Metabolomic Insights into the Antimicrobial Effects of Metschnikowia Yeast on Phytopathogens
Abstract
1. Introduction
2. Results and Discussion
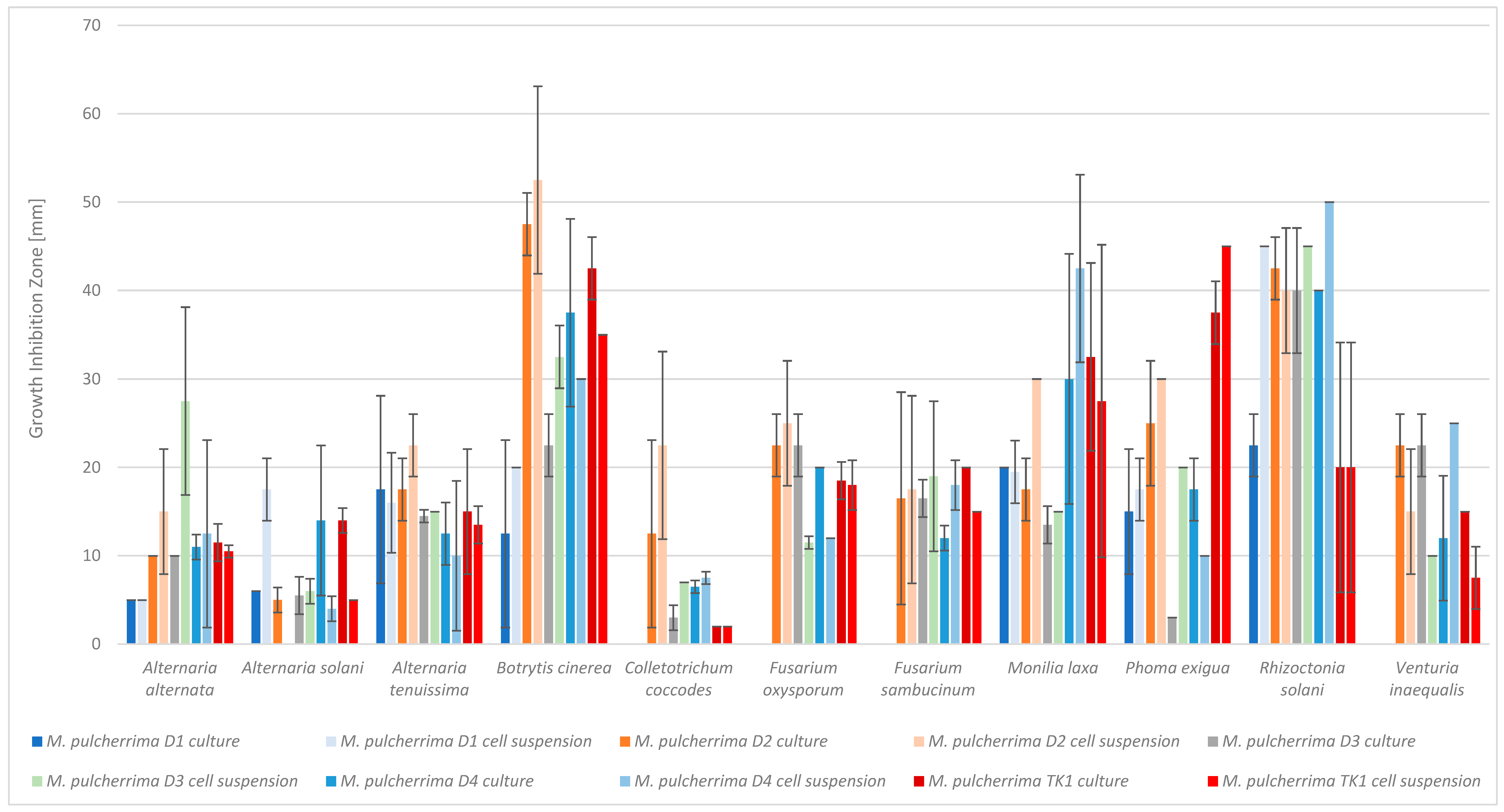
2.1. Antifungal Activity
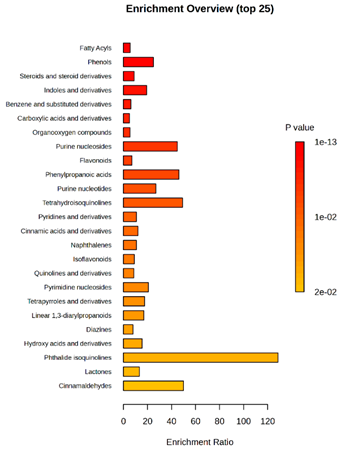
2.2. Metabolomic Analysis
2.2.1. Laser Ablation Remote Atmospheric Pressure Photoionization/Chemical Ionization Mass Spectrometry Imaging (LARAPPI/CI MSI)
2.2.2. Untargeted Ultra-High-Performance Liquid Chromatography–Quadrupole Time-of-Flight Ultra-High Resolution Mass Spectrometry and Tandem Mass Spectrometry (UHPLC-QToF-UHRMS and MS/MS)
3. Materials and Methods
3.1. Microorganisms
3.1.1. Metschnikowia pulcherrima Strains
3.1.2. Phytopathogen Strains
3.2. Antimicrobial Activity Assessment
3.3. Co-Cultivation of M. Pulcherrima and Phytopathogens
3.4. Metabolomic Analysis
3.4.1. Laser Ablation Remote Atmospheric Pressure Photoionization/Chemical Ionization Mass Spectrometry Imaging (LARAPPI/CI MSI)
3.4.2. Untargeted Ultra-High-Performance Liquid Chromatography–Quadrupole Time-of-Flight Ultra-High Resolution Mass Spectrometry and Tandem Mass Spectrometry (UHPLC-QToF-UHRMS)
3.5. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Morata, A.; Loira, I.; Escott, C.; del Fresno, J.M.; Bañuelos, M.A.; Suárez-Lepe, J.A. Applications of Metschnikowia pulcherrima in Wine Biotechnology. Fermentation 2019, 5, 63. [Google Scholar] [CrossRef]
- Fernandez-San Milan, A.; Gamir, J.; Farran, I.; Larraya, L.; Veramendi, J. Identification of new antifungal metabolites produced by the yeast Metschnikowia pulcherrima involved in the biocontrol of postharvest plant pathogenic fungi. Postharvest Biol. Technol. 2022, 192, 111995. [Google Scholar] [CrossRef]
- Lachance, M.A. Metschnikowia: Half tetrads, a regicide and the fountain of youth. Yeast 2016, 33, 563–574. [Google Scholar] [CrossRef]
- Sipiczki, M. Metschnikowia pulcherrima and Related Pulcherrimin-Producing Yeasts: Fuzzy Species Boundaries and Complex Antimicrobial Antagonism. Microorganisms 2020, 8, 1029. [Google Scholar] [CrossRef]
- Steglinska, A.; Kołtuniak, A.; Berłowska, J.; Czyżowska, A.; Szulc, J.; Cieciura-Włoch, W.; Okrasa, M.; Kręgiel, D.; Gutarowska, B. Metschnikowia pulcherrima as a Biocontrol Agent against Potato (Solanum tuberosum) Pathogens. Agronomy 2022, 12, 2546. [Google Scholar] [CrossRef]
- Sipiczki, M. Taxonomic Revision of the pulcherrima Clade of Metschnikowia (Fungi): Merger of Species. Taxonomy 2022, 2, 107–123. [Google Scholar] [CrossRef]
- Kręgiel, D.; Pawlikowska, E.; Antolak, H.; Dziekońska-Kubczak, U.; Pielech-Przybylska, K. Exploring Use of the Metschnikowia pulcherrima Clade to Improve Properties of Fruit Wines. Fermentation 2022, 8, 247. [Google Scholar] [CrossRef]
