Hair Levels of Lead, Cadmium, Selenium, and Their Associations with Neurotoxicity and Hematological Biomarkers in Children from the Mojana Region, Colombia
Abstract
1. Introduction
2. Results
2.1. General Characteristics and Dietary Habits of the Study Population
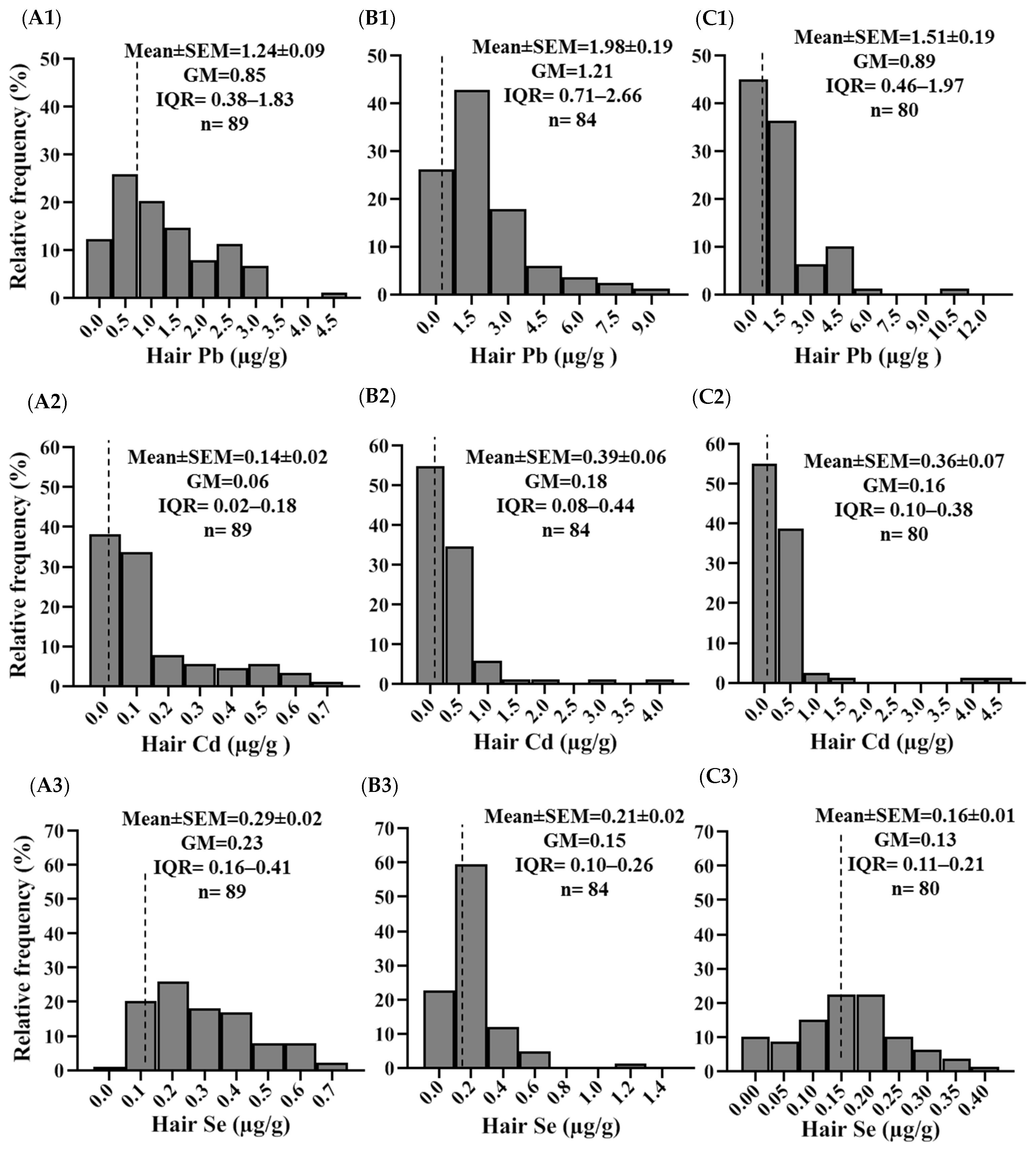
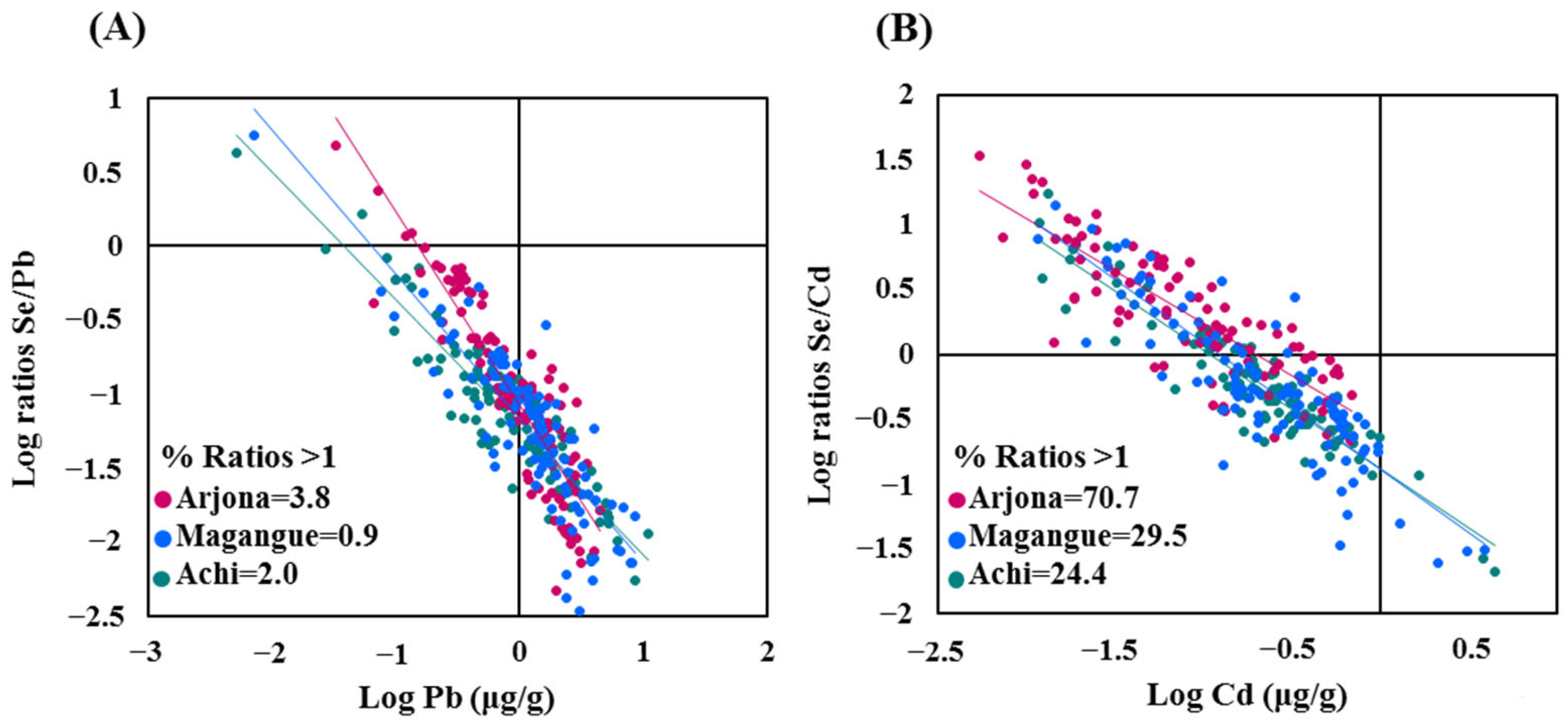
2.2. Concentrations of Pb, Cd, and Se in Hair
2.3. Hematological Parameters
2.4. Biochemical Markers
3. Discussion
4. Materials and Methods
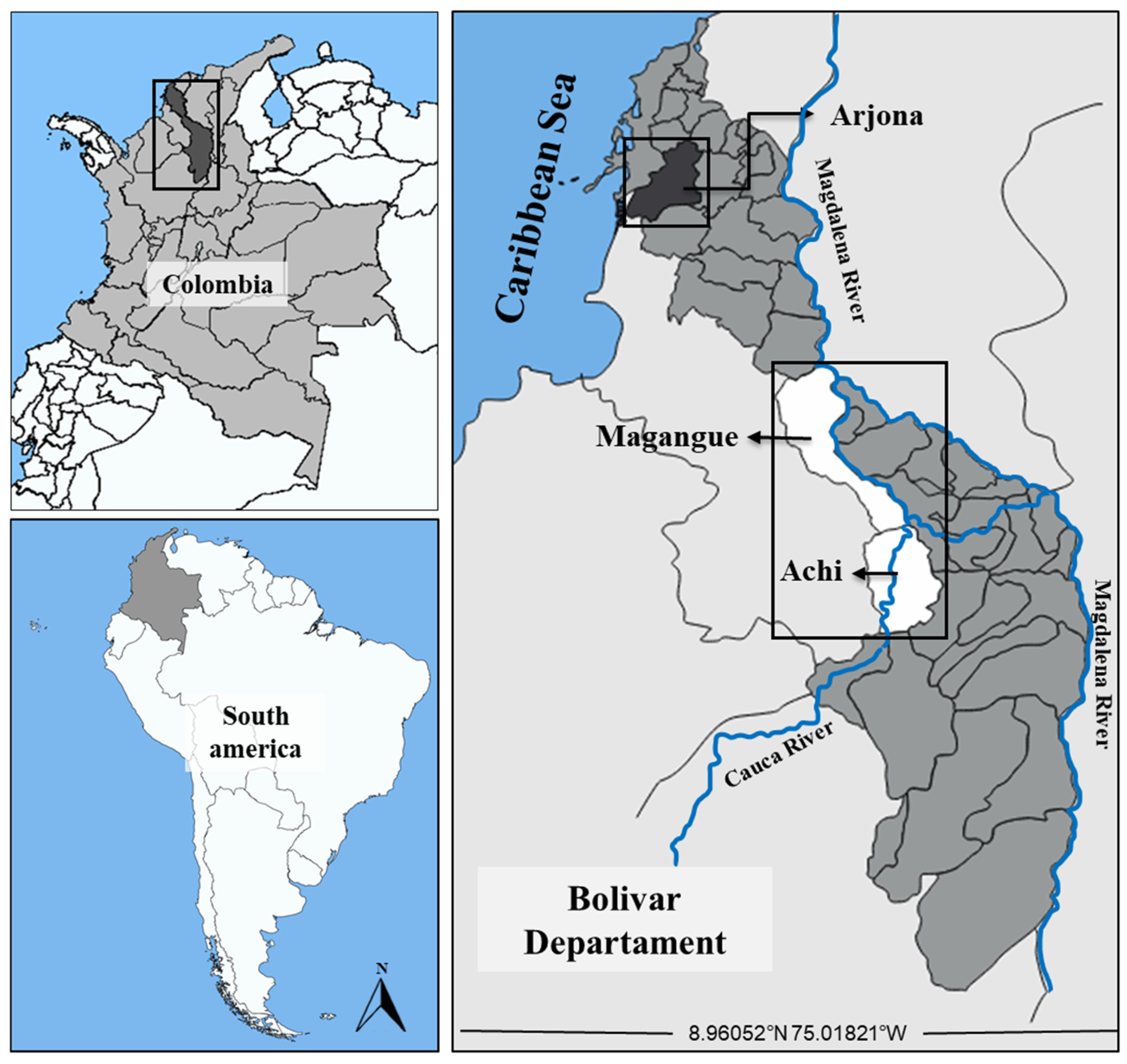
4.1. Study Area
4.2. Participant Selection and Data Collection
4.3. Collection of Hair and Blood Samples
4.4. Analysis of Lead, Cadmium, and Selenium in Hair
4.5. Quality Control
4.6. Determination of Se/Toxic Metal Molar Ratios
4.7. Measurement of Biochemical Markers
4.8. Hematological Analysis
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De La Guardia Gutiérrez, M.A.; Ruvalcaba Ledezma, J.C. La salud y sus determinantes, promoción de la salud y educación sanitaria. J. Negat. No Posit. Results 2020, 5, 81–90. [Google Scholar] [CrossRef]
- Rollin, H.B. Introduction to the special issue of IJERPH entitled “Prenatal exposure to environmental pollutants and other stressors: Impacts on fetal development, birth outcomes, children’s health and beyond”. Int. J. Environ. Res. Public Health 2022, 19, 8816. [Google Scholar] [CrossRef] [PubMed]
- Al Osman, M.; Yang, F.; Massey, I.Y. Exposure routes and health effects of heavy metals on children. Biometals 2019, 32, 563–573. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Xi, S. The effects of heavy metals on human metabolism. Toxicol. Mech. Methods 2020, 30, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Rashid, A.; Bhat, R.A.; Qadri, H.; Mehmood, M.A. Environmental and socioeconomic factors induced blood lead in children: An investigation from Kashmir, India. Environ. Monit. Assess. 2019, 191, 76. [Google Scholar] [CrossRef]
- Charkiewicz, A.E.; Backstrand, J.R. Lead toxicity and pollution in Poland. Int. J. Environ. Res. Public Health 2020, 17, 4385. [Google Scholar] [CrossRef]
- Zhao, B.; Zhao, J.; Zhou, S. Selenium and toxic metals in human hair of the Dashan Region, China: Concentrations, sources, and antagonism effect. Ecotoxicol. Environ. Saf. 2023, 250, 114479. [Google Scholar] [CrossRef]
- Heidari, S.; Mostafaei, S.; Razazian, N.; Rajati, M.; Saeedi, A.; Rajati, F. The effect of lead exposure on IQ test scores in children under 12 years: A systematic review and meta-analysis of case-control studies. Syst. Rev. 2022, 11, 106. [Google Scholar] [CrossRef]
- Alvarez-Ortega, N.; Caballero-Gallardo, K.; Olivero-Verbel, J. Low blood lead levels impair intellectual and hematological function in children from Cartagena, Caribbean coast of Colombia. J. Trace Elem. Med. Biol. 2017, 44, 233–240. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, A.; Cabral-Pinto, M.M.S.; Chaturvedi, A.K.; Shabnam, A.A.; Subrahmanyam, G.; Mondal, R.; Gupta, D.K.; Malyan, S.K.; Kumar, S.S.; et al. Lead toxicity: Health hazards, influence on food chain, and sustainable remediation approaches. Int. J. Environ. Res. Public Health 2020, 17, 2179. [Google Scholar] [CrossRef]
