In Situ Construction of Thiazole-Linked Covalent Organic Frameworks on Cu2O for High-Efficiency Photocatalytic Tetracycline Degradation
Abstract
1. Introduction
2. Results and Discussion
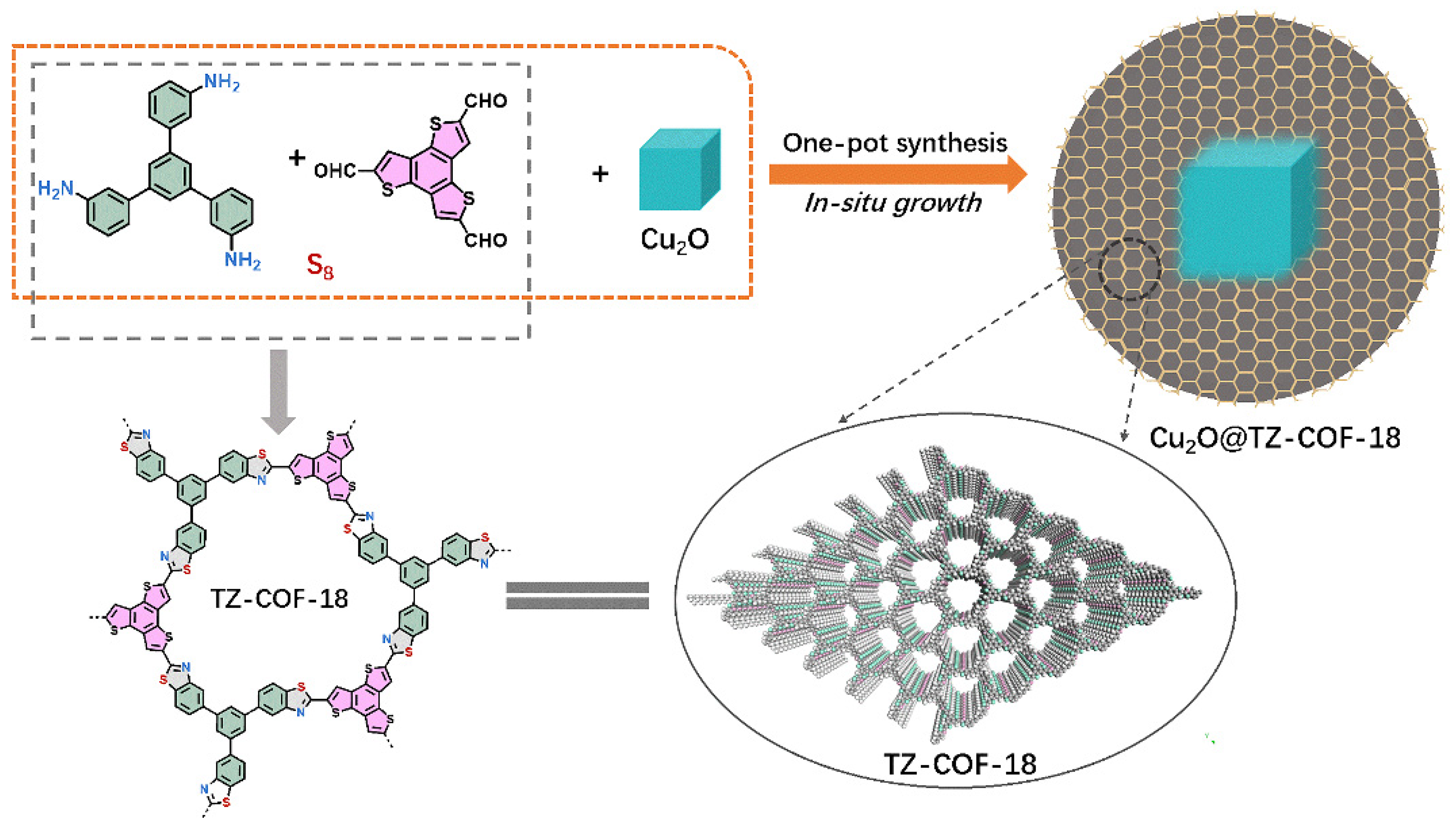
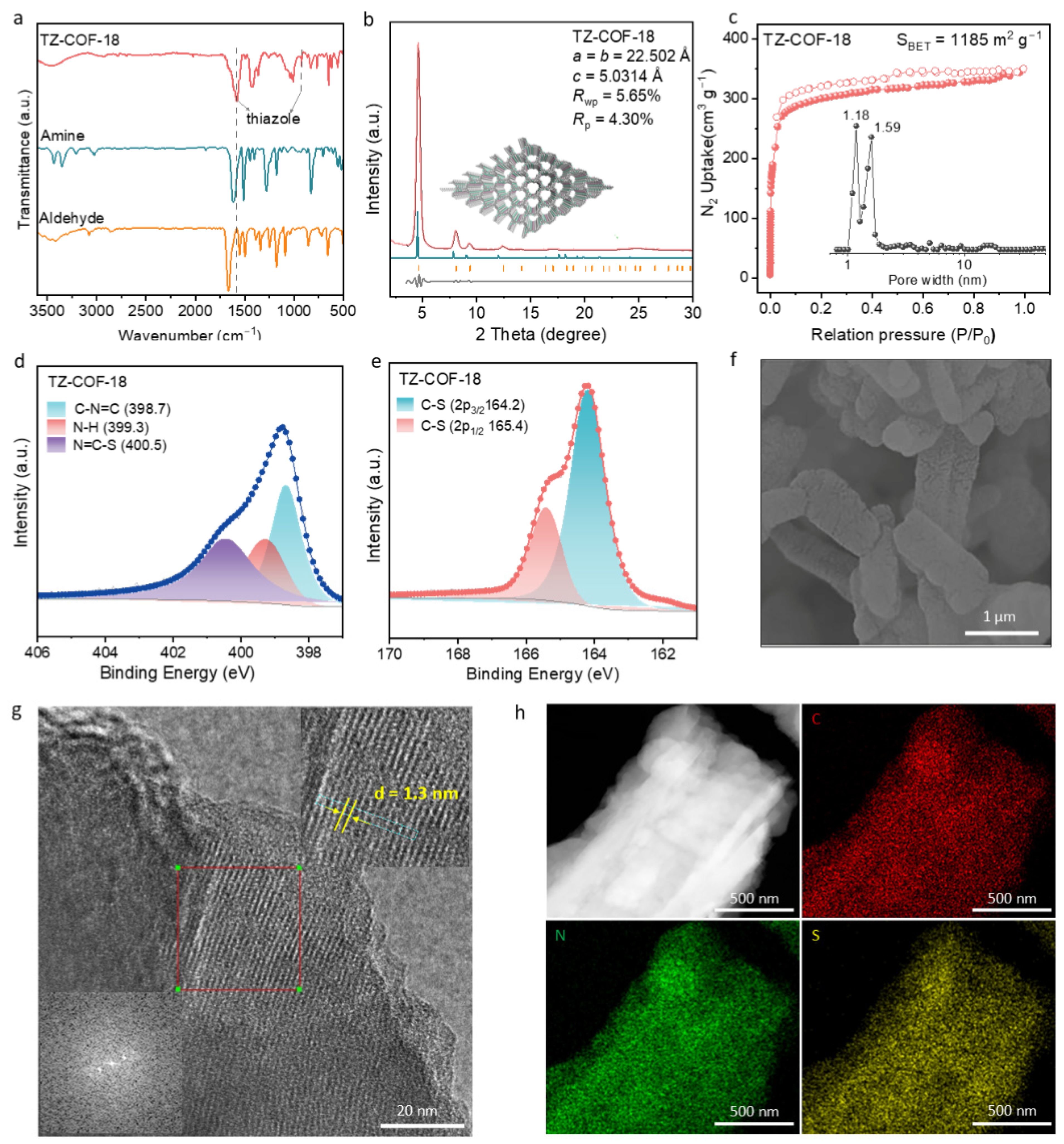
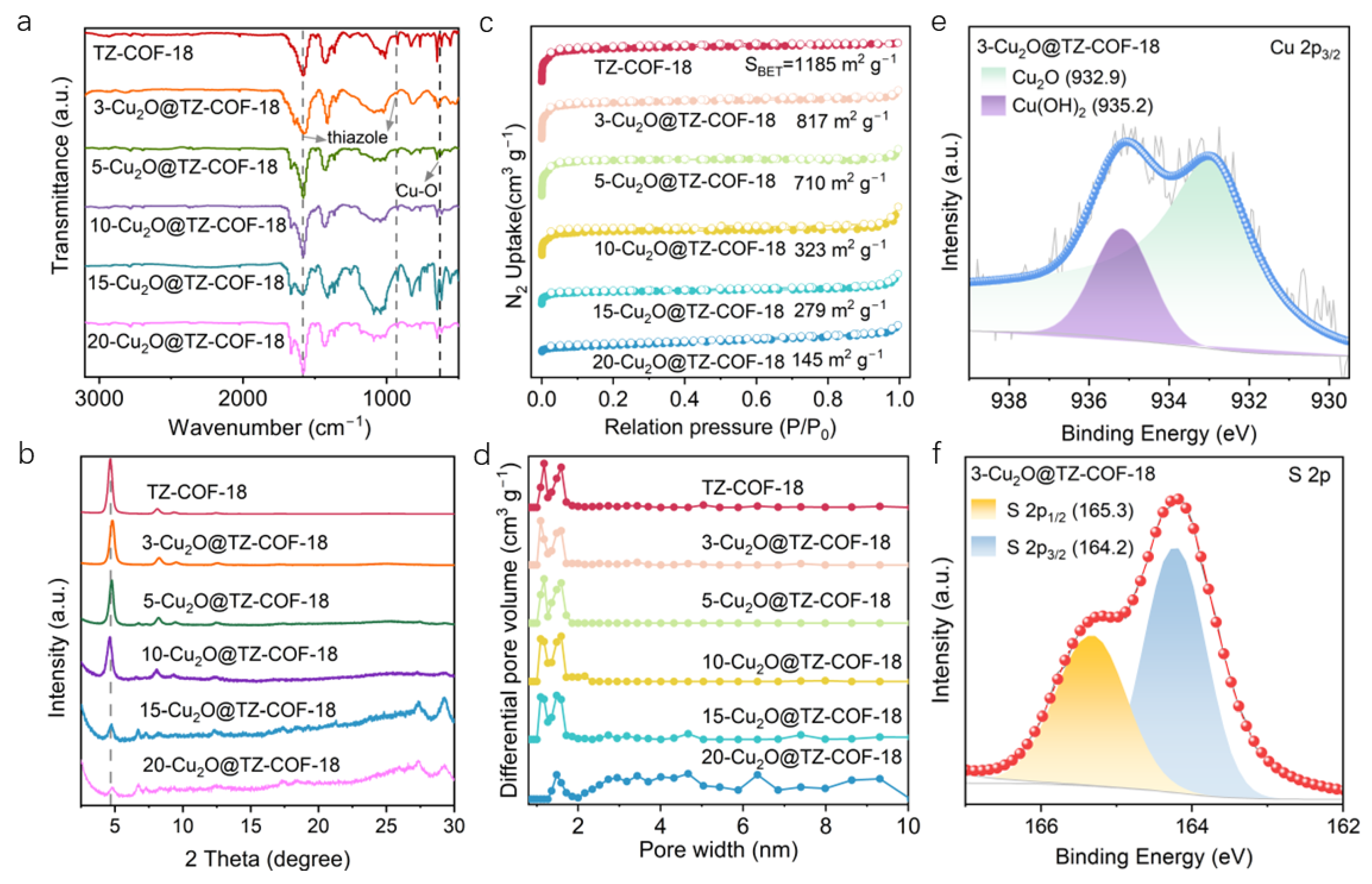
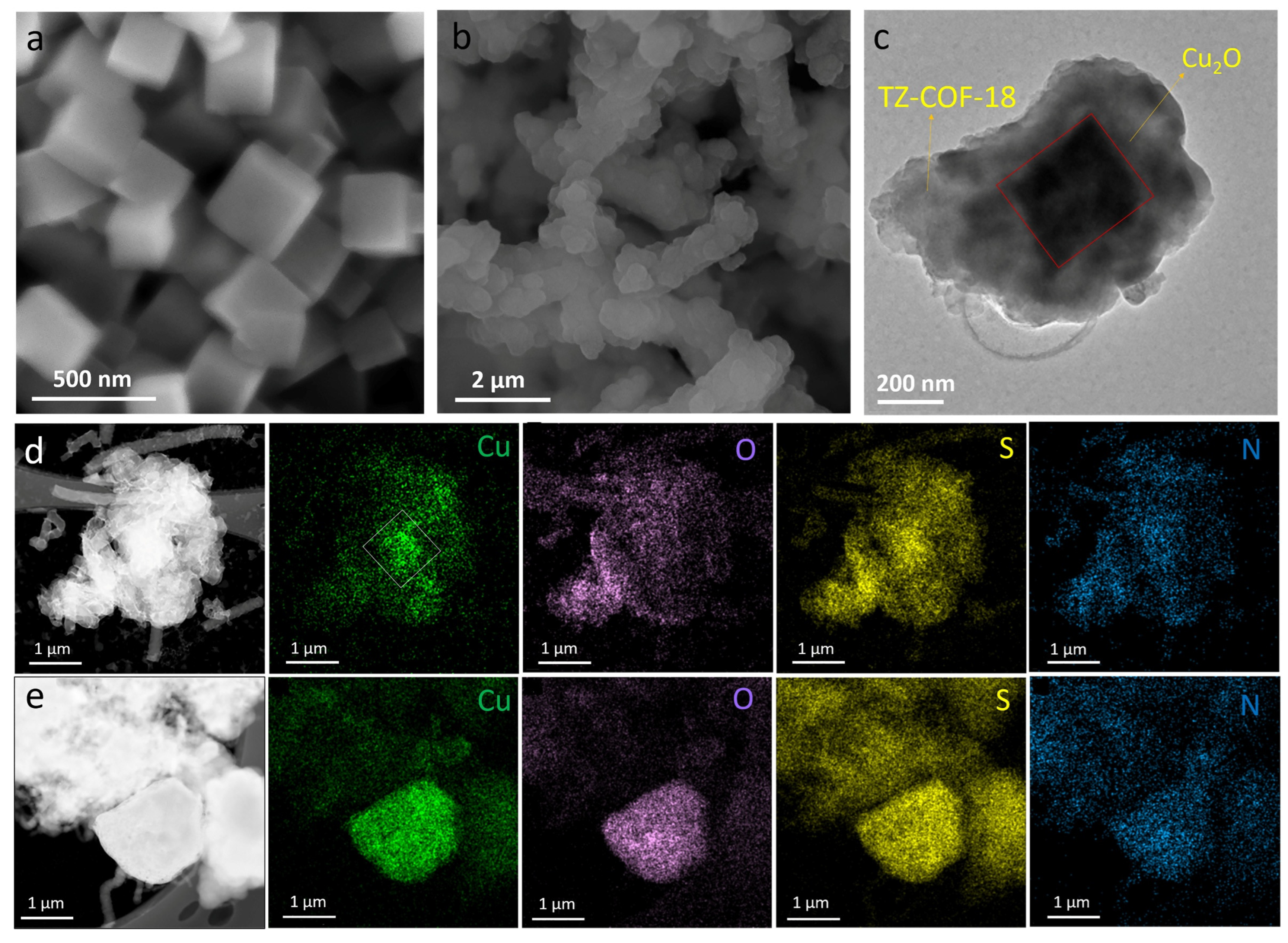
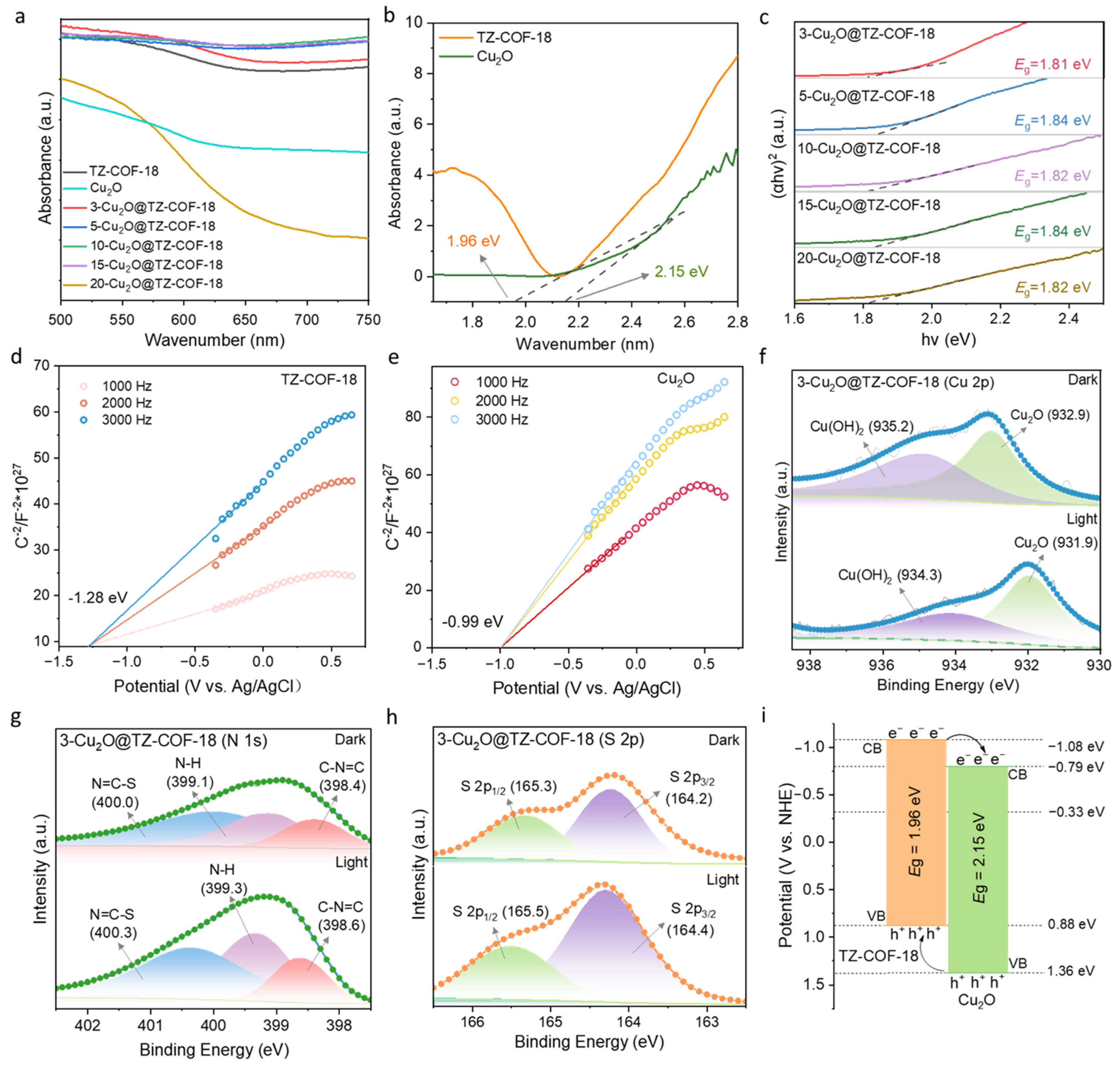
2.1. Characterizations
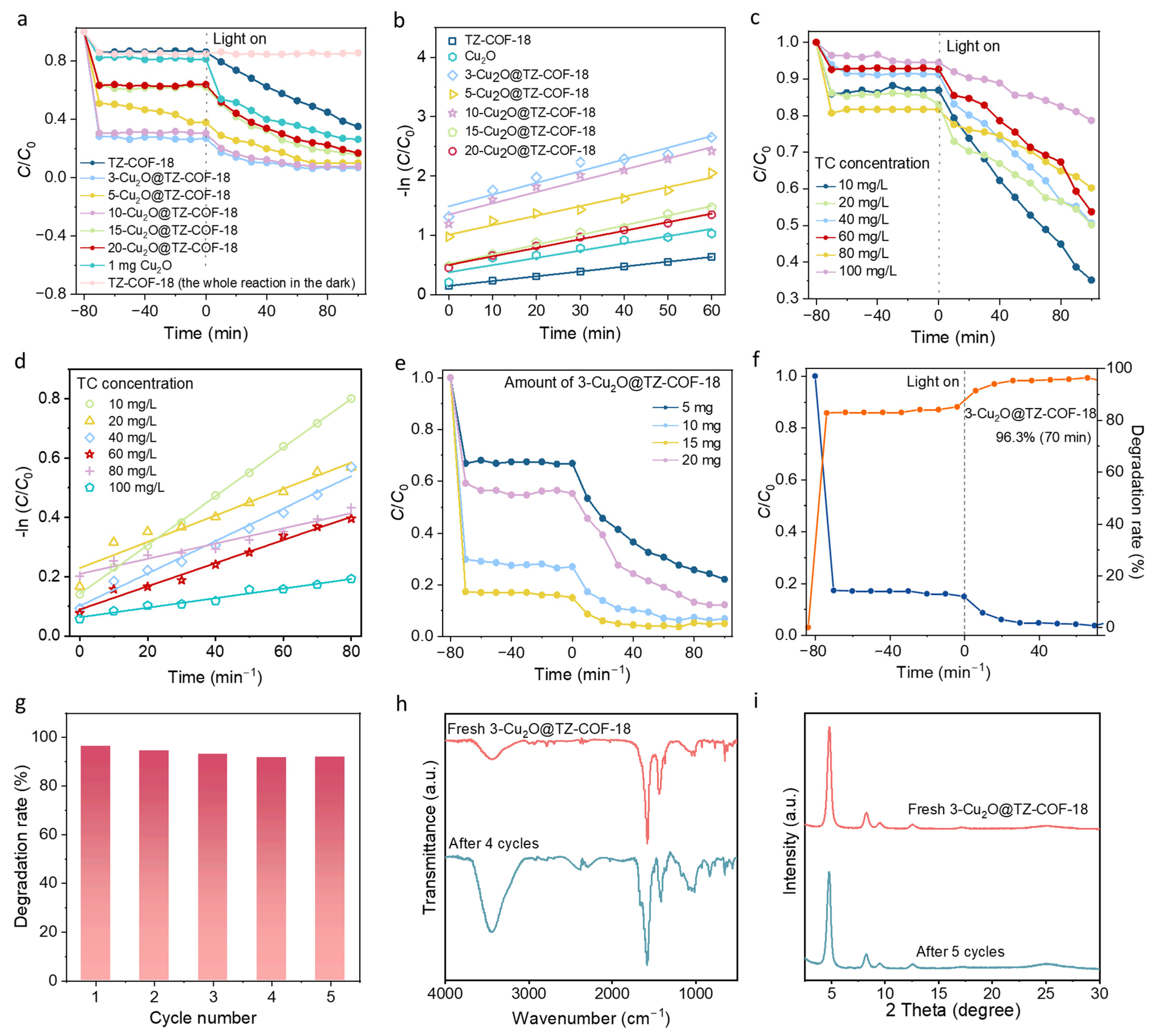
2.2. Photocatalytic Degradation Performance
2.3. Photocatalytic Degradation Mechanism
3. Experimental Section
3.1. Synthesis of TZ-COF-18
3.2. Synthesis of Cu2O@TZ-COF-18
3.3. Photocatalytic Degradation Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Feng, L.; Cheng, Y.; Zhang, Y.; Li, Z.; Yu, Y.; Feng, L.; Zhang, S.; Xu, L. Distribution and human health risk assessment of antibiotic residues in large-scale drinking water sources in Chongqing area of the Yangtze River. Environ. Res. 2020, 185, 109386. [Google Scholar] [CrossRef]
- Xing, W.; Xu, X.; Liu, C.; Wang, Y.; Wan, Z.; Han, J.; Wu, G.; Huang, Y. Rapid exciton dissociation and charge transfer endowed by reinforced interfacial interaction in a mixed-dimensional 2D/3D heterojunction nanocatalyst for high-efficiency photocatalysis. ACS Appl. Nano Mater. 2024, 7, 16725–16734. [Google Scholar] [CrossRef]
- Boyjoo, Y.; Wang, M.; Pareek, V.K.; Liu, J.; Jaroniec, M. Synthesis and applications of porous non-silica metal oxide submicrospheres. Chem. Soc. Rev. 2016, 45, 6013–6047. [Google Scholar] [CrossRef]
- Wei, M.; Huo, J.; Lun, N.; Ma, X.; Wen, S. A new semiconductor photocatalyst-cuprous oxide nanoparticles. Mater. Rev. 2007, 21, 130–133. [Google Scholar]
- Wang, J.; Li, S.; Ma, P.; Guo, Z.; Ma, Q.; Zhao, Q.; Guo, Y.; Zhao, J.; Guan, G. Carbon quantum dots/Cu2O S-scheme heterojunction for enhanced photocatalytic degradation of tetracycline. Colloids Surf. A Physicochem. Eng. Asp. 2024, 690, 133779. [Google Scholar] [CrossRef]
