Enhanced NiFe2O4 Catalyst Performance and Stability in Anion Exchange Membrane Water Electrolysis: Influence of Iron Content and Membrane Selection
Abstract
1. Introduction
2. Results
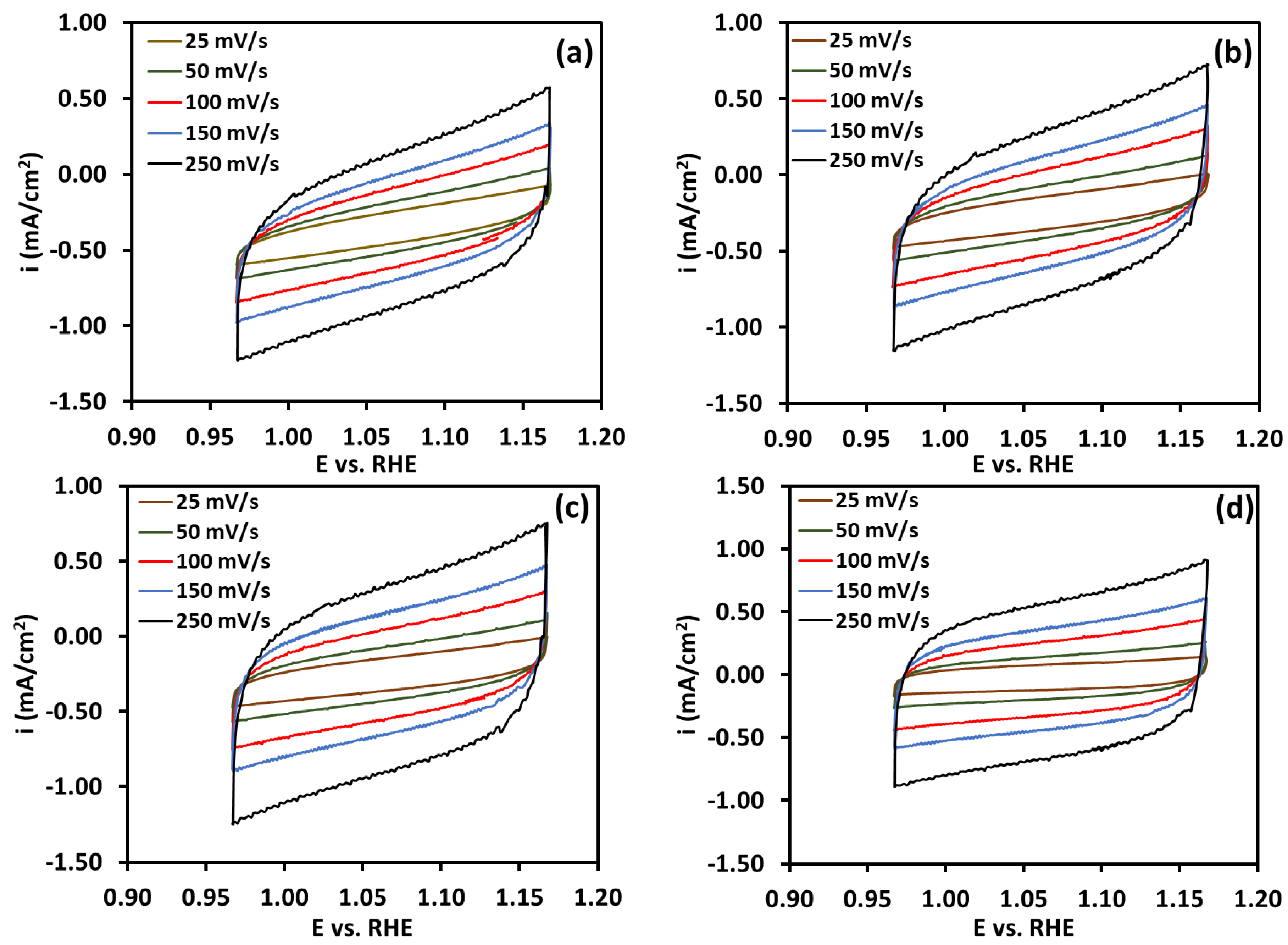
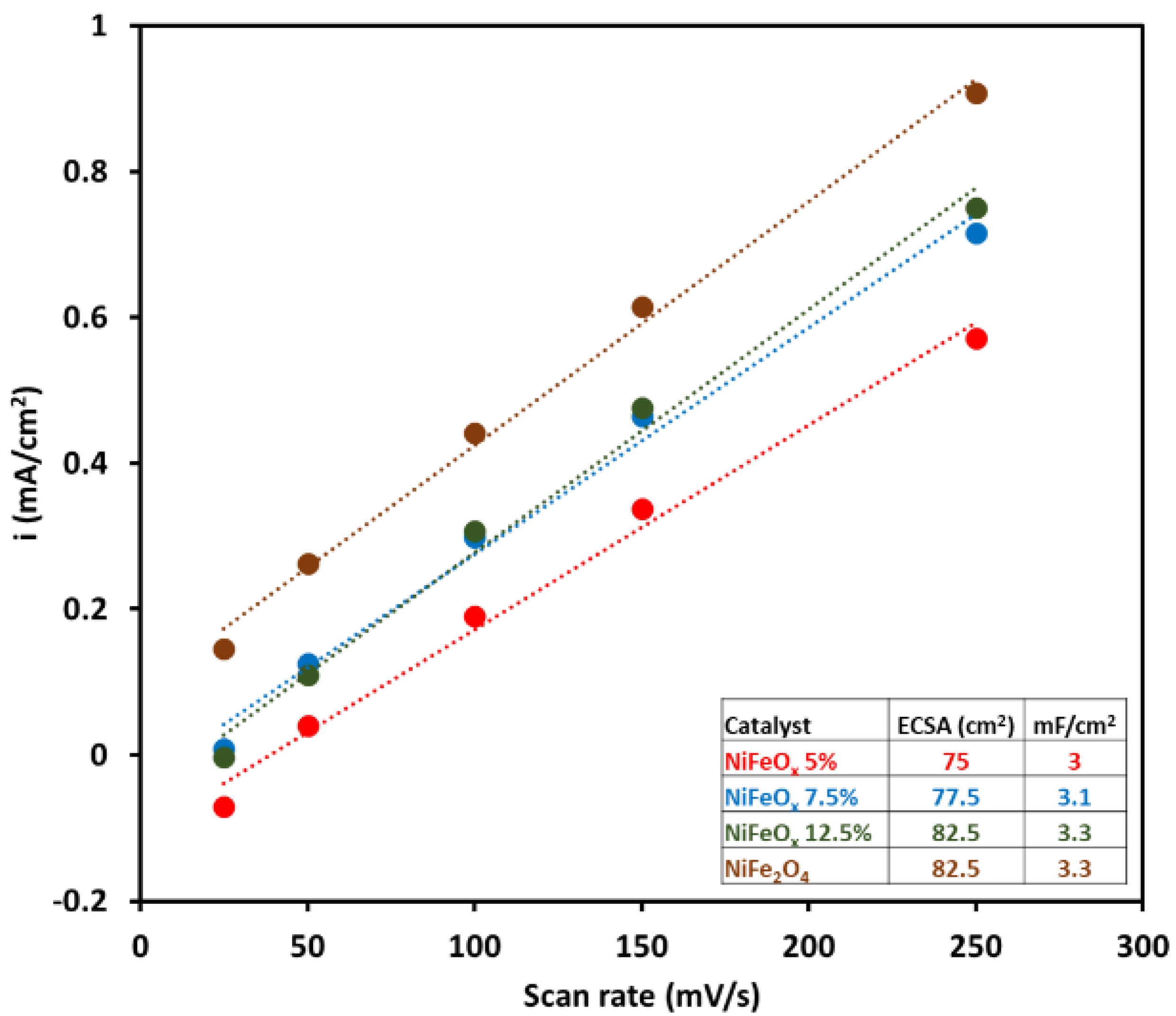
2.1. Oxygen Evolution Reaction
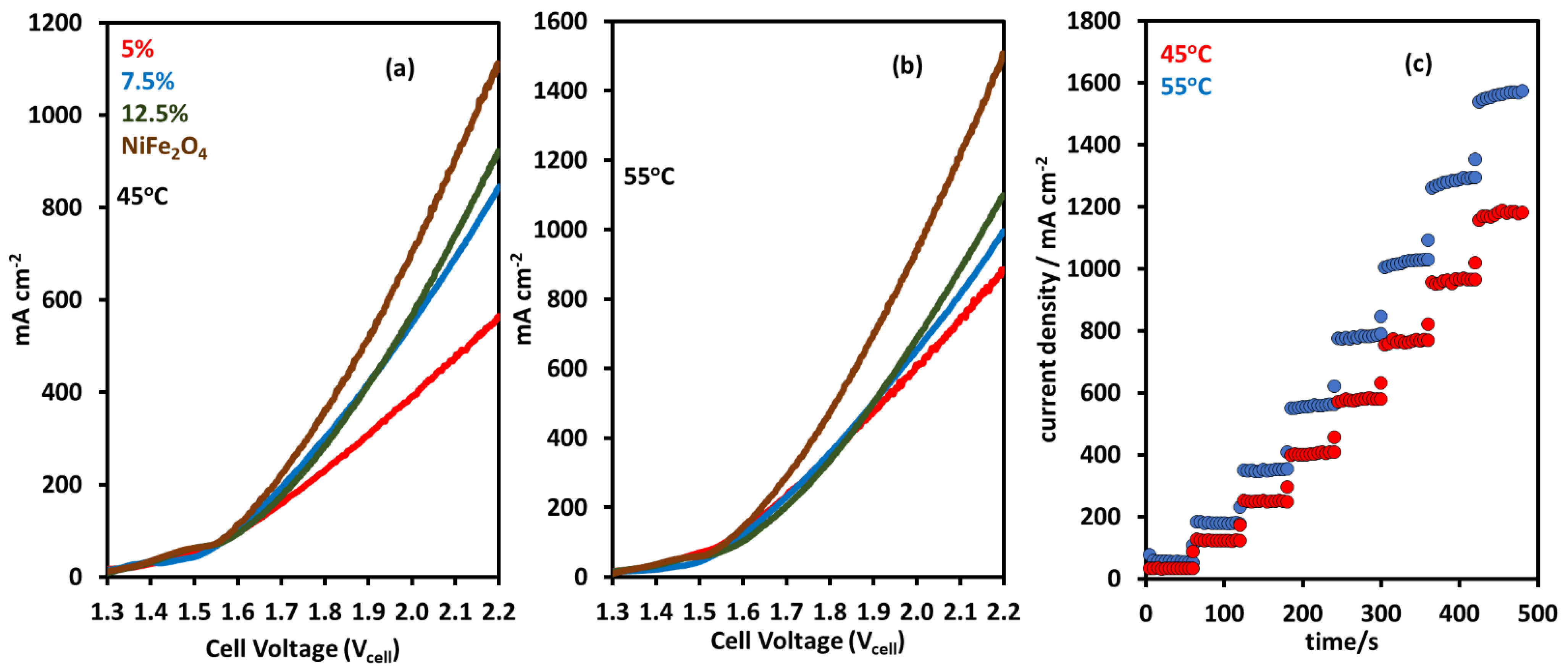
2.2. AEM Electrolyzer Tests with NiFeOx and NiFe2O4 Catalysts
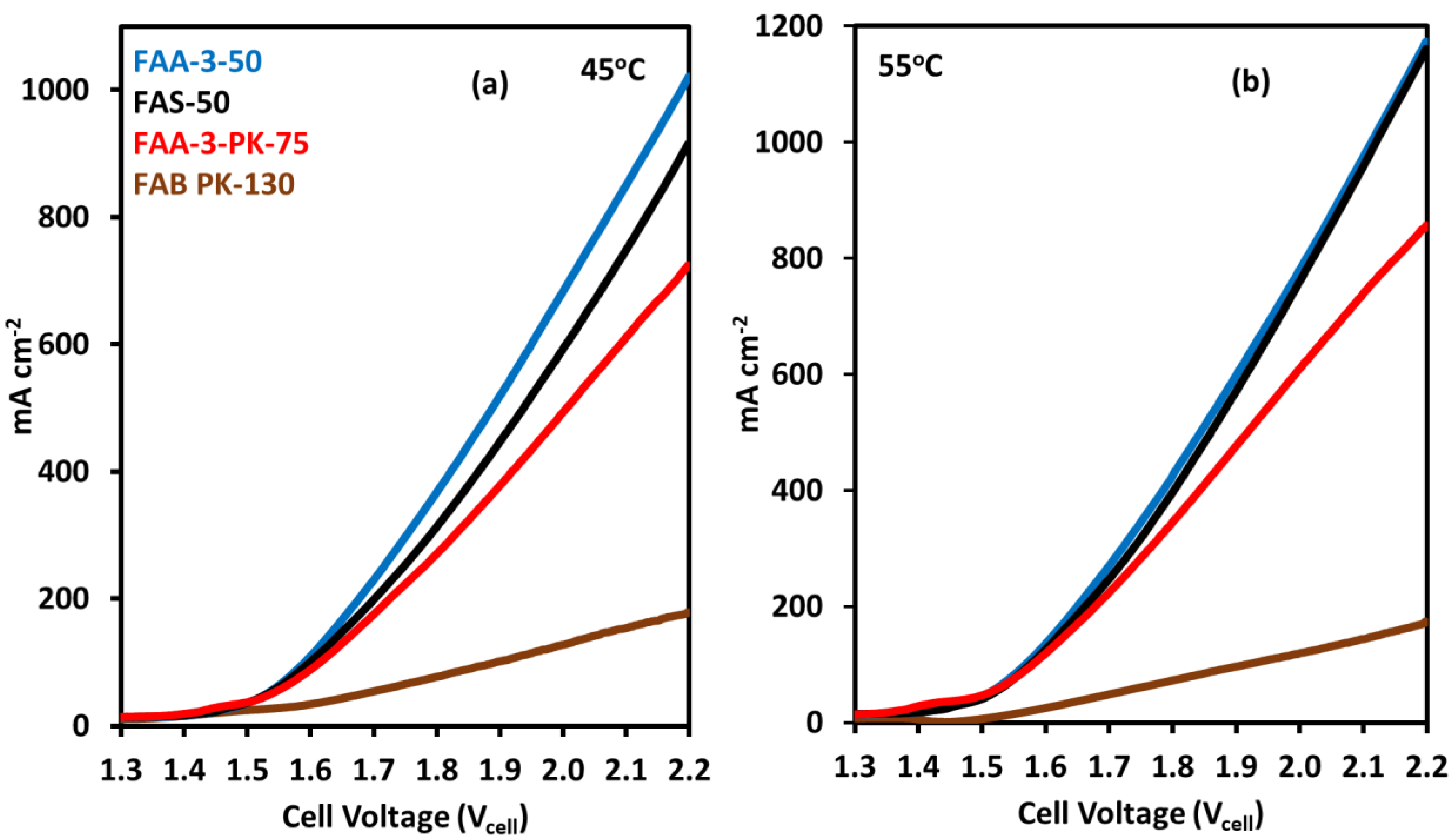
2.3. Effect of Anion Exchange Membranes on Electrolyzer Performance
2.3.1. EIS Effect of Voltage and Temperature
2.3.2. EIS with Different Anion Exchange Membranes
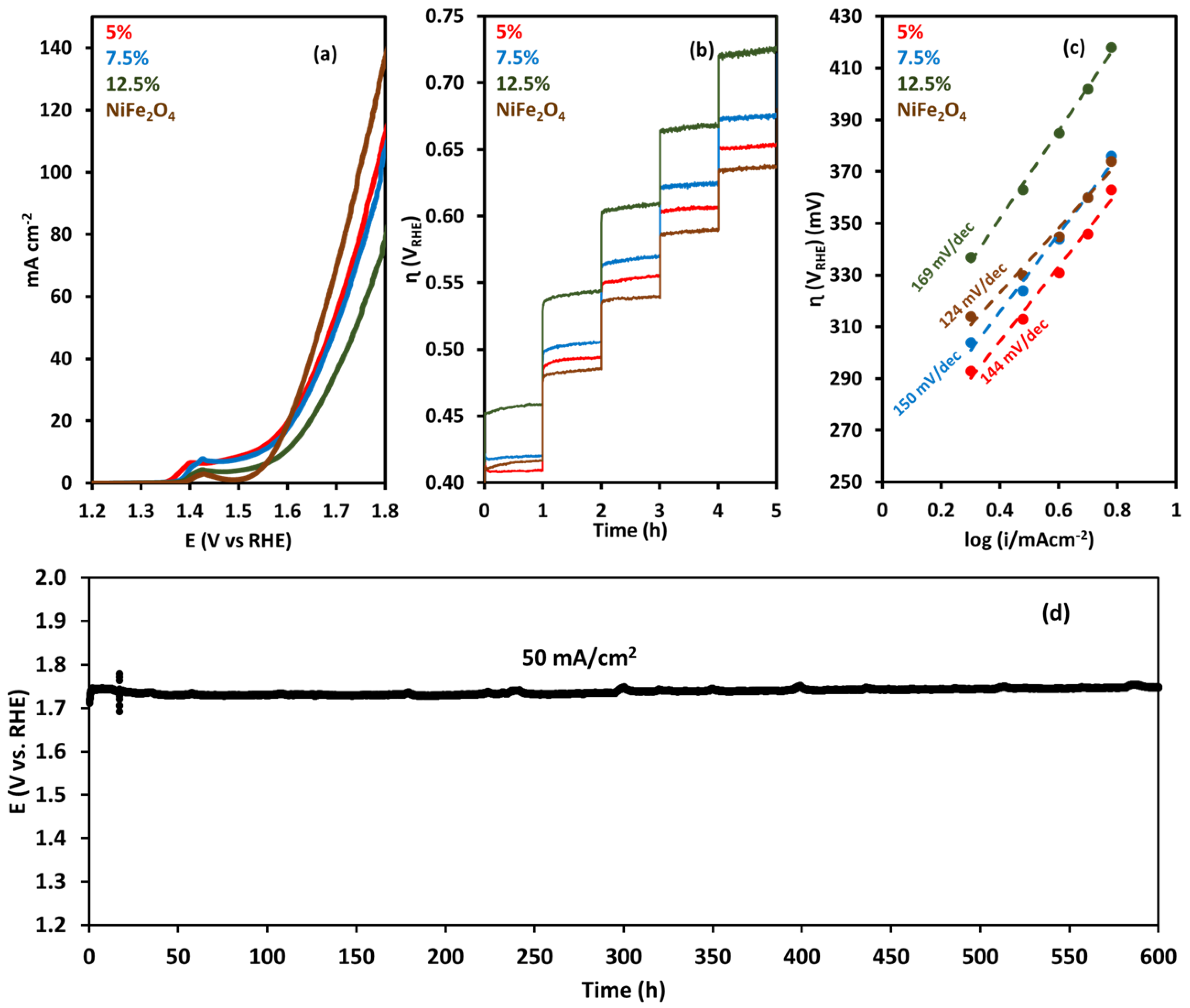
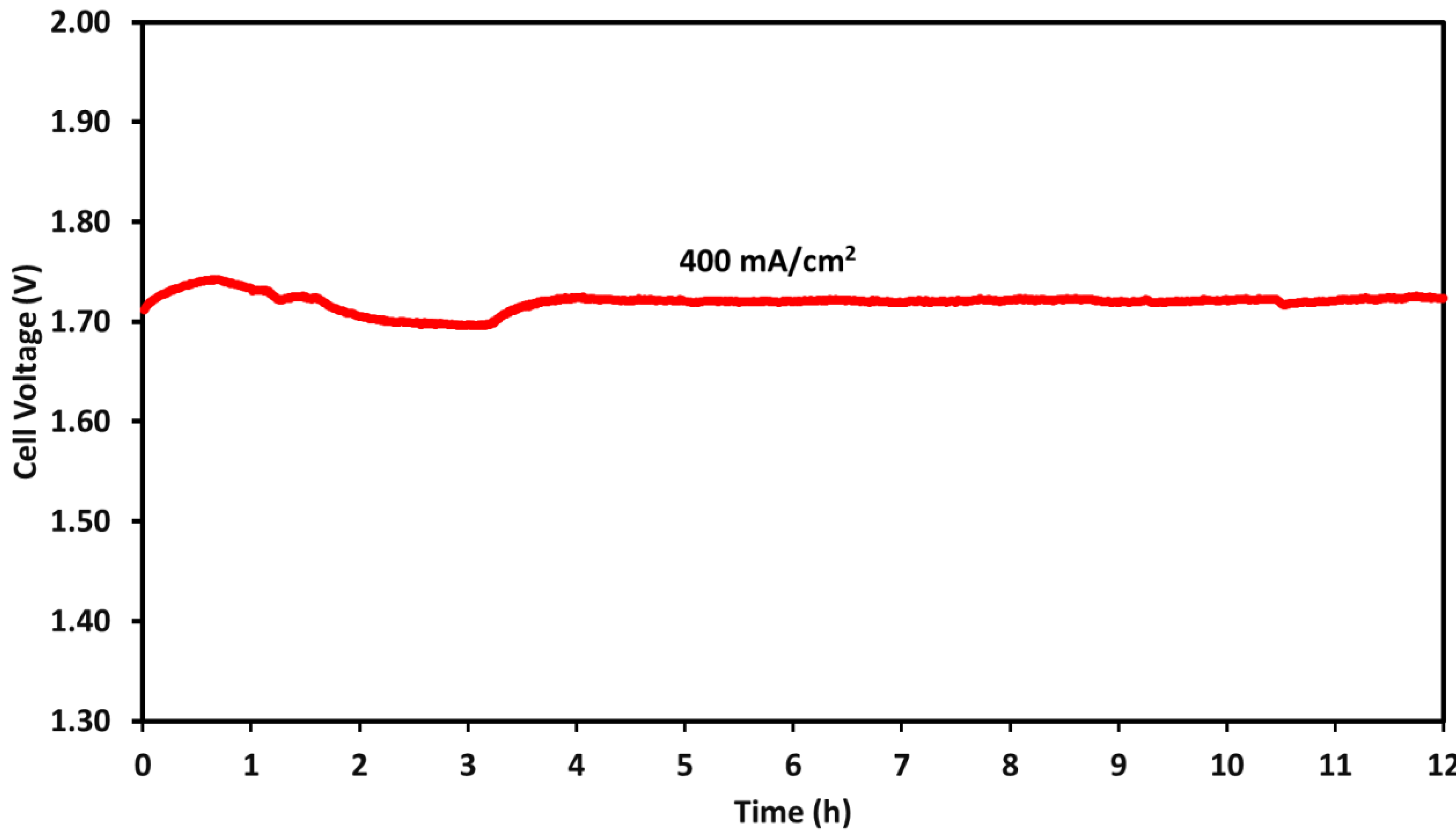
2.4. Durability Test
3. Experimental
3.1. Synthesis of NiFeOx Catalyst
3.2. Catalyst Testing in Three-Electrode System
3.3. Preparation of Gas Diffusion Electrode
3.4. AEM Electrolyzer Single-Cell Performance Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yue, M.; Lambert, H.; Pahon, E.; Roche, R.; Jemei, S.; Hissel, D. Hydrogen Energy Systems: A Critical Review of Technologies, Applications, Trends and Challenges. Renew. Sustain. Energy Rev. 2021, 146, 111180. [Google Scholar] [CrossRef]
- Züttel, A.; Remhof, A.; Borgschulte, A.; Friedrichs, O. Hydrogen: The Future Energy Carrier. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2010, 368, 3329–3342. [Google Scholar] [CrossRef]
- Mazloomi, K.; Gomes, C. Hydrogen as an Energy Carrier: Prospects and Challenges. Renew. Sustain. Energy Rev. 2012, 16, 3024–3033. [Google Scholar] [CrossRef]
- Johnston, B.; Mayo, M.C.; Khare, A. Hydrogen: The Energy Source for the 21st Century. Technovation 2005, 25, 569–585. [Google Scholar] [CrossRef]
- Tang, D.; Tan, G.-L.; Li, G.-W.; Liang, J.-G.; Ahmad, S.M.; Bahadur, A.; Humayun, M.; Ullah, H.; Khan, A.; Bououdina, M. State-of-the-Art Hydrogen Generation Techniques and Storage Methods: A Critical Review. J. Energy Storage 2023, 64, 107196. [Google Scholar] [CrossRef]
- Cho, H.H.; Strezov, V.; Evans, T.J. Environmental Impact Assessment of Hydrogen Production via Steam Methane Reforming Based on Emissions Data. Energy Rep. 2022, 8, 13585–13595. [Google Scholar] [CrossRef]
- Bhandari, R.; Trudewind, C.A.; Zapp, P. Life Cycle Assessment of Hydrogen Production via Electrolysis—A Review. J. Clean. Prod. 2014, 85, 151–163. [Google Scholar] [CrossRef]