- Ramirez Ortega, D.; González Esquivel, D.F.; Blanco Ayala, T. Cognitive Impairment induced by lead exposure during lifespan: Mechanisms of lead neurotoxicity. Toxics 2021, 9, 23. [Google Scholar] [CrossRef] [PubMed]
- Marianti, A.; Anies, A.; Abdurachim, H.R.S. Causality pattern of the blood lead, monoamine oxidase A, and serotonin levels in brass home industry workers chronically exposed to lead. Songklanakarin J. Sci. Technol. 2016, 38, 147–153. [Google Scholar] [CrossRef]
- Zink, L.; Wiseman, S.; Pyle, G.G. Single and combined effects of cadmium, microplastics, and their mixture on whole-body serotonin and feeding behaviour following chronic exposure and subsequent recovery in the freshwater leech, Nephelopsis obscura. Aquat. Toxicol. 2023, 259, 106538. [Google Scholar] [CrossRef] [PubMed]
- Owumi, S.E.; Adedara, I.A.; Otunla, M.T.; Owoeye, O. Influence of furan and lead co-exposure at environmentally relevant concentrations on neurobehavioral performance, redox-regulatory system and apoptotic responses in rats. Environ. Toxicol. Pharmacol. 2023, 97, 1–12. [Google Scholar] [CrossRef]
- Messaoudi, I.; El Heni, J.; Hammouda, F.; Saïd, K.; Kerkeni, A. Protective effects of selenium, zinc, or their combination on cadmium-induced oxidative stress in rat kidney. Biol. Trace Elem. Res. 2009, 130, 152–161. [Google Scholar] [CrossRef]
- Carranza-Lopez, L.; Alvarez-Ortega, N.; Caballero-Gallardo, K.; Gonzalez-Montes, A.; Olivero-Verbel, J. Biomonitoring of lead exposure in children from two fishing communities at Northern Colombia. Biol. Trace Elem. Res. 2021, 199, 850–860. [Google Scholar] [CrossRef]
- Yohannes, Y.B.; Nakayama, S.M.; Yabe, J. Glutathione S-transferase gene polymorphisms in association with susceptibility to lead toxicity in lead-and cadmium-exposed children near an abandoned lead-zinc mining area in Kabwe, Zambia. Environ. Sci. Pollut. Res. 2022, 29, 6622–6632. [Google Scholar] [CrossRef]
- Ebrahimi, M.; Khalili, N.; Razi, S. Effects of lead and cadmium on the immune system and cancer progression. J. Environ. Health Sci. Eng. 2020, 18, 335–343. [Google Scholar] [CrossRef]
- Joseph, N.; Kolok, A.S. Assessment of pediatric cancer and its relationship to environmental contaminants: An ecological study in Idaho. Geohealth 2022, 6, 548. [Google Scholar] [CrossRef]
- Cruz-Esquivel, Á.; Díez, S.; Marrugo-Negrete, J.L. Genotoxicity effects in freshwater fish species associated with gold mining activities in tropical aquatic ecosystems. Ecotoxicol. Environ. Saf. 2023, 253, 114670. [Google Scholar] [CrossRef]
- Chaali, N.; Bravo, D.; Ouazaa, S. New insights into arsenic and cadmium distribution and origin in paddy soils using electrical resistivity tomography. J. Appl. Geophys. 2022, 202, 104638. [Google Scholar] [CrossRef]
- Galeano-Páez, C.; Espitia-Pérez, P.; Jimenez-Vidal, L.; Pastor-Sierra, K.; Salcedo-Arteaga, S.; Hoyos-Giraldo, L.S.; Gioda, A.; Saint’pIerre, T.D.; García, S.C.; Brango, H.; et al. Dietary exposure to mercury and its relation to cytogenetic instability in populations from “La Mojana” region, northern Colombia. Chemosphere 2021, 265, 129066. [Google Scholar] [CrossRef]