- Geng, K.; He, T.; Liu, R.; Dalapati, S.; Tan, K.T.; Li, Z.; Tao, S.; Gong, Y.; Jiang, Q.; Jiang, D. Covalent organic frameworks: Design, synthesis, and functions. Chem. Rev. 2020, 120, 8814–8933. [Google Scholar] [CrossRef] [PubMed]
- Waller, P.J.; Gándara, F.; Yaghi, O.M. Chemistry of covalent organic frameworks. Acc. Chem. Res. 2015, 48, 3053–3063. [Google Scholar] [CrossRef]
- Guo, L.; Jin, S. Stable covalent organic frameworks for photochemical applications. ChemPhotoChem 2019, 3, 973–983. [Google Scholar] [CrossRef]
- Li, Y.; Chen, W.; Xing, G.; Jiang, D.; Chen, L. New synthetic strategies toward covalent organic frameworks. Chem. Soc. Rev. 2020, 49, 2852–2868. [Google Scholar] [CrossRef]
- Pan, J.; Guo, L.; Zhang, S.; Wang, N.; Jin, S.; Tan, B. Embedding carbon nitride into a covalent organic framework with enhanced photocatalysis performance. Chem. Asian J. 2018, 13, 1674–1677. [Google Scholar] [CrossRef]
- Ahmadijokani, F.; Ghaffarkhah, A.; Molavi, H.; Dutta, S.; Lu, Y.; Wuttke, S.; Kamkar, M.; Rojas, O.J.; Arjmand, M. COF and MOF hybrids: Advanced materials for wastewater treatment. Adv. Funct. Mater. 2023, 34, 2305527. [Google Scholar] [CrossRef]
- Chen, W.; Yang, Z.; Xie, Z.; Li, Y.; Yu, X.; Lu, F.; Chen, L. Benzothiadiazole functionalized D–A type covalent organic frameworks for effective photocatalytic reduction of aqueous chromium (VI). J. Mater. Chem. A 2019, 7, 998–1004. [Google Scholar] [CrossRef]
- Yang, L.; Wang, Y.; Yuan, J.; Wang, G.; Cao, Q.; Fei, H.; Li, M.; Shao, J.; Li, H.; Lu, J. Construction of covalent-integrated MOFs@COFs composite material for efficient synergistic adsorption and degradation of pollutants. Chem. Eng. 2022, 446, 137095. [Google Scholar] [CrossRef]
