Cellulose Ether/Citric Acid Systems Loaded with SrTiO3 Nanoparticles with Solvent-Tailored Features for Energy-Related Technologies
Abstract
1. Introduction
2. Results
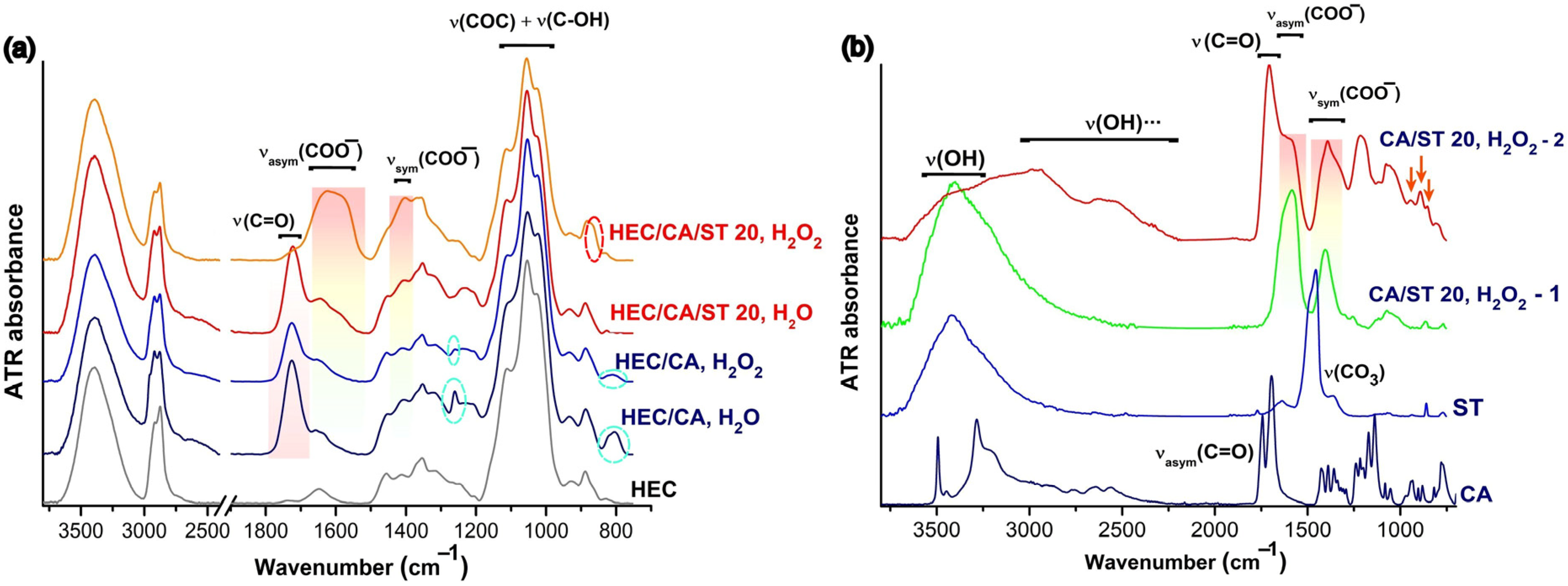
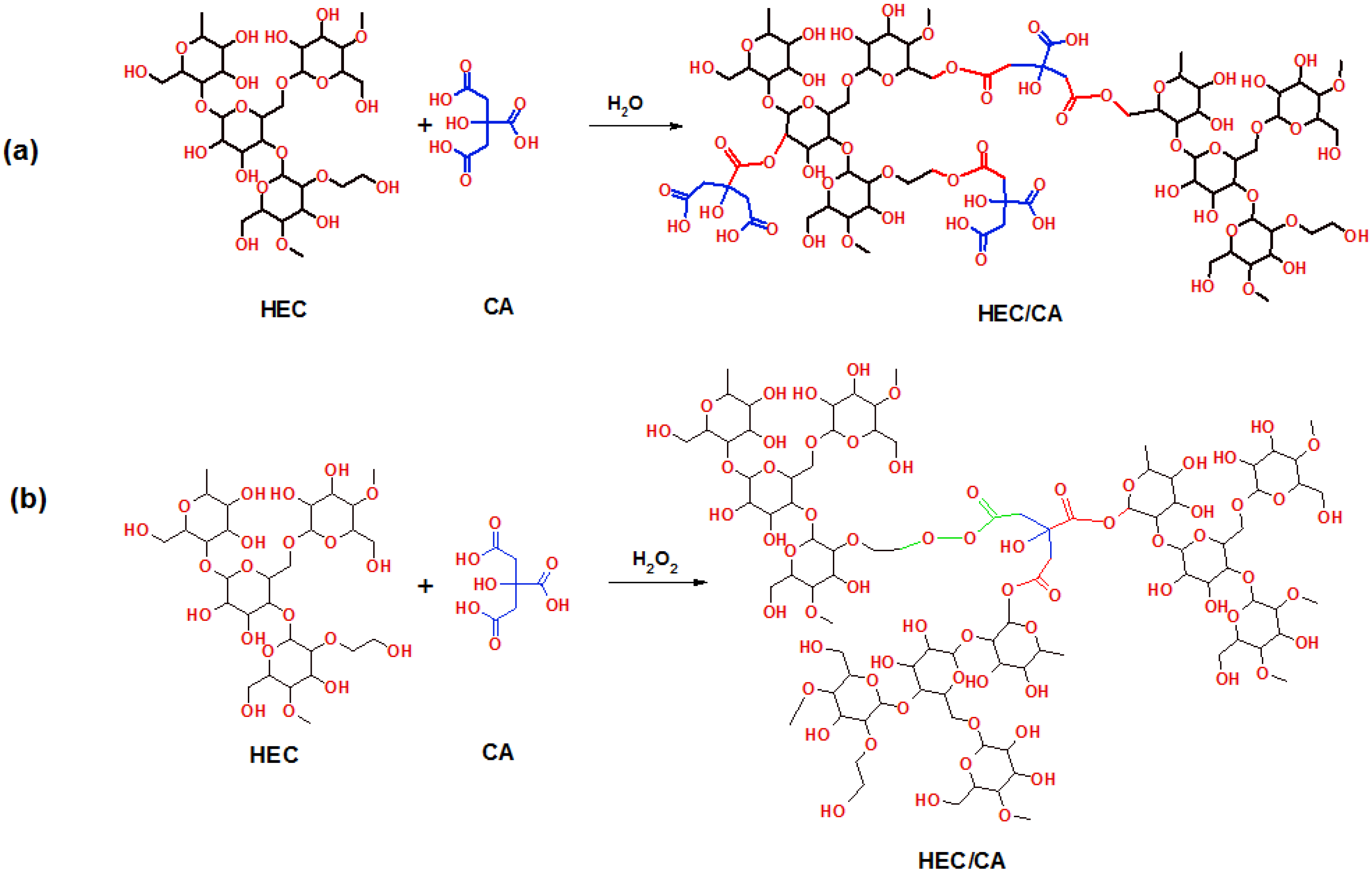
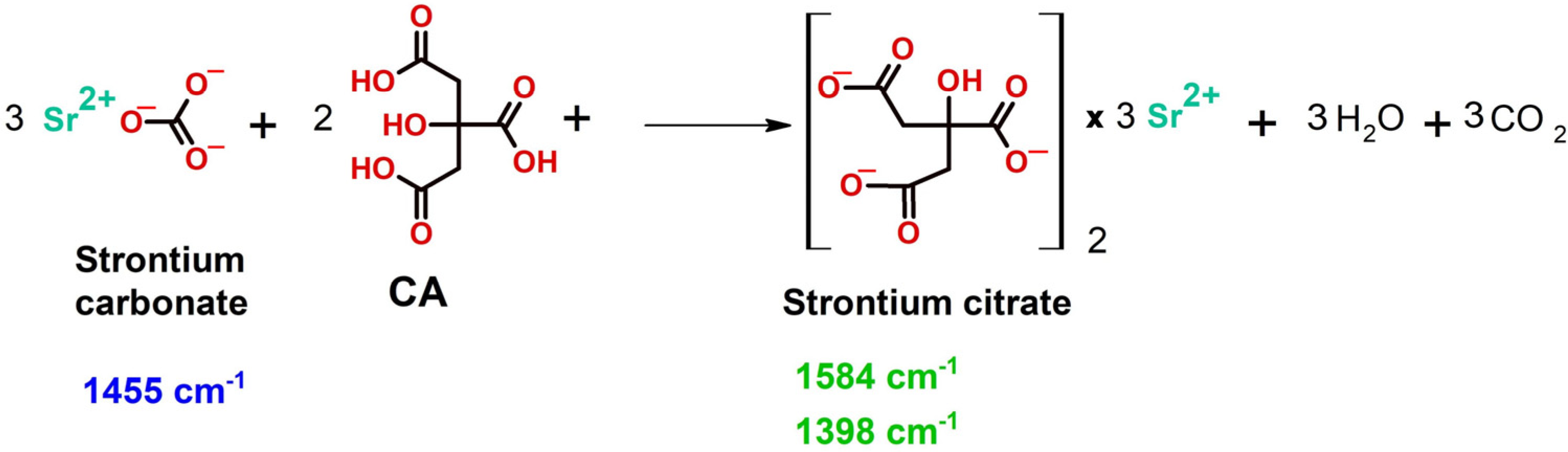
2.1. FTIR Investigations
 | (9) |
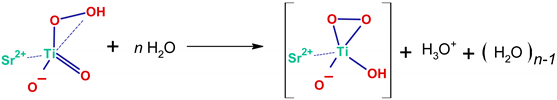 | (10) |
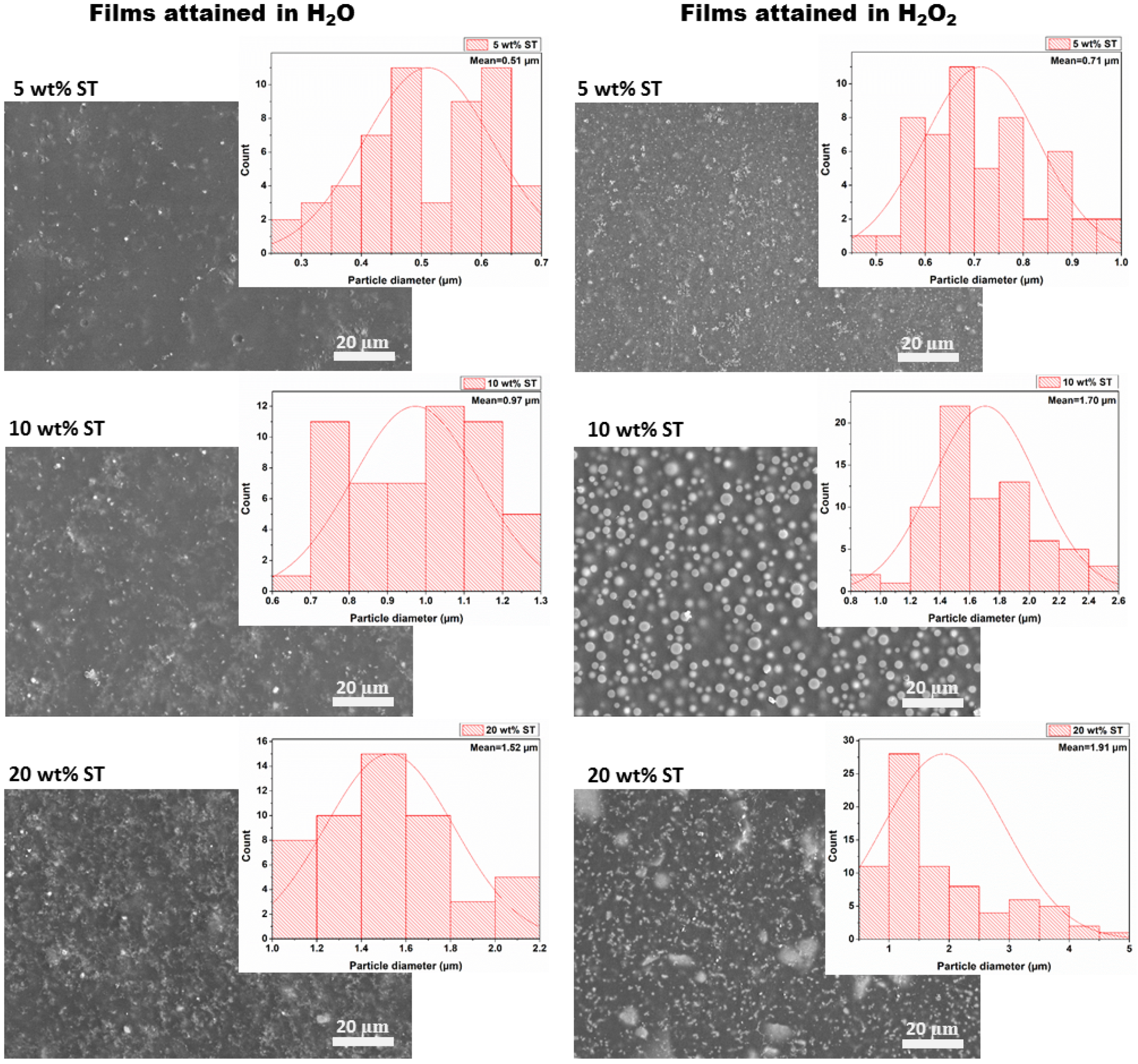
2.2. Morphological Analysis
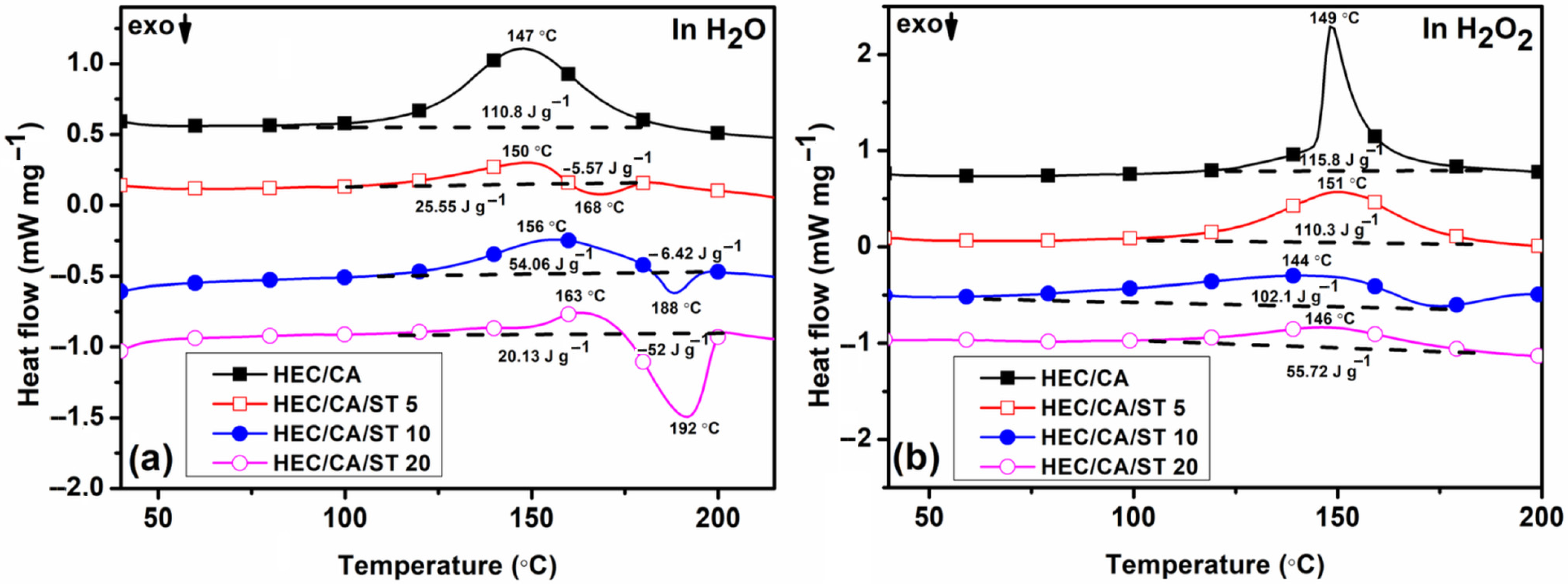
2.3. DSC Analysis
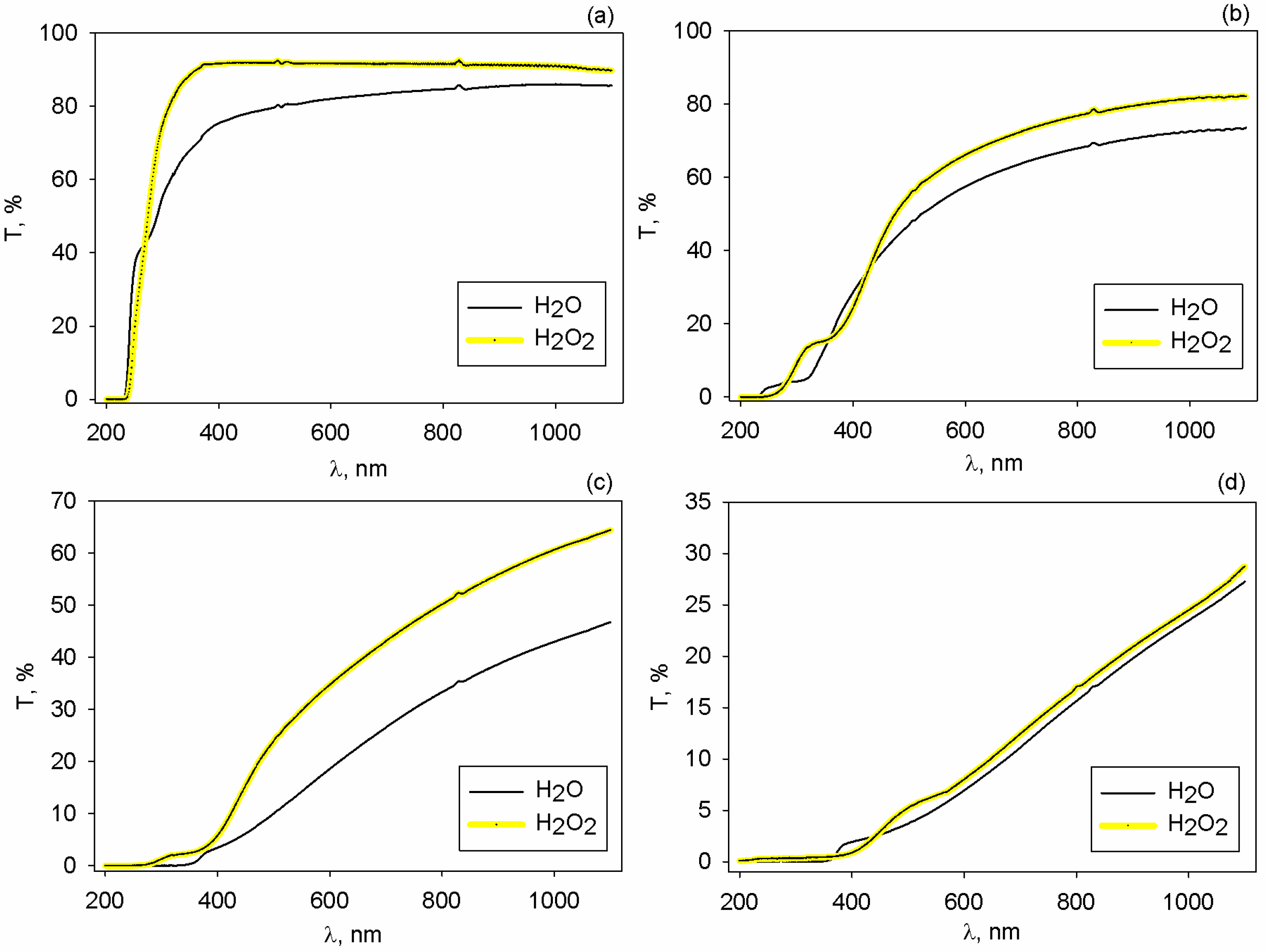
2.4. Optical Analysis
2.4.1. Absorption Edge and Band Gap Energy
2.4.2. Colorimetry Analysis
2.4.3. Refractive Index and Optical Dielectric Constant
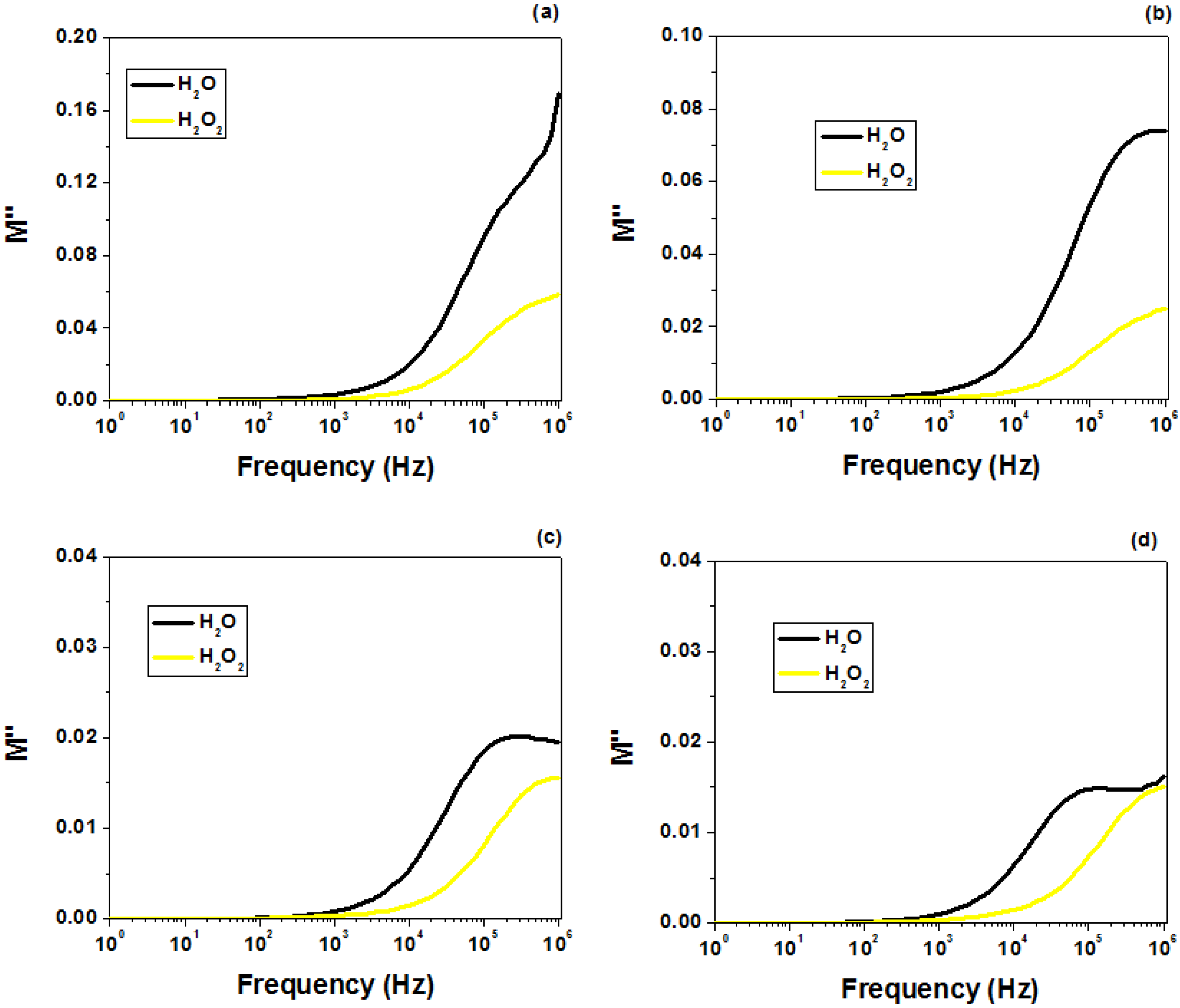
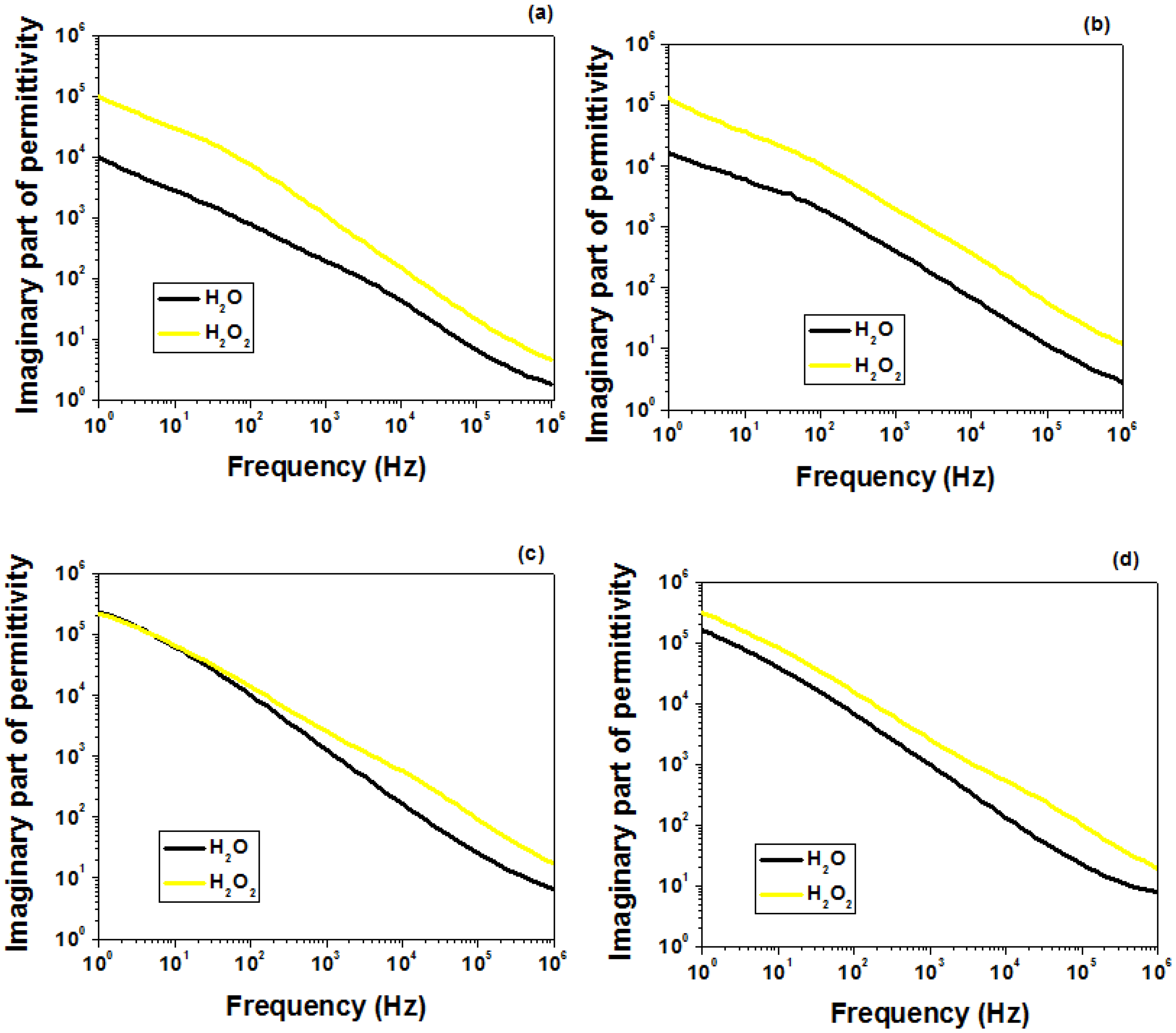
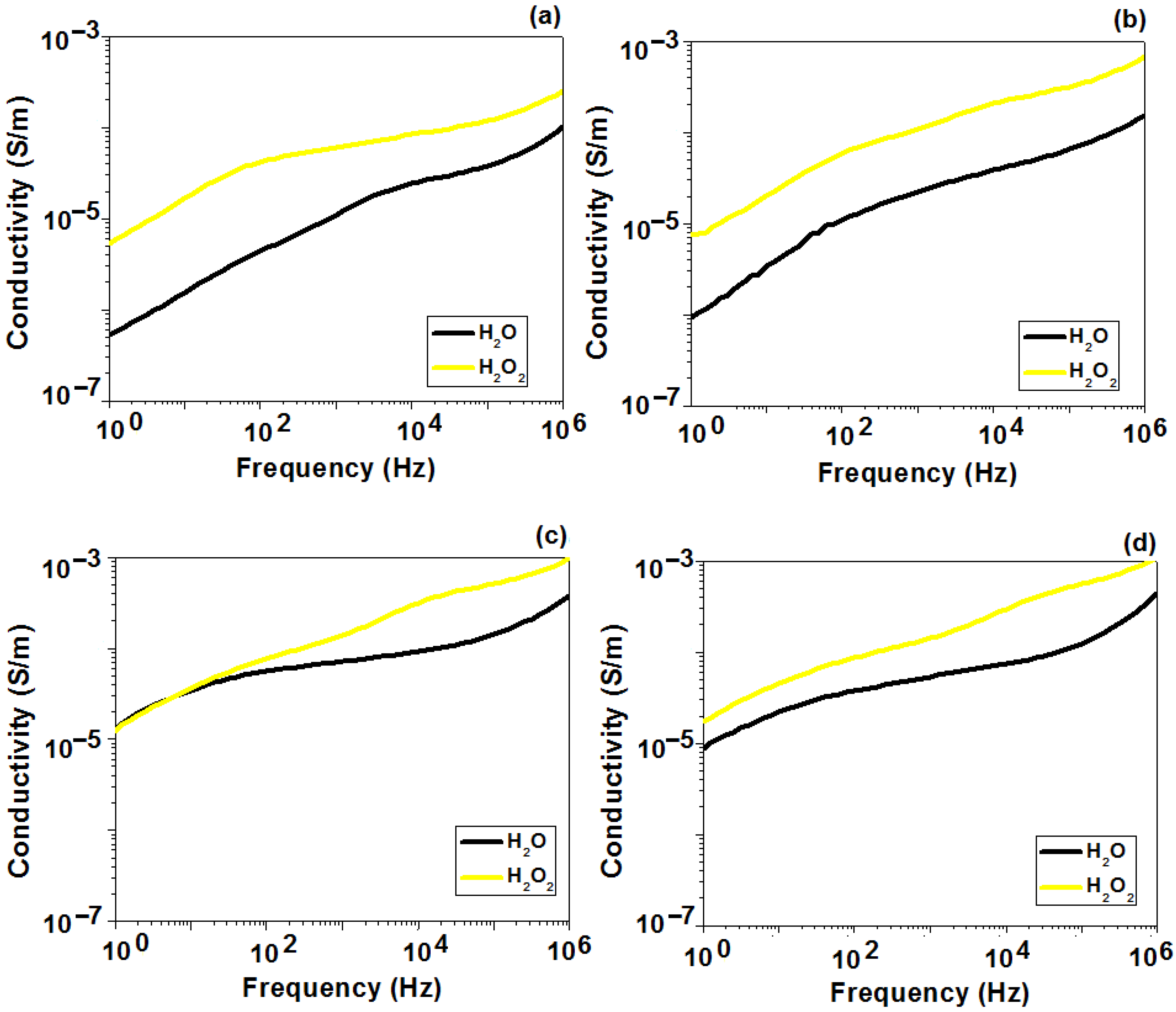
2.5. Dielectric Behavior Analysis
3. Materials and Methods
3.1. Basic Materials
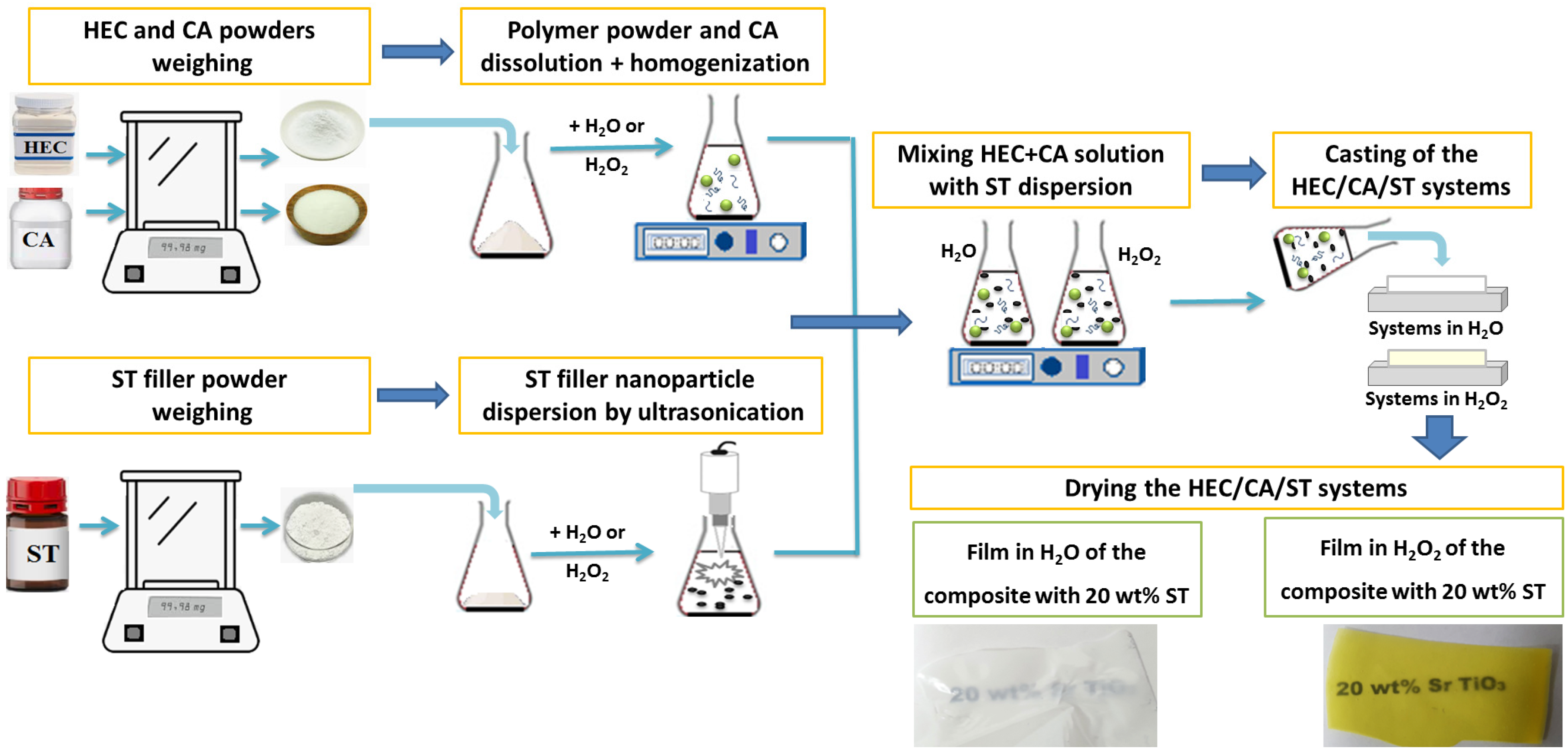
3.2. The Preparation of the Materials
3.3. Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Buga, C.S.; Viana, J.C. A Review on Materials and Technologies for Organic Large-Area Electronics. Adv. Mater. Technol. 2021, 6, 2001016. [Google Scholar] [CrossRef]
- Kumbhakar, P.; Jayan, J.S.; Sreedevi Madhavikutty, A.; Sreeram, P.R.; Saritha, A.; Ito, T.; Tiwary, C.S. Prospective applications of two-dimensional materials beyond laboratory frontiers: A review. iScience 2023, 26, 106671. [Google Scholar] [CrossRef]
- Cimatu, K.L.A.; Ambagaspitiya, T.D.; Premadasa, U.I.; Adhikari, N.M.; Kruse, A.; Robertson, E.; Guan, S.; Rong, L.; Advincula, R.; Bythell, B.J. Polymer-solvent interaction and conformational changes at a molecular level: Implication to solvent-assisted deformation and aggregation at the polymer surface. J. Colloid Interface Sci. 2022, 616, 221–233. [Google Scholar] [CrossRef]
- Aitken, H.M.; Jiang, Z.; Hampton, I.; O’Mara, M.L.; Connal, L.A. Polymer–solvent interactions as a tool to engineer material properties. Mol. Syst. Des. Eng. 2022, 7, 746–754. [Google Scholar] [CrossRef]
- Ma, G.; Li, Z.; Fang, L.; Xia, W.; Gu, X. Effect of solvent quality and sidechain architecture on conjugated polymer chain conformation in solution. Nanoscale 2024, 16, 6495–6506. [Google Scholar] [CrossRef]
- Zhang, M.; Biesold, G.M.; Choi, W.; Yu, J.; Deng, Y.; Silvestre, C.; Lin, Z. Recent advances in polymers and polymer composites for food packaging. Mater. Today 2022, 53, 134–161. [Google Scholar] [CrossRef]
- Li, L.; Han, L.; Hu, H.; Zhang, R. A review on polymers and their composites for flexible electronics. Mater. Adv. 2023, 4, 726–746. [Google Scholar] [CrossRef]
