Abstract
Diabetic retinopathy (DR) is a progressive, multifactorial complication of diabetes and one of the major global causes of visual impairment. Its pathogenesis involves chronic hyperglycaemia-induced oxidative stress, inflammation, mitochondrial dysfunction, neurodegeneration, and pathological angiogenesis, as well as emerging systemic contributors such as gut microbiota dysregulation. While current treatments, including anti-vascular endothelial growth factor (anti-VEGF) agents, corticosteroids, and laser photocoagulation, have shown clinical efficacy, they are largely limited to advanced stages of DR, require repeated invasive procedures, and do not adequately address early neurovascular and metabolic abnormalities. Resveratrol (RSV), a naturally occurring polyphenol, has emerged as a promising candidate due to its potent antioxidant, anti-inflammatory, neuroprotective, and anti-angiogenic properties. This review provides a comprehensive analysis of the molecular mechanisms by which RSV exerts protective effects in DR, including modulation of oxidative stress pathways, suppression of inflammatory cytokines, enhancement of mitochondrial function, promotion of autophagy, and inhibition of pathological neovascularisation. Despite its promising pharmacological profile, the clinical application of RSV is limited by poor aqueous solubility, rapid systemic metabolism, and low ocular bioavailability. Various routes of administration, including intravitreal injection, topical instillation, and oral and sublingual delivery, have been investigated to enhance its therapeutic potential. Recent advances in drug delivery systems, including nanoformulations, liposomal carriers, and sustained-release intravitreal implants, offer potential strategies to address these challenges. This review also explores RSV’s role in combination therapies, its potential as a disease-modifying agent in early-stage DR, and the relevance of personalised medicine approaches guided by metabolic and genetic factors. Overall, the review highlights the therapeutic potential and the key translational challenges in positioning RSV as a multi-targeted treatment strategy for DR.
1. Introduction
Diabetes mellitus and diabetic retinopathy (DR) are rapidly escalating global public health concerns. DR affects one in three individuals with diabetes and remains the leading cause of blindness among the adult working population, necessitating the provision of comprehensive eye care services for individuals living with diabetes [1,2]. According to the World Health Organization, the global number of people with diabetes is projected to be more than double, rising from 171 million in 2000 to 366 million by 2030, with a similar prevalence observed in both men and women, and the highest rates occurring in individuals aged 75–79 years. In 2021, global health expenditures related to diabetes were estimated at USD 966 billion, and are expected to increase to USD 1054 billion by 2045 [3,4]. Although the management of DR, a largely irreversible complication of diabetes mellitus, has become increasingly effective, it remains significantly more costly than the treatment of many other ocular diseases, placing a substantial burden on both patients and healthcare systems [2].
DR is a microvascular complication of diabetes mellitus that progresses gradually, typically presenting with worsening vision, the appearance of floaters, distorted visual perception, and, in advanced stages, partial or complete vision loss due to retinal detachment [5]. The severe forms of DR, which significantly affect vision, include proliferative DR, characterised by the abnormal growth of new retinal blood vessels, and diabetic macular oedema, marked by fluid accumulation and exudation in the central part of the retina [6]. The main risk factors for DR include chronic hyperglycaemia, hypertension, dyslipidaemia, obesity, duration of diabetes, and genetic predisposition, all of which contribute to the onset and progression of retinal microvascular damage [7,8,9,10,11]. In addition to managing and treating modifiable risk factors in patients with diabetes mellitus, the standard treatment for proliferative DR and/or diabetic macular oedema typically includes laser photocoagulation and/or intravitreal injections of anti-vascular endothelial growth factor (anti-VEGF) agents. These therapies often require multiple sessions to achieve and maintain optimal visual outcomes [12,13,14,15]. Intravitreal corticosteroid injections are commonly employed as second-line therapy. In more severe cases, complications such as persistent vitreous haemorrhage and tractional retinal detachment may necessitate a vitrectomy, a surgical procedure involving the removal of the vitreous humour and its replacement with a different solution [13].
Additionally, intravitreal aflibercept has received FDA approval for non-proliferative DR [14,15]. However, routine use of anti-VEGF therapy for non-proliferative DR remains unlikely as, despite initial regression of vascular lesions and temporary improvement in DR severity, studies indicate that underlying retinal ischemia remains unaltered and pathological changes often recur shortly after treatment cessation [16]. Anti-VEGF therapy is further limited by its short half-life and the need for frequent invasive intravitreal injections. While both photocoagulation and anti-VEGF agents have demonstrated efficacy in slowing disease progression, challenges such as drug resistance, suboptimal therapeutic responses, and the burdensome nature of repeated ocular injections continue to hinder treatment outcomes and patient adherence [17].
Given the current limitations of available DR therapies, primarily their invasive nature and limited efficacy in early disease stages, there is an urgent need to develop complementary or alternative therapeutic strategies [12]. These novel approaches should ideally target early pathogenic mechanisms such as oxidative stress, neuroinflammation, and mitochondrial dysfunction, and be delivered through non-invasive or less invasive routes to enhance patient compliance and therapeutic outcomes. Furthermore, interventions that improve the efficacy or durability of current treatments, while providing sustained protection against retinal neurovascular damage, are highly desirable. Accordingly, naturally derived bioactive compounds with pleiotropic properties have gained increasing attention. Among them, resveratrol (RSV), a polyphenol known for its antioxidant, anti-inflammatory, and neuroprotective effects, has emerged as a promising candidate for managing DR, either as an adjunct or a stand-alone therapy by modulating multiple pathological pathways involved in its progression [18,19,20].
RSV (3,4′,5-trihydroxy-trans-stilbene), a naturally occurring polyphenol found in grapes, red wine, berries, peanuts, and certain medicinal plants, has gained significant attention for its diverse health-promoting effects, including anti-inflammatory, antioxidant, anticarcinogenic, and lipid-modulating properties (Figure 1). Due to its natural origin and chemical structure, resveratrol is classified as a natural polyphenolic compound belonging to the stilbene group. Structurally, it comprises two benzene rings: one bearing hydroxyl groups at the 3 and 5 positions, and the other with a hydroxyl group at the 4′ position. This specific substitution pattern defines resveratrol as a polyphenol, contributing to its strong antioxidant properties. The two aromatic rings are connected via a carbon–carbon double bond (C=C), which forms the characteristic stilbene backbone of the molecule. Resveratrol naturally occurs in two geometric isomers, trans and cis. Among these, the trans isomer is both thermodynamically more stable and biologically more active, whereas the cis isomer primarily arises from photoisomerisation or the thermal conversion of the trans form under exposure to light, heat, or alkaline pH [21,22,23].
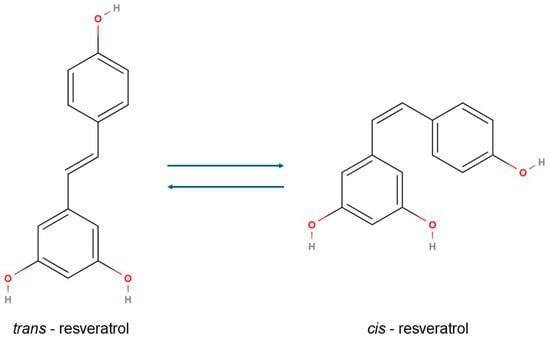
Figure 1.
The chemical structure of the cis and trans isomers of resveratrol.
RSV contributes to metabolic and vascular health by reducing vascular inflammation, enhancing vasodilation, inhibiting pathological angiogenesis, and improving glucose homeostasis. It also decreases insulin resistance, promotes autophagy, regulates lipid metabolism, protects pancreatic β-cells, alleviates diabetic cardiomyopathy, and upregulates glucose transporter type 4 (GLUT4) expression [24,25,26]. These pleiotropic actions are primarily mediated through activation of AMP-activated protein kinase (AMPK) and sirtuin 1 (SIRT1), which are key regulators of cellular energy homeostasis and stress resistance, thus supporting the prevention and management of diabetes-related complications [18]. In the retina, RSV has been shown to attenuate oxidative stress and prevent retinal ganglion cell apoptosis by activating the nuclear factor erythroid 2-related factor 2/heme oxygenase-1 (Nrf2/HO-1) signalling pathway [27]. As a master regulator of antioxidant defence, Nrf2 plays a central role in counteracting oxidative damage and maintaining glucose balance [28]. Despite these promising effects, the clinical translation of RSV has been hindered by its poor aqueous solubility, rapid metabolism, and low systemic bioavailability. Various nanotechnology-based drug delivery systems have been developed to overcome these challenges, including liposomes, polymeric nanoparticles, solid lipid nanoparticles, nanoemulsions, and exosome-based formulations. These strategies aim to improve RSV’s pharmacokinetic profile, increase retinal bioavailability, and enhance its therapeutic efficacy in vivo [29].
This review critically synthesises current evidence on the molecular mechanisms of RSV in DR, with an emphasis on its antioxidant, anti-inflammatory, and anti-angiogenic properties. It includes a discussion of preclinical findings, examines the results of emerging clinical studies, and analyses the potential of novel delivery systems to overcome existing pharmacological limitations. By synthesising recent advances, the aim is to assess the translational viability of RSV-based therapies in DR and outline prospective directions for future research and clinical application.
2. Pathophysiology of Diabetic Retinopathy
DR is a complex, multifactorial disease characterised by progressive neurovascular damage to the retina, driven by chronic hyperglycaemia, and exacerbated by oxidative stress, inflammation, neurodegeneration, and vascular dysfunction. While traditionally considered a microvascular complication of diabetes, recent advances have underscored the significant involvement of neurodegenerative processes, metabolic dysregulation, and gut-derived inflammatory mechanisms. Understanding these interconnected pathological pathways is essential for developing effective therapeutic strategies [30,31]. Table 1 summarises the key pathogenic mechanisms involved in DR, highlighting the principal molecular pathways, retinal impacts, and clinical relevance, along with the modulatory effects of RSV as a potential therapeutic agent [32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54].

Table 1.
Pathogenic Mechanisms and Retinal Changes in Diabetic Retinopathy: Resveratrol’s Therapeutic Relevance.
One of the earliest pathogenic mechanisms in DR is the overproduction of reactive oxygen species (ROS) due to hyperglycaemia-induced mitochondrial dysfunction. High glucose levels disrupt the mitochondrial electron transport chain (ETC), leading to excessive ROS generation and mitochondrial DNA damage, including double-strand breaks (DSBs), which trigger the overactivation of poly(ADP-ribose) polymerase (PARP) [35]. Hyperactivated PARP depletes cellular NAD+ and poly(ADP-ribosyl)ates glyceraldehyde-3-phosphate dehydrogenase (GAPDH), thereby inhibiting glycolysis and rerouting glucose metabolism into alternative damaging pathways, such as the polyol pathway, protein kinase C (PKC) activation, and hexosamine biosynthesis, all of which contribute to the accumulation of advanced glycation end-products (AGEs) and amplify oxidative damage in retinal vascular and neural cells [36,37].
To counteract oxidative insults, the transcription factor Nrf2 plays a pivotal role. Under oxidative stress conditions, Nrf2 dissociates from its cytoplasmic inhibitor Keap1, translocates into the nucleus, and binds to antioxidant response elements (AREs), initiating the transcription of detoxifying and antioxidant genes such as HO-1, NQO1, and glutathione S-transferases [38]. This Nrf2/ARE signalling pathway constitutes a vital endogenous defence mechanism that attenuates ROS-induced tissue injury and preserves retinal homeostasis. However, chronic hyperglycaemia may impair Nrf2 activation over time, weakening the antioxidant defence system in the diabetic retina [39,40].
Parallel to oxidative stress, low-grade chronic inflammation plays an important role in the pathogenesis of DR. The nuclear factor kappa B (NF-κB) pathway, a central regulator of immune and inflammatory responses, is activated under prolonged hyperglycaemic conditions, leading to the upregulation of pro-inflammatory cytokines and chemokines [41]. Microglial cells, the resident immune cells of the retina, shift to an activated amoeboid phenotype and migrate from the inner to the outer retina, where they secrete inflammatory mediators including interleukin-1β (IL-1β), IL-6, tumour necrosis factor-alpha (TNF-α), and monocyte chemoattractant protein-1 (MCP-1) [42]. These molecules disrupt the integrity of the blood–retinal barrier (BRB), attract peripheral immune cells, and exacerbate local inflammation. Notably, gliosis, reactive changes in Müller cells, and astrocytes, are strongly associated with early neuronal loss and microvascular damage [43]. Müller cells, which encase all retinal vessels, regulate vascular permeability and retinal metabolism, and modulate glial responses and cell survival through cytokine release and neurotrophic support [44,45].
Neurodegeneration, an early and critical component of DR, is evidenced by the loss of retinal ganglion cells (RGCs), the thinning of the retinal nerve fibre layer (RNFL), and synaptic dysfunction. RGC apoptosis can precede the development of detectable microvascular lesions, indicating that neuronal damage may be the initiating factor in DR [46]. Moreover, excitotoxicity driven by excessive extracellular glutamate further contributes to neurodegeneration. Glutamate overstimulation activates N-methyl-D-aspartate receptors (NMDARs), leading to intracellular calcium overload, osmotic imbalance caused by Na+ and Cl− influx, and ultimately neuronal swelling and death. In parallel, impaired autophagy, a cellular process essential for the clearance of damaged organelles and proteins, has been implicated in DR, further exacerbating oxidative damage and cell death [47,48,49].