- Wang, H.; Guan, L.; Liu, J.; Lei, T.; Xue, Y.; Qu, Z.; Jin, S.; Ma, H.; Guo, Z. A thiazolo [5,4-d] thiazole functionalized covalent triazine framework showing superior photocatalytic activity for hydrogen production and dye degradation. J. Mater. Chem. A 2022, 10, 16328–16336. [Google Scholar] [CrossRef]
- Wang, K.; Jia, Z.; Bai, Y.; Wang, X.; Hodgkiss, S.E.; Chen, L.; Chong, S.Y.; Wang, X.; Yang, H.; Xu, Y.; et al. Synthesis of stable thiazole-linked covalent organic frameworks via a multicomponent reaction. J. Am. Chem. Soc. 2020, 142, 11131–11138. [Google Scholar] [CrossRef]
- Wang, K.; Wu, Z.; Ji, N.; Wang, T.; Gu, Y.; Zhao, Z.; Guo, Y.; Wang, X.; Jia, Z.; Tan, B. Robust thiazole-linked covalent organic frameworks for water sensing with high selectivity and sensitivity. Molecules 2024, 29, 1677. [Google Scholar] [CrossRef]
- Liu, H.; Wang, D.; Yu, Z.; Chen, Y.; Li, X.; Zhang, R.; Chen, X.; Wu, L.; Ding, N.; Wang, Y.; et al. Fully conjugated two-dimensional sp2-carbon covalent organic frameworks for efficient photocatalytic hydrogen generation. Sci. China Mater. 2023, 66, 2283–2289. [Google Scholar] [CrossRef]
- Haase, F.; Troschke, E.; Savasci, G.; Banerjee, T.; Duppel, V.; Dörfler, S.; Grundei, M.M.J.; Burow, A.M.; Ochsenfeld, C.; Kaskel, S.; et al. Topochemical conversion of an imine- into a thiazole-linked covalent organic framework enabling real structure analysis. Nat. Comm. 2018, 9, 2600–2610. [Google Scholar] [CrossRef]
- Biswal, B.P.; Vignolo-González, H.A.; Banerjee, T.; Grunenberg, L.; Savasci, G.K.; Gottschling, K.; Nuss, J.R.; Ochsenfeld, C.; Lotsch, B.V. Sustained solar H2 evolution from a thiazolo [5,4-d] thiazole-bridged covalent organic framework and nickel-thiolate cluster in water. J. Am. Chem. Soc. 2019, 141, 11082–11092. [Google Scholar] [CrossRef]
- Xu, Q.; Zhang, L.; Cheng, B.; Fan, J.; Yu, J. S-Scheme heterojunction photocatalyst. Chem 2020, 6, 1543–1559. [Google Scholar] [CrossRef]