- Barzic, A.I.; Albu, R.M.; Stoica, I.; Hulubei, C. New shielding covers based on transparent polyimide/ferrous sulfide composites that reduce optical losses in solar cells. Compos. Sci. Technol. 2022, 218, 109140. [Google Scholar] [CrossRef]
- Li, X.; Li, P.; Wu, Z.; Luo, D.; Yu, H.-Y.; Lu, Z.-H. Review and perspective of materials for flexible solar cells. Mater. Rep. Energy 2021, 1, 100001. [Google Scholar] [CrossRef]
- Yadav, A.; Yadav, D.K.; Choudhary, B.L.; Jakhar, N.; Jain, S.K.; Tripathi, B. Study of dielectric response of BaTiO3/PVA composites for energy storage applications. Mater. Today Proc. 2022, 67, 894–899. [Google Scholar] [CrossRef]
- Asimakopoulos, I.A.; Psarras, G.C.; Zoumpoulakis, L. Barium titanate/polyester resin nanocomposites: Development, structure-properties relationship and energy storage capability. Express Polym. Lett. 2014, 8, 692–707. [Google Scholar] [CrossRef]
- Romeo, A. CdTe Solar Cells. In McEvoy’s Handbook of Photovoltaics; Elsevier: Amsterdam, The Netherlands, 2018; pp. 309–369. [Google Scholar]
- Feenstra, J.; Van Leest, R.H.; Smeenk, N.J.; Oomen, G.; Bongers, E.; Mulder, P.; Vlieg, E.; Schermer, J.J. Flexible shielding layers for solar cells in space applications. J. Appl. Polym. Sci. 2016, 133, 43661. [Google Scholar] [CrossRef]
- Allsopp, B.L.; Orman, R.; Johnson, S.R.; Baistow, I.; Sanderson, G.; Sundberg, P.; Stålhandske, C.; Grund, L.; Andersson, A.; Booth, J.; et al. Towards improved cover glasses for photovoltaic devices. Prog. Photovolt. Res. Appl. 2020, 28, 1187–1206. [Google Scholar] [CrossRef]
- Hulubei, C.; Albu, R.M.; Lisa, G.; Nicolescu, A.; Hamciuc, E.; Hamciuc, C.; Barzic, A.I. Antagonistic effects in structural design of sulfur-based polyimides as shielding layers for solar cells. Sol. Energy Mater. Sol. Cells 2019, 193, 219–230. [Google Scholar] [CrossRef]
- Barzic, A.I.; Albu, R.M.; Hulubei, C.; Mahmoud, S.F.; Abu Ali, O.A.; El-Bahy, Z.M.; Stoica, I. Polyimide Layers with High Refractivity and Surface Wettability Adapted for Lowering Optical Losses in Solar Cells. Polymers 2022, 14, 4049. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.-R.; Han, J.-W.; Lee, K.-M. Dielectric Properties of Polymer-ceramic Composites for Embedded Capacitors. Trans. Electr. Electron. Mater. 2009, 10, 116–120. [Google Scholar] [CrossRef]
- Yin, Y.; Zhang, C.; Chen, J.; Yu, W.; Shi, Z.; Xiong, C.; Yang, Q. Cellulose/BaTiO3 nanofiber dielectric films with enhanced energy density by interface modification with poly(dopamine). Carbohydr. Polym. 2020, 249, 116883. [Google Scholar] [CrossRef] [PubMed]
