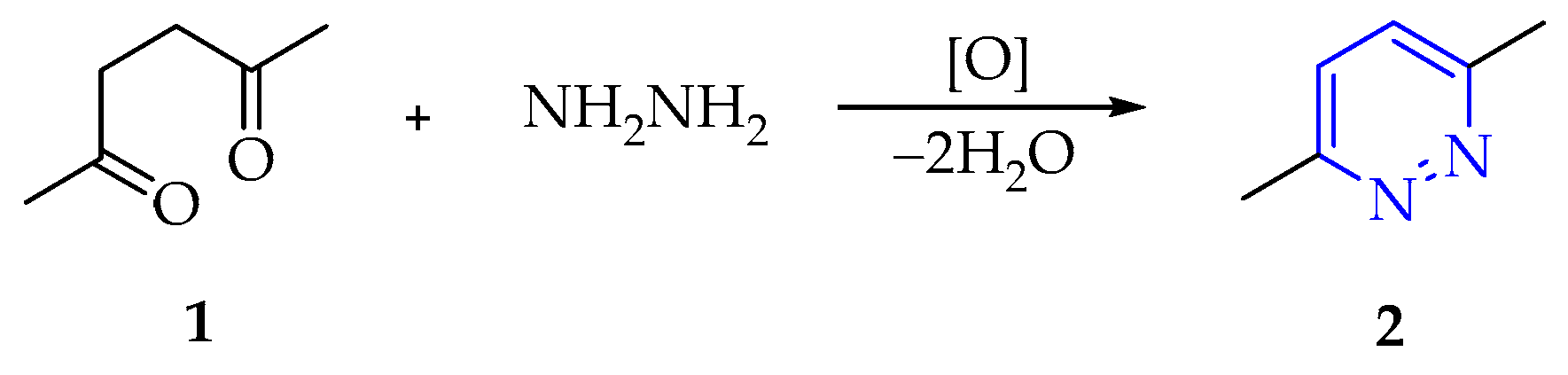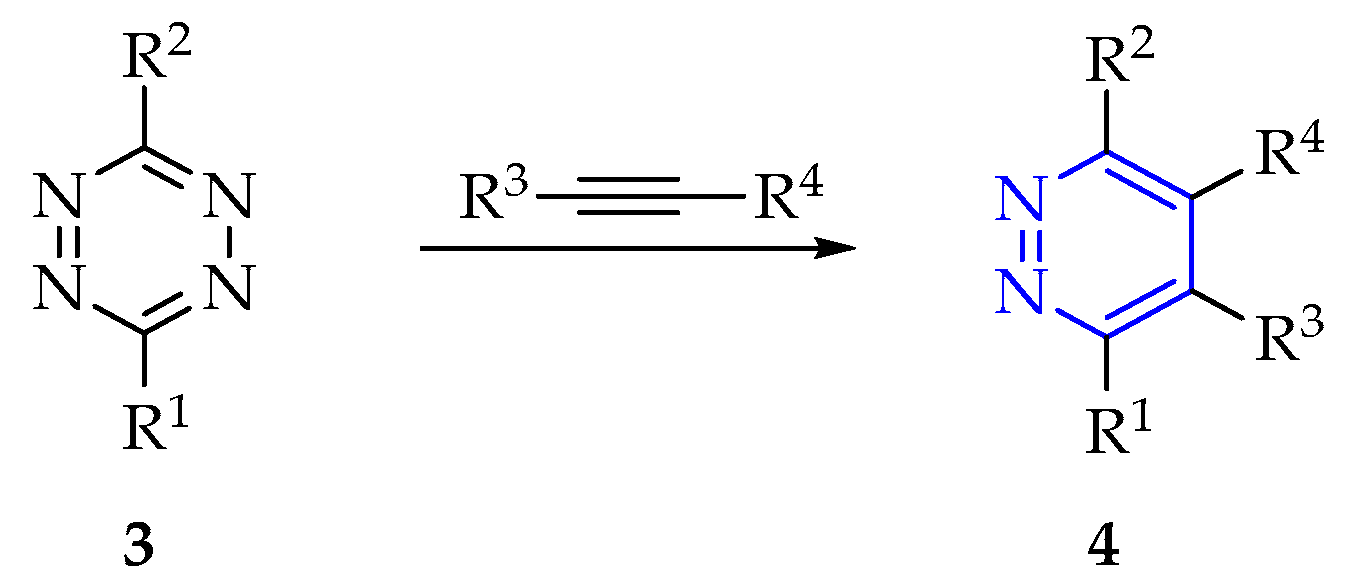Efficient Approaches to the Design of Six-Membered Polyazacyclic Compounds—Part 1: Aromatic Frameworks
Abstract
1. Introduction
2. Application of Six-Membered Polyazocyclic Compounds
2.1. Diazines
2.2. Triazines
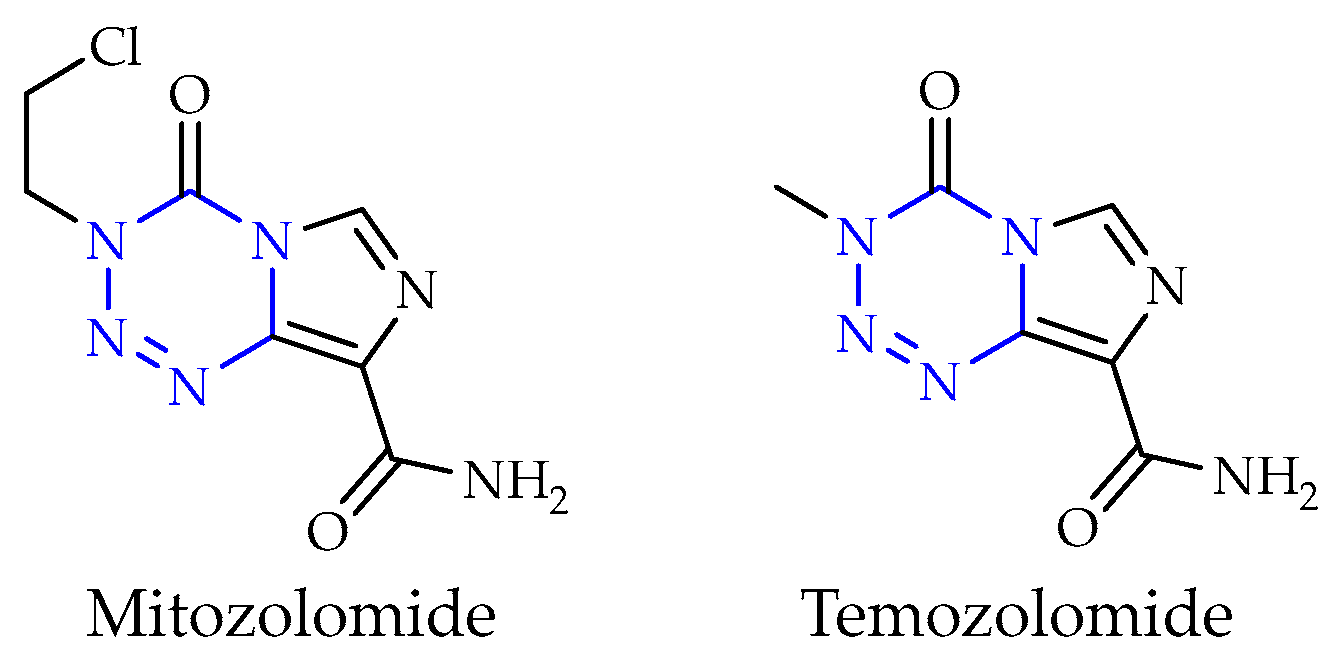
2.3. Tetrazines
3. Synthesis of Six-Membered Polyazocyclic Compounds
3.1. Synthesis of Diazine Derivatives
3.1.1. Synthesis of Pyridazine Derivatives
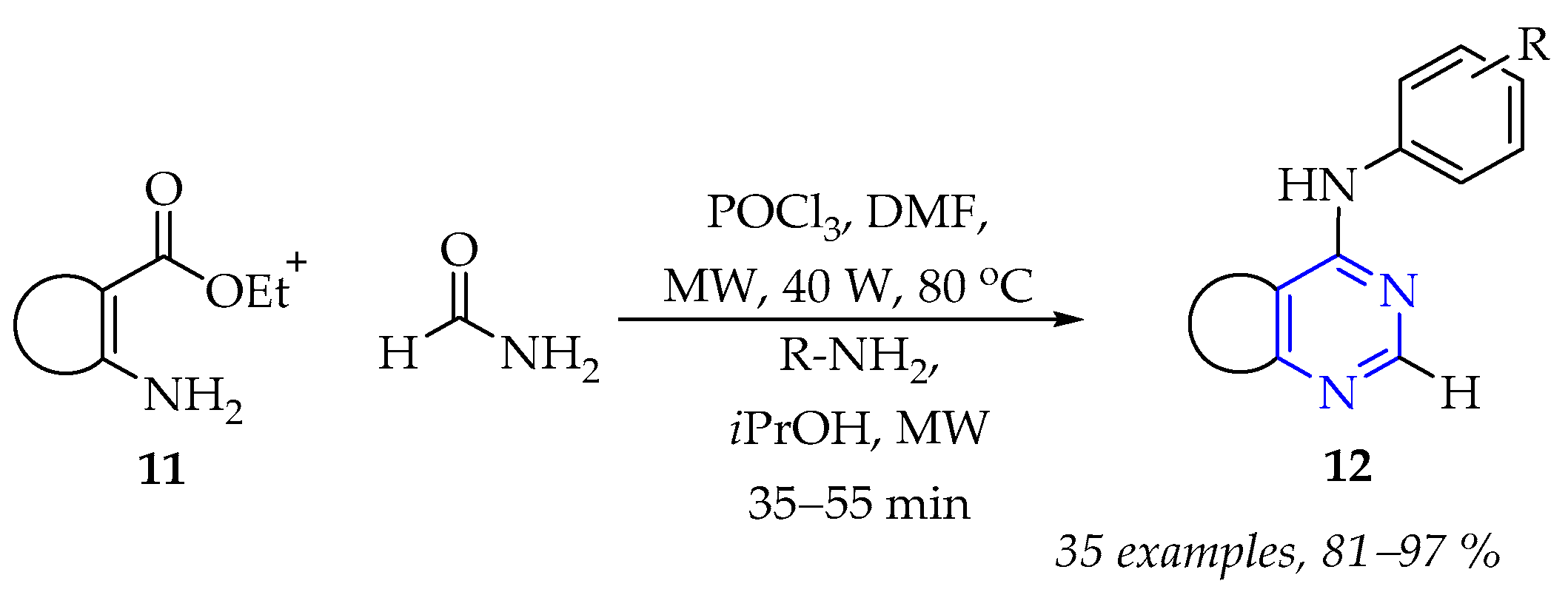
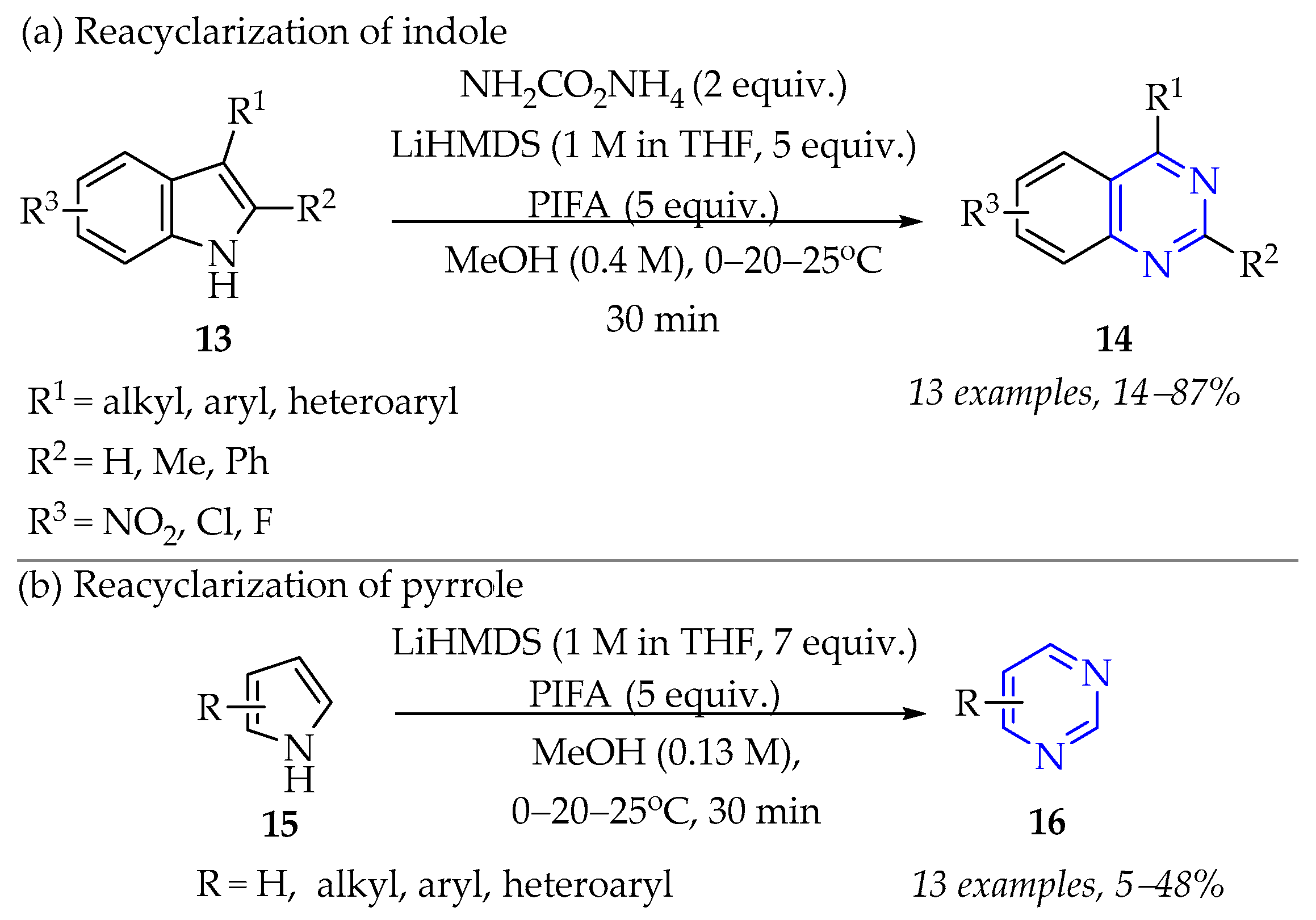
3.1.2. Synthesis of Pyrimidine Derivatives
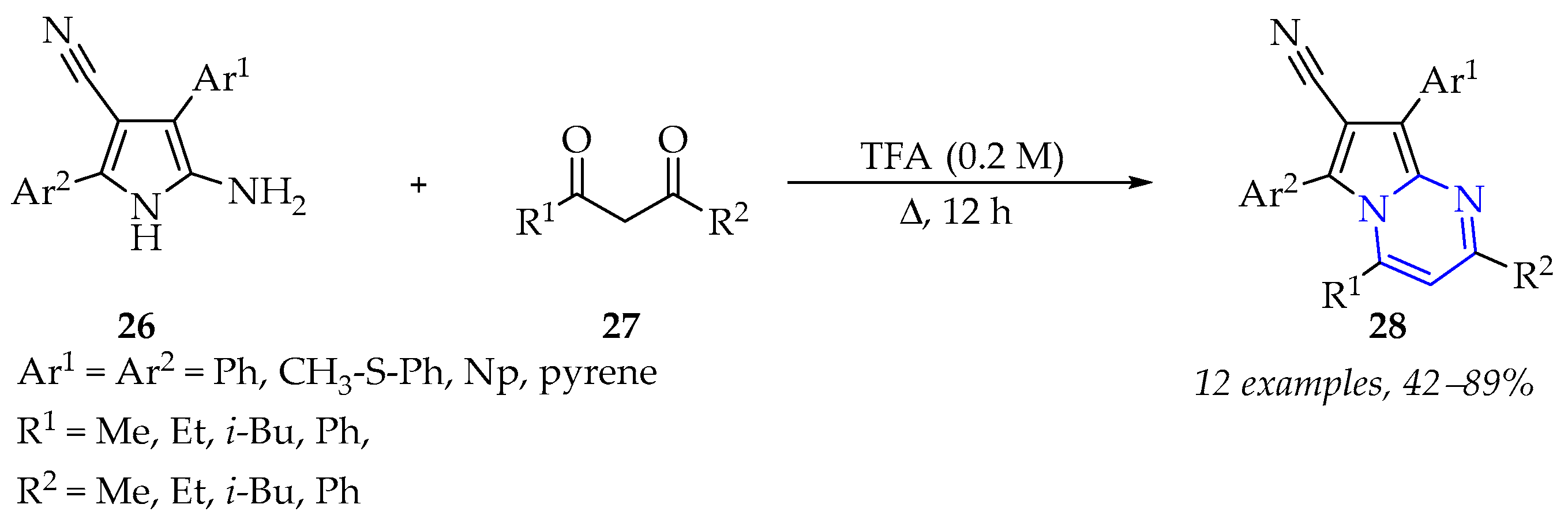
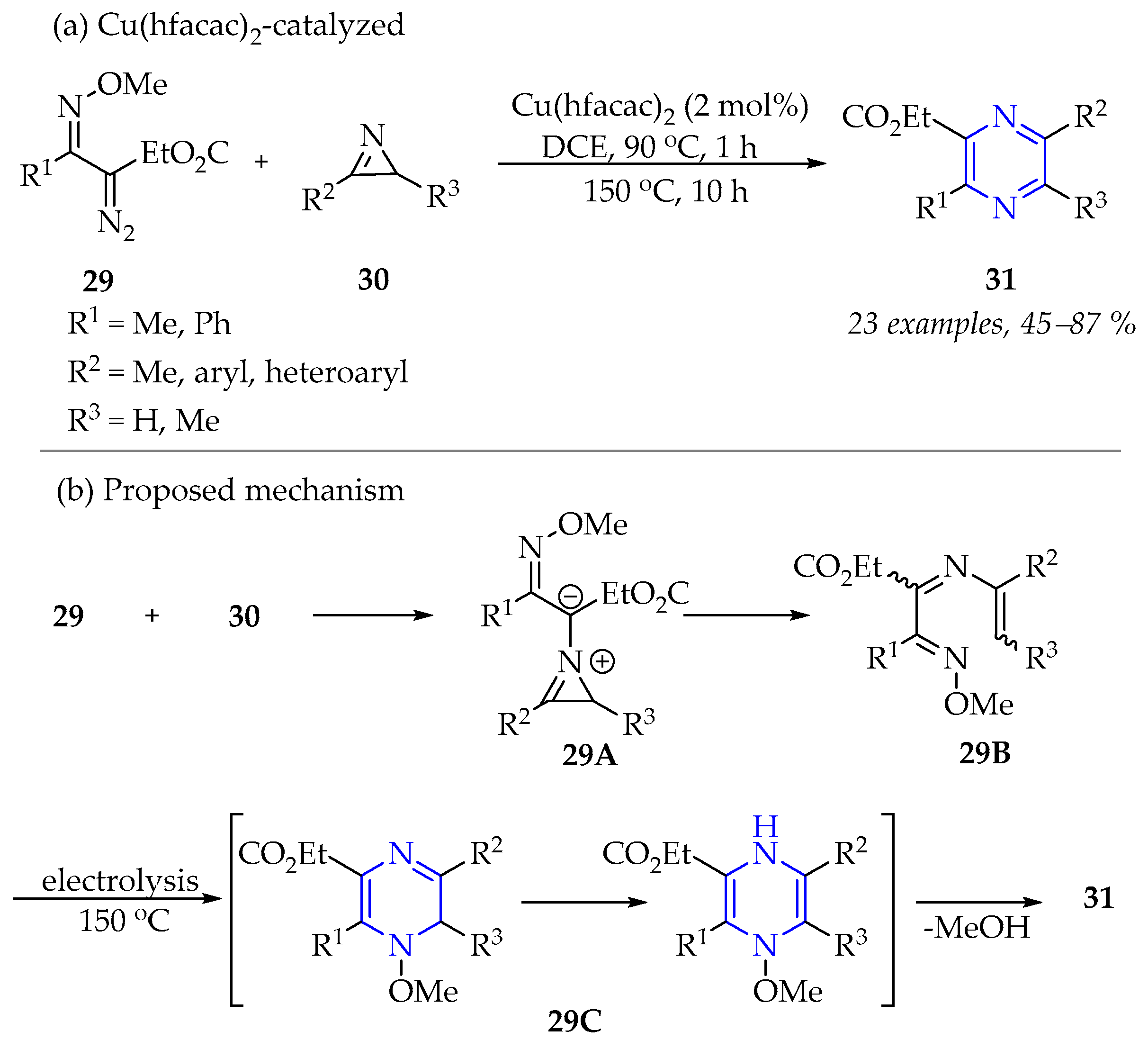
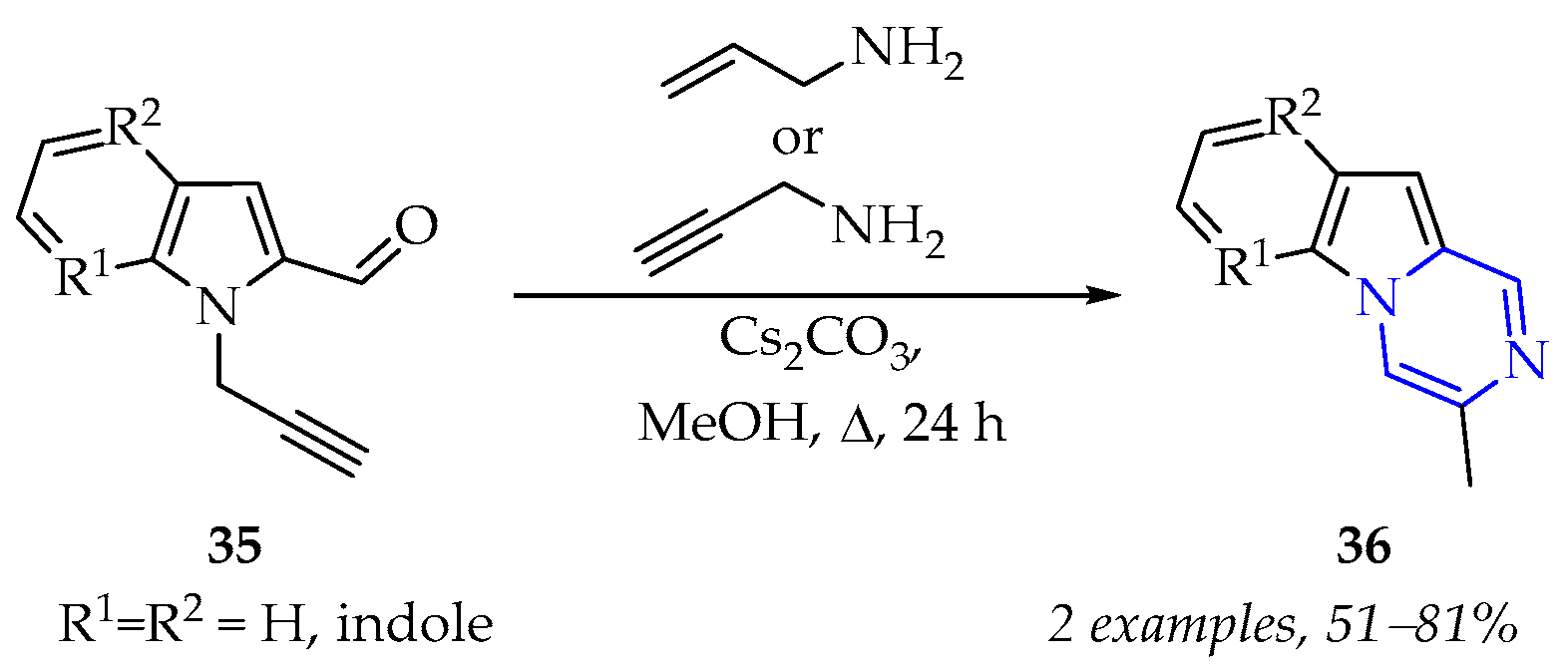
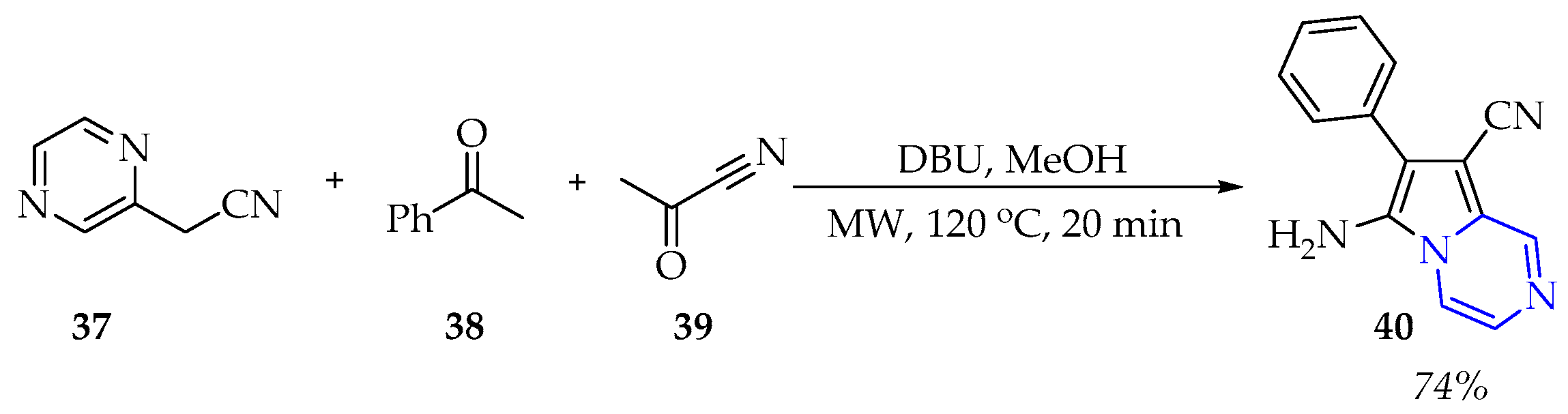
3.1.3. Synthesis of Pyrazine Derivatives
3.2. Synthesis of Triazine Derivatives
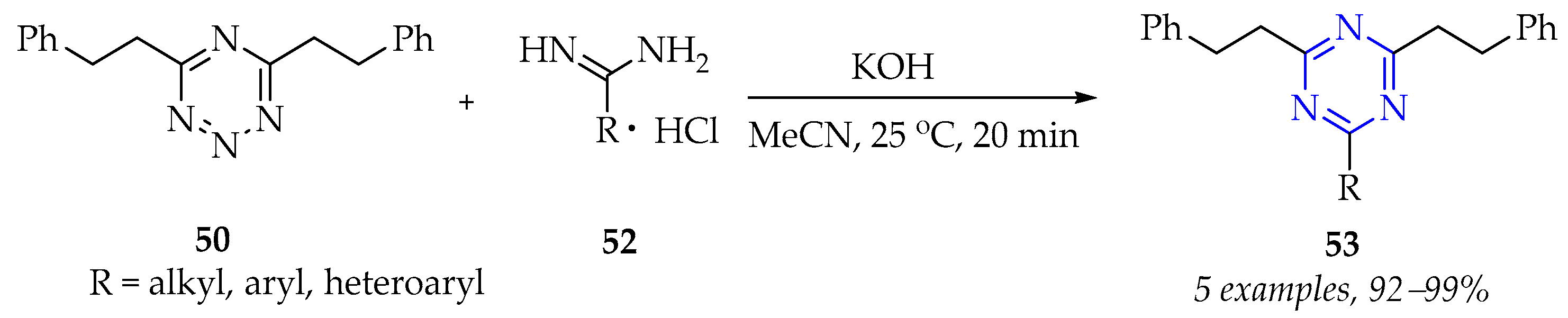
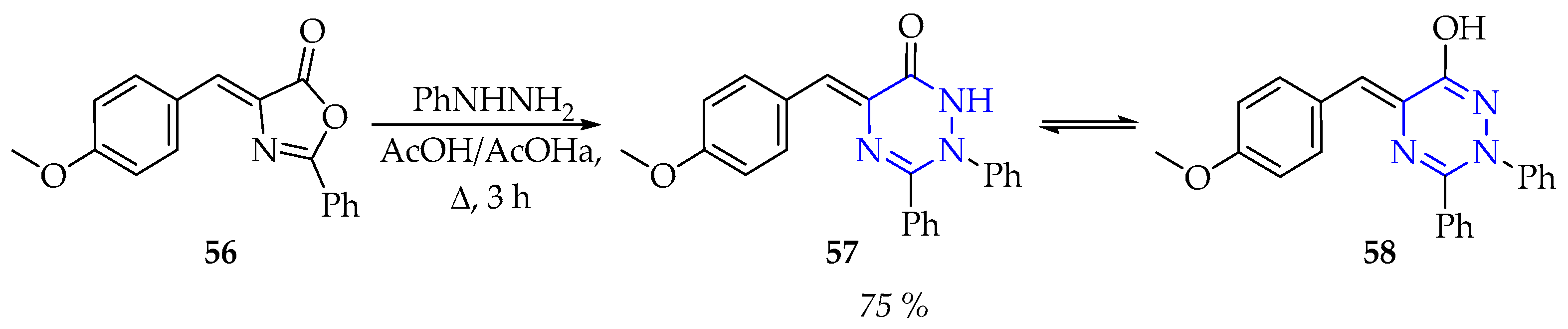
3.2.1. Synthesis of 1,3,5-Triazine Derivatives
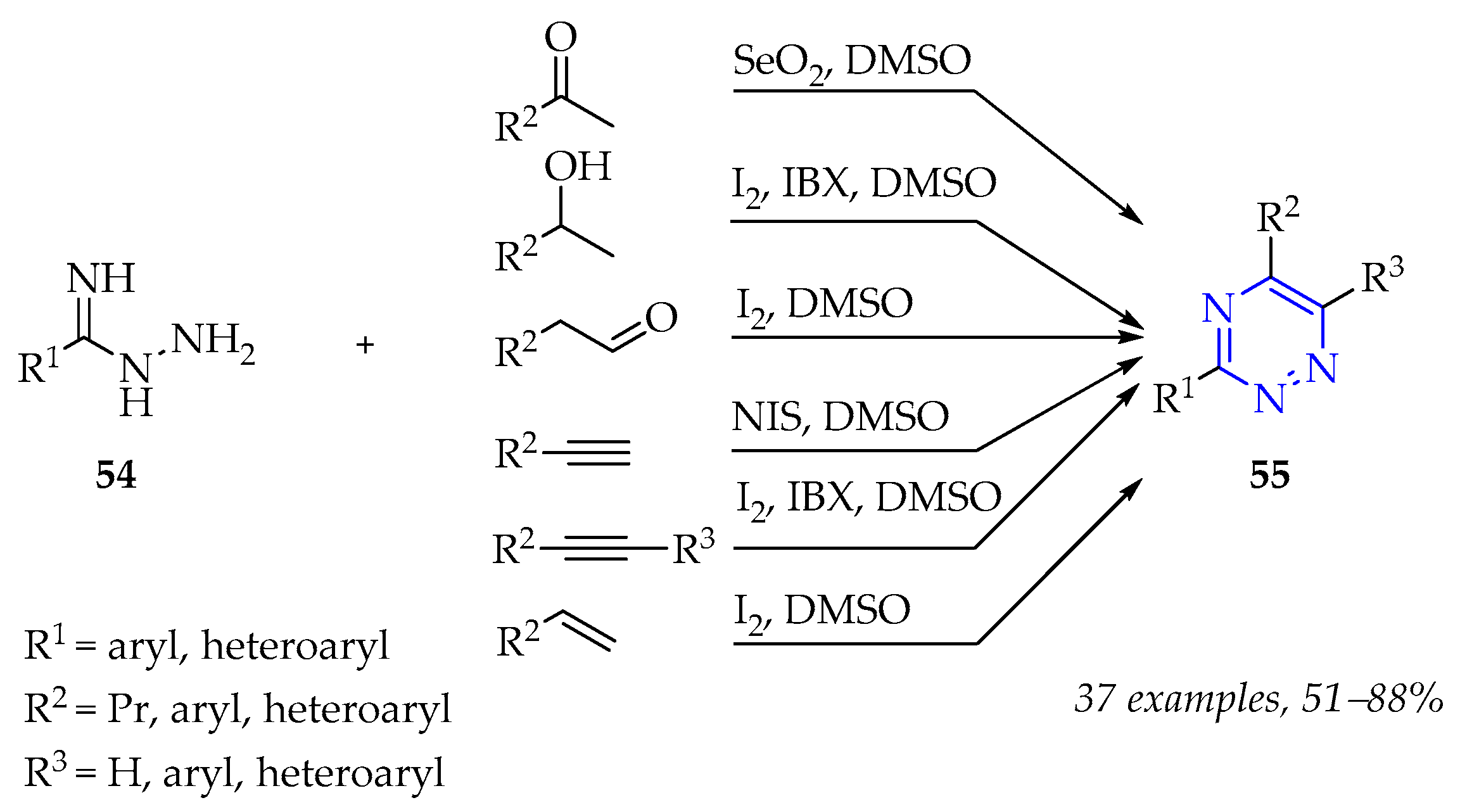
3.2.2. Synthesis of 1,2,4-Triazine Derivatives
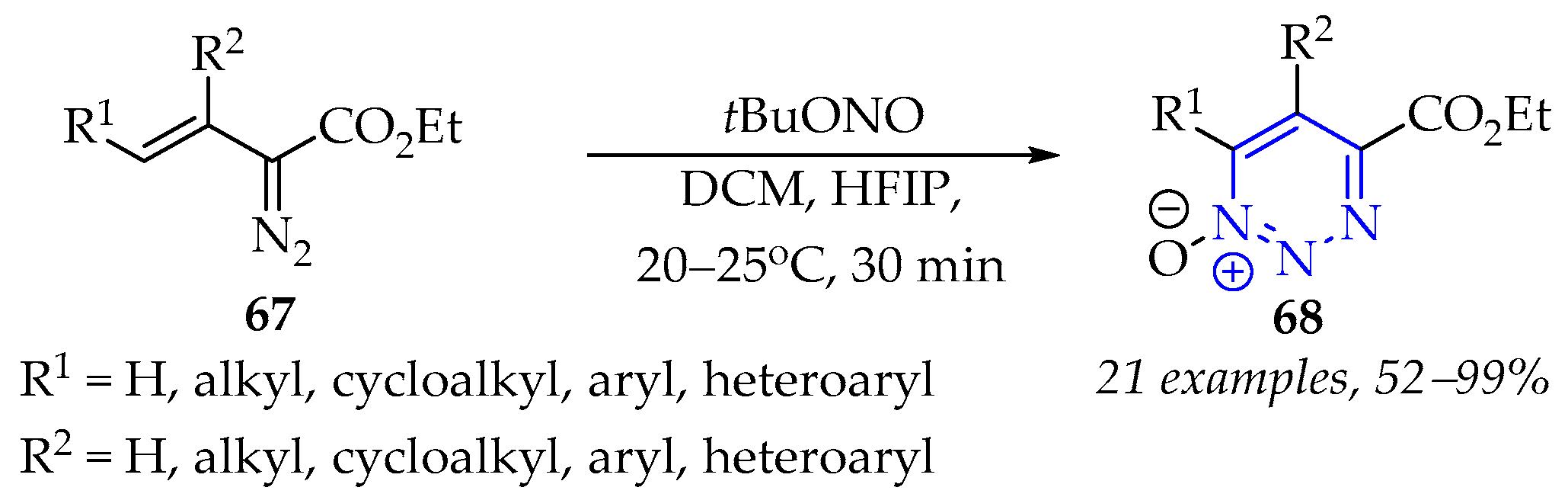
3.2.3. Synthesis of 1,2,3-Triazine Derivatives
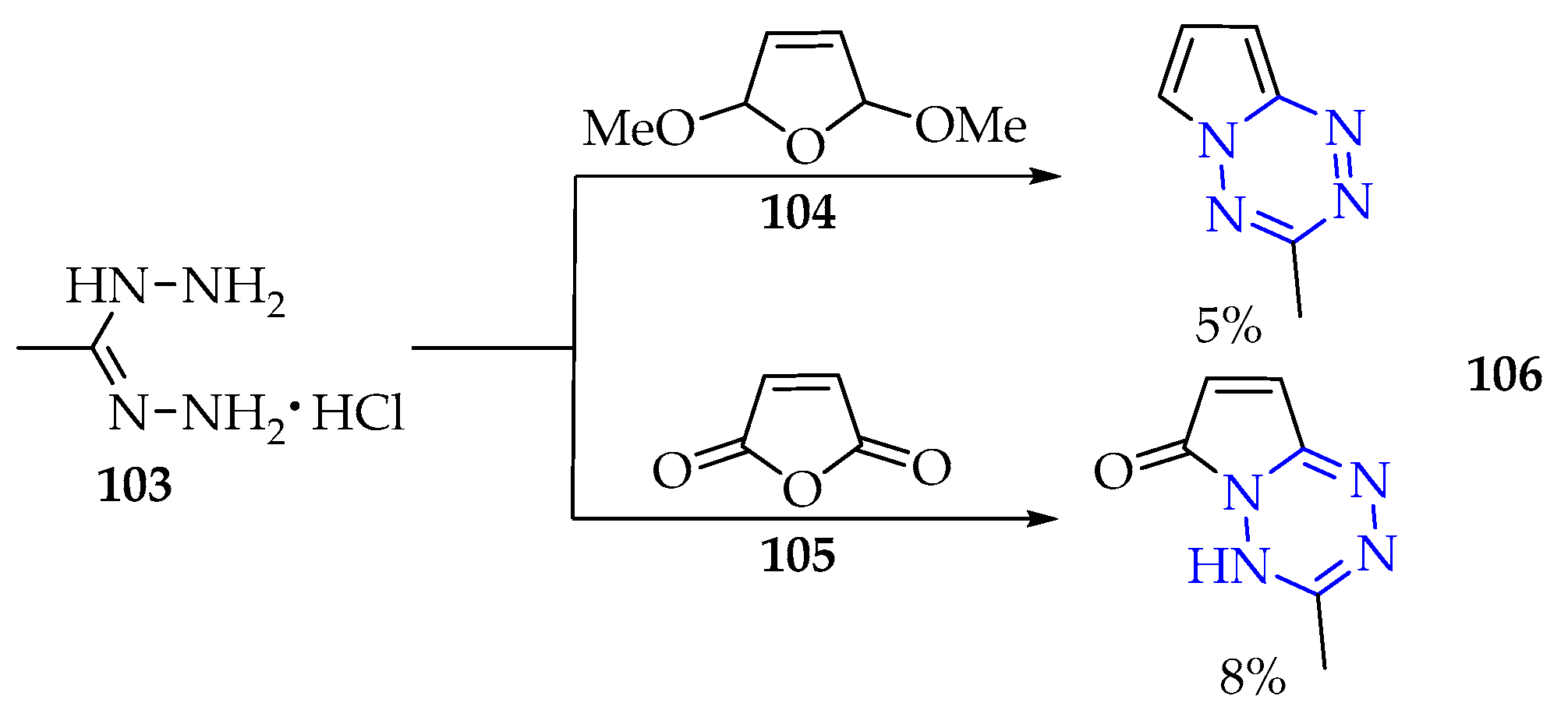
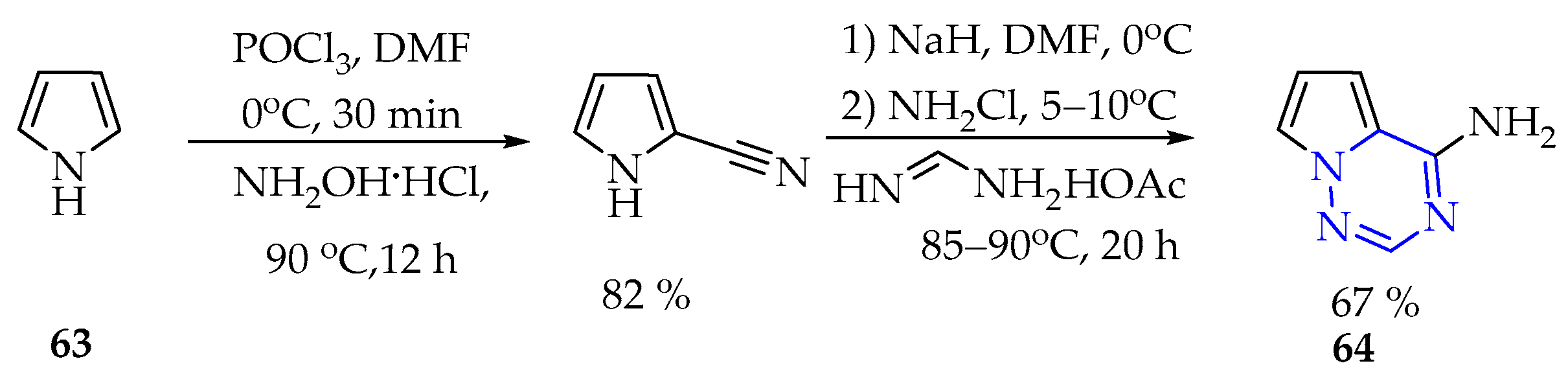
3.3. Synthesis of 1,2,3,4-Tetrazine Derivatives
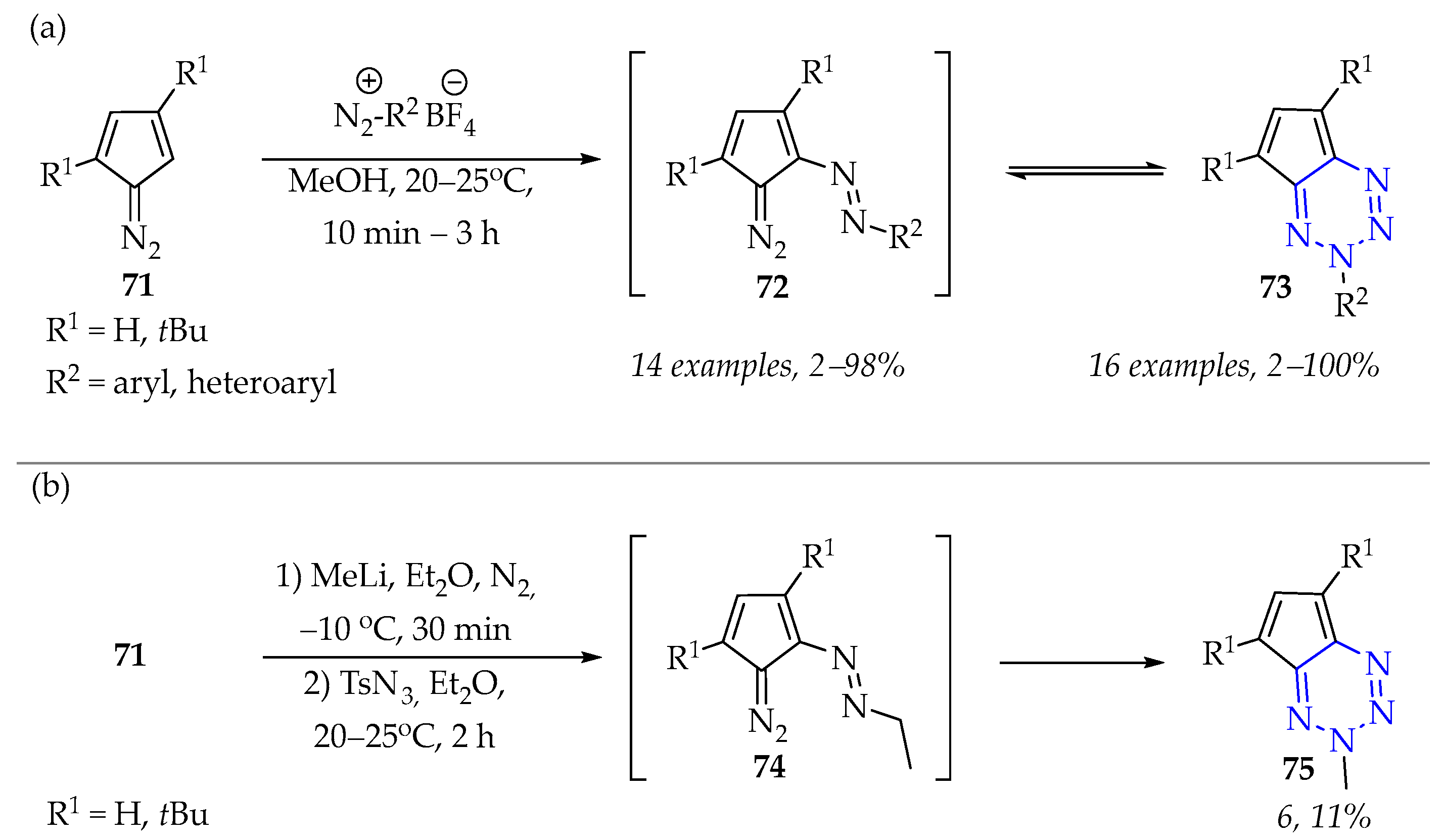
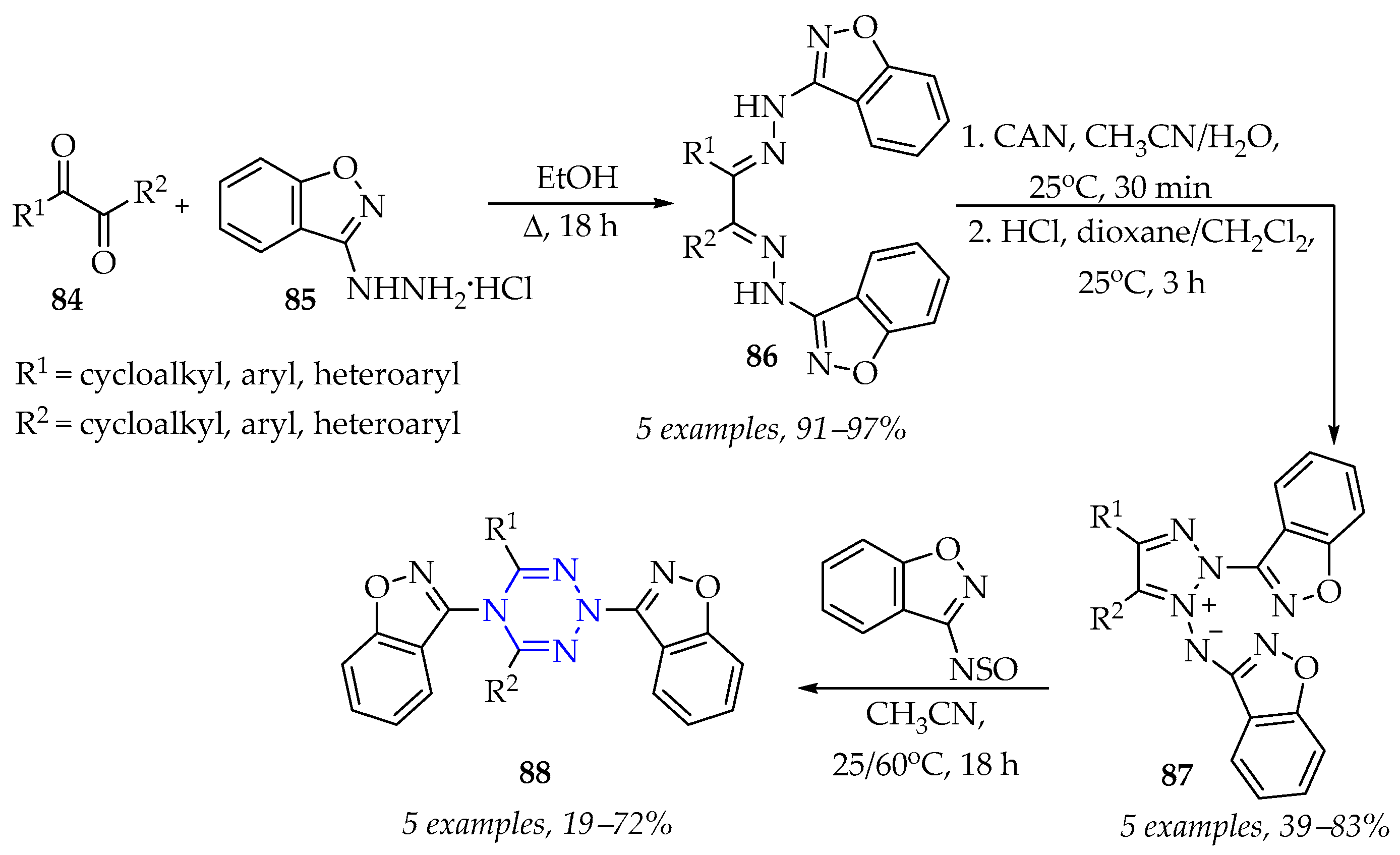
3.3.1. Synthesis of 1,2,3,4-Tetrazine Derivatives
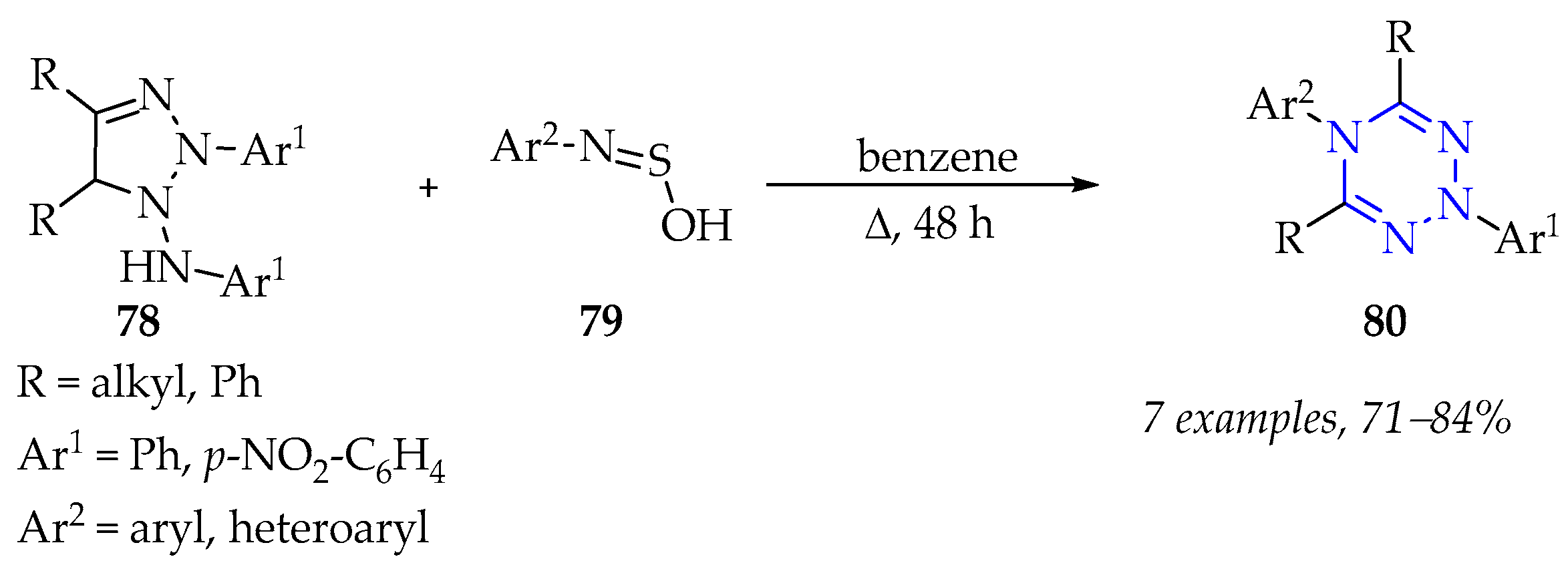
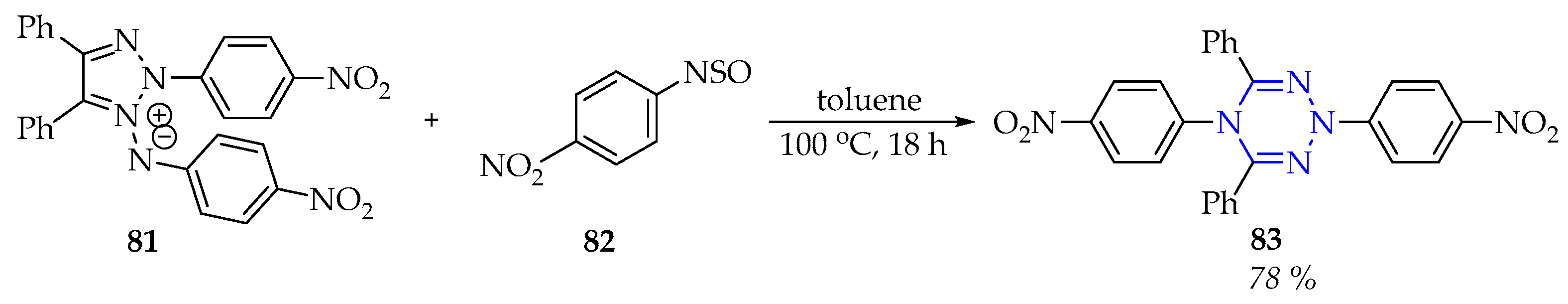
3.3.2. Synthesis of 1,2,3,5-Tetrazine Derivatives
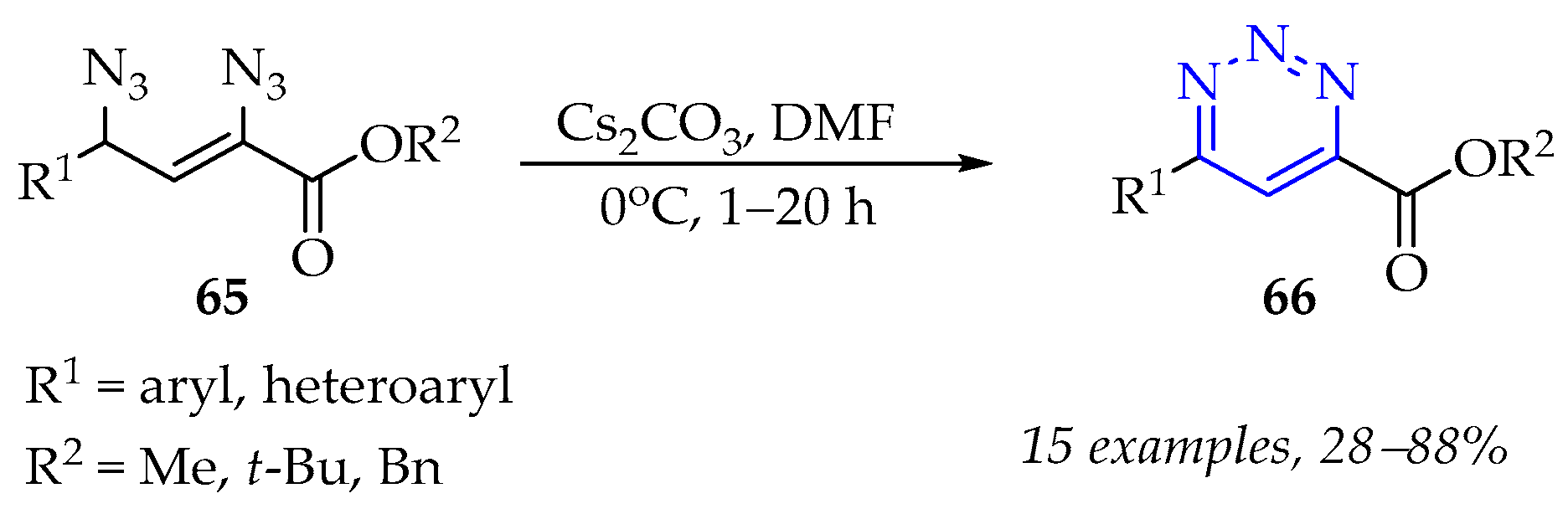
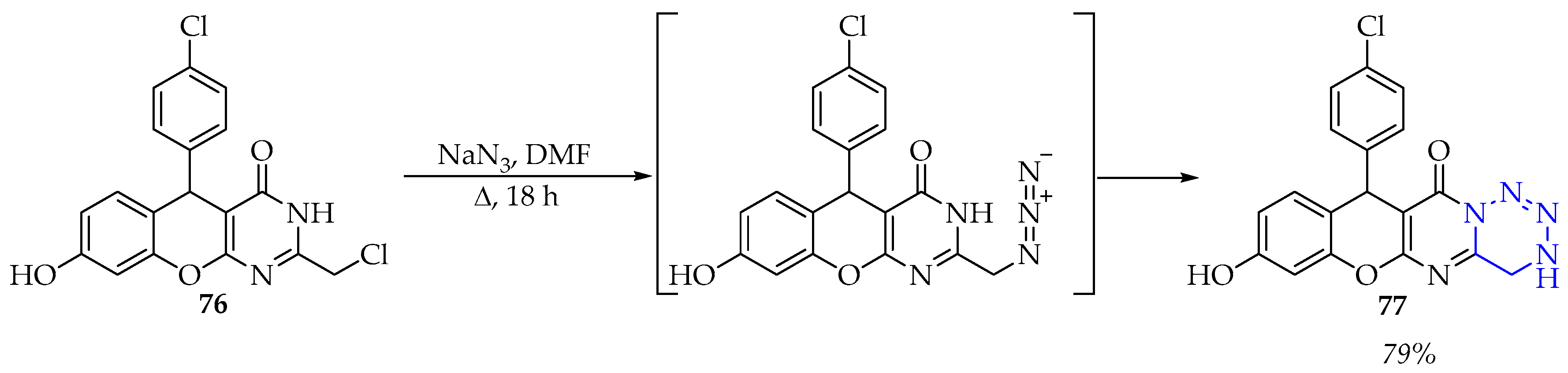
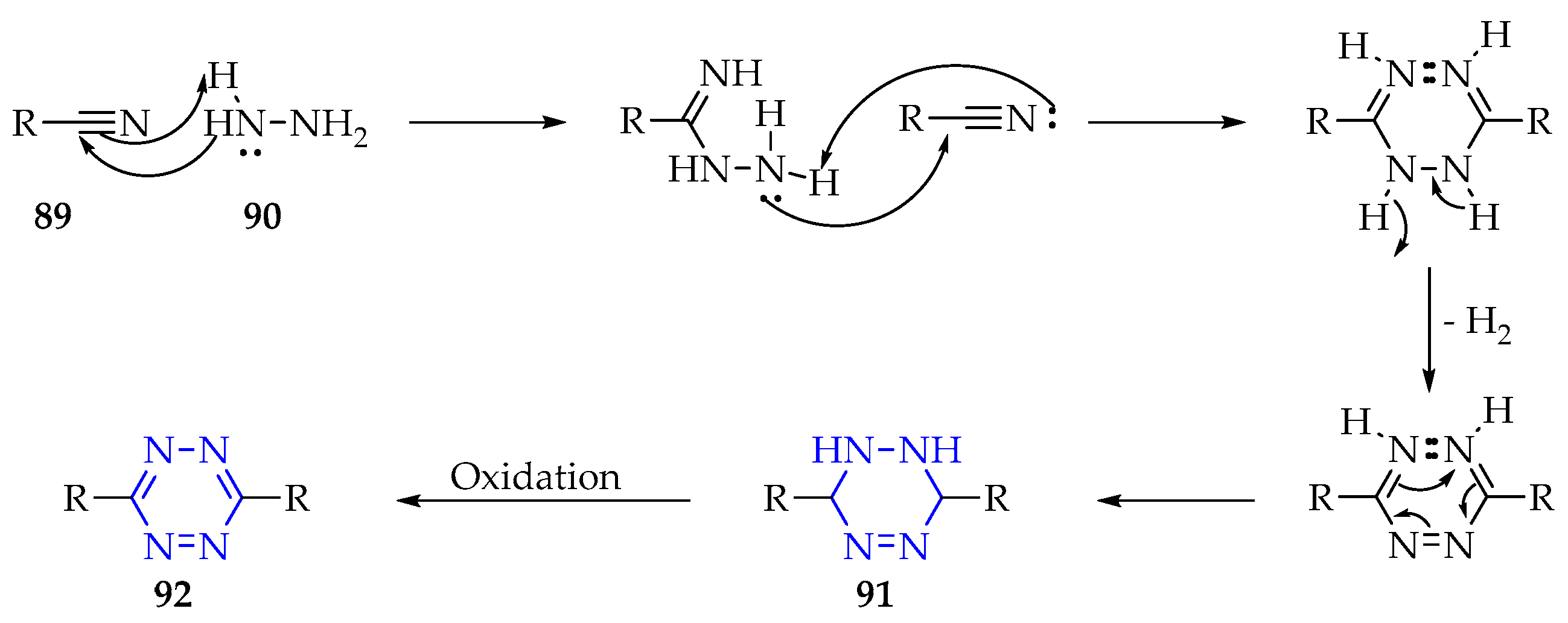
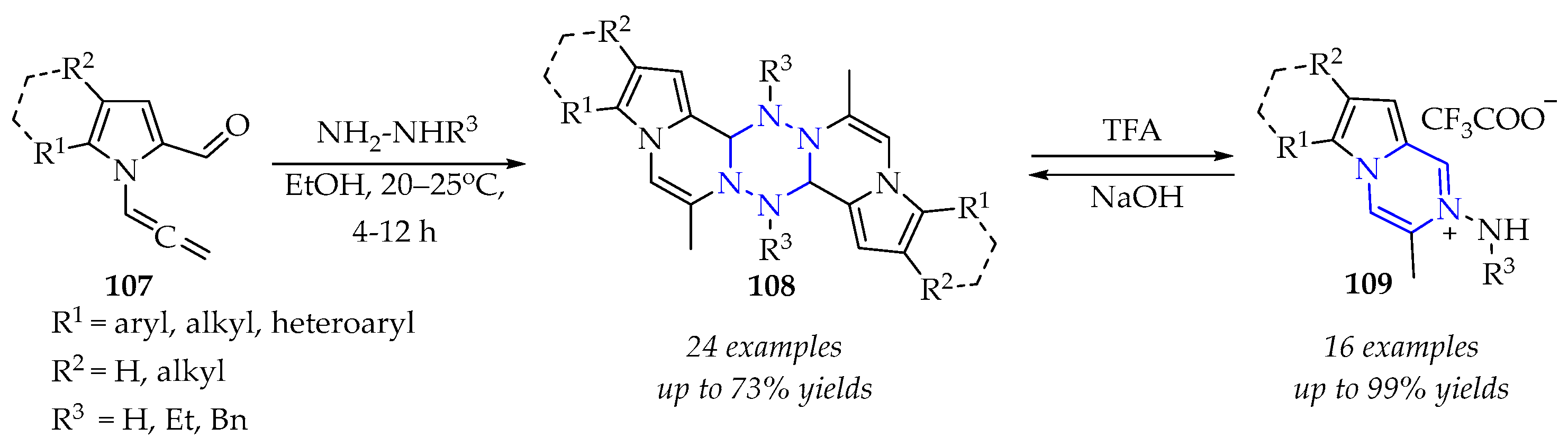
3.3.3. Synthesis of 1,2,4,5-Tetrazine Derivatives
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Δ | Boiling or reflux |
| Ac | Acetyl |
| AIDS | Acquired Immune Deficiency Syndromes. |
| Ar | Aryl |
| Bn | Benzyl |
| Boc | Tert-butoxycarbonyl group |
| Bu | Butyl |
| C(+)|Ni foam(–) | C(+)—graphite plate (cathode), Ni foam(–)—nickel foam (cathode) |
| CAN | Ceric(IV) ammonium nitrate |
| CRF | Corticotropin releasing factor |
| Cy | Cyclohexyl |
| DABCO | 1,4-Diazabicyclo [2.2.2]octane |
| DBU | Diazabicycloundecene |
| DCE | Dichloroethane |
| DMF | N,N-Dimethylformamide |
| DMSO | Dimethyl sulfoxide |
| Et | Ethyl |
| Fur | Furyl |
| IBX | 2-Iodoxybenzoic acid |
| Hfacac | Hexafluoroacetylacetonate |
| HFIP | 1,1,1,3,3,3-Hexafluoroisopropanol |
| Hx | Hexyl |
| LiHMDS | Lithium bis(trimethylsilyl)amide |
| NIS | N-Iodosuccinimide |
| Np | Naphtyl |
| Me | Metyl |
| MeCN | Acetonitrile |
| MS | Molecular sieves |
| MW | Microwave radiation |
| OTf | Trifluoromethanesulfonic acid |
| Ph | Phenyl |
| PIVA | Bis(tert-butylcarbonyloxy)iodobenzene |
| Pr | Propyl |
| Py | Pyridinyl |
| TBAClO4 | Tetrabutylammonium perchlorate |
| TBS | Tert-butyldimethylsilyl group |
| TFA | Trifluoroacetic acid |
| Th | Thienyl |
| TMS | Trimethylsilyl group |
| Tol | Tolyl |
| Ts | Toluenesulfonyl |
| UV | Ultraviolet radiation |
| Vin | Vinyl |
References
- Abida; Alam, M.T.; Asif, M. Pharmacological activities of pyridoxines and pyridazinone Derivatives: A Review on biologically active scaffold. SAR J. Pharm. Sci. 2019, 1, 16–37. [Google Scholar] [CrossRef]
- Liljebris, C. Synthesis and biological activity of a novel class of pyridazine analogues as non-competitive reversible inhibitors of protein tyrosine phosphatase 1B (PTP1B). Bioorg. Med. Chem. 2002, 10, 3197–3212. [Google Scholar] [CrossRef]
- Asif, M.; Alghamdi, S. A mini-review on pyridazine analogs: Chemical and pharmacological potentials. Mini Rev. Org. Chem. 2023, 20, 100–123. [Google Scholar] [CrossRef]
- Natarajan, R.; Anthoni Samy, H.N.; Sivaperuman, A.; Subramani, A. Structure-activity relationships of pyrimidine derivatives and their biological activity—A review. Med. Chem. 2022, 19, 10–30. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.B. Recent medicinal approaches of novel pyrimidine analogs: A review. Heliyon 2023, 9, e16773. [Google Scholar] [CrossRef]
- Chen, G.-Q.; Guo, H.-Y.; Quan, Z.-S.; Shen, Q.-K.; Li, X.; Luan, T. Natural products-pyrazine hybrids: A review of developments in medicinal chemistry. Molecules 2023, 28, 7440. [Google Scholar] [CrossRef]
- Huigens, R.W., 3rd; Brummel, B.R.; Tenneti, S.; Garrison, A.T.; Xiao, T. Pyrazine and phenazine heterocycles: Platforms for total synthesis and drug discovery. Molecules 2022, 27, 1112. [Google Scholar] [CrossRef]
- Xing, L.; An, Y.; Qin, Y.; Xin, H.; Deng, T.; Meng, K.; Liu, D.; Xue, W. Design, synthesis, and antiviral activities of myricetin derivatives containing pyridazinone. New J. Chem. 2024, 48, 117–130. [Google Scholar] [CrossRef]
- Flefel, E.M.; Tantawy, W.A.; El-Sofany, W.I.; El-Shahat, M.; El-Sayed, A.A.; Abd-Elshafy, D.N. Synthesis of some new pyridazine derivatives for anti-HAV evaluation. Molecules 2017, 22, 148. [Google Scholar] [CrossRef]
- Berhe, H.G.; Birhan, Y.S.; Beshay, B.Y.; Habib, H.J.; Hymete, A.; Bekhit, A.A. Synthesis, antileishmanial, antimalarial evaluation and molecular docking study of some hydrazine-coupled pyrazole derivatives. BMC Chem. 2024, 18, 9. [Google Scholar] [CrossRef]
- Tupare, S.D.; Sonawale, S.B. Synthesis of some novel isoxazoline from sec 3-amino pyridazine chalcones and their antimicrobial studies. Mediterr. J. Basic Appl. Sci. MJBAS 2023, 07, 133–140. [Google Scholar] [CrossRef]
- Bai, X.-Q.; Li, C.-S.; Cui, M.-Y.; Song, Z.-W.; Zhou, X.-Y.; Zhang, C.; Zhao, Y.; Zhang, T.-Y.; Jiang, T.-Y. Synthesis and molecular docking studies of novel pyrimidine derivatives as potential antibacterial agents. Mol. Divers. 2020, 24, 1165–1176. [Google Scholar] [CrossRef]
- Zhang, J.; Peng, J.-F.; Bai, Y.-B.; Wang, P.; Wang, T.; Gao, J.-M.; Zhang, Z.-T. Synthesis of pyrazolo[1,5-a]pyrimidine derivatives and their antifungal activities against phytopathogenic fungi in vitro. Mol. Divers. 2016, 20, 887–896. [Google Scholar] [CrossRef]
- Hilmy, K.M.H.; Khalifa, M.M.A.; Hawata, M.A.A.; Keshk, R.M.A.; el-Torgman, A.A. Synthesis of new pyrrolo[2,3-d]pyrimidine derivatives as antibacterial and antifungal agents. Eur. J. Med. Chem. 2010, 45, 5243–5250. [Google Scholar] [CrossRef] [PubMed]
- Alatawi, F.A.; Alrefaei, A.F.; Alqahtani, A.M.; Alsoliemy, A.; Katouah, H.A.; Abumelha, H.M.; Saad, F.A.; El-Metwaly, N.M. New thiazol-pyridazine derivatives as antimicrobial and antiviral candidates: Synthesis, and application. J. Saudi Chem. Soc. 2024, 28, 101830. [Google Scholar] [CrossRef]
- Ganai, A.M.; Pathan, T.K.; Mohite, S.B.; Vojáčková, V.; Řezníčková, E.; Kozlanská, K.; Kryštof, V.; Govender, K.; Mokoena, S.; Merugu, S.R.; et al. Design and synthesis of novel 1,2,4-triazolo[4,3-b]pyridazine derivatives with anti-cancer activity. J. Mol. Struct. 2023, 1291, 135938. [Google Scholar] [CrossRef]
- Paidi, K.R.; Tatipamula, V.B.; Kolli, M.K.; Pedakotla, V.R. Benzohydrazide incorporated imidazo[1,2-b]pyridazine: Synthesis, characterization and in vitro anti-tubercular activity. Int. J. Chem. Sci. 2017, 15, 172. [Google Scholar]
- Almehizia, A.A.; Khattab, A.E.A.; Darwish, A.M.; Al-Omar, M.A.; Naglah, A.M.; Bhat, M.A.; Kalmouch, A. Anti-inflammatory activity of novel derivatives of pyrazolo [3,4-d] pyridazine against digestive system inflammation. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2023, 396, 2729–2739. [Google Scholar] [CrossRef]
- Kandile, N.G.; Zaky, H.T. New pyrano[2,3-c]pyridazine derivatives with antimicrobial activity synthesized using piperidine as the organocatalyst. J. Enzym. Inhib. Med. Chem. 2015, 30, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, M.S.; Awad, S.M.; Sayed, A.I. Synthesis of certain pyrimidine derivatives as antimicrobial agents and anti-inflammatory agents. Molecules 2010, 15, 1882–1890. [Google Scholar] [CrossRef] [PubMed]
- Sönmez, M.; Berber, I.; Akbaş, E. Synthesis, antibacterial and antifungal activity of some new pyridazinone metal complexes. Eur. J. Med. Chem. 2006, 41, 101–105. [Google Scholar] [CrossRef]
- Rathish, I.G.; Javed, K.; Bano, S.; Ahmad, S.; Alam, M.S.; Pillai, K.K. Synthesis and blood glucose lowering effect of novel pyridazinone substituted benzenesulfonylurea derivatives. Eur. J. Med. Chem. 2009, 44, 2673–2678. [Google Scholar] [CrossRef]
- Alghamdi, S.; Asif, M. Role of pyridazine analogs as acetylcholinesterase inhibitor: An approach for management of alzheimer’s disease. Eurasian Chem. Commun. 2021, 3, 435–442. [Google Scholar] [CrossRef]