- Shiva Kumar, S.; Lim, H. An Overview of Water Electrolysis Technologies for Green Hydrogen Production. Energy Rep. 2022, 8, 13793–13813. [Google Scholar] [CrossRef]
- Xu, Q.; Zhang, L.; Zhang, J.; Wang, J.; Hu, Y.; Jiang, H.; Li, C. Anion Exchange Membrane Water Electrolyzer: Electrode Design, Lab-Scaled Testing System and Performance Evaluation. EnergyChem 2022, 4, 100087. [Google Scholar] [CrossRef]
- Zhang, K.; Liang, X.; Wang, L.; Sun, K.; Wang, Y.; Xie, Z.; Wu, Q.; Bai, X.; Hamdy, M.S.; Chen, H.; et al. Status and Perspectives of Key Materials for PEM Electrolyzer. Nano Res. Energy 2022, 1, e9120032. [Google Scholar] [CrossRef]
- Shiva Kumar, S.; Himabindu, V. Hydrogen Production by PEM Water Electrolysis—A Review. Mater. Sci. Energy Technol. 2019, 2, 442–454. [Google Scholar] [CrossRef]
- Yang, J.; Jang, M.J.; Zeng, X.; Park, Y.S.; Lee, J.; Choi, S.M.; Yin, Y. Non-Precious Electrocatalysts for Oxygen Evolution Reaction in Anion Exchange Membrane Water Electrolysis: A Mini Review. Electrochem. Commun. 2021, 131, 107118. [Google Scholar] [CrossRef]
- Liu, J.; Kang, Z.; Li, D.; Pak, M.; Alia, S.M.; Fujimoto, C.; Bender, G.; Kim, Y.S.; Weber, A.Z. Elucidating the Role of Hydroxide Electrolyte on Anion-Exchange-Membrane Water Electrolyzer Performance. J. Electrochem. Soc. 2021, 168, 054522. [Google Scholar] [CrossRef]
- Park, S.; Park, J.E.; Na, G.; Choi, C.; Cho, Y.-H.; Sung, Y.-E. Low-Cost and High-Performance Anion-Exchange Membrane Water Electrolysis Stack Using Non-Noble Metal-Based Materials. ACS Appl. Energy Mater. 2023, 6, 8738–8748. [Google Scholar] [CrossRef]
- Vincent, I.; Bessarabov, D. Low Cost Hydrogen Production by Anion Exchange Membrane Electrolysis: A Review. Renew. Sustain. Energy Rev. 2018, 81, 1690–1704. [Google Scholar] [CrossRef]
- Palmas, S.; Rodriguez, J.; Mais, L.; Mascia, M.; Herrando, M.C.; Vacca, A. Anion Exchange Membrane: A Valuable Perspective in Emerging Technologies of Low Temperature Water Electrolysis. Curr. Opin. Electrochem. 2023, 37, 101178. [Google Scholar] [CrossRef]
- Kang, S.Y.; Park, J.E.; Jang, G.Y.; Kim, O.-H.; Kwon, O.J.; Cho, Y.-H.; Sung, Y.-E. High-Performance and Durable Water Electrolysis Using a Highly Conductive and Stable Anion-Exchange Membrane. Int. J. Hydrogen Energy 2022, 47, 9115–9126. [Google Scholar] [CrossRef]
- Soltani, M.; Amin, H.M.A.; Cebe, A.; Ayata, S.; Baltruschat, H. Metal-Supported Perovskite as an Efficient Bifunctional Electrocatalyst for Oxygen Reduction and Evolution: Substrate Effect. J. Electrochem. Soc. 2021, 168, 034504. [Google Scholar] [CrossRef]
