Gold(III) Complexes with Aromatic Cyano-Substituted Bisdithiolate Ligands as Potential Anticancer and Antimicrobial Agents
Abstract
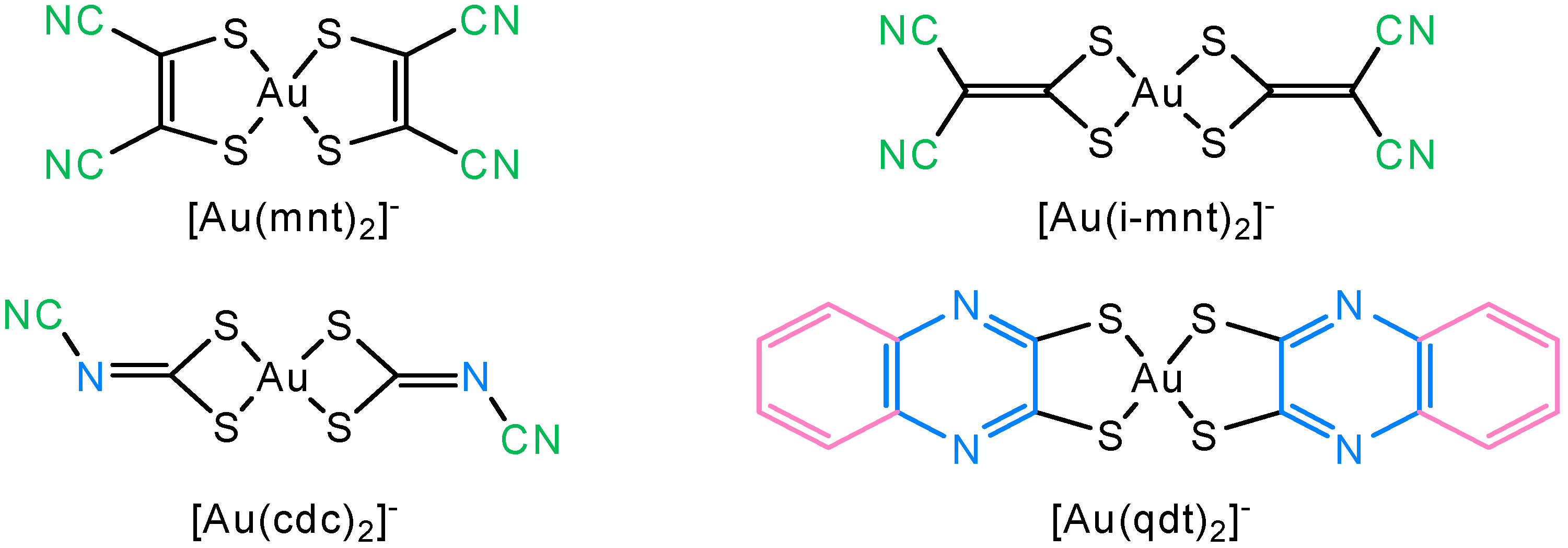
1. Introduction
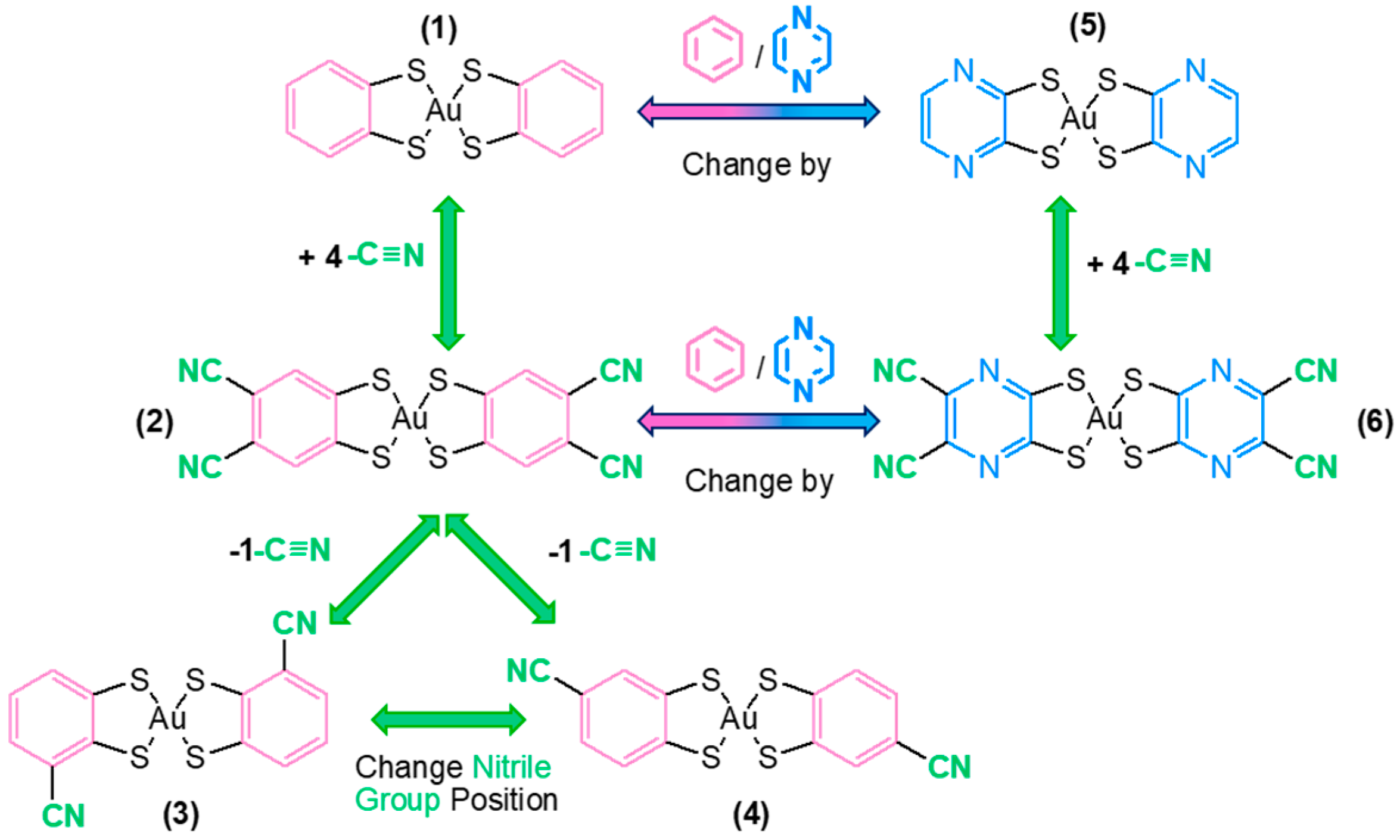
2. Results and Discussion
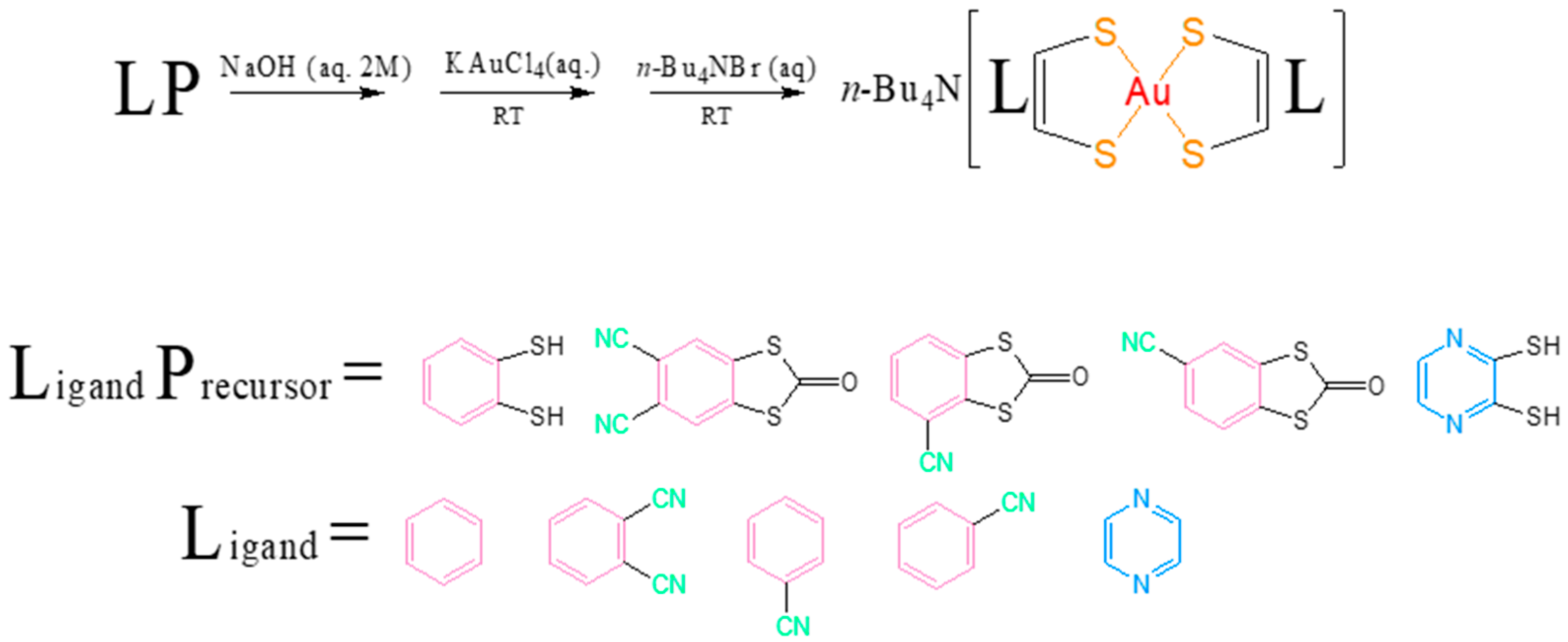
2.1. Syntheses and Characterization
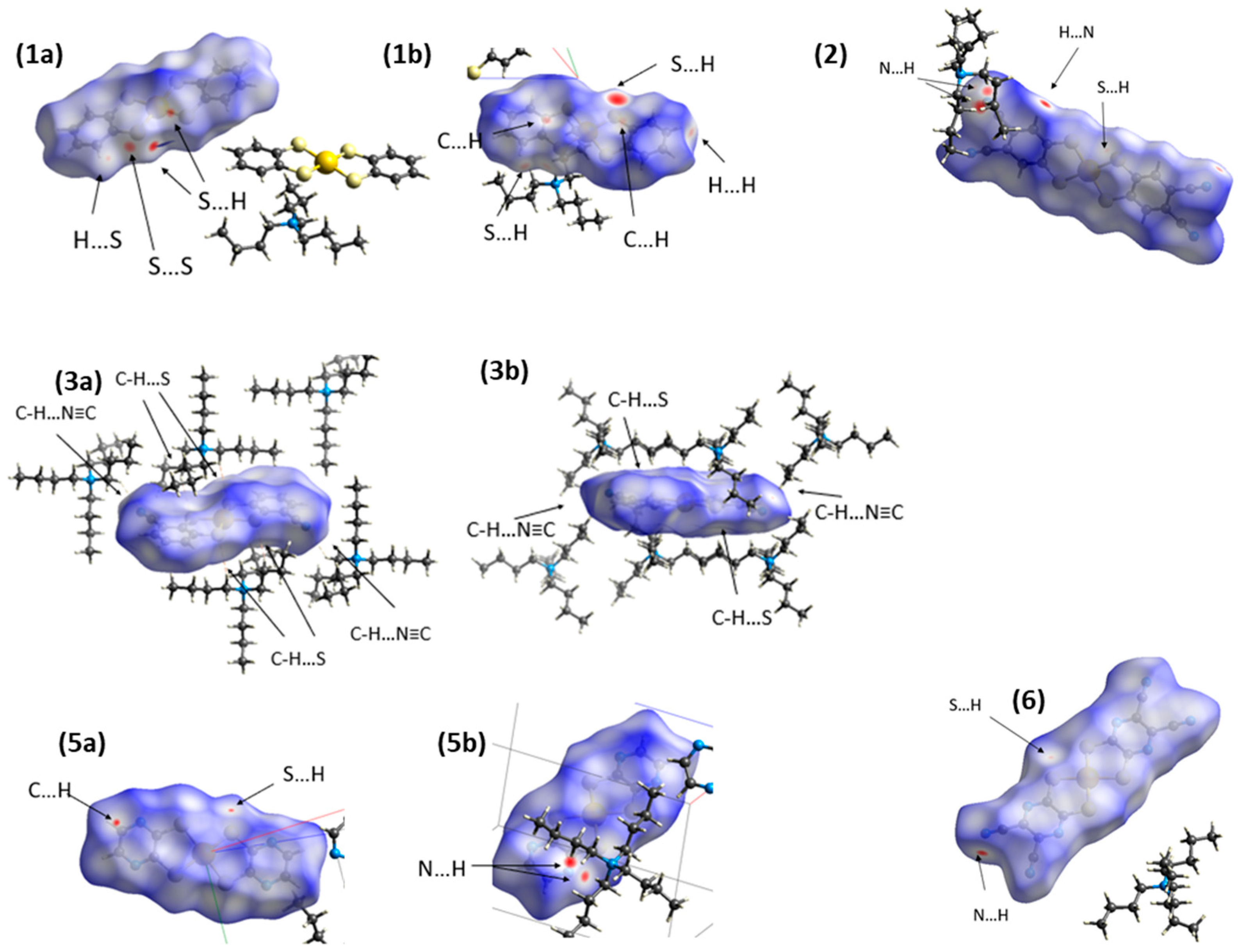
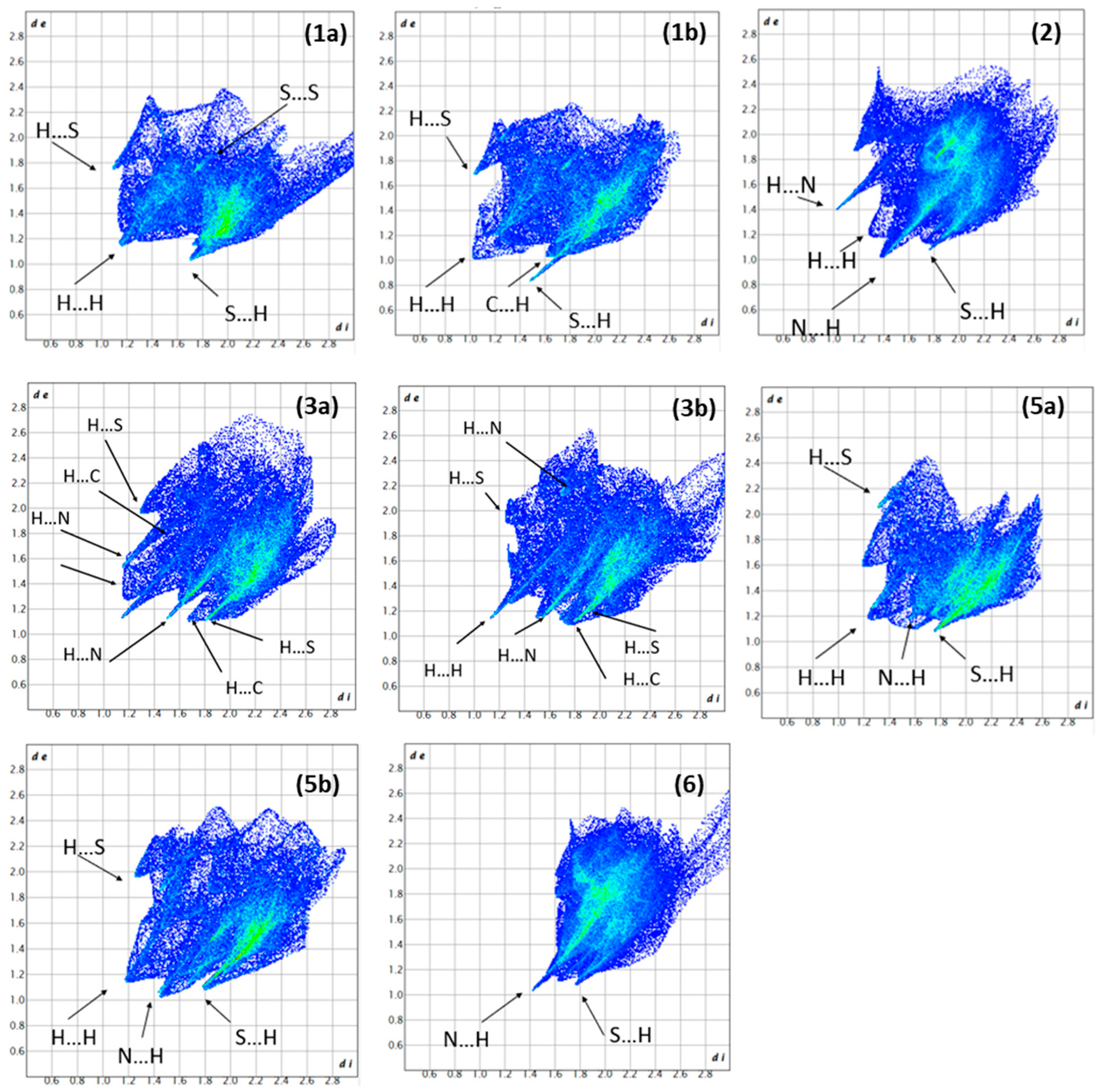
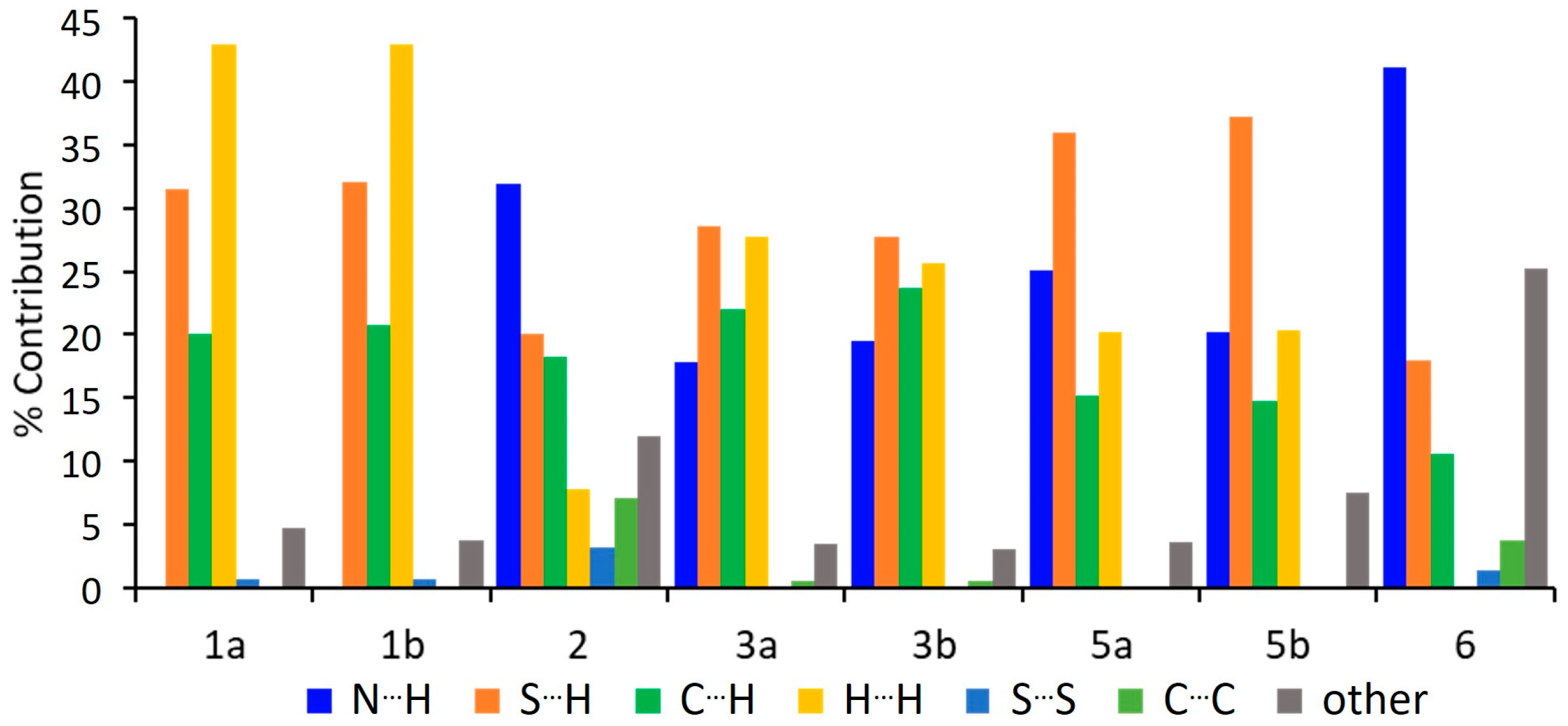
2.1.1. Hirshfeld Surface Analysis and Fingerprint Plots
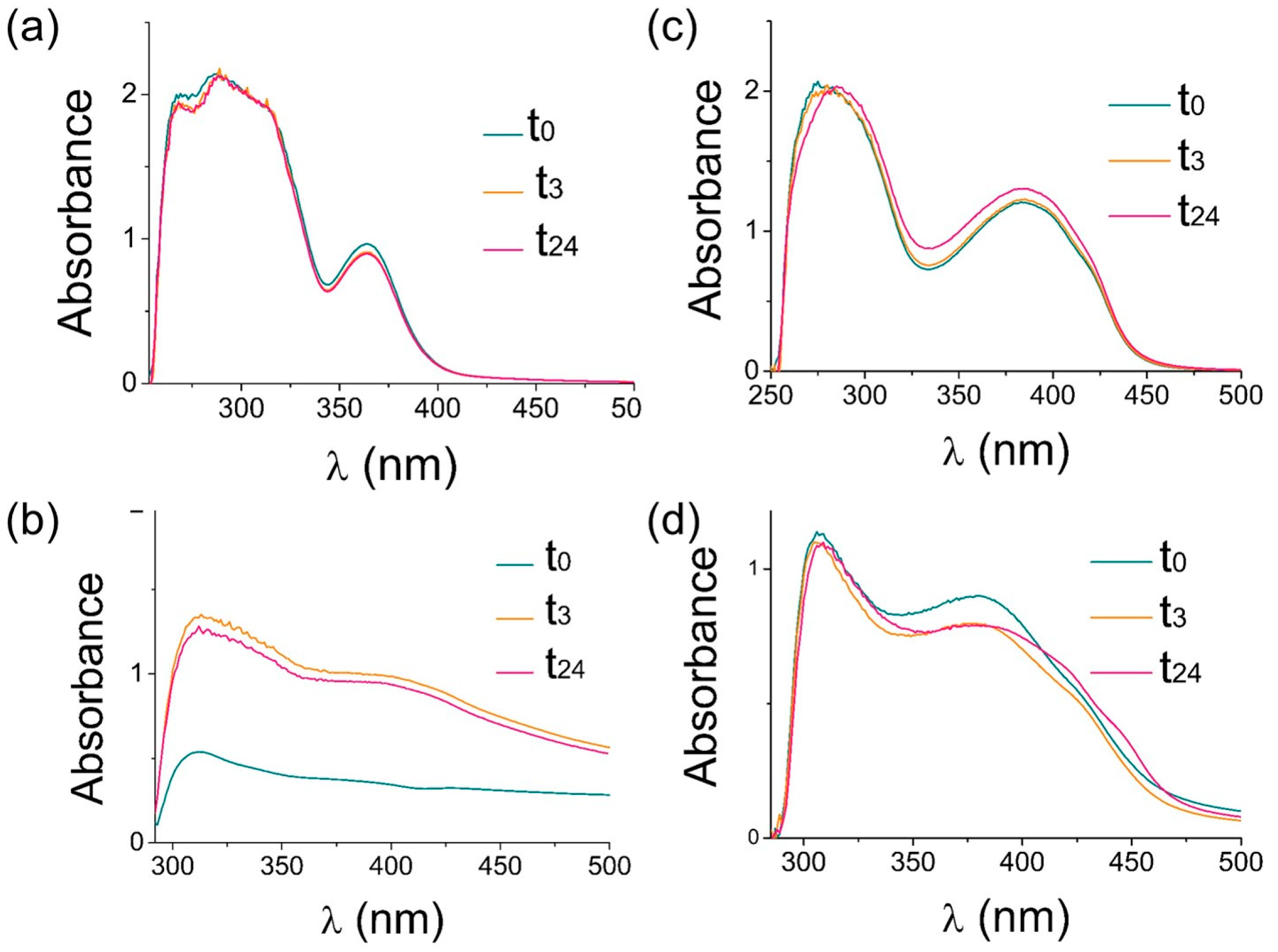
2.1.2. Studies on Complexes Stability in Solution by UV–Vis Spectroscopy
2.2. Biological Studies
2.2.1. Anticancer Activity Assessment
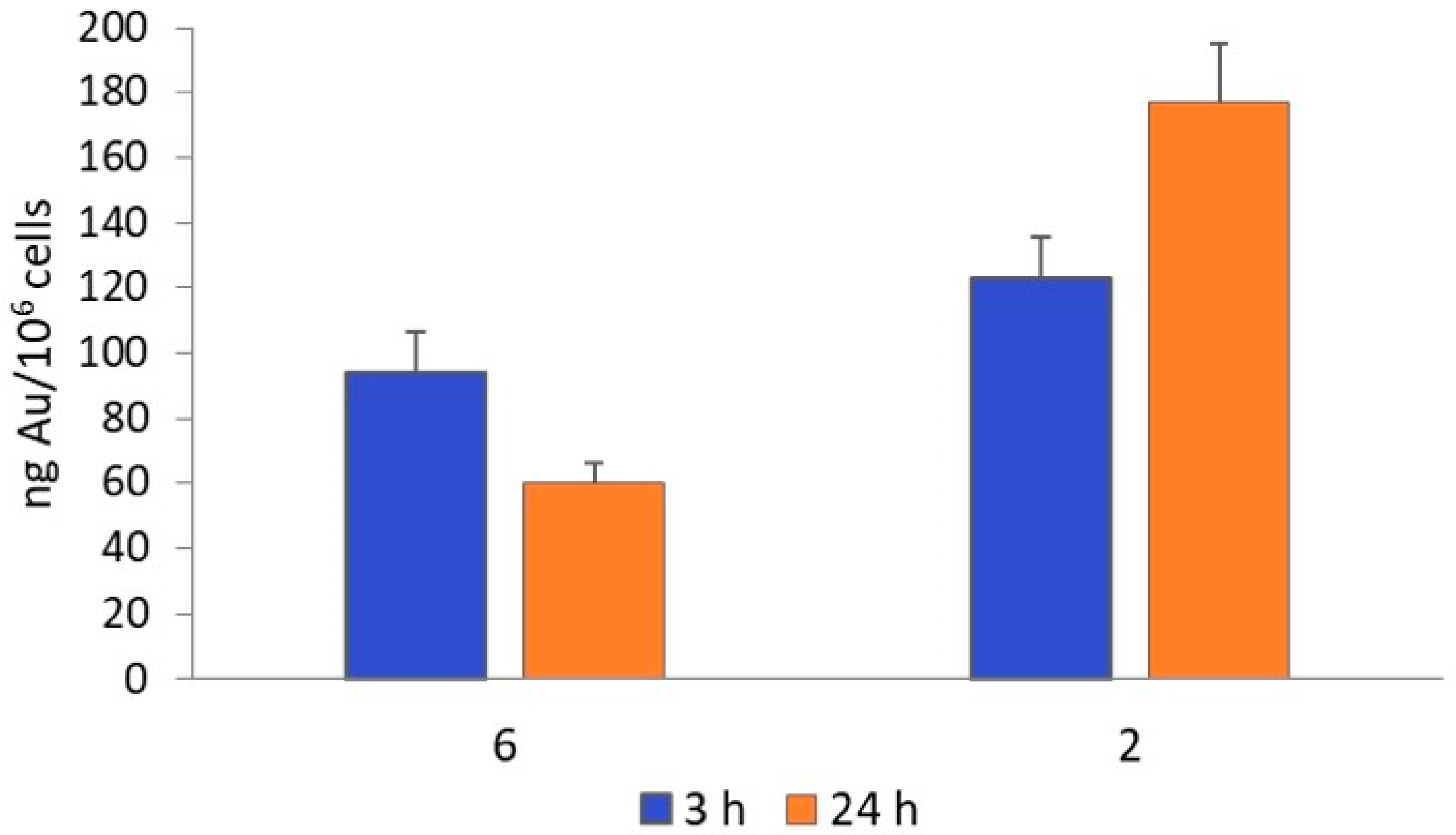
2.2.2. Cellular Uptake
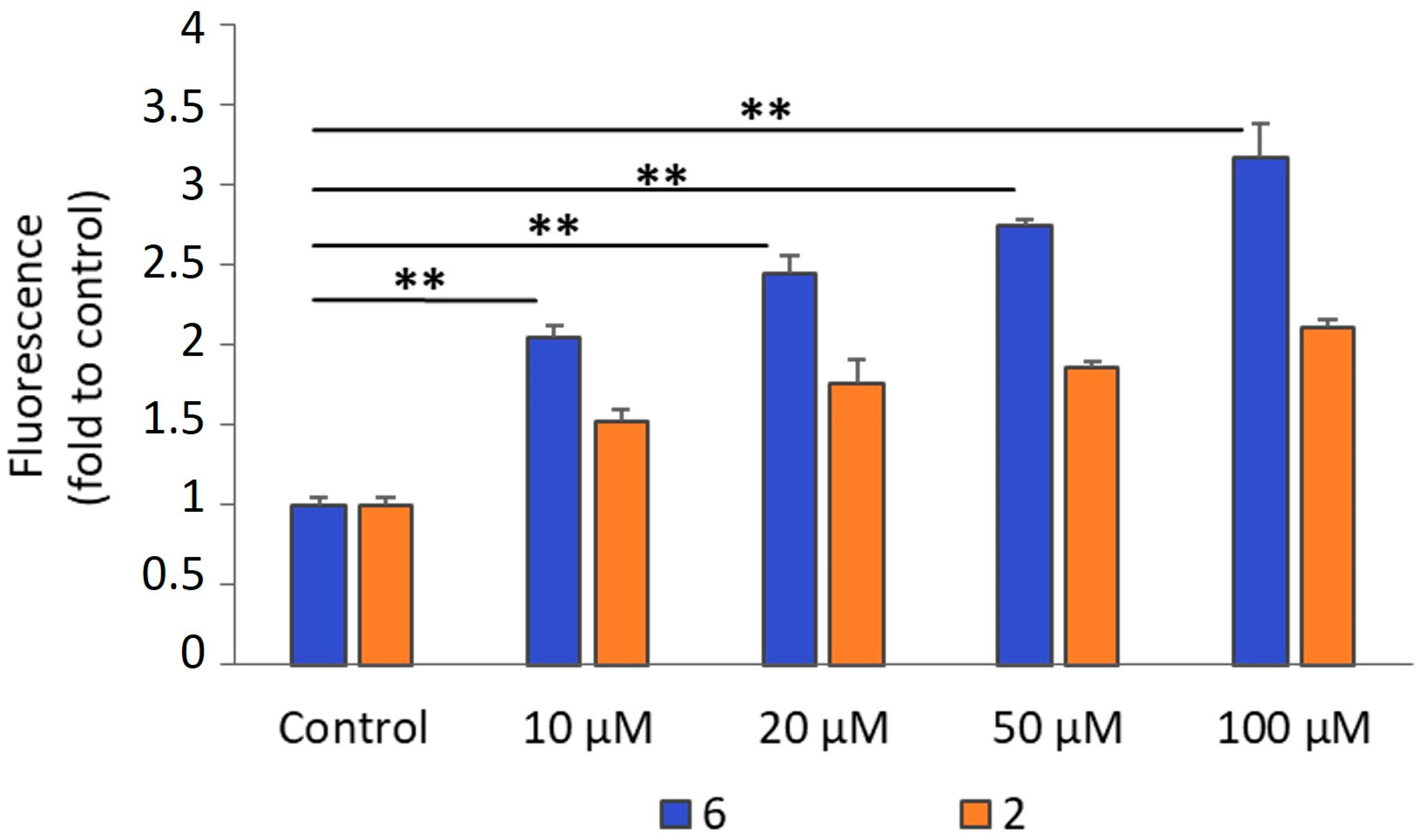
2.2.3. Production of Intracellular ROS
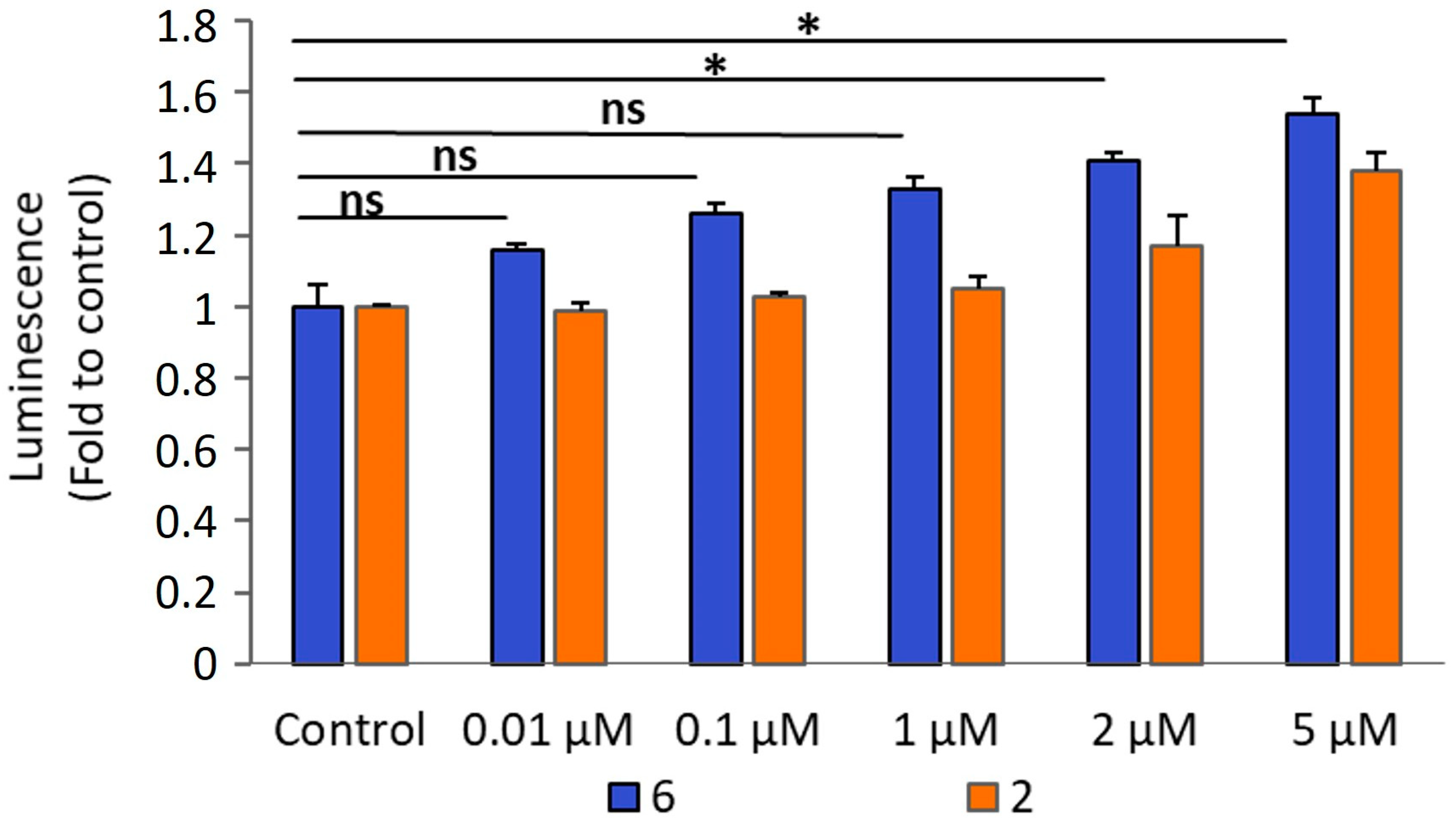
2.2.4. Apoptosis by Caspases 3/7 Activation
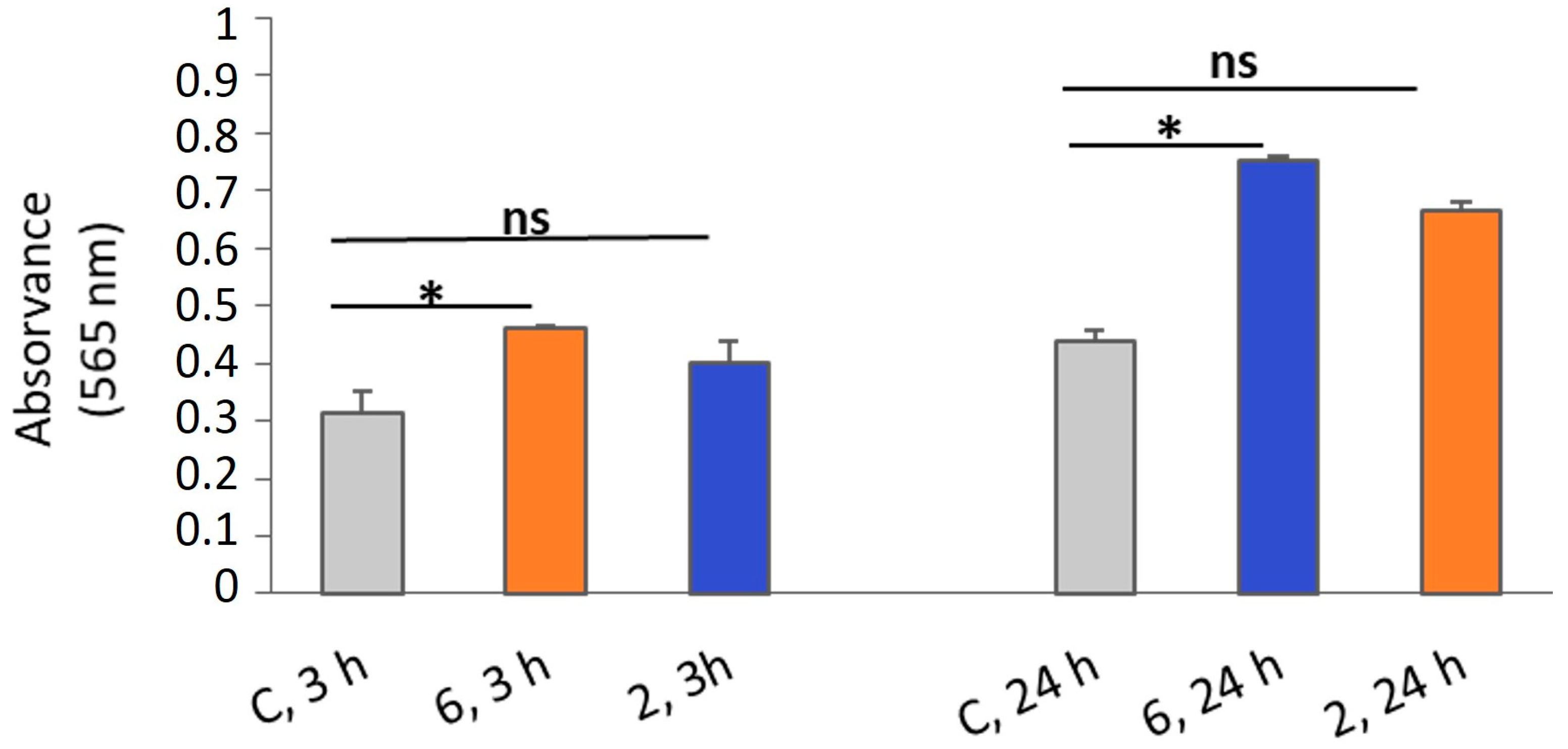
2.2.5. Necrosis by Lactate Dehydrogenase (LDH) Release
2.2.6. Antimicrobial Activity
3. Materials and Methods
3.1. Synthesis and Characterization of Complexes 1–6
3.2. Studies on Complexes Stability in Solution by UV–Vis Spectroscopy
3.3. Cytotoxic Activity Against Ovarian Cancer
3.4. Cellular Uptake
3.4.1. Cellular Uptake by PIXE
3.4.2. Cellular Uptake by ICP-MS
3.5. Intracellular ROS by H2DCF-DA
3.6. Apoptosis (Caspase 3/7)
3.7. Lactate Dehydrogenase Release
3.8. Antimicrobial Activity Determination
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics, 2024. CA Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef]
- Oh, J.K.; Weiderpass, E. Infection and cancer: Global distribution and burden of diseases. Ann. Glob. Health 2014, 80, 384–392. [Google Scholar] [CrossRef]
- De Martel, C.; Georges, D.; Bray, F.; Ferlay, J.; Clifford, G.M. Global burden of cancer attributable to infections in 2018: A worldwide incidence analysis. Lancet Glob. Health 2020, 8, e180–e190. [Google Scholar] [CrossRef]
- Liardo, E.R.L.; Borzì, A.M.; Spatola, C.; Martino, B.; Privitera, G.; Basile, F.; Biondi, A.; Vacante, M. Effects of infections on the pathogenesis of cancer. Indian J. Med. Res. 2021, 153, 431–445. [Google Scholar] [CrossRef]