- Riquelme, S.A.; Ramam, K.; Jaramillo, A.F. Ceramics fillers enhancing effects on the dielectric properties of poly(vinylidene fluoride) matrix composites prepared by the torque rheometer method. Results Phys. 2019, 15, 102800. [Google Scholar] [CrossRef]
- Li, W.; Liang, R.; Wu, C.; Yang, L.; Wang, F.; Liu, Z.; Chen, X.; Mao, H.; Zhang, W. Ceramic-Polymer Nanocomposites Design for Energy Storage Capacitor Applications. Adv. Mater. Interfaces 2022, 9, 2201257. [Google Scholar] [CrossRef]
- Yin, G.Z.; Yang, X.M. Biodegradable polymers: A cure for the planet, but a long way to go. J. Polym. Res. 2020, 27, 38. [Google Scholar] [CrossRef]
- Jain, M.; Kumar, D.; Chaudhary, J.; Kumar, S.; Sharma, S.; Singh Verma, A. Review on E-waste management and its impact on the environment and society. Waste Manag. Bull. 2023, 1, 34–44. [Google Scholar] [CrossRef]
- Kausar, A.; Ahmad, I.; Maaza, M.; Eisa, M.; Bocchetta, P. Cutting-Edge Green Polymer/Nanocarbon Nanocomposite for Supercapacitor—State-of-the-Art. J. Compos. Sci. 2022, 6, 376. [Google Scholar] [CrossRef]
- Fic, K.; Platek, A.; Piwek, J.; Frackowiak, E. Sustainable materials for electrochemical capacitors. Mater. Today 2018, 21, 437–454. [Google Scholar] [CrossRef]
- Raj, K.; Das, A.P. Lead pollution: Impact on environment and human health and approach for a sustainable solution. Environ. Chem. Ecotoxicol. 2023, 5, 79–85. [Google Scholar] [CrossRef]
- Patial, S.; Hasija, V.; Raizada, P.; Singh, P.; Khan Singh, A.A.P.; Asiri, A.M. Tunable photocatalytic activity of SrTiO3 for water splitting: Strategies and future scenario. J. Environ. Chem. Eng. 2020, 8, 103791. [Google Scholar] [CrossRef]
- Boga, B.; Steinfeldt, N.; Moustakas, N.G.; Peppel, T.; Lund, H.; Rabeah, J.; Pap, Z.; Cristea, V.-M.; Strunk, J. Role of SrCO3 on Photocatalytic Performance of SrTiO3-SrCO3 Composites. Catalysts 2022, 12, 978. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhong, L.; Duan, D. Single-step hydrothermal synthesis of strontium titanate nanoparticles from crystalline anatase titanium dioxide. Ceram. Int. 2015, 41, 13516–13524. [Google Scholar] [CrossRef]
- Yadav, A.; Yadav, D.K.; Jain, S.K.; Tripathi, B.; Katiyar, R.S. Dielectric Response of Strontium Titanate (SrTiO 3)/Polycarbonate Nanocomposite Electrodes for Energy Storage. Energy Environ. Focus 2023, 7, 60–64. [Google Scholar] [CrossRef]