- Khalil, N.A.; Ahmed, E.M.; Mohamed, K.O.; Nissan, Y.M.; Zaitone, S.A.-B. Synthesis and biological evaluation of new pyrazolone-pyridazine conjugates as anti-inflammatory and analgesic agents. Bioorg. Med. Chem. 2014, 22, 2080–2089. [Google Scholar] [CrossRef]
- Asif, M.; Imran, M. Antitubercular and Anticonvulsant Activities of Phenyl-di-hydropyridazinone Derivatives Containing 2-Substituted amin-1-yl-methyl and 2-(2-1H-amin-1-yl) ethyl Moieties. Anal. Chem. Lett. 2020, 10, 414–427. [Google Scholar] [CrossRef]
- Asif, M.; Allahyani, M.; Almehmadi, M.M.; Alsaiari, A.A. Applications and biological potential of substituted pyridazine analogs in medicinal and agricultural fields. Curr. Org. Chem. 2023, 27, 814–820. [Google Scholar] [CrossRef]
- Tucaliuc, R.A.; Mangalagiu, V.; Mangalagiu, I.I. Pyridazinic bioisosteres with potential applications in medicinal chemistry and agriculture. Processes 2023, 11, 2306. [Google Scholar] [CrossRef]
- Mohd, I.; Mohammad, A. Study of Various Pyridazine and Phthalazine Drugs with Diverse Therapeutical and Agrochemical Activities. Russ. J. Bioorg. Chem. 2020, 46, 745–767. [Google Scholar] [CrossRef]
- Food Safety Commission of Japan. Pyridachlometyl (pesticides). Food Saf. 2023, 11, 21–24. [Google Scholar] [CrossRef]
- Ovchinnikov, A.; Yu, N.A.M.; Dzhimsheleishvili, N.P.; Nikolaeva, Y.O. Principles of modern pharmacotherapy of allergic rhinitis: Efficiency, safety, reliability. Eff. Pharmacother. 2022, 1, 16–22. [Google Scholar] [CrossRef]
- Kao, Y.-H.; Cheng, C.-C.; Chen, Y.-C.; Chung, C.-C.; Lee, T.-I.; Chen, S.-A.; Chen, Y.-J. Hydralazine-induced promoter demethylation enhances sarcoplasmic reticulum Ca2+-ATPase and calcium homeostasis in cardiac myocytes. Lab. Investig. 2011, 91, 1291–1297. [Google Scholar] [CrossRef]
- Talitsky, K.A.; Bulkina, O.S. Adelfan-Ezidrex as an effective combination drug for the treatment of arterial hypertension: An unbiased view. Russ. Med. J. 2005, 19, 1250. [Google Scholar]
- Ohno, K.; Arima, K.; Tanaka, S.; Yamagata, T.; Tsurugi, H.; Mashima, K. Synthesis and Characterization of Proximal Dinuclear Complexes of Palladium Supported by 2,6-Bis (arylimino) phenoxy (aryl-2,6-diisopropylphenyl and 2,4,6-tri-tert-butylphenyl) and 3,6-Bis(imino (2, 6-diisopropylphenyl)) pyridazine Ligands. Organometallics 2009, 28, 3256–3263. [Google Scholar] [CrossRef]
- Szymczak, N.K.; Berben, L.A.; Peters, J.C. Redox rich dicobalt macrocycles as templates for multi-electron transformations. Chem. Commun. 2009, 2009, 6729–6731. [Google Scholar] [CrossRef]
- Wriedt, M.; Näther, C. Dimorphic Modification of the Trinuclear Nickel(II) Complex [Ni3(NCS)6(pdz)6]: Synthesis, Crystal Structure, Thermal, Magnetic and Spectroscopic Properties. Z. Anorg. Allg. Chem. 2009, 635, 2459–2464. [Google Scholar] [CrossRef]
- Krotkus, S.; Matulaitis, T.; Diesing, S.; Copley, G.; Archer, E.; Keum, C.; Cordes, D.B.; Slawin, A.M.Z.; Gather, M.C.; Zysman-Colman, E.; et al. Fast delayed emission in new pyridazine-based compounds. Front. Chem. 2020, 8, 572862. [Google Scholar] [CrossRef]
- Chmovzh, T.N.; Knyazeva, E.A.; Tanaka, E.; Popov, V.V.; Mikhalchenko, L.V.; Robertson, N.; Rakitin, O.A. [1,2,5]thiadiazolo[3,4-d]pyridazine as an internal acceptor in the D-A-π-A organic sensitizers for dye-sensitized solar cells. Molecules 2019, 24, 1588. [Google Scholar] [CrossRef]
- Zbancioc, G.N.; Mangalagiu, I.I. Microwave-Assisted Synthesis of Highly Fluorescent Pyrrolopyridazine Derivatives. Synlett 2006, 2006, 0804–0806. [Google Scholar] [CrossRef]
- Zbancioc, G.; Mangalagiu, I.I. Pyrrolopyridazine derivatives as blue organic luminophores: Synthesis and properties. Part 2. Tetrahedron 2010, 66, 278–282. [Google Scholar] [CrossRef]
- Zbancioc, G.N.; Huhn, T.; Groth, U.; Deleanu, C.; Mangalagiu, I.I. Pyrrolodiazine derivatives as blue organic luminophores: Synthesis and properties. Part 3. Tetrahedron 2010, 66, 4298–4306. [Google Scholar] [CrossRef][Green Version]
- Maftei, D.; Zbancioc, G.; Humelnicu, I.; Mangalagiu, I. Conformational effects on the lowest excited states of benzoyl-pyrrolopyridazine: Insights from PCM time-dependent DFT. J. Phys. Chem. A. 2013, 117, 3165–3175. [Google Scholar] [CrossRef]
- Moldoveanu, C.; Amariucai-Mantu, D.; Mangalagiu, V. Microwave Assisted Reactions of Fluorescent Pyrrolodiazine Building Blocks. Molecules 2019, 24, 3760. [Google Scholar] [CrossRef]
- Slagman, S.; Fessner, W.-D. Biocatalytic routes to anti-viral agents and their synthetic intermediates. Chem. Soc. Rev. 2021, 50, 1968–2009. [Google Scholar] [CrossRef]
- Khatavi, S.Y.; Kamanna, K. Facile and greener method synthesis of pyrano[2,3-d]pyrimidine-2,4,7-triones: Electrochemical and biological activity evaluation studies. J. Mol. Struct. 2022, 1250, 131708. [Google Scholar] [CrossRef]
- Ferazoddin, M.; Marupati, S.; Dasari, G.; Syeda, A.B.; Ali, M.I.; Manchal, R.; Bokkala, K.; Bandari, S. New quinoxaline-piperazine-oxazole conjugates: Synthesis, in vitro anticancer, in silico ADMET, and molecular docking studies. J. Heterocycl. Chem. 2024, 61, 627–641. [Google Scholar] [CrossRef]
- El-Gaby, M.S.A.; Gaber, A.M.; Atalla, A.A.; Abd Al-Wahab, K.A. Novel synthesis and antifungal activity of pyrrole and pyrrolo[2,3-d]pyrimidine derivatives containing sulfonamido moieties. Farmaco 2002, 57, 613–617. [Google Scholar] [CrossRef]
- Kaur, R.; Chaudhary, S.; Kumar, K.; Gupta, M.K.; Rawal, R.K. Recent synthetic and medicinal perspectives of dihydropyrimidinones: A review. Eur. J. Med. Chem. 2017, 132, 108–134. [Google Scholar] [CrossRef]
- Bassyouni, F.; Tarek, M.; Salama, A.; Ibrahim, B.; Salah El Dine, S.; Yassin, N.; Hassanein, A.; Moharam, M.; Abdel-Rehim, M. Promising antidiabetic and antimicrobial agents based on fused pyrimidine derivatives: Molecular modeling and biological evaluation with histopathological effect. Molecules 2021, 26, 2370. [Google Scholar] [CrossRef]
- Poletto, J.; da Silva, M.J.V.; Jacomini, A.P.; Bidóia, D.L.; Volpato, H.; Nakamura, C.V.; Rosa, F.A. Antiparasitic activities of novel pyrimidine N-acylhydrazone hybrids. Drug Dev. Res. 2021, 82, 230–240. [Google Scholar] [CrossRef]
- Sirakanyan, S.N.; Spinelli, D.; Geronikaki, A.; Kartsev, V.; Hakobyan, E.K.; Petrou, A.; Paronikyan, R.G.; Nazaryan, I.M.; Akopyan, H.H.; Hovakimyan, A.A. Synthesis and neurotropic activity of new heterocyclic systems: Pyridofuro[3,2-d]pyrrolo[1,2-a]pyrimidines, pyridofuro[3,2-d]pyrido[1,2-a]pyrimidines and pyridofuro[3′,2′:4,5]pyrimido[1,2-a]azepines. Molecules 2021, 26, 3320. [Google Scholar] [CrossRef]
- Chauhan, M.; Kumar, R. Medicinal attributes of pyrazolo[3,4-d]pyrimidines: A review. Bioorg. Med. Chem. 2013, 21, 5657–5668. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.; Pal, R.; Khan, S.A.; Kumar, B.; Akhtar, M.J. Insights into the structure activity relationship of nitrogen-containing heterocyclics for the development of antidepressant compounds: An updated review. J. Mol. Struct. 2021, 1237, 130369. [Google Scholar] [CrossRef]
- Abdel-Aziz, S.A.; Taher, E.S.; Lan, P.; El-Koussi, N.A.; Salem, O.I.A.; Gomaa, H.A.M.; Youssif, B.G.M. New pyrimidine/thiazole hybrids endowed with analgesic, anti-inflammatory, and lower cardiotoxic activities: Design, synthesis, and COX-2/sEH dual inhibition. Arch. Pharm. 2022, 355, e2200024. [Google Scholar] [CrossRef]
- Kazantseva, I.A. Clinical efficacy of acyclovir and valtresc in the treatment of recurrent herpes labialis in children. Educ. Bull. Conscious. 2006, 8, 326–327. [Google Scholar]
- Kłysik, K.; Pietraszek, A.; Karewicz, A.; Nowakowska, M. Acyclovir in the treatment of herpes viruses—A review. Curr. Med. Chem. 2020, 27, 4118–4137. [Google Scholar] [CrossRef]
- De Clercq, E. Fifty years in search of selective antiviral drugs. J. Med. Chem. 2019, 62, 7322–7339. [Google Scholar] [CrossRef]
- Lamb, Y.N. Rosuvastatin/ezetimibe: A review in hypercholesterolemia. Am. J. Cardiovasc. Drugs 2020, 20, 381–392. [Google Scholar] [CrossRef]
- Qazi, S.U.; Qamar, U.; Maqsood, M.T.; Gul, R.; Ansari, S.A.; Imtiaz, Z.; Noor, A.; Suheb, M.Z.K.; Zaheer, Z.; Andleeb, A.; et al. Efficacy of allopurinol in improving endothelial dysfunction: A systematic review and meta-analysis. High Blood Press. Cardiovasc. Prev. 2023, 30, 539–550. [Google Scholar] [CrossRef]
- Stojkovic, M.; Radmanovic, B.; Jovanovic, M.; Janjic, V.; Muric, N.; Ristic, D.I. Risperidone induced hyperprolactinemia: From basic to clinical studies. Front. Psychiatry 2022, 13, 874705. [Google Scholar] [CrossRef]