- Amin, H.M.A.; Apfel, U. Metal-Rich Chalcogenides as Sustainable Electrocatalysts for Oxygen Evolution and Reduction: State of the Art and Future Perspectives. Eur. J. Inorg. Chem. 2020, 2020, 2679–2690. [Google Scholar] [CrossRef]
- Amin, H.M.A.; Zan, L.; Baltruschat, H. Boosting the Bifunctional Catalytic Activity of Co3O4 on Silver and Nickel Substrates for the Alkaline Oxygen Evolution and Reduction Reactions. Surf. Interfaces 2024, 54, 105218. [Google Scholar] [CrossRef]
- Sathiyan, K.; Mukherjee, P.; Higashimine, K.; Taniike, T. Durability Meets Performance: A LaCoO3–MoS2 Composite for Electrochemical Overall Water Splitting. Energy Fuels 2025, 39, 13606–13617. [Google Scholar] [CrossRef]
- Dalai, N.; Mohanty, B.; Mitra, A.; Jena, B. Highly Active Ternary Nickel–Iron Oxide as Bifunctional Catalyst for Electrochemical Water Splitting. ChemistrySelect 2019, 4, 7791–7796. [Google Scholar] [CrossRef]
- Liu, G.; Wang, K.; Gao, X.; He, D.; Li, J. Fabrication of Mesoporous NiFe2O4 Nanorods as Efficient Oxygen Evolution Catalyst for Water Splitting. Electrochim. Acta 2016, 211, 871–878. [Google Scholar] [CrossRef]
- Liu, G.; Gao, X.; Wang, K.; He, D.; Li, J. Uniformly Mesoporous NiO/NiFe2O4 Biphasic Nanorods as Efficient Oxygen Evolving Catalyst for Water Splitting. Int. J. Hydrogen Energy 2016, 41, 17976–17986. [Google Scholar] [CrossRef]
- Feng, Z.; Wang, P.; Cheng, Y.; Mo, Y.; Luo, X.; Liu, P.; Guo, R.; Liu, X. Recent Progress on NiFe2O4 Spinels as Electrocatalysts for the Oxygen Evolution Reaction. J. Electroanal. Chem. 2023, 946, 117703. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, Q.; Zhao, P.; Chen, L.; Yang, S.; Yan, Q.; Li, H. NiFe2O4 @Co3O4 Heterostructure with Abundant Oxygen Vacancies as a Bifunctional Electrocatalyst for Overall Water Splitting. J. Alloys Compd. 2022, 918, 165705. [Google Scholar] [CrossRef]
- Amin, H.M.A.; Attia, M.; Tetzlaff, D.; Apfel, U. Tailoring the Electrocatalytic Activity of Pentlandite FexNi9-XS8 Nanoparticles via Variation of the Fe : Ni Ratio for Enhanced Water Oxidation. ChemElectroChem 2021, 8, 3863–3874. [Google Scholar] [CrossRef]
- Liu, Z.; Sajjad, S.D.; Gao, Y.; Yang, H.; Kaczur, J.J.; Masel, R.I. The Effect of Membrane on an Alkaline Water Electrolyzer. Int. J. Hydrogen Energy 2017, 42, 29661–29665. [Google Scholar] [CrossRef]