- Kręgiel, D.; Dziekonska-Kubczak, U.; Czarnecka-Chrebelska, K.; Pielech-Przybylska, K. Chemical fingerprints of honey fermented by conventional and non-conventional yeasts. Molecules 2025, 30, 2319. [Google Scholar] [CrossRef]
- Nemcová, A.; Szotkowski, M.; Samek, O.; Cagánová, L.; Sipiczki, M.; Márová, I. Use of Waste Substrates for the Lipid Production by Yeasts of the Genus Metschnikowia—Screening Study. Microorganisms 2021, 9, 2295. [Google Scholar] [CrossRef] [PubMed]
- Sipiczki, M. Metschnikowia Strains Isolated from Botrytized Grapes Antagonize Fungal and Bacterial Growth by Iron Depletion. Appl. Environ. Microbiol. 2006, 72, 6716–6724. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Li, S.J.; Yan, J.; Tang, Y.; Cheng, J.P.; Gao, A.J.; Yao, X.; Ruan, J.J.; Xu, B.L. Research Progress on Phytopathogenic Fungi and Their Role as Biocontrol Agents. Front. Microbiol. 2021, 12, 670135. [Google Scholar] [CrossRef]
- Synan, A.; Khaled, M.; Lam, S.T. Mechanisms and strategies of plant defense against Botrytis cinerea. Crit. Rev. Biotechnol. 2017, 37, 262–274. [Google Scholar] [CrossRef] [PubMed]
- Gould, M.; Nelson, L.N.; Waterer, D.; Hynes, R.K. Biocontrol of Fusarium sambucinum, dry rot of potato, by Serratia plymuthica 5–6. Biocontrol Sci. Technol. 2008, 18, 1005–1016. [Google Scholar] [CrossRef]
- González-Domínguez, E.; Armengol, J.; Rossi, V. Biology and Epidemiology of Venturia Species Affecting Fruit Crops: A Review. Front. Plant Sci. 2017, 8, 1496. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Aldwinckle, H.S.; Sutton, T.; Tsuge, T.; Kang, G.; Cong, P.H.; Cheng, Z.M. Interactions of Apple and the Alternaria alternata Apple Pathotype. Crit. Rev. Plant Sci. 2013, 32, 141–150. [Google Scholar] [CrossRef]
- Khezri, M.; Mohammadi, M. Identification and characterization of Pseudomonas syringae pv. syringae strains from various plants and geographical regions. J. Plant Protect. Res. 2018, 58, 354–361. [Google Scholar] [CrossRef]
- Dagher, F.; Olishevska, S.; Philion, V.; Zheng, J.; Deziel, E. Development of a novel biological control agent targeting the phytopathogen Erwinia amylovora. Heliyon 2020, 6, 10. [Google Scholar] [CrossRef]
- Gikas, G.D.; Parlakidis, P.; Mavropoulos, T.; Vryzas, Z. Particularities of Fungicides and Factors Affecting Their Fate and Removal Efficacy: A Review. Sustainability 2022, 14, 4056. [Google Scholar] [CrossRef]
- Kaur, R.; Mavi, G.K.; Raghav, S. Pesticides Classification and its Impact on Environment. Int. J. Curr. Microbiol. Appl. Sci. 2019, 8, 1889–1897. [Google Scholar] [CrossRef]