- Chen, K.; Cai, A.; Li, T.T. Covalent organic framework-semiconductor-based heterostructures for photocatalytic applications. ChemSusChem 2023, 16, 137095. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Wang, L.; Liu, G.; Liu, Y.; Zhang, S.; Wang, L.; Zheng, X.; Zhou, L.; Gao, J.; Shi, J.; et al. Hollow Rh-COF@COF S-scheme heterojunction for photocatalytic nicotinamide cofactor regeneration. ACS Catal. 2023, 13, 6619–6629. [Google Scholar] [CrossRef]
- Deng, M.; Sun, J.; Laemont, A.; Liu, C.; Wang, L.; Bourda, L.; Chakraborty, J.; Van Hecke, K.; Morent, R.; De Geyter, N.; et al. Extending the π-conjugation system of covalent organic frameworks for more efficient photocatalytic H2O2 production. Green Chem. 2023, 25, 3069–3076. [Google Scholar] [CrossRef]
- Singh, V.; Kim, J.; Kang, B.; Moon, J.; Kim, S.; Kim, W.Y.; Byon, H.R. Thiazole-linked covalent organic framework promoting fast two-electron transfer for lithium-organic batteries. Adv. Energy Mater. 2021, 11, 2003735. [Google Scholar] [CrossRef]
- Wang, J.; Zhuang, S. Covalent organic frameworks (COFs) for environmental applications. Coordin. Chem. Rev. 2019, 400, 213046. [Google Scholar] [CrossRef]
- Zhao, Q.; Wang, J.; Li, Z.; Qiao, Y.; Jin, C.; Guo, Y. Preparation of Cu2O/exfoliated graphite composites with high visible light photocatalytic performance and stability. Ceram. Int. 2016, 42, 13273–13277. [Google Scholar] [CrossRef]
- Hua, Q.; Chen, K.; Chang, S.; Ma, Y.; Huang, W. Crystal plane-dependent compositional and structural evolution of uniform Cu2O nanocrystals in aqueous ammonia solutions. J. Phys. Chem. 2011, 115, 20618–20627. [Google Scholar] [CrossRef]
- Yang, C.; Wan, S.; Zhu, B.; Yu, J.; Cao, S. Calcination-regulated microstructures of donor-acceptor polymers towards enhanced and stable photocatalytic H2O2 production in pure water. Angew. Chem. Int. Ed. 2022, 61, e202208438. [Google Scholar] [CrossRef]