Vascular abnormalities, including microaneurysms, capillary dropout, and neovascularisation, are hallmark features of DR. VEGF, a potent proangiogenic cytokine, is markedly upregulated in diabetic retinas and correlates with insulin resistance and elevated insulin-like growth factor-1 (IGF-1) levels [50,51]. VEGF functions with intercellular adhesion molecule-1 (ICAM-1) to promote leukocyte adhesion, endothelial dysfunction, and BRB breakdown. The expression of ICAM-1 is regulated through the PKC/endothelin (ET)/NF-κB axis, forming a signalling loop that perpetuates vascular inflammation and non-perfusion. Hypoxia-inducible factor-1 alpha (HIF-1α), stabilised under diabetic and hypoxic conditions, further amplifies VEGF expression and pathological angiogenesis, contributing to proliferative DR and vision-threatening complications [52,53,54].
Emerging research highlights the involvement of the gut–retina axis as a novel contributor to DR pathogenesis. Gut dysbiosis in diabetes disrupts intestinal barrier integrity, leading to metabolic endotoxemia and systemic inflammation. Circulating endotoxins, such as lipopolysaccharide (LPS), can cross the compromised BRB and activate retinal microglia and endothelial cells, further fuelling local oxidative and inflammatory responses. Altered gut microbiota may also affect host metabolism, short-chain fatty acid production, and immune modulation, indirectly impacting retinal health. Thus, gut-derived inflammatory signals and microbial metabolites could represent upstream modulators of DR pathophysiology, opening new avenues for therapeutic intervention-targeting systemic-metabolic crosstalk. Given its systemic influence, the gut–retina axis represents a promising therapeutic target. Modulating gut microbiota through prebiotics, probiotics, or dietary interventions may offer novel approaches to mitigate inflammation and improve retinal outcomes in DR [32,33,34].
3. Molecular Mechanisms of Resveratrol in Diabetic Retinopathy
RSV, a polyphenolic compound found in grapes, red wine, and various plant sources, exhibits significant therapeutic potential in addressing the complex pathophysiology of DR. Its effects span multiple molecular pathways, targeting oxidative stress, inflammation, neurodegeneration, angiogenesis, and metabolic dysfunction. By influencing key cellular signalling cascades, RSV offers a multifaceted approach to mitigating the progression of DR [18].
3.1. Antioxidant and Cytoprotective Effects
One of RSV’s central mechanisms in DR is its potent antioxidant activity. It exerts cytoprotective effects primarily through the activation of the Nrf2/ARE pathway. Activation of Nrf2 leads to the transcription of various antioxidant and phase II detoxification enzymes, which collectively diminish oxidative damage to cellular macromolecules, such as lipids, proteins, and DNA [55,56]. This contributes to reduced retinal oxidative stress, a key driver of DR pathogenesis.
Other naturally occurring Nrf2 activators, including sulforaphane, curcumin, allicin, epigallocatechin gallate (EGCG), quercetin, luteolin, and apigenin, have also been shown to offer cytoprotective effects by attenuating oxidative stress and inflammation [57,58,59,60]. However, despite their promising antioxidant potential, challenges related to low bioavailability and the risk of overactivating redox-sensitive transcription factors call for a cautious and regulated therapeutic approach [61].
Beyond the Nrf2/ARE pathway, RSV confers mitochondrial protection through activation of the AMPK/SIRT1/PGC-1α signalling axis. By stimulating AMPK and SIRT1, RSV enhances mitochondrial biogenesis and function, reduces reactive oxygen species (ROS) generation, and prevents apoptosis in retinal cells under hyperglycaemic conditions [62,63]. Moreover, AMPK activation contributes to improved insulin sensitivity and energy homeostasis, reinforcing its value in metabolic regulation [64,65]. The synergy between AMPK and SIRT1 underscores the comprehensive impact of RSV on mitochondrial health and metabolic control [66,67].
3.2. Anti-Inflammatory Mechanisms
Inflammation plays a pivotal role in the progression of DR, and RSV exerts strong anti-inflammatory actions by targeting key inflammatory mediators. One of the primary pathways influenced by RSV is the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) signalling pathway. By inhibiting NF-κB activation, RSV suppresses the expression of pro-inflammatory cytokines such as TNF-α, IL-1β, and IL-6, which are elevated in the diabetic retina and contribute to vascular damage and neural dysfunction [68].
Natural compounds, including bee venom, Vaccinium bracteatum extract, and licochalcone B, have also been reported to inhibit NF-κB activity, indicating that targeting this pathway may represent a viable anti-inflammatory strategy in DR [69,70,71].
RSV also modulates microglial activation, a central event in retinal neuroinflammation. Hyperactivation of microglia contributes to neuronal injury and degeneration in DR. Studies have shown that RSV and other agents, such as Nanog and Piper cubeba extract, suppress microglial activation by interfering with NF-κB signalling [72,73]. Moreover, RSV downregulates thioredoxin-interacting protein (TXNIP) and inhibits the NOD-like receptor pyrin 3 (NLRP3) inflammasome, a critical intracellular complex involved in the maturation of pro-inflammatory cytokines. This action reduces retinal vascular inflammation and protects against endothelial cell apoptosis [68].
3.3. Neuroprotection and Retinal Cell Survival
RSV contributes to the survival and protection of retinal neurons through several interconnected mechanisms. It significantly upregulates SIRT1, which helps mitigate oxidative and inflammatory insults. This upregulation supports the viability of retinal ganglion cells, as evidenced in both DR models and experimental optic neuritis [74,75,76].
Additionally, RSV promotes autophagy in Müller glial cells, which play a key role in maintaining retinal homeostasis. It achieves this through regulation of the miR-29b/SP1 signalling pathway, leading to increased expression of autophagy-related proteins such as LC3-I/II and Beclin-1 [77,78]. By enhancing autophagic flux, RSV reduces apoptotic cell death and preserves retinal structure and function. This autophagic activity is also modulated in part by SIRT1, indicating a synergistic effect between the two pathways in neuroprotection and cellular maintenance [79,80].
Furthermore, the activation of the Nrf2 pathway by RSV extends beyond antioxidant functions to confer direct neuroprotective and anti-ageing effects. Through this pathway, RSV shields retinal neurons from oxidative insults and supports long-term neural integrity [27].
3.4. Anti-Angiogenic Properties
A hallmark of advanced DR is pathological neovascularisation, driven primarily by upregulated VEGF and hypoxia-inducible factor 1-alpha (HIF-1α). RSV exerts anti-angiogenic effects by downregulating these key mediators, thereby impairing endothelial cell proliferation and migration, critical steps in aberrant angiogenesis [62].
Similar effects have been observed with bioactive agents such as nargenicin A1 and Persian shallot extract, which suppress VEGF signalling and inhibit pathological vascular responses [81,82]. Additionally, molecules such as death-associated protein kinase (DAPK) and suppressor of MEK1 (sMEK1) have been implicated in regulating HIF-1α expression, offering further insights into angiogenesis control mechanisms [70].
RSV also inhibits the endothelial-to-mesenchymal transition (EndMT), which contributes to vascular dysfunction in DR. This effect is achieved through inhibition of PKC-dependent NADPH oxidase activity, which limits ROS production and protects vascular cells from oxidative stress [83]. Furthermore, RSV modulates nitric oxide synthase (NOS) activity, supporting vascular tone and endothelial health. Nevertheless, some uncertainty persists regarding RSV’s dose-dependent effects on angiogenesis, with conflicting results in preclinical studies highlighting the need for standardised dosing protocols [84].
3.5. Broader Relevance in Retinal Vascular Pathology
Although primarily investigated in the context of DR, RSV also exhibits protective effects in other retinal vascular conditions. For instance, in models of retinal ischemia/reperfusion injury, RSV modulates the expression of matrix metalloproteinase-9 (MMP-9), inducible nitric oxide synthase (iNOS), and heme oxygenase-1 (HO-1), contributing to reduced vascular leakage and cell death [85]. These findings underscore the broader therapeutic applicability of RSV to various retinal diseases characterised by oxidative stress and vascular compromise.
RSV represents a promising multi-targeted therapeutic agent for DR. Through modulation of interconnected molecular pathways, such as Nrf2/ARE, AMPK/SIRT1, NF-κB, and VEGF/HIF-1α, RSV addresses the multifaceted nature of DR, influencing oxidative damage, inflammation, neurodegeneration, angiogenesis, and metabolic imbalances [79,80]. Despite encouraging preclinical outcomes, the translation of RSV into clinical practice demands further exploration of its pharmacokinetics, dosage optimisation, bioavailability, and long-term safety profiles.
3.6. Structural Activity Relationship (SAR) of Resveratrol: Structural Domains and Functional Implications
The biological activity of RSV in diabetic retinopathy is closely linked to specific structural features within its molecule. In the context of SAR analysis, the molecular framework of RSV can be conceptually divided into three key structural domains: the planar aromatic rings, the hydroxyl (phenolic) functional groups, and the central ethylene bridge. The 3,5,4-trihydroxy substitution pattern is central to its antioxidant potential, enabling efficient scavenging of ROS, which are typically elevated in diabetic retinal tissue. Substitution or removal of these hydroxyl groups significantly reduces RSV’s biological efficacy. Among these, the 4-hydroxyl group is particularly crucial for inhibiting pro-inflammatory cytokines such as IL-1β and TNF-α through modulation of the NF-κB signalling pathway [23,86]. Structural derivatives containing additional hydroxyl groups, such as piceatannol, have demonstrated enhanced inhibitory effects on COX-2 and NF-κB, both key mediators in the pathogenesis of diabetic retinopathy [87,88]. The planar aromatic rings are essential for π-π interactions with various biomolecular targets, including enzymes and membrane proteins involved in inflammation and oxidative stress pathways. The planar conjugated structure of RSV facilitates membrane permeability and allows it to modulate intracellular signalling cascades such as NF-κB, Nrf2/ARE, and VEGF/PI3K-Akt pathways—key regulators in the pathogenesis of diabetic retinopathy. The ethylene bridge, with its trans configuration, imparts structural rigidity and electronic delocalisation, which are crucial for maintaining bioactivity [23]. Furthermore, structural modifications such as methoxylation (e.g., pterostilbene) have been shown to improve lipophilicity and bioavailability, enhancing the compound’s apoptotic and antioxidant effects in retinal cells. Additionally, glycosylated derivatives like piceid (resveratrol-3-O-glucoside) offer increased in vivo stability and more effective systemic delivery, providing additional protection to retinal tissue [86,89,90].
Collectively, these structural features are responsible for RSV’s broad spectrum of biological activities, including anti-inflammatory, antioxidant, neuroprotective, and antiangiogenic effects, all of which are relevant in the pathophysiology of diabetic retinopathy.
4. Challenges in the Clinical Translation of Resveratrol
RSV, a naturally occurring polyphenol with demonstrated antioxidant, anti-inflammatory, and anti-angiogenic properties, has shown compelling preclinical efficacy in the treatment of retinal diseases such as DR. However, despite extensive preclinical evidence supporting its therapeutic potential, the clinical translation of RSV remains significantly limited. These limitations stem primarily from pharmacokinetic challenges, variability in treatment response, and a lack of robust clinical evidence, all of which hinder its integration into routine ocular therapeutics.
4.1. Poor Bioavailability and Rapid Metabolism
A fundamental challenge in the clinical application of RSV is its inherently poor bioavailability. RSV is classified as a BCS Class II compound, characterised by low aqueous solubility and high membrane permeability, which necessitates formulation strategies to enhance its bioavailability [91]. It has high oral absorption due to its lipophilicity; however, due to rapid and extensive metabolism in the liver and intestines, only small amounts of unchanged RSV reach systemic circulation. In the bloodstream, it appears in free form or as glucuronide and sulphate conjugates, with the free form primarily binding to serum albumin, which serves as a potential reservoir for RSV capable of binding it and thereby influencing its distribution and bioavailability [90]. The stability of the RSV–albumin complex and its cellular uptake via albumin and LDL receptors further confirms its absorption and transport mechanisms in the body [22,92]. This results in subtherapeutic concentrations at the target site, particularly in ocular tissues that are protected by the blood–retinal barrier. The inability of RSV to efficiently penetrate this barrier further reduces its therapeutic efficacy in the posterior segment of the eye [92].
These pharmacokinetic limitations are not unique to RVS. Compounds such as Akebia saponin D (ASD) also exhibit ultra-low bioavailability due to poor gastrointestinal permeability and extensive pre-absorption degradation [93]. Similarly, drugs like ketamine and clarithromycin are subject to significant hepatic metabolism [94,95]. In contrast, structural modification strategies have been employed to enhance the bioavailability of other therapeutic agents, such as chaplain inhibitors, which have demonstrated improved retinal penetration and sustained drug levels [96].
4.2. Innovations to Enhance Bioavailability
To overcome these limitations, various drug delivery innovations have been explored. Nanoencapsulation, chemical derivatisation, including hydroxylation and glycosylation, and lipid-based formulations have demonstrated improved solubility, enhanced systemic distribution, and increased metabolic stability of RSV [22,92]. Micronisation and nanotechnology-based delivery systems, such as liposomes and polymeric nanoparticles, have further enhanced its pharmacokinetic profile by improving tissue penetration and prolonging intraocular retention [97].