- Tefferi, A. Challenges facing JAK inhibitor therapy for myeloproliferative neoplasms. N. Engl. J. Med. 2012, 366, 844–846. [Google Scholar] [CrossRef]
- Samson, K. One cord blood unit effective, safer than two in younger leukemia patients. Oncol. Times 2014, 36, 39–40. [Google Scholar] [CrossRef]
- Kenmotsu, H.; Yamamoto, N.; Yamanaka, T.; Yoshiya, K.; Takahashi, T.; Ueno, T.; Goto, K.; Daga, H.; Ikeda, N.; Sugio, K.; et al. Randomized phase III study of pemetrexed plus cisplatin versus vinorelbine plus cisplatin for completely resected stage II to IIIA nonsquamous non-small-cell lung cancer. J. Clin. Oncol. 2020, 38, 2187–2196. [Google Scholar] [CrossRef]
- Rossi, A.; Ricciardi, S.; Maione, P.; de Marinis, F.; Gridelli, C. Pemetrexed in the treatment of advanced non-squamous lung cancer. Lung Cancer 2009, 66, 141–149. [Google Scholar] [CrossRef]
- Nishimura, S.; Harada, F.; Ikehara, M. The selective utilization of tubercidin triphosphate as an ATP analog in the DNA-dependent RNA polymerase system. Acta Biochim. Biophys. Sin. 1966, 129, 301–309. [Google Scholar] [CrossRef]
- Cohen, M.B.; Glazer, R.I. Comparison of the cellular and RNA-dependent effects of sangivamycin and toyocamycin in human colon carcinoma cells. Mol. Pharmacol. 1985, 27, 349–355. [Google Scholar] [CrossRef]
- Renau, T.E.; Lee, J.S.; Kim, H.; Young, C.G.; Wotring, L.L.; Townsend, L.B.; Drach, J.C. Relationship between cytotoxicity and conversion of thiosangivamycin analogs to toyocamycin analogs in cell culture medium. Biochem. Pharmacol. 1994, 48, 801–807. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Robins, R.K.; Revankar, G.R. Purine analogs and related nucleosides and nucleotides as antitumor agents. Med. Res. Rev. 1985, 5, 273–296. [Google Scholar] [CrossRef] [PubMed]
- Cousins, D.L.; Fricero, P.; Kopf, K.P.M.; McColl, E.J.; Czechtizky, W.; Lim, Y.H.; Harrity, J.P.A. Pyrimidin-6-yl Trifluoroborate Salts as Versatile Templates for Heterocycle Synthesis. Angew. Chem. Int. Ed. 2021, 60, 9412–9415. [Google Scholar] [CrossRef]
- Fischer, T.; Bamberger, J.; Mancheño, O.G. Asymmetric nucleophilic dearomatization of diazarenes by anion-binding catalysis. Org. Biomol. Chem. 2016, 14, 5794–5802. [Google Scholar] [CrossRef]
- Resch, V.; Seidler, C.; Chen, B.S.; Degeling, I.; Hanefeld, U. On the Michael Addition of Water to α,β-Unsaturated Ketones Using Amino Acids. Eur. J. Org. Chem. 2013, 2013, 7697–7704. [Google Scholar] [CrossRef]
- George, J.T.; Srivatsan, S.G. Bioorthogonal chemistry-based RNA labeling technologies: Evolution and current state. Chem. Commun. 2020, 56, 12307–12318. [Google Scholar] [CrossRef]
- Evers, W.J.; Matawan, J.S. Flavoring with an Oxocyclic Pyrimidine. US Patent 3857972, 31 December 1974. [Google Scholar]
- Chen, Y.-K.; Kuo, H.-H.; Luo, D.; Lai, Y.-N.; Li, W.-C.; Chang, C.-H.; Escudero, D.; Jen, A.K.Y.; Hsu, L.-Y.; Chi, Y. Phenyl- and Pyrazolyl-Functionalized Pyrimidine: Versatile Chromophore of Bis-Tridentate Ir(III) Phosphors for Organic Light-Emitting Diodes. Chem. Mater. 2019, 31, 6453–6464. [Google Scholar] [CrossRef]
- Navarro, J.A.R.; Barea, E.; Galindo, M.A.; Salas, J.M.; Romero, M.A.; Quirós, M.; Masciocchi, N.; Galli, S.; Sironi, A.; Lippert, B. Soft functional polynuclear coordination compounds containing pyrimidine bridges. J. Solid. State Chem. 2005, 178, 2436–2451. [Google Scholar] [CrossRef]
- Achelle, S.; Nouira, I.; Pfaffinger, B.; Ramondenc, Y.; Plé, N.; Rodríguez-López, J. V-Shaped 4,6-Bis(arylvinyl)pyrimidine Oligomers: Synthesis and Optical Properties. J. Org. Chem. 2009, 74, 3711–3717. [Google Scholar] [CrossRef]
- Patra, A.; Ralston, J.; Sedev, R.; Zhou, J. Design of Pyrimidine-Based Photoresponsive Surfaces and Light-Regulated Wettability. Langmuir 2009, 25, 11486–11494. [Google Scholar] [CrossRef]
- Meyer, D.; Taige, M.A.; Zeller, A.; Hohlfeld, K.; Ahrens, S.; Strassner, T. Palladium complexes with pyrimidine-functionalized N-heterocyclic carbene ligands: Synthesis, structure and catalytic activity. Organomet. Chem. 2009, 28, 2142–2149. [Google Scholar] [CrossRef]
- Saito, R.; Tokita, M.; Uda, K.; Ishikawa, C.; Satoh, M. Synthesis and in vitro evaluation of botryllazine B analogues as a new class of inhibitor against human aldose reductase. Tetrahedron 2009, 65, 3019–3026. [Google Scholar] [CrossRef]
- Taylor, E.C.; Perlman, K.L.; Sword, I.P.; Sequin-Frey, M.; Jacobi, P.A. Pteridines. XXVIII. New, general, and unequivocal pterin synthesis. J. Am. Chem. Soc. 1973, 95, 6407–6412. [Google Scholar] [CrossRef]
- Janssens, T.K.S.; Tyc, O.; Besselink, H.; de Boer, W.; Garbeva, P. Biological activities associated with the volatile compound 2,5-bis(1-methylethyl)-pyrazine. FEMS Microbiol. Lett. 2019, 366, fnz023. [Google Scholar] [CrossRef]
- Dickschat, J.S.; Reichenbach, H.; Wagner-Döbler, I.; Schulz, S. Novel Pyrazines from the Myxobacterium Chondromyces crocatus and Marine Bacteria. Eur. J. Org. Chem. 2005, 2005, 4141–4153. [Google Scholar] [CrossRef]
- Jeong, J.U.; Sutton, S.C.; Kim, S.; Fuchs, P.L. Biomimetic total syntheses of (+)-cephalostatin 7, (+)-cephalostatin 12, and (+)-ritterazine K. J. Am. Chem. Soc. 1995, 117, 10157–10158. [Google Scholar] [CrossRef]
- Chaignaud, M.; Gillaizeau, I.; Ouhamou, N.; Coudert, G. New highlights in the synthesis and reactivity of 1,4-dihydropyrazine derivatives. Tetrahedron 2008, 64, 8059–8066. [Google Scholar] [CrossRef]
- Wang, Y.-T.; Yang, P.-C.; Zhang, J.-Y.; Sun, J.-F. Synthetic Routes and Clinical Application of Representative Small-Molecule EGFR Inhibitors for Cancer Therapy. Molecules 2024, 29, 1448. [Google Scholar] [CrossRef]
- Yang, Y.; Zhu, Q.; Zhong, Y.; Cui, X.; Jiang, Z.; Wu, P.; Zheng, X.; Zhang, K.; Zhao, S. Synthesis, anti-microbial and anti-inflammatory activities of 18β-glycyrrhetinic acid derivatives. Bioorg. Chem. 2020, 101, 103985. [Google Scholar] [CrossRef]
- Müller, R.; Rappert, S. Pyrazines: Occurrence, formation and biodegradation. Appl. Microbiol. Biotechnol. 2010, 85, 1315–1320. [Google Scholar] [CrossRef]
- Green, J.B.; Bethel, M.A.; Armstrong, P.W.; Buse, J.B.; Engel, S.S.; Garg, J.; Josse, R.; Kaufman, K.D.; Koglin, J.; Korn, S.; et al. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N. Engl. J. Med. 2015, 373, 232–242. [Google Scholar] [CrossRef]
- Haddy, F.J.; Pamnani, M.B.; Swindall, B.T.; Johnston, J.; Cragoe, E.J. Sodium channel blockers are vasodilator as well as natriuretic and diuretic agents. Hypertension 1985, 7, I121–I126. [Google Scholar] [CrossRef]
- Helzlsouer, K.J.; Kensler, T.W. Cancer chemoprotection by oltipraz: Experimental and clinical considerations. Prev. Med. 1993, 22, 783–795. [Google Scholar] [CrossRef]
- Ala, M.; Mohammad Jafari, R.; Dehpour, A.R. Sildenafil beyond erectile dysfunction and pulmonary arterial hypertension: Thinking about new indications. Fundam. Clin. Pharmacol. 2021, 35, 235–259. [Google Scholar] [CrossRef]
- Sanders, O. Sildenafil for the treatment of Alzheimer’s disease: A systematic review. J. Alzheimers Dis. 2020, 4, 91–106. [Google Scholar] [CrossRef]
- Park, M.S.; Kim, B.-R.; Dong, S.M.; Lee, S.-H.; Kim, D.-Y.; Rho, S.B. The antihypertension drug doxazosin inhibits tumor growth and angiogenesis by decreasing VEGFR-2/Akt/mTOR signaling and VEGF and HIF-1α expression. Oncotarget 2014, 5, 4935–4944. [Google Scholar] [CrossRef][Green Version]
- Ghimire, S.; Van’t Boveneind-Vrubleuskaya, N.; Akkerman, O.W.; de Lange, W.C.; van Soolingen, D.; Kosterink, J.G.; van der Werf, T.S.; Wilffert, B.; Touw, D.J.; Alffenaar, J.W. Pharmacokinetic/pharmacodynamic-based optimization of levofloxacin administration in the treatment of MDR-TB. J. Antimicrob. Chemother. 2016, 71, 2691–2703. [Google Scholar] [CrossRef]
- Liou, J.-M.; Jiang, X.-T.; Chen, C.-C.; Luo, J.-C.; Bair, M.-J.; Chen, P.-Y.; Chou, C.-K.; Fang, Y.-J.; Chen, M.-J.; Chen, C.-C.; et al. Second-line levofloxacin-based quadruple therapy versus bismuth-based quadruple therapy for Helicobacter pylori eradication and long-term changes to the gut microbiota and antibiotic resistome: A multicentre, open-label, randomised controlled trial. Lancet Gastroenterol. Hepatol. 2023, 8, 228–241. [Google Scholar] [CrossRef]
- Kanth, S.; Malgar Puttaiahgowda, Y.; Gupta, S.; Swathi, T. Recent advancements and perspective of ciprofloxacin-based antimicrobial polymers. J. Biomater. Sci. Polym. Ed. 2023, 34, 918–949. [Google Scholar] [CrossRef]