- Spadaro, D.; Vola, R.; Piano, S.; Gullino, M.L. Mechanisms of action and efficacy of four isolates of the yeast Metschnikowia pulcherrima active against postharvest pathogens on apples. Postharvest Biol. Technol. 2002, 24, 123–134. [Google Scholar] [CrossRef]
- Nowak, A.; Steglińska, A.; Gutarowska, B.; Kręgiel, D. Cyto- and Genotoxicity of Selected Plant Extracts and Microbial Metabolites with Confirmed Activity Against Phytopathogens of Potato Seed (Solanum tuberosum L.). Molecules 2025, 30, 701. [Google Scholar] [CrossRef] [PubMed]
- Settier-Ramírez, L.; López-Carballo, G.; Hernández-Muñoz, P.; Fontana, A.; Strub, C.; Schorr-Galindo, S. New Isolated Metschnikowia pulcherrima Strains from Apples for Postharvest Biocontrol of Penicillium expansum and Patulin Accumulation. Toxins 2021, 13, 397. [Google Scholar] [CrossRef] [PubMed]
- Kregiel, D.; Nowacka, M.; Rygala, A.; Vadkertiová, R. Biological Activity of Pulcherrimin from the Meschnikowia pulcherrima Clade. Molecules 2022, 27, 1855. [Google Scholar] [CrossRef]
- Puyo, M.; Simonin, S.; Bach, B.; Klein, G.; Alexandre, H.; Tourdot-Maréchal, R. Bio-protection in oenology by Metschnikowia pulcherrima: From field results to scientific inquiry. Front. Microbiol. 2023, 14, 1133570. [Google Scholar] [CrossRef]
- Pawlikowska, E.; James, S.A.; Breierova, E.; Antolak, H.; Kregiel, D. Biocontrol capability of local Metschnikowia sp. isolates. Antonie Leeuwenhoek 2019, 112, 1425–1445. [Google Scholar] [CrossRef]
- Bauermeister, A.; Monnochio-Russo, H.; Costa-Lotufo, L.V.; Jarmusch, A.K. Mass spectrometry-based metabolomics in microbiome investigations. Nat. Rev. Microbiol. 2021, 20, 143–160. [Google Scholar] [CrossRef]
- Szulc, J.; Grzyb, T.; Gutarowska, B.; Nizioł, J.; Krupa, S.; Ruman, T. 3D Mass Spectrometry Imaging as a Novel Screening Method for Evaluating Biocontrol Agents. J. Agric. Food Chem. 2025, 73, 8225–8242. [Google Scholar] [CrossRef]
- Ruman, T.; Krupa, Z.; Nizioł, J. Direct Three-Dimensional Mass Spectrometry Imaging with Laser Ablation Remote Atmospheric Pressure Photoionization/Chemical Ionization. Anal. Chem. 2024, 96, 13326–13334. [Google Scholar] [CrossRef] [PubMed]
- Agarbati, A.; Canonico, L.; Pecci, T.; Romanazzi, G.; Ciani, M.; Comitini, F. Biocontrol of Non-Saccharomyces Yeasts in Vineyard against the Gray Mold Disease Agent Botrytis cinerea. Microorganisms 2022, 10, 200. [Google Scholar] [CrossRef]
- Ruiz-Moyano, S.; Martín, A.; Villalobos, M.C.; Calle, A.; Serradilla, M.J.; Córdoba, M.G.; Hernández, A. Yeasts isolated from figs (Ficus carica L.) as biocontrol agents of postharvest fruit diseases. Food Microbiol. 2016, 57, 45–53. [Google Scholar] [CrossRef]
