Progress and Challenges in the Process of Using Solid Waste as a Catalyst for Biodiesel Synthesis
Abstract
1. Introductory
2. Organic Solid Waste-Derived Catalysts
2.1. Biomass Organic Solid Waste
2.1.1. Plant Solid Waste
Agricultural Waste
Forestry Waste
2.1.2. Animal Solid Waste
2.1.3. Microbial Solid Waste
2.2. Industrial Organic Solid Waste
2.3. Municipal Organic Solid Waste
3. Inorganic Solid Waste Derived Catalysts
3.1. Metallurgical Solid Waste
3.2. Coal-Fired Solid Waste
3.3. Chemical Solid Waste
3.4. Construction Solid Waste
3.5. Mining Solid Waste
3.6. Restaurant Solid Waste
3.6.1. Eggshells
3.6.2. Shellfish
3.6.3. Animal Bone
| No. | Catalyst Source | Feedstock | Catalyst | Optimum Conditions | Yield (%) | Citation | |||
|---|---|---|---|---|---|---|---|---|---|
| A | B | C | D | ||||||
| 1 | Beef bone | Palm fatty acid distillate | CaSO4 | 20:1 | 5 | 180 | 70 | 81.5 | [157] |
| 2 | Goat bone | Goat fat oil | CaO | 12:1 | 2.5 | 120 | 60 | 87.2 | [158] |
| 3 | Camel bone | jujube seed oil | HAP | 7:1 | 4 | 180 | 75 | 89 | [159] |
| 4 | Chicken bone | UCO | CaO | 15:1 | 5 | 240 | 65 | 89.33 | [160] |
| 5 | Animal bones and teeth | Castor oil | CaO/P2O5 | 9:1 | 5 | 180 | 60 | 89.5 | [161] |
| 6 | Ostrich bone | UCO | HAP | 15:1 | 5 | 240 | 60 | 90.52 | [150] |
| 7 | Ox bone—Termite Mountain | UCO | CaO/SiO2 | 9:1 | 2 | 150 | 65 | 95.12 | [154] |
| 8 | Animal bones | Neem oil | CaO/K | 9:1 | 6 | 180 | 70 | 96.01 | [162] |
| 9 | Chicken bone | Rapeseed oil | CaO/Li-Cb | 18:1 | 4 | 35 | 60 | 96.6 | [155] |
| 10 | Animal bones | Neem oil | CaO/K | 12:1 | 5 | 240 | 65 | 96.82 | [163] |
| 11 | Chicken bones—catfish bones | Mahogany oil, Brazilian rubber tree oil, and Guinea rubber tree oil | CaO | 4.62:1 | 4.16 | 69.76 | 69.79 | 97.12 | [151] |
| 12 | Salmon bones | Sunflower oil | CaO/K | 10:1 | 10 | 180 | 65 | 99.13 | [152] |
4. Prospects for Solid Waste Applications
4.1. Cost Analysis
4.2. Environmental Analysis
4.3. Heterogeneity Analysis
4.4. Preparation Method Analysis
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| WOO | World Oil Outlook |
| UNEP | United Nations Environment Programme |
| EDS | Energy Dispersive Spectroscopy |
| FAME | Fatty Acid Methyl Esters |
| FESEM | field emission scanning electron microscope |
| FI-IR | fourier transform infrared spectrometer |
| XPS | X-ray photoelectron spectroscopy |
| EDX | Energy Dispersive X-Ray Spectroscopy |
| TGA | Thermal Gravimetric Analyzer |
| BET | Brunauer–Emmett–Teller |
| ICP | Inductively Coupled Plasma |
| CO2-TPD | Carbon Dioxide Temperature Programmed Desorption |
| GC-MS | Gas chromatography mass spectrometry |
| HAP | Hydroxyapatite |
| β-TCP | β-tricalcium phosphate |
| RSM | Response Surface Methodology |
| FCCD | face centered central composite design |
| MOF | metal–organic framework |
| TP | Tetra Pak |
| PWC | plastic waste char |
| UCO | Used Cooking Oil |
| ASTM | American Society of Testing Materials |
| IRI | infrared irradiation |
| LCA | life cycle assessment |
| NPV | Net Present Value |
References
- Naseef, H.H.; Tulaimat, R.H. Transesterification and esterification for biodiesel production: A comprehensive review of catalysts and palm oil feedstocks. Energy Convers. Manag. X 2025, 26, 100931. [Google Scholar] [CrossRef]
- The World Oil Outlook (WOO). 2024. Available online: https://www.opec.org/ (accessed on 1 March 2025).
- Galadima, A.; Muraza, O. Waste materials for production of biodiesel catalysts: Technological status and prospects. J. Clean. Prod. 2020, 263, 121358. [Google Scholar] [CrossRef]
- Soosai, M.R.; Sundaraman, S.; Deivasigamani, P.; Varalakshmi, P.; Ganeshmoorthy, I. Toward developing a reconcile solution for leaching of catalyst in biodiesel production by Harnessing prediction model and machine learning. Biomass Bioenergy 2025, 201, 108124. [Google Scholar] [CrossRef]
- Krishnan, S.G.; Pua, F.-l.; Zhang, F. A review of magnetic solid catalyst development for sustainable biodiesel production. Biomass Bioenergy 2021, 149, 106099. [Google Scholar] [CrossRef]
- Ao, S.; Dwivedi, P.; Chowdhury, A.P.; Lallianrawna, S.; Dhakshinamoorthy, A.; Rokhum, S.L. Paper waste-derived functionalized biochar catalyst for production of biodiesel using Jatropha curcas oil feedstock. Bioresour. Technol. Rep. 2025, 29, 102031. [Google Scholar] [CrossRef]
- Falowo, O.A.; Oladipo, B.; Adewole, O.; Ojumu, T.V. Enhancement of metal-oxides bifunctional catalyst applied in biodiesel production from waste cooking oil via microwave-aided and conventional heating methods. Process Saf. Environ. Prot. 2025, 200, 107349. [Google Scholar] [CrossRef]
- Roy, S.; Ahmaruzzaman, M. Waste eggshell derived CaO/metal-organic framework@ LDH for microwave-assisted biodiesel synthesis: Thermodynamics, mechanistic insights and life-cycle cost analysis. Energy Convers. Manag. 2025, 325, 119380. [Google Scholar] [CrossRef]
- Fernandez, I.A.P.; Liu, D.-H.; Zhao, J. LCA studies comparing alkaline and immobilized enzyme catalyst processes for biodiesel production under Brazilian conditions. Resour. Conserv. Recycl. 2017, 119, 117–127. [Google Scholar] [CrossRef]
- Alterkaoui, A.; Belibagli, P.; Arslan, H.; Dizge, N.; Balakrishnan, D. Heterogeneous catalyst production from waste cucumber stems and investigation of production potential in biodiesel. Process Saf. Environ. Prot. 2025, 196, 106842. [Google Scholar] [CrossRef]
- Rezki, B.; Essamlali, Y.; Amadine, O.; Sair, S.; Aadil, M.; Len, C.; Zahouily, M. A comprehensive review on apatite-derived catalysts for sustainable biodiesel production: Classification, features and challenges. J. Environ. Chem. Eng. 2024, 12, 111913. [Google Scholar] [CrossRef]
- Ao, S.; Changmai, B.; Vanlalveni, C.; Chhandama, M.V.L.; Wheatley, A.E.; Rokhum, S.L. Biomass waste-derived catalysts for biodiesel production: Recent advances and key challenges. Renew. Energy 2024, 223, 120031. [Google Scholar] [CrossRef]
- Parandi, E.; Safaripour, M.; Mosleh, N.; Saidi, M.; Nodeh, H.R.; Oryani, B.; Rezania, S. Lipase enzyme immobilized over magnetic titanium graphene oxide as catalyst for biodiesel synthesis from waste cooking oil. Biomass Bioenergy 2023, 173, 106794. [Google Scholar] [CrossRef]
- 2024 Global Waste Management Outlook. Available online: https://www.unep.org/zh-hans/resources/2024nianquanqiufeiwuguanlizhanwang (accessed on 1 March 2025).