- Olivero-Verbel, J.; Caballero-Gallardo, K.; Negrete-Marrugo, J. Relationship between localization of gold mining areas and hair mercury levels in people from Bolivar, north of Colombia. Biol. Trace Elem. Res. 2011, 144, 118–132. [Google Scholar] [CrossRef]
- Zheng, J.; Li, M.; Tang, B. Levels, spatial distribution, and impact factors of heavy metals in the hair of metropolitan residents in China and human health implications. Environ. Sci. Technol. 2021, 55, 10578–10588. [Google Scholar] [CrossRef]
- González-Álvarez, D.; Cabrera Jaramillo, A.; Cadavid Muñoz, N.; Agudelo-Echavarría, D.M.; Soto-Ospina, A.; Arango Ruiz, Á. Total mercury and methylmercury levels in eggs from laying hens in a mining area in Bajo Cauca, Antioquia, Colombia. Emerg. Contam. 2023, 9, 100230. [Google Scholar] [CrossRef]
- Cadavid Muñoz, N.; González-Álvarez, D.; Cabrera Jaramillo, A.; Soto-Ospina, A.; Arango Ruiz, Á. Toxicological risk in individuals exposed to methylmercury and total mercury through daily-consumed foodstuffs in one of the mining regions of Bajo Cauca, Antioquia, Colombia. Emerg. Contam. 2023, 9, 100226. [Google Scholar] [CrossRef]
- Córdoba-Tovar, L.; Marrugo-Negrete, J.; Barón, P.A.R.; Díez, S. Ecological and human health risk from exposure to contaminated sediments in a tropical river impacted by gold mining in Colombia. Environ. Res. 2023, 236, 116759. [Google Scholar] [CrossRef] [PubMed]
- Marrugo-Negrete, J.; Verbel, J.O.; Ceballos, E.L.; Benitez, L.N. Total mercury and methylmercury concentrations in fish from the Mojana region of Colombia. Environ. Geochem. Health 2008, 30, 21–30. [Google Scholar] [CrossRef]
- Palomares-Bolaños, J.C.; Caballero-Gallardo, K.; Olivero-Verbel, J. Hematological parameters and mercury exposure in children living along gold-mining-impacted rivers in the Mojana Region, Colombia. Biol. Trace Elem. Res. 2025, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, M.; Rajabi, S.; Eghbalian, M.; Suliburska, J.; Nasab, H. Demographic and anthropometric characteristics and their effect on the concentration of heavy metals (arsenic, lead, chromium, zinc) in children and adolescents. Heliyon 2023, 9, 13621. [Google Scholar] [CrossRef]
- Örün, E.; Yalçın, S.S.; Aykut, O. Lead, mercury, and cadmium levels in breast milk and infant hair in the late period of lactation in Ankara, Turkey. Int. J. Environ. Health Res. 2022, 32, 1950–1961. [Google Scholar] [CrossRef]
- Heng, Y.Y.; Asad, I.; Coleman, B.; Menard, L.; Benki-Nugent, S.; Were, F.H.; Karr, C.J.; McHenry, M.S. Heavy metals and neurodevelopment of children in low and middle-income countries: A systematic review. PLoS ONE 2022, 17, 0265536. [Google Scholar] [CrossRef] [PubMed]
- Manjarres-Suarez, A.; de la Rosa, J.; Gonzalez-Montes, A.; Galvis-Ballesteros, J.; Olivero-Verbel, J. Trace elements, peripheral blood film, and gene expression status in adolescents living near an industrial area in the Colombian Caribbean Coastline. J. Expo. Sci. Environ. Epidemiol. 2022, 32, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Liu, R.; Wang, R.; Ba, Y.; Yu, F.; Deng, Q.; Huang, H. Lead-induced neurodevelopmental lesion and epigenetic landscape: Implication in neurological disorders. J. Appl. Toxicol. 2023, 43, 1256–1271. [Google Scholar] [CrossRef] [PubMed]
