Design and Application of Atomically Dispersed Transition Metal–Carbon Cathodes for Triggering Cascade Oxygen Reduction in Wastewater Treatment
Abstract
1. Introduction
2. Mechanism and Performance Evaluation of Cascade ORR
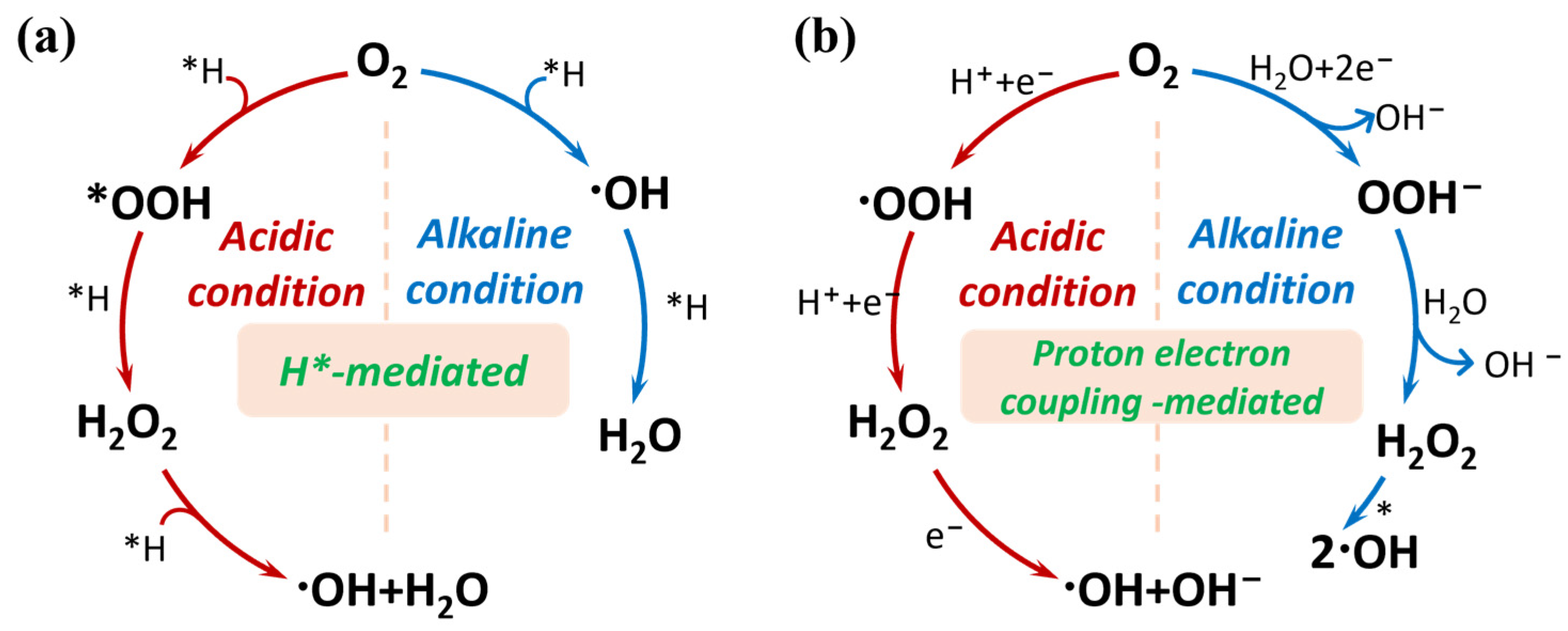
2.1. Fundamental Principles of Cascade ORR
- 2e− ORR:
- Habor–Weiss cycle:
- H2O2 reduction:
2.2. Generation of H2O2
2.3. Performance Evaluation
3. Design and Modulation of Active Sites in Cathode Materials
3.1. Synthesis Strategies for Atomically Dispersed Catalysts
3.1.1. Pre-Coordination Strategy
3.1.2. Spatial Confinement
3.1.3. CVD
3.1.4. Carbon-Assisted Flash Joule Heating
3.1.5. Biomass-Derived Carbon-Supported SACs
3.2. Modulation Strategies for Catalytic Performance
3.2.1. Increasing Active Sites
3.2.2. Heteroatom Doping
3.2.3. Modulating the Coordination Environment of Single Metal Stoms
4. Environmental Factors Influencing Cascade ORR
4.1. Inorganic Anions
4.2. Natural Organic Matter (NOM)
4.3. pH
5. Stability Challenges and Material Solutions
5.1. Enhancing the Metal–Support Interaction
5.2. Spatial Confinement Strategy
5.3. Constructing Self-Supporting Cathodes
5.4. Combining with More Stable Metal Centers
6. Recommendations for Future Research
6.1. Date-Driven Catalyst Design
6.2. Balancing Performance and Stability/Durability
6.3. Optimizing Reactors for Efficient Mass Transfer
6.4. Integration with Other Technologies
6.5. Assessing the Risk of Degradation Intermediates
Author Contributions
Funding
Conflicts of Interest
References
- Lu, S.; Lin, C.; Lei, K.; Wang, B.; Xin, M.; Gu, X.; Cao, Y.; Liu, X.; Ouyang, W.; He, M. Occurrence, spatiotemporal variation, and ecological risk of antibiotics in the water of the semi-enclosed urbanized Jiaozhou Bay in eastern China. Water Res. 2020, 184, 116187. [Google Scholar] [CrossRef]
- Huang, S.; Tian, F.; Dai, J.; Tian, X.; Li, G.; Liu, Y.; Chen, Z.; Chen, R. Highly efficient degradation of chlorophenol over bismuth oxides upon near-infrared irradiation: Unraveling the effect of Bi-O-Bi-O defects cluster and 1O2 involved process. Appl. Catal. B Environ. 2021, 298, 120576. [Google Scholar] [CrossRef]
- Lu, S.; Lin, C.; Lei, K.; Xin, M.; Gu, X.; Lian, M.; Wang, B.; Liu, X.; Ouyang, W.; He, M. Profiling of the spatiotemporal distribution, risks, and prioritization of antibiotics in the waters of Laizhou Bay, northern China. J. Hazard. Mater. 2022, 424, 127487. [Google Scholar] [CrossRef]
- Zhi, H.; Shen, G.; Cheng, H.; Tao, S. Uncovering the Dominant Contribution of Untreated Domestic Wastewater to Antimicrobials in the Lower Reach of the Lhasa River on the Tibetan Plateau. ACS ES&T Water 2023, 3, 2307–2317. [Google Scholar] [CrossRef]
- Yang, L.; Jiao, Y.; Xu, X.; Pan, Y.; Su, C.; Duan, X.; Sun, H.; Liu, S.; Wang, S.; Shao, Z. Superstructures with Atomic-Level Arranged Perovskite and Oxide Layers for Advanced Oxidation with an Enhanced Non-Free Radical Pathway. ACS Sustain. Chem. Eng. 2022, 10, 1899–1909. [Google Scholar] [CrossRef]
- Zhi, Y.; Lu, X.; Munoz, G.; Yeung, L.W.Y.; De Silva, A.O.; Hao, S.; He, H.; Jia, Y.; Higgins, C.P.; Zhang, C. Environmental Occurrence and Biotic Concentrations of Ultrashort-Chain Perfluoroalkyl Acids: Overlooked Global Organofluorine Contaminants. Environ. Sci. Technol. 2024, 58, 21393–21410. [Google Scholar] [CrossRef]
- Triñanes, S.; Casais, M.C.; Mejuto, M.C.; Cela, R. Selective determination of COXIBs in environmental water samples by mixed-mode solid phase extraction and liquid chromatography quadrupole time-of-flight mass spectrometry. J. Chromatogr. A 2015, 1420, 35–45. [Google Scholar] [CrossRef]
- Nath, S. Electrochemical Wastewater Treatment Technologies Through Life Cycle Assessment: A Review. ChemBioEng Rev. 2024, 11, e202400016. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, D.; Zhang, M.; Ma, H.; Wang, G. Highly active and selective H2O2 electrosynthesis in O-rich ZIF-67 derived Co-N/O-C cathode for ofloxacin oxidation. Appl. Catal. B Environ. 2022, 324, 122252. [Google Scholar] [CrossRef]
- Zhou, X.; Yang, J.; Guo, J.; Xiong, W.; Leung, M.K.H. Advances and Prospects in Electrocatalytic Processes for Wastewater Treatment. Processes 2024, 12, 1615. [Google Scholar] [CrossRef]
- Moreira, F.C.; Boaventura, R.A.R.; Brillas, E.; Vilar, V.J.P. Electrochemical advanced oxidation processes: A review on their application to synthetic and real wastewaters. Appl. Catal. B Environ. 2017, 202, 217–261. [Google Scholar] [CrossRef]
- Panizza, M.; Cerisola, G. Direct And Mediated Anodic Oxidation of Organic Pollutants. Chem. Rev. 2009, 109, 6541–6569. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Xiao, S.; Li, N.; Chen, J.; Zhou, X.; Qian, Y.; Huang, C.-H.; Zhang, Y. Water decontamination via nonradical process by nanoconfined Fenton-like catalysts. Nat. Commun. 2023, 14, 2881. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Tsuchimochi, T.; Ina, T.; Kumabe, Y.; Muto, S.; Ohara, K.; Yamada, H.; Ten-No, S.L.; Tachikawa, T. Binary dopant segregation enables hematite-based heterostructures for highly efficient solar H2O2 synthesis. Nat. Commun. 2022, 13, 1499. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Yang, X.; Wang, Z.; Han, Y.; Guo, J.; Yin, R.; Niu, S.; Shan, D.; Ding, L.; Wang, J.; et al. A nature-inspired metal-free electrocatalyst towards efficient electron transfer and robust cascade oxygen reduction for wastewater treatment. Water Res. 2025, 282, 123747. [Google Scholar] [CrossRef]
- Qin, X.; Cao, P.; Quan, X.; Zhao, K.; Chen, S.; Yu, H.; Su, Y. Highly Efficient Hydroxyl Radicals Production Boosted by the Atomically Dispersed Fe and Co Sites for Heterogeneous Electro-Fenton Oxidation. Environ. Sci. Technol. 2023, 57, 2907–2917. [Google Scholar] [CrossRef]
- Yin, J.; Zhang, H.; Luo, M.; Zhao, J.; Zhou, X.; Wang, F.; Zhou, H.; Yuan, Y.; Liu, Y.; Shi, Y.; et al. Electro-Fenton process for wastewater treatment: From selective H2O2 generation to efficient •OH conversion. Chem. Eng. J. 2025, 507, 160709. [Google Scholar] [CrossRef]
- Liu, X.; Ma, H.; Zhang, M.; Che, P.; Luo, Y.; Zhang, S.; Xu, J. Catalytic Activation of Molecular Oxygen Toward Producing Hydroxyl Radicals Controllably for Highly Selective Oxidation of Hydroxyl Compounds under Mild Conditions. ACS Catal. 2023, 13, 11104–11116. [Google Scholar] [CrossRef]
- Xiao, F.; Wang, Z.; Fan, J.; Majima, T.; Zhao, H.; Zhao, G. Selective Electrocatalytic Reduction of Oxygen to Hydroxyl Radicals via 3-Electron Pathway with FeCo Alloy Encapsulated Carbon Aerogel for Fast and Complete Removing Pollutants. Angew. Chem. Int. Ed. Engl. 2021, 60, 10375–10383. [Google Scholar] [CrossRef]
- Wang, Z.; Hu, N.; Wang, L.; Zhao, H.; Zhao, G. In Situ Production of Hydroxyl Radicals via Three-Electron Oxygen Reduction: Opportunities for Water Treatment. Angew. Chem. Int. Ed. Engl. 2024, 63, e202407628. [Google Scholar] [CrossRef]