- Zusso, M.; Lunardi, V.; Franceschini, D.; Pagetta, A.; Lo, R.; Stifani, S.; Frigo, A.C.; Giusti, P.; Moro, S. Ciprofloxacin and levofloxacin attenuate microglia inflammatory response via TLR4/NF-kB pathway. J. Neuroinflamm. 2019, 16, 148. [Google Scholar] [CrossRef]
- Van Tyle, J.H. Ketoconazole. Mechanism of action, spectrum of activity, pharmacokinetics, drug interactions, adverse reactions and therapeutic use. Pharmacotherapy 1984, 4, 343–373. [Google Scholar] [CrossRef]
- Wichniak, A.; Wierzbicka, A.E.; Jarema, M. Treatment of insomnia—effect of trazodone and hypnotics on sleep. Psychiatr. Pol. 2021, 55, 743–755. [Google Scholar] [CrossRef]
- Geoffroy, P.A.; Lejoyeux, M.; Rolland, B. Management of insomnia in alcohol use disorder. Expert Opin. Pharmacother. 2020, 21, 297–306. [Google Scholar] [CrossRef]
- Cox, J.H.; Cavanna, A.E. Aripiprazole for the treatment of Tourette syndrome. Expert Rev. Neurother. 2021, 21, 381–391. [Google Scholar] [CrossRef]
- Pozderac, I.; Lugović-Mihić, L.; Artuković, M.; Stipić-Marković, A.; Kuna, M.; Ferček, I. Chronic inducible urticaria: Classification and prominent features of physical and non-physical types. Acta Dermatovenerol. Alp. Pannonica Adriat. 2020, 29, 141–148. [Google Scholar] [CrossRef]
- Ferreri, M.; Hantouche, E.G. Recent clinical trials of hydroxyzine in generalized anxiety disorder. Acta Psychiatr. Scand. Suppl. 1998, 393, 102–108. [Google Scholar] [CrossRef]
- Louzada, L.L.; Machado, F.V.; Nóbrega, O.T.; Camargos, E.F. Zopiclone to treat insomnia in older adults: A systematic review. Eur. Neuropsychopharmacol. 2021, 50, 75–92. [Google Scholar] [CrossRef] [PubMed]
- von Brevern, M.; Lempert, T. Vestibular migraine: Treatment and prognosis. Semin. Neurol. 2020, 40, 83–86. [Google Scholar] [CrossRef]
- Edwards, C.A.; Thompson, A.R. Pesticides and the soil fauna. Residue Rev. 1973, 45, 1–79. [Google Scholar] [CrossRef]
- Ren, A.; Zhang, Y.; Bian, Y.; Liu, Y.-J.; Zhang, Y.-X.; Ren, C.-J.; Zhou, Y.; Zhang, T.; Feng, X.-S. Pyrazines in food samples: Recent update on occurrence, formation, sampling, pretreatment and analysis methods. Food Chem. 2024, 430, 137086. [Google Scholar] [CrossRef]
- Velusamy, M.; Huang, J.-H.; Hsu, Y.-C.; Chou, H.-H.; Ho, K.-C.; Wu, P.-L.; Chang, W.-H.; Lin, J.T.; Chu, C.-W. Dibenzo[f,h]thieno[3,4-b]quinoxaline-Based Small Molecules for Efficient Bulk-Heterojunction Solar Cells. Org. Lett. 2009, 11, 4898–4901. [Google Scholar] [CrossRef] [PubMed]
- Mastalerz, M.; Fischer, V.; Ma, C.-Q.; Janssen, R.A.J.; Bäuerle, P. Conjugated oligothienyl dendrimers based on a pyrazino[2,3-g]quinoxaline core. Org. Lett. 2009, 11, 4500–4503. [Google Scholar] [CrossRef]
- Zhang, C.Y.; Tour, J.M. Synthesis of highly functionalized pyrazines by ortho-lithiation reactions. Pyrazine ladder polymers. J. Am. Chem. Soc. 1999, 121, 8783–8790. [Google Scholar] [CrossRef]
- Ocheje, M.U.; Goodman, R.B.; Onge, P.B.J.S.; Malik, M.-N.; Yadiki, M.; He, Y.; Tao, Y.; Chu, T.-Y.; Rondeau-Gagné, S. Pyrazine as a noncovalent conformational lock in semiconducting polymers for enhanced charge transport and stability in thin film transistors. J. Mater. Chem. C Mater. Opt. Electron. Devices 2019, 7, 11507–11514. [Google Scholar] [CrossRef]
- Ma, S.; Wang, J.; Feng, K.; Zhang, H.; Wu, Z.; Wang, Y.; Liu, B.; Li, Y.; An, M.; Gonzalez-Nuñez, R.; et al. N-type polymer semiconductors based on dithienylpyrazinediimide. ACS Appl. Mater. Interfaces 2023, 15, 1639–1651. [Google Scholar] [CrossRef]
- Li, C.; Wang, Y.; Yin, Y.; Li, Y.; Li, J.; Sun, D.; Lu, J.; Zhang, G.; Sun, R. A comprehensive study of pyrazine-contained and low-temperature curable polyimide. Polymer 2021, 228, 123963. [Google Scholar] [CrossRef]
- Wang, H.; Wen, Y.; Yang, X.; Wang, Y.; Zhou, W.; Zhang, S.; Zhan, X.; Liu, Y.; Shuai, Z.; Zhu, D. Fused-ring pyrazine derivatives for n-type field-effect transistors. ACS Appl. Mater. Interfaces 2009, 1, 1122–1129. [Google Scholar] [CrossRef]
- dos Santos, P.L.; Chen, D.; Rajamalli, P.; Matulaitis, T.; Cordes, D.B.; Slawin, A.M.Z.; Jacquemin, D.; Zysman-Colman, E.; Samuel, I.D.W. Use of pyrimidine and pyrazine bridges as a design strategy to improve the performance of thermally activated delayed fluorescence organic light emitting diodes. ACS Appl. Mater. Interfaces 2019, 11, 45171–45179. [Google Scholar] [CrossRef]
- Chen, D.; Zhang, L.; Matulaitis, T.; Cordes, D.B.; Slawin, A.M.Z.; Zhang, X.-H.; Samuel, I.D.W.; Zysman-Colman, E. Tuning the emission and exciton utilization mechanisms of pyrazine-based multi-carbazole emitters and their use in organic light-emitting diodes. J. Mater. Chem. C Mater. Opt. Electron. Devices 2023, 11, 13095–13105. [Google Scholar] [CrossRef]
- Shahari, M.S.B.; Dolzhenko, A.V. A closer look at N2,6-substituted 1,3,5-triazine-2,4-diamines: Advances in synthesis and biological activities. Eur. J. Med. Chem. 2022, 241, 114645. [Google Scholar] [CrossRef]
- Li, F.; Wang, C.; Xu, Y.; Zhao, Z.; Su, J.; Luo, C.; Ning, Y.; Li, Z.; Li, C.; Wang, L. Efficient synthesis of unsymmetrical trisubstituted 1,3,5-triazines catalyzed by hemoglobin. J. Mol. Catal. 2021, 505, 111519. [Google Scholar] [CrossRef]
- Bai, L.; Wei, C.; Zhang, J.; Song, R. Design, synthesis, and anti-PVY biological activity of 1,3,5-triazine derivatives containing piperazine structure. Int. J. Mol. Sci. 2023, 24, 8280. [Google Scholar] [CrossRef]
- Cascioferro, S.; Parrino, B.; Spanò, V.; Carbone, A.; Montalbano, A.; Barraja, P.; Diana, P.; Cirrincione, G. An overview on the recent developments of 1,2,4-triazine derivatives as anticancer compounds. Eur. J. Med. Chem. 2017, 142, 328–375. [Google Scholar] [CrossRef]
- Abdel-Rahman, R.M.; Morsy, J.M.; Hanafy, F.; Amene, H.A. Synthesis of heterobicyclic nitrogen systems bearing the 1,2,4-triazine moiety as anti-HIV and anticancer drugs: Part I. Die Pharm. 1999, 54, 347–351. [Google Scholar]
- Schmitz, W.D.; Brenner, A.B.; Bronson, J.J.; Ditta, J.L.; Griffin, C.R.; Li, Y.W.; Lodge, N.J.; Molski, T.F.; Olson, R.E.; Zhuo, X.; et al. 5-arylamino-1,2,4-triazin-6(1H)-one CRF1 receptor antagonists. Bioorg. Med. Chem. Lett. 2010, 20, 3579–3583. [Google Scholar] [CrossRef] [PubMed]
- Hynes, J., Jr.; Dyckman, A.J.; Lin, S.; Wrobleski, S.T.; Wu, H.; Gillooly, K.M.; Kanner, S.B.; Lonial, H.; Loo, D.; McIntyre, K.W.; et al. Design, synthesis, and anti-inflammatory properties of orally active 4-(phenylamino)-pyrrolo[2,1-f][1,2,4]triazine p38alpha mitogen-activated protein kinase inhibitors. J. Med. Chem. 2008, 51, 4–16. [Google Scholar] [CrossRef]
- Cascioferro, S.; Parrino, B.; Spanò, V.; Carbone, A.; Montalbano, A.; Barraja, P.; Diana, P.; Cirrincione, G. Synthesis and antitumor activities of 1,2,3-triazines and their benzo- and heterofused derivatives. Eur. J. Med. Chem. 2017, 142, 74–86. [Google Scholar] [CrossRef]
- Cirrincione, G.; Almerico, A.M.; Barraja, P.; Diana, P.; Lauria, A.; Passannanti, A.; Musiu, C.; Pani, A.; Murtas, P.; Minnei, C.; et al. Derivatives of the new ring system indolo[1,2-c]benzo[1,2,3]triazine with potent antitumor and antimicrobial activity. J. Med. Chem. 1999, 42, 2561–2568. [Google Scholar] [CrossRef]
- Bondock, S.; Rabie, R.; Etman, H.A.; Fadda, A.A. Synthesis and antimicrobial activity of some new heterocycles incorporating antipyrine moiety. Eur. J. Med. Chem. 2008, 43, 2122–2129. [Google Scholar] [CrossRef]
- Migawa, M.T.; Drach, J.C.; Townsend, L.B. Design, synthesis and antiviral activity of novel 4,5-disubstituted 7-(beta-D-ribofuranosyl)pyrrolo[2,3-d][1,2,3]triazines and the novel 3-amino-5-methyl-1-(beta-D-ribofuranosyl)- and 3-amino-5-methyl-1-(2-deoxy-beta-D-ribofuranosyl)-1,5-dihydro-1,4,5,6,7,8-hexaazaacenaphthylene as analogues of triciribine. J. Med. Chem. 2005, 48, 3840–3851. [Google Scholar] [CrossRef] [PubMed]
- Viswanatha, G.L.; Akinapally, N.; Krishnadas, N.; Rangappa, S.; Janardhanan, S. Analgesic, Anti-Inflammatory and Antiarthritic Activity of Newly Synthesized Bicyclothieno 1,2,3-Triazines. Iran. J. Pharmacol. Ther. 2011, 10, 31–38. [Google Scholar]
- Raffa, D.; Migliara, O.; Maggio, B.; Plescia, F.; Cascioferro, S.; Cusimano, M.G.; Tringali, G.; Cannizzaro, C.; Plescia, F. Pyrazolobenzotriazinone derivatives as COX inhibitors: Synthesis, biological activity, and molecular-modeling studies. Arch. Pharm. 2010, 343, 631–638. [Google Scholar] [CrossRef]
- Viswanatha, G.L.; Priyadarshini, B.J.; Krishnadas, N.; Janardhanan, S.; Rangappa, S.; Hanumanthappa, S. Synthesis and antihistaminic activity of 3H-benzo[4,5]thieno[2,3-d][1,2,3]triazin-4-ones. Saudi Pharm. J. 2012, 20, 45–52. [Google Scholar] [CrossRef]