Additional strategies aimed at improving bioavailability include enhancing lipid solubility and intestinal permeability, as well as modulating gut flora to minimise presystemic metabolism [93,98]. However, the success of these strategies is highly dependent on the physicochemical and metabolic properties of the compound in question, and their applicability must be evaluated on a case-by-case basis.
4.3. Precision Dosing and Pharmacokinetic Variability
Effective therapy for retinal diseases such as DR necessitates precise dosing strategies due to the pharmacokinetic variability seen across patient populations. The presence of biological barriers, including the blood–retinal barrier and active efflux transporters, pose significant challenges to drug delivery in the posterior segment of the eye [59,99,100]. Moreover, interindividual differences in metabolism and systemic absorption can significantly alter therapeutic outcomes [99], underscoring the need for personalised dosing regimens. Personalised approaches that incorporate genetic, phenotypic, and metabolic profiles are essential to optimise treatment efficacy while minimising adverse effects [99,101].
4.4. Limitations in Clinical Evidence
Despite encouraging findings from preclinical models, the clinical application of RSV in retinal diseases remains largely theoretical due to the absence of large-scale, well-controlled clinical trials. Studies in animal models of age-related macular degeneration (AMD) and DR have shown antioxidant, anti-inflammatory, and anti-angiogenic effects [62,78,102], yet these results are not consistently replicated in human trials. Of the 165 clinical studies investigating RSV, the majority have focused on systemic metabolic conditions, with relatively few addressing ocular applications [103].
This gap in evidence is exacerbated by what has been termed the “Resveratrol Paradox”, a disconnect between its strong in vitro and animal model efficacy and its limited systemic availability and inconsistent performance in humans [104]. Furthermore, many preclinical studies utilise concentrations of RSV that are not physiologically achievable in humans, thereby limiting their translatability. The diversity in study methodologies and the use of proprietary formulations further complicate comparisons between trials and delay the development of standardised treatment protocols [105].
4.5. Safety Considerations and Systemic Implications
Although RSV is generally considered safe and well-tolerated, there is growing concern about its long-term safety and potential interactions with medications commonly used in DR patients, particularly those with diabetes. RSV may interact with antidiabetic drugs, potentially altering glycaemic control or affecting systemic metabolism and cardiovascular function [106]. Moreover, sudden improvements in glycaemic control, whether drug-induced or diet-mediated, have been linked to transient worsening of DR in some patients, further emphasising the need for caution when integrating RSV into treatment regimens.
Broader implications for DR management also need to be considered. Systemic therapies such as ACE inhibitors and angiotensin receptor blockers have demonstrated protective effects against DR progression [107], while anti-VEGF agents remain the cornerstone for managing neovascular complications [108,109]. Given the role of chronic inflammation and metabolic dysfunction in DR pathogenesis, systemic agents such as hypoglycaemics and lipid-lowering medications may provide ancillary benefits in reducing disease burden [106,110]. Nevertheless, any long-term strategy incorporating RSV must be rigorously tested in well-designed trials to confirm its safety and efficacy.
Although RSV holds substantial therapeutic promise, particularly due to its pleiotropic pharmacological effects, its clinical utility in retinal diseases remains limited by challenges related to poor bioavailability, rapid metabolism, dosing variability, and insufficient human data. Ongoing advances in drug delivery platforms, combined with personalised medicine approaches, offer avenues to overcome these barriers. However, the successful clinical translation of RSV will ultimately depend on the development of standardised formulations and the implementation of large-scale, randomised clinical trials that can validate its therapeutic potential in real-world patient populations.
5. Advances in Resveratrol Drug Delivery for Ocular Use
RSV’s therapeutic application in ocular diseases is limited by its pharmacokinetic properties and the anatomical barriers of the eye. Advances in drug delivery technologies aim to overcome these challenges by improving bioavailability, stability, and targeted release within ocular tissues [24,82,83,96,97,98].
5.1. Resveratrol Delivery Strategies and Routes of Administration in Ocular Use
Despite the promising effects of RSV in managing retinal dysfunction, its clinical translation has been significantly hindered by intrinsic pharmacokinetic limitations, including poor aqueous solubility, rapid systemic metabolism, and low oral bioavailability. These factors severely limit its therapeutic potential, especially in chronic conditions such as DR, where sustained exposure of the retina to active compounds is essential [24].
To overcome these limitations, various nanotechnology-based drug delivery systems have been developed. These include liposomes, polymeric nanoparticles, solid lipid nanoparticles, nanoemulsions, and exosome-based formulations. Such systems are designed to enhance RSV’s pharmacokinetic profile, increase its bioavailability in retinal tissues, and ultimately improve its therapeutic efficacy in vivo [24,82,83]. These advanced formulations also provide protection against premature degradation and offer controlled and targeted release features particularly advantageous in ocular drug delivery.
In addition to formulation strategies, multiple routes of administration have been investigated to optimise the delivery of RSV to retinal tissues. These include systemic, localised, and transmucosal approaches, each offering distinct benefits and presenting specific limitations.
Intravitreal injection delivers RSV directly into the vitreous cavity, achieving high local drug concentrations. Preclinical studies have demonstrated that intravitreal RSV administration significantly reduces vascular distortion and pathological neovascularisation in models of DR [38]. However, due to its invasive nature and potential for injection-related complications, this route may not be feasible for long-term or routine clinical use.
Topical instillation in the form of eye drops offers a non-invasive alternative. It has shown comparable benefits to intravitreal injection in diabetic models, including reduced retinal inflammation and preservation of retinal structure [38]. Nonetheless, effective transcorneal delivery remains a challenge, with limited retinal penetration and the need for frequent administration to maintain therapeutic levels.
Oral administration is the most patient-compliant route; however, it is associated with significantly reduced bioavailability due to extensive first-pass hepatic metabolism and poor intestinal absorption. Despite these limitations, long-term oral administration of RSV (5–10 mg/kg/day) has led to improvements in retinal function and vascular integrity in diabetic rats [107], suggesting that systemic delivery can still yield therapeutic benefits under optimised dosing regimens.
Sublingual delivery has emerged as a promising route to bypass hepatic first-pass metabolism and enhance systemic absorption of RSV. Novel formulations, such as fast-disintegrating sublingual mini-tablets, have been developed to improve bioavailability and enhance patient adherence [107]. Although still in the early stages of clinical development, this route offers a practical and less invasive alternative to oral and intravitreal administration.
Taken together, these alternative delivery strategies aim not only to overcome the pharmacokinetic challenges of RSV but also offer opportunities to tailor therapeutic approaches based on disease severity, patient-specific factors, and treatment goals. Importantly, by enhancing local or systemic bioavailability, RSV-based therapies have the potential to complement existing treatment modalities for DR, including anti-VEGF agents and corticosteroids, particularly in cases where neurovascular protection and long-term disease modulation are required.
5.2. Challenges of Topical Delivery and Novel Resveratrol-Based Ophthalmic Formulations
Topical ocular drug delivery, primarily through eye drops or ointments, accounts for approximately 90% of the global ophthalmic drug market and is the most widely used method for administering ophthalmic drugs due to its simplicity, non-invasiveness, and high patient compliance [111,112]. However, it has some limitations. The main disadvantage of conventional eye drops is the administration of an excessive fluid volume (20–70 µL), which exceeds the physiological capacity of the conjunctival sac (7–10 µL) [111,112]. This leads to overflow, reflex blinking, and increased drainage through the nasolacrimal duct. Additionally, rapid tear turnover significantly reduces the duration of drug contact with the ocular surface to 14–17 min, with most of the drug eliminated within the first 5 min [111,112].
Given that the drug concentration on the ocular surface decreases by approximately 16% per minute after administration, the formulation becomes diluted by a factor of 1:1000 within 40 min [111,112]. As a result, no more than 5% of the topically applied drug reaches the anterior ocular tissues, and usually less than 1% of the administered dose reaches the retina and vitreous [113]. This poor penetration is due to multiple physiological barriers, such as the tear film, blinking, nasolacrimal drainage, and the blood–retinal barrier. Therefore, posterior segment diseases such as DR are commonly treated with intravitreal injections or advanced delivery systems (e.g., nanoparticles, implants) that ensure prolonged and targeted drug release [111,112].
In response to these limitations, recent innovations in ocular drug delivery technologies have focused on overcoming low drug bioavailability, particularly for compounds like RSV, which exhibit poor solubility and rapid metabolism. By improving solubility, chemical stability, tissue permeability, and sustained release within ocular structures, these strategies enhance RSV’s therapeutic potential and clinical relevance in treating eye diseases such as DR. An overview of innovative topical formulations with RSV developed for ocular administration in the management of DR and other ocular diseases is presented in Table 2 [114,115,116,117,118,119,120,121,122,123,124,125,126,127].

Table 2.
Examples of innovative topical formulations with resveratrol in the management of diabetic retinopathy and other ocular diseases.
5.3. Nanoformulations for Improved Bioavailability
A significant area of progress lies in the development of nanoformulations, including liposomes, nanoemulsions, polymeric nanoparticles, and self-nanoemulsifying drug delivery systems (SNEDDS). These nanocarriers have demonstrated considerable promise in enhancing the pharmacokinetic profile of RSV.
Liposomes and nanoemulsions have been shown to significantly improve the permeability of RSV across ocular barriers, thereby increasing its bioavailability [29]. Polymeric nanoparticles offer the additional advantage of enabling controlled drug release and prolonged retention within ocular tissues, contributing to more effective and sustained therapeutic outcomes [121]. SNEDDSs, on the other hand, provide exceptional cytocompatibility and a capacity for prolonged release while protecting RSV from enzymatic degradation [125]. These systems support targeted ocular drug delivery, minimise systemic side effects, and significantly enhance drug stability [123]. However, despite these benefits, concerns about potential toxicity, the need for extensive in vivo validation, and regulatory hurdles remain pressing challenges for clinical translation.
5.4. Sustained-Release Intravitreal Implants
Another promising advancement in ocular drug delivery is the use of sustained-release intravitreal implants, particularly for managing chronic conditions like DR. These delivery systems, including microspheres and hydrogels, are designed to reduce the frequency of invasive intravitreal injections by providing extended and controlled drug release.
Microspheres are biodegradable carriers that encapsulate therapeutic agents and release them gradually over time. This allows for precise control of drug kinetics, enhanced entrapment efficiency, and improved therapeutic consistency. Clinical applications of microspheres include the delivery of anti-VEGF agents and corticosteroids such as triamcinolone acetonide, which have been shown to reduce inflammation and inhibit neovascularisation in DR patients [124,125].
Temperature-sensitive hydrogels also offer a viable approach for sustained intraocular drug delivery. These hydrogels exhibit high biocompatibility and, upon injection, form semi-solid structures within the vitreous cavity. These delivery systems provide sustained drug release over prolonged periods while maintaining compatibility with the intraocular environment [126,127]. Together, microspheres and hydrogels can significantly reduce the frequency of intravitreal injections, improving patient adherence and lowering the risk of complications associated with repeated ocular procedures [128]. Nevertheless, long-term safety, targeting efficiency, and the challenge of penetrating the blood–retinal barrier remain areas that require further research and technological refinement.
5.5. Combination Therapies with Existing DR Treatments
The use of combination therapies in the treatment of DR is gaining traction to enhance therapeutic outcomes by leveraging the synergistic effects of multiple interventions. One such example is the combination of intravitreal triamcinolone with phacoemulsification, which has demonstrated significant improvements in visual acuity and a reduction in macular thickness, with no increase in adverse events [129]. Another promising combination is panretinal photocoagulation (PRP) with anti-VEGF therapy. This strategy has demonstrated superior outcomes in terms of neovascular regression and improvement in visual function compared to PRP alone, with no additional complications reported [130,131].
Although recent findings are promising, variability in study design, patient populations, and follow-up durations complicates direct comparisons across studies [12,13,16,17,18,132,133,134,135,136,137,138,139,140,141,142]. Moreover, while combination therapies hold potential to reduce the frequency of anti-VEGF injections, definitive evidence supporting their efficacy is still lacking [133]. Therefore, large-scale, standardised clinical trials are essential to confirm these results and facilitate their integration into routine clinical practice. Current standard therapies for DR are primarily directed at advanced disease stages and often fail to adequately address early pathophysiological processes such as oxidative stress, inflammation, and neurodegeneration. RSV, with its broad spectrum of biological activity, has emerged as a promising adjunct by targeting multiple molecular pathways implicated in DR. Table 3 presents a comparative overview of the mechanisms, benefits, and limitations of existing DR therapies, along with the potential complementary or enhancing role of RSV [12,13,14,15,16,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,129,130,131,132,133].

Table 3.
Comparison of Current Therapies for Diabetic Retinopathy and the Potential Role of Resveratrol.
5.6. Need for Well-Designed Randomised Clinical Trials
To establish optimal dosage and treatment regimens for RSV-based and combination therapies, there is a critical need for well-designed randomised clinical trials (RCTs). These trials must address several key parameters, including controlling for variability in patient subgroups, follow-up durations, and study methodologies. Additionally, RCTs should rigorously evaluate the synergistic potential of combination treatments and assess their impact on injection frequency and long-term safety.