- Miao, J.; Jiang, Y.; Wang, X.; Li, X.; Zhu, Y.; Shao, Z.; Long, M. Correlating active sites and oxidative species in single-atom catalyzed Fenton-like reactions. Chem. Sci. 2024, 15, 11699–11718. [Google Scholar] [CrossRef]
- Rodríguez-Peña, M.; Pérez, J.A.B.; Llanos, J.; Sáez, C.; Rodrigo, M.A.; Barrera-Díaz, C.E. New insights about the electrochemical production of ozone. Curr. Opin. Electrochem. 2021, 27, 100697. [Google Scholar] [CrossRef]
- Liu, G.; Tian, Y.; Zou, H.; Ren, N.; You, S. Thermodynamic and Kinetic Investigation on Electrogeneration of Hydroxyl Radicals for Water Purification. ACS ES&T Eng. 2023, 3, 2161–2170. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, J. Electro-Fenton degradation of pefloxacin using MOFs derived Cu, N co-doped carbon as a nanocomposite catalyst. Environ. Pollut. 2024, 355, 124198. [Google Scholar] [CrossRef]
- Loeb, S.K.; Alvarez, P.J.J.; Brame, J.A.; Cates, E.L.; Choi, W.; Crittenden, J.; Dionysiou, D.D.; Li, Q.; Li-Puma, G.; Quan, X.; et al. The technology horizon for photocatalytic water treatment: Sunrise or sunset? Environ. Sci. Technol. 2019, 53, 2937–2947. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, B. Atomic regulations of single atom from metal-organic framework derived carbon for advanced water treatment. Nano Res. 2023, 16, 10326–10341. [Google Scholar] [CrossRef]
- Song, X.; Zhang, H.; Bian, Z.; Wang, H. In situ electrogeneration and activation of H2O2 by atomic Fe catalysts for the efficient removal of chloramphenicol. J. Hazard. Mater. 2021, 412, 125162. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.-Q.; Wu, B.-X.; Huang, X.-W.; Hu, Y.; Hou, D.; Ren, W.; Liu, L.-L.; Chen, Y.; Tian, L.; Zhang, L.-S.; et al. Highly selective in-situ generation of 1O2 via electronic structure regulation of single atom sites in electro-Fenton system. Appl. Catal. B Environ. 2025, 366, 125040. [Google Scholar] [CrossRef]
- Yang, Y.; Yan, Z.; Luo, X.; Cao, J.; Zheng, W.; Feng, C. Inhibition of inorganic chlorinated byproducts formation during electrooxidation treatment of saline phenolic wastewater via synergistic cathodic generation of H2O2. Chemosphere 2024, 367, 143542. [Google Scholar] [CrossRef]
- Peng, M.; He, J.; An, J.; Xie, T.; Zhao, T.; Li, G. Establishment of a reagent-free three-dimensional electro-Fenton system for high H2O2 production and efficient degradation of Roxarsone. Chem. Eng. J. 2023, 477, 146963. [Google Scholar] [CrossRef]
- Zhang, D.; Tang, Y.; Liu, H.; Wang, Z.; Liu, X.; Tang, H.; Zhang, H.; Wang, D.; Long, Y.; Liu, C. Electrocatalytic Deep Dehalogenation and Mineralization of Florfenicol: Synergy of Atomic Hydrogen Reduction and Hydroxyl Radical Oxidation over Bifunctional Cathode Catalyst. Environ. Sci. Technol. 2023, 57, 20315–20325. [Google Scholar] [CrossRef]
- Trench, A.B.; Moura, J.P.C.; Antonin, V.S.; Gentil, T.C.; Lanza, M.R.; Santos, M.C. Using a novel gas diffusion electrode based on Vulcan XC-72 carbon modified with Nb2O5 nanorods for enhancing H2O2 electrogeneration. J. Electroanal. Chem. 2023, 946, 117732. [Google Scholar] [CrossRef]
- Sun, L.; Sun, L.; Huo, L.; Zhao, H. Promotion of the Efficient Electrocatalytic Production of H2O2 by N,O-Co-Doped Porous Carbon. Nanomaterials 2023, 13, 1188. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; He, S.; Veder, J.; De Marco, R.; Yang, S.; Jiang, S.P. Atomically Dispersed Bimetallic FeNi Catalysts as Highly Efficient Bifunctional Catalysts for Reversible Oxygen Evolution and Oxygen Reduction Reactions. ChemElectroChem 2019, 6, 3478–3487. [Google Scholar] [CrossRef]
- Zhang, M.; Dai, Q.; Zheng, H.; Chen, M.; Dai, L. Novel MOF-Derived Co@N-C Bifunctional Catalysts for Highly Efficient Zn–Air Batteries and Water Splitting. Adv. Mater. 2018, 30, 1705431. [Google Scholar] [CrossRef]
- Tang, B.; Zhou, Y.; Ji, Q.; Zhuang, Z.; Zhang, L.; Wang, C.; Hu, H.; Wang, H.; Mei, B.; Song, F.; et al. A Janus dual-atom catalyst for electrocatalytic oxygen reduction and evolution. Nat. Synth. 2024, 3, 878–890. [Google Scholar] [CrossRef]
- Liu, H.; Jiang, L.; Liu, Y.; Lu, B.; Li, L.; Tang, Y.; Sun, Y.; Zhou, J. Construction of single-atomic Fe and Cu sites within nitrogen/sulfur co-doped carbon matrix for boosting the performance of zinc-air batteries. Appl. Catal. B Environ. 2024, 362, 124705. [Google Scholar] [CrossRef]
- El-Nagar, G.A.; Hassan, M.A.; Fetyan, A.; Kayarkatte, M.K.; Lauermann, I.; Roth, C. A promising N-doped carbon-metal oxide hybrid electrocatalyst derived from crustacean’s shells: Oxygen reduction and oxygen evolution. Appl. Catal. B Environ. 2017, 214, 137–147. [Google Scholar] [CrossRef]
- Li, X.; Liu, Y.; Wen, J.; Qing, B.; Yang, M.; Liu, B.; Chen, H.; Li, H. N,S-Codoped Carbon Nanostructures Encapsulated with FeNi Nanoparticles as a Bifunctional Electrocatalyst for Rechargeable Zn–Air Batteries. ACS Appl. Nano Mater. 2023, 6, 2719–2728. [Google Scholar] [CrossRef]
- Zhang, G.; Liu, X.; Wang, L.; Sun, F.; Yang, Y.; Tian, C.; Yu, P.; Pan, Q.; Fu, H. B,N-Doped Defective Carbon Entangled Fe3C Nanoparticles as the Superior Oxygen Reduction Electrocatalyst for Zn–Air Batteries. ACS Sustain. Chem. Eng. 2019, 7, 19104–19112. [Google Scholar] [CrossRef]
- Pang, Y.; Wang, K.; Xie, H.; Sun, Y.; Titirici, M.-M.; Chai, G.-L. Mesoporous Carbon Hollow Spheres as Efficient Electrocatalysts for Oxygen Reduction to Hydrogen Peroxide in Neutral Electrolytes. ACS Catal. 2020, 10, 7434–7442. [Google Scholar] [CrossRef]
- Hu, J.; Wang, S.; Yu, J.; Nie, W.; Sun, J.; Wang, S. Duet Fe3C and FeNx Sites for H2O2 Generation and Activation toward Enhanced Electro-Fenton Performance in Wastewater Treatment. Environ. Sci. Technol. 2021, 55, 1260–1269. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Liu, X.; Yao, H.; Wang, X.; Huang, M.; Jiang, H. Single-atom catalysis for oxygen reduction, what’s next? Next Mater. 2024, 6, 100464. [Google Scholar] [CrossRef]
- Cheng, Y.; Wang, H.; Qian, T.; Yan, C. Interfacial engineering of carbon-based materials for efficient electrocatalysis: Recent advances and future. EnergyChem 2022, 4, 100074. [Google Scholar] [CrossRef]
- Ma, D.; Lian, Q.; Zhang, Y.; Huang, Y.; Guan, X.; Liang, Q.; He, C.; Xia, D.; Liu, S.; Yu, J. Catalytic ozonation mechanism over M1-N3C1 active sites. Nat. Commun. 2023, 14, 7011. [Google Scholar] [CrossRef]
- Yin, S.; Solís, J.J.C.; Sandoval-Pauker, C.; Puerto-Diaz, D.; Villagrán, D. Advances in PFAS electrochemical reduction: Mechanisms, materials, and future perspectives. J. Hazard. Mater. 2025, 491, 137943. [Google Scholar] [CrossRef]
- Abdelghafar, F.; Xu, X.; Jiang, S.P.; Shao, Z. Designing single-atom catalysts toward improved alkaline hydrogen evolution reaction. Mater. Rep. Energy 2022, 2, 100144. [Google Scholar] [CrossRef]
- Hu, T.; Zhou, W.; Tang, W. Metal-organic framework-derived diatomic catalysts for environmental remediation: Synthesis, applications and improvement strategies. Coord. Chem. Rev. 2024, 526, 216357. [Google Scholar] [CrossRef]