- Rolston, K.V.I. Infections in cancer patients with solid tumors: A review. Infect. Dis. Ther. 2017, 6, 69–83. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, K.; Sampath, V.; Umar, S. Bacterial Infections and Cancer: Exploring this association and its implications for cancer patients. Int. J. Mol. Sci. 2023, 24, 3110. [Google Scholar] [CrossRef] [PubMed]
- Prestinaci, F.; Pezzotti, P.; Pantost, A. Antimicrobial resistance: A global multifaceted phenomenon. Pathog. Glob. Health 2015, 109, 309–318. [Google Scholar] [CrossRef]
- Mansoori, B.; Mohammadi, A.; Davudian, S.; Shirjang, S.; Baradaran, B. The different mechanisms of cancer drug resistance: A brief review. Adv. Pharm. Bull. 2017, 7, 339–348. [Google Scholar] [CrossRef]
- Bukowski, K.; Kciuk, M.; Kontek, R. Mechanisms of Multidrug Resistance in Cancer Chemotherapy. Int. J. Mol. Sci. 2020, 21, 3233. [Google Scholar] [CrossRef]
- Soo, V.W.; Kwan, B.W.; Quezada, H.; Castillo-Juárez, I.; Pérez-Eretza, B.; García-Contreras, S.J.; Martínez-Vázquez, M.; Wood, T.K.; García-Contreras, R. Repurposing of anticancer drugs for the treatment of bacterial infections. Curr. Top. Med. Chem. 2017, 17, 1157–1176. [Google Scholar] [CrossRef] [PubMed]
- Chifiriuc, M.C.; Filip, R.; Constantin, M.; Pircalabioru, G.G.; Bleotu, C.; Burlibasa, L.; Ionica, E.; Corcionivoschi, N.; Mihaescu, G. Common themes in antimicrobial and anticancer drug resistance. Front. Microbiol. 2022, 13, 960693. [Google Scholar] [CrossRef] [PubMed]
- Waters, J.E.; Stevens-Cullinane, L.; Siebenmann, L.; Hess, J. Recent advances in the development of metal complexes as antibacterial agents with metal-specific modes of action. Curr. Opin. Microbiol. 2023, 75, 102347. [Google Scholar] [CrossRef]
- Anthony, E.J.; Bolitho, E.M.; Bridgewater, H.E.; Carter, O.W.L.; Donnelly, J.M.; Imberti, C.; Lant, E.C.; Lermyte, F.; Needham, R.J.; Palau, M.; et al. Metallodrugs are unique: Opportunities and challenges of discovery and development. Chem. Sci. 2020, 11, 12888–12917. [Google Scholar] [CrossRef]
- Mjos, K.D.; Orvid, C. Metallodrugs in medicinal inorganic chemistry. Chem. Rev. 2014, 114, 4540–4563. [Google Scholar] [CrossRef]