Confirmation of therapeutic efficacy through such trials will be instrumental in transitioning current experimental successes into validated, evidence-based treatment protocols for DR.
5.7. Personalised Medicine Approaches
An emerging frontier in the treatment of DR involves personalised medicine approaches, which tailor interventions based on individual genetic, phenotypic, and clinical profiles. This strategy promises more targeted and effective treatment regimens by accounting for variability among patients.
Genetic research has identified specific variants associated with DR susceptibility, particularly those involved in angiogenesis and inflammatory pathways [134]. By identifying these markers early, clinicians can initiate preventive or therapeutic interventions tailored to individual risk profiles.
Moreover, the DR management increasingly involves a multifactorial approach, with systemic control of glucose, blood pressure, and lipid levels being essential for overall disease management [13,15,135]. Personalised treatment strategies account for comorbidities and genetic predispositions, thereby improving outcomes and reducing the risk of progression [142].
Emerging therapeutics are now targeting specific disease pathways and components of the retinal neurovascular unit, including VEGF inhibitors and agents that modulate interactions between neurons, glia, and vasculature [141,143]. Machine learning tools have further enabled the identification of biomarkers, such as HDL/LDL levels, that predict differential responses to intensive glycaemic control, providing a basis for extending similar approaches to DR therapies [144].
Notably, individuals with type 2 diabetes who exhibit specific metabolic traits may respond more favourably to RVS-based interventions, suggesting the importance of personalised selection criteria [135,145]. Despite these advancements, challenges remain in implementing personalised approaches at scale, including the integration of multi-omic data, high costs, and limited accessibility across diverse patient populations.
5.8. Exploring Synthetic Analogues and Prodrugs of Resveratrol
To address the intrinsic limitations of RSV, namely its low solubility, instability, and rapid metabolism, researchers have been exploring the development of synthetic analogues and prodrug formulations. These strategies aim to retain or enhance the biological activity of RSV while improving its stability and pharmacological properties.
Synthetic analogues are structurally modified compounds designed to replicate the therapeutic effects of RSV but with enhanced bioavailability and chemical stability [139]. Prodrugs, on the other hand, involve the chemical modification of RSV. Prodrugs are inactive or less active compounds that convert into active forms within the body. For RSV, prodrug strategies aim to overcome its poor oral bioavailability, low stability, and rapid metabolism. Key goals include protecting phenolic hydroxyl groups from oxidation, enhancing chemical stability, and enabling targeted delivery to tissues such as the brain, retina, or liver. Various resveratrol prodrugs have been developed and synthesised from RSV: ester derivatives (e.g., acetate, butyrate) improve membrane permeability, glycosylated forms like piceid increase solubility and act as reversible carriers, phosphate prodrugs enhance water solubility, lipid conjugates facilitate CNS delivery, and nanoparticulate systems improve stability and targeting. These approaches aim to improve systemic and ocular delivery by enhancing the compound’s resistance to metabolic degradation and potentially advance the therapeutic potential of resveratrol in clinical use [146,147,148,149]. Recent studies have investigated RSV prodrugs with improved retinal delivery in diabetic retinopathy. One example is piceid-octanoate (PIC-OCT), a lipophilic ester of piceid designed to enhance bioavailability and target retinal tissue. In vitro and in vivo models have shown that PIC-OCT reduces oxidative stress, protects photoreceptors, and modulates SIRT1 and PARP1 pathways, highlighting its potential as a retina-targeted therapeutic [135].
These developments represent a crucial step toward translating the promising outcomes observed in preclinical studies into clinically effective therapies. When used in conjunction with advanced drug delivery systems, such as nanoformulations and sustained-release platforms, synthetic analogues and prodrugs may significantly improve the clinical applicability of RSV for ocular diseases. To provide a synthesis of the pleiotropic therapeutic actions and translational strategies involving RSV in DR, Figure 2 summarises its multifaceted effects, including molecular mechanisms, delivery innovations, combinatory strategies, and directions toward personalised and clinical application [16,22,27,28,29,57,64,65,66,69,70,71,72,73,74,75,76,77,78,79,104,105,106,107,108,109,110,124,125,126,127,128,134,135,136,137,138].
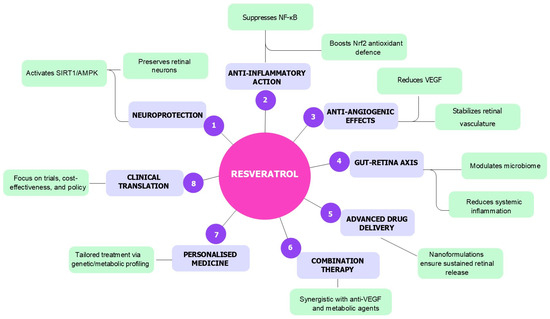
Figure 2.
Overview of the multifactorial therapeutic actions of RSV in DR: RSV exerts neuroprotective, anti-inflammatory, and anti-angiogenic effects through activation of SIRT1/AMPK and Nrf2 pathways, suppression of NF-κB and VEGF signalling, and modulation of the gut–retina axis. Advanced delivery systems, combination therapies, and personalised medicine approaches further support its translational potential. Clinical translation requires well-designed trials and policy integration.
6. Future Directions and Clinical Perspectives
Despite strong preclinical evidence supporting RSV as a multi-targeted compound with antioxidative, anti-inflammatory, neuroprotective, and anti-angiogenic properties, its clinical translation for DR remains limited. To bridge this gap, future research must focus on expanding insights, overcoming bioavailability barriers, validating therapeutic efficacy through clinical trials, and integrating RSV into personalised and combinatory treatment strategies [18,137].
Further elucidation of RSV’s molecular mechanisms is essential to unlock its full therapeutic potential in DR. While key signalling pathways, such as AMPK/SIRT1, NF-κB, Nrf2, and VEGF, are established mediators of RSV’s protective effects, additional regulatory layers remain underexplored [27,28,67,68]. Integrative multi-omic approaches, encompassing transcriptomics, proteomics, epigenomics, and metabolomics, should be employed to identify novel molecular signatures predictive of therapeutic response. Particular focus should be placed on mitochondrial biogenesis regulators, non-coding RNAs, and inflammation-resolution mediators, which may serve as next-generation targets for RSV-guided interventions in DR [20,138].
Emerging evidence positions DR as a neurovascular disease, where neurodegeneration often precedes and exacerbates microvascular dysfunction. RSV’s ability to protect retinal neurons, particularly ganglion cells, by modulating oxidative stress, enhancing autophagy, activating SIRT1, and suppressing microglial-mediated inflammation, supports its application as a neuroprotective agent [27,139,140]. Future preclinical and clinical studies should evaluate its capacity to preserve retinal function using electrophysiological metrics such as ERG, measurements of the OCT-based ganglion cell complex, and markers of retinal neuroinflammation. These endpoints may redefine RSV as a disease-modifying agent rather than merely symptomatic relief.
One of the primary limitations hindering the clinical applicability of RSV is its poor bioavailability caused by extensive hepatic metabolism and limited aqueous solubility [92,150]. To address this, several innovative drug delivery platforms are being developed, including liposomes, micelles, polymeric nanoparticles, nanoemulsions, and SNEDDS. Intravitreal sustained-release systems such as microspheres and hydrogels, as well as ROS/glucose-sensitive carriers, represent next-generation solutions for site-specific, long-acting retinal delivery [22,151,152,153]. These approaches must now be tested in long-term animal models and early-phase clinical studies to assess pharmacodynamics, retinal tissue penetration, and safety profiles under chronic use scenarios.
Another strategic avenue lies in integrating RSV into existing treatment regimens. Anti-VEGF agents, although effective, require frequent intravitreal injections and fail to address the oxidative and neuroinflammatory components of DR. RSV, by reducing VEGF expression and restoring BRB integrity, may reduce the frequency of injections and enhance long-term outcomes [154,155,156].
Moreover, the combination of RSV with neuroprotective agents, including citicoline, brimonidine, or metabolic modulators such as metformin and GLP-1 receptor agonists, may offer additive or synergistic effects. RSV could also mitigate corticosteroid-induced fibrosis by exerting anti-fibrotic effects [18,157]. These multidimensional therapeutic strategies represent a compelling frontier for clinical research. Future studies should explore the sequencing, dosing, and pharmacodynamic interactions of RSV with standard and emerging DR therapies to set future guideline-based implementation. To synthesise the clinical and molecular mechanisms underlying RSV’s therapeutic role in DR, Table 4 provides a structured overview of major pathophysiological pathways, molecular targets, proposed mechanisms of action, strategic therapeutic approaches, and their potential clinical implications [18,22,27,32,33,34,42,43,50,51,52,53,54,79,80,84,91,92,93,94,95,96,135,150,154,155,156,158].

Table 4.
Resveratrol in Diabetic Retinopathy: Pathophysiological Targets, Molecular Pathways, and Translational Potential.
Recent studies have linked diabetic gut dysbiosis with retinal pathology via the gut–retina axis. This bidirectional communication is driven by increased intestinal permeability, LPS-induced systemic inflammation, and altered production of short-chain fatty acids (SCFAs), all of which contribute to retinal endothelial dysfunction. RSV has demonstrated prebiotic properties, restoring microbial diversity, reducing metabolic endotoxemia, and attenuating systemic oxidative stress [33,34,159]. This systemic effect may indirectly protect the retina, opening new avenues for dual-targeted interventions that combine RSV with microbiota-directed therapies, including prebiotics, probiotics, and synbiotics [33,34,159,160]. Investigating gut-derived biomarkers such as SCFA profiles and endotoxin levels concerning RSV efficacy may provide a holistic framework for modulating retinal disease.
To date, the majority of RSV research in DR has been confined to preclinical models [135,150,161,162]. Existing clinical studies suffer from small sample sizes, heterogeneity in formulations, and a lack of standardised endpoints [18]. Future randomised controlled trials must stratify participants by DR stage, systemic comorbidities, and molecular biomarkers to assess efficacy more precisely.
Importantly, interindividual variability in RSV metabolism, systemic inflammation, and genetic background underscores the need for a precision medicine approach. Integrating metabolomic and genomic profiling with AI-assisted patient stratification may identify “RSV responders” and facilitate individualised therapeutic regimens. Additionally, simulation models based on systems biology could predict disease progression and optimise RSV-based intervention timing.
Although classified as a nutraceutical, RSV occupies a unique position at the interface between dietary supplements and pharmaceutical agents. Standardisation of formulation, quality control, and therapeutic dosing remains imperative before regulatory endorsement and inclusion in clinical guidelines. Incorporating RSV into eHealth and mHealth platforms for diabetes care may enhance treatment adherence, improve patient education, and enable remote monitoring of visual and systemic parameters [163,164]. Pharmacoeconomic studies should also evaluate the cost-effectiveness of RSV-based interventions in reducing DR-related morbidity and healthcare burden.
Given its favourable safety profile, RSV may be especially suitable for early-stage DR or high-risk diabetic populations with subclinical retinal changes. However, longitudinal data are needed to confirm its long-term impact on visual acuity, retinal integrity, and systemic metabolism.
7. Conclusions
DR remains a leading cause of vision impairment worldwide, driven by a complex interaction of metabolic, inflammatory, vascular, and neurodegenerative processes [1,2,3,4,25,26]. RSV, a bioactive polyphenol with multiple actions, stands out as a promising therapeutic candidate able to target several pathogenic pathways. RSV’s capacity to attenuate oxidative stress, modulate chronic inflammation, preserve neuronal integrity, regulate angiogenic signalling, and potentially influence gut–retina axis communication suggests that its effects extend beyond those of a conventional antioxidant [19,24,27,28,29]. These multifaceted actions position it as a systemically active, disease-modifying therapeutic agent. However, unlocking this potential requires overcoming key translational hurdles. Advanced drug delivery systems must address pharmacokinetic issues, while comprehensive clinical trials are needed to determine optimal dosing and long-term efficacy. Incorporating omics-based diagnostics and personalised medicine strategies will further improve patient selection and therapeutic predictability. Future research should also explore how RSV works synergistically with existing DR treatments, aiming to lessen treatment burden, improve functional outcomes, and probably delay disease progression.
With technological and scientific advances at hand, it is the right time to move RSV from promising preclinical findings to a validated part of integrated DR therapy. Supported by strong mechanistic understanding and emerging translational innovations, RSV could ultimately change how we prevent and treat the neurovascular complications of diabetes, potentially safeguarding vision and establishing a new approach to metabolic and retinal health.