- Du, B.; Zhou, J.; Lu, B.; Zhang, C.; Li, D.; Zhou, J.; Jiao, S.; Zhao, K.; Zhang, H. Environmental and human health risks from cadmium exposure near an active lead-zinc mine and a copper smelter, China. Sci. Total Environ. 2020, 720, 137585. [Google Scholar] [CrossRef]
- Nakaona, L.; Maseka, K.K.; Hamilton, E.M.; Watts, M.J. Using human hair and nails as biomarkers to assess exposure of potentially harmful elements to populations living near mine waste dumps. Environ. Geochem. Health 2020, 42, 1197–1209. [Google Scholar] [CrossRef]
- Shimo, N.A.; Salam, M.A.; Parvin, M.; Sultan, M.Z. Assessment of selected metals (chromium, lead and cadmium) in the hair of tannery workers at Hemayetpur, Bangladesh. J. Trace Elem. Miner. 2023, 4, 100056. [Google Scholar] [CrossRef]
- Genchi, G.; Sinicropi, M.S.; Lauria, G.; Carocci, A.; Catalano, A. The effects of cadmium toxicity. Int. J. Environ. Res. Public Health 2020, 17, 3782. [Google Scholar] [CrossRef]
- Pierron, F.; Daffe, G.; Daramy, F.; Heroin, D.; Barré, A.; Bouchez, O.; Clérendeau, C.; Romero-Ramirez, A.; Nikolski, M. Transgenerational endocrine disruptor effects of cadmium in zebrafish and contribution of standing epigenetic variation to adaptation. J. Hazard. Mater. 2023, 455, 131579. [Google Scholar] [CrossRef]
- Allali, S.; Brousse, V.; Sacri, A.S. Anemia in children: Prevalence, causes, diagnostic work-up, and long-term consequences. Expert Rev. Hematol. 2017, 10, 1023–1028. [Google Scholar] [CrossRef]
- López-Rodríguez, G.; Galván, M.; González-Unzaga, M.; Hernández Ávila, J.; Pérez-Labra, M. Blood toxic metals and hemoglobin levels in Mexican children. Environ. Monit. Assess. 2017, 189, 179. [Google Scholar] [CrossRef]
- Saha, S.; Dhara, K.; Chukwuka, A.V.; Pal, P.; Saha, N.C.; Faggio, C. Sub-lethal acute effects of environmental concentrations of inorganic mercury on hematological and biochemical parameters in walking catfish, Clarias batrachus. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2023, 264, 109511. [Google Scholar] [CrossRef] [PubMed]
- Bal, C.; Karakulak, U.; Gunduzoz, M.; Ercan, M.; Tutkun, E.; Yilmaz, O.M. Evaluation of subclinical inflammation with neutrophil lymphocyte ratio in heavy metal exposure. J. Clin. Anal. Med. 2016, 7, 643–647. [Google Scholar] [CrossRef]
- Xiong, L.; Fan, C.; Song, J.; Wan, Y.; Lin, X.; Su, Z.; Qiu, J.; Wu, W.; He, Z.; Wu, Y.; et al. Associations of long-term cadmium exposure with peripheral white blood cell subtype counts and indices in residents of cadmium-polluted areas. Chemosphere 2022, 308, 135946. [Google Scholar] [CrossRef] [PubMed]
- Prasanthi, R.J.; Devi, C.B.; Basha, D.C.; Reddy, N.S.; Reddy, G.R. Calcium and zinc supplementation protects lead (Pb)-induced perturbations in antioxidant enzymes and lipid peroxidation in developing mouse brain. Int. J. Dev. Neurosci. 2010, 28, 161–167. [Google Scholar] [CrossRef]
- Shin, C.Y.; Choi, J.W.; Choi, M.S.; Ryu, J.R.; Ko, K.H.; Cheong, J.H. Developmental changes of the activity of monoamine oxidase in pre-and postnatally lead exposed rats. Environ. Toxicol. Pharmacol. 2007, 24, 5–10. [Google Scholar] [CrossRef]