- Miao, W.; Chen, H.; Pan, Z.; Pei, X.; Li, L.; Li, P.; Liu, J.; Zhai, J.; Pan, H. Enhancement thermal stability of polyetherimide-based nanocomposites for applications in energy storage. Compos. Sci. Technol. 2021, 201, 108501. [Google Scholar] [CrossRef]
- Wang, J.; Liu, S.-H.; Zhou, M.; Hao, H.-S.; Zhai, J.-W. Effects of suface hydroxylated strontium titanate nanofibers on dielectric and energy storage properties of polyvinylidene fluoride composites. Acta Phys. Sin. 2020, 69, 218101. [Google Scholar] [CrossRef]
- Etale, A.; Onyianta, A.J.; Turner, S.R.; Eichhorn, S.J. Cellulose: A Review of Water Interactions, Applications in Composites, and Water Treatment. Chem. Rev. 2023, 123, 2016–2048. [Google Scholar] [CrossRef]
- Madhushree, M.; Vairavel, P.; Mahesha, G.; Bhat, K.S. A Comprehensive Review of Cellulose and Cellulose-Based Materials: Extraction, Modification, and Sustainable Applications. J. Nat. Fibers 2024, 21, 2418357. [Google Scholar] [CrossRef]
- Desmarais, A.J.; Wint, R.F. Hydroxyalkyl and Ethyl Ethers of Cellulose. In Industrial Gums; Elsevier: Amsterdam, The Netherlands, 1993; pp. 505–535. [Google Scholar]
- McMullen, R.L.; Ozkan, S.; Gillece, T. Physicochemical Properties of Cellulose Ethers. Cosmetics 2022, 9, 52. [Google Scholar] [CrossRef]
- Mahmoud, K.H. Optical properties of hydroxyethyl cellulose film treated with nitrogen plasma. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2016, 157, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Bozova, N.; Petrov, P.D. Highly Elastic Super-Macroporous Cryogels Fabricated by Thermally Induced Crosslinking of 2-Hydroxyethylcellulose with Citric Acid in Solid State. Molecules 2021, 26, 6370. [Google Scholar] [CrossRef]
- Liedermann, K.; Lapčík, L., Jr. Dielectric relaxation in hydroxyethyl cellulose. Carbohydr. Polym. 2000, 42, 369–374. [Google Scholar] [CrossRef]
- Ulutas, K.; Yakut, S.; Bozoglu, D.; Tankul, M.I.; Deger, D. Thickness-dependent glass transition temperature of hydroxyethyl cellulose thin films based on dielectric properties. J. Appl. Polym. Sci. 2024, 141, e55185. [Google Scholar] [CrossRef]
- Gupta, S.; Varshney, P.K. Effect of plasticizer concentration on structural and electrical properties of hydroxyethyl cellulose (HEC)-based polymer electrolyte. Ionics 2017, 23, 1613–1617. [Google Scholar] [CrossRef]
- Baidya, S. Poly-vinyl alcohol/hydroxy-ethyl cellulose/montmorillonite nanoclay-based food packaging film for dielectric heating of vegetable sandwiches. Int. J. Biol. Macromol. 2025, 304, 140817. [Google Scholar] [CrossRef] [PubMed]