Author Contributions
Conceptualisation, S.K. (Snježana Kaštelan) and A.Š.P.; methodology, T.M.; formal analysis, S.K. (Suzana Konjevoda); investigation, S.Č., A.S., I.U. and I.L.; resources, S.K. (Snježana Kaštelan); data curation, A.S. and T.M.; writing—original draft preparation, S.K. (Snježana Kaštelan), S.K. (Suzana Konjevoda), A.S., I.U. and I.L.; writing—review and editing, S.K. (Snježana Kaštelan) and A.Š.P.; visualisation, S.Č. and T.M.; supervision, S.K. (Snježana Kaštelan); project administration, S.K. (Snježana Kaštelan) and A.Š.P.; funding acquisition, S.K. (Snježana Kaštelan). All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Acknowledgments
We acknowledge Angela Budimir, University Hospital Dubrava, for proofreading the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Wong, T.Y.; Tan, T.E. The Diabetic Retinopathy “Pandemic” and Evolving Global Strategies: The 2023 Friedenwald Lecture. Investig. Ophthalmol. Vis. Sci. 2023, 64, 47. [Google Scholar] [CrossRef]
- Benhamza, M.; Dahlui, M.; Said, M.A. Determining Direct, Indirect Healthcare and Social Costs for Diabetic Retinopathy Management: A Systematic Review. BMC Ophthalmol. 2024, 24, 424. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.N.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, Regional and Country-Level Diabetes Prevalence Estimates for 2021 and Projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar] [CrossRef] [PubMed]
- Teo, Z.L.; Tham, Y.-C.; Yu, M.; Chee, M.L.; Rim, T.H.; Cheung, N.; Bikbov, M.M.; Wang, Y.X.; Tang, Y.; Lu, Y.; et al. Global Prevalence of Diabetic Retinopathy and Projection of Burden through 2045. Ophthalmology 2021, 128, 1580–1591. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, N. Progress of Nanotechnology in Diabetic Retinopathy Treatment. Int. J. Nanomed. 2021, 16, 1391–1403. [Google Scholar] [CrossRef]
- Li, H.; Liu, X.; Zhong, H.; Fang, J.; Li, X.; Shi, R.; Yu, Q. Research Progress on the Pathogenesis of Diabetic Retinopathy. BMC Ophthalmol. 2023, 23, 372. [Google Scholar] [CrossRef]
- Zhou, X.; Yang, N.; Xu, W.; Li, X.; Spiliopoulou, A.; Theodoratou, E. Associations of Genetic Factors with Vascular Diabetes Complications: An Umbrella Review. J. Glob. Health 2025, 15, 04081. [Google Scholar] [CrossRef] [PubMed]
- Wong, T.Y.; Cheung, C.M.G.; Larsen, M.; Sharma, S.; Simó, R. Diabetic Retinopathy. Nat. Rev. Dis. Primer 2016, 2, 16012. [Google Scholar] [CrossRef]
- Kaštelan, S.; Salopek Rabatić, J.; Tomić, M.; Gverović Antunica, A.; Ljubić, S.; Kaštelan, H.; Novak, B.; Orešković, D. Body Mass Index and Retinopathy in Type 1 Diabetic Patients. Int. J. Endocrinol. 2014, 2014, 387919. [Google Scholar] [CrossRef]
- Kaštelan, S.; Tomić, M.; Gverović Antunica, A.; Ljubić, S.; Salopek Rabatić, J.; Karabatić, M. Body Mass Index: A Risk Factor for Retinopathy in Type 2 Diabetic Patients. Mediat. Inflamm. 2013, 2013, 436329. [Google Scholar] [CrossRef]
- Tomić, M.; Ljubić, S.; Kaštelan, S.; Gverović Antunica, A.; Jazbec, A.; Poljičanin, T. Inflammation, Haemostatic Disturbance, and Obesity: Possible Link to Pathogenesis of Diabetic Retinopathy in Type 2 Diabetes. Mediat. Inflamm. 2013, 2013, 818671. [Google Scholar] [CrossRef]
- Wong, T.Y.; Sun, J.; Kawasaki, R.; Ruamviboonsuk, P.; Gupta, N.; Lansingh, V.C.; Maia, M.; Mathenge, W.; Moreker, S.; Muqit, M.M.K.; et al. Guidelines on Diabetic Eye Care. Ophthalmology 2018, 125, 1608–1622. [Google Scholar] [CrossRef] [PubMed]
- Chabba, N.; Silwal, P.R.; Bascaran, C.; Murphy, R.; Gordon, I.; Mwangi, N.; Bhatta, S.; Pant, N.; Burton, M.J.; Keel, S.; et al. Measures of Diabetic Retinopathy Treatment Coverage: Protocol for a Methodological Review. BMJ Open 2025, 15, e092081. [Google Scholar] [CrossRef] [PubMed]
- Bahr, T.A.; Bakri, S.J. Update on the Management of Diabetic Retinopathy: Anti-VEGF Agents for the Prevention of Complications and Progression of Nonproliferative and Proliferative Retinopathy. Life 2023, 13, 1098. [Google Scholar] [CrossRef]
- Brown, D.M.; Wykoff, C.C.; Boyer, D.; Heier, J.S.; Clark, W.L.; Emanuelli, A.; Higgins, P.M.; Singer, M.; Weinreich, D.M.; Yancopoulos, G.D.; et al. Evaluation of Intravitreal Aflibercept for the Treatment of Severe Nonproliferative Diabetic Retinopathy: Results From the PANORAMA Randomized Clinical Trial. JAMA Ophthalmol. 2021, 139, 946. [Google Scholar] [CrossRef]
- Tan, T.E.; Wong, T.Y. Diabetic Retinopathy: Looking Forward to 2030. Front. Endocrinol. 2023, 13, 1077669. [Google Scholar] [CrossRef]
- Seo, H.; Park, S.-J.; Song, M. Diabetic Retinopathy (DR): Mechanisms, Current Therapies, and Emerging Strategies. Cells 2025, 14, 376. [Google Scholar] [CrossRef]
- Koushki, M.; Farahani, M.; Yekta, R.F.; Frazizadeh, N.; Bahari, P.; Parsamanesh, N.; Chiti, H.; Chahkandi, S.; Fridoni, M.; Amiri-Dashatan, N. Potential Role of Resveratrol in Prevention and Therapy of Diabetic Complications: A Critical Review. Food Nutr. Res. 2024, 68, 9731. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, D.; Sahu, P.K.; Purohit, S.; Ranajit, S.K.; Acharya, B.; Sangam, S.; Shrivastava, A.K. Therapeutic Interventions for Diabetes Mellitus-Associated Complications. Curr. Diabetes Rev. 2025, 21, e030524229631. [Google Scholar] [CrossRef]
- Roy, D.; Ghosh, M.; Rangra, N.K. Herbal Approaches to Diabetes Management: Pharmacological Mechanisms and Omics-Driven Discoveries. Phytother. Res. 2024, ptr.8410. [Google Scholar] [CrossRef]
- Bohara, R.A.; Tabassum, N.; Singh, M.P.; Gigli, G.; Ragusa, A.; Leporatti, S. Recent Overview of Resveratrol’s Beneficial Effects and Its Nano-Delivery Systems. Molecules 2022, 27, 5154. [Google Scholar] [CrossRef]
- Salla, M.; Karaki, N.; El Kaderi, B.; Ayoub, A.J.; Younes, S.; Abou Chahla, M.N.; Baksh, S.; El Khatib, S. Enhancing the Bioavailability of Resveratrol: Combine It, Derivatize It, or Encapsulate It? Pharmaceutics 2024, 16, 569. [Google Scholar] [CrossRef] [PubMed]
- Pop, R.; Daescu, A.; Rugina, D.; Pintea, A. Resveratrol: Its Path from Isolation to Therapeutic Action in Eye Diseases. Antioxidants 2022, 11, 2447. [Google Scholar] [CrossRef] [PubMed]
- Bola, C.; Bartlett, H.; Eperjesi, F. Resveratrol and the Eye: Activity and Molecular Mechanisms. Graefes Arch. Clin. Exp. Ophthalmol. 2014, 252, 699–713. [Google Scholar] [CrossRef]
- Rauf, A.; Imran, M.; Butt, M.S.; Nadeem, M.; Peters, D.G.; Mubarak, M.S. Resveratrol as an Anti-Cancer Agent: A Review. Crit. Rev. Food Sci. Nutr. 2018, 58, 1428–1447. [Google Scholar] [CrossRef]
- Tsai, H.-Y.; Ho, C.-T.; Chen, Y.-K. Biological Actions and Molecular Effects of Resveratrol, Pterostilbene, and 3′-Hydroxypterostilbene. J. Food Drug Anal. 2017, 25, 134–147. [Google Scholar] [CrossRef]
- Yuan, D.; Xu, Y.; Xue, L.; Zhang, W.; Gu, L.; Liu, Q. Resveratrol Protects against Diabetic Retinal Ganglion Cell Damage by Activating the Nrf2 Signaling Pathway. Heliyon 2024, 10, e30786. [Google Scholar] [CrossRef]
- Ebrahimi, R.; Mohammadpour, A.; Medoro, A.; Davinelli, S.; Saso, L.; Miroliaei, M. Exploring the Links between Polyphenols, Nrf2, and Diabetes: A Review. Biomed. Pharmacother. 2025, 186, 118020. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, Z.; Lei, H.; Zhang, D. Recent Progress in Nanotechnology-Based Drug Carriers for Resveratrol Delivery. Drug Deliv. 2023, 30, 2174206. [Google Scholar] [CrossRef]
- Wei, L.; Sun, X.; Fan, C.; Li, R.; Zhou, S.; Yu, H. The Pathophysiological Mechanisms Underlying Diabetic Retinopathy. Front. Cell Dev. Biol. 2022, 10, 963615. [Google Scholar] [CrossRef]
- Shyam, M.; Sidharth, S.; Veronica, A.; Jagannathan, L.; Srirangan, P.; Radhakrishnan, V.; Sabina, E.P. Diabetic Retinopathy: A Comprehensive Review of Pathophysiology and Emerging Treatments. Mol. Biol. Rep. 2025, 52, 380. [Google Scholar] [CrossRef]
- Zhang, H.; Mo, Y. The Gut-Retina Axis: A New Perspective in the Prevention and Treatment of Diabetic Retinopathy. Front. Endocrinol. 2023, 14, 1205846. [Google Scholar] [CrossRef]
- Cai, Y.; Kang, Y. Gut Microbiota and Metabolites in Diabetic Retinopathy: Insights into Pathogenesis for Novel Therapeutic Strategies. Biomed. Pharmacother. 2023, 164, 114994. [Google Scholar] [CrossRef]
- Schiavone, N.; Isoldi, G.; Calcagno, S.; Rovida, E.; Antiga, E.; De Almeida, C.V.; Lulli, M. Exploring the Gut Microbiota-Retina Axis: Implications for Health and Disease. Microorganisms 2025, 13, 1101. [Google Scholar] [CrossRef]
- Li, S.; Deng, J.; Sun, D.; Chen, S.; Yao, X.; Wang, N.; Zhang, J.; Gu, Q.; Zhang, S.; Wang, J.; et al. FBXW7 Alleviates Hyperglycemia-Induced Endothelial Oxidative Stress Injury via ROS and PARP Inhibition. Redox Biol. 2022, 58, 102530. [Google Scholar] [CrossRef] [PubMed]
- Szabó, C.; Biser, A.; Benkő, R.; Böttinger, E.; Suszták, K. Poly(ADP-Ribose) Polymerase Inhibitors Ameliorate Nephropathy of Type 2 Diabetic Lepr Db/Db Mice. Diabetes 2006, 55, 3004–3012. [Google Scholar] [CrossRef]
- Giacco, F.; Brownlee, M. Oxidative Stress and Diabetic Complications. Circ. Res. 2010, 107, 1058–1070. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Deng, A.; Liu, J.; Hou, W. The Role of Keap1-Nrf2-ARE Signal Pathway in Diabetic Retinopathy Oxidative Stress and Related Mechanisms. Int. J. Clin. Exp. Pathol. 2018, 11, 3084–3090. [Google Scholar]
- Wang, K.; Chen, Z.; Huang, L.; Meng, B.; Zhou, X.; Wen, X.; Ren, D. Naringenin Reduces Oxidative Stress and Improves Mitochondrial Dysfunction via Activation of the Nrf2/ARE Signaling Pathway in Neurons. Int. J. Mol. Med. 2017, 40, 1582–1590. [Google Scholar] [CrossRef]
- Tabei, Y.; Murotomi, K.; Umeno, A.; Horie, M.; Tsujino, Y.; Masutani, B.; Yoshida, Y.; Nakajima, Y. Antioxidant Properties of 5-Hydroxy-4-Phenyl-Butenolide via Activation of Nrf2/ARE Signaling Pathway. Food Chem. Toxicol. 2017, 107, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-κB Signaling in Inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef]
- Altmann, C.; Schmidt, M. The Role of Microglia in Diabetic Retinopathy: Inflammation, Microvasculature Defects and Neurodegeneration. Int. J. Mol. Sci. 2018, 19, 110. [Google Scholar] [CrossRef]