- Urazova, M.; Satenova, A.; Askarova, M.; Tuyakova, A.; Abilkhadirov, A.; Shaikhin, S. Biocontrol Activity of Metschnikowia pulcherrima Strains Isolated from Local Varieties of Apples in Kazakhstan. Int. J. Agric. Biosci. 2025, 14, 265–275. [Google Scholar]
- Türkel, S.; Korukluoglu, M.; Yavuz, M. Biocontrol Activity of the Local Strain of Metschnikowia pulcherrima on Different Postharvest Pathogens. Biotechnol. Res. Int. 2014, 2014, 397167. [Google Scholar] [CrossRef]
- Aragno, J.; Fernandes-Valle, P.; Thiriet, A.; Grondin, C.; Legras, J.-L.; Camarasa, C.; Bloem, A. Two-Stage Screening of Metschnikowia spp. Bioprotective Properties: From Grape Juice to Fermented Must by Saccharomyces cerevisiae. Microorganisms 2024, 12, 8. [Google Scholar] [CrossRef] [PubMed]
- Sova, M. Antioxidant and Antimicrobial Activities of Cinnamic Acid Derivatives. Mini-Reviews. Med. Chem. 2012, 12, 749–767. [Google Scholar]
- Boz, H. p-Coumaric acid in cereals: Presence, antioxidant and antimicrobial effects. Int. J. Food Sci. Technol. 2015, 50, 2290–2298. [Google Scholar] [CrossRef]
- Tivari, S.R.; Kokate, S.V.; Delgado-Alvarado, E.; Gayke, M.S.; Kotmale, A.; Patel, H.; Sobhia, E.M.; Kumar, S.G.; Gracia Lara, B.; Jain, V.D.; et al. A novel series of dipeptide derivatives containing indole-3-carboxylic acid conjugates as potential antimicrobial agents: The design, solid phase peptide synthesis, in vitro biological evaluation, and molecular docking study. RSC Adv. 2023, 35, 24250–24263. [Google Scholar] [CrossRef]
- Sun, P.; Huang, Y.; Yang, X.; Liao, A.; Wu, J. The role of indole derivative in the growth of plants: A review. Front. Plant Sci. 2023, 13, 1120613. [Google Scholar] [CrossRef]
- Wang, C.; Chang, T.; Yang, H.; Cui, M. Antibacterial mechanism of lactic acid on physiological and morphological properties of Salmonella Enteritidis, Escherichia coli and Listeria monocytogenes. Food Control 2015, 47, 231–236. [Google Scholar] [CrossRef]
- Dilika, F.; Bremner, P.D.; Mayer, J.J.M. Antibacterial activity of linoleic and oleic acids isolated from Helichrysum pedunculatum: A plant used during circumcision rites. Fitoterapia 2000, 71, 450–452. [Google Scholar] [CrossRef]
- Kachroo, A.; Fu, D.Q.; Havens, W.; Navarre, D.; Kachroo, P.; Ghabrial, S.A. An Oleic Acid-Mediated Pathway Induces Constitutive Defense Signaling and Enhanced Resistance to Multiple Pathogens in Soybean. Mol. Plant-Microbe Interact. 2008, 21, 564–575. [Google Scholar] [CrossRef]