- Zhao, Q.; Wang, J.; Li, Z.; Guo, Y.; Wang, J.; Tang, B.; Abudula, A.; Guan, G. Heterostructured graphitic-carbon-nitride-nanosheets/copper(I) oxide composite as an enhanced visible light photocatalyst for decomposition of tetracycline antibiotics. Sep. Purif. Technol. 2020, 250, 117238. [Google Scholar] [CrossRef]
- Bi, H.; Liu, J.; Wu, Z.; Zhu, K.; Suo, H.; Lv, X.; Fu, Y.; Jian, R.; Sun, Z. Construction of Bi2WO6/ZnIn2S4 with Z-scheme structure for efficient photocatalytic performance. Chem. Phys. Lett. 2021, 769, 138449. [Google Scholar] [CrossRef]
- Zhao, Q.; Guo, Z.; Li, S.; Wang, J.; Li, Z.; Jia, Z.; Wang, K.; Guo, Y. Cu2O/Bi2MoO6 Z-type heterojunction: Construction and photocatalytic degradation properties. Chinese J. Inorg. Chem. 2024, 40, 885–894. [Google Scholar]
- Hu, Z.; Luo, Y.; Wang, L.; Wang, Y.; Wang, Q.; Jiang, G.; Zhang, Q.; Cui, F. Synthesis of pyrene-based covalent organic frameworks for photocatalytic tetracycline degradation. ACS Appl. Polym. 2023, 5, 9263–9273. [Google Scholar] [CrossRef]
- Hou, Y.; Liu, F.; Nie, C.; Li, Z.; Tong, M. Boosting exciton dissociation and charge transfer in triazole-based covalent organic frameworks by increasing the donor unit from one to two for the efficient photocatalytic elimination of emerging contaminants. Environ. Sci. Technol. 2023, 57, 11675–11686. [Google Scholar] [CrossRef]
- Qi, L.; Zhou, Y.; Qi, J.; Yang, Y.; Zhu, Z.; Xiao, C.; Yan, X.; Li, J. Enhanced generation and effective utilization of Cr(V) for simultaneous removal of coexisting pollutants via MOF@COF photocatalysts. ACS ES T Eng. 2024, 4, 870–881. [Google Scholar] [CrossRef]
- Hu, Z.; Wang, Y.; Wang, L.; Wang, Q.; Zhang, Q.; Cui, F.; Jiang, G. Synthesis of S-type heterostructure π-COF for photocatalytic tetracycline degradation. Chem. Eng. J. 2024, 479, 147534. [Google Scholar] [CrossRef]