- Hu, Q.; Zhang, X.; Peng, H.; Guan, J.; Huang, Z.; Jiang, B.; Sun, D. A New Modulator of Neuroinflammation in Diabetic Retinopathy: USP25. Inflammation 2024, 47, 1520–1535. [Google Scholar] [CrossRef]
- Viganò, I.; Galbiati, S.; Aragona, E.; Gabellini, D.; Lattanzio, R.; Pedon, V.; Basile, G.; Arrigo, A.; Bandello, F.; Zerbini, G. Diabetes-Driven Retinal Neurodegeneration: Its Role in the Pathogenesis of Diabetic Retinopathy. Biomedicines 2025, 13, 1328. [Google Scholar] [CrossRef]
- Arrigo, A.; Cremona, O.; Aragona, E.; Casoni, F.; Consalez, G.; Dogru, R.M.; Hauck, S.M.; Antropoli, A.; Bianco, L.; Parodi, M.B.; et al. Müller Cells Trophism and Pathology as the next Therapeutic Targets for Retinal Diseases. Prog. Retin. Eye Res. 2025, 106, 101357. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Zhou, C. Observation on the Changes of Visual Field and Optic Nerve Fiber Layer Thickness in Patients with Early Diabetic Retinopathy. Photodiagn. Photodyn. Ther. 2024, 47, 104197. [Google Scholar] [CrossRef]
- Neves, D.; Salazar, I.L.; Almeida, R.D.; Silva, R.M. Molecular Mechanisms of Ischemia and Glutamate Excitotoxicity. Life Sci. 2023, 328, 121814. [Google Scholar] [CrossRef]
- Daniele, S.G.; Trummer, G.; Hossmann, K.A.; Vrselja, Z.; Benk, C.; Gobeske, K.T.; Damjanovic, D.; Andrijevic, D.; Pooth, J.-S.; Dellal, D.; et al. Brain Vulnerability and Viability after Ischaemia. Nat. Rev. Neurosci. 2021, 22, 553–572. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.W. Excitotoxicity: Still Hammering the Ischemic Brain in 2020. Front. Neurosci. 2020, 14, 579953. [Google Scholar] [CrossRef]
- El-Sehrawy, A.A.; Elkhamisy, E.M.; Badawi, A.E.; Elshahawy, H.A.; Elsayed, E.; Mohammed, N.T.; El-Eshmawy, M.M. Subclinical Hypothyroidism in Patients with Diabetic Retinopathy: Role of Vascular Endothelial Growth Factor. Endocr. Metab. Immune Disord. Drug Targets 2022, 22, 502–509. [Google Scholar] [CrossRef] [PubMed]
- Batsos, G.; Christodoulou, E.; Christou, E.E.; Galanis, P.; Katsanos, A.; Limberis, L.; Stefaniotou, M. Vitreous Inflammatory and Angiogenic Factors on Patients with Proliferative Diabetic Retinopathy or Diabetic Macular Edema: The Role of Lipocalin2. BMC Ophthalmol. 2022, 22, 496. [Google Scholar] [CrossRef]
- Jain, A.; Saxena, S.; Khanna, V.K.; Shukla, R.K.; Meyer, C.H. Status of Serum VEGF and ICAM-1 and Its Association with External Limiting Membrane and Inner Segment-Outer Segment Junction Disruption in Type 2 Diabetes Mellitus. Mol. Vis. 2013, 19, 1760–1768. [Google Scholar]
- Zhang, M.; Zhou, M.; Cai, X.; Zhou, Y.; Jiang, X.; Luo, Y.; Hu, Y.; Qiu, R.; Wu, Y.; Zhang, Y.; et al. VEGF Promotes Diabetic Retinopathy by Upregulating the PKC/ET/NF-κB/ICAM-1 Signaling Pathway. Eur. J. Histochem. EJH 2022, 66, 3522. [Google Scholar] [CrossRef] [PubMed]
- Gaonkar, B.; Prabhu, K.; Rao, P.; Kamat, A.; Rao Addoor, K.; Varma, M. Plasma Angiogenesis and Oxidative Stress Markers in Patients with Diabetic Retinopathy. Biomarkers 2020, 25, 397–401. [Google Scholar] [CrossRef]
- Chen, C.-H. Nrf2-ARE Pathway: Defense Against Oxidative Stress. In Xenobiotic Metabolic Enzymes: Bioactivation and Antioxidant Defense; Springer International Publishing: Cham, Switzerland, 2020; pp. 145–154. ISBN 978-3-030-41678-2. [Google Scholar]
- Park, C.; Lee, H.; Han, M.H.; Jeong, J.-W.; Kim, S.O.; Jeong, S.-J.; Lee, B.; Kim, G.; Park, E.K.; Jeon, Y.; et al. Cytoprotective Effects of Fermented Oyster Extracts against Oxidative Stress-Induced DNA Damage and Apoptosis through Activation of the Nrf2/HO-1 Signaling Pathway in MC3T3-E1 Osteoblasts. EXCLI J. 2020, 9, 1102–1119. [Google Scholar] [CrossRef]
- Abiko, Y.; Toriba, A.; Kumagai, Y. Phytochemicals to Regulate Oxidative and Electrophilic Stress through Nrf2 Activation. Redox Exp. Med. 2022, 2023, e220021. [Google Scholar] [CrossRef]
- Chang, H.-Y.; Lin, C.-W.; Yang, C.-M.; Yang, C.-H. Nrf-2 Activator Sulforaphane Protects Retinal Cells from Oxidative Stress-Induced Retinal Injury. J. Funct. Foods 2020, 71, 104023. [Google Scholar] [CrossRef]
- Thiruvengadam, M.; Venkidasamy, B.; Subramanian, U.; Samynathan, R.; Ali Shariati, M.; Rebezov, M.; Girish, S.; Thangavel, S.; Dhanapal, A.R.; Fedoseeva, N.; et al. Bioactive Compounds in Oxidative Stress-Mediated Diseases: Targeting the NRF2/ARE Signaling Pathway and Epigenetic Regulation. Antioxidants 2021, 10, 1859. [Google Scholar] [CrossRef] [PubMed]
- Truong, V.; Jun, M.; Jeong, W. Role of Resveratrol in Regulation of Cellular Defense Systems against Oxidative Stress. BioFactors 2018, 44, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Shah, P.; Patel, J. Resveratrol and Its Biological Actions. Int. J. Green Pharm. 2010, 4, 15. [Google Scholar] [CrossRef]
- Bryl, A.; Falkowski, M.; Zorena, K.; Mrugacz, M. The Role of Resveratrol in Eye Diseases—A Review of the Literature. Nutrients 2022, 14, 2974. [Google Scholar] [CrossRef]
- Li, J.; Yu, S.; Ying, J.; Shi, T.; Wang, P. Resveratrol Prevents ROS-Induced Apoptosis in High Glucose-Treated Retinal Capillary Endothelial Cells via the Activation of AMPK/Sirt1/PGC-1 α Pathway. Oxid. Med. Cell Longev. 2017, 2017, 7584691. [Google Scholar] [CrossRef]
- Jîtcă, G. The Role of AMPK Activation in Metabolic Regulation, Energy Homeostasis and Aging: A Comprehensive Overview. INNOSC Theranostics Pharmacol. Sci. 2024, 8, 1–15. [Google Scholar] [CrossRef]
- Marin, T.L.; Gongol, B.; Zhang, F.; Martin, M.; Johnson, D.A.; Xiao, H.; Wang, Y.; Subramaniam, S.; Chien, S.; Shyy, J.Y.-J. AMPK Promotes Mitochondrial Biogenesis and Function by Phosphorylating the Epigenetic Factors DNMT1, RBBP7, and HAT1. Sci. Signal. 2017, 10, eaaf7478. [Google Scholar] [CrossRef] [PubMed]
- Duarte, F.; Amorim, J.; Palmeira, C.; Rolo, A. Regulation of Mitochondrial Function and Its Impact in Metabolic Stress. Curr. Med. Chem. 2015, 22, 2468–2479. [Google Scholar] [CrossRef] [PubMed]
- Malik, N.; Ferreira, B.I.; Hollstein, P.E.; Curtis, S.D.; Trefts, E.; Weiser Novak, S.; Yu, J.; Gilson, R.; Hellberg, K.; Fang, L.; et al. Induction of Lysosomal and Mitochondrial Biogenesis by AMPK Phosphorylation of FNIP1. Science 2023, 380, eabj5559. [Google Scholar] [CrossRef]
- Jiang, T.; Gu, J.; Chang, Q. Resveratrol Suppresses “Metabolic Memory” by Inhibiting Inflammation and Apoptosis Through the ROS/TXNIP/NLRP3 Signaling Pathway. Pharmacogn. Mag. 2024, 20, 898–907. [Google Scholar] [CrossRef]
- Im, E.J.; Kim, S.J.; Hong, S.B.; Park, J.-K.; Rhee, M.H. Anti-Inflammatory Activity of Bee Venom in BV2 Microglial Cells: Mediation of MyD88-Dependent NF- κ B Signaling Pathway. Evid. Based Complement. Alternat. Med. 2016, 2016, 3704764. [Google Scholar] [CrossRef]
- Kim, J.-K.; Jun, J.-G. Licochalcone B Exhibits Anti-Inflammatory Effects via Modulation of NF-κB and AP-1. Biomed. Sci. Lett. 2015, 21, 218–226. [Google Scholar] [CrossRef]
- Kwon, S.-H.; Ma, S.-X.; Ko, Y.-H.; Seo, J.-Y.; Lee, B.-R.; Lee, T.H.; Kim, S.Y.; Lee, S.-Y.; Jang, C.-G. Vaccinium Bracteatum Thunb. Exerts Anti-Inflammatory Activity by Inhibiting NF-κB Activation in BV-2 Microglial Cells. Biomol. Ther. 2016, 24, 543–551. [Google Scholar] [CrossRef]
- Qomaladewi, N.P.; Aziz, N.; Kim, M.-Y.; Cho, J.Y. Piper Cubeba L. Methanol Extract Has Anti-Inflammatory Activity Targeting Src/Syk via NF- κ B Inhibition. Evid. Based Complement. Alternat. Med. 2019, 2019, 1548125. [Google Scholar] [CrossRef]
- Zhou, H.; Chen, S.; Wang, W.; Wang, Z.; Wu, X.; Zhang, Z. Nanog Inhibits Lipopolysaccharide-Induced Expression of pro-Inflammatory Cytokines by Blocking NF-κB Transcriptional Activity in Rat Primary Microglial Cells. Mol. Med. Rep. 2011, 5, 842–844. [Google Scholar] [CrossRef][Green Version]
- McDougald, D.S.; Dine, K.E.; Zezulin, A.U.; Bennett, J.; Shindler, K.S. SIRT1 and NRF2 Gene Transfer Mediate Distinct Neuroprotective Effects Upon Retinal Ganglion Cell Survival and Function in Experimental Optic Neuritis. Investig. Opthalmol. Vis. Sci. 2018, 59, 1212. [Google Scholar] [CrossRef] [PubMed]
- Ross, A.G.; Chaqour, B.; McDougald, D.S.; Dine, K.E.; Duong, T.T.; Shindler, R.E.; Yue, J.; Liu, T.; Shindler, K.S. Selective Upregulation of SIRT1 Expression in Retinal Ganglion Cells by AAV-Mediated Gene Delivery Increases Neuronal Cell Survival and Alleviates Axon Demyelination Associated with Optic Neuritis. Biomolecules 2022, 12, 830. [Google Scholar] [CrossRef]
- Mimura, T.; Hidetaka, N.; Funatsu, H.; Aki, K.; Matsubara, M. Retinal Neuroprotective Effect of Sirtuins. SM Ophthalmol. J. 2014, 2, 1016. [Google Scholar]
- Zeng, K.; Wang, Y.; Yang, N.; Wang, D.; Li, S.; Ming, J.; Wang, J.; Yu, X.; Song, Y.; Zhou, X.; et al. Resveratrol Inhibits Diabetic-Induced Müller Cells Apoptosis through MicroRNA-29b/Specificity Protein 1 Pathway. Mol. Neurobiol. 2017, 54, 4000–4014. [Google Scholar] [CrossRef] [PubMed]
- Zeng, K.; Wan, Z.-W.; Sun, P.; Wang, Y.; Song, Y.; Yu, X.-M.; Deng, B. Resveratrol Improves Diabetic Retinopathy via Regulating MicroRNA-29b/Specificity Protein 1/Apoptosis Pathway by Enhancing Autophagy 2024. Eur. J. Nutr. 2025, 64, 232. [Google Scholar]
- Luo, J.; He, T.; Yang, J.; Yang, N.; Li, Z.; Xing, Y. SIRT1 Is Required for the Neuroprotection of Resveratrol on Retinal Ganglion Cells after Retinal Ischemia-Reperfusion Injury in Mice. Graefes Arch. Clin. Exp. Ophthalmol. 2020, 258, 335–344. [Google Scholar] [CrossRef]
- Shindler, K.S.; Ventura, E.; Rex, T.S.; Elliott, P.; Rostami, A. SIRT1 Activation Confers Neuroprotection in Experimental Optic Neuritis. Investig. Opthalmol. Vis. Sci. 2007, 48, 3602. [Google Scholar] [CrossRef]
- Arshadi, D.; Mansouri, K.; Khodarahmi, R.; Seyfi, P.; Shakiba, Y.; Mostafaie, A. In Vitro Anti-Angiogenic Activity of Persian Shallot (Allium Hirtifolium) Extract Is Mediated through Inhibition of Endothelial Cell Proliferation/Migration and down-Regulation of VEGF and MMP Expression. J. Rep. Pharm. Sci. 2014, 3, 65. [Google Scholar] [CrossRef]