- Orlo, E.; Russo, C.; Nugnes, R.; Lavorgna, M.; Isidori, M. Natural Methoxyphenol Compounds: Antimicrobial Activity against Foodborne Pathogens and Food Spoilage Bacteria, and Role in Antioxidant Processes. Foods 2021, 10, 1807. [Google Scholar] [CrossRef]
- Li, H.; Wu, X.; Li, X.; Cao, X.; Li, Y.; Cao, H.; Men, Y. Multistage Extraction of Star Anise and Black Pepper Derivatives for Antibacterial, Antioxidant, and Anticancer Activity. Front. Chem. 2021, 9, 660138. [Google Scholar] [CrossRef]
- Spaggiari, C.; Annunziato, G.; Spadini, C.; Montanaro, S.L.; Iannarelli, M.; Cabassi, C.S.; Costantino, G. Extraction and Quantification of Azelaic Acid from Different Wheat Samples (Triticum durum) Desf. and Evaluation of Their Antimicrobial and Antioxidant Activities. Molecules 2023, 28, 2134. [Google Scholar] [CrossRef]
- Rolim, T.D.S.; Sampaio, A.L.F.; Mazzei, J.L.; Moreira, D.L.; Siani, A.C. Synthesis, Bioproduction and Bioactivity of Perillic Acid—A Review. Molecules 2025, 30, 528. [Google Scholar] [CrossRef]
- Shi, C.; Sun, Y.; Zheng, Z.; Zhang, X.; Song, K.; Jia, Z.; Chen, Y.; Yang, M.; Liu, X.; Dong, R.; et al. Antimicrobial activity of syringic acid against Cronobacter sakazakii and its effect on cell membrane. Food Chem. 2016, 15, 197. [Google Scholar] [CrossRef]
- Cook, S.D. An Historical Review of Phenylacetic Acid. Plant Cell Physiol. 2019, 60, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Lobiuc, A.; Pavăl, N.-E.; Mangalagiu, I.I.; Gheorghita, R.; Teliban, G.-C.; Amariucai-Mantu, D.; Stoleru, V. Future Antimicrobials: Natural and Functionalized Phenolics. Molecules 2023, 28, 1114. [Google Scholar] [CrossRef] [PubMed]
- Abeln, F.; Chuck, C.J. Achieving a high-density oleaginous yeast culture: Comparison of four processing strategies using Metschnikowia pulcherrima. Biotechnol. Bioeng. 2019, 116, 3200–3214. [Google Scholar] [CrossRef] [PubMed]
- Middelhoven, W.J. Catabolism of benzene compounds by ascomycetous and basidiomycetous yeasts and yeastlike fungi. A literature review and an experimental approach. Antonie Leeuwenhoek 1993, 63, 125–144. [Google Scholar] [CrossRef]
- Schweigert, N.; Zehnder, A.J.B.; Eggen, R.I.L. Chemical properties of catechols and their molecular modes of toxic action in cells, from microorganisms to mammals. Environ. Microbiol. 2021, 3, 81–91. [Google Scholar] [CrossRef]
- Neto, A.C.; Luiz, C.; Maraschin, M.; Di Piero, R.M. Efficacy of salicylic acid to reduce Penicillium expansum inoculum and preserve apple fruits. Int. J. Food Microbiol. 2016, 221, 54–60. [Google Scholar] [CrossRef]
- Panahirad, S.; Zaare-Nahandi, F.; Safaralizadeh, R.; Alizadeh-Salteh, S. Postharvest control of Rhizopus stolonifer in peach (Prunus persica L. Batsch) fruits using salicylic acid. J. Food Saf. 2012, 32, 502–507. [Google Scholar] [CrossRef]