- Kutz, R.B.; Chen, Q.; Yang, H.; Sajjad, S.D.; Liu, Z.; Masel, I.R. Sustainion Imidazolium-Functionalized Polymers for Carbon Dioxide Electrolysis. Energy Technol. 2017, 5, 929–936. [Google Scholar] [CrossRef]
- Noor Azam, A.M.I.; Ragunathan, T.; Zulkefli, N.N.; Masdar, M.S.; Majlan, E.H.; Mohamad Yunus, R.; Shamsul, N.S.; Husaini, T.; Shaffee, S.N.A. Investigation of Performance of Anion Exchange Membrane (AEM) Electrolysis with Different Operating Conditions. Polymers 2023, 15, 1301. [Google Scholar] [CrossRef]
- Vincent, I.; Lee, E.-C.; Kim, H.-M. Comprehensive Impedance Investigation of Low-Cost Anion Exchange Membrane Electrolysis for Large-Scale Hydrogen Production. Sci. Rep. 2021, 11, 293. [Google Scholar] [CrossRef] [PubMed]
- Vincent, I.; Kruger, A.; Bessarabov, D. Development of Efficient Membrane Electrode Assembly for Low Cost Hydrogen Production by Anion Exchange Membrane Electrolysis. Int. J. Hydrogen Energy 2017, 42, 10752–10761. [Google Scholar] [CrossRef]
- Park, J.E.; Kang, S.Y.; Oh, S.-H.; Kim, J.K.; Lim, M.S.; Ahn, C.-Y.; Cho, Y.-H.; Sung, Y.-E. High-Performance Anion-Exchange Membrane Water Electrolysis. Electrochim. Acta 2019, 295, 99–106. [Google Scholar] [CrossRef]
- Chakik, F.E.; Kaddami, M.; Mikou, M. Optimization of Physico-Chemical Parameters of Hydrogen Production by Electrolysis of Water. In Proceedings of the 2018 Renewable Energies, Power Systems & Green Inclusive Economy (REPS-GIE), Casablanca, Morocco, 23–24 April 2018. [Google Scholar]
- Motealleh, B.; Liu, Z.; Masel, R.I.; Sculley, J.P.; Richard Ni, Z.; Meroueh, L. Next-Generation Anion Exchange Membrane Water Electrolyzers Operating for Commercially Relevant Lifetimes. Int. J. Hydrogen Energy 2021, 46, 3379–3386. [Google Scholar] [CrossRef]
- Chen, M.; Mandal, M.; Groenhout, K.; McCool, G.; Tee, H.M.; Zulevi, B.; Kohl, P.A. Self-Adhesive Ionomers for Durable Low-Temperature Anion Exchange Membrane Electrolysis. J. Power Sources 2022, 536, 231495. [Google Scholar] [CrossRef]
- Caprì, A.; Gatto, I.; Lo Vecchio, C.; Trocino, S.; Carbone, A.; Baglio, V. Anion Exchange Membrane Water Electrolysis Based on Nickel Ferrite Catalysts. ChemElectroChem 2022, 10, e202201056. [Google Scholar] [CrossRef]
- Henkensmeier, D.; Najibah, M.; Harms, C.; Žitka, J.; Hnát, J.; Bouzek, K. Overview: State-of-the Art Commercial Membranes for Anion Exchange Membrane Water Electrolysis. J. Electrochem. Energy Convers. Storage 2020, 18, 024001. [Google Scholar] [CrossRef]