- David, M.; Turi, N.; Ain, Q.U.; Rahman, H.; Jahan, S. Evaluation of environmental effects of heavy metals on biochemical profile and oxidative stress among children at brick kiln sites. Arch. Environ. Occup. Health 2021, 76, 441–449. [Google Scholar] [CrossRef]
- Tsentsevitsky, A.N.; Petrov, A.M. Synaptic mechanisms of cadmium neurotoxicity. Neural Regen. Res. 2021, 16, 1762–1763. [Google Scholar] [CrossRef]
- Fairweather-Tait, S.J.; Bao, Y.; Broadley, M.R.; Collings, R.; Ford, D.; Hesketh, J.E.; Hurst, R. Selenium in human health and disease. Antioxid. Redox Signal. 2011, 14, 1337–1383. [Google Scholar] [CrossRef]
- Stoffaneller, R.; Morse, N.L. A review of dietary selenium intake and selenium status in Europe and the Middle East. Nutrients 2015, 7, 1494–1537. [Google Scholar] [CrossRef]
- Combs, G.F., Jr. Biomarkers of selenium status. Nutrients 2015, 7, 2209–2236. [Google Scholar] [CrossRef]
- Corvalán, C.; Garmendia, M.L.; Jones-Smith, J.; Lutter, C.K.; Miranda, J.J.; Pedraza, L.S.; Popkin, B.M.; Ramirez-Zea, M.; Salvo, D.; Stein, A.D. Nutrition status of children in Latin America. Obes. Rev. 2017, 18, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Rayman, M.P. Selenium and human health. Lancet 2012, 379, 1256–1268. [Google Scholar] [CrossRef] [PubMed]
- Nuttall, K.L. Evaluating selenium poisoning. Ann. Clin. Lab. Sci. 2006, 36, 409–420. [Google Scholar] [PubMed]
- Błażewicz, A.; Klatka, M.; Astel, A.; Korona-Glowniak, I.; Dolliver, W.; Szwerc, W.; Kocjan, R. Serum and urinary selenium levels in obese children: A cross-sectional study. J. Trace Elem. Med. Biol. 2015, 29, 116–122. [Google Scholar] [CrossRef]
- Mistry, H.D.; Williams, P.J. The importance of antioxidant micronutrients in pregnancy. Oxidative Med. Cell. Longev. 2011, 2011, 841749. [Google Scholar] [CrossRef]
- Zeng, H.; Combs, G.F., Jr. Selenium as an anticancer nutrient: Roles in cell proliferation and tumor cell invasion. J. Nutrl. Biochem. 2008, 19, 1–7. [Google Scholar] [CrossRef]
- Klotz, L.O. Reactive oxygen species as initiators and mediators of cellular signaling processes. In Studies on Experimental Toxicology and Pharmacology; Springer International Publishing: Cham, Switzerland, 2015; pp. 149–171. [Google Scholar] [CrossRef]
- Zwolak, I. The role of selenium in arsenic and cadmium toxicity: An updated review of scientific literature. Biol. Trace Elem. Res. 2020, 193, 44–63. [Google Scholar] [CrossRef]
- Batyrova, G.; Taskozhina, G.; Umarova, G.; Umarov, Y.; Morenko, M.; Iriskulov, B.; Kudabayeva, K.; Bazargaliyev, Y. Unveiling the role of selenium in child development: Impacts on growth, neurodevelopment and immunity. J. Clin. Med. 2025, 14, 1274. [Google Scholar] [CrossRef]
- DANE. Censo Nacional De Población Y Vivienda Colombia, 2018. Available online: https://www.dane.gov.co/index.php/estadisticas-por-tema/demografia-y-poblacion/censo-nacional-de-poblacion-y-vivenda-2018 (accessed on 15 May 2025).
- PDEA. Plan de Desarrollo 2016–2019. Available online: https://www.bolivar.gov.co/archivos/?p=Planes_de_Desarrollo%2FPlan_de_Desarrollo_2016-2019 (accessed on 27 January 2024).
- PDM. Plan de Desarrollo Municipal de Achí, Bolívar, 2020–2023. Available online: https://www.achi-bolivar.gov.co/tema/planes/plan-de-desarrollo (accessed on 25 May 2024).