- Ferraro, G.; Merlino, A. Metallodrugs: Mechanisms of action, molecular targets and biological activity. Int. J. Mol. Sci. 2022, 23, 3504. [Google Scholar] [CrossRef]
- Peña, Q.; Wang, A.; Zaremba, O.; Shi, Y.; Scheeren, H.W.; Metselaar, J.M.; Kiessling, F.; Pallares, R.M.; Wuttke, S.; Lammers, T. Metallodrugs in cancer nanomedicine. Chem. Soc. Rev. 2022, 51, 2544–2582. [Google Scholar] [CrossRef] [PubMed]
- Lucaciu, R.L.; Hangan, A.C.; Sevastre, B.; Oprean, L.S. Metallo-drugs in cancer therapy: Past, present and future. Molecules 2022, 27, 6485. [Google Scholar] [CrossRef] [PubMed]
- Joyce, K.; Saxena, S.; Williams, A.; Damurjian, C.; Auricchio, N.; Aluotto, S.; Tynan, H.; Demain, A.L. Antimicrobial spectrum of the antitumor agent, cisplatin. J. Antibiot. 2010, 63, 530–532. [Google Scholar] [CrossRef]
- Ghosh, S. Cisplatin: The first metal based anticancer drug. Bioorg. Chem. 2019, 88, 102925. [Google Scholar] [CrossRef]
- Liang, J.; Sun, D.; Yang, Y.; Li, M.; Li, H.; Chen, L. Discovery of metal-based complexes as promising antimicrobial agents. Eur. J. Med. Chem. 2021, 224, 113696. [Google Scholar] [CrossRef]
- Sharma, B.; Shukla, S.; Rattan, R.; Fatima, M.; Goel, M.; Bhat, M.; Dutta, S.; Ranjan, R.K.; Sharma, M. Antimicrobial agents based on metal complexes: Present situation and future prospects. Int. J. Biomater. 2022, 2022, 6819080. [Google Scholar] [CrossRef]
- Zhang, C.; Xu, C.; Gao, X.; Yao, Q. Platinum-based drugs for cancer therapy and anti-tumor strategies. Theranostics 2022, 12, 2115–2132. [Google Scholar] [CrossRef]
- Lazarević, T.; Rilak, A.; Bugarčić, Ž.D. Platinum, palladium, gold and ruthenium complexes as anticancer agents: Current clinical uses, cytotoxicity studies and future perspectives. Eur. J. Med. Chem. 2017, 142, 8–31. [Google Scholar] [CrossRef] [PubMed]
- Hanif, M.; Hartinger, C.G. Anticancer metallodrugs: Where is the next cisplatin? Future Med. Chem. 2018, 10, 615–617. [Google Scholar] [CrossRef]