- Santoro, C.; Lavacchi, A.; Mustarelli, P.; Di Noto, V.; Elbaz, L.; Dekel, D.R.; Jaouen, F. What Is Next in Anion-Exchange Membrane Water Electrolyzers? Bottlenecks, Benefits, and Future. ChemSusChem 2022, 15, e202200027. [Google Scholar] [CrossRef]
- Pushkareva, I.V.; Pushkarev, A.S.; Grigoriev, S.A.; Modisha, P.; Bessarabov, D.G. Comparative Study of Anion Exchange Membranes for Low-Cost Water Electrolysis. Int. J. Hydrogen Energy 2020, 45, 26070–26079. [Google Scholar] [CrossRef]
- Lo Vecchio, C.; Carbone, A.; Gatto, I.; Baglio, V. Investigation of Fumasep® FAA3-50 Membranes in Alkaline Di-rect Methanol Fuel Cells. Polymers 2023, 15, 1555. [Google Scholar] [CrossRef]
- Fumasep FAA-3-50. Available online: https://www.fuelcellstore.com/fumasep-faa-3-50 (accessed on 27 October 2023).
- Fumasep FAS-50. Available online: https://www.fuelcellstore.com/fumasep-fas-50 (accessed on 27 October 2023).
- Lee, S.A.; Kim, J.; Kwon, K.C.; Park, S.H.; Jang, H.W. Anion Exchange Membrane Water Electrolysis for Sustaina-ble Large-scale Hydrogen Production. Carbon Neutralization 2022, 1, 26–48. [Google Scholar] [CrossRef]
- Fumasep FAA-3-PK-75. Available online: https://www.fuelcellstore.com/fumasep-faa-3-pk-75 (accessed on 27 October 2023).
- Fumasep FAB-PK-130. Available online: https://www.fuelcellstore.com/fumasep-fab (accessed on 27 October 2023).
- Caprì, A.; Martínez-Lázaro, A.; Béjar, J.; Gatto, I.; Álvarez-Contreras, L.; Gurrola, M.P.; Ledesma-García, J.; Bag-lio, V.; Arriaga, L.G. Three-Dimensionally Ordered Macroporous Trimetallic Spinel for Anion Exchange Mem-brane Water Electrolysis. Electrochim. Acta 2023, 463, 142851. [Google Scholar] [CrossRef]
- Siracusano, S.; Trocino, S.; Briguglio, N.; Baglio, V.; Aricò, A.S. Electrochemical Impedance Spectroscopy as a Diagnostic Tool in Polymer Electrolyte Membrane Electrolysis. Materials 2018, 11, 1368. [Google Scholar] [CrossRef] [PubMed]
- van der Merwe, J.; Uren, K.; van Schoor, G.; Bessarabov, D. Characterisation Tools Development for PEM Electro-lysers. Int. J. Hydrogen Energy 2014, 39, 14212–14221. [Google Scholar] [CrossRef]
- Kang, Z.; Yang, G.; Mo, J.; Yu, S.; Cullen, D.A.; Retterer, S.T.; Toops, T.J.; Brady, M.P.; Bender, G.; Pivovar, B.S.; et al. Developing Titanium Micro/Nano Porous Layers on Planar Thin/Tunable LGDLs for High-Efficiency Hydrogen Production. Int. J. Hydrogen Energy 2018, 43, 14618–14628. [Google Scholar] [CrossRef]