- Barzic, A.I.; Stoica, I.; Asandulesa, M.; Albu, R.M.; Oprisan, B. Bentonite/hydroxyethylcellulose as eco-dielectrics with potential utilization in energy storage. E-Polymers 2023, 23, 20230073. [Google Scholar] [CrossRef]
- Albu, R.M.; Barzic, A.I.; Asandulesa, M.; Rusu, B.-G.; Stoica, I.; Sava, I. Insights into Interfacial Features of Metal/Eco-Composites Designed for Energy Storage. Coatings 2023, 13, 1390. [Google Scholar] [CrossRef]
- Albu, R.M.; Asandulesa, M.; Stoica, I.; Rusu, B.-G.; Avadanei, M.I.; Varganici, C.-D.; Barzic, A.I. Hydroxyethyl cellulose loaded with nettle leaf ash: Introspective on the microstructure changes for adapting electrical performance. Cellulose 2025, under revision. [Google Scholar]
- Ayouch, I.; Kassem, I.; Kassab, Z.; Barrak, I.; Barhoun, A.; Jacquemin, J.; Draoui, K.; Achaby, M. El Crosslinked carboxymethyl cellulose-hydroxyethyl cellulose hydrogel films for adsorption of cadmium and methylene blue from aqueous solutions. Surf. Interfaces 2021, 24, 101124. [Google Scholar] [CrossRef]
- Socrates, G. The Carbonyl Group: C=O. In Infrared and Raman Characteristic Group Frequencies: Tables and Charts; John Wiley & Sons Ltd.: Chichester, UK, 2001; pp. 115–156. [Google Scholar]
- Özen, M.; Mertens, M.; Cool, P. Exploring barium-titanium-peroxo-hydroxide as an excellent single-source precursor for crystallographically diverse barium titanate ceramics—parametric study. Ceram. Int. 2024, 50, 53209–53221. [Google Scholar] [CrossRef]
- Tozzola, G.; Mantegazza, M.A.; Ranghino, G.; Petrini, G.; Bordiga, S.; Ricchiardi, G.; Lamberti, C.; Zulian, R.; Zecchina, A. On the Structure of the Active Site of Ti-Silicalite in Reactions with Hydrogen Peroxide: A Vibrational and Computational Study. J. Catal. 1998, 179, 64–71. [Google Scholar] [CrossRef]
- Kakihana, M.; Kobayashi, M.; Tomita, K.; Petrykin, V. Application of Water-Soluble Titanium Complexes as Precursors for Synthesis of Titanium-Containing Oxides via Aqueous Solution Processes. Bull. Chem. Soc. Jpn. 2010, 83, 1285–1308. [Google Scholar] [CrossRef]
- Kakihana, M.; Tada, M.; Shiro, M.; Petrykin, V.; Osada, M.; Nakamura, Y. Structure and Stability of Water Soluble (NH4)8[Ti4(C6H4O7)4(O2)4]·8H2O. Inorg. Chem. 2001, 40, 891–894. [Google Scholar] [CrossRef]
- Dakanali, M.; Kefalas, E.T.; Raptopoulou, C.P.; Terzis, A.; Voyiatzis, G.; Kyrikou, I.; Mavromoustakos, T.; Salifoglou, A. A New Dinuclear Ti(IV)−Peroxo−Citrate Complex from Aqueous Solutions. Synthetic, Structural, and Spectroscopic Studies in Relevance to Aqueous Titanium(IV)−Peroxo−Citrate Speciation. Inorg. Chem. 2003, 42, 4632–4639. [Google Scholar] [CrossRef] [PubMed]