- Cui, J.; Ye, L.; Chen, X.; Li, J.; Yang, B.; Yang, M.; Yang, Q.; Yun, D.; Sun, S. Simultaneously promoting adsorption and charge separation in Z-scheme ZnO/Cu2O heterojunctions for efficient removal of tetracycline. Appl. Surf. Sci. 2023, 638, 158046. [Google Scholar] [CrossRef]
- Xu, X.; Feng, F.; Wan, Z.; Wang, Y.; Yu, M.; Han, X.; Wu, G.; Xing, W. Rapid electron transfer reinforced by interfacial Co-O bonding in MOF/COF hybrids for highly efficient degrade tetracycline by activating peroxymonosulfate. Colloids Surf. A Physicochem. Eng. Asp. 2024, 689, 133686. [Google Scholar] [CrossRef]
- Shi, Z.; Chen, Z.; Zhang, Y.; Wang, X.; Lu, T.; Wang, Q.; Zhan, Z.; Zhang, P. COF TzDa/Ag/AgBr Z-scheme heterojunction photocatalyst for efficient visible light driven elimination of antibiotics tetracycline and heavy metal ion Cr(VI). Sep. Purif. Technol. 2022, 288, 120717. [Google Scholar] [CrossRef]
- Guo, X.; Yin, D.; Khaing, K.K.; Wang, J.; Luo, Z.; Zhang, Y. Construction of MOF/COF hybrids for boosting sunlight-induced fenton-like photocatalytic removal of organic pollutants. Inorg. Chem. 2021, 60, 15557–15568. [Google Scholar] [CrossRef]
- Khaing, K.K.; Yin, D.; Ouyang, Y.; Xiao, S.; Liu, B.; Deng, L.; Li, L.; Guo, X.; Wang, J.; Liu, J.; et al. Fabrication of 2D–2D heterojunction catalyst with covalent organic framework (COF) and MoS2 for highly efficient photocatalytic degradation of organic pollutants. Inorg. Chem. 2020, 59, 6942–6952. [Google Scholar] [CrossRef]
- Wang, J.; Yin, D.; Guo, X.; Luo, Z.; Tao, L.; Ren, J.; Zhang, Y. Fabrication of a covalent organic framework-based heterojunction via coupling with ZnAgInS nanosphere with high photocatalytic activity. Langmuir 2022, 38, 4680–4691. [Google Scholar] [CrossRef]