- Perspicace, E.; Jouan-Hureaux, V.; Ragno, R.; Ballante, F.; Sartini, S.; La Motta, C.; Da Settimo, F.; Chen, B.; Kirsch, G.; Schneider, S.; et al. Design, synthesis and biological evaluation of new classes of thieno[3,2-d]pyrimidinone and thieno[1,2,3]triazine as inhibitor of vascular endothelial growth factor receptor-2 (VEGFR-2). Eur. J. Med. Chem. 2013, 63, 765–781. [Google Scholar] [CrossRef]
- Hunt, J.C.A.; Briggs, E.; Clarke, E.D.; Whittingham, W.G. Synthesis and SAR studies of novel antifungal 1,2,3-triazines. Bioorg. Med. Chem. Lett. 2007, 17, 5222–5226. [Google Scholar] [CrossRef]
- Keldsen, N.; Havsteen, H.; Vergote, I.; Bertelsen, K.; Jakobsen, A. Altretamine (hexamethylmelamine) in the treatment of platinum-resistant ovarian cancer: A phase II study. Gynecol. Oncol. 2003, 88, 118–122. [Google Scholar] [CrossRef]
- Malik, I.A. Altretamine is an Effective Palliative Therapy of Patients with Recurrent Epithelial Ovarian Cancer. Jpn. J. Clin. Oncol. 2001, 31, 69–73. [Google Scholar] [CrossRef]
- Chan, J.K.; Loizzi, V.; Manetta, A.; Berman, M.L. Oral altretamine used as salvage therapy in recurrent ovarian cancer. Gynecol. Oncol. 2004, 92, 368–371. [Google Scholar] [CrossRef]
- Damia, G.; D’Incalci, M. Clinical pharmacokinetics of altretamine. Clin. Pharmacokinet. 1995, 28, 439–448. [Google Scholar] [CrossRef]
- Kang, Y.-K.; Yook, J.H.; Park, Y.-K.; Lee, J.S.; Kim, Y.-W.; Kim, J.Y.; Ryu, M.-H.; Rha, S.Y.; Chung, I.J.; Kim, I.-H.; et al. PRODIGY: A phase III study of neoadjuvant docetaxel, oxaliplatin, and S-1 plus surgery and adjuvant S-1 versus surgery and adjuvant S-1 for resectable advanced gastric cancer. J. Clin. Oncol. 2021, 39, 2903–2913. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Tian, X.; Wei, Y.; Deng, H.; Ma, L.; Chen, Z. Activity and safety of tegafur, gimeracil, and oteracil potassium for nasopharyngeal carcinoma: A systematic review and meta-analysis. J. Oncol. 2021, 2021, 6690275. [Google Scholar] [CrossRef] [PubMed]
- Jack, G.; Doyle, L.; Palejwalla, M.M. Tretamine in the treatment of inoperable lung cancer. Lancet 1960, 275, 206–207. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, Y.; Kotake, K.; Watanabe, N.; Fujiwara, T.; Sakamoto, S. Lamotrigine in the maintenance treatment of bipolar disorder. Cochrane Database Syst. Rev. 2021, 9, CD013575. [Google Scholar] [CrossRef]
- Liu, C.-H.; Peng, C.-M.; Hwang, J.-I.; Liang, P.-C.; Chen, P.-J.; Abi-Jaoudeh, N.; Giiang, L.-H.; Tyan, Y.-S. Phase I dose-escalation study of tirapazamine chemoembolization for unresectable early- and intermediate-stage hepatocellular carcinoma. J. Vasc. Interv. Radiol. 2022, 33, 926–933.e921. [Google Scholar] [CrossRef]
- Sztanke, K.; Rzymowska, J.; Niemczyk, M.; Dybała, I.; Kozioł, A.E. Novel derivatives of methyl and ethyl 2-(4-oxo-8-aryl-2H-3,4,6,7-tetrahydroimidazo[2,1-c][1,2,4]triazin-3-yl)acetates from biologically active 1-aryl-2-hydrazinoimidazolines: Synthesis, crystal structure and antiproliferative activity. Eur. J. Med. Chem. 2006, 41, 1373–1384. [Google Scholar] [CrossRef]
- Thieu, T.; Sclafani, J.A.; Levy, D.V.; McLean, A.; Breslin, H.J.; Ott, G.R.; Bakale, R.P.; Dorsey, B.D. Discovery and process synthesis of novel 2,7-pyrrolo[2,1-f][1,2,4]triazines. Org. Lett. 2011, 13, 4204–4207. [Google Scholar] [CrossRef] [PubMed]
- Sathiyan, G.; Sakthivel, P. Synthesis and characterization of triazine linked carbazole derivatives green-light-emitting molecules. Dye. Pigment. 2017, 143, 444–454. [Google Scholar] [CrossRef]
- Sathiyan, G.; Venkatesan, G.; Ramasamy, S.K.; Lee, J.; Barathi, S. Recent progress in triazine-based fluorescent probes for detecting hazardous nitroaromatic compounds. J. Environ. Chem. Eng. 2024, 12, 112804. [Google Scholar] [CrossRef]
- Zhao, G.; Wei, G.; Yan, Z.; Guo, B.; Guang, S.; Wu, R.; Xu, H. A multiple fluorescein-based turn-on fluorophore (FHCS) identified for simultaneous determination and living imaging of toxic Al3+ and Zn2+ by improved Stokes shift. Anal. Chim. Acta 2020, 1095, 185–196. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, Y.; Liu, D.; Wang, Y.; Wang, G.; Hua, J. Ultrafast relaxation processes of multi-branched compounds based on 1,3,5-triazine: An investigation of the causes of a high fluorescence quantum yield after modification with perfluoroalkyl chains. J. Lumin. 2017, 190, 89–93. [Google Scholar] [CrossRef]
- Wang, S.; Miao, S.; Lu, Y.; Li, C.; Li, B. A C-type lectin (CTL2) mediated both humoral and cellular immunity against bacterial infection in Tribolium castaneum. Pestic. Biochem. Physiol. 2024, 201, 105852. [Google Scholar] [CrossRef]
- Saracoglu, N. Recent advances and applications in 1,2,4,5-tetrazine chemistry. ChemInform 2007, 38, 4199–4236. [Google Scholar] [CrossRef]
- Churakov, A.M.; Tartakovsky, V.A. Progress in 1,2,3,4-tetrazine chemistry. Chem. Rev. 2004, 104, 2601–2616. [Google Scholar] [CrossRef]
- Almerico, A.M.; Mingoia, F.; Diana, P.; Barraja, P.; Lauria, A.; Montalbano, A.; Cirrincione, G.; Dattolo, G. 1-Methyl-3H-pyrazolo[1,2-a]benzo[1,2,3,4]tetrazin-3-ones. Design, synthesis, and biological activity of new antitumor agents. J. Med. Chem. 2005, 48, 2859–2866. [Google Scholar] [CrossRef]
- Ratnikov, M.O.; Lipilin, D.L.; Churakov, A.M.; Strelenko, Y.A.; Tartakovsky, V.A. Release of nitrosating species in the course of reduction of benzo-1,2,3,4-tetrazine 1,3-dioxides. Org. Lett. 2002, 4, 3227–3229. [Google Scholar] [CrossRef]
- Koukourakis, G.V.; Kouloulias, V.; Zacharias, G.; Papadimitriou, C.; Pantelakos, P.; Maravelis, G.; Fotineas, A.; Beli, I.; Chaldeopoulos, D.; Kouvaris, J. Temozolomide with radiation therapy in high grade brain gliomas: Pharmaceuticals considerations and efficacy; a review article. Molecules 2009, 14, 1561–1577. [Google Scholar] [CrossRef]
- Newlands, E.S.; Stevens, M.F.; Wedge, S.R.; Wheelhouse, R.T.; Brock, C. Temozolomide: A review of its discovery, chemical properties, pre-clinical development and clinical trials. Cancer Treat. Rev. 1997, 23, 35–61. [Google Scholar] [CrossRef]
- Bleehen, N.M.; Newlands, E.S.; Lee, S.M.; Thatcher, N.; Selby, P.; Calvert, A.H.; Rustin, G.J.; Brampton, M.; Stevens, M.F. Cancer Research Campaign phase II trial of temozolomide in metastatic melanoma. J. Clin. Oncol. 1995, 13, 910–913. [Google Scholar] [CrossRef] [PubMed]
- Newlands, E.S.; Blackledge, G.R.; Slack, J.A.; Rustin, G.J.; Smith, D.B.; Stuart, N.S.; Quarterman, C.P.; Hoffman, R.; Stevens, M.F.; Brampton, M.H.; et al. Phase I trial of temozolomide. Br. J. Cancer 1992, 65, 287–291. [Google Scholar] [CrossRef]
- Kaim, W. The coordination chemistry of 1,2,4,5-tetrazines. Coord. Chem. Rev. 2002, 230, 127–139. [Google Scholar] [CrossRef]
- Wu, Z.-C.; Boger, D.L. 1,2,3,5-tetrazines: A general synthesis, cycloaddition scope, and fundamental reactivity patterns. J. Org. Chem. 2022, 87, 16829–16846. [Google Scholar] [CrossRef] [PubMed]
- Talawar, M.B.; Sivabalan, R.; Senthilkumar, N.; Prabhu, G.; Asthana, S.N. Synthesis, characterization and thermal studies on furazan- and tetrazine-based high energy materials. J. Hazard. Mater. 2004, 113, 11–25. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Pan, Y.; Xia, X.; Shao, Y.; Zhu, W.; Xiao, H. Theoretic design of 1,2,3,4-tetrazine-1,3-dioxide-based high-energy density compounds with oxygen balance close to zero. Struct. Chem. 2013, 24, 1579–1590. [Google Scholar] [CrossRef]
- Muravyev, N.V. Thermal stability vs. energy content of compound: Thermolysis of tetrazino-tetrazine 1,3,6,8-tetraoxide (TTTO) from a broad perspective. Chem. Eng. J. 2023, 472, 145032. [Google Scholar] [CrossRef]
- Sauer, J.; Pabst, G.R.; Holland, U.; Kim, H.-S.; Loebbecke, S. 3,6-Bis(2H-tetrazol-5-yl)-1,2,4,5-tetrazine: A Versatile Bifunctional Building Block for the Synthesis of Linear Oligoheterocycles. Eur. J. Org. Chem. 2001, 2001, 697–706. [Google Scholar] [CrossRef]
- Hoogenboom, R.; Moore, B.C.; Schubert, U.S. Microwave-assisted synthesis of 3,6-di(pyridin-2-yl)pyridazines: Unexpected ketone and aldehyde cycloadditions. J. Org. Chem. 2006, 71, 4903–4909. [Google Scholar] [CrossRef]
- Jiang, H.; Gong, Q.; Zhang, R.; Yuan, H. Tetrazine-based metal-organic frameworks. Coord. Chem. Rev. 2024, 499, 215501. [Google Scholar] [CrossRef]
- Audebert, P.; Clavier, G.; Allain, C. Chapter 6.3—Triazines, Tetrazines, and Fused Ring Polyaza Systems. In Progress in Heterocyclic Chemistry; Gribble, G.W., Joule, J.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; Volume 28, pp. 493–521. [Google Scholar]