- De la Ossa, C.A.; Ramírez-Giraldo, A.F.; Arroyo-Alvis, K.; Marrugo-Negrete, J.; Díez, S. Neuropsychological effects and cognitive deficits associated with exposure to mercury and arsenic in children and adolescents of the Mojana region, Colombia. Environ. Res. 2023, 216, 114467. [Google Scholar] [CrossRef]
- Manjarres-Suarez, A.; Olivero-Verbel, J. Hematological parameters and hair mercury levels in adolescents from the Colombian Caribbean. Environ. Sci. Pollut. Res. 2020, 27, 14216–14227. [Google Scholar] [CrossRef]
- Aljumaili, O.I.; Ewais, E.E.D.A.; El-Waseif, A.A.; Suleiman, A.A. Determination of hair lead, iron, and cadmium in a sample of autistic Iraqi children: Environmental risk factors of heavy metals in autism. Mater. Today Proc. 2023, 80, 2712–2715. [Google Scholar] [CrossRef]




| Variable | Measurement | Total n = 253 | Sampling Sites | Statistic | p-Value | ||
|---|---|---|---|---|---|---|---|
| Arjona n = 89 | Magangue n = 84 | Achi = 80 | |||||
| Age (years) | Mean ± SEM | 9.1 ± 0.1 | 8.8 ± 0.2 | 9.5 ± 0.2 | 9.1 ± 0.2 | KW = 5.3 | 0.07 |
| GM | 8.9 | 8.6 | 9.3 | 8.9 | |||
| Sex | Male | 138 (54.5) | 43 (48.3) | 43 (51.2) | 52 (65.0) | χ2 = 5.9 | 0.05 |
| Female | 115 (45.5) | 46 (51.7) | 41 (48.8) | 28 (35.0) | |||
| Weight (kg) | Mean ± SEM | 30.6± 0.6 | 31.3 ± 0.9 | 29.2 ± 0.8 | 31.2 ± 1.0 | KW = 2.4 | 0.29 |
| GM | 29.4 | 29.9 | 28.3 | 30.0 | |||
| Height (cm) | Mean ± SEM | 134.2 ± 0.8 | 135.9 ± 1.4 | 132.9 ± 1.3 | 133.6 ± 1.3 | KW = 2.5 | 0.29 |
| GM | 133.6 | 135.2 | 132.4 | 133.1 | |||
| BMI (kg/m2) | Mean ± SEM | 16.7 ± 0.2 | 16.7 ± 0.3 | 16.3 ± 0.2 | 17.2 ± 0.3 | KW = 3.0 | 0.22 |
| GM | 16.5 | 16.4 | 16.1 | 16.9 | |||
| Variable | Measurement | Study Area | Statistic | p-Value | |||
|---|---|---|---|---|---|---|---|
| Total n = 253 | Arjona n = 89 | Magangue n = 84 | Achi = 80 | ||||
| Age (years) | Mother Mean ± SEM GM | 33.6 ± 0.4 33.1 | 34.6 ± 0.7 34.0 | 33.0 ± 0.7 32.4 | 33.3 ± 0.6 32.8 | KW = 3.2 | 0.21 |
| Father Mean ± SEM GM | 38.9 ± 0.5 38.1 | 38.0 ± 0.7 37.4 | 38.9 ± 1.0 37.9 | 40.0 ± 0.9 39.2 | KW = 1.9 | 0.38 | |
| Father occupation | Agriculture | 17 (6.7) | 5 (5.6) | 0 (0.0) | 12 (15.0) | χ2 = 46.1 | <0.01 * |
| Fishing | 12 (4.7) | 0 (0.0) | 10 (11.9) | 2 (2.5) | |||
| Various occupations | 97 (38.3) | 25 (28.1) | 30 (35.7) | 42 (52.5) | |||
| Others (commerce, bricklaying, mechanics) | 127 (50.3) | 59 (66.3) | 44 (52.4) | 24 (30.0) | |||
| Alcohol consumption Father and mother | Yes | 210 (44.8) | 89 (51.4) | 77 (54.6) | 44 (28.4) | χ2 = 25.5 | <0.01 * |
| No | 259 (55.2) | 84 (48.6) | 64 (45.4) | 111 (71.6) | |||
| Tobacco consumption Father and mother | Yes | 19 (4.1) | 11 (6.4) | 7 (5.0) | 1 (0.6) | χ2 = 7.3 | 0.03 * |
| No | 450 (95.9) | 162 (93.6) | 134 (95.0) | 154 (99.4) | |||
| Variable | Category | Sampling Sites | Statistic χ2 | p-Value | |||
|---|---|---|---|---|---|---|---|
| Total n = 180 | Arjona n = 79 | Magangue n = 55 | Achi = 46 | ||||
| Fish (meals/week) | 0–4 | 146 (81.1) | 79 (100.0) | 27 (49.1) | 40 (87.0) | 61.9 | <0.01 * |
| 5–10 | 21 (11.7) | 0 (0.0) | 17 (30.9) | 4 (8.7) | |||
| >10 | 13 (7.2) | 0 (0.0) | 11 (20.0) | 2 (4.3) | |||
| Tubers (meals/week) | 0–4 | 118 (65.6) | 50 (63.3) | 35 (63.6) | 33 (71.7) | 7.1 | 0.13 |
| 5–10 | 53 (29.4) | 28 (35.4) | 15 (27.3) | 10 (21.7) | |||
| >10 | 9 (5.0) | 1 (1.3) | 5 (9.1) | 3 (6.5) | |||
| Rice (meals/week) | 0–4 | 6 (3.3) | 3 (3.8) | 3 (5.5) | 0 (0.0) | 67.6 | <0.01 * |
| 5–10 | 74 (41.1) | 57 (72.2) | 7 (12.7) | 10 (21.7) | |||
| >10 | 100 (55.6) | 19 (24.0) | 45 (81.8) | 36 (78.3) | |||
| Cereal grains (Corn, wheat) (meals/week) | 0–4 | 164 (91.1) | 71 (89.9) | 49 (89.1) | 44 (95.7) | 1.57 | 0.46 |
| 5–10 | 16 (8.9) | 8 (10.1) | 6 (10.9) | 2 (4.3) | |||
| >10 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |||
| Grains (lentils, beans) (meals/week) | 0–4 | 171 (95.0) | 79 (100.0) | 53 (96.4) | 39 (84.8) | 16.0 | <0.01 * |