- Surh, Y.J. The 50-year war on cancer revisited: Should we continue to fight the enemy within? J. Cancer Prev. 2021, 26, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Glišić, B.Đ.; Djuran, M.I. Gold complexes as antimicrobial agents: An overview of different biological activities in relation to the oxidation state of the gold ion and the ligand structure. Dalton Trans. 2014, 43, 5950–5969. [Google Scholar] [CrossRef]
- Casini, A.; Sun, R.W.; Ott, I. Medicinal Chemistry of gold anticancer metallodrugs. In Metal Ions in Life Sciences; Routledge & CRC Press: Oxford, UK, 2018; Volume 18. [Google Scholar] [CrossRef]
- APatil, S.; PHoagland, A.; APatil, S.; Bugarin, A. N-heterocyclic carbene-metal complexes as bio-organometallic antimicrobial and anticancer drugs, an update (2015–2020). Future Med. Chem. 2020, 12, 2239–2275. [Google Scholar] [CrossRef]
- Feng, L.; Pomel, S.; Latre de Late, P.; Taravaud, A.; Loiseau, P.M.; Maes, L.; Cho-Ngwa, F.; Bulman, C.A.; Fischer, C.; Sakanari, J.A.; et al. Repurposing auranofin and evaluation of a new gold(I) compound for the search of treatment of human and cattle parasitic diseases: From protozoa to helminth infections. Molecules 2020, 25, 5075. [Google Scholar] [CrossRef] [PubMed]
- Abdalbari, F.H.; Telleria, C.M. The gold complex auranofin: New perspectives for cancer therapy. Discov. Oncol. 2021, 12, 42. [Google Scholar] [CrossRef]
- Bertrand, B.; Williams, M.R.M.; Bochmann, M. Gold(III) complexes for antitumor applications: An overview. Chemistry 2018, 24, 11840–11851. [Google Scholar] [CrossRef]
- Tong, K.C.; Hu, D.; Wan, P.K.; Lok, C.N.; Che, C.M. Anticancer Gold(III) Compounds with porphyrin or N-heterocyclic carbene ligands. Front. Chem. 2020, 8, 587207. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Aodeng, G.; Ga, L.; Hai, W.; Ai, J. Research progress of metal anticancer drugs. Pharmaceutics 2023, 15, 2750. [Google Scholar] [CrossRef]
- Abás, E.; Aguirre-Ramírez, D.; Laguna, M.; Grasa, L. Selective anticancer and antimicrobial metallodrugs based on gold(III) dithiocarbamate complexes. Biomedicines 2021, 9, 1775. [Google Scholar] [CrossRef]