- Vitaku, E.; Smith, D.T.; Njardarson, J.T. Analysis of the structural diversity, substitution patterns, and frequency of nitrogen heterocycles among U.S. FDA approved pharmaceuticals. J. Med. Chem. 2014, 57, 10257–10274. [Google Scholar] [CrossRef]
- Coates, W.J.; Kennerley, E.A.R.; Rees, C.W.; Scriven, E.F.V. Comprehensive Heterocyclic Chemistry II: A Review of the Literature 1982–1995: The Structure, Reactions, Synthesis, and Uses of Heterocyclic Compounds; Elsevier: Oxford, UK, 1996. [Google Scholar]
- Sergeev, P.G.; Nenajdenko, V.G. Recent advances in the chemistry of pyridazine—an important representative of six-membered nitrogen heterocycles. Russ. Chem. Rev. 2020, 89, 393–429. [Google Scholar] [CrossRef]
- Plieva, A.T. Methods for the synthesis of pyrrolo[1,2-b]pyridazine and pyrrolo[1,2-b]cinnoline derivatives (microreview). Chem. Heterocycl. Compd. 2019, 55, 199–201. [Google Scholar] [CrossRef]
- Curtius, T. 20. Hydrazide und Azide organischer Säuren I. Abhandlung. J. Prakt. Chem. 1894, 50, 275–294. [Google Scholar] [CrossRef]
- Sayed, G.; Hamed, A.; Meligi, G.; Boraie, W.; Shafik, M. The use of 4-(3,4-dichlorophenyl)-4-oxo-2-(4-antipyrinyl)-butanoic acid in the preparation of some new heterocyclic compounds with expected biological activity. Molecules 2003, 8, 322–332. [Google Scholar] [CrossRef]
- Tamayo, N.; Liao, L.; Goldberg, M.; Powers, D.; Tudor, Y.-Y.; Yu, V.; Wong, L.M.; Henkle, B.; Middleton, S.; Syed, R.; et al. Design and synthesis of potent pyridazine inhibitors of p38 MAP kinase. Bioorg. Med. Chem. Lett. 2005, 15, 2409–2413. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Zhu, Y.; Lian, M.; Liu, M.; Yuan, J.; Yin, G.; Wu, A. Unexpected C-C bond cleavage: A route to 3,6-diarylpyridazines and 6-arylpyridazin-3-ones from 1,3-dicarbonyl compounds and methyl ketones. J. Org. Chem. 2012, 77, 9865–9870. [Google Scholar] [CrossRef]
- El-Hameed Hassan, A.A. Heterocyclic synthesis via enaminones: Synthesis and molecular docking studies of some novel heterocyclic compounds containing sulfonamide moiety. Int. J. Org. Chem. 2014, 04, 68–81. [Google Scholar] [CrossRef]
- Islam, M.; Sethi, V.A. Synthesis and antiviral activity of 3-substituted phenyl-6-substituted phenyl (1,2,4) triazolo(4,3-b) pyridazin. Pharm. Chem. J. 2017, 4, 28–33. [Google Scholar]
- Meresz, O.; Foster-Verner, P.A. Synthesis of 3-monosubstituted s-tetrazines and their reactions with monosubstituted acetylenes. J. Chem. Soc. Chem. Commun. 1972, 950–951. [Google Scholar] [CrossRef]
- Sauer, J.; Heldmann, D.K. Synthesis of 3,5-disubstituted pyridazines by regioselective [4+2] cycloadditions with ethynyltributyltin and subsequent replacement of the organotin substituent. Tetrahedron 1998, 54, 4297–4312. [Google Scholar] [CrossRef]
- Sauer, J.; Heldmann, D.K.; Hetzenegger, J.; Krauthan, J.; Sichert, H.; Schuster, J. 1,2,4,5-tetrazine: Synthesis and reactivity in [4+2] cycloadditions. Eur. J. Org. Chem. 1998, 1998, 2885–2896. [Google Scholar] [CrossRef]
- Yamamoto, C.; Suzuki, M.; Yoshida, S. Pyridazine Synthesis from 1,2,4,5-Tetrazines and Alkynes in 1,1,1,3,3,3-Hexafluoro-2-propanol through the Inverse Electron Demand Diels–Alder Reaction. Bull. Chem. Soc. Jpn. 2022, 95, 1741–1746. [Google Scholar] [CrossRef]
- Kodama, T.; Sasaki, I.; Sugimura, H. Synthesis of pyridazine derivatives via Aza-Diels-Alder reactions of 1,2,3-triazine derivatives and 1-propynylamines. J. Org. Chem. 2021, 86, 8926–8932. [Google Scholar] [CrossRef]
- Ghozlan, S.A.S.; Abdelhamid, I.A.; Hassaneen, H.M.; Elnagdi, M.H. Studies with enamines and azaenamines: A novel efficient route to 6-amino-1,4-dihydropyridazines and their condensed derivatives. J. Heterocycl. Chem. 2007, 44, 105–108. [Google Scholar] [CrossRef]
- Aziz, S.I.; Anwar, H.F.; El-Apasery, M.A.; Elnagdi, M.H. Studies with pyridazines and condensed pyridazines: Routes for synthesis of 3-amino-5-aryl-2,5-dihydro-pyridazine, 10aH-pyridazino[1,6-a]quinazoline and thieno[3,4-d]pyridazinone. J. Heterocycl. Chem. 2007, 44, 877–881. [Google Scholar] [CrossRef]
- Wu, Q.; Shao, P.-L.; He, Y. Synthesis of 1,4,5,6-tetrahydropyridazines and pyridazines via transition-metal-free (4 + 2) cycloaddition of alkoxyallenes with 1,2-diaza-1,3-dienes. RSC Adv. 2019, 9, 21507–21512. [Google Scholar] [CrossRef]
- Liu, Z.; Lou, J.; Xiao, J. TBAI/K2S2O8-promoted [4+2] annulation of ketene N,S-acetals and N-tosylhydrazones toward pyridazines. Org. Lett. 2021, 23, 1606–1610. [Google Scholar] [CrossRef] [PubMed]
- Huo, H.; Runa, A.; Gong, Y. Formal [3+3] cycloaddition reactions between electron-deficient cyclopropenes and hydrazones: A route to alkyl 1,4,5,6-tetrahydropyridazine-3-carboxylates. J. Org. Chem. 2019, 84, 2093–2101. [Google Scholar] [CrossRef] [PubMed]
- Suleman, M.; Xie, J.; Wang, Z.; Lu, P.; Wang, Y. Base promoted three-component annulation of 4-diazoisochroman-3-imines with dimethylsulfonium ylides: Synthesis of highly functionalized isochromeno[4,3-c]pyridazines. J. Org. Chem. 2021, 86, 455–465. [Google Scholar] [CrossRef]
- Sheibani, H.; Esfandiarpoor, Z. Multicomponent synthesis of pyridazine and pyridazinoquinazoline derivatives in the presence of catalysts such as magnesium oxide (MgO) and 12-tungstophosphoric acid (PW). J. Heterocycl. Chem. 2011, 48, 1122–1125. [Google Scholar] [CrossRef]
- Bel Abed, H.; Mammoliti, O.; Bande, O.; Van Lommen, G.; Herdewijn, P. Strategy for the synthesis of pyridazine heterocycles and their derivatives. J. Org. Chem. 2013, 78, 7845–7858. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.-Y.; Lu, Y.-J.; Cheng, Y.-C. In(OTf)3-mediated synthesis of substituted pyridazines. Tetrahedron 2015, 71, 6840–6845. [Google Scholar] [CrossRef]
- Hashem, H.E.; Haneen, D.S.A.; Saied, K.F.; Youssef, A.S.A. Synthesis of new annulated pyridazine derivatives and studying their antioxidant and antimicrobial activities. Synth. Commun. 2019, 49, 3169–3180. [Google Scholar] [CrossRef]
- Moore, J.A.; Volker, E.J.; Kopay, C.M. Heterocyclic studies. 34. Tolylsulfonyl derivatives of 2,3-dihydro-5-methyl-6-phenyl-1,2-diazepin-4-one. Rearrangement to a 1,4-dihydropyridazine. J. Org. Chem. 1971, 36, 2676–2680. [Google Scholar] [CrossRef]
- Gradl, S.; Köckenberger, J.; Oppl, J.; Schiller, M.; Heinrich, M.R. Synthetic Route to Phenyl Diazenes and Pyridazinium Salts from Phenylazosulfonates. J. Org. Chem. 2021, 86, 6228–6238. [Google Scholar] [CrossRef]
- Flitsch, W.; Krämer, U. Synthesen und reaktionen von 5-Aza-indolizinen und 5-Aza-cycl[3.2.2]azin-derivaten. Tetrahedron Lett. 1968, 9, 1479–1484. [Google Scholar] [CrossRef]
- Flitsch, W.; Krämer, U.; Zimmermann, H. Cyclische Verbindungen mit Heterobrückenatomen, V. Zur Chemie der 1-Amino-pyrrole. Chem. Ber. 1969, 102, 3268–3276. [Google Scholar] [CrossRef]
- Kuhla, D.E.; Lombardino, J.O. Pyrrolodiazines with a Bridgehead Nitrogen. In Advances in Heterocyclic Chemistry; Katritzky, A.R., Boulton, A.J., Eds.; Academic Press: Cambridge, MA, USA, 1977; Volume 21, pp. 1–63. [Google Scholar]
- Sagyam, R.R.; Buchikonda, R.; Pitta, J.P.; Vurimidi, H.; Padi, P.R.; Ghanta, M.R. An expedient and new synthesis of pyrrolo[1,2-b]pyridazine derivatives. Beilstein J. Org. Chem. 2009, 5, 66. [Google Scholar] [CrossRef]
- Xiang, H.-Y.; Chen, J.-Y.; Huan, X.-J.; Chen, Y.; Gao, Z.-B.; Ding, J.; Miao, Z.-H.; Yang, C.-H. Identification of 2-substituted pyrrolo[1,2-b]pyridazine derivatives as new PARP-1 inhibitors. Bioorg. Med. Chem. Lett. 2021, 31, 127710. [Google Scholar] [CrossRef] [PubMed]
- Merugu, R.; Garimella, S.; Balla, D.; Sambaru, K. Synthesis and Biological Activities of Pyrimidines: A Review. Int. J. PharmTech Res. 2016, 8, 88–93. [Google Scholar] [CrossRef]
- Mahfoudh, M.; Abderrahim, R.; Leclerc, E.; Campagne, J.-M. Recent approaches to the synthesis of pyrimidine derivatives. Eur. J. Org. Chem. 2017, 2017, 2856–2865. [Google Scholar] [CrossRef]
- Díaz-Fernández, M.; Calvo-Losada, S.; Quirante, J.J.; Sarabia, F.; Algarra, M.; Pino-González, M.S. Catalyzed methods to synthesize pyrimidine and related heterocyclic compounds. Catalysts 2023, 13, 180. [Google Scholar] [CrossRef]
- Müller, T.J.; Karpov, A.S. Straightforward novel one-pot enaminone and pyrimidine syntheses by coupling-addition-cyclocondensation sequences. Synthesis 2003, 2003, 2815–2826. [Google Scholar] [CrossRef]
- Zhichkin, P.; Fairfax, D.J.; Eisenbeis, S.A. A general procedure for the synthesis of 2-substituted pyrimidine-5-carboxylic esters. Synthesis 2002, 2002, 720–722. [Google Scholar] [CrossRef]