| 5–10 | 9 (5.0) | 0 (0.0) | 2 (3.6) | 7 (15.2) | |||
| >10 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |||
| Bread (meals/week) | 0–4 | 89 (48.4) | 40 (50.6) | 16 (29.1) | 33 (71.7) | 19.5 | <0.01 * |
| 5–10 | 77 (42.8) | 32 (40.5) | 34 (61.8) | 11 (23.9) | |||
| >10 | 14 (7.8) | 7 (8.9) | 5 (9.1) | 2 (4.3) | |||
| Vegetables (meals/week) | 0–4 | 115 (63.9) | 51 (64.6) | 37 (67.3) | 27 (58.7) | 4.2 | 0.38 |
| 5–10 | 51 (28.3) | 20 (25.3) | 17 (30.9) | 14 (30.4) | |||
| >10 | 14 (7.8) | 8 (10.1) | 1 (1.8) | 5 (10.9) | |||
| Fruits (meals/week) | 0–4 | 121 (67.2) | 43 (54.4) | 49 (89.1) | 29 (63.0) | 20.2 | <0.01 * |
| 5–10 | 57 (31.7) | 35 (44.3) | 5 (9.1) | 17 (37.0) | |||
| >10 | 2 (1.1) | 1 (1.3) | 1 (1.8) | 0 (0.0) | |||
| Meat (meals/week) | 0–4 | 107 (59.4) | 30 (37.9) | 51 (92.7) | 26 (56.5) | 46.4 | <0.01 * |
| 5–10 | 61 (33.9) | 42 (53.2) | 4 (7.3) | 15 (32.6) | |||
| >10 | 12 (6.7) | 7 (8.9) | 0 (0.0) | 5 (10.9) | |||
| Eggs (meals/week) | 0–4 | 111 (61.7) | 48 (60.8) | 34 (61.8) | 29 (63.0) | 1.75 | 0.81 |
| 5–10 | 63 (35.0) | 29 (36.7) | 18 (32.7) | 16 (34.8) | |||
| >10 | 6 (3.3) | 2 (2.5) | 3 (5.5) | 1 (2.2) | |||
| Dairy products (meals/week) | 0–4 | 99 (55.0) | 29 (36.7) | 38 (69.1) | 32 (69.6) | 17.9 | <0.01 * |
| 5–10 | 79 (43.9) | 49 (62.0) | 17 (30.9) | 13 (28.3) | |||
| >10 | 2 (1.1) | 1 (1.3) | 0 (0.0) | 1 (2.2) | |||
| Selenium Level (µg/g) * | Classification | Arjona n = 89 | Magangue n = 84 | Achi n = 80 | |||
|---|---|---|---|---|---|---|---|
| Male n (%) | Female n (%) | Male n (%) | Female n (%) | Male n (%) | Female n (%) | ||
| <0.2 | Se-deficient | 16 (18.0) | 20 (22.5) | 29 (34.5) | 21 (25.0) | 37 (46.2) | 20 (25.0) |
| 0.2–0.25 | Se-marginal | 1 (1.1) | 5 (5.6) | 3(3.6) | 6 (7.1) | 5 (6.3) | 4 (5.0) |
| 0.25–0.5 | Se-sufficient | 20 (22.5) | 13 (14.6) | 9 (10.7) | 11 (13.1) | 10 (12.5) | 4 (5.0) |
| 0.5–3 | Se-rich | 6 (6.7) | 8 (9.0) | 2 (2.4) | 3 (3.6) | 0 (0.0) | 0 (0.0) |
| >3 | Se-excessive | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Variable | Unit | Reference Value | Arjona n = 89 | Magangue n = 84 | Achi n = 80 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± SEM | Below Reference (%) | Above Reference (%) | Mean ± SEM | Below Reference (%) | Above Reference (%) | Mean ± SEM | Below Reference (%) | Above Reference (%) | |||
| Red blood cell | |||||||||||
| RBC | 1012/L | 4.1–5.6 | 4.7 ± 0.04 | 1.1 | 0.0 | 4.7 ± 0.05 | 0.0 | 0.0 | 4.7 ± 0.04 | 2.5 | 0.0 |
| HGB | g/dL | 11.5–16.3 | 13.3 ± 0.1 | 2.2 | 0.0 | 12.8 ± 0.1 * | 2.4 | 0.0 | 12.4 ± 0.1 * | 8.8 | 0.0 |
| HTC | % | 34.4–48.3 | 37.7 ± 0.3 | 9.0 | 0.0 | 37.5 ± 0.3 | 9.5 | 1.2 | 37.3 ± 0.2 | 8.8 | 0.0 |
| MCV | fL | 74.3–93 | 79.9 ± 0.5 | 10.1 | 0.0 | 80.4± 0.3 | 4.8 | 0.0 | 80.1 ± 0.5 | 6.3 | 0.0 |
| MCH | pg | 24.3–31.7 | 28.3 ± 0.2 | 2.2 | 0.0 | 27.6 ± 0.2 * | 1.2 | 0.0 | 26.6 ± 0.2 * | 6.3 | 1.3 |
| MCHC | g/dL | 31.9–35.1 | 35.4 ± 0.1 | 3.4 | 0.0 | 34.4 ± 0.1 * | 1.2 | 21.4 | 33.2 ± 0.1 * | 1.3 | 1.3 |
| RDW | % | 11.7–18.3 | 16.0 ± 0.1 | 0.0 | 0.0 | 16.9 ± 0.1 * | 0.0 | 5.9 | 17.3 ± 0.1 * | 0.0 | 11.3 |
| White blood cell | |||||||||||
| WBC | 109/L | 3.7–11.9 | 6.6 ± 0.2 | 0.0 | 0.0 | 8.5 ± 0.2 * | 0.0 | 5.9 | 9.1 ± 0.3 * | 0.0 | 12.5 |
| LYM | 109/L | 2.2–6.8 | 2.9 ± 0.1 | 18.0 | 1.1 | 3.0 ± 0.1 | 14.3 | 0.0 | 3.2 ± 0.1 * | 3.8 | 2.5 |
| GRA | 109/L | 2.5–7.5 | 3.4 ± 0.1 | 23.6 | 0.0 | 4.9 ± 0.2 * | 2.4 | 5.9 | 4.8 ± 0.2 * | 10.0 | 11.3 |
| LYM | % | 20–56.6 | 43.4 ± 1.2 | 1.1 | 18.0 | 35.6 ± 0.9 * | 2.4 | 0.0 | 37.3 ± 1.0 * | 1.3 | 1.3 |
| GRA | % | 33.2–74.7 | 49.5 ± 1.2 | 11.2 | 1.1 | 57.9 ± 1.0 * | 0.0 | 3.6 | 52.2 ± 1.1 | 2.5 | 1.3 |
| Platelet | |||||||||||
| PLT | 109/L | 194–477 | 294.9 ± 5.9 | 2.2 | 0.0 | 313.6 ± 8.0 | 5.9 | 2.4 | 285.1 ± 6.9 | 5.0 | 0.0 |
| PCT | % | 0.1–0.5 | 0.2 ± 0.01 | 0.0 | 0.0 | 0.2 ± 0.01 | 0.0 | 0.0 | 0.2 ± 0.01 | 1.3 | 0.0 |