- Ratia, C.; Ballén, V.; Gabasa, Y.; Soengas, R.G.; Velasco-de Andrés, M.; Iglesias, M.J.; Cheng, Q.; Lozano, F.; Arnér, E.S.J.; López-Ortiz, F.; et al. Novel gold(III)-dithiocarbamate complex targeting bacterial thioredoxin reductase: Antimicrobial activity, synergy, toxicity, and mechanistic insights. Front. Microbiol. 2023, 14, 1198473. [Google Scholar] [CrossRef]
- Sousa, S.A.; Leitão, J.H.; Silva, R.A.L.; Belo, D.; Santos, I.C.; Guerreiro, J.F.; Martins, M.; Fontinha, D.; Prudêncio, M.; Almeida, M.; et al. On the path to gold: Monoanionic Au bisdithiolate complexes with antimicrobial and antitumor activities. J. Inorg. Biochem. 2020, 202, 110904. [Google Scholar] [CrossRef]
- Fontinha, D.; Sousa, S.A.; Morais, T.S.; Prudêncio, M.; Leitão, J.H.; Le Gal, Y.; Lorcy, D.; Silva, R.A.L.; Velho, M.F.G.; Belo, D.; et al. Gold(III) bis(dithiolene) complexes: From molecular conductors to prospective anticancer, antimicrobial and antiplasmodial agents. Metallomics 2020, 12, 974–987. [Google Scholar] [CrossRef]
- Le Gal, Y.; Filatre-Furcate, A.; Lorcy, D.; Jeannin, O.; Roisnel, T.; Dorcet, V.; Fontinha, D.; Francisco, D.; Prudncio, M.; Martins, M.; et al. Broad spectrum functional activity of structurally related monoanionic Au(III) bis(dithiolene) complexes. Int. J. Mol. Sci. 2022, 23, 7146. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, Y.; Li, X.; Yu, Z.; Song, C.; Du, Y. Nitrile-containing pharmaceuticals: Target, mechanism of action, and their SAR studies. RSC Med. Chem. 2021, 12, 1650–1671. [Google Scholar] [CrossRef]
- Schiødt, N.C.; Sommer-Larsen, P.; Bjørnholm, T.; Nielsen, M.F.; Larsen, J.; Bechgaard, K. Preparation and Electronic Structure of Substituted Aromatic Dithiolene Complexes of Gold(III). Inorg. Chem. 1995, 34, 3688–3694. [Google Scholar] [CrossRef]
- Kennedy, S.R.; Kozar, M.N.; Yennawar, H.P.; Lear, B.J. Synthesis and characterization of the gold dithiolene monoanion, (nBu4N)[Au(pdt=2,3-pyrazinedithiol)2]. Polyhedron 2016, 103, 100–104. [Google Scholar] [CrossRef]
- Cerdeira, A.C.; Afonso, M.L.; Santos, I.C.; Pereira, L.C.J.; Coutinho, J.T.; Rabaça, S.; Simão, D.; Henriques, R.T.; Almeida, M. Synthesis, structure and physical properties of transition metal bis 4-cyanobenzene-1,2-dithiolate complexes [M(cbdt)2]z-(M = Zn, Co, Cu, Au, Ni, Pd, z = 0, 1, 2). Polyhedron 2012, 44, 228–237. [Google Scholar] [CrossRef]