- Hiroki, A.; LaVerne, J.A. Decomposition of Hydrogen Peroxide at Water−Ceramic Oxide Interfaces. J. Phys. Chem. B 2005, 109, 3364–3370. [Google Scholar] [CrossRef]
- Chang, S.-J.; Liao, W.-S.; Ciou, C.-J.; Lee, J.-T.; Li, C.-C. An efficient approach to derive hydroxyl groups on the surface of barium titanate nanoparticles to improve its chemical modification ability. J. Colloid Interface Sci. 2009, 329, 300–305. [Google Scholar] [CrossRef] [PubMed]
- Li, C.-C.; Chang, S.-J.; Lee, J.-T.; Liao, W.-S. Efficient hydroxylation of BaTiO3 nanoparticles by using hydrogen peroxide. Colloids Surf. A Physicochem. Eng. Asp. 2010, 361, 143–149. [Google Scholar] [CrossRef]
- Bonino, F.; Damin, A.; Ricchiardi, G.; Ricci, M.; Spanò, G.; D’Aloisio, R.; Zecchina, A.; Lamberti, C.; Prestipino, C.; Bordiga, S. Ti-Peroxo Species in the TS-1/H2O2/H2O System. J. Phys. Chem. B 2004, 108, 3573–3583. [Google Scholar] [CrossRef]
- Luxová, J.; Dohnalová, Ž.; Šulcová, P.; Reinders, N. Mn-doped SrTiO3 perovskite: Synthesis and characterisation of a visible light-active semiconductor. Mater. Sci. Eng. B 2025, 313, 117889. [Google Scholar] [CrossRef]
- Tauc, J.; Menth, A. States in the gap. J. Non. Cryst. Solids 1972, 8–10, 569–585. [Google Scholar] [CrossRef]
- Davis, E.A.; Mott, N.F. Conduction in non-crystalline systems V. Conductivity, optical absorption and photoconductivity in amorphous semiconductors. Philos. Mag. 1970, 22, 0903–0922. [Google Scholar] [CrossRef]
- Makuła, P.; Pacia, M.; Macyk, W. How To Correctly Determine the Band Gap Energy of Modified Semiconductor Photocatalysts Based on UV-Vis Spectra. J. Phys. Chem. Lett. 2018, 9, 6814–6817. [Google Scholar] [CrossRef]
- Fareed, I.; Farooq, M.u.H.; Khan, M.D.; Ali, Z.; Butt, F.K. Band gap engineering of Strontium Titanate (SrTiO3) for improved photocatalytic activity and excellent bio-sensing aptitude. Mater. Sci. Semicond. Process. 2024, 177, 108327. [Google Scholar] [CrossRef]
- Alsubaie, A.S.A. Characterization and Optical Studies of Hydroxyethyl Cellulose-Copper Oxide Nanocomposites. J. Spectrosc. 2022, 2022, 8422803. [Google Scholar] [CrossRef]
- Jiang, H.F.; Lei, H.C.; Zhu, X.B.; Li, G.; Yang, Z.R.; Song, W.H.; Dai, J.M.; Sun, Y.P.; Fu, Y.K. Effects of citric acid on properties of single phase CuAlO2 thin films derived by chemical solution deposition. J. Alloys Compd. 2009, 487, 404–408. [Google Scholar] [CrossRef]