- Li, J.; Tan, T.; Xie, Y.; Chu, J.; Li, L.; Ouyang, B.; Kan, E.; Zhang, W. Bimetal-MOF and bacterial cellulose-derived three-dimensional N-doped carbon sheets loaded Co/CoFe nanoparticles wrapped graphite carbon supported on porous carbon nanofibers: An efficient multifunctional electrocatalyst for Zn-air batteries and overall water splitting. J. Colloid Interface Sci. 2023, 640, 78–90. [Google Scholar] [CrossRef]
- Guo, J.; Song, G.; Zheng, Y.; Gu, J.; Li, S.; Zhou, M. Single iron atoms embedded in MOF-derived nitrogen-doped carbon as an efficient heterogeneous electro-Fenton catalyst for degradation of carbamazepine over a wide pH. Sep. Purif. Technol. 2022, 302, 122141. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, H.; Du, Y.; Tang, H.; Tang, Y.; Chen, Y.; Wang, Z.; Sun, X.; Liu, C. Highly efficient production of hydroxyl radicals from oxygen reduction over Ni-Fe dual atom electrocatalysts for removing emerging contaminants in wastewater. Chem. Eng. Sci. 2023, 278, 118914. [Google Scholar] [CrossRef]
- Jiang, X.; Zhou, B.; Yang, W.; Chen, J.; Miao, C.; Guo, Z.; Li, H.; Hou, Y.; Xu, X.; Zhu, L.; et al. Precise coordination of high-loading Fe single atoms with sulfur boosts selective generation of nonradicals. Proc. Natl. Acad. Sci. USA 2024, 121, e2309102121. [Google Scholar] [CrossRef] [PubMed]
- Deng, F.; Olvera-Vargas, H.; Zhou, M.; Qiu, S.; Sirés, I.; Brillas, E. Critical Review on the Mechanisms of Fe2+ Regeneration in the Electro-Fenton Process: Fundamentals and Boosting Strategies. Chem. Rev. 2023, 123, 4635–4662. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Wang, P.; Zheng, W.; Zhan, S.; Xia, Y.; Liu, Y.; Yang, W.; Li, Y. The strong metal–support interactions induced electrocatalytic three-electron oxygen reduction to hydroxyl radicals for water treatment. Proc. Natl. Acad. Sci. USA 2023, 120, e2307989120. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Cao, J.; Zhang, Y.; Liu, X.; Li, J.; Yang, B.; Lv, W.; Yang, Q.; Xing, M. Selective adsorption of high ionization potential value organic pollutants in wastewater. Proc. Natl. Acad. Sci. USA 2024, 121, e2403766121. [Google Scholar] [CrossRef]
- Wang, W.-L.; Gan, L.; Huang, Y.; Shuai, D.; Lee, M.-Y.; Wu, Q.-Y. Self-replenishing neutral Fenton-like treatment for emerging contaminants through single Fe atom electron configuration regulation. Water Res. 2025, 276, 123251. [Google Scholar] [CrossRef]
- Chu, Y.; Luo, E.; Wei, Y.; Zhu, S.; Wang, X.; Yang, L.; Gao, N.; Wang, Y.; Jiang, Z.; Liu, C.; et al. Dual single-atom catalyst design to build robust oxygen reduction electrode via free radical scavenging. Chem. Catal. 2023, 3, 100532. [Google Scholar] [CrossRef]
- Mou, H.; Yang, H.; Qu, S.; Yang, A.; Hu, X. Enhanced phthalate degradation in sewage plant by heterogeneous electro-fenton modified bimetallic single-atom catalyst. Process Saf. Environ. Prot. 2024, 194, 119–128. [Google Scholar] [CrossRef]
- Zhu, Y.; Chen, Z.; Wang, H.; Ma, A.; Li, J. Continuous flow-through electro-Fenton membrane reactor with Fe–N4-doped carbon membrane as cathode for efficient removal of dimethylacetamide. Sep. Purif. Technol. 2024, 354, 129290. [Google Scholar] [CrossRef]
- Zhao, K.; Quan, X.; Su, Y.; Qin, X.; Chen, S.; Yu, H. Enhanced Chlorinated Pollutant Degradation by the Synergistic Effect between Dechlorination and Hydroxyl Radical Oxidation on a Bimetallic Single-Atom Catalyst. Environ. Sci. Technol. 2021, 55, 14194–14203. [Google Scholar] [CrossRef]
- Ren, R.; Shang, X.; Song, Z.; Li, C.; Wang, Z.; Qi, F.; Ikhlaq, A.; Kumirska, J.; Siedlecka, E.M.; Ismailova, O. Active electronic structure derived by Fe-Cl-C coordination of single-atom cathode applied in antibiotics degradation by electro-Fenton: Enhanced transformation of oxygen to hydroxyl radicals via 3-electron pathway. Chem. Eng. J. 2023, 474, 145545. [Google Scholar] [CrossRef]
- Zhang, D.; Yin, K.; Tang, Y.; Wei, Y.; Tang, H.; Du, Y.; Liu, H.; Chen, Y.; Liu, C. Hollow sea-urchin-shaped carbon-anchored single-atom iron as dual-functional electro-Fenton catalysts for degrading refractory thiamphenicol with fast reaction kinetics in a wide pH range. Chem. Eng. J. 2022, 427, 130996. [Google Scholar] [CrossRef]
- Tan, Z.; Cao, P.; Chen, S.; Yu, H.; Su, Y.; Quan, X. Highly efficient electrocatalysis of oxygen to hydroxyl radical by FeN2O2 single-atom catalyst for refractory organic pollutant removal. Appl. Catal. B Environ. 2024, 355, 124170. [Google Scholar] [CrossRef]
- Zhao, L.; Murrieta, M.F.; Padilla, J.A.; Lanzalaco, S.; Cabot, P.L.; Sirés, I. Bimetallic FeCu-MOF derivatives as heterogeneous catalysts with enhanced stability for electro-Fenton degradation of lisinopril. Sci. Total Environ. 2024, 953, 176110. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, L.; Sun, W.; Yang, Z.; Jin, J.; Huang, Y.; Liu, G. Boron Bifunctional Catalysts for Rapid Degradation of Persistent Organic Pollutants in a Metal-Free Electro-Fenton Process: O2 and H2O2 Activation Process. Environ. Sci. Technol. 2023, 57, 15693–15702. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Fan, X.; Yu, Y.; Liu, Y.; Quan, X. Regulating the Electronic Structure of Cu Single-Atom Catalysts toward Enhanced Electro-Fenton Degradation of Organic Contaminants via 1O2 and •OH. Environ. Sci. Technol. 2024, 58, 19545–19554. [Google Scholar] [CrossRef]
- Liu, Y.; Cui, K.; Wang, L.; Yao, Y.; Mu, R. Efficient degradation of sulfamethoxazole by a three-dimensional electro-Fenton system based on B-Fe@BC particle catalysts: A new non-radical dominated mechanism. Sep. Purif. Technol. 2025, 371, 133269. [Google Scholar] [CrossRef]
- Shi, X.; Chen, S.; Zhao, K.; Wu, S.; Ye, F.; Yu, H.; Zhang, Y.; Chen, X.; Liang, Y.; Niu, J. Nanoconfinement-mediated non-radical enhanced pollutant degradation on Fe single-atom electrocatalyst. J. Hazard. Mater. 2025, 490, 137764. [Google Scholar] [CrossRef]
- Hu, T.; Deng, F.; Feng, H.; Zhang, J.; Shao, B.; Feng, C.; Tang, W.; Tang, L. Fe/Co bimetallic nanoparticles embedded in MOF-derived nitrogen-doped porous carbon rods as efficient heterogeneous electro-Fenton catalysts for degradation of organic pollutants. Appl. Mater. Today 2021, 24, 101161. [Google Scholar] [CrossRef]
- Hou, X.; Wang, H.; Jia, L.; Li, M.; Yu, W.; Bian, Z. Nitrogen-doped carbon bilayer flow-through electrocatalytic membrane based on transition metal single atoms: Simultaneous generation and activation of H2O2 for ibuprofen degradation. Chem. Eng. J. 2025, 513, 162950. [Google Scholar] [CrossRef]
- Feng, Z.; Chen, M.; Yang, Q.; Wang, Z.; Li, L.; Zhao, H.; Zhao, G. New Insights into Selective Singlet Oxygen Production via the Typical Electroactivation of Oxygen for Water Decontamination. Environ. Sci. Technol. 2023, 57, 17123–17131. [Google Scholar] [CrossRef]
