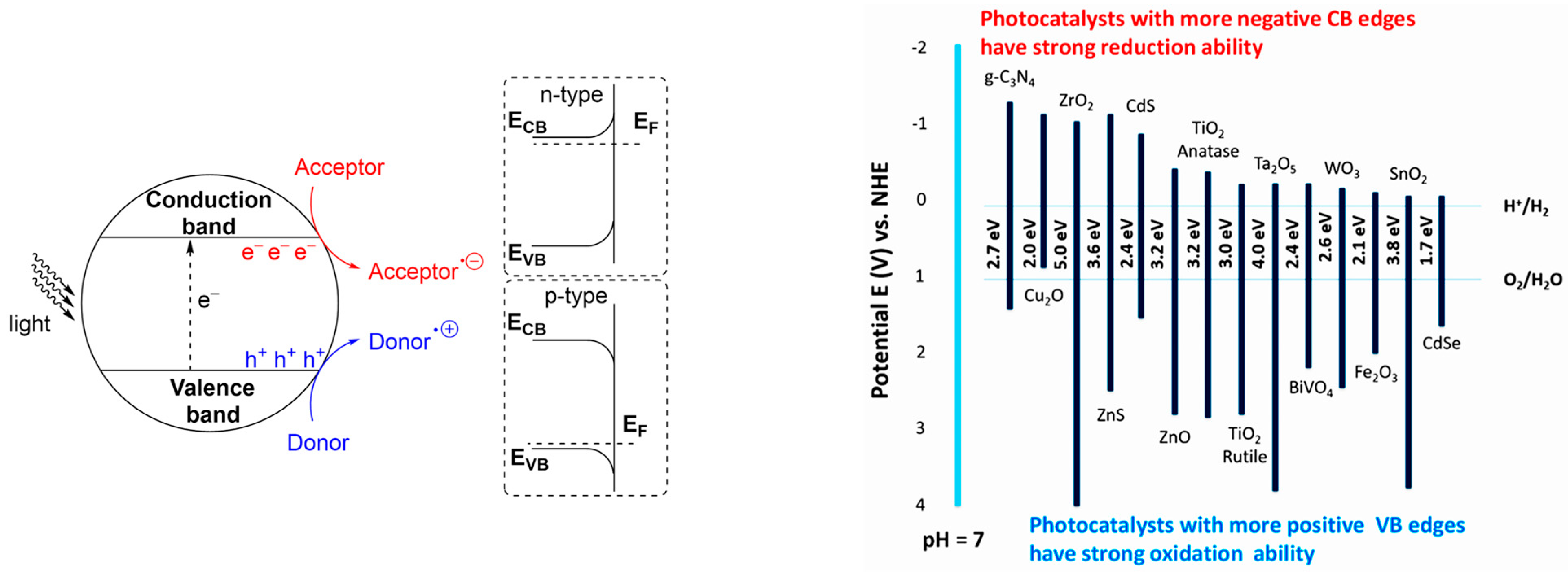
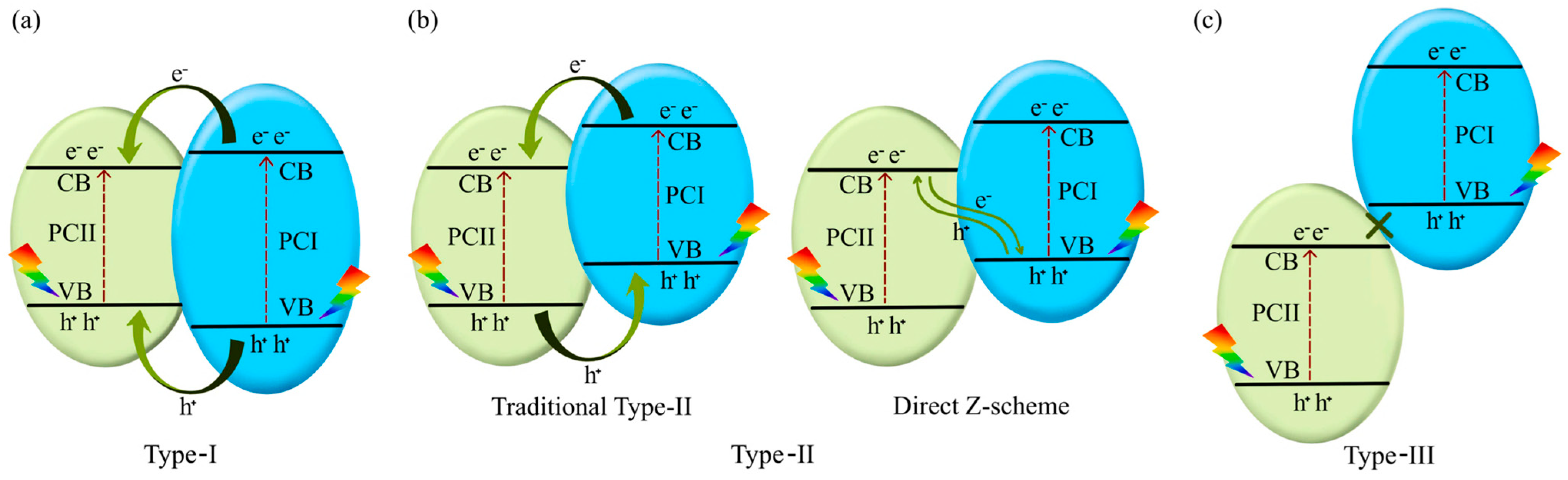
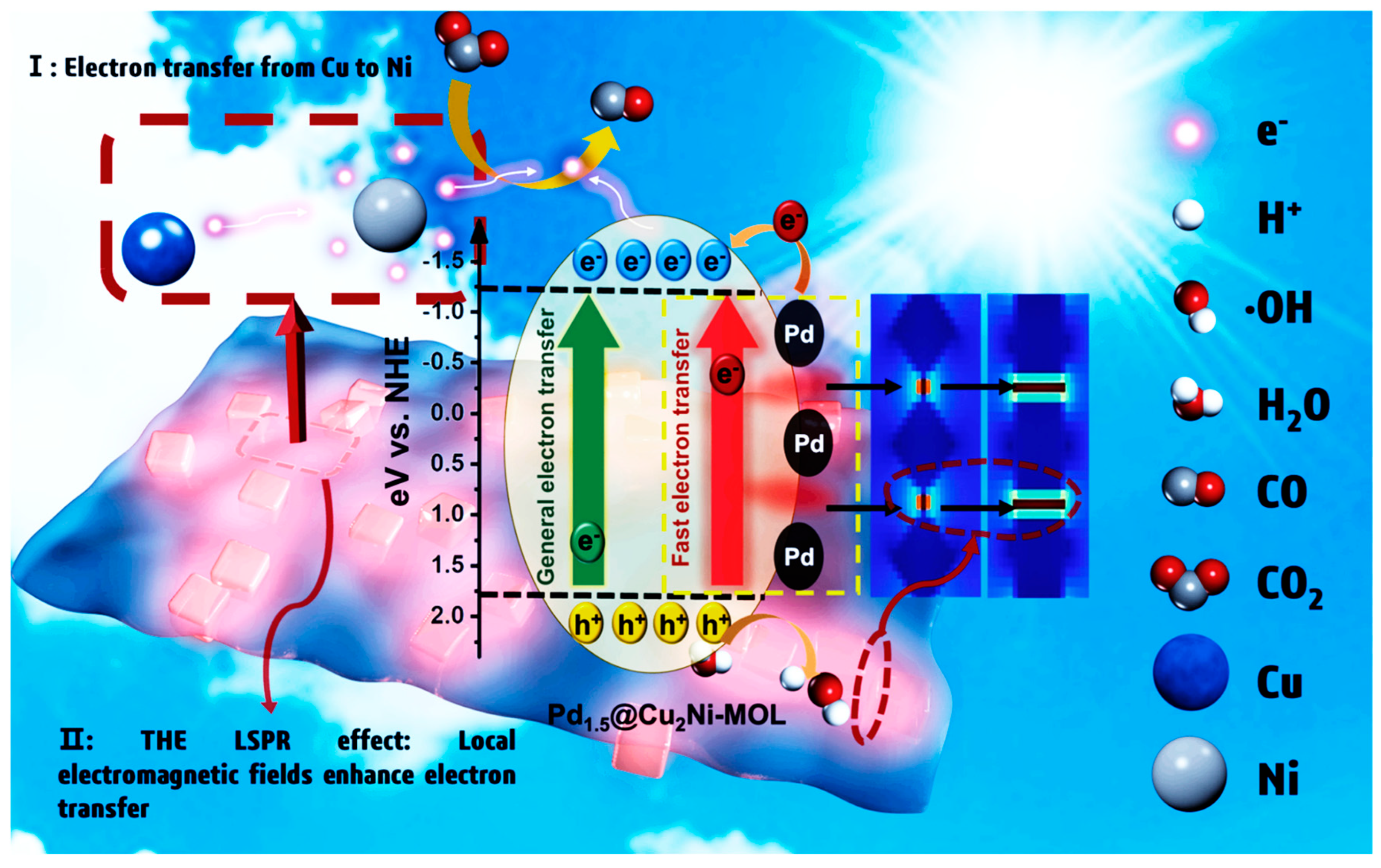
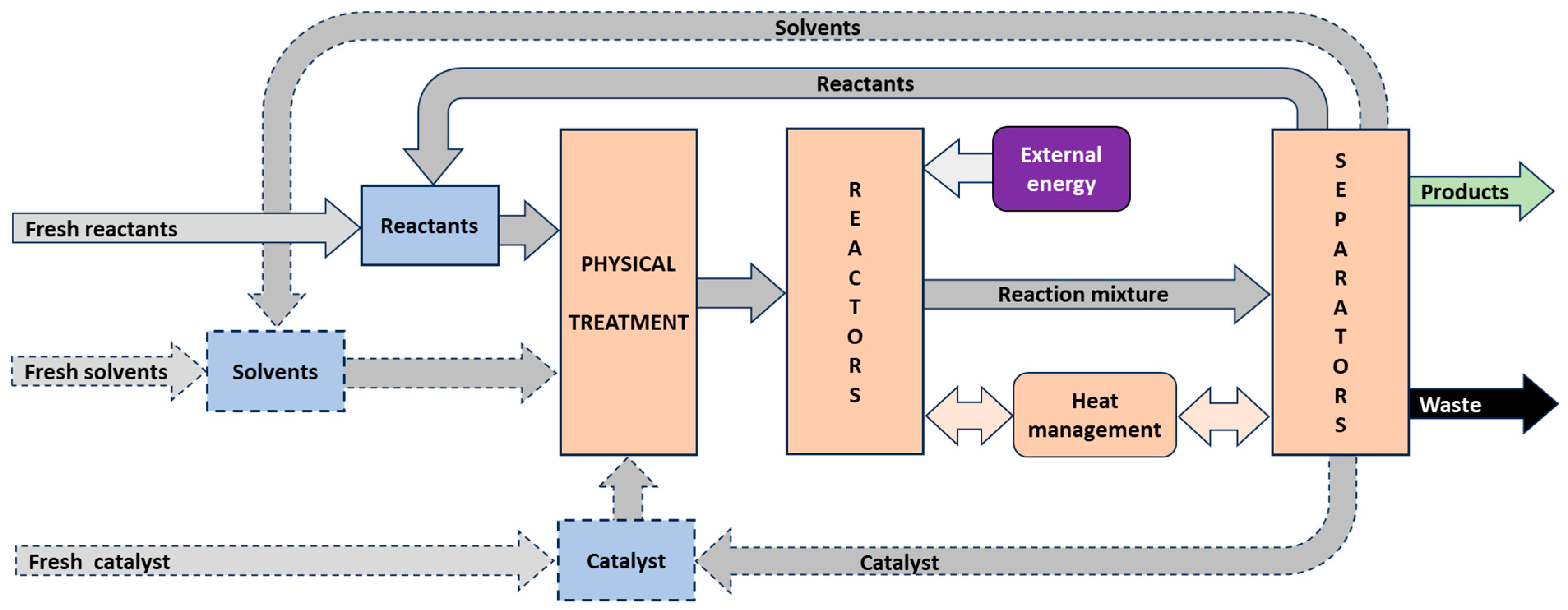

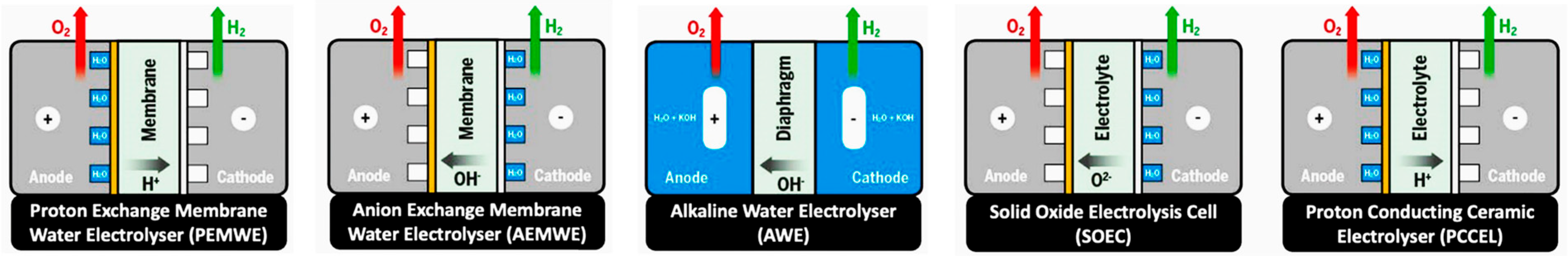
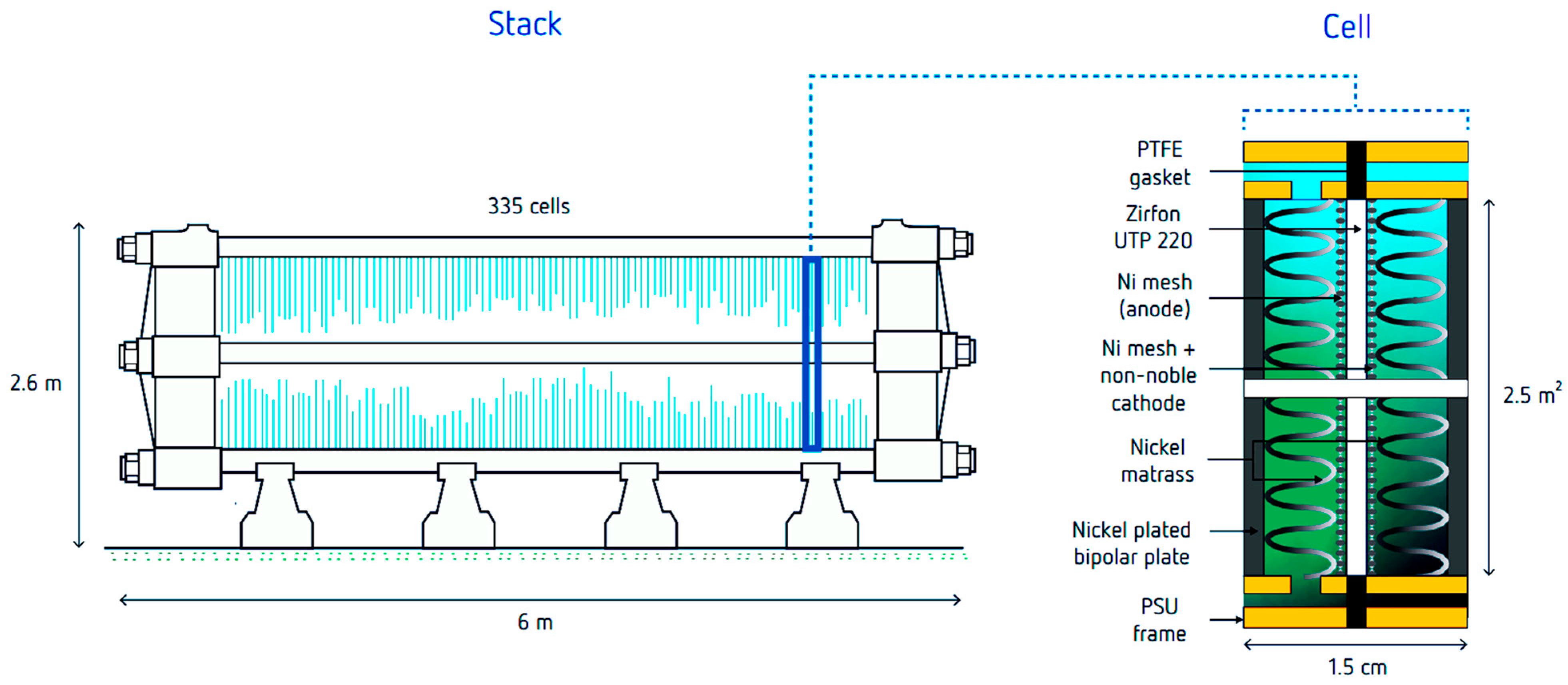
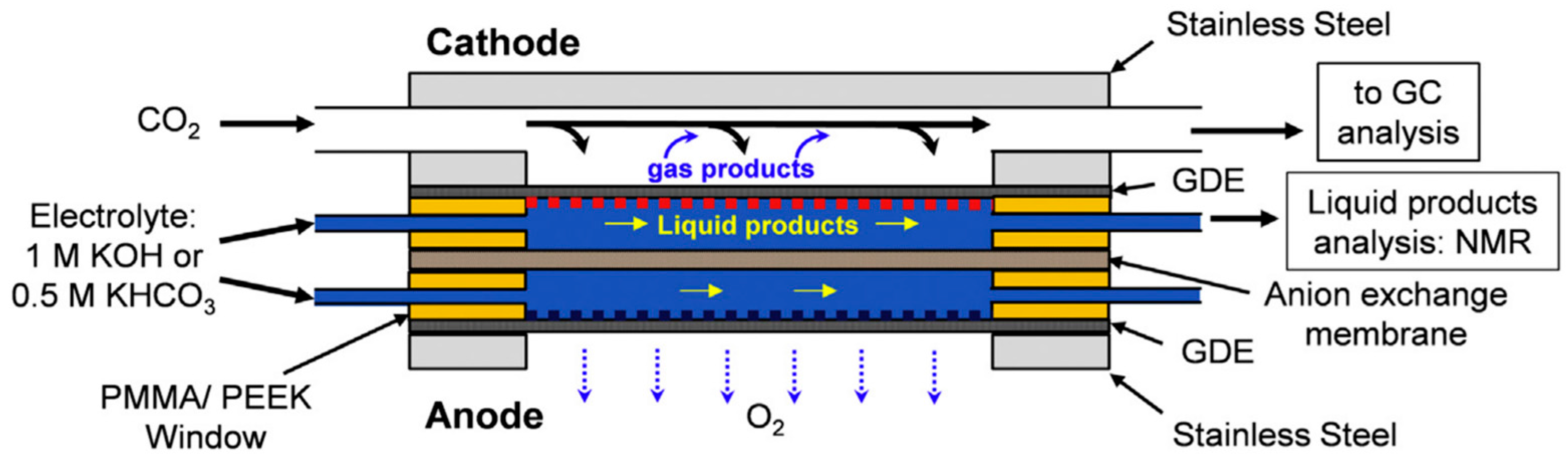
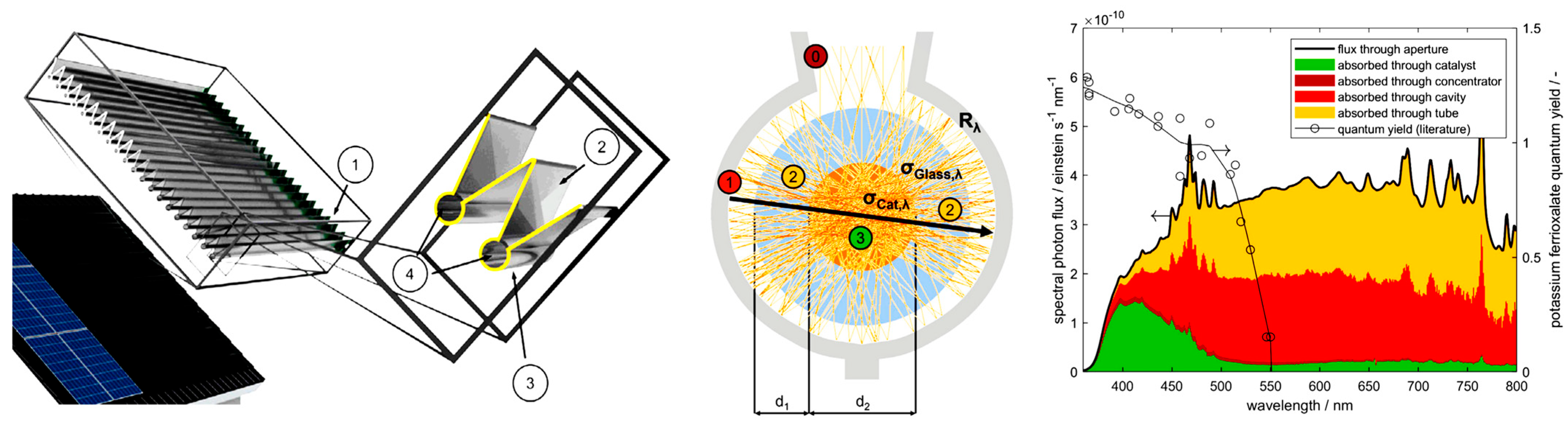
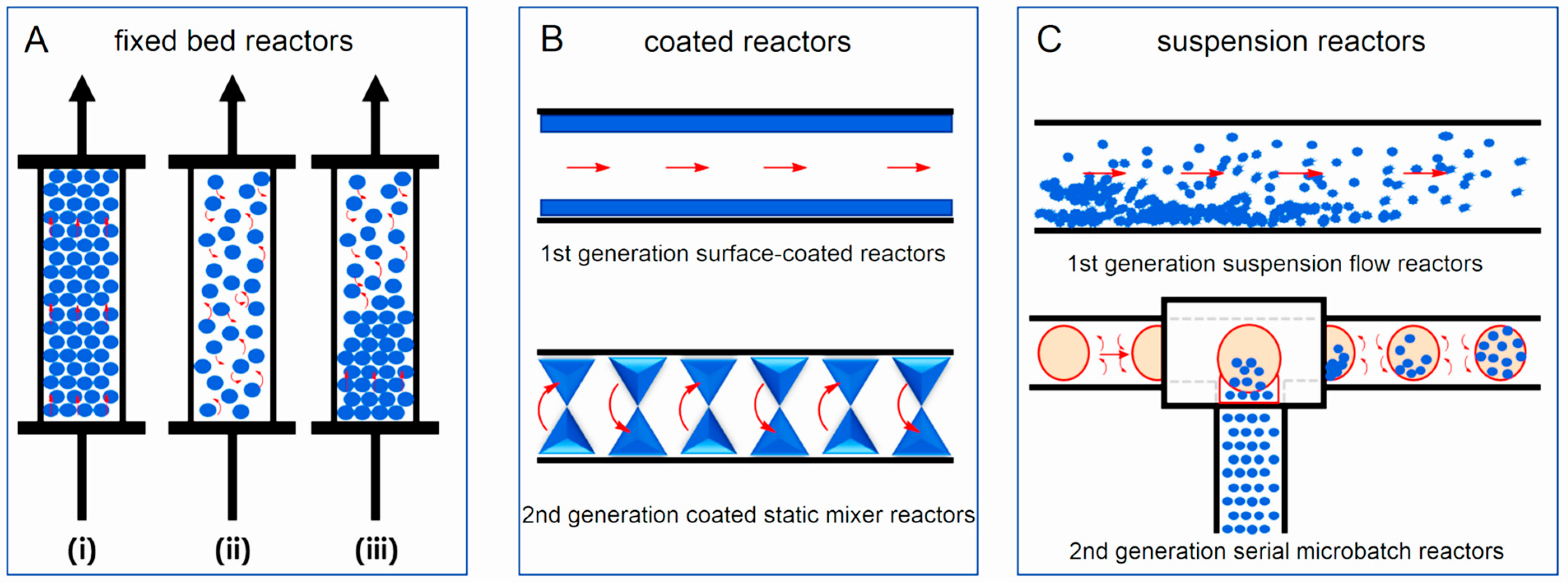
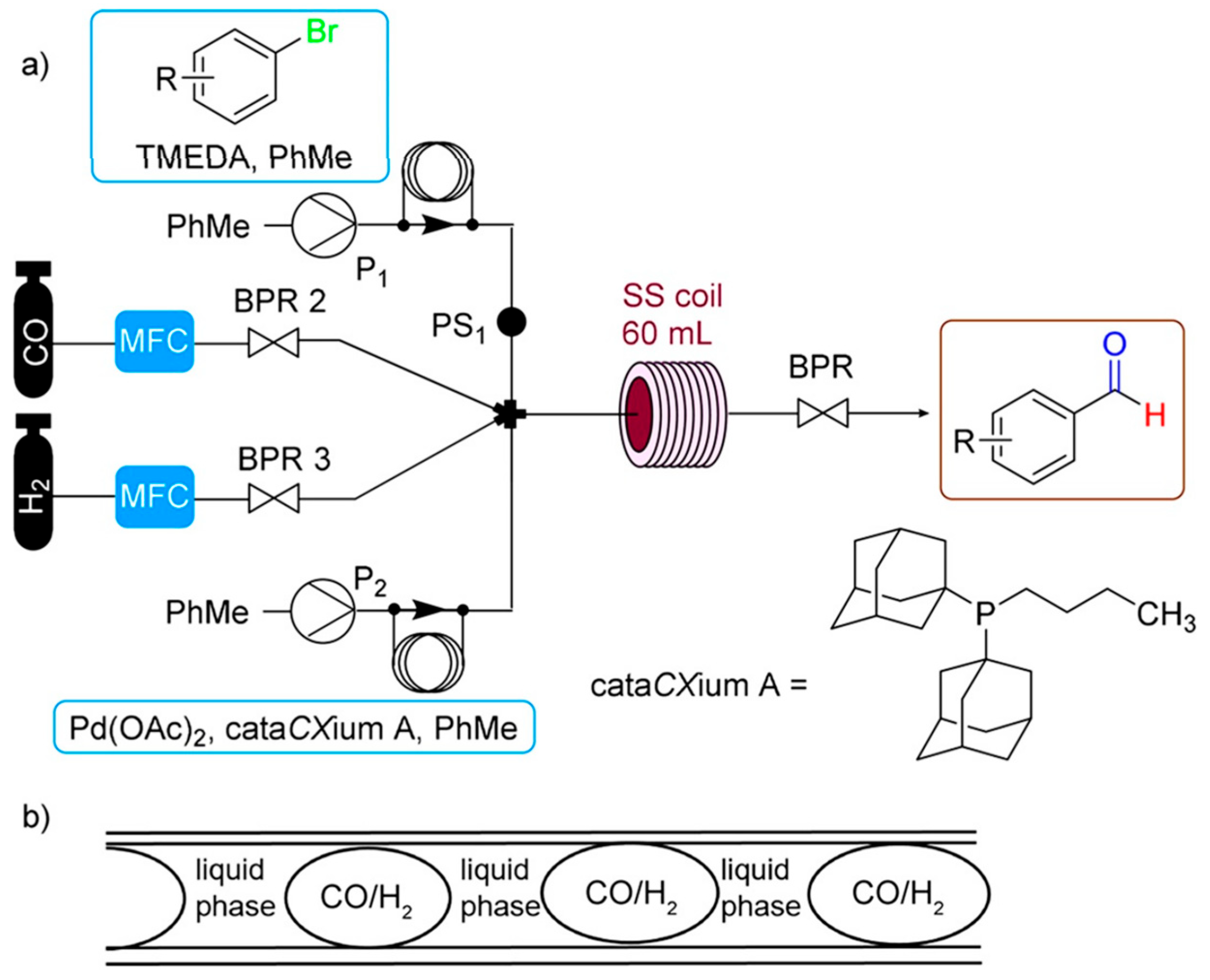
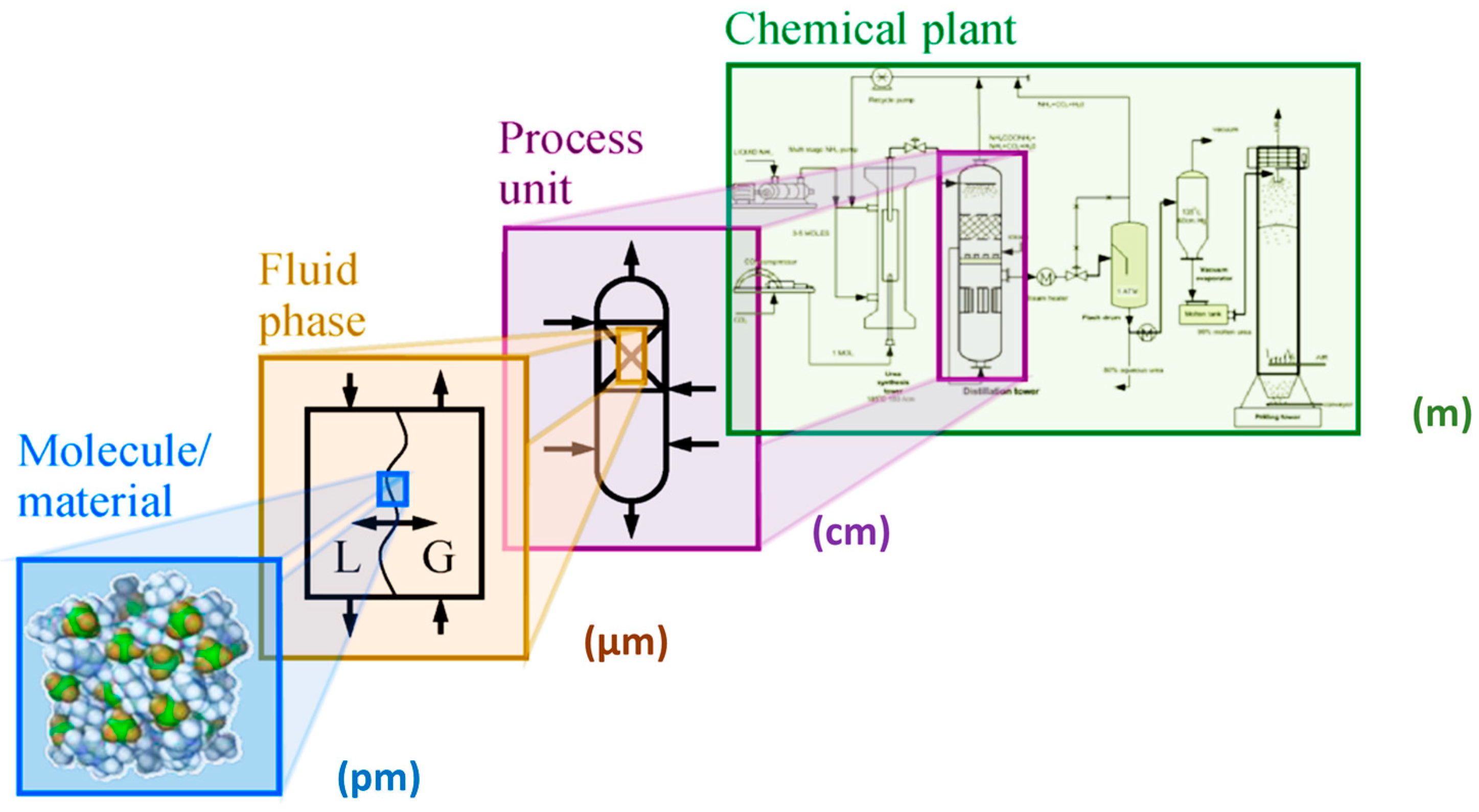
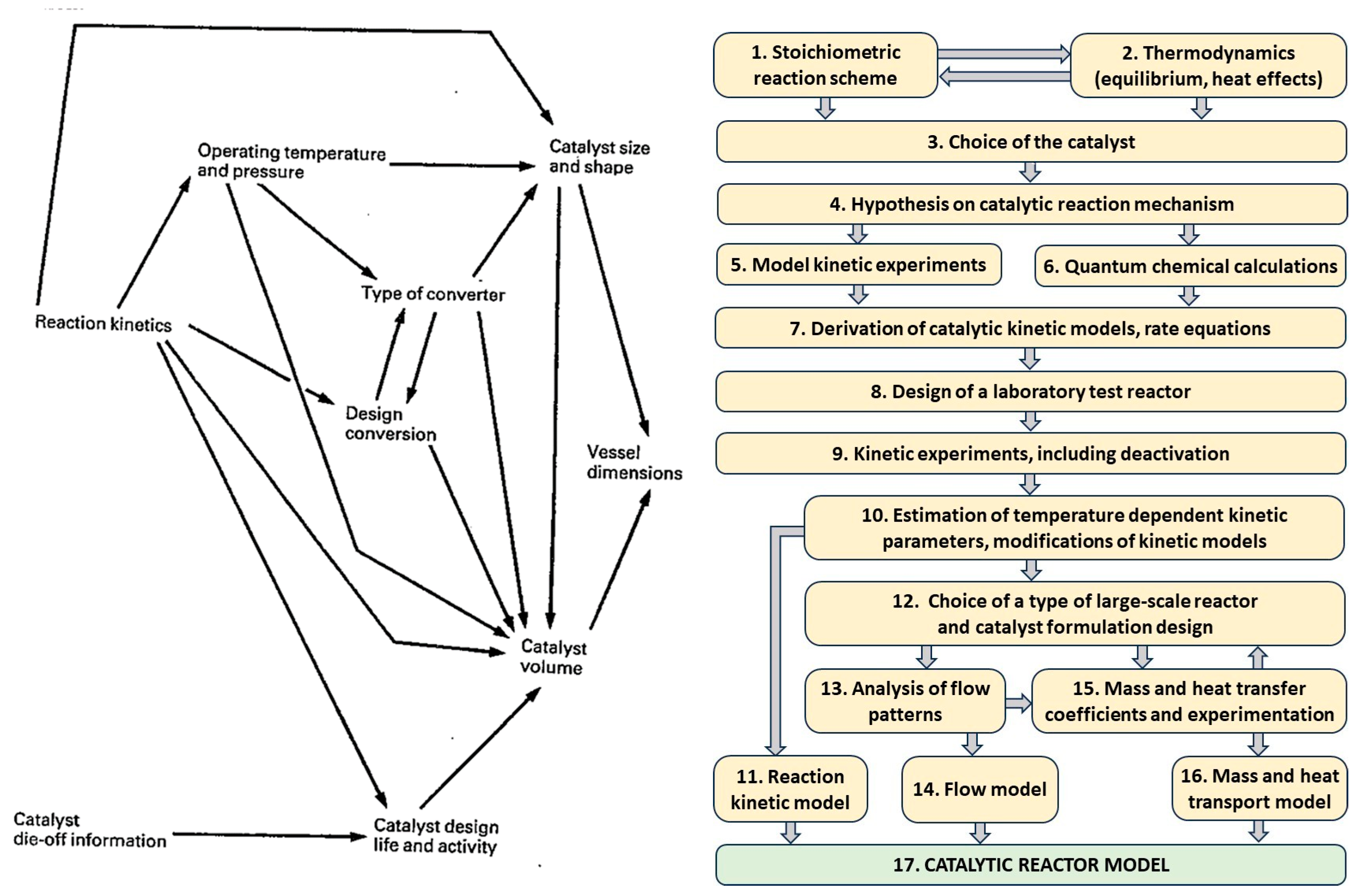
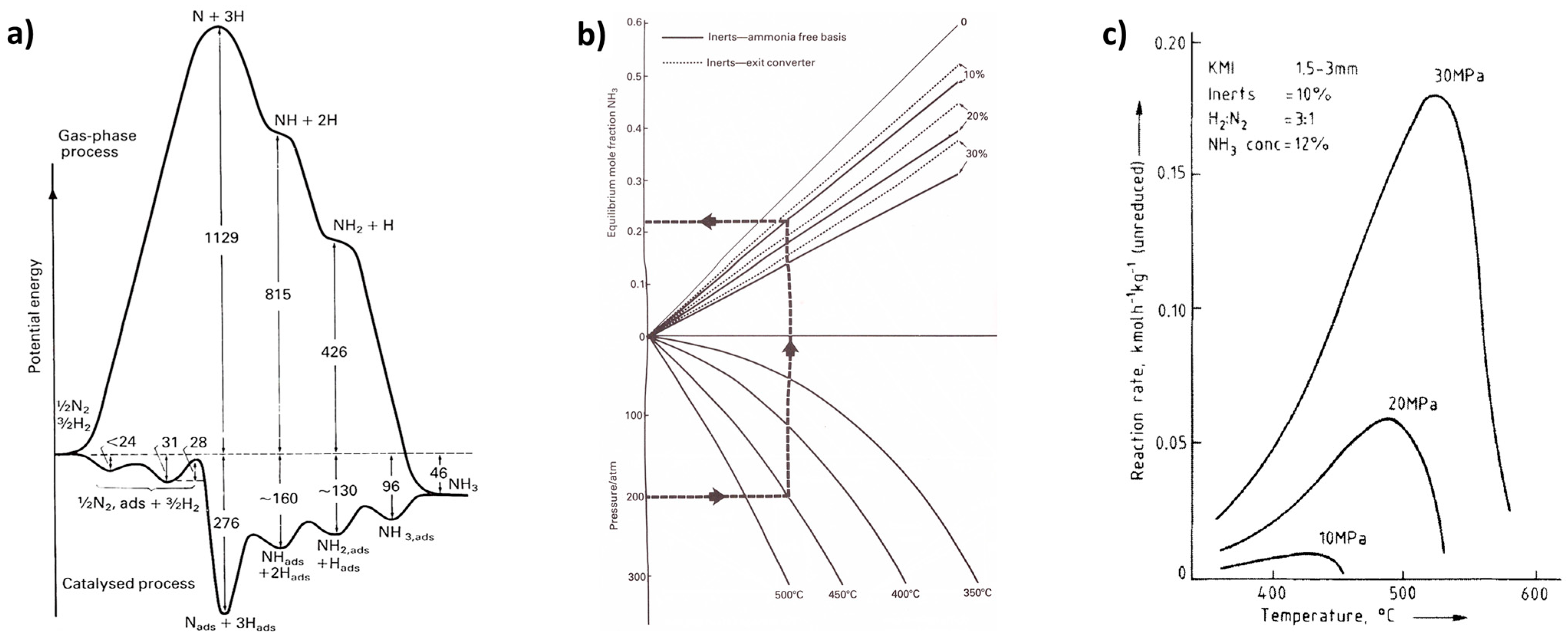
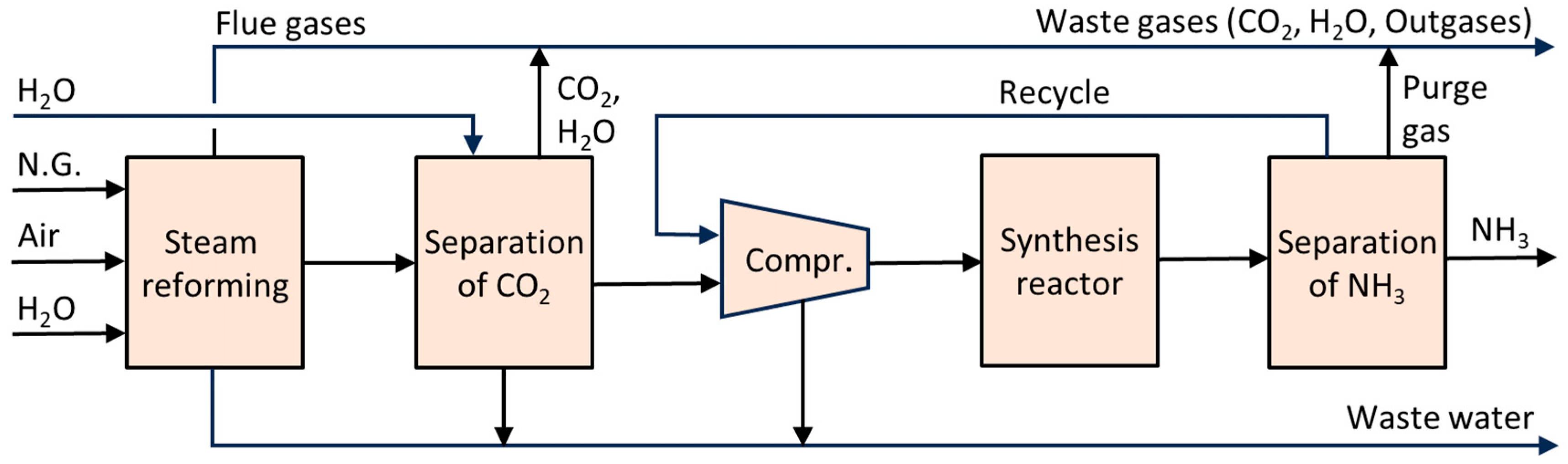
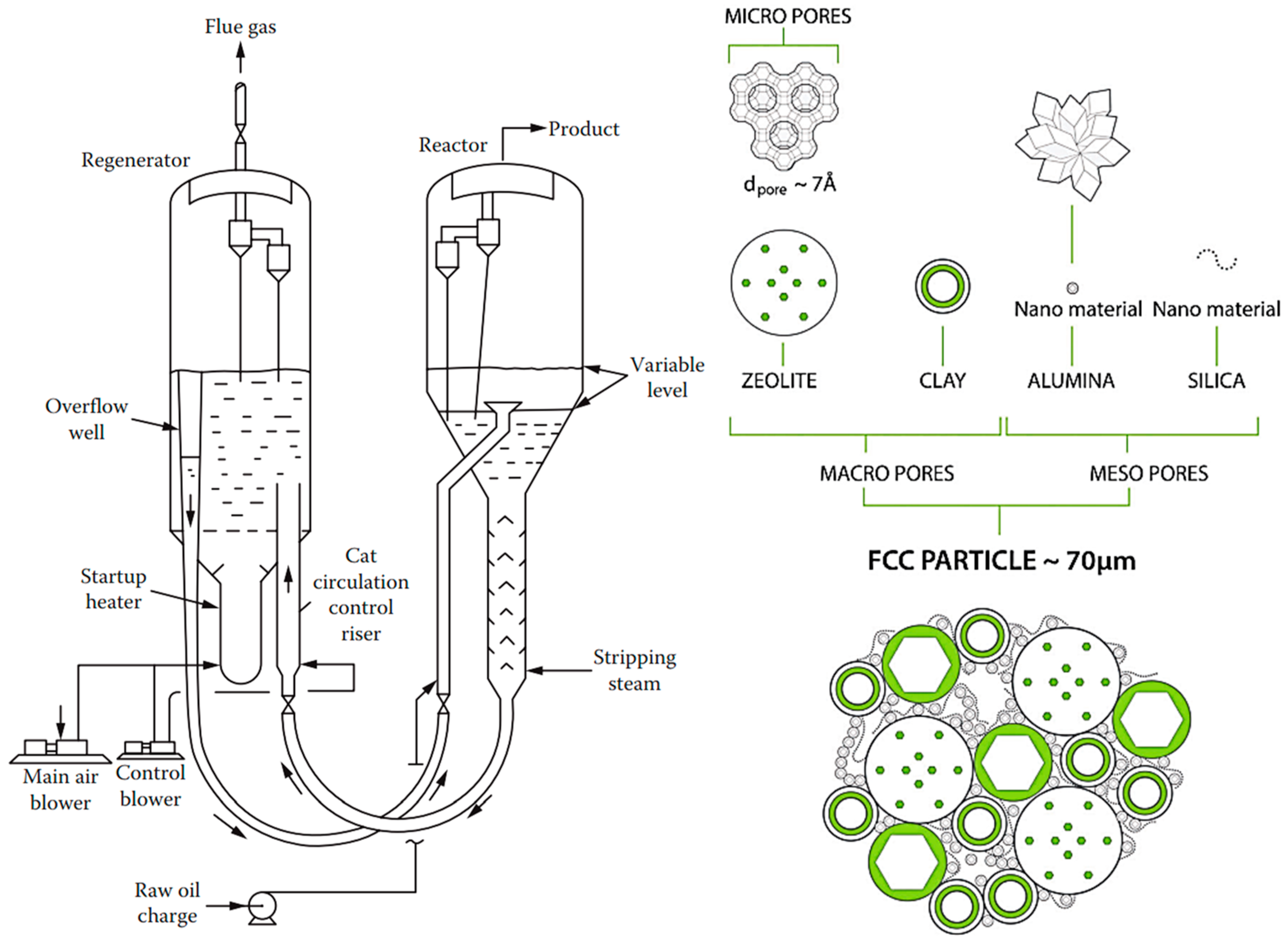
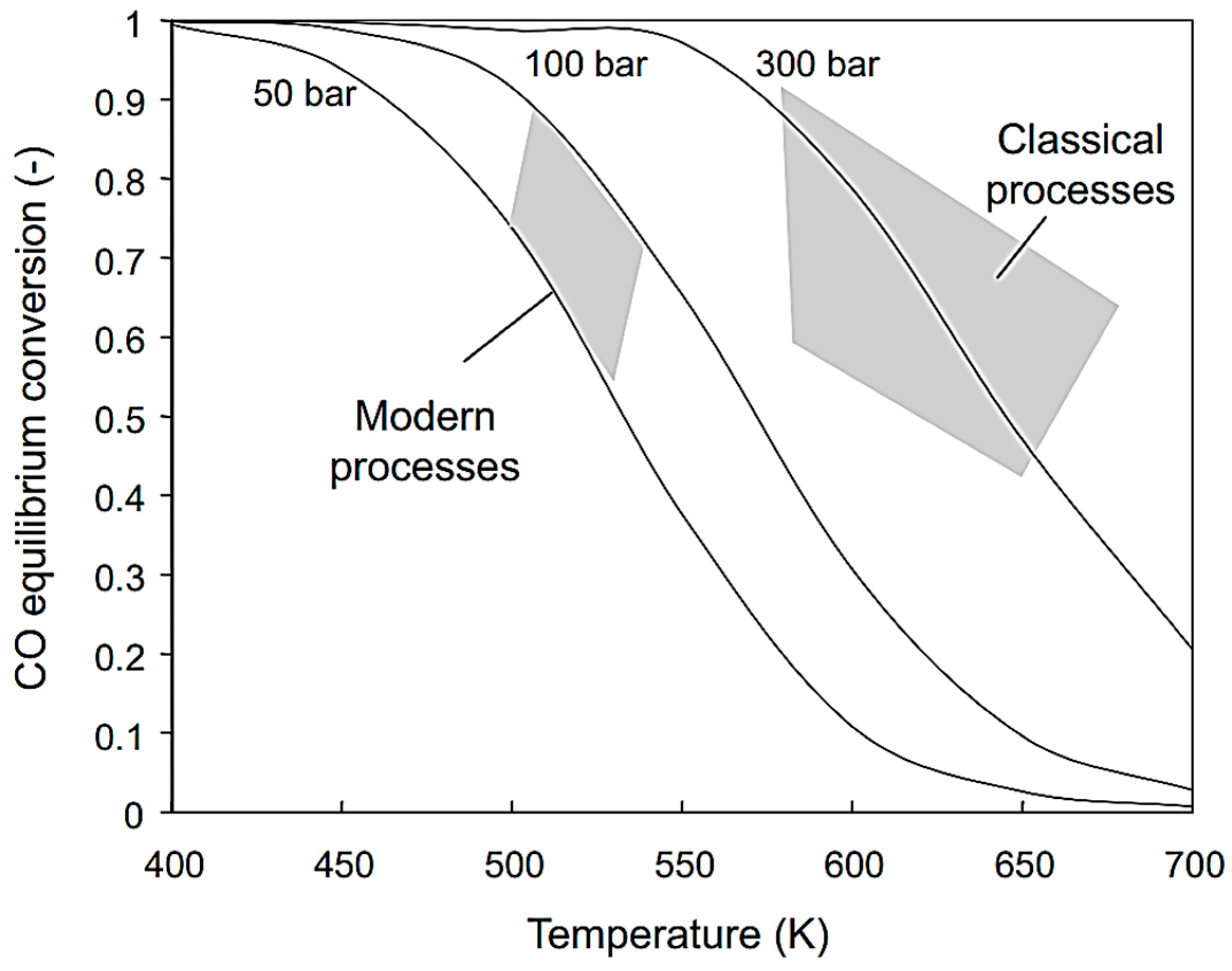
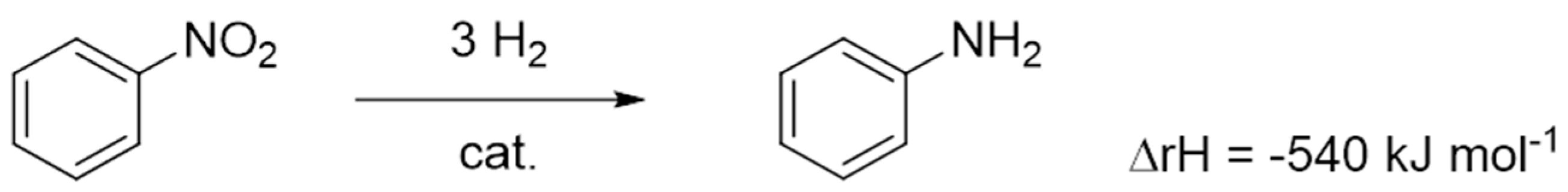
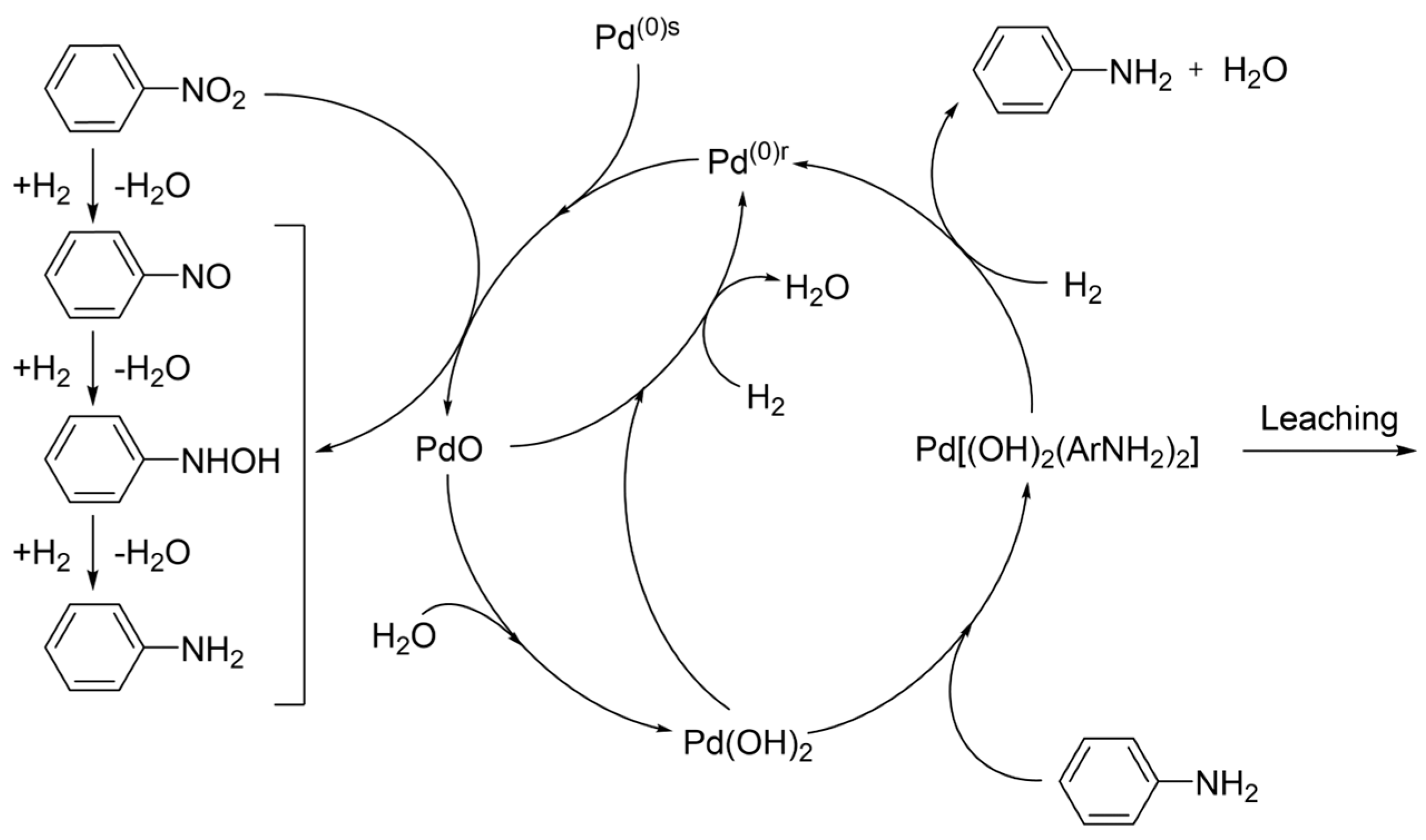
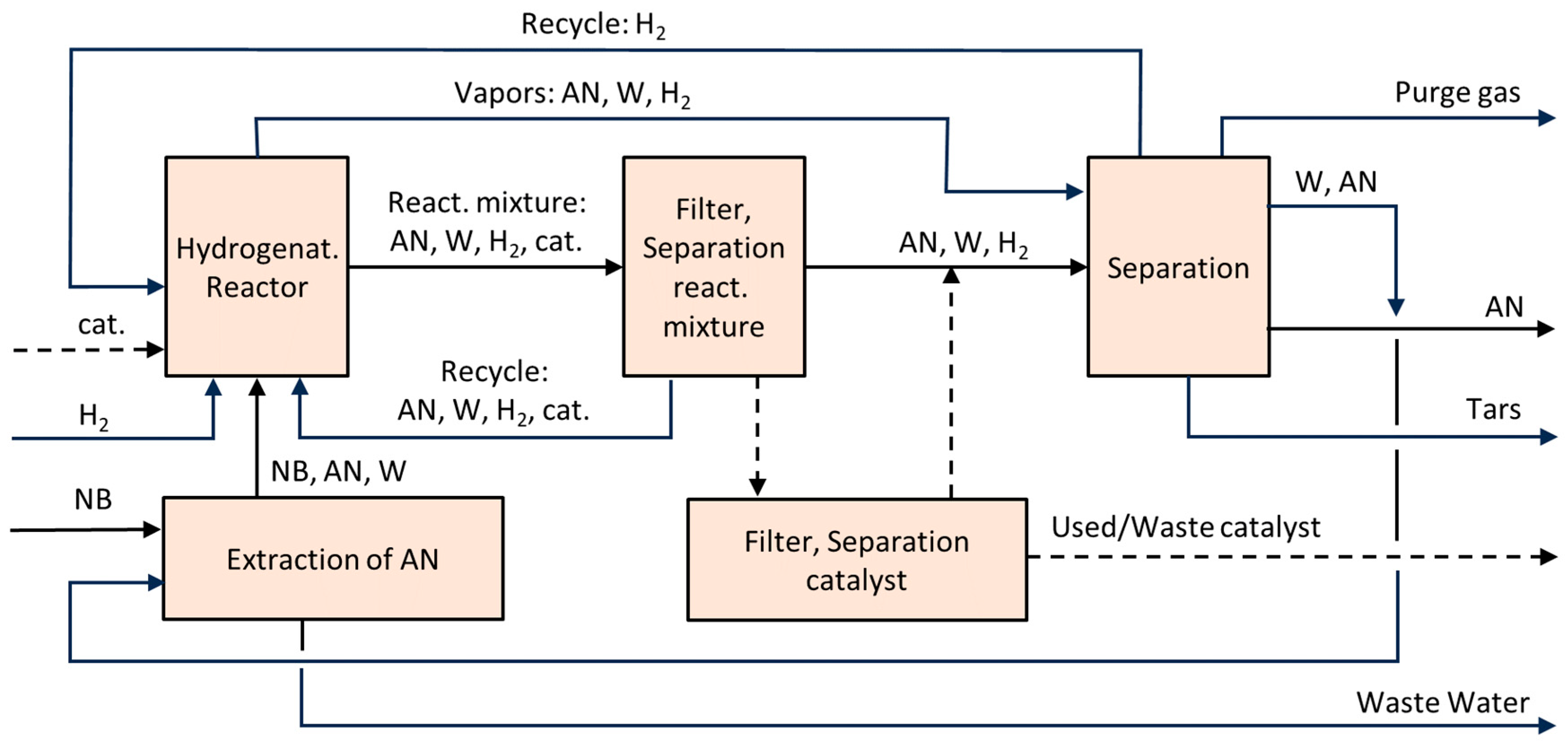