- Morsi, M.A.; Abdelaziz, M.; Oraby, A.H.; Mokhles, I. Structural, optical, thermal, and dielectric properties of polyethylene oxide/carboxymethyl cellulose blend filled with barium titanate. J. Phys. Chem. Solids 2019, 125, 103–114. [Google Scholar] [CrossRef]
- Aziz, S.B.; Rasheed, M.A.; Hussein, A.M.; Ahmed, H.M. Fabrication of polymer blend composites based on [PVA-PVP] (1−x):(Ag 2 S) x (0.01 ≤ x ≤ 0.03) with small optical band gaps: Structural and optical properties. Mater. Sci. Semicond. Process. 2017, 71, 197–203. [Google Scholar] [CrossRef]
- Choudhury, A.K.R. Using instruments to quantify colour. In Principles of Colour and Appearance Measurement; Elsevier: Amsterdam, The Netherlands, 2014; pp. 270–317. [Google Scholar]
- Schanda, J. Colorimetry: Understanding the CIE System; John Wiley & Sons: Hoboken, NJ, USA, 2007; pp. 1–459. [Google Scholar] [CrossRef]
- Ignaczak, W.; Ladegaard Skov, A.; El Fray, M. Interfacial Polarization in Thermoplastic Basalt Fiber-Reinforced Composites. Polymers 2020, 12, 1486. [Google Scholar] [CrossRef] [PubMed]
- Padurariu, L.; Horchidan, N.; Ciomaga, C.E.; Curecheriu, L.P.; Lukacs, V.A.; Stirbu, R.S.; Stoian, G.; Botea, M.; Florea, M.; Maraloiu, V.A.; et al. Influence of Ferroelectric Filler Size and Clustering on the Electrical Properties of (Ag–BaTiO3)–PVDF Sub-Percolative Hybrid Composites. ACS Appl. Mater. Interfaces 2023, 15, 5744–5759. [Google Scholar] [CrossRef] [PubMed]
- Barzic, A.I.; Soroceanu, M.; Rotaru, R.; Doroftei, F.; Asandulesa, M.; Tugui, C.; Dascalu, I.A.; Harabagiu, V. Cellulose derivative/barium titanate composites with high refractive index, conductivity and energy density. Cellulose 2022, 29, 863–878. [Google Scholar] [CrossRef]
- Hodge, I.M.; Ingram, M.D.; West, A.R. Impedance and modulus spectroscopy of polycrystalline solid electrolytes. J. Electroanal. Chem. Interfacial Electrochem. 1976, 74, 125–143. [Google Scholar] [CrossRef]
- Gerhardt, R. Impedance and dielectric spectroscopy revisited: Distinguishing localized relaxation from long-range conductivity. J. Phys. Chem. Solids 1994, 55, 1491–1506. [Google Scholar] [CrossRef]
- Jonscher, A.K. A new understanding of the dielectric relaxation of solids. J. Mater. Sci. 1981, 16, 2037–2060. [Google Scholar] [CrossRef]



| System | ST, wt% | X | Y | Z | L* | a* | b* |
|---|---|---|---|---|---|---|---|
| HEC/CA/ST films prepared in H2O | 0 | 384.4128 | 399.9580 | 429.7695 | 93.0786 | 0.2584 | 0.3728 |
| 5 | 370.4469 | 386.3166 | 399.3975 | 91.8241 | −0.1016 | 2.7398 | |
| 10 | 329.0398 | 343.4064 | 336.4649 | 87.6743 | −0.2152 | 5.7599 | |
| 20 | 249.2407 | 259.9917 | 247.3358 | 78.4905 | −0.1275 | 6.7915 | |