- Hussain, M.; Hamid, M.I.; Ghazanfar, M.U. Salicylic acid induced resistance in fruits to combat against postharvest pathogens: A review. Arch. Phytopathol. Plant Prot. 2015, 48, 34–42. [Google Scholar] [CrossRef]
- Dalavaye, N.; Nicholas, M.; Pillai, M.; Erridge, S.; Sodergren, M.H. The Clinical Translation of α-humulene—A Scoping Review. Planta Medica 2024, 90, 664–674. [Google Scholar] [CrossRef]
- Mączka, W.; Duda-Madej, A.; Górny, A.; Grabarczyk, M.; Wińska, K. Can Eucalyptol Replace Antibiotics? Molecules 2021, 26, 4933. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.; Goossens, A.; Lacchini, E. Jasmonate: A hormone of primary importance for plant metabolism. Curr. Opin. Plant Biol. 2022, 67, 102197. [Google Scholar] [CrossRef]
- Rao, I.R.; Punitha, P.; Premalatha, B.; Suresh, M. Synthesis, structure identification, antioxidant and antimicrobial activities of some novel quinoline derivatives. Discover Chem. 2024, 1, 65. [Google Scholar] [CrossRef]
- Kuppala, R.; Govindarajan, M.; Tambat, R.; Patel, N.; Nandanwar, H.; Bhutani, K.K.; Kartha, K.P.R. Synthesis and antibacterial activity of ricinoleic acid glycosides. RSC Adv. 2016, 6, 3700–3713. [Google Scholar] [CrossRef]
- Ghany, S.S.H.A.E.; Ibrahem, R.A.; EL-Gendy, A.O.; Mahmoud, R.; El-Baky, A.; Mustafa, A.; Azmy, A.F. Novel synergistic interactions between monolaurin, a mono-acyl glycerol and β lactam antibiotics against Staphylococcus aureus: An in vitro study. BMC Infect Dis. 2024, 24, 379. [Google Scholar] [CrossRef]

| No | Compounds | M. pulcherrima D2 | M. pulcherrima D4 | M. pulcherrima TK1 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | B. cinerea | R. solani | Control | B. cinerea | R. solani | Control | B. cinerea | R. solani | A. alternata | M. laxa | ||
| 1 | 2-Ketobutyric acid a,b | − | −/− | +/− | + | +/− | +/− | + | +/+ | +/+ | +/− | +/+ |
| 2 | Serine a,b | − | +/+ | +/− | + | +/+ | +/+ | + | −/+ | −/− | +/− | −/− |
| 3 | Proline a,b,c,d | − | −/− | +/− | + | +/+ | +/+ | − | +/− | −/− | +/+ | +/− |
| 4 | Indole a,b,c,d | − | +/− | +/− | n.d. | n.d. | n.d. | − | +/− | − | +/− | +/− |
| 5 | 3-Hydroxyisovaleric acid a,b | + | +/− | +/− | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| 6 | 2-Hydroxy-2-methylbutyric acid a,b | n.d. | n.d. | n.d. | + | +/− | +/− | + | +/− | −/− | +/− | +/− |
| 7 | Threonine a,b | + | +/+ | +/− | + | +/+ | +/+ | − | +/− | − | +/− | +/+ |
| 8 | Histidine a,b | n.d. | n.d. | n.d. | − | +/− | +/− | n.d. | n.d. | n.d. | n.d. | n.d. |
| 9 | Phenylalanine a,b | n.d. | n.d. | n.d. | + | +/+ | +/+ | − | +/+ | −/− | +/− | +/− |