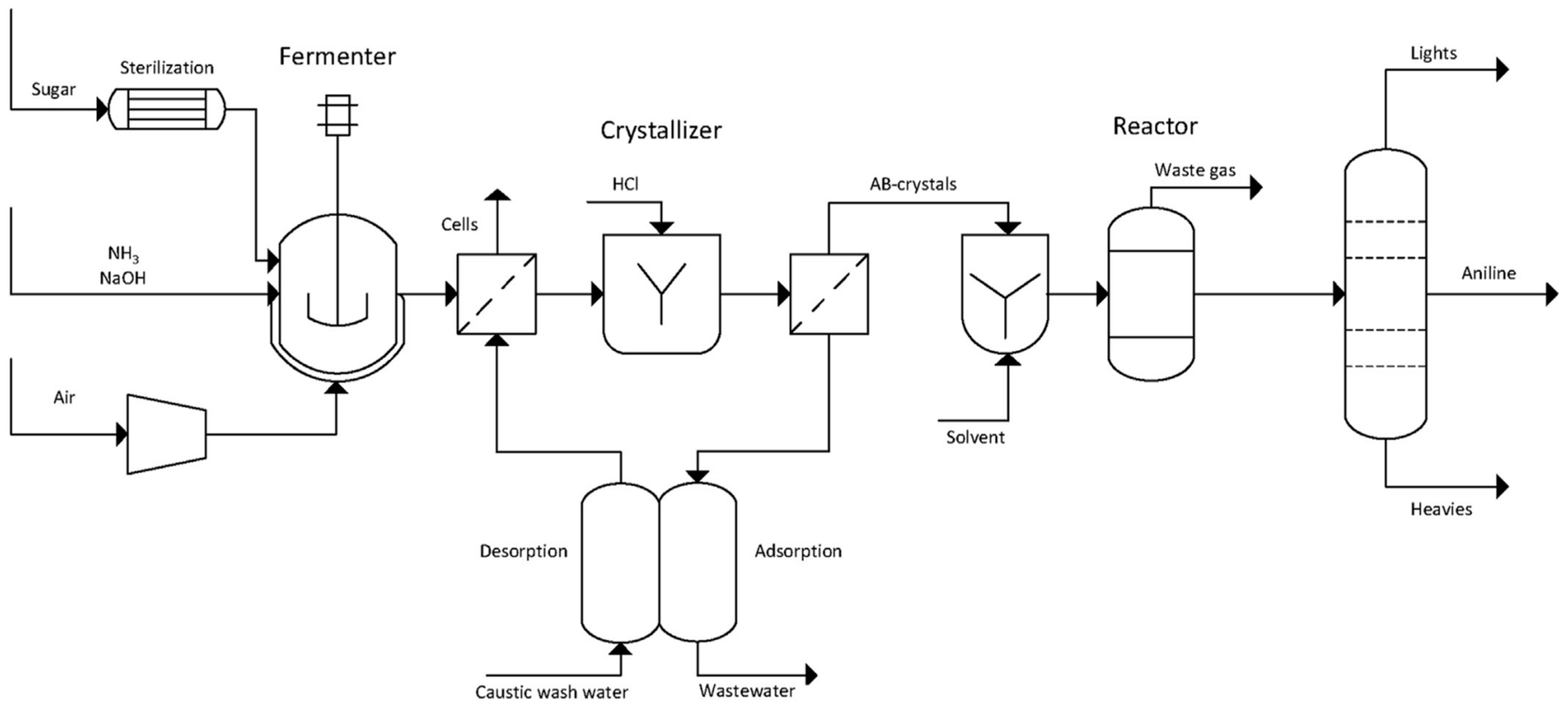

| Catalyst | Process | CLT (1), Deactivation |
|---|---|---|
| Fe/Al2O3/CaO/K Hollow extrudates | NH3 synth. (N2 + 3H2 -> 2NH3), 400–550 °C, 150–500 bar | 5–10 years, slow sintering |
| Pt-Pd-Ru-Rh alloy gauze | Oxidation of NH3 to NOx, production of HNO3, 800–940 °C, 1–10 bar | 1–6 months, deterioration/ evaporation of atoms from wires |
| V2O5/K2SO4/SiO2 extrudates | Sulfuric acid (2SO2 + O2 -> 2SO3) 400–600 °C, 1 bar | 5–10 years, slow physical deterioration (dust), pressure drop |
| Cu/ZnO/Al2O3 pellets | CH3OH synth. (CO + 2H2 -> CH3OH) 200–300 °C, 50–200 bar | 2–8 years, slow sintering |
| Co/Mo sulf./Al2O3 extrudates | Petrochem. Desulf. (R2S + 2H2 -> 2RH + H2S) 300–400 °C, 20–40 bar | 2–8 years, slow coking, pressure drop |