- Wu, Q.; Yang, Y.-Y.; Dong, Z.-T.; Liu, H.-Y.; Feng, H.-P.; Guo, H.; Sui, L.; Tian, Y.; Yan, M.; Niu, C.-G. One-step phosphidation-prepared FeP@ECC achieves efficient H2O2 generation and activation in the electro-Fenton system for the degradation of sulfamethoxazole: Enhanced active sites and electron transfer efficiency. Chem. Eng. J. 2024, 500, 157126. [Google Scholar] [CrossRef]
- Huo, S.; Zhao, Q.; Song, L.; Su, R.; Wang, Y.; Wu, X.; Gao, M. Novel aeration-free electro-Fenton system based on N, P self-doped carbon cathode and oxygen defect-rich Mn1.1Fe1.9O4 particle electrodes for efficient H2O2 generation and tetracycline degradation. J. Clean. Prod. 2025, 507, 145544. [Google Scholar] [CrossRef]
- Lv, Z.; Miao, J.; Wei, X.; Zhou, X.; Zhang, N.; Xu, H.; Peng, S. Graphene oxide enhanced Iron-carbon aerogel electrodes for heterogeneous electro-Fenton oxidation of phenol. J. Electroanal. Chem. 2025, 985, 119084. [Google Scholar] [CrossRef]
- Chen, X.; Wang, Y.; Fan, X.; Zhu, G.; Liu, Y.; Quan, X. Efficient electro-Fenton degradation of organic pollutants via the synergistic effect of 1O2 and •OH generated on single Fe-N4 sites. Sci. Total Environ. 2024, 932, 173042. [Google Scholar] [CrossRef]
- Zhang, G.; Zhou, L.; Tan, X.; Fang, Y.; Du, C.; Bao, X.; Zeng, Y.; Ma, W.; Yan, Z. Endogenous iron-enriched biochar loaded nickel-foam cathode in electro-Fenton for ciprofloxacin degradation: Performance, mechanism and DFT calculation. Chem. Eng. J. 2024, 498, 155446. [Google Scholar] [CrossRef]
- Sun, Y.; Silvioli, L.; Sahraie, N.R.; Ju, W.; Li, J.; Zitolo, A.; Li, S.; Bagger, A.; Arnarson, L.; Wang, X.; et al. Activity–Selectivity Trends in the Electrochemical Production of Hydrogen Peroxide over Single-Site Metal–Nitrogen–Carbon Catalysts. J. Am. Chem. Soc. 2019, 141, 12372–12381. [Google Scholar] [CrossRef]
- Wu, Z.; Wang, T.; Zou, J.-J.; Li, Y.; Zhang, C. Amorphous Nickel Oxides Supported on Carbon Nanosheets as High-Performance Catalysts for Electrochemical Synthesis of Hydrogen Peroxide. ACS Catal. 2022, 12, 5911–5920. [Google Scholar] [CrossRef]
- Song, M.; Liu, W.; Zhang, J.; Zhang, C.; Huang, X.; Wang, D. Single-Atom Catalysts for H2O2 Electrosynthesis via Two-Electron Oxygen Reduction Reaction. Adv. Funct. Mater. 2023, 33, 2212087. [Google Scholar] [CrossRef]
- Jin, L.; Sun, M.; Yang, J.; Huang, Y.; Liu, Y. Janus photoelectrocatalytic filter for sustainable water decontamination. Appl. Catal. B Environ. 2023, 339, 123150. [Google Scholar] [CrossRef]
- Wang, B.; Yang, X.; Xie, C.; Liu, H.; Ma, C.; Zhang, Z.; Zhuang, Z.; Han, A.; Zhuang, Z.; Li, L.; et al. A General Metal Ion Recognition Strategy to Mediate Dual-Atomic-Site Catalysts. J. Am. Chem. Soc. 2024, 146, 24945–24955. [Google Scholar] [CrossRef]
- Guo, X.; Lin, S.; Gu, J.; Zhang, S.; Chen, Z.; Huang, S. Simultaneously Achieving High Activity and Selectivity toward Two-Electron O2 Electroreduction: The Power of Single-Atom Catalysts. ACS Catal. 2019, 9, 11042–11054. [Google Scholar] [CrossRef]
- Jung, E.; Shin, H.; Lee, B.-H.; Efremov, V.; Lee, S.; Lee, H.S.; Kim, J.; Antink, W.H.; Park, S.; Lee, K.-S.; et al. Atomic-level tuning of Co–N–C catalyst for high-performance electrochemical H2O2 production. Nat. Mater. 2020, 19, 436–442. [Google Scholar] [CrossRef]
- Liu, K.; Li, F.; Zhan, H.; Zhan, S. Recent progress in two-dimensional materials for generation of hydrogen peroxide by two-electron oxygen reduction reaction. Mater. Today Energy 2024, 40, 101500. [Google Scholar] [CrossRef]
- Liu, L.; Kang, L.; Feng, J.; Hopkinson, D.G.; Allen, C.S.; Tan, Y.; Gu, H.; Mikulska, I.; Celorrio, V.; Gianolio, D.; et al. Atomically dispersed asymmetric cobalt electrocatalyst for efficient hydrogen peroxide production in neutral media. Nat. Commun. 2024, 15, 4079. [Google Scholar] [CrossRef]
- Wan, C.; Duan, X.; Huang, Y. Molecular Design of Single-Atom Catalysts for Oxygen Reduction Reaction. Adv. Energy Mater. 2020, 10, 1903815. [Google Scholar] [CrossRef]
- Liu, M.; Zhao, Z.; Duan, X.; Huang, Y. Nanoscale Structure Design for High-Performance Pt-Based ORR Catalysts. Adv. Mater. 2018, 31, e1802234. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Zheng, Y.; Jaroniec, M.; Qiao, S.-Z. Determination of the Electron Transfer Number for the Oxygen Reduction Reaction: From Theory to Experiment. ACS Catal. 2016, 6, 4720–4728. [Google Scholar] [CrossRef]
- Shinagawa, T.; Garcia-Esparza, A.T.; Takanabe, K. Insight on Tafel slopes from a microkinetic analysis of aqueous electrocatalysis for energy conversion. Sci. Rep. 2015, 5, 13801. [Google Scholar] [CrossRef]
- Wei, X.; Song, S.; Cai, W.; Luo, X.; Jiao, L.; Fang, Q.; Wang, X.; Wu, N.; Luo, Z.; Wang, H.; et al. Tuning the spin state of Fe single atoms by Pd nanoclusters enables robust oxygen reduction with dissociative pathway. Chem 2022, 9, 181–197. [Google Scholar] [CrossRef]
- Malko, D.; Kucernak, A.; Lopes, T. In situ electrochemical quantification of active sites in Fe–N/C non-precious metal catalysts. Nat. Commun. 2016, 7, 13285. [Google Scholar] [CrossRef]
- Xia, C.; Kim, J.Y.; Wang, H. Recommended practice to report selectivity in electrochemical synthesis of H2O2. Nat. Catal. 2020, 3, 605–607. [Google Scholar] [CrossRef]
- Hu, H.; Wang, J.; Tao, P.; Song, C.; Shang, W.; Deng, T.; Wu, J. Stability of single-atom catalysts for electrocatalysis. J. Mater. Chem. A 2021, 10, 5835–5849. [Google Scholar] [CrossRef]
- Tauster, S.J.; Fung, S.C.; Garten, R.L. Strong metal-support interactions. Group 8 noble metals supported on titanium dioxide. J. Am. Chem. Soc. 1978, 100, 170–175. [Google Scholar] [CrossRef]
- Li, J.; Guan, Q.; Wu, H.; Liu, W.; Lin, Y.; Sun, Z.; Ye, X.; Zheng, X.; Pan, H.; Zhu, J.; et al. Highly Active and Stable Metal Single-Atom Catalysts Achieved by Strong Electronic Metal–Support Interactions. J. Am. Chem. Soc. 2019, 141, 14515–14519. [Google Scholar] [CrossRef]
- Tang, C.; Jin, R.; Xia, Y.; Zhang, Q.; Wang, J.; Wu, M.; Cui, X.; Yang, Y. Asymmetric low-coordination tailoring of single-atom cobalt catalysts enabling efficient oxygen reduction reaction. Nano Energy 2025, 137, 110776. [Google Scholar] [CrossRef]
- Liu, M.; Dong, H.; Wang, G.; Zhao, J. Enhancing Zinc–Air Flow Batteries: Single-Atom Catalysis within Cobalt-Encapsulated Carbon Nanotubes for Superior Efficiency. Nano Lett. 2024, 24, 12102–12110. [Google Scholar] [CrossRef]
- Li, J.; Luo, L.; Wang, S.; Song, H.; Jiang, B. Recent advances in Joule-heating synthesis of functional nanomaterials for photo and electrocatalysis. PhotoMat 2024. [Google Scholar] [CrossRef]
- Lu, T.; Liu, Y.; Li, Y.; Chen, J.; Chen, S.; Liao, X.; Xia, W.; Zhou, T.; Wang, W.; Chen, Z.; et al. Identifying catalytic sites in oxygen-modified cobalt single atoms for H2O2 electrosynthesis and in situ realizing electro-Fenton process in acid. Appl. Catal. B Environ. 2024, 365, 124949. [Google Scholar] [CrossRef]