- Han, J.M.; Choi, Y.S.; Dhakal, D.; Sohng, J.K.; Jung, H.J. Novel Nargenicin A1 Analog Inhibits Angiogenesis by Downregulating the Endothelial VEGF/VEGFR2 Signaling and Tumoral HIF-1α/VEGF Pathway. Biomedicines 2020, 8, 252. [Google Scholar] [CrossRef]
- Giordo, R.; Nasrallah, G.K.; Posadino, A.M.; Galimi, F.; Capobianco, G.; Eid, A.H.; Pintus, G. Resveratrol-Elicited PKC Inhibition Counteracts NOX-Mediated Endothelial to Mesenchymal Transition in Human Retinal Endothelial Cells Exposed to High Glucose. Antioxidants 2021, 10, 224. [Google Scholar] [CrossRef] [PubMed]
- Toro, M.D.; Nowomiejska, K.; Avitabile, T.; Rejdak, R.; Tripodi, S.; Porta, A.; Reibaldi, M.; Figus, M.; Posarelli, C.; Fiedorowicz, M. Effect of Resveratrol on In Vitro and In Vivo Models of Diabetic Retinophathy: A Systematic Review. Int. J. Mol. Sci. 2019, 20, 3503. [Google Scholar] [CrossRef]
- Liu, X.-Q.; Wu, B.-J.; Pan, W.H.T.; Zhang, X.-M.; Liu, J.-H.; Chen, M.-M.; Chao, F.-P.; Chao, H.-M. Resveratrol Mitigates Rat Retinal Ischemic Injury: The Roles of Matrix Metalloproteinase-9, Inducible Nitric Oxide, and Heme Oxygenase-1. J. Ocul. Pharmacol. Ther. 2013, 29, 33–40. [Google Scholar] [CrossRef]
- Meng, T.; Xiao, D.; Muhammed, A.; Deng, J.; Chen, L.; He, J. Anti-Inflammatory Action and Mechanisms of Resveratrol. Molecules 2021, 26, 229. [Google Scholar] [CrossRef]
- Al-Jaber, H.I.; Shakya, A.K.; Al-Qudah, M.A.; Barhoumi, L.M.; Abu-Sal, H.E.; Hasan, H.S.; Al-Bataineh, N.; Abu-Orabi, S.; Mubarak, M.S. Piceatannol, a Comprehensive Review of Health Perspectives and Pharmacological Aspects. Arab. J. Chem. 2024, 17, 105939. [Google Scholar] [CrossRef]
- Ashikawa, K.; Majumdar, S.; Banerjee, S.; Bharti, A.C.; Shishodia, S.; Aggarwal, B.B. Piceatannol Inhibits TNF-Induced NF-κB Activation and NF-κB-Mediated Gene Expression Through Suppression of IκBα Kinase and P65 Phosphorylation. J. Immunol. 2002, 169, 6490–6497. [Google Scholar] [CrossRef]
- Liu, X.; Pei, J.; Li, J.; Zhu, H.; Zheng, X.; Zhang, X.; Ruan, B.; Chen, L. Recent Advances in Resveratrol Derivatives: Structural Modifications and Biological Activities. Molecules 2025, 30, 958. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Jia, Y.; Ren, F. Multidimensional Biological Activities of Resveratrol and Its Prospects and Challenges in the Health Field. Front. Nutr. 2024, 11, 1408651. [Google Scholar] [CrossRef] [PubMed]
- Amri, A.; Chaumeil, J.C.; Sfar, S.; Charrueau, C. Administration of Resveratrol: What Formulation Solutions to Bioavailability Limitations? J. Control. Release 2012, 158, 182–193. [Google Scholar] [CrossRef] [PubMed]
- Radeva, L.; Yoncheva, K. Resveratrol—A Promising Therapeutic Agent with Problematic Properties. Pharmaceutics 2025, 17, 134. [Google Scholar] [CrossRef]
- Li, P.; Peng, J.; Li, Y.; Gong, L.; Lv, Y.; Liu, H.; Zhang, T.; Yang, S.; Liu, H.; Li, J.; et al. Pharmacokinetics, Bioavailability, Excretion and Metabolism Studies of Akebia Saponin D in Rats: Causes of the Ultra-Low Oral Bioavailability and Metabolic Pathway. Front. Pharmacol. 2021, 12, 621003. [Google Scholar] [CrossRef]
- Chong, C.; Schug, S.; Page-Sharp, M.; Ilett, K. Bioavailability of Ketamine After Oral or Sublingual Administration. Pain Med. 2006, 7, 469. [Google Scholar] [CrossRef]
- Chu, S.Y.; Deaton, R.; Cavanaugh, J. Absolute Bioavailability of Clarithromycin after Oral Administration in Humans. Antimicrob. Agents Chemother. 1992, 36, 1147–1150. [Google Scholar] [CrossRef][Green Version]
- Shirasaki, Y.; Yamaguchi, M.; Miyashita, H. Retinal Penetration of Calpain Inhibitors in Rats After Oral Administration. J. Ocul. Pharmacol. Ther. 2006, 22, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.-C.; Chen, Y.-H.; Lu, D.-W. Overview of Recent Advances in Nano-Based Ocular Drug Delivery. Int. J. Mol. Sci. 2023, 24, 15352. [Google Scholar] [CrossRef]
- Azman, M.; Sabri, A.H.; Anjani, Q.K.; Mustaffa, M.F.; Hamid, K.A. Intestinal Absorption Study: Challenges and Absorption Enhancement Strategies in Improving Oral Drug Delivery. Pharmaceuticals 2022, 15, 975. [Google Scholar] [CrossRef]
- Minichmayr, I.K.; Shebley, M.; Van Der Graaf, P.H.; Venkatakrishnan, K. Getting the Dosage Right: A Vital Role for Clinical Pharmacology in the Era of Precision Medicine. Clin. Pharmacol. Ther. 2024, 116, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Thrimawithana, T.; Young, S.; Bunt, C.R.; Green, C.R.; Alany, R.G. Drug Delivery to the Posterior Segment of the Eye: Challenges and Opportunities. Drug Deliv. Lett. 2011, 1, 40–44. [Google Scholar] [CrossRef] [PubMed]
- Diress, M.; Wagle, S.R.; Lim, P.; Foster, T.; Kovacevic, B.; Ionescu, C.M.; Mooranian, A.; Al-Salami, H. Advanced Drug Delivery Strategies for Diabetic Retinopathy: Current Therapeutic Advancement, and Delivery Methods Overcoming Barriers, and Experimental Modalities. Expert Opin. Drug Deliv. 2024, 21, 1859–1877. [Google Scholar] [CrossRef] [PubMed]
- Pintea, A.M.; Rugină, D.O. Resveratrol and the Human Retina. In Handbook of Nutrition, Diet and the Eye; Elsevier: Amsterdam, The Netherlands, 2014; pp. 481–491. ISBN 978-0-12-401717-7. [Google Scholar]
- Rutkovsky, A.C. Resveratrol Clinical Trials: Number of Studies per Condition 2020. Available online: https://zenodo.org/records/4058712 (accessed on 12 July 2025).
- Tomé-Carneiro, J.; Larrosa, M.; González-Sarrías, A.; Tomás-Barberán, F.; García-Conesa, M.; Espín, J. Resveratrol and Clinical Trials: The Crossroad from In Vitro Studies to Human Evidence. Curr. Pharm. Des. 2013, 19, 6064–6093. [Google Scholar] [CrossRef]
- Smoliga, J.M.; Vang, O.; Baur, J.A. Challenges of Translating Basic Research Into Therapeutics: Resveratrol as an Example. J. Gerontol. A Biol. Sci. Med. Sci. 2012, 67, 158–167. [Google Scholar] [CrossRef]
- Simó-Servat, O.; Hernández, C.; Simó, R. Diabetic Retinopathy in the Context of Patients with Diabetes. Ophthalmic Res. 2019, 62, 211–217. [Google Scholar] [CrossRef]
- Silva, P.S.; Cavallerano, J.D.; Sun, J.K.; Aiello, L.M.; Aiello, L.P. Effect of Systemic Medications on Onset and Progression of Diabetic Retinopathy. Nat. Rev. Endocrinol. 2010, 6, 494–508. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Jiang, Y.; Jaganathan, R.; Hao, Y. Current Advances in Pharmacotherapy and Technology for Diabetic Retinopathy: A Systematic Review. J. Ophthalmol. 2018, 2018, 1694187. [Google Scholar] [CrossRef]
- Maheshwari, S.Y.; Kumar, S.; Sinha, A.H.; Kumar, M. Diabetic Retinopathy: A Pharmacological Consideration. Cureus 2023, 15, e46842. [Google Scholar] [CrossRef]
- Magri, C.J.; Fava, S. The Diabetic Eye: A Window to the Heart & Vascular System. J. Diabetes Metab. 2012, 03. [Google Scholar] [CrossRef]
- Jumelle, C.; Gholizadeh, S.; Annabi, N.; Dana, R. Advances and Limitations of Drug Delivery Systems Formulated as Eye Drops. J. Control. Release 2020, 321, 1–22. [Google Scholar] [CrossRef]
- Ahmed, S.; Amin, M.M.; Sayed, S. Ocular Drug Delivery: A Comprehensive Review. AAPS PharmSciTech 2023, 24, 66. [Google Scholar] [CrossRef]
- Kannan, R.M.; Pitha, I.; Parikh, K.S. A New Era in Posterior Segment Ocular Drug Delivery: Translation of Systemic, Cell-Targeted, Dendrimer-Based Therapies. Adv. Drug Deliv. Rev. 2023, 200, 115005. [Google Scholar] [CrossRef]
- Pandian, S.; Jeevanesan, V.; Ponnusamy, C.; Natesan, S. RES-Loaded Pegylated CS NPs: For Efficient Ocular Delivery. IET Nanobiotechnol. 2017, 11, 32–39. [Google Scholar] [CrossRef]
- Buosi, F.S.; Alaimo, A.; Di Santo, M.C.; Elías, F.; García Liñares, G.; Acebedo, S.L.; Castañeda Cataña, M.A.; Spagnuolo, C.C.; Lizarraga, L.; Martínez, K.D.; et al. Resveratrol Encapsulation in High Molecular Weight Chitosan-Based Nanogels for Applications in Ocular Treatments: Impact on Human ARPE-19 Culture Cells. Int. J. Biol. Macromol. 2020, 165, 804–821. [Google Scholar] [CrossRef]
- Li, M.; Zhang, L.; Li, R.; Yan, M. New Resveratrol Micelle Formulation for Ocular Delivery: Characterization and in Vitro/in Vivo Evaluation. Drug Dev. Ind. Pharm. 2020, 46, 1960–1970. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, P.; Fnu, G.; Bhatia, D.; Shahid, A.; Sutariya, V. Nanodelivery of Resveratrol-Loaded PLGA Nanoparticles for Age-Related Macular Degeneration. AAPS PharmSciTech 2020, 21, 291. [Google Scholar] [CrossRef]
- Chakole, C.M. Resveratrol Loaded Nanostructured Lipid Carrier for Enhanced Ocular Delivery: Formulation, In Vitro and Ex Vivo Evaluation. Afr. J. Biol. Sci. 2024, 6, 1215–1221. [Google Scholar]
- Saha, M.; Saha, D.R.; Ulhosna, T.; Sharker, S.M.; Shohag, M.H.; Islam, M.S.; Ray, S.K.; Rahman, G.M.S.; Reza, H.M. QbD Based Development of Resveratrol-Loaded Mucoadhesive Lecithin/Chitosan Nanoparticles for Prolonged Ocular Drug Delivery. J. Drug Deliv. Sci. Technol. 2021, 63, 102480. [Google Scholar] [CrossRef]
- Li, M.; Yu, X.; Zhu, L.; Jin, Y.; Wu, Z. Ocular Lamellar Crystalline Gels for Sustained Release and Enhanced Permeation of Resveratrol against Corneal Neovascularization. Drug Deliv. 2021, 28, 206–217. [Google Scholar] [CrossRef]
- Krstić, L.; Jarho, P.; Ruponen, M.; Urtti, A.; González-García, M.J.; Diebold, Y. Improved Ocular Delivery of Quercetin and Resveratrol: A Comparative Study between Binary and Ternary Cyclodextrin Complexes. Int. J. Pharm. 2022, 624, 122028. [Google Scholar] [CrossRef] [PubMed]
- Vivero-Lopez, M.; Sparacino, C.; Quelle-Regaldie, A.; Sánchez, L.; Candal, E.; Barreiro-Iglesias, A.; Huete-Toral, F.; Carracedo, G.; Otero, A.; Concheiro, A.; et al. Pluronic®/Casein Micelles for Ophthalmic Delivery of Resveratrol: In Vitro, Ex Vivo, and in Vivo Tests. Int. J. Pharm. 2022, 628, 122281. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.; Chen, P.; Liu, X.; Lian, Y.; Xi, J.; Li, J.; Song, J.; Li, X. Trimethylated Chitosan-Coated Flexible Liposomes with Resveratrol for Topical Drug Delivery to Reduce Blue-Light-Induced Retinal Damage. Int. J. Biol. Macromol. 2023, 252, 126480. [Google Scholar] [CrossRef]
- De Luca, I.; Di Cristo, F.; Conte, R.; Peluso, G.; Cerruti, P.; Calarco, A. In-Situ Thermoresponsive Hydrogel Containing Resveratrol-Loaded Nanoparticles as a Localized Drug Delivery Platform for Dry Eye Disease. Antioxidants 2023, 12, 993. [Google Scholar] [CrossRef]