- Liu, F.; Zhang, X.; Qian, Q.; Yang, C. A concise and efficient approach to 2,6-disubstituted 4-fluoro-pyrimidines from α-CF3 aryl ketones. Synthesis 2020, 52, 273–280. [Google Scholar] [CrossRef]
- Romanov, A.R.; Rulev, A.Y.; Popov, A.V.; Kondrashov, E.V.; Zinchenko, S.V. Reaction of Bromoenones with Amidines: A Simple Catalyst-Free Approach to Trifluoromethylated Pyrimidines. Synthesis 2020, 52, 1512–1522. [Google Scholar] [CrossRef]
- Mondal, R.; Chakraborty, G.; Guin, A.K.; Sarkar, S.; Paul, N.D. Iron-Catalyzed Alkyne-Based Multicomponent Synthesis of Pyrimidines under Air. J. Org. Chem. 2021, 86, 13186–13197. [Google Scholar] [CrossRef]
- Qin, Z.; Ma, Y.; Li, F. Construction of a Pyrimidine Framework through [3+2+1] Annulation of Amidines, Ketones, and N,N-Dimethylaminoethanol as One Carbon Donor. J. Org. Chem. 2021, 86, 13734–13743. [Google Scholar] [CrossRef]
- Sagir, H.; Rai, P.; Neha, S.; Singh, P.K.; Tiwari, S.; Siddiqui, I.R. S-Nanoparticle/SDS: An efficient and recyclable catalytic system for synthesis of substituted 4H-pyrido[1,2-a]pyrimidines in aqueous admicellar medium. RSC Adv. 2016, 6, 73924–73932. [Google Scholar] [CrossRef]
- Jain, K.S.; Kathiravan, M.K.; Bariwal, J.B.; Chaskar, P.K.; Tompe, S.S.; Arya, N. Novel dual use of formamide-POCl3 mixture for the efficient, one-pot synthesis of condensed 2H-pyrimidin-4-amine libraries under microwave irradiation. Synth. Commun. 2013, 43, 719–727. [Google Scholar] [CrossRef]
- Nguyen, T.K.; Titov, G.D.; Khoroshilova, O.V.; Kinzhalov, M.A.; Rostovskii, N.V. Light-induced one-pot synthesis of pyrimidine derivatives from vinyl azides. Org. Biomol. Chem. 2020, 18, 4971–4982. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, R.; Zhao, Q.; Yao, J.; Miao, M. Palladium/Silver Cocatalyzed Divergent Dimerization of 2H-Azirines for Regioselective Synthesis of Tetrasubstituted Pyrroles and Pyrimidines. Org. Lett. 2023, 25, 3978–3983. [Google Scholar] [CrossRef]
- Reisenbauer, J.C.; Paschke, A.-S.K.; Krizic, J.; Botlik, B.B.; Finkelstein, P.; Morandi, B. Direct access to quinazolines and pyrimidines from unprotected indoles and pyrroles through nitrogen atom insertion. Org. Lett. 2023, 25, 8419–8423. [Google Scholar] [CrossRef]
- Dutta, J.; Ghosh, S.; Hassan, A.; De Sarkar, S. Concomitant (3+3) Annulation/Fragmentation of Triazinanes with Enamines: Electrosynthesis of Multisubstituted Dihydropyrimidines. Org. Lett. 2025, 27, 2682–2686. [Google Scholar] [CrossRef]
- Minguez, J.; Vaquero, J.J.; García-Navio, J.; Alvarez-Builla, J. Improved synthesis of pyrrolo[1,2-c]pyrimidine and derivatives. Tetrahedron Lett. 1996, 37, 4263–4266. [Google Scholar] [CrossRef]
- Alizadeh, A.; Ghasemzadeh, H.; Xiao, H.-P. A highly efficient synthesis of pyrrolo[1,2-a]pyrimidine derivatives containing 1,3-indandione skeleton. ChemistrySelect 2019, 4, 7446–7449. [Google Scholar] [CrossRef]
- Li, T.; Chiou, M.-F.; Li, Y.; Ye, C.; Su, M.; Xue, M.; Yuan, X.; Wang, C.; Wan, W.-M.; Li, D.; et al. Synthesis of unsymmetrically tetrasubstituted pyrroles and studies of AIEE in pyrrolo[1,2-a]pyrimidine derivatives. Chem. Sci. 2022, 13, 5667–5673. [Google Scholar] [CrossRef]
- Ong, K.T.; Liu, Z.-Q.; Tay, M.G. Review on the synthesis of pyrazine and its derivatives. Borneo J. Resour. Sci. Technol. BJRST 2017, 7, 60–75. [Google Scholar] [CrossRef]
- Staedel, W.; Rügheimer, L. Ueber die Einwirkung von Ammoniak auf Chloracetylbenzol. Ber. Dtsch. Chem. Ges. 1876, 9, 563–564. [Google Scholar] [CrossRef]
- Gutknecht, H. Ueber nitrosoäthylmethylketon. Ber. Dtsch. Chem. Ges. 1879, 12, 2290–2292. [Google Scholar] [CrossRef]
- Gastaldi, C. Sulle pirazine (On pyrazines). Gazz. Chim. Ital. 1921, 51, 233–255. [Google Scholar]
- Loy, N.S.Y.; Kim, S.; Park, C.-M. Synthesis of unsymmetrical pyrazines based on α-diazo oxime ethers. Org. Lett. 2015, 17, 395–397. [Google Scholar] [CrossRef]
- Cho, C.S.; Oh, S.G. A new ruthenium-catalyzed approach for quinoxalines from o-phenylenediamines and vicinal-diols. Tetrahedron Lett. 2006, 47, 5633–5636. [Google Scholar] [CrossRef]
- Hille, T.; Irrgang, T.; Kempe, R. The synthesis of benzimidazoles and quinoxalines from aromatic diamines and alcohols by iridium-catalyzed acceptorless dehydrogenative alkylation. Chem. Eur. J. 2014, 20, 5569–5572. [Google Scholar] [CrossRef]
- Shee, S.; Ganguli, K.; Jana, K.; Kundu, S. Cobalt complex catalyzed atom-economical synthesis of quinoxaline, quinoline and 2-alkylaminoquinoline derivatives. Chem. Commun. 2018, 54, 6883–6886. [Google Scholar] [CrossRef]
- Daw, P.; Kumar, A.; Espinosa-Jalapa, N.A.; Diskin-Posner, Y.; Ben-David, Y.; Milstein, D. Synthesis of pyrazines and quinoxalines via acceptorless dehydrogenative coupling routes catalyzed by manganese pincer complexes. ACS Catal. 2018, 8, 7734–7741. [Google Scholar] [CrossRef]
- Yang, H.-R.; Hu, Z.-Y.; Li, X.-C.; Wu, L.; Guo, X.-X. Cobalt-catalyzed effective access to quinoxalines with insights in annulation of terminal alkynes and o-phenylenediamines. Org. Lett. 2022, 24, 8392–8396. [Google Scholar] [CrossRef]
- Tran, T.M.C.; Lai, N.D.; Bui, T.T.T.; Mac, D.H.; Nguyen, T.T.T.; Retailleau, P.; Nguyen, T.B. DABCO-catalyzed DMSO-promoted sulfurative 1,2-diamination of phenylacetylenes with elemental sulfur and o-phenylenediamines: Access to quinoxaline-2-thiones. Org. Lett. 2023, 25, 7225–7229. [Google Scholar] [CrossRef]
- Chaubey, T.N.; Borpatra, P.J.; Pandey, S.K. Elemental sulfur-mediated synthesis of quinoxalines from sulfoxonium ylides. Org. Lett. 2023, 25, 5329–5332. [Google Scholar] [CrossRef]
- Terenin, V.I.; Kabanova, E.V.; Feoktistova, E.S. Synthesis and properties of pyrrolo[1,2-a]pyrazines (review). Chem. Heterocycl. Compd. 1991, 27, 1037–1048. [Google Scholar] [CrossRef]
- Dehnavi, F.; Alizadeh, S.R.; Ebrahimzadeh, M.A. Pyrrolopyrazine derivatives: Synthetic approaches and biological activities. Med. Chem. Res. 2021, 30, 1981–2006. [Google Scholar] [CrossRef]
- Voznesenskaia, N.G.; Shmatova, O.I.; Nenajdenko, V.G. Pictet–Spengler synthesis of perfluoroalkylated tetrahydro-γ-carbolines and tetrahydropyrrolopyrazines. Synthesis 2020, 52, 263–272. [Google Scholar] [CrossRef]
- Seregin, I.V.; Schammel, A.W.; Gevorgyan, V. Multisubstituted N-fused heterocycles via transition metal-catalyzed cycloisomerization protocols. Tetrahedron 2008, 64, 6876–6883. [Google Scholar] [CrossRef]
- Chen, W.; Hu, M.; Wu, J.; Zou, H.; Yu, Y. Domino approach for the synthesis of pyrrolo[1,2-a]pyrazine from vinyl azides. Org. Lett. 2010, 12, 3863–3865. [Google Scholar] [CrossRef]
- Martynovskaya, S.V.; Budaev, A.B.; Ushakov, I.A.; Borodina, T.N.; Ivanov, A.V. Solvent moisture-controlled self-assembly of fused benzoimidazopyrrolopyrazines with different ring’s interposition. Molecules 2022, 27, 2460. [Google Scholar] [CrossRef]
- Ivanov, A.V.; Martynovskaya, S.V.; Shcherbakova, V.S.; Ushakov, I.A.; Borodina, T.N.; Bobkov, A.S.; Vitkovskaya, N.M. Ambient access to a new family of pyrrole-fused pyrazine nitrones via 2-carbonyl-N-allenylpyrroles. Org. Chem. Front. 2020, 7, 4019–4025. [Google Scholar] [CrossRef]
- Trofimov, B.; Sobenina, L.; Sagitova, E.; Ushakov, I. Transition-metal-free synthesis of pyrrolo[1,2-a]pyrazines via intramolecular cyclization of N-propargyl(pyrrolyl)enaminones. Synthesis 2017, 49, 4065–4081. [Google Scholar] [CrossRef]
- Sari, O.; Seybek, A.F.; Kaya, S.; Menges, N.; Erdem, S.S.; Balci, M. Mechanistic insights into the reaction of N-propargylated pyrrole- and indole-carbaldehyde with ammonia, alkyl amines, and branched amines: A synthetic and theoretical investigation. Eur. J. Org. Chem. 2019, 2019, 5261–5274. [Google Scholar] [CrossRef]
- Martinez-Ariza, G.; Mehari, B.T.; Pinho, L.A.G.; Foley, C.; Day, K.; Jewett, J.C.; Hulme, C. Synthesis of fluorescent heterocycles via a Knoevenagel/[4 + 1]-cycloaddition cascade using acetyl cyanide. Org. Biomol. Chem. 2017, 15, 6076–6079. [Google Scholar] [CrossRef]
- Pinner, A. Ueber Diphenyloxykyanidin. Ber. Dtsch. Chem. Ges. 1890, 23, 2919–2922. [Google Scholar] [CrossRef]
- Kelarev, V.I.; Karakhanov, R.A.; Polivin, Y.N.; Remizov, A.S. Synthesis of 1,3,5-triazine derivatives on the basis of carboxylic acid imino esters (review). Chem. Heterocycl. Compd. 1992, 28, 1357–1373. [Google Scholar] [CrossRef]
- Díaz-Ortiz, A.; de la Hoz, A.; Moreno, A.; Sánchez-Migallón, A.; Valiente, G. Synthesis of 1,3,5-triazines in solvent-free conditions catalysed by silica-supported lewis acids. Green Chem. 2002, 4, 339–343. [Google Scholar] [CrossRef]