| 10 | Perillic acid a,b,c | n.d. | n.d. | n.d. | + | +/+ | +/− | + | +/− | +/− | +/− | +/− |
| 11 | o-Tyrosine a,b,c | n.d. | n.d. | n.d. | − | +/− | +/− | n.d. | n.d. | n.d. | n.d. | n.d. |
| 12 | Pipecolic acid a,b | − | −/− | +/− | + | +/+ | +/− | n.d. | n.d. | n.d. | n.d. | n.d. |
| 13 | 3-Methylindole a,c,d | − | +/+ | +/− | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| 14 | Glutamic acid a | + | +/+ | +/− | + | +/+ | +/+ | + | +/− | +/− | +/− | +/− |
| 15 | 2-Hydroxycaproic acid a,b,c | + | +/− | +/− | + | +/+ | +/+ | − | +/+ | +/− | +/− | +/− |
| 16 | Malic acid a,b | − | −/− | +/− | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| 17 | Lactic acid a | n.d. | n.d. | n.d. | + | +/− | +/− | n.d. | n.d. | n.d. | n.d. | n.d. |
| 18 | 3,4-Dimethylbenzaldehyde a,c,d | + | +/− | +/− | n.d. | n.d. | n.d. | + | −/− | −/+ | +/− | +/− |
| 19 | Phenylacetic acid a,b,c | − | +/− | +/− | − | +/+ | +/+ | − | +/− | +/+ | −/− | +/− |
| 20 | Syringic acid a,b | n.d. | n.d. | n.d. | + | +/− | +/− | + | +/− | +/− | +/− | +/− |
| 21 | 4-Hydroxyquinoline a,c,d | − | −/+ | +/+ | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| 22 | Cinnamic acid a,d | + | +/+ | +/− | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| 23 | Hydrocinnamic acid a,b,d | + | +/− | +/− | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| 24 | 4-Methylcinnamic acid a,d | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | − | −/− | −/+ | −/− | −/+ |
| 25 | p-Anisic acid a,b | + | +/− | +/− | + | +/− | +/− | + | +/− | +/− | +/− | +/− |
| 26 | 3,5-Dimethoxyphenol a,b,c | + | +/− | +/− | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| 27 | m-Coumaric acid a,b,c | − | +/+ | +/− | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| 28 | 4-Pyridoxic acid a,b | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | − | −/+ | −/− | −/− | −/+ |
| 29 | 5-Hydroxytryptophan a,b | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | + | +/− | +/− | +/− | +/− |
| 30 | Pulcherriminic acid a | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | − | −/− | −/− | +/− | −/− |
| 31 | Oleic acid a,c | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | − | +/− | +/− | +/− | +/− |
| Name of Chemical Compound | Ion Image | Name of Chemical Compound | Ion Image |
|---|---|---|---|
| Metschnikowia pulcherrima yeast D2 (center) and Alternaria alternata culture grown on PDA medium—control pre-ablation |  | Metschnikowia pulcherrima yeast D4 (center) and Monilia laxa culture grown on PDA medium—control pre-ablation |  |
| Acetic acid | 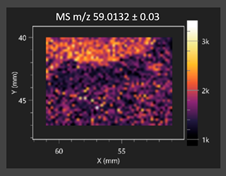 | Acetic acid | 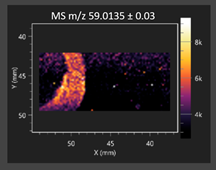 |