- Khan, S.; Rizvi, S.M.A.; Urooj, S. Equivalent Circuit Modelling Using Electrochemical Impedance Spectroscopy for Different Materials of SOFC. In Proceedings of the 2016 3rd International Conference on Computing for Sustainable Global Development (INDIACom), New Delhi, India, 16–18 March 2016; pp. 1563–1567. [Google Scholar]
- Zeng, L.; Zhao, T.S. Integrated Inorganic Membrane Electrode Assembly with Layered Double Hydroxides as Ion-ic Conductors for Anion Exchange Membrane Water Electrolysis. Nano Energy 2015, 11, 110–118. [Google Scholar] [CrossRef]
- Seetharaman, S.; Balaji, R.; Ramya, K.; Dhathathreyan, K.S.; Velan, M. Graphene Oxide Modified Non-Noble Met-al Electrode for Alkaline Anion Exchange Membrane Water Electrolyzers. Int. J. Hydrogen Energy 2013, 38, 14934–14942. [Google Scholar] [CrossRef]
- Lim, A.; Cho, M.K.; Lee, S.Y.; Kim, H.-J.; Yoo, S.J.; Sung, Y.-E.; Jang, J.H.; Park, H.S. A Review of Industrially De-veloped Components and Operation Conditions for Anion Exchange Membrane Water Electrolysis. J. Electrochem. Sci. Technol. 2017, 8, 265–273. [Google Scholar] [CrossRef]
- Wu, Z.; Zou, Z.; Huang, J.; Gao, F. NiFe2O4 Nanoparticles/NiFe Layered Double-Hydroxide Nanosheet Hetero-structure Array for Efficient Overall Water Splitting at Large Current Densities. ACS Appl. Mater. Interfaces 2018, 10, 26283–26292. [Google Scholar] [CrossRef]
- Ahmed, K.W.; Jang, M.J.; Habibpour, S.; Chen, Z.; Fowler, M. NiFeOx and NiFeCoOx Catalysts for Anion Exchange Membrane Water Electrolysis. Electrochem 2022, 3, 843–861. [Google Scholar] [CrossRef]
- Kong, T.-H.; Thangavel, P.; Shin, S.; Kwon, S.; Choi, H.; Lee, H.; Park, N.; Woo, J.-J.; Kwon, Y. In-Situ Ionomer-Free Catalyst-Coated Membranes for Anion Exchange Membrane Water Electrolyzers. ACS Energy Lett. 2023, 8, 4666–4673. [Google Scholar] [CrossRef]
- Li, D.; Motz, A.R.; Bae, C.; Fujimoto, C.; Yang, G.; Zhang, F.-Y.; Ayers, K.E.; Kim, Y.S. Durability of Anion Exchange Membrane Water Electrolyzers. Energy Environ. Sci. 2021, 14, 3393–3419. [Google Scholar] [CrossRef]
- Du, N.; Roy, C.; Peach, R.; Turnbull, M.; Thiele, S.; Bock, C. Anion-Exchange Membrane Water Electrolyzers. Chem. Rev. 2022, 122, 11830–11895. [Google Scholar] [CrossRef] [PubMed]
- Mustain, W.E.; Chatenet, M.; Page, M.; Kim, Y.S. Durability Challenges of Anion Exchange Membrane Fuel Cells. Energy Environ. Sci. 2020, 13, 2805–2838. [Google Scholar] [CrossRef]