- Belo, D.; Santos, I.C.; Almeida, M. 5,6-Dicyano-2,3-dithiopyrazine (dcdmp) chemistry: Synthesis and crystal structure of Au(III)(dcdmp)2 complexes and 2,3,7,8-tetracyano-1,4,6,9-tetraazothianthrene. Polyhedron 2004, 23, 1351–1359. [Google Scholar] [CrossRef]
- Costa, A.G.; Vees, P.M.P.; Lopes, G.; Santos, I.C.; Simão, D.; Baudron, S.A.; Almeida, M.; Rabaça, S. Influence of Cation Size on the Self-Assembly of Gold Bis(dithiolate) Complexes: From Discrete Cations to 1D Coordination Polymers. J. Mol. Struct. 2025, 1344, 142940. [Google Scholar] [CrossRef]
- Alves, H.; Simão, D.; Lopes, E.B.; Belo, D.; Gama, V.; Duarte, M.T.; Novais, H.; Henriques, R.T.; Almeida, M. Structure and physical properties of (n-Bu4N)2 [Au(dcbdt)2]5. Synth. Met. 2001, 120, 1011–1012. [Google Scholar] [CrossRef]
- Mehri, H.; Gholiee, Y. Quantitative assessment of the nature and strength of Au—dithiolate bond in gold(III) bis(1,2-dithiolate) homoleptic complexes. Transit. Met. Chem. 2024, 49, 253–260. [Google Scholar] [CrossRef]
- Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Spackman, P.R.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer17; University of Western Australia: Perth, WA, Australia, 2017. [Google Scholar]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- Zaidieh, T.; Smith, J.R.; Ball, K.E.; An, Q. ROS as a novel indicator to predict anticancer drug efficacy. BMC Cancer 2019, 19, 1224. [Google Scholar] [CrossRef]
- Ahrweiler-Sawaryn, M.C.; Biswas, A.; Frias, C.; Frias, J.; Wilke, N.L.; Wilke, N.; Berkessel, A.; Prokop, A. Novel gold(I) complexes induce apoptosis in leukemia cells via the ROS-induced mitochondrial pathway with an upregulation of Harakiri and overcome multi drug resistances in leukemia and lymphoma cells and sensitize drug resistant tumor cells to apoptosis in vitro. Biomed. Pharmacother. 2023, 161, 114507. [Google Scholar] [CrossRef]