| Pyruvaldehyde | 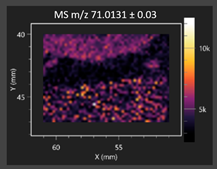 | Pyruvaldehyde | 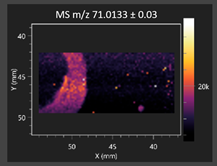 |
| Lactic acid | 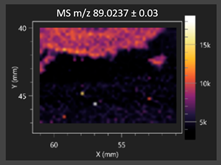 | Lactic acid | 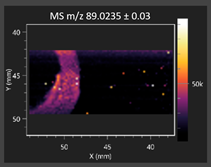 |
| 3-Phenyllactic acid | 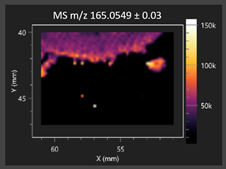 | Succinic acid semialdehyde | 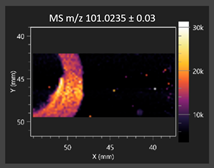 |
| Azelaic acid | 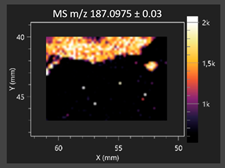 | Cinnamic acid | 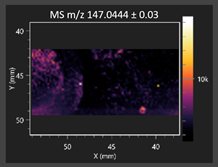 |
| Samples Compared | Number of Metabolic Pathways Identified * | Number of Chemical Compounds Identified # |
|---|---|---|
| M.p D2 | 63 | 1002 |
| M.p D4 | 64 | 2458 |
| M.p TK1 | 61 | 713 |
| M.p D2/P9 | 46 | 344 # |
| M.p D4/P9 | 49 | 855 # |
| M.p TK1/P9 | 47 | 830 # |
| M.p D2 vs. M.p D4 vs. M.p TK1 | 17–19 * | 336 # |
| M.p D2/P5 vs. M.p D4/P5 vs. M.p TK1/P5 | 23–26 * | 477 # |
| M.p D2/P9 vs. M.p D4/P9 vs. M.p TK1/P9 | 26–30 * | 511 # |
| M.p D2/P10 vs. M.p D4/P10 vs. M.p TK1/P10 | 24–25 * | 479 # |
| M.p D2/P11 vs. M.p D4/P11 vs. M.p TK1/P11 | 24–28 * | 519 # |
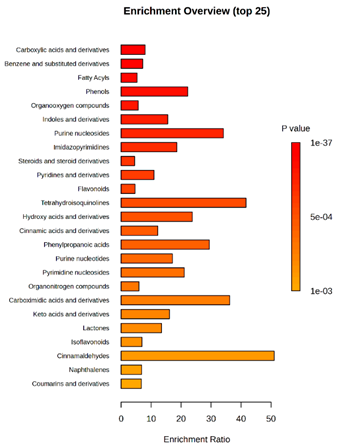 (KD2 vs. D2/P5, D2/P9, D2/P10, D2/P11) | 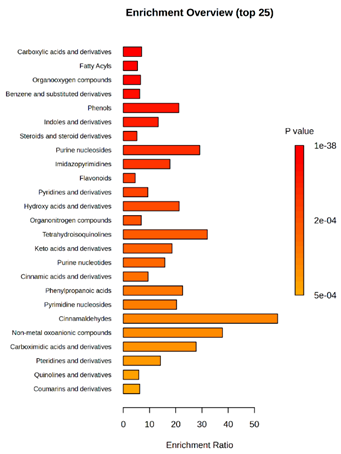 KD4 vs. D4/P5, D4/P9, D4/P10, D4/P11) |
 (KTK1 vs. TK1/P5, TK1/P9, TK1/P10, TK1/P11) | 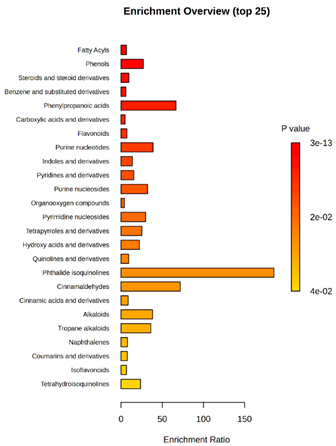 D2 vs. D4 vs. TK1 |
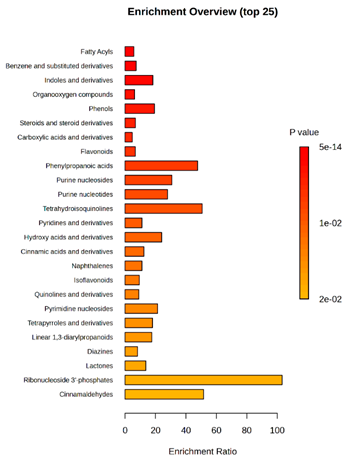 D2/P5 vs. D4/P5 vs. TK1/P5 | 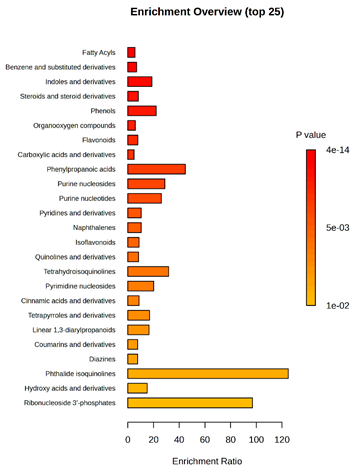 D2/P9 vs. D4/P9 vs. TK1/P9 |
 D2/P10 vs. D4/P10 vs. TK1/P10 | 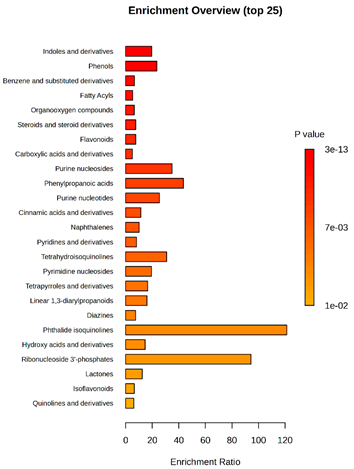 D2/P11 vs. D4/P11 vs. TK1/P11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perek, Z.; Krupa, S.; Nizioł, J.; Kręgiel, D.; Ruman, T.; Gutarowska, B. Metabolomic Insights into the Antimicrobial Effects of Metschnikowia Yeast on Phytopathogens. Molecules 2025, 30, 3268. https://doi.org/10.3390/molecules30153268
Perek Z, Krupa S, Nizioł J, Kręgiel D, Ruman T, Gutarowska B. Metabolomic Insights into the Antimicrobial Effects of Metschnikowia Yeast on Phytopathogens. Molecules. 2025; 30(15):3268. https://doi.org/10.3390/molecules30153268
Chicago/Turabian StylePerek, Zofia, Sumi Krupa, Joanna Nizioł, Dorota Kręgiel, Tomasz Ruman, and Beata Gutarowska. 2025. "Metabolomic Insights into the Antimicrobial Effects of Metschnikowia Yeast on Phytopathogens" Molecules 30, no. 15: 3268. https://doi.org/10.3390/molecules30153268
APA StylePerek, Z., Krupa, S., Nizioł, J., Kręgiel, D., Ruman, T., & Gutarowska, B. (2025). Metabolomic Insights into the Antimicrobial Effects of Metschnikowia Yeast on Phytopathogens. Molecules, 30(15), 3268. https://doi.org/10.3390/molecules30153268