- Wierzbicki, S.; Douglin, J.C.; Kostuch, A.; Dekel, D.R.; Kruczała, K. Are Radicals Formed During Anion-Exchange Membrane Fuel Cell Operation? J. Phys. Chem. Lett. 2020, 11, 7630–7636. [Google Scholar] [CrossRef]
- Couture, G.; Alaaeddine, A.; Boschet, F.; Ameduri, B. Polymeric Materials as Anion-Exchange Membranes for Alkaline Fuel Cells. Prog. Polym. Sci. 2011, 36, 1521–1557. [Google Scholar] [CrossRef]
- Arges, C.G.; Ramani, V. Two-Dimensional NMR Spectroscopy Reveals Cation-Triggered Backbone Degradation in Polysulfone-Based Anion Exchange Membranes. Proc. Natl. Acad. Sci. USA 2013, 110, 2490–2495. [Google Scholar] [CrossRef]
- Merle, G.; Wessling, M.; Nijmeijer, K. Anion Exchange Membranes for Alkaline Fuel Cells: A Review. J. Membr. Sci. 2011, 377, 1–35. [Google Scholar] [CrossRef]
- Li, D.; Park, E.J.; Zhu, W.; Shi, Q.; Zhou, Y.; Tian, H.; Lin, Y.; Serov, A.; Zulevi, B.; Baca, E.D.; et al. Highly Quaternized Polystyrene Ionomers for High Performance Anion Exchange Membrane Water Electrolysers. Nat. Energy 2020, 5, 378–385. [Google Scholar] [CrossRef]
- Leonard, D.P.; Lehmann, M.; Klein, J.M.; Matanovic, I.; Fujimoto, C.; Saito, T.; Kim, Y.S. Phenyl-Free Poly-norbornenes for Potential Anion Exchange Ionomers for Fuel Cells and Electrolyzers. Adv. Energy Mater. 2022, 13, 2203488. [Google Scholar] [CrossRef]
- Lei, C.; Yang, K.; Wang, G.; Wang, G.; Lu, J.; Xiao, L.; Zhuang, L. Impact of Catalyst Reconstruction on the Durability of Anion Exchange Membrane Water Electrolysis. ACS Sustain. Chem. Eng. 2022, 10, 16725–16733. [Google Scholar] [CrossRef]
- Chen, R.; Hung, S.; Zhou, D.; Gao, J.; Yang, C.; Tao, H.; Yang, H.B.; Zhang, L.; Zhang, L.; Xiong, Q.; et al. Layered Structure Causes Bulk NiFe Layered Double Hydroxide Unstable in Alkaline Oxygen Evolution Reaction. Adv. Mater. 2019, 31, 1903909. [Google Scholar] [CrossRef]
- Jang, H.; Chung, S.; Lee, J. In Situ Demonstration of Anodic Interface Degradation during Water Electrolysis: Corrosion and Passivation. Electrochim. Acta 2021, 365, 137276. [Google Scholar] [CrossRef]
- Singh, S.K.; Takeyasu, K.; Paul, B.; Sharma, S.K.; Nakamura, J. CoOx Electro-Catalysts Anchored on Nitro-gen-Doped Carbon Nanotubes for the Oxygen Evolution Reaction. Sustain. Energy Fuels 2021, 5, 820–827. [Google Scholar] [CrossRef]
- Berenguer, R.; La Rosa-Toro, A.; Quijada, C.; Morallón, E. Origin of the Deactivation of Spinel CuxCo3−xO4/Ti Anodes Prepared by Thermal Decomposition. J. Phys. Chem. C 2008, 112, 16945–16952. [Google Scholar] [CrossRef]