- Rao, P.; Han, X.; Sun, H.; Wang, F.; Liang, Y.; Li, J.; Wu, D.; Shi, X.; Kang, Z.; Miao, Z.; et al. Precise Synthesis of Dual-Single-Atom Electrocatalysts through Pre-Coordination-Directed in Situ Confinement for CO2 Reduction. Angew. Chem. Int. Ed. Engl. 2024, 64, e202415223. [Google Scholar] [CrossRef]
- Yang, L.; Cheng, H.; Li, H.; Sun, G.; Liu, S.; Ma, T.; Zhang, L. Atomic Confinement Empowered CoZn Dual-Single-Atom Nanotubes for H2O2 Production in Sequential Dual-Cathode Electro-Fenton Process. Adv. Mater. 2024, 36, e2406957. [Google Scholar] [CrossRef]
- Liu, L.; Shi, J.; Li, W.; Zheng, K.; Yuan, H.; Liang, F.; Liu, R.; Yang, Y.; Yang, F.; Cheng, S.; et al. Rapid preparation of graphene-skinned glass fiber fabric based on propane as carbon source. Nano Res. 2025, 18, 94907217. [Google Scholar] [CrossRef]
- Yao, Y.; Huang, Z.; Xie, P.; Wu, L.; Ma, L.; Li, T.; Pang, Z.; Jiao, M.; Liang, Z.; Gao, J.; et al. High temperature shockwave stabilized single atoms. Nat. Nanotechnol. 2019, 14, 851–857. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Chai, S.; Zhu, Y.; Yao, X.; Yang, L.; Du, C.; Ma, X.; Cao, C.; Zou, M. Pre-coordination anchoring strategy for modulating the coordination structure of iron single atoms and ORR performance. Chem. Eng. J. 2024, 502, 157943. [Google Scholar] [CrossRef]
- Zhang, L.; Zong, L.; Lu, F.; Wang, C.; Liu, Z.; Qi, J.; Wang, L. Metal-saloph complexes pre-coordination for Fe single atom catalyst towards oxygen reduction reaction in rechargeable quasi-solid-state Zn-air battery. Appl. Catal. B Environ. 2025, 370, 125189. [Google Scholar] [CrossRef]
- Xu, Q.; Hu, J.; Zhou, C.; Zhang, L.; Kong, Q.; Gao, S.; Hu, G. High pyridine nitrogen regulates the d-band center in single atom iron electrocatalyst for promoted oxygen reduction. Chem. Eng. J. 2024, 499, 156425. [Google Scholar] [CrossRef]
- Cao, F.; Zhao, Q.; Tan, X.; Xu, Q.; Wang, L.; Zhu, B.; Yan, Y.; Kong, D.; Zhi, L.; Wu, M. A Pre-Coordinated Strategy Precisely Tailors the Coordination Structure of Single-Atom Sites Toward Efficient Catalysis. Adv. Funct. Mater. 2025, 35, 2423398. [Google Scholar] [CrossRef]
- Zhao, T.; Huang, X.; Cui, R.; Han, W.; Zhang, G.; Tang, Z. Design of confined catalysts and applications in environmental catalysis: Original perspectives and further prospects. J. Clean. Prod. 2023, 390, 136125. [Google Scholar] [CrossRef]
- Yao, Y.; Xu, Z.; Cheng, F.; Li, W.; Cui, P.; Xu, G.; Xu, S.; Wang, P.; Sheng, G.; Yan, Y.; et al. Unlocking the potential of graphene for water oxidation using an orbital hybridization strategy. Energy Environ. Sci. 2018, 11, 407–416. [Google Scholar] [CrossRef]
- Wang, K.; Wang, X.; Liang, X. Synthesis of High Metal Loading Single Atom Catalysts and Exploration of the Active Center Structure. ChemCatChem 2020, 13, 28–58. [Google Scholar] [CrossRef]
- Lu, G.; Li, S.; Guo, Z.; Farha, O.K.; Hauser, B.G.; Qi, X.; Wang, Y.; Wang, X.; Han, S.; Liu, X.; et al. Imparting functionality to a metal–organic framework material by controlled nanoparticle encapsulation. Nat. Chem. 2012, 4, 310–316. [Google Scholar] [CrossRef]
- Zhanga, S.; Gao, H.; Xu, X.; Cao, R.; Yang, H.; Xu, X.; Li, J. MOF-derived CoN/N-C@SiO2 yolk-shell nanoreactor with dual active sites for highly efficient catalytic advanced oxidation processes. Chem. Eng. J. 2020, 381, 122670. [Google Scholar] [CrossRef]
- Muñoz, R.; Gómez-Aleixandre, C. Review of CVD Synthesis of Graphene. Chem. Vap. Depos. 2013, 19, 297–322. [Google Scholar] [CrossRef]
- Wang, M.; Kim, Y.C.; Meng, Y.; Chatterjee, S.; Bakharev, P.; Luo, D.; Gong, Y.; Abadie, T.; Kim, M.H.; Sitek, J.; et al. Growth Kinetics of Graphene on Cu(111) Foils from Methane, Ethyne, Ethylene, and Ethane. Angew. Chem. Int. Ed. Engl. 2024, 63, e202412131. [Google Scholar] [CrossRef] [PubMed]
- Yamane, Y.; Miyahara, M.T.; Tanaka, H. High-Performance Carbon Molecular Sieves for the Separation of Propylene and Propane. ACS Appl. Mater. Interfaces 2022, 14, 17878–17888. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Liu, H.; Sun, X.; Shi, J.; Jing, Z.; Sun, X.; Song, Y.; Yin, W.; Zhang, G.; Sun, L.; et al. Theoretical investigations on hydroxyl carbon precursor fueled growth of graphene on transition metal substrates. Nano Res. 2024, 17, 10235–10241. [Google Scholar] [CrossRef]
- Sun, Z.; Yan, Z.; Yao, J.; Beitler, E.; Zhu, Y.; Tour, J.M. Growth of graphene from solid carbon sources. Nature 2010, 468, 549–552. [Google Scholar] [CrossRef]
- Chen, K.; Shi, L.; Zhang, Y.; Liu, Z. Scalable chemical-vapour-deposition growth of three-dimensional graphene materials towards energy-related applications. Chem. Soc. Rev. 2018, 47, 3018–3036. [Google Scholar] [CrossRef]
- Shen, M.; Qi, J.; Gao, K.; Duan, C.; Liu, J.; Liu, Q.; Yang, H.; Ni, Y. Chemical vapor deposition strategy for inserting atomic FeN4 sites into 3D porous honeycomb carbon aerogels as oxygen reduction reaction catalysts in high-performance Zn-air batteries. Chem. Eng. J. 2023, 464, 142719. [Google Scholar] [CrossRef]
- Hu, H.; Zhang, C.; Liu, W.; Qi, H.; Wang, H.; Wang, X.; Zhang, L.; Liu, L.; Bao, L.; Alomar, M.; et al. Coaxial Metal-Nitrogen–Carbon Single-Atom Catalysts Boost Acid Hydrogen Peroxide Production. Adv. Funct. Mater. 2024, 35, 2419220. [Google Scholar] [CrossRef]
- Dong, Q.; Yao, Y.; Cheng, S.; Alexopoulos, K.; Gao, J.; Srinivas, S.; Wang, Y.; Pei, Y.; Zheng, C.; Brozena, A.H.; et al. Programmable heating and quenching for efficient thermochemical synthesis. Nature 2022, 605, 470–476. [Google Scholar] [CrossRef] [PubMed]
- Xing, L.; Liu, R.; Gong, Z.; Liu, J.; Liu, J.; Gong, H.; Huang, K.; Fei, H. Ultrafast Joule heating synthesis of hierarchically porous graphene-based Co-N-C single-atom monoliths. Nano Res. 2021, 15, 3913–3919. [Google Scholar] [CrossRef]
- Wang, H.; Man, S.; Wang, H.; Presser, V.; Yan, Q.; Zhang, Y. Grave-to-cradle upcycling of harmful algal biomass into atomically dispersed iron catalyst for efficient ammonia electrosynthesis from nitrate. Appl. Catal. B Environ. 2023, 332, 122778. [Google Scholar] [CrossRef]
- Li, X.; Hu, K.; Huang, Y.; Gu, Q.; Chen, Y.; Yang, B.; Qiu, R.; Luo, W.; Weckhuysen, B.M.; Yan, K. Upcycling biomass waste into Fe single atom catalysts for pollutant control. J. Energy Chem. 2022, 69, 282–291. [Google Scholar] [CrossRef]
- Ma, L.; Hu, X.; Min, Y.; Zhang, X.; Liu, W.; Lam, P.K.S.; Li, M.M.J.; Zeng, R.J.; Ye, R. Microalgae-derived single-atom oxygen reduction catalysts for zinc-air batteries. Carbon 2022, 203, 827–834. [Google Scholar] [CrossRef]
- Zhong, S.; Zhu, Z.-S.; Zhou, H.; Wang, S.; Duan, X. Perspectives of Wood-Inspired Electro-Fenton Catalysis for Energy-Efficient Wastewater Decontamination. Energy Fuels 2023, 37, 17932–17950. [Google Scholar] [CrossRef]
- Wang, X.; Du, J.; Zhang, Q.; Gu, L.; Cao, L.; Liang, H.-P. In situ synthesis of sustainable highly efficient single iron atoms anchored on nitrogen doped carbon derived from renewable biomass. Carbon 2020, 157, 614–621. [Google Scholar] [CrossRef]