| Ag granules, 1–3 mm | Oxidat. (CH3OH + 1/2O2 -> HCHO + H2O) 500–600 °C, 1 bar | 0.3–1 year, coking, loss of selectivity |
| Raney Ni skeletal particles | Hydrogenation, e.g., vegetable oils, 100–200 °C, 3–30 bar | 1–10 days, deactivation by sticking of side products on the surface |
| Zeolites/SiO2-Al2O3 microspheroids | Fluid catalytic cracking (FCC), 350–550 °C, 1–5 bar | 1–10 s, permanent loss of activity, reactivation by burning coke |
| Support | S (1) (m2 g−1) | Vp (2) (cm3 g−1) | Other Properties | Top.max (3) (°C) |
|---|---|---|---|---|
| Graphite [103] | 10–100 | 0.01−0.1 | Mesopor. Struct., high el. (4) and heat conduct. (5) | 500 |
| Activated carbon [103] | 200–3000 | 0.6−2 | Micropor. Struct., low el. and heat conduct. | 400 |
| Carbon nanotubes, [103] | 50–500 | 2–2.5 | Micropor. Struct., high el. and heat conduct. | 300 |
| Graphene sheets [103] | 1500–3000 | 2–3.5 | Micropor. Struct., low el. and heat conduct. | 300 |
| Nickel (Raney) [104] | 5–30 | 0.01–0.05 | Low micropor. struct. high el. and heat conduct. | 300 |
| γ-Al2O3 [79] | 50–300 | 0.4–0.8 | Meso-micropor. Struct., mild el. and heat conduct. | 800 |
| α-Al2O3 [79] | 0.3–5 | 0.01–0.05 | Low meso-micropor. Struct., mild el. Conduct., good heat conduct. | 1100 |
| SiO2—gels [105,106] | 100–800 | 0.2–0.6 | Micropor. Struct., mild el. and heat conduct. | 400 |
| CaCO3—precipitated [107,108] | 5–40 | 0.01–0.05 | Micropor. Struct., mild el. and heat conduct. | 400 |