- Ahmed, K.W.; Habibpour, S.; Chen, Z.; Fowler, M. Investigation of NiCoOx Catalysts for Anion Exchange Membrane Water Electrolysis: Performance, Durability, and Efficiency Analysis. J. Energy Storage 2024, 79, 110149. [Google Scholar] [CrossRef]
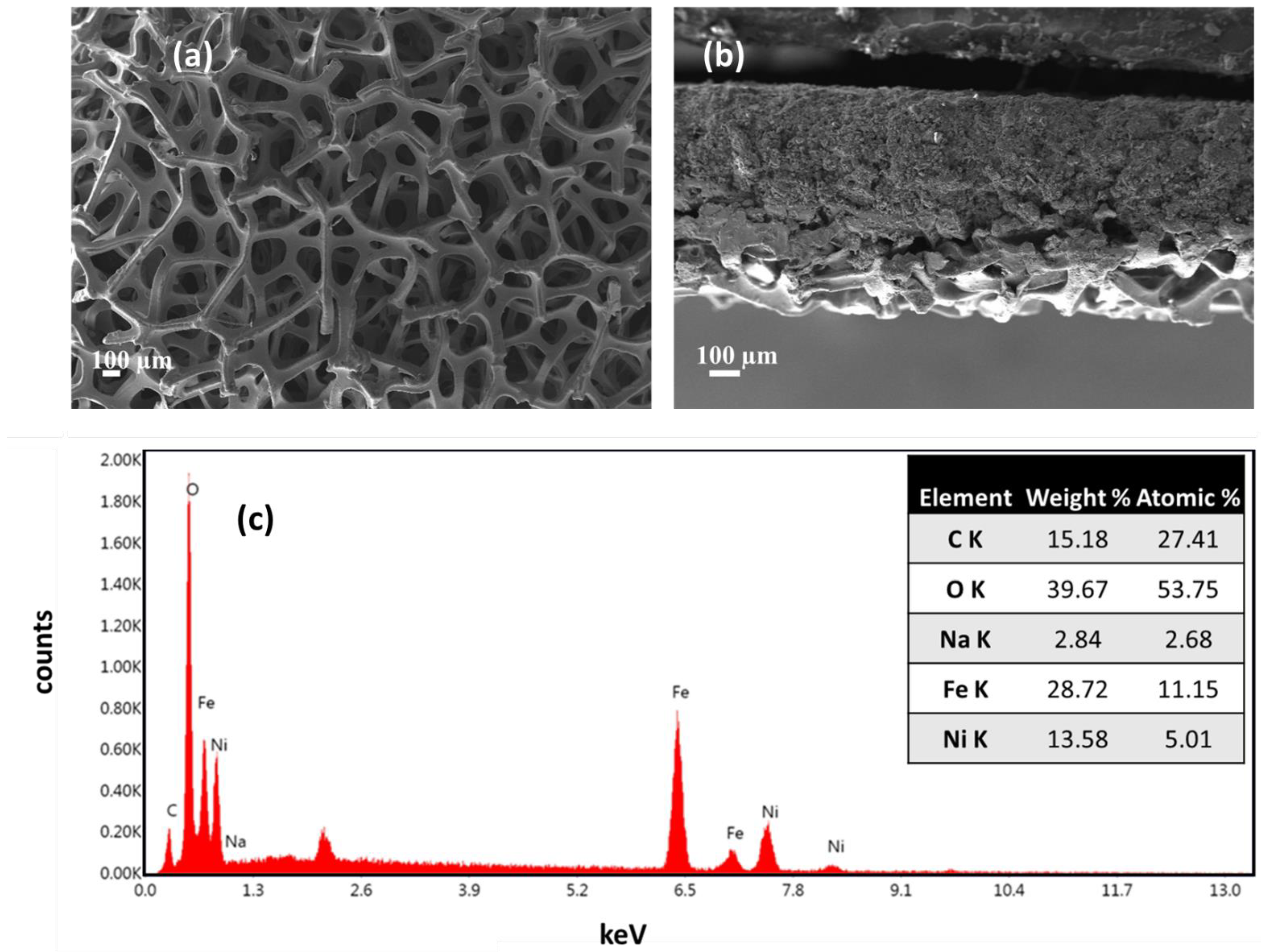



| Membrane | Thickness ( μm) | Specific Conductivity in Cl− (mS cm−1) | Specific Area Resistance (Ω cm−2) |
|---|---|---|---|
| FAA-3-50 | 45–55 | 3–8 | 0.6–1.5 |
| FAS-50 | 45–55 | 3–8 | 0.6–1.5 |
| FAA-3-PK-75 | 70–80 | 4.5–6.5 | 1.2–2 |
| FAB PK-130 | 110–140 | 1–2.5 | 5–9 |
| Temperature | Ohmic Resistance | Activation Resistance |
|---|---|---|
| Rohm (Ω) | Ranode + Rcathode (Ω) | |
| 1.7 V | ||
| 45 °C | 0.127 | 0.101 |
| 55 °C | 0.108 | 0.075 |
| 1.8 V | ||
| 45 °C | 0.133 | 0.077 |
| 55 °C | 0.093 | 0.071 |
| 1.9 V | ||
| 45 °C | 0.134 | 0.051 |
| 55 °C | 0.116 | 0.036 |
| 2 V | ||
| 45 °C | 0.134 | 0.055 |
| 55 °C | 0.117 | 0.031 |
| Membranes | Ohmic Resistance | Activation Resistance |
|---|---|---|
| Rohm (Ω) | Ranode + Rcathode (Ω) | |
| FAA-3-50 | 0.108 | 0.0756 |
| FAS-50 | 0.092 | 0.0918 |
| FAA-3-PK-75 | 0.16 | 0.113 |
| FAB-PK-130 | 0.777 | 0.199 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, K.W.; Dobson, A.; Habibpour, S.; Fowler, M. Enhanced NiFe2O4 Catalyst Performance and Stability in Anion Exchange Membrane Water Electrolysis: Influence of Iron Content and Membrane Selection. Molecules 2025, 30, 3228. https://doi.org/10.3390/molecules30153228
Ahmed KW, Dobson A, Habibpour S, Fowler M. Enhanced NiFe2O4 Catalyst Performance and Stability in Anion Exchange Membrane Water Electrolysis: Influence of Iron Content and Membrane Selection. Molecules. 2025; 30(15):3228. https://doi.org/10.3390/molecules30153228
Chicago/Turabian StyleAhmed, Khaja Wahab, Aidan Dobson, Saeed Habibpour, and Michael Fowler. 2025. "Enhanced NiFe2O4 Catalyst Performance and Stability in Anion Exchange Membrane Water Electrolysis: Influence of Iron Content and Membrane Selection" Molecules 30, no. 15: 3228. https://doi.org/10.3390/molecules30153228
APA StyleAhmed, K. W., Dobson, A., Habibpour, S., & Fowler, M. (2025). Enhanced NiFe2O4 Catalyst Performance and Stability in Anion Exchange Membrane Water Electrolysis: Influence of Iron Content and Membrane Selection. Molecules, 30(15), 3228. https://doi.org/10.3390/molecules30153228