- Peng, L.; Duan, X.; Shang, Y.; Gao, B.; Xu, X. Engineered carbon supported single iron atom sites and iron clusters from Fe-rich Enteromorpha for Fenton-like reactions via nonradical pathways. Appl. Catal. B Environ. 2021, 287, 119963. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, J.; Wang, S.; Yuan, Y.; Yu, W.; Fang, S.; Kong, F.; Qiu, J.; Wang, S.; Zhang, J. Coordination dependent photocatalytic peroxymonosulfate activation on biomass derived Fe single atom catalysts for atrazine degradation. Chem. Eng. J. 2024, 499, 156625. [Google Scholar] [CrossRef]
- Li, J.; Le, Q.; Nan, Z. Improvement Catalytic Efficiency of the Fenton-Like Reaction via the Interaction among Fe Species Encapsulated in N-Doped Carbon Materials. Langmuir 2025, 41, 7684–7696. [Google Scholar] [CrossRef]
- Tang, C.; Chen, L.; Li, H.; Li, L.; Jiao, Y.; Zheng, Y.; Xu, H.; Davey, K.; Qiao, S.-Z. Tailoring Acidic Oxygen Reduction Selectivity on Single-Atom Catalysts via Modification of First and Second Coordination Spheres. J. Am. Chem. Soc. 2021, 143, 7819–7827. [Google Scholar] [CrossRef]
- Ding, S.; Barr, J.A.; Lyu, Z.; Zhang, F.; Wang, M.; Tieu, P.; Li, X.; Engelhard, M.H.; Feng, Z.; Beckman, S.P.; et al. Effect of Phosphorus Modulation in Iron Single-Atom Catalysts for Peroxidase Mimicking. Adv. Mater. 2023, 36, e2209633. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Wu, Y.; Gu, W.; Zhu, C. Atomically Dispersed Metal Interfaces for Analytical Chemistry. Accounts Chem. Res. 2025, 58, 1366–1378. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Fu, N.; Yao, S.; Li, Z.; Li, Y. The Progress and Outlook of Metal Single-Atom-Site Catalysis. J. Am. Chem. Soc. 2022, 144, 18155–18174. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Zhu, Y.; Yao, X.; Yang, L.; Du, C.; Lv, Z.; Hou, M.; Zhang, S.; Ma, X.; Cao, C. Chemical vapor deposition towards atomically dispersed iron catalysts for efficient oxygen reduction. J. Mater. Chem. A 2023, 11, 5288–5295. [Google Scholar] [CrossRef]
- Hai, X.; Xi, S.; Mitchell, S.; Harrath, K.; Xu, H.; Akl, D.F.; Kong, D.; Li, J.; Li, Z.; Sun, T.; et al. Scalable two-step annealing method for preparing ultra-high-density single-atom catalyst libraries. Nat. Nanotechnol. 2021, 17, 174–181. [Google Scholar] [CrossRef]
- Jiang, Y.; Chen, Z.; Peng, T.; Jiao, L.; Pan, X.; Jiang, H.; Bao, X. Single-Atom Fe Catalysts With Improved Metal Loading for Efficient Ammonia Synthesis Under Mild Conditions. Angew. Chem. Int. Ed. Engl. 2025, 64, e202501190. [Google Scholar] [CrossRef]
- Wang, Y.; Li, C.; Han, X.; Bai, J.; Wang, X.; Zheng, L.; Hong, C.; Li, Z.; Bai, J.; Leng, K.; et al. General negative pressure annealing approach for creating ultra-high-loading single atom catalyst libraries. Nat. Commun. 2024, 15, 5675. [Google Scholar] [CrossRef]
- Li, D.; Zhang, F.; Luo, L.; Shang, Y.; Yang, S.; Wang, J.; Chen, W.; Liu, Z. A universal strategy for green synthesis of biomass-based transition metal single-atom catalysts by simple hydrothermal and compression treatment. Chem. Eng. J. 2023, 461, 142104. [Google Scholar] [CrossRef]
- Yang, S.; Zhi, L.; Tang, K.; Feng, X.; Maier, J.; Müllen, K. Efficient Synthesis of Heteroatom (N or S)-Doped Graphene Based on Ultrathin Graphene Oxide-Porous Silica Sheets for Oxygen Reduction Reactions. Adv. Funct. Mater. 2012, 22, 3634–3640. [Google Scholar] [CrossRef]
- Chen, Y.; Ji, S.; Wang, Y.; Dong, J.; Chen, W.; Li, Z.; Shen, R.; Zheng, L.; Zhuang, Z.; Wang, D.; et al. Isolated Single Iron Atoms Anchored on N-Doped Porous Carbon as an Efficient Electrocatalyst for the Oxygen Reduction Reaction. Angew. Chem. Int. Ed. Engl. 2017, 56, 6937–6941. [Google Scholar] [CrossRef]
- Jiang, K.; Back, S.; Akey, A.J.; Xia, C.; Hu, Y.; Liang, W.; Schaak, D.; Stavitski, E.; Nørskov, J.K.; Siahrostami, S.; et al. Highly selective oxygen reduction to hydrogen peroxide on transition metal single atom coordination. Nat. Commun. 2019, 10, 3997. [Google Scholar] [CrossRef]
- He, T.; Yu, B.; Zhang, Y.; Ouyang, X.; Chen, S. Rational design of carbon-supported single and dual atom catalysts for bifunctional oxygen electrocatalysis. Curr. Opin. Electrochem. 2022, 37, 101197. [Google Scholar] [CrossRef]
- Bhoyate, S.D.; Kim, J.; de Souza, F.M.; Lin, J.; Lee, E.; Kumar, A.; Gupta, R.K. Science and engineering for non-noble-metal-based electrocatalysts to boost their ORR performance: A critical review. Co-ord. Chem. Rev. 2022, 474, 214854. [Google Scholar] [CrossRef]
- Bin Yang, H.; Miao, J.; Hung, S.-F.; Chen, J.; Tao, H.B.; Wang, X.; Zhang, L.; Chen, R.; Gao, J.; Chen, H.M.; et al. Identification of catalytic sites for oxygen reduction and oxygen evolution in N-doped graphene materials: Development of highly efficient metal-free bifunctional electrocatalyst. Sci. Adv. 2016, 2, e1501122. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Xue, Y.; Xu, X.; Hu, W.; Wu, Y.; Shen, M.; Liu, X. Electrocatalytic Oxygen Self-Sufficiency System Enables Singlet Oxygen Production for Water Decontamination. ACS ES&T Water 2024, 4, 5880–5889. [Google Scholar] [CrossRef]
- Yu, D.; Zhang, Q.; Dai, L. Highly Efficient Metal-Free Growth of Nitrogen-Doped Single-Walled Carbon Nanotubes on Plasma-Etched Substrates for Oxygen Reduction. J. Am. Chem. Soc. 2010, 132, 15127–15129. [Google Scholar] [CrossRef]
- Yang, W.; Zhou, M.; Mai, L.; Ou, H.; Oturan, N.; Oturan, M.A.; Zeng, E.Y. Generation of hydroxyl radicals by metal-free bifunctional electrocatalysts for enhanced organics removal. Sci. Total Environ. 2021, 791, 148107. [Google Scholar] [CrossRef]
- Xu, J.; Meng, Y.; Qiu, X.; Zhong, H.; Liu, S.; Zhang, L.; Zhang, J.; Hou, P.; Beckman, S.P.; Wu, F.; et al. Honeycomb-like single-atom catalysts with FeN3Cl sites for high-performance oxygen reduction. Adv. Powder Mater. 2025, 4, 100298. [Google Scholar] [CrossRef]
- Mo, F.; Zhou, Q.; Li, C.; Tao, Z.; Hou, Z.; Zheng, T.; Wang, Q.; Ouyang, S.; Zhan, S. Diatomic catalysts for Fenton and Fenton-like reactions: A promising platform for designing/regulating reaction pathways. Chem. Sci. 2023, 14, 7818–7827. [Google Scholar] [CrossRef]
- Zhao, Z.; Hu, M.; Nie, T.; Zhou, W.; Pan, B.; Xing, B.; Zhu, L. Improved Electronic Structure from Spin-State Reconstruction of a Heteronuclear Fe–Co Diatomic Pair to Boost the Fenton-like Reaction. Environ. Sci. Technol. 2023, 57, 4556–4567. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Gao, J.; Hu, Y.; Jin, Z.; Hu, K.; Reddy, K.M.; Yuan, Q.; Lin, X.; Qiu, H.-J. Theoretically Revealed and Experimentally Demonstrated Synergistic Electronic Interaction of CoFe Dual-Metal Sites on N-doped Carbon for Boosting Both Oxygen Reduction and Evolution Reactions. Nano Lett. 2022, 22, 3392–3399. [Google Scholar] [CrossRef]