- Pang, D.; Moliner, C.; Wang, T.; Sun, J.; Zhang, X.; Pang, Y.; Zhao, X.; Song, Z.; Wang, Z.; Mao, Y.; et al. A Mini Review on AI-driven Thermal Treatment of Solid Waste: Emission Control and Process Optimization. Green Energy Resour. 2025, 3, 100132. [Google Scholar] [CrossRef]
- Maalouf, A.; Mavropoulos, A. Re-assessing global municipal solid waste generation. Waste Manag. Res. 2023, 41, 936–947. [Google Scholar] [CrossRef]
- Guo, D.; Zhang, S.; Dai, Z.; Hou, H.; Wang, G.; Xu, H. Synergistic benefits of pollution and carbon reduction in collaborative domestic solid waste disposal: A life cycle perspective. Environ. Impact Assess. Rev. 2025, 114, 107892. [Google Scholar] [CrossRef]
- Wang, S.; Yan, W.; Zhao, F. Recovery of solid waste as functional heterogeneous catalysts for organic pollutant removal and biodiesel production. Chem. Eng. J. 2020, 401, 126104. [Google Scholar] [CrossRef]
- Betiku, E.; Oraegbunam, J.C.; Falowo, O.A.; Ojumu, T.V.; Latinwo, L.M. Sustainable microwave-supported biodiesel production using sandbox oil and its waste shell as a nanoparticle green alkali heterogeneous catalyst. Process Biochem. 2024, 142, 1–12. [Google Scholar] [CrossRef]
- Falowo, O.A.; Oladipo, B.; Taiwo, A.E.; Olaiya, A.T.; Oyekola, O.O.; Betiku, E. Green heterogeneous base catalyst from ripe and unripe plantain peels mixture for the transesterification of waste cooking oil. Chem. Eng. J. Adv. 2022, 10, 100293. [Google Scholar] [CrossRef]
- Basumatary, B.; Brahma, S.; Nath, B.; Basumatary, S.F.; Das, B.; Basumatary, S. Post-harvest waste to value-added materials: Musa champa plant as renewable and highly effective base catalyst for Jatropha curcas oil-based biodiesel production. Bioresour. Technol. Rep. 2023, 21, 101338. [Google Scholar] [CrossRef]
- Abukhadra, M.R.; Mostafa, M.; El-Sherbeeny, A.M.; Soliman, A.T.A.; Abd Elgawad, A.E.E. Effective transformation of waste sunflower oil into biodiesel over novel K+ trapped clay nanotubes (K+/KNTs) as a heterogeneous catalyst; response surface studies. Microporous Mesoporous Mater. 2020, 306, 110465. [Google Scholar] [CrossRef]
- Rajak, A.K.; Harikrishna, M.; Mahato, D.L.; Anandamma, U.; Pothu, R.; Sarangi, P.K.; Sahoo, U.K.; Vennu, V.; Boddula, R.; Karuna, M.S. Valorising orange and banana peels: Green catalysts for transesterification and biodiesel production in a circular bioeconomy. J. Taiwan Inst. Chem. Eng. 2024, 14, 105804. [Google Scholar] [CrossRef]
- Gonçalves, M.A.; dos Santos, H.C.L.; Ribeiro, T.S.; Viegas, A.d.C.; Filho, G.N.d.R.; da Conceição, L.R.V. Boosting biodiesel production of waste frying oil using solid magnetic acid catalyst from agro-industrial waste. Arab. J. Chem. 2024, 17, 105521. [Google Scholar] [CrossRef]
- Boro, S.; Das, B.; Brahma, S.; Basumatary, B.; Basumatary, S.F.; Basumatary, S. Biodiesel production using areca nut (Areca catechu L.) leaf ash-K2CO3 catalyst via transesterification from an oil blend of three different feedstocks. Sustain. Chem. Environ. 2024, 8, 100164. [Google Scholar] [CrossRef]
- Ribeiro, T.S.; Sobrinho, I.d.A.; Gonçalves, M.A.; Lima, V.d.S.; Figueira, B.A.M.; Filho, G.N.d.R.; da Conceição, L.R.V. Green synthesis of biodiesel from magnetic basic biochar derived from Amazonian murici residual biomass: Optimization, kinetic, thermodynamic, and environmental studies. J. Environ. Chem. Eng. 2024, 12, 114725. [Google Scholar] [CrossRef]
- Saikia, K.; Das, A.; Sema, A.H.; Basumatary, S.; Moyon, N.S.; Mathimani, T.; Rokhum, S.L. Response surface optimization, kinetics, thermodynamics, and life cycle cost analysis of biodiesel production from Jatropha curcas oil using biomass-based functional activated carbon catalyst. Renew. Energy 2024, 229, 120743. [Google Scholar] [CrossRef]
- Mulkan, A.; Zulkifli, N.W.M.; Husin, H.; Dahlan, I.; Syafiie, S. Development of jackfruit (Artocarpus heterophyllus) peel waste as a new solid catalyst: Biodiesel synthesis, optimization and characterization. Process Saf. Environ. Prot. 2023, 177, 152–168. [Google Scholar] [CrossRef]
- Maleki, B.; Esmaeili, H.; Venkatesh, Y.K.; Amruth, E. Valorization of dairy waste scum oil and rice husk ash-supported CuO nanocatalyst towards cleaner production of biodiesel: A waste-to-energy approach. Process Saf. Environ. Prot. 2024, 192, 1393–1407. [Google Scholar] [CrossRef]
- Husin, H.; Riza, M.; Faisal, M. Biodiesel production using waste banana peel as renewable base catalyst. Mater. Today Proc. 2023, 87, 214–217. [Google Scholar] [CrossRef]
- Akwenuke, O.; Okwelum, C.; Balogun, T.; Nwadiolu, R.; Okolotu, G.; Chukwuma, I.; Adepoju, T.; Essaghah, A.; Ibimilua, A.; Taiga, A. Biodiesel production from agricultural biomass wastes: Duroc breed fat oil, Citrillus lanatus rind, and Sorghum Bagasse. MethodsX 2024, 13, 102948. [Google Scholar] [CrossRef]
- Novita, L.; de Freitas, F.A.; Fauzia, S.; Zein, R. Enhanced conversion of used palm cooking oil to biodiesel by a green and recyclable palm kernel shell ash-derived catalyst: Process optimization by response surface methodology. Case Stud. Chem. Environ. Eng. 2024, 9, 100678. [Google Scholar] [CrossRef]
- Nyorere, O.; Oluka, S.; Onoji, S.; Nwadiolu, R.; Adepoju, T. Production of biodiesel from biocatalysis of agro-wastes in acidic environment. Sci. Afr. 2024, 24, e02154. [Google Scholar] [CrossRef]
- Yadav, G.; Yadav, N.; Ahmaruzzaman, M. Microwave-assisted sustainable synthesis of biodiesel on Oryza sativa catalyst derived from agricultural waste by esterification reaction. Chem. Eng. Process.-Process Intensif. 2023, 187, 109327. [Google Scholar] [CrossRef]
- Yadav, N.; Yadav, G.; Ahmaruzzaman, M. Fabrication of surface-modified dual waste-derived biochar for biodiesel production by microwave-assisted esterification of oleic acid: Optimization, kinetics, and mechanistic studies. Renew. Energy 2023, 218, 119308. [Google Scholar] [CrossRef]
- Rocha, P.D.; Oliveira, L.S.; Franca, A.S. Sulfonated activated carbon from corn cobs as heterogeneous catalysts for biodiesel production using microwave-assisted transesterification. Renew. Energy 2019, 143, 1710–1716. [Google Scholar] [CrossRef]
- Valizadeh, S.; Valizadeh, B.; Khani, Y.; Jae, J.; Ko, C.H.; Park, Y.-K. Production of biodiesel via esterification of coffee waste-derived bio-oil using sulfonated catalysts. Bioresour. Technol. 2024, 404, 130908. [Google Scholar] [CrossRef]
- Priambodo, R.; Chen, T.-C.; Lu, M.-C.; Gedanken, A.; Liao, J.-D.; Huang, Y.-H. Novel technology for bio-diesel production from cooking and waste cooking oil by microwave irradiation. Energy Procedia 2015, 75, 84–91. [Google Scholar] [CrossRef]
- Oladipo, B.; Qasana, S.; Zini, S.C.; Menemene, N.; Ojumu, T.V. Microwave-assisted biodiesel synthesis from waste cooking oil: Exploring the potential of carob pod-derived solid base catalyst. Fuel Process. Technol. 2024, 266, 108161. [Google Scholar] [CrossRef]
- Akream, N.S.; Hamd, M.I.; Gheni, S.A.; Al-Sudani, F.T.; Mohammed, A.E.; Mohammed, H.R.; Ali, M.M.; Ahmed, S.M.; Karakullukçu, N.T.; Tahah, A.K. High-yield activated carbon based ZnO-Ce bifunctional catalyst for production of biodiesel from waste cooking oil. Energy Convers. Manag. 2024, 321, 119054. [Google Scholar] [CrossRef]
- Soltani, S.; Khanian, N.; Choong, T.S.Y.; Asim, N.; Zhao, Y. Microwave-assisted hydrothermal synthesis of sulfonated TiO2-GO core–shell solid spheres as heterogeneous esterification mesoporous catalyst for biodiesel production. Energy Convers. Manag. 2021, 238, 114165. [Google Scholar] [CrossRef]