| MPV | fL | 6.6–9.9 | 8.2 ± 0.1 | 0.0 | 1.1 | 7.8 ± 0.1 * | 3.6 | 2.4 | 7.9 ± 0.1 * | 3.8 | 3.8 |
| PDW | % | 25–50 | 38.7 ± 0.2 | 0.0 | 0.0 | 38.2 ± 0.2 | 0.0 | 0.0 | 38.1 ± 0.2 * | 0.0 | 0.0 |
| Variables | Hair Pb (µg/g) | Hair Cd (µg/g) | Hair Se (µg/g) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Not Adjusted | Adjusted | Not Adjusted | Adjusted | Not Adjusted | Adjusted | |||||||
| β (p Value) | 95% CI | β (p-Value) | 95% CI | β (p-Value) | 95% CI | β (p-Value) | 95% CI | β (p-Value) | 95% CI | β (p-Value) | 95% CI | |
| Serotonin | −2.575 (0.022) * | −4.775; −0.375 | −2.495 (0.042) * | −5.021; −0.031 | −5.337 (0.100) | −11.71; 1.035 | −4.867 (0.179) | −12.00; 2.263 | 24.20 (0.025) * | 2.929; 45.48 | 24.78 (0.027) * | 2.846; 46.72 |
| MAO-A | −0.416 (0.275) | −1.166; 0.333 | −0.372 (0.343) | −1.144; 0.399 | −2.279 (0.048) * | −4.534; −0.024 | −1.584 (0.177) | −3.885; 0.717 | 7.617 (0.038) * | 0.424; 14.81 | 6.647 (0.073) | −0.645; 13.94 |
| AChE | 0.851 (0.652) | −2.859; 4.561 | 0.529 (0.787) | −3.319; 4.376 | 1.979 (0.709) | −8.485; 12.44 | 0.808 (0.884) | −10.08; 11.69 | −21.67 (0.222) | −56.60; 13.26 | −21.24 (0.245) | −57.21; 14.73 |
| WBC | 0.193 (0.043) * | −0.003; 0.389 | 0.174 (0.086) | −0.025; 0.373 | 0.206 (0.489) | −0.380; 0.722 | 0.161 (0.597) | −0.439; 0.761 | −1.393 (0.150) | −3.297; 0.510 | −1.125 (0.248) | −3.041; 0.790 |
| HGB | −0.032 (0.341) | −0.099; 0.034 | −0.026 (0.448) | −0.092; 0.041 | −0.157 (0.118) | −0.354; 0.040 | −0.159 (0.114) | −0.357; 0.038 | 0.792 (0.015) * | 0.154; 1.431 | 0.739 (0.021) * | 0.109; 1.370 |
| MCV | −0.115 (0.483) | −0.438; 0.208 | −0.067 (0.683) | −0.390; 0.256 | 0.579 (0.234) | −0.377; 1.536 | 0.664 (0.176) | −0.300; 1.629 | 3.472 (0.028) * | 0.376; 6.568 | 3.302 (0.035) * | 0.233; 6.371 |
| MCH | −0.012 (0.865) | −0.151; 0.127 | 0.009 (0.895) | −0.129; 0.147 | −0.127 (0.541) | −0.539; 0.283 | −0.140 (0.504) | −0.553; 0.272 | 2.288 (0.001) * | 0.976; 3.599 | 2.105 (0.002) * | 0.808; 3.402 |
| MCHC | 0.039 (0.524) | −0.081; 0.159 | 0.046 (0.459) | −0.077; 0.169 | −0.381 (0.035) * | −0.736; 0.027 | −0.431 (0.020) * | −0.796; −0.067 | 1.441 (0.014) * | 0.289; 2.593 | 1.281 (0.031) | 0.114; 2.448 |
| PLT | 3.533 (0.177) | −1.602; 8.668 | 2.529 (0.342) | −2.716; 7.774 | −0.625 (0.935) | −15.92; 14.67 | −5.080 (0.525) | −20.81; 10.65 | 30.49 (0.228) | −19.20; 80.17 | 22.70 (0.374) | −27.58; 72.98 |
| RDW | 0.028 (0.523) | −0.058; 0.114 | 0.010 (0.819) | −0.076; 0.097 | 0.254 (0.048) * | 0.001; 0.508 | 0.260 (0.048) * | 0.002; 0.518 | −1.204 (0.004) * | −2.023; −0.385 | −1.100 (0.008) | −1.920; −0.279 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palomares-Bolaños, J.; Olivero-Verbel, J.; Caballero-Gallardo, K. Hair Levels of Lead, Cadmium, Selenium, and Their Associations with Neurotoxicity and Hematological Biomarkers in Children from the Mojana Region, Colombia. Molecules 2025, 30, 3227. https://doi.org/10.3390/molecules30153227
Palomares-Bolaños J, Olivero-Verbel J, Caballero-Gallardo K. Hair Levels of Lead, Cadmium, Selenium, and Their Associations with Neurotoxicity and Hematological Biomarkers in Children from the Mojana Region, Colombia. Molecules. 2025; 30(15):3227. https://doi.org/10.3390/molecules30153227
Chicago/Turabian StylePalomares-Bolaños, Jenny, Jesus Olivero-Verbel, and Karina Caballero-Gallardo. 2025. "Hair Levels of Lead, Cadmium, Selenium, and Their Associations with Neurotoxicity and Hematological Biomarkers in Children from the Mojana Region, Colombia" Molecules 30, no. 15: 3227. https://doi.org/10.3390/molecules30153227
APA StylePalomares-Bolaños, J., Olivero-Verbel, J., & Caballero-Gallardo, K. (2025). Hair Levels of Lead, Cadmium, Selenium, and Their Associations with Neurotoxicity and Hematological Biomarkers in Children from the Mojana Region, Colombia. Molecules, 30(15), 3227. https://doi.org/10.3390/molecules30153227