- Cao, P.; Quan, X.; Zhao, K.; Chen, S.; Yu, H.; Su, Y. High-Efficiency Electrocatalysis of Molecular Oxygen toward Hydroxyl Radicals Enabled by an Atomically Dispersed Iron Catalyst. Environ. Sci. Technol. 2020, 54, 12662–12672. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Zhao, X.; Guo, N.; Lyu, Z.; Du, Y.; Xi, S.; Guo, R.; Chen, C.; Chen, Z.; Liu, W.; et al. Atomic engineering of high-density isolated Co atoms on graphene with proximal-atom controlled reaction selectivity. Nat. Commun. 2018, 9, 3197. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Ai, J.; Zhang, H. The mechanism of degradation of bisphenol A using the magnetically separable CuFe2O4/peroxymonosulfate heterogeneous oxidation process. J. Hazard. Mater. 2016, 309, 87–96. [Google Scholar] [CrossRef]
- Lei, M.; Tong, J.; Wang, S.; Zhang, Z.; Chen, J.; Li, L.; Li, J.; Wu, D.; Ma, Z. Synergistic reduction of iron single-atom and clusters enhances chloramphenicol degradation: Implications of surface functional groups absorbing reactive hydrogen. J. Hazard. Mater. 2025, 489, 137604. [Google Scholar] [CrossRef]
- Wei, R.; Pei, S.; Yu, Y.; Zhang, J.; Liu, Y.; You, S. Water Flow-Driven Coupling Process of Anodic Oxygen Evolution and Cathodic Oxygen Activation for Water Decontamination and Prevention of Chlorinated Byproducts. Environ. Sci. Technol. 2023, 57, 17404–17414. [Google Scholar] [CrossRef]
- Puyang, C.; Han, J.; Guo, H. Degradation of emerging contaminants in water by a novel non-thermal plasma/periodate advanced oxidation process: Performance and mechanisms. Chem. Eng. J. 2024, 483, 149194. [Google Scholar] [CrossRef]
- Yang, Y.; Pignatello, J.J.; Ma, J.; Mitch, W.A. Comparison of Halide Impacts on the Efficiency of Contaminant Degradation by Sulfate and Hydroxyl Radical-Based Advanced Oxidation Processes (AOPs). Environ. Sci. Technol. 2014, 48, 2344–2351. [Google Scholar] [CrossRef]
- Fang, J.; Fu, Y.; Shang, C. The Roles of Reactive Species in Micropollutant Degradation in the UV/Free Chlorine System. Environ. Sci. Technol. 2014, 48, 1859–1868. [Google Scholar] [CrossRef]
- Yang, X.; Rosario-Ortiz, F.L.; Lei, Y.; Pan, Y.; Lei, X.; Westerhoff, P. Multiple Roles of Dissolved Organic Matter in Advanced Oxidation Processes. Environ. Sci. Technol. 2022, 56, 11111–11131. [Google Scholar] [CrossRef]
- Jin, Q.; Liu, W.; Dong, Y.; Lu, Y.; Yang, C.; Lin, H. Single atom catalysts for degradation of antibiotics from aqueous environments by advanced oxidation processes: A review. J. Clean. Prod. 2023, 423, 138688. [Google Scholar] [CrossRef]
- Jiang, W.-L.; Xia, X.; Han, J.-L.; Ding, Y.-C.; Haider, M.R.; Wang, A.-J. Graphene Modified Electro-Fenton Catalytic Membrane for in Situ Degradation of Antibiotic Florfenicol. Environ. Sci. Technol. 2018, 52, 9972–9982. [Google Scholar] [CrossRef]
- Zeng, H.; Zhang, G.; Ji, Q.; Liu, H.; Hua, X.; Xia, H.; Sillanpää, M.; Qu, J. pH-Independent Production of Hydroxyl Radical from Atomic H*-Mediated Electrocatalytic H2O2 Reduction: A Green Fenton Process without Byproducts. Environ. Sci. Technol. 2020, 54, 14725–14731. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhong, G.; Yu, H.; Wang, H.; Peng, F. O2 and H2O2 transformation steps for the oxygen reduction reaction catalyzed by graphitic nitrogen-doped carbon nanotubes in acidic electrolyte from first principles calculations. Phys. Chem. Chem. Phys. 2015, 17, 21950–21959. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Lin, S.; Ma, X.; Xu, Z.; Yang, H.; Wang, M.; Piao, J.; Zhang, J.; Dong, P.; Zhao, C. Enhanced mass transfer by 3D printing to promote the degradation of organic pollutants in electro-Fenton system. Chem. Eng. J. 2024, 495, 153720. [Google Scholar] [CrossRef]
- Jia, X.; Wang, C.; Zhao, X.; Li, Y.; He, H.; Wu, J.; Yang, Y. Levofloxacin degradation in electro-Fenton system with FeMn@GF composite electrode. J. Environ. Chem. Eng. 2024, 12, 114625. [Google Scholar] [CrossRef]
- Dong, P.; Ma, X.; Li, M.; Xu, Z.; Zhang, J.; Ge, B.; Wang, Y.; Zhao, C.; Sun, H. Efficient electro-Fenton processes on a novel self-supported single-atom Fe electrode: Mechanism and practical application. Sep. Purif. Technol. 2023, 325, 124599. [Google Scholar] [CrossRef]
- Du, L.; Prabhakaran, V.; Xie, X.; Park, S.; Wang, Y.; Shao, Y. Low-PGM and PGM-Free Catalysts for Proton Exchange Membrane Fuel Cells: Stability Challenges and Material Solutions. Adv. Mater. 2020, 33, 1908232. [Google Scholar] [CrossRef]
- Liu, J.; Yang, J.; Dou, Y.; Liu, X.; Chen, S.; Wang, D. Deactivation Mechanism and Mitigation Strategies of Single-Atom Site Electrocatalysts. Adv. Mater. 2025, 37, e2420383. [Google Scholar] [CrossRef]
- Sun, M.; Gong, S.; Zhang, Y.-X.; Niu, Z. A perspective on the PGM-free metal–nitrogen–carbon catalysts for PEMFC. J. Energy Chem. 2022, 67, 250–254. [Google Scholar] [CrossRef]
- Liu, P.; Zhao, Y.; Qin, R.; Mo, S.; Chen, G.; Gu, L.; Chevrier, D.M.; Zhang, P.; Guo, Q.; Zang, D.; et al. Photochemical route for synthesizing atomically dispersed palladium catalysts. Science 2016, 352, 797–800. [Google Scholar] [CrossRef]
- Zhu, C.; Cun, F.; Fan, Z.; Nie, Y.; Du, Q.; Liu, F.; Yang, W.; Li, A. Heterogeneous Fe-Co dual-atom catalyst outdistances the homogeneous counterpart for peroxymonosulfate-assisted water decontamination: New surface collision oxidation path and diatomic synergy. Water Res. 2023, 241, 120164. [Google Scholar] [CrossRef]
- Liu, Z.; Du, Y.; Zhang, P.; Zhuang, Z.; Wang, D. Bringing catalytic order out of chaos with nitrogen-doped ordered mesoporous carbon. Matter 2021, 4, 3161–3194. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, M.; Ma, W.; Ma, T.; Wang, S.; Duan, X. Nanoconfinement in ordered mesopores materials for catalytic wastewater purification. Chem. Eng. J. 2024, 504, 156407. [Google Scholar] [CrossRef]
- Xu, Z.; Ma, X.; He, F.; Lu, M.; Zhang, J.; Wang, S.; Dong, P.; Zhao, C. In situ generated iron oxide nanosheet on iron foam electrode for enhanced electro-Fenton performance toward pharmaceutical wastewater treatment. J. Hazard. Mater. 2023, 465, 133193. [Google Scholar] [CrossRef]
- Qi, H.; Sun, X.; Sun, Z. Porous graphite felt electrode with catalytic defects for enhanced degradation of pollutants by electro-Fenton process. Chem. Eng. J. 2021, 403, 126270. [Google Scholar] [CrossRef]
- Giulimondi, V.; Mitchell, S.; Pérez-Ramírez, J. Challenges and Opportunities in Engineering the Electronic Structure of Single-Atom Catalysts. ACS Catal. 2023, 13, 2981–2997. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, L.; Zheng, Y.; Liu, Z.; Wang, L.; Yang, R.; Liu, Z.; Wang, Y.; Chen, Z.; Yang, C. Enhanced catalytic performance through a single-atom preparation approach: A review on ruthenium-based catalysts. Nanoscale 2024, 16, 16744–16768. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Liu, H.; Zou, P.; Zhang, R.; Wang, C.; Xin, H.L. Altering Ligand Fields in Single-Atom Sites through Second-Shell Anion Modulation Boosts the Oxygen Reduction Reaction. J. Am. Chem. Soc. 2022, 144, 2197–2207. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.H.; Lim, H.-K.; Chung, M.W.; Chon, G.; Sahraie, N.R.; Altin, A.; Sougrati, M.-T.; Stievano, L.; Oh, H.S.; Park, E.S.; et al. The Achilles’ heel of iron-based catalysts during oxygen reduction in an acidic medium. Energy Environ. Sci. 2018, 11, 3176–3182. [Google Scholar] [CrossRef]