- Amjad, R.; Ahmad, M.; Sultana, S.; Munir, M.; Ali, M.I.; El-Toony, M.M.; Usmankulovna, N.M.; Avutkhanov, B.; Mustafa, A. Exploring the potential of novel feedstock (Caesalpinia bonduc seeds) for circular biodiesel production using seed shell-derived green nanocatalysts. Biomass Bioenergy 2025, 197, 107847. [Google Scholar] [CrossRef]
- Zia, U.; Ahmad, M.; Alsahli, A.A.; Faiz, I.; Sultana, S.; Caicedo-Paz, A.V.; Mussagy, C.U.; Mustafa, A. Integrating environmental remediation with biodiesel production from toxic non-edible oil seeds (Croton bonplandianus) using a sustainable phyto-nano catalyst. Biomass Bioenergy 2024, 190, 107406. [Google Scholar] [CrossRef]
- Rozina; Emmanuel, O.; Ahmad, M.; Duduyemi, A.; Ahmad, S.; Khan, A.; Esiaba, R.; Elekwachi, C. Valorization of waste seed oil from Cupressus macrocarpa L. for biodiesel production via green-synthesized iron oxide nanoparticles: A sustainable approach toward decarbonization. Next Energy 2025, 7, 100218. [Google Scholar] [CrossRef]
- Ahmad, M.; Ezeji, T.C.; Emmanuel, O.; Qureshi, N.; Khan, A. Utilization of waste seed oil from Cestrum nocturnum as a novel source for cleaner production of biodiesel using green nano-catalyst of antimony oxide. Fuel 2024, 364, 131124. [Google Scholar] [CrossRef]
- Okezie, E.; Ugbogu, E.; Uwadinachi, A.; Okeke, S.; Achulonu, V. A comparative study on micronutrients and anti-nutrients of leaf extracts of Pterocarpus soyauxii and Pterocarpus santalinoides. J. Complement. Med. Res. 2018, 7, 131–137. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, Z. Comparison of hydrochar-and pyrochar-based solid acid catalysts from cornstalk: Physiochemical properties, catalytic activity and deactivation behavior. Bioresour. Technol. 2020, 297, 122477. [Google Scholar] [CrossRef] [PubMed]
- Naeem, M.M.; Al-Sakkari, E.G.; Boffito, D.C.; Rene, E.R.; Gadalla, M.A.; Ashour, F.H. Single-stage waste oil conversion into biodiesel via sonication over bio-based bifunctional catalyst: Optimization, preliminary techno-economic and environmental analysis. Fuel 2023, 341, 127587. [Google Scholar] [CrossRef]
- Aliyu, M.; Moser, B.R.; Alharthi, F.A.; Rashid, U. Efficient production of biodiesel from palm fatty acid distillate using a novel hydrochar-based solid acid catalyst derived from palm leaf waste. Process Saf. Environ. Prot. 2024, 187, 1126–1139. [Google Scholar] [CrossRef]
- Lokman, I.M.; Rashid, U.; Taufiq-Yap, Y.H. Meso-and macroporous sulfonated starch solid acid catalyst for esterification of palm fatty acid distillate. Arab. J. Chem. 2016, 9, 179–189. [Google Scholar] [CrossRef]
- Zhang, F.; Tian, X.; Shah, M.; Yang, W. Synthesis of magnetic carbonaceous acids derived from hydrolysates of Jatropha hulls for catalytic biodiesel production. RSC Adv. 2017, 7, 11403–11413. [Google Scholar] [CrossRef]
- Zhang, F.; Fang, Z.; Wang, Y.-T. Biodiesel production direct from high acid value oil with a novel magnetic carbonaceous acid. Appl. Energy 2015, 155, 637–647. [Google Scholar] [CrossRef]
- Brindhadevi, K.; Kim, P.; Chinnathambi, A.; Kamarudin, S.; Pugazhendhi, A. Comparison of photocatalytic vs conventional transesterification for biodiesel preparation from fish waste oil using green TiO2/SiO2 catalyst. Process Saf. Environ. Prot. 2024, 188, 167–176. [Google Scholar] [CrossRef]
- Chakraborty, R.; Bepari, S.; Banerjee, A. Application of calcined waste fish (Labeo rohita) scale as low-cost heterogeneous catalyst for biodiesel synthesis. Bioresour. Technol. 2011, 102, 3610–3618. [Google Scholar] [CrossRef]
- Liu, X.; Piao, X.; Wang, Y.; Zhu, S.; He, H. Calcium methoxide as a solid base catalyst for the transesterification of soybean oil to biodiesel with methanol. Fuel 2008, 87, 1076–1082. [Google Scholar] [CrossRef]
- Rhrissi, I.; Abouliatim, Y.; Hlaibi, M.; Kamil, N. Moroccan sardine scales as a novel and renewable source of heterogeneous catalyst for biodiesel production using palm fatty acid distillate. Renew. Energy 2023, 217, 119223. [Google Scholar] [CrossRef]
- Bosoy, S.; Intachai, S.; Sumanatrakul, P.; Kongsune, P.; Loiha, S.; Khaorapapong, N. Novel magnetic composite: NiFe-layered double oxide/ferric oxyhydroxide/activated carbon for optimizing biodiesel production from used cooking oil. Biomass Bioenergy 2024, 183, 107096. [Google Scholar] [CrossRef]
- Çakırca, E.E.; Akın, A.N. Study on heterogeneous catalysts from calcined Ca riched hydrotalcite like compounds for biodiesel production. Sustain. Chem. Pharm. 2021, 20, 100378. [Google Scholar] [CrossRef]
- Maneerung, T.; Kawi, S.; Dai, Y.; Wang, C.-H. Sustainable biodiesel production via transesterification of waste cooking oil by using CaO catalysts prepared from chicken manure. Energy Convers. Manag. 2016, 123, 487–497. [Google Scholar] [CrossRef]
- Jung, S.; Kim, M.; Jung, J.-M.; Kwon, E.E. Valorization of swine manure biochar as a catalyst for transesterifying waste cooking oil into biodiesel. Environ. Pollut. 2020, 266, 115377. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, H.; Chen, X.; Sun, Z.; Peng, L.; Si, H. Study on simplifying the synthesis method of CaO/ZrO2 catalyst and optimizing its performance in catalytic transesterification to prepare biodiesel. Biomass Bioenergy 2025, 200, 108023. [Google Scholar] [CrossRef]
- Maafa, I.M.; Alahl, A.A.S.; Abd El-Magied, M.O.; Cui, X.; Dhmees, A.S. Eco-friendly self-terminated process for preparation of CaO catalyst based on chitosan production wastes for biodiesel production. J. Mater. Res. Technol. 2024, 30, 1217–1227. [Google Scholar] [CrossRef]
- EN 14214:2010; Liquid Petroleum Products—Fatty Acid Methyl Esters (FAME) for Use in Diesel Engines and Heating Applications—Requirements and Test Methods. Standard Norge: Oslo, Norway, 2010.
- Farobie, O.; Santosa, N.F.; Fatriasari, W.; Karimah, A.; Amrullah, A.; Suseno, S.H.; Nandiyanto, A.B.D.; Hartulistiyoso, E. Harnessing macroalgae Sargassum plagiophyllum-derived heterogeneous catalyst for biodiesel production. Bioresour. Technol. Rep. 2024, 25, 101768. [Google Scholar] [CrossRef]
- Shen, Y.; Zhang, Q.; Sun, X.; Zhang, Y.; Cai, Q.; Deng, W.; Rao, S.; Wu, X.; Ye, Q. Conversion of wet microalgae to biodiesel with microalgae carbon based magnetic solid acid catalyst. Energy Convers. Manag. 2023, 286, 117022. [Google Scholar] [CrossRef]
- Yameen, M.Z.; Juchelková, D.; Naqvi, S.R.; Noor, T.; Ali, A.M.; Shahzad, K.; Rashid, M.I.; Mahpudz, A.B. Biodiesel production from marine macroalgae Ulva lactuca lipids using novel Cu-BTC@ AC catalyst: Parametric analysis and optimization. Energy Convers. Manag. X 2024, 23, 100628. [Google Scholar] [CrossRef]
- ASTM D6751-24; Standard Specification for Biodiesel Fuel Blend Stock (B100) for Middle Distillate Fuels. ASTM International: West Conshohocken, PA, USA, 2024.
- Foroutan, R.; Mohammadi, R.; Razeghi, J.; Ramavandi, B. Biodiesel production from edible oils using algal biochar/CaO/K2CO3 as a heterogeneous and recyclable catalyst. Renew. Energy 2021, 168, 1207–1216. [Google Scholar] [CrossRef]
- Roschat, W.; Phewphong, S.; Thangthong, A.; Moonsin, P.; Yoosuk, B.; Kaewpuang, T.; Promarak, V. Catalytic performance enhancement of CaO by hydration-dehydration process for biodiesel production at room temperature. Energy Convers. Manag. 2018, 165, 1–7. [Google Scholar] [CrossRef]