| HEC/CA/ST films prepared in H2O2 | 0 | 414.9604 | 431.9948 | 458.6668 | 95.9166 | 0.1706 | 1.1499 |
| 5 | 396.2388 | 420.8070 | 396.2108 | 94.9420 | −2.9978 | 8.6038 | |
| 10 | 357.8094 | 393.8825 | 279.7866 | 92.5235 | −8.4612 | 244371 | |
| 20 | 311.7227 | 352.9012 | 173.3759 | 88.6211 | −12.2736 | 41.6947 |
| System | ST, wt% | n | εopt | ||||
|---|---|---|---|---|---|---|---|
| 486 nm | 589 nm | 656 nm | 486 nm | 589 nm | 656 nm | ||
| HEC/CA/ST films prepared in H20 | 0 | 1.5049 | 1.4977 | 1.4968 | 2.2647 | 2.2431 | 2.2404 |
| 5 | 1.5152 | 1.5063 | 1.5022 | 2.2958 | 2.2689 | 2.2566 | |
| 10 | 1.5190 | 1.5177 | 1.5118 | 2.3073 | 2.3034 | 2.2855 | |
| 20 | 1.5646 | 1.5471 | 1.5334 | 2.4479 | 2.3935 | 2.3513 | |
| HEC/CA/ST films prepared in H202 | 0 | 1.5090 | 1.4993 | 1.4981 | 2.2771 | 2.2479 | 2.2443 |
| 5 | 1.5229 | 1.5189 | 1.5132 | 2.3192 | 2.3071 | 2.2898 | |
| 10 | 1.5843 | 1.5705 | 1.5606 | 2.5100 | 2.4665 | 2.4355 | |
| 20 | 1.5914 | 1.5811 | 1.5763 | 2.5325 | 2.4999 | 2.4847 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Albu, R.M.; Avadanei, M.I.; Curecheriu, L.P.; Turcanu, G.; Stoica, I.; Soroceanu, M.; Rusu, D.; Varganici, C.-D.; Cojocaru, V.; Barzic, A.I. Cellulose Ether/Citric Acid Systems Loaded with SrTiO3 Nanoparticles with Solvent-Tailored Features for Energy-Related Technologies. Molecules 2025, 30, 3271. https://doi.org/10.3390/molecules30153271
Albu RM, Avadanei MI, Curecheriu LP, Turcanu G, Stoica I, Soroceanu M, Rusu D, Varganici C-D, Cojocaru V, Barzic AI. Cellulose Ether/Citric Acid Systems Loaded with SrTiO3 Nanoparticles with Solvent-Tailored Features for Energy-Related Technologies. Molecules. 2025; 30(15):3271. https://doi.org/10.3390/molecules30153271
Chicago/Turabian StyleAlbu, Raluca Marinica, Mihaela Iuliana Avadanei, Lavinia Petronela Curecheriu, Gabriela Turcanu, Iuliana Stoica, Marius Soroceanu, Daniela Rusu, Cristian-Dragos Varganici, Victor Cojocaru, and Andreea Irina Barzic. 2025. "Cellulose Ether/Citric Acid Systems Loaded with SrTiO3 Nanoparticles with Solvent-Tailored Features for Energy-Related Technologies" Molecules 30, no. 15: 3271. https://doi.org/10.3390/molecules30153271
APA StyleAlbu, R. M., Avadanei, M. I., Curecheriu, L. P., Turcanu, G., Stoica, I., Soroceanu, M., Rusu, D., Varganici, C.-D., Cojocaru, V., & Barzic, A. I. (2025). Cellulose Ether/Citric Acid Systems Loaded with SrTiO3 Nanoparticles with Solvent-Tailored Features for Energy-Related Technologies. Molecules, 30(15), 3271. https://doi.org/10.3390/molecules30153271