- Holmgren, G.; Synnergren, J.; Bogestål, Y.; Améen, C.; Åkesson, K.; Holmgren, S.; Lindahl, A.; Sartipy, P. Identification of novel biomarkers for doxorubicin-induced toxicity in human cardiomyocytes derived from pluripotent stem cells. Toxicology 2015, 328, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Chan, F.K.; Moriwaki, K.; De Rosa, M.J. Detection of necrosis by release of lactate dehydrogenase activity. Methods Mol. Biol. 2013, 979, 65–70. [Google Scholar] [CrossRef]
- Kabakov, A.E.; Gabai, V.L. Cell Death and Survival Assays. Chaperones 2017, 1709, 107–127. [Google Scholar] [CrossRef]
- Feng, Y.; Xiong, Y.; Qiao, T.; Li, X.; Jia, L.; Han, Y. Lactate dehydrogenase A: A key player in carcinogenesis and potential target in cancer therapy. Cancer Med. 2018, 7, 6124–6136. [Google Scholar] [CrossRef] [PubMed]
- Vitré, C.; Le Gal, Y.; Vacher, A.; Roisnel, T.; Lorcy, D.; Santana, S.; Prudêncio, M.; Pinheiro, T.; Marques, F. Structure-activity relationship of anticancer and antiplasmodial gold bis(dithiolene) complexes. Dalton Trans. 2024, 53, 11903–11913. [Google Scholar] [CrossRef] [PubMed]
- The European Committee on Antimicrobial Susceptibility Testing. MIC Determination of Non-Fastidious and Fastidious Organisms. Available online: https://www.eucast.org/ast_of_bacteria/mic_determination (accessed on 25 January 2025).
- The European Committee on Antimicrobial Susceptibility Testing. Susceptibility Testing of Yeasts. Available online: https://www.eucast.org/astoffungi/methodsinantifungalsusceptibilitytesting/susceptibility_testing_of_yeasts (accessed on 25 January 2025).
- Lambert, R.J.W.; Pearson, J. Susceptibility testing: Accurate and reproducible minimum inhibitory concentration (MIC) and non-inhibitory concentration (NIC) values. J. Appl. Microbiol. 2000, 88, 784–790. [Google Scholar] [CrossRef] [PubMed]
| Compound | E1/2 (mV) [M(L)2]0/[M(L)2]+ | E1/2 (mV) [M(L)2]−/[M(L)2]0 | E1/2 (mV) [M(L)2]−/[M(L)5]−2 | E1/2 (mV) [M(L)2]−2/[Au(L)2]− | Ref. |
|---|---|---|---|---|---|
| 1 (a) | _ | 365 | _ | _ | [40] |
| 2 (b) | _ | +1200 * | +400 | −1330 | [45] |
| 3 (c) | _ | +530 | _ | _ | [44] |
| 4 (d) | +1410 | +490 | _ | −1520 | [42] |
| 5 (e) | +1500 * | +720 * | _ | −1665 | [41] |
| 6 (f) | _ | _ | _ | −629 | [43] |
| Compound | A2780 | A2780cisR | |||
|---|---|---|---|---|---|
| 3 h | 24 h | 48 h | 24 h | 48 h | |