| Clays [109] | 250–800 | 0.1–0.3 | Micropore, mild el. and heat conduct. Used mainly as an acid catalyst (H+ form), after calcination (>400 °C) mixed oxides are formed—basic catalysts | 200 |
| Hydrotalcites [110] | 100–300 | 0.1–0.3 | Micropore., mild el. and heat conduct. Used mainly as a base catalyst (OH+ form), after calcination (>400 °C) mixed oxides are formed—basic catalysts | 200 |
| Zeolites [88] | 300–800 | 0.5–2 | Regular micropore structure 0.5–2 nm, mild el. and heat conduct, shape selectivity, in H+ form very efficient acid catalysts, e.g., in FCC | 900 |
| Cordierite, 2MgO·2Al2O3·5SiO2 Skeleton [101,102], | 1–8 | 0.01–0.05 | Mild el. and heat conduct. (semiconductor), high mechanical strength and toughness | 900 |
| TiO2 [79] | 20–400 | 0.1–0.6 | Mild el. and heat conduct. (semiconductor), photocatalytic act. | 400 |
| Perovskites—“ABO3”, [111,112] CaTiO3 as a representative | 5–40 | 0.05–0.6 | Mild el. and heat conduct. (semiconductor), photocatalytic act. | 300 |
| Glasses [79,113] | 0.01–0.1 | 1–40 | Low el. and heat conduct. Surface treatment is needed | 400 |
| Property | Fixed Bed | Multitube | Fluidized | Moving Bed |
|---|---|---|---|---|
| Dcat (mm) | 2–30 | 1–5 | 0.1–0.5 | 2–30 |
| V (m3) | 3–20 | 1–10 | 3–20 | 3–20 |
| Pmax (MPa) | 15 | 5 | 3 | 5 |
| Tmax (°C) | 1000 | 1000 | 800 | 600 |
| Mass_transport | Average | Good | Very good | Average |
| Heat_transport | Bad | Very good | Very good | Bad |
| CAPEX | Low | Very high | Average | Average |
| OPEX | Low | Very high | Very High | High |
| Examples | Oxidation, hydrogenation, alkylation, hydrocracking | Oxidation | Polymerization, cracking | Hydrotreatment |