- Zingale, E.; Bonaccorso, A.; D’Amico, A.G.; Lombardo, R.; D’Agata, V.; Rautio, J.; Pignatello, R. Formulating Resveratrol and Melatonin Self-Nanoemulsifying Drug Delivery Systems (SNEDDS) for Ocular Administration Using Design of Experiments. Pharmaceutics 2024, 16, 125. [Google Scholar] [CrossRef]
- Gonzalez-Perez, J.; Lopera-Echavarría, A.M.; Arevalo-Alquichire, S.; Araque-Marín, P.; Londoño, M.E. Development of a Resveratrol Nanoformulation for the Treatment of Diabetic Retinopathy. Materials 2024, 17, 1420. [Google Scholar] [CrossRef]
- Krstić, L.; Vallejo, R.; Rodriguez-Rojo, S.; González-García, M.J.; Arias, F.J.; Girotti, A.; Diebold, Y. Effective Ocular Delivery of Antioxidant Polyphenols Using Elastin-like Polymer Nanosystems Developed by Sustainable Process. Int. J. Pharm. 2025, 678, 125691. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Z.; Wang, C.; Xie, X.; Ma, Y.; Wang, Y. Biodegradable and Dissolvable Resveratrol Nanocrystals Non-Silicon Microneedles for Transdermal Drug Delivery. J. Drug Deliv. Sci. Technol. 2023, 86, 104653. [Google Scholar] [CrossRef]
- Lynch, C.; Kondiah, P.P.D.; Choonara, Y.E.; Du Toit, L.C.; Ally, N.; Pillay, V. Advances in Biodegradable Nano-Sized Polymer-Based Ocular Drug Delivery. Polymers 2019, 11, 1371. [Google Scholar] [CrossRef]
- Saleem, Z.; Rehman, K.; Hamid Akash, M.S. Role of Drug Delivery System in Improving the Bioavailability of Resveratrol. Curr. Pharm. Des. 2022, 28, 1632–1642. [Google Scholar] [CrossRef]
- Taheri, S.L.; Poorirani, S.; Mostafavi, S.A. Intraocular Drug Delivery Systems for Diabetic Retinopathy: Current and Future Prospective. BioImpacts 2024, 15, 30127. [Google Scholar] [CrossRef]
- Yilmaz, T.; Cordero-Coma, M.; Lavaque, A.J.; Gallagher, M.J.; Padula, W.V. Triamcinolone and Intraocular Sustained-Release Delivery Systems in Diabetic Retinopathy. Curr. Pharm. Biotechnol. 2011, 12, 337–346. [Google Scholar] [CrossRef]
- Rafael, D.; Guerrero, M.; Marican, A.; Arango, D.; Sarmento, B.; Ferrer, R.; Durán-Lara, E.F.; Clark, S.J.; Schwartz, S. Delivery Systems in Ocular Retinopathies: The Promising Future of Intravitreal Hydrogels as Sustained-Release Scaffolds. Pharmaceutics 2023, 15, 1484. [Google Scholar] [CrossRef]
- Wykoff, C.C.; Kuppermann, B.D.; Regillo, C.D.; Chang, M.; Hariprasad, S.M.; Duker, J.S.; Altaf, S.; Saïm, S. Extended Intraocular Drug-Delivery Platforms for the Treatment of Retinal and Choroidal Diseases. J. Vitreoretin. Dis. 2024, 8, 577–586. [Google Scholar] [CrossRef]
- Rathi, S.A.; Dhoke, A.P. A Comprehensive Review of Innovations for Diabetic Retinopathy and Next-Generation Intraocular Drug Delivery. Int. J. Sci. Technol. 2025, 16, 1504. [Google Scholar] [CrossRef]
- Zaher Addeen, S.; Shaddoud, I. Combined Phacoemulsification Surgery and Intravitreal Triamcinolone Injection versus Stand-Alone Surgery in Patients with Type 2 Diabetes: A Prospective Randomized Trial. BMC Ophthalmol. 2022, 22, 445. [Google Scholar] [CrossRef]
- Riaz, S.; Ahmed, N.; Khan, A.; Jabran, A.; Raza, I.; Afzal, t. Panretinal Photocoagulation Plus Intravitreal Bevacizumab Versus Panretinal Photocoagulation Alone for Proliferative Diabetic Retinopathy. Biol. Clin. Sci. Res. J. 2024, 2024, 1313. [Google Scholar] [CrossRef]
- Zhang, W.; Geng, J.; Sang, A. Effectiveness of Panretinal Photocoagulation Plus Intravitreal Anti-VEGF Treatment Against PRP Alone for Diabetic Retinopathy: A Systematic Review With Meta-Analysis. Front. Endocrinol. 2022, 13, 807687. [Google Scholar] [CrossRef]
- Fallico, M.; Maugeri, A.; Lotery, A.; Longo, A.; Bonfiglio, V.; Russo, A.; Avitabile, T.; Pulvirenti, A.; Furino, C.; Cennamo, G.; et al. Intravitreal Anti-vascular Endothelial Growth Factors, Panretinal Photocoagulation and Combined Treatment for Proliferative Diabetic Retinopathy: A Systematic Review and Network Meta-analysis. Acta Ophthalmol. 2021, 99, e795–e805. [Google Scholar] [CrossRef]
- Pei, X.; Huang, D.; Li, Z. Genetic Insights and Emerging Therapeutics in Diabetic Retinopathy: From Molecular Pathways to Personalized Medicine. Front. Genet. 2024, 15, 1416924. [Google Scholar] [CrossRef]
- Hammes, H.P. Optimal Treatment of Diabetic Retinopathy. Ther. Adv. Endocrinol. Metab. 2013, 4, 61–71. [Google Scholar] [CrossRef]
- Agarwal, A.; Soliman, M.K.; Sepah, Y.J.; Do, D.V.; Nguyen, Q.D. Diabetic Retinopathy: Variations in Patient Therapeutic Outcomes and Pharmacogenomics. Pharmacogenom. Pers. Med. 2014, 7, 399–409. [Google Scholar] [CrossRef][Green Version]
- Duh, E.J.; Sun, J.K.; Stitt, A.W. Diabetic Retinopathy: Current Understanding, Mechanisms, and Treatment Strategies. JCI Insight 2017, 2, e93751. [Google Scholar] [CrossRef]
- Kianmehr, H.; Zhang, P.; Ospina, N.S.; Shi, L.; Fonseca, V.; Guo, J.; Shao, H. 418-P: Certain Patient Subgroups with Type 2 Diabetes May Benefit from Intensive Glycemic and Blood Pressure Control from Reductions in Major Adverse Cardiovascular Events (MACE): A Machine Learning–Based Post Hoc Analysis of ACCORD Trial Data. Diabetes 2022, 71, 418. [Google Scholar] [CrossRef]
- Dhruval, N.; Manohar, M.; Gowda, D.V. Recent Review on Role of Resveratrol in Diabetes and Its Complication. Int. J. Res. Pharm. Sci. 2020, 11, 1692–1700. [Google Scholar] [CrossRef]
- Vallianou, N.G.; Evangelopoulos, A.; Kazazis, C. Resveratrol and Diabetes. Rev Diabet Stud 2013, 10, 236–242. [Google Scholar] [CrossRef]
- Moshtaghion, S.M.; Caballano-Infantes, E.; Plaza Reyes, Á.; Valdés-Sánchez, L.; Fernández, P.G.; De La Cerda, B.; Riga, M.S.; Álvarez-Dolado, M.; Peñalver, P.; Morales, J.C.; et al. Piceid Octanoate Protects Retinal Cells against Oxidative Damage by Regulating the Sirtuin 1/Poly-ADP-Ribose Polymerase 1 Axis In Vitro and in Rd10 Mice. Antioxidants 2024, 13, 201. [Google Scholar] [CrossRef]
- Trombino, S.; Cassano, R.; Di Gioia, M.L.; Aiello, F. Emerging Trends in Green Extraction Techniques, Chemical Modifications, and Drug Delivery Systems for Resveratrol. Antioxidants 2025, 14, 654. [Google Scholar] [CrossRef]
- Intagliata, S.; Modica, M.N.; Santagati, L.M.; Montenegro, L. Strategies to Improve Resveratrol Systemic and Topical Bioavailability: An Update. Antioxidants 2019, 8, 244. [Google Scholar] [CrossRef]
- Robertson, I.; Wai Hau, T.; Sami, F.; Sajid Ali, M.; Badgujar, V.; Murtuja, S.; Saquib Hasnain, M.; Khan, A.; Majeed, S.; Tahir Ansari, M. The Science of Resveratrol, Formulation, Pharmacokinetic Barriers and Its Chemotherapeutic Potential. Int. J. Pharm. 2022, 618, 121605. [Google Scholar] [CrossRef]
- Dikmetas, D.N.; Yenipazar, H.; Can Karaca, A. Recent Advances in Encapsulation of Resveratrol for Enhanced Delivery. Food Chem. 2024, 460, 140475. [Google Scholar] [CrossRef]
- Harwansh, R.K.; Yadav, P.; Deshmukh, R. Current Insight into Novel Delivery Approaches of Resveratrol for Improving Therapeutic Efficacy and Bioavailability with Its Clinical Updates. Curr. Pharm. Des. 2023, 29, 2921–2939. [Google Scholar] [CrossRef]
- Ye, X.; Fung, N.S.K.; Lam, W.C.; Lo, A.C.Y. Nutraceuticals for Diabetic Retinopathy: Recent Advances and Novel Delivery Systems. Nutrients 2024, 16, 1715. [Google Scholar] [CrossRef]
- Delmas, D.; Cornebise, C.; Courtaut, F.; Xiao, J.; Aires, V. New Highlights of Resveratrol: A Review of Properties against Ocular Diseases. Int. J. Mol. Sci. 2021, 22, 1295. [Google Scholar] [CrossRef]
- Hu, W.H.; Zhang, X.-Y.; Leung, K.-W.; Duan, R.; Dong, T.-X.; Qin, Q.-W.; Tsim, K.W.-K. Resveratrol, an Inhibitor Binding to VEGF, Restores the Pathology of Abnormal Angiogenesis in Retinopathy of Prematurity (ROP) in Mice: Application by Intravitreal and Topical Instillation. Int. J. Mol. Sci. 2022, 23, 6455. [Google Scholar] [CrossRef]
- Uludag, G.; Hassan, M.; Matsumiya, W.; Pham, B.H.; Chea, S.; Trong Tuong Than, N.; Doan, H.L.; Akhavanrezayat, A.; Halim, M.S.; Do, D.V.; et al. Efficacy and Safety of Intravitreal Anti-VEGF Therapy in Diabetic Retinopathy: What We Have Learned and What Should We Learn Further? Expert Opin. Biol. Ther. 2022, 22, 1275–1291. [Google Scholar] [CrossRef]
- Zhu, H.; Li, B.; Huang, T.; Wang, B.; Li, S.; Yu, K.; Cai, L.; Ye, Y.; Chen, S.; Zhu, H.; et al. Update in the Molecular Mechanism and Biomarkers of Diabetic Retinopathy. Biochim. Biophys. Acta Mol. Basis Dis. 2025, 1871, 167758. [Google Scholar] [CrossRef]
- Seong, E.J.; Kim, Y.; Su, Z.-Y.; Kang, H.-T.; Lee, J.H. Combined Treatment of Metformin and Resveratrol Promotes Myogenesis Through Increased Irisin Release in C2C12 Cells. Pharm. Res. 2025, 42, 419–428. [Google Scholar] [CrossRef]
- Chung, J.Y.; Jeong, J.-H.; Song, J. Resveratrol Modulates the Gut-Brain Axis: Focus on Glucagon-Like Peptide-1, 5-HT, and Gut Microbiota. Front. Aging Neurosci. 2020, 12, 588044. [Google Scholar] [CrossRef]
- Berzack, S.; Galor, A. Microbiome-Based Therapeutics for Ocular Diseases. Clin. Exp. Optom. 2025, 108, 115–122. [Google Scholar] [CrossRef]
- Danışman, B.; Ercan Kelek, S.; Aslan, M. Resveratrol in Neurodegeneration, in Neurodegenerative Diseases, and in the Redox Biology of the Mitochondria. Psychiatry Clin. Psychopharmacol. 2023, 33, 147–155. [Google Scholar] [CrossRef]
- Taurone, S.; De Ponte, C.; Rotili, D.; De Santis, E.; Mai, A.; Fiorentino, F.; Scarpa, S.; Artico, M.; Micera, A. Biochemical Functions and Clinical Characterizations of the Sirtuins in Diabetes-Induced Retinal Pathologies. Int. J. Mol. Sci. 2022, 23, 4048. [Google Scholar] [CrossRef]
- Çevik, D.; Yildirim, E.B.; Güzelmeriç, E.; Yeşilada, E. Inconsistency between Declared versus Determined Transresveratrol and/or Quercetin Contents in Food Supplements. J. Res. Pharm. 2024, 28, 2078–2091. [Google Scholar] [CrossRef]
- Khattar, S.; Khan, S.A.; Zaidi, S.A.A.; Darvishikolour, M.; Farooq, U.; Naseef, P.P.; Kurunian, M.S.; Khan, M.Z.; Shamim, A.; Khan, M.M.U.; et al. Resveratrol from Dietary Supplement to a Drug Candidate: An Assessment of Potential. Pharmaceuticals 2022, 15, 957. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).