| [Au(bdt)2]− (1) | >100 | 31.6 ± 4.2 | 12.1 ± 2.2 | 51.4 ± 6.4 | 48.7 ± 10 |
| [Au(dcbdt)2]− (2) | 80.6 ± 16 | 5.20 ± 1.8 | 0.11 ± 0.04 | 5.62 ± 2.1 | 1.81 ± 0.7 |
| [Au(3-cbdt)2]− (3) | >100 | 8.9 ± 2.0 | 2.61 ± 1.3 | 35.3 ± 4.6 | 18.9 ± 3.6 |
| [Au(4-cbdt)2]− (4) | >100 | 17.4 ± 3.6 | 5.89 ± 1.3 | 13.5 ± 2.2 | 7.13 ± 1.0 |
| [Au(pdt)2]− (5) | 65.3 ± 17 | 30.9 ± 4.4 | 13.6 ± 4.2 | 59.5 ± 6.0 | 53.5 ± 11 |
| [Au(dcdmp)2]− (6) | 21.8 ± 5.3 | 5.10 ± 0.9 | 3.77 ± 0.9 | 3.90 ± 1.0 | 11.7 ± 2.8 |
| Cisplatin | …… | 21.1 ± 5.0 * | 3.6 ± 1.3 * | 45.0 ± 18 | 35.8 ± 13 * |
| MIC (µg/mL) | ||
|---|---|---|
| 2 | 6 | |
| S. aureus Newman | >125 | 60 ± 4.3 |
| E. coli ATCC25922 | >125 | >125 |
| B. contaminans IST408 | >125 | >125 |
| C. glabrata CBS138 | >62.5 | >62.5 |
| C. albicans SC5134 | >62.5 | >62.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belo, D.; Rabaça, S.; Fava, S.G.; Sousa, S.A.; Coelho, D.; Leitão, J.H.; Pinheiro, T.; Fernandes, C.; Marques, F. Gold(III) Complexes with Aromatic Cyano-Substituted Bisdithiolate Ligands as Potential Anticancer and Antimicrobial Agents. Molecules 2025, 30, 3270. https://doi.org/10.3390/molecules30153270
Belo D, Rabaça S, Fava SG, Sousa SA, Coelho D, Leitão JH, Pinheiro T, Fernandes C, Marques F. Gold(III) Complexes with Aromatic Cyano-Substituted Bisdithiolate Ligands as Potential Anticancer and Antimicrobial Agents. Molecules. 2025; 30(15):3270. https://doi.org/10.3390/molecules30153270
Chicago/Turabian StyleBelo, Dulce, Sandra Rabaça, Sara G. Fava, Sílvia A. Sousa, Diogo Coelho, Jorge H. Leitão, Teresa Pinheiro, Célia Fernandes, and Fernanda Marques. 2025. "Gold(III) Complexes with Aromatic Cyano-Substituted Bisdithiolate Ligands as Potential Anticancer and Antimicrobial Agents" Molecules 30, no. 15: 3270. https://doi.org/10.3390/molecules30153270
APA StyleBelo, D., Rabaça, S., Fava, S. G., Sousa, S. A., Coelho, D., Leitão, J. H., Pinheiro, T., Fernandes, C., & Marques, F. (2025). Gold(III) Complexes with Aromatic Cyano-Substituted Bisdithiolate Ligands as Potential Anticancer and Antimicrobial Agents. Molecules, 30(15), 3270. https://doi.org/10.3390/molecules30153270