- Farobie, O.; Aslamah, R.; Amrullah, A.; Fatriasari, W.; Nandiyanto, A.B.D.; Sucahyo, L.; Hartulistiyoso, E. A novel mesoporous catalyst from Ulva lactuca for biodiesel production from waste cooking oil using a microwave-assisted technique. Fuel 2025, 386, 134261. [Google Scholar] [CrossRef]
- Russo, D.; Portarapillo, M.; Di Benedetto, A. Flash point of biodiesel/glycerol/alcohol mixtures for safe processing and storage. J. Loss Prev. Process Ind. 2023, 83, 105077. [Google Scholar] [CrossRef]
- Ponce, S.; Gangotena, P.A.; Anthony, C.; Rodriguez, Y.; Bazani, H.A.; Keller, M.H.; Souza, B.S.; Vizuete, K.; Debut, A.; Mora, J.R. Aluminum-based catalysts prepared in the presence of pectin for low-energy biodiesel production. Fuel 2024, 361, 130691. [Google Scholar] [CrossRef]
- Keasavan, T.; Loh, S.K.; Jaafar, N.F.; Rahmawati, Z.; Abdullah, W.N.W. Synthesis of biodiesel from residual oil extracted from spent bleaching earth using spent bleaching earth-supported catalyst. Chem. Eng. Res. Des. 2023, 200, 716–728. [Google Scholar] [CrossRef]
- Elgharbawy, A.S.; Osman, A.I.; El Demerdash, A.G.M.; Sadik, W.A.; Kasaby, M.A.; Ali, S.E. Enhancing biodiesel production efficiency with industrial waste-derived catalysts: Techno-economic analysis of microwave and ultrasonic transesterification methods. Energy Convers. Manag. 2024, 321, 118945. [Google Scholar] [CrossRef]
- Abou-Elyazed, A.S.; Shaban, A.K.; Osman, A.I.; Heikal, L.A.; Mohamed, H.F.; Hassan, W.M.; El-Nahas, A.M.; Keshta, B.E.; Hamouda, A.S. Comparative catalytic efficacy of cost-effective MIL-101 (Cr) based PET waste for biodiesel production. Curr. Res. Green Sustain. Chem. 2024, 8, 100401. [Google Scholar] [CrossRef]
- Ooi, C.-H.; Cheah, W.-K.; Sim, Y.-L.; Pung, S.-Y.; Yeoh, F.-Y. Conversion and characterization of activated carbon fiber derived from palm empty fruit bunch waste and its kinetic study on urea adsorption. J. Environ. Manag. 2017, 197, 199–205. [Google Scholar] [CrossRef]
- Hazmi, B.; Rashid, U.; Kawi, S.; Mokhtar, W.N.A.W.; Yaw, T.C.S.; Moser, B.R.; Alsalme, A. Palm fatty acid distillate esterification using synthesized heterogeneous sulfonated carbon catalyst from plastic waste: Characterization, catalytic efficacy and stability, and fuel properties. Process Saf. Environ. Prot. 2022, 162, 1139–1151. [Google Scholar] [CrossRef]
- Veitía-De-Armas, L.; Reynel-Ávila, H.E.; Villalobos-Delgado, F.J.; Duran-Valle, C.J.; Adame-Pereira, M.; Bonilla-Petriciolet, A. A circular economy approach to produce low-cost biodiesel using agro-industrial and packing wastes from Mexico: Valorization, homogeneous and heterogeneous reaction routes and product characterization. Renew. Energy 2024, 237, 121684. [Google Scholar] [CrossRef]
- Man, Y.; Habibi, M.; Maleki, B. Biodiesel synthesis from waste coconut scum oil utilizing SnFe2O4/cigarette butt-derived biochar as a magnetic nanocatalyst: Optimization, kinetic and thermodynamic study. Chem. Eng. Res. Des. 2024, 210, 311–327. [Google Scholar] [CrossRef]
- Liu, J.; Lin, T.; Niu, S.; Zhu, J.; Yang, Z.; Geng, J.; Liu, S.; Zheng, Y.; Liang, B.; Sun, X.; et al. Transesterification of acidic palm oil using solid waste/CaO as a bifunctional catalyst. Fuel 2024, 362, 130913. [Google Scholar] [CrossRef]
- Araujo, R.O.; Santos, V.O.; Ribeiro, F.C.; Chaar, J.d.S.; Pereira, A.M.; Falcão, N.P.; de Souza, L.K. Magnetic acid catalyst produced from acai seeds and red mud for biofuel production. Energy Convers. Manag. 2021, 228, 113636. [Google Scholar] [CrossRef]
- Ali, J.; Rutto, H.; Seodigeng, T.; Kiambi, S. Thermal activation of basic oxygen furnace slag as a heterogeneous catalyst in transesterification: Characterization and catalytic activity studies. Results Eng. 2024, 24, 102899. [Google Scholar] [CrossRef]
- Ali, J.; Rutto, H.; Seodigeng, T.; Kiambi, S. Alkali-impregnated blast furnace slag heterogeneous catalyst for biodiesel production. Results Eng. 2024, 22, 102082. [Google Scholar] [CrossRef]
- Liu, Q.; Xin, R.; Li, C.; Xu, C.; Yang, J. Application of red mud as a basic catalyst for biodiesel production. J. Environ. Sci. 2013, 25, 823–829. [Google Scholar] [CrossRef]
- Liu, K.; Wei, G.; Zhu, Y.; Zhang, L.; He, Z. A clean route of biodiesel production using red mud-based potassium catalyst. J. Environ. Chem. Eng. 2023, 11, 111015. [Google Scholar] [CrossRef]
- Abu-Ghazala, A.H.; Abdelhady, H.H.; Mazhar, A.A.; El-Deab, M.S. Enhanced low-temperature production of biodiesel from waste cooking oil: Aluminum industrial waste as a precursor of efficient CaO/Al2O3 nano-catalyst. Fuel 2023, 351, 128897. [Google Scholar] [CrossRef]
- Ba, J.; Wei, G.; Li, Z.; Zhang, L.; Pei, R.; Xu, J.; Zhou, Y. Castor oil transesterification catalyzed by a new red mud based LiAlO2-LiFeO2 composite. Energy Convers. Manag. 2022, 254, 115214. [Google Scholar] [CrossRef]
- Abu-Ghazala, A.H.; Abdelhady, H.H.; Mazhar, A.A.; El-Deab, M.S. Exceptional room temperature catalytic transesterification of waste cooking oil to biodiesel using environmentally-benign K2CO3/γ-Al2O3 nano-catalyst. Chem. Eng. J. 2023, 474, 145784. [Google Scholar] [CrossRef]
- Wang, F.-P.; Kang, L.-L.; Wang, Y.-J.; Wang, Y.-R.; Wang, Y.-T.; Li, J.-G.; Jiang, L.-Q.; Ji, R.; Chao, S.; Zhang, J.-B.; et al. Magnetic biochar catalyst from reed straw and electric furnace dust for biodiesel production and life cycle assessment. Renew. Energy 2024, 227, 120570. [Google Scholar] [CrossRef]
- Barbarey, M.S.; Seleman, M.M.E.-S.; El Kheshen, A.; Zawrah, M. Utilization of ladle furnace slag for fabrication of geopolymer: Its application as catalyst for biodiesel production. Constr. Build. Mater. 2024, 411, 134226. [Google Scholar] [CrossRef]
- Mukhtar, A.; Saqib, S.; Lin, H.; Shah, M.U.H.; Ullah, S.; Younas, M.; Rezakazemi, M.; Ibrahim, M.; Mahmood, A.; Asif, S.; et al. Current status and challenges in the heterogeneous catalysis for biodiesel production. Renew. Sustain. Energy Rev. 2022, 157, 112012. [Google Scholar] [CrossRef]
- Guo, F.; Liang, S.; Zhao, X.; Jia, X.; Peng, K.; Jiang, X.; Qian, L. Catalytic reforming of biomass pyrolysis tar using the low-cost steel slag as catalyst. Energy 2019, 189, 116161. [Google Scholar] [CrossRef]
- Marvila, M.T.; Azevedo, A.R.G.d.; Vieira, C.M.F. Reaction mechanisms of alkali-activated materials. Rev. IBRACON Estrut. Mater. 2021, 14, e14309. [Google Scholar] [CrossRef]
- Liu, K.; Wang, R.; Yu, M. An efficient, recoverable solid base catalyst of magnetic bamboo charcoal: Preparation, characterization, and performance in biodiesel production. Renew. Energy 2018, 127, 531–538. [Google Scholar] [CrossRef]
- Mansoorsamaei, Z.; Mowla, D.; Esmaeilzadeh, F.; Dashtian, K. Sustainable biodiesel production from waste cooking oil using banana peel biochar-Fe2O3/Fe2K6O5 magnetic catalyst. Fuel 2024, 357, 129821. [Google Scholar] [CrossRef]
- Zhang, F.; Wu, X.-H.; Yao, M.; Fang, Z.; Wang, Y.-T. Production of biodiesel and hydrogen from plant oil catalyzed by magnetic carbon-supported nickel and sodium silicate. Green Chem. 2016, 18, 3302–3314. [Google Scholar] [CrossRef]
- Ibrahim, N.A.; Rashid, U.; Taufiq-Yap, Y.H.; Yaw, T.C.S.; Ismail, I. Synthesis of carbonaceous solid acid magnetic catalyst from empty fruit bunch for esterification of palm fatty acid distillate (PFAD). Energy Convers. Manag. 2019, 195, 480–491. [Google Scholar] [CrossRef]