- Xu, X.; Shao, W.; Tai, G.; Yu, M.; Han, X.; Han, J.; Wu, G.; Xing, W. Single-atomic Co-N site modulated exciton dissociation and charge transfer on covalent organic frameworks for efficient antibiotics degradation via peroxymonosulfate activation. Sep. Purif. Technol. 2024, 333, 125890. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, W.; Ma, B.; Qian, S.; Wu, Y.; Zhang, X.; Kadasala, N.R.; Jiang, Y.; Liu, Y. A novel all-solid-state Z-scheme BiVO4/Ag/Cu2O heterojunction: Photocatalytic and photothermal synergistic catalysis of tetracycline under simulated sunlight irradiation. J. Alloys Compd. 2024, 1005, 176191. [Google Scholar] [CrossRef]
- Meng, X.; Yan, L.; Wei, M.; Wang, T.; Xu, T.; Yan, Y.; Cheng, S. A novel Co(OH)2/Cu2O nanocomposite-activated peroxydisulfate for the enhanced degradation of tetracycline. New J. Chem. 2021, 45, 16705–16713. [Google Scholar] [CrossRef]
- Zhou, Y.; Feng, S.; Duan, X.; Wu, W.; Ye, Z.; Dai, X.; Wang, Y.; Cao, X. Stable self-assembly Cu2O/ZIF-8 heterojunction as efficient visible light responsive photocatalyst for tetracycline degradation and mechanism insight. J. Solid State Chem. 2022, 305, 122628. [Google Scholar] [CrossRef]
- Liu, B.; Wu, Y.; Zhang, J.; Han, X.; Shi, H. Visible-light-driven g-C3N4/Cu2O heterostructures with efficient photocatalytic activities for tetracycline degradation and microbial inactivation. J. Photoch. Photobio. 2019, 378, 1–8. [Google Scholar] [CrossRef]
- Niu, J.; Song, Z.; Gao, X.; Ji, Y.; Zhang, Y. Construction of Bi2WO6 composites with carbon-coated Cu2O for effective degradation of tetracycline. J. Alloy. Compd. 2021, 884, 161282. [Google Scholar] [CrossRef]
- Zhu, Y.; Pan, Y.; Zhang, E.; Dai, W. A self-assembled urchin-like TiO2@Ag–CuO with enhanced photocatalytic activity toward tetracycline hydrochloride degradation. New J. Chem. 2020, 44, 11076–11084. [Google Scholar] [CrossRef]
- He, X.; Fang, H.; Gosztola, D.J.; Jiang, Z.; Jena, P.; Wang, W. Mechanistic insight into photocatalytic pathways of MIL-100(Fe)/TiO2 composites. ACS Appl. Mater. Interfaces 2019, 11, 12516–12524. [Google Scholar] [CrossRef] [PubMed]