- Yang, Q.; Huang, H.; Li, K.; Wang, Y.; Wang, J.; Zhang, X. Ibuprofen removal from drinking water by electro-peroxone in carbon cloth filter. Chem. Eng. J. 2021, 415, 127618. [Google Scholar] [CrossRef]
- Liu, Z.; Li, K.; Liu, L.; Song, H.; Zhang, Y.; Tebyetekerwa, M.; Zhang, X.; Wang, K.; Xu, L.; Wang, J. Stable H2O2 electrosynthesis at industrially-relevant currents by a membrane-based electrode with high oxygen accessibility. Appl. Catal. B Environ. 2024, 357, 124311. [Google Scholar] [CrossRef]
- Zhou, Y.; Chai, Y.; Sun, H.; Li, X.; Liu, X.; Liang, Y.; Gong, X.; Wu, Z.; Liu, C.; Qin, P. Design strategies and mechanisms of g–C3N4–based photoanodes for photoelectrocatalytic degradation of organic pollutants in water. J. Environ. Manag. 2023, 344, 118545. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Liang, S.; Jiang, C.; Gu, M.; Zhang, Q.; Abdelhafiz, A.; Zhang, Z.; Han, Y.; Yang, Y.; Zhang, X.; et al. Electric field–confined synthesis of single atomic TiOxCy electrocatalytic membranes. Sci. Adv. 2025, 11, eads7154. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, J.; Yang, B.; Li, J.; Liang, Z.; Liu, X.; Bao, Y.; Cao, J.; Xing, M. Organic carbon transfer process in advanced oxidation systems for water clean-up. Nat. Water 2025, 3, 334–344. [Google Scholar] [CrossRef]
- Shi, J.; Jiang, J.; Chen, Q.; Wang, L.; Nian, K.; Long, T. Production of higher toxic intermediates of organic pollutants during chemical oxidation processes: A review. Arab. J. Chem. 2023, 16, 104856. [Google Scholar] [CrossRef]






| Catalyst | Operation Conditions | Active Sites | Pollutant | Treatment Performance * | Major ROSs | Ref. |
|---|---|---|---|---|---|---|
| FeN6/CN | 0.025 mol·L−1 Na2SO4, potential: −0.8 V vs. Ag/AgCl, pH 6 | FeN6 | 0.1 mmol·L−1 4-CP | 90% ΔD and 59% ΔT within 120 min | 1O2, •OH | [28] |
| Ni0.5-Fe0.5-NC | 0.1 mol·L−1 Na2SO4, potential: −0.5 V vs. SCE, pH 7 | FeNi-N6 | 55.9 μmol·L−1 florfenicol | 67.75% ΔT within 20 min | •OH | [51] |
| FeCo@NPC | Potential: −0.7 V vs. Ag/AgCl, pH 7 | FeCo-N8 | phthalate | 93%ΔD and 46% ΔT within 300 min | •OH, •O2−, | [58] |
| FeCMs | 15 mg/L Na2SO4 | Fe-N4 | 5.74 mmol·L−1 dimethylacetamide | 42.1% ΔD and 3.1% ΔT within 10 min | •OH | [59] |
| FeCuSA-NPC | 0.05 mol·L−1 Na2SO4 | Fe-N4, Cu-N4 | 15.6 μmol·L−1 4-CP | 95% ΔD and 41% ΔT within 60 min | •OH | [60] |
| FeCl2Cx/PC | 0.05 mol·L−1 Na2SO4, pH 7.41, current density: 15 mA cm−2 | Fe-Cl2C2 | 2.74 μmol·L−1 amoxicillin | 98.12% ΔD and 62.5% ΔC within 15 min | •OH | [61] |
| SAFe@HSC | 0.1 mol·L−1 K2SO4, current density: 20 mA·cm−2, pH 7 | Fe-N4 | 56.0 μmol ·L−1 Thiamphenicol | 100% ΔD and 67.8% ΔT within 40 min | •OH, •O2− | [62] |
| CoFe DAC | 0.1 mol·L−1 Na2SO4, potential: −0.6 V vs. SCE, pH 6 | Fe-N4, Co-N4 | 53.1 μmol·L−1 phenol | 100% ΔD and 87.2% ΔT within 120 min | •OH | [16] |
| Cu-N@C-700 | 0.05 mol·L−1 Na2SO4, current density: 50 mA·cm−2 | Cu0/Cu+/Cu2+ cycle | 60.0 μmol·L−1 pefloxacin | 100% ΔD and 48.6% ΔT within 60 min | •OH | [24] |
| FeN2O2 | 0.05 mol·L−1 Na2SO4, potential: −0.4 V vs. SCE, pH 7 | Fe-N2O2 | 213 μmol·L−1 phenol | 100% ΔD and 75.2% ΔT within 90 min | •OH | [63] |
| FeCu/NC | 0.05 mol·L−1 Na2SO4, current density: 33 mA·cm−2, pH 5.9 | - | 39.7 μmol·L−1 lisinopril | 100% ΔD and 37.1% ΔT within 120 min | •OH | [64] |
| Boron-modified porous carbon | 0.05 mol·L−1 Na2SO4, current density: 33 mA·cm−2 | C=O and BC2O act on H2O2 generation, BCO2 acts on •OH generation | 319 μmol·L−1 phenol | 100% ΔD within 20 min and 73% ΔC within 180 min | •OH | [65] |
| CuBN-HCMs | 0.1 mol·L−1 Na2SO4, potential: −0.4 to −0.7 V vs. Ag/AgCl, pH 1–9 | CuN4-B | 213 μmol·L−1 phenol | 100% ΔD within 60 min and 74.8% ΔC within 180 min | 1O2, •OH | [66] |
| B-Fe@BC | 0.05 mol·L−1 Na2SO4 | - | 106 μmol·L−1 phenol, 39.5 μmol·L−1 sulfamethoxazole | 100% within 40 min and 70.7% ΔC within 60 min | 1O2, •OH | [67] |
| FexHCS | 0.1 mol·L−1 Na2SO4, potential: 0 V vs. RHE, pH 7 | - | 55.4 μmol·L−1 ofloxacin | 72.7% ΔT within 60 min | 1O2, •OH | [68] |
| Fe2Co1/NPC | 0.05 mol·L−1 Na2SO4, current density: 100 mA·cm−2, pH 7 | - | 45.0 μmol·L−1 tetracycline | 91% ΔD within 60 min | •OH | [69] |
| Co2-NC/Fe3-C3N4 | 0.05 mol·L−1 Na2SO4, current density: 10 mA·cm−2, pH 7 | Fe-N4, Co-N/O5 | 97.0 μmol·L−1 ibuprofen | 93.1% ΔD and 58.8% ΔT within 60 min | •OH | [70] |
| Self-supporting N@C-CC | 0.05 mol·L−1 Na2SO4, potential: −0.7 V vs. SCE, pH 7 | N-C | 0.100 μmol·L−1 phenol | 100% ΔD within 60 min and 75.5% ΔT within 180 min | 1O2 | [29] |
| Cu-C aerogel | 0.05 mol·L−1 Na2SO4, current density: 2.5 mA·cm−2, pH 7 | CuN4 | 43.8 μmol·L−1 bisphenol A | 100% ΔD within 30 min and 72.3% ΔT within 120 min | 1O2 | [71] |
| FeP@ECC | 0.05 mol·L−1 Na2SO4, current density: 50 mA·cm−2, pH 7 | Fe3+/Fe2+ | 79.0 μmol·L−1 sulfamethoxazole | 100% ΔD within 20 min | •OH | [72] |
| N, P self-doped carbon | 0.05 mol·L−1 Na2SO4, current density: 20 mA·cm−2, pH 7 | - | 90.0 μmol·L−1 tetracycline | 7.97 mM H2O2 produced within 60 min | •OH | [73] |
| SA-FeNGA/CF | 0.05 mol·L−1 Na2SO4, current density: 1–5 mA·cm−2 | Fe-Nx | 1.063 mmol·L−1 phenol | 100% ΔD within 120 min and 95.5% ΔT within 240 min | •OH | [74] |
| Fe-NSC | 0.05 mol·L−1 Na2SO4, potential: −0.6 V vs. Ag/AgCl, pH 6 | Fe-N4 | 213 μmol·L−1 phenol | 100% ΔD and 56% ΔT within 50 min | 1O2, •OH | [75] |
| Endogenous iron-enriched biochar @Ni-Foam | 0.1 mol·L−1 Na2SO4, current density: 30 mA·cm−2, pH 3 | Fe3+/Fe2+ | 151 μmol·L−1 ciprofloxacin | 86% ΔD within 150 min | •OH | [76] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, S.; Lyu, G.; Zhang, C.; Lin, C.; Cheng, H. Design and Application of Atomically Dispersed Transition Metal–Carbon Cathodes for Triggering Cascade Oxygen Reduction in Wastewater Treatment. Molecules 2025, 30, 3258. https://doi.org/10.3390/molecules30153258
Huang S, Lyu G, Zhang C, Lin C, Cheng H. Design and Application of Atomically Dispersed Transition Metal–Carbon Cathodes for Triggering Cascade Oxygen Reduction in Wastewater Treatment. Molecules. 2025; 30(15):3258. https://doi.org/10.3390/molecules30153258
Chicago/Turabian StyleHuang, Shengnan, Guangshuo Lyu, Chuhui Zhang, Chunye Lin, and Hefa Cheng. 2025. "Design and Application of Atomically Dispersed Transition Metal–Carbon Cathodes for Triggering Cascade Oxygen Reduction in Wastewater Treatment" Molecules 30, no. 15: 3258. https://doi.org/10.3390/molecules30153258
APA StyleHuang, S., Lyu, G., Zhang, C., Lin, C., & Cheng, H. (2025). Design and Application of Atomically Dispersed Transition Metal–Carbon Cathodes for Triggering Cascade Oxygen Reduction in Wastewater Treatment. Molecules, 30(15), 3258. https://doi.org/10.3390/molecules30153258