- Díaz-Ortiz, Á.; Elguero, J.; Foces-Foces, C.; de la Hoz, A.; Moreno, A.; del Carmen Mateo, M.; Sánchez-Migallón, A.; Valiente, G. Green synthesis and self-association of 2,4-diamino-1,3,5-triazine derivatives. New J. Chem. 2004, 28, 952–958. [Google Scholar] [CrossRef]
- Shie, J.-J.; Fang, J.-M. Microwave-assisted one-pot tandem reactions for direct conversion of primary alcohols and aldehydes to triazines and tetrazoles in aqueous media. J. Org. Chem. 2007, 72, 3141–3144. [Google Scholar] [CrossRef]
- Gautret, P.; Oudir, S.; Rigo, B.; Hénichart, J.-P. A convenient method for the conversion of a carboxy group into a 4,6-di-methoxy-1,3,5-triazine group: Application to N-benzylpyroglutamic acids. Synthesis 2006, 2006, 2845–2848. [Google Scholar] [CrossRef]
- Zhang, C.; Ban, M.-T.; Zhu, K.; Zhang, L.-Y.; Luo, Z.-Y.; Guo, S.-N.; Cui, D.-M.; Zhang, Y. Copper-catalyzed synthesis of substituted 2,4-diamino-1,3,5-triazines from 1,1-dibromoalkenes and biguanides. Org. Lett. 2017, 19, 3947–3949. [Google Scholar] [CrossRef]
- Arshad, M.; Khan, T.A.; Khan, M.A. 1,2,4-triazine derivatives: Synthesis and biological applications. ChemInform 2015, 46. [Google Scholar] [CrossRef]
- Rai, G.S.; Maru, J.J. Synthetic strategies for pyrrolo[2,1-f][1,2,4]triazine: The parent moiety of antiviral drug remdesivir. Chem. Heterocycl. Compd. 2020, 56, 1517–1522. [Google Scholar] [CrossRef]
- Tang, D.; Wang, J.; Wu, P.; Guo, X.; Li, J.-H.; Yang, S.; Chen, B.-H. Synthesis of 1,2,4-triazine derivatives via [4+2] domino annulation reactions in one pot. RSC Adv. 2016, 6, 12514–12518. [Google Scholar] [CrossRef]
- Hashem, H.E.; Abo-Bakr, A.M. Synthesis of some new 1,2,4-triazine and 1,2,5-oxadiazine derivatives with antimicrobial activity. Heteroat. Chem. 2019, 2019, 2326514. [Google Scholar] [CrossRef]
- Ji, Z.; Dai, Y.; Abad-Zapatero, C.; Albert, D.H.; Bouska, J.J.; Glaser, K.B.; Marcotte, P.A.; Soni, N.B.; Magoc, T.J.; Stewart, K.D.; et al. Exploration of diverse hinge-binding scaffolds for selective Aurora kinase inhibitors. Bioorg. Med. Chem. Lett. 2012, 22, 4528–4531. [Google Scholar] [CrossRef]
- Roy, S.; Yadaw, A.; Roy, S.; Sirasani, G.; Gangu, A.; Brown, J.D.; Armstrong, J.D., 3rd; Stringham, R.W.; Gupton, B.F.; Senanayake, C.H.; et al. Facile and scalable methodology for the pyrrolo[2,1-f][1,2,4]triazine of remdesivir. Org. Process Res. Dev. 2022, 26, 82–90. [Google Scholar] [CrossRef]
- Sugimura, H.; Takeuchi, R.; Ichikawa, S.; Nagayama, E.; Sasaki, I. Synthesis of 1,2,3-triazines using the base-mediated cyclization of (Z)-2,4-diazido-2-alkenoates. Org. Lett. 2018, 20, 3434–3437. [Google Scholar] [CrossRef]
- De Angelis, L.; Zheng, H.; Perz, M.T.; Arman, H.; Doyle, M.P. Intermolecular [5+1]-cycloaddition between vinyl diazo compounds and tert-butyl nitrite to 1,2,3-triazine 1-oxides and their further transformation to isoxazoles. Org. Lett. 2021, 23, 6542–6546. [Google Scholar] [CrossRef]
- Klenov, M.S.; Voronin, A.A.; Churakov, A.M.; Tartakovsky, V.A. Advances in the chemistry of 1,2,3,4-tetrazines. Russ. Chem. Rev. 2023, 92, RCR5089. [Google Scholar] [CrossRef]
- Nelsen, S.F.; Fibiger, R. 1,4-Dimethyl-1,4,5,6-tetrahydro-1,2,3,4-tetrazine. Cyclic cis-2-tetrazene. J. Am. Chem. Soc. 1972, 94, 8497–8501. [Google Scholar] [CrossRef]
- Kaihoh, T.; Itoh, T.; Yamaguchi, K.; Ohsawa, A. First synthesis of a 1,2,3,4-tetrazine. Chem. Commun. 1988, 1608–1609. [Google Scholar] [CrossRef]
- Mackert, P.J.; Hafner, K.; Nimmerfroh, N.; Banert, K. Synthesis, structure and reactivity of cyclopenta-annulated 1,2,3,4-tetrazines. Chem. Ber. 1994, 127, 1479–1488. [Google Scholar] [CrossRef]
- Moustafa, A.H.; Mohammed, S.M.; El-Salam, E.A.A.; El-Sayed, H.A. Synthesis and antimicrobial activity of new 3H-chromeno[2,3-d]pyrimidine derivatives. Russ. J. Gen. Chem. 2020, 90, 1566–1572. [Google Scholar] [CrossRef]
- Gao, Y.; Lam, Y. Synthesis of pyrazolo[5,1-d][1,2,3,5]tetrazine-4(3H)-ones. J. Comb. Chem. 2010, 12, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Butler, R.N.; Cunningham, D.; McArdle, P.; O’Halloran, G.A. 1,2,3,5-Tetrazines and 1,2,3-triazaspiro[4.4]nonanes: Remarkable products from 1,3-dipolar cycloadditions of N-sulphinylamines with substituted triazolium imides. Chem. Commun. 1988, 3, 232–234. [Google Scholar] [CrossRef]
- Wu, Z.-C.; Boger, D.L. Synthesis, characterization, and cycloaddition reactivity of a monocyclic aromatic 1,2,3,5-tetrazine. J. Am. Chem. Soc. 2019, 141, 16388–16397. [Google Scholar] [CrossRef]
- Neunhoeffer, H. 2.21—Tetrazines and Pentazines. In Comprehensive Heterocyclic Chemistry; Katritzky, A.R., Rees, C.W., Eds.; Pergamon: Oxford, UK, 1984; pp. 531–572. [Google Scholar]
- Sauer, J. 6.21—1,2,4,5-Tetrazines. In Comprehensive Heterocyclic Chemistry II; Katritzky, A.R., Rees, C.W., Scriven, E.F.V., Eds.; Pergamon: Oxford, UK, 1996; pp. 901–955. [Google Scholar]
- Wu, H.; Devaraj, N.K. Advances in tetrazine bioorthogonal chemistry driven by the synthesis of novel tetrazines and dienophiles. Acc. Chem. Res. 2018, 51, 1249–1259. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.X.; Rao, G.W.; Sun, Y.Q. Synthesis and antitumor activity of s-tetrazine derivatives. Bioorg. Med. Chem. Lett. 2004, 14, 1177–1181. [Google Scholar] [CrossRef]
- Pinner, A. Ueber die Einwirkung von Hydrazin auf Imidoäther. Ber. Dtsch. Chem. Ges. 1893, 26, 2126–2135. [Google Scholar] [CrossRef]
- Yang, J.; Karver, M.R.; Li, W.; Sahu, S.; Devaraj, N.K. Metal-catalyzed one-pot synthesis of tetrazines directly from aliphatic nitriles and hydrazine. Angew. Chem. Int. Ed. 2012, 51, 5222–5225. [Google Scholar] [CrossRef]
- Coburn, M.D.; Buntain, G.A.; Harris, B.W.; Hiskey, M.A.; Lee, K.Y.; Ott, D.G. An improved synthesis of 3,6-diamino-1,2,4,5-tetrazine. II. From triaminoguanidine and 2,4-pentanedione. J. Heterocycl. Chem. 1991, 28, 2049–2050. [Google Scholar] [CrossRef]
- Alghamdi, Z.S.; Klausen, M.; Gambardella, A.; Lilienkampf, A.; Bradley, M. Solid-phase synthesis of s-tetrazines. Org. Lett. 2023, 25, 3104–3108. [Google Scholar] [CrossRef]
- Maj, A.; Kudelko, A.; Świątkowski, M. Synthesis and luminescent properties of s-tetrazine derivatives conjugated with the 4H-1,2,4-triazole ring. Molecules 2022, 27, 3642. [Google Scholar] [CrossRef] [PubMed]
- Neunhoeffer, H.; Degen, H.-J. Hydrazidine, II. Die Synthese von Pyrrolo[1,2-b]-1,2,4,5-tetrazine. Chem. Ber. 1975, 108, 3509–3517. [Google Scholar] [CrossRef]
- Degen, H.-J.; Haller, S.; Und, K.H.; Neunhoeffer, H. Hydrazidine, III. Synthese von 1,2,4,5-tetrazino [3,2-a]isoindolen. Chem. Ber. 1979, 112, 1981–1990. [Google Scholar] [CrossRef]
- Neunhoeffer, H.; Karafiat, U.; Köhler, G.; Sowa, B.; Hydrazidine, V. Synthese und Reaktionen aromatischer und aliphatischer Hydrazidine und deren N1-substituierter Derivate. Liebigs Ann. Chem. 1992, 1992, 115–126. [Google Scholar] [CrossRef]
- Martynovskaya, S.V.; Gyrgenova, E.A.; Ushakov, I.A.; Borodina, T.N.; Ivanov, A.V. Synthesis of Tetrazines from N-Allenylpyrrole-2-carbaldehydes and pH-Controlled Reversible Fragmentation. Org. Lett. 2024, 26, 132–136. [Google Scholar] [CrossRef] [PubMed]





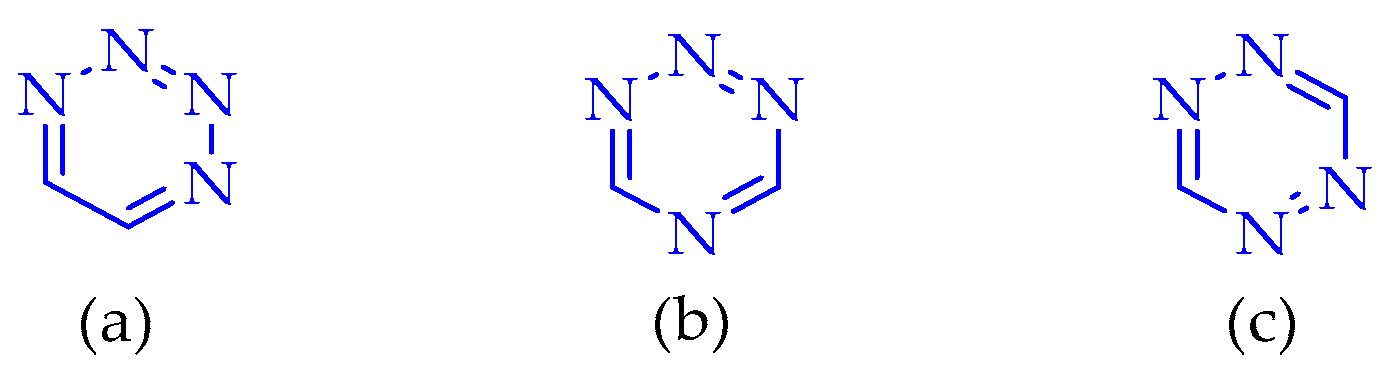
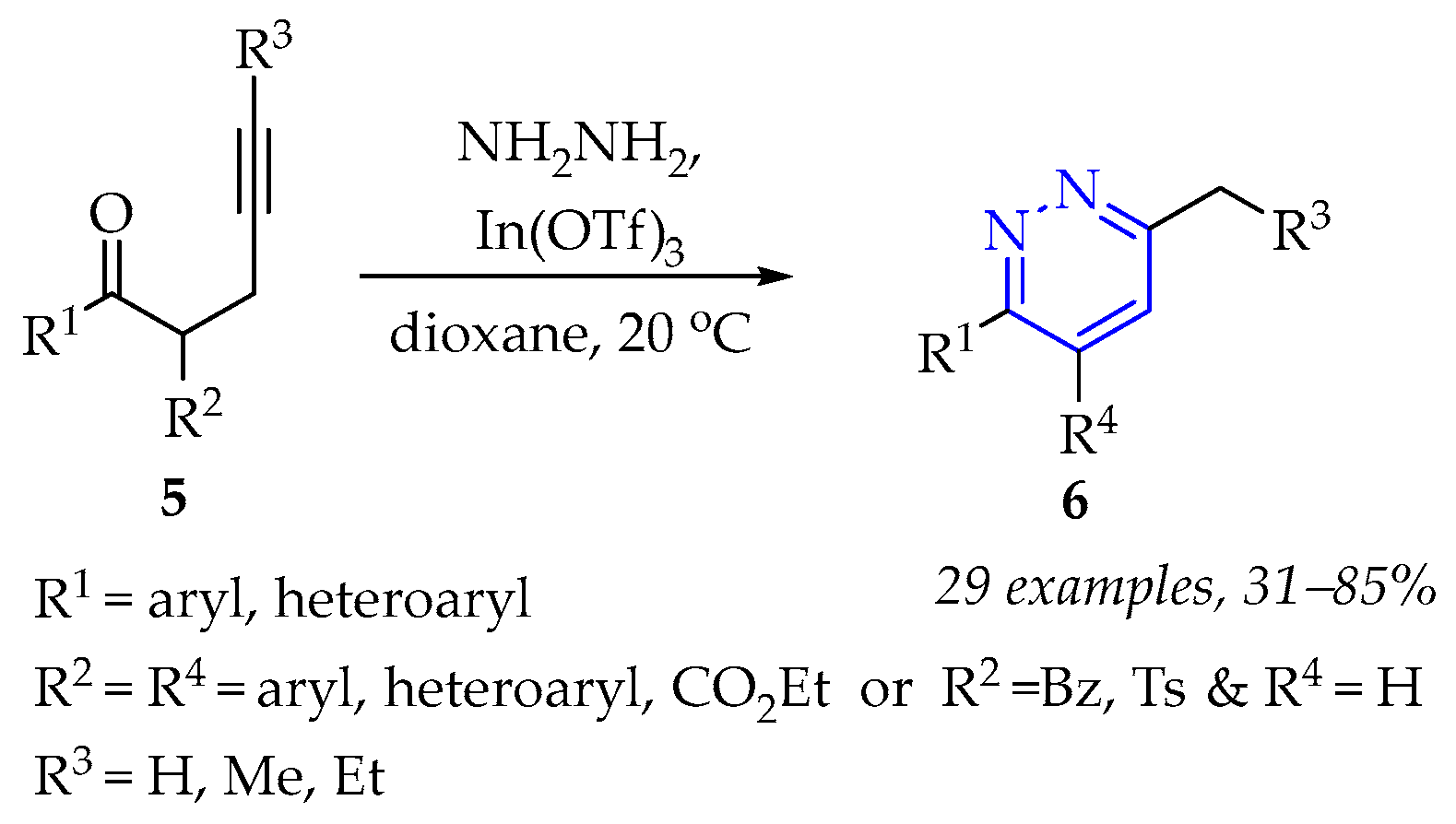


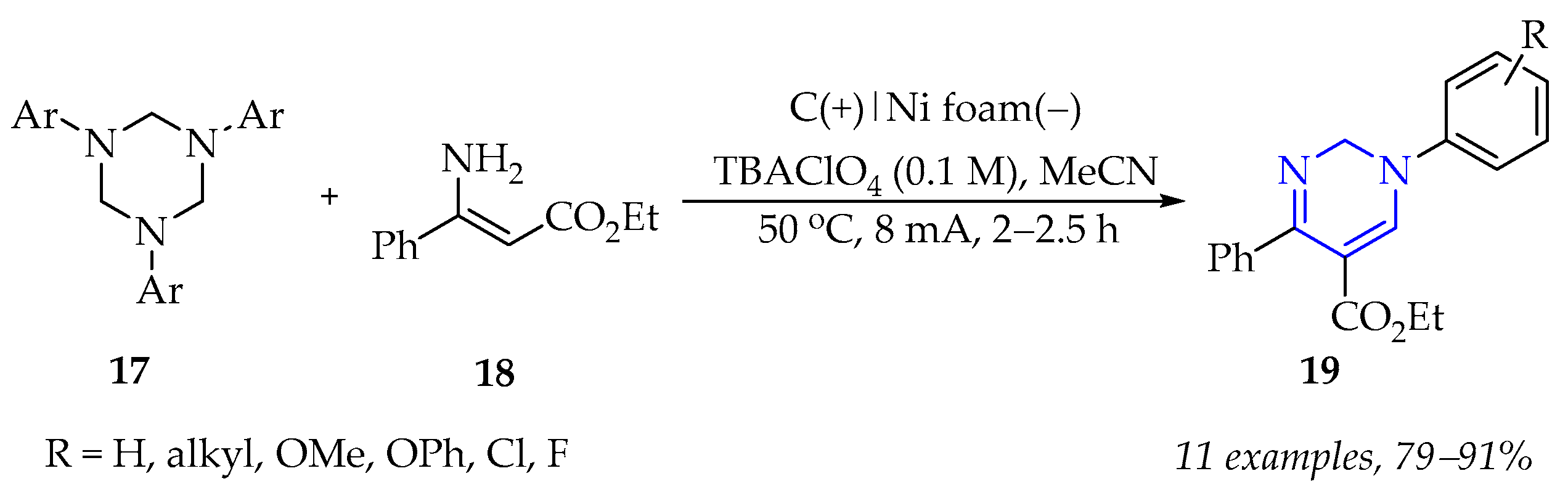


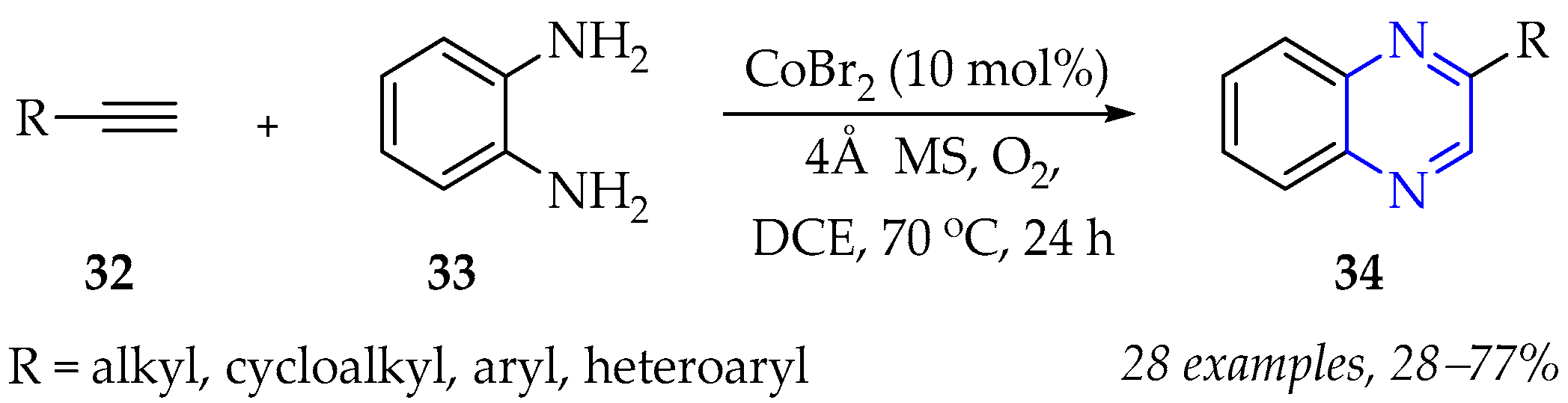


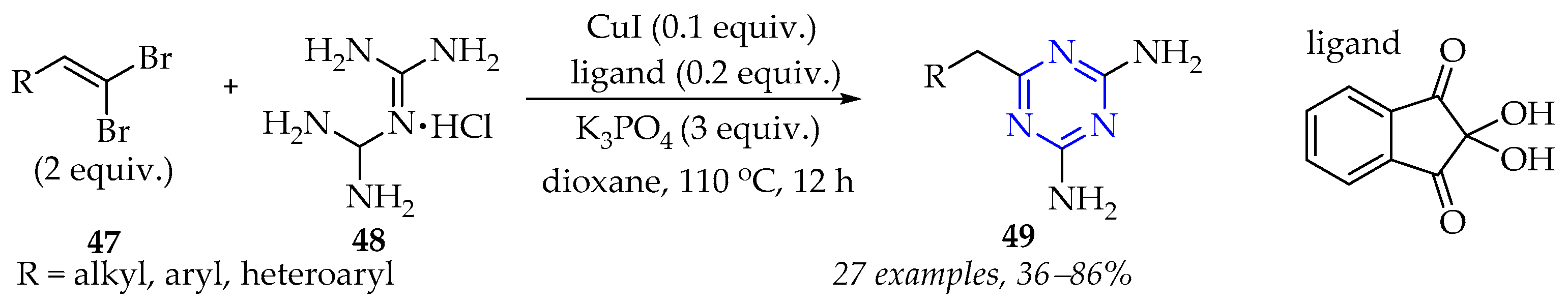

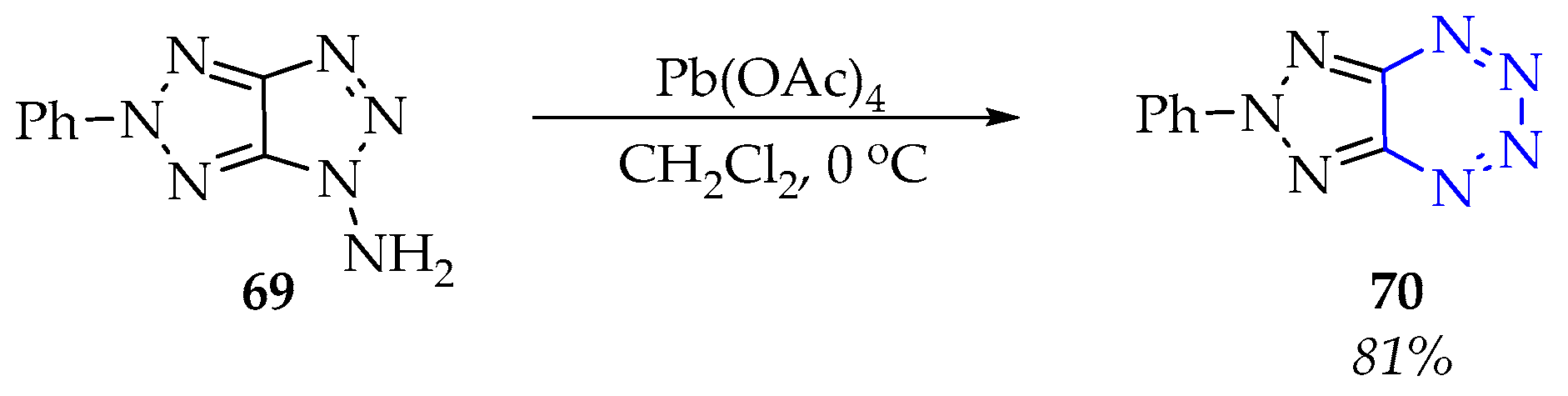
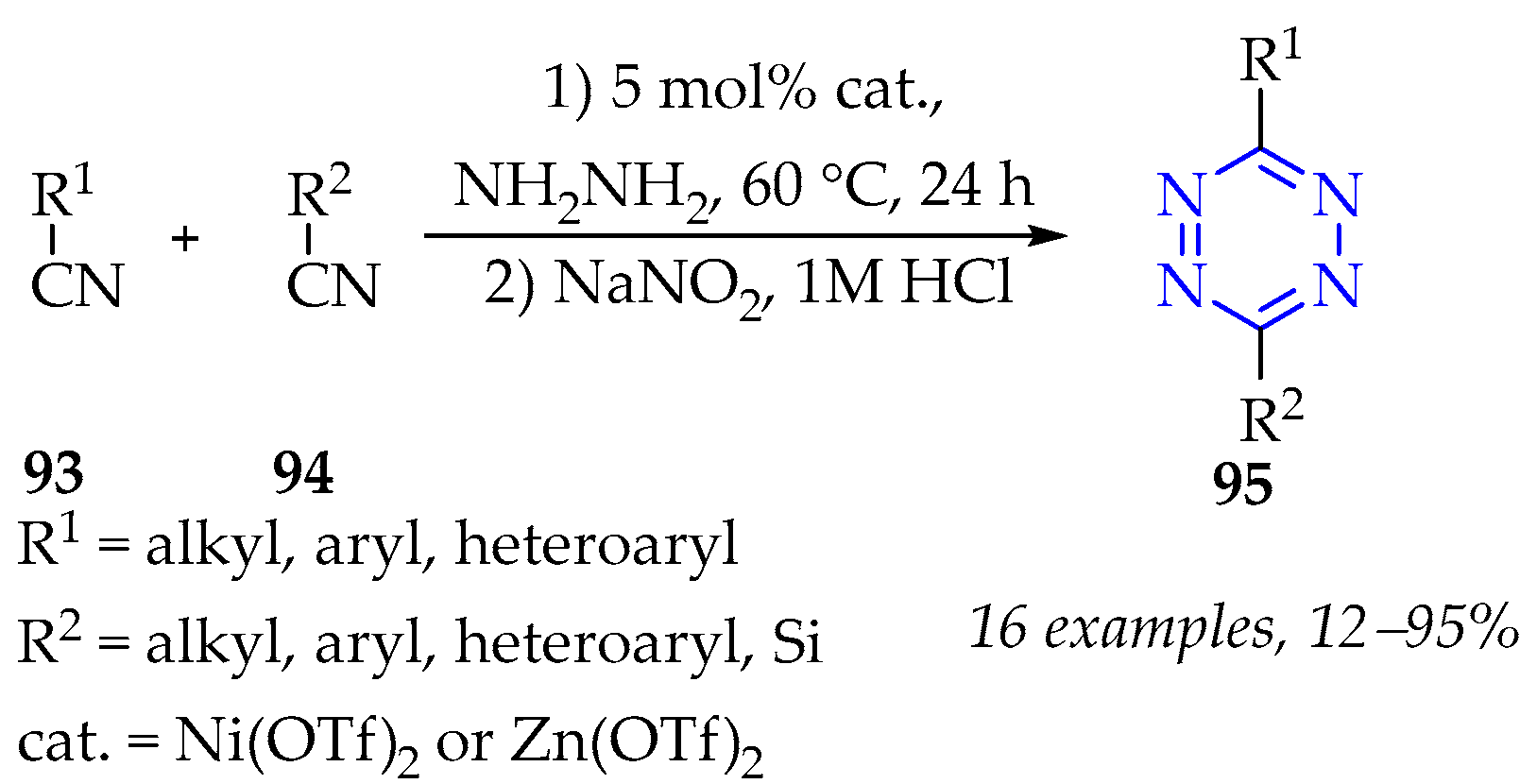



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gyrgenova, E.A.; Titova, Y.Y.; Ivanov, A.V. Efficient Approaches to the Design of Six-Membered Polyazacyclic Compounds—Part 1: Aromatic Frameworks. Molecules 2025, 30, 3264. https://doi.org/10.3390/molecules30153264
Gyrgenova EA, Titova YY, Ivanov AV. Efficient Approaches to the Design of Six-Membered Polyazacyclic Compounds—Part 1: Aromatic Frameworks. Molecules. 2025; 30(15):3264. https://doi.org/10.3390/molecules30153264
Chicago/Turabian StyleGyrgenova, Elena A., Yuliya Y. Titova, and Andrey V. Ivanov. 2025. "Efficient Approaches to the Design of Six-Membered Polyazacyclic Compounds—Part 1: Aromatic Frameworks" Molecules 30, no. 15: 3264. https://doi.org/10.3390/molecules30153264
APA StyleGyrgenova, E. A., Titova, Y. Y., & Ivanov, A. V. (2025). Efficient Approaches to the Design of Six-Membered Polyazacyclic Compounds—Part 1: Aromatic Frameworks. Molecules, 30(15), 3264. https://doi.org/10.3390/molecules30153264