- Zabeti, M.; Daud, W.M.A.W.; Aroua, M.K. Biodiesel production using alumina-supported calcium oxide: An optimization study. Fuel Process. Technol. 2010, 91, 243–248. [Google Scholar] [CrossRef]
- Yusuff, A.S.; Bhonsle, A.K.; Trivedi, J.; Bangwal, D.P.; Singh, L.P.; Atray, N. Synthesis and characterization of coal fly ash supported zinc oxide catalyst for biodiesel production using used cooking oil as feed. Renew. Energy 2021, 170, 302–314. [Google Scholar] [CrossRef]
- Ho, W.W.S.; Ng, H.K.; Gan, S.; Tan, S.H. Evaluation of palm oil mill fly ash supported calcium oxide as a heterogeneous base catalyst in biodiesel synthesis from crude palm oil. Energy Convers. Manag. 2014, 88, 1167–1178. [Google Scholar] [CrossRef]
- Arora, S.; Gosu, V.; Subbaramaiah, V.; Hameed, B. Lithium loaded coal fly ash as sustainable and effective catalyst for the synthesis of glycerol carbonate from glycerol. J. Environ. Chem. Eng. 2021, 9, 105999. [Google Scholar] [CrossRef]
- Song, X.; Wu, Y.; Cai, F.; Pan, D.; Xiao, G. High-efficiency and low-cost Li/ZnO catalysts for synthesis of glycerol carbonate from glycerol transesterification: The role of Li and ZnO interaction. Appl. Catal. A: Gen. 2017, 532, 77–85. [Google Scholar] [CrossRef]
- Xiang, Y.; Wang, L.; Jiao, Y. Ultrasound strengthened biodiesel production from waste cooking oil using modified coal fly ash as catalyst. J. Environ. Chem. Eng. 2016, 4, 818–824. [Google Scholar] [CrossRef]
- Izidoro, J.d.C.; Fungaro, D.A.; dos Santos, F.S.; Wang, S. Characteristics of Brazilian coal fly ashes and their synthesized zeolites. Fuel Process. Technol. 2012, 97, 38–44. [Google Scholar] [CrossRef]
- Manique, M.C.; Lacerda, L.V.; Alves, A.K.; Bergmann, C.P. Biodiesel production using coal fly ash-derived sodalite as a heterogeneous catalyst. Fuel 2017, 190, 268–273. [Google Scholar] [CrossRef]
- Pavlović, S.M.; Marinković, D.M.; Kostić, M.D.; Janković-Častvan, I.M.; Mojović, L.V.; Stanković, M.V.; Veljković, V.B. A CaO/zeolite-based catalyst obtained from waste chicken eggshell and coal fly ash for biodiesel production. Fuel 2020, 267, 117171. [Google Scholar] [CrossRef]
- Zdujić, M.; Lukić, I.; Kesić, Ž.; Janković-Častvan, I.; Marković, S.; Jovalekić, Č.; Skala, D. Synthesis of CaOSiO2 compounds and their testing as heterogeneous catalysts for transesterification of sunflower oil. Adv. Powder Technol. 2019, 30, 1141–1150. [Google Scholar] [CrossRef]
- Tahmasebi-Boldaji, R.; Rashidi, S.; Rajabi-Kuyakhi, H.; Tahmasebi-Boldaji, N.; Baghdadi, M.; Karbassi, A. Application of pharmaceutical waste as a heterogeneous catalyst for transesterification of waste cooking oil: Biofuel production and its modeling using predictive tools. Biofuels 2024, 15, 415–431. [Google Scholar] [CrossRef]
- Rashidi, S.; Tahmasebi-Boldaji, R.; Baghbadarani, A.A.; Baghdadi, M.; Tavakoli, O.; Karbassi, A.; Avami, A. Biodiesel production through transesterification of waste Pistacia-Terebinthus Oil by pharmaceutical waste as a heterogeneous catalyst: A sustainable solution for reducing external costs. Heliyon 2024, 10, e34404. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, C.; Luo, B.; He, P.; Li, L.; Wu, G. Biodiesel production by transesterification of waste cooking oil in the presence of graphitic carbon nitride supported molybdenum catalyst. Fuel 2023, 332, 126309. [Google Scholar] [CrossRef]
- Olutoye, M.; Wong, S.; Chin, L.; Amani, H.; Asif, M.; Hameed, B. Synthesis of fatty acid methyl esters via the transesterification of waste cooking oil by methanol with a barium-modified montmorillonite K10 catalyst. Renew. Energy 2016, 86, 392–398. [Google Scholar] [CrossRef]
- Mekonnen, K.D.; Hailemariam, K. Valorization of calcium hypochlorite precipitate as a new source of heterogeneous catalyst development for biodiesel production: A preliminary experiment. Heliyon 2023, 9, e21959. [Google Scholar] [CrossRef] [PubMed]
- Davoodbasha, M.; Pugazhendhi, A.; Kim, J.-W.; Lee, S.-Y.; Nooruddin, T. Biodiesel production through transesterification of Chlorella vulgaris: Synthesis and characterization of CaO nanocatalyst. Fuel 2021, 300, 121018. [Google Scholar] [CrossRef]
- Somasundaram, S.; Jeon, T.-W.; Kang, Y.-Y.; Kim, W.-I.; Jeong, S.-K.; Kim, Y.-J.; Yeon, J.-M.; Shin, S.K. Characterization of wastes from construction and demolition sector. Environ. Monit. Assess. 2015, 187, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Singh, B.; Banerjee, A.; Chatterjee, S. Cement wastes as transesterification catalysts for the production of biodiesel from Karanja oil. J. Clean. Prod. 2018, 183, 26–34. [Google Scholar] [CrossRef]
- Wang, J.-X.; Chen, K.-T.; Wen, B.-Z.; Liao, Y.-H.B.; Chen, C.-C. Transesterification of soybean oil to biodiesel using cement as a solid base catalyst. J. Taiwan Inst. Chem. Eng. 2012, 43, 215–219. [Google Scholar] [CrossRef]
- Kouzu, M.; Tsunomori, M.; Yamanaka, S.; Hidaka, J. Solid base catalysis of calcium oxide for a reaction to convert vegetable oil into biodiesel. Adv. Powder Technol. 2010, 21, 488–494. [Google Scholar] [CrossRef]
- Fiala, K.; Rublaim, A.; Leesing, R. Boosting wet yeast-based biodiesel production by in-situ transesterification using a novel acid-base bifunctional carbon-based catalyst derived from spent coffee ground and cement residue. Renew. Energy 2025, 241, 122341. [Google Scholar] [CrossRef]
- Zhu, Z.; Liu, Y.; Cong, W.; Zhao, X.; Janaun, J.; Wei, T.; Fang, Z. Soybean biodiesel production using synergistic CaO/Ag nano catalyst: Process optimization, kinetic study, and economic evaluation. Ind. Crops Prod. 2021, 166, 113479. [Google Scholar] [CrossRef]
- Okoduwa, I.G.; Oiwoh, O.; Amenaghawon, A.N.; Okieimen, C.O. A biobased mixed metal oxide catalyst for biodiesel production from waste cooking oil: Reaction conditions modeling, optimization and sensitivity analysis study. J. Eng. Res. 2024, 13, 1344–1357. [Google Scholar] [CrossRef]
- Prates, C.D.; Ballotin, F.C.; Limborço, H.; Ardisson, J.D.; Lago, R.M.; Teixeira, A.P.d.C. Heterogeneous acid catalyst based on sulfated iron ore tailings for oleic acid esterification. Appl. Catal. A Gen. 2020, 600, 117624. [Google Scholar] [CrossRef]
- Niu, S.; Zhang, X.; Ning, Y.; Zhang, Y.; Qu, T.; Hu, X.; Gong, Z.; Lu, C. Dolomite incorporated with cerium to enhance the stability in catalyzing transesterification for biodiesel production. Renew. Energy 2020, 154, 107–116. [Google Scholar] [CrossRef]
- Widiarti, N.; Holilah, H.; Bahruji, H.; Nugraha, R.E.; Suprapto, S.; Ni’MAh, Y.L.; Prasetyoko, D. Coprecipitation and hydrothermal synthesis of CaO from dolomite in the presence of Sapindus rarak extract for biodiesel production: Catalysts characterization and optimization. RSC Adv. 2024, 14, 23332–23340. [Google Scholar] [CrossRef]
- Takase, M.; Chen, Y.; Liu, H.; Zhao, T.; Yang, L.; Wu, X. Biodiesel production from non-edible Silybum marianum oil using heterogeneous solid base catalyst under ultrasonication. Ultrason. Sonochemistry 2014, 21, 1752–1762. [Google Scholar] [CrossRef]
- Xiao, Y.; Hao, Y.; Yan, L.; Xu, Z.; Sui, Z.; Pan, Y.; Wang, C.; Bian, H.; Wang, X. Mechanism on surface hydrophobically modification of fibrous wollastonite and its reinforcement of natural rubber. J. Polym. Res. 2022, 29, 342. [Google Scholar] [CrossRef]