- Kondrakov, A.; Ignatev, A.; Lunin, V.; Frimmel, F.; Bräse, S.; Horn, H. Roles of water and dissolved oxygen in photocatalytic generation of free OH radicals in aqueous TiO2 suspensions: An isotope labeling study. Appl. Cat. B Environ. 2016, 182, 424–430. [Google Scholar] [CrossRef]
- Wang, H.; Quan, X.; Xiong, Q.; Yin, L.; Tian, Y.; Zhang, J. Enhanced performance of β-cyclodextrin modified Cu2O nanocomposite for efficient removal of tetracycline and dyes: Synergistic role of adsorption and photocatalysis. Appl. Surf. Sci. 2023, 621, 156735. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, Z.; Wang, T.; Wu, Z.; Razzaque, S.; Zhao, Z.; Cai, J.; Xie, W.; Wang, J.; Zhao, Q.; Wang, K. In Situ Construction of Thiazole-Linked Covalent Organic Frameworks on Cu2O for High-Efficiency Photocatalytic Tetracycline Degradation. Molecules 2025, 30, 3233. https://doi.org/10.3390/molecules30153233
Jia Z, Wang T, Wu Z, Razzaque S, Zhao Z, Cai J, Xie W, Wang J, Zhao Q, Wang K. In Situ Construction of Thiazole-Linked Covalent Organic Frameworks on Cu2O for High-Efficiency Photocatalytic Tetracycline Degradation. Molecules. 2025; 30(15):3233. https://doi.org/10.3390/molecules30153233
Chicago/Turabian StyleJia, Zhifang, Tingxia Wang, Zhaoxia Wu, Shumaila Razzaque, Zhixiang Zhao, Jiaxuan Cai, Wenao Xie, Junli Wang, Qiang Zhao, and Kewei Wang. 2025. "In Situ Construction of Thiazole-Linked Covalent Organic Frameworks on Cu2O for High-Efficiency Photocatalytic Tetracycline Degradation" Molecules 30, no. 15: 3233. https://doi.org/10.3390/molecules30153233
APA StyleJia, Z., Wang, T., Wu, Z., Razzaque, S., Zhao, Z., Cai, J., Xie, W., Wang, J., Zhao, Q., & Wang, K. (2025). In Situ Construction of Thiazole-Linked Covalent Organic Frameworks on Cu2O for High-Efficiency Photocatalytic Tetracycline Degradation. Molecules, 30(15), 3233. https://doi.org/10.3390/molecules30153233