| Property | Trickle Bed | Mechan. Stirred | Bubble Column | Ebullated | Loop |
|---|---|---|---|---|---|
| Dcat (mm) | 2–30 | 0.01–1 | 0.01–0.2 | 0.01–0.2 | 0.01–0.1 |
| V (m3) | 3–20 | 10−4–1000 | 1–20 | 1–20 | 0.05–5 |
| Pmax (MPa) | 5 | 15 | 5 | 3 | 3 |
| Tmax (°C) | 600 | 500 | 400 | 400 | 300 |
| Mass_transport | Average | Very good | Average | Average | Excellent |
| Heat_transport | Bad | Very good | Average | Good | Excellent |
| CAPEX | Low | High | Low | High | Very high |
| OPEX | Low | High | High | Very high | Very high |
| Examples | Hydrotreatment | All processes | Hydrogenation | Hydrotreatment | Hydrogenation, Oxidation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Králik, M.; Koóš, P.; Markovič, M.; Lopatka, P. Research and Developments of Heterogeneous Catalytic Technologies. Molecules 2025, 30, 3279. https://doi.org/10.3390/molecules30153279
Králik M, Koóš P, Markovič M, Lopatka P. Research and Developments of Heterogeneous Catalytic Technologies. Molecules. 2025; 30(15):3279. https://doi.org/10.3390/molecules30153279
Chicago/Turabian StyleKrálik, Milan, Peter Koóš, Martin Markovič, and Pavol Lopatka. 2025. "Research and Developments of Heterogeneous Catalytic Technologies" Molecules 30, no. 15: 3279. https://doi.org/10.3390/molecules30153279
APA StyleKrálik, M., Koóš, P., Markovič, M., & Lopatka, P. (2025). Research and Developments of Heterogeneous Catalytic Technologies. Molecules, 30(15), 3279. https://doi.org/10.3390/molecules30153279