- Zhu, J.; Qu, T.; Niu, S.; Liu, J.; Liu, S.; Geng, J.; Yang, Z.; Abulizi, A. Preparation and characterization of a novel bifunctional heterogeneous Sr–La/wollastonite catalyst for biodiesel production. Mater. Today Sustain. 2024, 26, 100716. [Google Scholar] [CrossRef]
- Chen, L.; He, L.; Zheng, B.; Wei, G.; Li, H.; Zhang, H.; Yang, S. Bifunctional acid-activated montmorillonite catalyzed biodiesel production from non-food oil: Characterization, optimization, kinetic and thermodynamic studies. Fuel Process. Technol. 2023, 250, 107903. [Google Scholar] [CrossRef]
- Qu, T.; Niu, S.; Gong, Z.; Han, K.; Wang, Y.; Lu, C. Wollastonite decorated with calcium oxide as heterogeneous transesterification catalyst for biodiesel production: Optimized by response surface methodology. Renew. Energy 2020, 159, 873–884. [Google Scholar] [CrossRef]
- Amal, R.; Nadeem, R.; Intisar, A.; Rouf, H.; Hussain, D.; Kousar, R. An insight into the catalytic properties and process optimization of Fe, Ni doped eggshell derived CaO for a green biodiesel synthesis from waste chicken fat. Catal. Commun. 2024, 187, 106848. [Google Scholar] [CrossRef]
- Falowo, O.A.; Ojediran, O.J.; Moses, O.; Eghianruwa, R.; Betiku, E. A bifunctional catalyst from waste eggshells and its application in biodiesel synthesis from waste cooking oil. Results Eng. 2024, 23, 102613. [Google Scholar] [CrossRef]
- Tshizanga, N.; Aransiola, E.F.; Oyekola, O. Optimisation of biodiesel production from waste vegetable oil and eggshell ash. S. Afr. J. Chem. Eng. 2017, 23, 145–156. [Google Scholar] [CrossRef]
- Lani, N.S.; Ngadi, N.; Haron, S.; Inuwa, I.M.; Opotu, L.A. The catalytic effect of calcium oxide and magnetite loading on magnetically supported calcium oxide-zeolite catalyst for biodiesel production from used cooking oil. Renew. Energy 2024, 222, 119846. [Google Scholar] [CrossRef]
- Gupta, A.R.; Rathod, V.K. Waste cooking oil and waste chicken eggshells derived solid base catalyst for the biodiesel production: Optimization and kinetics. Waste Manag. 2018, 79, 169–178. [Google Scholar] [CrossRef]
- Yusuff, A.S.; Gbadamosi, A.O.; Atray, N. Development of a zeolite supported CaO derived from chicken eggshell as active base catalyst for used cooking oil biodiesel production. Renew. Energy 2022, 197, 1151–1162. [Google Scholar] [CrossRef]
- Khatibi, M.; Khorasheh, F.; Larimi, A. Biodiesel production via transesterification of canola oil in the presence of Na–K doped CaO derived from calcined eggshell. Renew. Energy 2021, 163, 1626–1636. [Google Scholar] [CrossRef]
- Khan, S.G.; Hassan, M.; Anwar, M.; Khan, U.M.; Zhao, C. Mussel shell based CaO nano-catalyst doped with praseodymium to enhance biodiesel production from castor oil. Fuel 2022, 330, 125480. [Google Scholar] [CrossRef]
- Chingakham, C.; David, A.; Sajith, V. Fe3O4 nanoparticles impregnated eggshell as a novel catalyst for enhanced biodiesel production. Chin. J. Chem. Eng. 2019, 27, 2835–2843. [Google Scholar] [CrossRef]
- Mohammed, A.S.; Ancha, V.R.; Atnaw, S.M. Optimization of biodiesel production from Croton Macrostachyus seed oil with calcium oxide (CaO) catalyst and Characterization: Potential assessment of seed and kernel. Energy Convers. Manag. X 2024, 24, 100791. [Google Scholar] [CrossRef]
- Wang, S.; Fu, H.; Abulizi, A.; Okitsu, K.; Maeda, Y.; Nulahong, A.; Ren, T.; Niu, S. Biodiesel production from sheep fat catalyzed by CaO-SrO (x)/BCs (y) acid-base bifunctional catalyst and process optimization. Renew. Energy 2025, 238, 121881. [Google Scholar] [CrossRef]
- Ashine, F.; Kiflie, Z.; Prabhu, S.V.; Tizazu, B.Z.; Varadharajan, V.; Rajasimman, M.; Joo, S.-W.; Vasseghian, Y.; Jayakumar, M. Biodiesel production from Argemone mexicana oil using chicken eggshell derived CaO catalyst. Fuel 2023, 332, 126166. [Google Scholar] [CrossRef]
- Li, L.; Li, L.; Ma, J.; Xie, F. Biodiesel production from the transesterification of waste cooking oil via CaO/HAP/MnFe@ K magnetic nanocatalyst derived from eggshells and chicken bones: Diesel engine and kinetic studies. Renew. Energy 2024, 237, 121563. [Google Scholar] [CrossRef]
- Sai, B.A.; Subramaniapillai, N.; Mohamed, M.S.B.K.; Narayanan, A. Optimization of continuous biodiesel production from rubber seed oil (RSO) using calcined eggshells as heterogeneous catalyst. J. Environ. Chem. Eng. 2020, 8, 103603. [Google Scholar] [CrossRef]
- Chen, G.; Shan, R.; Shi, J.; Yan, B. Ultrasonic-assisted production of biodiesel from transesterification of palm oil over ostrich eggshell-derived CaO catalysts. Bioresour. Technol. 2014, 171, 428–432. [Google Scholar] [CrossRef]
- Zhang, M.; Ramya, G.; Brindhadevi, K.; Alsehli, M.; Elfasakhany, A.; Xia, C.; Chi, N.T.L.; Pugazhendhi, A. Microwave assisted biodiesel production from chicken feather meal oil using Bio-Nano Calcium oxide derived from chicken egg shell. Environ. Res. 2022, 205, 112509. [Google Scholar] [CrossRef]
- Karkal, S.S.; Rathod, D.R.; Jamadar, A.S.; Mamatha, S.; Kudre, T.G. Production optimization, scale-up, and characterization of biodiesel from marine fishmeal plant oil using Portunus sanguinolentus crab shell derived heterogeneous catalyst. Biocatal. Agric. Biotechnol. 2023, 47, 102571. [Google Scholar] [CrossRef]
- Singh, W.R.; Singh, H.N. CCD-RSM optimization of biodiesel production from waste cooking oil using Angulyagra oxytropis and Bellamya crassa snail shell-based heterogeneous catalysts. Fuel 2024, 378, 132953. [Google Scholar] [CrossRef]
- Jindapon, W.; Jaiyen, S.; Ngamcharussrivichai, C. Seashell-derived mixed compounds of Ca, Zn and Al as active and stable catalysts for the transesterification of palm oil with methanol to biodiesel. Energy Convers. Manag. 2016, 122, 535–543. [Google Scholar] [CrossRef]
- Amesho, K.T.; Lin, Y.-C.; Chen, C.-E.; Cheng, P.-C.; Shangdiar, S. Kinetics studies of sustainable biodiesel synthesis from Jatropha curcas oil by exploiting bio-waste derived CaO-based heterogeneous catalyst via microwave heating system as a green chemistry technique. Fuel 2022, 323, 123876. [Google Scholar] [CrossRef]
- Kumar, G.; Singh, V.; Kumar, D. Ultrasonic-assisted continuous methanolysis of Jatropha curcas oil in the appearance of biodiesel used as an intermediate solvent. Ultrason. Sonochemistry 2017, 39, 384–391. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.M.; Iqbal, T.; Ali, C.H.; Javaid, A.; Cheema, I.I. Sustainable biodiesel production from waste cooking oil utilizing waste ostrich (Struthio camelus) bones derived heterogeneous catalyst. Fuel 2020, 277, 118091. [Google Scholar] [CrossRef]
- Adepoju, T.; Ibeh, M.; Asuquo, A. Elucidate three novel catalysts synthesized from animal bones for the production of biodiesel from ternary non-edible and edible oil blend: A case of Jatropha curcus, Hevea brasiliensis, and Elaeis guineensis oil. S. Afr. J. Chem. Eng. 2021, 36, 58–73. [Google Scholar] [CrossRef]
- Mohebolkhames, E.; Kazemeini, M.; Sadjadi, S. Utilization of Salmon fish bone wastes as a novel bio-based heterogeneous catalyst-support toward the production of biodiesel: Process optimizations and kinetics studies. Mater. Chem. Phys. 2024, 311, 128522. [Google Scholar] [CrossRef]
- Maleki, H.; Kazemeini, M.; Larimi, A.S.; Khorasheh, F. Transesterification of canola oil and methanol by lithium impregnated CaO–La2O3 mixed oxide for biodiesel synthesis. J. Ind. Eng. Chem. 2017, 47, 399–404. [Google Scholar] [CrossRef]
- Babatunde, E.O.; Bamidele, S.H.; Aderibigbe, F.A.; Yusuff, A.S.; Karmakar, B.; Rokhum, S.L.; Halder, G. Valorization of restaurant waste oil and cow-bone doped siliceous termite hills towards biodiesel production: Kinetics and thermodynamics. Sustain. Chem. Pharm. 2022, 30, 100895. [Google Scholar] [CrossRef]
- AlSharifi, M.; Znad, H. Development of a lithium based chicken bone (Li-Cb) composite as an efficient catalyst for biodiesel production. Renew. Energy 2019, 136, 856–864. [Google Scholar] [CrossRef]
- Chakraborty, R.; Mukhopadhyay, P.; Kumar, B. Optimal biodiesel-additive synthesis under infrared excitation using pork bone supported-Sb catalyst: Engine performance and emission analyses. Energy Convers. Manag. 2016, 126, 32–41. [Google Scholar] [CrossRef]
- Kader, S.; Jusoh, M.; Zakaria, Z. Palm fatty acid distillate-based biodiesel with sulfonated chicken and cow bone catalyst. Mater. Today: Proc. 2022, 57, 1053–1060. [Google Scholar] [CrossRef]
- Sunday, U.C.; Chizoo, E.; Blessing, I.N.; Kingsley, A.A.; John, O.I.; Chukwu, E.G.; Nonso, U.C.; Blessing, E.C.; Mbamalu, E.E.; Uwaoma, O.A. Bioresource integration approach of sustainable green-diesel production from waste mutton using waste goat bone as biohetrogeneous catalyst. Therm. Sci. Eng. Prog. 2024, 53, 102711. [Google Scholar] [CrossRef]
- Alsaiari, R.A.; Musa, E.M.; Rizk, M.A. Biodiesel production from date seed oil using hydroxyapatite-derived catalyst from waste camel bone. Heliyon 2023, 9, e15606. [Google Scholar] [CrossRef]
- Farooq, M.; Ramli, A.; Naeem, A. Biodiesel production from low FFA waste cooking oil using heterogeneous catalyst derived from chicken bones. Renew. Energy 2015, 76, 362–368. [Google Scholar] [CrossRef]
- Mengistu, T.G.; Reshad, A.S. Synthesis and characterization of a heterogeneous catalyst from a mixture of waste animal teeth and bone for castor seed oil biodiesel production. Heliyon 2022, 8, e09724. [Google Scholar] [CrossRef] [PubMed]
- Nisar, J.; Razaq, R.; Farooq, M.; Iqbal, M.; Khan, R.A.; Sayed, M.; Shah, A.; Rahman, I.U. Enhanced biodiesel production from Jatropha oil using calcined waste animal bones as catalyst. Renew. Energy 2017, 101, 111–119. [Google Scholar] [CrossRef]
- Jayakumar, M.; Gebeyehu, K.B.; Selvakumar, K.V.; Parvathy, S.; Kim, W.; Karmegam, N. Waste Ox bone based heterogeneous catalyst synthesis, characterization, utilization and reaction kinetics of biodiesel generation from Jatropha curcas oil. Chemosphere 2022, 288, 132534. [Google Scholar] [CrossRef]
- Krishnan, M.G.; Rajkumar, S.; Devasagar, T. The sustainable prospect of biodiesel production: Transformative technologies, catalysts from bio-wastes, and techno-economic assessment. Mater. Today Proc. 2024, 451, 142112. [Google Scholar] [CrossRef]
- Oke, E.O.; Adeyi, O.; Okolo, B.I.; Ude, C.J.; Adeyi, J.A.; Salam, K.K.; Nwokie, U.; Nzeribe, I. Heterogeneously catalyzed biodiesel production from Azadiricha Indica oil: Predictive modelling with uncertainty quantification, experimental optimization and techno-economic analysis. Bioresour. Technol. 2021, 332, 125141. [Google Scholar] [CrossRef]
- Foroutan, R.; Peighambardoust, S.J.; Foroughi, M.; Peighambardoust, N.S.; Maleki, B.; Ramavandi, B. Recycling the powder of spent alkaline batteries as a sustainable and reusable catalyst in producing biodiesel from waste cooking oil. Environ. Res. 2025, 271, 121028. [Google Scholar] [CrossRef]
- Hlaibi, M.; Kamil, N. Highly efficient sustainable heterogeneous catalyst derived from onion peels (Allium sepa L.) for the ecological biodiesel production using non-edible feedstock. Energy Convers. Manag. 2024, 315, 118801. [Google Scholar] [CrossRef]
- Rhithuparna, D.; Ghosh, N.; Khatoon, R.; Rokhum, S.L.; Halder, G. Evaluating the commercial potential of Cocos nucifera derived biochar catalyst in biodiesel synthesis from Kanuga oil: Optimization, kinetics, thermodynamics, and process cost analysis. Process Saf. Environ. Prot. 2024, 183, 859–874. [Google Scholar] [CrossRef]
- Osagiede, C.A.; Aisien, F.A. Biochar-based bi-functional catalyst derived from rubber seed shell and eggshell for biodiesel production from waste cooking oil. Fuel 2024, 358, 130076. [Google Scholar] [CrossRef]
- Mawlid, O.A.; Abdelhady, H.H.; El-Moghny, M.G.A.; Hamada, A.; Abdelnaby, F.; Kased, M.; Al-Bajouri, S.; Elbohy, R.A.; El-Deab, M.S. Clean approach for catalytic biodiesel production from waste frying oil utilizing K2CO3/Orange peel derived hydrochar via RSM Optimization. J. Clean. Prod. 2024, 442, 140947. [Google Scholar] [CrossRef]
- Fu, H.; Xiao, Y.; Abulizi, A.; Okitsu, K.; Maeda, Y.; Ren, T. Surfactant-modified ZnFe2O4/CaOPS porous acid-base bifunctional catalysts for biodiesel production from waste cooking oil and process optimization. J. Environ. Chem. Eng. 2024, 12, 114234. [Google Scholar] [CrossRef]
- Gaurav, A.; Ng, F.T.; Rempel, G.L. A new green process for biodiesel production from waste oils via catalytic distillation using a solid acid catalyst–Modeling, economic and environmental analysis. Green Energy Environ. 2016, 1, 62–74. [Google Scholar] [CrossRef]
- Ruatpuia, J.V.; Halder, G.; Mohan, S.; Gurunathan, B.; Li, H.; Chai, F.; Basumatary, S.; Rokhum, S.L. Microwave-assisted biodiesel production using ZIF-8 MOF-derived nanocatalyst: A process optimization, kinetics, thermodynamics and life cycle cost analysis. Energy Convers. Manag. 2023, 292, 117418. [Google Scholar] [CrossRef]
- Roy, S.; Yadav, G.; Ahmaruzzaman, M. Engineering of metal-organic framework functionalized by Fe (III) exchanged keggin unit for microwave-assisted sustainable production of biodiesel: Kinetics, thermodynamics, mechanistic insights and life-cycle cost analysis. Energy Convers. Manag. 2024, 313, 118625. [Google Scholar] [CrossRef]
- Hou, S.; Xie, W. Three-dimensional hierarchical meso/macroporous Mo/Ce/TiO2 composites enhances biodiesel production from acidic soybean oil by transesterification-esterifiications. Energy Convers. Manag. 2024, 305, 118273. [Google Scholar] [CrossRef]
- Sánchez, N.; Encinar, J.M.; Nogales, S.; González, J.F. Biodiesel production from castor oil by two-step catalytic transesterification: Optimization of the process and economic assessment. Catalysts 2019, 9, 864. [Google Scholar] [CrossRef]
- Yang, J.; Cong, W.-J.; Zhu, Z.; Miao, Z.-D.; Wang, Y.-T.; Nelles, M.; Fang, Z. Microwave-assisted one-step production of biodiesel from waste cooking oil by magnetic bifunctional SrO–ZnO/MOF catalyst. J. Clean. Prod. 2023, 395, 136182. [Google Scholar] [CrossRef]
- Sangeetha, B.; Baskar, G. Process design, kinetics, simulation, and techno-economic analysis of biodiesel production from Pongamia pinnata seed oil using a magnetically recyclable acidic ionic liquid catalyst. Energy Convers. Manag. 2024, 301, 118040. [Google Scholar] [CrossRef]
- Yadav, M.; Sharma, Y.C. Transesterification of used vegetable oil using BaAl2O4 spinel as heterogeneous base catalyst. Energy Convers. Manag. 2019, 198, 111795. [Google Scholar] [CrossRef]
- Roy, T.; Sahani, S.; Sharma, Y.C. Green synthesis of biodiesel from Ricinus communis oil (castor seed oil) using potassium promoted lanthanum oxide catalyst: Kinetic, thermodynamic and environmental studies. Fuel 2020, 274, 117644. [Google Scholar] [CrossRef]
- Malik, M.A.I.; Zeeshan, S.; Khubaib, M.; Ikram, A.; Hussain, F.; Yassin, H.; Qazi, A. A review of major trends, opportunities, and technical challenges in biodiesel production from waste sources. Energy Convers. Manag. X 2024, 23, 100675. [Google Scholar] [CrossRef]
- Corral-Bobadilla, M.; Lostado-Lorza, R.; Sabando-Fraile, C.; Íñiguez-Macedo, S. An artificial intelligence approach to model and optimize biodiesel production from waste cooking oil using life cycle assessment and market dynamics analysis. Energy 2024, 307, 132712. [Google Scholar] [CrossRef]
- Hosseinzadeh-Bandbafha, H.; Tan, Y.H.; Kansedo, J.; Mubarak, N.; Liew, R.K.; Yek, P.N.Y.; Aghbashlo, M.; Ng, H.S.; Chong, W.W.F.; Lam, S.S.; et al. Assessing biodiesel production using palm kernel shell-derived sulfonated magnetic biochar from the life cycle assessment perspective. Energy 2023, 282, 128758. [Google Scholar] [CrossRef]
- Ao, S.; Gouda, S.P.; Selvaraj, M.; Boddula, R.; Al-Qahtani, N.; Mohan, S.; Rokhum, S.L. Active sites engineered biomass-carbon as a catalyst for biodiesel production: Process optimization using RSM and life cycle assessment. Energy Convers. Manag. 2024, 300, 117956. [Google Scholar] [CrossRef]
- Ao, S.; Gouda, S.P.; Selvaraj, M.; Boddula, R.; Al-Qahtani, N.; Mohan, S.; Rokhum, S.L. Transesterification of Jatropha curcas oil to biodiesel using highly porous sulfonated biochar catalyst: Optimization and characterization dataset. Data Brief 2024, 53, 110096. [Google Scholar] [CrossRef] [PubMed]



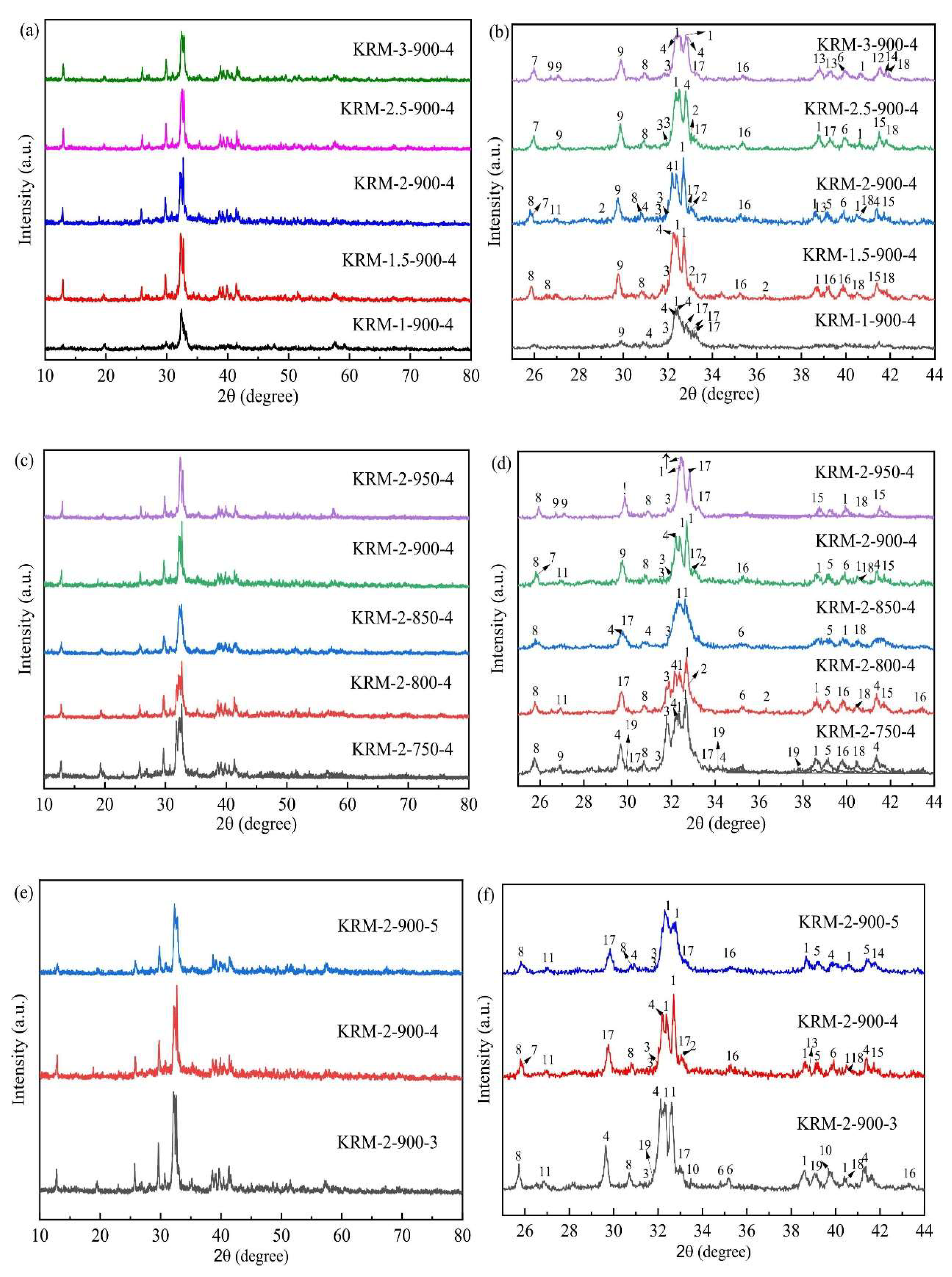
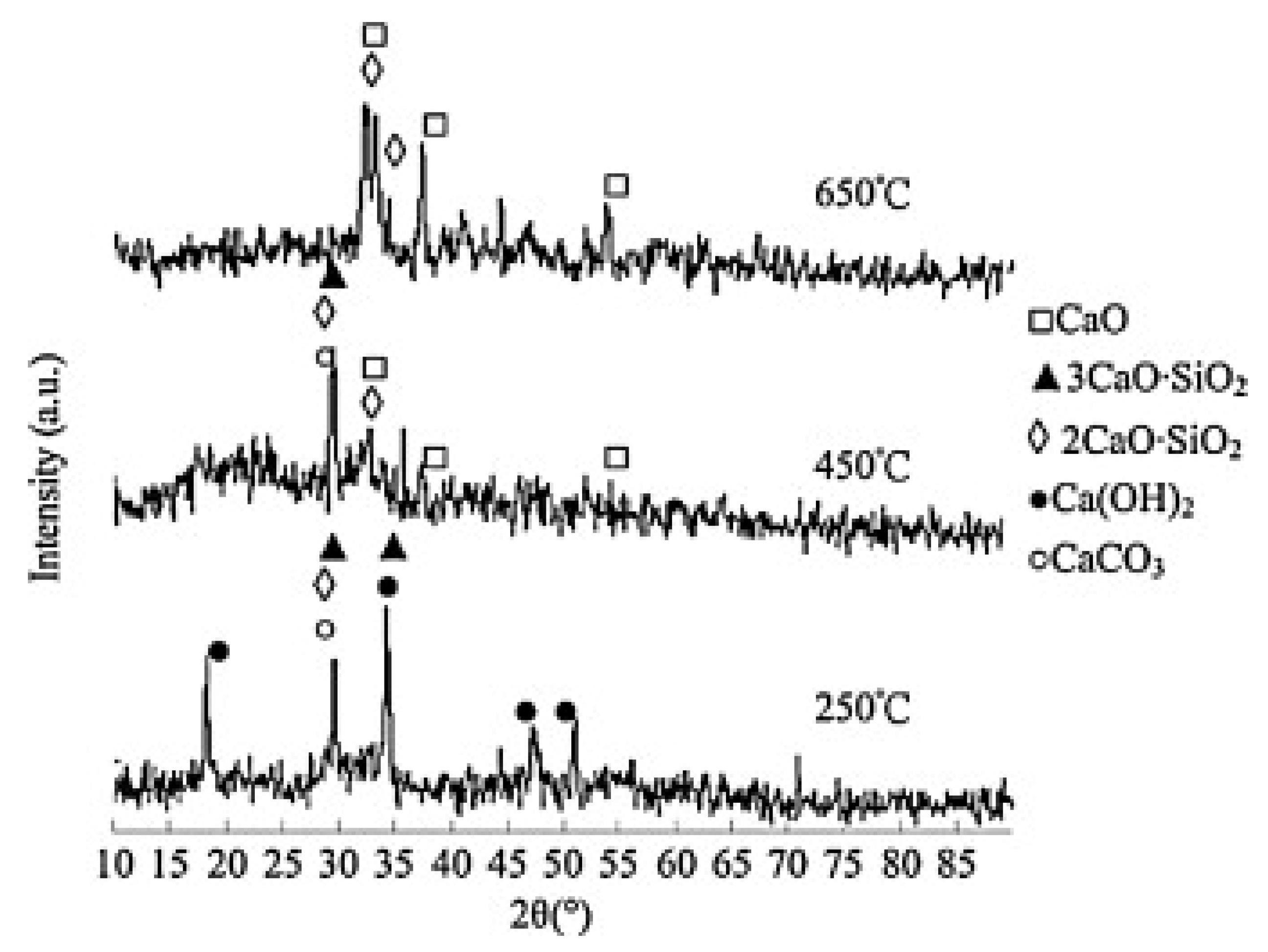



| No. | Catalyst Source | Feedstock | Catalyst | Optimum Conditions | Yield (%) | Citation | |||
|---|---|---|---|---|---|---|---|---|---|
| A | B | C | D | ||||||
| 1 | Rice husk | UCO | MoO3/RHA-CoFe2O4 | 20:1 | 6 | 240 | 160 | 94.60 | [24] |
| 2 | Betel leaf | Soybean oil, jatropha oil, and pongamia oil | Biochar/K2CO3 | 9:1 | 15 | 201 | 65 | 96.57 | [25] |
| 3 | Murici seeds | Soybean oil | Biochar/CoFe2O4 | 17:1 | 7 | 84 | 90 | 97.11 | [26] |
| 4 | Banana peel | Safflower oil | C/Zn | 22.4:1 | 6.63 | 53.55 | 85 | 97.12 | [27] |
| 5 | Jackfruit peel | UCO | K/Ca/Mg | 9:1 | 12 | 105 | 65 | 97.42 | [28] |
| 6 | Rice husk | Dairy waste oil | CuO/RHA | 11.12:1 | 2.76 | 171 | 62.36 | 97.42 | [29] |
| 7 | Banana peel | Palm oil | K2O/Na2O | 6:1 | 2 | 90 | 65 | 98.06 | [30] |
| 8 | Banana peel, stem, rhizome | Neem oil | K2O/CaO | 9:1 | 5 | 10 | 65 | 98.27 | [21] |
| 9 | Sorghum-sugarcane bagasse | Pork fat oil | K/Ca/Mg | 8.57:1 | 3.15 | 69.96 | 79.93 | 98.52 | [31] |
| 10 | Orange peel—Banana peel | Flaxseed oil | CaO | 10:1 | 3 | 50 | 70 | 98.78 | [23] |
| 11 | Palm kernel shell | Used palm oil | Na2O/SiO2 | 15:1 | 6 | 90 | 50 | 99.01 | [32] |
| 12 | Corn pod | Papaya oil | CaO/K2O | 5.99:1 | 3.96 | 72.42 | 70 | 99.06 | [33] |
| 13 | Rice husk | Oleic acid | Biochar/H2SO4 | 24:1 | 8 | 60 | 80 | 99.60 | [34] |
| 14 | Bamboo—Coconut shell | Oleic acid | Biochar/H2SO4 | 9:1 | 3 | 40 | 80 | 99.60 | [35] |
| No. | Catalyst Source | Feedstock | Catalyst | Optimum Conditions | Yield (%) | Citation | |||
|---|---|---|---|---|---|---|---|---|---|
| A | B | C | D | ||||||
| 1 | Blast furnace dust | Palm oil | Fe/CaO | 15.24:1 | 7.96 | 120 | 148.95 | 87.67 | [80] |
| 2 | Red mud—Brazilian berry seeds | Oleic acid | Fe/H2SO4 | 12:1 | 5 | 60 | 100 | 88 | [81] |
| Blast furnace slag | UCO | SFCA | 20:1 | 20 | 720 | 60 | 90.77 | [82] | |
| 4 | Blast furnace slag | UCO | K2SiO3 | 15:1 | 5 | 180 | 60 | 93.15 | [83] |
| 5 | Red mud | Soybean oil | Ca/Ti/K | 24:1 | 4 | 180 | 65 | 94 | [84] |
| 6 | Red mud | Castor oil | RM/K | 18:1 | 5 | 150 | 65 | 95 | [85] |
| 7 | Aluminum industry scrap | UCO | CaO/Al2O3 | 7:1 | 3 | 180 | 45 | 95 | [86] |
| 8 | Red mud | Castor oil | RM/Li | 15:1 | 5 | 150 | 65 | 96.32 | [87] |
| 9 | Aluminum industry scrap | UCO | KAlO | 9:1 | 5.8 | 120 | 25 | 98.7 | [88] |
| 10 | Electric furnace dust—reed straw | Soybean oil | Fe/Na2CO3 | 14.06:1 | 7.75 | 249.6 | 74.15 | 99.89 | [89] |
| 11 | Steel ladle furnace slag—kaolin | Soybean oil | CaSiO3 | 7.5:1 | 3 | 240 | 60 | [90] | |
| No. | Catalyst Source | Feedstock | Catalyst | Optimum Conditions | Yield (%) | Citation | |||
|---|---|---|---|---|---|---|---|---|---|
| A | B | C | D | ||||||
| 1 | Eggshell | Chicken fat oil | CaO/Fe | 15:1 | 3 | 300 | 65 | 83 | [129] |
| 2 | Eggshell | UCO | CaSO4/Fe2O3 | 5:1 | 15 | 201 | 65 | 89.45 | [130] |
| 3 | Eggshell | UCO | CaO | 22.5:1 | 4 | 45 | 70 | 91 | [131] |
| 4 | Eggshell | UCO | CaO-ZSM/Fe | 5:1 | 3.5 | 330 | 65 | 91 | [132] |
| 5 | Eggshell | UCO | CaO/MeOH | 10:1 | 1.5 | 90 | 60 | 93.1 | [133] |
| 6 | Eggshell-Zeolite | UCO | CaO | 9.7:1 | 2.1 | 238.8 | 69.1 | 93.7 | [134] |
| 7 | Eggshell | Rapeseed oil | CaO/Na-K | 9:1 | 3 | 180 | 50 | 97.6 | [135] |
| 8 | Eggshell | Rubber seed oil | CaO | 9:1 | 5 | 240 | 60 | 97.84 | [136] |
| 9 | Eggshell | P. pinnata oil | CaO/Fe | 12:1 | 2 | 120 | 65 | 98 | [137] |
| 10 | Eggshell | Castor bean seed oil | CaO | 8.44:1 | 2.78 | 108 | 60 | 98.31 | [138] |
| 11 | Eggshell—Poplar Leaf | Sheep fat oil | CaO-SrO/C | 8:1 | 6 | 90 | 65 | 98.83 | [139] |
| 12 | Eggshell | Argemone mexicana oil | CaO | 9.7:1 | 3.05 | 180 | 60 | 99.07 | [140] |
| 13 | Eggshells—chicken bones | UCO | CaO-HAp/MnFe-K | 15.24:1 | 2.97 | 175.72 | 67.72 | 99.1 | [141] |
| No. | Feedstock | Catalyst | Price (T/USD) | Citation |
|---|---|---|---|---|
| 1 | UCO | Used alkaline batteries | 579 | [166] |
| 2 | Soybean oil | Eggshell/MOF@ZnCo-LDH | 420 | [8] |
| 3 | Sheep fat oil | Poplar leaves/eggshells | 522 | [139] |
| 4 | Palm fatty acid distillate | Onion peel/sulfuric acid | 114 | [167] |
| 5 | Safflower oil | Banana peel/sulfuric acid | 814 | [27] |
| 6 | Kanuga oil | Coconut shell/sulfuric acid | 833 | [168] |
| 7 | UCO | Rubber seed shells/eggshells | 370 | [169] |
| 8 | UCO | Orange peel | 1120 | [170] |
| 9 | UCO | Eggshell/ZnFe2O4 | 584 | [171] |
| 10 | UCO | Corn cob/KOH | 776 | [48] |
| 11 | Castor oil | Red mud/K2CO3 | 718 | [85] |
| 12 | Neem oil | Animal skeleton | 1060 | [165] |
| 13 | FFA | Al2O3/HSiW | 2240 | [172] |
| 14 | Soybean oil | CaO/ZnO | 1110 | [173] |
| 15 | OA | FPW-HK | 593 | [174] |
| 16 | Soybean oil | Mo/Ce/H-TiO | 1090 | [175] |
| 17 | Castor oil | NaOH | 1496 | [176] |
| 18 | Soybean oil | CaO | 1294 | [119] |
| 19 | UCO | SrO–ZnO/MOF | 710 | [177] |
| 20 | P. pinnata oil | Fe3O4/SiO2/PAIL | 980 | [178] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, Z.; Dong, K.; Li, H.; Zhang, L.; Wang, Y. Progress and Challenges in the Process of Using Solid Waste as a Catalyst for Biodiesel Synthesis. Molecules 2025, 30, 3243. https://doi.org/10.3390/molecules30153243
Dong Z, Dong K, Li H, Zhang L, Wang Y. Progress and Challenges in the Process of Using Solid Waste as a Catalyst for Biodiesel Synthesis. Molecules. 2025; 30(15):3243. https://doi.org/10.3390/molecules30153243
Chicago/Turabian StyleDong, Zhaolin, Kaili Dong, Haotian Li, Liangyi Zhang, and Yitong Wang. 2025. "Progress and Challenges in the Process of Using Solid Waste as a Catalyst for Biodiesel Synthesis" Molecules 30, no. 15: 3243. https://doi.org/10.3390/molecules30153243
APA StyleDong, Z., Dong, K., Li, H., Zhang, L., & Wang, Y. (2025). Progress and Challenges in the Process of Using Solid Waste as a Catalyst for Biodiesel Synthesis. Molecules, 30(15), 3243. https://doi.org/10.3390/molecules30153243








